March 6, 2022 by Shahriar Lahouti.
CONTENTS
- Introduction
- Pathophysiology and causes
- Diagnostic consideration
- General approach to management
- Blood pressure management
- Coagulopathy reversal
- Neurosurgical intervention
- Other supportive measures
- Prognosis
- Appendix
- Media
- Going further
- References
Introduction
Spontaneous intracerebral hemorrhage (ICH) is a medical emergency and comprises 15% of all cerebrovascular accident cases worldwide. It is associated with higher mortality and long-term disability compared with ischemic stroke.
The phrase “time is brain” was derived for patients with large vessel occlusion ischemic stroke in which approximately 1.9 million neurons are lost every minute. Similarly, this statement holds true for ICH patients due to a high volume of neurons that are damaged at initial onset and during hematoma expansion 1.
- This mathematical model of primary neuronal loss in ICH underscores the significance of rapid identification and stabilization (ABC of acute hemorrhage stabilization) of patients with intracerebral hemorrhage.
Pathophysiology and causes
Intracerebral hemorrhage causes injury through various mechanisms including:
- Hematoma expansion: Up to 73% of patients will experience hemorrhage expansion, with associated increases in morbidity and mortality. This is most common within the first 6 hours (yet it can occur within ~48 hours in coagulopathic patients).
- The risk of volume expansion is associated with larger hematomas, earlier presentation, anticoagulant use, and the presence of a “spot sign” (see below) on CTA.
- Secondary edema: Breakdown of the blood-brain barrier contributes to the formation of perihematomal edema. Edema volume may peak after ~5-6 days. In addition to decreased cerebral perfusion pressure, this process may theoretically result in perihematomal ischemia, further worsening outcomes.
- Increased intracranial pressure: Intracranial pressure may increase due to the mass effect of the hemorrhage, as well as blood accumulation in the ventricles (if the hematoma extends into the ventricles). Elevated ICP hinder blood flow (cerebral perfusion pressure), and may lead to herniation.
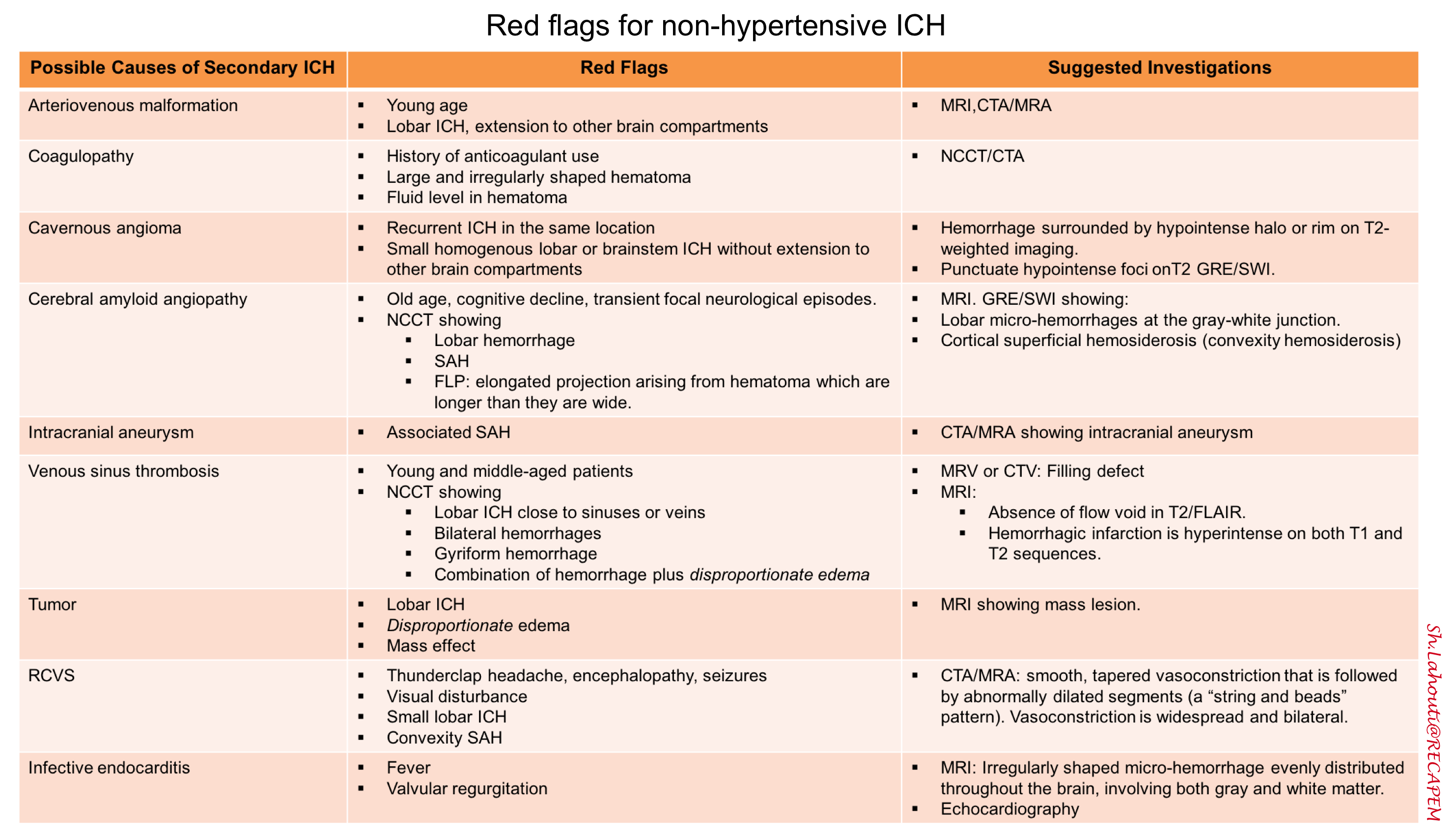
ICH is classified as primary (80%) or secondary (20%). Primary ICH is typically attributed to chronic hypertension or amyloid angiopathy, while secondary ICH can be caused by vascular malformation, tumor, coagulopathy, vasculitis, drug abuse, cerebral venous thrombosis, or the use of anticoagulants.
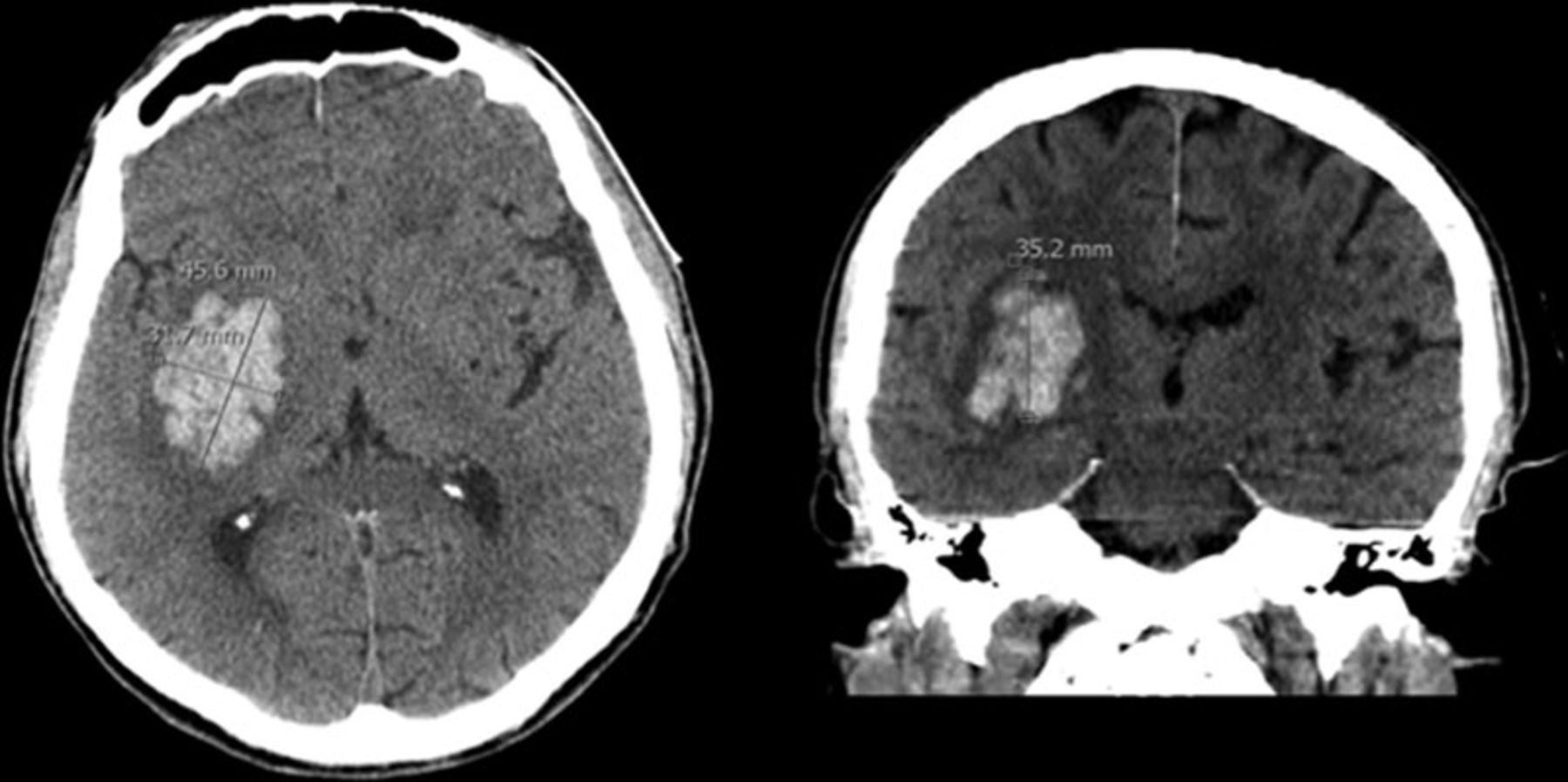
Hypertensive ICH
- The most common cause of spontaneous ICH.
- Patients with a SBP ≥160 mmHg or a DBP ≥110 mmHg have a ~5.5 times increased rate of ICH, compared with normotensive patients 2.
- Hypertension associated microvasculopathy occurs at deep sites in the brain. Most common locations are basal ganglia, thalamus, cerebellum, and brainstem, especially the pons.
- Supportive clues for presence of hypertensive ICH include other evidence of hypertensive end-organ damage e.g. left ventricular hypertrophy and chronic kidney disease 2.
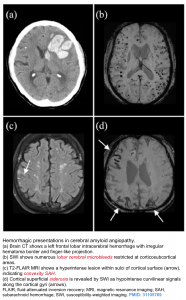
Cerebral amyloid angiopathy (CAA)
- CAA is caused by deposition of β-amyloid (the same peptide involved in Alzheimer’s disease) in cerebral arterioles activating late complement, which destroys vascular smooth muscle rendering arterioles brittle and unable to autoregulate blood flow 2.
- The genetic risk allele ApoE-ε4, famous for its association with Alzheimer’s disease risk, and to a lesser degree ApoE-ε2 are associated with ICH risk and are linked to CAA.
- It is a major cause of hemorrhage in elderly. ICH related to CAA typically occur in lobar locations at the junction of the cortical gray and white matter, sometimes with subarachnoid extension or finger-like projections of the hemorrhage.
- Importantly, however, one-half of lobar ICH in the elderly are not caused by CAA, so these two factors alone do not establish the underlying mechanism of the hemorrhage 2.
- It is often provoked by anticoagulation or thrombolysis.
- Diagnosis is based on MRI (GRE/SWI sequences) demonstrating
- A pattern of cerebral micro-hemorrhages typical of CAA, primarily in or near the cortical ribbon, associated with white matter hyperintensities; and
- Cortical superficial hemosiderosis (possibly represents oozing of blood products into the subarachnoid space on the surface of the brain) 2.
- Cortical hemosiderosis results from deposition of hemosiderin at leptomeningeal coverage of the brain.
- Distinguishing CAA from hypertensive ICH is important because patients with ICH due to CAA have a much higher risk of recurrence (8–10% vs. 1–2%) and have a higher risk of post-stroke dementia.
Cerebral aneurysm rupture
- ICH associated with cerebral aneurysms typically occurs in the subarachnoid space, but may occasionally rupture directly into the brain parenchyma, particularly when the aneurysm is large or distally situated in the vascular tree 2.
Vascular malformation
- Arteriovenous malformations. It may lead to devastating hemorrhages because of the arterial pressure entering the venous circulation; after a hemorrhage they need to be promptly diagnosed, usually with conventional and/or computed tomography (CT) angiography, and surgically treated.
- Cavernous malformations (also called cavernomas or cavernous hemangiomas). These consist of abnormal capillaries with relatively low pressure, so hemorrhages related to these lesions often produce less-severe symptoms than would be expected 2.
Ischemic stroke with hemorrhagic transformation
- Hemorrhagic transformation may result from natural evolution of ischemic stroke. The term hemorrhagic transformation is somewhat variably used and collectively refers to the following entities:
- Petechial hemorrhages: Most hemorrhagic transformations are small, petechial hemorrhages of little clinical significance.
- Asymptomatic hematomas: Small hematomas can occur within infarcted brain. If the hematoma is small and it occurs in a region of brain tissue which is already nonfunctional, this may not affect clinical outcome.
- Symptomatic hematomas: Large hematomas may exert mass effect, cause vasogenic edema, and worsen clinical outcomes.
- When it occurs, it is usually within the first 24–48 hours and, in almost all cases, is present 4–5 days after stroke (it is very rare in the first 12h after stroke onset).
- Risk factors for hemorrhagic transformation may include old age, uncontrolled hypertension and hyperglycemia, large infarct size, cardioembolic stroke etiology, coagulopathy (e.g. therapeutic anticoagulation or tPA) 3.
- Radiographically it may be classified as 4:
- Hemorrhagic infarct type 1: Petechial hemorrhages at the margins.
- Hemorrhagic infarct type 2: Petechial hemorrhages throughout the infarct, no mass effect.
- Parenchymal hematoma type 1: Hemorrhage of <30% of the stroke volume with mild mass effect.
- Parenchymal hematoma type 2: Hemorrhage of >30% of the stroke volume with substantial mass effect.
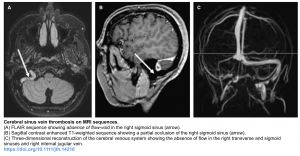
Cerebral venous thrombosis (CVT) with secondary hemorrhage
- It accounts for 5% of ICH in younger patients.
- Risk factors include hypercoagulability, inflammatory bowel disease, dehydration, pregnancy, parameningeal infection, lupus and thyroid disease 5, 7
- Imaging findings
- Imaging findings may be divided between direct signs (visualizing the clot itself) and indirect signs (hemorrhage or edema as a consequence of the clot).
- Direct sings of CVT may include: 👇Hemorrhage (~ 25% of patients), diffuse cerebral edema and venous infarction.
- Certain patterns of hemorrhage should suggest venous thrombosis 6:
- Bilateral hemorrhages, for example:
- Bilateral frontoparietal lobar hemorrhages due to superior sagittal sinus thrombosis.
- Bilateral thalamic hemorrhages due to thrombosis of the vein of Galen or straight sinus.
- Gyriform hemorrhage 8.
- Combination of hemorrhage plus disproportionate edema.
- Bilateral hemorrhages, for example:
- Diagnostic modality of choice: MRV/CTV.
- MR venography/CT venography allow for direct visualization of luminal filling defect.
👉MRI findings
- Normally, dural sinus can be seen as flow voids (e.g. on T2/FLAIR). Absence of these flow voids may suggest thrombosis.
- Hemorrhagic infarction is hyperintense on both T1 and T2 sequences.
Tumor
- Primary CNS tumor e.g. glioblastoma.
- Metastasis
- The metastases most frequently associated with brain hemorrhage are melanoma, renal cell carcinoma, papillary thyroid carcinoma, and germ cell tumors, non-small cell lung cancer 2.
- Metastases to the brain preferentially occur at the junction between the gray and white matter, so hemorrhagic metastases often present as lobar hemorrhages 2.
- The hemorrhage products may completely obscure the underlying tumor on initial imaging, so a high index of suspicion and follow-up neuroimaging is necessary to ensure that a malignancy is not missed.
- An underlying malignancy should especially be suspected when brain edema is out of proportion to the hemorrhage or present at an early time point when hemorrhage-related edema would not typically be expected 2 .
Coagulopathy
- Anticoagulation is a definite risk factor for intracerebral hemorrhage. Many such hemorrhages occur in the typical sites of ICH associated with hypertension.
- Therapeutic, acquired, or inherited coagulopathies may cause a large and irregularly shaped hematoma. In addition coagulopathy is associated with fluid level to form in the hematoma 2. This finding is specific but only moderately sensitive for the presence of a coagulopathy.
Less common causes
- Trauma
- Cerebral vasculitis
- Reversible cerebral vasoconstriction syndrome (RCVS)
Table.1. Summary of red flags for non-hypertensive ICH
Neuroimaging features of various mechanisms of intracerebral hemorrhage
Diagnostic considerations
Clinical presentation
The clinical presentation of intracerebral hemorrhage depends on the involved brain region, but it often presents with the sudden onset of a focal neurologic deficit. Symptoms can include sudden onset of headache, decreased level of consciousness, and vomiting 1 .
-
Unresponsiveness, neck pain or stiffness, severe headache, vomiting, seizures, and diastolic blood pressure >110 mm Hg all increase the probability of hemorrhagic vs. ischemic stroke in a patient with neurologic deficits 9.
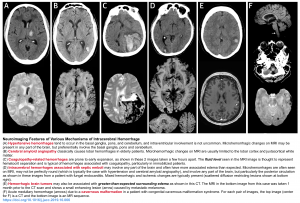
- Optic nerve sheath diameter (ONSD) on POCUS may help increase your suspicion for ICH in the setting of abrupt onset neurologic symptoms. Raised ICP is more in keeping with ICH than with ischemic stroke. An optic nerve sheath diameter of >6 mm is highly specific for raised ICP while an optic nerve sheath diameter of <5 mm is highly sensitive for ruling out raised ICP. See the following figure.
☠️ Hypertension, bradycardia, and an abnormal respiratory pattern, collectively known as “Cushing’s Triad,” are indications of intracranial hypertension.
Non-contrast CT
Non-contrast head CT is the recommended imaging modality to rapidly evaluate for intracranial hemorrhage. Fortunately, acute blood is markedly hyperdense compared to brain parenchyma, and as such usually poses little difficulty in diagnosis (provided the amount of blood is large enough, and the scan is performed early).
In the context of ICH, the following points should be considered on NCCT:
- Location: ICH location can provide important clues in the identification of ICH etiology.
- Deep ICHs are more commonly associated with long standing hypertension.
- Lobar bleedings are traditionally associated with CAA in the right clinical context.
- Remeber that the term lobar refer to the location of hematoma to the cortex and subcortical white matter.
- Hematoma volume
- The volume of hematoma may be estimated as the multiple of the diameters divided by two (ABC/2).
- A is the largest hematoma diameter on any axial CT image.
- B is the largest diameter perpendicular to A, within that axial CT slice.
- C is the number of axial CT slices showing hemorrhage, multiplied by the slice thickness (which may vary from 3-10 mm depending on the scanner). Alternatively, this may be measured as the height of the hematoma, when measured in sagittal or coronal plane.
- All measurements should be made in centimeters. This will yield a volume measured in milliliters (mL).
- Volume >60 ml suggests a worse prognosis
- The volume of hematoma may be estimated as the multiple of the diameters divided by two (ABC/2).
- Shape (irregular vs regular) and density (homogeneous vs heterogeneous)
- Edema: Presence/absence of substantial surrounding edema that may indicate an underlying tumor
- Presence/absence of intraventricular hemorrhage and hydrocephalus
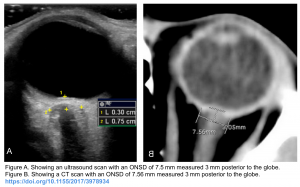
- Markers of hemorrhage expansion on non-contrast CT 10
- Black hole sign
- Defined as a relatively hypo-attenuated area within a hematoma that is not connected with adjacent brain tissue. It predicts hematoma growth with 32% sensitivity and 94% specificity.
- Fluid-fluid level: Fluid level in a hematoma suggests the presence of coagulopathy. This finding is only moderately sensitive, but it is specific 2.
- Blend sign: Hemorrhage contains hyperdense and hypodense regions.
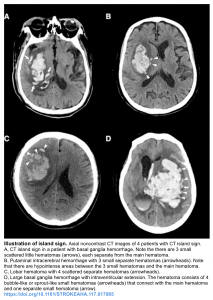
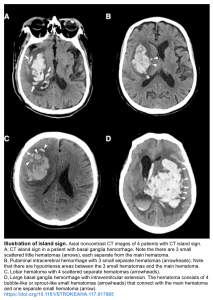
- Island sign: Is defined as ≥3 scattered small hematomas all separate from the main hematoma or ≥4 small hematomas some or all of which may connect with the main hematoma 11.
- Black hole sign
Evaluation for underlying etiology
Once intracerebral hemorrhage is diagnosed, one should consider the etiology of the bleed. In an elderly patient with an obvious rounded hemorrhage in the deep cerebral tissue, it may be adequate to presume a hypertensive bleed in the correct clinical context.
In patients without typical risk factors and those demonstrating alternative presentations, further investigation is warranted to tailor appropriate treatment.
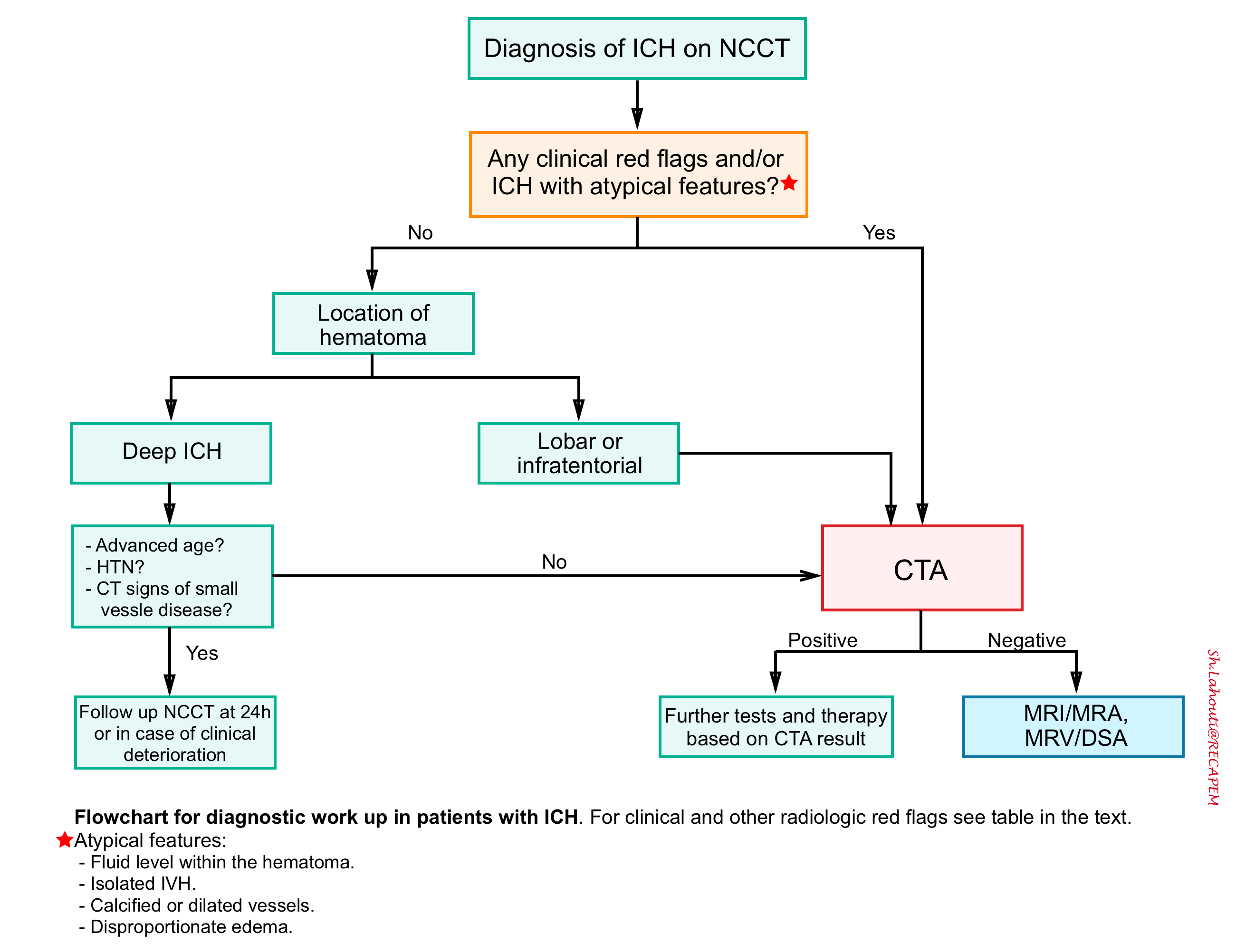
When to suspect to underlying vascular pathology
- Factors which increase the likelihood of an underlying vascular pathology include 12:
- Imaging findings on non-contrast CT scan
- Atypical location for hypertensive ICH (e.g. lobar or infratentorial hemorrhage).
- Fluid level within the hematoma.
- Isolated intraventricular hemorrhage.
- Calcified or dilated vessels.
- Dispropotionate edema surrounding the hematoma (e.g. more edema than might be expected).
- Epidemiological findings
- Younger age (e.g. <65 years old).
- Female sex.
- No history of hypertension.
- No coagulopathy.
- Imaging findings on non-contrast CT scan
Flowchart for diagnostic work up in patients with ICH is shown below.
CT angiography / venography
- CTA is the recommended imaging modality used to assess for the vascular underlying cause, due to its ease of acquisition and high sensitivity (96%) and specificity (99%).
- It may be helpful for prognostication of the risk of hematoma expansion as well.
- The spot sign reveals leakage of contrast into the hematoma (predicting expansion with 51% sensitivity and 85% specificity). The spot sign must not be in anatomic continuity with any adjacent blood vessels.
- Spot sign is a predictor of early neurologic deterioration and mortality and may be useful for targeting aggressive blood pressure management and/or clinical decision-making in certain settings.
- When central venous thrombosis is suspected, CT venography is the test of choice.
MRI
- Because of logistic barriers, this is not immediately performed.
- MRI may be superior for detecting certain underlying pathologies 13:
- Ischemic stroke with hemorrhagic transformation.
- Amyloid angiopathy with multiple occult cerebral micro-bleeds.
- Underlying hypertensive microangiopathy (may be suggested by white matter lesions and small lacunar strokes).
- Tumor
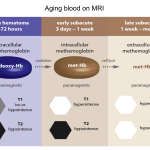
👉Note that Hemorrhage on MRI has highly variable imaging characteristics that depend on both the age of the blood, the type of hemoglobin present (oxy- deoxy- or met-), on whether or not the red blood cell walls are intact and the specifics of the MRI sequence (see appendix 1).
General approach to management
The major priorities associated with acute care of critical patients with ICH involves:
- Cardiopulmonary resuscitation if required.
- Assess airway, breathing and circulation upon arrival. Intubation may be required for airway protection. Care is required to avoid stimulation and hypertension, which could worsen intracranial pressure elevation.
- ABC of acute hemorrhage stabilization 2
- A. Assess Alertness. If decreased due to mass effect, initiate rescue hyperosmolar therapy (if the patient is to be transferred, a rescue hyperosmolar agent should be available in the ambulance in case the patient deteriorates). If there is hydrocephalus or impending obstruction, CSF diversion should be rapidly achieved (typically via external ventricular drain or, for large infratentorial hemorrhages, suboccipital craniectomy and clot evacuation).
- B. Optimize Blood pressure. Acutely reduce blood pressure to a target SBP <180 mm Hg in most patients using titratable drug if initial blood pressure is significantly elevated. Avoid hypotension.
- C. Correct Coagulopathy. If the patient is taking anticoagulants, this should be reversed using the fastest, most effective available agent (see below).
- Move the patient to a neurological intensive care unit as soon as feasible.
- Consultation with neurosurgical team for appropriate cases.
- Document hemorrhage stability at 6 hours with repeat neuroimaging (sooner if there is any worsening of neurological exam).
- Investigate and address the underlying cause of the hemorrhage, discussed above.
Blood pressure management
Blood pressure should be managed aggressively, with particular attention to the goal targeted to the individual patient.
- Background: Acute hypertension is a major driver of early hematoma expansion, so aggressive blood pressure control could theoretically reduce hematoma expansion. This would be expected to be most effective within the period that the hematoma is expanding (typically the first six hours, but potentially longer in coagulopathic patients).
- Risks: Reduced blood pressure could impair perfusion of penumbra surrounding the hematoma. This could cause necrosis, leading to worsening edema and intracranial pressure (leading to a cascade of secondary brain insults).
- Target: A systolic blood pressure < 180 mm Hg is consistent with available evidence and the most recent guidelines 14.
- If SBP >220 mm Hg, lower by 25%, with a goal of <180 mm Hg.
- Agents: Fast-acting titratable agents are preferred e.g. nicardipine, clevidipine or labetalol.
- Nicardipine 5 mg/h IV, increase by 2.5 mg/h q10min (max 15 mg/h) until the target blood pressure is achieved and then immediately titrate down to maintenance infusion of 3-5 mg/hr. If BP falls below target, decrease infusion to 2.5 mg/hr or stop entirely.
- Labetalol. Start with sequential pushes of 20mg, 40mg, 80mg, 80mg, 80mg (q15 min PRN). Max 300 mg total dose.
⚠️Avoid antihypertensive agents that can increase intracranial pressure, e.g. hydralazine, nitroprusside, and nitroglycerin.
Coagulopathy reversal
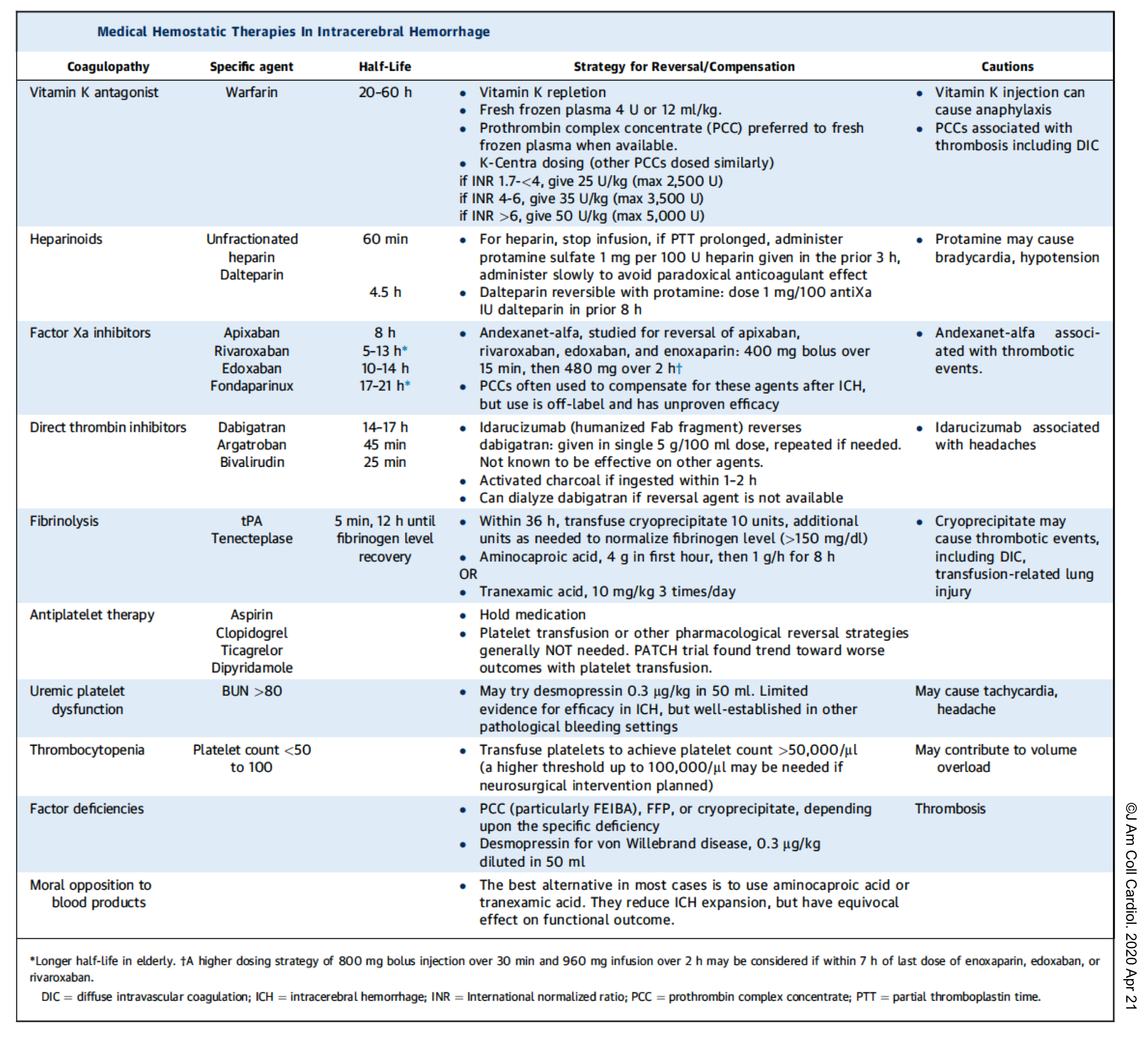
Anticoagulant use should be aggressively reversed. The following table summarize effective strategies for reversing the coagulopathy.
👉Keep in mind that thrombocytopenia should be treated, to maintain a platelet count >100,000 if possible.
- However, there is no benefit in platelet transfusion for patients who had been treated with aspirin or anti-platelet agents (PATCH trial), with the possible exception of patients undergoing neurosurgery.
Neurosurgical intervention
Surgical hematoma evacuation for supratentorial hemorrhage
- Possible indications for surgery
- Large hematoma with significant midline shift.
- Elevated ICP refractory to medical management.
- Hemorrhage secondary to an underlying lesion that could benefit from resection (e.g. arteriovenous malformation or tumor).
Surgical hematoma evacuation for cerebellar hemorrhage
- In the presence of following conditions, surgical evacuation may be considered
- Hematoma >3 cm diameter.
- Mass effect causing brainstem compression or herniation.
- Hydrocephalus due to ventricular obstruction.
- Note that management with an external ventricular drain alone in this situation may lead to upward herniation of the posterior fossa. Thus, an external ventricular drain should be combined with hematoma evacuation.
External ventricular drain (EVD)
- Consider ventriculostomy placement in the setting of intraventricular hemorrhage, hydrocephalus, mass effect, or brainstem herniation with neurologic deterioration.
Other supportive measures
Fever
- Fever is common after ICH and may be due to systemic infection from secondary aspiration pneumonia, or inflammation from the ICH, especially if IVH is present (neurogenic fever) 1.
- Source identification for the infection should be the goal. Neurogenic fever is essentially a diagnosis of exclusion.
- Any fever should be treated aggressively (e.g. with scheduled acetaminophen). If fever is refractory to antipyretics, an external cooling device may be used to achieve normothermia.
Seizure
- Seizure occurs with an incidence of ~7%. Risk factors for seizure include non-hypertensive ICH and cortical involvement.
- Evaluation for seizure with continuous EEG may be indicated for patients with impaired consciousness that is out of proportion to what would be expected based on the CT scan. Evaluation is also indicated based on any history or clinical signs of seizure.
- Seizure prophylaxis is generally not warranted. Anti-epileptic therapy is indicated for a patient with witnessed seizure or electroencephalographic seizure.
Glucose control
- The current recommendation for blood glucose level is 110-180 mg/d 1.
Elevated ICP
-
Consider osmotic therapy using hypertonic saline or mannitol if invasive monitoring indicates elevated ICP. Ideally its used as a bridge to more definitive therapy (e.g. an external ventricular drain).
-
Mannitol: 1-1.5 g/kg 20% solution, followed by bolus doses of 0.25-1 g/kg to a target osmolarity of 300-320 mOsm/kg.
-
Hypertonic sodium chloride: 23.4% 30 mL IV over 2-20 min or 0.43 mL/kg IV over 15 min.
-
DVT prophylaxis
- Intermittent pneumatic compression should be used initially for DVT prophylaxis.
- Heparin for DVT prophylaxis is generally safe >48 hours after initial bleed, if there has been no hematoma expansion on repeat CT scan.
Prognosis
Mortality of ICH is high with approaching to 70% at 5 year post-incident. Morbidity is high, with less than 40% of patients reaching functional independence.
Poor predictor of outcome
- Location: Deep or infratentorial
- The mortality of ICH varies, depending on the location of the bleed. Brainstem bleeds have the highest mortality (60%), followed by lobar (46%), deep (44%), and cerebellar (34%).
- Size: Hematoma volume > 60 ml.
- Intraventricular extension
- GCs <8.
- Elderly
- The most widely used tool for assessing prognosis, which predicts mortality based on hemorrhage size, patient age, Glasgow coma score, hemorrhage location (infratentorial or supratentorial), and the presence of intraventricular hemorrhage 2.
- It is designed to predict survival, rather than independent survival or quality of life.
- ⚠️Do not prognosticate within the first 24 hours. Early prognostication for individual patients is often challenging and difficult to predict. Withdrawal of care should generally not be decided in the ED
- Estimates the likelihood of functional independence at 90 days, potentially a more meaningful endpoint.
Appendix
Media
Hypertensive hemorrhage
Lobar hemorrhage
Hemorrhagic transformation of ischemic stroke
Going further
- Emergency Management of Intracerebral Hemorrhage – The Golden Hour (EMCases )
- Intracerebral hemorrhage (IBCC)
Peer reviewed by Mojtaba Chardoli.
References
1. O’Carroll CB, Brown BL, Freeman WD. Intracerebral Hemorrhage: A Common yet Disproportionately Deadly Stroke Subtype. Mayo Clin Proc. 2021 Jun;96(6):1639-1654. doi: 10.1016/j.mayocp.2020.10.034. Epub 2021 May 2. PMID: 33952393.
2. Schrag M, Kirshner H. Management of Intracerebral Hemorrhage: JACC Focus Seminar. J Am Coll Cardiol. 2020 Apr 21;75(15):1819-1831. doi: 10.1016/j.jacc.2019.10.066. PMID: 32299594.
3. Álvarez-Sabín J, Maisterra O, Santamarina E, Kase CS. Factors influencing haemorrhagic transformation in ischaemic stroke. Lancet Neurol. 2013 Jul;12(7):689-705. doi: 10.1016/S1474-4422(13)70055-3. Epub 2013 May 31. PMID: 23726850.
4. Larrue V, von Kummer R R, Müller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke. 2001 Feb;32(2):438-41. doi: 10.1161/01.str.32.2.438. PMID: 11157179.
5. Ropper AH, Klein JP. Cerebral Venous Thrombosis. N Engl J Med. 2021 Jul 1;385(1):59-64. doi: 10.1056/NEJMra2106545. PMID: 34192432.
6. Fink KR, Benjert JL. Imaging of Nontraumatic Neuroradiology Emergencies. Radiol Clin North Am. 2015 Jul;53(4):871-90, x. doi: 10.1016/j.rcl.2015.02.004. PMID: 26046515.
7. Canedo-Antelo M, Baleato-González S, Mosqueira AJ, Casas-Martínez J, Oleaga L, Vilanova JC, Luna-Alcalá A, García-Figueiras R. Radiologic Clues to Cerebral Venous Thrombosis. Radiographics. 2019 Oct;39(6):1611-1628. doi: 10.1148/rg.2019190015. PMID: 31589585.
8. Hollingsworth J, Mirabelli MM. Neurologic Emergencies on Computed Tomography of the Head. Semin Ultrasound CT MR. 2017 Aug;38(4):384-398. doi: 10.1053/j.sult.2017.02.005. Epub 2017 Feb 20. PMID: 28865528.
9. de Oliveira Manoel AL, Goffi A, Zampieri FG, Turkel-Parrella D, Duggal A, Marotta TR, Macdonald RL, Abrahamson S. The critical care management of spontaneous intracranial hemorrhage: a contemporary review. Crit Care. 2016 Sep 18;20:272. doi: 10.1186/s13054-016-1432-0. PMID: 27640182; PMCID: PMC5027096.
10. Boulouis G, Morotti A, Charidimou A, Dowlatshahi D, Goldstein JN. Noncontrast Computed Tomography Markers of Intracerebral Hemorrhage Expansion. Stroke. 2017 Apr;48(4):1120-1125. doi: 10.1161/STROKEAHA.116.015062. Epub 2017 Mar 13. PMID: 28289239; PMCID: PMC5378158.
11. Li Q, Liu QJ, Yang WS, Wang XC, Zhao LB, Xiong X, Li R, Cao D, Zhu D, Wei X, Xie P. Island Sign: An Imaging Predictor for Early Hematoma Expansion and Poor Outcome in Patients With Intracerebral Hemorrhage. Stroke. 2017 Nov;48(11):3019-3025. doi: 10.1161/STROKEAHA.117.017985. Epub 2017 Oct 10. PMID: 29018128.
12. Reyes R, Viswanathan M, Aiyagari V. An update on neurocritical care for intracerebral hemorrhage. Expert Rev Neurother. 2019 Jun;19(6):557-578. doi: 10.1080/14737175.2019.1618709. Epub 2019 May 21. PMID: 31092052.
13. Sporns PB, Psychogios MN, Boulouis G, Charidimou A, Li Q, Fainardi E, Dowlatshahi D, Goldstein JN, Morotti A. Neuroimaging of Acute Intracerebral Hemorrhage. J Clin Med. 2021 Mar 5;10(5):1086. doi: 10.3390/jcm10051086. PMID: 33807843; PMCID: PMC7962049.
14. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018 Jun;71(6):1269-1324. doi: 10.1161/HYP.0000000000000066. Epub 2017 Nov 13. Erratum in: Hypertension. 2018 Jun;71(6):e136-e139. Erratum in: Hypertension. 2018 Sep;72(3):e33. PMID: 29133354.
15.





Add comment