30 July, 2020 by Shahriar Lahouti. Last update 5 February, 2023.
CONTENTS
- Preface
- Anatomy
- Patthophysiology and definition
- Classification
- Epidemiology and risk factors
- Clinical presentation
- Differentials
- Evaluation
- Labs
- ECG
- Imaging
- Modalities and diagnostic performance
- Artifacts
- Diagnostic findings
- Approach to diagnosis and management
- Treatment
- Specific condition
- Prognostication
- RECAP
- Media
- Going further
- References
Preface
Acute aortic syndromes include a wide range of life-threatening aortic conditions with high probability of misdiagnosis1 ,which requires immediate diagnosis and management. It encompasses aortic dissection (AD), intramural hematoma (IMH), penetrating atherosclerotic ulcer (PAU) and traumatic aortic injury (TAI),aortic rupture, and ruptured aortic aneurysm.2
AAA has its unique pathophysiology, presentation and management as such is not included in this post (More to come on this).
Anatomy
The gross anatomy of the aorta is divided into the following segments2: (1) aortic root; (2) sinotubular junction; (3) ascending aorta; (4) aortic arch; (5) isthmus and descending (thoracic) aorta; and (6) abdominal aorta.
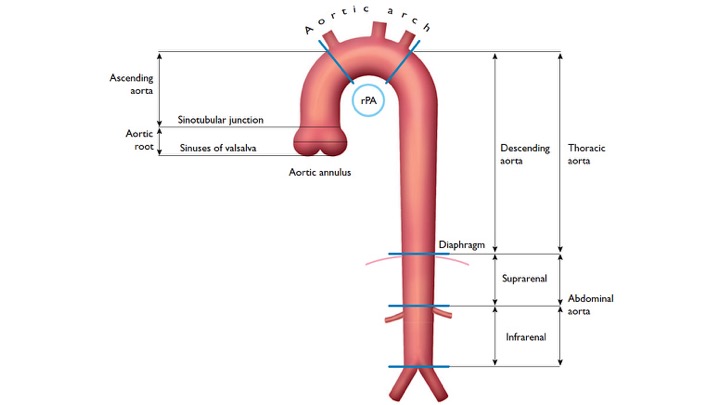
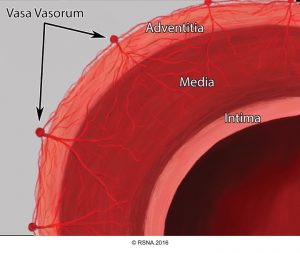
- The aortic root (Light blue bellow picture) is intrapericardial and consists of three rounded dilations (sinuses) at the aortic valve leaflet level that allows the aortic valve leaflets to open appropriately3. Normally, the left coronary ostium arises from the superior left coronary sinus, and the right coronary ostium arises from the superior right coronary Sinus. Aortic root can be involved in a variety of pathologic conditions. In aortic dissection with root involvement, following consequences are possible:
- Disruption of aorta and rupture into pericardial sac resulting in hemopericardium.
- Dynamic obstruction of coronary ostium, most commonly on the right side, resulting in ACS.
- Dilation of the root leads to reduced suspension of the leaflets resulting in acute aortic valve insufficiency.
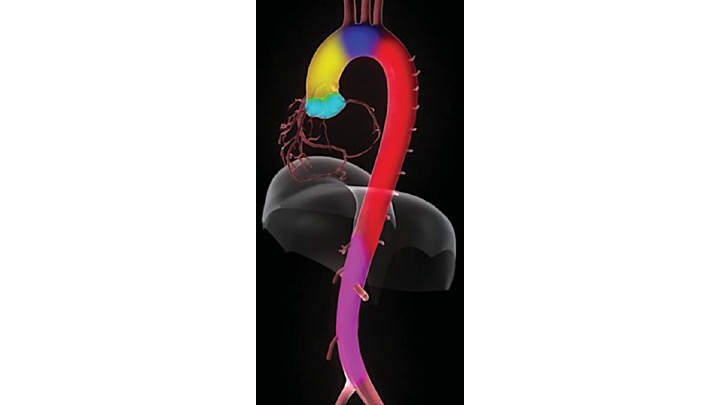
2.The ascending aorta is the segment of the aorta from the sinotubular junction to the first great arch vessel. This segment is also intrapericardial and frequently involved with the same pathologic processes as the aortic root.
3. Aortic arch is the transverse portion of the aorta from the first arch vessel to the left subclavian artery Ostium and is mostly extrapericardial.
4. Isthmus: a short portion of the aorta after the left subclavian artery to the present or former ductus arteriosus.
5. Descending aorta: the vertically oriented portion of the aorta continuing from the isthmus to the level of the diaphragm. Branch vessels include intercostal, spinal, and bronchial arteries. Rupture of the descending aorta is either into the mediastinum or into the large left pleural cavity, which is catastrophic
6. Abdominal aorta: is the infradiaphragmatic segment down to the aortic bifurcation. Branch vessels include:
- Inferior phrenic arteries
- Celiac artery branches (hepatic artery, gastroepiploic artery, and splenic artery)
- Renal arteries
- Superior mesenteric artery
- Inferior mesenteric artery
- Lumbar and spinal arteries
- iliac arteries (internal iliac artery: anterior division [obturator artery, inferior gluteal artery, internal pudendal artery, and visceral branches] and posterior division [superior gluteal artery, iliolumbar artery, and lateral sacral arteries]; and external iliac artery).
The predominant disease processes involving the abdominal aorta include: atherosclerosis and aneurysms.
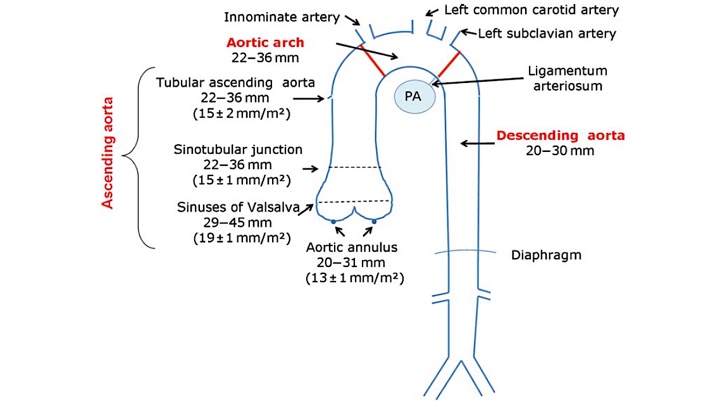
Normal diameter of different segments of aorta is shown below: 4
The basic components of the aorta are the intima, media, and adventitia.5 Each contributes to specific properties of aorta as the major conduit in the body, and their functional alteration during pathologic processes result in certain pathophysiological features shown in following table.3
| Layer | Physiologic function | Dysfunction in pathologic states |
| Intima | -Anti-thrombotic -Anti-atherosclerotic | Atherosclerotic plaque, thrombosis, ulcer, stenosis |
| Media | -Elastic tissue layer enables accommodation of stroke volume during systole and propelling blood forward in diastole -Fibrous tissue resists overdistension and rupture | -Atherosclerosis, and overdistension (HTN) lead to excessive stiffness →↑Pulse pressure -Weakness →Aneurysm, dissection, IMH, rupture |
| Adventitia | Wrap, Nutritive layer | Rupture |
👉Tip
- Calcification of atherosclerotic plaque, a subtype of atheroma complication, increases with each decade of advancing age, until it is present in the vast majority of the elderly in their 80s. There’s a twofold increase in cardiovascular death if atherosclerotic calcification of the aorta is present before 65 years old. In individuals younger than 70 years old, the risk of cardiovascular events, e.g., CAD, Stroke, approximates the plaque burden of the aorta (> 4mm plaque is 90% sensitive for significant CAD).3
- On imaging, calcified aortic intimal atheroma is a marker of intimal location radiographically, and can help to distinguish certain disease states (below, diagnostic findings in imaging).
Pathophysiology and definition
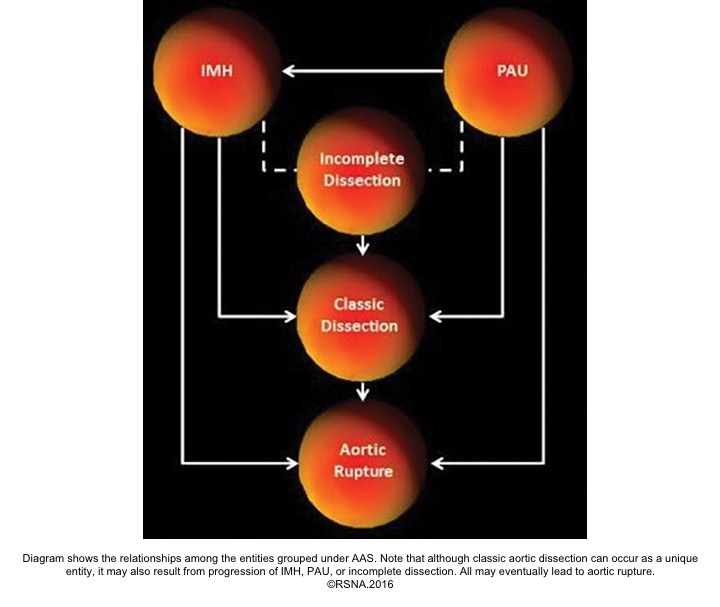
Acute aortic syndromes, defined as life-threatening conditions with similar clinical features involving the aorta. It encompasses aortic dissection, intramural hematoma (IMH), ruptured aneurysm, and penetrating atherosclerotic ulcer (PAU).6
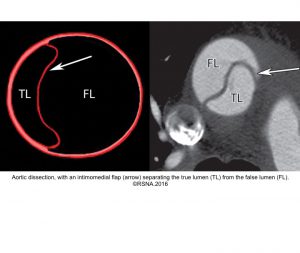
- Aortic dissection: It could be spontaneous or iatrogenic/traumatic. In spontaneous form, intramural bleeding causes disruption of the medial layer, which results in the formation of a false lumen (FL) partitioned from the true lumen(TL) by an intimal flap. In most cases, the inciting event is an intimal tear or an ulcer, but sometimes the rupture of vasa vasorum causes a bleed within the media. The dissection can propagate proximally or distally from an intimal tear to involve the aortic root (aortic valve, coronary arteries), side branches of the aortic arch, and descending aorta.7
- Aortic intramural hematoma (IMH): defined as a hematoma within the medial layer of the aorta in the absence of detectable intimal tears. Recent studies show that ‘microscopic’ tears in the intima are the possible mechanism of bleeding into the media layer of the aorta. Therefore, there’s no imaging evidence of intimal flap or false lumen within the aorta. The presence of blood within the media layer provokes an inflammatory response. This weakens the components of the aortic wall, and consequently, it may lead to aortic rupture.
3.Penetrating aortic ulcer(PAU): refers to the region of the aorta where the aortic intima is denuded, with the lesion progressing through a variable depth of the aortic wall, over which there may or may not be overlying thrombus. Penetrating aortic ulcers are typically associated with atherosclerotic changes of the adjacent aortic wall. It may be associated with thrombus formation within the media (IMH), or may progress through the adventitia to form a pseudoaneurysm, and finally, perforation or aortic dissection may happen.
- Periaortic hematoma represents a ‘contained aortic rupture’ due to slow oozing from the damaged aorta at or near the site of aortic injury. The relationship among the entities grouped under the acute aortic syndrome is shown below:
Anatomic classification
Acute aortic syndromes are classified based on their location and extent of involvement. There are two different anatomic classification systems:
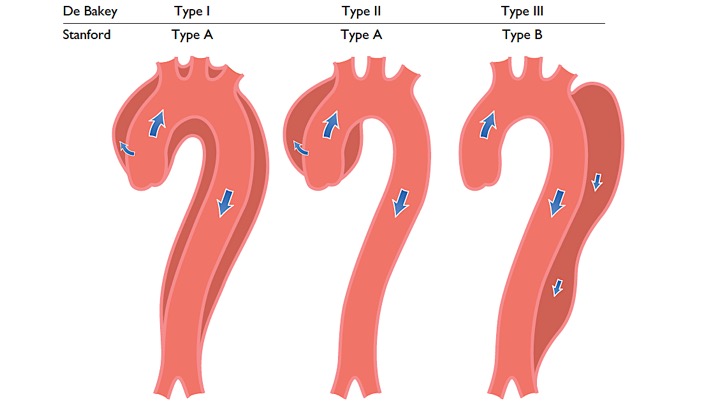
- DeBakey system: is based upon the site of the origin of the tear, whereas type 1 originating in the ascending aorta and propagating to at least the aortic arch, type 2 originating in and confined to the ascending aorta, and type 3 originating in the descending aorta and extending distally or proximally, but not proximal to the left subclavian artery
- Stanford system: classifies aortic dissections that involve the ascending aorta as type A, regardless of the site of the primary intimal tear, and all other dissections as type B
Other classifications
Aortic dissection can also be classified according to the disease phase (acute, subacute, and chronic phases), to the status of blood flow in the FL (communicating and non-communicating types), or to the presence of associated complications (complicated vs. uncomplicated)
Disease phase: By convention, acute disease is distinguished from chronic disease at an arbitrary time point of two weeks from initial clinical presentation.
- A new classification system by the International Registry of Acute Aortic Dissection (IRAD) takes into account that the morbidity and mortality associated with acute dissection are highest in the first 2 weeks, especially the first 24 to 48 hours.
- The IRAD classification includes hyperacute (<24 hours), acute (2 to 7 days), subacute (8 to 30 days), and chronic (>30 days).8
Status of the flow in false lumen
- Complete ‘FL’ thrombosis (absence of flow and presence of thrombus within the FL) is the key to long-term clinical success and long-term survival.9
Complications
- The mortality of acute aortic syndromes is a consequence of its complications. Aortic dissection, as the prototype of this syndrome, can give rise to a variety of organ dysfunction either by rupture or via malperfusion. The latter may be dynamic, static, or mixed.
- Dynamic malperfusion is the most common and results from the overpressurized false lumen pushing the septum toward the true lumen, leading to collapse of the true lumen and obstruction of branch vessels.
- Static malperfusion results from stenosis or occlusion of a branch artery caused by the dissection flap, hematoma, embolism, or thrombosis.
| Organ system | Complications |
| Cardiovascular | Hemothorax (commonly on the left side) Pleural effusion (more on the left side) Hemoptysis (from aortotracheal or bronchial fistula) |
| Pulmonary | GI bleeding (from aortoenteric fistula) Mesenteric ischemia/infarction |
| Neurologic | Hypoxic encephalopathy Horner syndrome Ischemic stroke TIA Spinal cord ischemia Ischemic neuropathy Coma |
| Renal | AKI Renvoascular hypertension Renal ischemia/infarction |
| GI | GI bleeding (from aortoenteric fistula) Mesenteric ischemia / infarction |
| Peripheral vascular | Upper or lower extremity ischemia |
| Systemic | Fever |
| Tip The presence of blood within the aortic wall layer can cause localized inflammation. This inflammatory response not only weakens the aortic wall, which leads to rupture, pseudoaneurysm, fistula formation with adjacent structures, but also may provoke an inflammatory reaction within adjacent anatomic structures, such as in pleura (causing pleural effusion), the pericardium (causing pericarditis). |
- Complications of IMH
- Aortic dissection: about 8% to 16% evolve into aortic dissection.
- Rupture: The risk of rupture is greater than aortic dissection as the outer adventitia is thinner.
- Periaortic hematoma: more common in IMH relative to aortic dissection.
- Complications of PAU
- IMH
- Rupture (Rare)
- Aortic dissection
Epidemiology and risk factors
The overall incidence of acute aortic syndromes ranges from two to four cases per 100,000 individuals, with most cases being ‘acute aortic dissection’. Aortic dissection is the most common form of aortic catastrophe, and twice that of abdominal aortic aneurysm rupture.
Aortic intramural hematoma accounts for 5 to 20 percent of patients with symptoms consistent with acute aortic syndrome, while penetrating aortic ulcers account for 2 to 7 percent of acute aortic syndromes 10
Aortic dissection has a bimodal age distribution. The first peak involves younger patients with specific predisposing conditions such as connective tissue disorders or crack cocaine use. The second peak occurs in those aged > 50 years with chronic hypertension and/or ischemic heart disease.
Risk factors associated with aortic dissection
- Hypertension: the most important risk factor
- Atherosclerosis
- Gender: more common in men than women
- Known aneurysm
- Congenital diseases: Bicuspid aortic valve, coarctation of the aorta, tetralogy of Fallot.
- Connective tissue disorder, e.g., Marfan syndrome.
- Substance abuse e.g,. cocaine, methamphetamine.
- Inflammation/infectious diseases: Giant cell arteritis, Takayasu arteritis, Behçet disease, Syphilis.
- Pregnancy: Possibly attributed to hemodynamic and hormonal milieu changes during pregnancy
- Trauma, blunt or iatrogenic e.g., cardiac, or aortic surgery
AD more frequently occurs between 6 a.m. and 12 p.m., peaking at 9 a.m., and during the winter months, with a peak in January.
Clinical presentation
The clinical presentation of acute aortic syndromes depends upon the extent of dissection and the affected cardiovascular structures
- Pain
- Location: Chest/back/abdomen. In the presence of abdominal pain, have a high index of suspicion for mesenteric vascular compromise.
- Onset: abrupt onset, with maximal intensity at its inception
- Severity: It’s severe (in 90% of patients)
- Other features:
- May be accompanied by a ‘sense of doom’
- Quality of pain is often described as sharp, severe, or stabbing, and adjectives such as tearing or ripping are less commonly used.
- Pain is migratory (17% of patients) and tends to follow the pathway of dissection through the aorta.
- Pitfalls:
- Pain may lessen or resolve, which makes diagnosis even more challenging.
- In some patients, the symptoms related to complications dominate, and pain is not mentioned or is downplayed.
- The description of pain is not universal because patients may also present with a more gradual onset of mild pain that is similar in description to musculoskeletal conditions of the thorax, back, or groin.
- Painless AD is more likely to present with neurological symptoms.11
- Syncope: 5 to 10% of patients with aortic dissection present with syncope. It’s more common in type A rather than type B aortic dissection. Syncope happens either due to the development of cardiac tamponade or involvement of the brachiocephalic vessels, and carries an increased mortality rate 12 13
- Focal neurologic deficit
- Acute paraplegia
- Ischemic stroke and altered consciousness (6%)13
- Horner syndrome
- Hoarseness
- Cardiac arrest and sudden death
Physical findings
- Findings are highly variable and range from virtually unremarkable to full cardiac arrest secondary to tamponade or rupture. The findings may signify complications related to dissection. The presence of these findings must raise clinical suspicion for aortic dissection, but their absence does not exclude dissection and should not deter pursuit of the diagnosis when suspected.
- Hypotension:14 Presence of hypotension in type B signifies rupture; while hypotension, especially in type A aortic dissection,n should prompt you to consider the following:
- Acute aortic valve insufficiency (<10%)13
- Cardiac tamponade: the most common cause of death. More common in women 15
- Hemothorax: can happen on both sides, though more commonly it occurs on the left side.
- Hemoperitoneum
- Acute Coronary syndrome(1-2%): more commonly involves RCA, though left-sided STEMI is not uncommon. Virtually any patient with STEMI is not safe from ‘AD ’- induced myocardial infarction. Aortic dissection may not be suspected as a cause of coronary ischemia, and misdiagnosis may lead to inappropriate therapy and delays in treatment.
- Pseudo-hypotension: The dissection flap has cut off the limb’s perfusion.
- Troubleshooting: switch the BP cuff to the other side.
- Hypotension:14 Presence of hypotension in type B signifies rupture; while hypotension, especially in type A aortic dissection,n should prompt you to consider the following:
-
- Hypertension (70%): more common in patients with descending ‘AD’. The underlying pathophysiology could be attributed to either hyperadrenergic response to pain, or renal arterial occlusion, or renal ischemia.14
- Pulse deficit: A pulse deficit is reported in 31% of type A dissections and 19% of type B dissections.
- Aortic regurgitation (41%-76%) is a key diagnostic feature of type A dissection.
- Neurologic findings related to ischemic stroke (6%), TIA, spinal cord ischemia, ischemic neuropathy, hypoxic encephalopathy, and seizure. Coma and cerebral malperfusion are associated with poor outcomes.
Differential diagnosis of acute aortic dissection
- Clinical DDx:
- ACS
- Pulmonary embolism
- Pneumothorax
- Aortic regurgitation without dissection
- Esophageal rupture
- Tamponade
- Pericarditis
- Peripheral arterial disease
- Imaging DDx:16
- Artifacts
- Aortic pulsation ‘motion artefact’ (typically left anterior and right posterior aspects of ascending aorta)
- Contrast streaks on CECT
- Reverberation artifacts in the ascending aorta on TEE
- Adjacent atelectasis
- Pericardial recess
- Innominate vein
- Remnant of a non-patent PDA
- IMH
- Periaortic fat and hemiazygos sheath may mimic IMH
- Mural thrombus within an aneurysm
- PAU
- Prior surgery of the aorta
- Atheromatous plaque
- Aortitis
- Artifacts

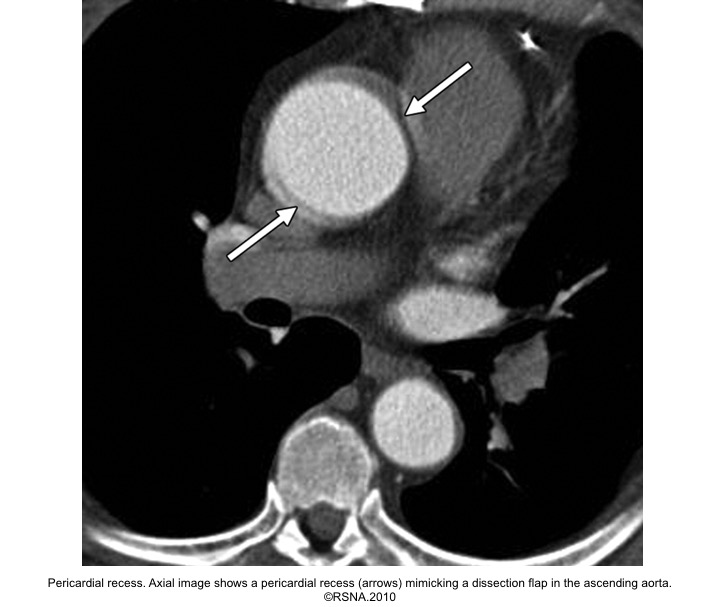
Evaluation
Labs
In patients admitted to the hospital with chest pain and suspicion of AD, the following laboratory tests are required for differential diagnosis or detection of complications:
- RBC count
- To detect blood loss, anemia.
- Troponin
- Almost 18% of patients with ‘AD’ have elevated troponin. Chest pain with an elevated troponin does not rule out ‘AD’.
- Renal function test
- To detect signs of renal failure (existing or developing).
- Liver function test
- To scan for signs of liver ischemia, liver disease.
- Serum lactate
- In contrast to traditional belief holding that elevated serum lactate is a marker of organ hypoperfusion (anaerobic metabolism); several studies (in patients with septic shock) show that this is not true.17, 18
- Elevated serum lactate represents endogenous catecholamine release, stimulating beta-2 receptors. This stimulation upregulates glycolysis, generating more pyruvate than can be used by mitochondria through the TCA cycle. Excess pyruvate is converted into lactate.
- Tox screen
- May be considered in young patients without apparent clinical risk factors for stimulant drug abuse. The results of toxicology screening may alter the pharmacological agents chosen for acute medical management.
- D-dimer
- Within the context of acute aortic syndromes, a positive D-dimer possibly signifies activation of the extrinsic coagulation cascade within the aortic media.
- Disadvantages of D-dimer as a diagnostic tool:
- Very low specificity: For D-dimer <500 ng/mL, the pooled estimate of the sensitivity 97%, specificity 56%, and negative predictive value 96% was reported. A positive D-dimer can be seen in a variety of conditions.19
- Lack of standardized testing protocols.
- Variability of levels from the time since the onset of the symptoms.
- In a recent prospective multicenter study that included 1850 patients, 8 patients (0.4%) with acute aortic syndrome had a negative D-dimer.20
- its usage may be limited to patients at low risk for having aortic dissection, but in whom ‘AD’ remains a clinical diagnostic uncertainty.21
- ACEP Clinical Guidelines have issued a Level C recommendation cautioning physicians not to rely on the D-dimer assay alone to exclude a diagnosis of acute AD.22
- Type and screen with crossmatch
- Essential for operative management and should be collected in the ED.
ECG
Presence of ECG findings consistent with acute myocardial infarction, do not rule out acute aortic dissection, as ‘AD’ can cause myocardial ischemia/infarction.
- ‘AD’ is notorious for causing right-sided STEMI/ischemia!
- However, it’s not true in the sense that left-sided events can happen.23 Virtually no one with STEMI is safe from AD-induced STEMI.
- Other findings, such as evidence of left heart strain, are seen in patients with uncontrolled HTN.24
More 👉 ECG Interpretation in Aortic Dissection (Emergency Medicine Cases)
Imaging
Modalities and diagnostic performance
The diagnosis of acute aortic syndromes may be suspected clinically, based on high-risk clinical features (discussed below), but confirmation of the diagnosis, determining the type of acute aortic syndromes, as well as the location and extent of the pathology, and finally delineating any anatomic complications, requires cardiovascular imaging.
Multiple imaging modalities are available, however, each has its advantages and disadvantages.
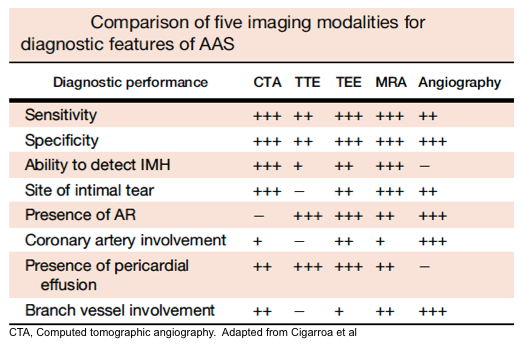
- CT angiography of the aorta is the diagnostic imaging modality of choice in hemodynamically stable patients.
- Advantages include: availability, accuracy (roughly 100% sensitive and specific), ability to depict rupture, and ability to identify differential diagnostic concerns e.g., PAU, IMH, PE.
- Disadvantages include: inability to identify cardiac complications of AAS, e.g., AR, and impractical to perform in patients impending to crash.
- TTE: applicable in unstable patients and can identify AR, hemorrhagic pericardial effusion, and tamponade, as well as regional wall motion abnormalities. However, a negative TTE cannot rule out aortic dissection due to its insensitivity.
- ACEP Clinical Guidelines recommend not relying on an abnormal bedside TTE for the definitive diagnosis of thoracic AD (ACEP Clinical Guidelines, Level B). However, the guidelines do provide a consensus recommendation that if an AD is identified on limited bedside ultrasound, then surgical consultation and/or transfer to a higher level of care capable of definitively managing AD should occur (ACEP Clinical Guidelines, Level C).
- TEE: despite having reasonable sensitivity (86%-100 %) and specificity (90%-100%), it requires specialized equipment, an experienced clinician, and sedation.
- MRA: Highly sensitive and specific, however, it can cause a delay in diagnosis due to its limited availability as well as longer image acquisition time.
Artifacts
- The aortic root and ascending aorta move about 1.5 cm per cardiac cycle, contributing to motion artifact in nongated imaging with low temporal resolution. High-resolution imaging of the ascending aorta requires either high temporal resolution (e.g., multidetector computed tomography [MDCT]) or electrocardiography (ECG) gating 3.
- High-attenuation streak artifacts from concentrated contrast dye in the adjacent SVC, brachiocephalic veins are common and clinically important problems because these streak artifacts can contaminate the depiction of the ascending aorta and arch, respectively. Pacemaker leads in the SVC, and the brachiocephalic vein may also project artifacts across the ascending aorta and arch correspondingly.
- The descending and abdominal aorta are less subject to motion artifacts.
- More on echocardiographic artifacts, below.
Diagnostic findings on imaging
Diagnosis of acute aortic syndrome requires cardiovascular imaging, therefore, it is important to recognize the imaging signs of acute aortic syndromes and be capable of distinguishing imaging findings from artifacts.
Acute aortic dissection diagnostic findings 25
- The diagnosis of AD is based on the presence of an intimal flap separating a false lumen (FL) from a true lumen (TL), and imaging signs of associated findings or complications such as:
- Thrombus in FL
- Aortic regurgitation (on echo)
- Pericardial effusion
- Branched vessel involvement
- Aortic enlargement
More on aortic dissection, here.
| Tip It is important to identify and differentiate ‘TL’ from ‘FL’, since in a situation where intervention is warranted, placing the stent within the ‘FL’ is devastating! |
- True Lumen (TL) is the one that is ‘smaller’, has ‘outer wall calcification’, and gives rise to the origin of the celiac trunk, the superior mesenteric artery, and the right renal artery.
- False Lumen (FL) has the following radiographic features:
- ‘FL’ is larger (due to higher systolic pressure within the FL).
- Has no outer wall calcification.
- On NECT, FL appears darker due to slower blood flow.
- On CECT, it often appears as a lower density due to delayed enhancement, i.e., opacification.
- May contain thrombus (more common in chronic AD). A thrombus appears as a low-density area on NECT.
- Beak sign: Wedges around the ‘TL’.
- Cobwebs: Collagenous media remnants.
- Circular configuration (due to persistent systolic pressure).
- Forms the outer curve of the aortic arch.
- The origin of the left renal artery is usually from the ‘FL’.
- Surround ‘TL’ in type A aortic dissection.
- On color doppler, it shows flow in acute AD, while in chronic AD may lack flow due to the formation of thrombus.
- ‘FL’ is at risk of rupture due to reduced elastic recoil and dilation.
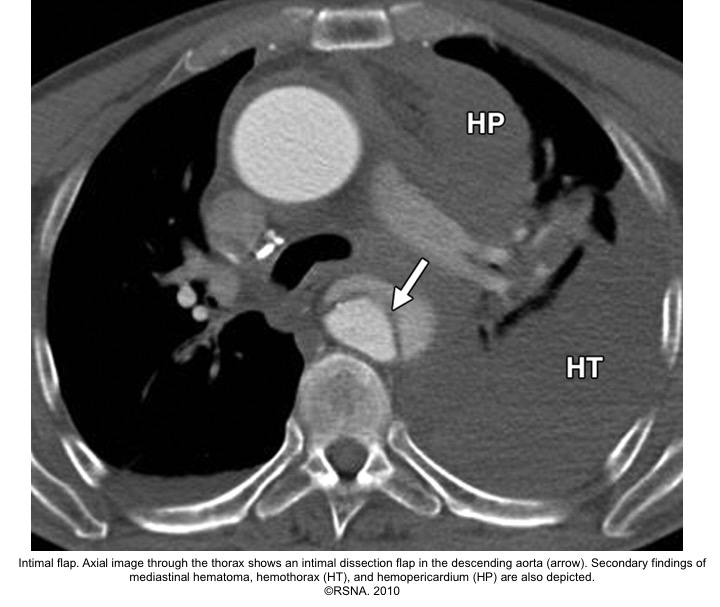
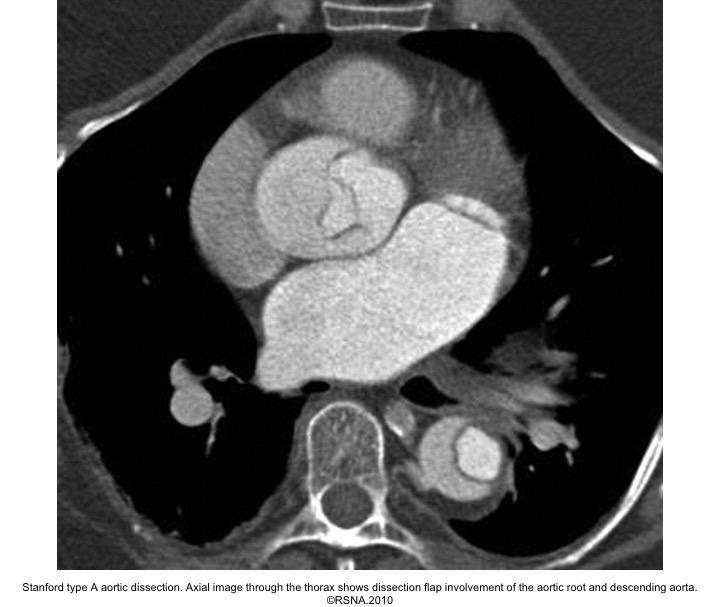
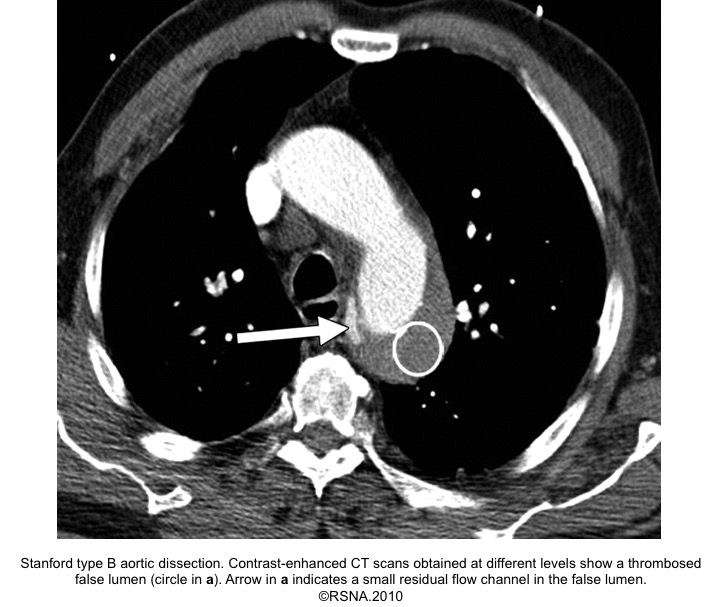
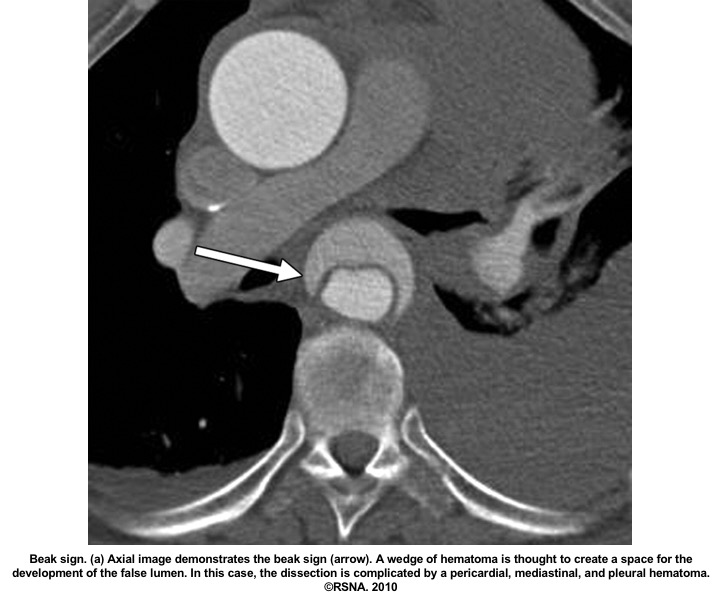
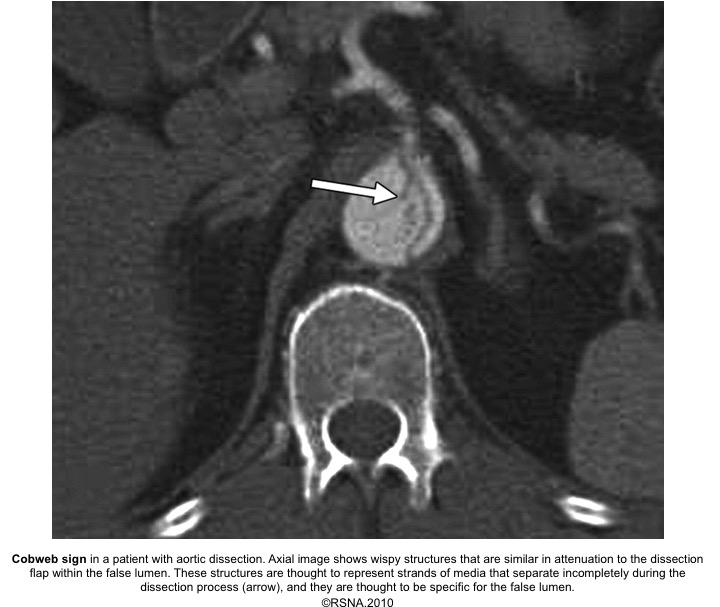
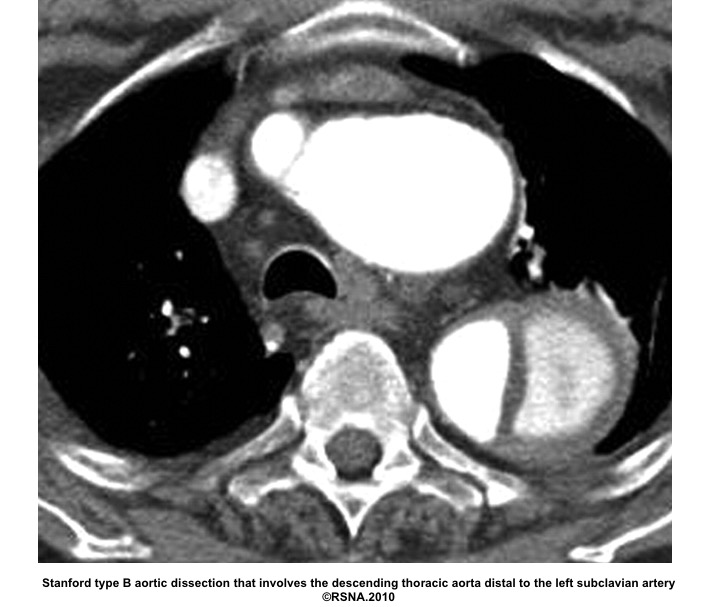
Echocardiographic findings in acute AD:16
- Recognizing Direct evidence of AD:
- The diagnostic hallmark of AD is a mobile dissection flap that separates the true and false lumens. Important features of the dissection flap include:
- Oscillation or motion that is independent of the aorta itself.
- Visualization in more than one view.
- Clear distinction of the flap from reverberations from other structures, such as a calcified aortic wall, catheter in the right ventricular outflow tract, pacemaker wire, or pericardial fluid in the transverse oblique sinus.
- Other main direct evidence of AD includes, e.g., identifying ‘TL’ vs. ‘FL’(below table).
- The diagnostic hallmark of AD is a mobile dissection flap that separates the true and false lumens. Important features of the dissection flap include:
- Identifying Indirect evidence of ‘AD’ (complications of AD):
- Dilatation of the aorta.
- Compression of the left atrium.
- AR (more on aortic regurgitation, here).
- Pericardial and/or pleural effusion.
- Involvement of the coronary arteries.
The following table summarizes the main diagnostic findings on echo in ‘AD’
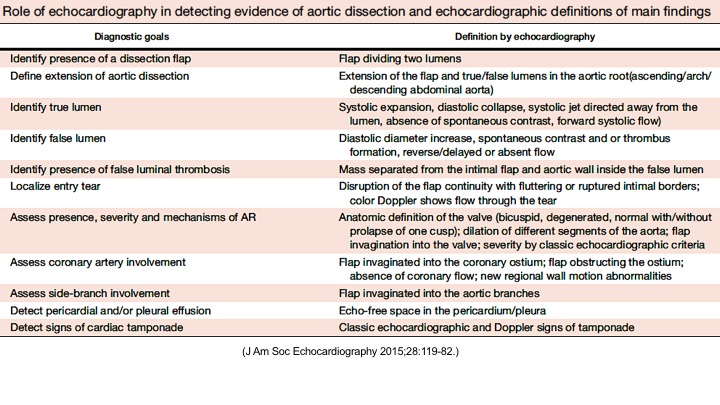
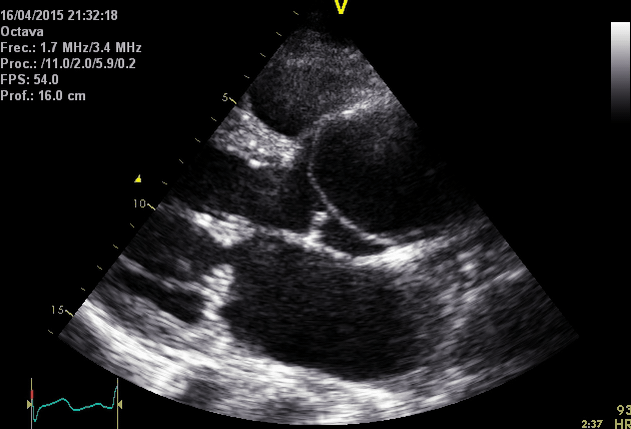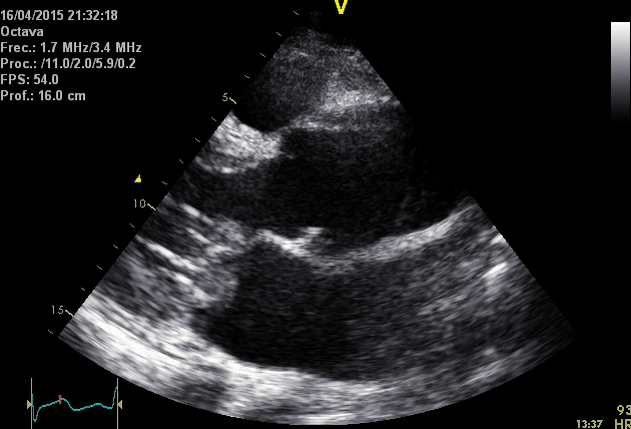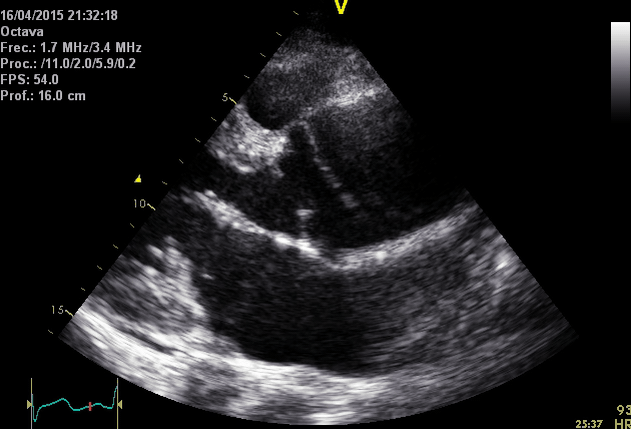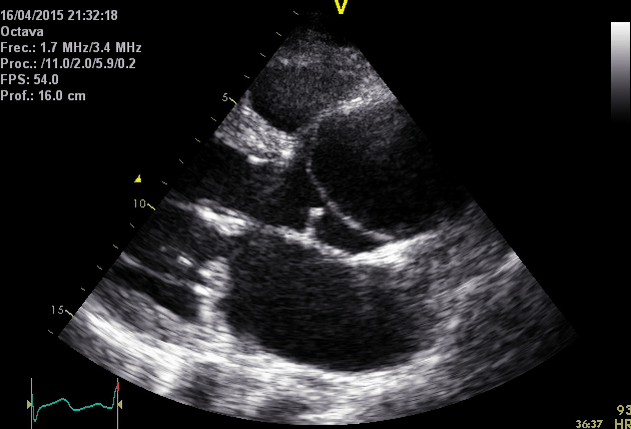
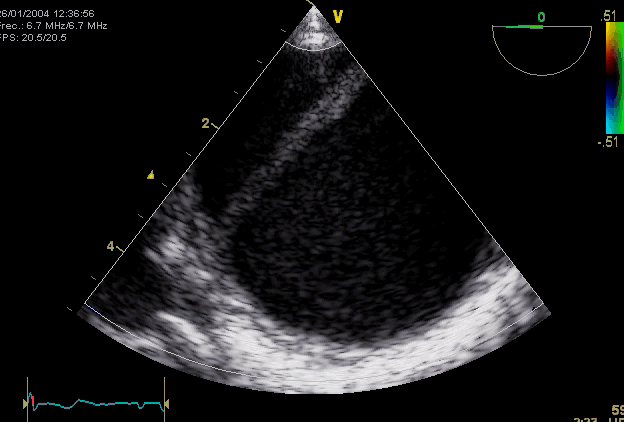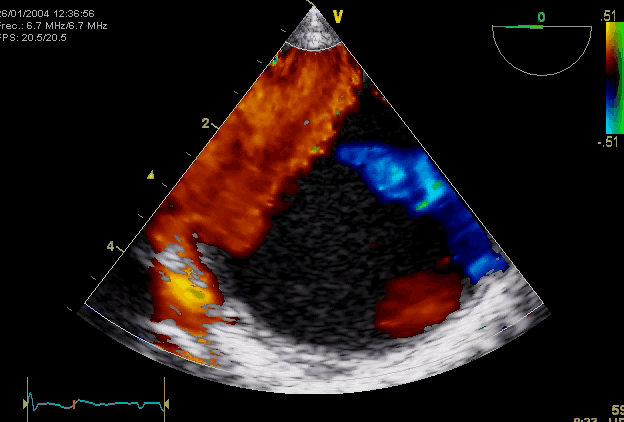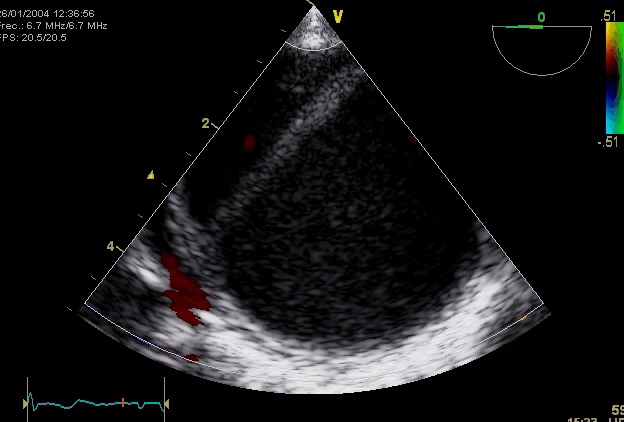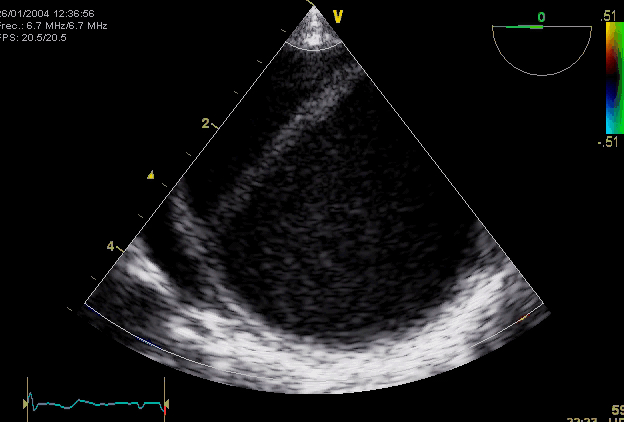
Right. Type B dissection diagnosed by colour Doppler TEE showing a turbulent flow through a secondary tear from the true lumen to false lumen © British Society of Echocardiography.2019
Techniques
- Use the high right/left parasternal long axis views to achieve a better window of the ascending aorta.
- Substernal view gives valuable information regarding the involvement of the aortic arch and branched vessels.
- A short-axis view of the descending aorta can be imaged posteriorly to the left atrium in the parasternal long-axis view.
- The use of M-mode echo may improve diagnostic accuracy by demonstrating a lack of relation between the movement of the intimal flap and the aortic wall.
- Scanning the neck vessels (carotid artery) can be informative, especially in those with neurological findings.
- Remember that the hallmark of ‘AD’ is the detection of undulating linear echoes of an intimal flap.
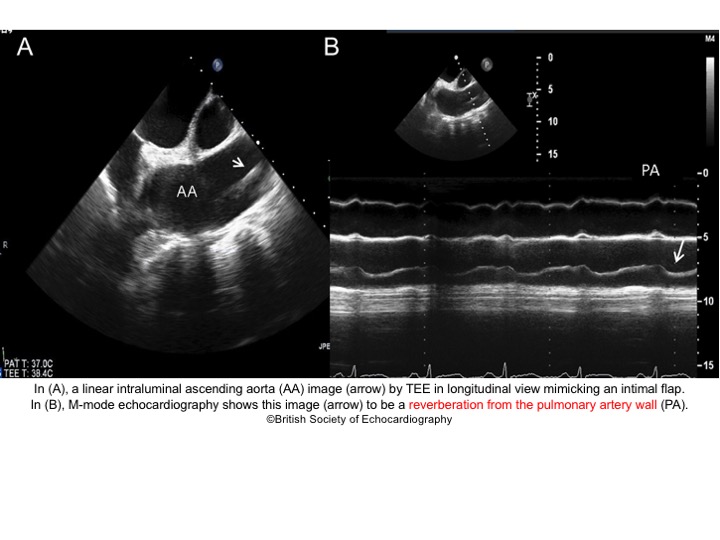
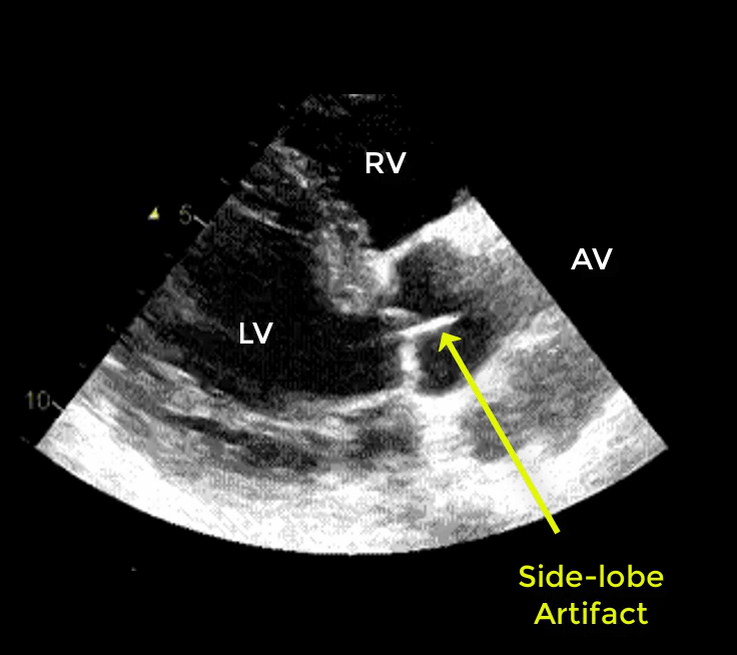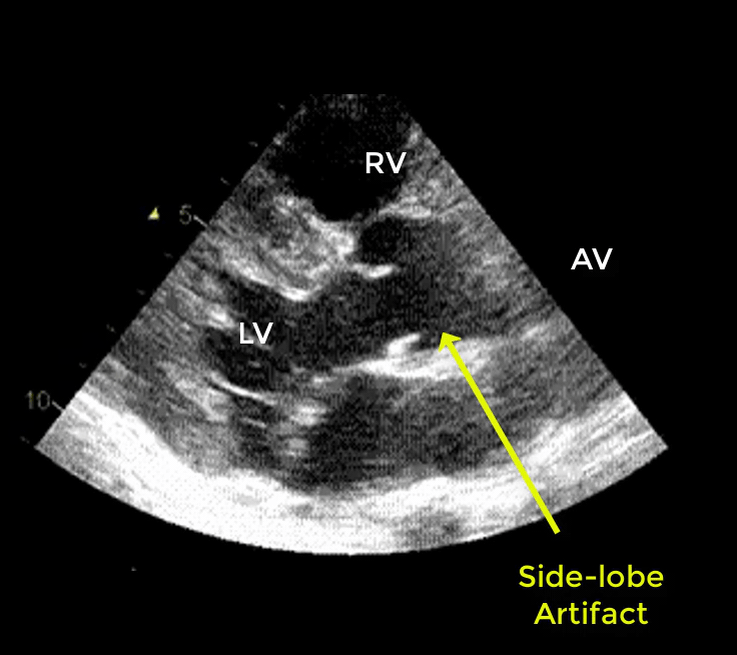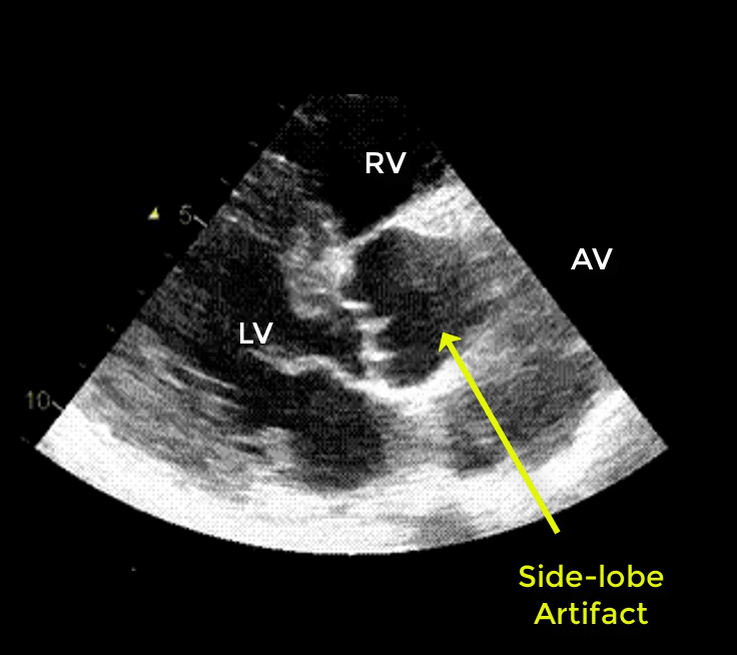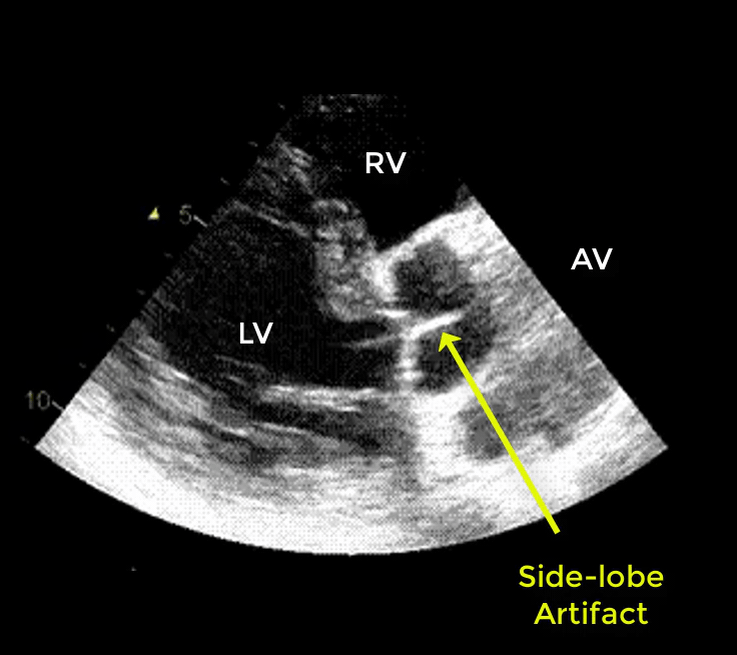
- Artifacts mimicking intimal flaps are common in the ascending aorta and are a potential diagnostic challenge.
| There are 2 types of artifacts: 👉Side lobe artifact: A curvilinear echodensity along a sector arc which is Not Undulating. It is caused by calcification of aortic root, aortic valve, and sinotubular junction. 👉Reverberation artifact: It’s the duplication of a linear echo signal along the pathway of ultrasound beam. It may originate from the left atrial wall, Rt pulmonary artery. Linear artifacts are more common when aortic root diameter is > left atrium. |
🔐Troubleshooting
- These Artifacts can be easily recognized by using M-mode to assess their movement and color flow Doppler to assess their flow characteristics on either side of the linear echodensity.
- M-mode proves that the artifact has the same motion as its originating structure.
- Color flow Doppler proves the presence of homogenous color flow on both sides of the curvilinear echodensity without communicating jets.

IMH diagnostic findings 27
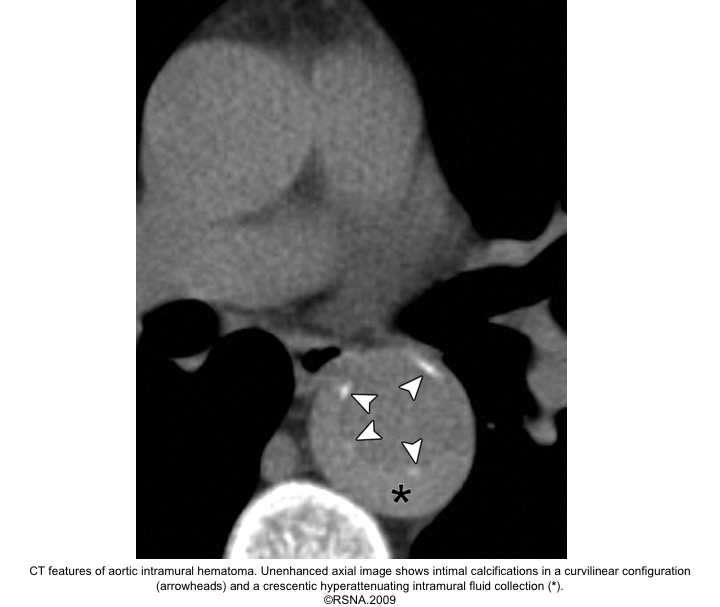
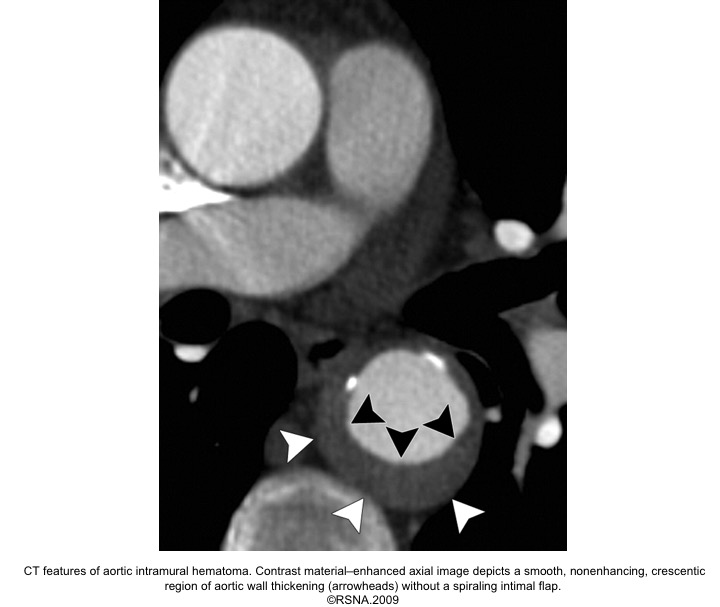
The diagnosis of IMH is based on:
- Focal crescentic thickening of the aortic wall of ≧5 mm in aortic wall with a smooth inner surface.
- Displaced intimal calcification inwardly.
- Absence of flap, intimal Tear, or flow within the hematoma.
- NCECT: a hyperattenuating rim or crescent along the curvature of an aortic wall.
- CECT: non-enhancing low attenuation rim
More on IMH, here.

| Tips: Imaging findings that affect the prognosis of “IMH” should be noticed and include: 👉Stanford type 👉Maximal aortic diameter 👉IMH thickness 👉Presence of an ulcer-like projection |
Echocardiographic signs for diagnosis of IMH:16
- Areas of echolucency within the aortic wall and no intimal flap or flow in the aortic wall28
- Focal thickening of the aortic wall:
- Normal aortic wall thickness is < 3mm. Wall thickness must be > 5-7mm to be diagnostic for IMH.
- Presence of mixed echogenicity within the aortic wall (predominantly echodense with scattered internal echo lucencies).
- Absence of intimal flap, and false lumen.
- Absence of detectable internal flow: employment of color doppler flow is important to differentiate IMH from AD, as the TL of AD will demonstrate systolic flow, and variable flow patterns m/b present in the ‘FL’, which tends to expand in size during diastole.
- The luminal surface in IMH tends to be smooth and continuous.
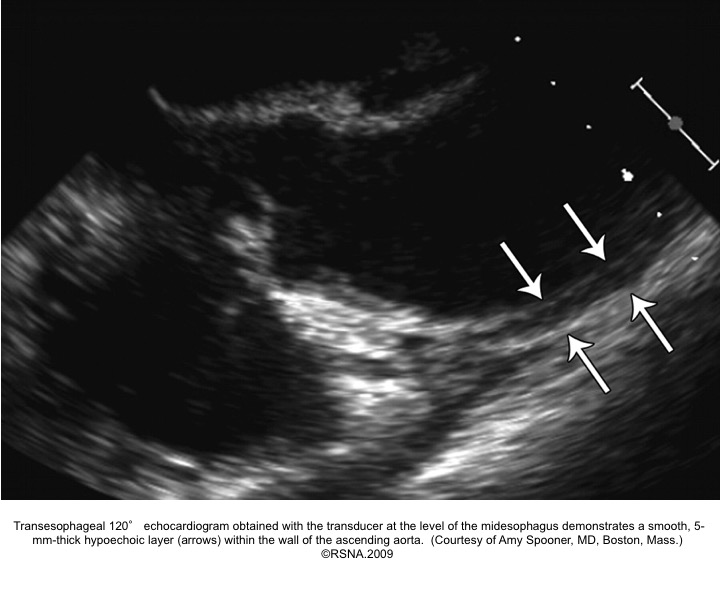
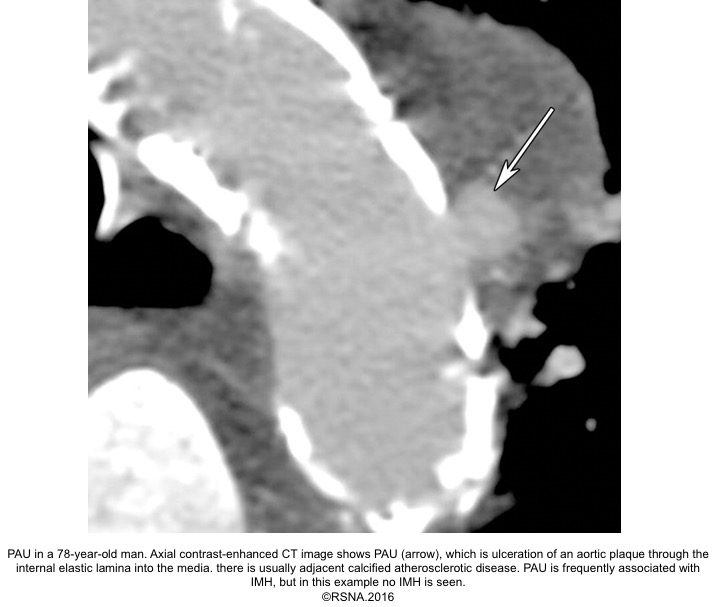
PAU diagnostic findings 16
- Extensive atherosclerosis with severe intimal calcifications and atherosclerotic plaques.
- Focally displaced and separated intimal calcifications.
- Crater and/or contrast extravasation.
- Focal IMH, with longitudinal spread limited by media fibrosis.
- Possibly enhancing the aortic wall.
More on PAU, here.
Approach to diagnosis and management
- Identify critically ill patients.
- Employ simultaneous diagnostic and resuscitative measures.
- Identify and distinguish acute aortic syndromes from other potential life-threatening diseases.
- Recognize and differentiate type A from type B in AAS.
- Identify the presence of end-organ malperfusion.
- AAS encompasses potentially life-threatening conditions that can happen in patients without apparent risk factors and can present with a wide variety of clinical pictures, from virtually unremarkable to cardiac arrest.
- The large differential diagnosis for the complaint of chest pain, plus the many end-organ ischemic manifestations associated with aortic dissections, makes the diagnosis challenging.
- Such errors in diagnosis not only delay timely treatment of aortic catastrophe, but also can lead to using inappropriate treatments (e.g., antithrombotic agents) which increase the risk of bleeding and other serious complications in such patients.
- Factors associated with delay in diagnosis have been reported as
- Female gender
- Transfer from another hospital
- fever
- Normal BP.31
- Factors associated with delay in diagnosis have been reported as
- It is significantly important to rapidly identify situations where emergent cardiac surgery is warranted:
- Identifying type A (which calls for emergent surgical procedures), vs. type B (which often can be managed medically in hemodynamically stable patients).
- Recognition of end-organ complications, upon which cardiac surgery is required, regardless of the anatomic type of aortic dissection.
Diagnostic approaches
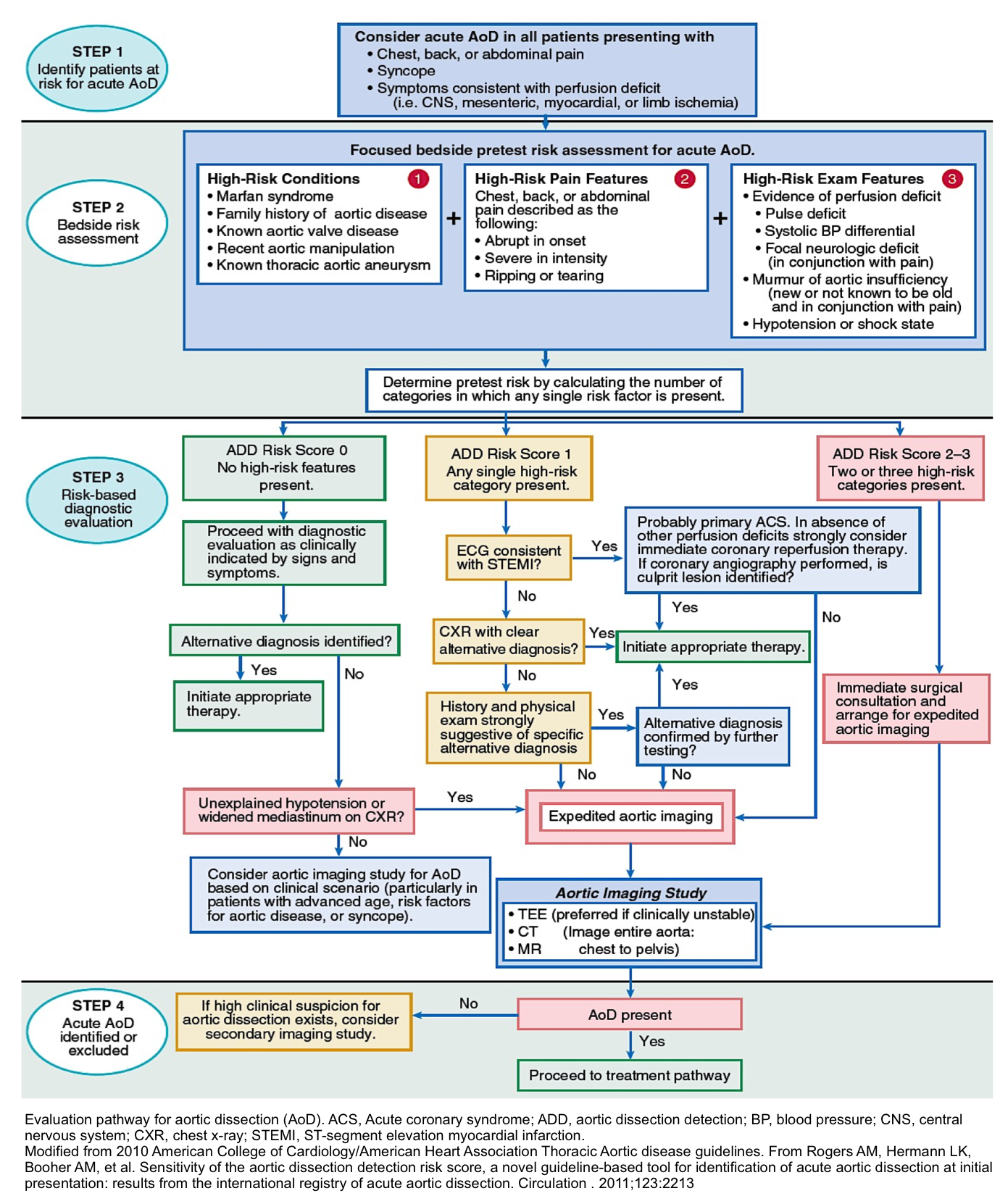
Diagnostic Evaluation and Management based on Acute Aortic Dissection Detection Risk Score (ADD-RS) 2 32
- Step 1: Identify patients at risk for acute AD
- Collect information by taking medical history, exam, ECG.
- Consider Acute AD in all patients presenting with:
- Chest/back/abdominal pain
- Syncope
- Symptoms consistent with perfusion deficit i.e. CNS, mesenteric, myocardial, limb ischemia.
- Consider Acute AD in all patients presenting with:
- Collect information by taking medical history, exam, ECG.
- Step 2: Bedside risk assessment (pretest risk):
- The Aortic Dissection Detection Risk Score (ADD-RS) is based on the presence of one or more of the following high-risk features (table below).
- The presence of ≥1 marker within each of these groups is given a score of 1, with a maximum cumulative score of 3, if all three are present. In a review from the IRAD registry, high ADD-RS effectively stratified the risk for acute aortic dissection.33
- The Aortic Dissection Detection Risk Score (ADD-RS) is based on the presence of one or more of the following high-risk features (table below).
| High risk conditions | High-risk conditions | High-risk pain features |
| -Marfan syndrome or other connective tissue disease -Family history of aortic disease -Known aortic valve disease e.g. BAV -Known thoracic aortic aneurysm | Chest, back, or abdominal pain described as any of the following: -Abrupt onset -Severe intensity -Ripping or tearing | Evidence of perfusion deficit -Pulse deficit -SBP difference -Focal neurologic deficit (with pain) Aortic diastolic murmur (new and with pain) Hypotension or shock |
- Step 3: Risk-based diagnostic evaluation
- Employ the ADD-RS score to direct your diagnostic evaluation, management accordingly (see following algorithm).
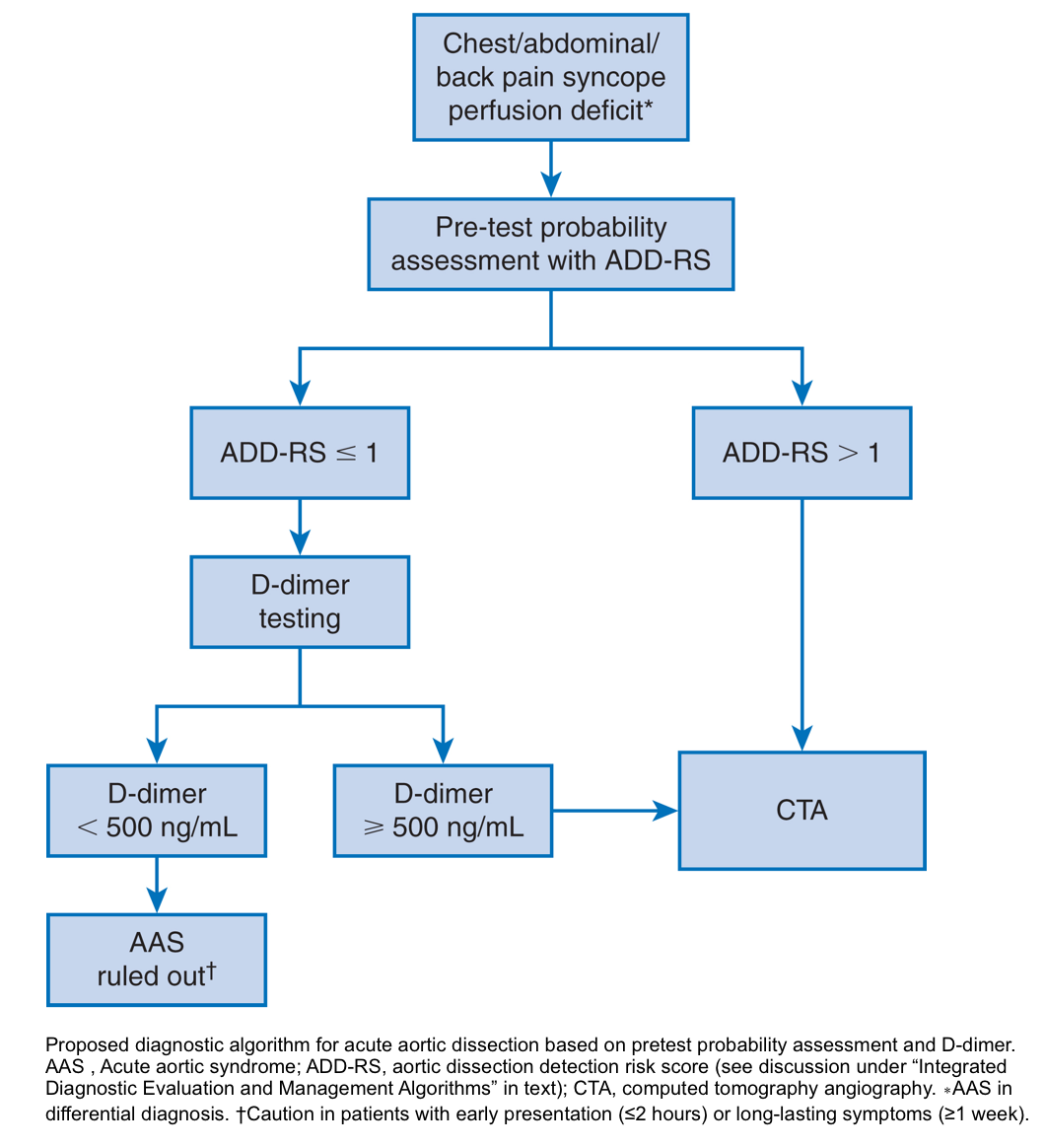
Diagnostic algorithm for acute aortic dissection based on pretest probability assessment and D-dimer
- Used a combination of the ADD-RS and D-dimer as a diagnostic tool for acute aortic syndromes (ADD-RS Plus D-dimer) 34.
- The combination of ADD-RS (0 to 1) and negative d-dimer (<500 mg/dL) effectively ruled out AAS with a failure rate of less than 1 in 300 patients.
- Based on these results, about 60% of patients with a low probability for AAS might be spared from unnecessary conclusive vascular imaging.
- For ADD-RS >1, D-dimer was not discriminatory, requiring conclusive imaging.
- However, additional validation in a broader patient population is required before routine use of this combination as a diagnostic tool.
- See more on D-dimer for aortic dissection: the evidence (First10EM blog)
Treatment
General principles of treatment :
- Treatment strategy for acute ‘AD’ and ‘IMH’ depends on:
- Type
- Location
- Presence of complication (end-organ ischemia
- Progression
- Rupture
- Universal therapeutic measures
- Make sure the patient is NPO.
- Place two large-bore IVs.
- Monitor HR and BP continuously, preferentially using an arterial line to adequately titrate antihypertensive medications (put your arterial line on the side with the stronger pulse).
- Provide analgesia to control the pain.
- Control HR and BP: Keep HR < 60 bpm and SBP between 100-120 mmHg.
- Place a Foley catheter for monitoring urine output and renal perfusion.
- Surgical consultation (cardiothoracic, vascular surgery) as soon as diagnosis is strongly suspected or confirmed (regardless of location).
- Stanford Type A aortic dissection is a cardiac surgical emergency.
- Stanford Type B aortic dissection can be managed medically in the ICU.
- 🚩Urgent aortic stent-grafting or surgery is warranted in the presence of the following conditions:
- Evidence of end-organ malperfusion
- Rupture or impending rupture, e.g., periaortic hematoma
- Hemorrhagic pleural effusion
- Refractory hypertension
- Refractory pain
- Aneurysmal dilation (>55 mm)
- Rapid increase in diameter
- Recurrent symptoms
- 🚩Urgent aortic stent-grafting or surgery is warranted in the presence of the following conditions:
- 🚑Transfer
- If appropriate surgical services are not available, initiate emergent transfer to the nearest available cardiovascular center (the most expeditious means is encouraged, i.e., air).
- If sudden cardiac arrest occurs, perform POCUS to evaluate for likely etiologies such as tamponade, hemothorax.
- Despite that, pericardiocentesis is technically difficult in tamponade due to aortic dissection (as often blood clots), draining even a small amount of fluid (e.g., even 4cc!) may potentially be associated with ROSC. More on this, here.
- IMH
- The mortality rate is similar in patients with IMH compared to patients with aortic dissection. Therefore, they should be treated similarly to an individual with a true AD at the level of the hematoma, that is to say:
- Stanford Type A IMH is a cardiac surgical emergency.
- Stanford Type B IMH: This can be managed medically in the ICU.
- 🚩Surgical or endovascular treatment is considered in the presence of the following situations:
- Hemodynamic instability
- Persistence of chest pain despite medical management
- Signs of aortic rupture e.g., periaortic hematoma, enlarging pleural effusion
- Presence of a large ulcer-like projection (Depth > 10 mm}
- Maximal aortic diameter > 55 mm
- Rapid aortic diameter growth during in-hospital stay
- 🚩Surgical or endovascular treatment is considered in the presence of the following situations:
- The mortality rate is similar in patients with IMH compared to patients with aortic dissection. Therefore, they should be treated similarly to an individual with a true AD at the level of the hematoma, that is to say:
Treatment
Principles
◾️The initial primary goal of care in the emergency department setting is to minimize the dissection extension.
- The aortic root and ascending aorta move about 1.5 cm per cardiac cycle. Anti-impulse therapy aims to reduce the velocity of left ventricular contraction, thereby decreasing pulse pressure-related shear stress (Δp/Δt) and minimizing lesion progression. Three goals are deserved:
- ↓HR
- ↓Intopry
- ↓Afterload
| Clinical targets * 🔴Pulse rate of 60–80 beats/min. 🔴Systolic blood pressure of 100-120 mm Hg. 🔴Adequate organ perfusion, i.e., intact mental status, and adequate urine output. 🔴Pain control |
💡Notes:
- Hemodynamic targets may need to be individualized based on chronic baseline blood pressure and organ perfusion *.
- ⚠️Caution: If there is evidence of acute aortic insufficiency, significant caution must be used when initiating beta blockers, because in this context, a slow heart rate may lead to increased diastolic time, and blood spends more time regurgitating, leading to cardiovascular collapse.32
Pain control
◾️Pain induces a sympathetic system response, resulting in a hyperadrenergic state, which could potentiate the progression of AAS. Adequate analgesia is of paramount importance.
- Preferred choice: fentanyl
⚠️Pain refractory to optimal medical management has been associated with worse outcomes in patients with aortic dissection.
- In patients with recurrent pain despite optimal medication, request for radiographic studies, as it may be due to progression of the disease (rupture, effusions, etc).
Heart rate control
- Intravenous beta blocker can be initiated first to reduce shear stress on the aortic wall, reduce contractility, and prevent rupture of the diseased aortic wall in AAD not complicated by aortic regurgitation *.
- Beta blocker use has been associated with improved survival in the International Registry of Acute Aortic Dissection database.
- Agents
- Esmolol: 250 to 500 mcg/kg loading dose over one minute, then infuse at 25 to 50 mcg/kg/minute; maximum of 300 mcg/kg/minute. Notice that prior to each upward dose titration, a re-bolus should be given.
- Advantages include: Short half-life, ability to titrate to the effect perfectly, controls both cardiac HR and inotropy, and clearance is not dependent on renal or hepatic function.
- Disadvantages: Nausea, flushing, bronchospasm, bradycardia, first-degree heart block, infusion site pain, and half-life prolongation in the setting of anemia.
- Labetalol: 20 mg initially, followed by 20 to 80 mg every 10 minutes to a total dose of 300 mg or as an infusion (0.5 to 2 mg/minute).
- Disadvantage: It’s predominantly a negative inotropy, but not a great chronotropic agent, and despite its alpha blocking properties, it’s not a great afterload reducer either. It’s not a perfectly titratable agent.
- Advantages: It’s a combined alpha and beta-blocker that may enable management with a single agent.
- Metoprolol: Start with 5 mg IV, which should take effect within ~ 5 minutes. Additional doses may be given every 5 minutes, titrating to effect. Generally, no more than 15 mg total will be used initially.
- Advantage
- It may allow for control of heart rate without causing a substantial reduction in blood pressure (this is beneficial in patients with tachycardia but only mild hypertension).
- Advantage
- Esmolol: 250 to 500 mcg/kg loading dose over one minute, then infuse at 25 to 50 mcg/kg/minute; maximum of 300 mcg/kg/minute. Notice that prior to each upward dose titration, a re-bolus should be given.
◾️ Nondihydropyridine calcium channel blockers
- Diltiazem: Give an initial bolus of 0.25 mg/kg over two minutes with subsequent infusion of 5-20 mg/hr.
- Indication
- It could be used if beta-blockers are contraindicated (e.g., acute cocaine intoxication).
- Respiratory distress in severe asthma, COPD patients.
Management of blood pressure (add-on to beta blockade)
◾️If after beta blockade, the SBP remains elevated, adding an antihypertensive (to ↓afterload) medication is warranted; preferred medications are listed below in sequence as:
- Nicardipine IV 5 mg/h; may titrate up by 2.5 mg/h every 5-15 min to a max of 15 mg/h.
- Clevidipine: IV initial dose 1-2 mg/h IV infusion, titrate up by doubling the dose at 90 sec intervals initially; once approaching target blood pressure, increase by less than double the dose and lengthen dose interval to 5-10 min, to a max of 32 mg/h.
- Advantages: Perfectly titratable to the effect, short time to onset of action, it is a pure afterload reducer without any effects on heart rate and inotropy (the same as for nicardipine), and clearance is not dependent on renal or hepatic function.
- Disadvantages: lipids contain potential allergens (eg, soy, egg), atrial fibrillation, and nausea.
- Sodium nitroprusside IV 0.25 to 0.5 mcg/kg per minute to a maximum of 10 mcg/kg per minute for a maximum duration of 10 minutes.
- Disadvantages: a tiny dose adjustment leads to massive BP change, making titration very difficult, definitely needs an arterial line for BP monitoring, has deleterious effects on cerebral perfusion pressure, cyanide and thiocyanate toxicity, nausea, vomiting, muscle spasm, flushing, and sweating.
- Nitroglycerin: IV infusion at 5 to 200 mcg/minute.
- Advantages: It is useful in patients with acute pulmonary edema and coronary ischemia, available in most centers.
- Disadvantages: tachycardia (reflex sympathetic activation), headache, vomiting, flushing, methemoglobinemia, tolerance with prolonged use.
⚠️Caution: Avoid other direct vasodilators like hydralazine as they increase aortic wall shear stress and provide less accurate and reversible control of the BP.
| Differential diagnosis of resistant hypertension in aortic dissection 👉Inadequate pain management 👉Inappropriate antihypertensive medication/dosage👉Renal ischemia 👉Progression of aortic dissection 👉Consider other possible coexisting conditions e.g., delirium, drug withdrawal, urinary obstruction. |
Intubation
Consider the same way as you intubate the patients with high ICP/high BP.
- If you choose to intubate the patient (e.g. when deciding to transfer the patient to another facility, in the presence of any deterioration in mental status), follow the hemodynamically safe intubation, that is bringing HR and BP down to the safe range with aforementioned medications, and only then proceeding to intubation, perfectly using video-guided approach.
- For a more comprehensive lecture on this, see: Neurocritical Care Intubation (EMCrit RACC)
Specific condition
Cocaine intoxication
- Cocaine toxicity results from central monoamine reuptake inhibition and its sodium channel blockade properties.
- Therapeutic considerations
- Decreasing central-mediated sympathetic tone is achieved by administration of benzodiazepines.
- Non-selective beta blockers are generally avoided in such patients as they may lead to unopposed alpha stimulation and worsen the situation.
- However, for patients with acute “AD”, antihypertensive medications are usually started before the Tox screen results become available, and as such, many patients do receive beta blockers.
- In an IRAD review of cocaine-related dissection, there were no differences in the type of treatment received comparing cocaine and non-cocaine users, including beta blocker therapy.35 However, when more than one antihypertensive agent was used, it was not stated which medication was used first.
- However, for patients with acute “AD”, antihypertensive medications are usually started before the Tox screen results become available, and as such, many patients do receive beta blockers.
- Management
- BZD: diazepam (5 mg IV) or lorazepam (1 mg IV); may repeat q 5 minutes until sedated
- Selective beta blockers such as esmolol or mixed agents like labetalol may be reasonable for treating patients with acute aortic syndrome when needed.
- When additional antihypertensive medication is needed, nitroglycerin or calcium channel blockers can be considered.
Prognostication of acute aortic syndrome
- Mortality is estimated to increase by 1% to 2% per hour following the onset of symptoms, calling for a timely diagnosis and intervention.
- Poor prognostic indicators for Stanford Type A ADs include:
- previous history of aortic valve replacement
- migrating chest pain
- presenting in shock or with tamponade physiology
- limb ischemia
- hypotension
- Poor prognostic indicators for Stanford Type B ADs include:
- Older age
- Presence of mesenteric ischemia
- Presenting with hypotension or shock
- Acute renal failure
- an aorta diameter larger than 5.5 cm
- Periaortic hematoma
- Limb ischemia
RECAP
- Remember that chest pain with an elevated troponin does not rule out ‘AD’.
- Patients with ‘AD’ can present with neurologic deficit, chest pain etc. that may initially make you think about ischemic CVA, type 1 STEMI. Be cautious! Have a low threshold for ‘AD’ when patients present with weird symptoms like neurologic deficit plus abdominal pain. Do not give antithrombotic meds until appropriate diagnostic imaging is performed (which is variable in different circumstances).
- By enlarge; do not rely on the D-dimer assay alone to exclude a diagnosis of acute AD. A negative D-dimer may have diagnostic utility in patients with low probability of ‘AD’.
- Artifacts on CECT and echo, can mimik imaging findings of ‘AAS’.
- Analgesics and anti-impulse therapy are the cornerstone of medical management of patients with ‘AAS’.
- The knee jerk response to development of hypotension in patients with suspected or confirmed diagnosis of aortic dissection is to perform POCUS, looking for possible tamponade, hemorrhagic complications,catastrophic aortic regurgitation, all of which can be appreciated by bedside ultrasound.
Media
Aortic dissection with hemothorax
Aortic dissection or not?
Acute aortic syndromes, CT evaluation
Going further
- 📚Acute Aortic Syndrome Revisited: JACCState-of-the-Art Review
- Nontraumatoc thoracic aortic dissection (Wikiem)
- Aortic Dissection (Core ultrasound)
- Aortic Dissection (EM:RAP)
- Aortic Dissection Live from The EM Cases Course (Emergency Medicine Cases)
- The ADvISED Trial: A Novel Clinical Algorithm for the Diagnosis of Acute Aortic Syndromes (REBELEM)
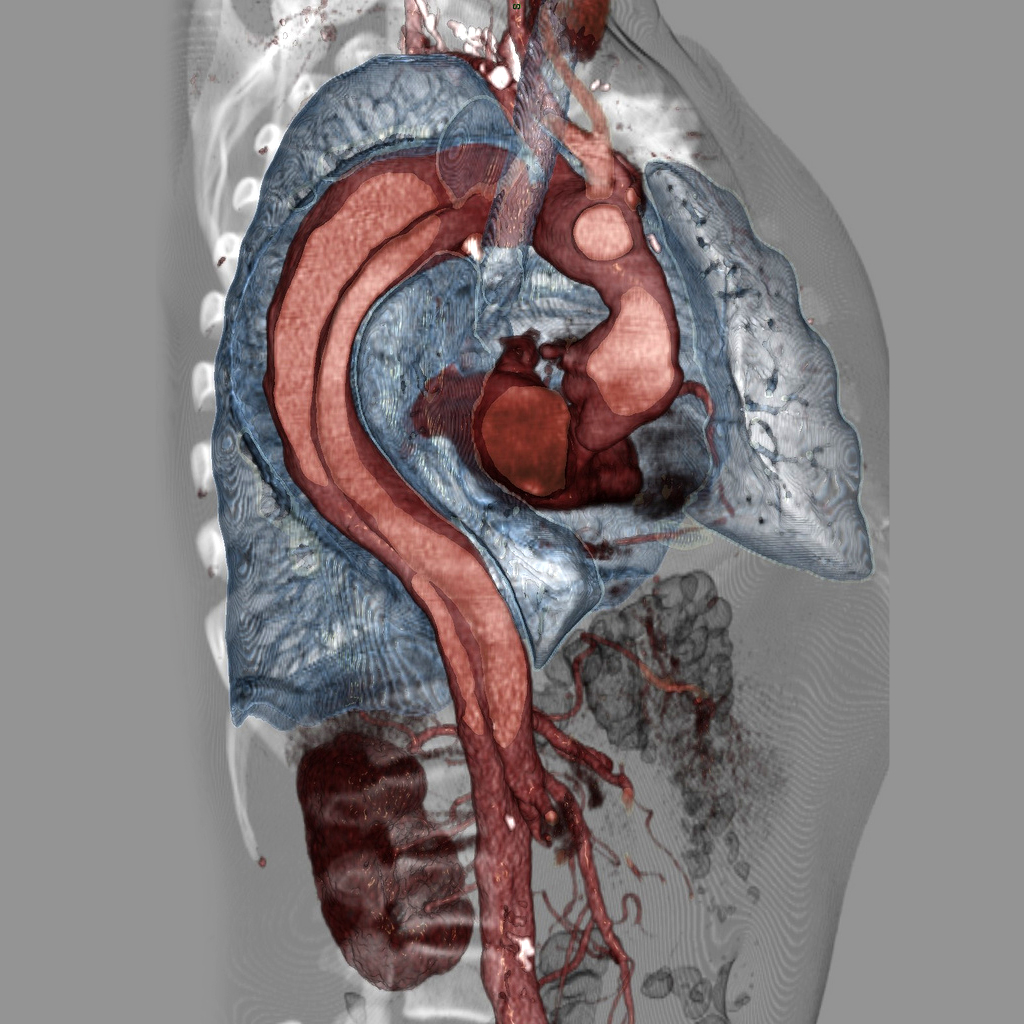
Top image: Case courtesy of Assoc Prof Frank Gaillard, Radiopaedia.org, rID: 9068
References
1. Zhan, S., Hong, S., Shan-Shan, L., Chen-Ling, Y., Lai, W., Dong-Wei, S., Chao-Yang, T., Xian-Hong, S., & Chun-Sheng, W. (2012). Misdiagnosis of Aortic Dissection: Experience of 361 Patients. Journal of Clinical Hypertension, 14(4), 256–260. https://doi.org/10.1111/j.1751-7176.2012.00590.x
2. Erbel, R., Aboyans, V., Boileau, C., Bossone, E., Di Bartolomeo, R., Eggebrecht, H., Evangelista, A., Falk, V., Frank, H., Gaemperli, O., Grabenwöger, M., Haverich, A., Iung, B., Manolis, A. J., Meijboom, F., Nienaber, C. A., Roffi, M., Rousseau, H., Sechtem, U., … Vlachopoulos, C. (2014). 2014 ESC guidelines on the diagnosis and treatment of aortic diseases. European Heart Journal, 35(41), 2873–2926. https://doi.org/10.1093/eurheartj/ehu281
3. Hutchison, Stuart J. Aortic diseases: clinical diagnostic imaging atlas. Clinical diagnostic imaging atlas, Philadelphia, Saunders, Elsevier, 2009
4. Evangelista, A., Flachskampf, F. A., Erbel, R., Antonini-Canterin, F., Vlachopoulos, C., Rocchi, G., Sicari, R., Nihoyannopoulos, P., Zamorano, J., Pepi, M., Breithardt, O. A., & Płońska-Gościniak, E. (2010). Echocardiography in aortic diseases: EAE recommendations for clinical practice. European Journal of Echocardiography, 11(8), 645–658. https://doi.org/10.1093/ejechocard/jeq056
5. Mussa, F. F., Horton, J. D., Moridzadeh, R., Nicholson, J., Trimarchi, S., & Eagle, K. A. (2016). Acute aortic dissection and intramural hematoma a systematic review. JAMA – Journal of the American Medical Association, 316(7), 754–763. https://doi.org/10.1001/jama.2016.10026
6. Erbel, R., Alfonso, F., Boileau, C., Dirsch, O., Eber, B., Haverich, A., Rakowski, H., Struyven, J., Radegran, K., Sechtem, U., Taylor, J., Zollikofer, C., Klein, W. W., Mulder, B., & Providencia, L. A. (2001). Diagnosis and management of aortic dissection: Recommendations of the Task Force on Aortic Dissection, European Society of Cardiology. European Heart Journal, 22(18), 1642–1681. https://doi.org/10.1053/euhj.2001.2782
7. Nienaber, C. A., & Eagle, K. A. (2003). Aortic dissection: New frontiers in diagnosis and management. Part I: From etiology to diagnostic strategies. Circulation, 108(5), 628–635. https://doi.org/10.1161/01.CIR.0000087009.16755.E4
8. Booher, A. M., Isselbacher, E. M., Nienaber, C. A., Trimarchi, S., Evangelista, A., Montgomery, D. G., Froehlich, J. B., Ehrlich, M. P., Oh, J. K., Januzzi, J. L., O’Gara, P., Sundt, T. M., Harris, K. M., Bossone, E., Pyeritz, R. E., & Eagle, K. A. (2013). The IRAD classification system for characterizing survival after aortic dissection. American Journal of Medicine, 126(8), 730.e19-730.e24. https://doi.org/10.1016/j.amjmed.2013.01.020
9. Valente, T., Rossi, G., Lassandro, F., Rea, G., Marino, M., Muto, M., Molino, A., & Scaglione, M. (2016). MDCT evaluation of acute aortic syndrome (AAS). British Journal of Radiology, 89(1061), 1–19. https://doi.org/10.1259/bjr.20150825
10. Evangelista, A., Isselbacher, E. M., Bossone, E., Gleason, T. G., Di Eusanio, M., Sechtem, U., Ehrlich, M. P., Trimarchi, S., Braverman, A. C., Myrmel, T., Harris, K. M., Hutchinson, S., O’Gara, P., Suzuki, T., Nienaber, C. A., & Eagle, K. A. (2018). Insights from the international registry of acute aortic dissection: A 20-year experience of collaborative clinical research. Circulation, 137(17), 1846–1860. https://doi.org/10.1161/CIRCULATIONAHA.117.031264
11. Imamura, H., Sekiguchi, Y., Iwashita, T., Dohgomori, H., Mochizuki, K., Aizawa, K., Aso, S. ichi, Kamiyoshi, Y., Ikeda, U., Amano, J., & Okamoto, K. (2011). Painless acute aortic dissection -Diagnostic, prognostic and clinical implications -. Circulation Journal, 75(1), 59–66. https://doi.org/10.1253/circj.CJ-10-0183
12. Nallamothu, B. K., Mehta, R. H., Saint, S., Llovet, A., Bossone, E., Cooper, J. V., Sechtem, U., Isselbacher, E. M., Nienaber, C. A., Eagle, K. A., & Evangelista, A. (2002). Syncope in acute aortic dissection: Diagnostic, prognostic, and clinical implications. American Journal of Medicine, 113(6), 468–471. https://doi.org/10.1016/S0002-9343(02)01254-8
13. Hagan, P. G., Nienaber, C. A., Isselbacher, E. M., Bruckman, D., Karavite, D. J., Russman, P. L., Evangelista, A., Fattori, R., Suzuki, T., Oh, J. K., Moore, A. G., Malouf, J. F., Pape, L. A., Gaca, C., Sechtem, U., Lenferink, S., Deutsch, H. J., Diedrichs, H., Marcos y Robles, J., … Eagle, K. A. (2000). The International Registry of Acute Aortic Dissection (IRAD). Jama, 283(7), 897. https://doi.org/10.1001/jama.283.7.897
14. Pape, L. A., Awais, M., Woznicki, E. M., Suzuki, T., Trimarchi, S., Evangelista, A., Myrmel, T., Larsen, M., Harris, K. M., Greason, K., Di Eusanio, M., Bossone, E., Montgomery, D. G., Eagle, K. A., Nienaber, C. A., Isselbacher, E. M., & O’Gara, P. (2015). Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the international registry of acute aortic dissection. Journal of the American College of Cardiology, 66(4), 350–358. https://doi.org/10.1016/j.jacc.2015.05.029
15. Nienaber, C. A., Fattori, R., Mehta, R. H., Richartz, B. M., Evangelista, A., Petzsch, M., Cooper, J. V., Januzzi, J. L., Ince, H., Sechtem, U., Bossone, E., Fang, J., Smith, D. E., Isselbacher, E. M., Pape, L. A., & Eagle, K. A. (2004). Gender-related differences in acute aortic dissection. Circulation, 109(24), 3014–3021. https://doi.org/10.1161/01.CIR.0000130644.78677.2C
16. Goldstein, S. A., Evangelista, A., Abbara, S., Arai, A., Asch, F. M., Badano, L. P., Bolen, M. A., Connolly, H. M., Cuéllar-Calàbria, H., Czerny, M., Devereux, R. B., Erbel, R. A., Fattori, R., Isselbacher, E. M., Lindsay, J. M., McCulloch, M., Michelena, H. I., Nienaber, C. A., Oh, J. K., … Schepens, M. (2015). Multimodality imaging of diseases of the thoracic aorta in adults: From the American society of echocardiography and the european association of cardiovascular imaging: Endorsed by the society of cardiovascular computed tomography and society for cardiova. Journal of the American Society of Echocardiography, 28(2), 119–182. https://doi.org/10.1016/j.echo.2014.11.015
17. Garcia-Alvarez, M., Marik, P., & Bellomo, R. (2014). Sepsis-associated hyperlactatemia. Critical Care, 18(5), 1–11. https://doi.org/10.1186/s13054-014-0503-3
18. Adeva-Andany M, López-Ojén M, Funcasta-Calderón R, et al. Comprehensive review on lactate metabolism in human health. Mitochondrion. 2014;17:76-100. doi:10.1016/j.mito.2014.05.007
19. Suzuki, T., Distante, A., Zizza, A., Trimarchi, S., Villani, M., Uriarte, J. A. S., De Luca Tupputi Schinosa, L., Renzulli, A., Sabino, F., Nowak, R., Birkhahn, R., Hollander, J. E., Counselman, F., Vijayendran, R., Bossone, E., & Eagle, K. (2009). Diagnosis of acute aortic dissection by D-dimer: The international registry of acute aortic dissection substudy on biomarkers (IRAD-bio) experience. Circulation, 119(20), 2702–2707. https://doi.org/10.1161/CIRCULATIONAHA.108.833004
20. Nazerian, P., Mueller, C., De Matos Soeiro, A., Leidel, B. A., Salvadeo, S. A. T., Giachino, F., Vanni, S., Grimm, K., Oliveira, M. T., Pivetta, E., Lupia, E., Grifoni, S., Morello, F., Capretti, E., Castelli, M., Gualtieri, S., Trausi, F., Battista, S., Bima, P., … Soeiro, A. M. (2018). Diagnostic accuracy of the aortic dissection detection risk score plus D-dimer for acute aortic syndromes the ADvISED prospective multicenter study. Circulation, 137(3), 250–258. https://doi.org/10.1161/CIRCULATIONAHA.117.029457
21. Marill, K. A. (2008). Serum D-Dimer is a Sensitive Test for the Detection of Acute Aortic Dissection: A Pooled Meta-Analysis. Journal of Emergency Medicine, 34(4), 367–376. https://doi.org/10.1016/j.jemermed.2007.06.030
22. Diercks, D. B., Promes, S. B., Schuur, J. D., Shah, K., Valente, J. H., & Cantrill, S. V. (2015). Clinical policy: Critical issues in the evaluation and management of adult patients with suspected acute nontraumatic thoracic aortic dissection from the american college of emergency physicians clinical policies subcommittee (writing committee) on thorac. Annals of Emergency Medicine, 65(1), 32-42.e12. https://doi.org/10.1016/j.annemergmed.2014.11.002Lentini S, Perrotta S. Aortic dissection with concomitant acute myocardial infarction: From diagnosis to management. J Emerg Trauma Shock. 2011;4(2):273-278. doi:10.4103/0974-2700.82221
23. Kawahito, K., Adachi, H., Murata, S. I., Yamaguchi, A., & Ino, T. (2003). Coronary Malperfusion Due to Type A Aortic Dissection: Mechanism and Surgical Management. Annals of Thoracic Surgery, 76(5), 1471–1476. https://doi.org/10.1016/S0003-4975(03)00899-3
24. Lentini S, Perrotta S. Aortic dissection with concomitant acute myocardial infarction: From diagnosis to management. J Emerg Trauma Shock. 2011;4(2):273-278. doi:10.4103/0974-2700.82221
25. McMahon, M. A., & Squirrell, C. A. (2010). Multidetector ct of aortic dissection: A pictorial review. Radiographics, 30(2), 445–460. https://doi.org/10.1148/rg.302095104
26. Quien, M. M., & Saric, M. (2018). Ultrasound imaging artifacts: How to recognize them and how to avoid them. Echocardiography, 35(9), 1388–1401. https://doi.org/10.1111/echo.14116
27. Gutschow, S. E., Walker, C. M., Martínez-Jiménez, S., Rosado-de-Christenson, M. L., Stowell, J., & Kunin, J. R. (2016). Emerging concepts in intramural hematoma imaging. Radiographics, 36(3), 660–674. https://doi.org/10.1148/rg.2016150094
28. Chao, C. P., Walker, T. G., & Kalva, S. P. (2009). Natural history and CT appearances of aortic intramural hematoma. Radiographics, 29(3), 791–804. https://doi.org/10.1148/rg.293085122
29. Chua, M., Ibrahim, I., Neo, X., Sorokin, V., Shen, L., & Ooi, S. B. S. (2012). Acute aortic dissection in the ED: Risk factors and predictors for missed diagnosis. American Journal of Emergency Medicine, 30(8), 1622–1626. https://doi.org/10.1016/j.ajem.2011.11.017
30. Hansen, M. S., Nogareda, G. J., & Hutchison, S. J. (2007). Frequency of and Inappropriate Treatment of Misdiagnosis of Acute Aortic Dissection. American Journal of Cardiology, 99(6), 852–856. https://doi.org/10.1016/j.amjcard.2006.10.055
31. Nienaber, C. A., & Clough, R. E. (2015). Management of acute aortic dissection. The Lancet, 385(9970), 800–811. https://doi.org/10.1016/S0140-6736(14)61005-9
32. Hiratzka, L. F., Bakris, G. L., Beckman, J. A., Bersin, R. M., Carr, V. F., Casey, D. E., Eagle, K. A., Hermann, L. K., Isselbacher, E. M., Kazerooni, E. A., Kouchoukos, N. T., Lytle, B. W., Milewicz, D. M., Reich, D. L., Sen, S., Shinn, J. A., Svensson, L. G., Williams, D. M., Jacobs, A. K., … Yancy, C. W. (2010). 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: Executive summary: A report of the american college of cardiology foundation/american heart association task force on practice guidelines, american association for thoracic surgery, american college of radiology, american stroke association. Circulation, 121(13), 266–369. https://doi.org/10.1161/CIR.0b013e3181d4739e
33. Rogers, A. M., Hermann, L. K., Booher, A. M., Nienaber, C. A., Williams, D. M., Kazerooni, E. A., Froehlich, J. B., O’Gara, P. T., Montgomery, D. G., Cooper, J. V., Harris, K. M., Hutchison, S., Evangelista, A., Isselbacher, E. M., & Eagle, K. A. (2011). Sensitivity of the aortic dissection detection risk score, a novel guideline-based tool for identification of acute aortic dissection at initial presentation: Results from the international registry of acute aortic dissection. Circulation, 123(20), 2213–2218. https://doi.org/10.1161/CIRCULATIONAHA.110.988568
34. Nazerian, P., Mueller, C., De Matos Soeiro, A., Leidel, B. A., Salvadeo, S. A. T., Giachino, F., Vanni, S., Grimm, K., Oliveira, M. T., Pivetta, E., Lupia, E., Grifoni, S., Morello, F., Capretti, E., Castelli, M., Gualtieri, S., Trausi, F., Battista, S., Bima, P., … Soeiro, A. M. (2018). Diagnostic accuracy of the aortic dissection detection risk score plus D-dimer for acute aortic syndromes the ADvISED prospective multicenter study. Circulation, 137(3), 250–258. https://doi.org/10.1161/CIRCULATIONAHA.117.029457
35. Dean, J. H., Woznicki, E. M., O’Gara, P., Montgomery, D. G., Trimarchi, S., Myrmel, T., Pyeritz, R. E., Harris, K. M., Suzuki, T., Braverman, A. C., Hughes, G. C., Kline-Rogers, E., Nienaber, C. A., Isselbacher, E. M., Eagle, K. A., & Bossone, E. (2014). Cocaine-related aortic dissection: Lessons from the international registry of acute aortic dissection. American Journal of Medicine, 127(9), 878–885. https://doi.org/10.1016/j.amjmed.2014.05.005






Add comment