December 10, 2021 via Shahriar Lahouti

CONTENTS
- Preface
- Pathophysiology
- Causes
- Reversibility of the underlying cause
- Clinical presentation
- Complications of hyponatremia
- Risk stratification
- Diagnostic considerations
- Diagnostic criteria of SIADH
- Indication for hospitalization
- Principles of hyponatremia management
- Hyponatremia with persistent causes
- Management of sodium overcorrection
- RECAP
- Going further
- References
Preface
Hyponatremia is the most common electrolyte abnormality seen in medical practice 1 and is associated with increased mortality, morbidity and length of hospital stay in patients presenting with a range of conditions. Hyponatremia is therefore both common and important.
Pathophysiology
Because hyponatremia can result from a varied spectrum of conditions, based on different mechanisms, it would be useful to outlines some of the pathophysiological principles encountered in hyponatremia in brief.
Hyponatremia is primarily a disorder of water balance, with a relative excess of body water compared to total body sodium and potassium content. It is usually associated with a disturbance in the hormone that governs water balance, vasopressin (also called antidiuretic hormone). Even in disorders associated with (renal) sodium loss, vasopressin activity is generally required for hyponatremia to develop.
Changes in serum osmolality are primarily determined by changes in the serum concentration of sodium and its associated anions. It is important to differentiate the concepts of “total osmolality” and “effective osmolality” or tonicity.
- Total osmolality is defined as the concentration of all solutes in a given weight of water, regardless of whether or not the osmoles can move across biological membranes.
- Effective osmolality or tonicity refers to the number of osmoles that contribute water movement between the intracellular and extracellular compartment. It is a function of the relative solute permeability properties of the membranes separating the intracellular and extracellular fluid compartments. Only effective solutes (mainly sodium) create osmotic pressure gradients across cell membranes leading to osmotic movement of water between the intracellular and extracellular fluid compartment.
As the serum sodium concentration is determined by the amount of extracellular water relative to the amount of sodium, it can be regulated by changing intake or output of water. The major mechanisms responsible for regulating water metabolism are thirst and the pituitary secretion and renal effects of vasopressin.
- The sodium level is primarily controlled by thirst regulation. As the sodium level increases, most commonly due to the loss of free water in insensible losses such as sweat, respiration, and excretion, the patient feels the need to replace free water through consumption.
- Sodium is also regulated in the kidneys. Serum osmolarity is regulated by vasopressin or antidiuretic hormone (ADH). As serum osmolarity increases, ADH is secreted. ADH works in the distal convoluted tubule of the kidney to reabsorb free water, decreasing serum osmolarity 2. As serum osmolarity decreases, ADH secretion ceases and free water is excreted by the kidney, increasing serum osmolarity.
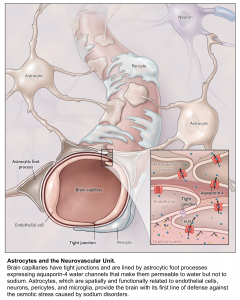
Cells are essentially suspended in a solute bath containing water, potassium, and sodium. Sodium is primarily extracellular and excluded from the cell by an Na+-K+ ATPase in favor of potassium, which is primarily intracellular. Water can move across the cell membrane via aquaporin channels; in the normal physiologic state, osmotic gradients are quickly eliminated by the diffusion of water across the cell membrane 3.
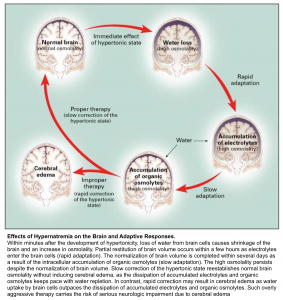
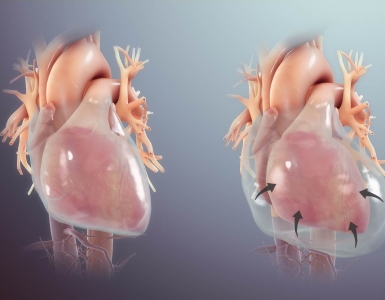
Sodium can easily cross capillary membranes of blood vessels in the periphery; however, capillaries in the brain have tight endothelial junctions that sodium cannot cross. Changes in sodium level will lead to the diffusion of water into brain cells, without the ability of sodium to diffuse out, leading to swelling or shrinkage of brain cells in response to changes in plasma sodium level (see following figures).
Causes
The body attempts to avoid the development of hyponatremia through the kidneys by diluting the urine and excreting free water. Hyponatremia develops when there is an excess of free water intake in conjunction with an underlying condition that impairs the ability of the kidneys to dilute the urine and preserve normal plasma sodium levels.
The etiology of hyponatremia can be broken down into 4 categories (however in practice, hyponatremia is often multifactorial):
1.Pseudohyponatremia (serum osmolality is not actually low): It is an errant sodium measurement caused by the presence of other osmotically active particles in the serum such as:
- Severe hyperglycemia (i.e. in DKA, Hyperosmolar hyperglycemic states)
- Triglyceride level >1,500 mg/dL.
- High protein level (multiple myeloma, IVIG).
- Exogenous osmoles:
- Contrast dye.
- Mannitol administration.
- Maltose (from IVIG).
- Sorbitol/glycine (used for surgical irrigation)
2.Hypovolemic hyponatremia: It is usually caused by volume depletion and a subsequent decrease in extracellular volume associated with losses of sodium. The etiologies are categorized into renal loss and loss of body fluid.
- Non-renal losses
- GI loss (vomiting, diarrhea, gastric tube drainage)
- Hemorrhage, sweating
- Third-spacing: burns, rhabdomyolysis, bowel obstruction, pancreatitis, sepsis. Markedly reduced effective circulating blood volume through fluid leakage from blood vessels causes baroreceptor activation and vasopressin release, which may result in hyponatremia.
- Renal losses
- Diuretics: The traditional explanation is that urinary sodium loss can cause volume depletion and, if sufficiently severe, trigger vasopressin release, however this would require a substantial loss of sodium and body weight!
- Thiazides: It is assumed that thiazides directly induce the release of vasopressin or increase the response of the collecting duct to circulatory vasopressin.
- Loop diuretics: Despite the potential for causing more urinary sodium loss, loop diuretics only rarely cause hyponatremia because they reduce osmolality in the renal medulla and thus limit the kidney’s ability to concentrate urine.
- Renal tubular acidosis
- Post-obstructive diuresis
- Adrenal insufficiency (late): hypoaldosteronism causes renal sodium loss, contracted extracellular fluid volume and hyponatraemia.
- Cerebral salt wasting: Renal sodium loss has been documented in patients with intracranial disorders such as subarachnoid hemorrhage. The most likely pathophysiology may be aldosterone deficiency, which occurs in some patients with SAH 14. This would explain the efficacy of exogenous fludrocortisone or hydrocortisone for these patients (as both medications possess mineralocorticoid effects) 15. The recognition of cerebral salt wasting is important because its treatment requires volume resuscitation rather than water restriction (more on this here).
- Diuretics: The traditional explanation is that urinary sodium loss can cause volume depletion and, if sufficiently severe, trigger vasopressin release, however this would require a substantial loss of sodium and body weight!
3.Hypervolemic hyponatremia: The patient will have signs of hypervolemia, and the hyponatremia will be a result of excess free water and/or loss of sodium. The most common causes are:
- Congestive heart failure with low cardiac output
- Cirrhosis
- Nephrotic syndrome
- Renal failure (if severe e.g. when GFR <15 ml/min)
4.Euvolemic hyponatremia: Patients are clinically euvolemic but have increased total body free water, leading to hyponatremia. The most common etiologies include:
- Adrenal insufficiency (early)
- Hypothyroidism: Although included in many diagnostic algorithms, hypothyroidism very rarely causes hyponatremia. Moreover it has been observed that only severe cases of clinically manifest hypothyroidism resulted in clinically important hyponatremia. Development of hyponatremia may be related to myxedema, resulting from a reduction in cardiac output and glomerular filtration rate16 .
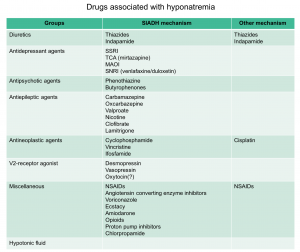
- SIADH: is the most common etiology of hyponatremia in hospitalized and ambulatory patients 4. Vasopressin increases water permeability and reabsorption in the renal collecting system, leading to increased total body water. In SIADH, vasopressin secretion is inappropriate because it occurs independently from effective serum osmolality or circulating volume. The resulting antidiuresis causes progressive hyponatremia until the expression of vasopressin V2 receptors and aquaporin-2 water channels is down-regulated, a process appropriately called ‘vasopressin escape’. In SIADH, even with increased total body water, most of the water is intracellular and, as such, these patients are euvolemic and have no stigmata of hypervolemia such as ascites, pulmonary congestion, or peripheral edema. Increased ADH may result from increased release by the pituitary gland, ectopic production of ADH, increased activity of vasopressin in the collecting duct or from a gain-of-function mutation in its type 2 receptor. These can be caused by a variety medications and other disorders 5.
- Drug induced (see below table)
- Malignancy (especially small cell lung carcinoma)
- Neuropsychiatric disorder (virtually any)
- Severe respiratory failure
- Pain or nausea (especially post-operative, or due to extreme exercise especially marathons)
👉Water intoxication (water intake > solute intake) is a rare cause of euvolemic hyponatremia seen in following conditions
- Psychogenic polydipsia (especially in schizophrenia)
- Very rapid water intake (e.g. fraternity hazing, or water loading prior to a drug screen)
- Beer potomania (excessive beer intake with reduced solute intake)
- Elderly patients who eat a “tea-and-toast” diet, or anorexia (low solute intake)
Reversibility of underlying cause
Determining the reversibility of the underlying cause of hyponatremia has significant impact on management of the patient (more on this below).
By enlarge, some etiologies of hyponatremia are rapidly reversible including:
- Hyponatremia due to true volume depletion (in such patients, correction of hypovolemia inhibits secretion of antidiuretic hormone (ADH), thereby leading to a water diuresis)
- Hyponatremia due to adrenal insufficiency (in such patients, administration of adrenal steroids inhibits ADH secretion and produces a water diuresis)
- Hyponatremia due to the syndrome of inappropriate antidiuretic hormone (SIADH; including postsurgical patients or SIADH due to pain or a drug)
In contrast, some other causes of hyponatremia is irreversible including
- SIADH secondary to e.g. malignancy, severe brain injury etc.
- Cirrhosis
- Heart failure
Clinical presentation
The symptoms are vague and nonspecific for hyponatremia. Patients with hyponatremia may present asymptomatically or with symptoms. There are typically two factors which influence how symptomatic a patient will become as a result of hyponatremia:
- Severity of hyponatremia (level of serum sodium)
- Acuity of onset
👉The lower the sodium and the faster the fall, the more symptomatic a patient will become.
Determine the severity (degree) of hyponatremia 12
- Mild hyponatremia: Serum sodium 130-134 mEq/L
- Moderate hyponatremia: Serum sodium 120-129 mEq/L
- Severe hyponatremia: Serum sodium <120 mEq/L
- Complications of untreated hyponatremia and complications from overcorrection of hyponatremia are most common among patients with severe hyponatremia
Determine the duration of hyponatremia: Therapy for hyponatremia depends in part upon the acuity:
- Acute: If the hyponatremia has developed over a period of less than 48 hours, it is called acute 12. Hyponatremia is usually a chronic condition and should be presumed as such unless there is a clear history that suggests an acute drop in serum sodium level. Historical factors that suggest an acute drop:
- Recent surgery with infusion of IV fluids
- Patients presenting with acute over-ingestion of water (competitive runners, psychotic patients with polydipsia, and users of ecstasy)
- Certain medications
- Recent gastrointestinal illness with marked vomiting and diarrhea
- Chronic: If it is known that hyponatremia has been present for more than 48 hours, or if the duration is unclear (such as in patients who develop hyponatremia at home), it is called chronic 12 .
👉The more acute the hyponatremia, the greater the risk of complications (such as cerebral edema and seizures) and the greater the need for aggressive therapy. The more chronic the hyponatremia and the lower the serum sodium concentration, the greater the risk of complications from overaggressive therapy and the greater the need for monitoring to avoid overcorrection.
Determine the severity of symptoms
Non-severe symptoms. Moderately symptomatic hyponatremia is defined as any biochemical degree of hyponatremia in the presence of non-severe symptoms including 12:
- Fatigue
- Headache
- Nausea, vomiting
- Irritability, lethargy, agitation
- Forgetfulness, mild confusion
- Dizziness, gait disturbances (leading to a fall)
- Tremor, multifocal myoclonus
- Hyperreflexia, muscle cramps
👉This is most commonly seen with chronic hyponatremia that is severe (serum sodium concentration less than 120 mEq/L).
👉Note that if the serum sodium concentration is extremely low (less than 110 mEq/L), then such symptoms may be a prelude to seizures. In addition, mild to moderate symptoms in patients with acute hyponatremia, even those with sodium concentrations of greater than 120 mEq/L, should be considered ominous and may evolve without warning to seizures, respiratory arrest, and herniation.
Severe symptoms. Severely symptomatic hyponatremia is defined as any biochemical degree of hyponatremia in the presence of severe symptoms including 12:
👉This is relatively common in patients with an acute and marked reduction in the serum sodium concentration. By contrast, seizures and other severe neurologic manifestations are relatively uncommon in patients with chronic hyponatremia (even among those with severely decreased serum sodium concentrations).
Asymptomatic. Many hyponatremic patients appear to be asymptomatic. However, “asymptomatic” patients, particularly those with chronic hyponatremia of moderate severity (120 to 129 mEq/L), may have subtle impairments in mentation and gait and an increased risk of falls and fractures . Thus, such patients are often offered chronic therapies to normalize the serum sodium concentration in an effort to treat these subtle manifestations and to avoid the possibility that the serum sodium concentration will fall further and produce more serious symptoms.
- Chronic therapies that are used include oral urea, salt tablets and furosemide, or vasopressin antagonists. However, there is no evidence proving that these therapies decrease the risk of falls and fractures.
Complications of hyponatremia
Cerebral edema
Water passes from the hypotonic serum into the hypertonic brain cells, causing development of cerebral edema. It should be considered in all patients with either severe hyponatremia or a rapid lowering of serum sodium concentration and altered level of consciousness.
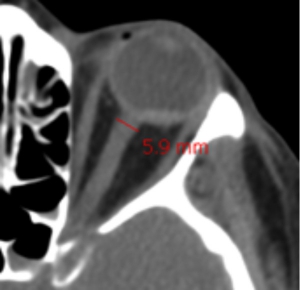
👉Surrogate of cerebral edema: Measurement of the optic nerve diameter with point-of-care ultrasound (POCUS) or CT scan of the head. When measured 3 mm behind the eye:
- <5 mm is normal.
- 5-6 mm is a grey zone.
- >6 mm suggests abnormal ICP.
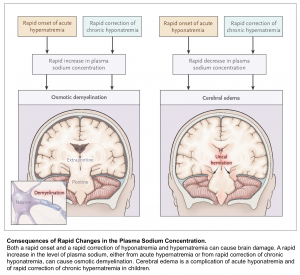
Osmotic Demyelination Syndrome (ODS)
ODS is a devastating condition which can occur after rapid overcorrection of hyponatremia. It is a clinical diagnosis with a delayed presentation up to 7 days after the rapid correction. The symptoms can vary and are dependent on which anatomical structure in the brain demyelinates. ODS most commonly affects the pons, however other structures can be affected including the cerebellum or basal ganglia. Commonly described symptoms include ataxia, quadriplegia, cranial nerve palsies, and the locked-in syndrome.
Risk stratification
Generally there are two things to worry about with a patient with hyponatremia. First, hyponatremia may worsen, leading to severe complications (e.g. seizure, cerebral edema, and herniation). Second, hyponatremia may overcorrect too rapidly, leading to osmotic demyelination.
Risk for severe complications of hyponatremia (e.g. seizures, herniation)
- Acute hyponatremia
- Symptomatic hyponatremia
- Brain substrate:
- Premenopausal women (little extra space in the cranium for the brain to swell)
- Intracranial pathology (e.g. space-occupying lesion, cerebral edema)
- Known seizure disorder
Risk for osmotic demyelination syndrome (due to rapid overcorrection)
- Chronicity & severity
- Severe, asymptomatic hyponatremia is the most worrisome (especially Na <110 mM).
- Osmotic demyelination is rare if the initial sodium is >120-125 mM 8
- Reversible cause of hyponatremia
- Hypovolemia, adrenal insufficiency
- SIADH due to a transient factor (e.g. pain, nausea, or medication)
- Water intoxication (e.g. psychogenic polydipsia, etc.)
- High rate of sodium rise
- >8-10 mM per day on average may cause osmotic demyelination
- The greatest risk occurs if patients transition from chronic hyponatremia to hypernatremia. Hypernatremia should be aggressively avoided in patients who are recovering from hyponatremia.
- Other risk factors for osmotic demyelination syndrome 9 10
- Hypokalemia
- Liver disease
- Alcoholism
- Malnutrition
Diagnostic consideration
Hyponatremia is a lab diagnosis and the level of serum sodium is diagnostic (i.e. <135 mmol/l).
- Keep in mind that you do NOT need to figure out the etiology of hyponatremia in the ED in order to provide the definitive emergency treatment. However, diagnostic work up can be started in ED for cases of unclear etiology.
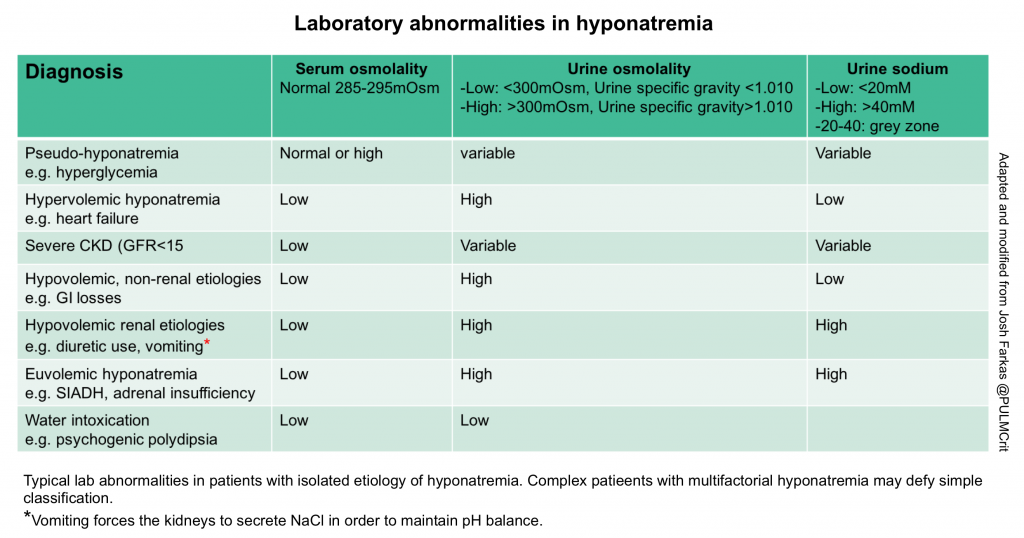
Traditional diagnostic algorithms often fail, because patients frequently have multifactorial hyponatremia (especially in crtically ill patients). Therefore, patients have a tendency to break the rules and fall outside the boxes. Nonetheless, laboratory tests can often point us in the correct direction (see the table below). Labs should always be combined with the history, medication evaluation, and physical examination. Obtaining following lab test are useful:
- Pseudohyponatremia? evaluate by checking lipid and protein, and blood glucose levels.
- Basic metabolic panel
- Serum osmolality (normal or increased level indicates pseudohyponatremia)
- TSH
- Cortisol level
- Urine osmolarity: The preferred way to measure this is a direct measurement of urine osmolality. However, a crude estimate can be obtained by looking at the urine specific gravity. A specific gravity <1.010 suggests dilute urine, whereas a specific gravity >1.010 suggests concentrated urine 11.
- Concentrated urine
- Diagnostic significance
- Most hyponatremic patients will have a urine osmolality of >300 mOsm.
- This is nonspecific, revealing relatively little about the diagnosis.
- Therapeutic significance
- Concentrated urine indicates that the kidney is still retaining water. This indicates that the patient is not going to auto-correct their sodium.
- A very concentrated urine suggests that giving isotonic fluid could potentially worsen the hyponatremia (because the kidney may respond by retaining water and excreting sodium.
- Diagnostic significance
- Dilute urine
- Diagnostic significance:
- The kidney is working correctly, that is secreting water! This suggests one of the following situations:
- Hyponatremia due to water intoxication
- A patient in the recovery phase from any other cause of hyponatremia (e.g. the patient initially had hypovolemic hyponatremia, received volume resuscitation prior to urinalysis, and is currently auto-correcting their own sodium levels.)
- The kidney is working correctly, that is secreting water! This suggests one of the following situations:
- Therapeutic significance:
- Secretion of dilute urine will cause the patient’s sodium to rise, so these patients will correct their own sodium levels.
- Production of large volumes of dilute urine is often a sign that the sodium is about to over-correct.
- Diagnostic significance:
- Concentrated urine
- Urine sodium
- Low urine sodium (<20 mEq/L) suggests:
- Hypovolemic hyponatremia due to extra-renal volume loss.
- Hypervolemic hyponatremia (e.g. heart failure, cirrhosis).
- Intermediate urine sodium (~20-40 mEq/L)
- This represents a grey zone, providing no clear information.
- High urine sodium (>40 mEq/L) suggests:
- Euvolemic hyponatremia (SIADH, hypothyroidism, or adrenal insufficiency).
- Hypovolemic hyponatremia due to renal salt wasting (e.g. diuretics, vomiting, or cerebral salt wasting).
- Low urine sodium (<20 mEq/L) suggests:
Diagnostic criteria for the SIADH
Importantly SIADH is a diagnosis of exclusion. The originally proposed diagnostic criteria include:
Essential criteria
- Effective serum osmolality <275 mOsm/kg
- Urine osmolality >100 mOsm/kg at some level of decreased effective osmolality
- Clinical euvolemia
- Urine sodium concentration >30 mmol/l with normal dietary salt and water intake
- Absence of adrenal, thyroid, pituitary or renal insufficiency
- No recent use of diuretic agents
Supplemental criteria
- Serum uric acid < 4mg/dl
- Serum urea < 21.6 mg/dl
- Failure to correct hyponatremia after 0.9% saline infusion
- Fractional sodium excretion >0.5%
- Fractional urea excretion >55%
- Fractional uric acid excretion >12%
- Correction of hyponatremia through fluid restriction
Indication for hospitalization
- Acute and symptomatic
- Patients with severe symptoms and those who needs hypertonic therapy require ICU admission.
- Chronic hyponatremia: depends on severity of hyponatremia and symptoms
- Severe symptoms regardless of the sodium level requires ICU admission.
- Severe hyponatremia (i.e. serum sodium <120 mEq/L). Admit per local protocols (some hospitals have a sodium cut-off for automatic ICU admission).
Most patients with symptomatic hyponatremia should be treated in hospital settings that allow frequent assessments of the patient’s neurologic condition, accurate measurements of urine output, and frequent measurements of the serum sodium concentration. By contrast, patients with mild hyponatremia and asymptomatic patients with moderate hyponatremia usually do not require hospitalization.
Principles of hyponatremia management
The treatment of hyponatremia in hospitalized patients has four important goals 12 :
- To relieve symptoms of hyponatremia
- To prevent further declines in the serum sodium concentration
- To decrease intracranial pressure in patients at risk for developing brain herniation
- To avoid excessive correction of hyponatremia in patients at risk for osmotic demyelination syndrome
The approach to attaining these goals, both during initial therapy (i.e. the first six hours after recognition of the electrolyte disturbance) and during subsequent therapy (the first several days), is presented below.
Management in ED
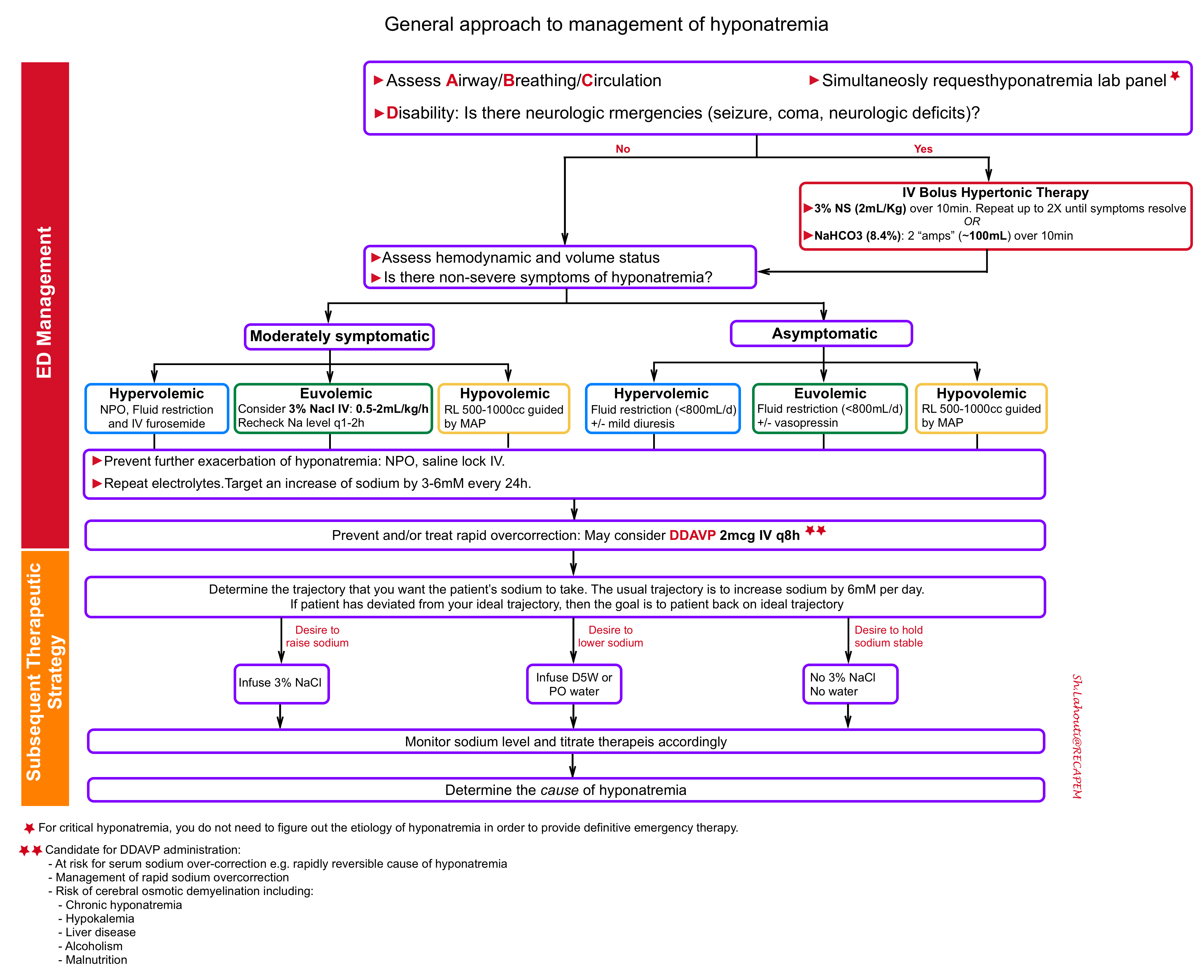
The principles of critical hyponatremia management in ED involves several steps:
1.Treat neurologic emergencies related to hyponatremia
- Note that due to the time needed to obtain laboratory test results, the sodium level will not be known immediately; thus, treat seizures and evaluate for causes of altered mental status in the usual manner.
- Treat neurologic emergencies with IV bolus hypertonic treatment (see below)
- Indications for IV bolus hypertonic treatment 12:
- Empiric treatment: If the patient has a known sodium level lower than 120 mEq/L with seizures or altered mental status
- Sever symptomatic hyponatremia ( seizure, coma, cerbral herniation, focal neurologic deficits)
- Options for providing IV bolus hypertonic therapy
- Hypertonic saline: administer 3% sodium chloride in a dose of 2 ml/kg body weight (e.g. ~ 150 ml) over 10 min. Can repeat twice as needed if severe symptoms persist 12 .
- Each 100mL raise serum sodium by 2mEq/L.
- Repeat a full electrolyte panel 20min after administration of hypertonic therapy.
- The goal is to increase the Na by about 3-5 mM, which should cause clinical improvement.
- If symptoms persist:
- If the sodium has increased by <4 mM, then an additional round of hypertonic therapy may be needed (with a goal of increasing the sodium by ~4-6 mM total).
- If the sodium has increased by 6 mM and the symptoms have not resolved, then hyponatremia isn’t the cause of the patient’s symptoms. Evaluate further for alternative or additional problems.
- Sodium bicarbonate: is an alternative if hypertonic saline is not available. Give 100 cc of 8.4% sodium bicarbonate (2 “ampules” of crash cart bicarb) over 10 minutes.
- 100mL of hypertonic bicarbonate is equivalent to giving ~200 ml of 3% saline, which will raise the serum sodium by ~3 mM).
- For smaller patients, 1 or 1.5 ampules (50-75 ml) might be more appropriate (depending also on the clinical context and the urgency of increasing the sodium).
- Hypertonic saline: administer 3% sodium chloride in a dose of 2 ml/kg body weight (e.g. ~ 150 ml) over 10 min. Can repeat twice as needed if severe symptoms persist 12 .
2. Evaluate and defend intravascular volume
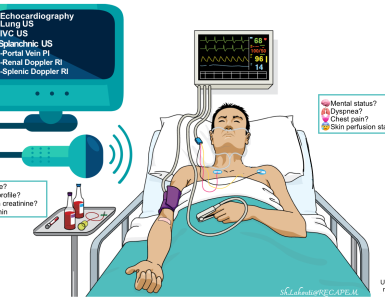
Assess patient’s volume status, e.g. patient’s history, heart rate, blood pressure and point-of-care ultrasound (POCUS), to make a rough determination of whether the patient is significantly hypovolemic or significantly hypervolemic.
- Hypovolemic and hyponatremic: the priority is to restore adequate circulating volume. In particular, restoring adequate circulating volume takes priority over any concerns that the hyponatremia might be corrected too rapidly and lead to osmotic demyelination syndrome.
- Fluid of choice: Ringer’s lactate has a sodium concentration of 128mmol/L which will be more isotonic to the hyponatremic patient. Although it has not been shown in clinical studies, administering Ringer’s lactate will likely result in a slower rise in serum serum sodium than Normal Saline, and therefore have a lower risk of potentiating osmotic demyelination syndrome.
- Hypervolemic hyponatremia: Focus on sodium and free water restriction plus diuretics.
- Euvolemic hyponatremia: they have an appropriate volume status, and so do not require any particular treatment to defend intravascular volume, and management should concentrate on preventing worsening hyponatremia.
- For moderately symptomatic hyponatremia: Consider 3% Nacl 0.5-2ml/kg/h infusion. Recheck sodium q1-2h 17.
- For asymptomatic patient: Consider free water restriction (<800cc/d) +/- vasopressin.
📖A prospective multi-center randomized trial including 148 patients with symptomatic hyponatremia demonstrated that slow continuous infusion (SCI) and rapid intermittent bolus (RIB) are equally safe therapies, with no difference in the risk of overcorrection. RIB is preferred given lower rates of therapeutic relowering treatment and better efficacy within 1 hour 18 .
3.Prevent worsening hyponatremia
Once baseline volume status has correctly identified and managed, the goal becomes preventing further exacerbation of the hyponatremia. This is achieved through strict fluid restriction and saline locking the IV.
4.Prevent rapid overcorrection
Excessively rapid correction of sodium runs a risk of osmotic demyelination. The key concept to appropriately manage hyponatremia and prevent overcorrection is to understand the physiology of sodium overcorrection. The most common cause of over-correction is “reversal of underlying cause of hyponatremia”.
- General concept: Once the underlying cause of hyponatremia is reversed, the body suddenly excretes a lot of free water, thereby causing the sodium to rise. 👉 Therefore, the fluid itself that you have given to your patient is NOT the cause of a rapid increase in the serum sodium, but rather, the free water diuresis that results shortly afterwards is the culprit.
Strategies to prevent overcorrection
- Target rates of sodium correction: Rule of 6s
- 6 in 6hrs for sever symptoms and stop (maximum Na increase of 6 mEq/L in 6 hours)
- Note that if the sodium was increased by 4-6 mM acutely, this is included within the 6 mM increase which is desired over the first 24 hours. For example: If you raise the sodium by 6 mM to treat symptomatic hyponatremia, the goal over the next 24 hours is to maintain the sodium at that same level without any further rise.
- 6-a-day makes sense for safety and no more than 8 mEq/L (goal maximum of 6mEq/L in 24 hours).
- 6 in 6hrs for sever symptoms and stop (maximum Na increase of 6 mEq/L in 6 hours)
- Prevent over-correction: Rule of 100s
- Insert a foley catheter and monitor intakes, outputs.
- f urine output >100cc/hour, send STAT urine osmolarity and urine sodium
- If urine osmolarity<100, consider administering DDAVP IV. Consider DDAVP for selected patients (see below).
-
Use caution with predictive equations: equations can be used to predict the effect of a fluid on the patient’s sodium level (e.g. MDCalc). These equations treat the body as a passive receptacle which receives IV fluid and does nothing with it. This assumption would work well for patients in anuric renal failure. Predictive equations will fail most for patients with reversible hyponatremia. In this scenario, patients may rapidly excrete lots of dilute urine and thereby over-correct their sodium spontaneously (much faster than would be predicted).
-
In contrast, predictive equations tend to work much better for patients on the DDAVP, because these patients won’t produce much urine.
-
Subsequent therapeutic strategy
In patients at risk of sodium overcorrection (see above) or those with already developed over-correction, administration of DDAVP may be considered.
Administration of DDAVP prevents the kidneys from secreting free water. This takes the patient’s kidneys out of the equation, preventing the patient from auto-correcting. The patient essentially becomes like a passive container, with very predictable behavior:
- Administration of hypotonic fluid (e.g. water, juice) will cause the sodium to decrease.
- Administration of hypertonic fluids (e.g. 3% saline, potassium tablets) will cause the sodium to increase.
- If there are no inputs, the sodium should stay roughly stable
DDAVP administration
First: Give DDAVP as 2micrograms IV q8 hours scheduled.
Second: The patient’s fluid intake must be strictly controlled.
- With DDAVP, if the patient drinks too much they will lower their sodium.
- To begin with, restrict any fluid intake (dry foods are OK). After 6-12 hours you may liberalize a bit.
- Note that the patient’s sense of thirst is due to hyponatremia, so there is no safe way to get rid of this immediately.
Third: Calculate the rate of 3% saline required to achieve your target sodium.
- Figure out how much more you want the sodium to increase over the next 24 hours.
- The effect of 3% sodium on your patient’s sodium can be calculated using MDCalc equation
Fourth: Follow electrolytes every 4-6 hours to ensure that the sodium is on track.
- If the sodium is too low, increase the rate of 3% saline.
- If the sodium is too high, calculate the amount of free water required to decrease it back to target using this formula. This volume of free water may be given intravenously (as D5W) or orally (if the patient is able to drink).
Discontinuing the DDAVP
- Once the sodium is close to normal (e.g. >125 mM), the DDAVP may be discontinued. About 10 hours later, the patient’s diet may be gradually liberalized.
- One of two things will happen at this point:
- If the cause of hyponatremia has been eliminated (e.g. hypovolemia), then the sodium will rapidly increase to normal and stay within a normal range. You’re all set.
- If the patient still has a cause for hyponatremia (e.g. chronic SIADH), their sodium may start falling again. At this point, they would require maintenance therapy for SIADH (e.g. furosemide and oral salt tabs, or oral urea).
Hyponatremia due to persistent underlying causes
SIADH with persistent cause
Treatment of SIADH with reversible cause (medications, nausea) is described above. Management of hyponatremia secondary to an ongoing cause which is difficult or impossible to remove (e.g. malignancy, severe brain injury) involves two treatment strategies (In either case, a moderate amount of fluid restriction should also be employed):
- Loop diuretic plus sodium, or
- Oral urea
1.Loop diuretic plus sodium
General concept:
- These patients are usually in sodium balance, so they shouldn’t retain sodium. As sodium is excreted, it will pull water out of the body along with it. The amount of water loss is equal to the osmotic load of the sodium divided by the urine osmolarity.
- Continuous exposure to a loop diuretic will wash out the concentration gradient in the kidney, causing the urine osmolarity to decrease. Thus, even though the kidney is “trying” to retain water, it’s less able to achieve that. Washing out the kidney concentration gradient will increase the amount of water which is pulled out of the body due to sodium administration.
-Loop diuretic (e.g. furosemide): should be given frequently enough so that the kidney doesn’t escape in between doses (e.g. 20 mg IV furosemide q6hr). As patients stabilize, this may be weaned down or off (furosemide will augment the efficacy of the NaCl, but it’s not mandatory for it to work).
-Sodium intake: It should be increased. In acutely ill patients, this is usually achieved with a 3% NaCl infusion. As chronic maintenance therapy or in less emergent situations, oral salt tabs may also be used (a typical dose would be 3 grams TID with meals).
Monitor fluid balance, to make sure that the patient doesn’t become volume overloaded or depleted.
2.Oral urea
The kidneys will excrete any exogenous urea in the urine, along with water. Thus, oral urea intake functions as an aquaretic that forces the net elimination of water from the body. The volume of water removed is proportional to the amount of urea administered, leading to a finite and controlled removal of water (equation above). Urea is generally cleared from the body within ~12 hours.
Contraindications to urea are as follows:
- Inability to tolerate PO intake.
- Cirrhosis with hepatic encephalopathy.
- Severe renal failure.
Dosing: The starting dose is 15-30 grams (with a dosing range of 7.5-90 grams/day).
Heart failure
Approximately 20–30% of patients with chronic heart failure New York Heart Association (NYHA) classes III and IV have hyponatremia.
Physiology
- Inadequate cerebral perfusion stimulates the brain to produce antidiuretic hormone (ADH), leading to water retention.
- Thus, hyponatremia in heart failure is a reflection of poor systemic perfusion. This may explain why hyponatremia is a prognostic sign of poor long-term outcome.
Hyponatremia due to heart failure is usually chronic and is not a life-threatening process. Heart failure rarely causes profound hyponatremia, rather there is usually a fairly stable sodium of ~120-135 mM.
Treatment
This depends on a clinical assessment of the patient’s perfusion and volume status.
- Sometimes acute hyponatremia may be a feature of cardiogenic shock (deteriorating systemic perfusion).
- Treatment here will depend on systemic hemodynamic assessment.
- Sometimes hypoperfusion may be due to volume depletion. Such patients may benefit from judicious volume repletion.
- Some hypoperfused patients could benefit from therapies such as inotropes (or withdrawal of beta-blockers).
- Chronic hyponatremia is often associated with volume overload.
- For patients with marked volume overload, loop diuretics are preferred over thiazides. Furosemide stimulates the production of dilute urine, causing loss of both water and sodium (but generally more water than sodium). This will improve the volume status and also increase the sodium concentration.
- For patients with hyponatremia and severe refractory congestion that doesn’t respond to conventional doses of loop diuretics, a combination of simultaneous loop diuretic and hypertonic saline may be tried (i.e. typically achieved by administering 150 ml 3% saline plus 250 mg furosemide IV every 12 hours) 13. More on this here.
Liver failure
Hyponatremia in liver failure is also associated with poor survival. Similar to heart failure, cirrhosis rarely causes severe hyponatremia, rather there is usually a fairly stable sodium of ~120-135 mM.
Physiology
-
Systemic vasodilation and arteriovenous shunting of blood may reduce the effective arterial blood volume. As in heart failure, this reduction can lead to neurohumoral activation and water retention due to baroreceptor mediated vasopressin release.
-
In addition, mineralocorticoid receptor blockers such as spironolactone, which either alone or in combination with loop diuretics, are frequently used to reduce sodium retention in liver failure, can contribute to hyponatremia.
Treatment
As in heart failure, assessment of perfusion and volume status should be performed.
- Rare patients may be hypovolemic, in which case judicious volume resuscitation may be considered.
- Loop diuretics are often a good option for patients with volume overload.
- Oral lactulose is a good option for patients with any degree of hepatic encephalopathy. Lactulose causes wasting of water via the gut (which will improve the hyponatremia), while simultaneously treating hepatic encephalopathy.
Management of sodium overcorrection
Overcorrection occasionally will happen, however with appropriate management, it can be promptly fixed and patient will do fine. The patients who develop osmotic demyelination are patients who over-correct and nothing is done about it.
Causes
As explained earlier, over-correcting the sodium is almost never due to the practitioner’s giving too much sodium chloride. Common causes of over-correction:
- The underlying cause of hyponatremia is reversed.
- KCl administration, without taking into account the effect this will have on sodium
- Administration of KCl to patients with hypokalemic hyponatremia will increase the sodium concentration just as much as NaCl would (they have the same impact on tonicity). Remember that potassium entry into the cells is paired with the exit of sodium, such that the sodium levels will rise.
- The impact of oral potassium supplementation on the serum sodium can be estimated by following formula.
- Estimated rise in serum sodium = mEq of oral potassium / 0.55 (weight in Kg)
- Therefore administration of 50 mEq of oral KCl will have about the same effect as 100 ml of 3% NaCl.
- Treatment of hyponatremia with vaptans. Vaptans block aquaporin water channels in the kidneys, causing uncontrolled excretion of water by the kidneys. Indeed, this medications are not recommended for treatment of hyponatremia because of the following risks12
- Water loss is uncontrolled and unpredictable. Patients can correct their sodium much faster than would be desired.
- Patients may develop overshoot hypernatremia. This is particularly dangerous regarding the risk of osmotic demyelination.
Management
- The best approach is immediate initiation of the DDAVP. Start DDAVP 2 micrograms IV q8hr.
- Calculate the amount of free water needed to bring the patient back down to their target sodium level. This should be given fairly quickly, with a goal of bringing the patient back to their target sodium as soon as possible.
- Follow the sodium closely. Use either water or hypertonic sodium to adjust the sodium to your target trajectory.
RECAP
- Hyponatremia is a disorder of water balance.
- Traditional diagnostic algorithms often fail, because patients frequently have multifactorial hyponatremia (especially critically ill patients). Therefore, patients have a tendency to break the rules and fall outside the boxes.
- Management of clinically significant hyponatremia involves two phase:
- Initial therapy (i.e. the first six hours after recognition of the electrolyte disturbance).
- Subsequent therapy (the first several days).
- Initial therapy
- Correct acute, severe hyponatremia with bolus(es) of hypertonic saline or sodium bicarbonate.
- Goal: Raise sodium by 4–6 mmol/L and stop.
- 3% NaCl: 2mL/kg over 10min. If persistent symptoms, may repeat 2X until symptoms resolve.
- NaHCO3: 100 cc of 8.4% sodium bicarbonate (2 “ampules” of crash cart bicarb) over 10 minutes.
- Evaluate and defend volume status.
-
- Hypovolemic hyponatremic patients: restore circulating volume.
- Hypervolemic hyponatremic patients: Free water restriction plus IV diuretic.
- DO treat moderately symptomatic hyponatremia with 3% NaCl (0.5–2 mL/kg/h) infusion and monitor serum sodium every 1-2h.
-
- Prevent worsening of hyponatremia: strict fluid restriction and saline locking the IV; applied to all hyponatremic patients.
- Prevent Rapid Overcorrection:
-
- Target rates of sodium correction: Rule of 6s. Six in six hours for severe symptoms, then stop. Six a day makes sense for safety.”
- Prevent overcorrection: Rule of 100s.
- Insert a foley catheter and monitor intake and outputs.
- If urine output >100cc/hour, send STAT urine osmolarity and urine sodium.
- If urine osmolarity<100, consider administering DDAVP IV. Consider DDAVP for appropriate candidates.
- Use caution with predictive equations
-
- Correct acute, severe hyponatremia with bolus(es) of hypertonic saline or sodium bicarbonate.
- Subsequent management
- Monitor sodium and serum electrolyte as well as clinical response frequently.
- Identify the underlying cause(s) of hyponatremia.
- Treat overcorrection. Patients with rapidly rising sodium may look fine for a while, but later develop ODS. If the patient’s sodium over-corrects, give them DDAVP and water, and reduce the sodium to the appropriate target.
- Don’t use vaptans.
Further going
- Emergency Management of Hyponatremia (EM Cases)
- Severe hyponatremia in ED (EMCrit RACC)
- Severe Hyponatremia (CoreEM)
- Severe hyponatremia (EM:RAP)
- Management of hyponatremia (EM:RAP)
References
1.Liamis G, Rodenburg EM, Hofman A, Zietse R, Stricker BH, Hoorn EJ. Electrolyte disorders in community subjects: prevalence and risk factors. Am J Med. 2013 Mar;126(3):256-63. doi: 10.1016/j.amjmed.2012.06.037. Epub 2013 Jan 18. PMID: 23332973.
2. Zieg J. Pathophysiology of Hyponatremia in Children. Front Pediatr. 2017 Oct 16;5:213. doi: 10.3389/fped.2017.00213. PMID: 29085814; PMCID: PMC5650627
3. Sterns RH. Disorders of plasma sodium–causes, consequences, and correction. N Engl J Med. 2015 Jan 1;372(1):55-65. doi: 10.1056/NEJMra1404489. PMID: 25551526.
4. Peri A, Grohé C, Berardi R, Runkle I. SIADH: differential diagnosis and clinical management. Endocrine. 2017 Jan;55(1):311-319. doi: 10.1007/s12020-016-0936-3. Epub 2016 Mar 30. PMID: 27025948
5. Moritz ML. Syndrome of Inappropriate Antidiuresis. Pediatr Clin North Am. 2019 Feb;66(1):209-226. doi: 10.1016/j.pcl.2018.09.005. PMID: 30454744.
6. Stefanko G, Lancashire B, Coombes JS, Fassett RG. Pulmonary oedema and hyponatraemia after an ironman triathlon. BMJ Case Rep. 2009;2009:bcr04.2009.1764. doi:10.1136/bcr.04.2009.1764
7. Moritz ML, Ayus JC. New aspects in the pathogenesis, prevention, and treatment of hyponatremic encephalopathy in children. Pediatr Nephrol. 2010;25(7):1225-1238. doi:10.1007/s00467-009-1323-6
8. Rondon-Berrios H. Therapeutic Relowering of Plasma Sodium after Overly Rapid Correction of Hyponatremia: What Is the Evidence? Clin J Am Soc Nephrol. 2020 Feb 7;15(2):282-284. doi: 10.2215/CJN.04880419. Epub 2019 Oct 10. PMID: 31601554; PMCID: PMC7015090
9. Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RW, Sterns RH, Thompson CJ. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013 Oct;126(10 Suppl 1):S1-42. doi: 10.1016/j.amjmed.2013.07.006. PMID: 24074529
10. Rondon-Berrios H, Tandukar S, Mor MK, Ray EC, Bender FH, Kleyman TR, Weisbord SD. Urea for the Treatment of Hyponatremia. Clin J Am Soc Nephrol. 2018 Nov 7;13(11):1627-1632. doi: 10.2215/CJN.04020318. Epub 2018 Sep 4. PMID: 30181129; PMCID: PMC6237061
11. Imran S, Eva G, Christopher S, Flynn E, Henner D. Is specific gravity a good estimate of urine osmolality? J Clin Lab Anal. 2010;24(6):426-30. doi: 10.1002/jcla.20424. PMID: 21089176; PMCID: PMC6647580
12. Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D, Decaux G, Fenske W, Hoorn EJ, Ichai C, Joannidis M, Soupart A, Zietse R, Haller M, van der Veer S, Van Biesen W, Nagler E; Hyponatraemia Guideline Development Group. Clinical practice guideline on diagnosis and treatment of hyponatremia. Eur J Endocrinol. 2014 Feb 25;170(3):G1-47. doi: 10.1530/EJE-13-1020. Erratum in: Eur J Endocrinol. 2014 Jul;171(1):X1. PMID: 24569125.
13. Lafrenière G, Béliveau P, Bégin JY, et al. Effects of hypertonic saline solution on body weight and serum creatinine in patients with acute decompensated heart failure. World J Cardiol. 2017;9(8):685-692. doi:10.4330/wjc.v9.i8.685
14. Berendes E, Walter M, Cullen P, Prien T, Van Aken H, Horsthemke J, Schulte M, von Wild K, Scherer R. Secretion of brain natriuretic peptide in patients with aneurysmal subarachnoid haemorrhage. Lancet. 1997 Jan 25;349(9047):245-9. doi: 10.1016/s0140-6736(96)08093-2. PMID: 9014912.
15. Katayama Y, Haraoka J, Hirabayashi H, Kawamata T, Kawamoto K, Kitahara T, Kojima J, Kuroiwa T, Mori T, Moro N, Nagata I, Ogawa A, Ohno K, Seiki Y, Shiokawa Y, Teramoto A, Tominaga T, Yoshimine T. A randomized controlled trial of hydrocortisone against hyponatremia in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007 Aug;38(8):2373-5. doi: 10.1161/STROKEAHA.106.480038. Epub 2007 Jun 21. PMID: 17585086
16. CURTIS RH. Hyponatremia in primary myxedema. Ann Intern Med. 1956 Feb;44(2):376-85. doi: 10.7326/0003-4819-44-2-376. PMID: 13292846.
17. Aylwin S, Burst V, Peri A, Runkle I, Thatcher N. ‘Dos and don’ts’ in the management of hyponatremia. Curr Med Res Opin. 2015;31(9):1755-61. doi: 10.1185/03007995.2015.1072706. Epub 2015 Sep 4. PMID: 26173050.
18. Baek SH, Jo YH, Ahn S, Medina-Liabres K, Oh YK, Lee JB, Kim S. Risk of Overcorrection in Rapid Intermittent Bolus vs Slow Continuous Infusion Therapies of Hypertonic Saline for Patients With Symptomatic Hyponatremia: The SALSA Randomized Clinical Trial. JAMA Intern Med. 2021 Jan 1;181(1):81-92. doi: 10.1001/jamainternmed.2020.5519. PMID: 33104189; PMCID: PMC7589081



Add comment