18 December by Mojtaba Chardoli. Peer reviewed by Shahriar Lahouti. Last update 12 September 2023.
CONTENTS
- Preface
- Case scenario
- Background
- Scope of discussion
- Mitral valve apparatus
- Terminology
- Mitral regurgitation
- Differential diagnosis
- Bedside echo
- Interpretation of echo findings
- Case scenario revisited
- Management
- Multiple and Mixed Valvular Heart Disease
- Media
- Going further
- References
2D, two-dimensional
ACC,American college of cardiology
ACS, acute coronary syndrome
AF, atrial fibrillation
ASE,American Society of Echocardiography
AV, aortic valve
AR, aortic regurgitation
AS, aortic stenosis
CM, cardiomyopathy
CWD, Continuous wave Doppler
CFD, color flow Doppler
EAE, European Association of Echocardiography
EF, ejection fraction
EROA, Effective regurgitant orifice area
LA, left atrium
LAE, left atrial enlargement
LAP, left atrial pressure
LV, left ventricle
LVE, left ventricle enlargement
LVOTO, left ventricular outflow tract obstruction
MV, mitral valve
MS, mitral stenosis
MR, mitral regurgitation
MVP, mitral valve prolapse
PAP, pulmonary arterial pressure
PH, pulmonary hypertension
POCUS, point of care ultrasound
PWD, pulse wave Doppler
RA, right atrium
RV, right ventricle
TTE, transthoracic echocardiography
TEE, transesophageal echocardiography
TV, tricuspid valve
TR, tricuspid regurgitation
VHD, valvular heart disease
Preface
Mitral regurgitation (MR) is a common valvular disorder that can arise from abnormalities of any part of the mitral valve apparatus. Patients with acute and, or severe mitral regurgitation are often critically ill with significant hemodynamic abnormalities that require urgent medical and usually surgical treatment.
Case scenario
In a busy shift; a 45-year-old woman with no significant past medical history presented to the hospital with acute onset of shortness of breath and scattered cough (which she admits to cigarette smoking). Initial vitals BP 140/95mm Hg, HR 120, RR 28, SO2 91% room air. ECG was unremarkable. No fever, leg swelling, or size discrepancy was noticed. My enthusiastic resident put the patient on the typical protocol for COPD exacerbation and gave a 500cc bolus crystalloid. Two hours later her clinical condition deteriorated. She was sitting bolt upright, looked pale, and struggling for breath. Despite receiving supplemental oxygen and medications, her oxygen saturation trended down to 88%. What could have been done for this patient upon entry?
Background
Patients who present with a clinical picture of cardiorespiratory failure require an expedited and precise assessment at the bedside. In such patients, bedside echo is crucially important and can shift the diagnostic paradigm.
Evaluation of the LV and RV systolic and diastolic function, heart rate rhythm, and preload are intertwined with valvular examination.
- Since the essence of POCUS is to expeditiously identify life-threatening conditions, screening for valvular pathologies in the ED is mostly focused on identifying those valvular diseases that can put your patient’s life at risk.
The purpose of this series of discussions is to review bedside echocardiographic evaluation of adult valvular emergencies in ED. At the end of the day, you should feel comfortable in the evaluation of critical valvular lesions! 😀
Scope of the discussion
The major concern in ED is to rapidly identify life-threatening valvular lesions. Three essential questions should be kept in mind when screening for valvular emergencies in ED:
| 1. Is there a “severe” valvular dysfunction? 2. Does this explain my patient’s clinical presentation? 3. How can this finding change my management plan? |
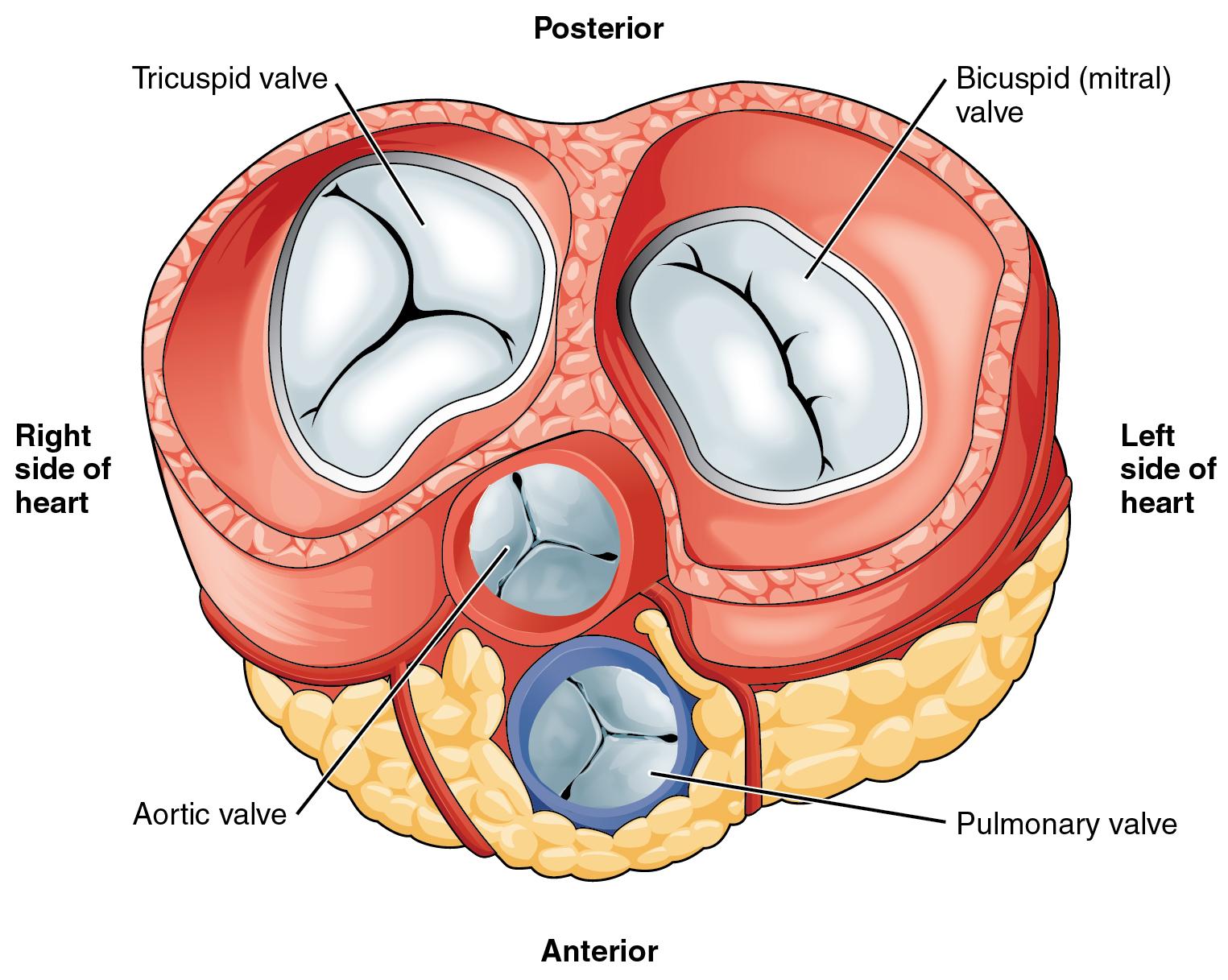
Mitral valve apparatus
Knowledge of the valve anatomy is essential for identifying valvular pathologies. The mitral valve apparatus includes *, *
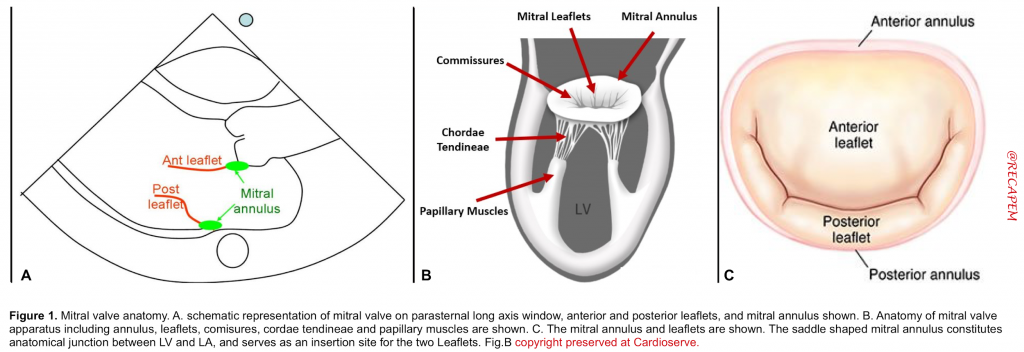
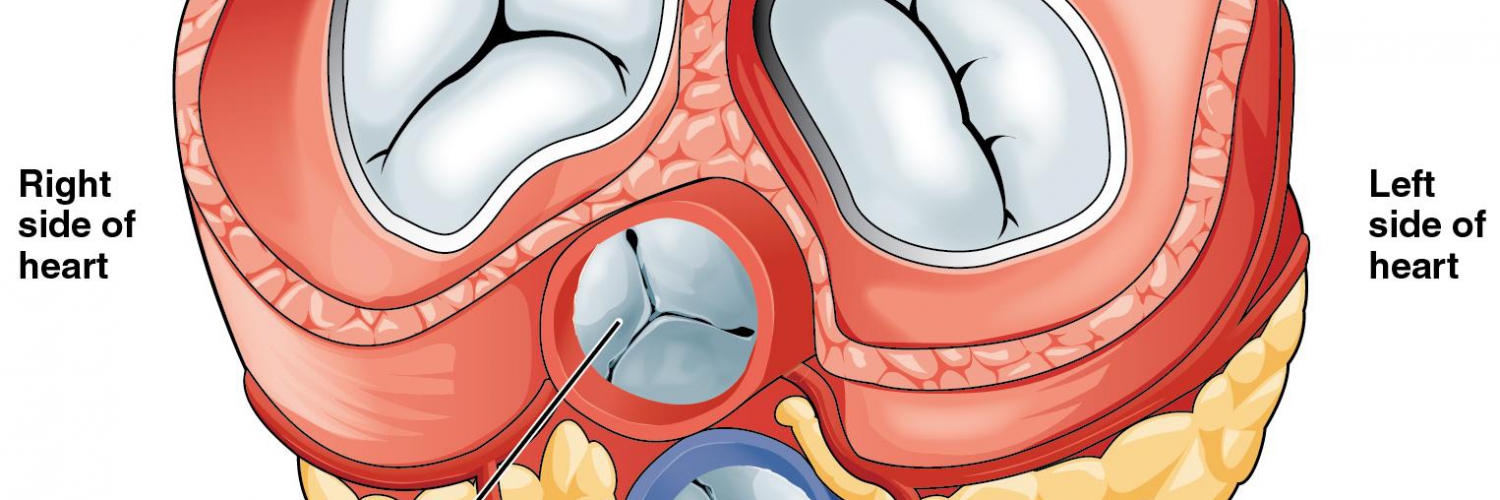
- Mitral annulus: A fibroelastic structure separating LA from LV (figure 1.C)
- Mitral leaflets: There are two thin leaflets. One is located anteriorly and is thicker and larger. The other is posteriorly located and is thinner & more flexible (figure 1.A,B)
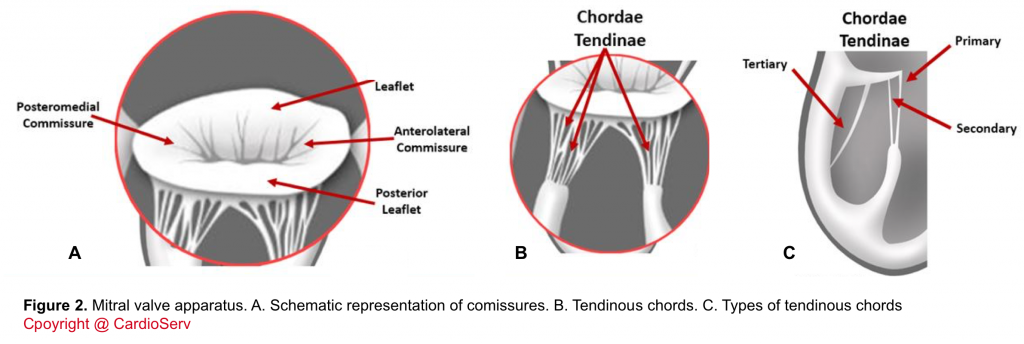
- Comissures: These are the sites where the leaflets are inserted and joined to the annulus (figure 2.A)
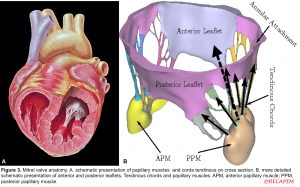
- Tendinous chords: Fibrous strings that attach specific portions of mitral leaflets to papillary muscle tips (figure 2.B,C).
- There are 3 types of chords based on the location of insertion:
- Primary (marginal): attaches at the leaflet tip. Its primary function is to maintain the coaptation of leaflets. Failure of the primary chords, either elongated which leads to leaflet prolapse, or primary chord rupture leading to flail leaflet are concerning.
- Secondary: attaches at mid-body of leaflets.
- Tertiary: attaches at the base of leaflets and provides structural support.
- There are 3 types of chords based on the location of insertion:
5. Papillary muscles (PM): Large trabeculated muscles that connect tendinous chords to the mitral leaflets (figure 3. A,B)
-
- Anterolateral PM (APM): The anatomic location is shown below. It receives a dual blood supply (LAD & Cx) and therefore is relatively resistant to ischemia.
- Posteromedial PM (PPM): It receives a single blood supply (either RCA or LCX) and is prone to injury from ischemia.
Terminology
The common terms which are frequently used in the word of valvular diseases are *, *:
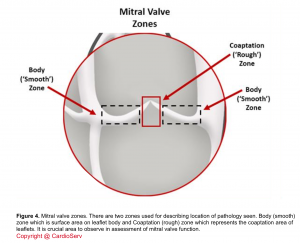
- Coaptation
- Denotes how well the leaflets come together and touch each other.
- In a healthy state, the leaflets nicely coapt and there’s no gap between them during systole. The coaptation zone on the leaflet surface shown below (figure 4).
- Poor coaptation results in regurgitation.
- Excursion
- Denotes how smoothly and freely leaflets open up well during diastole.
- Poor excursion can be seen in stenotic valvular lesions.
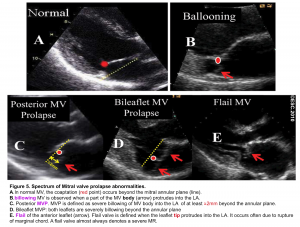
- MV billowing
- In normal MV, the leaflet tips meet each other (coaptation zone) beyond the annular plane (figure 5.A).
- Billowing MV is defined as when a part of the MV body protrudes into the LA (figure 5.B).
- MVP
- Prolapse is defined as a severe billowing of the MV (≧ 2mm) into the LA (figure 5.C). It can occur in one or both leaflets (figure 5.D).
- Flail valve
- It occurs when the leaflet edge protrudes into the LA (figure 5.E).
- Most commonly it is due to a rupture of the marginal chord so that the leaflet tip is no longer being supported and it freely moves into the LA during systole.
- The presence of a flail valve always denotes a severe regurgitation.
- A papillary muscle rupture is an extreme form of flail valve wherein both the leaflet tip and papillary muscle move freely into the LA during systole.
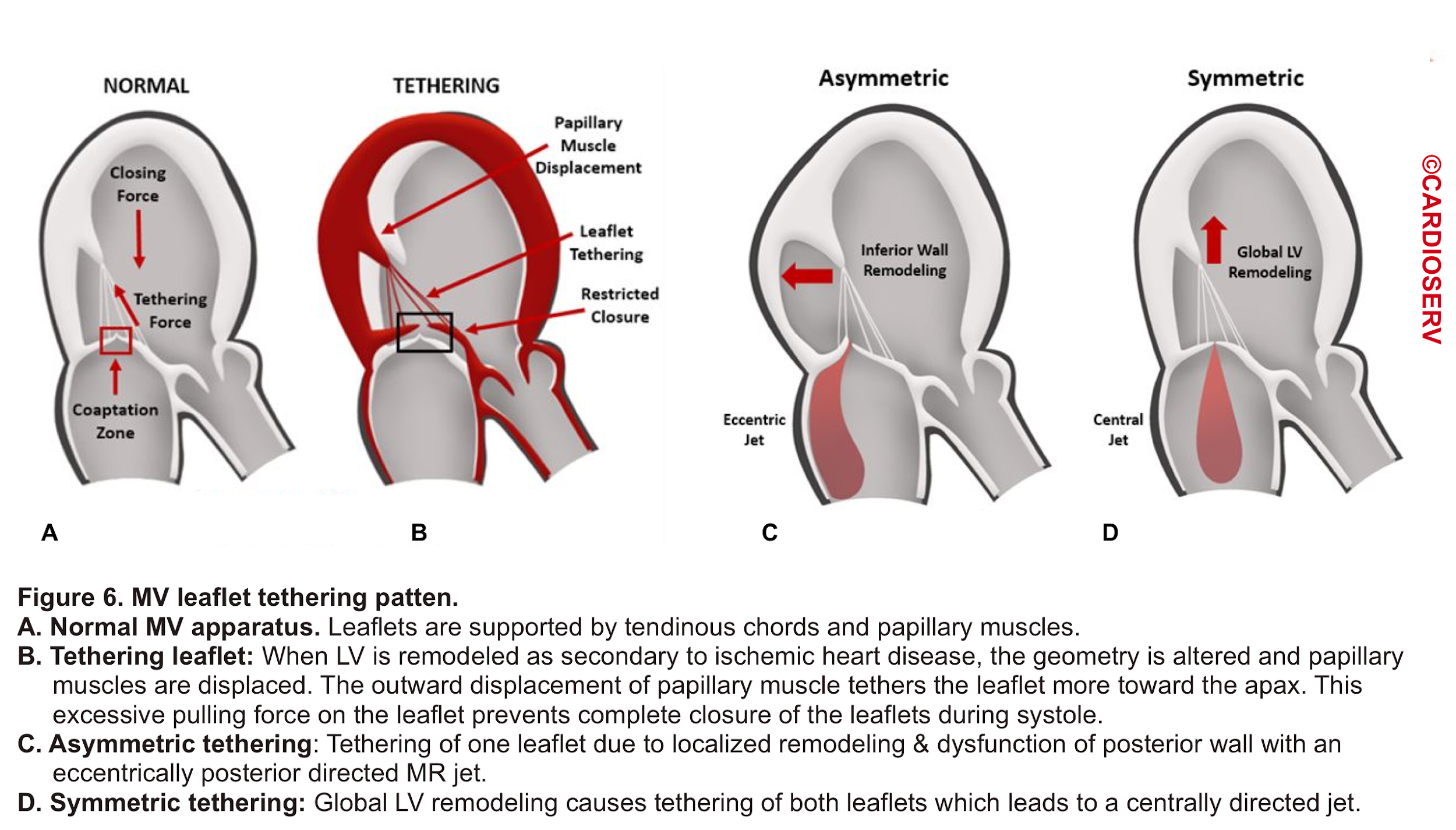
- Leaflet Tethering
- In a Normal MV, the tip of the leaflet comes into close contact during systole. As mentioned earlier, the leaflets are supported by tendinous chords and papillary muscles (figure 6.A).
- When LV remodeling happens (e.g. after MI), the LV geometry is altered which results in the displacement of papillary muscles. This will put excessive force on the corresponding leaflet (tethers the leaflet) and prevent complete closure (mal-coaptation) of MV during systole (figure 6.B).
- Depending on the involved etiology, tethering can be asymmetric (figure 6.C) as following ‘MI or symmetric (figure 6.D) as in global LV remodeling secondary to ‘dilated CM’ *.
Mitral regurgitation
Definition
- MR is defined as blood regurgitation from LV to LA during systole due to poor coaptation of the MV leaflets.
Clinical presentation
- The clinical presentation of MR is variable. Development of signs and symptoms depends on multiple factors including mechanism and etiology, course of the disease (rate of progression), “severity” of MR, the hemodynamic impact of MR, and presence of other coexisting cardiac diseases.
- By and large, clinical presentation ranges from asymptomatic patients to those with exercise intolerance and in severe cases full-blown pulmonary edema and cardiogenic shock.
- Other symptoms such as thromboembolism, hemoptysis, and right-sided heart failure do occur but are less common than with mitral stenosis. However, there is an increased risk of infective endocarditis in patients with moderate to severe MR.
| Clinical question #1 Let’s say that you have a patient who complains of acute dyspnea and POCUS reveals MR. >Does this finding explain your patient’s symptoms?🤔 Let’s walk through and define each piece of the puzzle that you need to answer this key question. |
Mechanism of MR
Identification of the MR mechanism is important and has therapeutic implications.
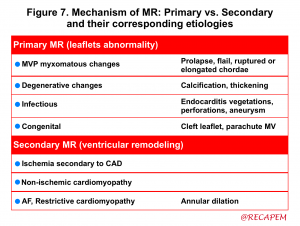
- Since the MV is an active valve (in contrast to the aortic valve, which is passive) with aforementioned muscles and chords originating from the left ventricle and supporting mitral leaflets, the dysfunction of the mitral valve can happen either primarily due to intrinsic valve pathology or secondary to left ventricular disease.
- Defining the mechanism of MR explains how MV dysfunction has happened (figure 7) *.
| Primary (organic) MR |
Intrinsic abnormality of one or more of the 4 components of the valve apparatus (leaflets, tendinous cords, papillary muscles, and annulus) is the culprit mechanism of poor coaptation. Primary MR is suggested in 2D echo by visualizing:
-
- Leaflet thickening, calcification, prolapse or flail.
- Preserved annulus dynamic function.
- Absence of tethering pattern.
- Mitral valve prolapse (1)
(1). The definition of MV prolapse (MVP) is explored earlier. Prolapse of the MV can be due to a variety of etiologies classified as primary or secondary.
- Primary MVP is the most common cause of primary MR. Primary MVP is a myxomatous degenerative disease that involves the MV leaflets either in a focal or diffuse fashion.
- Focal disease
- In the focal form, there’s fibroelastic deficiency which results in focal and segmental leaflet thinning.
- Frequently associated findings include chordal rupture and mild annular enlargement.
- Diffuse (Barlow’s disease)
- This results in diffuse thickening with redundant leaflets.
- Associated findings include chordal elongation and rupture and severe annular dilation.
- Focal disease
| Secondary (functional) MR |
Secondary MR is suggested in 2D echo by the presence of the following findings:
- Absent or mild leaflet thickening, calcification.
- Note that in some patients primary and secondary MT may coexist.
- Tethering or tenting of mitral leaflets caused by papillary muscle displacement, regional and/or global LV remodeling, and mitral annular dilation (figure 6).
- A non-valvular heart disease (e.g. cardiomyopathy etc.) can primarily alter LV geometry (LV dilation) via a remodeling process. This causes displacement of papillary muscles resulting in an imbalance between closure and tethering force on the MV as well as dilatation of the MV annulus *.
- Impaired closing as a result of impaired LV contractility, LV dyssynchrony, papillary muscle dyssynchrony, and/or reduced mitral annular contraction.
- Annular enlargement: It can happen secondary to
- Mitral valve prolapse (MVP)
- Secondary MVP can happen following myocardial infarction, hypertrophic cardiomyopathy, or annular calcification.
- Systolic anterior motion (SAM) of mitral valve. See media below
- For more on dynamic LVOTO, 👉here.
Systolic anterior motion (SAM) of mitral valve
Etiology of MR
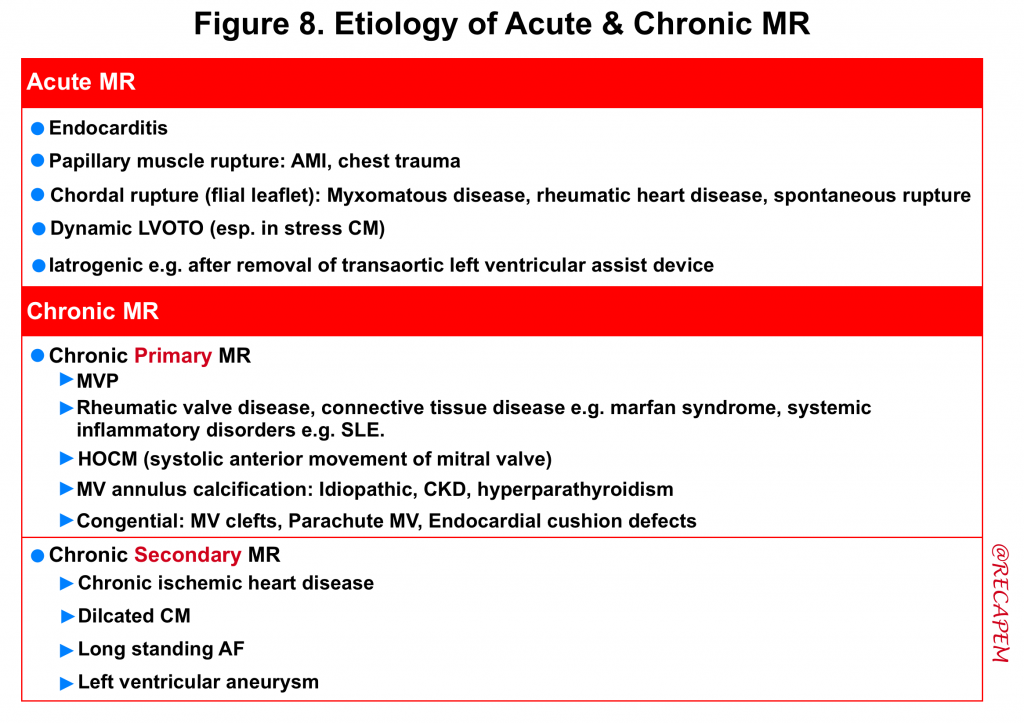
Always consider what possible pathologic processes (e.g. infectious, inflammatory, ischemic, degenerative) could be involved in the development of MR. The etiology of acute and chronic MR is summarized below (figure 8).
| Etiology of Primary (organic) MR |
- Degenerative mitral valve disease (mitral valve prolapse, partial flail, and flail leaflet) includes a range of disorders ranging from myxomatous mitral valve disease (aka. myxomatous degeneration, with redundancy of anterior and posterior mitral leaflets and the chords), seen primarily in younger populations, and fibroelastic deficiency disease, seen primarily in older populations.
- Spontaneous rupture of the tendinous chord has been reported in several studies as well! *
- Rheumatic heart disease
- Infective endocarditis
- Congenital causes of MR include cleft mitral valve, which may be isolated or associated with other anomalies
- Mitral annular calcification
- Irradiation
| Etiology of Secondary (functional) MR |
Based on the phase in the cardiac cycle, functional MR can be further divided into systolic mitral regurgitation (SMR) and diastolic mitral regurgitation (DMR) *. Diastolic MR can be missed easily or confused with systolic MR. Therefore, we will review both in brief.
| Secondary (functional) Systolic MS (SMR) |
- Coronary heart disease
- Postinfarction MR. Regional and/or global LV systolic dysfunction and adverse LV remodeling cause restricted leaflet motion and failure of leaflet coaptation.
- MR secondary to papillary muscle rupture is a life-threatening complication of acute MI.
- Reversible ischemic MR can be caused by acute myocardial ischemia (e.g., acute coronary syndrome or exercise-induced ischemia).
- Dilated (nonischemic) cardiomyopathy
- Hypertrophic cardiomyopathy
- Right ventricular pacing (particularly apical pacing) creates LV asynchrony, which can cause worsening or de novo secondary MR.
| Secondary (functional) Diastolic MR (DMR) |
Diastolic mitral regurgitation (DMR) is a type of functional MR. Its occurrence in the diastolic phase of the cardiac cycle renders DMR an easily ignored entity and confusing it with systolic MR (SMR) occasionally happens *.
- Like SMR, the DMR also increases the volume load of LA. However, the degree is relatively slight because of the low-pressure gradient in diastole.
- The inability to distinguish DMR from SMR and sometimes a confusingly large jet area of DMR can together lead to erroneous judgment of the severity of regurgitation.
- Generally visualizing DMR has an incremental value for diagnosing and evaluating the underlying cardiovascular disease.
Pathophysiology of DMR
- Severely elevated LVEDP
- Under normal conditions, the LV pressure is maintained lower than the left atrial pressure in diastole. This keeps a forward flow from LA to the LV through the MV during diastole. A reversal of this pressure gradient establishes the hemodynamic basis for DMR *.
- Lack of effective LV contraction
- Generally, a full closure of the normal MV requires effective LV contraction with enough closing force.
- This is true that normally after LA contraction, LV diastolic pressure will increase above LA pressure and drive MV leaflets to approximate each other. However, this small pressure gradient is not enough to achieve a full closure of MV. In fact, LV contraction with enough closing force is required for the complete closure of a normal MV.
Mechanism and etiology of DMR. DMR is more likely to happen when several of the following mechanisms are present concurrently *.
- Delayed LV contraction (“overdue” left ventricular systole)
- DMR may happen whenever an LA contraction is not followed by an immediate LV contraction. Simply saying, this can happen in the following conditions:
- Atrioventricular block (AVB)
- DMR may happen during any degree of AVB where LA contraction is not followed by LV contraction immediately.
- Atrial fibrillation or flutter with a long pause (e.g. atrial flutter with 4:1 AV conduction ratio)
- Atrioventricular block (AVB)
- DMR may happen whenever an LA contraction is not followed by an immediate LV contraction. Simply saying, this can happen in the following conditions:
- LV systolic asynchrony
- Complete left bundle branch block
- In the state of LV systolic dyssynchrony, A few myocardial segments may perform post-systolic shortening after the closure of the aortic valve, which might cause a paradoxical increase of LV pressure during early diastole.
- Complete left bundle branch block
- Elevation of LV diastolic pressure
Severity of MR
The essential component of bedside evaluation of VHD in ED is to determine the severity of the disease. The patient’s tolerance to MR is contingent upon the acuteness of the process and severity of the MR among the list.
- The course of the disease and rate of progression
- An acute severe MR is a devastating illness. On the other hand, chronic MR often progresses through a time course.
- Acute Severe MR is not tolerable and the development of acute pulmonary edema and, or cardiogenic shock is inevitable.
- Note that the natural history of degenerative primary MVP is often benign and complications (e.g. sudden cardiac death, endocarditis, requirement for surgery, cerebrovascular accident) rarely develop.
- Patients with compensated chronic MR are mildly symptomatic, while those with decompensated chronic MR present with pulmonary edema.
- There’s a stage in between among patients with chronic MR, which is called a transitional stage. Such patients greatly benefit from possible corrective operation before their cardiac geometry becomes severely altered.
- An acute severe MR is a devastating illness. On the other hand, chronic MR often progresses through a time course.
- Underlying substrate and presence of other cardiovascular disease
- The presence of preexisting cardiopulmonary disorders has a great impact on the patient’s tolerance to mitral regurgitation.
- Patients with poor physiologic reserve or those with systolic dysfunction of the heart cannot tolerate even a moderate degree of new MR and clinical deterioration happens.
- The presence of preexisting cardiopulmonary disorders has a great impact on the patient’s tolerance to mitral regurgitation.
Hemodynamic impact of MR
💡The burden of MR on the heart is volume overload.
- Acute Severe MR
- This causes a surplus of volume within the left atrium, building up a high pressure, and therefore left atrial pressure (LAP) is acutely increased. This will result in the development of acute pulmonary edema and cardiogenic shock.
- Chronic MR
- Here, cardiac remodeling takes place over time as a compensatory mechanism in response to chronic volume overload, leading to the development of left atrial and left ventricular enlargement.
- The more severe the MR, the more severe the LV geometry alteration would be.
- While during earlier stages of chronic MR, the remodeling process is accommodative and patients remain asymptomatic (at the expense of left-chambers dilation), later on during advanced stages of chronic MR, the high build-up of pressure within the enlarged chambers puts a patient at risk of developing acute pulmonary edema and cardiogenic shock following a clinical trigger.
- In chronic primary MR, the presence of the following factors implies the severity of the MR:
- LAE, LVE
- PH (type 2). More on this here.
- AF
- Here, cardiac remodeling takes place over time as a compensatory mechanism in response to chronic volume overload, leading to the development of left atrial and left ventricular enlargement.
Differential diagnosis
Patients with MR present with varying degrees of signs and symptoms of heart failure. Acute heart failure syndrome (AHFS) is synonymous with pulmonary edema in ED and most commonly manifests with shortness of breath. AHFS has different hemodynamic phenotypes (figure 9).
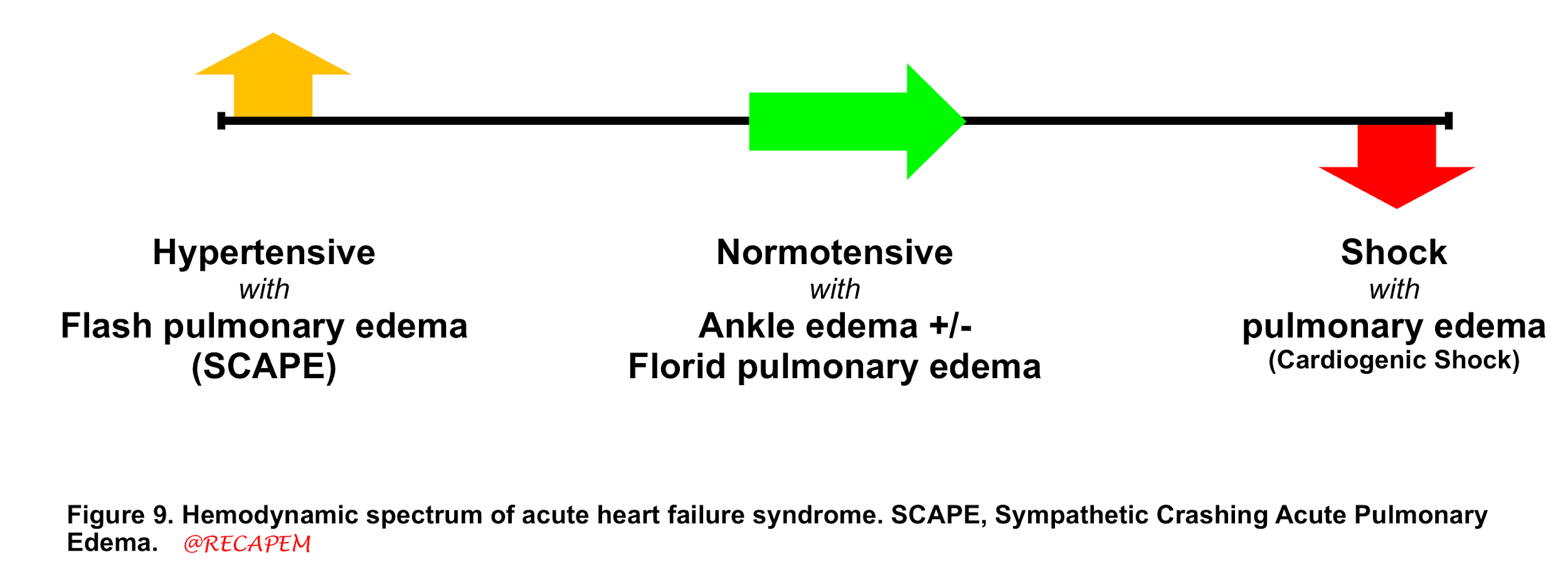
- Having different phenotypes with a myriad of etiologies makes AHFS a challenging condition as the treatment is different for each corresponding etiology.
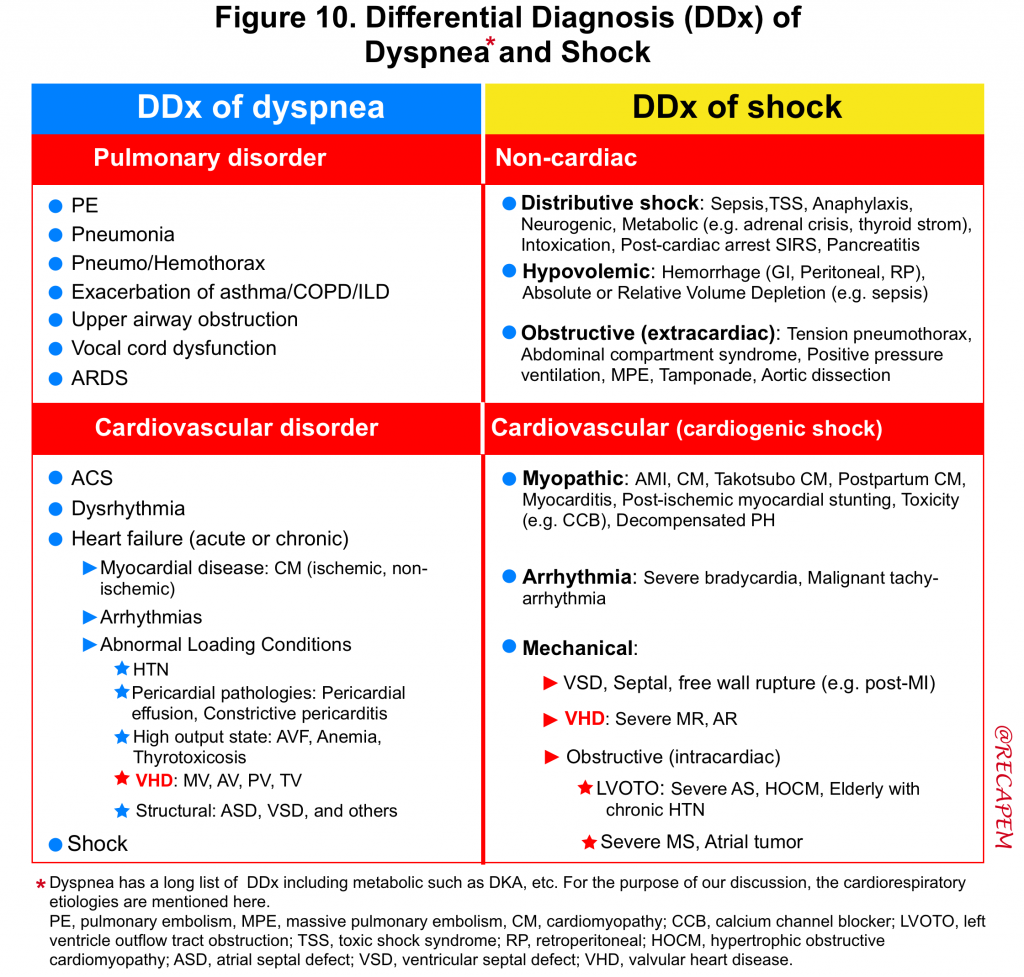
- The DDx of dyspnea and cardiogenic shock is summarized below *. (figure 10).
- Despite this long list of differentials, POCUS is your friend and can significantly help you to identify the culprit etiology and manage your patient appropriately.
- 🔴Acute life-threatening VHD is often overlooked in clinical practice because the clinical history may mimic other disease entities such as acute pulmonary process (e.g. pneumonia, ARDS).
- Physicians should include acute valvular disease in the differential diagnosis of any patient presenting with cardiopulmonary decompensation and consider bedside echo promptly to exclude or confirm this possibility.
Bedside echo in the evaluation of severe MR in ED
Scope of the examination
- There are several qualitative and quantitative methods for a comprehensive assessment of valvular dysfunction (guidelines from ASE * and EAE *), however, these require both time and expertise.
- Moreover, it is claimed that a comprehensive exam (quantitative assessment) rarely adds to the acute management of a severe valvular dysfunction that is readily apparent by a qualitative assessment *.
- The best approach to diagnose severe native valve failure in the ED is ‘practical, simple’ and incorporates 2D, CFD, and PWD echocardiography which is explored below.
Examination technique
2D exam
- It is your first technique. Try to obtain a focused window of the interested valve (MV) in multiple views and catch the possible valvular pathologies by eyeball.
- Clinical usefulness: In 2D examination, the following pathologies can be discerned. These can give you a hint regarding the presence of MR, the severity of regurgitation as well as possible etiology of MR (i.e. primary vs. secondary MR).
- Leaflets: Pay specific attention and look for:
- Morphology: Leaflet thickening, calcification, vegetation?
- Leaflet Motion: Valve coaptation, prolapse, flail, restriction, leaflet tethering?
- Annulus: Size, dilation, motion.
- Other echocardiographic data which can be discerned in 2D:
- Chambers size (LV/LA enlargement), right-sided chamber enlargement.
- LV, RV hypertrophy.
- LV and RV systolic function.
- 🔴Note that all measures of LV systolic function such as fractional shortening, and end-point septal separation are load-dependent.
- 🔴The LV may appear hyperdynamic in the presence of severe MR. More on hyperdynamic LV, 👉here.
- Leaflets: Pay specific attention and look for:
CFD assessment
It is the most essential part of the examination for any suspected valvular lesions (especially regurgitation). However, clinicians must be aware of important considerations and common pitfalls when using this mode.
- Basic
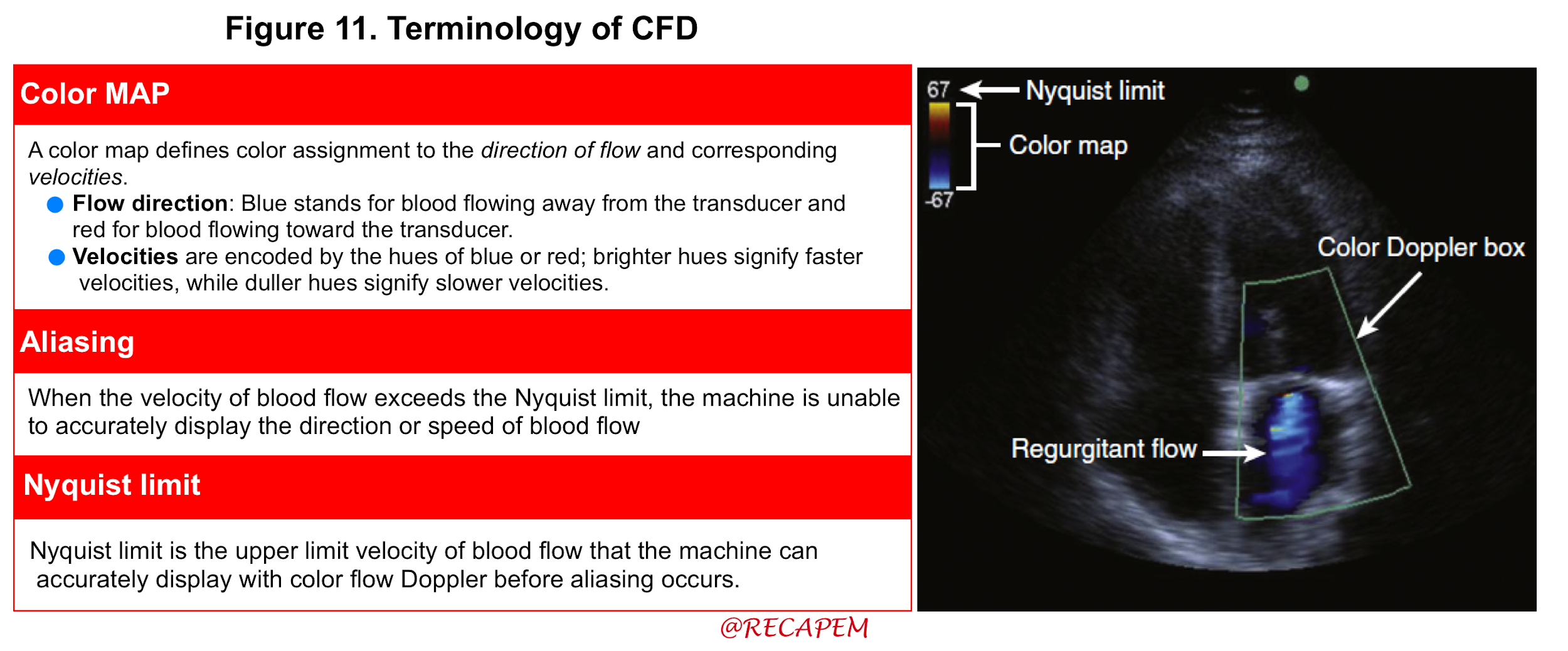
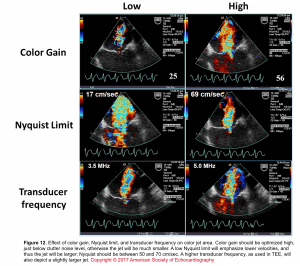
- Understanding key concepts, such as Nyquist limit, and aliasing (Figure 11) are requisite starting points before using CFD.
- Technique: The following recommendations can help optimize the performance and accuracy when assessing regurgitation with CFD:
- Minimize imaging depth.
- Narrow image sector size.
- Set color gain appropriately: Use the gain bottom to adjust the gain.
- Turn up that color gain to get random speckles. When you see speckles, turn them down until speckles disappear.
- Turn gain down; If you have too much color outside of the vessel or too much aliasing.
- Align the imaging plane to be parallel with blood flow.
- Set the Nyquist limit to 40–70 cm/s
- Use the smallest color box that reasonably includes the valve and receiving chamber of interest.
- The effect of color gain, Nyquist time, and transducer frequency on color jet area is shown below (Figure 12)
- Clinical usefulness
- Diagnosis of MR by identifying the presence of the regurgitant flow.
- Determining the severity of MR by:
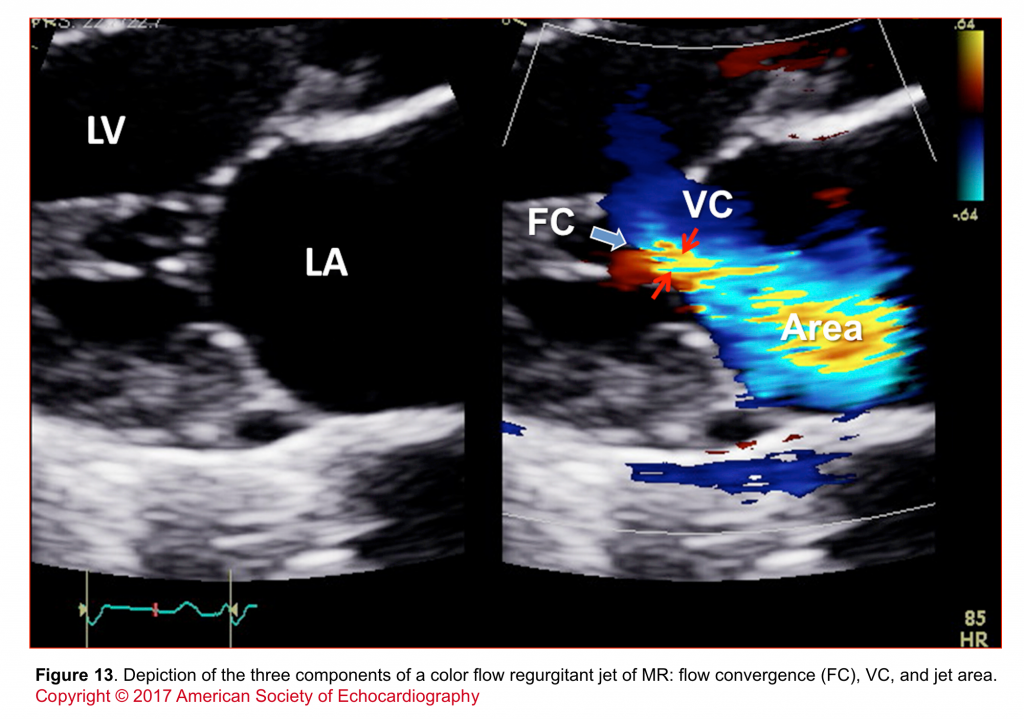
- Assessment of components of jet (flow convergence, VC, jet area; shown in figure 13)
- Showing the direction of regurgitant flow (central, eccentric, multiple jets in unpredictable directions).
- Presence of regurgitant flow
- CFD is able to directly display the high-velocity backflow of blood into the LA in the form of a jet.
- The dominant color of the jet corresponds to the direction of flow (BART, standing for Blue away and Red toward the transducer).
- As velocities in such jets exceed the Nyquist limit and because of turbulent flow, there will also be an array of other colors in the jet. It is this aliased flow that makes jets easily visible.
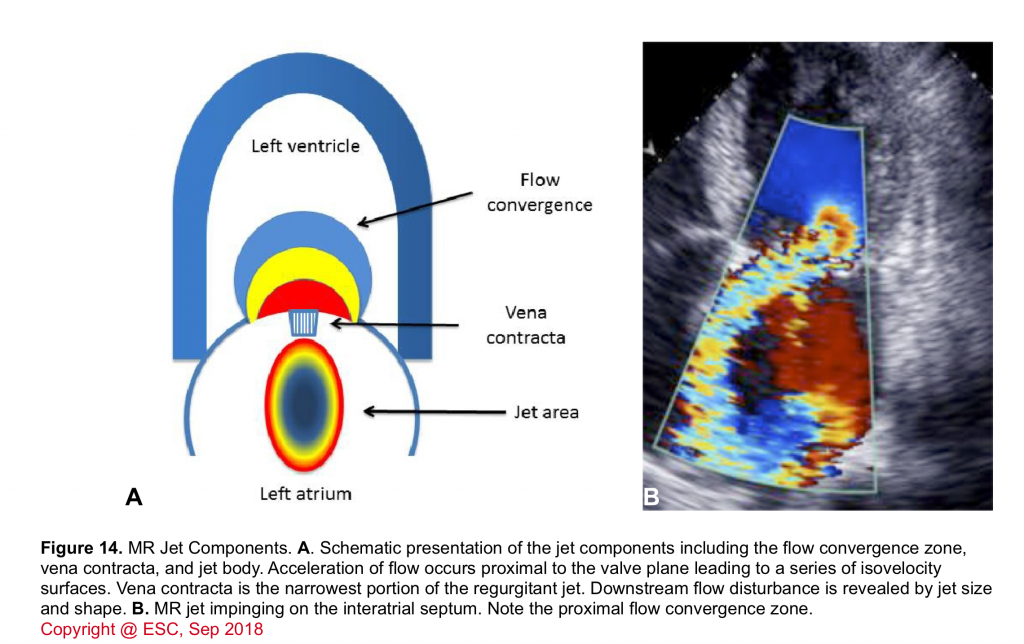
- The various components of a regurgitant jet are described below (figure 14).
- Flow convergence zone
- It is the zone of increased flow velocity before the regurgitant orifice just like the swirls of water one sees in a bathtub close to the outlet. The velocity of flow increases towards the regurgitant orifice in concentric shells, causing a more or less semicircular region of aliased flow. The size of this region corresponds to the magnitude of blood flow and the size of the regurgitant orifice. Thus, it can be used to quantify the severity of regurgitation.
- The actual measurement of this area is somehow difficult, but one can make a crude estimate of this zone as:
- Absence of flow convergence zone: Reflects mild MR.
- Visible but small (<4 mm) flow convergence zone: Moderate MR.
- Large (>1.0 cm) flow convergence zone: Suggests severe MR.
- Vena contracta (VC)
- It corresponds to the region in which blood passes through the valve. Velocity is highest here.
- The width of the vena contracta is a good marker of the severity of MR because it corresponds to the diameter of the regurgitant orifice area.
- The vena contracta width (VCW) is measured perpendicular to the color flow jet in the neck between the proximal flow convergence (PFC) and distal jet expansion.
- VCW ≧ 0.7cm indicates severe MR.
- VCW <0.3cm indicates mild MR.
- VCW is a measurement largely independent of driving pressures and flow rates for a fixed orifice however, its accuracy with multiple jets is uncertain.
- Jet area
- The portion of the jet that is seen in the receiving chamber (LA) is the jet body. Its size also corresponds to the severity of MR.
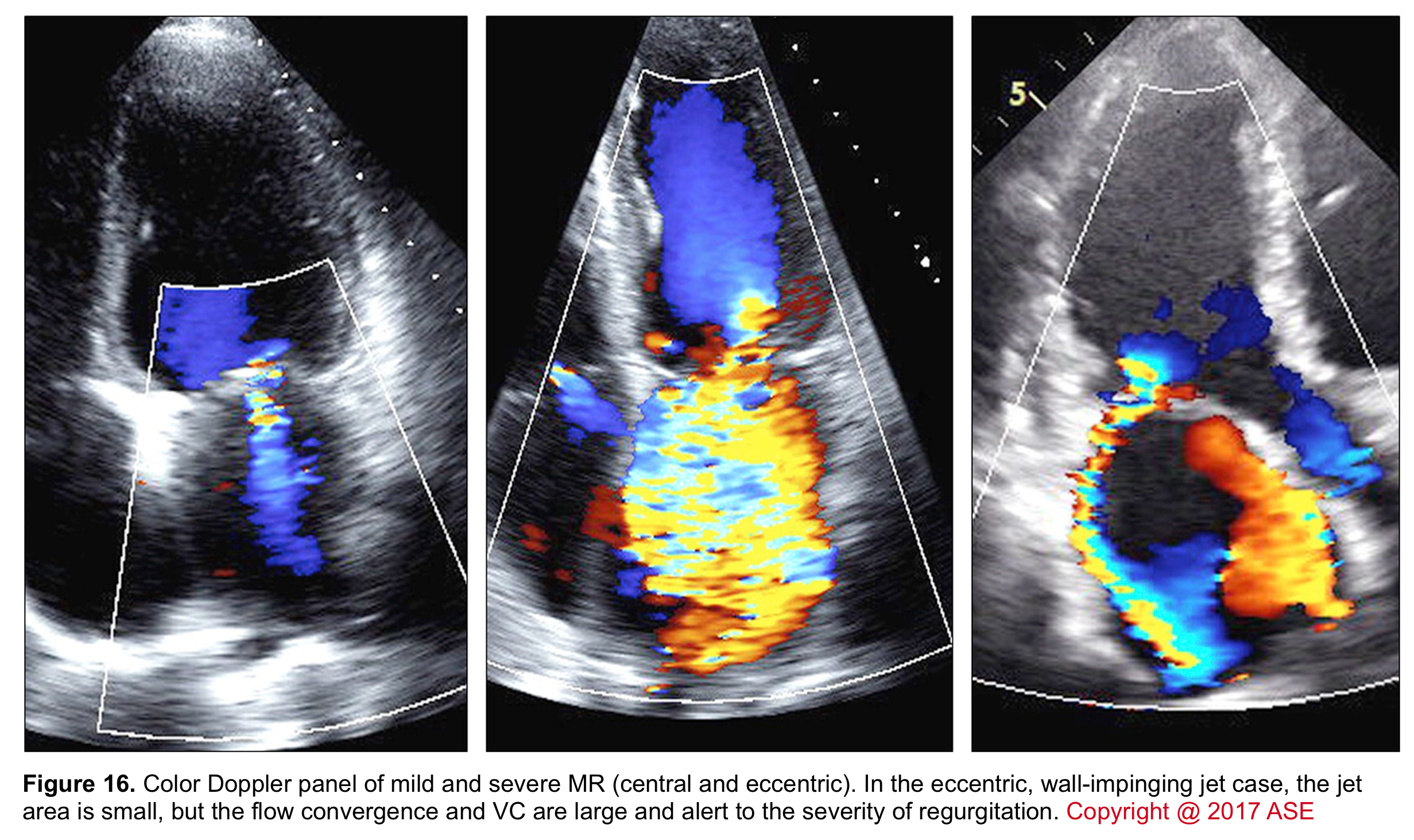
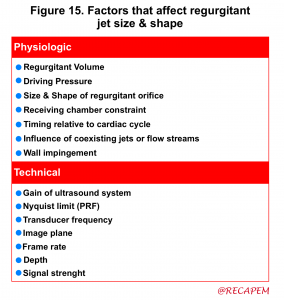
- The jet area (the area that the jet body captures in the receiving chamber; the LA) can be estimated roughly by eyeball although assessment of the jet area is dependent on several variables (figure 15) and is not highly accurate.
- A large central jet (occupying >50% of the LA) can suggest severe MR.
Mitral Regurgitation for #firstecho #MedEd. All 👀 on the blue colour entering Left Atrium during Systole👇🏻Video adapted from the fabulous https://t.co/cfjU4Az75T @echocardiac @pocusmeded @pocusfoamed @IM_POCUS @The_echo_lady @POCUSIAPN @ABCDEcografiapic.twitter.com/o1UqXFCxIo
— POCUSmedicine (@POCUSpeek) June 5, 2022
- The direction of the regurgitant flow
- The jet body is directed into the receiving chamber (LA) in one of the following patterns. Identifying the direction of the jet not only can assess severity but also can give you a hint regarding possible mechanisms and etiology of MR.
- Centrally directed jet: often seen in MR associated with global LV dysfunction (functional MR).
- Eccentric jet: The jet body is directed towards the left atrial walls. The presence of an eccentric jet (of variable size) is a more accurate measure for defining the severity of MR than the jet area. An eccentric jet suggests severe MR with a high probability.
- Multiple jets in unpredictable directions: often seen in endocarditis.
- The jet body is directed into the receiving chamber (LA) in one of the following patterns. Identifying the direction of the jet not only can assess severity but also can give you a hint regarding possible mechanisms and etiology of MR.
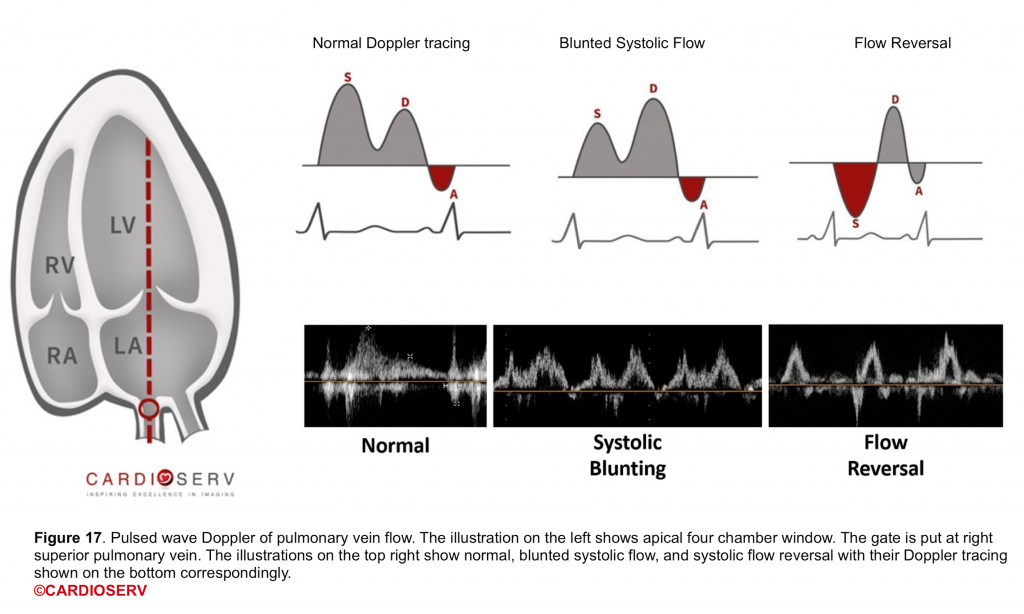
PWD of pulmonary venous flow
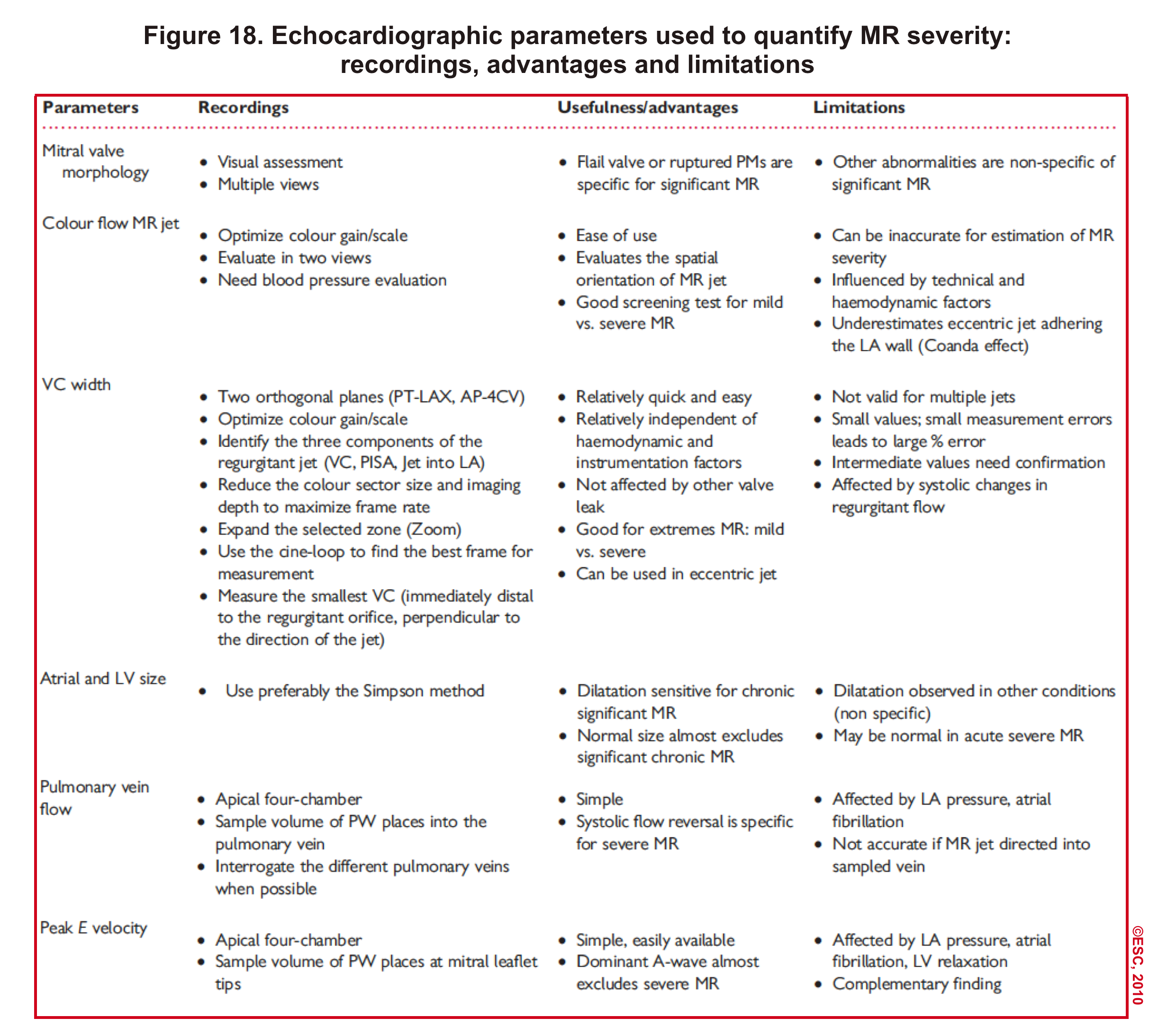
- Technique: Below table (figure 18).
- Clinical usefulness
- It gives useful additional information regarding the severity of MR and the hemodynamic consequences of MR.
- The normal Doppler tracing of pulmonary venous flow shows dominant systolic components (figure 17).
- With increasing severity of MR, there is reduced systolic velocity, and in severe MR systolic flow reversal is seen (more on this here).
- Since each echocardiographic parameter has its own advantages and limitations, try to evaluate and determine the severity of MR by multiple methods. The following table (figure 18) summarizes the usefulness and limitations of each method.
Interpretation of echo findings
| Clinical question #2 I have performed POCUS in my critically ill patient and other diagnostic possibilities are less likely. Valvular dysfunction is on top of my differential diagnosis list. Is this a severe MR?👇 |
In the presence of ≧4 following strong parameters, MR is defined as severe and no further sophisticated and time-consuming quantitative assessment is warranted.😀
- In 2D echo: Specific valvular lesions with high positive predictive value for a severe MR include any of the following: Flail leaflet, ruptured papillary muscle (figure 19), severe retraction, large perforation, and tethering leaflet.
- Keep in mind that these findings are highly specific but their absence does not exclude the presence of severe MR.
- In CFD: the presence of the following findings are specific for a severe MR:
- VCW ≧ 0.7cm
- Flow convergence: Large flow convergence present throughout the systole
- Jet area: An eccentric jet adhering, swirling, and reaching the posterior wall of the LA or a Large central jet occupying > 50% of the LA
- In PWD: Pulmonary vein flow tracing of systolic flow reversal pattern
In the presence of ≧4 following strong parameters, MR is defined as mild, and no further quantitative assessment is needed 😀
- 2D: The presence of following finding is valuable:
- Normal or mild leaflet abnormality (Mild thickening, calcification, or prolapse)
- CFD:
- Color flow jet area is small, central, narrow, and often brief
- Flow convergence: Not visible, transient of small
- VC <0.3
- Mitral inflow: A-wave dominant
- PWD: Pulmonary vein flow tracing of systolic dominance pattern (maybe blunted in LV dysfunction or AF)
👉If 2-3 criteria for either mild or severe MR were met, then it’s “probably” moderate, and further quantitative assessment is required which is beyond the scope of our discussion.
👉Keep in mind that in the absence of coexisting cardiopulmonary disease, a mild-moderate valvular dysfunction is Not often the main problem of your critical patient. Look for other possible diagnoses!
Case scenario revisited
Perform POCUS for any patients with signs and symptoms of cardiorespiratory dysfunction and do not attribute shortness of breath to common diagnoses such as COPD exacerbation or pneumonia despite the presence of clinical risk factors. In this case, POCUS (figure below) showed flail anterior MV. A flail leaflet in 2D echo is a concerning finding for severe MR. Lung US showed bilateral B-profile.
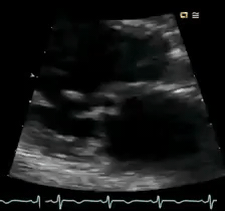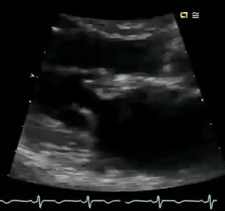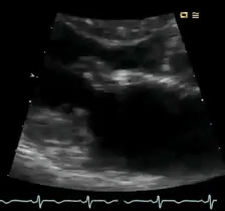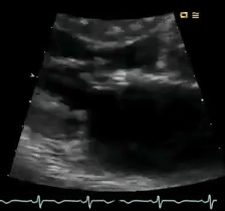
Management: General strategy in the ED
| Clinical question #3 How has my management plan been changed by identifying a severe MR? |
Patients with acute severe MR (and decompensated chronic MR) are critically ill with significant abnormalities that require urgent medical and sometimes surgical treatment *, *.
Acute severe MR with low blood pressure (cold and wet) *
- Oxygenation
- Patients in cardiogenic shock often respond to BiPAP.
- It has been shown in several studies that BiPAP reduces intubation and mortality.
- It also reduces cardiac preload and afterload which are deserved in severe MR.
- Put your patient acutely on BiPAP and titrate up the pressure as tolerated. The possible up-titration of pressure is exemplified as:
- Start: iPAP 10 cmH2O / ePAP 5 cmH2O
- Increase: iPAP 15 cmH2O / ePAP 10 cmH2O
- Increase: iPAP 18 cmH2O / ePAP 14 cmH2O
- Patients in cardiogenic shock often respond to BiPAP.
- Intubation
- Intubation in cardiogenic shock has a high risk of hypotension and cardiac arrest.
- If you’re in doubt about the need for intubation, initiate BiPAP and resuscitate before intubation.
- Protect MAP
- Hypotension requires prompt treatment to defend the coronary and end-organ perfusion.
- Norepinephrine
- Although it can improve the BP, it carries the risk that excessive afterload will increase the regurgitant flow back into the LA and worsen pulmonary edema.
- Low dose epinephrine (e.g. <8mcg/min)
- It works predominantly as an inotrope. However, unlike dobutamine, epinephrine doesn’t cause vasodilation and hypotension. The net effect of low-dose epinephrine is often an improvement in blood pressure and cardiac output, without affecting systemic vascular resistance much.
- Norepinephrine
- Hypotension requires prompt treatment to defend the coronary and end-organ perfusion.
- Pure inotrop (e.g dobutamine)
- If a patient does not respond to the above tier of treatments, it may be used for patients with reduced ejection fraction and refractory cardiogenic shock.
- Dysrhythmias
- Patient with cardiogenic shock does not tolerate arrhythmias.
- AF may need to be terminated by cardioversion. More on this here.
- Digoxin can be considered. It has inotropic properties, though with intravenous loading, improvement may occur over several hours!
Acute severe MR with normal blood pressure (Warm and wet)
- Oxygenation: The same rules are applied here as mentioned above.
- Reduce preload and afterload
- Afterload reduction reduces regurgitant flow into the LA and improves cardiac output.
- Nitroglycerine
- In the acute phase, a high-dose nitroglycerine infusion is the safest vasodilator.
- High doses (up to 250 mcg/min) may be needed to achieve arterial vasodilation, titrated against the patient’s blood pressure.
- Nitroglycerine
- Afterload reduction reduces regurgitant flow into the LA and improves cardiac output.
- Dysrhythmias: Try to terminate dysrhythmia.
👉Involve Cardiothoracic surgery early in patients with severe valvular disease.
👉Consult Interventional Cardiology for possible intra-aortic balloon pump in patients with acute severe MR as a temporizing measure.
- ACS
- Patients with acute MR due to ACS will benefit from revascularization.
- This may be sufficient to reverse acute MR, with the exception of MR caused by tendinous chord/papillary muscle rupture.
- Consult Interventional cardiology early for possible left heart catheterization and revascularization if indicated and possible *, *.
- Endocarditis
- Patients with acute MR due to endocarditis require broad-spectrum antibiotic treatment.
- Operative intervention may be required as well *.
Acute decompensation of chronic MR
- While the same principle of management as mentioned above would be applied here, pay attention and try to identify and correct possible clinical factors that may have triggered decompensation of chronic MR.
- Factors that can trigger acute decompensation of chronic MR include:
- New onset arrhythmia such as AF
- Decompensated coexisting conditions such as pulmonary hypertension. More on this here.
- These patients benefit from inhaled pulmonary vasodilators.
- Volume overload.
- New insult to myocardial systolic performance such as AMI.
- Factors that can trigger acute decompensation of chronic MR include:
🔴Summary of principles of hemodynamic resuscitation in valvular emergencies
- Patients with critical valvular disease usually fail to respond to medical therapy alone *.
- The goal of medical therapy and mechanical circulatory support (MCS) is stabilization prior to definitive correction of the lesion.
- Generally, patients with regurgitant lesions require aggressive afterload reduction and inotropic support *, while those with stenotic lesions may paradoxically require β-blockade and vasoconstrictors (table below) *.
Multiple and mixed valvular heart disease
- Multiple VHD refers to the combination of stenotic or regurgitant lesions occurring on ≥2 cardiac valves.
- Mixed VHD refers to the combination of stenotic and regurgitant lesions on the same valve *.
- The hemodynamic and clinical consequences of any given valvular lesion may be modulated by the concomitant presence of mixed or multiple VHD. These hemodynamic interactions can promote, exacerbate, or, in contrast, blunt the clinical expression of each singular lesion *.
- Conditions that reduce the effective LV forward flow in patients with MR:
- Increasing LV volume loading: Concomitant AR
- Concomitant MS
- ↑Afterload (e.g. Hypertension, aortic stenosis)
- Tachycardia can worsen MR, “only if” the etiology of MR is “SAM” (systolic anterior motion) of mitral valve.
- Hypotension→↓Coronary perfusion pressure→ myocardial ischemia
- How presence of MR can affect the measurement of systolic indices of LV?
- In patients with MR, LVEF overestimates the LV function while the patient could have a picture of heart failure *.
- In MR, LVEF is overestimated because it does not only reflect the blood volume pumped into the aorta but also that is pumped into the low-pressure left atrium. Measurement of effective forward flow (stroke volume) will be helpful.
- In patients with MR, LVEF overestimates the LV function while the patient could have a picture of heart failure *.
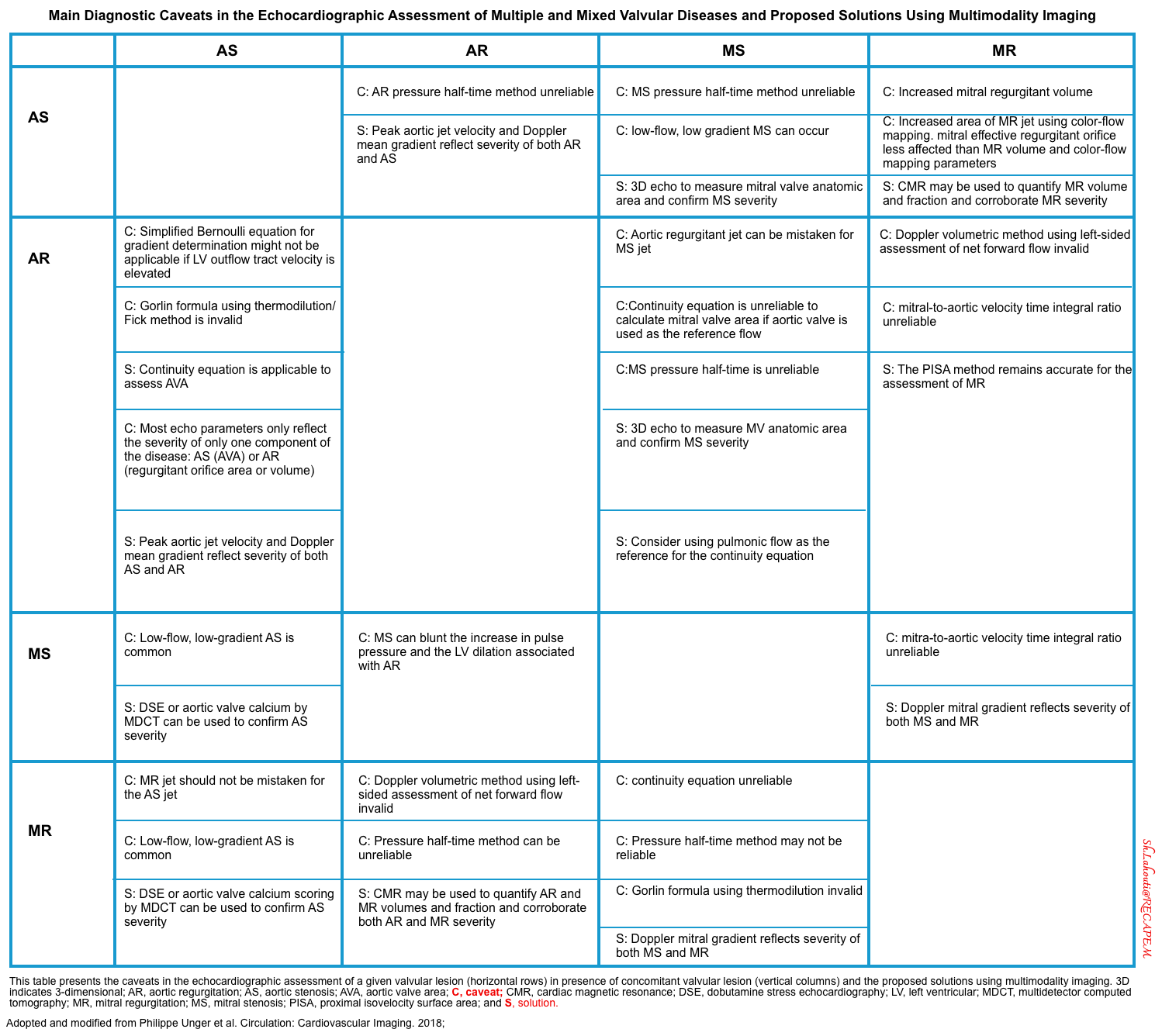
- The main diagnostic caveats in the echocardiographic assessment of multiple and mixed valvular diseases are summarized below.
Media
Going further
- Mitral regurgitation (Radiopaedia)
- Ultrasound of cardiac valves (part 1)
- Ultrasound of cardiac valves (part 2)
- Cardiogenic Shock (EM:RAP)
- Resuscitation of CHF with Cardiogenic Shock (EM:RAP)
References
1. Silverman NH. Echocardiography of congenital mitral valve disorders: echocardiographic-morphological comparisons. Cardiol Young. 2014 Dec;24(6):1030-48. doi: 10.1017/S1047951114002157. PMID: 25647377.
2. Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017 Apr;30(4):303-371. doi: 10.1016/j.echo.2017.01.007. Epub 2017 Mar 14. PMID: 28314623.
3. Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL; Scientific Document Committee of the European Association of Cardiovascular Imaging. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2013 Jul;14(7):611-44. doi: 10.1093/ehjci/jet105. Epub 2013 Jun 3. PMID: 23733442.
4. Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C, Hagendorff A, Monin JL, Badano L, Zamorano JL; European Association of Echocardiography. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr. 2010 May;11(4):307-32. doi: 10.1093/ejechocard/jeq031. PMID: 20435783
5. American Association For Thoracic Surgery Ischemic Mitral Regurgitation Consensus Guidelines Writing Committee, Kron IL, LaPar DJ, Acker MA, Adams DH, Ailawadi G, Bolling SF, Hung JW, Lim DS, Mack MJ, O’Gara PT, Parides MK, Puskas JD. 2016 update to The American Association for Thoracic Surgery consensus guidelines: Ischemic mitral valve regurgitation. J Thorac Cardiovasc Surg. 2017 May;153(5):1076-1079. doi: 10.1016/j.jtcvs.2016.11.068. Epub 2017 Jan 17. PMID: 28190606.
6. Dal-Bianco JP, Beaudoin J, Handschumacher MD, Levine RA. Basic mechanisms of mitral regurgitation. Can J Cardiol. 2014;30(9):971-981. doi:10.1016/j.cjca.2014.06.022
7. Silbiger JJ. Mechanistic insights into atrial functional mitral regurgitation: Far more complicated than just left atrial remodeling. Echocardiography. 2019 Jan;36(1):164-169. doi: 10.1111/echo.14249. PMID: 30620100
8. Gertz ZM, Raina A, Saghy L, Zado ES, Callans DJ, Marchlinski FE, Keane MG, Silvestry FE. Evidence of atrial functional mitral regurgitation due to atrial fibrillation: reversal with arrhythmia control. J Am Coll Cardiol. 2011 Sep 27;58(14):1474-81. doi: 10.1016/j.jacc.2011.06.032. PMID: 21939832.
9. Gabbay U, Yosefy C. The underlying causes of chordae tendinae rupture: a systematic review. Int J Cardiol. 2010 Aug 20;143(2):113-8. doi: 10.1016/j.ijcard.2010.02.011. Epub 2010 Mar 7. PMID: 20207434
10. Aydin A, Gurol T, Soylu O, Dagdeviren B. Intermittent symptomatic functional mitral regurgitation illustrated by two cases. Cardiovasc J Afr. 2015 Jul 23;26(4):e12-4. doi: 10.5830/CVJA-2015-026. PMID: 26407328; PMCID: PMC4683338.
11. Kodamag M, Koiyama S, Yamaguchi T, Hanawa H, Zhang S, Yokoyama A, Tsuda T, Izumi T, Shibata A, Masani F. Diastolic mitral regurgitation in intact mitral valve detected by color Doppler echocardiography in a patient with acute aortic regurgitation. Korean J Intern Med. 1992 Jan;7(1):64-7. doi: 10.3904/kjim.1992.7.1.64. PMID: 1477033; PMCID: PMC4532101.
12. Rokutanda T, Misumi I, Usuku H, Kusuhara K, Akahoshi R, Matsumoto M, Akahoshi G, Yasuda H, Kaikita K, Hokimoto S, Sugiyama S, Ogawa H. Deceased left ventricular compliance contributing to diastolic mitral regurgitation in a patient with atrioventricular block. J Echocardiogr. 2013 Dec;11(4):167-8. doi: 10.1007/s12574-013-0187-8. Epub 2013 Jun 23. PMID: 27278771.
13. Agrawal A, Parikh M, Verma I, Thyagarajan B. Diastolic mitral regurgitation in a patient with coronary artery disease and anaemia. BMJ Case Rep. 2016 Mar 14;2016:bcr2016214473. doi: 10.1136/bcr-2016-214473. PMID: 26976838; PMCID: PMC4800209
14. Li Q, Liu Y, Zuo W, Chen H, Zhao W, Dong L, Pan C, Shu X. Mechanisms, features, and significance of diastolic mitral regurgitation: a case series. Eur Heart J Case Rep. 2020 Sep 19;4(5):1-8. doi: 10.1093/ehjcr/ytaa203. PMID: 33426438; PMCID: PMC7780436
15. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016 Aug;18(8):891-975. doi: 10.1002/ejhf.592. Epub 2016 May 20. PMID: 27207191
16. Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, Horton K, Ogunyankin KO, Palma RA, Velazquez EJ. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019 Jan;32(1):1-64. doi: 10.1016/j.echo.2018.06.004. Epub 2018 Oct 1. PMID: 30282592
17. Lancellotti P, Price S, Edvardsen T, Cosyns B, Neskovic AN, Dulgheru R, Flachskampf FA, Hassager C, Pasquet A, Gargani L, Galderisi M, Cardim N, Haugaa KH, Ancion A, Zamorano JL, Donal E, Bueno H, Habib G. The use of echocardiography in acute cardiovascular care: recommendations of the European Association of Cardiovascular Imaging and the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care. 2015 Feb;4(1):3-5. doi: 10.1177/2048872614568073. Epub 2015 Jan 29. PMID: 25635106
18. Stout KK, Verrier ED. Acute valvular regurgitation. Circulation. 2009 Jun 30;119(25):3232-41. doi: 10.1161/CIRCULATIONAHA.108.782292. PMID: 19564568
19. Thiele H, Ohman EM, Desch S, Eitel I, de Waha S. Management of cardiogenic shock. Eur Heart J. 2015 May 21;36(20):1223-30. doi: 10.1093/eurheartj/ehv051. Epub 2015 Mar 1. PMID: 25732762
20. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: Lifeline. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 2017 Oct 17;136(16):e232-e268. doi: 10.1161/CIR.0000000000000525. Epub 2017 Sep 18. PMID: 28923988
21. Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, Hollenberg SM, Kapur NK, O’Neill W, Ornato JP, Stelling K, Thiele H, van Diepen S, Naidu SS. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019 Jul 1;94(1):29-37. doi: 10.1002/ccd.28329. Epub 2019 May 19. PMID: 31104355
22. Rab T, Ratanapo S, Kern KB, Basir MB, McDaniel M, Meraj P, King SB 3rd, O’Neill W. Cardiac Shock Care Centers: JACC Review Topic of the Week. J Am Coll Cardiol. 2018 Oct 16;72(16):1972-1980. doi: 10.1016/j.jacc.2018.07.074. Erratum in: J Am Coll Cardiol. 2018 Nov 27;72(21):2685. PMID: 30309475
23. Truesdell AG, Tehrani B, Singh R, et al. ‘Combat’ Approach to Cardiogenic Shock. Interv Cardiol. 2018;13(2):81-86. doi:10.15420/icr.2017:35:3
24. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL; ESC Scientific Document Group. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015 Nov 21;36(44):3075-3128. doi: 10.1093/eurheartj/ehv319. Epub 2015 Aug 29. PMID: 26320109.
25. Akodad M, Schurtz G, Adda J, Leclercq F, Roubille F. Management of valvulopathies with acute severe heart failure and cardiogenic shock. Arch Cardiovasc Dis. 2019 Dec;112(12):773-780. doi: 10.1016/j.acvd.2019.06.009. Epub 2019 Sep 3. PMID: 31492536.
26. Bernard S, Deferm S, Bertrand PB. Acute valvular emergencies. Eur Heart J Acute Cardiovasc Care. 2022 Aug 9;11(8):653-665. doi: 10.1093/ehjacc/zuac086. PMID: 35912478.
27. Jentzer JC, Ternus B, Eleid M, Rihal C. Structural Heart Disease Emergencies. J Intensive Care Med. 2021 Sep;36(9):975-988. doi: 10.1177/0885066620918776. Epub 2020 Apr 21. PMID: 32314662
28. Unger P, Pibarot P, Tribouilloy C, Lancellotti P, Maisano F, Iung B, Piérard L; European Society of Cardiology Council on Valvular Heart Disease. Multiple and Mixed Valvular Heart Diseases. Circ Cardiovasc Imaging. 2018 Aug;11(8):e007862. doi: 10.1161/CIRCIMAGING.118.007862. PMID: 30354497.
29. Unger P, Clavel MA, Lindman BR, Mathieu P, Pibarot P. Pathophysiology and management of multivalvular disease. Nat Rev Cardiol. 2016 Jul;13(7):429-40. doi: 10.1038/nrcardio.2016.57. Epub 2016 Apr 28. PMID: 27121305; PMCID: PMC5129845.
30. Yousef, H.A., Hamdan, A.E.S., Elminshawy, A. et al. Corrected calculation of the overestimated ejection fraction in valvular heart disease by phase-contrast cardiac magnetic resonance imaging for better prediction of patient morbidity. Egypt J Radiol Nucl Med 51, 11 (2020). https://doi.org/10.1186/s43055-019-0130-8



Add comment