21 Feb, 2022 via Shahriar Lahouti. Last update 30 December 2024.

CONTENTS
- Introduction
- Background
- Pathophysiology of RV failure
- Definition
- Causes of PH
- Precipitating factors
- Clinical presentation
- Management of PH
- Specific conditions
- Media
- Appendix
- References
CHD congenital heart disease
CO cardiac output
COPD chronic obstructive pulmonary disease
CT computed tomography
CTD connective tissue disease
CVP central venous pressure
IVC inferior vena cava
LA left atrium/atrial
LHD left heart disease
LV left ventricle/ventricular
MAP mean arterial pressure
mPAP mean pulmonary arterial pressure
PA pulmonary artery
PAH pulmonary arterial hypertension
PAP pulmonary arterial pressure
PAWP pulmonary artery wedge pressure
PASP pulmonary artery systolic pressure
PE pulmonary embolism
PH pulmonary hypertension
PVH pulmonary venous hypertension
PVR pulmonary vascular resistance
RA right atrium
RAP right atrial pressure
RHC right heart catheterization
RV right ventricle/ventricular
Introduction
Pulmonary hypertension (PH) is a feature of a variety of diseases and continues to harbor high morbidity and mortality. The main consequence of PH is right-sided heart failure which can be very difficult to correct once deterioration begins.
Given the tenuous hemodynamic state of PH, small physiologic insults can result in RV failure; hence a tiny mistake in treatment is unforgivable.
Background
Three mechanisms can contribute to right ventricle failure 1 :
- Pump failure – RV muscle failure e.g. RV MI.
- Pipe problem – Pressure overload (i.e. excessive afterload) on the RV (pulmonary hypertension).
- Tank problem – Volume overload e.g. massive volume resuscitation, ASD.
The most common cause for RV dysfunction/failure in practice is pressure overload with decreased contractility at second place 2.
- In patients with PH, the mean pulmonary arterial pressure is increased. The RV dysfunction secondary to increased right ventricular afterload can result in a syndrome with the clinical signs and symptoms of heart failure 3.
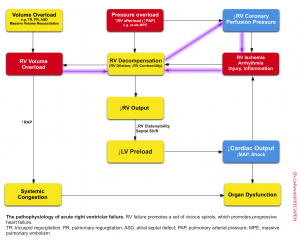
Pathophysiology of RV failure
◾️Vicious cycle
- Any type of primary derangement to RV (pressure and/or volume overload, ↓contractility) will further deteriorate RV performance via downward complex chains of events that reinforce themselves through a feedback loop (vicious cycle). This is extremely dangerous because if left untreated, cardiovascular collapse and death will be the final outcome.
- These spirals explain why patients with RV failure can sometimes suddenly die (a phenomenon often seen in patients with massive PE or following intubation).
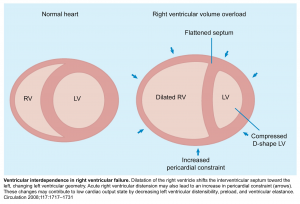
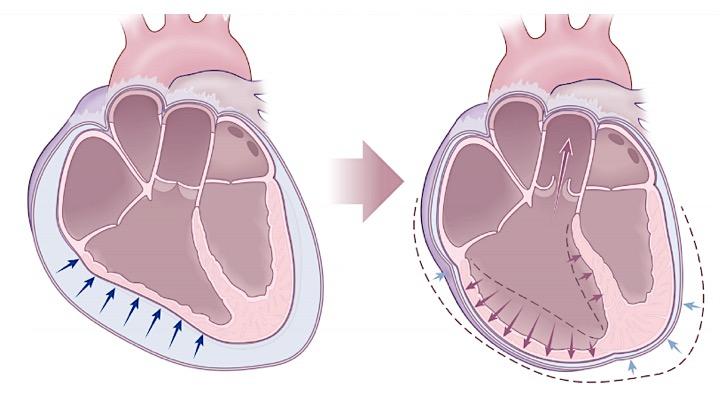
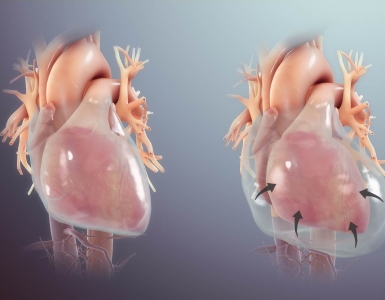
- A central feature of RV failure is RV dilation which causes shifting of the interventricular septum, as well as functional tricuspid regurgitation.
- The pulmonary vascular bed is normally a high-flow, low-pressure system. When there is an increase in pulmonary vascular resistance, the RV cannot adapt as well as the LV, leading to dilation.
- The relationship between the RV and LV cavities is known as interventricular dependence (figure below). When the RV bulges with volume and pressure overload into the LV, the LV has decreased filling 2.
◾️RV Perfusion principles
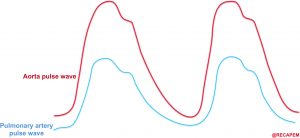
- Unlike the left ventricle, the RV is normally perfused during both systole and diastole 4.
- Perfusion pressure within the right coronary artery is equal to (aortic pressure minus pulmonary arterial pressure) 5. The aortic and pulmonary arterial pulse waves are illustrated above. The gap between these two represents the perfusion pressure within the right coronary artery.
- In the context of PH, this gap is narrowed. The right coronary artery perfusion pressure is decreased and RV ischemia eventually happens.
- Defending the RV myocardial perfusion (widening the gap) requires interventions that increase the systemic blood pressure and decrease the pulmonary artery pressure.
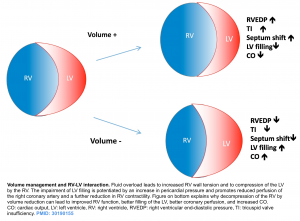
◾️Volume Overload
- Adequate right-sided filling pressure is essential in maintaining cardiac output in patients with acute RV failure.
- RV preload requirements differ substantially based on whether afterload is normal or increased.
- When RV failure occurs in the setting of normal pulmonary vascular resistance, such as in right-sided myocardial infarction, RV end-diastolic pressure often needs to be increased above normal levels to maintain cardiac output.
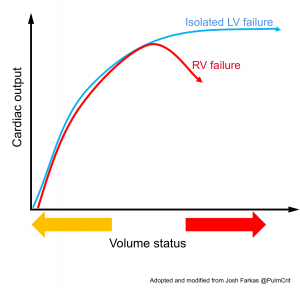
- On the other hand, when RV failure occurs in the setting of increased RV afterload, volume loading can result in displacement of the interventricular septum toward the LV and impaired LV diastolic filling and cardiac output. At the same time, RV dilation increases free wall tension, resulting in decreased RV perfusion. Impaired RV function in the context of volume overload is referred to as falling off the Starling curve (figure below). In this setting, intravascular volume need to be decreased.
- Note that this phenomenon probably doesn’t occur acutely in patients with isolated LV failure, because the left ventricle is a thick-walled chamber with a fairly fixed volume. Thus, the LV doesn’t dilate acutely in response to volume overload.
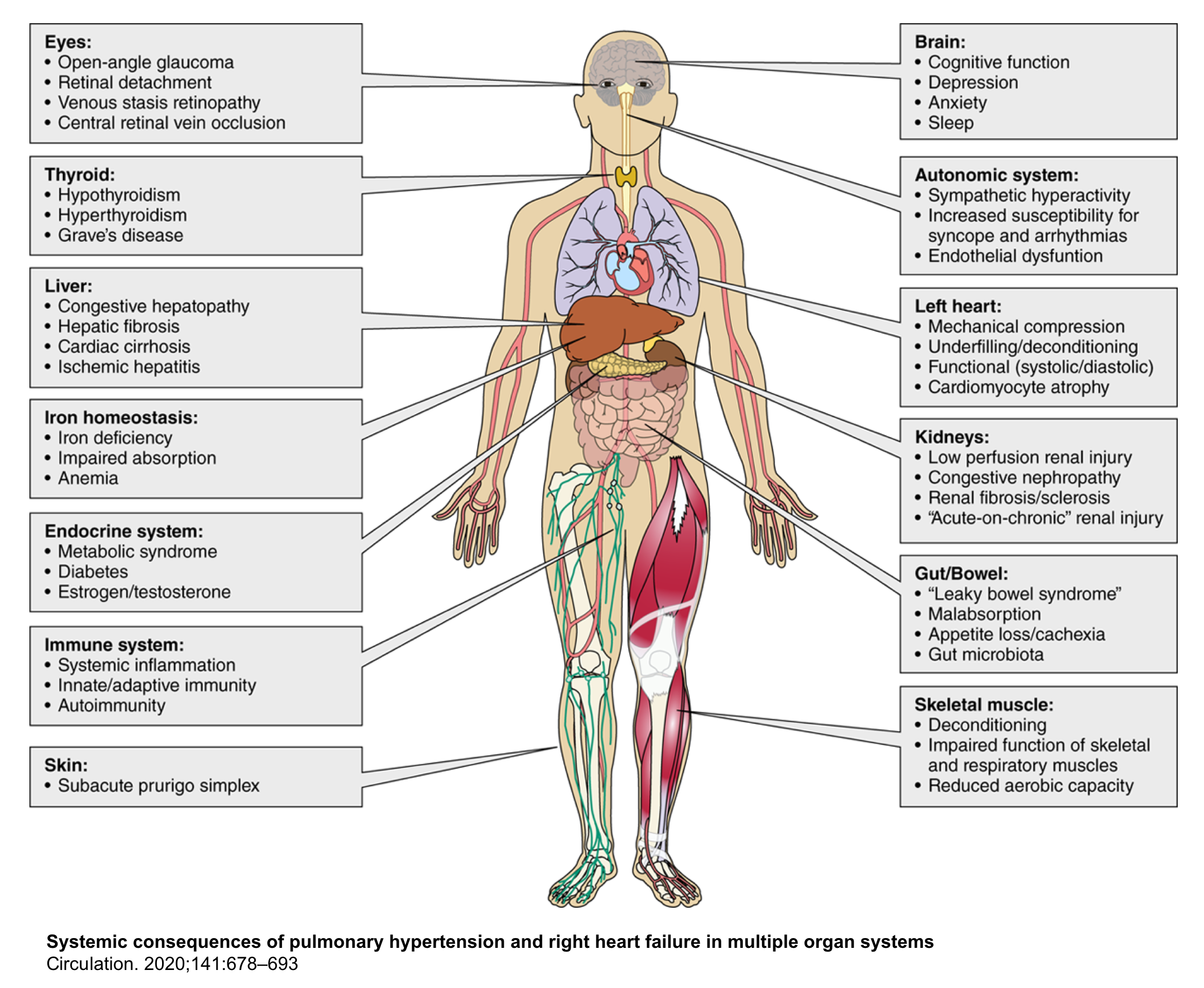
◾️Systemic consequence of RHF
Systemic Perfusion Pressure = (MAP – RAP)
Hypoperfusion can happen as a result of hypotension (decreased MAP), advanced congestion (increased RAP), or both. More on this here.
- In right ventricular failure, volume overload can result in systemic congestion (with an elevated RAP).
- Advanced congestion with significant elevation of RAP may lead to organ malperfusion because it reduces the systemic perfusion pressure. Organs that are particularly affected include the brain, kidneys, liver, and bowel 3.
- If patients deteriorate further and develop overt hypotension, visceral organs will suffer a double hit due to both the reduced MAP plus the elevated RAP.
The systemic consequences of pulmonary hypertension and right heart failure are shown below.
- ⚠️The systemic character of PH and right-sided HF is often neglected or underestimated since systemic congestion doesn’t result in any vital sign abnormality or dramatic symptomatology until late when it is profound.
Definition
Pulmonary hypertension (PH) has been previously defined as a sustained increase in mean pulmonary arterial pressure (mPAP) to >25 mmHg at rest, measured by right heart catheterization (RHC) 6.
- PH has been recently redefined (by the Sixth World Symposium on Pulmonary Hypertension) as a mean pulmonary artery pressure of >20 mm Hg along with a pulmonary vascular resistance (PVR) of 3 or more Wood units for precapillary disease 7.
- Note that echocardiographic measurement of pulmonary arterial systolic pressure (PASP) roughly correlates with RHC value by a factor of 10.
- Therefore, a PASP >35 mm Hg (or a tricuspid jet velocity > 3.4 m/s) on echocardiography suggests PH.
Causes of PH
The causes of PH can be classified into 2 lines: causes of chronic pulmonary hypertension and causes of acute pulmonary hypertension which encompasses acute severe diseases that can cause acute pulmonary hypertension.
◾️Chronic Pulmonary Hypertension 7, 8, 9
- Group 1: Pulmonary Arterial Hypertension (PAH)
- Idiopathic
- Hereditary
- Drug and toxin induced (e.g. anorexigens, cocaine, chemotherapy).
- Pulmonary Arterial Hypertension associated with:
- Connect tissue diseases such as SLE, scleroderma
- HIV
- Portal hypertension
- Congenital right-to-left shunt (e.g. atrial septal defect)
- Schistosomiasis
- Long-term responders to calcium channel blockers
- Pulmonary veno-occlusive disease / pulmonary capillary hemangiomatosis.
- Persistent PH of the newborn syndrome
- Group 2: Pulmonary Venous Hypertension (PVH). LV systolic and/or diastolic disease, valvular disease, congenital or acquired left heart inflow/outflow tract obstructions.
- Group 3: Pulmonary Hypertension (PH) due to underlying lung disease and/or hypoxia; e.g. COPD, ILD, obstructive sleep apnea, obesity hypoventilation syndrome.
- Group 4: Pulmonary Hypertension (PH) due to pulmonary artery obstruction e.g. chronic thromboembolic pulmonary artery hypertension (CTEPH).
- Group 5: Pulmonary Hypertension (PH) secondary to multifactorial or unknown mechanisms.
- Hematologic disorders (e.g. chronic hemolytic anemia, myeloproliferative disorders, splenectomy, sickle cell anemia).
- End-stage renal disease requiring dialysis.
- External compression of the pulmonary arteries.
◾️Acute Pulmonary Hypertension 10
- A severe, acute insult may cause new-onset pulmonary hypertension. These insults can also be superimposed upon chronic pulmonary hypertension, causing an acute decompensation. Acute insults include:
- Massive pulmonary embolism (MPE)
- ARDS
- Septic shock
Precipitating factors
These factors are typically not severe enough to cause pulmonary hypertension by themselves. However, they may serve to destabilize patients who already have pulmonary hypertension due to the causes listed above.
- Hypervolemia: is a common precipitant of RV failure 10.
- Pulmonary embolism: even a subsegmental PE may be sufficient to cause decompensation among patients with chronic PH.
- Infection
- Any lung disease (any cause of hypoxemia, hypercapnia, acidosis, diminished/excessive lung volumes) 11,12,13.
- Parenchymal lung disease: Pneumonia.
- Lung overdistension: Excessive PEEP or auto-PEEP.
- Lung under-distention: Atelectasis, pneumothorax, pleural effusion.
- Arrhythmia: Patients with RV failure are susceptible to alteration in cardiac rhythm and ventricular synchrony 14.
- Supraventricular arrhythmias (especially atrial fibrillation) are more common than ventricular arrhythmias in PAH. Atrial fibrillation is especially problematic due to the loss of atrial kick.
- Bradycardia is also poorly tolerated and suggestive of imminent death in some contexts e.g. massive pulmonary embolism.
- Medications
- Negative inotropes (e.g. beta-blockers, calcium channel blockers).
- Alpha-agonists
- Systemic vasodilators
Clinical presentation
◾️History
- PH is often under-recognized and under-documented.
- Some patients may have a history of chronic symptoms such as dyspnea (due to PH) which has remained unrecognized.
- Many other patients have asymptomatic PH. They may clinically manifest with signs and symptoms of RV failure upon exposure to a precipitating factor. The overall clinical picture here is often a patient with an acute problem who gets much sicker than would be expected.
◾️Clinical signs and symptoms of PH result from progressive RV dysfunction/failure.
- Shortness of breath, fatigue, weakness, angina, and syncope.
- These are initial symptoms and are typically induced by exertion. Symptoms at rest occur only in advanced cases.
- The following symptoms may be seen with increasing severity of the disease:
- Peripheral congestion
- These result from failure of the right ventricle to decongest the peripheral circulation and are invariably present in patients with chronic progressive PH. In patients with acute PH (e.g. massive PE), these signs are usually absent. Signs and symptoms of peripheral congestion include:
- Peripheral edema.
- Ascites.
- Pleural effusion.
- Pericardial effusion.
- Congestive hepatomegaly may result in right upper abdominal pain.
- These result from failure of the right ventricle to decongest the peripheral circulation and are invariably present in patients with chronic progressive PH. In patients with acute PH (e.g. massive PE), these signs are usually absent. Signs and symptoms of peripheral congestion include:
- Systemic hypoperfusion (poor perfusion can be caused by low ‘MAP’ or elevated ‘RAP’).
- Hypoperfusion due to systemic congestion (elevated RAP): The following organ malfunction can happen in the absence of frank hypotension.
- Congestive encephalopathy with delirium (agitation, drowsiness)
- Congestive nephropathy, with elevated creatinine and decreased urine output 3.
- Leaky bowel syndrome: Bowel wall edema may promote bacterial translocation and systemic inflammation, adding a component of vasodilatory shock 3.
- Congestive hepatopathy: Congestion is indicated by elevations in alkaline phosphatase and bilirubin 15.
- In severe cases of acute RV failure, transaminases may be elevated from shock liver (AST > ALT) 16.
- The classic finding in shock liver is an ALT: LDH ratio < 1:1.5.
- Hypoperfusion due to systemic hypotension: In the most severe cases, RV failure will cause overt hypotension resulting in multiple organ dysfunction. This is extremely dangerous because it causes a ‘double hit’ to the systemic perfusion.
- Hypoperfusion due to systemic congestion (elevated RAP): The following organ malfunction can happen in the absence of frank hypotension.
- Peripheral congestion
- Other less common presentations of patients with PH include:
- Hemoptysis. it’s related to the rupture of hypertrophied bronchial arteries.
- Hoarsens, wheezing due to mechanical compression of enlarged pulmonary artery on the recurrent laryngeal nerve and large airway.
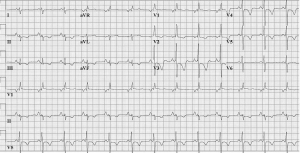
ECG
ECG in PH is non-sensitive and non-specific, in part due to the heterogeneity of the condition. ECG may provide supportive evidence of PH, but a normal ECG does not exclude the diagnosis. Findings may include 6:
- Right atrial enlargement: Peaked P wave (>2.5mm in amplitude) in inferior leads.
- Right ventricular hypertrophy: R/S ratio >1 or an R wave >7 mm in lead V1.
- RV strain: T wave inversions in the precordial leads, especially V2-3.
- Other findings: Right bundle branch block, right axis deviation.
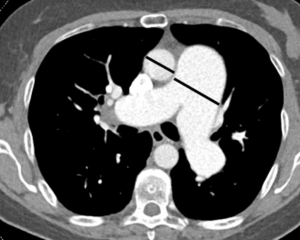
Chest CT
It can be useful for evaluating the heart and pulmonary vascular system. A review of archival CT images can also help sort out acute versus chronic pathology. Findings may include 17:
- Pulmonary artery (PA) dilation
- In PH, the diameter of the main pulmonary artery tends to be > 29 mm and may be larger than that of the aorta in the same section.
- The best location to measure the PA is at the level of the PA bifurcation perpendicular to its long axis 18.
- RV changes
- RV hypertrophy is defined as RV wall thickness >4 mm at the mid-ventricle level (figure below).
- RV dilation is considered when the RV: LV ratio is >1 at the mid-ventricular level.
- Straightening or leftward bowing of the interventricular septum (IVS).
- Contrast reflux into the inferior vena cava and hepatic veins suggest right ventricular insufficiency (indicating tricuspid regurgitation and intolerance of the RV to handle additional preload).
- Pericardial effusion
⭐️Chest CT can also assist in identifying associated conditions or etiologies of PH. For example, CT may demonstrate evidence of chronic thromboembolic PH or interstitial lung disease.
Echocardiography
◾️Qualitative signs of pulmonary hypertension
- Chamber dilation
- RV enlargement (RVE)
- Right atrial dilation.
- Pulmonary artery dilation (either pulmonary artery diameter > aortic root diameter, or pulmonary artery diameter >25 mm).
- RV enlargement (RVE)
- RV systolic failure: TAPSE (tricuspid annular plane systolic excursion) < 18 mm
- TAPSE is assessed via M-mode from apical four chambers, measuring the amount of longitudinal motion of the annulus at peak systole. Normal range of TAPSE: >18 mm.
- Mild RV dysfunction: 10-17 mm.
- Moderate RV dysfunction: 5-10 mm.
- Severe RV dysfunction: < 5 mm.
- TAPSE is assessed via M-mode from apical four chambers, measuring the amount of longitudinal motion of the annulus at peak systole. Normal range of TAPSE: >18 mm.
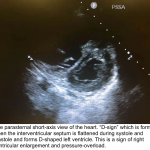
- RV Septal flattening (D-sign)
- The D-sign refers to interventricular septal flattening observed in short-axis views of the heart.
- D-configuration in diastole suggests volume overload.
- D-configuration in systole suggests pressure overload (so this is especially suggestive of pulmonary hypertension).
- D-configuration throughout the cardiac cycle suggests a combination of both volume and pressure overload (as is often seen in advanced pulmonary hypertension). Image 2.
- Systemic congestion
- Plethoric IVC
- Elevated hepatic venous pressure (more on this, here)
◾️Quantitative signs of pulmonary hypertension
- Pulmonary artery systolic pressure (PASP)
- PA systolic pressure (PASP) can be estimated based on the right atrial pressure (RAP) plus the maximal tricuspid regurgitant (TR) jet velocity, (more on this, here).
- PASP = RAP + 4 (TR jet velocity in m/s)2
- PASP may allow estimation of the likelihood of PH:
- sPAP < 36 mm Hg: PH is unlikely.
- sPAP 36-50 mm Hg: Grey zone.
- sPAP >50 mm Hg: PH is likely.
- Method
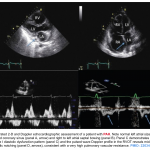
- The tricuspid regurgitant jet can be measured by using a continuous wave doppler (CW) placed across the tricuspid valve (Image 3).
- Note that tricuspid regurgitation is often absent in patients with proven pulmonary hypertension. Thus, the absence of a measurable tricuspid regurgitant jet doesn’t exclude pulmonary hypertension.
- ⚠️Pitfalls in estimating PASP (by TRJV method): Inaccuracies in the estimation of PASP
- TR is underestimated in compensated RV (for high ventricular pressure).
- Severe (free flow) TR → equalization of right atrial and ventricular pressures.
- Pulmonary stenosis.
- Severe RV systolic dysfunction.
- Misalignment of CW Doppler signal: e.g., eccentric jets.
- Suboptimal CWD envelope.
- 💡Tip: IV agitated saline can be used to improve the TR envelope.
- The tricuspid regurgitant jet can be measured by using a continuous wave doppler (CW) placed across the tricuspid valve (Image 3).
- Changes in sPAP over time don’t necessarily track disease improvement or deterioration *.
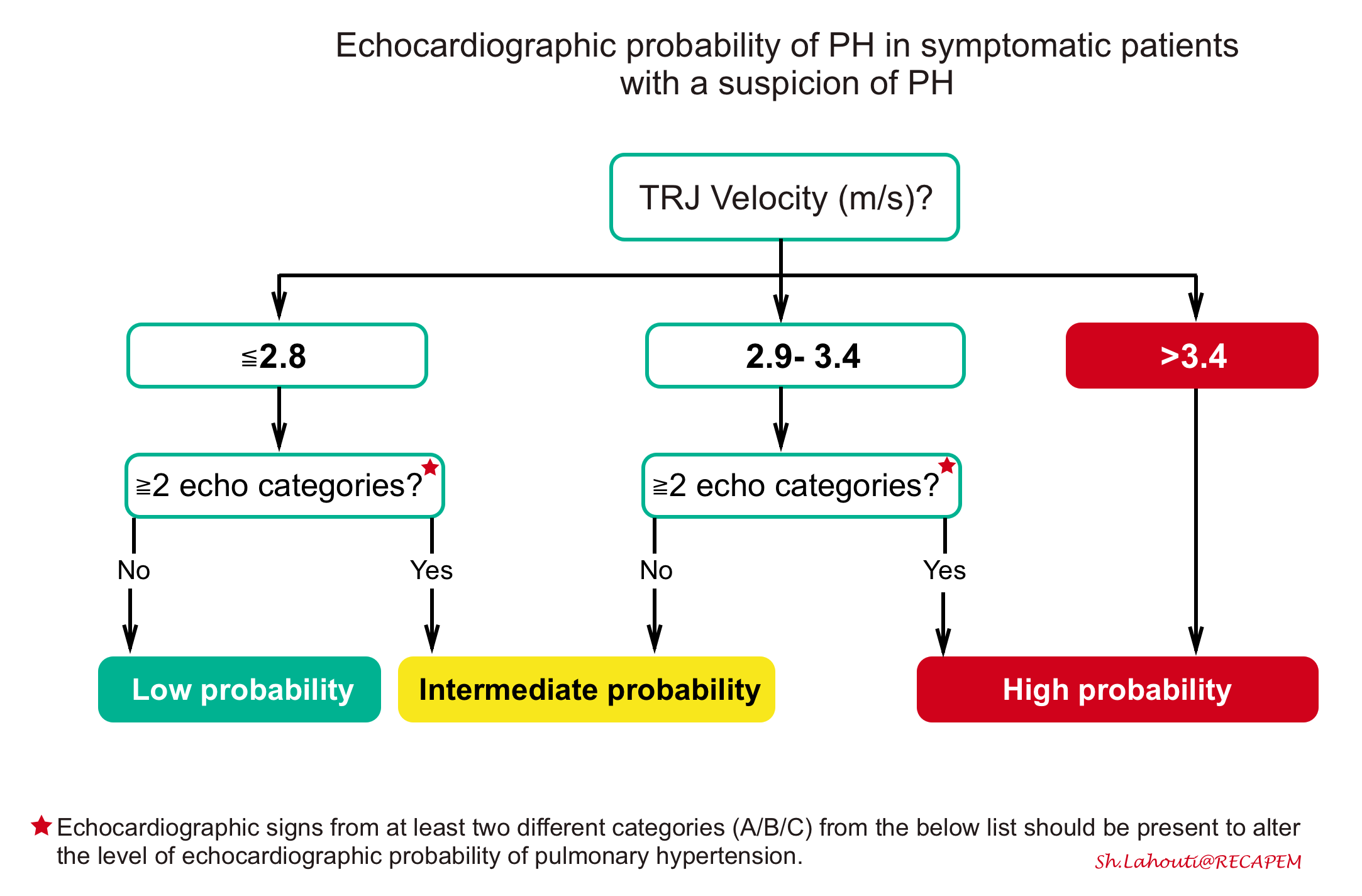
◾️Probability of pulmonary hypertention
- Tricuspid regurgitant velocity
- Echocardiographic calculation of systolic PA pressure (explained above) requires estimation of the right atrial pressure, a process which is relatively inaccurate. Thus, it may be more accurate to focus solely on the tricuspid regurgitant velocity *.
- TRV may be used to estimate the likelihood of PH:
- Peak tricuspid regurgitant velocity < 2.8 m/s: PH is unlikely
- Peak tricuspid regurgitant velocity 2.9-3.4 m/s: Grey zone.
- Peak tricuspid regurgitant velocity >3.4 m/s: PH is likely
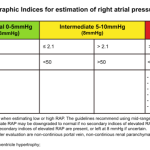
- Integrated approach
- The ESC guidelines recommend a combination of tricuspid valve velocity plus additional signs of pulmonary hypertension, as shown below.
- Pulmonary hypertension is highly likely if 20:
- PASP >35 mm Hg (or a tricuspid jet velocity >3.4 m/s); OR
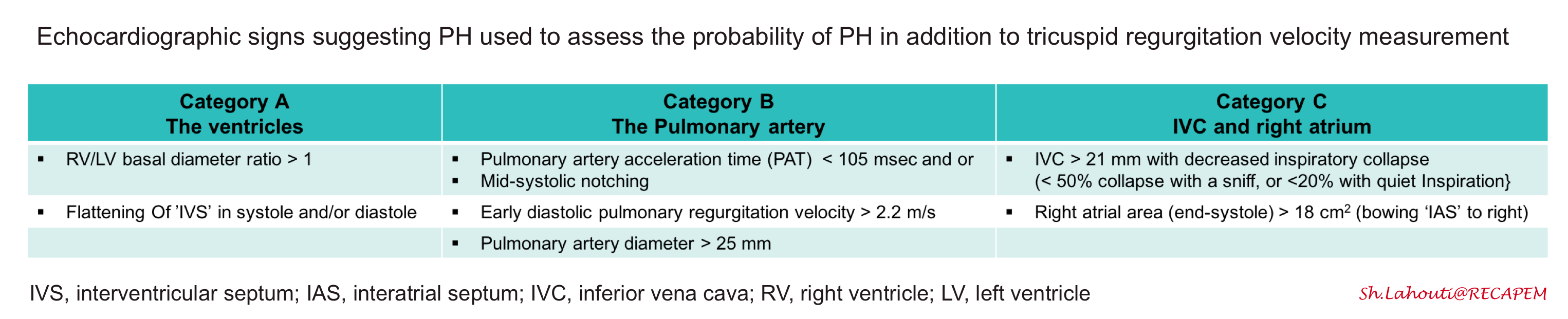
- TR jet is 2.9-3.4 m/s PLUS echocardiographic signs from at least 2 or more different categories (A/B/C) should be present (table below).
- For example a TRJV of 3m/s plus RV/LV size ratio>1 (category A) plus RVOT mid-systolic notching of pulmonary venous flow (category B) or any of category C findings.
◾️Aditional echocardiographic signs in pulmonary hypertension
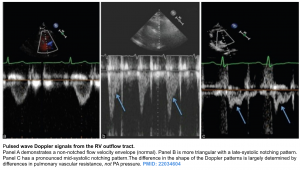
- Mid-systolic notching
- Background
- A PW Doppler measurement is taken after positioning the sample volume just below the pulmonic cusp on the RV side in the RV outflow tract. Normal pulmonary systolic flow does not have notching.
- Increased pulmonary vascular resistance can cause a reflection of waves that return toward the RV during systole. This impedes RV ejection and causes “notching” of the Doppler profile.
- Diagnostic value
- The presence of a pulmonary systolic notch is considered a marker of raised pulmonary arterial pressure and pulmonary vascular resistance.
- The absence of a systolic notch does not rule out pulmonary hypertension. In fact, systolic notching may be absent in isolated post-capillary pulmonary hypertension (PH due to left heart disease) 21.
- Background
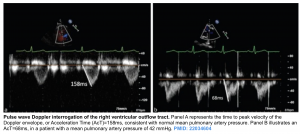
- Pulmonary acceleration time (PAT)
- A pulsed wave (PW) Doppler measurement taken after positioning the sample volume just below the pulmonic cusp on the RV side in the RV outflow tract.
- Measure at end-expiration from the onset of flow to peak flow velocity. As pulmonary artery pressure (PAP) increases, the acceleration time of the RV ejection into the PA shortens.
- An acceleration time of < 105 ms is considered a marker of raised PAP.
Signs of chronic pulmonary hypertension
- Pericardial effusion
- Severe, chronic pulmonary arterial hypertension may cause a small pericardial effusion. This is a poor prognostic factor 20.
- RV wall thickening
- RV-free wall thickness can be measured at end-diastole by M-mode or 2D echocardiography from the subcostal window, preferably at the level of the tip of the anterior tricuspid leaflet or left parasternal windows.
- RV wall thickness > 5 mm measured at end-diastole suggests chronic pulmonary hypertension 19.
- 💡However, wall thickening may increase within 48 hours, so this can develop rapidly in the context of acute pulmonary hypertension 22.
- RV wall thickening >10 mm suggests that the PA pressure may be approaching systemic pressures *.
- PA systolic pressure > 60 mm Hg
- The thin-wall RV is generally unable to generate high pressure against acute rises in afterload (e.g. after massive pulmonary embolism), thus a PASP > 60 mm Hg is more suggestive of a chronic process 23.
Management of pulmonary hypertension
Principles
By enlarge the traditional resuscitation techniques of “airway, breathing, and circulation”, referring to early intubation, positive-pressure ventilation, and IV fluid administration, should be avoided in patients with PH. The principles of “RV-friendly” resuscitation are shown below.

- Hypoxemia and hypercapnia both can cause pulmonary vasoconstriction and should be corrected through non-invasive means, including a high-flow nasal cannula.
- Drain moderate-large pleural effusion.
- Avoid intubation if possible. Patients with PH cannot tolerate sedation and positive pressure ventilation.
- If intubation is required, hemodynamically neutral intubation is the ideal.
- Hemodynamic optimization prior to intubation is recommended with “RV-friendly” agents (e.g. epinephrine plus vasopressin).
- Consider the administration of a nebulized pulmonary vasodilator prior to intubation.
- Induce the patient with hemodynamically stable agents (e.g. ketamine or etomidate plus rocuronium). Awake intubation with topical topical anesthetics is an alternative 8.
- Avoid aggressively bagging the patient.
- Set the BiPAP device to provide apneic ventilation as the patient begins to become sedated until you are about to insert the endotracheal tube.
- Start pulmonary vasodilator immediately following intubation.
- Provide lung protective ventilation
- Ventilator settings should target 6–8 mL/kg of ideal body weight and plateau pressures < 30 cm H2O.
- Avoid excessive PEEP, however using adequate PEEP to prevent atelectasis remains beneficial, as this will recruit lung tissue and thereby reduce the pulmonary vascular resistance.
- Do not leave the room following a successful intubation! Follow the patient’s hemodynamics very closely for the following 15-30 minutes.
- Check a blood gas. Hypoxemia and hypercapnia should be avoided.
Correct any precipitating factors
- Discontinue medications (e.g. negative inotropes such as beta-blockers; alpha-agonists; systemic vasodilators if hypotensive).
- Control AF (cardioversion of new AF)
- Observational studies suggest improved survival with a rhythm control strategy
- Rate control with calcium channel blockers or β-blockers is not advised, as these medications further impair RV function. If indicated, digoxin can be considered.
- All antiarrhythmic medications have a propensity to lower blood pressure and are not tolerated in the context of PH.
- In individuals with atrial arrhythmias lasting >48 h, anticoagulation is advised prior to cardioversion 33.
- Treat other active processes (e.g. infection, pulmonary embolism)
- Identify and treat underlying etiologies. In patients with acute exacerbation of underlying conditions such as COPD or left-sided chronic heart failure, treat the condition aggressively.
- Background
- Classical teaching has long been that the failing RV is preload dependent. However, this is only for patients with acute right heart failure from a right-sided myocardial infarction. Patients with chronic heart failure, including PH, are more likely to be volume-overloaded than dry due to upregulation of the renin-angiotensin-aldosterone system.
- Hypervolemia in the context of failing RV is problematic.
- Increased preload will cause excessive RV dilation, which impairs RV and LV function (decreasing cardiac output by shifting the interventricular septum to the left).
- Systemic congestion impairs systemic organ perfusion (explained above).
- Target
- CVP might be helpful in the evaluation of right-sided filling pressure 24.
- Consensus suggests that a reasonable target might be a CVP of ~8-12 cm (i.e. slightly elevated filling pressures)25.
-
Standard assessments of volume-responsiveness (e.g. pulse pressure variability) usually fail in patients with RV dysfunction. Given the chronic venous congestion, ultrasound measurements of the inferior vena cava are of limited use.
- Management
- Diuretic: For patients not previously receiving oral diuretics, an initial dose of 20–40 mg IV furosemide is recommended for hypervolemia. In individuals utilizing home diuretic therapy, the initial IV dose should be at least equivalent to the oral dose. Consultation may be required for ultrafiltration in patients who are resistant to diuretic therapy.
- Volume: Fluid should not be administered unless there is an especially good reason (e.g. frank volume loss plus echocardiographic evidence of a low CVP). In hypovolemic patients, volume should be delivered conservatively, with boluses of 250 mL over 15–30 min.
- Combined diuretic and vasopressor: Patients with systemic congestion and low MAP may require a combination of simultaneous vasoconstrictors (to maintain an adequate MAP and perfusion pressure, which is equal to MAP-RAP) along with diuresis (to promote decongestion and optimization of right ventricular preload).
- Once the patient has been adequately decongested, vasoconstrictors can often be weaned. At this point, the patient may be able to clinically tolerate a lower MAP without hypoperfusion (because, due to the lower CVP, the systemic perfusion pressure is now adequate at a lower MAP).
- Background
- An adequate MAP will promote perfusion of the right ventricular myocardium and thereby maintain contractility.
- A higher MAP will increase the LV afterload, which may counteract septal flattening and thereby promote normal cardiac geometry 26 .
- An adequate MAP is also necessary to perfuse the kidneys (allowing for diuresis, if needed).
- Among patients with a patent foramen ovale and right-to-left shunting, elevation of left-sided pressures may help reduce the amount of shunting.
- Target
- There have been no studies regarding a target MAP goal in RV failure leading to cardiogenic shock, but 65 mm Hg is a commonly accepted target 27 .
- Patients with markedly elevated CVP might need a higher MAP target to achieve adequate systemic perfusion pressures.
- To ensure RV perfusion, the systemic systolic blood pressure should be kept well above the RV systolic blood pressure (SBP >> RVSP) 28 .
- There have been no studies regarding a target MAP goal in RV failure leading to cardiogenic shock, but 65 mm Hg is a commonly accepted target 27 .
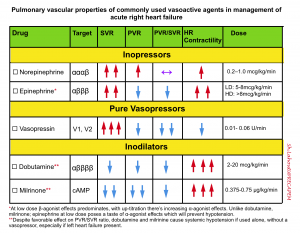
- Agents and dosage
- The ideal vasopressor for RHF would preferentially increase the systemic vascular resistance while having minimal effect on the PVR.
- Norepinephrine
- Advantage: Easily titratable, safe for peripheral administration. First-line agent in mildly unstable patients.
- Disadvantage: May increase PVR due to its alpha-agonist activity
- Dose: 8-40 mcg/min. Rapidly titrate as needed. Higher initial doses may be needed.
- Vasopressin
- Advantage: Increase MAP while simultaneously reducing pulmonary vascular resistance. Also appropriate for patients with significant tachycardia
- Disadvantages: difficult to titrate, not safe for peripheral administration, often insufficient among patients with profound hypotension.
- Dose: 0.01 to 0.06 units/min (typically not titrated above 0.06 units/min).
- Epinephrine
- Advantage: Titratable, safe to administer via a large bore peripheral line. Predominant beta-agonist activity at a lower dose, will reduce the pulmonary vascular resistance and support right ventricular function, thereby improving the MAP without worsening RV afterload. It is the drug of choice for the sickest patients with overt hypotension.
- Dose: 2-10 µg/min, titrated as needed.
- Norepinephrine
- The vascular properties of commonly used agents are shown in the following table.
- The ideal vasopressor for RHF would preferentially increase the systemic vascular resistance while having minimal effect on the PVR.
- ⚠️Medications which should be avoided: Phenylephrine and dopamine
- Pure alpha agonists such as phenylephrine should not be used in unstable PH patients as they increase pulmonary vascular resistance and may induce reflex bradycardia.
- Dopamine should be also avoided given β2-mediated decreases in systemic vascular resistance and possible arrhythmias.
- Background
- In chronic PH, the major pathology lies in increased afterload, also known as pulmonary vascular resistance (PVR).
- Keep in mind that hypoxemia, hypercapnia, and acidosis increase PVR, hence correcting pulmonary function is key to reducing RV afterload.
- Positive pressure ventilation also increases RV afterload, hence intubation should be avoided (if possible).
- Inhaled pulmonary vasodilators
- Reduce PVR and therefore improve RV failure in the context of pulmonary hypertension.
- These agents exert local effects (dilating pulmonary artery without having a systemic vasodilatory effect).
- Contraindication: Severe left ventricular failure or pulmonary veno-occlusive disease.
- In these patients, pulmonary vasodilators may precipitate cardiogenic pulmonary edema by increasing blood flow through the right ventricle and hence increasing left ventricular preload.
- Suggested agents and dosage include:
- Epoprostenol at 0.05mcg/kg/min.
- Nitric Oxide at 20 ppm.
- Milrinone (1mg/ml), inhale 5mg over 15 minutes.
- Nitroglycerin (1mg/ml), inhale 5mg over 15 minutes.
- More on inhaled pulmonary vasodilators here.
- Indication
- Once the MAP is optimized, the patient should be reassessed for their perfusion. In cases of continued poor perfusion, inotropes should be added.
- Medications
- Dobutamine
- At low doses (5–10 μg/kg/min), dobutamine improves RV contractility and increases cardiac output through β1 receptor stimulation. Higher doses should be avoided, given increased stimulation of β2 receptors, causing vasodilation and hypotension.
- Epinephrine
- Epinephrine stimulates cardiac beta-1 and beta-2 receptors, which may allow it to be a more powerful inotrope than dobutamine (which selectively affects beta-1 receptors).
- At low doses is largely an inotrope and at higher doses, it has a vasoconstrictor effect.
- Epinephrine has some vasoconstrictor effect even at low doses, so hypotension is unlikely.
- Dobutamine
Other therapeutic consideration
-
Intravenous pulmonary vasodilators
-
PH patients may be on a chronic infusion of a pulmonary vasodilator. A malfunction of these pumps is an emergency requiring prompt restoration of medication to prevent rebound PH and cardiopulmonary collapse.
- If a patient has not been using IV pulmonary vasodilators, then do not initiate these agents in ED since incorrect administration can precipitate cardiovascular collapse. As such, these agents should only be initiated by a PH specialist in the ICU.
-
- Patients with PH are often on oral medications, such as phosphodiesterase-5 inhibitors (tadalafil or sildenafil), or endothelin receptor antagonists (e.g. macitentan, bosentan).
- These medications will never be initiated by an emergency physician, and a full discussion is beyond the scope of this work. However, ED patients may need to take these medications during their ED visit, and the dosages should not be missed if at all possible. If a patient is unable to take oral medications, discussion with the prescribing physician to develop an alternative plan is essential.
Special conditions
Pericardial effusion in PH
- Pathophysiology
- Elevated RAP in the context of PH will impair venous and lymphatic drainage of the parietal pericardium resulting in pericardial fluid accumulation 29.
- The development of pericardial effusion in PAH is a compensatory response.
- Pericardial effusion restrains distension of the high-pressured right ventricle in PH patients. Therefore IVS and IAS bowing to the left is minimal.
- Diagnostic challenge
- 👉Note that echocardiographic diagnosis of tamponade in patients with PAH is challenging since right-sided chamber collapse may be absent.
- In PAH or any other conditions (RVH, severe TR) where RV end-diastolic pressure is very high, right-sided chamber collapse may be absent.
- 👉Note that echocardiographic diagnosis of tamponade in patients with PAH is challenging since right-sided chamber collapse may be absent.
- General management strategy
- ⚠️By and large, drainage of hemodynamically stable pericardial effusion in PAH is not warranted and is dangerous 30,31
- If the patient’s hemodynamic is stable; a gentle trial of diuretic may be tried.
- In unstable patients, measures to ↓RV afterload to optimize the RV pressure-volume curve should be attempted before disturbing the integrity of the protective pericardial restraint mechanism.
- If indicated as a last resort, removing a minimal amount of fluid is enough. Gradual drainage is advised.
PH in left heart disease (LHD)
Left heart disease (LHD) accounts for 65–80% of PH cases. Development of PH in the context of left heart disease (LHD), negatively impacts symptoms, exercise capacity, and outcome. The proper distinction between pulmonary arterial hypertension and PH-LHD may be challenging, however, it has direct therapeutic consequences 32
◾️Hemodynamic phenotypes of PH
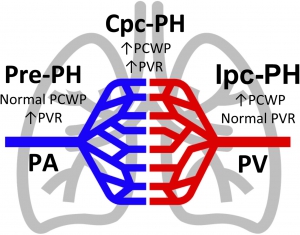
- PH is hemodynamically characterized by estimates of pulmonary arterial pressures (using pulmonary vascular resistance) and left ventricular filling pressures (using pulmonary capillary wedge pressure). Using these measures, PH can be further stratified into the following subtypes (see the figure below) 6 :
- Pre-capillary PH (Pre-PH) or pulmonary arterial hypertension (PAH)
- It is associated with
- Elevated pulmonary vascular resistance (PVR) and
- Normal pulmonary capillary wedge pressure (PCWP=LAP ≤15 mm Hg).
- It is associated with
- Isolated post-capillary PH (Ipc-PH) or pulmonary venous hypertension (PVH)
- Defined as
- Pulmonary capillary wedge pressure (PCWP=LAP >15 mm Hg) and
- Normal PVR
- Defined as
- Combined pre-and post-capillary PH (Cpc-PH)
- It occurs when both (PCWP=LAP) and PVR are elevated. (aka. reactive, out-of-proportion, or mixed PH).
- Pre-capillary PH (Pre-PH) or pulmonary arterial hypertension (PAH)
Schematic of pulmonary hypertension subtypes. PMID: 31732231
◾️Pathophysiology
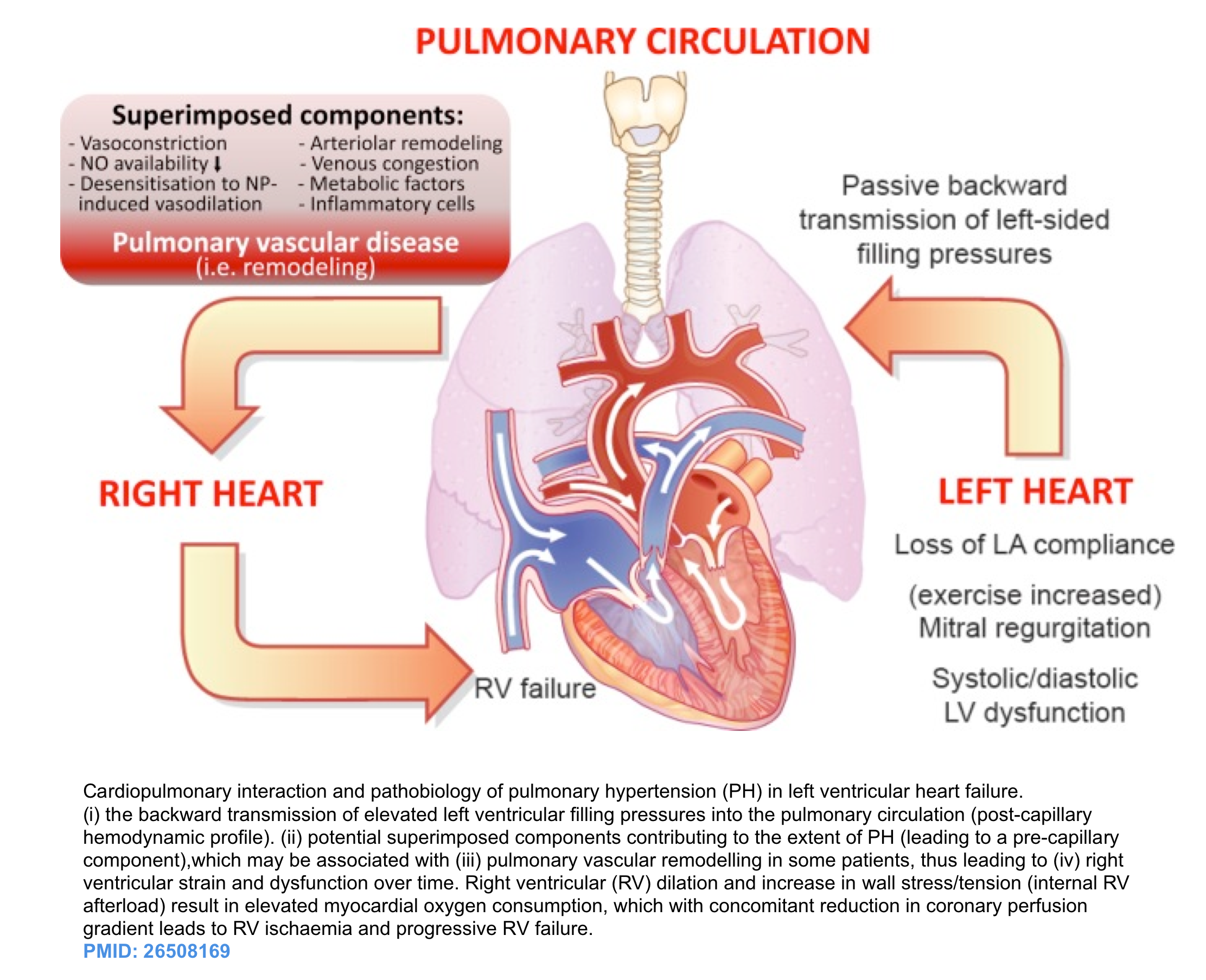
- Systolic or diastolic LV dysfunction leads to elevated left-sided filling pressure. This results in a “proportionate” increase of the PAP (via passive backward transmission of elevated left-sided filling pressures) so that a normal transpulmonary pressure gradient is maintained.
- Chronic progressive LV systolic or diastolic dysfunction will cause left atrial dysfunction as well. This alteration of LA systolic and diastolic properties will further increase PAP.
- Chronic and consistent elevation of left-sided filling pressure is associated with neurohormonal and other mediator activation which may cause excess pulmonary arterial vasoconstriction leading to a “disproportionate” increase of the PAP. This has been described as “reactive”, “out-of-proportion”, or “mixed” PH 32.
◾️Development of PH due to LHD is multifactorial.
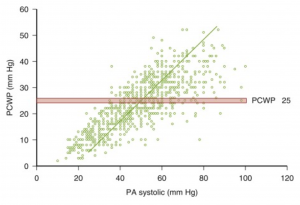
- It is still unclear why patients with the same degree of elevated LAP (PCWP) will progress to develop mixed PH (out-of-proportionate PH) while other patients do not. The diagram below represents a wide range of distribution of PASP ranging from 20 to 60 mm Hg at PCWP of 25mm Hg.
- This heterogeneous pulmonary vascular response is probably multifactorial and is an area of further investigation and research.
◾️Causes of PH-LHD
- LV systolic and/or diastolic disease.
- Mitral and aortic valve disease.
- Congenital or acquired left heart inflow/outflow tract obstructions.
◾️Helpful Clues to differentiate PAH vs. PVH
- A comprehensive non-invasive work-up incorporating medical history, ECG, and echocardiography data helps to differentiate PAH from PH-LHD.
- Clinical feature
- PAH: Younger age, familial cases.
- Risk factors for PAH: connective tissue disease, HIV, portal hypertension, severe liver disease, congenital heart disease.
- History of progressive dyspnea over a couple of months.
- PVH: Older age.
- Risk factors for PVH: hypertension, DM, coronary arterial disease, pulmonary congestion.
- History of pulmonary edema, orthopnea.
- PAH: Younger age, familial cases.
- ECG
- PAH: right axis deviation, RVH, RV strain pattern.
- PVH: left axis deviation, LVH, atrial fibrillation.
- Chest CT
- PAH: Dilated pulmonary artery, signs of associated conditions for example ILD.
- PVH: Pulmonary congestion, pleural effusion
- Echocardiography: Helpful findings are summarized below. See Images 4 and 5 for comparison.
Video on the left of the screen (pre-capillary PH). A4C view showing right atrial enlargement with bowing of inter-atrial septum to the left. Video on the right of the screen (post-capillary PH). A4C view showing left ventricular hypertrophy left atrial enlargement, and bowing of inter-atrial septum to the right.
◾️Table. Summary of distinguishing features of PAH vs. PVH
👉Pearl
- In PAH, a significant increase in PASP and RV dilation will cause reduced LV compliance (through ventricular interdependence physiology) with resulting grade 1 diastolic dysfunction.
- In PVH, diastolic dysfunction ≥ grade 2 is usually present due to primary LV diastolic dysfunction. More on diastology, here.
◾️Therapeutic Pearls
- The treatment goal is to reduce left-sided pressure (LAP=PCWP). This may include medical treatment (e.g. adjustment of diuretics) or interventional therapies (CRT, ICD, repair of mitral regurgitation).
- Note that in the presence of severe LV failure, inhaled pulmonary vasodilators are contraindicated.
- These agents by increasing blood flow through the right ventricle, may increase the left ventricular preload. This could precipitate cardiogenic pulmonary edema among patients with left ventricular dysfunction.
Media
Appendix
References
1. Lahm T, McCaslin CA, Wozniak TC, Ghumman W, Fadl YY, Obeidat OS, Schwab K, Meldrum DR. Medical and surgical treatment of acute right ventricular failure. J Am Coll Cardiol. 2010 Oct 26;56(18):1435-46. doi: 10.1016/j.jacc.2010.05.046. PMID: 20951319.
2. Harjola VP, Mebazaa A, Čelutkienė J, Bettex D, Bueno H, Chioncel O, Crespo-Leiro MG, Falk V, Filippatos G, Gibbs S, Leite-Moreira A, Lassus J, Masip J, Mueller C, Mullens W, Naeije R, Nordegraaf AV, Parissis J, Riley JP, Ristic A, Rosano G, Rudiger A, Ruschitzka F, Seferovic P, Sztrymf B, Vieillard-Baron A, Yilmaz MB, Konstantinides S. Contemporary management of acute right ventricular failure: a statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur J Heart Fail. 2016 Mar;18(3):226-41. doi: 10.1002/ejhf.478. PMID: 26995592.
3. Rosenkranz S, Howard LS, Gomberg-Maitland M, Hoeper MM. Systemic Consequences of Pulmonary Hypertension and Right-Sided Heart Failure. Circulation. 2020 Feb 25;141(8):678-693. doi: 10.1161/CIRCULATIONAHA.116.022362. Epub 2020 Feb 24. PMID: 32091921.
4. Konstam MA, Kiernan MS, Bernstein D, Bozkurt B, Jacob M, Kapur NK, Kociol RD, Lewis EF, Mehra MR, Pagani FD, Raval AN, Ward C; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; and Council on Cardiovascular Surgery and Anesthesia. Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement From the American Heart Association. Circulation. 2018 May 15;137(20):e578-e622. doi: 10.1161/CIR.0000000000000560. Epub 2018 Apr 12. PMID: 29650544.
5. Price LC, Wort SJ, Finney SJ, Marino PS, Brett SJ. Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review. Crit Care. 2010;14(5):R169. doi: 10.1186/cc9264. Epub 2010 Sep 21. PMID: 20858239; PMCID: PMC3219266.
6. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M; ESC Scientific Document Group. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016 Jan 1;37(1):67-119. doi: 10.1093/eurheartj/ehv317. Epub 2015 Aug 29. PMID: 26320113.
7. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019 Jan 24;53(1):1801913. doi: 10.1183/13993003.01913-2018. PMID: 30545968; PMCID: PMC6351336.
8. Simon E, Bridwell RE, Montrief T, Koyfman A, Long B. Evaluation and management of pulmonary hypertension in the emergency department setting. Am J Emerg Med. 2020 Jun;38(6):1237-1244. doi: 10.1016/j.ajem.2020.02.041. Epub 2020 Feb 20. PMID: 32115291.
9. Aryal S, King CS. Critical care of patients with pulmonary arterial hypertension. Curr Opin Pulm Med. 2020 Sep;26(5):414-421. doi: 10.1097/MCP.0000000000000713. PMID: 32740380.
10. Harjola VP, Mebazaa A, Čelutkienė J, Bettex D, Bueno H, Chioncel O, Crespo-Leiro MG, Falk V, Filippatos G, Gibbs S, Leite-Moreira A, Lassus J, Masip J, Mueller C, Mullens W, Naeije R, Nordegraaf AV, Parissis J, Riley JP, Ristic A, Rosano G, Rudiger A, Ruschitzka F, Seferovic P, Sztrymf B, Vieillard-Baron A, Yilmaz MB, Konstantinides S. Contemporary management of acute right ventricular failure: a statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur J Heart Fail. 2016 Mar;18(3):226-41. doi: 10.1002/ejhf.478. PMID: 26995592.
11. Stengl M, Ledvinova L, Chvojka J, Benes J, Jarkovska D, Holas J, Soukup P, Sviglerová J, Matejovic M. Effects of clinically relevant acute hypercapnic and metabolic acidosis on the cardiovascular system: an experimental porcine study. Crit Care. 2013 Dec 30;17(6):R303. doi: 10.1186/cc13173. PMID: 24377654; PMCID: PMC4056780.
12. Dunham-Snary KJ, Wu D, Sykes EA, Thakrar A, Parlow LRG, Mewburn JD, Parlow JL, Archer SL. Hypoxic Pulmonary Vasoconstriction: From Molecular Mechanisms to Medicine. Chest. 2017 Jan;151(1):181-192. doi: 10.1016/j.chest.2016.09.001. Epub 2016 Sep 16. PMID: 27645688; PMCID: PMC5310129.
13. Hrymak C, Strumpher J, Jacobsohn E. Acute Right Ventricle Failure in the Intensive Care Unit: Assessment and Management. Can J Cardiol. 2017 Jan;33(1):61-71. doi: 10.1016/j.cjca.2016.10.030. Epub 2016 Nov 11. PMID: 28024557.
14. Goldstein JA, Harada A, Yagi Y, Barzilai B, Cox JL. Hemodynamic importance of systolic ventricular interaction, augmented right atrial contractility and atrioventricular synchrony in acute right ventricular dysfunction. J Am Coll Cardiol. 1990 Jul;16(1):181-9. doi: 10.1016/0735-1097(90)90477-7. PMID: 2193048.
15. Nikolaou M, Parissis J, Yilmaz MB, Seronde MF, Kivikko M, Laribi S, Paugam-Burtz C, Cai D, Pohjanjousi P, Laterre PF, Deye N, Poder P, Cohen-Solal A, Mebazaa A. Liver function abnormalities, clinical profile, and outcome in acute decompensated heart failure. Eur Heart J. 2013 Mar;34(10):742-9. doi: 10.1093/eurheartj/ehs332. Epub 2012 Oct 22. PMID: 23091203.
16. Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005 Feb 1;172(3):367-79. doi: 10.1503/cmaj.1040752. PMID: 15684121; PMCID: PMC545762.
17. Peña E, Dennie C, Veinot J, Muñiz SH. Pulmonary hypertension: how the radiologist can help. Radiographics. 2012 Jan-Feb;32(1):9-32. doi: 10.1148/rg.321105232. PMID: 22236891.
18. Altschul E, Remy-Jardin M, Machnicki S, Sulica R, Moore JA, Singh A, Raoof S. Imaging of Pulmonary Hypertension: Pictorial Essay. Chest. 2019 Aug;156(2):211-227. doi: 10.1016/j.chest.2019.04.003. Epub 2019 Apr 11. PMID: 30981724.
19. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010 Jul;23(7):685-713; quiz 786-8. doi: 10.1016/j.echo.2010.05.010. PMID: 20620859.
20. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M; ESC Scientific Document Group. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016 Jan 1;37(1):67-119. doi: 10.1093/eurheartj/ehv317. Epub 2015 Aug 29. PMID: 26320113.
21. Arkles JS, Opotowsky AR, Ojeda J, Rogers F, Liu T, Prassana V, Marzec L, Palevsky HI, Ferrari VA, Forfia PR. Shape of the right ventricular Doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am J Respir Crit Care Med. 2011 Jan 15;183(2):268-76. doi: 10.1164/rccm.201004-0601OC. Epub 2010 Aug 13. PMID: 20709819.
22. Hrymak C, Strumpher J, Jacobsohn E. Acute Right Ventricle Failure in the Intensive Care Unit: Assessment and Management. Can J Cardiol. 2017 Jan;33(1):61-71. doi: 10.1016/j.cjca.2016.10.030. Epub 2016 Nov 11. PMID: 28024557.
23. Hockstein MA, Haycock K, Wiepking M, Lentz S, Dugar S, Siuba M. Transthoracic Right Heart Echocardiography for the Intensivist. J Intensive Care Med. 2021 Sep;36(9):1098-1109. doi: 10.1177/08850666211003475. Epub 2021 Apr 15. PMID: 33853435.
24. Hoeper MM, Benza RL, Corris P, de Perrot M, Fadel E, Keogh AM, Kühn C, Savale L, Klepetko W. Intensive care, right ventricular support and lung transplantation in patients with pulmonary hypertension. Eur Respir J. 2019 Jan 24;53(1):1801906. doi: 10.1183/13993003.01906-2018. PMID: 30545979; PMCID: PMC6351385.
25. Olsson KM, Halank M, Egenlauf B, Fistera D, Gall H, Kaehler C, Kortmann K, Kramm T, Lichtblau M, Marra AM, Nagel C, Sablotzki A, Seyfarth HJ, Schranz D, Ulrich S, Hoeper MM, Lange TJ. Decompensated right heart failure, intensive care and perioperative management in patients with pulmonary hypertension: Updated recommendations from the Cologne Consensus Conference 2018. Int J Cardiol. 2018 Dec 1;272S:46-52. doi: 10.1016/j.ijcard.2018.08.081. Epub 2018 Aug 26. PMID: 30190155.
26. Mullin CJ, Ventetuolo CE. Critical Care Management of the Patient with Pulmonary Hypertension. Clin Chest Med. 2021 Mar;42(1):155-165. doi: 10.1016/j.ccm.2020.11.009. PMID: 33541609.
27. Levy B, Bastien O, Karim B, Cariou A, Chouihed T, Combes A, Mebazaa A, Megarbane B, Plaisance P, Ouattara A, Spaulding C, Teboul JL, Vanhuyse F, Boulain T, Kuteifan K. Experts’ recommendations for the management of adult patients with cardiogenic shock. Ann Intensive Care. 2015 Dec;5(1):52. doi: 10.1186/s13613-015-0052-1. Epub 2015 Jul 1. Erratum in: Ann Intensive Care. 2015 Dec;5(1):26. Benjelid, Karim [corrected to Karim, Bendjelid]; Splaulding, Christian [corrected to Spaulding, Christian]. PMID: 26152849; PMCID: PMC4495097.
28. Cassady SJ, Ramani GV. Right Heart Failure in Pulmonary Hypertension. Cardiol Clin. 2020 May;38(2):243-255. doi: 10.1016/j.ccl.2020.02.001. PMID: 32284101.
29. Sahay S, Tonelli AR. Pericardial effusion in pulmonary arterial hypertension. Pulm Circ. 2013;3(3):467-477. doi:10.1086/674302.
30. Hemnes AR, Gaine SP, Wiener CM. Poor outcomes associated with drainage of pericardial effusions in patients with pulmonary arterial hypertension. South Med J. 2008 May;101(5):490-4. doi: 10.1097/SMJ.0b013e31816c0169. PMID: 18414173.
31. Adams JR, Tonelli AR, Rokadia HK, Duggal A. Cardiac tamponade in severe pulmonary hypertension. A therapeutic challenge revisited. Ann Am Thorac Soc. 2015 Mar;12(3):455-60. doi: 10.1513/AnnalsATS.201410-453CC. PMID: 25786153; PMCID: PMC5466174.
32. Rosenkranz S, Gibbs JS, Wachter R, De Marco T, Vonk-Noordegraaf A, Vachiéry JL. Left ventricular heart failure and pulmonary hypertension. Eur Heart J. 2016 Mar 21;37(12):942-54. doi: 10.1093/eurheartj/ehv512. Epub 2015 Oct 27. PMID: 26508169; PMCID: PMC4800173.
33. Wanamaker B, Cascino T, McLaughlin V, Oral H, Latchamsetty R, Siontis KC. Atrial Arrhythmias in Pulmonary Hypertension: Pathogenesis, Prognosis and Management. Arrhythm Electrophysiol Rev. 2018;7(1):43-48. doi:10.15420/aer.2018.3.2.











Add comment