19 February 2024 by Shahriar Lahouti.
CONTENTS
- Preface
- Pharmacology
- Opioid intoxication
- Treatment
- Naloxone
- Management
- Treatment of toxicity of specific opioids
- References
Preface
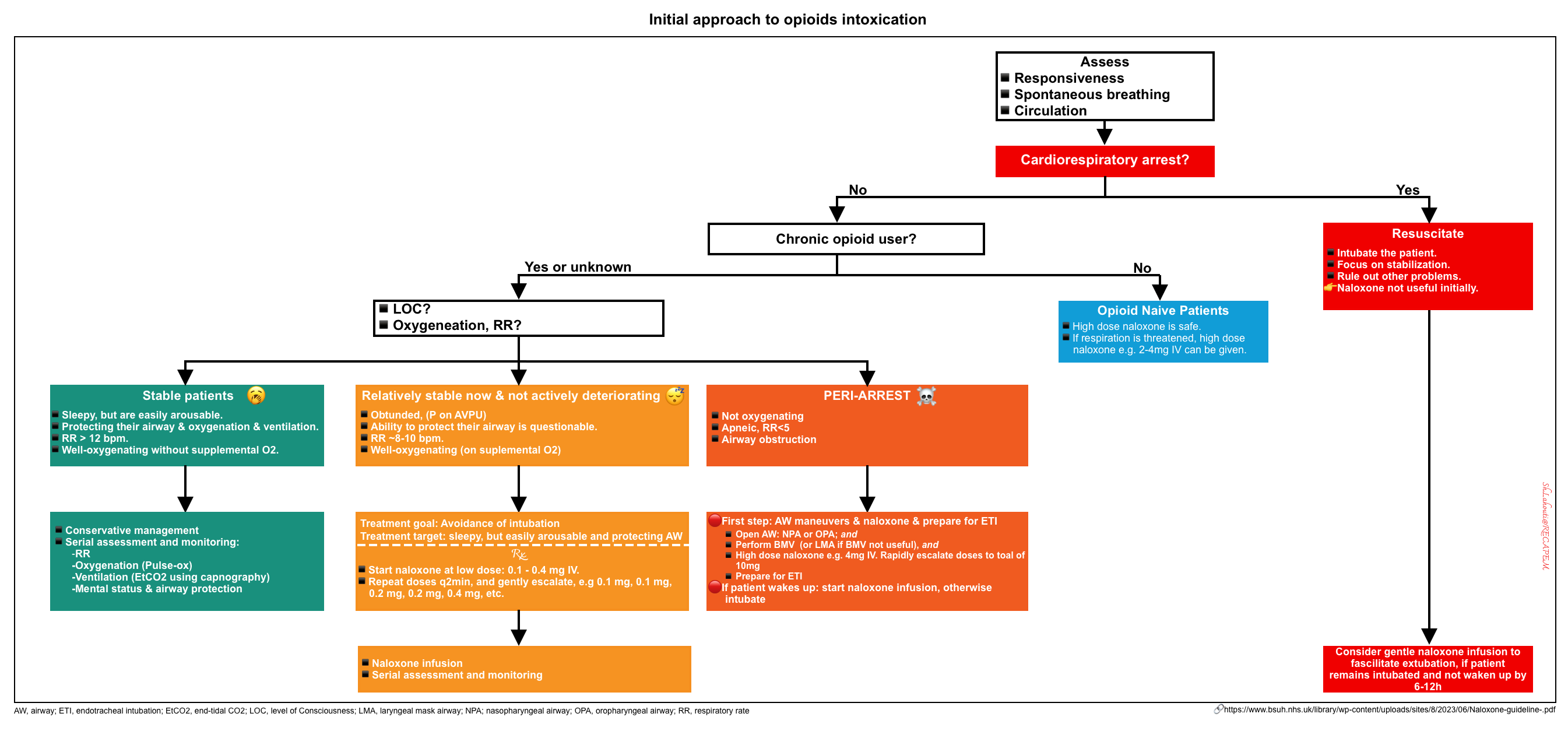
Opioid intoxication is a potentially lethal condition with toxic effects in multiple organ systems leading to a wide range of clinical findings. Although the therapeutic and toxic doses are difficult to predict because of the development of tolerance with chronic use, the primary adverse event from excessive dosing is respiratory depression. Thus ventilatory support, or administration of opioid antagonists such as naloxone, should be adequate for initial therapy.
Pharmacology
▪️Opioids are broadly referred to as all compounds related to opium that possess analgesic and sedative properties. There are three primary opioid receptors: μ (mu), κ (kappa), and δ (delta).
- Stimulation of the μ-receptors results in analgesia, sedation, miosis, respiratory depression, cough suppression, euphoria, and decreased GI motility.
- Stimulation of κ-receptors results in weaker analgesia, sedation, miosis, decreased intestinal motility, dysphoria, and hallucinations.
- Stimulation of the δ-receptors results in some analgesia and antidepressant effects.
▪️There is interplay between opioid receptors and other transmembrane receptors found in the nervous system.
- Analgesic effects result from the inhibition of nociceptive information at multiple points of its transmission from the peripheral nerve to the spinal cord to the brain.
- The analgesic effect of morphine is enhanced in the presence of N-methyl-d-aspartate receptor blockers such as amantadine.
- Euphoria results from opioid binding to μ-receptors in the mesolimbic system (nucleus accumbens) leading to a localized release of dopamine (dopamine pleasure pathway).
- Anxiolysis results from effects on noradrenergic neurons in the locus ceruleus.
- Mast cell histamine release can be induced by morphine and meperidine.
Some opioids are agonists at all opioid receptors (e.g. morphine and hydromorphone), whereas others are partial agonists–antagonists (e.g. pentazocine, butorphanol, and nalbuphine) at the opioid receptors. Buprenorphine is a partial agonist at opioid receptors (more on this below).
Opioid intoxication
Clinical features
▪️Key features of opioid toxidrome include *
- Altered mental status ranging from mild euphoria or lethargy to coma
- Mental status can range from euphoria to coma, or be nearly normal.
- The depression in mental status can be profound.
- Mental status can range from euphoria to coma, or be nearly normal.
- Respiratory depression
- Slow and shallow respirations can produce hypercarbia, hypoxia, and cyanosis.
- A decreased respiratory rate is the most notable vital sign abnormality *.
- Respiratory rate < 12/minute is the best predictor of opioid toxicity and can predict response to naloxone in virtually all patients *.
- Use pulse oximetry in every patient.
- However, keep in mind that mild hypercapnia can be present in the setting of normal oxygen saturation when breathing room air and can be particularly severe when the patient is placed on supplemental oxygen.
- Use capnography to monitor end-tidal CO (EtCO2 ), and thereby ventilation, directly.
- When increased, EtCO2 often predicts respiratory complications, although a normal value does not exclude such problems.
- In a prospective cohort study of 201 patients poisoned with respiratory depressants, noninvasive end-tidal EtCO2 >50 mmHg predicted complications of hypoventilation with 46 percent sensitivity and 86 percent specificity *.
- Miosis
- Normal pupil examination does not exclude opioid toxicity.
- Some opioids have serotonergic effects that dilate the pupils (e.g. meperidine and tramadol).
- Opioids with predominantly δ-agonist effects, such as pentazocine, may not result in miosis.
- The presence of coingestants (such as sympathomimetics or anticholinergics) makes pupils appear normal or large.
- Similarly, patients ingesting diphenoxylate and atropine (Lomotil) or those using scopolamine-adulterated heroin typically develop mydriasis.
- Speedball (the combination of heroin and cocaine) may produce virtually any size pupil, depending on the relative contribution by each xenobiotic.
- Severe cerebral hypoxia can cause mydriasis.
- Severe opioid intoxication causes hypercapnia, triggering a sympathetic response that may normalize the pupil size *.
- Normal pupil examination does not exclude opioid toxicity.
▪️Other possible findings include
- Hypothermia 🥶
- Low to normal heart rate and blood pressure (low blood pressure from histamine release)
- Localized urticaria (histamine release)
- Nausea and vomiting (especially in opioid-naïve patients)🤮
- Bronchospasm (histamine release)
- Pulmonary edema
- Ileus (↓GI motility)
- Urinary retention (↑vesical sphincter tone)
- Rhabdomyolysis, compartment syndrome, myoglobinuric renal failure *.
- ⚡️Seizures typically occur in the setting of tramadol, propoxyphene, and meperidine overdose, or as a result of hypoxia from any opioid.
▪️Toxicities of specific opioids
- Serotonin syndrome and seizure can be caused by meperidine, tramadol *, or dextromethorphan.
- Note that naloxone is not effective in treating opioid-induced serotonin syndrome.
- QT prolongation and torsade de pointes can happen following overdose of methadone, and loperamide.
Differential diagnosis
▪️Intoxicologic with different agents that can produce similar clinical findings
- Clonidine (alpha-2 receptor agonists intoxication)
- Coma, bradycardia, hypotension, miosis; but without bradypnea.
- Organophosphates and carbamates
- Cholinergic toxidrome: miosis, muscle fasciculations, profuse vomiting, diarrhea, and sweating.
- Phenothiazines and atypical antipsychotic medications
- Neurologic depression and miosis from a decreased adrenergic tone.
- Gamma hydroxybutyrate (GHB) intoxication
- Profound CNS depression, bradypnea, and, occasionally, miosis.
- Sedative-hypnotic medications and Carbon monoxide
- Profound neurologic depression, but is not usually associated with miosis.
- The sedative-hypnotics result in less respiratory depression than opioids, especially when taken orally.
▪️Polysubstance intoxication
- The presence of coingestants often confounds the diagnosis of opioid toxicity.
- Opioids are often coingested with acetaminophen when patients overdose on combination pills (e.g. Percocet or Vicodin).
- Opioids may be coingested with a sympathomimetic such as cocaine or amphetamine (a combination known as a “speedball”).
- In this case, administration of naloxone will unmask the effects of sympathomimetic intoxication, often leading to marked agitation.
▪️Any medical condition that produces coma 📖
- Any medical condition that produces coma may be mistaken for (or occur in conjunction with) opioid toxicity.
- The most important conditions to exclude are those in which a delay in diagnosis will delay definitive care, such as stroke, electrolyte abnormality, hypoglycemia, and sepsis.
▪️Acute pathology in opioid-dependent patients
- Many patients are on chronic opioids (either therapeutically or recreationally). The superposition of an acute illness (e.g. stroke) may create a confusing picture:
- Patients will often have evidence of opioid ingestion.
- Patients generally have a partial response to naloxone (e.g. with pupil dilation and agitation), but often without complete normalization of mental status. Increased arousal following naloxone is due to elicitation of opioid withdrawal, rather than reversal of opioid intoxication.
- The key to the diagnosis may be features that don’t otherwise fit with an opioid toxidrome (e.g. fever, focal neurological findings, failure to improve over time, incomplete response to naloxone).
Investigations
- Fingerstick glucose
- Hypoglycemia is prevalent, easily detectable, rapidly correctable, and potentially confused with opioid toxicity.
- Acetaminophen & salicylate levels.
- Creatine kinase (to exclude rhabdomyolysis in the patient presenting after prolonged immobilization and if there is concern regarding compartment syndrome).
- A metabolic panel (can demonstrate acute kidney injury, which may be due to rhabdomyolysis or evidence of metabolic acidosis, both of which should prompt further evaluation).
- Serum ethanol level.
- ECG.
⚠️Urine toxicologic screens should not be routinely obtained.
- Acute opioid toxicity is a clinical diagnosis; the management of a patient with an opioid toxidrome is unchanged by the result of a urine opioid screen.
- A positive test indicates recent use but does not confirm acute toxicity and may even represent a false positive. Conversely, many opioids, especially synthetic drugs, will produce false-negative results in many urine screens.
Diagnosis
The combination of an altered level of consciousness, a respiratory rate of <12 breaths/min, miosis, and circumstantial evidence of opioid use (drug paraphernalia, needle marks, presence of a tourniquet, bystander corroboration) is highly sensitive for opioid overdose.
- Listen for auscultatory findings suggestive of pulmonary edema.
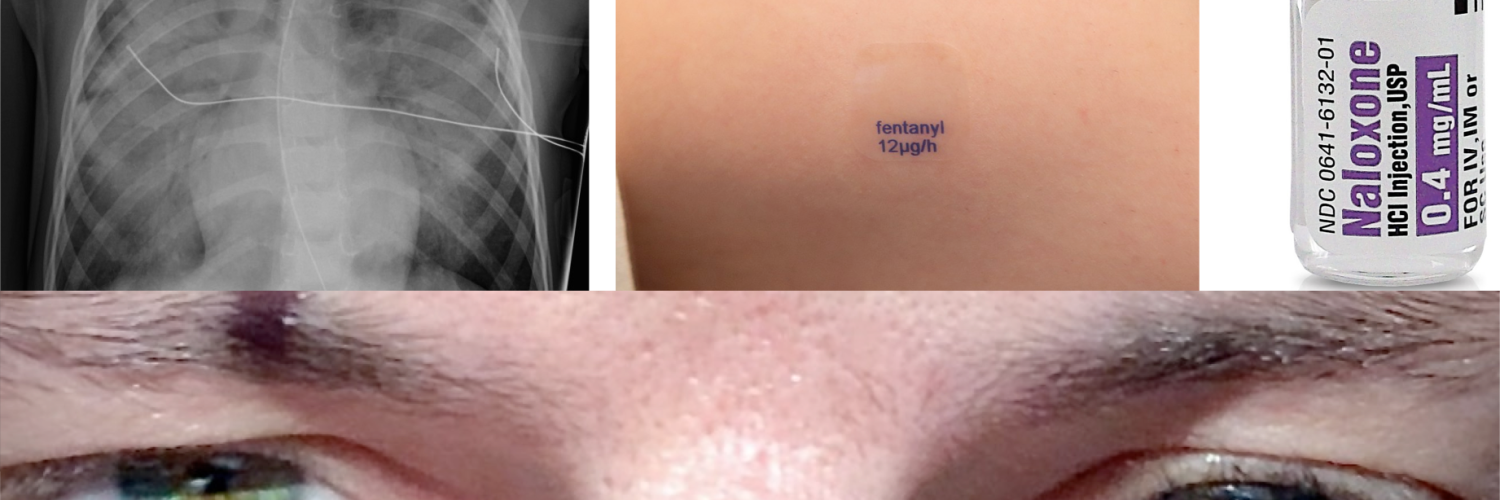
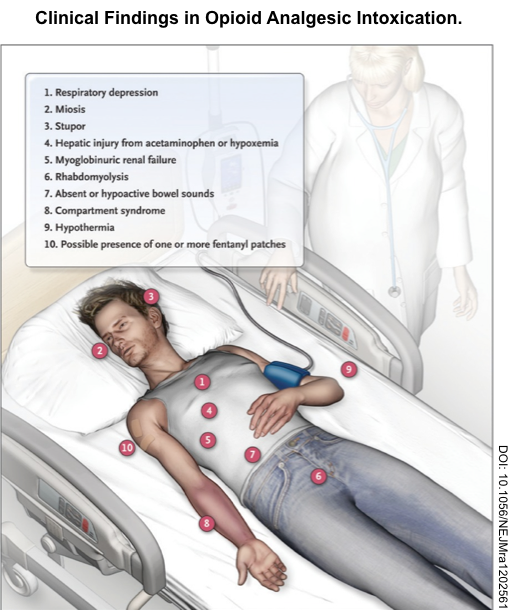
- Undress the patient completely and look for hidden opioids or drug-use paraphernalia, check for fentanyl patches on all parts of the body, including mucous cavities.
- Palpate muscle groups to detect signs of compartment syndrome.
▪️Does a clinical response of a stuprous patient to naloxone indicate opioid intoxication?
- Any opioid-dependent patient will experience pain and agitation after naloxone, due to withdrawal of opioid effects. This does not prove that their stupor/coma is caused solely by opioids unless they can wake up and mentate normally.
Treatment
Naloxone
▪️Basic principles
- Naloxone is a pure competitive antagonist at all opioid receptors, with a particular affinity for μ-receptors.
- It fully reverses all the effects of opioids, including respiratory and CNS depression, miosis, and analgesia.
- Naloxone also antagonizes opioid-induced seizures, except those induced by meperidine, propoxyphene, or tramadol.
- Giving naloxone (even in massive doses) to someone not on opioids will have minimal effect.
- Side effects of naloxone: Precipitated opioid withdrawal manifested as:
- In opioid-naive patients with acute intoxication, administering high doses of naloxone is safe.
- For patients who are on chronic opioids with acute-on-chronic intoxication, giving high doses of naloxone may induce an immediate state of opioid withdrawal.
▪️Pharmacology
- Route of administration: Nebulized, intramuscular (IM), or intravenous (IV).
- The onset of action following IV administration is 1-2 minutes.
- Duration of action: 20-90 minutes (shorter duration of action, if a large amount of opioid agonist is present).
- Most opioids have a longer half-life than naloxone, so the naloxone will wear off before the opioid intoxication resolves. Therefore, repeated doses or an infusion of naloxone are often needed to prevent re-sedation.
▪️Initial dosing of naloxone
- The starting dose is somewhat controversial.
- General principles
- In patients with less critical conditions, e.g. patients who present with mental status depression (obtunded) but with minimal respiratory depression:
- 🎯The goal is to achieve adequate ventilation and airway protection (not necessarily a full state of arousal).
- Follow the low-dose protocol.
- In opioid-dependent patients: 0.1 mg IV.
- In non–non-opioid-dependent patients: 0.4mg IV
- If naloxone isn’t working, successively larger doses may be given q3-5 minutes (e.g. 0.1 mg, 0.2 mg, 0.4 mg, 1 mg, etc.)
- In patients with critical conditions, e.g. apnea or near-apnea and cyanosis:
- Follow the high-dose protocol, “regardless of drug use history“: 4 mg IV or IM bolus.
- Escalate naloxone doses rapidly, e.g. initial 4mg →8mg if no appropriate response is seen after 2min → 2-4 mg if no appropriate response is seen after 2min; up to a total of ~10 mg.
- Patients who may require larger-than-ordinary-doses:
- Exposures to synthetic opioids, such as propoxyphene, pentazocine, dextromethorphan, or fentanyl *.
- Methadone intoxication
- Sustained-release preparations
- Toxicity from leaking opioid-containing packets in the intestinal tract (i.e. in “body packers”) can be extremely severe, and such patients require large and sustained naloxone doses until the drug-containing packets are expelled or removed.
- In patients with less critical conditions, e.g. patients who present with mental status depression (obtunded) but with minimal respiratory depression:
▪️Naloxone infusion
- Rational
- The duration of action of naloxone is often shorter than that of the offending opioid, so naloxone infusions are occasionally required to support respiration over several hours as the opioid is metabolized. This is especially true for certain long-acting opioids, such as
- Buprenorphine
- Methadone
- Propoxyphene
- Sustained-release preparations
- Ingestions of dermal patches
- The duration of action of naloxone is often shorter than that of the offending opioid, so naloxone infusions are occasionally required to support respiration over several hours as the opioid is metabolized. This is especially true for certain long-acting opioids, such as
- Indication
- A continuous infusion should be considered only if the patient responded to the initial naloxone bolus and subsequently required repeat administration.
- ‘Good Response’ implies improvement of both respiratory rate to >12 bpm AND improvement of level of consciousness to at least V on the AVPU scale.
- Naloxone continuous infusion dose
- Stop as soon as the patient improves to a point where they are protecting their airway and ventilating adequately (e.g. respiratory rate >12/minute).
- Determine the cumulative “wake-up dose” and administer two-thirds of that dose per hour by IV infusion (e.g., if the patient responds well to 1 mg, then start the infusion at 0.6 mg/hour).
- Adjustment of infusion:
- If the patient develops respiratory depression: Repeat a bolus and increase the infusion rates.
- If the patient develops agitation, or withdrawal symptoms (patients on chronic opioids, as their acute intoxication wears off the naloxone will start to induce a state of withdrawal):
- Hold or down-titrate the naloxone (wait for naloxone to wear off, which should occur within an hour).
- Avoid giving an opioid, as this leads to an ongoing vicious spiral.
- Watch for re-sedation, and if respiratory depression recurs, start the infusion at half the original rate.
▪️The patient is medically stable for transfer or discharge when their mental status and ventilation remain normal for more than one hour after cessation of naloxone.
Management
Principles
🟥Care of the opioid-poisoned patient should focus on airway and breathing.
🟥Naloxone is the antidote for opioid toxicity and rapidly reverses all drug effects. Primary indication:
- Reversal of life-threatening respiratory depression, e.g. apnea, severe bradypnea (e.g. RR<5), hypoxia, and/or severe hypercapnia.
- Reduced consciousness (P or U on AVPU) due to opioid toxicity.
🟥Gastrointestinal decontamination: Activated charcoal and gastric emptying are almost never indicated in opioid toxicity. Gastrointestinal decontamination should be reserved for patients presenting with potentially life-threatening coingestants, not for opioids alone, and should be performed only if the airway is secure.
🟥Extracorporeal removal (dialysis) is not effective for opioids because of their large volume of distribution.
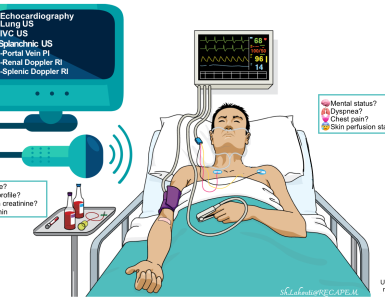
🟥Serial assessment & monitoring
- Respiratory rate (RR)
- This is the most useful parameter to trend.
- A falling RR < ~ 8-10 breaths/minute is concerning for hypoventilation.
- Oxygenation
- Deteriorating oxygenation is highly worrisome, as these patients should oxygenate well once given naloxone.
- A chest x-ray may be needed to evaluate for aspiration (common) or opioid-induced noncardiogenic pulmonary edema (rare).
- Mental status & airway protection
- Ideally, patients should be easily arousable and able to protect their airways (A or V on AVPU).
- EtCO2
- If available, a nasal cannula capable of measuring end-tidal CO2 may be attached and used to trend the patient’s CO2.
- Note, however, that these patients still require close attention to the other parameters listed above.
- Mild hypercapnia is acceptable as long as the hypercapnia is roughly stable and the patient is clinically arousable and protecting their airway.
Overview
An overview of the initial approach to management is presented below *.
Individualized approach to management
| Stable intoxicated patients |
- These patient are protecting their airway, oxygenating, and ventilating.
- Patients may be sleepy but are easily arousable (A or V on AVPU).
- The respiratory rate is roughly >12 bpm.
- Management
- The general condition of these patients is stable.
- They just need to sleep off the opioid. Careful observation is needed, with a focus on respiratory rate and airway protection.
- If deterioration occurs, naloxone administration is warranted.
| Obtunded patients |
- These patients are usually obtunded (P on AVPU), with questionable ability to protect their airways.
- Their respiratory rate is low, roughly 8-10 bpm
- Oxygenation is appropriate (probably on supplemental O2)
- Management
- 🎯Therapeutic goal: Avoidance of intubation.
- It is to give just enough naloxone so that the patient protects their airway.
- The therapeutic target is thus a patient who is comfortable and sleepy, but easily awakens and protects their airway (V on AVPU)
- Note that the patient should not be given too much naloxone to bring the patient back to a normal state of consciousness. In opioid-dependent patients, trying to “normalize” their mental status may provoke a withdrawal state.
- Naloxone dosing and continuous infusion are discussed above.
- Give antiemetics to prevent vomiting (when naloxone is administered).
- 🎯Therapeutic goal: Avoidance of intubation.
| Peri-arrest patients |
- These patients are apneic, or near-apneic and cyanotic.
- Management
- Perform basic airway maneuvers, give high-dose naloxone, and prepare for intubation.
- Basic airway management
- Open the airway. Nasopharyngeal or oropharyngeal airways may be used to open the airway.
- Perform bag-mask ventilation (BMV).
- If BMV isn’t successful, move immediately to the placement of a laryngeal mask airway (LMA).
- Give high-dose naloxone with rapidly escalating doses (discussed above).
- Prepare for intubation.
- Basic airway management
- If the patient awakens following naloxone administration, then the need for intubation is averted.
- Intubation is indicated if either of the following occurs:
- Failure of basic airway maneuvers to achieve adequate ventilation AND the patient doesn’t immediately respond to naloxone.
- Basic airway maneuvers succeed in achieving adequate ventilation, but despite escalating doses of naloxone, the patient fails to awaken.
- Perform basic airway maneuvers, give high-dose naloxone, and prepare for intubation.
| Intubated patients |
- There is generally no immediate need for naloxone in intubated patients.
- Rather focus on stabilizing the patient, and excluding other problems (e.g. head CT scan, toxicology evaluation).
- Long-acting sedatives should be avoided.
- Propofol is appropriate for the initial sedation of intubated patients.
- If the patient starts requiring any significant dose of sedation, consider whether they may be extubated.
- Naloxone may be indicated to facilitate extubation.
- Rationale: Earlier extubation may avoid complications associated with mechanical ventilation.
- A gentle naloxone titration may be indicated for patients who fail to metabolize their opioids after 6-12 hours.
- If the patient responds well to a naloxone infusion, they may be extubated and continued on a naloxone infusion.
- Candidates for this strategy should meet the following criteria:
- Uncomplicated opioid intoxication with a long-acting agent.
- No active lung disease (e.g. chest X-ray shows no aspiration).
- Any tests which may be desired (e.g. LP, MRI) have already been done.
Treatment of toxicity of specific opioids
Loperamide
- Loperamide is an antidiarrheal agent, which may exert opioid effects when taken in large quantities, typically 30 to 40 pills or more.
- The opioid toxidrome of loperamide intoxication should be treated as usual (e.g. with naloxone).
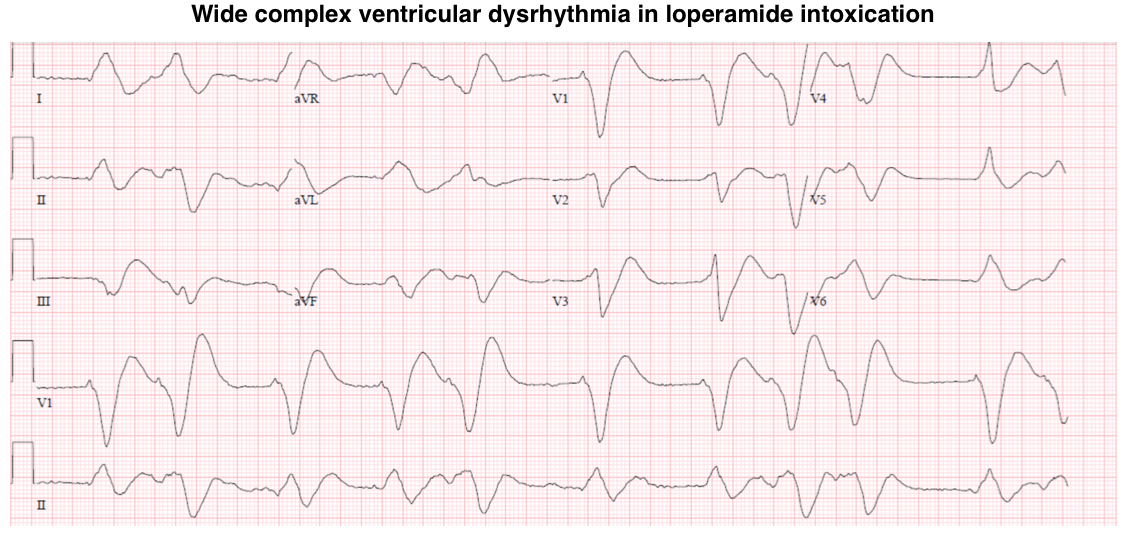
- The specific feature of loperamide intoxication is cardiotoxicity.
- Loperamide blocks hERG potassium channels, potentially causing a prolongation of the QT interval and torsade de pointes.
- Management
- Follow the QT interval with serial ECGs. If the QTi > 500 msec, monitor the patient on telemetry for 24 hours.
- Aggressively replete electrolytes to target a magnesium level >3 mg/dL and a potassium level >4 mEq/L.
- If torsade de pointes occurs, consider the initiation of a magnesium infusion. More on the treatment of torsade de pointes 📖.
- Management
- Loperamide may also block sodium channels, causing a prolongation of the QRS interval that can lead to monomorphic ventricular tachycardia.
- Management: If QRS is substantially prolonged (QRS>100 ms in a patient with normal or presumably normal baseline QRS, then:
- Hypertonic bicarbonate should be loaded initially. Give 1-2 meq/kg of sodium bicarbonate IV (~ 2 amps IV push).
- Watch for hypokalemia induced by the bicarbonate infusion, as this may predispose to torsade de pointes.
- If bicarbonate causes an improvement in the ECG, this confirms the presence of clinical sodium channel blockade, and bicarbonate infusion should be started.
- Isotonic bicarbonate for maintenance infusion. Mix 3 amp of sodium bicarbonate (150meq) with 1L of D5W.
- Infusion rate: ~150-250 ml/h.
- Lidocaine may be considered for recurrent VT despite bicarbonate, or persistent hypotension with prolonged QRS interval.
- Lipid emulsion may have a role in cardiovascular collapse unresponsive to other measures.
- Hypertonic bicarbonate should be loaded initially. Give 1-2 meq/kg of sodium bicarbonate IV (~ 2 amps IV push).
- Management: If QRS is substantially prolonged (QRS>100 ms in a patient with normal or presumably normal baseline QRS, then:
- Loperamide blocks hERG potassium channels, potentially causing a prolongation of the QT interval and torsade de pointes.
Methadone
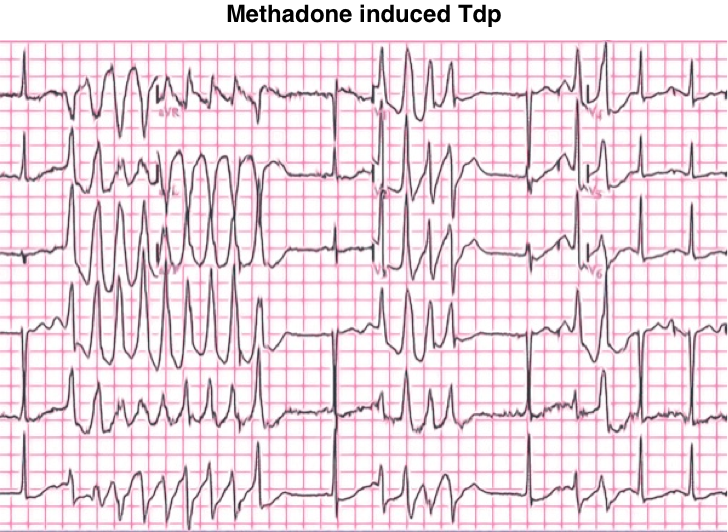
- Methadone increases the QT interval and can promote torsade de pointes.
- The opioid toxidrome of loperamide intoxication should be treated as usual (e.g. with naloxone), however, methadone has a long half-life and intoxication may require a prolonged naloxone infusion.
- Management of cardiotoxicity is the same as the treatment of loperamide-induced QTc prolongation and Tdp, discussed above.
Buprenorphine
Pharmacologic properties
- Buprenorphine is a partial agonist at μ-receptors, with a decreased intrinsic activity that causes its clinical effects to plateau at higher dosages *.
- Buprenorphine has a high affinity for and slow dissociation from the μ-receptor, which results in a long duration of action.
- Furthermore, other opioid agonists (such as heroin) or antagonists (such as naloxone) cannot easily displace buprenorphine from the μ-receptor.
Buprenorphine can be associated with three distinct clinical scenarios.
- If an opioid-naïve patient overdoses on buprenorphine, the toxidrome includes mental status depression, nausea, vomiting, miosis, and respiratory depression (usually with a plateau).
- In this situation, naloxone may not be fully effective in reversing mental status and respiratory depression.
- Re-administration of naloxone and naloxone infusion is frequent due to the long duration of action of buprenorphine.
- If an opioid-dependent patient who is still under the influence of an opioid agonist is exposed to buprenorphine, an opioid withdrawal state happens.
- This happens because the partial agonist buprenorphine behaves like an antagonist in the presence of an agonist.
- The management of buprenorphine-induced withdrawal is symptom-driven therapy, including antiemetics, nonopioid analgesics, antidiarrheals, and nonbenzodiazepine sedatives for insomnia.
- If an opioid-dependent patient is undergoing withdrawal, buprenorphine exposure can alleviate the symptoms of opioid withdrawal.
- This is due to the partial agonist activity of buprenorphine.
- This forms the basis for buprenorphine detoxification and maintenance therapy *.
References
1. PMID: 22784117. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012 Jul 12;367(2):146-55. doi: 10.1056/NEJMra1202561.
2. PMID: 1996818. Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Med. 1991 Mar;20(3):246-52. doi: 10.1016/s0196-0644(05)80933-3.
3. PMID: 26810758. Viglino D, et al. Noninvasive End Tidal CO2 Is Unhelpful in the Prediction of Complications in Deliberate Drug Poisoning. Ann Emerg Med. 2016 Jul;68(1):62-70.e1. doi: 10.1016/j.annemergmed.2015.11.037. Epub 2016 Jan 23.
4. PMID: 25068603. Rollins MD, Feiner JR, Lee JM, Shah S, Larson M. Pupillary effects of high-dose opioid quantified with infrared pupillometry. Anesthesiology. 2014 Nov;121(5):1037-44. doi: 10.1097/ALN.0000000000000384.
5. PMID: 28958275. Benns M, Miller K, Harbrecht B, Bozeman M, Nash N. Heroin-Related Compartment Syndrome: An Increasing Problem for Acute Care Surgeons. Am Surg. 2017 Sep 1;83(9):962-965.
6. PMID: 29752906. Hassamal S, Miotto K, Dale W, Danovitch I. Tramadol: Understanding the Risk of Serotonin Syndrome and Seizures. Am J Med. 2018 Nov;131(11):1382.e1-1382.e6. doi: 10.1016/j.amjmed.2018.04.025. Epub 2018 May 10.
7. PMID: 25645123. Spinner HL, Lonardo NW, Mulamalla R, Stehlik J. Ventricular tachycardia associated with high-dose chronic loperamide use. Pharmacotherapy. 2015 Feb;35(2):234-8. doi: 10.1002/phar.1540. Epub 2015 Feb 3.
8. PMID: 27022002. Wightman RS, Hoffman RS, Howland MA, Rice B, Biary R, Lugassy D. Not your regular high: cardiac dysrhythmias caused by loperamide. Clin Toxicol (Phila). 2016 Jun;54(5):454-8. doi: 10.3109/15563650.2016.1159310. Epub 2016 Mar 29.
9. PMID: 27747471. Lameijer H, Azizi N, Ligtenberg JJ, Ter Maaten JC. Ventricular Tachycardia After Naloxone Administration: a Drug Related Complication? Case Report and Literature Review. Drug Saf Case Rep. 2014 Dec;1(1):2. doi: 10.1007/s40800-014-0002-0.
10. PMID: 20223408. Lemesle F, Lemesle F, Nicola W, Pierre Jonville-Béra A. First case of stress cardiomyopathy as a result of methadone withdrawal secondary to drug-drug interaction. Am J Emerg Med. 2010 Mar;28(3):387.e5-6. doi: 10.1016/j.ajem.2009.07.007.
11. PMID: 29749582. Jiwa N, Sheth H, Silverman R. Naloxone-Induced Non-Cardiogenic Pulmonary Edema: A Case Report. Drug Saf Case Rep. 2018 May 10;5(1):20. doi: 10.1007/s40800-018-0088-x.
12. PMID: 29103795. Love JS, Perrone J, Nelson LS. Should Buprenorphine Be Administered to Patients With Opioid Withdrawal in the Emergency Department? Ann Emerg Med. 2018 Jul;72(1):26-28. doi: 10.1016/j.annemergmed.2017.10.002. Epub 2017 Nov 3.



Add comment