12 July 2020 by Shahriar Lahouti. Last update May 12, 2025.
CONTENTS
- Preface
- Anatomy of Pericardium
- Physiology of tamponade
- Spectrum of Tamponade Severity
- Causes
- Clinical Presentation
- Subtypes of tamponade
- Differential diagnosis
- Bedside Evaluation
- Approach to diagnosis and management
- Objectives
- Diagnostic approach
- Management
- Tamponade in cardiac arrest
- General Principles
- Specific situation
- General Principles
- Tamponade with atrial fibrillation and rapid ventricular response
- Recap
- References
Preface
Cardiac tamponade is a potentially life-threatening disease process with myriad clinical presentations, frequently resulting in misdiagnosis and mismanagement.
In clinical practice, cardiac tamponade is not an “all-or-none” phenomenon, but rather a continuum of hemodynamic impairment. Several misconceptions regarding subacute tamponade can make it a challenging diagnosis *. Prompt diagnosis and appropriate management of tamponade is possible only when a clinician grasps the pathophysiology of the disease and the diagnostic value of related findings in clinical exam and echocardiography.
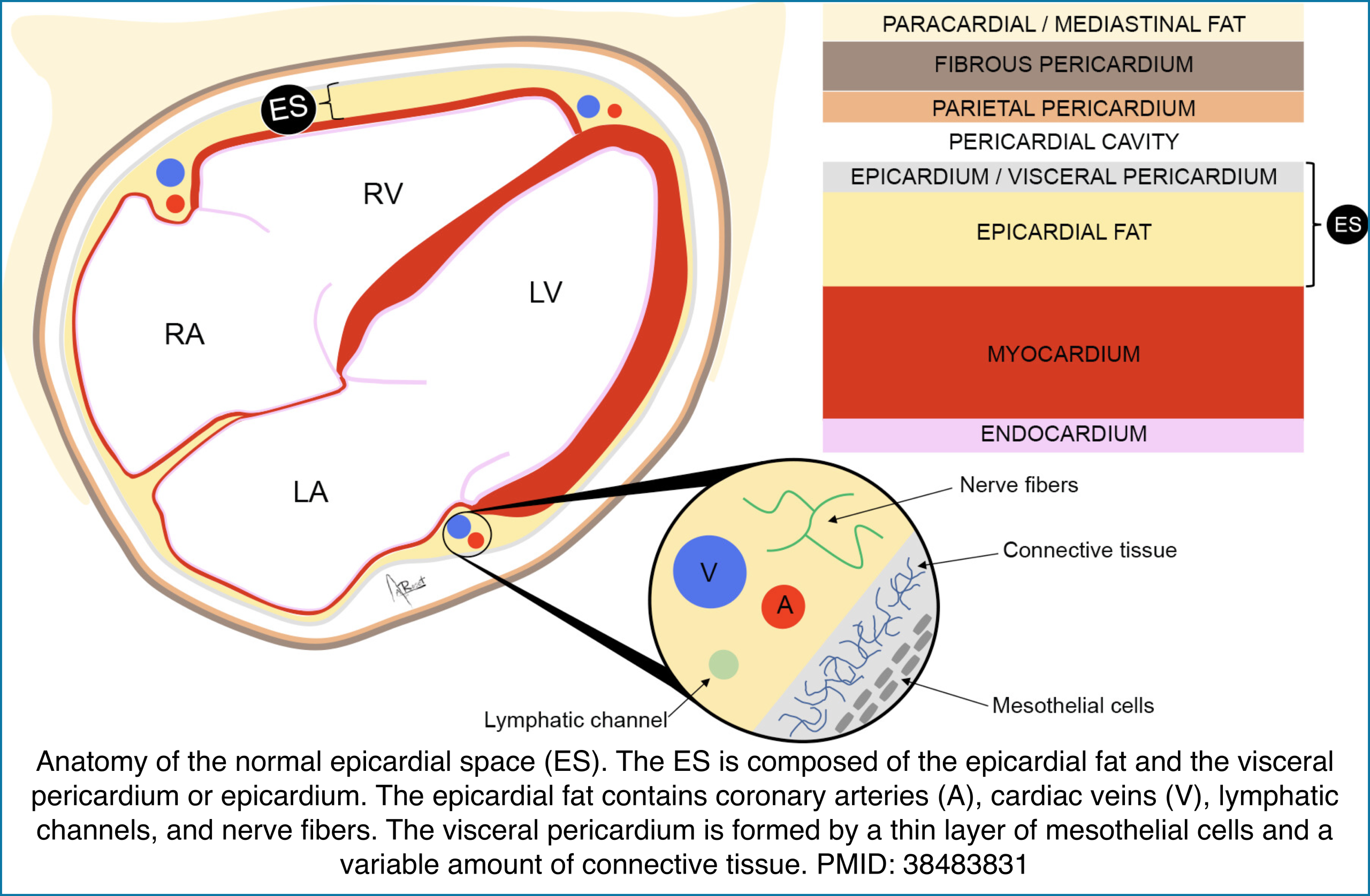
Anatomy
The pericardial space is contained by the parietal and visceral layers. Arterial supply to the whole pericardium is via branches of the thoracic aorta, and venous flow drains into the superior vena cava. Drainage of pericardial fluid occurs through the parietal pericardial lymphatic.
The parietal pericardium covers the proximal parts of all the great vessels, which is why the rupture of the ascending aorta leads to cardiac tamponade.
- Understanding the circulatory dynamics of pericardial fluid helps to grasp the development of pericardial effusion in specific conditions, e.g., pulmonary arterial hypertension (discussed later)
⎮Epicardial space (ES) is the anatomic region located between the myocardium and the pericardium.
- This space includes the visceral pericardium and the epicardial fat that contains the epicardial coronary arteries, cardiac veins, lymphatic channels, and
nerves. - ⚠️Don’t mistake pericardial effusion with epicardial fat (see below).
Physiology of tamponade
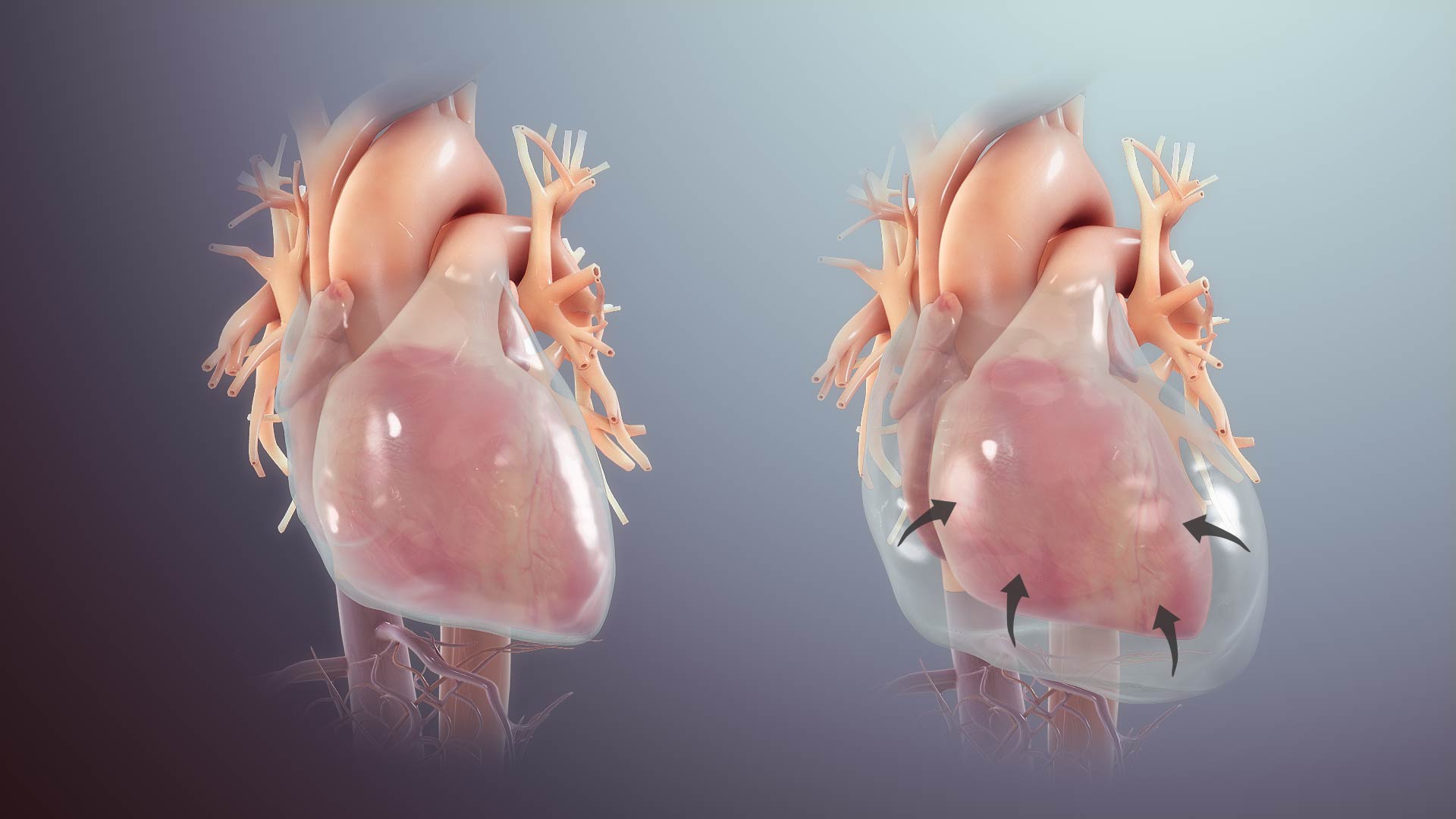
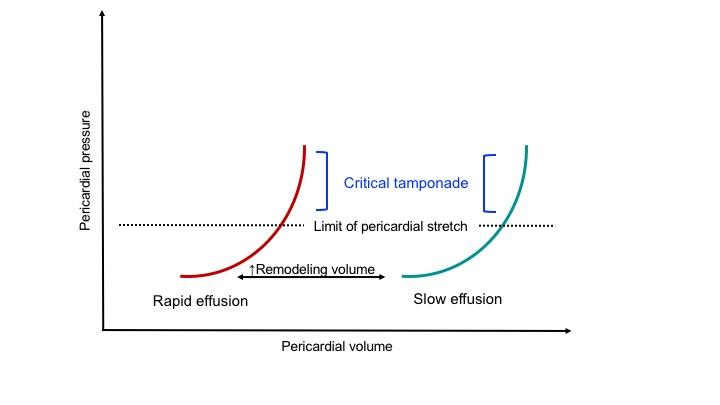
Normally, there’s roughly 50cc of pericardial fluid. The normal intrapericardial pressure is -4 to 1 mm Hg. The unique physical properties of the pericardium are conferred by the parietal pericardium, the majority of which is composed of stiff collagen fiber, and only a modest amount of elastin, imparting a small amount of potential stretch, so-called the ‘pericardial reserve volume’ *. The nature of the pericardial pressure-to-volume relationship is shown below *.
When pericardial fluid accumulation occurs beyond the critical point of exhaustion of elastic reserve and remodeling capacity, the intrapericardial pressure (IPP) rapidly rises and impairs the heart’s diastolic filling.
- Acute tamponade occurs at a much lesser volume than chronic tamponade, as the pericardium has no time for remodeling the matrix of the pericardium.
- In chronic tamponade, the pericardium has undergone remodeling, augmenting its stretching capacity; however, a definite amount of elastin will only allow a fixed reserve volume.
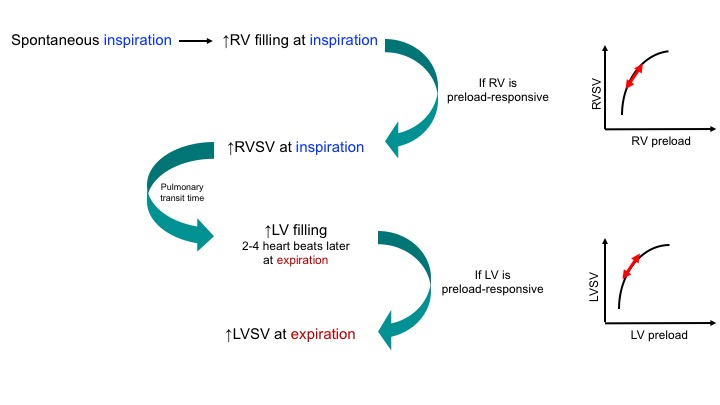
In a healthy physiologic state, the preload of all four chambers changes by inspiration and expiration, provided that the chambers are operating normally (not on a flat portion of the Starling curve). As shown below, SBP rises during expiration in a physiologic state:
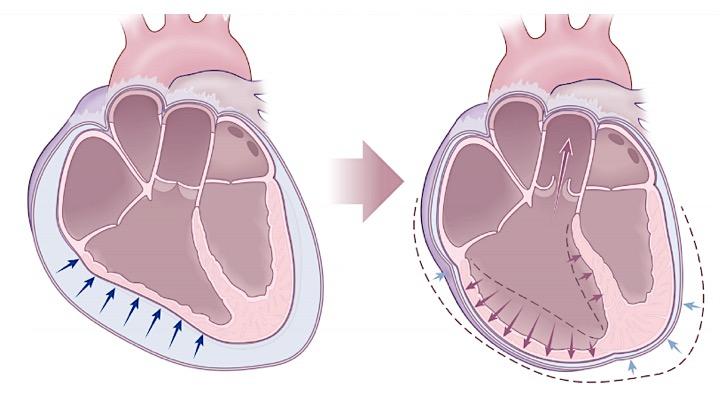
During tamponade physiology, there is a progressive increase in the IPP, and cardiac chambers shrink, resulting in reduced diastolic compliance. The following changes happen progressively:
- Progressive decline in SBP during inspiration (ventricular interdependence)
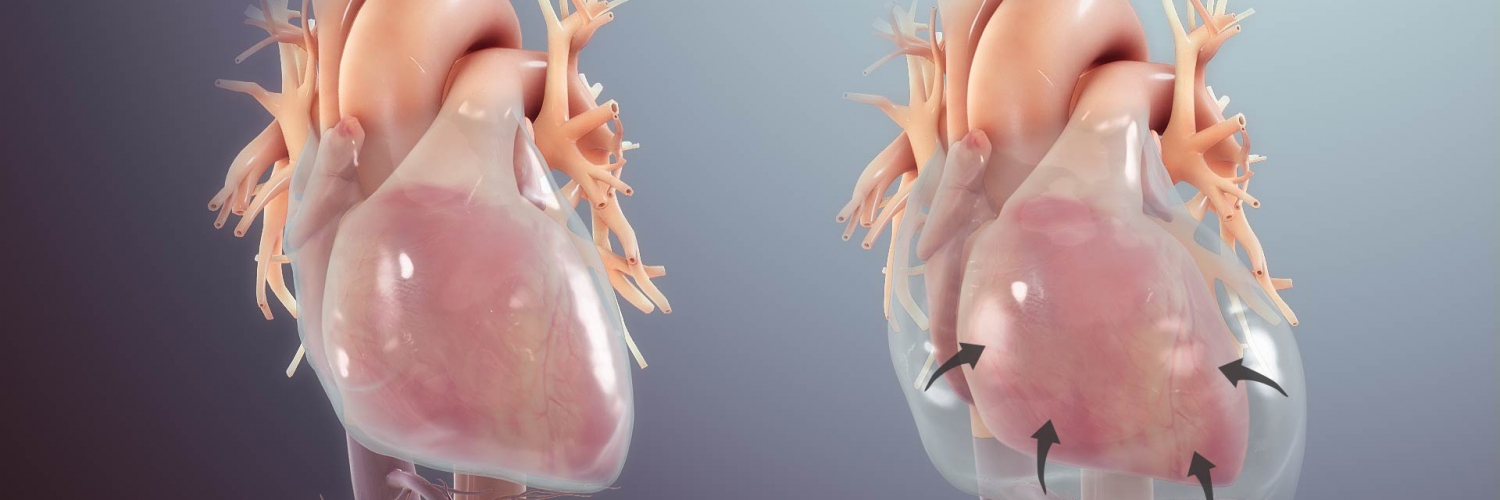
- Since the RV diastolic filling is jeopardized by the impact of pericardial effusion, the right heart fills during inspiration at the expense of the left heart; therefore the interatrial and interventricular septum bow to the left (picture on the left below). This diminishes LV diastolic filling during inspiration, and therefore LV cardiac output and SBP drop during inspiration (a component of pulsus paradoxus in tamponade).
- Progressive reduction in cardiac output
- The ongoing shrinkage of cardiac chambers (right-sided chambers collapse earlier than the left side as the right heart filling pressure is lower) during diastole, and reduction in venous inflow to the right atrium, lead to a progressive decline in cardiac output and a narrower pulse pressure.
Spectrum of Tamponade Severity
Cardiac tamponade is Not an “all-or-none” phenomenon, rather, it has a hemodynamic spectrum ranging from what is called “hemodynamic tamponade” on one extreme to the obstructive shock at the other end *, *. If the latter is not recognized and managed promptly, it can end up with a bradycardic response and eventually cardiac arrest (PEA).
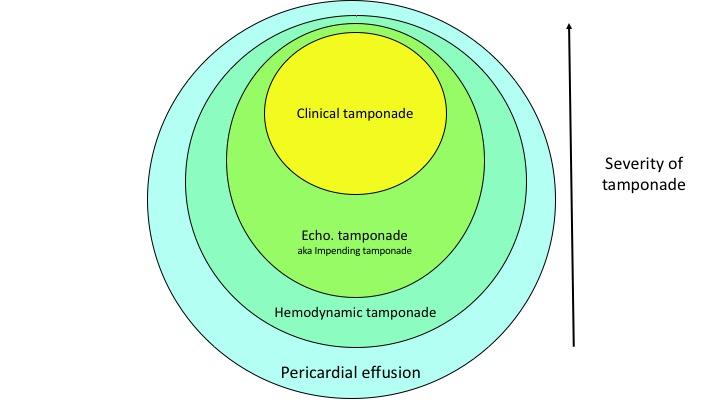
◾️Hemodynamic tamponade *
- IPP is increased but remains less than 10 mmHg
- IPP equilibrates with right atrial pressure (RAP) but is lower than left atrial pressure(LAP).
- Chamber collapse does not occur. There’s a minimal decrease in cardiac output and SBP during inspiration, but still lying within the normal range
◾️Echocardiographic tamponade, aka Impending tamponade
- 10mmHg< IPP <20mmHg
- IPP equilibrates with LAP.
- Collapsing right-sided chambers happen (RA collapses earlier than the RV)
- Further decrease in SBP during inspiration meets the definition of pulsus paradoxus.
- Cardiac output decreases further, but there’s no clinical s/s of shock
◾️Clinical tamponade (obstructive shock)
- IPP>20mmHg
- Severe Cardiac output reduction causes s/s of frank shock
◾️Debunking a common myth: This pericardial effusion is not hemodynamically significant!
- Tamponade walks through a spectrum of hemodynamic impairment *. Almost all pericardial effusions impair hemodynamics to some extent. Some patients remain clinically asymptomatic through their course and often effusion fades away following the resolution of possibly benign associated conditions, e.g., pericardial inflammation, while others may progress to clinical tamponade manifested as frank shock.
- Studies have shown that drainage of pericardial fluid, even in asymptomatic patients, will decrease RAP and PCWP, debunking the myth that asymptomatic pericardial effusion is not hemodynamically significant *.
- This does not imply that all pericardial effusions should be drained. A large pericardial effusion may be’ conservatively managed. (Indications for urgent pericardial drainage will be discussed below.)
Causes
◾️Pericardial effusion can be associated with virtually all causes of pericarditis, but those etiologies with a higher risk for progression to tamponade are *:
- Infectious: TB, bacterial, fungal, HIV-associated infections.
- Neoplastic disease.
- Hemopericardium:
- Chest trauma.
- Post-MI LV wall rupture.
- Complications of cardiac procedures, such as cardiac surgery, pacemaker insertion, atrial fibrillation ablation, and PCI with coronary artery perforation.
- Aortic dissection.
- Uremia (may cause hemopericardium too)
◾️Other causes of pericardial effusion that are less likely to progress to tamponade:
- Systemic autoimmune disease.
- Hypo-hyperthyroidims
- Early and late pericarditis in the setting of acute myocardial infarction.
◾️Finally, some conditions rarely progress to tamponade:
- Pericardial transudate due to hemodynamic causes such as heart failure, pulmonary hypertension, and hypoalbuminaemia.
- Pericardial transudate in the last trimester of pregnancy.
Clinical presentation
Tamponade is not a clinical diagnosis *. In fact, clinical diagnosis of tamponade is tricky since none of the suggested symptoms and signs are sensitive or specific. Moreover, clinical presentation varies based on severity and subtypes of tamponade.
Tempo
◾️The onset of the disease could be classified into:
- Acute: Acute cardiac tamponade occurs within minutes, due to trauma, rupture of the heart or aorta, or as a complication of an invasive cardiac procedure.
- This results in a picture resembling cardiogenic shock that, if not timely managed, leads to cardiac arrest and death.
- Subacute: occurs over days to weeks and is associated with neoplastic, uremic, or idiopathic pericarditis.
- Symptoms include fatigue, chest tightness, shortness of breath, and peripheral edema.
Clinical symptoms of tamponade
- Shortness of breath (despite normal oxygen saturation):
- This is the most sensitive symptom (66-90%) *.
- Exertional fatigue (3-14%)
- Chest tightness (12-74%)
- Syncope or presyncope upon standing or walking (3-6%)
- Abdominal pain (12-61%)
- Palpitation (3%)
- Orthopnea (23-51%)
- Peripheral edema (in subacute tamponade)
- Fever (7-70%)
- Severe tamponade can present with signs and symptoms of shock, e.g., altered mental status, and multiorgan failure.
- An untreated tamponade ends up with a bradycardic response (impending to crash) and ultimately cardiac arrest (PEA).
Physical findings
- Tachycardia
- It is part of a compensatory response to diminished cardiac output and, in contrast to hypotension, it is almost invariably present in all patients with impending to tamponade, except for patients taking medications that alter the HR, as well as when tamponade is associated with hypothyroidism.
- Bradycardia
- In terminal tamponade (impending to crash), the paradoxical bradycardic response may supervene.
- Tachypnea
- By and large, it’s not a specific sign but highly sensitive to the presence of occult critical illness.
- Bradypnea
- A crashing patient impending to arrest develops bradypnea and agonal breathing.
- Hypotension (e.g. MAP< 65 mmHg)
- It is a late finding in subacute cardiac tamponade. On the other hand, not all patients with subacute tamponade are hypotensive.
- Studies show that 27% to 43% of patients presenting with subacute tamponade are hypertensive *.
- Shock index (HR/SBP)
- SI is a more reliable index than tachycardia or BP alone to reveal underlying occult instability. Shock index > 1 suggests possible shock.
- Narrow Pulse Pressure
- It could be an early sign of dropping cardiac output.
- Elevated JVP
- Diaphoresis, cool clammy skin, mottling
- Suggest poor tissue perfusion and shock state.
Pulsus paradoxus
◾️Physiological background:
- Normally, during inspiration, increased venous inflow to the right ventricle causes an increase in right ventricle size that stimulates a reciprocal compression of the left ventricle (secondary to pericardial restraint), leading to reduced cardiac output. This will cause a drop in systolic blood pressure by ~10 mmHg.
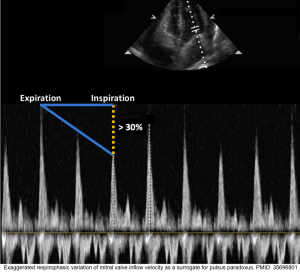
◾️Definition: Pulsus paradoxus is present when the decrease in systolic arterial pressure is >10 mmHg during inspiration (shown below).
◾️Nomenclature: Pulsus paradoxus is not a ‘paradoxical’ phenomenon *, rather it is an exaggeration of physiological decrease in SBP with inspiration
◾️Measurement: It can be measured most accurately by examining the arterial line tracing over several respiratory cycles.
-
Pulsus paradoxus correlates with 👉 variation in echocardiographic flow velocities. see below
◾️Cutoff: In tamponade, the inspiratory drop in SBP is generally >10 mm. However, there is some disagreement about the optimal cutoff value.
- For patients with severe hypotension, a lower cutoff value will have superior sensitivity.
- Even a systolic drop in blood pressure of 8 mmHg may be enough to call it ‘impending tamponade’.
◾️Specificity: An elevated pulsus is not entirely specific for tamponade. Other causes include *:
- Obesity
- Asthma, COPD
- RVMI
- PE
- CHF
- Tension pneumothorax
- Bilateral pleural effusion
- Hypovolemic shock
◾️Sensitivity: An elevated pulsus is reasonably sensitive, with sources quoting sensitivity rates of 75-98%.
- However, there are several causes of a falsely normal pulsus despite the presence of tamponade, including:
- Low-pressure tamponade.
- Aortic regurgitation.
- Atrial septal defect.
- RVH without pulmonary arterial hypertension.
- Cardiogenic shock due to severe tamponade .
- Decreased LV compliance, i.e., diastolic dysfunction.
- Local pericardial adhesion.
- Positive pressure ventilation.
Kussmaul’s sign
◾️ Physiological background: Normally, during inspiration, jugular venous pressure decreases due to negative intrathoracic pressure.
◾️Definition: Kussmaul’s sign refers to a paradoxical increase in JVP during inspiration. See Media 🎥
◾️Pathophysiologic basis: During inspiration, downward diaphragmatic excursion compresses the abdominal contents.
- This increases venous return from the abdomen into the thorax.
- If the RV is unable to accommodate this influx of blood, then increased blood entry into the chest will cause intrathoracic venous congestion, which, in turn, increases the jugular vein pressure.
◾️Etiology
- Right ventricular failure (either acute or chronic), for example:
- Right ventricular myocardial infarction.
- Pulmonary embolism.
- Constrictive pericarditis or restrictive cardiomyopathy.
◾️Clinical values
- Diagnostic value
- The presence of Kussmaul’s sign indicates RV dysfunction and requires appropriate investigations for possible causes.
- Therapeutic value
- Kussmaul’s sign reflects an inability of the RV to handle additional preload *.
- This might theoretically imply that additional volume administration will not be beneficial.
Subtypes of tamponade
- Hypertensive hyperadrenergic tamponade *
- Low-pressure tamponade
- Tamponade with atypical regional pericardial effusion
- Tamponade with atypical chamber collapse
Hypertensive hyperadrenergic tamponade
- Most commonly seen in patients with a history of hypertension and advanced CKD *,*.
- Mechanism: Excessive sympathetic-adrenergic response to distress.
- Systolic blood pressure may reach 200 mmHg, exemplifying the poor correlation of SBP with cardiac output and tissue perfusion
- ⚠️The most serious treatment error is to treat the hypertension without draining the pericardial fluid since antihypertensive medications will blunt physiologic compensatory response, and cause worsening hemodynamic status.
Low-pressure tamponade
- In one study, 20% of patients who met catheterization criteria for tamponade were identified to have a lower IPP (< 7 mmHg) before pericardiocentesis.
- This can happen when ‘severe hypovolemia’ is superimposed on tamponade *.
- Volume loss can be caused by severe traumatic hemorrhage in patients with acute tamponade, or by long-term diuretic use, excessive dialysis, and dehydration in patients with subacute tamponade.
- Clinically, their most common presenting finding is dyspnea on exertion.
- Some classic findings of tamponade are absent such as JVD, pulsus paradoxus.
- Echo: Findings do not differ from classic tamponade, except that:
- IVC is not enlarged.
- Pulsus paradoxus may be absent
- They fulfill catheterization criteria for diagnosis of tamponade, however, their IPP has modest elevation.
- Their hemodynamic response, e.g., cardiac output improvement to therapeutic pericardial fluid drainage, is excellent.
- However, the effect of volume resuscitation on the improvement of their hemodynamic indices is not well-established.
- Oftentimes, the initial step in management involves volume resuscitation, e.g., crystalloid or blood, depending on the clinical context. This may have one of two general consequences:
- The patient stabilizes and no longer has any significant tamponade.
- Such patients remain at risk for subsequently developing tamponade, but they don’t represent an immediate emergency.
- The central filling pressures increase, but this doesn’t cause clinical improvement.
- At this point, the patient has transitioned to a typical state of tamponade (aka, “high-pressure tamponade”).
- The patient stabilizes and no longer has any significant tamponade.
Tamponade with atypical regional effusion
- Etiology: After cardiac surgery, pericardiotomy *.
- Localized effusion, if limited around the left heart, may not show some typical clinical and echocardiographic findings of classic tamponade.
- For example, the jugular vein distension and right-sided chamber collapse on echo could be absent.
Tamponade with atypical chamber collapse
- The likelihood of cardiac chamber collapse is influenced by *,*,*, *, *
- Net balance between the two opposing forces, the IPP, which puts restraints on cardiac chambers’ distensibility during diastole, and the intracardiac end-diastolic pressure within each chamber.
- The wall thickness of cardiac chambers.
- Right-sided chamber collapse may be absent in conditions where either right ventricle end-diastolic pressure is very high, or the RV wall has been thickened, including:
- Right ventricular hypertrophy (RVH)
- Tricuspid regurgitation (TR)
- Right-sided paced chamber
- Coexisting severe LV dysfunction
- Pulmonary arterial hypertension (PAH) 👇
Tamponade in Pulmonary Arterial Hypertension
◾️Pathogenesis of pericardial effusion in PAH
- Elevated pulmonary arterial pressure leads to increased right ventricular and subsequently right atrial filling pressure.
- Elevated RAP will impair venous and lymphatic drainage of the parietal pericardium, resulting in pericardial fluid accumulation.
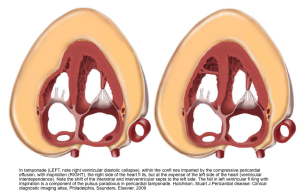
💡Development of pericardial effusion in PAH is a compensatory response, restraining distensibility of the high-pressured right ventricle and therefore preventing intraventricular and interatrial septal bowing to the left. Thereby, it preserves left-sided filling pressure and cardiac output to some degree (right figure below).
⚠️It is extremely dangerous to remove pericardial fluid in the context of PAH, as following drainage, restraint to the RV is eliminated, and septal bowing to the left occurs, leading to rapid hemodynamic decompensation of the patient (picture on the left below).
◾️General management strategies:
- Generally, drainage of hemodynamically stable pericardial effusion in PAH is not warranted and is risky.
- If the patient’s hemodynamic status is stable, a gentle trial of a diuretic may be tried.
- In unstable patients:
- Measures to ↓RV afterload to optimize the RV pressure-volume curve should be attempted before disturbing the integrity of the protective pericardial restraint mechanism.
- This may include the administration of pulmonary artery vasodilators such as nitric oxide, nitroglycerin *, or milrinone * via inhalation, and optimizing oxygenation and ventilation (as hypoxia and acidosis cause pulmonary artery vasoconstriction).
- As a last resort, removing a minimal amount of fluid is enough. Gradual drainage is advised.
- Development of pericardial effusion in patients with PAH is a poor prognostic sign *.
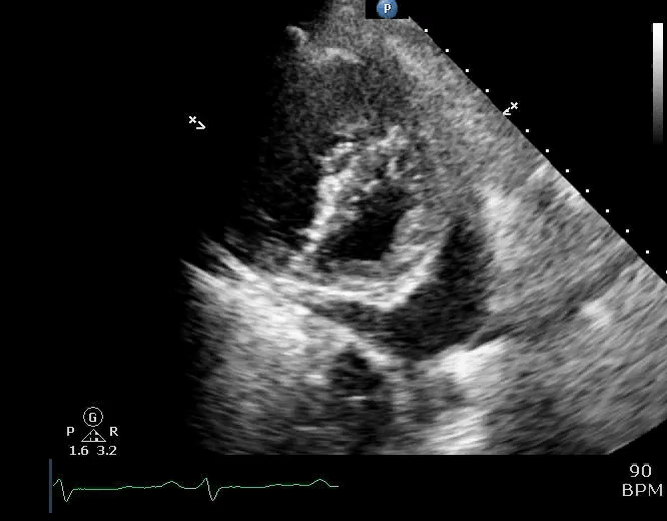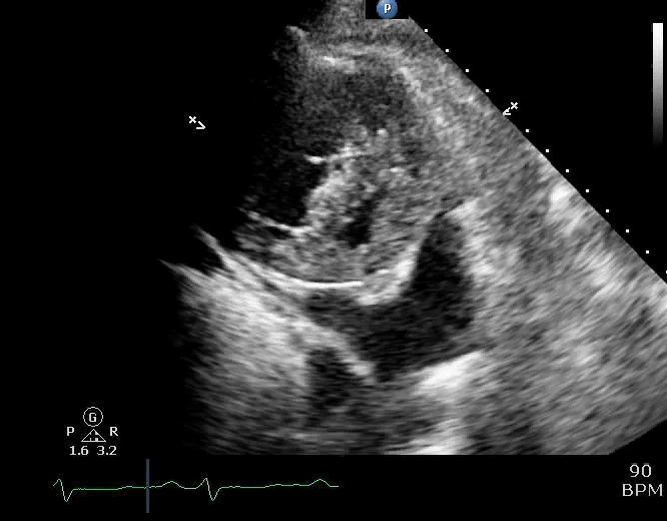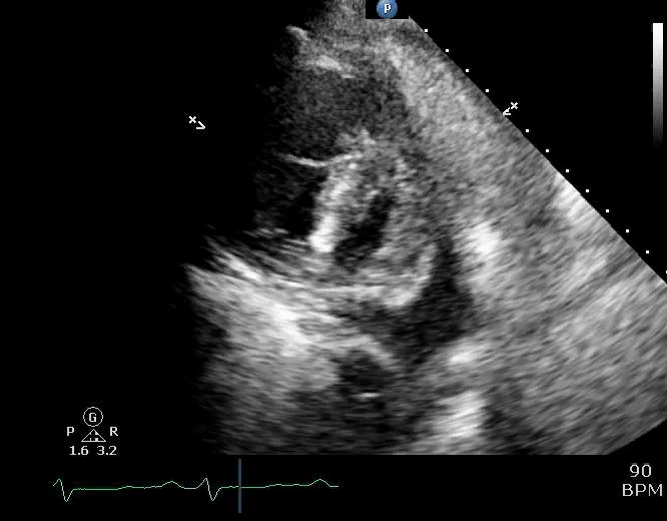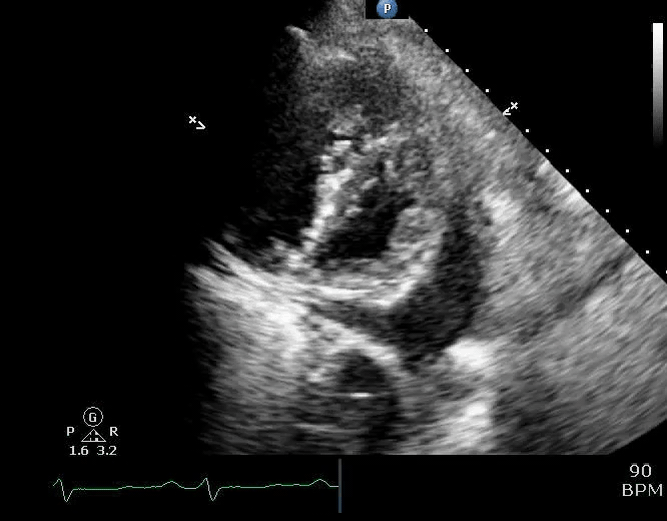
Parasternal short-axis view showing a large pericardial effusion, systolic and diastolic flattening of the interventricular septum, and severely enlarged right ventricle 16
Differential diagnosis
The differential diagnosis of cardiac tamponade depends upon the clinical presentation:
- Acute cardiac tamponade. The Ddx includes:
- AMI (especially with RV involvement)
- Massive PE
- Aortic dissection
- Tension pneumothorax
- Subacute cardiac tamponade. The Ddx includes:
- Heart failure
- Cirrhosis
- Constrictive pericarditis
- Large pleural effusion *
- Corpulmonale
Bedside Evaluation
ECG
◾️Not a sensitive or specific diagnostic tool *. Possible findings may include
- Sinus tachycardia
- Low voltage (max QRS amplitude < 0.5 mV in the limb leads)
- Electrical alternans, and
- No features of acute myocardial ischemia *.
Labs
◾️Tamponade is not diagnosed with lab findings.
- Identifying the cause of the pericardial effusion is often suggested by
- The clinical setting in which it occurs
- The size of the effusion
- Associated symptoms.
- If not suggestive, labs may be obtained, including
- CBC, CMP, coagulation studies, thyroid function, inflammatory markers (CRP, ESR), troponin (only if myopericardial syndrome is suspected), ANA.
- 👉The diagnostic yield of pericardial fluid analysis is low.
Bedside Evaluation
Echocardiography
◾️Although Bedside echo is by far the most useful diagnostic tool in recognizing tamponade and plays a crucial life-saving role in diagnostic approaches to crashing patients, echocardiographic signs of tamponade are not virtually diagnostic for tamponade in some cases *.
- It is important to interpret echocardiographic signs in the context of a patient’s clinical picture and comorbidities.
Pericardial effusion
◾️Echo delineates the size, location, and possibly composition of pericardial fluid.
◾️A pericardial effusion should be classified by its hemodynamic impact, size, distribution, onset, and composition *.
◾️Size
- The size of effusion should be estimated by the end-diastolic diameter between the visceral and parietal pericardium (i.e., the parietal separation distance) *.
- It is important to acquire multiple cardiac views.
- Pericardial fluid generally appears first posteriorly to the left ventricle (LV), moves apically and anteriorly as the fluid volume increases, and finally progresses laterally and posteriorly to the left atrium (LA) *.
- An effusion may appear in one cardiac view but not in another, and what can appear as a small effusion in one view could appear large in another, and vice versa *.
- In Tamponade, a moderate to large effusion is usually present, and swinging of the heart within the effusion may be seen.
- 👉However, the pericardial effusion may be small in the setting of acute accumulation of pericardial fluid.
- The effusion may be loculated or circumferential. The latter is graded into *:
- Small: Echo-free space in diastole < 10 mm (~ 300 mL).
- Moderate: 10-20 mm (~ 400-600 mL).
- Large: > 20 mm (~ > 700 mL).
◾️Composition: Compared to simple serous fluid that appears anechoic, hemorrhagic or purulent contents may appear more echogenic.
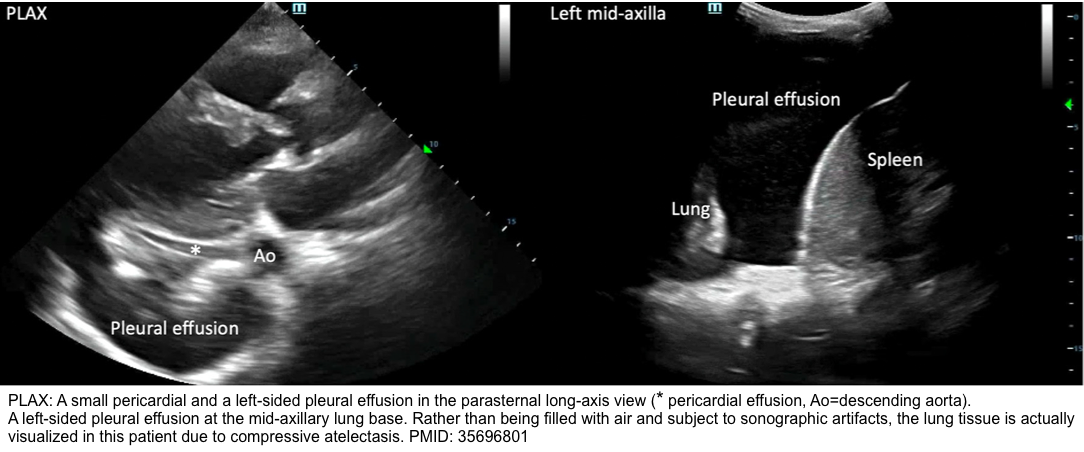
◾️Differential diagnosis of pericardial effusion on echo *:
- A large left-sided pleural effusion.
- In the PLAX view, a pericardial effusion will appear anterior to the descending aorta, whereas a pleural effusion will appear posterior to it *.
- As confirmation, assess the left lung base for the presence of the pleural effusion.
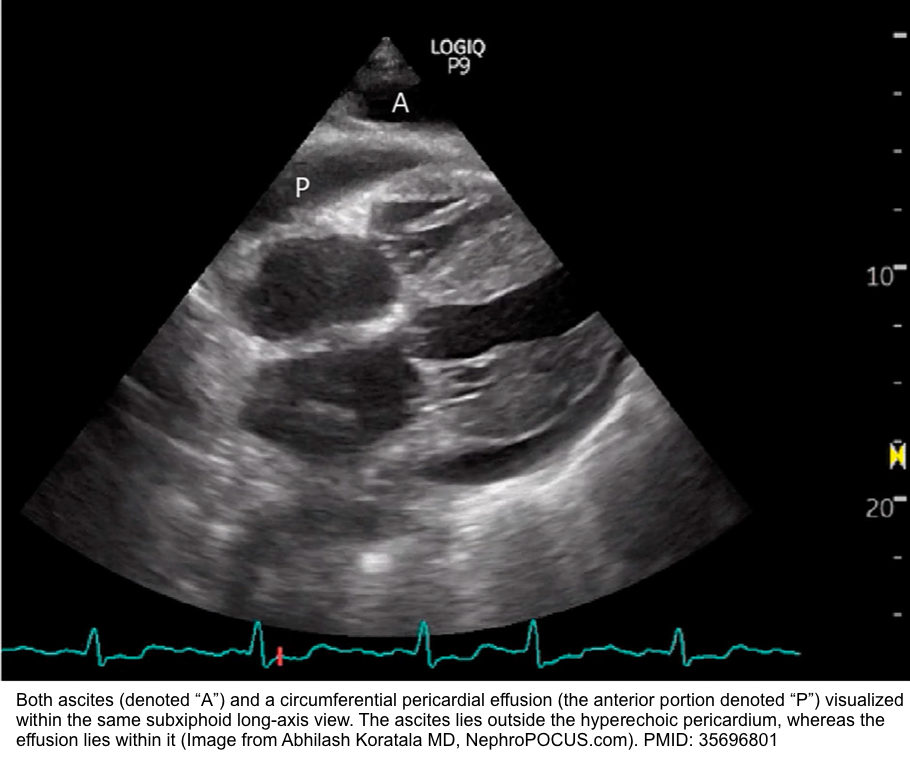
- Abdominal ascites
- In the SXLA view, abdominal ascites may mimic a pericardial effusion, but they can be differentiated by their locations outside and within the pericardium, respectively.
- Get other abdominal views of ascites to further point to the presence of ascites.
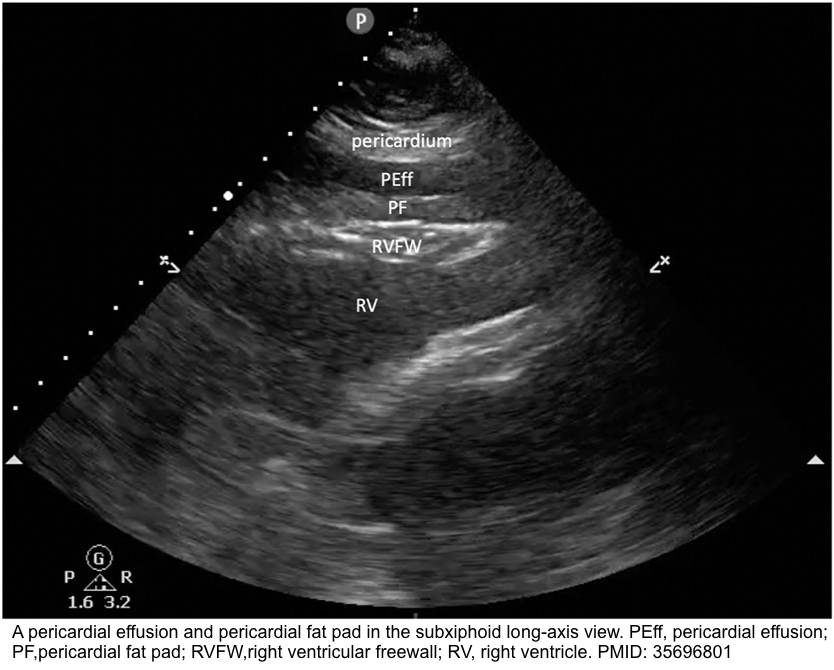
- Pericardial fat
- Pericardial fat appears “stippled” and is located in the anterior atrioventricular groove, best visualized in the parasternal long-axis (PLAX) *.
- In contrast, an effusion (usually anechoic) will typically collect in the most dependent pericardial location.
- It adheres to the myocardium and therefore moves in synchrony with the heart throughout the cardiac cycle.
- In contrast, an effusion will alternately appear smaller and larger as the adjacent cardiac chamber expands and contracts within the pericardium, whose space they share.
- Pericardial fat appears “stippled” and is located in the anterior atrioventricular groove, best visualized in the parasternal long-axis (PLAX) *.
- Cardiac tumors
- They can appear as echo-free spaces along the epicardium.
- Similar to a pericardial fat pad, these tumors will move in concert with the myocardium throughout the cardiac cycle *.
Pleural fluid can mimic pericardial fluid on #Echo. Key is finding pericardium. Look for the bright line just anterior to descending aorta! pic.twitter.com/WNt271sGAg
— Sam Ghali, M.D. (@EM_RESUS) August 1, 2017
Classic finding of Tamponade
Chamber Collapse
- Any cardiac chamber may collapse when intrapericardial pressure exceeds intracardiac pressure within the chamber.
- Both the right atrium (RA) and RV are compliant structures, so increased intrapericardial pressure causes their collapse when intracavitary pressures are only slightly exceeded by those in the pericardium *.
- When timely echocardiography is performed in patients with cardiac tamponade, cardiac chamber collapse is typically present before hemodynamic collapse.
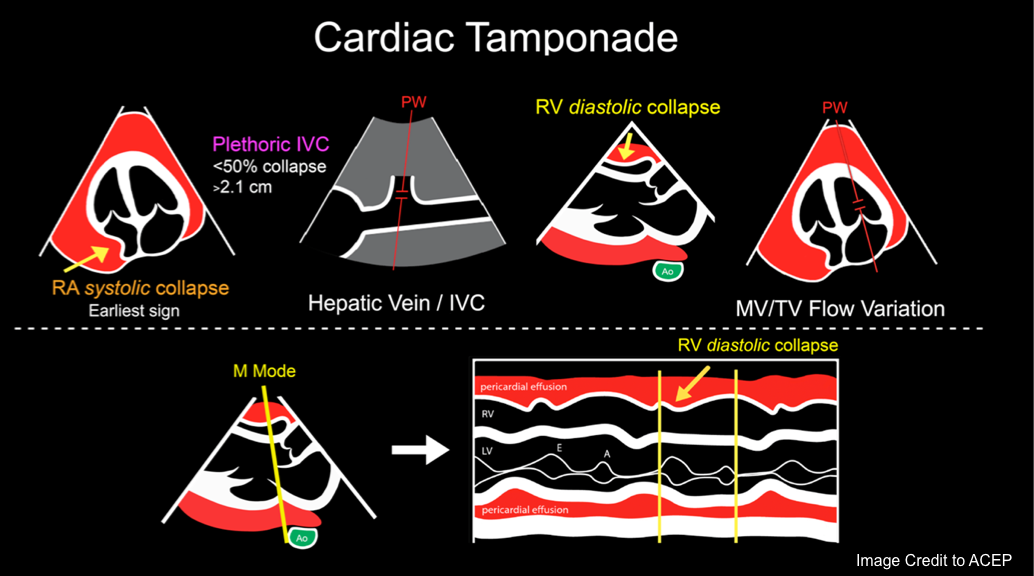
Right Atrial Diastolic Collapse
◾️RA collapse during atrial diastole (when tricuspid and mitral valves are closed) may be considered as an ‘earliest sign’ of tamponade, though it’s not a highly specific finding.
- The duration of RA collapse (RA Collapse > 1/3 cardiac cycle) is a more accurate sign for tamponade *.
- Although RA collapse is a sensitive sign, the predictive value of this sign is heavily influenced by the clinically derived pretest probability of tamponade.
- For example, in a patient with aortic dissection and pericardial effusion (High PTP), the presence of RA collapse on echo has a positive predictive value of 89%.
- On the other hand, in a patient with hypothyroidism and small pericardial effusion, the presence of RA collapse has a PPV of 8%.
🟥Pitfalls:
- False positive finding in large pleural effusion, and severe dehydration.
- False negative finding in states of ↑ RAP.
- RA diastolic collapse could be absent in the context of elevated right atrial pressure, such as in patients with pulmonary arterial hypertension.
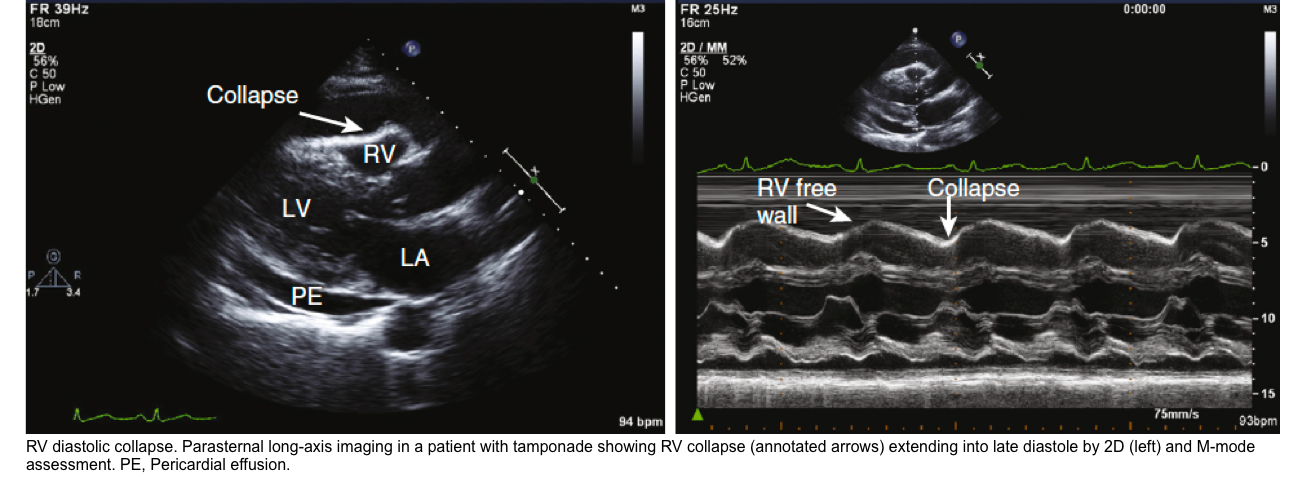
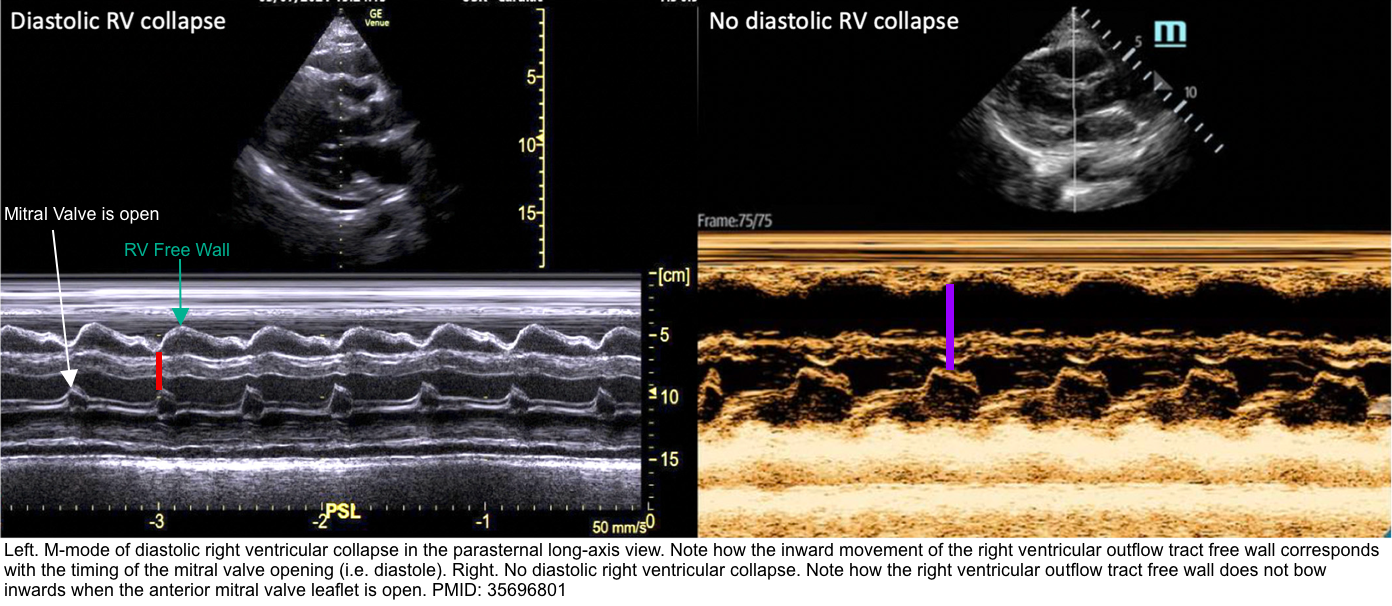
Right Ventricular Diastolic Collapse
- RV collapse during ventricular diastole is a later finding in tamponade but is a more specific sign than RA collapse.
- It’s critically important to identify whether RV collapses in diastole (which is pathologic) or in systole (which is normal).
🟥Pitfalls:
- RV diastolic collapse is relatively sensitive (~ 60%), however, it may be absent if other pathologies increase right-sided pressures (e.g., pulmonary hypertension, right ventricular myocardial infarction, tricuspid regurgitation).
- In this situation, the collapse of the left atrium may become a useful diagnostic sign.
- In this situation, the collapse of the left atrium may become a useful diagnostic sign.
- RV diastolic collapse has a high specificity (90%), but it may also be seen in low-pressure tamponade or a large left pleural effusion *.
- In the presence of low-pressure tamponade or a large left-sided pleural effusion, either of these conditions should be treated. The former with IV fluid, and the latter with drainage of pleural effusion.
- If RV diastolic collapse persists, that is more likely to indicate for presence of tamponade.
BEDSIDE ECHO IN CARDIAC TAMPONADE#FOAMed pic.twitter.com/AVWazhRrNs
— Sam Ghali, M.D. (@EM_RESUS) March 13, 2016
Left-sided chamber collapse
- Left atrial collapse is seen in approximately 25% of patients with hemodynamic compromise and is very specific for cardiac tamponade *.
- Left ventricular collapse is less common since the wall of the left ventricle is more muscular, but it can be seen in cases of regional cardiac tamponade *.
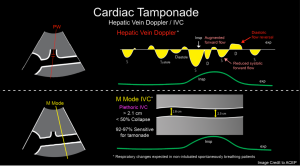
IVC Plethora
◾️Plethoric IVC (size > 2.1 cm with collapsibility < 50%) is reasonably sensitive (95%) but lacks specificity for tamponade *.
🟥Pitfalls
- False negative findings in hypovolemic states.
- In low-pressure tamponade (hypovolemic tamponade), IVC can be small or normal.
- False positive finding in ↑ RAP.
- An engorged IVC is not specific for tamponade *. This can be seen in conditions such as “Massive PE, PAH, RVMI, Volume Overload, Positive pressure ventilation”.
◾️Hepatic venous flow abnormalities (blunting or frank reversal of diastolic flow with expiration and systolic venous flow predominance) have high positive and negative
predictive values for cardiac tamponade, but cannot be adequately assessed in approximately one-third of patients *.
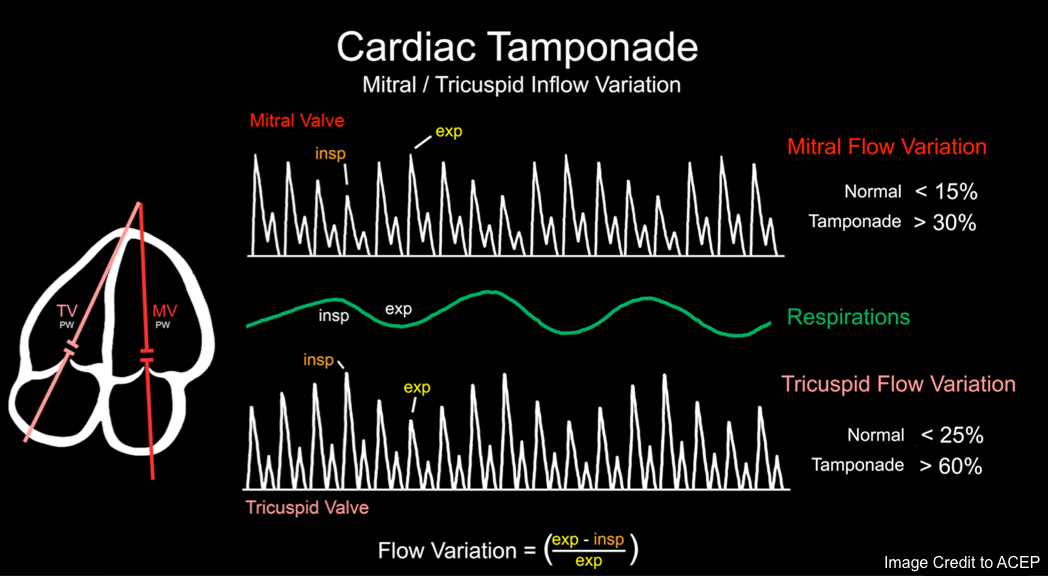
Respiratory variation in volume and flow
Mitral and Tricuspid valve inflow variation
◾️Tamponade will cause increased respirophasic variation across all the cardiac valves
- It’s an echocardiographic sign of pulsus paradoxus in tamponade *. See above.
- Normal physiologic respiratory variation in RV inflow and LV inflow velocity is < 20%-25%.
- However, in cardiac tamponade, the respiratory variations are *
- Mitral flow variation is > 30%.
- Tricuspid valve flow variation is > 60% *.
- Increased respiratory variation of carotid arterial and aortic blood flow may also be seen *.
🟥Pitfall
- The causes of false-positive or false-negative results are essentially the same as for pulsus paradoxus (discussed above).
- Respiratory variation of flows alone should not be used to diagnose tamponade in the absence of accompanying chamber collapse or abnormal hepatic vein flow.
Other imaging
- A large pericardial effusion may be suggested by an enlarged and globular heart.
- A CT scan should not be obtained to evaluate tamponade (rather, echocardiography is utilized for that).
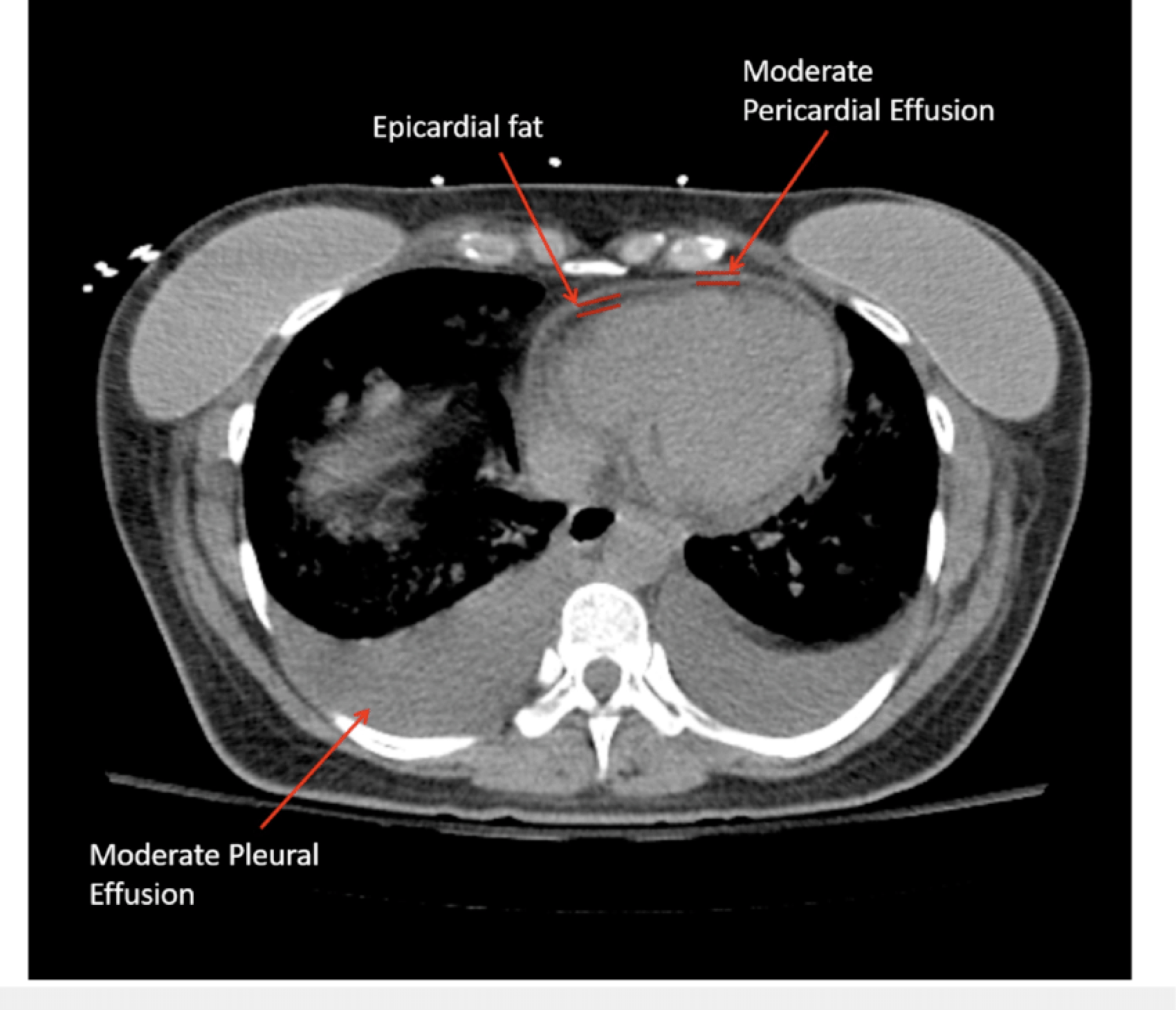
- A chest CT scan is highly sensitive for a pericardial effusion *.
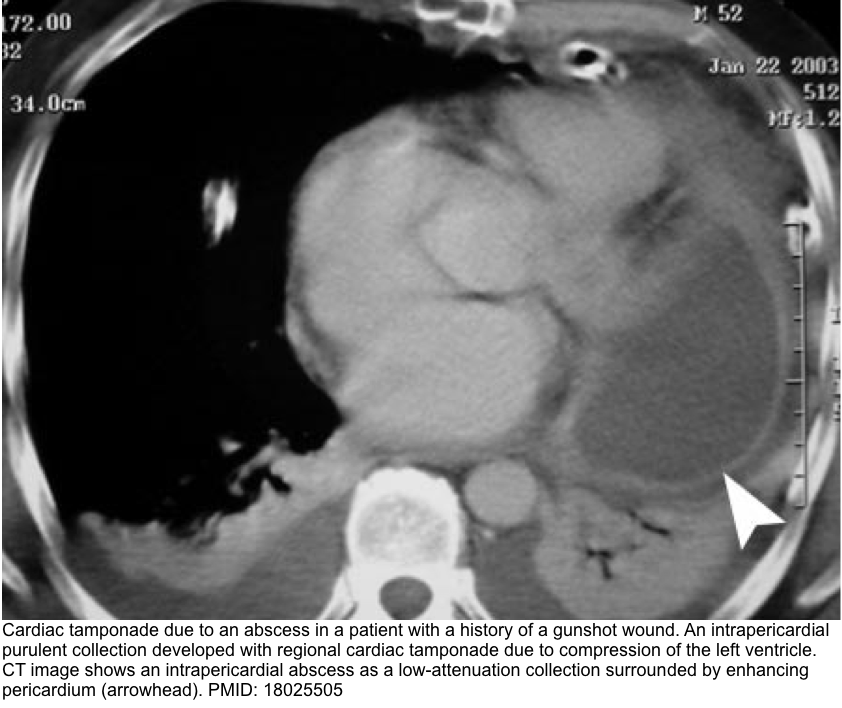
- A CT scan may reveal the presence of posterior loculated effusion, clot, or abscess, which may not be visible via transthoracic echo.
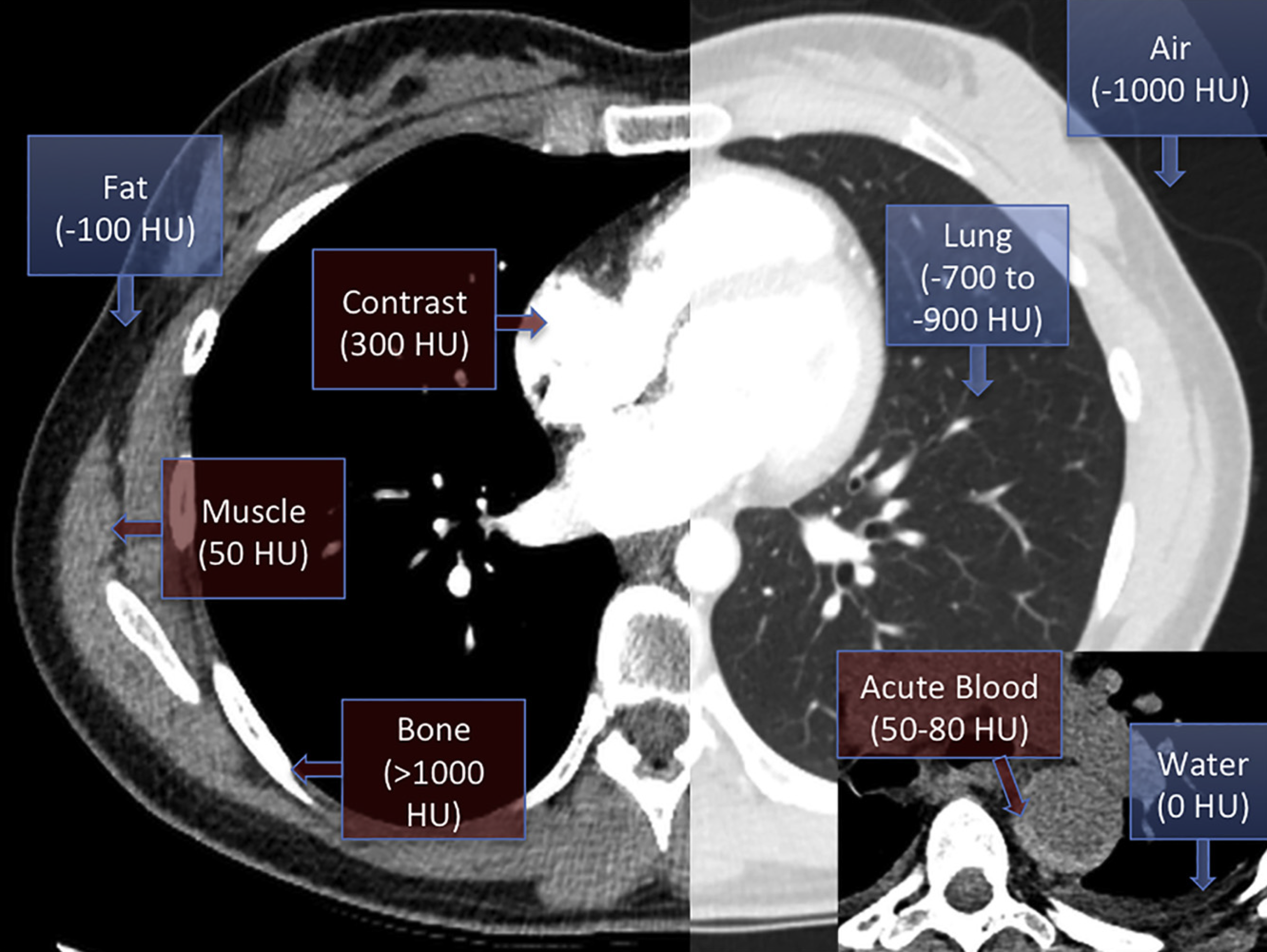
- CT also provides valuable information about the possible nature of pericardial effusions based on the attenuation measurements of the collection *.
- Pericardial fluid with relatively low attenuation values close to that of water likely represents a simple serous effusion from underlying heart failure or nonhemorrhagic carcinomatous involvement.
- An attenuation value greater than that of water suggests hemopericardium, malignancy, purulent exudates, or myxedematous effusion associated with hypothyroidism.
- Other reported findings may include the following *
- Note that each of these findings by itself is not specific for tamponade, but a constellation of findings, especially in the presence of a large pericardial effusion, can highly suggest tamponade.
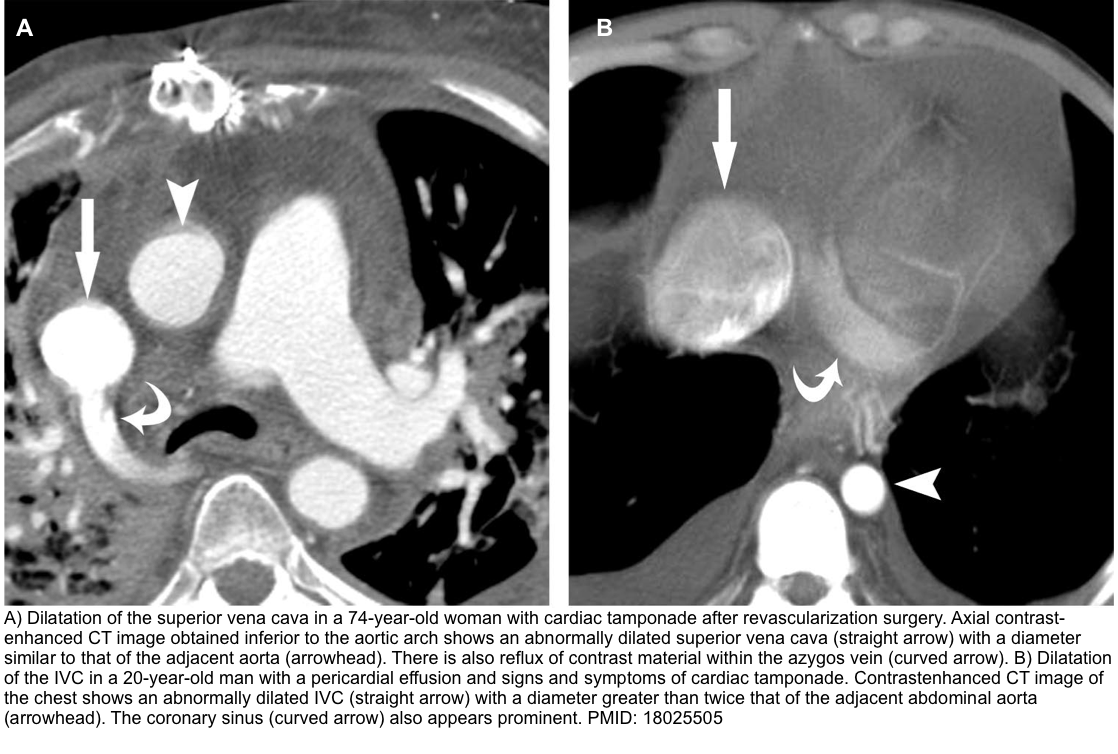
- Enlargement of the superior vena cava with a diameter ≥ adjacent thoracic aorta.
- Enlargement of the IVC with a diameter greater than twice that of the adjacent abdominal aorta.
- Reflux of contrast material within the IVC and the azygos vein.
- Periportal lymphedema.
- Enlargement of the hepatic and renal veins.
- Note that each of these findings by itself is not specific for tamponade, but a constellation of findings, especially in the presence of a large pericardial effusion, can highly suggest tamponade.
- Echocardiography has several limitations that are not typically seen with CT. Some advantages of CT over TTE:
- Assessment of the entire chest and detection of associated abnormalities in the mediastinum, lungs, and adjacent structures.
- Ability to demonstrate pericardial calcification, allowing measurement of its thickness, location, and extent.
- Lack of echocardiographic limitations such as:
- High rate of false-positive findings of echo due to adjacent pathologic conditions that may simulate pericardial effusions (ie, pleural effusions, lower lobe atelectasis, pericardial and intracardiac masses, or other mediastinal lesions).
- CT is better in identifying clotting or clots within the pericardium
- CT provides a better differentiation of small fluid collections from pericardial thickening, differentiation of fluid in the anterior and posterior spaces around the heart from epicardial fat, and identification of loculations in complex pericardial collections.
🔎 Epicardial fat vs. pericardial effusion.
- In CT, the density (HU) of epicardial fat vs pericardial effusion is shown below. In echo, see here 🎥.
Approach to diagnosis and management
Objectives
- Recognize critically ill patients
- Prompt mindful resuscitative efforts.
- Immediately perform POCUS in unstable patients to identify the possible causes that could explain patient hemodynamic instability
- In the presence of pericardial effusion on bedside echo, determine the likelihood probability of tamponade by a constellation of findings from history, presenting a clinical picture, and specific echocardiographic signs of tamponade *.
- Determine the safest, and most logical methods for drainage of TP, if ever needed
Diagnostic approach
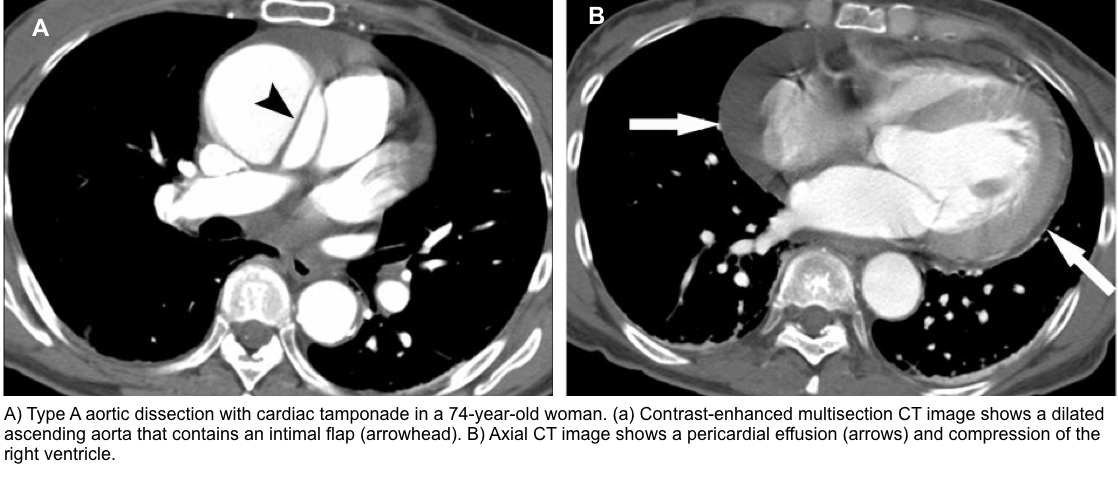
Rapid recognition of patients with tamponade is critical since the underlying hemodynamic derangement can rapidly progress, leading to patient death. Likewise, identifying life-threatening etiologies of tamponade, e.g., aortic dissection and myocardial rupture, is important as these warrant appropriate intervention.
It is also crucial to search for other disease processes that could otherwise explain the hemodynamic instability (innocent pericardial effusion).
Not uncommon in practice, patients presenting with shock may have multifactorial hemodynamic insults, i.e., mixed shock. For example, the development of septic shock in a patient with underlying chronic pericardial effusion can precipitate the development of obstructive tamponade physiology in addition to the distributive nature of septic shock.
Integrative information obtained from patients’ history, physical exam, ECG, and most importantly, bedside echocardiography is critical in diagnostic and therapeutic decision-making.
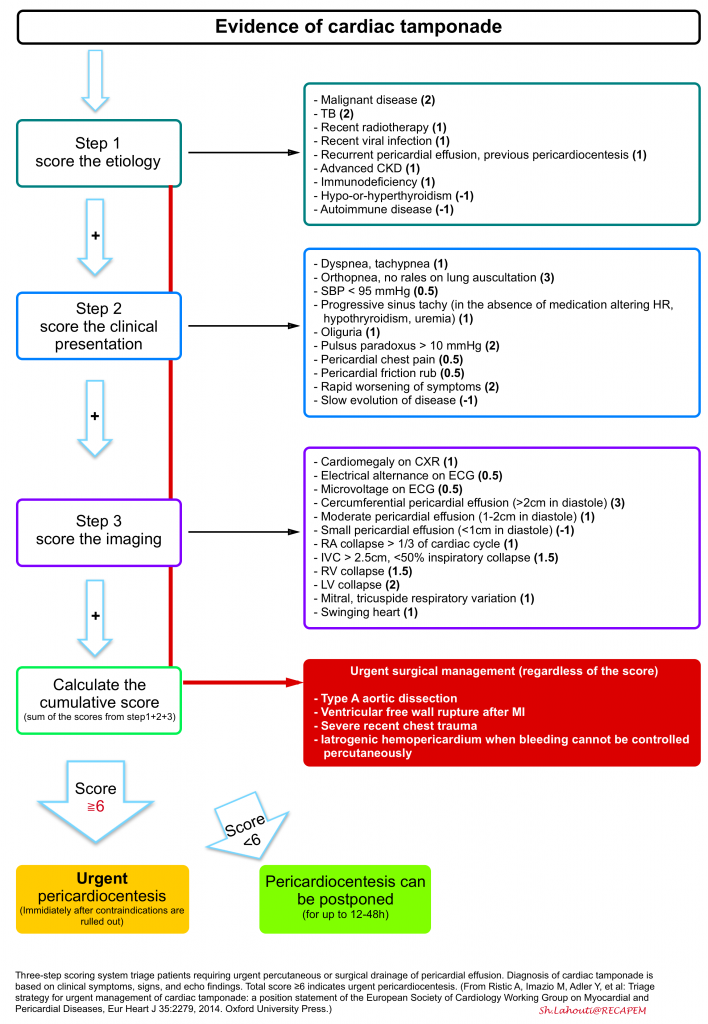
A tree-step scoring system has been proposed and can be employed for triage and appropriate therapeutic decision-making for patients with pericardial effusion who are not in cardiac arrest or impending to arrest.
In the presence of indications for surgical drainage, e.g., type A aortic dissection, there’s no need to use this scoring system, as patients obviously need urgent intervention *.
Management
Pericardial drainage – Background
- Pericardial drainage is the only definitive treatment of tamponade if indicated.
- Choice of pericardial fluid drainage
- Emergent Percutaneous Pericardiocentesis
- Surgical procedure (open surgical drainage with or without a pericardial window or video-assisted thoracoscopic pericardial window).
- There are no absolute contraindications for emergent percutaneous pericardiocentesis when the patient is in cardiac arrest or when hemodynamic status is worsening *.
- In crashing patients with cardiac tamponade, percutaneous pericardiocentesis should be attempted as a bridge to surgical intervention (even in the presence of relative contraindications).
- Pericardiocentesis under echo guidance is a relatively safe procedure for the experienced hand.
Relative contraindications for emergent percutaneous pericardiocentesis
- If the ultrasound shows only clotted blood within the pericardium.
- This generally is not amenable to needle drainage; either cardiothoracic surgery or thoracotomy may be needed.
- Coagulopathy.
- In a large case series, neither elevated INR nor thrombocytopenia correlated with increased risk of bleeding *.
- Severe pulmonary arterial hypertension (PAP >70 mmHg).
- In the following conditions, cardiothoracic surgery is indicated *:
- Type A aortic dissection.
- Ventricular free wall rupture following AMI.
- Traumatic hemopericardium
- Iatrogenic hemopericardium.
- Recent cardiothoracic surgery with pericardial clot.
- Failure or inability to achieve nonoperative drainage.
- Recurrent malignant effusion
- Suspicion of purulent pericarditis.
Complications of pericardial fluid removal
◾️Pericardial fluid removal by pericardiocentesis or surgical drainage is generally well tolerated and effective.
- Major complications of percutaneous pericardiocentesis include:
- Bleeding during pericardiocentesis
- Myocardial Perforation/Laceration
- Coronary Artery/Vein Laceration/Perforation
- Reaccumulation
- Pericardial decompression syndrome
- Pneumopericardium
- Pneumothorax
- Arhythmia
- Infection
- Injury to Abdominal Viscera
- Bleeding during pericardiocentesis
- Major complications of surgical pericardial drainage
- Reaccumulation
- Pericardial decompression syndrome
- Infection
- Arrhythmia
◾️Pericardial Decompression Syndrome (PDS)
- PDS is a potentially fatal consequence of pericardial drainage characterized by hemodynamic instability, pulmonary edema, and cardiogenic shock *.
- The time of onset ranges from immediately after the procedure to 48 hours later *.
- Risk factors *:
- Malignancy
- TB
- Inflammation
- Infection
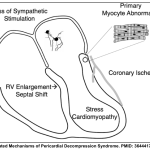
- Pathogenesis: PDS can occur secondary to multiple pathogenic processes (See Appendix). Postulated mechanisms include:
- Myocardial ischaemia
- Overexpansion of the right heart
- Autonomic imbalance from the sudden removal of sympathetic stimulation.
- Differential diagnosis *
- Cardiac perforation
- Vessel injury
- Pneumothorax
- Echocardiographic signs may include the acute development of hypokinesis of left, right, or both ventricles, without reaccumulation of pericardial fluid.
- In PDS, usually the right heart is expanded with an increase in venous return.
- Management
- Generally, management has to be multifaceted, given the potentially multifactorial pathogenesis.
- The treatment goal is to provide supportive care and minimize complications until full recovery.
- Prevention
- Gradual removal of pericardial fluid has not been shown to prevent the development of PDS *.
- Drainage of pericardial fluid should be attempted in a unit capable of airway management and inotropic support.
- Prognosis
- PDS is associated with high mortality (~22%) *.
Initial Resuscitation
The pivotal management of tamponade is drainage of the effusion, and this should be the priority. However, in some situations, it may not be possible to immediately drain the pericardium. The following measures may provide a bridge to definitive treatment (pericardial drainage). Follow 7 crucial principles to keep your patient alive! *
- Do not over-resuscitate your patients with fluid in the absence of clinically overt volume loss.
- The teaching that tamponade is a preload-dependent condition, requiring volume loading, is overly simplistic. Ventricular interdependence plays a much more critical role in determining LV cardiac output than does RV preload *. Several studies show that fluid is not always beneficial in tamponade *.
- Volume resuscitation should be performed under extreme caution.
- Patients who are volume-depleted are particularly susceptible to tamponade (low-pressure tamponade). Such patients can be stabilized by fluid administration. In other situations, be extremely cautious and administer fluid (if ever required) under careful monitoring.
- The appearance of Kussmaul’s sign may be an indicator that the limit of pericardial constraint has been exceeded, and additional fluid is not beneficial *.
- Avoid diuretics except in certain conditions, such as hemodynamically stable patients with PAH, wherein gentle diuretics may be considered.
- Vasopressor support (e.g., norepinephrine) may be used as a transient bridge to pericardiocentesis.
- Avoid inotropic medications (if possible) since endogenous inotropic stimulation is often maximal in such patients.
- The cardiac output of patients with tamponade is strongly dependent on “Heart Rate”.
- These patients have a limitation on diastolic filling and stroke volume. Therefore, their cardiac output is heart rate-dependent.
- These patients have a limitation on diastolic filling and stroke volume. Therefore, their cardiac output is heart rate-dependent.
- Most patients will develop compensatory tachycardia, except those who are taking medications that alter the HR, patients with conduction system disease, as well as when tamponade is associated with hypothyroidism.
- Speed up the heart rate; if the patient’s heart rate is inappropriately normal or bradycardia.
- Epinephrine may be a reasonable choice to accelerate the heart rate, without causing systemic vasodilation *.
- The cardiac output of patients with tamponade is strongly dependent on “Heart Rate”.
- Avoid Intubation and mechanical ventilation (if possible), since positive pressure ventilation can further impair cardiac filling.
- If the patient requires intubation for some other reason, it is generally preferable to drain the pericardium before intubation.
- Employ hemodynamically neutral intubation *. Use minimal PEEP and low inspiratory pressure.
- Avoid vasodilators, e.g., nitrates, since this will compromise the vasoconstrictor compensatory response.
- Reduce intrapericardial pressure. It is essential to identify and rapidly reverse other contributing factors leading to elevated external pressure compressing diastolic cardiac chambers. Some adjunctive interventions may indirectly reduce the pericardial pressure:
- Drainage of a large pleural effusion.
- Reduction of intra-abdominal pressure.
- Avoiding excessively high PEEP (among intubated patients).
- Anticoagulation reversal
- Anticoagulation reversal has potential benefits in certain clinical situations:
- If pericardiocentesis is anticipated (this will increase the procedure’s safety).
- In patients with hemopericardium, this is a crucial step.
- Anticoagulation reversal has potential benefits in certain clinical situations:
Percutaneous Pericardiocentesis: Methods
◾️Ideally, drainage will typically be performed by cardiology (usually with the placement of a pericardial drain) or cardiothoracic surgery (with the creation of a pericardial window in some patients). However, patients in shock who cannot immediately receive these procedures will require emergent drainage. This section discusses emergent pericardiocentesis, with the caveat that this isn’t the ideal procedure for most patients. Different patients may accumulate the deepest pockets of fluid in various locations.
- The subxiphoid approach may generally be less desirable for the following reasons:
- The needle needs to traverse a longer distance (especially in obese patients). This may make it impossible to reach the effusion using the finder needle from a central line or pigtail catheter kit. A lumbar puncture (LP) needle may be needed to remove fluid from the subxiphoid position (but this generally doesn’t allow passage of a guidewire through it).
- A subxiphoid approach introduces a risk of liver laceration, causing hemoperitoneum.
- Parasternal or apical approaches (through the chest wall) may allow fluid to be reached more easily.
-
- If a parasternal approach is taken, avoid the internal mammary artery, which often runs vertically a few centimeters lateral to the sternum. Ideally, the internal mammary artery may be identified using Doppler ultrasonography with a linear probe and specifically avoided.
- Elevating the head of the bed may cause fluid to shift into a more anterior distribution, facilitating pericardiocentesis from a parasternal or apical approach.
- The needle should be inserted just above a rib to avoid the neurovascular bundle (similar to a thoracentesis).
- Needle insertion should ideally be performed with direct visualization (using an in-plane approach). Continuous negative pressure should be used to aspirate fluid as soon as the needle punctures the pericardium. The tip of the needle should ideally be seen entering the pericardial space.
- If amber fluid returns, then fluid may be therapeutically aspirated (or a wire & catheter may be inserted).
- If bloody fluid returns, there may be some confusion regarding whether the needle is in the pericardium or the ventricle. This can be rapidly sorted out by injecting agitated saline with ultrasound guidance. Ideally, the bubbles will show up within the pericardium, confirming intrapericardial location.
Tamponade in cardiac arrest
General Principles
- Chest compressions alone are unlikely to be beneficial in this situation.
- The only viable options include:
- Immediate pericardiocentesis.
- Immediate thoracotomy.
- Drainage of the pericardium should probably be prioritized ahead of chest compressions.
Specific Situations
Spontaneously Resolving Pericardial Effusion During CPR
- For a cardiac arrest caused by cardiac tamponade, it is possible that injury to the pericardium from high-quality CPR would decompress the pericardial space into the pleural space, resulting in new pleural effusions *.
- This will increase the likelihood of achieving ROSC, since this inadvertent drainage is analogous to a percutaneous pericardiocentesis.
- Read more: 📖
Tamponade and atrial fibrillation with rapid ventricular response
AF with rapid ventricular response (RVR) is defined as a ventricular rate of more than 100 bpm. Patients with underlying AF rhythm can have RVR during high catecholamine states, fever, etc. However, AF is seldom the cause for instability (innocent bystander) unless the heart rate is more than 130-150 bpm.
Patients with tamponade are poorly tolerant of atrial fibrillation. Why?
- Tamponade causes RV early diastolic collapse → ↓RV diastolic filling during the passive phase.
- Therefore, RV filling is more tightly dependent on the atrial kick. This atrial contraction is lost in AF.
Although such patients are poorly tolerant to AF, trial of rate control medications is not safe, as almost all of these drugs have vasodilator properties that can blunt the compensatory response to tamponade, resulting in patient deterioration.
Rhythm control often is not helpful in chronic AF since even if the rhythm is converted to sinus rhythm, the cardiac output will not significantly improve (atrial stunting).
AF with RVR will slow down often when the acute physiologic insult is resolved.
👉Pearl: The presence of AF does not distort the interpretation of mitral and tricuspid inflow variation on the Doppler study.
RECAP
- Do not be fooled by BP! Hypotension is a late finding in a shock state. Moreover, Hypertensive tamponade presents with high BP. Shock Index is a better marker to identify ↑HR in relation to SBP.
- Not all pericardial effusions are enemies. Pericardial effusion in PAH is a compensatory response of our body to preserve LV cardiac output. Often, draining effusion results in poor outcomes.
- Do not mistake pleural effusion and pericardial fat for pericardial effusion.
- Remember that a plumped IVC is not specific for tamponade and is also seen in the state of RV strain (e.g., PAH, RVMI, MPE, biventricular heart failure)
- If you notice any chamber collapse on echo, pause there and identify whether it is collapsing during systole or diastole. Systolic collapse per se can happen during a hypovolemic state.
- During tamponade, ‘ventricular interdependence’ is more tightened. This explains the development of the pulsus paradoxus.
- Mitral and tricuspid valve inflow variation on Doppler is the echocardiographic sign of pulsus paradoxus in tamponade.
- Always think about the etiology that has caused tamponade. It might be more life-threatening than tamponade by itself.
- When tamponade is highly suspected based on a constellation of findings, the job is not done yet! Mixed shock is not uncommon in practice. Perform echocardiography on every shock patient.
- The most critically ill patients deserve the most aggressive management. Pericardiocentesis is not contraindicated for crashing patients or those in cardiac arrest. In traumatic tamponade, resuscitative thoracotomy is warranted for such patients.
- The benefit of performing chest compression for patients in cardiac arrest with tamponade is under question.
- Always calibrate your management to patient physiology. Patients who are on the end-rope of their physiologic reserve are extremely susceptible to even a minor fault. Rate-controlling patients with RVR AF may be counterproductive in such situations.
- Do not over-resuscitate your patients with fluid. It may be counterproductive as it worsens interventricular septum bulging to the left, further impairing LV cardiac output.
Appendix
Pathogenesis of pericardial decompression syndrome
Going further
- The Pericardium (FOAMcast)
- Acute Myopericardial Syndromes (EM:RAP)
- The Hemodynamically Neutral Intubation (EMcrit RACC)
- Pericardial Tamponade (Core Ultrasound)
References
1. Mekontso Dessap A, Chew MS. Cardiac tamponade. Intensive Care Med. 2018;44(6):936-939. doi:10.1007/s00134-018-5191-z
2. Ariyarajah, V., & Spodick, D. H. (2007). Cardiac tamponade revisited: A postmortem look at a cautionary case. Texas Heart Institute Journal, 34(3), 347–351.
3. APA Bodson, Laurent; Bouferrache, Koceïla; Vieillard-Baron, Antoine Cardiac tamponade, Current Opinion in Critical Care: October 2011 – Volume 17 – Issue 5 – p 416-424 doi: 10.1097/MCC.0b013e3283491f27
4. Ojeda, W., & Martínez-Toro, J. A. (2006). Diagnosis and management of pericardial effusions. Puerto Rico Health Sciences Journal, 25(3), 255–258. https://doi.org/10.4330/wjc.v3.i5.135
5. Schairer, J. R., Biswas, S., Keteyian, S. J., & Ananthasubramaniam, K. (2011). A systematic approach to evaluation of pericardial effusion and cardiac tamponade. Cardiology in Review, 19(5), 233–238. https://doi.org/10.1097/CRD.0b013e31821e202c
6. Argulian, E., & Messerli, F. (2013). Misconceptions and facts about pericardial effusion and tamponade. American Journal of Medicine, 126(10), 858–861. https://doi.org/10.1016/j.amjmed.2013.03.022
7. Reddy, P. S., Curtiss, E. I., & Uretsky, B. F. (1990). Spectrum of hemodynamic changes in cardiac tamponade. The American Journal of Cardiology, 66(20), 1487–1491. https://doi.org/10.1016/0002-9149(90)90540-H
8. Imazio, M., & Adler, Y. (2013). Management of pericardial effusion. European Heart Journal, 34(16), 1186–1197. https://doi.org/10.1093/eurheartj/ehs372
9. Argulian, E., Herzog, E., Halpern, D. G., & Messerli, F. H. (2012). Paradoxical hypertension with cardiac tamponade. American Journal of Cardiology, 110(7), 1066–1069. https://doi.org/10.1016/j.amjcard.2012.05.042
10. Vakamudi, S., Ho, N., & Cremer, P. C. (2017). Pericardial Effusions: Causes, Diagnosis, and Management. Progress in Cardiovascular Diseases, 59(4), 380–388. https://doi.org/10.1016/j.pcad.2016.12.009
11. Shabetai, R. (2004). Pericardial effusion: Haemodynamic spectrum. Heart, 90(3), 255–256. https://doi.org/10.1136/hrt.2003.024810
12. Kapoor, T., Locurto, M., Farina, G. A., & Silverman, R. (2012). Hypotension is uncommon in patients presenting to the emergency department with non-traumatic cardiac tamponade. Journal of Emergency Medicine, 42(2), 220–226. https://doi.org/10.1016/j.jemermed.2010.05.071
13. Rowan, S. B., & Krantz, M. J. (2006). Paradoxical decrease in blood pressure after relief of cardiac tamponade: The role of sympathetic activity. Medical Science Monitor, 12(2), 16–20.
14. Sagristà-Sauleda, J., Angel, J., Sambola, A., Alguersuari, J., Permanyer-Miralda, G., & Soler-Soler, J. (2006). Low-pressure cardiac tamponade: Clinical and hemodynamic profile. Circulation, 114(9), 945–952. https://doi.org/10.1161/CIRCULATIONAHA.106.634584
15. Grumann, A., Baretto, L., Dugard, A., Morera, P., Cornu, E., Amiel, J. B., & Vignon, P. P. (2012). Localized cardiac tamponade after open-heart surgery. Annals of Thoracic and Cardiovascular Surgery, 18(6), 524–529. https://doi.org/10.5761/atcs.oa.11.01855
16. Adams, J. R., Tonelli, A. R., Rokadia, H. K., & Duggal, A. (2015). Cardiac tamponade in severe pulmonary hypertension: A therapeutic challenge revisited. Annals of the American Thoracic Society, 12(3), 455–460. https://doi.org/10.1513/AnnalsATS.201410-453CC
17. Fenstad, E. R., Le, R. J., Sinak, L. J., Maradit-Kremers, H., Ammash, N. M., Ayalew, A. M., … Kane, G. C. (2013). Pericardial effusions in pulmonary arterial hypertension: Characteristics, prognosis, and role of drainage. Chest, 144(5), 1530–1538. https://doi.org/10.1378/chest.12-3033
18. Plotnick, G. D., Rubin, D. C., Feliciano, Z., & Ziskind, A. A. (1995). Pulmonary hypertension decreases the predictive accuracy of echocardiographic clues for cardiac tamponade. Chest, 107(4), 919–924. https://doi.org/10.1378/chest.107.4.919
19. Plotnick, G. D., Rubin, D. C., Feliciano, Z., & Ziskind, A. A. (1995). Pulmonary hypertension decreases the predictive accuracy of echocardiographic clues for cardiac tamponade. Chest, 107(4), 919–924. https://doi.org/10.1378/chest.107.4.919
20. Sahay, S., & Tonelli, A. R. (2013). Pericardial effusion in pulmonary arterial hypertension. Pulmonary Circulation, 3(3), 467–477. https://doi.org/10.1086/674302
21. Yurtseven, N., Karaca, P., Kaplan, M., Ozkul, V., Tuygun, A. K., Aksoy, T., Canik, S., & Kopman, E. (2003). Effect of nitroglycetin inhalation on patients with pulmonary hypertension undergoing mitral valve replacement surgery. Anesthesiology, 99(4), 855–858. https://doi.org/10.1097/00000542-200310000-00017
22. Sablotzki, A., Startzmann, W., Scheubel, R., Grond, S., & Czeslick, E. G. (2005). Selective pulmonary vasodilation with inhaled aerosolized milrinone in heart transplant candidates. Canadian Journal of Anesthesia, 52(10), 1076–1082. https://doi.org/10.1007/bf03021608
23. Opotowsky, A. R., Ojeda, J., Rogers, F., Prasanna, V., Clair, M., Moko, L., … Forfia, P. R. (2012). A simple echocardiographic prediction rule for hemodynamics in pulmonary hypertension. Circulation: Cardiovascular Imaging, 5(6), 765–775. https://doi.org/10.1161/CIRCIMAGING.112.976654
24. Vaska, K., Wann, L. S., Sagar, K., & Klopfenstein, H. S. (1992). Pleural effusion as a cause of right ventricular diastolic collapse. Circulation, 86(2), 609–617. https://doi.org/10.1161/01.CIR.86.2.609
25. Merce, J., Sagrista-Sauleda, J., Permanyer-Miralda, G., Evangelista, A., & Soler- Soler, J. (1999). Correlation between clinical and Doppler echocardiographic findings in patients with moderate and large pericardial effusion: Implications for the diagnosis of cardiac tamponade. American Heart Journal, 138(4 I), 759–764. https://doi.org/10.1016/S0002-8703(99)70193-6
26. Klein, A. L., Abbara, S., Agler, D. A., Appleton, C. P., Asher, C. R., Hoit, B., … White, R. D. (2013). American society of echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: Endorsed by the society for cardiovascular magnetic resonance and society of cardiovascular computed tomography. Journal of the American Society of Echocardiography, 26(9), 965-1012.e15. https://doi.org/10.1016/j.echo.2013.06.023
27. Lancellotti, P., Price, S., Edvardsen, T., Cosyns, B., Neskovic, A. N., Dulgheru, R., Flachskampf, F. A., Hassager, C., Pasquet, A., Gargani, L., Galderisi, M., Cardim, N., Haugaa, K. H., Ancion, A., Zamorano, J. L., Donal, E., Bueno, H., & Habib, G. (2015). The use of echocardiography in acute cardiovascular care: recommendations of the European Association of Cardiovascular Imaging and the Acute Cardiovascular Care Association. European Heart Journal. Acute Cardiovascular Care, 4(1), 3–5. https://doi.org/10.1177/2048872614568073
28.Imazio, M., & Adler, Y. (2013). Management of pericardial effusion. European Heart Journal, 34(16), 1186–1197. https://doi.org/10.1093/eurheartj/ehs372
29. Sherbino, J. (2009). Does This Patient With a Pericardial Effusion Have Cardiac Tamponade? Annals of Emergency Medicine, 53(3), 390–391. https://doi.org/10.1016/j.annemergmed.2008.02.015
30. Sagristà-Sauleda, J., Mercé, J., Permanyer-Miralda, G., & Soler-Soler, J. (2000). Clinical clues to the causes of large pericardial effusions. American Journal of Medicine, 109(2), 95–101. https://doi.org/10.1016/S0002-9343(00)00459-9
31. Ristić, A. D., Imazio, M., Adler, Y., Anastasakis, A., Badano, L. P., Brucato, A., … Charron, P. (2014). Triage strategy for urgent management of cardiac tamponade: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. European Heart Journal, 35(34), 2279–2284. https://doi.org/10.1093/eurheartj/ehu217
32. Cruz, I., Stuart, B., Caldeira, D., Morgado, G., Gomes, A. C., Almeida, A. R., … Pereira, H. (2015). Controlled pericardiocentesis in patients with cardiac tamponade complicating aortic dissection: experience of a centre without cardiothoracic surgery. European Heart Journal. Acute Cardiovascular Care, 4(2), 124–128. https://doi.org/10.1177/2048872614549737
33. EMCrit RACC Podcast 216 – The Hemodynamically Neutral Intubation. (n.d.). Retrieved July 11, 2020, from https://emcrit.org/emcrit/hemodynamically-neutral-intubation/
34. Konstam, M. A., Kiernan, M. S., Bernstein, D., Bozkurt, B., Jacob, M., Kapur, N. K., … Ward, C. (2018). Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement From the American Heart Association. Circulation (Vol. 137). https://doi.org/10.1161/CIR.0000000000000560
35. Sagristá-Sauleda, J., Angel, J., Sambola, A., & Permanyer-Miralda, G. (2008). Hemodynamic effects of volume expansion in patients with cardiac tamponade.
36. Circulation, 117(12), 1545–1549. https://doi.org/10.1161/CIRCULATIONAHA.107.737841






Add comment