November 14, 2021, by Shahriar Lahouti.
CONTENTS
- Introduction with a case
- Definition and Diagnosis of AF
- Hemodynamic impact of AF
- Etiology of New onset AF
- Evaluation
- Approach to management of AF in critical illness
- Emergent Cardioversion
- Common therapeutic measures for AF in unstable patients
- Hemodynamic optimization
- Treatment of inciting factors
- Rate vs. rhythm control strategy
- Rate control strategy for critically ill patients
- Rhythm control strategy for critically ill patients
- Anticoagulation
- Back to drawing case
- RECAP
- AF Tool
- Media
- Further going
- References
Introduction
The charge nurse asks you to see a 60-year-old patient in the ED ICU, who she thinks has ST. You walk into the room and see a pale, diaphoretic man with moderate respiratory discomfort. The chart in your hand says that he had a severe pancreatitis with acute kidney injury but no prior medical problems. The monitor reveals a heart rate of approximately 143 bpm, but it jumps around a lot from beat to beat. His blood pressure is 85/46 (MAP 59). He is satting 89% on a nonrebreather. The ECG is narrow complex and irregularly irregular. What is the best therapeutic approach for him?
Atrial fibrillation (AF) is the most common sustained arrhythmia encountered in clinical practice 1. In critically ill patients, the incidence of new onset AF is about 10% but little is known about its treatment. Regrettably, our understanding of AF treatment is based almost exclusively on studies of outpatients with chronic, recurrent AF occurring spontaneously, which is the polar opposite of AF in critically ill patients. This article will focus on the management of AF in the critically ill patients.
Definition and Diagnosis
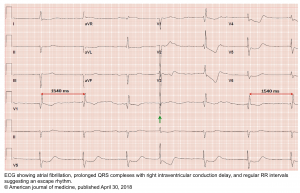
AF may be suspected on the basis of an irregularly irregular heart rate (either on clinical examination or telemetry). However the diagnosis of AF should always be confirmed with a rhythm documentation with a 12-lead ECG tracing. By convention, an episode lasting at least 30s is diagnostic for clinical AF 2.
Diagnostic criteria for AF on ECG
- Irregularly irregular RR intervals: The RR intervals follow no repetitive pattern (hence labeled as irregularly irregular). There should be no regularity 3.
- At very high rates, the heart rate may appear to be regular (“pseudo-regularization”).
- When in doubt, calipers may help determine whether there is any regularity.
- No discernible P waves: instead these may be replaced by fibrillation waves.
- Fibrillation waves may be best seen in the inferior and right-sided precordial leads.
- In some patients, fibrillation waves may be small and difficult to distinguish from artifacts.
👉One exception to these criteria is that if AF is combined with heart block, then the ventricular response may be regular (see below)
Classification
AF has been traditionally classified based on following patterns 4
- Episode persistence: These patterns (paroxysmal, persistent, long standing, permanent) 5 have been used to characterize the severity of disease, define patient populations in clinical trials, and to form the basis of therapeutic recommendations regarding pharmacological and invasive arrhythmia management.
- Pathophysiologic pattern of AF: The likelihood of developing AF varies across physiological and pathological states. Within this context AF may be considered as:
- Primary: If AF has an established pathophysiologic process.
- Secondary: If AF is caused by a self-limited or acutely reversible (or at least partially reversible) precipitant. Common causes of secondary AF are described below.
Heart rate among patients with AF
- During AF, electrical activity in the atria can exceed 400 beats/min. The majority of these impulses do not conduct to the ventricles because of the electrical properties of the atrioventricular (AV) node. The ventricular rate is controlled by the conduction properties of the AV node. In the typical patient with untreated AF, the ventricular rate varies between 90 and 170 beats/min (~ 150).
- 👉Clinical entities that can cause accelerated ventricular response ( HR > ~110) in patients with AF:
- Catecholamine excess or parasympathetic withdrawal
- Hyperthyroidism
- State of shock (e.g. sepsis, hemorrhagic shock etc.)
- Pulmonary embolism
- Acute flash pulmonary edema
- Acute coronary syndrome
- Alcohol/drug withdrawal
- Acute exacerbation of COPD
- Decompensated pulmonary hypertension
- Worsening valvulopathy (e.g. mitral stenosis, mitral regurgitation)
- Myocarditis, pericarditis
- Acute infection: pneumonia, UTI, etc.
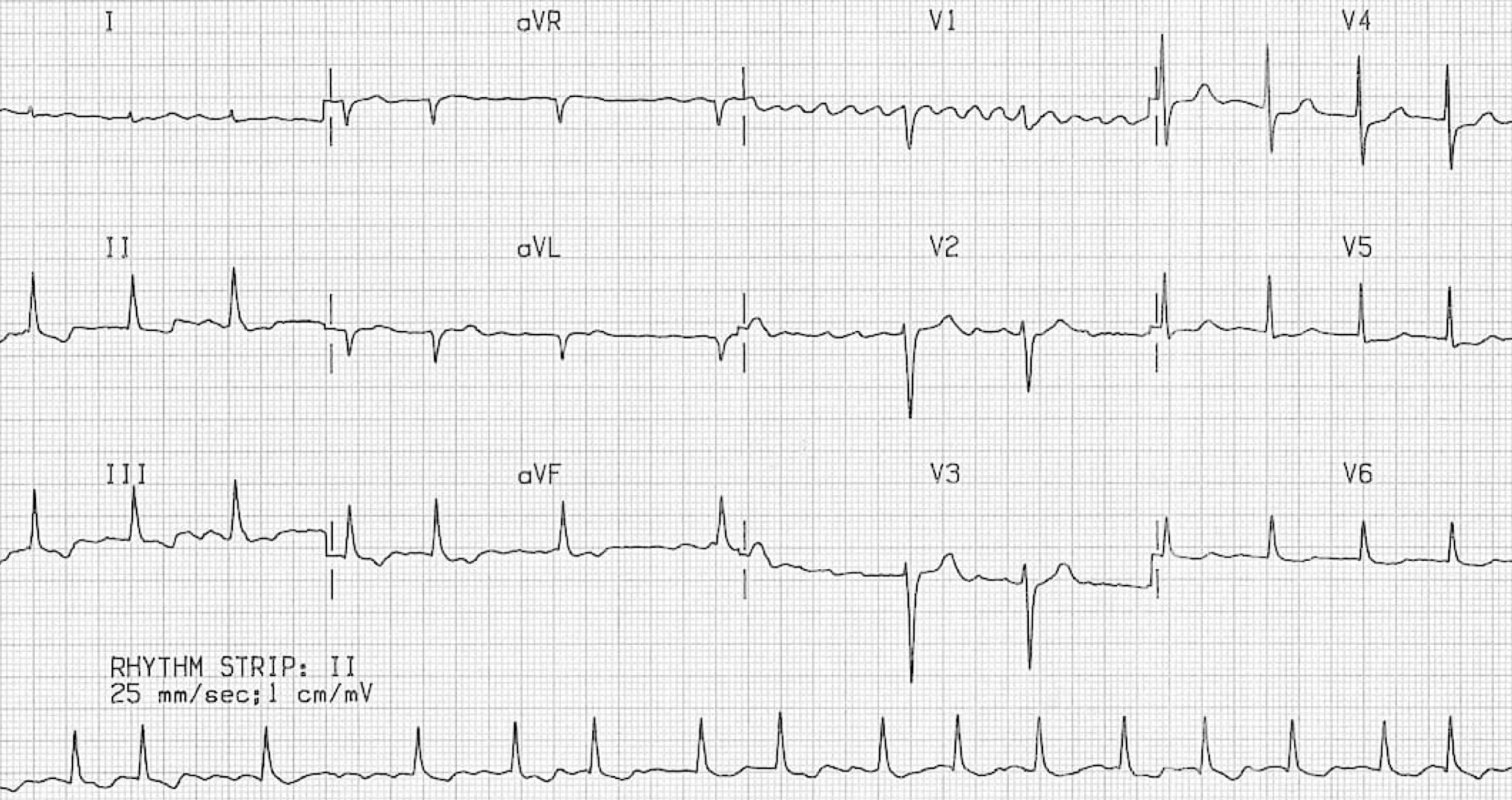
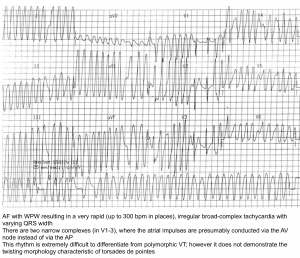
- AF plus Wolf Parkinson White: an accessory pathway as occurs in preexcitation syndrome (If the heart rate is >250, consider the possibility of an accessory tract) 6.
- Normally, when in AF the heart rate is limited by the refractory period of the AV node. Although the AV node may allow for a fast heart rate (e.g. ~120-180), these heart rates are usually tolerated well.
- When AF occurs in the context of an accessory pathway, both the AV node and the accessory pathway can transmit beats to the ventricles. Since the accessory pathway often has a shorter refractory period than the AV node, it may drive the ventricle very rapidly (e.g. >250). This is dangerous because the extremely fast and uncoordinated contractions of the ventricle can promote ventricular tachycardia or cardiovascular collapse.
- AF with an accessory pathway produces a distinctive pattern of ECG findings:
- Irregularly irregular heart rate that may be extremely fast (e.g. >250).
- Wide-complex beats (resulting from transmission over the accessory pathway).
- QRS Morphology varies between different beats.
- Catecholamine excess or parasympathetic withdrawal
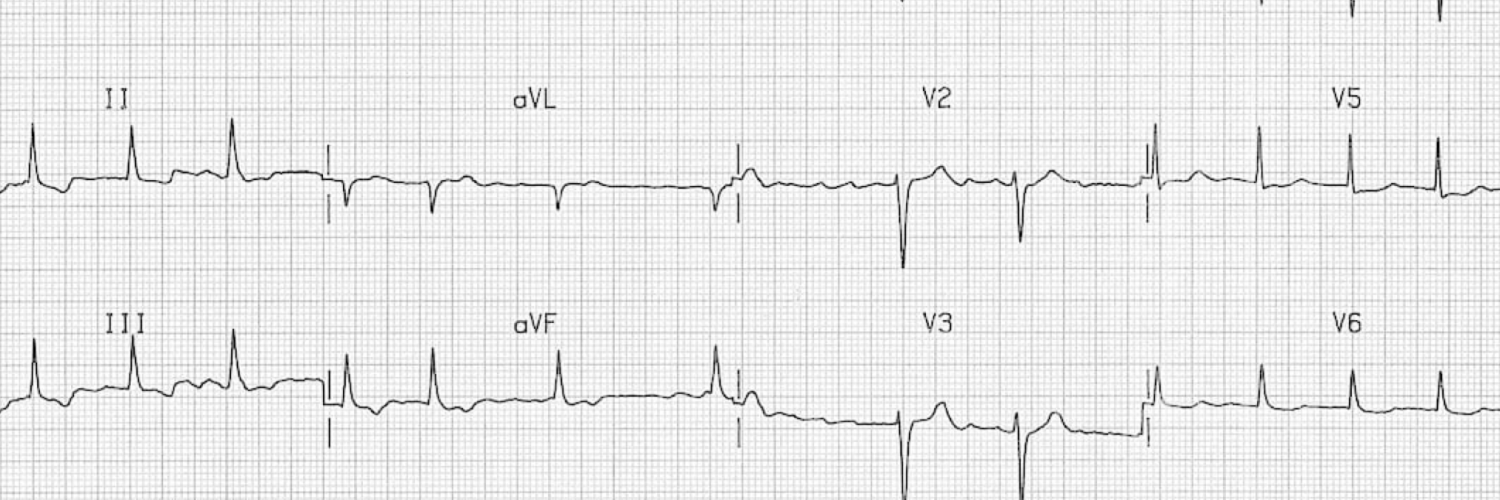
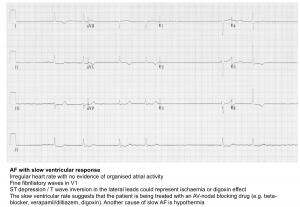
- 👉The ventricular rate may be slower (e.g. <60 bpm) in the following settings:
- Hyperkalemia
- Increased vagal tone
- Drugs that affect AV nodal conduction
- AV nodal disease, which should be suspected if the ventricular rate is below 60 bpm in the absence of a drug that slows AV conduction. Although this relates to AV node dysfunction, AF with slow rates is generally included as a manifestation of sinus node dysfunction.
👉Be careful when cardioverting patients with a heart rate <100, as there may be an increased risk of bradycardia.
- 👉Regularized AF:
- AF is an irregularly irregular rhythm. Remember that a rhythm is irregular if ’R-R’ variation in single cardiac cycle > 10%
- Regularized AF can be caused by 7
- Digoxin intoxication
- Complete heart block
New onset of AF (NOAF) in critical illness
NOAF in critical illness is used to refer to patients with no prior history of AF who are admitted in sinus rhythm and subsequently develop new-onset AF while being treated for critical illness 8. A common example is in patients with septic shock, wherein the prevalence of NOAF is ~10% 9. The main differential diagnostic consideration in patients with acute critical illness and AF are:
- A patient with chronic AF develops critical illness.
- Preexisting AF is highly prevalent among older patients with chronic conditions who are at risk for critical illness.
- A patient who was previously in sinus rhythm develops new-onset AF secondary to the physiologic stress of critical illness (e.g. secondary to sepsis, massive GI bleeding). Features that would support a diagnosis of NOAF rather than chronic, paroxysmal AF may include symptomatic AF or severe physiologic stress triggering transition into AF (e.g. AF immediately following an epinephrine bolus).
In a recent systematic review and meta analysis several risk factors were associated with development of AF in critical ill patients 10:
- Age
- Shock
- Sepsis
- Use of a pulmonary artery catheter
- Mechanical ventilation
- Increased fluid loading
- Organ failures
Prognosis and follow up
An increased risk of adverse outcomes (increased risk of stroke, ICU length of stay and mortality) in association with new-onset AF secondary to MI, sepsis, or surgery has been reported 4. However, it remains unclear whether the NOAF causes worse outcomes or the NOAF merely is a marker of sicker patients, or both 11.
- The natural history of NOAF is usually to revert to sinus rhythm on its own, as the underlying critical illness resolves.
- Irrespective of the cause of secondary AF, long-term recurrence of AF is frequently observed 12 13.
At present, there is limited data to predict which patients will develop recurrent AF beyond an assessment of the traditional risk factors for AF. As such, it is recommended that patients with secondary AF undergo careful reassessment and follow up.
Hemodynamic impact of AF
AF can lead to a fall in cardiac output with consequent decrease in blood pressure and pulmonary congestion. The following factors may contribute to the adverse hemodynamic changes in AF.
- Loss of atrial systole (aka. atrial “kick”): Contraction of the left atrium injects a volume of blood under pressure into the left ventricle, leading to increments in ventricular diastolic volume and stroke volume. Loss of atrial kick can therefore diminish the stroke volume. This may be particularly important in the following conditions:
-
- Impaired left ventricular compliance: LV compliance is reduced in diastolic dysfunction. In such patients, there is a relative shift of left ventricular filling to the later part of diastole with a greater dependence upon atrial contraction. Clinical examples in which AF can lead to hemodynamic deterioration in patients with diastolic dysfunction include advanced aortic stenosis, which often produces a hypertrophied, poorly compliant left ventricle, and hypertrophic cardiomyopathy.
- Mitral stenosis: Among such patients, the onset of AF can lead to hemodynamic deterioration. Two factors contribute to this adverse effect: the loss in atrial systole, which plays an important role in the generation of adequate left atrial pressure to maintain blood flow across the stenotic valve and may also reduce effective mitral valve area; and the rapid ventricular response which, due to the reduction in the duration of diastole, diminishes the time available for filling of the left ventricle
- Pulmonary hypertension: As left atrial and pulmonary venous pressures increase due to diastolic dysfunction, this increases the pulmonary artery pressure through passive back-transmission of this hydrostatic pressure. With more advanced stages of HFpEF, there may also be changes in pulmonary vascular structure and function leading to a “precapillary” component where pulmonary vascular resistance increases.
2. Rapid ventricular response
- While moderate tachycardia often leads to increased cardiac output (CO= HR x SV), severe tachycardia (e.g. HR>150) tends to decrease stroke volume as the LV diastolic filling is reduced.
- The dependence of cardiac output on heart rate is greatest in patients with left ventricular dysfunction, which is often associated with a relatively fixed stroke volume. As an example, the cardiac output is optimal over a narrow range of heart rates in patients with a myocardial infarction, falling significantly with slower or faster heart rates. A rapid ventricular response is also particularly deleterious in patients with mitral stenosis in whom the reduction in the duration of diastole diminishes the time available for filling of the left ventricle across the stenotic valve. The optimal heart rate for patients in permanent AF is discussed separately.
3. Other hemodynamic consequences
- It has been shown in several studies that AF can lead to increased mitral annular dilatation and mitral regurgitation (MR) 14.
- The presence of AF can create a cycle of worsening AF burden and increasing MR, which can be reversed with a persistent return to normal sinus rhythm.
- Increased left atrial size in the presence of normal left ventricular function has been associated with increased mitral annular size and more severe MR in some studies, but not in all.
Etiology of NOAF
- Electrolyte abnormalities (especially hypokalemia and hypomagnesemia)
- Endocrine disorder: thyrotoxicosis, pheochromocytoma
- Adrenergic overstimulation:
- Medications: Beta-agonists (norepinephrine, epinephrine, dobutamine), theophylline
- Stress (pain, anxiety), including physiologic stress e.g. shock
- Alcohol withdrawal
- Substance use (especially cocaine, amphetamine, methamphetamine)
- Primary neurologic disorders (intracranial hemorrhage, ischemic stroke)
- Inappropriate oxygen delivery to the myocardium
- Myocardial ischemia
- Hypovolemia
- Anemia e.g. GI bleeding
- Respiratory failure: Pulmonary embolism, pneumonia, COPD, hypoxemia, hypercapnia
- Systemic and local inflammation
- Heart-Lung machine
- Sepsis
- Myocarditis
- Myocardial stretch (atrial hypertension, atrial dilatation, reduced contractility)
- Fluid overload 15
- Acute mitral insufficiency
- Mitral stenosis
- Surgery: cardiac or non-cardiac surgery
- Electrocution and Swan-Ganz catheterization
- Hypothermia
Evaluation
Basic evaluation
- ECG
- Electrolytes, including magnesium
- Complete blood count
- Review of medication list
- Review of the presence of any indwelling cardiac devices
- Point of care ultrasound/echo (see media below):
- Is there an underlying abnormal substrate (cardiac abnormality)?
- LV EF? (systolic dysfunction)
- Diastolic dysfunction
- Valvular heart disease esp. mitral stenosis/regurgitation, aortic stenosis
- Chamber size: left and right atrial diameter
- RV function
- Pulmonary arterial pressure
- Are there any other causes of instability?
- Heart: massive pulmonary embolism, acute myocardial infarction, cardiac tamponade?
- Abdomen: aortic dissection? hemorrhage?
- Lungs: pulmonary edema? empyema/pneumonia?
- IVC: volume status? 👉volume depletion with absent B-lines strongly suggests that the hemodynamic instability is NOT the result of rapid ventricular response, but that rapid ventricular response is the result of volume depletion.
- The TTE may also identify left atrial thrombus, although the sensitivity is low
- Is there an underlying abnormal substrate (cardiac abnormality)?
Additional tests as clinically warranted. For example:
- Troponin: only If review of ECG and history suggests ischemia.
- TSH should be considered if
- There is no obvious cause of AF.
- If other clinical features suggest thyrotoxicosis.
- New onset of AF especially in age >55 yo (since in this population, classic signs and symptoms of hyperthyroidism could be absent).
- If rapid ventricular response is difficult to control despite appropriate measures.
- When clinical status of the patient worsen following administration of amiodarone.
Approach to management of AF in critical illness
General principles
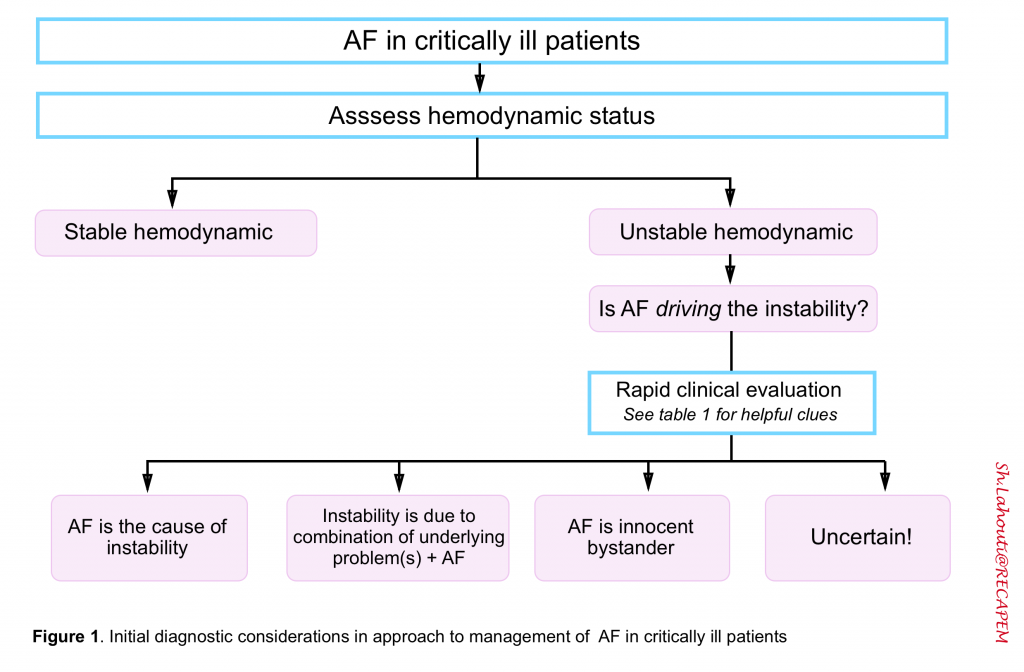
The first priority when evaluating AF in a critically ill patient is an assessment of hemodynamic status (figure 1). Hemodynamic instability confer an imminent risk of further deterioration, irreversible organ injury, or cardiac arrest and manifested as 16
- Acute altered mental status
- SBP < 90 mmHg (MAP<65 mmHg)
- Ischemic chest pain
- Dyspnea from pulmonary congestion
- HR in extremis
- Signs and symptoms of organ hypoperfusion e.g. low urine output, mottled/cold clammy skin.
Circulatory failure (i.e. shock) is often multifactorial in practice, resulting from multiple overlapping derangements in hemodynamic parameters (i.e. Pump failure, tank or pipe problems).
In the context of tachy-or-bradyarrhythmias, the rule of “Primacy of the Heart Rate” implies that heart rate at extremis should be corrected at the first place even in the presence of multiple overlapping perfusion problems.
- However this rule often is not true for patients with “AF plus instability”. Therefore the key question in approach to unstable patients with AF is: What is driving the instability? (figure 1 below)
- Is the AF causing the patient to be unstable? or is AF merely triggered by underlying instability?
Why is it crucial to sort out if the AF is the cause of hemodynamic instability?
- Because if tachycardia is due to an underlying process, then overly aggressive attempts to reduce the heart rate to a “normal” range may make the condition worse (remember that a mild degree of tachycardia may actually have a compensatory, beneficial effect). More on this below.
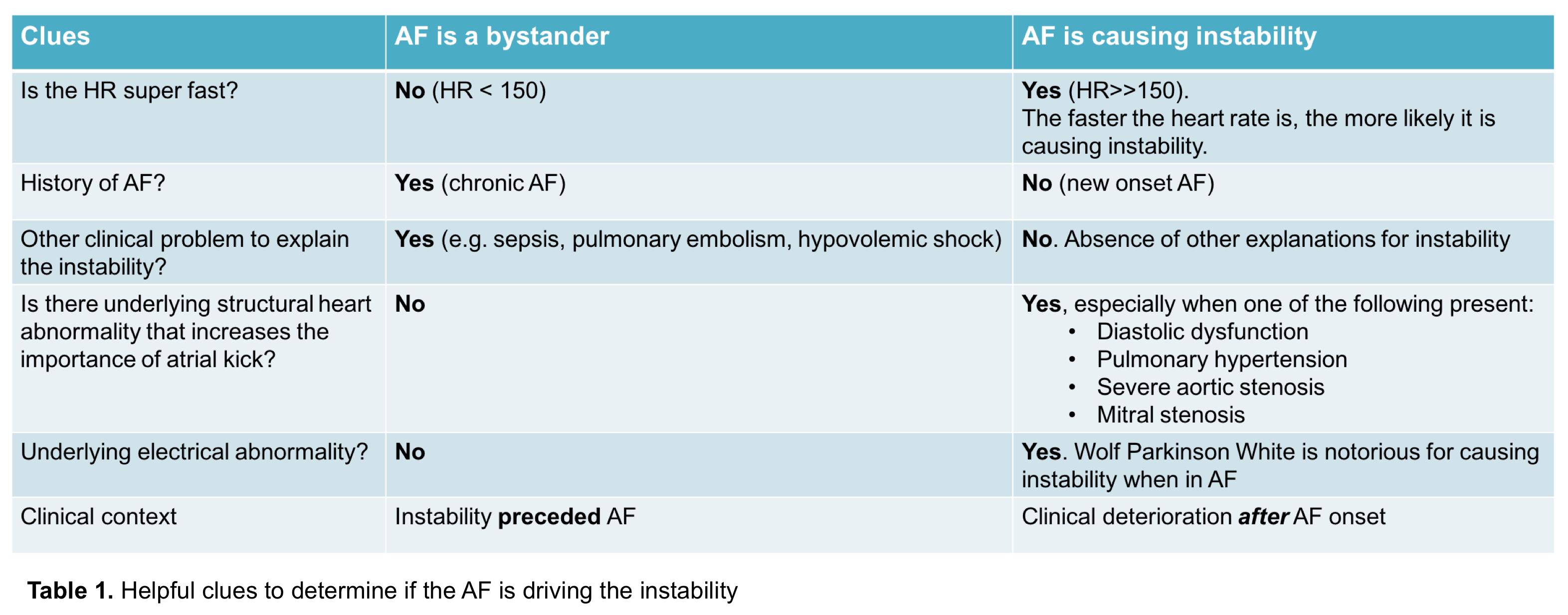
Is this AF an innocent bystander or criminal in a patient with hemodynamic instability? Helpful clues to sort out if the atrial fibrillation is driving the hemodynamic instability are summarized in the following table. 34
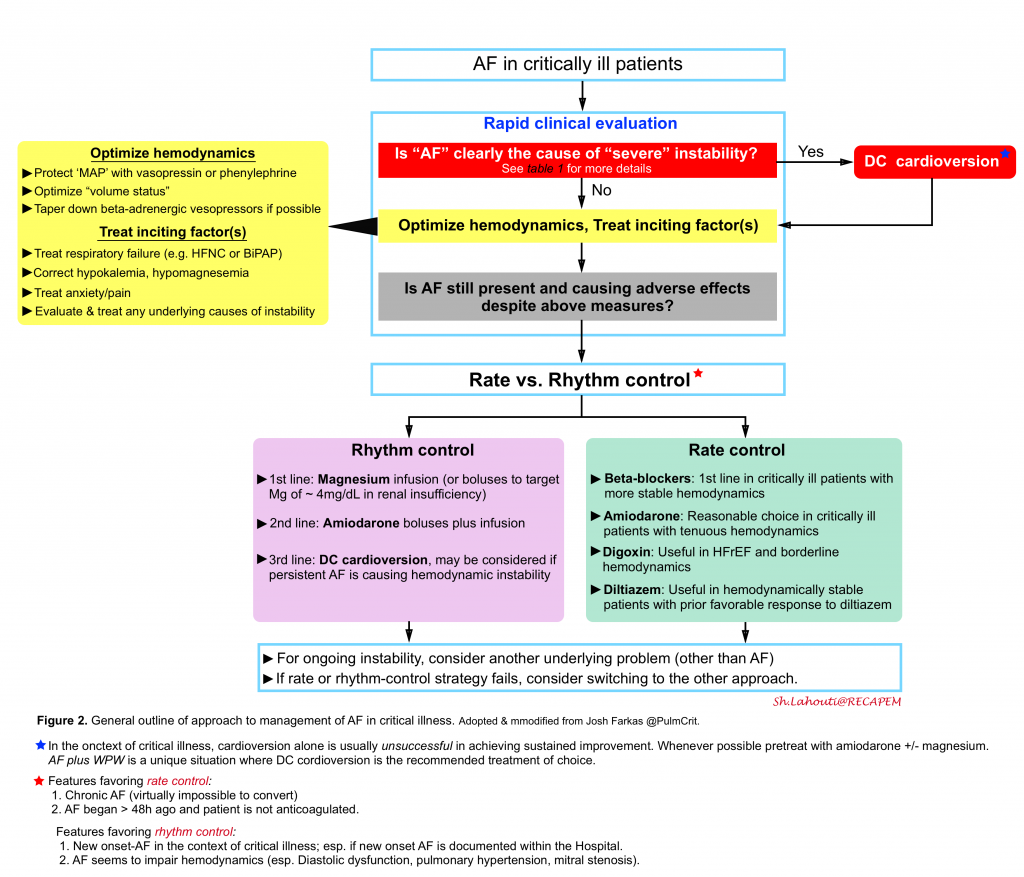
Overview of management: Algorithm
The general outline to management of AF in critical illness is illustrated in the following figure.
Emergent cardioversion
- DC cardioversion will stabilize the patient only if the AF is causing the instability. Therefore it is only indicated if new-onset AF clearly caused the patient to be severely unstable.
- 👉This is unusual for most critically ill patients, the AF isn’t the primary driver of instability.
- Even if cardioversion was successful, most patients with critical illness will revert to AF subsequently 18.
- If possible, pretreatment or post-treatment with amiodarone +/- magnesium may enhance the likelihood of achieving and maintaining sinus rhythm.
- AF with accessory pathway (WPW) should be treated with DC cardioversion.
- If a patient with AF and an accessory pathway is displaying instability, proceeding directly to DC cardioversion is indicated.
- AF with an accessory tract shouldn’t be treated with medications that impair the AV node (e.g. beta-blockers, calcium channel blockers, or amiodarone). Blockade of the AV node may merely cause a greater dominance of the accessory pathway, exacerbating matters (to a certain extent, the AV node and the accessory pathway are competing for control of the ventricle).
- Antiarrhythmics which may be used are procainamide or ibutilide.
Common therapeutic measures for AF in unstable patients
The most important intervention for critically ill patients with AF is usually treating the causes of AF. There is a risk of getting overly focused on antiarrhythmics and cardioversion, but the most important interventions are often as follows.
Hemodynamic optimization
Optimize volume status
- AF may be caused by volume overload, which causes atrial dilation. If volume overload is present, diuresis may be beneficial.
- If frank hypovolemia is present, then volume administration may be beneficial.
Protect MAP
- If hypotensive, add vasopressor without beta-adrenergic activity e.g. vasopressin, phenylephrine.
- Phenylephrine is a good choice, because it will give you alpha-1 vasoconstriction, without stimulating the beta-receptors and raising the rate. More on phenylephrine here.
- Dose (push): 50-200 mcg q1-2 mins PRN (goal DBP ≥ 60)
- Dose (drip): 40-180 mcg/min, titrated to goal
Discontinue beta-adrenergic vasopressors, especially epinephrine, dobutamine and dopamine may increase heart rate and should be weaned if possible
Treatment of inciting factors
Correct electrolyte abnormalities
- Hypokalemia and hypomagnesemia may trigger AF, so if present these should be treated aggressively.
- The magnesium level should be checked, but administration of magnesium doesn’t need to wait until the level returns. If magnesium hasn’t been checked recently, it is reasonable to empirically give 2-4 grams of magnesium sulfate provided that the patient’s renal function is normal.
Treatment of pain/anxiety/withdrawal
- Untreated distress can drive sympathetic tone and aggravate AF.
- Pain, anxiety should always be treated adequately.
Respiratory support
- Hypoxemia or respiratory distress may be drivers of AF.
- Respiratory failure should be aggressively treated (e.g. CPAP for heart failure, BiPAP for COPD, HFNC for pneumonia).
Evaluation and treatment of other causes of hemodynamic instability
- Avoid anchoring excessively on AF as the cause of the patient’s hemodynamic instability.
- For ongoing instability, evaluate broadly and treat appropriately. For example, uncontrolled AF could be a manifestation of sepsis, PE, thyrotoxicosis, or any cause of shock.
- If AF is being driven by another underlying process, focusing solely on suppressing the heart rate with medications will fail and may actually make the patient worse.
Rate control vs. rhythm control
No RCTs have been performed comparing rate control vs. rhythm control in a general ICU population. Available studies show no differences in hard endpoints (e.g. mortality or stroke). However, this doesn’t exclude the possibility that some subgroups of patients might benefit more from one strategy.
By enlarge, both strategies are reasonable and the choice may depend on patient specifics.
- The decision to provide rate or rhythm control for AF depends on the clinical context and the risk-benefit balance must take into consideration the underlying medical condition.
Factors favoring a rate-control strategy
- Chronic AF is perhaps the strongest indicator for a rate-control strategy. AF leads to electrical remodeling, which leaves the atria less able to convert to sinus rhythm. The longer patients are in AF, the harder it is to convert to sinus rhythm.
- Onset of AF >48 hours previously, in a patient who isn’t anticoagulated: In this situation, conversion to sinus rhythm may theoretically increase the risk of stroke due to dislodging a clot from the atrial appendage.
Factors favoring rhythm control strategy
- Inability to tolerate AF hemodynamically: AF can cause hemodynamic instability, especially in patients with chronic pulmonary hypertension, mitral stenosis, or diastolic dysfunction (situations where the atrial kick may be especially beneficial).
- New-onset AF: Patients often develop new AF in the context of critical illness. If this occurs while being monitored in the hospital, it may warrant an attempt at rhythm control.
- Atrial flutter: This is often a transitional state, as the atria is deciding whether to settle down into sinus rhythm or atrial fibrillation. Atrial flutter is often difficult to treat using rate control, since the rate tends to be stubbornly stuck at around 150
In the absence of high quality evidence regarding the optimal approach to NOAF in the context of critical illness (e.g. sepsis), several consideration should be made here:
- Most patients with NOAF will eventually revert back into sinus rhythm on their own. However, the risk of stroke may relate to the duration of time that the patient spends in AF (e.g. >48 hours in NOAF may increase stroke risks). If we can convert patients out of NOAF rapidly, this ought to reduce the risk of stroke.
- Not all patients with NOAF will spontaneously revert back into sinus rhythm. The longer AF is allowed to continue, the less likely it is to revert to sinus rhythm. Ongoing atrial fibrillation causes electrical remodeling of the atria, which perpetuates the atrial fibrillation (hence the clinical adage, “AF begets AF”). Prompt cardioversion out of NOAF could theoretically increase the likelihood that the patient could successfully revert to sinus rhythm and stay in sinus rhythm on a long-term basis
- AF may impair cardiac function in a subset of patients, due to impaired atrial kick. Cardioversion out of NOAF might therefore improve cardiac function in some patients, allowing them to compensate better for their acute critical illness.
Rate control strategy for critically ill patients
The target heart rate for outpatients is often regarded to be <110 19. However the optimal HR for critical patients have not been well studied.
- Keep in mind that at very fast HR e.g. >150, the diastolic filling impairment may drop the stroke volume and BP.
- However, at heart rates < 150, the diastolic filling is often preserved, so the dominant driver of cardiac output may be the heart rate. Thus, for example, causing a drop in the heart rate from 130 to 90 may often cause a drop in the cardiac output.
- Trying to “normalize” the heart rate (e.g. targeting a rate below 100) may increase the risk of iatrogenic harm in patients with tenuous hemodynamics.
- For many critically ill patients, a heart rate goal below ~130 might be reasonable. The target may vary depending on patient specifics and clinical response. The optimal target may also vary over time. For example, initially a target of <130 may be reasonable, but as the patient recovers a lower target may become appropriate
Generally, four medications are commonly used for rate control: amiodarone, beta-blocker, diltiazem, digoxin. Several factors should be considered when selecting a rate-controller among the list (see table below).
- The most important consideration is how stable the patient is (since most of these agents can cause hypotension).
- Effect on likelihood of cardioversion
- Comorbidities
🛠Troubleshooting: If the patient continues to have a fast ventricular response, one should consider the following conditions
- Re-evaluate the patient for possible inappropriately managed of underlying problem e.g. hypovolemia, pulmonary edema, sepsis, pulmonary embolism, thyrotoxicosis
- Consider adding additional magnesium.
- Try a different agent: Avoid combining beta-blockers and calcium-channel blockers (overlapping these agents may increase the risk of hypotension). Adding on amiodarone is often useful here (it is more hemodynamically stable and less likely to cause synergistic hypotension in combination with other agents).
- Atrial flutter: May consider flutter as heart rate control is often difficult. The heart rate has a tendency to get “stuck” at ~150 (2:1 transmission through the AV node) in atrial flutter.
Mechanism of action: broad spectrum antiarrhythmic action by blockade of certain types of potassium channels i.e. Ikr (prolongs the action potential duration; QT interval on ECG) and the inactivated sodium channels (depolarized cells). It has weak beta-adrenergic and calcium channel-blocking actions (slowing down the HR and AV conduction).
Indication: May be used for rate-control in AF with RVR in patients with potential hemodynamic instability.
- Achieving rate control may require reloading with 150 mg amiodarone 2-3 times.
- Don’t conclude that amiodarone has failed to work without re-bolusing adequately.
There is a theoretical risk of causing stroke among patients who aren’t anticoagulated and might cardiovert into sinus rhythm. However, for critically ill patients who are hemodynamically tenuous, this theoretical risk may often be superseded by the need to achieve hemodynamic stability.
Onset of action (IV route)
- Electrophysiologic effects occur within hours, while antiarrhythmic effects take 3 days to 1-3 weeks.
-
Mean onset of effect may be shorter in children vs adults and in patients receiving IV loading doses;
Amiodarone dosing
- Initial IV dosing
- Load with 150 mg bolus, then infuse at 1 mg/minute.
- May re-load 1-2 times if inadequate response (for a total of 150-450 mg given in the form of boluses) 20.
- Conversion to oral
- Infusion may be converted to oral administration after >24 hours.
- Start at 400 mg PO BID, until the patient has received a total of 10 grams cumulative dose (both IV and PO). Subsequently, the dose may be decreased to 200 mg daily.
- Eventually transition to another agent
- Chronic use of amiodarone causes a host of side effects.
- After patients recover from their critical illness, they should be transitioned to a safer long-term regimen (e.g., a beta-blocker)
Mechanism of action: Beta-blockade activity slows down atrioventricular conduction with an increased PR interval and AVN conduction velocity therefore slowing down ventricular response rate in AF. They poses negative inotropic and chronotropic effects. The β-receptor–blocking drugs differ in their relative affinities for β1 and β2 receptors
- Metoprolol, esmolol: β1>>β2 receptor (however, no currently available β1-selective antagonist is sufficiently specific to completely avoid interactions with β2-adrenoceptors).
- Propranolol: β1=β2 receptor
Selection of beta blocker
- IV metoprolol is usually the agent of choice.
- IV esmolol infusion may be used if it’s unclear whether the patient will tolerate a beta-blocker. This has the advantage that if it causes hypotension, it can be stopped and will wear off fairly rapidly (over ~10 minutes). If the patient responds well to esmolol, they may be transitioned to a longer-acting beta-blocker.
- Blockade of the β2 receptors in bronchial smooth muscle may lead to an increase in airway resistance, particularly in patients with asthma.
- These drugs should generally be avoided in patients with asthma.
- However, some patients with chronic obstructive pulmonary disease (COPD) may tolerate β1-selective blockers, and the benefits, for example in patients with concomitant ischemic heart disease, may outweigh the risks.
Metoprolol dosing
- Initial IV loading
- Usually start with 5 mg IV, which should take effect within ~5 minutes.
- Additional doses may be given every 5 minutes up to a total of 15 mg as needed. Dosing should be titrated to effect (reduction in heart rate, without causing hypotension).
- Ongoing IV therapy
- For patients without enteral access, scheduled IV doses may be required.
- The intravenous form wears off faster than the oral form, so more frequent dosing may be required than is usual with oral metoprolol (e.g. the initial loading dose may be repeated q4hr-q6hr, depending on heart rate and blood pressure).
- Transition to oral administration (PDF)
- The initial oral dose may be estimated based on the IV dose required, based on a 1 to 2.5 conversion from IV to PO:
- Response to 5 mg IV → Start metoprolol tartrate 12.5 mg PO q6hr.
- Response to 10 mg IV → Start metoprolol tartrate 25 mg PO q6hr.
- Response to 15 mg IV → Start metoprolol tartrate 37.5 mg PO q6hr.
- The first oral dose can be started 20 minutes after the initial IV dose.
- Metoprolol tartrate usually isn’t given every six hours. However, starting with more frequent dosing may allow more flexibility in dose adjustment.
- The initial oral dose may be estimated based on the IV dose required, based on a 1 to 2.5 conversion from IV to PO:
Esmolol dosing
- Starting esmolol: Load with 0.5 mg/kg IV over 1 minute. Then start the infusion at 0.05 mg/kg/min for 4 minutes.
- Assess the response to this initial infusion rate: it may be a rough indication of the responsiveness of the ventricular rate.
- If effective, continue with infusion at 50 mcg/kg/minute.
- If ineffective, the infusion may be up-titrated as follows:
- Reload with 0.5 mg/kg IV
- Increase the infusion rate by 0.05 mcg/kg/min
- Up-titration may be performed about every half hour as needed (up to a maximal infusion rate of 0.2 mg/kg/min).
Propranolol: 1 mg IV over one minute. It may be repeated up to three doses at two-minute intervals.
Diltiazem, verapamil: diltiazem may have less pronounced negative inotropic and hypotensive effects than verapamil. Diltiazem is more frequently used for AF with RVR.
Indication: It may be useful in patients who are hypertensive or more hemodynamically robust, especially patients who have been chronically treated with diltiazem as outpatients.
Diltiazem dosing 21
- Initial loading dose
- Start with 0.25 mg/kg (max dose 25 mg) IV bolus.
- if the first dose is tolerated but does not produce the desired response (20% reduction in HR from the baseline) a second bolus of 0.35 mg/kg (max dose dose 25 mg) is given once after 15 minutes
- Maintenance infusion
- In those who respond to the first or second bolus, a continuous infusion at a rate of 5 to 15 mg/h is initiated.
- Consider a reduction in the infusion rate after the target heart rate is reached. Especially with hepatic dysfunction, diltiazem may accumulate.
- Transition to oral
- Start diltiazem extended release (diltiazem-ER) at a dose roughly equal to 10 [3(infusion rate in mg/hr) +3]. When in doubt, round down. For example:
- 3 mg/hour →120 mg/day diltiazem-ER
- 5 mg/hour →180 mg/day diltiazem-ER
- 7.5 mg/hour → 260 mg/day diltiazem-ER
- 10 mg/hour → 330 mg/day diltiazem-ER
- 15 mg/hour →480 mg/day diltiazem-ER
- Wean off infusion over the next few hours.
- Start diltiazem extended release (diltiazem-ER) at a dose roughly equal to 10 [3(infusion rate in mg/hr) +3]. When in doubt, round down. For example:
Mechanism of action
- It inhibits the sodium-potassium pump, increasing the calcium availability to the contractile apparatus.
- Through centrally mediated vagal stimulation (vagotonic effect), it directly controls the ventricular response via action on AV node.
Disadvantages
- The benefit of digoxin is limited in hyperadrenergic states, limiting its efficacy in critically ill patients.
- Narrow therapeutic index, so a small variation in plasma concentration may rapidly result in toxic or subtherapeutic concentration.
Indications: Potential candidates for digitalization include
- HFrEF
- Digoxin has positive inotropic effects
- Uniquely beneficial for patients with heart failure whose hemodynamics are very tenuous, who may have difficulty tolerating a negative inotrope.
- Mild or moderate tachycardia for which immediate control isn’t necessary
- Digoxin takes several hours to work and it’s not tremendously potent.
- Chronic AF
- Digoxin tends to perpetuate AF, rather than favoring cardioversion back to normal sinus rhythm
Loading dose (digitalization): Digoxin takes a little while to work, but if given intravenously it may take effect within several hours. When initiated for critically ill patients, digoxin will nearly always be started with intravenous loading doses.
👉Total IV loading dose 22
- Normal renal function: 8-12 mcg/kg ideal body weight (usually ~ 600-1,000 mcg).
- Renal insufficiency: 6-10 mcg/kg ideal body weight.
- Err on the lower end in patients with renal dysfunction, hypothyroidism, and/or reduced muscle mass.
- Typically, 50% of the total loading dose is given initially, followed by 25% given twice, every six hours. The first IV dose (typically ~400-600 mcg) takes effect within roughly 1-4 hours.
- Monitor for effect. If an adequate heart rate is achieved, then subsequent doses may be omitted. If bradycardia occurs, further administration should be held.
👉Maintenance doses
- Patients <70 years old with normal renal function: 250 mcg daily.
- Patients over 70 years old or with renal dysfunction: 125 mcg daily.
- Patients who are both >70 yo and have renal dysfunction: 62.5 mcg daily.
- Digoxin has a long half-life (~36-48 hours, or longer in renal insufficiency). Therefore, steady state may not be reached until about a week after a dose adjustment.
Monitoring digoxin levels
- Drug level must be checked several hours after the last digoxin dose, to allow for distribution (e.g. > 8 hours after an oral dose). Ideally this should be a trough level.
- A therapeutic level is ~ 0.5-2 ng/mL
- 0.5-1 ng/mL might be the optimal concentration for outpatients.
- 1-2 ng/mL levels may improve contractility, so these aren’t unreasonable levels for closely monitored ICU patients.
If digoxin fails
- Digoxin is not an extremely powerful agent, so it may fail to achieve optimal heart rate control.
- If digoxin does fail, it may be combined with a beta-blocker or diltiazem.
- The presence of digoxin may reduce the required dose of beta-blocker or diltiazem, thereby improving hemodynamic stability.
- In particular, the combination of digoxin plus a beta-blocker may work well for some patients with systolic heart failure.
Rhythm control strategy in critically ill patients
- Among critically ill patients, magnesium seems to have similar efficacy when compared to other antiarrhythmics 23
- It has an excellent safety profile 24
- Given its efficacy and safety profile, magnesium is the first line agent among critically ill patients 9.
- Potential benefits of magnesium even if it fails cardioversion:
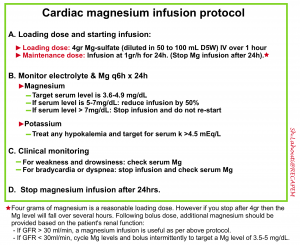
- Dosing: For patients with adequate renal function (GFR > 30 ml/min), a protocoled magnesium infusion may be used as shown below 29.
- For patients with renal insufficiency, intermittent boluses of magnesium may be utilized (targeting a level of 3-4 mg/dL).
Amiodarone is usually the preferred antiarrhythmic for ICU patients due to its ability to both cardiovert patients and subsequently prevent recurrent AF.
Dosing
Start with ~150-300 mg amiodarone load and an infusion of 1 mg/min. If unsuccessful, one or more reloading doses may be given (up to a total of ~450-600 mg in the form of IV boluses).
- Note that the dose of amiodarone required for chemical cardioversion is often large (e.g. some sources recommend 5-7 mg/kg infused over an hour) 30. Many patients won’t cardiovert in response to a 150 mg loading dose of amiodarone, but may nonetheless respond to additional loading doses.
👉Even if amiodarone fails to work immediately, continue the infusion, as some patients may have delayed cardioversion (e.g. within the first 24 hours after starting the amiodarone infusion).
👉If the patient does cardiovert in response to amiodarone, consider continuing the amiodarone infusion until they are substantially improved (e.g. for about a week). If amiodarone is stopped early (while the patient is still critically ill), patients are likely to revert into AF.
For patients in whom amiodarone is contraindicated, ibutilide may be considered.
The main drawback of ibutilide is that, unlike amiodarone, it doesn’t provide ongoing antiarrhythmic support to prevent AF recurrence.
The advantage of ibutilide over amiodarone is that it less frequently causes a drop in BP.
Ibutilide may be superior to procainamide among critically ill patients, because it has greater efficacy and no negative hemodynamic effects 31 32
Contraindications: QT prolongation, hypokalemia, hypomagnesemia, ejection fraction <30%, or severe LV hypertrophy.
Dosing: The standard dose is 1 mg infused over 10 minutes (or 0.01 mg/kg for patients <60 kg). This can be repeated once if needed. If cardioversion occurs while ibutilide is being infused, the infusion should be stopped. Patients should be monitored for ~4 hours subsequently (although arrhythmias will generally occur within the first hour after administration).
Most patients with NOAF will cardiovert in response to infusion of magnesium and amiodarone (especially if ~24 hours are allowed for the infusions to take effect).
Failure of magnesium and amiodarone to work suggests that the patient’s heart doesn’t want to go into a sinus rhythm (e.g. perhaps due to significant chronic atrial dilation, underlying structural heart disease, or profound systemic inflammation). In this situation, it’s difficult to know whether to accept AF or continue efforts towards conversion to normal sinus rhythm.
Technique
- When available, a biphasic defibrillator is preferred due to greater efficacy.
- For atrial fibrillation, 120 to 200 joules for biphasic devices and 200 joules for monophasic devices.
- Use full dose electricity on the first attempt (e.g. 200 joules on biphasic machine).
- Positioning: An anterior-posterior pad positioning is probably better than anterior-lateral 33
- Sedation: If you have time to sedate before delivering the shock, it will be greatly appreciated. The choice of agent is difficult since all sedatives will drop the blood pressure, which could be catastrophic. Etomidate and ketamine are viable options.
- Etomidate: Short acting, making assessment of mental status in patients with shock feasible. Start with 0.1mg/kg (~7-10mg) IV; repeat if needed.
- Ketamine: Longer acting than etomidate which make assessment of mental status in unstable patient problematic. Start with 0.5mg/kg IV; repeat if needed.
- May try 2 or 3 shocks in rapid sequence, but if the first is not successful, then success on a second or third one is low.
Anticoagulation
Critically ill patients often have systemic inflammation (which may increase their risk for thromboembolic stroke compared to outpatients). On the other hand they also have numerous risk factors for bleeding (e.g. renal dysfunction, use of antiplatelet medications, invasive procedures).
Risk scores for bleeding and thrombosis haven’t been validated in the ICU (e.g. CHA₂DS₂-VASc Score for AF Stroke Risk, HAS-BLED Score for Major Bleeding Risk). This makes it difficult to balance the risks versus benefits of anticoagulation accurately.
Possible recommendation for anticoagulation among critically ill patients with AF
- New onset AF
- The Canadian 2020 guidelines state that “In some cases, such as sepsis, the acute administration of intravenous anticoagulation increases the risk of bleeding, but does not appear to reduce the risk of ischemic events.
- If the AF persists for weeks, then it becomes increasingly likely that the patient may develop ongoing AF. In this situation, anticoagulation may become beneficial
- Chronic AF
- For patients who appear to have chronic AF, anticoagulation may be considered similarly to that of outpatients (e.g., based on CHA2D2-VASC scores).
- Among patients with long-standing AF who have been previously on anticoagulation, this will often be continued (unless it is necessary to hold it for a procedure or bleeding).
Choice of anticoagulation
Oral anticoagulants include warfarin and direct oral anticoagulants (DOAC), which were previously known as novel oral anticoagulants.
DOAC
Most patients should receive a DOAC (apixaban, dabigatran, edoxaban, or rivaroxaban) in preference to warfarin when OAC therapy is indicated for patients with non-valvular AF.
- DOAC include either direct thrombin inhibitors (dabigatran) or Factor Xa inhibitors (apixaban, edoxaban, rivaroxaban).
- DOACs have largely replaced warfarin due to their ease of use, which includes the elimination of bridging, monitoring, and dietary restrictions. Concerns regarding the current inability to reverse DOACs in case of bleeding have gradually decreased given the continued introduction of reversal agents.
- Dosing is dependent on and may be limited by the patient’s renal and hepatic function (see table 2 below).
Warfarin
It should be used for patients with a mechanical prosthetic valve and those with AF and moderate to severe mitral stenosis (when indicated).
- Initiate warfarin: 5 mg daily; (1–2 mg daily if frail, low weight, Asian descent).
- Heparin bridging not required unless TEE-guided CV.
- Arrange for INR blood test and review after 3 or 4 doses of warfarin. Subsequent warfarin doses should be communicated to patient on the day of the INR test.
Back to drawing case
Immediate trial of DC cardioversion with 200J by biphasic machine followed by second and third shocks was unsuccessful!
POCUS shows bilateral B-profile suggestive for pulmonary edema and moderate pleural effusion as well as massive ascites.
- Further investigation reveals that the patient has received six liters of fluid initially, and has been running net positive 3 liters daily since then (for a total of about 10 liters net positive).
Following administration of PUSH dose phenylephrine, hemodynamically neutral intubation was performed and the patient hooked to mechanical ventilator. After 12 hours of resuscitative measures including ventilation and hemodynamic support, atrial fibrillation converted to sinus rhythm!
RECAP
- Do NOT fall into a trap of AF with RVR in hypotensive patients; assuming that AF is the cause of instability. In some patients, this may be a “sinus tach equivalent” which is due to an underlying problem (e.g. sepsis, PE). Merely trying to slow down the heart rate can be dangerous among these patients, as it may suppress a compensatory tachycardia. 👉Avoid rate control before addressing and treating the underlying etiology. 34
- In patients with AF and shock, always look for other causes of hemodynamic instability. One helpful clue is that if the HR is <150, the hypotension is likely caused by something other than the arrhythmia. If the HR is >150, the arrhythmia is more likely to be the culprit. Other helpful clues are summarized in table 1.
- If another cause of shock is identified (e.g. hypovolemia, hemorrhage, sepsis, PE, etc.), treat the underlying cause; treating the compensatory tachycardia may worsen the situation.
- ACLS guidelines typically recommend immediate cardioversion for unstable patients with AF. However, among critically ill patients this has a low success rate.
- Support the hemodynamic and treat the inciting factors!
- Be aware of AF in WPW. If your patient has HR>250 and irregular (in rate and shape and amplitude) wide complex tachycardia, think WPW and avoid nodal block medication. Immediate DC cardioversion is advised.
AF Tool
The American College of Emergency Physicians “AF Tool”, exists as a valuable guide for the care of ED patients with AF.
Media
Atrial fibrillation on echo
10 simple rule of atrial fibrillation on echo
Primary AF causing functional MR
Post Peer Reviewed By: Mojtaba Chardoli.
Further going
- Treatment of hemodynamically stable new-onset AF in critical illness (PULMCRIT)
- The Crashing Atrial Fibrillation Patient (EMCrit)
- Approach to shocky patient in AF with RVR (PulmCrit)
References
1. Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014 Apr 25;114(9):1453-68. doi: 10.1161/CIRCRESAHA.114.303211. PMID: 24763464
2. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021 Feb 1;42(5):373-498. doi: 10.1093/eurheartj/ehaa612. Erratum in: Eur Heart J. 2021 Feb 1;42(5):507. Erratum in: Eur Heart J. 2021 Feb 1;42(5):546-547. Erratum in: Eur Heart J. 2021 Oct 21;42(40):4194. PMID: 32860505
3. Oishi ML, Xing S. Atrial fibrillation: management strategies in the emergency department. Emerg Med Pract. 2013 Feb;15(2):1-26; quiz 27. Epub 2013 Jan 20. PMID: 23369365
4. Andrade JG, Aguilar M, Atzema C, Bell A, Cairns JA, Cheung CC, Cox JL, Dorian P, Gladstone DJ, Healey JS, Khairy P, Leblanc K, McMurtry MS, Mitchell LB, Nair GM, Nattel S, Parkash R, Pilote L, Sandhu RK, Sarrazin JF, 5. Sharma M, Skanes AC, Talajic M, Tsang TSM, Verma A, Verma S, Whitlock R, Wyse DG, Macle L; Members of the Secondary Panel. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Comprehensive Guidelines for the Management of Atrial Fibrillation. Can J Cardiol. 2020 Dec;36(12):1847-1948. doi: 10.1016/j.cjca.2020.09.001. Epub 2020 Oct 22. PMID: 33191198
5. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014 Dec 2;130(23):e199-267. doi: 10.1161/CIR.0000000000000041. Epub 2014 Mar 28. Erratum in: Circulation. 2014 Dec 2;130(23):e272-4. PMID: 24682347; PMCID: PMC4676081
6. Silverman A, Taneja S, Benchetrit L, Makusha P, McNamara RL, Pine AB. Atrial Fibrillation in a Patient With an Accessory Pathway. J Investig Med High Impact Case Rep. 2018;6:2324709618802870. Published 2018 Sep 28. doi:10.1177/2324709618802870
7. Manne JRR. Regularized Atrial Fibrillation. Am J Med. 2018 Sep;131(9):e361-e363. doi: 10.1016/j.amjmed.2018.04.010. Epub 2018 May 1. PMID: 29723516
8. Bosch NA, Cimini J, Walkey AJ. Atrial Fibrillation in the ICU. Chest. 2018 Dec;154(6):1424-1434. doi: 10.1016/j.chest.2018.03.040. Epub 2018 Apr 6. PMID: 29627355; PMCID: PMC6335260
9. Arunachalam K, Kalyan Sundaram A, Jha K, Thakur L, Pond K. Evaluation of Anticoagulation Practice With New-Onset Atrial Fibrillation in Patients with Sepsis and Septic Shock in Medical Intensive Care Unit: A Retrospective Observational Cohort Study. Cureus. 2020 Aug 25;12(8):e10026. doi: 10.7759/cureus.10026. PMID: 32983720; PMCID: PMC7515803
10. Yoshida T, Fujii T, Uchino S, Takinami M. Epidemiology, prevention, and treatment of new-onset atrial fibrillation in critically ill: a systematic review. Journal of Intensive Care. 2015 ;3(1):19. DOI: 10.1186/s40560-015-0085-4. PMID: 25914828; PMCID: PMC4410002.
11. Wetterslev M, Haase N, Hassager C, Belley-Cote EP, McIntyre WF, An Y, Shen J, Cavalcanti AB, Zampieri FG, Guimaraes HP, Granholm A, Perner A, Møller MH. New-onset atrial fibrillation in adult critically ill patients: a scoping review. Intensive Care Med. 2019 Jul;45(7):928-938. doi: 10.1007/s00134-019-05633-x. Epub 2019 May 14. PMID: 31089761.
12. Walkey AJ, Hammill BG, Curtis LH, Benjamin EJ. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest. 2014 Nov;146(5):1187-1195. doi: 10.1378/chest.14-0003. PMID: 24723004; PMCID: PMC4219336
13. Topaz G, Flint N, Steinvil A, Finkelstein A, Banai S, Keren G, Shacham Y, Yankelson L. Long term prognosis of atrial fibrillation in ST-elevation myocardial infarction patients undergoing percutaneous coronary intervention. Int J Cardiol. 2017 Aug 1;240:228-233. doi: 10.1016/j.ijcard.2017.03.060. Epub 2017 Mar 16. PMID: 28390742
14. Kihara T, Gillinov AM, Takasaki K, Fukuda S, Song JM, Shiota M, Shiota T. Mitral regurgitation associated with mitral annular dilation in patients with lone atrial fibrillation: an echocardiographic study. Echocardiography. 2009 Sep;26(8):885-9. doi: 10.1111/j.1540-8175.2009.00904.x. Epub 2009 Jun 22. PMID: 19552671
15. Arrigo M, Bettex D, Rudiger A. Management of atrial fibrillation in critically ill patients. Crit Care Res Pract. 2014;2014:840615. doi:10.1155/2014/840615
16. Neumar RW, Otto CW, Link MS, Kronick SL, Shuster M, Callaway CW, Kudenchuk PJ, Ornato JP, McNally B, Silvers SM, Passman RS, White RD, Hess EP, Tang W, Davis D, Sinz E, Morrison LJ. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010 Nov 2;122(18 Suppl 3):S729-67. doi: 10.1161/CIRCULATIONAHA.110.970988. Erratum in: Circulation. 2011 Feb 15;123(6):e236. Erratum in: Circulation. 2013 Dec 24;128(25):e480. PMID: 20956224.
17. Kanji S, Williamson DR, Yaghchi BM, Albert M, McIntyre L; Canadian Critical Care Trials Group. Epidemiology and management of atrial fibrillation in medical and noncardiac surgical adult intensive care unit patients. J Crit Care. 2012 Jun;27(3):326.e1-8. doi: 10.1016/j.jcrc.2011.10.011. Epub 2012 Jan 4. PMID: 22226423
18. Mayr A, Ritsch N, Knotzer H, Dünser M, Schobersberger W, Ulmer H, Mutz N, Hasibeder W. Effectiveness of direct-current cardioversion for treatment of supraventricular tachyarrhythmias, in particular atrial fibrillation, in surgical intensive care patients. Crit Care Med. 2003 Feb;31(2):401-5. doi: 10.1097/01.CCM.0000048627.39686.79. PMID: 12576943
19. Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM, Hillege HL, Bergsma-Kadijk JA, Cornel JH, Kamp O, Tukkie R, Bosker HA, Van Veldhuisen DJ, Van den Berg MP; RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010 Apr 15;362(15):1363-73. doi: 10.1056/NEJMoa1001337. Epub 2010 Mar 15. PMID: 20231232.
20. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014 Dec 2;130(23):2071-104. doi: 10.1161/CIR.0000000000000040. Epub 2014 Mar 28. Erratum in: Circulation. 2014 Dec 2;130(23):e270-1. PMID: 24682348
21. Salerno DM, Dias VC, Kleiger RE, Tschida VH, Sung RJ, Sami M, Giorgi LV. Efficacy and safety of intravenous diltiazem for treatment of atrial fibrillation and atrial flutter. The Diltiazem-Atrial Fibrillation/Flutter Study Group. Am J Cardiol. 1989 May 1;63(15):1046-51. doi: 10.1016/0002-9149(89)90076-3. PMID: 2650517.
22. Pawlosky N, Macdonald E, Patel R, Kanji S. Evaluation of digoxin concentration after loading dose in patients with renal dysfunction. Can J Hosp Pharm. 2013 Mar;66(2):104-9. doi: 10.4212/cjhp.v66i2.1232. PMID: 23616674; PMCID: PMC3633494
23. Moran JL, Gallagher J, Peake SL, Cunningham DN, Salagaras M, Leppard P. Parenteral magnesium sulfate versus amiodarone in the therapy of atrial tachyarrhythmias: a prospective, randomized study. Crit Care Med. 1995 Nov;23(11):1816-24. doi: 10.1097/00003246-199511000-00005. PMID: 7587256
24. O’Bryan LJ, Redfern OC, Bedford J, Petrinic T, Young JD, Watkinson PJ. Managing new-onset atrial fibrillation in critically ill patients: a systematic narrative review. BMJ Open. 2020 Mar 24;10(3):e034774. doi: 10.1136/bmjopen-2019-034774. PMID: 32209631; PMCID: PMC7202704.
25. Malviya A, Kapoor M, Sivam RKN, Khan SA, Pandey R, Kumar U, Ete T, Mishra A. Ibutilide with magnesium for conversion of atrial fibrillation or flutter in rheumatic heart disease patients: Ibutilide with magnesium for chemical cardioversion of atrial fibrillation or flutter. Indian Heart J. 2020 Jul-Aug;72(4):283-288. doi: 10.1016/j.ihj.2020.07.008. Epub 2020 Jul 15. PMID: 32861384; PMCID: PMC7474117
26. Sultan A, Steven D, Rostock T, Hoffmann B, Müllerleile K, Servatius H, Drewitz I, Lüker J, Meyer P, Salukhe T, Willems S. Intravenous administration of magnesium and potassium solution lowers energy levels and increases success rates electrically cardioverting atrial fibrillation. J Cardiovasc Electrophysiol. 2012 Jan;23(1):54-9. doi: 10.1111/j.1540-8167.2011.02146.x. Epub 2011 Aug 4. PMID: 21815963
27. Ganga HV, Noyes A, White CM, Kluger J. Magnesium adjunctive therapy in atrial arrhythmias. Pacing Clin Electrophysiol. 2013 Oct;36(10):1308-18. doi: 10.1111/pace.12189. Epub 2013 Jun 3. PMID: 23731344
28. Davey MJ, Teubner D. A randomized controlled trial of magnesium sulfate, in addition to usual care, for rate control in atrial fibrillation. Ann Emerg Med. 2005 Apr;45(4):347-53. doi: 10.1016/j.annemergmed.2004.09.013. PMID: 15795711.
29. Sleeswijk ME, Tulleken JE, Van Noord T, Meertens JH, Ligtenberg JJ, Zijlstra JG. Efficacy of magnesium-amiodarone step-up scheme in critically ill patients with new-onset atrial fibrillation: a prospective observational study. J Intensive Care Med. 2008 Jan-Feb;23(1):61-6. doi: 10.1177/0885066607310181. PMID: 18320707
30. Slavik RS, Tisdale JE, Borzak S. Pharmacologic conversion of atrial fibrillation: a systematic review of available evidence. Prog Cardiovasc Dis. 2001 Sep-Oct;44(2):121-52. doi: 10.1053/pcad.2001.26966. PMID: 11568824.
31. Stambler BS, Wood MA, Ellenbogen KA. Antiarrhythmic actions of intravenous ibutilide compared with procainamide during human atrial flutter and fibrillation: electrophysiological determinants of enhanced conversion efficacy. Circulation. 1997 Dec 16;96(12):4298-306. doi: 10.1161/01.cir.96.12.4298. PMID: 9416896
32. Volgman AS, Carberry PA, Stambler B, Lewis WR, Dunn GH, Perry KT, Vanderlugt JT, Kowey PR. Conversion efficacy and safety of intravenous ibutilide compared with intravenous procainamide in patients with atrial flutter or fibrillation. J Am Coll Cardiol. 1998 May;31(6):1414-9. doi: 10.1016/s0735-1097(98)00078-3. PMID: 9581743.
33. Kirkland S, Stiell I, AlShawabkeh T, Campbell S, Dickinson G, Rowe BH. The efficacy of pad placement for electrical cardioversion of atrial fibrillation/flutter: a systematic review. Acad Emerg Med. 2014 Jul;21(7):717-26. doi: 10.1111/acem.12407. Epub 2014 Aug 12. PMID: 25117151
34. Stiell IG, de Wit K, Scheuermeyer FX, Vadeboncoeur A, Angaran P, Eagles D, Graham ID, Atzema CL, Archambault PM, Tebbenham T, McRae AD, Cheung WJ, Parkash R, Deyell MW, Baril G, Mann R, Sahsi R, Upadhye S, Brown E, Brinkhurst J, Chabot C, Skanes A. 2021 CAEP Acute Atrial Fibrillation/Flutter Best Practices Checklist. CJEM. 2021 Sep;23(5):604-610. doi: 10.1007/s43678-021-00167-y. Epub 2021 Aug 12. PMID: 34383280; PMCID: PMC8423652




Add comment