April 14, 2021, by Shahriar Lahouti .
CONTENTS
- Preface
- Basic physiology
- Measures of oxygenation
- Pathophysiology and Histopathology of ARDS
- Etiology
- Clinical Features
- Imaging
- Labs
- Diagnosis
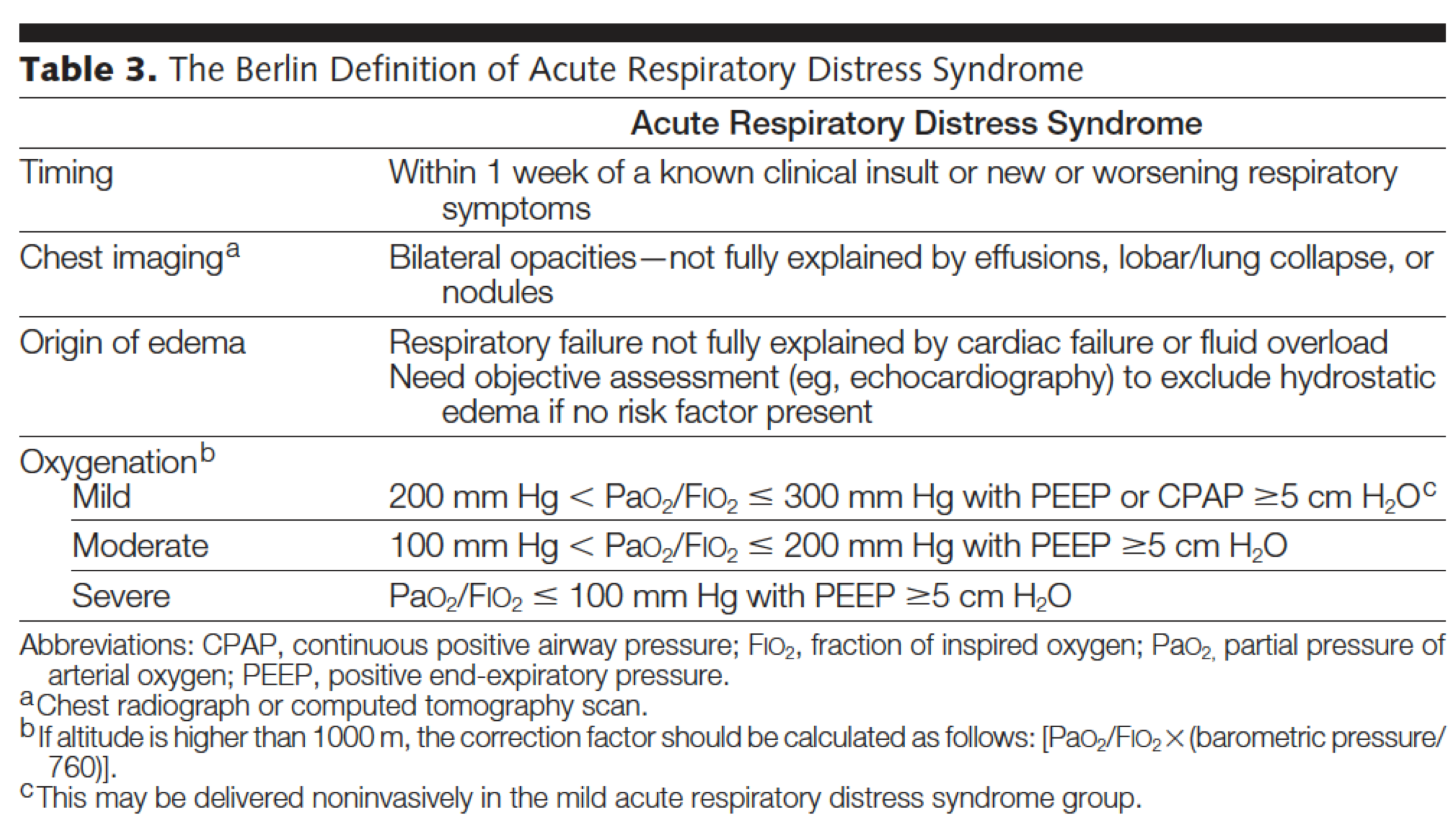
- Clinical Diagnosis: Berlin Definition of ARDS!
- Caveats
- Differential diagnosis
- Bedside Ultrasound
- Approach to diagnosis of hypoxic respiratory failure
- Management strategies
- Invasive mechanical ventilation
- Options for non-responding patients
- Supportive care
- Going further
- References
Preface
Adult Respiratory Distress Syndrome is a distinct type of acute hypoxic respiratory failure (shock lungs) which was first recognized during 1960s 1 and then was defined by the American-European Consensus Conference on ARDS (published in 1992)2. This has recently been replaced by the Berlin definition of ARDS (published in 2012)3 4 .
Despite all efforts, there’s still ongoing confusion regarding the definition of ARDS. From this perspective, ARDS is similar to shock state; i.e different clinicians use these terms to refer to different things!
What is your definition of circulatory shock?
- You visit a middle aged woman who doesn’t look good; altered with cold extremities. Her initial BP is 180/120 and HR of 140 b.p.m. Do you suspect shock or you will rule out shock just because her initial BP is high for the moment?! Don’t you ask for other relevant clinical information to reach a better decision?
- You visit another patient in the next room! A young man who is altered but not in any distress.His initial BP is 85/50 and HR of 60 b.p.m. Do you rule in shock confidently just because his initial SBP is< 90 mmHg?
One may become worried if SBP is read at 89 mmHg in triage and may be reassured if SBP is read at 91mmHg for another patient! How a certain index with magic cutoff can be extrapolated to all individual patients for a moment.
If a number per se does matter, the interpretation of the number in physiologic terms and in appropriate clinical context as well as it’s trajectory matter more.
More to the point, the diagnostic and prognostication value of certain parameters matter too.
Parting shot: I apologize for this long introduction, but other things need to be clarified.Well in fact, it is crucial to consider “WHO IS YOUR PATIENT RATHER THAN WHAT DISEASE DOES HE/SHE HAS” AT THE FIRST PLACE? (i.e patient’s age, comorbidities, risk factors for certain conditions, clinical presentation should come into your attention first).
In this post adult respiratory distress syndrome is reviewed and the Berlin definition which is in part the source of confusion and consternation around the ARDS is challenged.
Adult Respiratory Distress Syndrome is a distinct type of acute hypoxic respiratory failure (shock lungs) which was first recognized during 1960s 1 and then was defined by the American-European Consensus Conference on ARDS (published in 1992)2. This has recently been replaced by the Berlin definition of ARDS (published in 2012)3 4 .
Despite all efforts, there’s still ongoing confusion regarding the definition of ARDS. From this perspective, ARDS is similar to shock state; i.e different clinicians use these terms to refer to different things!
What is your definition of circulatory shock?
- You visit a middle aged woman who doesn’t look good; altered with cold extremities. Her initial BP is 180/120 and HR of 140 b.p.m. Do you suspect shock or you will rule out shock just because her initial BP is high for the moment?! Don’t you ask for other relevant clinical information to reach a better decision?
- You visit another patient in the next room! A young man who is altered but not in any distress.His initial BP is 85/50 and HR of 60 b.p.m. Do you rule in shock confidently just because his initial SBP is< 90 mmHg?
One may become worried if SBP is read at 89 mmHg in triage and may be reassured if SBP is read at 91mmHg for another patient! How a certain index with magic cutoff can be extrapolated to all individual patients for a moment.
If a number per se does matter, the interpretation of the number in physiologic terms and in appropriate clinical context as well as it’s trajectory matter more.
More to the point, the diagnostic and prognostication value of certain parameters matter too.
Parting shot: I apologize for this long introduction, but other things need to be clarified.Well in fact, it is crucial to consider “WHO IS YOUR PATIENT RATHER THAN WHAT DISEASE DOES HE/SHE HAS” AT THE FIRST PLACE? (i.e patient’s age, comorbidities, risk factors for certain conditions, clinical presentation should come into your attention first).
In this post adult respiratory distress syndrome is reviewed and the Berlin definition which is in part the source of confusion and consternation around the ARDS is challenged.
Basic Physiology
Understanding the physiology and course of the disease are more important than the numbers!
The physiology of oxygenation and ventilation was discussed here. For the purpose of this discussion, a brief review of fundamental concepts is mentioned here. The availability of O2 at the tissue level depends on 5 :
- Oxygenation at alveolar-capillary unit (blood O2 tension; PaO2)
- Hemoglobin concentration
- Cardiac output (Perfusion of peripheral tissue)
- Systemic oxygen consumption (cellular metabolic demand)
Impaired oxygenation (#1 above) is predominantly the result of V/Q mismatching (Blood passing through parts of the lung that are not adequately ventilated) and shunt physiology (blood does not ever meet O2 at alveolar interface either because alveoli are filled with puss, blood, etc. or as a result of extrapulmonary bypass tract such as ventricular septal defect).
- The compensatory mechanism working to correct V/Q mismatching is hypoxic pulmonary vasoconstriction 6. That is pulmonary vasoconstriction in regions where alveoli are filled with anything other than air.
Impaired CO2 elimination (ventilation): although oxygenation and ventilation oftentimes run together, it is physiologically reasonable to consider CO2 elimination as a separate process. The key variable in CO2 elimination is dead space; which is part of the air moving in and out with each breath without ever meeting alveoli. Excess dead space can arise due to following reasons:
- Minor alveolar-capillaries obstruction or destruction such as scarred alveoli in ARDS, fibrotic interstitial fibrosis, microthrombi in various inflammatory diseases.
- Major pulmonary obstruction such as segmental pulmonary embolism
- The compensatory mechanism to correct rising PCO2 level is tachypnea
Work of breathing (WOB): the level of effort for one to move in and out each tidal breath. This is a critical parameter for assessing severity of respiratory distress and can be determined at bedside (e.g. accessory muscle use, intercostal retraction etc).It turns out that the work of breathing is most strongly affected by the drive to clear CO2. This is influenced by:
- Airway resistance: obstruction to airflow (too narrow the bellows) can cause increased WOB.
- Lung compliance: If the lung is stiff (basketball lung), then it takes tremendous effort to inflate and deflate it.
- Dead space: Increasing dead space (wasted ventilation) equates to “one should breath more air in and out to compensate for the wasted part”.
👉Clinical implication: Some patients with COVID-19 infection present with profound hypoxemia (poorly responsive to supplemental oxygen) without dyspnea early in the disease course (called silent /happy hypoxemia). How could this be explained physiologically?
Poorly responsive profound hypoxemia without dyspnea can occur if all of the following condition are met 7 8:
- Presence of shunt physiology. This accounts for unresponsive hypoxemia of the presentation which is this condition is probably contributed by atelectasia. Dysregulated ‘HPV’ is also suggested as a contributing factor 9.
- Absence of excessive dead space. In the early disease course, there’s a fair amount of functional lung volume, as such CO2 clearance remains normal.
- Resistance to airflow remains relatively normal.
- Compliance of the lung remains reasonably normal. In the early disease process, compliance of the lung remains “relatively” normal.
The most simple example of happy hypoxemia is a patient with an intracardiac right-to-left shunt. Such a patient may have normal lungs and thus no difficulty clearing CO2 (with a normal work of breathing). However, the patient may be quite hypoxemic. This represents perhaps the most pure divorce between oxygenation and CO2 clearance.
The pathophysiology of COVID-19 disease is still in its nascent stages and there are conflicting data regarding advanced management of these patients.
Measures of oxygenation
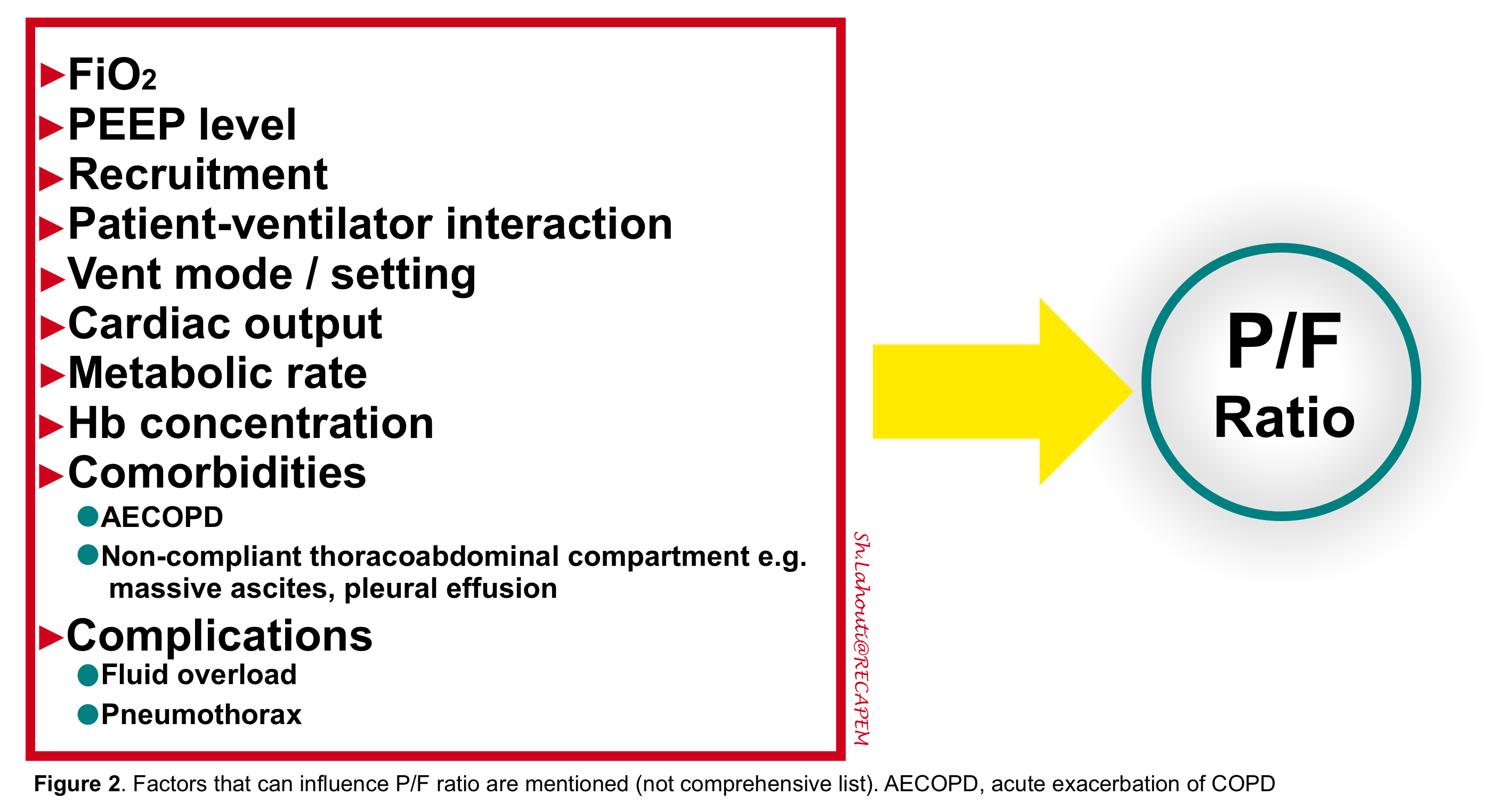
There are several ways to measure oxygenation (e.g. PaO2 on ABG, SaO2 on pulse-ox, (A-a) O2 gradient) with their own advantages and disadvantages. Here the PaO2/FiO2 (P/F ratio) is discussed. In the absence of a direct reliable marker of lung injury, the P/F ratio has been used for many years as an indicator for lung function (and also PEEP titration, changing different ventilator strategies and making decisions regarding the need for more advanced managements like recruitment maneuver). Therefore it is fair to discuss in brief what factors can change P/F ratio independent to lung function.
NUMBER 1: FiO2
Increasing the FiO2 tends to increase the P/F ratio. This occurs because increasing the FiO2 will increase the mixed venous oxygen saturation, which minimizes the impact of shunting deoxygenated blood through the lungs. It has been shown that increasing the FiO2 to 100% caused ~20% of patients with moderate-severe ARDS to be reclassified into a milder diagnostic category 10.
NUMBER 2: PEEP LEVEL
”True” ARDS is characterized by decreased lung compliance which is contributed in part by flooded alveolar units with exudate and alveolar units which have been collapsed (atelectasia). These two pathologies both share in blood shunting (leading to hypoxemia) however they are dissimilar in that, units with atelectasia are recruitable with appropriate PEEP titration. Therefore applied best individualized PEEP level in patients with recruitable lung can improve oxygenation and increases the P/F ratio leading to downgrade ARDS severity. In fact some patients may not even meet the ARDS definition anymore (P/F ratio on appropriate PEEP level) 11.
NUMBER 3: PERFUSION FACTORS
As discussed previously (here) the alveolar capillaries intuitively vasoconstrict in response to regional alveolar hypoxia, so that blood flow is redirected to areas where alveoli are well-ventilated. This V/Q matching response is called hypoxic pulmonary vasoconstriction (HPV) which is a compensatory response to hypoxia in order to preserve arterial SaO2. Interestingly but sadly some factors can hamper this adaptive response. For instance, sepsis 12, alkalemia 13, a high cardiac output during positive pressure ventilation (but not during spontaneous breathing), medications such as intravenous vasodilators tend to blunt the hypoxic response and increase the degree of venous admixture 14. Patients with cirrhosis often have poor HVP responses too. In contrast, inhaled vasodilators 15 or proning 16 17 help to redistribute blood flow to the aerated lung and thus to reduce venous admixture.Factors that alter ‘HPV’ can indirectly impact P/F ratio and upgrade or downgrade ARDS severity.
Some studies have shown that “HPV” becomes dysregulated in patients with COVID-19 infection.
NUMBER 4: CARDIOVASCULAR AND METABOLIC FACTORS
Other factors which affect P/F ratio include cardiac output, metabolic rate, and hemoglobin concentration. These variables can independently and strongly affect the PaO2 as previously explored (here). For example, a drop in cardiac output would decrease the mixed venous oxygen saturation and thereby decrease the P/F ratio 18.
Bottom line: P/F ratio is a very labile measurement and may not track linearly the degree of lung injury severity.There are several other factors completely independent of the lung injury (e.g. cardiac output) which can change P/F ratio (figure 2). It is wise to say that P/F ratio provides only a piece of the puzzle if interpreted in appropriate clinical context and the urge to build diagnosis and treatment strategy on any single measurement is unreasonable. This becomes especially dangerous when one does not consider the elemental factors which determine P/F ratio and ignore the trajectory of it over time.
HOW TO OVERCOME P/F RATIO MEASUREMENT PITFALLS? A more consistent measurement of lung function might be achieved as follows:
- Measurement over time (the trajectory of P/F ratio)
- Greater effort to account for variations in PEEP and recruitment
- Measurement of the shunt fraction (more on this here), rather than the P/F ratio may theoretically overcome many of these pitfalls associated with P/F ratio.
Pathophysiology and Histopathology of ARDS
Understanding pathophysiology and histopathology of a disease provides useful indirect clues for possible therapeutic options for future clinical trials.
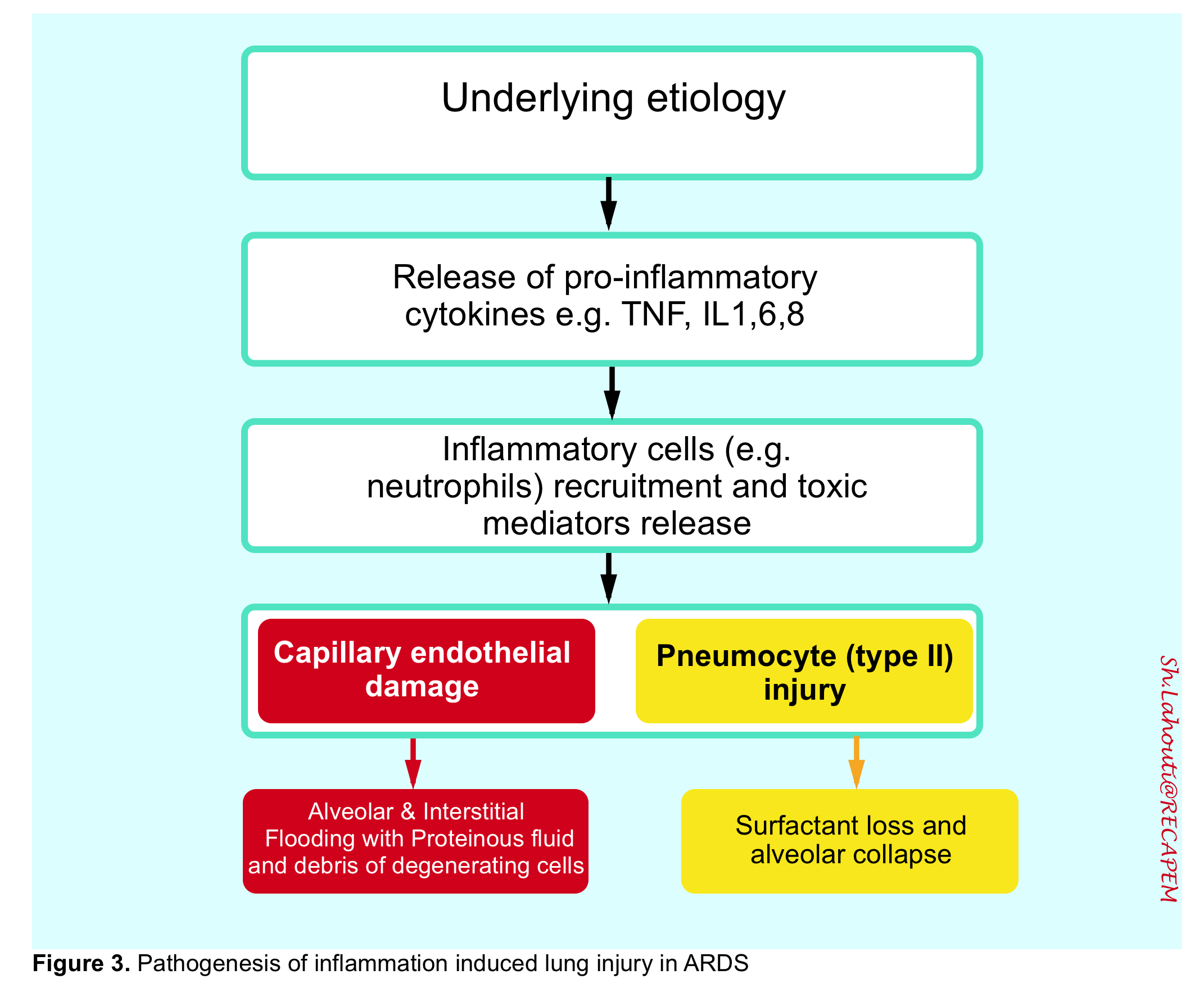
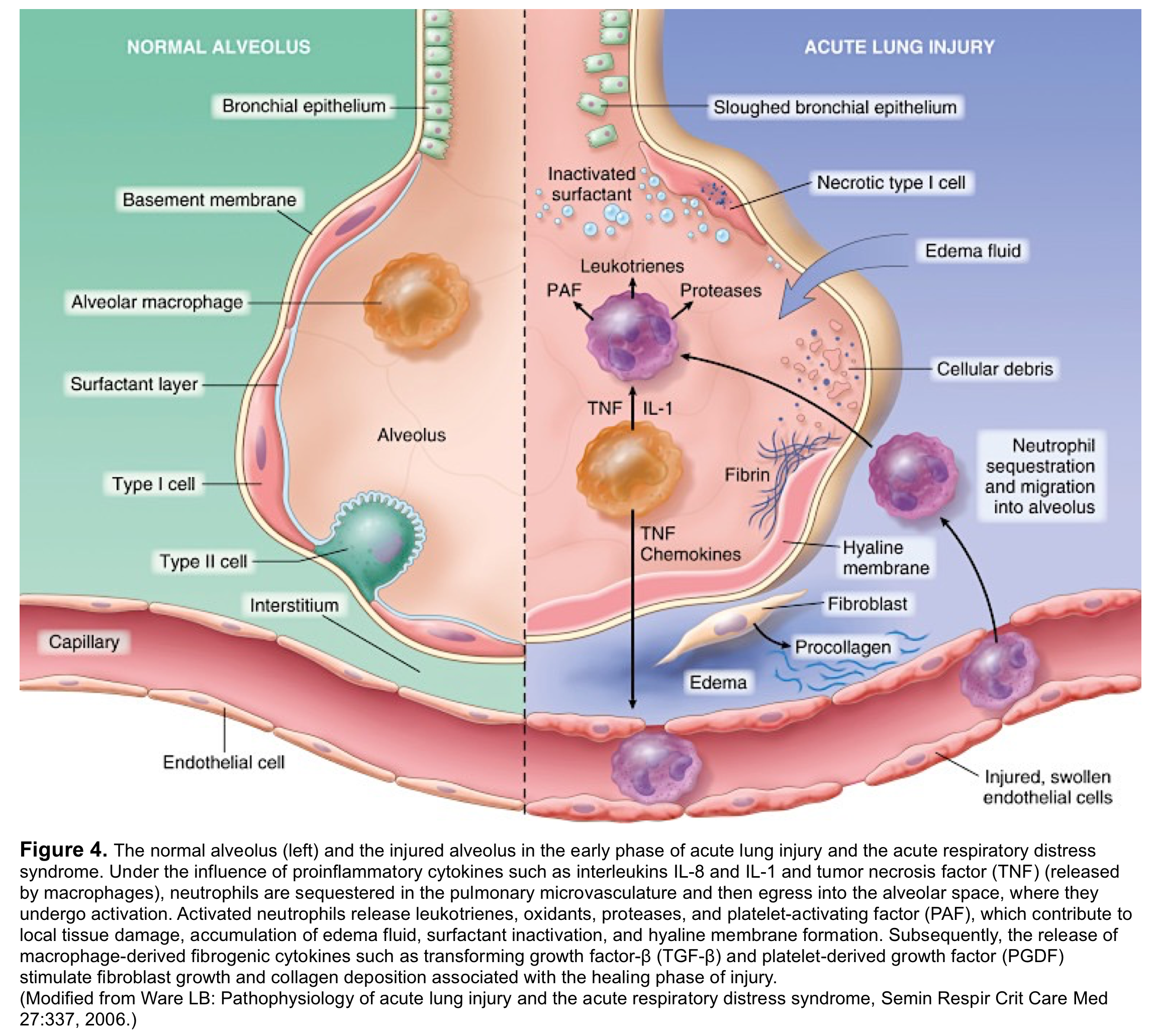
ARDS is caused by an insult to the alveolar-capillary membrane that results in increased permeability and subsequent interstitial and alveolar edema. This injury eventually involves both pulmonary capillary endothelium and the alveolar epithelium through a characteristic diffuse inflammatory process 19 20 (figure 3,4).
This inflammatory reaction interrupts the lung principal of homeostasis with consequent excess fluid accumulation in both alveolar and interstitial space. During this exudative stage diffuse alveolar damage (DAD) takes place.
👉DAD is a nonspecific reaction 21 to a variety of causes and is histologically characterized by interstitial edema, acute and chronic inflammation, type II cell hyperplasia and “hyaline membrane formation”. Thrombi within small arteries are also common. Some key points should be mentioned here:
- Presence of “DAD” provides a histologic clue for diagnosis of ARDS, however its absence does NOT rule out ARDS. In fact other non-vascular lung diseases with various histological findings other than ‘DAD’ have been reported in cases with ARDS (e.g. pneumonia, other ILD 22 and ventilator induced lung injury 23), collectively called “Non-DAD ARDS”.
- Presence of “DAD” does NOT provide a clue for specific etiology of ARDS (it’s a nonspecific reaction to inciting lung injury).
Does the presence of “DAD” provide a prognostic clue? Probably yes; since it has been reported in 10% of patients with mild ARDS, 40% of moderate ARDS, and 60% of patients with severe ARDS 24.
👉The pathophysiology of COVID-19 disease is complicated and still in question. Within the context of ARDS, most clinicians refer to ‘DAD’ (typical ARDS); while this is just one side of the coin. In fact many primary etiologies of ARDS cause different histopathology; collectively termed here to “Non-DAD ARDS”. Considering histopathology of lung injury is extremely helpful in choosing therapeutic modalities.
COVID-19 is part of a family of coronavirus.It binds to angiotensin-converting enzyme 2 (ACE2) receptor located diffusely on type II alveolar cells, vascular endothelium and intestinal epithelia 25 26 just like other family member of coronavirus e.g. SARS. The primary organ failure (hypoxic respiratory failure) is possibly due to reduced surfactant levels (causing atelectasis and derecruitment) rather than purely hyperinflammatory injury 27. Other unique features of COVID-19 include thrombogenic processes, vascular remodeling triggered by endotheliitis, and a cytokine release storm 9. In a series of 6 autopsies from patients who died from COVID infection, lymphocytic pneumonia (in one patient who died five days after onset of fever) and acute fibrinous and organizing pneumonia (AFOP) was found in the other five patients who died 20 days after symptoms onset 28. Despite numerous limitation in this small study, some possible conclusion can be made:
- Whether Lymphocytic pneumonia, AFOP or DAD 29 were predominant histopathologic patterns, the overall compliance of the lung is reduced.
- If AFOP does emerge as a common histological pattern, this would provide indirect support for the use of steroid in COVID-19 patients with hypoxemic respiratory failure. Of course, the use of steroids should ideally be based on direct clinical investigation (i.e., an RCT), rather than indirect histological evidence
For more on pathophysiology of COVID-19 see here.
Etiology
ARDS is a wastebasket of non-vascular lung diseases with a variety of histological and radiological patterns.However it is useful to think of pathogenesis of ARDS as a result of two different pathways: a direct insult to lung cells (e.g. pneumonia,inhalation injury like vaping 30) and an indirect insult as a result of an acute systemic inflammatory response (e.g. sepsis, acute pancreatitis) 31.
Clinical Features of True ARDS
True ARDS is a clinical syndrome which is histologically defined as typical diffuse alveolar damage (DAD) throughout the lungs 32 with slow recovery over a period of several days, often with residual fibrosis 33.
Remember that ARDS has a subacute progressive course often over a week following inciting insult.This is in contrast to some other etiologies of respiratory distress such as acute flash pulmonary edema, pulmonary embolism and fat embolism which usually have an acute course.
The clinical manifestation of ARDS behaves as:
👉IMPAIRED GAS EXCHANGE 34
- Severe hypoxemia which doesn’t disappear following recruitment over 12-24 hours.
- Increased dead space (inefficient CO2 clearance, requiring high minute ventilation).
👉DECREASED LUNG COMPLIANCE
- Decreased pulmonary compliance is one of the hallmarks of ARDS resulting from the stiffness of poorly or non-aerated lung 35 (basketball lung🏀)
👉PULMONARY HYPERTENSION
- It has been reported to occur in 25% of patients with ARDS who undergo mechanical ventilation 36 and has been associated with increased risk of death 37. Possible contributors may include pulmonary vasoconstriction mediated by hypoxia (HPV), hypercapnia and vasoactive mediator imbalance. Other possible causes may include parenchymal destruction,airway collapse, vascular compression induced by positive pressure ventilation, vessel obliteration, microthrombosis 38 39.
👉SLOW RECOVERY OVER PERIOD OF SEVERAL DAYS
Imaging
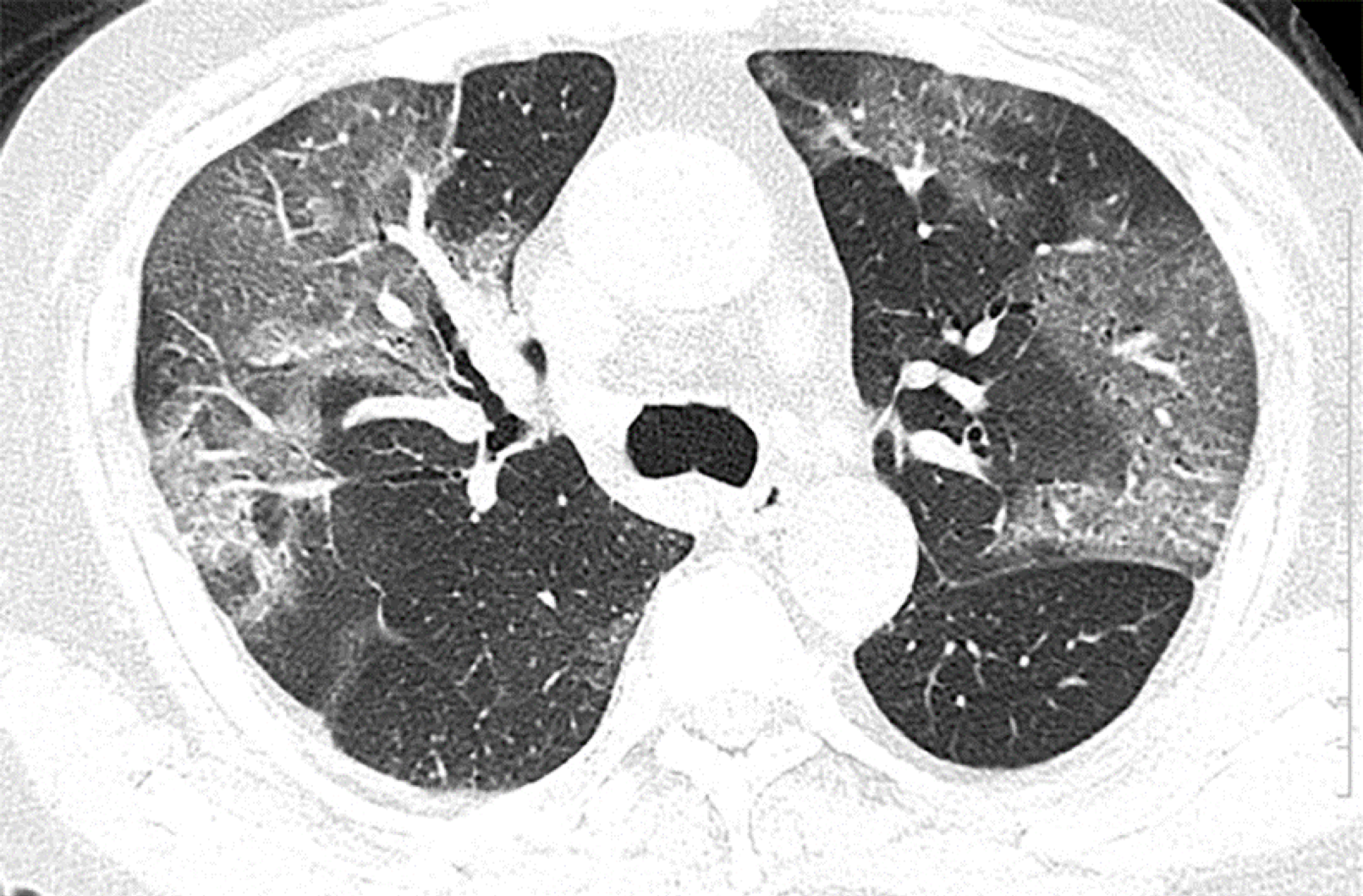
The radiologic manifestations of ARDS are variable and depend on the course and severity of ARDS. Chest CT may show widespread patchy and/or coalescent airspace opacities that are usually more apparent in the dependent lung zones. The opacities can be subtle (eg, patchy ground glass), particularly in early ARDS, but can become consolidative in appearance as severity worsens. Preliminary studies of bedside ultrasound report an 83 to 92 percent sensitivity for the diagnosis of ARDS compared with CT chest 40.
Labs
Except for blood gas analysis (ABG, VBG) which possibly provide useful information regarding any type of respiratory failure other laboratory tests are not specific. Besides hypoxemia, blood gas analysis in patients with ARDS initially often shows acute respiratory alkalosis.
Pearls:
👉The development of acute hypercapnic respiratory acidosis is an ominous sign and may represent severe ARDS with impending respiratory arrest.
👉Metabolic acidosis from hypoxemia is unusual and, if present, is more likely to be due to the precipitating etiology (eg, sepsis) or associated organ injury (eg, acute kidney injury).
A complete blood count and routine chemistry and coagulation studies are generally requested. BNP or NT-proBNP: in patients with suspected ARDS, BNP alone in general is not a reliable indicator for distinguishing ARDS from cardiogenic edema.
Diagnosis
By enlarge, ARDS is a diagnosis of exclusion. Although ARDS can be diagnosed histopathologically (eg, classically DAD in the early stages), clinicians typically only perform lung biopsy to confirm or exclude other important etiologies that can cause or mimic ARDS (e.g. Diffuse alveolar hemorrhage, Acute eosinophilic pneumonia). The presumptive diagnosis of ARDS relies on a combination of clinical, radiographic and physiologic criteria.This becomes problematic where these criteria allow the inclusion of a diverse group of patients in the trials. The clinical diagnosis of ARDS (Berlin definition) 3 4 is presented below and it requires that all of the following criteria be present for diagnosis.
Caveats
Arguably the Berlin Definition of ARDS is Clinically Useless 41 for a Couple of Reasons:
- In practice, the Berlin definition is extremely broad. Almost all intubated patients with bilateral infiltrates may meet this definition 42. Enrollment of unselected patients may negatively affect the prognostic enrichment and contribute to the failure of therapeutic trials 43.
- The Berlin definition of ARDS has over reliance on a measurement (PaO2/FiO2) which is very labile and can be influenced by multiple factors 44 discussed before.
- Although P/F ratio is an integral part of the assessment of patients with ARDS; it was not used under a standard procedure (e.g. ventilatory setting). Several studies have shown that many patients who initially meet the definition of severe ARDS by the Berlin criteria, were downgraded to mild or no ARDS by 24hrs of standard 45 physiologically reasonable procedure 46 47.
- In essence the Berlin definition of ARDS is the same as the previous published definition on ARDS (published in 1992)2 except that a PEEP of 5 cmH2O is added to the criterion. However a PEEP of 5 is not often enough to resolve the atelectasis component of ARDS 48.
- Last but not the least, the Berlin definition of ARDS criteria has never been used as a primary inclusion criteria for any clinical trial. Clinical trials have been far more mindful and selective about who to include in their trials.
- It is worth mentioning here the PROSEVA trial (a major RCT that showed benefit from proning) 49 which before they include the patients into the study, patients were optimized on ventilator for 12-24 hrs and If the P/F ratio increased to >150 during this period, the patient was excluded from this trial. So they included those patients into the study whose P/F ratio remains low despite optimization on the ventilator for 24hrs.
Bottom line: Disease definition does matter! If employing aggressive management strategies shall be based upon definition of the disease (i.e. ARDS) and it’s severity; then it comes to matter that one knows the caveats of Berlin definition and how possibly management strategies should be proceeded.
Differential Diagnosis
The term PseudoARDS 50 or rapidly improving ARDS 43 refers to those patients whose P/F ratio increases above 150 after 12-24 hrs of optimization on mechanical ventilation (without proning). These are “CLINICALLY” meaningful terms since upon resolving those confounding factors which can imitate ARDS (e.g. atelectasia, pleural effusion, volume overload) patients do not require prone positioning or other invasive management strategies.
The most important and common clinical conditions which can mimik ARDS (pseudoARDS) are listed below:
- Hydrostatic pulmonary edema
- A small bilateral pneumonia with a large pleural effusion
- Bilateral atelectasis
It is imperative that clinicians recognize these common ARDS imitators and employ appropriate therapeutic measures to resolve the underlying primary cause of respiratory insufficiency.
👉Remember that some of these patients may technically meet the Berlin definition of ARDS initially while in fact they do NOT.
Ok, let’s briefly discuss the major ARDS mimickers on the table.
NUMBER 1# Hydrostatic pulmonary edema: It can be caused by heart failure, renal insufficiency, volume overload or a combination of all! Sometimes it becomes challenging to differentiate these disease entities from “True” ARDS 51.
- Cardiogenic pulmonary edema(CPE): It is usually due to left ventricular systolic or diastolic dysfunction. TTE is extremely useful to look for evidence of cardiac systolic or diastolic dysfunction, acute severe valvular insufficiency (e.g. Acute Severe MR, AR).
- Keep in mind that ARDS can coexist with “CPE” and therefore even if initial studies were in favor of “CPE”, it is essential to evaluate response to treatment and assess for ARDS if still a patient is not satisfactorily improved.
- Volume overload: This pattern is often the result of aggressive volume resuscitation in patients with an initial injury causing noncardiogenic pulmonary edema (e.g. pneumonia, pancreatitis). For example a patient with moderate pneumonia is aggressively resuscitated by volume (according to survival sepsis campaign guidelines!!) upon which the patient deteriorates and ends up to be intubated. Bedside Ultrasound can be performed here to identify and monitor volume status of the patients.
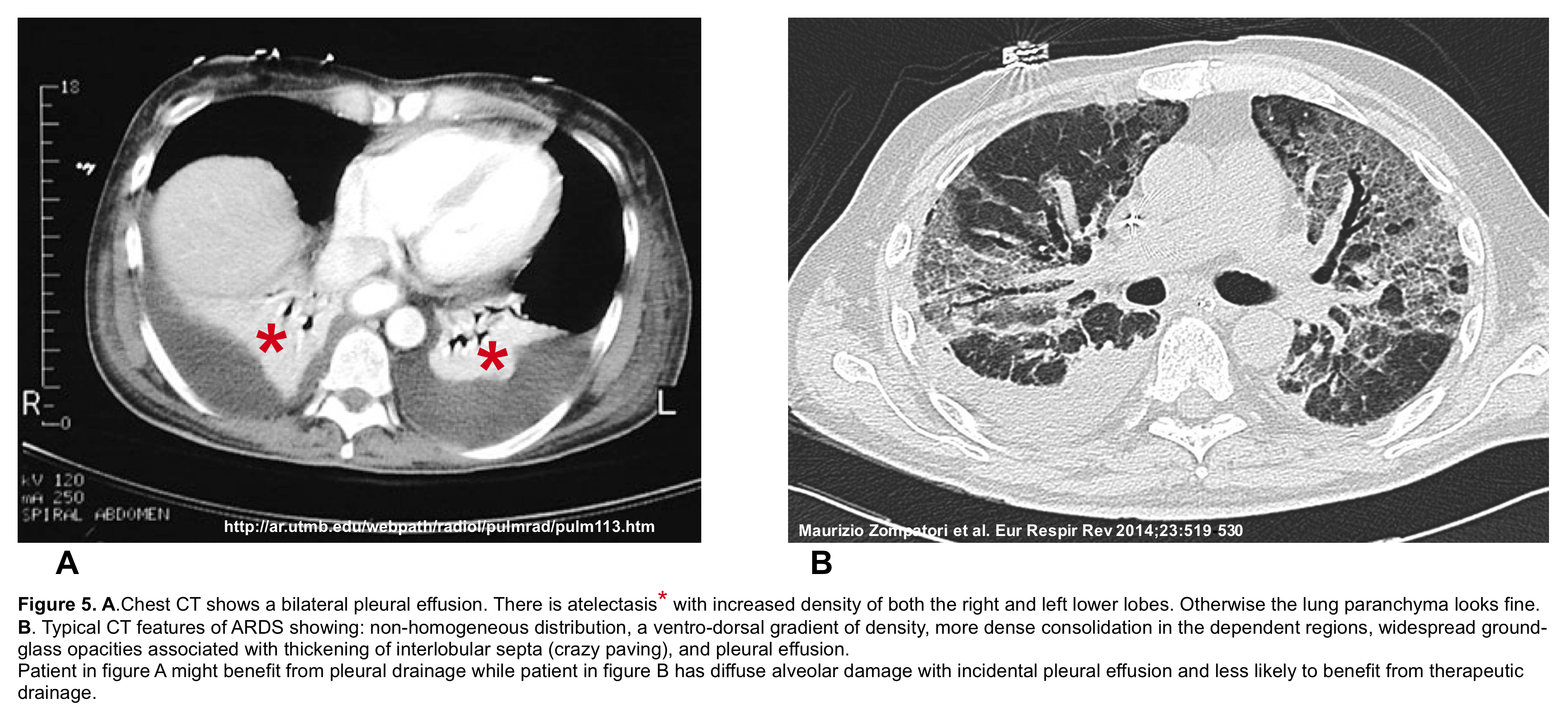
NUMBER 2 # Small bilateral pneumonia with large pleural effusion: patient with bilateral infiltrates who seems to have ARDS, but whose respiratory failure is in fact largely due to significant effusions and adjacent atelectasis (such as in a patient with small pneumonia and a large pleural effusion). Use of bedside ultrasound and CT may be helpful to sort this out (figure 5). Under such conditions where lung parenchyma looks otherwise fine, and significant pleural effusion is present, it is reasonable to drain the pleural effusion and reassess the patient 52 53 54.
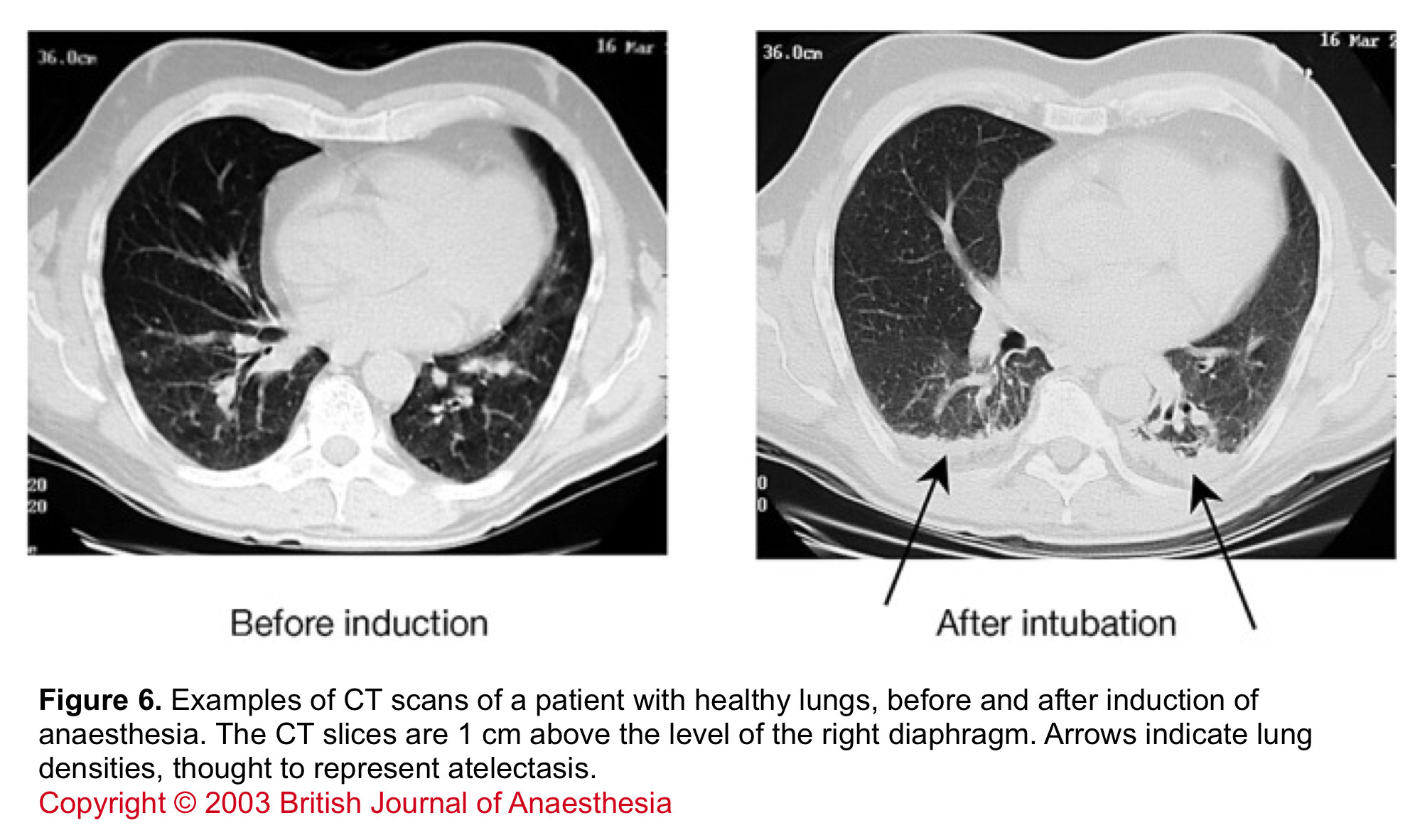
NUMBER 3# Atelectasia: The most common form of this pattern occurs in morbidly obese patient post-anesthesia for surgery 55 or intubated patients. The atelectasia often involves the dependent lung zone (figure 6), however diagnosis becomes challenging when atelectasia does not follow an easily identifiable lobar pattern 56 57. In the latter form, the initial CT can look nearly similar to ARDS and the only way to reliably diagnose this pattern is resolution of atelectasia upon ventilation with high PEEP. However, 5 cmH2O of PEEP (which is proposed by the Berlin definition of ARDS) is insufficient to cause recruitment (especially among morbidly obese patients).
Bedside Ultrasound
Bedside ultrasound and echocardiography is potentially useful in diagnosis of ARDS and exclusion of confounding factors (pleural effusion, volume overload, and atelectasis) which can imitate ARDS. Only after taking these items off the table, one will get to know what is happening there. As explained above the most crucial differential diagnosis for ARDS is decompensated heart failure.
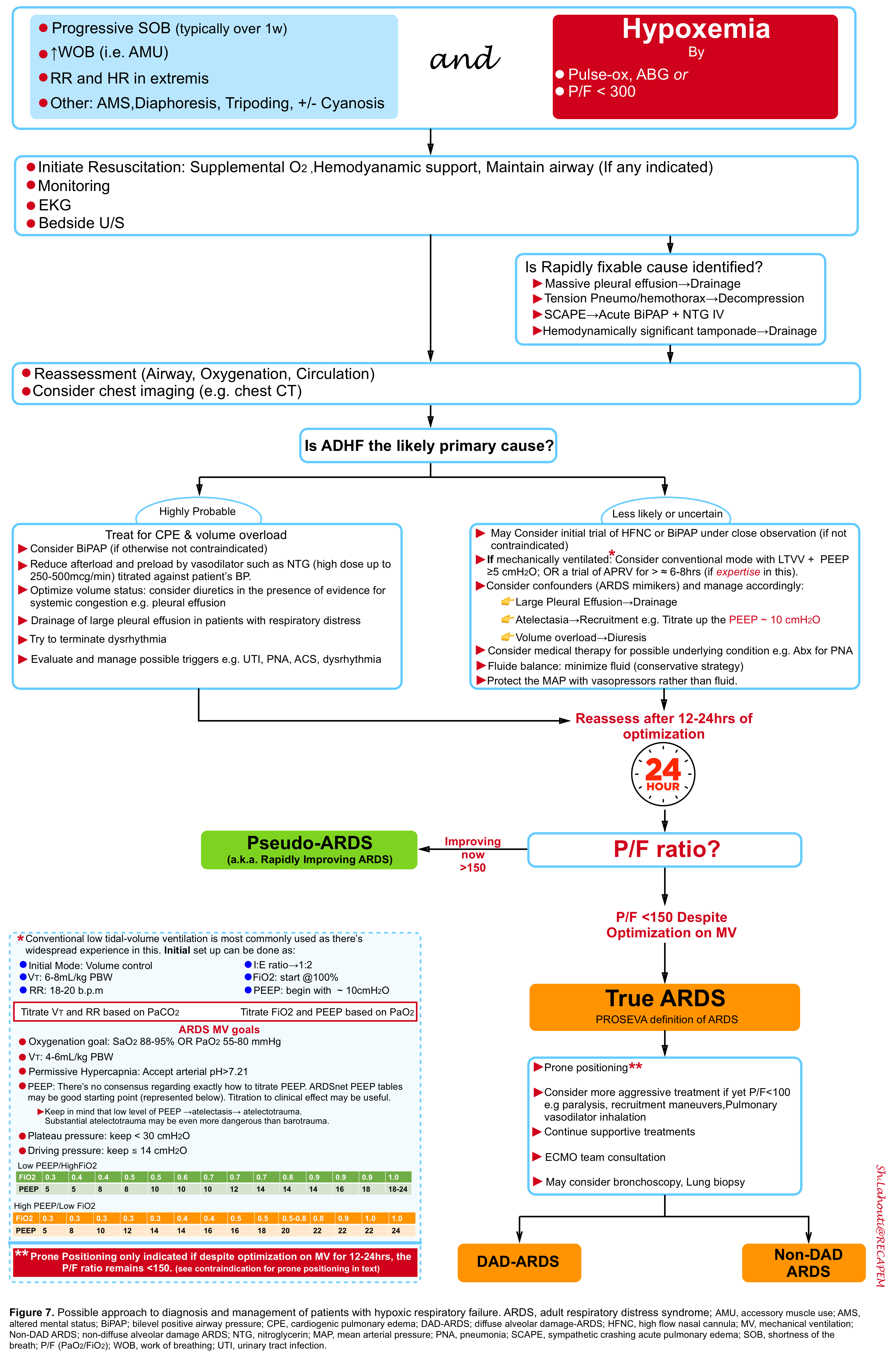
Approach to diagnosis of hypoxic respiratory failure
Patients with respiratory insufficiency or failure are critically ill and require prompt evaluation and simultaneous resuscitation. The general schema of approach to diagnosis and management of patients with hypoxic respiratory failure is presented below (figure 7).
Management strategies
Initial resuscitation is directed to achieve the following goals (not necessarily in following order):
- Airway protection
- Support oxygenation and ventilation
- Hemodynamic support
- Avoiding further injury!
Examples of Indication for immediate intubation:
- Inability to protect airway (e.g. expanding neck hematoma, bleeding into the airway {drowned airway}, persistent low GCS score, worsening upper airway obstruction)
- Increased work of breathing (AMU, tachypnea, terminal bradypnea) and severe hypoxemia
- In some conditions patients may look terrible but will do fine with non-invasive respiratory supports and direct medical treatment. These conditions may include: Acute Flash Pulmonary Edema, Bronchospasm (AECOPD, asthma)
- Cardiac arrest (or impending to arrest)
- Logistic consideration (patients need to be transferred, scanned, scoped)
Options for initial oxygenation and ventilation
By definition patients with ARDS are severely hypoxemic. In patients who are not in severe respiratory distress, and are hemodynamically stable and are not victims of multiorgan failure, an initial trial of noninvasive respiratory support may be tried under close monitoring (If not otherwise contraindicated).
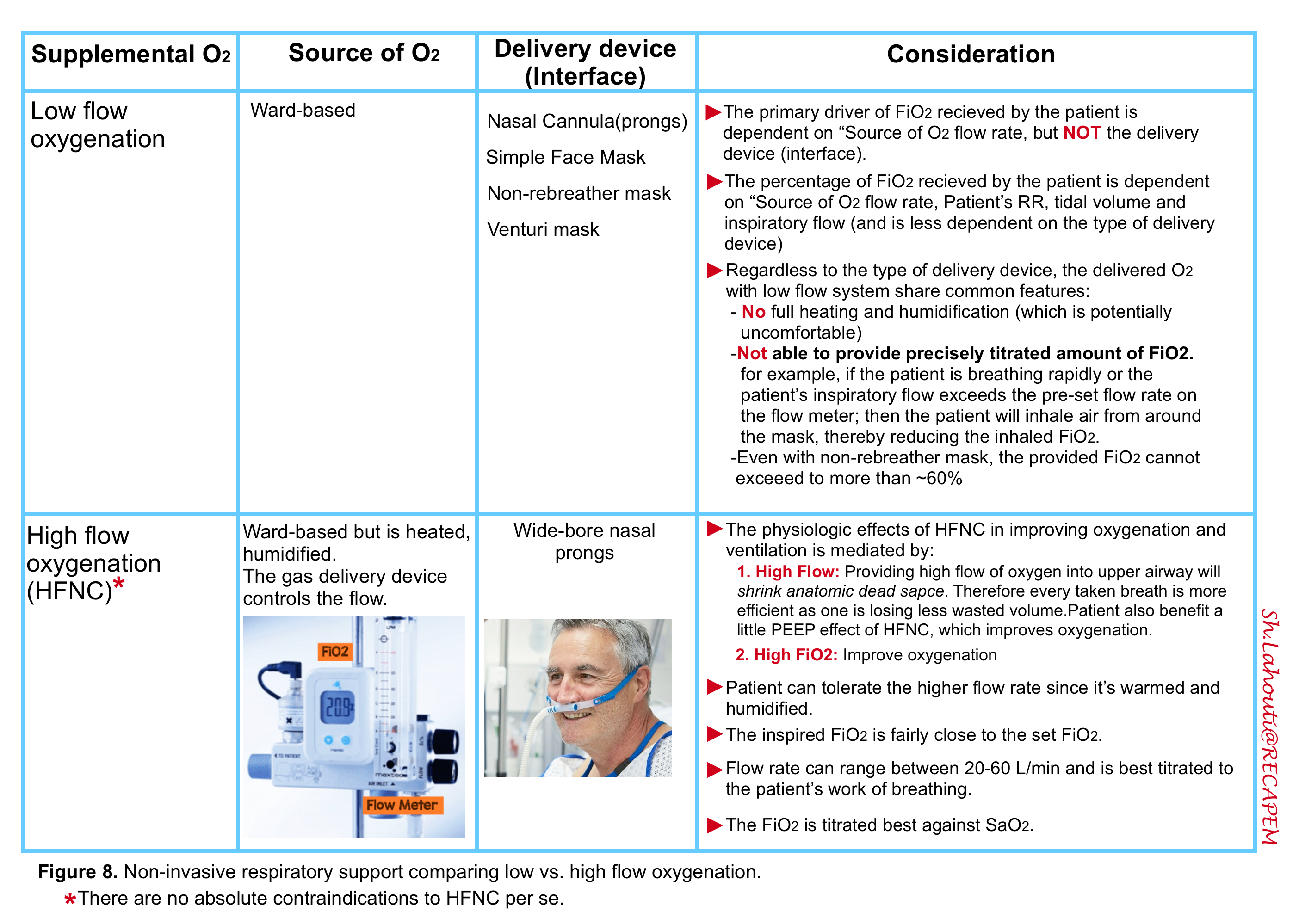
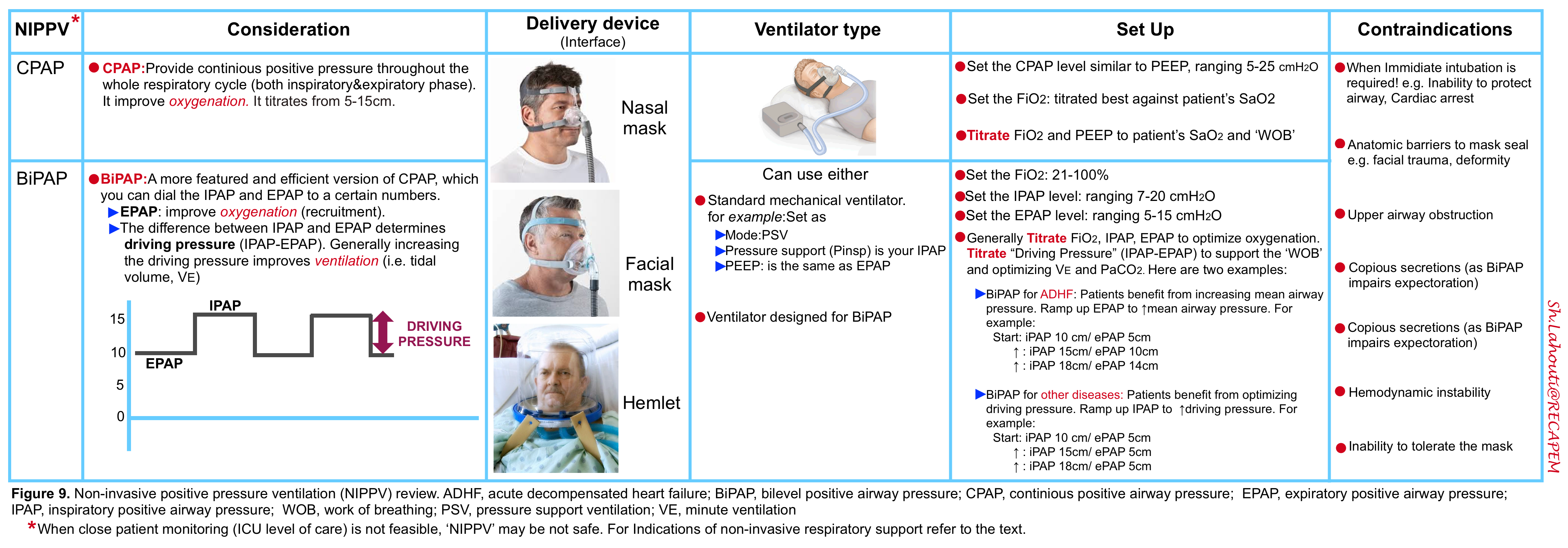
The non-invasive respiratory support refers to application of CPAP, BiPAP (video below) and HFNC. In the following tables (figure 8,9) the major features of each method are summarized.
The principles of applying non-invasive respiratory support include:
👉The goal is to borrow time for other therapies to work while supporting oxygenation (SaO2>88%) and/or ventilation (improving work of breathing and making patients more comfortable).
👉When close monitoring of the patient is not feasible, then these modalities are unsafe. The goal of monitoring the patients while being on non-invasive support is to determine and track the following parameters:
- Airway protection and risk of aspiration
- Ongoing improvement in work of breathing
- By evaluating use of accessory muscles of respiration, intercostal retraction, respiratory rate and pattern, tripoding and abdominal paradoxical breathing pattern.
- Mental status (a patient who is arousable and mentating does NOT have critical hypercapnia)
- BiPAP monitor: Trend the tidal volume and minute ventilation. As discussed here; read the upper bar on the ventilator screen to see what is actually happening. If the delivered tidal volume and minute ventilation is low (for each individual patient), it is a worrisome sign suggestive for hypoventilation.
- Oxygen saturation (monitored by pulse oximetry is appropriate if it shows good waveform).
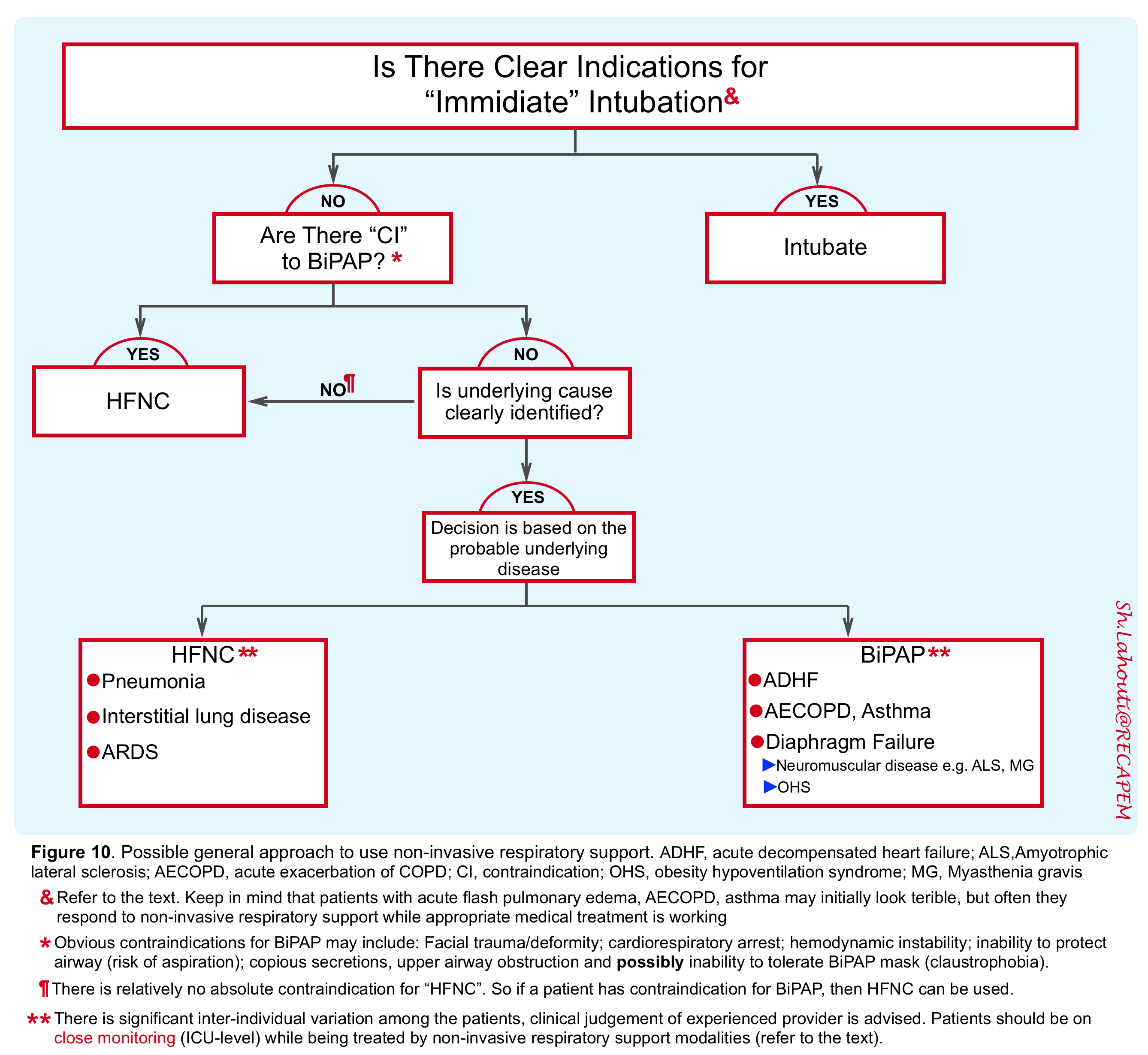
👉In the presence of clear indications for immediate intubation or presence of certain contraindications for use of “BiPAP, obviously non-invasive respiratory support should not be practiced (see figure 10).
👉If non-invasive respiratory supports were indicated, the selection of possible modalities mostly depends on the patient’s underlying disease rather than level of blood gases (figure 10).
- Keep in mind that neither invasive mechanical ventilation nor non-invasive support works for patients whose respiratory distress is primarily caused by pleural disease (e.g. pneumothorax or massive pleural effusion) or pericardial disease (e.g tamponade).These should be drained.
- Several studies have shown the clear benefit of BiPAP for patients with ADHF 58, Bronchospasm ( AECOPD, Asthma) 59 and ventilatory failure secondary to respiratory muscle fatigue (weak bellows) such as neuromuscular weakness in ALS, MG, or obesity hypoventilation syndrome.
- For patients with primary parenchymal lung disease such as pneumonia, ILD and ARDS; an initial trial of HFNC is reasonable 60 61 62.
- The practice of noninvasive respiratory support in patients whose respiratory failure is likely due to decreased central respiratory drive such as patients with opioid intoxication is not safe and not recommended.
Invasive Mechanical Ventilation
if the patient is not an appropriate candidate for non-invasive respiratory support or the patient’s respiratory and hemodynamic status are deteriorating on non-invasive respiratory treatment, then intubation and mechanical ventilation is considered.
The principles of mechanical ventilation and initial set up have been discussed here. For the purpose of this discussion, it suffices to say that practicing protective lung strategy and applying best individualized PEEP level are essential for patients with hypoxic respiratory failure. As elucidated in this post, a subset of patients with hypoxic respiratory failure will improve during initial 12-24hrs of optimization on mechanical ventilator (If appropriate PEEP level was set to account for atelectasia, pleural effusion drained if deemed significant, and other supportive therapies were appropriately administered). These patients are called rapidly resolving ARDS (aka. pseudoARDS) and do not require further aggressive interventions and treatments (according to PROSEVA trial).
On the other hand some patients with more severe underlying lung problems do not satisfactorily respond to the all taken measures (P/F ratio remains consistently < 150). These patients (i.e. TRUE ARDS) are candidates for prone positioning (as per PROSEVA trial 49) and/or other more aggressive interventions and treatments such as recruitment maneuver, inhaled pulmonary vasodilators and other possible pharmacologic treatments.
Options for non-responding patients
Recruitment
Recruitment refers to the concept that positive pressure can be used to open up collapsed alveolar units 63. It can be achieved by various methods such as prone positioning or applying PEEP level.
Recruitment maneuver may be performed as an independent maneuver (e.g. for a patient who has been disconnected from mechanical ventilator for a while as during tubing changes or transport) or it can be performed as part of open lung ventilation strategy (referring to using low tidal volume and high level of PEEP).
As of this time, there is no unified definition and agreement in terms of time frame and cycling of recruitment maneuver. The high PEEP level can be used as either of the following methods:
- Immediate recruitment: Denotes using a very high level of PEEP (e.g. 35 cmH2O) for a very brief time period (e.g. 40seconds). The optimal timing and frequency are also unknown.
- Gradual recruitment: Manipulation of PEEP or airway pressures causes an improvement over several hours (which sounds more physiologic).
For the purpose of this discussion, prone positioning is explored here. The physiology of proning is largely based on the following factors 64:
- Recruitment of posterior lung region which often become atelectatic
- Improved ventilation/perfusion matching
- Improved secretion clearance
Patients who are either awake (not intubated) 65 or those who are intubated and mechanically ventilated (as per PROSEVA trial) may benefit from proning under intensive monitoring. 👉Example for the former condition may include patients with COVID-19 pneumonia (isolated hypoxic respiratory failure) who are otherwise doing fine on non-invasive respiratory support (e.g. HFNC). Such reasonable candidates should meet the following criteria 66 :
- Absence of hemodynamic instability or multiorgan failure
- Normal mental status (being able to communicate their distress)
- Presuming that patient has a reversible lung injury (that could be easily recruited)
- No hypercapnia or substantial dyspnea
- No anticipation of difficult airway (because proning could delay intubation until the patient has deteriorated further and is more profoundly hypoxemic.Such a delayed intubation could increase the risk of peri-intubation desaturation.In order to avoid this, close monitoring could be used with prompt intubation if the patient were continuing to decline.
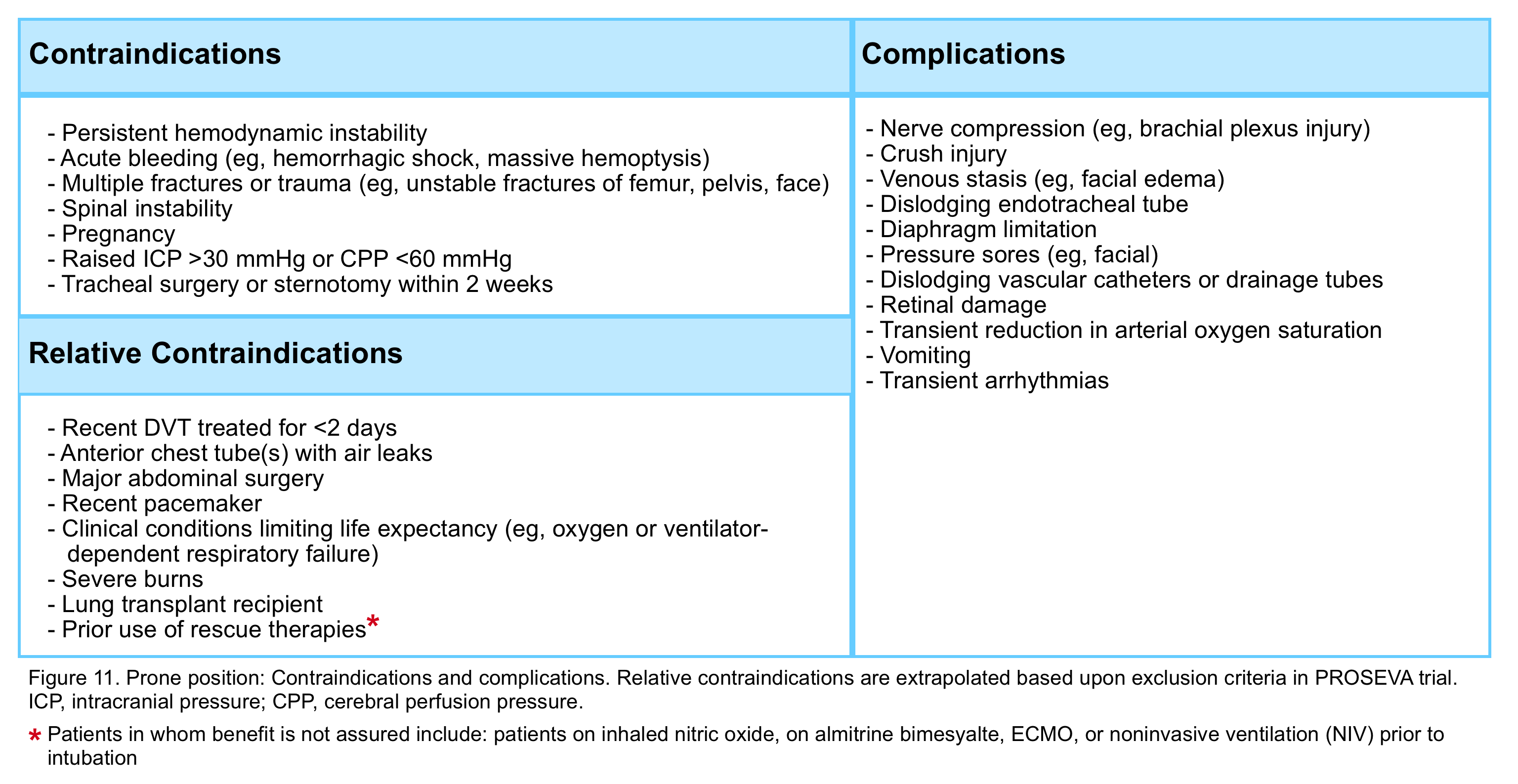
In patients who are intubated and mechanically ventilated, proning may be indicated provided that following criteria are met 49:
- Persistent P/F ratio < 150 despite 12-24hrs of optimization on mechanical ventilation
- Absence of following contraindication (figure 11)
Pharmacologic agents
Patients with moderate-severe ARDS may require other treatments and intervention in addition to ventilator optimization.
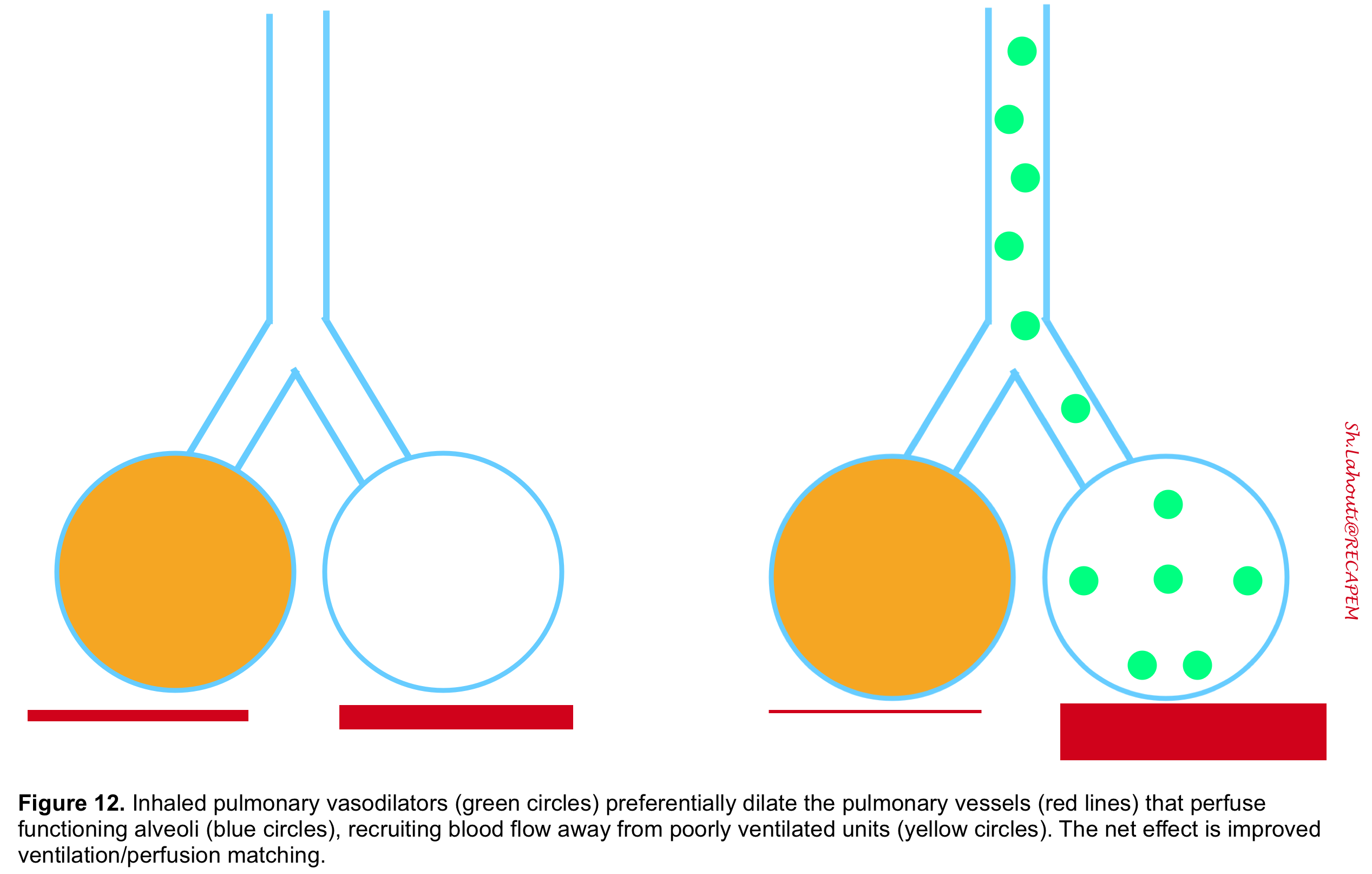
Pulmonary vasodilators: In the context of ARDS, Inhaled vasodilators (e.g. NO, nitroglycerin, prostacyclin, prostaglandin E1) selectively dilate the vessels that perfuse the well-ventilated lung zones, resulting in improved oxygenation due to better ventilation/perfusion (V/Q) matching and the amelioration of pulmonary hypertension 67 (figure 12). These medications have few systemic effects and rarely cause systemic hypotension since they act locally and have short half-lives.
It is worth mentioning here, other physiologic effects of pulmonary vasodilators that some patients may potentially benefit from it.
- These agents reduce pulmonary vascular resistance, hence reduce the right ventricle afterload. This physiologic effect is beneficial for patients with pulmonary hypertension and/or impaired RV function (such as massive pulmonary embolism).
- Patients with intracardiac right-to-left shunt (e.g. eisenmenger physiology such as a patient with pulmonary hypertension and a patent foramen ovale) potentially benefit from RV afterload reducing effect of pulmonary vasodilators.
For more on select agents and dosing see here.
Steroids: Glucocorticoid may be considered for patients with persistent moderate to severe ARDS (P/F ratio <200) who are relatively early in the disease course (e.g. within 14 days of onset) and who fail standard therapies including low tidal volume ventilation.
Glucocorticoid administration is not recommended for the following conditions:
- Patients with less severe ARDS
- Very late in the disease course
- Immunocompromised patients
- patients with certain viral infections like ARDS secondary to influenza 68
When indicated; possible regimen may include dexamethasone 20 mg IV once daily for five days, then 10 mg once daily for five days 69 ; however the efficacy of different regimens appears to be similar 70.
👉Glucocorticoid for COVID-19 patients: based on RECOVERY trial 71; a mortality benefit using dexamethasone 6 mg daily for up to 10 days among hospitalized patients requiring supplemental O2 or mechanical ventilation was shown. Indications for steroid include the following:
- Acute hypoxemic respiratory failure (true increase in oxygen requirement compared to baseline)
- Requirement for mechanical ventilation
- Another accepted indication for steroid (e.g. COVID plus asthma or COPD exacerbation)
For patients with COVID-19 infection who have normal oxygenation, or are early in the course of infection, steroid administration is not recommended.
Other therapeutic aspect:
As has been explained before, the hypoxemia in ARDS is the function of four variables among which pulmonary shunting and possible treatment strategies (e.g. PEEP, proning, etc) have been discussed. However other contributing factors to systemic oxygenation can be optimized in patient with non-responding ARDS including:
- Increasing (optimizing) cardiac output
- Increasing Hb concentration (if it is reduced)
- Decreasing systemic oxygen consumption (e.g. temperature control)
Supportive care
Patients with ARDS require meticulous supportive care including hemodynamic management, intelligent use of sedatives and analgesics, prophylaxis against GI bleeding and deep vein thrombosis, blood glucose control, nutritional support and directed treatment for the likely underlying disease (e.g. antibiotic for pneumonia). In this post, the principles of adequate pain control and sedation are reviewed.
Endotracheal tube, intravascular catheter, suctioning, immobility and other procedures done for critically ill patients are painful. Pain potentiates vicious cycles of delirium and also interrupts the ease of lung protective ventilation employment. Poor sedation strategy (from the very early moment of postintubation in ED) has been shown to be related with delirium in ICU and delirium associates with adverse outcome in critically ill patients 72 73.
It is important to remind you that putting the patient into sleep does not eliminate the pain, hence appropriate use of analgesics has priority over the use of sedatives 74.
Important factors to achieve this goal are:
- Considering each individual patient’s physiology and comorbidities.
- Knowledge of pharmacokinetics and pharmacodynamics of various agents that are commonly used.
- Frequent bedside assessment for sedation adequacy (as by using a validated sedation scale such as Richmond Agitation-Sedation Scale 75). Download here.
- Protocolised use of sedatives and analgesics.
Download the protocol for management of pain, agitation and delirium (PAD) in mechanically ventilated patients here.
Going further:
- Physiology:
- P/F ratio and Shunt (LITFL)
- ARDS Definition
- First Read This Article! here
- ARDS vs. PseudoARDS (PulmCrit)
- ARDS Physiology: Oxygenation goal (PulmCrit)
- I don’t like the Berlin ARDS definition (IFA)
- NIV:
- Noninvasive Respiratory Support (IBCC)
- Mastering the dark arts of BiPAP & HFNC (Pulmcrit)
- MV:
- Driving Pressure (EMCrit)
- Proning:
- Proning the non-intubated patient (Pulmcrit)
- The Logistics of Proning for ARDS(EMCrit)
- APRV:
- The APRV Part 1 and part 2 (EMCrit)
- APRV Guideline (PulmCrit)
- APRV: Resurrection of the open-lung strategy? (Pulmcrit)
- Inhaled pulmonary vasodilators
- Inhaled Pulmonary Vasodilator(IBCC)
- COVID-19:
- A Theoretical Model of the Pathophysiology of COVID-19 with Farid Jalali (EmCrit)
- Understanding happy hypoxemia physiology(PulmCrit)
- COVID-19 (IBCC)
- Defining ARDS & recruitability, with implications for COVID treatment(PulmCrit))
- Is COVID-19 ARDS, pseudoARDS, L, or H? (PulmCrit)
- Guide to APRV for COVID (IBCC)
Post Peer Reviewed By: Mojtaba Chardoli. MD, Darab Zohri. MD
References :
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967 Aug 12;2(7511):319-23. doi: 10.1016/s0140-6736(67)90168-7. PMID: 4143721
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994 Mar;149(3 Pt 1):818-24. doi: 10.1164/ajrccm.149.3.7509706. PMID: 7509706
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012 Jun 20;307(23):2526-33. doi: 10.1001/jama.2012.5669. PMID: 22797452.
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994 Mar;149(3 Pt 1):818-24. doi: 10.1164/ajrccm.149.3.7509706. PMID: 7509706
- Leach RM, Treacher DF. The pulmonary physician in critical care * 2: oxygen delivery and consumption in the critically ill. Thorax. 2002;57(2):170-177. doi:10.1136/thorax.57.2.170
- Petersson J, Glenny RW. Gas exchange and ventilation-perfusion relationships in the lung. Eur Respir J. 2014 Oct;44(4):1023-41. doi: 10.1183/09031936.00037014. Epub 2014 Jul 25. PMID: 25063240.
- Bickler PE, Feiner JR, Lipnick MS, McKleroy W. “Silent” Presentation of Hypoxemia and Cardiorespiratory Compensation in COVID-19 [published online ahead of print, 2020 Sep 24]. Anesthesiology. 2020;10.1097/ALN.0000000000003578. doi:10.1097/ALN.0000000000003578
- Dhont, S., Derom, E., Van Braeckel, E. et al. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir Res 21, 198 (2020). https://doi.org/10.1186/s12931-020-01462-5
- Karmouty-Quintana H, Thandavarayan RA, Keller SP, Sahay S, Pandit LM, Akkanti B. Emerging Mechanisms of Pulmonary Vasoconstriction in SARS-CoV-2-Induced Acute Respiratory Distress Syndrome (ARDS) and Potential Therapeutic Targets. Int J Mol Sci. 2020 Oct 29;21(21):8081. doi: 10.3390/ijms21218081. PMID: 33138181; PMCID: PMC7662604
- Villar J, Pérez-Méndez L, López J, Belda J, Blanco J, Saralegui I, Suárez-Sipmann F, López J, Lubillo S, Kacmarek RM; HELP Network. An early PEEP/FIO2 trial identifies different degrees of lung injury in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007 Oct 15;176(8):795-804. doi: 10.1164/rccm.200610-1534OC. Epub 2007 Jun 21. PMID: 17585106
- Grasso S, Fanelli V, Cafarelli A, Anaclerio R, Amabile M, Ancona G, Fiore T. Effects of high versus low positive end-expiratory pressures in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005 May 1;171(9):1002-8. doi: 10.1164/rccm.200407-940OC. Epub 2005 Jan 21. PMID: 15665322
- Fischer LG, Freise H, Hilpert JH, Wendholt D, Lauer S, Van Aken H, Sielenkämper AW. Modulation of hypoxic pulmonary vasoconstriction is time and nitric oxide dependent in a peritonitis model of sepsis. Intensive Care Med. 2004 Sep;30(9):1821-8. doi: 10.1007/s00134-004-2351-0. Epub 2004 Jun 15. PMID: 15375651
- Brimioulle S, Lejeune P, Vachiery JL, Leeman M, Melot C, Naeije R. Effects of acidosis and alkalosis on hypoxic pulmonary vasoconstriction in dogs. Am J Physiol. 1990 Feb;258(2 Pt 2):H347-53. doi: 10.1152/ajpheart.1990.258.2.H347. PMID: 2309902
- Moloney ED, Evans TW. Pathophysiology and pharmacological treatment of pulmonary hypertension in acute respiratory distress syndrome. Eur Respir J. 2003 Apr;21(4):720-7. doi: 10.1183/09031936.03.00120102. PMID: 12762363
- Rossaint R, Gerlach H, Falke KJ. Inhalation of nitric oxide–a new approach in severe ARDS. Eur J Anaesthesiol. 1994 Jan;11(1):43-51. PMID: 8143713.
- Gattinoni L, Busana M, Giosa L, Macrì MM, Quintel M. Prone Positioning in Acute Respiratory Distress Syndrome. Semin Respir Crit Care Med. 2019 Feb;40(1):94-100. doi: 10.1055/s-0039-1685180. Epub 2019 May 6. PMID: 31060091.
- Kallet RH. A Comprehensive Review of Prone Position in ARDS. Respir Care. 2015 Nov;60(11):1660-87. doi: 10.4187/respcare.04271. PMID: 26493592
- Takala J. Hypoxemia due to increased venous admixture: influence of cardiac output on oxygenation. Intensive Care Med. 2007 May;33(5):908-911. doi: 10.1007/s00134-007-0546-x. Epub 2007 Mar 7. PMID: 17342520
- Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, Wheeler AP; NHLBI Acute Respiratory Distress Syndrome Clinical Trials Network. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005 Jan;33(1):1-6; discussion 230-2. doi: 10.1097/01.ccm.0000149854.61192.dc. PMID: 15644641
- Windsor AC, Mullen PG, Fowler AA, Sugerman HJ. Role of the neutrophil in adult respiratory distress syndrome. Br J Surg. 1993 Jan;80(1):10-7. doi: 10.1002/bjs.1800800106. PMID: 8428262
- Katzenstein AL, Myers JL, Mazur MT. Acute interstitial pneumonia. A clinicopathologic, ultrastructural, and cell kinetic study. Am J Surg Pathol. 1986 Apr;10(4):256-67. PMID: 3706612.
- Hughes KT, Beasley MB. Pulmonary Manifestations of Acute Lung Injury: More Than Just Diffuse Alveolar Damage. Arch Pathol Lab Med. 2017 Jul;141(7):916-922. doi: 10.5858/arpa.2016-0342-RA. Epub 2016 Sep 21. PMID: 27652982.
- International consensus conferences in intensive care medicine: Ventilator-associated Lung Injury in ARDS. This official conference report was cosponsored by the American Thoracic Society, The European Society of Intensive Care Medicine, and The Societé de Réanimation de Langue Française, and was approved by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 1999 Dec;160(6):2118-24. doi: 10.1164/ajrccm.160.6.ats16060. PMID: 10588637
- Guerin C, Bayle F, Leray V, Debord S, Stoian A, Yonis H, Roudaut JB, Bourdin G, Devouassoux-Shisheboran M, Bucher E, Ayzac L, Lantuejoul S, Philipponnat C, Kemeny JL, Souweine B, Richard JC. Open lung biopsy in nonresolving ARDS frequently identifies diffuse alveolar damage regardless of the severity stage and may have implications for patient management. Intensive Care Med. 2015 Feb;41(2):222-30. doi: 10.1007/s00134-014-3583-2. Epub 2014 Dec 5. Erratum in: Intensive Care Med. 2015 Feb;41(2):387. PMID: 25476984
- Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005 Aug;11(8):875-9. doi: 10.1038/nm1267. Epub 2005 Jul 10. PMID: 16007097; PMCID: PMC7095783
- Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004 Jun;203(2):631-7. doi: 10.1002/path.1570. PMID: 15141377; PMCID: PMC7167720
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 Apr;8(4):420-422. doi: 10.1016/S2213-2600(20)30076-X. Epub 2020 Feb 18. Erratum in: Lancet Respir Med. 2020 Feb 25;: PMID: 32085846; PMCID: PMC7164771.
- Copin MC, Parmentier E, Duburcq T, Poissy J, Mathieu D; Lille COVID-19 ICU and Anatomopathology Group. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 2020 Jun;46(6):1124-1126. doi: 10.1007/s00134-020-06057-8. Epub 2020 Apr 23. PMID: 32328726; PMCID: PMC7178098
- Hwang, D., Chamberlain, D., Poutanen, S. et al. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod Pathol 18, 1–10 (2005). https://doi.org/10.1038/modpathol.3800247
- Lilly CM, Khan S, Waksmundzki-Silva K, Irwin RS. Vaping-Associated Respiratory Distress Syndrome: Case Classification and Clinical Guidance. Crit Care Explor. 2020;2(2):e0081. Published 2020 Feb 24. doi:10.1097/CCE.0000000000000081
- Villar J. What is the acute respiratory distress syndrome? Respir Care. 2011 Oct;56(10):1539-45. doi: 10.4187/respcare.01395. PMID: 22008395
- Piantadosi CA, Schwartz DA. The acute respiratory distress syndrome. Ann Intern Med. 2004 Sep 21;141(6):460-70. doi: 10.7326/0003-4819-141-6-200409210-00012. PMID: 15381520.
- Radermacher P, Maggiore SM, Mercat A. Fifty Years of Research in ARDS. Gas Exchange in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2017 Oct 15;196(8):964-984. doi: 10.1164/rccm.201610-2156SO. Erratum in: Am J Respir Crit Care Med. 2017 Dec 15;196(12):1619. PMID: 28406724.
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000 May 4;342(18):1334-49. doi: 10.1056/NEJM200005043421806. PMID: 10793167
- Roupie E, D’ambrosio M, Servillo G, Mentec H, el Atrous S, Beydon L, Brun-Buisson C, Lemaire F, Brochard L. Titration of tidal volume and induced hypercapnia in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995 Jul;152(1):121-8. doi: 10.1164/ajrccm.152.1.7599810. PMID: 7599810
- Vieillard-Baron A, Schmitt JM, Augarde R, Fellahi JL, Prin S, Page B, Beauchet A, Jardin F. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis. Crit Care Med. 2001 Aug;29(8):1551-5. doi: 10.1097/00003246-200108000-00009. Erratum in: Crit Care Med 2002 Mar;30(3):726. PMID: 11505125
- Monchi M, Bellenfant F, Cariou A, Joly LM, Thebert D, Laurent I, Dhainaut JF, Brunet F. Early predictive factors of survival in acute respiratory distress syndrome. A multivariate analysis. Am J Respir Crit Care Med. 1998 Oct;158(4):1076-81. doi: 10.1164/ajrccm.158.4.9802009. PMID: 9769263
- Price LC, Wort SJ. Pulmonary hypertension in ARDS: inflammation matters! Thorax. 2017 May;72(5):396-397. doi: 10.1136/thoraxjnl-2016-209199. Epub 2017 Feb 23. PMID: 28232508
- Moloney ED, Evans TW. Pathophysiology and pharmacological treatment of pulmonary hypertension in acute respiratory distress syndrome. Eur Respir J. 2003 Apr;21(4):720-7. doi: 10.1183/09031936.03.00120102. PMID: 12762363
- Chiumello D, Umbrello M, Sferrazza Papa GF, Angileri A, Gurgitano M, Formenti P, Coppola S, Froio S, Cammaroto A, Carrafiello G. Global and Regional Diagnostic Accuracy of Lung Ultrasound Compared to CT in Patients With Acute Respiratory Distress Syndrome. Crit Care Med. 2019 Nov;47(11):1599-1606. doi: 10.1097/CCM.0000000000003971. Erratum in: Crit Care Med. 2020 Mar;48(3):e268. PMID: 31464770
- Villar J, Pérez-Méndez L, Kacmarek RM. The Berlin definition met our needs: no. Intensive Care Med. 2016 May;42(5):648-650. doi: 10.1007/s00134-016-4242-6. Epub 2016 Mar 23. PMID: 27007104
- Villar J, Kacmarek RM, Guérin C. Clinical trials in patients with the acute respiratory distress syndrome: burn after reading. Intensive Care Med. 2014 Jun;40(6):900-2. doi: 10.1007/s00134-014-3288-6. Epub 2014 Apr 10. PMID: 24718644
- Schenck EJ, Oromendia C, Torres LK, Berlin DA, Choi AMK, Siempos II. Rapidly Improving ARDS in Therapeutic Randomized Controlled Trials. Chest. 2019 Mar;155(3):474-482. doi: 10.1016/j.chest.2018.09.031. Epub 2018 Oct 22. PMID: 30359616; PMCID: PMC6414787
- Broccard, Alain. (2013). Making sense of the PaO2/FIO2 ratio in patients with acute respiratory distress syndrome.. Open Access Critical Care. 1. 9.
- Villar J, Blanco J, del Campo R , et al. Assessment of PaO2/FiO2 for stratification of patients with moderate and severe acute respiratory distress syndrome.BMJ Open 2015;5:e006812. doi: 10.1136/bmjopen-2014-006812
- Villar J, Pérez-Méndez L, Blanco J, Añón JM, Blanch L, Belda J, Santos-Bouza A, Fernández RL, Kacmarek RM; Spanish Initiative for Epidemiology, Stratification, and Therapies for ARDS (SIESTA) Network. A universal definition of ARDS: the PaO2/FiO2 ratio under a standard ventilatory setting–a prospective, multicenter validation study. Intensive Care Med. 2013 Apr;39(4):583-92. doi: 10.1007/s00134-012-2803-x. Epub 2013 Jan 31. PMID: 23370826
- Villar J, Kacmarek RM, Guérin C. Clinical trials in patients with the acute respiratory distress syndrome: burn after reading. Intensive Care Med. 2014 Jun;40(6):900-2. doi: 10.1007/s00134-014-3288-6. Epub 2014 Apr 10. PMID: 24718644
- Villar J, Pérez-Méndez L, López J, Belda J, Blanco J, Saralegui I, Suárez-Sipmann F, López J, Lubillo S, Kacmarek RM; HELP Network. An early PEEP/FIO2 trial identifies different degrees of lung injury in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007 Oct 15;176(8):795-804. doi: 10.1164/rccm.200610-1534OC. Epub 2007 Jun 21. PMID: 17585106
- PERSOVA: Guérin C, Reignier J, Richard JC, Beuret P, Gacoin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013 Jun 6;368(23):2159-68. doi: 10.1056/NEJMoa1214103. Epub 2013 May 20. PMID: 23688302
- Schrier RW, Abraham E. Aggressive volume expansion and pseudo-ARDS. Hosp Pract (1995). 1995 Jun 15;30(6):19, 23. doi: 10.1080/21548331.1995.11443205. PMID: 7782392
- GlueckerT, Capasso P, Schnyder P, Guichet F, Schaller MD, Revelly JP, Chiolero R, Vock P, Wicky S. Clinical and radiologic features of pulmonary edema. Radiographics. 1999 Nov-Dec;19(6):1507-31; discussion 1532-3. doi: 10.1148/radiographics.19.6.g99no211507. PMID: 10555672
- Frost N, Ruwwe-Glösenkamp C, Raspe M, Brünger M, Temmesfeld-Wollbrück B, Suttorp N, Witzenrath M. Indwelling pleural catheters for non-malignant pleural effusions: report on a single centre’s 10 years of experience. BMJ Open Respir Res. 2020 Jan;7(1):e000501. doi: 10.1136/bmjresp-2019-000501. PMID: 31958272; PMCID: PMC7011895
- Thomas R, Jenkins S, Eastwood PR, Lee YC, Singh B. Physiology of breathlessness associated with pleural effusions. Curr Opin Pulm Med. 2015 Jul;21(4):338-45. doi: 10.1097/MCP.0000000000000174. PMID: 25978627; PMCID: PMC5633324
- DeBiasi EM, Feller-Kopman D. Physiologic Basis of Symptoms in Pleural Disease. Semin Respir Crit Care Med. 2019 Jun;40(3):305-313. doi: 10.1055/s-0039-1693648. Epub 2019 Sep 16. PMID: 31525806
- Magnusson L, Spahn DR. New concepts of atelectasis during general anaesthesia. Br J Anaesth. 2003 Jul;91(1):61-72. doi: 10.1093/bja/aeg085. PMID: 12821566
- Khan AN, Al-Jahdali H, Al-Ghanem S, Gouda A. Reading chest radiographs in the critically ill (Part II): Radiography of lung pathologies common in the ICU patient. Ann Thorac Med. 2009 Jul;4(3):149-57. doi: 10.4103/1817-1737.53349. PMID: 19641649; PMCID: PMC2714572
- Bentz MR, Primack SL. Intensive care unit imaging. Clin Chest Med. 2015 Jun;36(2):219-34, viii. doi: 10.1016/j.ccm.2015.02.006. Epub 2015 Apr 1. PMID: 26024601
- Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, Navalesi P Members Of The Steering Committee, Antonelli M, Brozek J, Conti G, Ferrer M, Guntupalli K, Jaber S, Keenan S, Mancebo J, Mehta S, Raoof S Members Of The Task Force. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017 Aug 31;50(2):1602426. doi: 10.1183/13993003.02426-2016. PMID: 28860265
- Bond KR, Horsley CA, Williams AB. Non-invasive ventilation use in status asthmaticus: 16 years of experience in a tertiary intensive care. Emerg Med Australas. 2018 Apr;30(2):187-192. doi: 10.1111/1742-6723.12876. Epub 2017 Nov 12. PMID: 29131536
- Ou X, Hua Y, Liu J, Gong C, Zhao W. Effect of high-flow nasal cannula oxygen therapy in adults with acute hypoxemic respiratory failure: a meta-analysis of randomized controlled trials. CMAJ. 2017 Feb 21;189(7):E260-E267. doi: 10.1503/cmaj.160570. PMID: 28246239; PMCID: PMC5318211.
- Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, Prat G, Boulain T, Morawiec E, Cottereau A, Devaquet J, Nseir S, Razazi K, Mira JP, Argaud L, Chakarian JC, Ricard JD, Wittebole X, Chevalier S, Herbland A, Fartoukh M, Constantin JM, Tonnelier JM, Pierrot M, Mathonnet A, Béduneau G, Delétage-Métreau C, Richard JC, Brochard L, Robert R; FLORALI Study Group; REVA Network. High-flow oxygen through the nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015 Jun 4;372(23):2185-96. doi: 10.1056/NEJMoa1503326. Epub 2015 May 17. PMID: 25981908
- Messika J, Ben Ahmed K, Gaudry S, Miguel-Montanes R, Rafat C, Sztrymf B, Dreyfuss D, Ricard JD. Use of High-Flow Nasal Cannula Oxygen Therapy in Subjects With ARDS: A 1-Year Observational Study. Respir Care. 2015 Feb;60(2):162-9. doi: 10.4187/respcare.03423. Epub 2014 Nov 4. PMID: 25371400.
- Recruitment Maneuvers and PEEP Titration, Dean R Hess, Respiratory Care Nov 2015, 60 (11) 1688-1704; DOI: 10.4187/respcare.04409
- Gattinoni L, Busana M, Giosa L, Macrì MM, Quintel M. Prone Positioning in Acute Respiratory Distress Syndrome. Semin Respir Crit Care Med. 2019 Feb;40(1):94-100. doi: 10.1055/s-0039-1685180. Epub 2019 May 6. PMID: 31060091
- Ding, L., Wang, L., Ma, W. et al. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care 24, 28 (2020). https://doi.org/10.1186/s13054-020-2738-5
- Awake Prone Positioning for Non-intubated Oxygen Dependent COVID-19 Pneumonia Patients, Ziqin Ng, Woo Chiao Tay, Choon Heng Benjamin Ho. European Respiratory Journal Jan 2020, 2001198; DOI: 10.1183/13993003.01198-2020
- Moloney ED, Evans TW. Pathophysiology and pharmacological treatment of pulmonary hypertension in acute respiratory distress syndrome. Eur Respir J. 2003 Apr;21(4):720-7. doi: 10.1183/09031936.03.00120102. PMID: 12762363
- Rodrigo C, Leonardi-Bee J, Nguyen-Van-Tam J, Lim WS. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2016 Mar 7;3:CD010406. doi: 10.1002/14651858.CD010406.pub2. Update in: Cochrane Database Syst Rev. 2019 Feb 24;2:CD010406. PMID: 26950335
- Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, Aguilar G, Alba F, González-Higueras E, Conesa LA, Martín-Rodríguez C, Díaz-Domínguez FJ, Serna-Grande P, Rivas R, Ferreres J, Belda J, Capilla L, Tallet A, Añón JM, Fernández RL, González-Martín JM; dexamethasone in ARDS network. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020 Mar;8(3):267-276. doi: 10.1016/S2213-2600(19)30417-5. Epub 2020 Feb 7. PMID: 32043986.
- Lewis SR, Pritchard MW, Thomas CM, Smith AF. Pharmacological agents for adults with acute respiratory distress syndrome. Cochrane Database Syst Rev. 2019 Jul 23;7(7):CD004477. doi: 10.1002/14651858.CD004477.pub3. PMID: 31334568; PMCID: PMC6646953
- RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19 – Preliminary Report. N Engl J Med. 2020 Jul 17:NEJMoa2021436. doi: 10.1056/NEJMoa2021436. Epub ahead of print. PMID: 32678530; PMCID: PMC7383595
- Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr, Inouye SK, Bernard GR, Dittus RS. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004 Apr 14;291(14):1753-62. doi: 10.1001/jama.291.14.1753. PMID: 15082703
- Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009 Dec 1;180(11):1092-7. doi: 10.1164/rccm.200904-0537OC. Epub 2009 Sep 10. PMID: 19745202; PMCID: PMC2784414
- Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, Watson PL, Weinhouse GL, Nunnally ME, Rochwerg B, Balas MC, van den Boogaard M, Bosma KJ, Brummel NE, Chanques G, Denehy L, Drouot X, Fraser GL, Harris JE, Joffe AM, Kho ME, Kress JP, Lanphere JA, McKinley S, Neufeld KJ, Pisani MA, Payen JF, Pun BT, Puntillo KA, Riker RR, Robinson BRH, Shehabi Y, Szumita PM, Winkelman C, Centofanti JE, Price C, Nikayin S, Misak CJ, Flood PD, Kiedrowski K, Alhazzani W. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 2018 Sep;46(9):e825-e873. doi: 10.1097/CCM.0000000000003299. PMID: 30113379
- Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002 Nov 15;166(10):1338-44. doi: 10.1164/rccm.2107138. PMID: 12421743.





Add comment