6 September 2024, by Shahriar Lahouti.
CONTENTS
- Preface
- Pharmacokinetic & toxicokinetic
- Pathophysiology
- Clinical manifestation
- Differential diagnosis
- Diagnosis
- Treatment
- Management
- Specific population
- Going further
- References
-
-
Preface
Acetaminophen (paracetamol) poisoning is the leading cause of acute liver failure worldwide, accounting for 46% of ALF cases in some areas. Liver toxicity may result from an acute overdose as well as from repeated supratherapeutic ingestion. In overdose, acetaminophen causes dose-dependent hepatotoxicity. Fortunately, N-acetyl-cysteine (NAC) is a very effective antidote for APAP overdose when administered early, during acetaminophen metabolism. Therefore, identification of acetaminophen overdose is critical, as significant morbidity and mortality may be prevented with early therapy.
Diagnosis could be challenging as early manifestations of acute acetaminophen hepatotoxicity are nonspecific and patients with repeated overdose (chronic poisoning) tend to present late, and such hepatotoxicity may have already evolved.
Recently, many changes to acetylcysteine treatment regimens have emerged to treat better high-risk overdoses {e.g. high-risk ingestion (>30 g), sustained-release acetaminophen overdoses, repeated supratherapeutic ingestions) and to minimize adverse reactions.
In this review, the mechanism of hepatotoxicity from acetaminophen, diagnosis, and treatments are explored
Pharmacokinetics
▪️Pharmacology
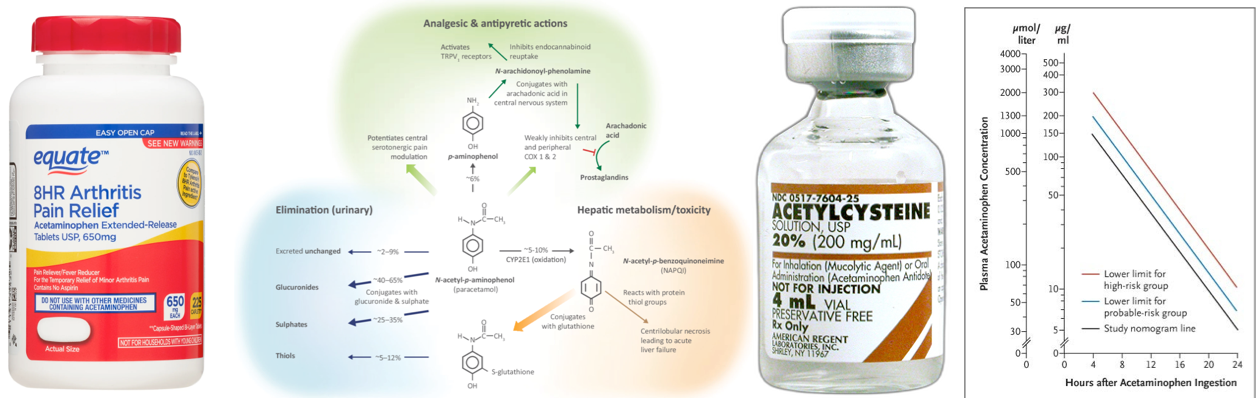
- Acetaminophen (aka. N-acetyl-p-aminophenol or APAP) is called in the U.S. and Canada, and paracetamol outside the United States. Acetaminophen is an analgesic and antipyretic with weak peripheral antiinflammatory properties *.
- The mechanism of action of paracetamol is beyond the scope of this discussion. However, it is worth mentioning a few points here.
- Antipyretic effects are thought to be due to the central (CNS) inhibition of COX-1 and COX-2 variant enzymes.
- Acetaminophen does not have remarkable peripheral inhibitory effects on prostaglandin production *.
- Analgesic effects may also be due to interference with descending serotonergic pain pathways through their activation.
- Acetaminophen is first metabolized to p-aminophenol, which easily crosses the blood-brain barrier and is converted to AM404 by fatty acid amide hydrolase. AM404 is widely known to be the most important mediator of acetaminophen metabolite-induced analgesia through its effect on various central receptors (see appendix) *.
- Antipyretic effects are thought to be due to the central (CNS) inhibition of COX-1 and COX-2 variant enzymes.
▪️Therapeutic dose
- Acetaminophen is available in both immediate-release and sustained-release formulations.
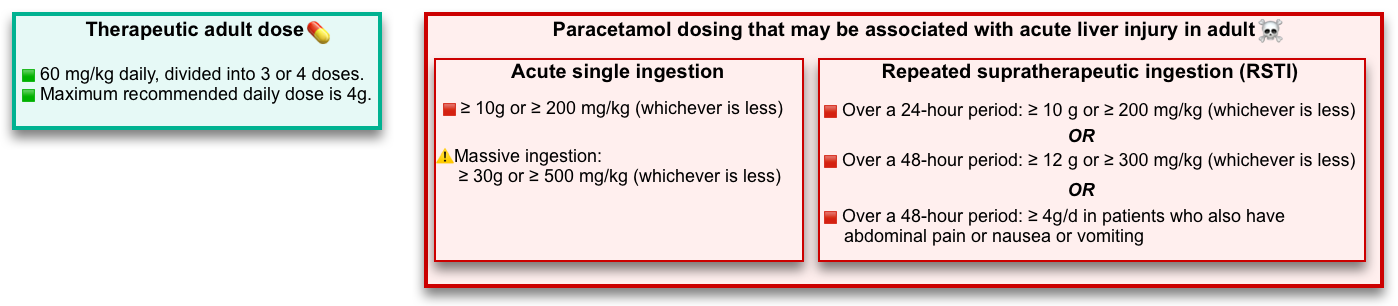
- Therapeutic dose in adults: 60 mg/kg daily, divided into 3 or 4 doses. The maximum recommended daily dose is 4g in adults *.
- Therapeutic serum concentrations for the antipyretic effect of acetaminophen range from 10 to 20 mcg/mL *.
▪️Pharmacokinetics and toxicokinetics
- Peak serum concentrations are usually achieved within 30 minutes to 2 hours following therapeutic dose *.
- Delayed peak serum concentrations may be seen in acetaminophen overdose.
- In an overdose of immediate-release preparations peak serum concentrations are reached within ~4 hours.
- In high-risk overdose (>30g), an overdose of extended-release preparations, or with co-ingestions that delay gastric emptying peak (e.g. opioid or antimuscarinic medications) serum concentrations may be delayed beyond 4 hours *,*.
- Delayed peak serum concentrations may be seen in acetaminophen overdose.
- In therapeutic amounts, acetaminophen has nearly the following pharmacokinetic properties *:
- Bioavailability: IV 100% *, whereas the oral bioavailability is 60-98% *
- Protein binding ~20% (that does not change in overdose) *.
- Volume of distribution of 0.9 L/kg.
- Elimination half-life of 2 to 4 hours.
- Prolonged elimination may be seen in:
- Extended-release preparations overdose.
- Intoxicated patients with hepatotoxicity.
- Serum half-lives >4 hours are considered a marker of poor prognosis *.
- Prolonged elimination may be seen in:
Pathophysiology
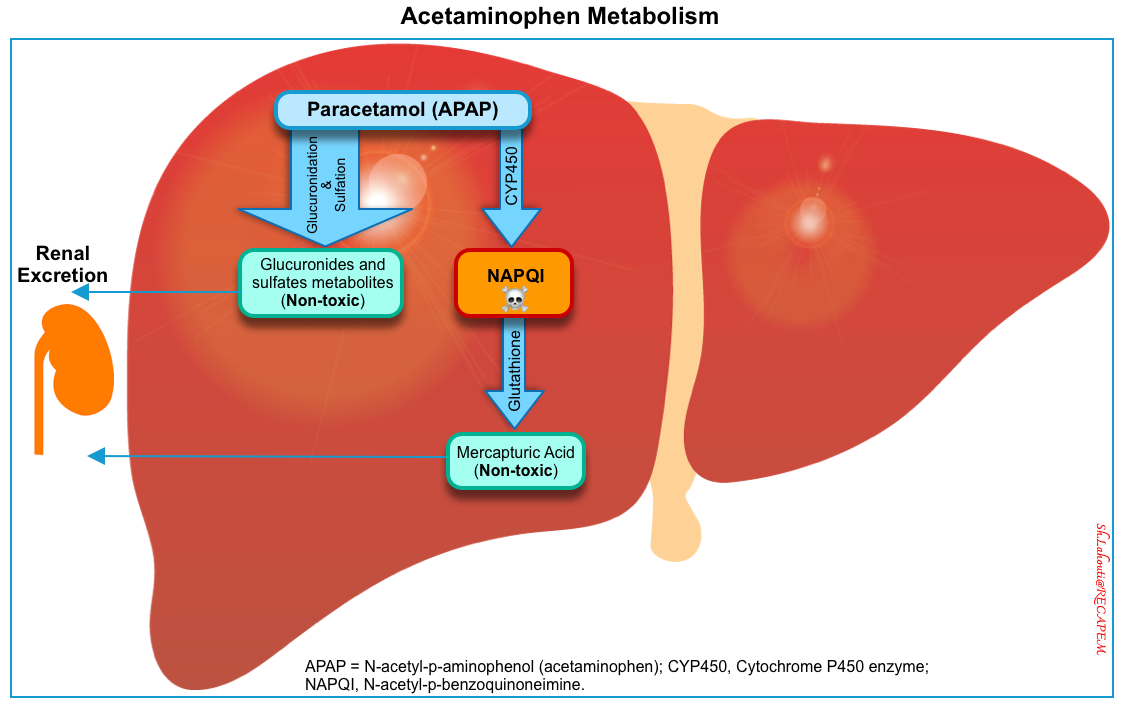
Acetaminophen metabolism
- Metabolism of acetaminophen occurs within the hepatic microsomes. At therapeutic doses, it follows as (left below figure):
- 90% of APAP is metabolized to sulfate (~32%) and glucuronide (58%) conjugates which are then excreted in the urine *.
- ~5% of APAP is excreted unchanged in the urine.
- ~5% of APAP is metabolized via oxidation by the hepatic cytochrome P450 (mainly CYP2E1) into a toxic, highly reactive intermediate metabolite N-acetyl-p-benzoquinoneimine (NAPQI) *.
- Glutathione (GSH) quickly combines with NAPQI, and the resulting complexes are converted to nontoxic cysteine or mercaptate conjugates, which are eliminated in the urine.
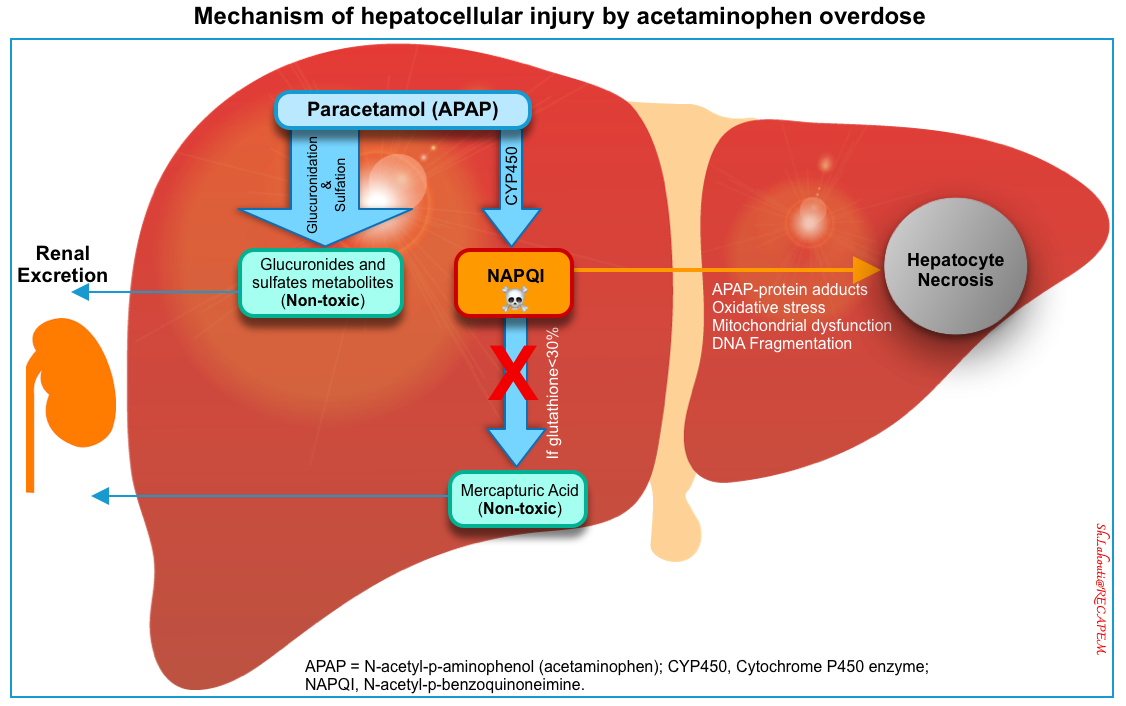
- In overdose, normal pathways for acetaminophen metabolism are saturated. Therefore a larger proportion is oxidized by the CYP450 to NAPQI *.
- When hepatic glutathione stores are decreased to < 30% of normal, NAPQI binds to cellular proteins (adduct formation). This adduct formation (especially in mitochondria) leads to oxidative stress and mitochondrial dysfunction (e.g. cessation of ATP formation, and mitochondrial membrane rupture) resulting in cellular necrosis (right below figure) *,*.
- Paracetamol is also a direct mitochondrial toxin and at very high concentrations can result in central nervous system depression *.
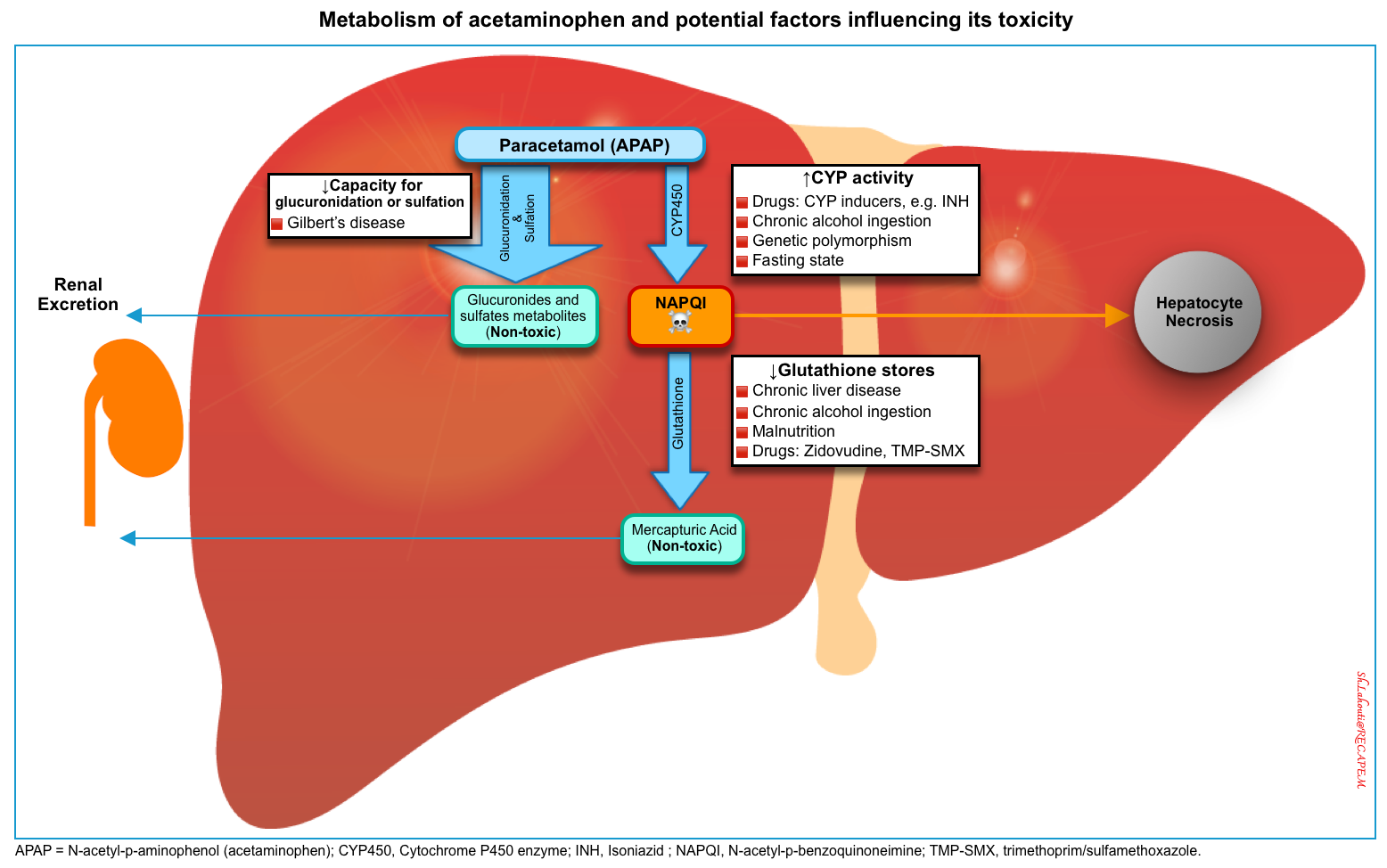
Risk factors for hepatotoxicity
Several risk factors may increase the propensity of acetaminophen to cause hepatotoxicity mediated by NAPQI (figure below) *,*
🔴Excessive intake of acetaminophen (most important).
- ⚠️Ingestion of ‘massive’ overdoses (>30 g) is associated with ~ 18% risk of hepatotoxicity. Hepatotoxicity may occur despite early NAC treatment within 8 hours of the ingestion *,*,*.
🔴Delayed presentation
- Rational
- ⚠️Repeated supratherapeutic ingestion (RSTI)
🔴Hepatic depletion of glutathione
- ⚠️Chronic alcohol ingestion (not acute use) *,*.
- Chronic heavy alcohol use may be associated with an increased risk of hepatotoxicity following acetaminophen overdose *. This may be due to the CYP2E1 induction, or poor nutrition and lower glutathione stores.
- In contrast, acute ingestion of alcohol at the time of paracetamol overdose is protective against the development of toxicity *.
- Ethanol is also a substrate for the CYP enzymes responsible for NAPQI generation and competes with acetaminophen for CYP2E1, reducing the amount of NAPQI being produced *.
- ⚠️Catabolic states
- Pathogenesis
- Catabolic states →↑CYP2E1 activity, shunting hepatic metabolism to gluconeogenesis →↓Glucose precursors for glucuronidation →↑Production of NAPQI) *
- Causes: Malnutrition, post-surgical patients, AIDS.
- Pathogenesis
- ⚠️Chronic liver disease *.
🔴Diminished hepatic capacity for glucuronidation
🔴Excessive cytochrome P450 activity
- Pathogenesis
- Inducers of CYP2E1 (↑metabolism of acetaminophen into toxic NAPQI).
- Inducers of CYP2E1 (↑metabolism of acetaminophen into toxic NAPQI).
- ⚠️Drugs. See 👉 Cytochrome P450 Drug Interaction Table 📚
🔴Sustained-release preparations (⚠️extended and modified-release formulations, more on this below).
🔴Other risk factors
- ⚠️Hepatitis C virus infection *
- ⚠️Nonalcoholic fatty liver disease (NAFLD) *
- ⚠️Genetic polymorphisms in enzymes responsible for acetaminophen metabolism *.
Clinical manifestation
▪️Hepatotoxicity
- Generally, the predominant clinical features of patients with acetaminophen poisoning are caused by APAP-induced hepatotoxicity.
- Acetaminophen-induced hepatotoxicity is generally defined as either AST or ALT > 1000 IU/L.
- Historically, an aminotransferase >1000 IU/L was empirically chosen to define hepatotoxicity. This was the upper limit of quantitation of assays performed in the early days of research regarding paracetamol poisoning and has empirically been maintained as the definition for liver injury since then *.
- Patients with peak AST/ALT levels <1000 U/L usually don’t develop clinically significant hepatic dysfunction *.
- APAP hepatotoxicity is acute and characterized by marked elevation of serum aminotransferase (often >3000 IU/L), which typically starts increasing within 24 to 36 hours, and peaks around 72 hours after overdose.
▪️Nephrotoxicity
- The overall incidence of kidney dysfunction among all APAP-poisoned patients (including those with minimal disease) is ~2% *.
- The incidence of renal dysfunction is related to the severity of the acetaminophen ingestion *:
- Patients with liver involvement but no hepatic failure: ~5%
- Patients with severe poisoning:10%
- Patients with acute hepatic failure: ~50%
- Pathogenesis *
- Direct toxicity by toxic metabolites of APAP, oxidized by CYP in the kidney.
- Hepatorenal syndrome (more on this, here).
- Acute renal failure often becomes evident around 1 to 3 days after ingestion.
- It manifests as acute tubular necrosis, either alone or in combination with hepatic necrosis (hepatorenal syndrome).
- AKI is manifested by elevations of BUN and creatinine along with proteinuria, hematuria, and granular and epithelial cell casts on urinalysis.
- Renal function spontaneously returns to the previous baseline within 1-4 weeks, although dialysis may be required during the acute episode *.
- There is no evidence that acetylcysteine, which is given to minimize hepatotoxicity, has any protective effect on the kidney *.
▪️Other organ injury
- Acetaminophen may also cause acute, extrahepatic toxic effects, presumably because of the presence of CYP450 or similar enzymes (e.g. prostaglandin H synthase) in other organs.
- In rare cases, isolated renal injury (discussed above), cardiac toxicity, and pancreatitis may occur.
Clinical course
The clinical course of APAP hepatotoxicity in patients with a single overdose can be classically divided into 4 sequential stages (table below), however, it should be noted that the course is variable and may not follow the “four-stage” sequential pattern seen after a single oral ingestion.
| The clinical course is influenced by: 🟧Dose (see massive intoxication) 🟧Formulation 🟧Co-ingested drugs 🟧Preexisting liver disease |
Four stages of acetaminophen toxicity
| Stage 1 =Preclinical toxic effects | Stage 2 (latent period) =Hepatic injury | Stage 3 =Maximal stage of hepatotoxicity = Hepatic failure | Stage 4=Recovery | |
| Timing | First 24 h | 24-72 hours | 72-96 hours | 96 hours- 2 weeks |
| Clinical manifestations | -Anorexia. -N&V🤮 -Diaphoresis 😥 -Malaise. | -Resolution of stage 1 symptoms. +/- RUQ abdominal pain 😫 | -Recurrence of anorexia, N&V🤮 -Encephalopathy 😔🤡 -Anuria -Jaundice🤢 -Bleeding 🩸 | -Resolution of stage 1 symptoms. +/- RUQ abdominal pain. |
| Laboratory abnormalities | -AST/ALT are normal -In massive poisoning, AST/ALT may rise 8-12h from ingestion. | -Subclinical AST/ALT elevation -↑Bilirubin and ↑PT if severe. -Nephrotoxicity may occur. | -Peak AST/ALT -Hypoglycemia -Hyperammonemia – ↑Total bilirubin (primarily indirect) >4 -Lactic acidosis -Prolonged PT -Rising serum creatinine -Amylase w/wo clinical pancreatitis | Improvement and resolution or Continued deterioration |
▪️Stage I (0-24 hours)=Incubation=Preclinical toxic effects
- Clinical features
- Patients are generally asymptomatic; if symptoms are present, they are related to GI irritation (anorexia, nausea, vomiting, diaphoresis).
- Generally, the initial manifestation of APAP poisoning is mild and does not reliably predict subsequent risk of hepatotoxicity.
- 👉Other symptoms during this period usually suggest massive ingestion or coingestion of other agents.
- ⚠️Massive ingestion (>30 g) may present with mental status alteration and lactic acidosis within 12 hours of ingestion *.
- The early onset of altered mental status and lactic acidosis are directly related to acetaminophen’s toxic effect on oxidative phosphorylation and the electron transport chain. This is unrelated to hepatic injury or failure *.
- These patients should be considered for specific treatment (discussed below).
- Coingestants, e.g. opioids, diphenhydramine, ASA.
- ⚠️Massive ingestion (>30 g) may present with mental status alteration and lactic acidosis within 12 hours of ingestion *.
- Labs
- The hepatic injury does not happen in this stage, and therefore laboratory analyses of liver injury (AST/ALT) and synthetic function (PT/INR) are typically normal.
▪️Stage II (24-72 hours) = Latent period=Hepatic injury
- Clinical features
- Stage I symptoms usually improve or resolve (so-called latent period) *.
- RUQ abdominal pain can occur.
- Labs
- AST/ALT begin to rise.
- Nephrotoxicity may occur.
▪️Stage III (72-96 hours) = Maximal stage of hepatotoxicity = Hepatic failure
- Clinical features
- Recurrence of systemic symptoms (anorexia, nausea, vomiting, malaise)
- Emerging signs & symptoms of hepatic failure:
- Encephalopathy, jaundice, coagulopathy, hypoglycemia, lactic acidosis, shock.
- Labs
- AST/ALT
- Acetaminophen-induced liver injury causes marked elevations in aminotransferases, in comparison to most other causes of liver injury.
- Transaminases peak 3-4 days after ingestion.
- The degree of aminotransferase elevation correlates roughly with the degree of hepatocellular damage.
- Marked elevation of serum aminotransferase (often >3000 IU/L) is typically seen.
- In patients with severe toxicity, aminotransferase concentrations > 10,000 IU/L are possible *.
- ALT and AST typically rise in a 1:1 ratio between presentation and aminotransferase peak *.
- AST rise happens first, but ALT is generally slower to fall than AST.
- If the AST: ALT ratio is > 2 at presentation, this may herald worse outcomes and also has been associated with chronic excessive ethanol use *.
- An AST: ALT ratio < 0.4 indicates that aminotransferases have peaked and are falling.
- Increased severity of hepatotoxicity is also associated with a slower fall in AST as well as in the AST: ALT ratio *.
- Rising PT/INR.
- ↑Bilirubin
- Mild total bilirubin elevation (primarily of indirect type).
- Dramatic elevations in the total bilirubin concentration (>1 mg/dL) are uncommon following acetaminophen overdose (unless fulminant hepatic failure is established).
- Total bilirubin > 4 is a marker of severity for hepatic dysfunction.
- Mild total bilirubin elevation (primarily of indirect type).
- Lactic acidosis
- Rising creatinine may occur.
- AST/ALT
- The greatest risk of death during this period (with common causes being cerebral edema, septic shock, or less commonly hemorrhage).
▪️Stage IV (4 days-2 weeks)= Recovery
- If the patient survives, this is the final stage, including complete hepatic recovery.
Differential diagnosis
🔵Altered mental status (more on this here).
- Co-formulated with other medications that may cause impairments in these domains (e.g. opioids, anticholinergics).
- APAP massive ingestion.
- Late-presenting APAP toxicity
- Alteration in mental status could be due to hepatic or renal failure.
- Neurologic: Cerebrovascular disease, intracranial infections, etc.
🔵Acute liver failure (more on this, here)
- Alcohol-related hepatitis
- Aminotransferase values are also markedly lower in patients with alcohol-related hepatitis and rarely exceed 500 IU/L.
- Viral hepatitis
- Ischemic hepatitis (“shock liver”), usually follows a period of severe prolonged hypotension.
Click on the image to enlarge
Diagnosis
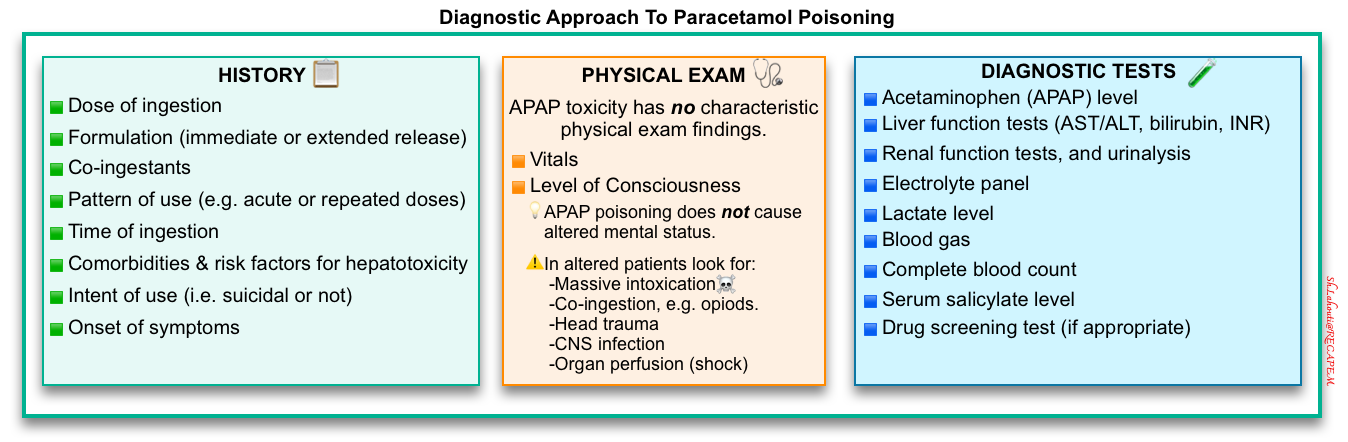
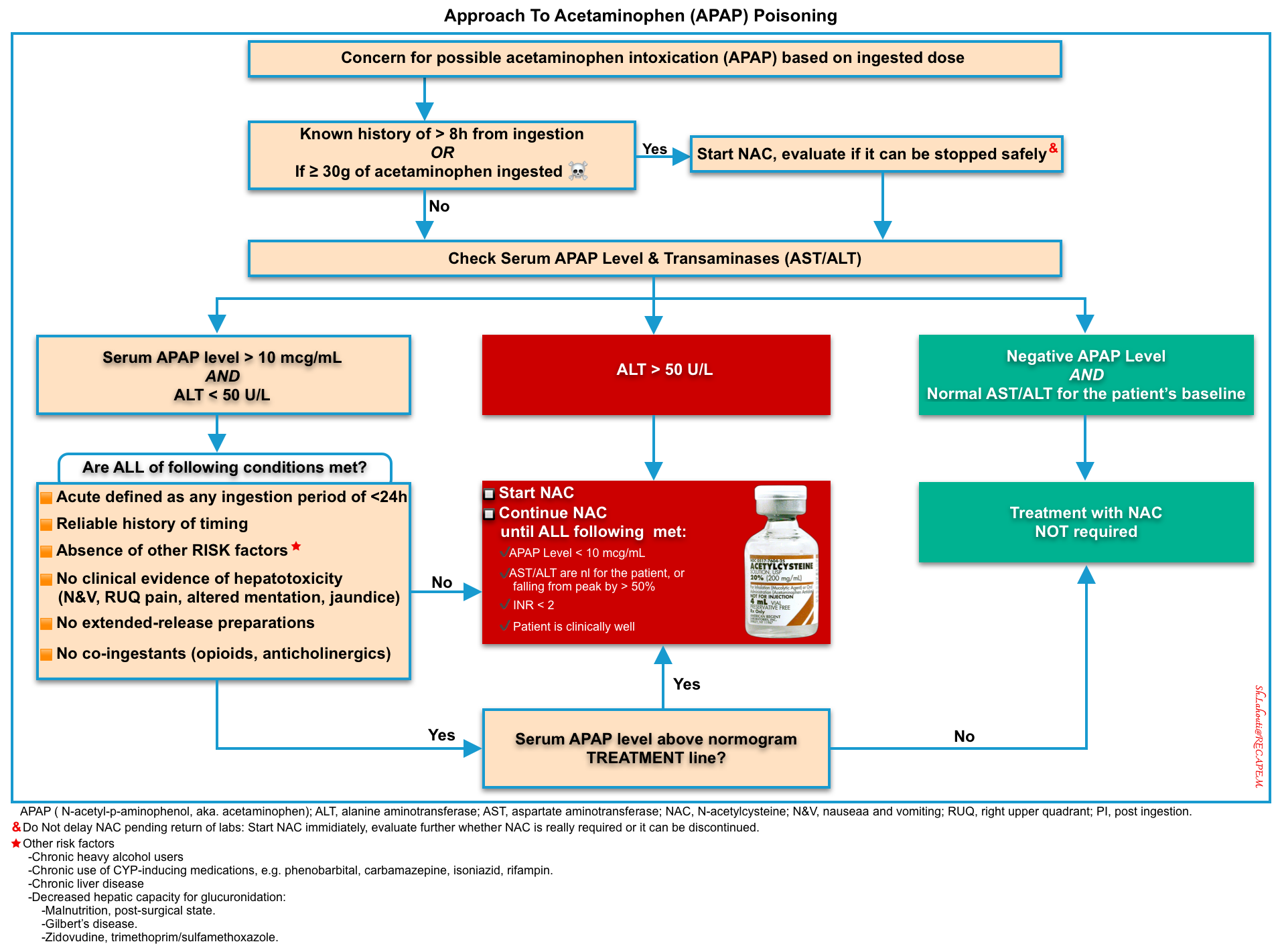
🔴A high index of suspicion is often required to diagnose acetaminophen poisoning.
- History should be obtained to elicit the dose, the pattern of use, the time of the ingestion, the presence of coingestants, and the existence of comorbid conditions that may predispose to the development of hepatic injury.
- A serum acetaminophen concentration should be measured in every patient suspected of an intentional or unintentional overdose. This provides a basis for diagnosing acute acetaminophen poisoning and determining the need for treatment.
- Liver function tests
History
🔵The pattern of use (e.g. acute or repeated doses)
- Repeated supratherapeutic dosing
- Accidental: Patients may accidentally develop clinically significant poisoning from repeated ingestions of supratherapeutic doses of acetaminophen in an attempt to relieve pain or treat fever.
- Dental pain appears to be associated with repeated supratherapeutic ingestions when compared to other types of pain *.
- Deliberate (with self-harm intent) often termed ‘staggered’ overdoses
- Accidental: Patients may accidentally develop clinically significant poisoning from repeated ingestions of supratherapeutic doses of acetaminophen in an attempt to relieve pain or treat fever.
- Repeated therapeutic dosing
- Subclinical toxicity (mild, self-limited, clinically insignificant transaminitis) may develop following repeated therapeutic dosing.
- An observational study of 24 healthy adult volunteers treated with 4 g/day of acetaminophen for 10 days demonstrated subclinical ALT elevations in 58% of study participants *.
- In a randomized, controlled trial of 145 healthy adults taking maximal therapeutic doses of acetaminophen (4 g/day) for 10 days, ~37% developed a maximum ALT > x3 the ULN (>120 U/L) as compared with 0% in the control group *.
- Subclinical toxicity (mild, self-limited, clinically insignificant transaminitis) may develop following repeated therapeutic dosing.
🔵Formulation (immediate-release vs. extended-release).
🔵Time of the ingestion
- Acute ingestion
- Defined as any pattern of acetaminophen ingestion as long as the serum concentration can be measured within 24 hours of swallowing the first dose *.
- Repeated supratherapeutic ingestion(RSTI)
- Defined as multiple ingestions during a period greater than 24 hours *.
🔵Intent of use (i.e. suicidal or not).
🔵Co-ingestants for example
- Agents that delay the absorption of acetaminophen such as anticholinergic agents or opioids *.
- Agents that may potentiate acetaminophen intoxication.
🔵Comorbid conditions that may predispose to the development of hepatic injury (e.g. chronic alcohol use, Gilbert’s disease, chronic liver disease).
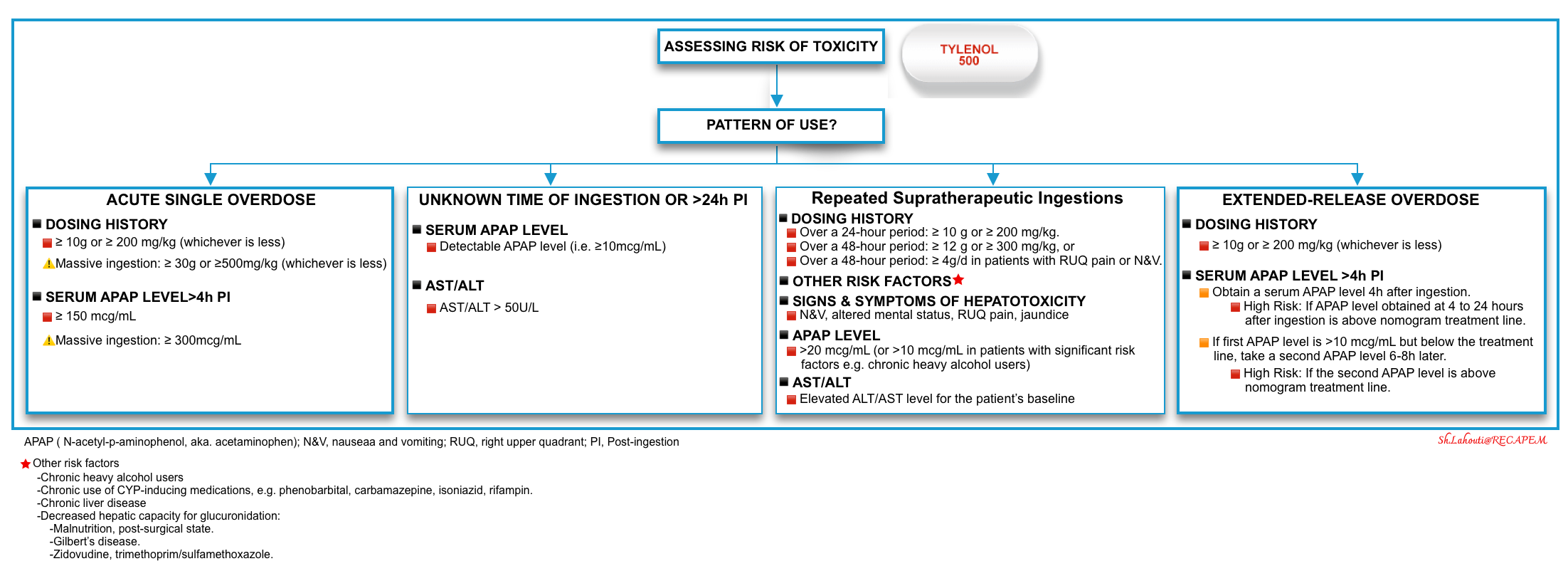
🔵Dosage (the following values are empiric and not validated in human trials, but they are widely used as recommendations for emergency evaluation):
- ⚠️A toxic exposure to acetaminophen is likely suggested in adults by following toxic doses:
- Single-ingestion hepatotoxic dose: ≥10 g, or ≥ 200 mg/kg (whichever is less).
- Repeated supratherapeutic ingestion:
- Over a 24-hour period: ≥10 g or ≥200 mg/kg (whichever is less), or
- Over a 48-hour period: ≥ 12 g or ≥ 300 mg/kg (whichever is less), or
- ≥ A daily therapeutic dose per day for > 48 hours in patients who also have abdominal pain, nausea, vomiting, or jaundice.
- ☠️Massive ingestion: Defined as patients with any of the following:
Serum acetaminophen level
The serum acetaminophen concentration is the basis for diagnosing acute acetaminophen poisoning and determining the need for treatment.
▪️Rationale to measure serum acetaminophen level in patients with unknown poisoning *
- Widespread availability of acetaminophen-containing products.
- Limited accuracy of the medical history *.
- Delayed nature of clinical manifestations after overdose with acetaminophen.
- Serious complications of acute toxicity without antidotal therapy.
▪️Diagnostic Considerations
- An APAP before 2 hours should not be interpreted because of several cases of low or undetectable APAP in this period.
- Measurement of the APAP between 2 and 4 hours after ingestion is only helpful to exclude ingestion of APAP.
- If an APAP is undetectable in this time frame, significant APAP overdose is likely excluded *.
- However, an APAP that is detectable between 2 and 4 hours cannot be definitively interpreted. Repeat testing at 4 hours is recommended.
- ⚠️Marked hyperbilirubinemia (>10 mg/dL) may cause a false-positive acetaminophen level, usually in the low range (0-30 ug/ml) *.
- However, bilirubin elevation in this range usually is not due to acetaminophen, so other causes of liver injury should be considered.
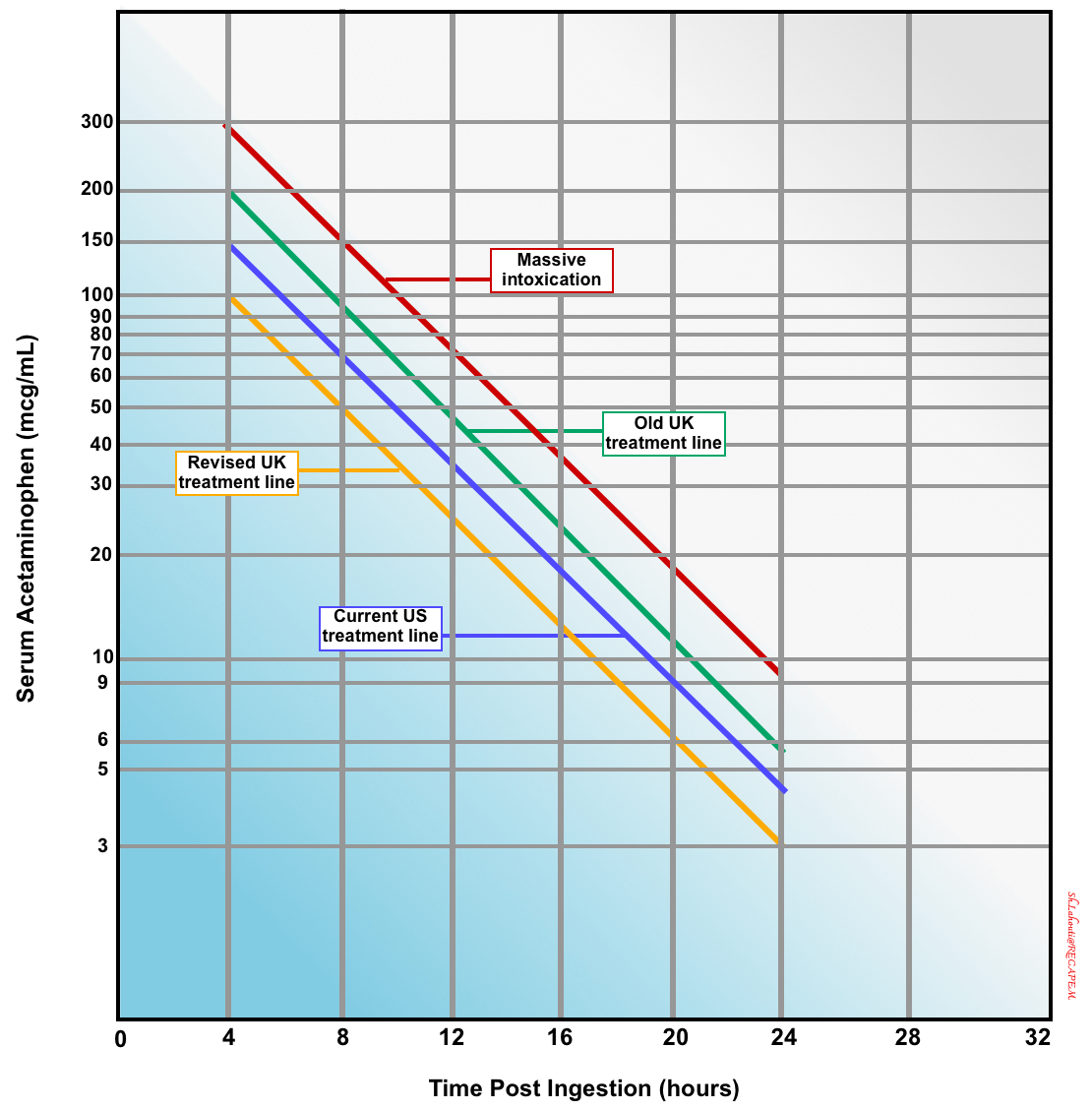
The implication of a measured acetaminophen concentration is determined by plotting the value on the Rumack-Matthew nomogram (below) *.
Rumack-Matthew nomogram
▪️Background
- This nomogram predicts the likelihood of hepatic failure based on acetaminophen level following a one-time oral ingestion (figure below) *.
- It is used to help in estimating the risk of liver toxicity, and thus whether treatment with N-acetylcysteine (NAC) is indicated.
- There’s disagreement regarding the ideal cutoff used in the nomogram:
- The original nomogram line separating possible toxicity from unlikely toxicity was based on a 4-hour acetaminophen concentration of 200 mcg/mL.
- This line was subsequently modified by moving the line to a 4-hour acetaminophen concentration of 150 mcg/mL (current US cutoff) to increase the safety margin for treatment decisions.
- To err on the side of caution, it may be safest to use the United Kingdom treatment line, i.e., a 4-hour APAP level of 100 mcg/mL (yellow line, figure below).
- ☠️If the acetaminophen level falls over the 300-line, the patient has a high risk of hepatotoxicity (massive intoxication), and a higher acetylcysteine dose may be considered.
An acetaminophen level is obtained 4-24 hours after an acetaminophen ingestion. The original nomogram line separating possible toxicity from unlikely toxicity was based on a 4-hour acetaminophen concentration of 200 mcg/mL (green line), but was subsequently modified by moving the line to a 4-hour acetaminophen concentration of 150 mcg/mL (yellow line) to increase the safety margin for treatment decisions. If the acetaminophen levels fall over the “300-line” (red line above), the patient has a massive intoxication and higher doses of acetylcysteine may be considered.
▪️Implication
- The nomogram only directly applies to an acetaminophen concentration obtained after a single oral exposure and during the window between 4 to 24 hours after ingestion.
⚠️Pitfalls
- The nomogram may fail to predict the risk of hematotoxicity when any of the following confounders are present:
- Misleading history about the time of intoxication.
- This nomogram cannot be applied to acetaminophen concentrations obtained outside the 4 to 24-hour window after ingestion, or when the time of ingestion is unknown.
- Multiple ingestions or chronic acetaminophen use *.
- Factors that increase the risk of acetaminophen-induced hepatotoxicity (explained above).
- Altered pharmacokinetics:
- Sustained-release acetaminophen preparations.
- Delayed gastric emptying (e.g. co-ingesting with opioids, anticholinergics).
- Misleading history about the time of intoxication.
Labs
In those with established toxicity, or those predicted to develop toxicity based on history and initial serum acetaminophen concentration, additional laboratory tests should include the following:
🔵Liver function tests (AST/ALT, INR, bilirubin).
- AST is a sufficient indication of hepatic conditions, and no additional testing is initially needed. ALT increases after AST and is not necessary to determine early toxicity.
- INR/PT
- Elevated PT has a strong predictive value for the development of hepatotoxicity. However, some confounders affect the predictive value of the PT in the development of hepatotoxicity *:
- Concurrent anticoagulant use.
- Pre-existing liver disease.
- Direct inhibition of factor VII activity by acetaminophen (minor prolongations resulting in a PT that usually is < twice the control).
- Acetaminophen overdose often leads to minor prolongation of PT even without causing hepatotoxicity. This most commonly occurs between 4 and 24 hours after ingestion and is the result of NAPQI-related inhibition of vitamin K–dependent γ-carboxylation of factors II, VII, IX, and X.
- These minor prolongations are rarely clinically relevant, are not evidence of hepatotoxicity, and should not be used as prognostic factors or indications for NAC treatment.
- NAC treatment
- Treatment with NAC also prolongs PT by interfering with the PT assay, by reversing an APAP/NAPQI effect, or by direct NAC effects *.
- Elevated PT has a strong predictive value for the development of hepatotoxicity. However, some confounders affect the predictive value of the PT in the development of hepatotoxicity *:
🔵Arterial or venous blood gas, and lactate (in critically ill patients with altered mental status, or following high-risk ingestions).
🔵Electrolytes including renal function, urinalysis.
- Mild AKI rarely occurs without hepatotoxicity. However, at least minimal elevation of the AST generally precedes evidence of clinically significant nephrotoxicity. Exceptions are rare, and routine screening of kidney function in the absence of an elevated AST is probably unnecessary.
🔵Other
- Amylase, lipase.
- Complete blood count.
- Toxic screening of blood and urine (in patients with intentional ingestions or unreliable histories).
- Pregnancy test (if appropriate).
Assessing the risk of toxicity
The risk factors of hepatotoxicity are discussed above. For acute overdose in typical circumstances, a systematic approach with demonstrated efficacy can be outlined, but for issues related to repeated supratherapeutic APAP dosing, uncertain circumstances, and new APAP formulations a validated strategy is lacking.
▪️The central concept of a one-approach basis for risk assessment of acetaminophen overdose is presented here by separating different categories of APAP exposure (figure below).
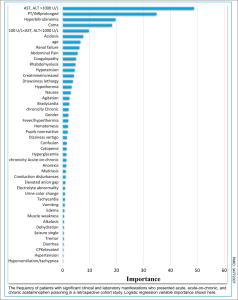
☠️ Other factors on presentation that are associated with poor outcomes or death include *:
- Altered level of consciousness: drowsiness/lethargy, agitation, coma.
- Hypothermia, and hyperthermia.
- Bradycardia.
- Hypoglycemia.
- Coagulopathy.
- Lactic acidosis.
- AKI/Rhabdomyolysis.
Click on the image to enlarge
▪️The paracetamol-aminotransferase multiplication product
- The multiplication product of ALT and paracetamol concentration has been proposed as a risk-stratification tool for predicting hepatotoxicity following acetaminophen overdose *.
- Advantages: This can be used when the Rumack-Matthew nomogram is not applicable, including:
- Unknown exact time of ingestion.
- Supratherapeutic or staggered overdoses.
- Modified-released paracetamol overdose *.
- The diagnostic accuracy of this product has been confirmed.
- ☠️An initial multiplication product of > 10,000 mg/L x IU/L is strongly associated with hepatotoxicity *.
- 😎Patients with a product of <1500 mg/L x IU/L were very unlikely to develop hepatotoxicity.
- A recent systematic review has concluded that the paracetamol-aminotransferase multiplication product can complement the Rumack-Matthew nomogram, especially for delayed presentations and staggered ingestions *.
Treatment
Decontamination
Activated charcoal should be considered in patients with acute ingestion of a potentially liver-toxic amount of acetaminophen if the patient can control the airway (or is intubated) and has no contraindications to its use.
▪️Indications: Give AC (in the absence of contraindications) for:
- Acute immediate-release acetaminophen ingestion of < 2 hours.
- Acute ingestions of sustained-release formulations, especially massive acetaminophen poisoning, and even later time points (e.g. 4 hours) should be considered *.
⛔️AC is not recommended for patients with Repeated Supratherapeutic Ingestion of Acetaminophen.
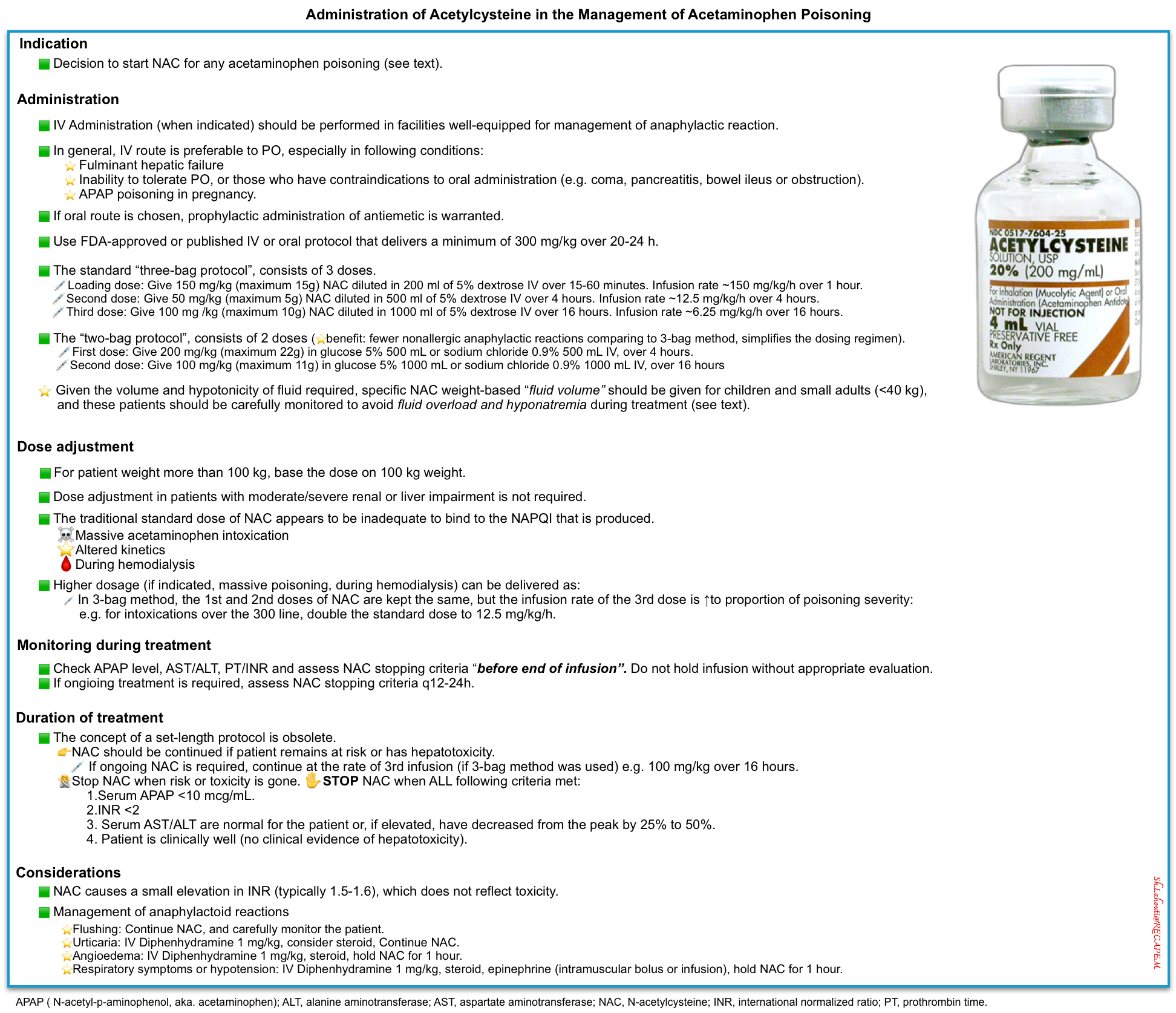
N-acetylcysteine (NAC)
N-acetylcysteine (NAC) is the cornerstone of therapy for patients with potentially lethal acetaminophen (APAP) overdoses.
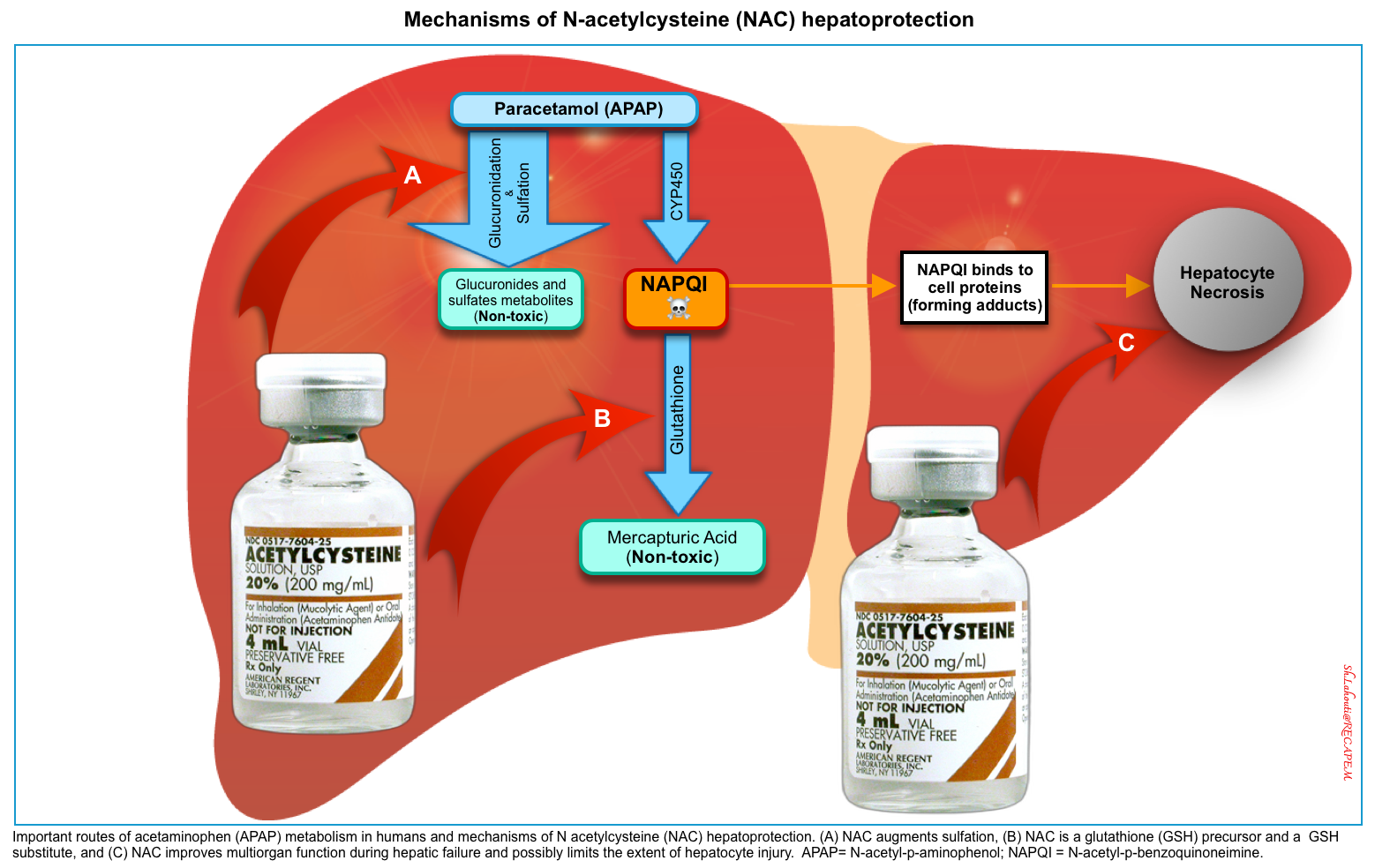
▪️Mechanism of Action
- During the metabolism of APAP to NAPQI, NAC prevents toxicity by limiting the formation of NAPQI *
- It can prevent the formation of the toxic NAPQI if administered early.
- NAC supplies cysteine and augments sulfation of APAP, allowing less metabolism by oxidation to NAPQI (A. Below figure) *.
- It increases the capacity to detoxify the NAPQI that is formed.
- NAC is a glutathione (GSH) precursor and a GSH substitute. It combines with NAPQI and augments the conversion of NAPQI to cysteine and mercaptate conjugates (B. Below figure) *.
- In fulminant hepatic failure, NAC treats toxicity through nonspecific mechanisms that preserve multiorgan function (C. Below figure) *
- It can prevent the formation of the toxic NAPQI if administered early.
▪️Efficacy
- NAC efficacy is nearly 100% as long as it is initiated within 8 hours of an acute overdose *,*,*.
- If administered early, NAC can prevent APAP-induced hepatotoxicity (as explained above).
- NAC effectively prevents APAP-induced hepatotoxicity if it is given before GSH stores are depleted to 30% of normal. This level of depletion occurs ~6 to 8 hours after toxic APAP ingestion *.
- In this preventive role, NAC acts primarily as a precursor for the synthesis of GSH *. The availability of cysteine is the rate-limiting step in the synthesis of GSH, and NAC is effective in replenishing diminished supplies of both cysteine and GSH.
- In this way, NAC concentration has a direct relationship with the amount of NAPQI detoxified and therefore higher NAPQI (and presumably APAP requires higher NAC).
- The longer the initiation of acetylcysteine therapy is delayed beyond 8 hours after ingestion, the greater the risk of developing hepatotoxicity *.
- Even if administration is delayed up to 24 hours, acetylcysteine reduces the risk of hepatotoxicity compared to historical controls *.
- If administered early, NAC can prevent APAP-induced hepatotoxicity (as explained above).
- The effectiveness of NAC even after NAPQI formation and binding has been shown in several studies. Therefore even if NAC is administered after the onset of hepatotoxicity, it improves outcomes and decreases mortality.
- NAC reverses NAPQI oxidation in hepatocytes and diminishes hepatocyte injury even after cell injury is initiated (discussed above) *,*.
- A prospective, randomized trial found that even after fulminant hepatic failure was evident, IV NAC diminished the need for vasopressors and the incidences of cerebral edema and death *.
- NAC reverses NAPQI oxidation in hepatocytes and diminishes hepatocyte injury even after cell injury is initiated (discussed above) *,*.
▪️Administration-Route: Oral vs. IV route?
- NAC can be administered via the oral or IV routes, each has its advantages and disadvantages. Because no controlled studies have compared IV with PO NAC, conclusions about the relative benefit of each are difficult.
- IV and PO NAC administration are equally efficacious in treating patients with APAP toxicity *, and the route of NAC should not be a factor when considering efficacy. The decision of which route to use should not be affected by questions of efficacy but should depend on the rate of adverse events, safety, availability, and ease of use *.
- However, there are 3 situations for which the available information suggests IV NAC is preferable to PO NAC:
- Fulminant hepatic failure.
- Inability to tolerate NAC orally.
- APAP poisoning in pregnancy.
- However, there are 3 situations for which the available information suggests IV NAC is preferable to PO NAC:
- Oral route
- Nausea and vomiting occur in up to 20% of patients treated with PO NAC compared with 7% with the traditional three-bag IV NAC protocol.
- Oral acetylcysteine has an unpleasant smell and taste (smells like rotten eggs) and makes the patient vomit (~20%) *.
- Prolonged duration of treatment (72-hour protocol)
- Nausea and vomiting occur in up to 20% of patients treated with PO NAC compared with 7% with the traditional three-bag IV NAC protocol.
- IV route (is now more commonly used)
- Benefits
- Less vomiting.
- Greater patient acceptance.
- Equivalent efficacy.
- Shorter duration of treatment.
- Disadvantages
- Anaphylactoid reaction (4% to 17%).
- Medication errors *
- Benefits
▪️Administration-Protocole
- N-acetylcysteine is administered via the oral or IV routes and in protocols that have historically varied in length. The most common regimens are a 12-hour IV infusion, a 21-hour IV infusion, and a 72-hour oral dosing protocol.
- However, the concept of a set-length protocol is obsolete.
- Conceptually, practitioners should start NAC when the patient is at risk of toxicity, continue NAC while the patient remains at risk or has hepatotoxicity, and stop NAC when that risk or toxicity is gone.
- Most institutions now use either oral or IV NAC in a variable-length protocol, using indicators of patient toxicity rather than a set protocol length.
▪️Administration- IV Protocol
- IV acetylcysteine is commercially available as a 20% solution and requires dilution to a 2% solution for infusion into a peripheral vein. Both 5% dextrose in water and half-normal saline can be used as diluents.
- The standard dosing regimen (total dose of 300 mg/kg) can be given through different protocols.
- The traditional “three-bag protocol”, consists of 3 doses. This can be calculated 🧮here.
- Loading dose: Give 150 mg/kg (maximum 15g) NAC diluted in 200 ml of 5% dextrose IV over 15-60 minutes. Infuse at 150 mg/kg/hour over 1 hour.
- Second dose: Give 50 mg/kg (maximum 5g) NAC diluted in 500 ml of 5% dextrose IV over 4 hours. Infuse at 12.5 mg/kg/hour over 4 hours.
- Third dose: Give 100 mg /kg (maximum 10g) NAC diluted in 1000 ml of 5% dextrose IV over 16 hours. Infuse at 6.25 mg/kg/hour over 16 hours
- If ongoing acetylcysteine is required, continue at the rate of the third infusion (e.g. 100 mg/kg over 16 hours) until the Stopping Criteria are met.
- The “two-bag protocol”, consists of 2 doses.
- Advantage
- Dosing
- First dose: Give 200 mg/kg (maximum 22g) in glucose 5% 500 mL or sodium chloride 0.9% 500 mL IV, over 4 hours.
- Second dose: Give 100 mg/kg (maximum 11g) in glucose 5% 1000 mL or sodium chloride 0.9% 1000 mL IV, over 16 hours
- If ongoing acetylcysteine is required, continue at the rate of the second infusion (e.g. 100 mg/kg over 16 hours) until the Stopping Criteria are met.
- The “one-bag” protocol: 30 g in 1 L of D5W (30 mg/mL) *
- The two or three-bag regimens can also be combined into one infusion bag given with variable rates, which may reduce the risk of gaps in treatment *.
- Bolus from bag 150 mg/kg over 1 hour.
- Infuse at 12.5 mg/kg/hour over the next 20 hours.
- The two or three-bag regimens can also be combined into one infusion bag given with variable rates, which may reduce the risk of gaps in treatment *.
- The traditional “three-bag protocol”, consists of 3 doses. This can be calculated 🧮here.
▪️It may still be appropriate in certain patients, such as those at high risk for anaphylactoid responses to the IV formulation and asthmatics.
▪️Oral loading dose: 140 mg/kg oral loading dose followed by
▪️Oral Maintenance dose: 70 mg/kg q4 hours for 17 additional doses (1330 mg/kg total dose) over 72 hours is the U.S. FDA-approved protocol, although many toxicologists recommend shorter durations (eg, 20 hours) based on clinical response (eg, ALT <50% of peak values, INR <2, and unmeasurable APAP level).
▪️If the dose is vomited within the first hour, repeat the dose.
▪️Diluting N-acetylcysteine 10%-20% (Mucomyst) to 5% in juice or soda is recommended and may improve tolerance and palatability.
▪️Administration-Dosage
- Background
- Although the route of NAC does not affect efficacy, the dosage of NAC does.
- N-acetylcysteine must be supplied in a quantity that is consistent with the amount of NAPQI that is being produced.
- In the following rare but lethal conditions, the dose of NAC in the traditional three-bag system appears to be inadequate to bind to the NAPQI that is produced.
- Massive acetaminophen intoxication
- Altered kinetics
- Tradition dosage is discussed above.
- Higher dosage
- Indications
- Massive acetaminophen poisoning * (more on this below).
- Acetylcysteine neutralizes NAPQI in a 1:1 molar ratio, so the dose of acetylcysteine should be scaled up in proportion to the amount of acetaminophen.
- During hemodialysis *
- If dialysis is used, this will remove acetylcysteine, thereby aggravating the mismatch between acetylcysteine dose vs. NAPQI levels. Intermittent hemodialysis may remove up to 50% of N-acetylcysteine.
- Massive acetaminophen poisoning * (more on this below).
- Risks of high-dose acetylcysteine
- Toxicity from high-dose NAC may cause hemolysis, thrombocytopenia, acute renal failure, and cerebral edema.
- Protocol
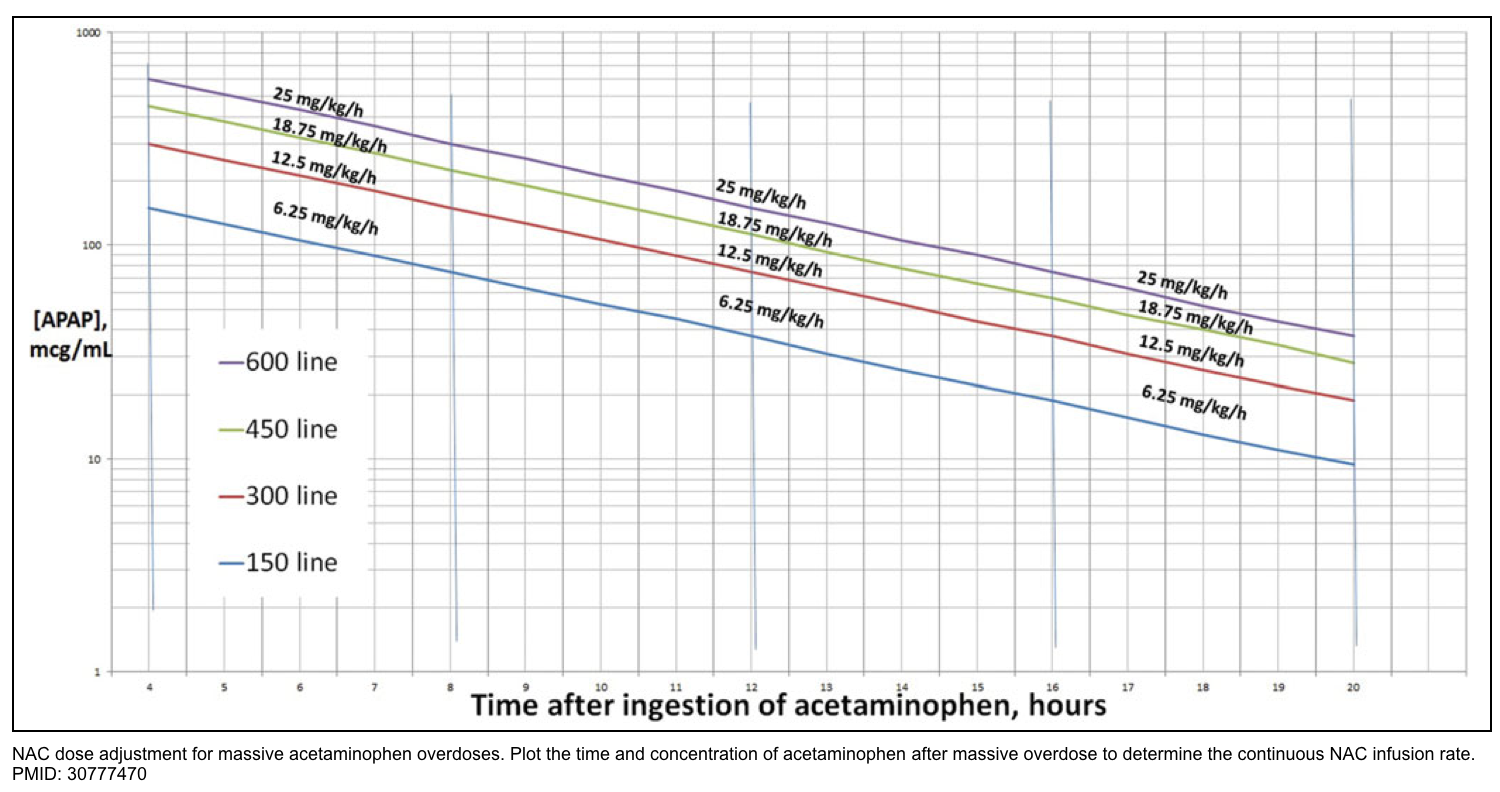
- One possible approach to acetylcysteine dosing has been recently proposed: *
- The first and second doses of acetylcysteine are kept the same, but the infusion rate of the third dose is increased in proportion to the severity of intoxication.
- Intoxications over the 300 line (red line below): Third dose of 12.5 mg/kg/hr (double the standard dose).
- Intoxications over the 450 line (green line below): Third dose of 18.75 mg/kg/hr (triple the standard dose).
- Intoxications over the 600 line (purple line below): Third dose of 25 mg/kg/hr (quadruple the standard dose).
- The first and second doses of acetylcysteine are kept the same, but the infusion rate of the third dose is increased in proportion to the severity of intoxication.
- This algorithm hasn’t been prospectively validated, so consider discussing the case with poison control or a local toxicologist. The practice of doubling the infusion rate is increasingly accepted, but tripling or quadrupling the dose remains more controversial *.
- One possible approach to acetylcysteine dosing has been recently proposed: *
- Indications
▪️Acetylcystein dosing in special population
- Weight > 100kg
- Calculation of acetylcysteine dose should be capped at 100 kg of body weight.
- Evidence is scant regarding the added benefit of acetylcysteine doses calculated with weights above these maximum amounts.
- Calculation of acetylcysteine dose should be capped at 100 kg of body weight.
- During hemodialysis
- For patients on hemodialysis, consider doubling the acetylcysteine dose compared to what you would otherwise use (but do not increase it any higher than 25 mg/kg/hr).
- Give a dose of at least 12.5 mg/kg/hour during hemodialysis.
▪️Duration of therapy and stopping criteria
- Treatment should continue until the Stopping Criteria are met.
- It is recommended not to adhere to a pre-defined duration (e.g. 21 hours) and not to stop acetylcysteine without reassessing the patient *.
- Before the end of the infusion, measure the serum acetaminophen concentration, serum transaminases, and PT/INR.
- If the stopping criteria are met discontinue acetylcysteine therapy.
- If the stopping criteria are not met:
- Continue at the same rate, equal to the rate of the third IV dose in the protocol (100 mg/kg infused over 16 hours repeatedly).
- Measure the serum acetaminophen concentration, serum transaminases, and PT/INR every 12 to 24 hours in addition to other clinical variables (eg, factor V, presence of encephalopathy); once stopping criteria are met, treatment may be discontinued.
- ✋Stopping Criteria: Acetylcysteine can generally be stopped once all the following criteria are met:
- Serum acetaminophen concentration <10 mcg/mL.
- INR <2
- Serum transaminases are normal for the patient or, if elevated, have decreased from the peak by 25% to 50%.
- For patients with hepatic failure, acetylcysteine should be continued until the liver is making a robust recovery (e.g. transaminases are definitively falling and the INR is <2).
- Note that small fluctuations in ALT (e.g. +/- 20 U/L or +/- 10%) are common and don’t necessarily indicate the need for ongoing N-acetylcysteine, especially if the ALT level is low.
- The patient is clinically well (no clinical evidence of hepatotoxicity).
▪️Adverse effect
- Anaphylactoid reaction from IV “NAC” (4% to 17%)
- Rapid administration of acetylcysteine can cause an anaphylactoid reaction.
- This involves histamine release due to a direct action of the medication (not an IgE-mediated allergic reaction) *.
- These reactions are not an allergic reaction.
- Acetylcysteine can be continued or resumed (perhaps at a lower rate initially).
- Anaphylactoid reactions are uncommon (especially if the initial dose is infused more slowly, over 60 minutes).
- They invariably occur within six hours of initiation of acetylcysteine, most often within the first two hours.
- When they do occur, they are usually mild (involving the skin only).
- Treatment may be as follows *:
- The only symptom is flushing: continue acetylcysteine, and carefully monitor the patient.
- Urticaria: IV diphenhydramine 1 mg/kg, consider steroid, continue acetylcysteine.
- Angioedema: IV diphenhydramine 1 mg/kg, steroid, hold acetylcysteine for 1 hour.
- Respiratory symptoms or hypotension: IV diphenhydramine 1 mg/kg, steroid, hold acetylcysteine for 1 hour, epinephrine (intramuscular bolus or infusion).
- Rapid administration of acetylcysteine can cause an anaphylactoid reaction.
- Note: N-acetylcysteine causes a small elevation in INR (typically 1.5-1.6), which does not reflect toxicity *.
Review: Administration of Acetylcysteine in the Management of Acetaminophen Poisoning
Hemodialysis
- APAP has a low volume of distribution and is not highly protein-bound, making APAP dialyzable *.
- Dialysis can remove both acetaminophen and toxic metabolites (NAPQI). This may be beneficial in massive poisoning, where acetylcysteine won’t necessarily work. Hemodialysis can also correct metabolic acidosis.
- Dialysis is not an alternative to acetylcysteine. In fact, patients who are dialyzed require higher doses of acetylcysteine (e.g. double the dose in patients undergoing intermittent hemodialysis) *.
- Indications for dialysis based on the EXTRIP guidelines 👉 📚
- If the APAP >1000 mcg/mL and NAC is NOT administered.
- If the patient presents with altered mental status, metabolic acidosis, elevated lactate, and an APAP is >700 mcg/mL, and NAC is NOT administered.
- If the patient presents with an altered mental status, metabolic acidosis, elevated lactate, and an APAP is >900 mcg/mL even if NAC is administered.
Management
Overview
An overview of the therapeutic approach to acetaminophen poisoning is shown below. This will be explored in detail as follows.
Acute Ingestion of Immediate-Release (IR) Acetaminophen
▪️Definition
- Acute ingestion includes any ingestion period of < 24 hours regardless of the ingestion pattern.
▪️Management
- An acetaminophen concentration sample drawn before 4 hours after ingestion cannot be used to risk-stratify patients on the acetaminophen nomogram.
- A nondetectable concentration at 2 to 4 h after ingestion typically excludes significant ingestion, but consultation with a poison center or toxicologist is recommended.
- INR activity should be measured in patients with elevated transaminase activity or clinical suspicion of liver injury.
- Note that INR elevation within the first 20 hours following exposure does not reflect developing synthetic dysfunction or fulminant hepatic failure and does not carry prognostic value.
- Both therapeutic acetylcysteine serum concentrations and high concentrations of acetaminophen can elevate the INR.
- These elevations are usually mild (INR not greater than 1.7), occur between 4 and 20 hours after ingestion, and resolve as treatment is continued.
- Note that INR elevation within the first 20 hours following exposure does not reflect developing synthetic dysfunction or fulminant hepatic failure and does not carry prognostic value.
- Treatment with charcoal (see here) and N-acetylcysteine (here).
1. The patient is at risk of hepatic injury if >10g or 200mg/kg (whichever is less) of paracetamol has been ingested.
2. Give activated charcoal within 2 hours if 10 to 30g ingested or within 4 hours if ≥30g ingested.
3. Measure serum paracetamol concentration and ALT/AST > 4 hours post ingestion.
4. If paracetamol concentration result can be determined before 8 hours, wait to start acetylcysteine and plot paracetamol concentration against the nomogram. If a paracetamol concentration cannot be determined within 8 hours, start NAC until the results are available.
5. In those who require acetylcysteine, 2 hours before completion of infusion check ALT/AST and paracetamol concentration (in those with high initial paracetamol concentrations greater than double the 150mg at 4 h nomogram line) to determine need for ongoing acetylcysteine.
6. Acetylcysteine should be continued in those patients who have a persistently high paracetamol concentration > 10 mg/L (66 μmol/L) OR ALT is > 50 U/L and increasing. Note: small fluctuations in ALT (e.g. +/- 20 U/L or +/-10%) are common and do not on their own indicate the need for ongoing acetylcysteine.
1. The patient is at risk of hepatic injury if >10g or 200mg/kg (whichever is less) of paracetamol has been ingested.
2. Commence NAC infusion
3. Measure paracetamol concentration and ALT/AST levels
4. If the paracetamol concentration is under the treatment nomogram line and ALT/AST is <50 IU/L cease acetylcysteine. Otherwise, continue and complete 20-hour acetylcysteine infusion.
5. In those who require 20 h of acetylcysteine, 2 h before completion of acetylcysteine infusion check ALT/AST and paracetamol concentration (in those with high initial paracetamol concentrations greater than double the 150mg at 4 h nomogram line) to determine need for ongoing acetylcysteine.
6. Acetylcysteine should be continued in those patients who have a persistently high paracetamol concentration of> 10 mg/L (66 μmol/L) OR ALT is > 50 U/L and increasing. [note: small fluctuations in ALT (e.g. +/- 20 U/L or +/-10%)] are common and do not on their own indicate the need for ongoing acetylcysteine).
1. The patient is at risk of hepatic injury if >10g or 200mg/kg (whichever is less) of paracetamol has been ingested.
2. Start acetylcysteine.
3. If the paracetamol concentration is > 10mg/L or the ALT/AST is >50 IU/L complete the 20-hour acetylcysteine infusion.
4. In those who require 20 h of acetylcysteine, 2 h before completion of acetylcysteine infusion check ALT/AST and paracetamol concentration (in those with high initial paracetamol concentrations greater than double the 150mg at 4 h nomogram line) to determine need for ongoing acetylcysteine.
5. Acetylcysteine should be continued in those patients who have a persistently high paracetamol concentration of> 10 mg/L (66 μmol/L) OR ALT is > 50 U/L and increasing. [note: small fluctuations in ALT (e.g. +/- 20 U/L or +/-10%)] are common and do not on their own indicate the need for ongoing acetylcysteine).
1. The patient is at risk of hepatic injury if >10g or 200mg/kg (whichever is less) of paracetamol has been ingested.
2. One published strategy here is to treat the patient as if they took all of their tablets at the first (earliest) point in time and treat them along the same strategies as above.
3. However, if the first paracetamol concentration was measured within 2 hours of the last ingested paracetamol dose it should be repeated after 2 hours to ensure there is no ongoing absorption. If either concentration is above the nomogram line (using time from the earliest ingestion), start/continue treatment with acetylcysteine.
Example 1: Staggered overdose < 8 hours e.g. 4g at 1 pm, 4g at 2 pm, and 4g at 3 pm and it is now 4 pm use the <8hr guideline above and time anchor the paracetamol concentration to 1 pm. This allows for the worst-case scenario. If you time anchor to 3 pm you may have a lower concentration and underestimate the overdose. The paracetamol concentration should be repeated at 6 pm (2 h after the first concentration) and time anchor concentration to 1 pm.
Example 2: If the patient has a staggered overdose throughout the day over 8 hours, e.g. 4g at 0800 then 4g at 1200, and 4g at 1700, treat them as per the 8-24 hr guideline above. Start acetylcysteine and time anchor the paracetamol concentration to 0800. Repeat the paracetamol concentration if the first paracetamol concentration was measured within 2 hours of the last ingested paracetamol dose.
1. Treat as per an acute ingestion.
2. The patient is at risk of hepatic injury if >10g or 200mg/kg (whichever is less) of paracetamol has been ingested.
3. Start acetylcysteine.
4. If the paracetamol concentration is > 10mg/L or the ALT/AST is >50 IU/L complete the 20-hour acetylcysteine infusion.
5. In those who require 20 h of acetylcysteine, 2 h before completion of acetylcysteine infusion check ALT/AST and paracetamol concentration (in those with high initial paracetamol concentrations greater than double the 150mg at 4 h nomogram line) to determine need for ongoing acetylcysteine.
6. Acetylcysteine should be continued in those patients who have a persistently high paracetamol concentration of> 10 mg/L (66 μmol/L) OR ALT is > 50 U/L and increasing. [note: small fluctuations in ALT (e.g. +/- 20 U/L or +/-10%)] are common and do not on their own indicate the need for ongoing N-acetylcysteine).
Massive acetaminophen poisoning
- Massive poisoning may be defined as patients with any of the following:
▪️Clinical presentation
- A patient with high-risk ingestion may be critically ill early following the overdose and before hepatic failure develops due to parent-compound toxicity *.
- These patients develop mitochondrial dysfunction very early (usually within <12 hours of ingestion) before any liver damage occurs. This may cause early development of lactic acidosis and altered mental status.
- Supportive measures may be required.
- Treatment
- Charcoal.
- AC may be useful in these patients (if patients present within < 4 hours of ingestion, especially if there is evidence of ongoing absorption such as an increasing acetaminophen concentration or if they have ingested sustained-release tablets).
- Early and high-dose administration of acetylcysteine.
- In a patient with a history of high-risk acetaminophen ingestion (>30 g) or signs of parent-compound toxicity (e.g. hemodynamic instability,
altered mental status, metabolic acidosis), giving acetylcysteine as rapidly as possible instead of waiting for the serum acetaminophen concentration to result is recommended *. - A higher dose of acetylcysteine may be required for high-risk overdoses (explained above) *,*,*.
- In a patient with a history of high-risk acetaminophen ingestion (>30 g) or signs of parent-compound toxicity (e.g. hemodynamic instability,
- Dialysis (discussed above).
- Charcoal.
Unknown time of ingestion, or >24 hours after ingestion
▪️Risk assessment
- For patients in whom the time of acetaminophen ingestion remains unknown or is >24 hours or for those with suggestive clinical findings of acetaminophen poisoning, a serum acetaminophen concentration and serum transaminase, bilirubin, and prothrombin time tests should be determined.
- In this scenario, a detectable acetaminophen concentration (>10 mcg/mL) suggests that the patient may be at risk for developing hepatotoxicity.
- Similarly, elevated serum transaminases suggest the possibility of ongoing hepatic toxicity.
▪️Management
- Initiate NAC therapy as soon as possible while awaiting laboratory results.
- Continue NAC if:
- APAP level is measurable (i.e. >10mcg/mL).
- Elevated serum transaminases (AST/ALT>50U/L)
- If the APAP is undetectable and the AST is normal, there is little evidence that subsequent consequential hepatic injury is possible, and NAC can be discontinued.
Repeated Supratherapeutic Ingestion
▪️Definition
- Repeated supratherapeutic ingestion is defined as multiple ingestions for a period of > 24 hours.
- Over a 24-hour period: >10g or 200mg/kg (whichever is less).
- Over a 48-hour period: ≥ 12 g or ≥ 300 mg/kg, or
- Over a 48-hour period: ≥ 4g/d in patients with RUQ pain or N&V.
▪️Assessing risk of hepatotoxicity
- Background
- RSTI carries an increased risk of hepatotoxicity and death when compared to acute single overdose *,*,*.
- Later presentation and higher prevalence of chronic alcohol use disorder are likely contributing to the increased morbidity *.
- Repeated overdoses can also occur with self-harm intent (often termed ‘staggered’ overdoses), and similarly carry an increased risk of hepatotoxicity when compared to acute self-harm overdoses *.
- RSTI carries an increased risk of hepatotoxicity and death when compared to acute single overdose *,*,*.
- Evaluation
- To determine the risk of hepatotoxicity, evaluate the patient’s risk based on the following factors:
- Dosing history
- The risk of hepatotoxicity is likely proportional to both the total amount of APAP ingested and the duration of the exposure; however, exact cutoffs for safe dosing are difficult to determine and are likely subject to factors related to the individual.
- Other risk factors (see above).
- APAP level
- Assess patients for clinical signs and symptoms of hepatotoxicity.
- Liver function tests (AST, ALT, INR, Bilirubin).
- Other tests may be indicated such as lactate, electrolytes, and serum creatinine.
- Dosing history
- To determine the risk of hepatotoxicity, evaluate the patient’s risk based on the following factors:
▪️Diagnostic and therapeutic considerations
- The revised nomogram should not be used for assessment of repeated ingestions that span more than 24 hours.
- Unlike the patient with acute ingestion, management is determined by the patient’s presentation (N&V, abdominal pain, altered mental status).
- Indications to start NAC treatment
- If the acetaminophen concentration is >20 mcg/mL (or >10 mcg/mL in patients with significant risk factors, such as chronic alcohol consumption), or
- If the AST or ALT level is abnormal (for the patient’s baseline).
- Continue NAC treatment until stopping criteria are met.
1. Patients who ingest excessive paracetamol for a therapeutic purpose (e.g. pain, viral illness) or ingest therapeutic doses of paracetamol and have symptoms of acute liver injury (e.g. abdominal pain, nausea, and vomiting) are managed as RSTI
2. The paracetamol nomogram is not useful
3. Management of RSTI varies worldwide from the definition of toxic ingestion to treatment with acetylcysteine.
4. Patients at risk:
≥ 10 g or ≥ 200 mg/kg (whichever is less) over a single 24 h period.
OR
≥ 12 g or ≥ 300 mg/kg (whichever is less) over a single 48 h period.
OR
≥ a daily therapeutic dose^ per day for more than 48 hours in those who also have abdominal pain nausea or vomiting.
Note: The therapeutic daily dose of paracetamol in adults is a total dose of 60 mg/kg over 24 h and up to a maximum dose of 4 g/day.
5. If the above criteria are met, then measure ALT/AST and paracetamol concentration:
6. If paracetamol concentration is >20 mcg/L or the ALT or AST is > 50 IU/L start acetylcysteine.
7. Repeat ALT/AST and paracetamol concentration 8 hours after the initial sampling. Those with significant acute liver injury secondary to paracetamol will have a very high and/or rapidly rising ALT. Those with a paracetamol concentration > 10mcg/L or ALT that is increasing should complete the 20 h course of acetylcysteine
8. Small fluctuations in ALT (e.g. +/- 20 U/L or +/-10%) are common and do not on their own indicate the need for ongoing acetylcysteine.
9. Those with an ALT or AST > 1000 U/L should receive at least a full 20 h course of acetylcysteine.
10. In those that receive 20 h of acetylcysteine, 2 h before completion of acetylcysteine infusion check ALT/AST to determine the need for ongoing acetylcysteine.
Acetylcysteine should be continued in those patients with an ALT > 50 U/L and is increasing.
Ingestion of Sustained-Release Acetaminophen Preparations
▪️Sustained-release preparations
- Sustained-release formulations of acetaminophen include ER (extended-release) acetaminophen and MR (modified-release).
- 🇺🇸ER acetaminophen is available on the US market and contains 650 mg acetaminophen, 50% immediate release, and 50% slow release.
- 🇦🇺MR acetaminophen is available in Australia and New Zealand and contains 665 mg paracetamol, 31% immediate release, and 69% slow release.
- Similarity
- Both MR and ER formulations are designed to provide pain relief for up to 8 hours.
- Both the above products result in the immediate release of APAP with delayed release of an additional dose.
- Delayed peaks, prolonged absorption, and multiple peaks are associated with both preparations resulting in “nomogram crossing’
- The nomogram line crossing refers to the condition where the initial level is below the treatment line, and subsequent levels are above the line.
- Note that this “nomogram crossing” is not unique to the sustained-release products and occurs in up to 10% of patients with acute ingestions of immediate-release APAP * and those with ingestions of APAP with diphenhydramine or opioids *.
- Differences
- MR acetaminophen has a longer Tmax than ER acetaminophen *.
- Different formulation
- MR tablets use a hydroxypropylmethylcellulose coating.
- ER tablets are bi-layered tablets where one side contains immediate release and the other slow-release paracetamol (in a matrix formulation).
▪️Risk assessment Available evidence
- Background
- Evidence
- A retrospective review found that in Australia deaths from MR formulation accounted for a third of the total cases recorded between 2009 and 2017 *.
- For ER paracetamol, early data suggested a similar risk between immediate release and ER acetaminophen overdose *.
- Given that the nomogram cannot be safely used for risk assessment in MR and ER overdose, it is unclear whether the documented risks for MR
acetaminophen extend to the ER formulation.
- Given that the nomogram cannot be safely used for risk assessment in MR and ER overdose, it is unclear whether the documented risks for MR
- More studies are needed to ascertain the risks of ER paracetamol compared to immediate release and MR formulations.
⚠️Caution. In the absence of these, it is prudent to assume that the risk is higher for all sustained-release acetaminophen preparations.
▪️Management
- Management is the same as for ingestion of other acetaminophen products with a few exceptions and considerations:
- Charcoal may be useful longer than 4 hours after ingestion if evidence of ongoing absorption is present (e.g. an increasing acetaminophen concentration).
- If the acetaminophen concentration from samples drawn 4 to 24 hours after ingestion is above the nomogram treatment line, acetylcysteine is needed.
- If the concentration from samples drawn 4 to 12 hours after ingestion is >10 mcg/mL but below the treatment line, it should be measured again 4 to 6 hours after the first measurement. A full course of acetylcysteine therapy should be instituted (or continued if already started) if the second acetaminophen concentration is above the nomogram treatment line.
Coingestion of Anticholinergics or Opioid

- Acetaminophen–opioid combination products have been implicated in chronic overuse, likely due to an increasing opioid requirement leading to concomitantly increasing acetaminophen exposure.
- The clinical concern is that acetaminophen absorption may be delayed or prolonged.
- Patients with poisoning may present with early onset alteration in mental status or signs and symptoms of opioid intoxication such as respiratory depression (see here).
▪️Management
- Management is the same as with other acetaminophen products with a few exceptions and considerations:
- If the first acetaminophen concentration measured at 4 to 24 hours after ingestion is ≤10 μg/mL, another measurement need not be taken and acetylcysteine treatment is not needed. If any concentration is above the treatment line, acetylcysteine is needed.
- If the acetaminophen concentration measured 4 to 24 hours after ingestion is > 10 μg/mL but less than the treatment line on the revised nomogram and the patient has anticholinergic or opioid clinical effects, another measurement should be taken 4 to 6 hours after the first measurement.
- The dose and duration of acetylcysteine treatment are the same as with other acetaminophen ingestions.
Management of hepatic failure
- Hepatic failure is defined as a severe liver injury in a patient who has impaired synthetic function (INR ≥1.5) with “hepatic encephalopathy” (HE). For a detailed discussion on the management of hepatic failure, see👉 here.
- The mortality rate for patients with acetaminophen-induced fulminant hepatic failure without acetylcysteine therapy is significant.
- Most fatalities occur on days 3 to 5 after overdose and are attributed to hepatic complications such as cerebral edema, hemorrhage, shock, acute lung injury, sepsis, and multiorgan failure.
- Patients who survive fulminant hepatic failure generally begin to show evidence of recovery by days 5 to 7. Survivors will eventually develop complete hepatic regeneration without any persistence of hepatic impairment.
- Acetylcysteine
- Acetylcysteine still provides benefits, even if delayed until after hepatic failure has been established (~ 28% mortality benefit).
- Acetylcysteine infusion should be started and continued until the patient recovers, receives a liver transplant, or dies.
- First, give the standard 24-hour regimen and then continue the infusion at a rate equal to the third IV dose in the protocol (100 mg/kg infused over 16 hours repeatedly).
- ⚠️ Don’t allow the acetylcysteine infusion to stop until the liver is improving and the acetaminophen level is zero.
- The King’s College Criteria can be used to assess the potential need for a liver transplant. 🧮MDCalc.
- The criteria include:
- Arterial pH <7.30 despite fluid and hemodynamic resuscitation
- Or a combination of
- PT>100 seconds (INR > 6.5 is commonly used), and
- Renal insufficiency (creatinine >3.3 mg/dL), and
- Grade III or IV hepatic encephalopathy.
- Other predictors of poor prognosis without transplant
- Lactate >3.5 mmol/L after fluid resuscitation (<4 hrs) OR lactate >3 mmol/L after full fluid resuscitation (12 hours).
- Phosphate >3.75 mg/dL (1.2 mmol/L) at 48-96 hours
- The criteria include:
- The survival rate of patients who meet the KCC but are not transplanted is 25% to 40%.
- Survival rates have significantly increased since the original studies, likely because of the utilization of prolonged NAC therapy and improved supportive care for patients with acute hepatic failure.
- Unfortunately, patients often meet the KCC and lactate criteria quite late in the course of the disease, so these criteria are not useful as early predictors or as standards for transfer to a facility that performs the hepatic transplant.
- Patients with acute hepatic failure can be candidates for transplantation, even if they are recently suicidal.
- Consult with the poison center and/or medical toxicologist to discuss the need for transfer to the transplant center.
Specific population
Pediatric poisoning
Pediatric patients may be at decreased risk of hepatotoxicity and severe APAP poisoning compared to adults based on the predominant sulfation pathway in patients. However, the Rumack-Matthew nomogram is used the same way in pediatric patients as in adult patients.
▪️N-acetylcysteine
- Due to the concern of fluid overload in pediatric patients, volume adjustments are made.
- Loading dose: 150 mg/kg over at least 45-60 minutes.
- The volume of diluent (D5W or 0.45% sodium chloride) needed to be adjusted based on weight to avoid fluid overload, and hyponatremia:
- 5–20 kg: 3 mL/kg 5% dextrose in water infused over 60 min.
- 21–40 kg: 100 mL 5% dextrose in water infused over 60 min.
- >40 kg = 200 mL 5% dextrose in water
- The volume of diluent (D5W or 0.45% sodium chloride) needed to be adjusted based on weight to avoid fluid overload, and hyponatremia:
- Second dose: 50mg/kg over 4h
- The volume of diluent (D5W or 0.45% sodium chloride) needed to be adjusted as:
- 5–20 kg:7 mL/kg 5% dextrose in water
- 21–40 kg: 250 mL 5% dextrose in water
- >40 kg = 500 mL 5% dextrose in water
- The volume of diluent (D5W or 0.45% sodium chloride) needed to be adjusted as:
- Third dose: 100mg/kg over 16h
- The volume of diluent (D5W or 0.45% sodium chloride) needed to be adjusted as:
- 5–20 kg: 14 mL/kg 5% dextrose.
- 21–40 kg: 500 mL 5% dextrose in water
- >40 kg = 1000 mL 5% dextrose in water
- The volume of diluent (D5W or 0.45% sodium chloride) needed to be adjusted as:
Poisoning in pregnancy
- Acetaminophen poses a risk of hepatic failure to both mother and fetus.
- Acetylcysteine is safe and beneficial in pregnancy.
- In a prospective observational study of pregnant women, delayed treatment with acetylcysteine was associated with an increased risk of miscarriage and fetal death *.
- IV acetylcysteine may be especially preferable because it achieves higher serum drug levels and avoids vomiting. If IV acetylcysteine is unavailable, then oral acetylcysteine may be used.
Going further
- Paracetamol toxicity (Life in the Fast Lane)
- Acetaminophen Poisoning – Pitfalls in Assessment and Management (Emergency Medicine Cases)
Appendix
Analgesic mechanism of acetaminophen






Add comment