March 17, 2022 by Shahriar Lahouti
CONTENTS
- Preface
- Anatomy
- Pathophysiology
- Risk factors
- Clinical presentation
- Imaging findings
- Laboratory tests
- Differential diagnosis
- Treatment
- Neurological deterioration
- Prognosis
- Going further
- References
Preface
Cerebral venous thrombosis (CVT) represent approximately 0.5-1% of all cases of cerebrovascular disease worldwide and is associated with an increased mortality rate. It is often difficult to diagnose clinically since clinical manifestation is broad and non-specific. Early recognition of symptoms and treatment improves overall outcome in these patients.
Anatomy
Unlike systemic veins, cerebral veins lack valves and do not follow cerebral arterial territory. This will account for unusual patterns of infarction and hemorrhage associated with CVT (see below).
The most prominent dural venous channel is the superior sagittal sinus and transverse sinus which are the most frequently involved venous systems in CVT 1 (See the following slides).
Pathophysiology
Obstruction of the venous vessels induce the following consequences 2
- Elevation of venous pressure can cause vasogenic edema (due to blood–brain barrier disruption)
-
- Although this can impairs the brain function, it is reversible if the venous occlusion can resolve.
- Continued blood stasis and elevation of venous pressure can impair cerebral perfusion pressure leading to ischemic infarction and cytotoxic edema, which may become irreversible.
- As intravenous pressure continues to increase, venous hemorrhage may occur due to venous or capillary rupture, leading to hemorrhagic transformation of the infarction.
- Normally the, CSF absorption occurs in the arachnoid granulation, which drain CSF into the superior sagittal sinus. CVT can also block cerebrospinal fluid absorption through the arachnoid villi, which then leads to raised intracranial pressure.
- Therefore CVT may lead to communicating hydrocephalus (excessive CSF causes dilation of the ventricles and elevated intracranial pressure) especially if the superior sagittal sinus is involved.
Risk factors
Cerebral venous thrombosis (CVT) is an important cause of stroke (~1% of all strokes) in young adults (mean age 33 years with a two-thirds female preponderance).
CVT is a multifactorial disease with at least one risk factor implicated in 85% of affected adults. The major risk factors for CVT in adult include 3:
- Prothrombotic conditions
- Pregnancy and puerperium (usually late in pregnancy or in the first months following delivery).
- Dehydration
- Oral contraceptive pills (increases risk six-fold, or 30-fold when combined with obesity), hormone replacement therapy.
- Factor V Leiden mutation
- Antithrombin III deficiency
- Protein C/S deficiency
- G20210A prothrombin gene mutation
- Antiphospholipid syndrome
- Heparin Induced Thrombocytopenia (HIT).
- Nephrotic syndrome
- Sickle cell disease
- Hematologic: Polycythemia, thrombocythemia, severe anemia including paroxysmal nocturnal hemoglobinuria
- Inflammatory diseases e.g. Inflammatory bowel disease, SLE, Bechet’s disease, Temporal arteritis.
- Malignancy: Central nervous system, solid tumors outside the CNS, hematologic
- Infection of the head and neck
- Intracranial: Meningitis, cerebral abscess
- Local: otitis media, mastoiditis, oral / neck infection and sinusitis
- Systemic: HIV, tuberculosis, and sepsis
- Mechanical trauma
- Head trauma (especially skull base fractures that cross venous sinuses)
- Jugular vein catherization
- Neurosurgical procedures
- Lumbar puncture, intrathecal methotrexate
- Other disorders
- Drugs: Androgens, glucocorticoids, tamoxifen, chemotherapy with L-asparaginase, cyclosporine, thalidomide.
- CNS disorders: Dural fistula, arteriovenous malformation, tumors.
- Thyroid disease
- Congenital heart disease
Clinical presentation
The clinical presentation in CVT is highly variable; depending upon several factors, including patient age and sex, the site and number of occluded sinuses and veins, the presence of parenchymal brain lesions, and the interval from CVT onset to presentation (acute, subacute, chronic) 4.
Symptoms and signs of CVT can be grouped into three major syndromes:
- Isolated intracranial hypertension syndrome (headache with or without vomiting, papilledema, and visual problems).
- Focal syndrome (focal deficits, seizures, or both).
- Encephalopathy (multifocal signs, mental status changes, stupor, or coma).
Headache
- Headache is the most frequent symptom of CVT (~ 89% of patients).
- It is usually the first symptom of CVT, and can be the only symptom, or can precede other symptoms and signs by days or weeks.
- The features of CVT-related headache are quite variable.
- Head pain may be localized or diffuse 5.
- Pain usually starts gradually (although it may occasionally cause a “thunderclap” headache, especially if CVT leads to a secondary subarachnoid hemorrhage) 2, 6.
- Headache caused by intracranial hypertension from CVT is typically characterized by severe head pain that worsens with Valsalva maneuvers and with lying down.
- The site of the headache has no relationship with the localization of the occluded sinus or the parenchymal lesions.
- There may be other signs and symptoms associated with intracranial hypertension
- Nausea, vomiting
- Vision changes, diplopia
- Papilledema
- Cranial nerve 6 palsy (inability to abduct the eye).
1. Presence of CVT risk factors (see text).
2. New headache or head pain with different features in patients with previous primary headache
3. Symptoms or signs of raised intracranial pressure (e.g. papilledema, CN VI deficit )
4. New focal neurological signs
5. Altered consciousness
6. Seizures
Focal deficits
- Onset is usually not as abrupt as a typical stroke due to arterial occlusion.
- Weakness with monoparesis or hemiparesis; sometimes bilateral is the most frequent (37% of patients) focal deficit.
- Aphasia (especially when left lateral sinus is affected).
- Visual field defects.
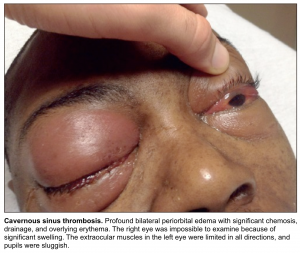
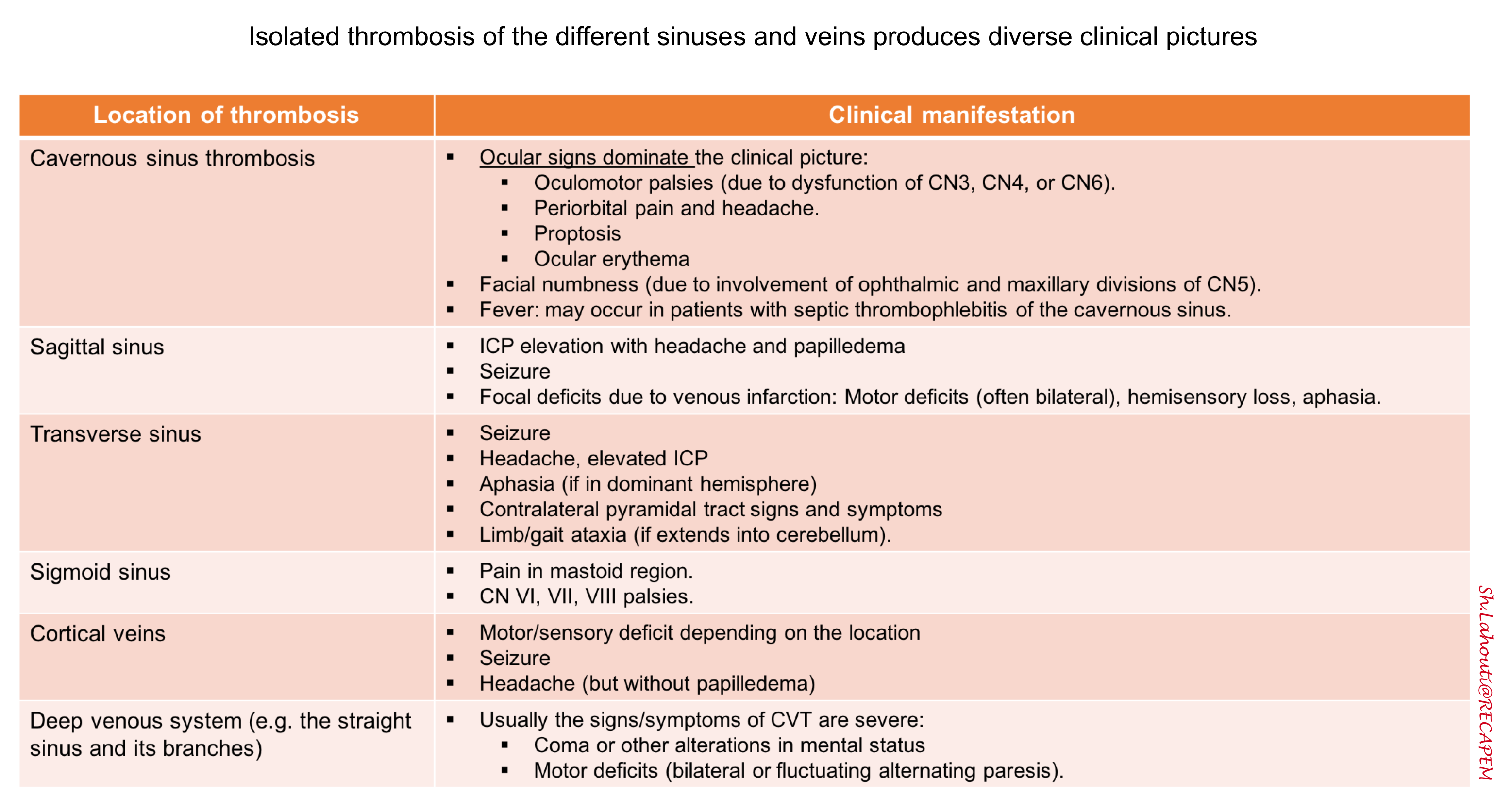
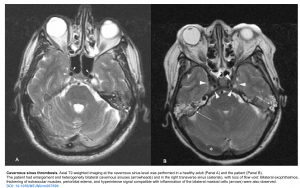
- Location of the thrombosis can be associated with various focal deficits (see table below) 2 ,for example isolated cavernous sinus thrombosis can cause cavernous sinus syndrome manifested as painful ophthalmoplegia, proptosis, chemosis.
Seizure
- Seizures are more frequent in CVT than in other cerebrovascular disorders (40%) 7.
- Usually focal, or focal with secondary generalization.
- Risk factors associated with seizure include 18:
- Supratentorial lesions with involvement of the cortex.
- Focal edema or infarcts (ischemic/hemorrhagic).
- Focal neurological deficits (eg. focal sensory deficits).
Encephalopathy
- Severe cases of CVT can cause altered level of consciousness and cognitive dysfunction, such as delirium, apathy, a frontal lobe syndrome, multifocal deficits, or seizures.
- In the context of CVT, diffuse encephalopathy can happen due to
- Communicating hydrocephalus.
- Occlusion of the deep venous system causing bilateral thalamic dysfunction (may cause some unusual findings such as mutism, amnesia) 19.
- Intracranial hemorrhage or edema, causing herniation.
Table. Summary of isolated focal neurologic deficits related to location of the clot in CVT.
Imaging findings
Patients with suspected CVT require urgent neuroimaging to confirm the diagnosis, using either CT or MR venography. Radiologic findings can be divided into two sets of signs as 8:
- Direct signs: refers to visualizing the “clot” itself
- Indirect signs: refers to signs related to consequences of the venous clot; e.g. edema, infarction, hemorrhage.
Direct signs
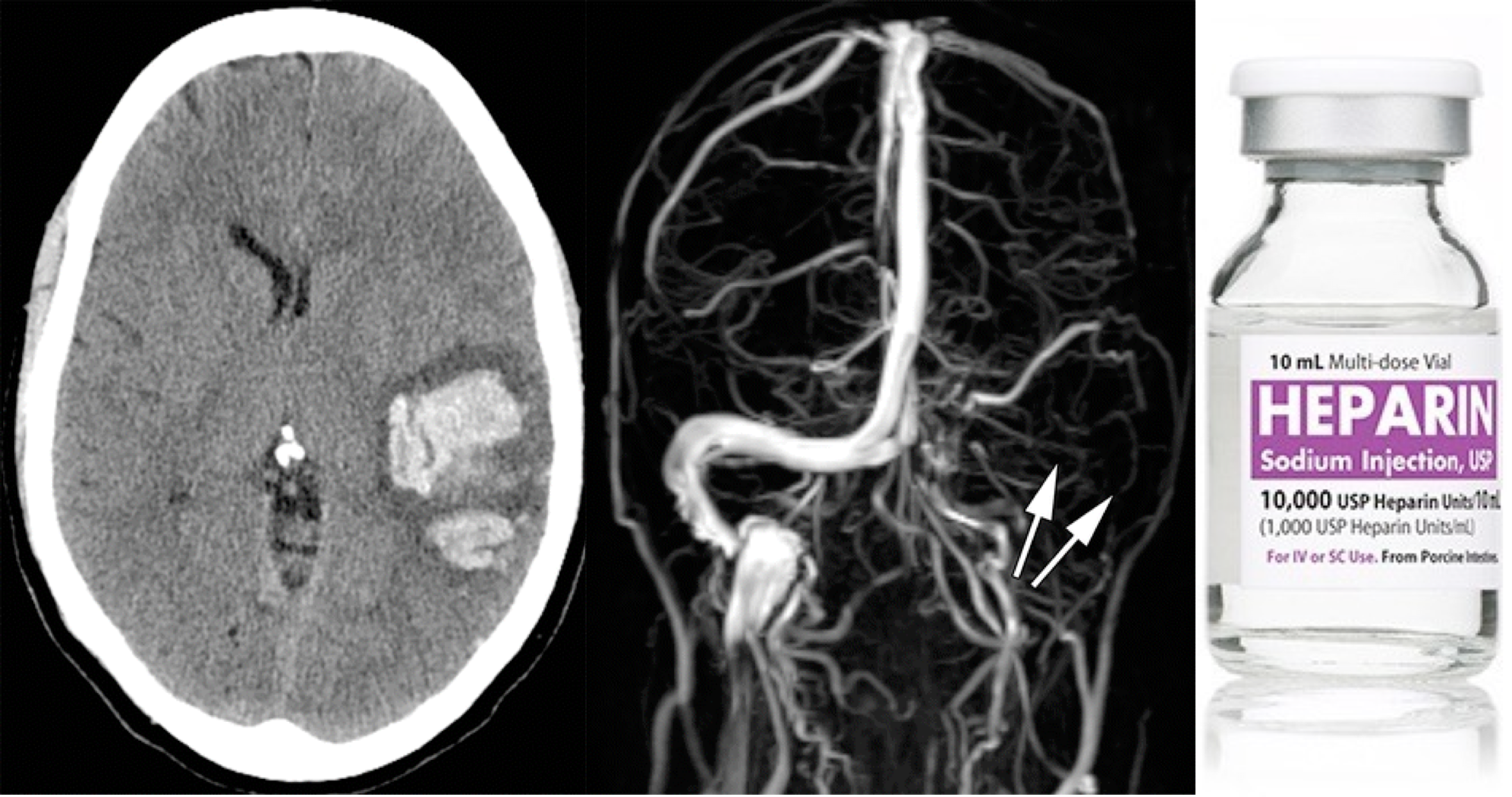
- NCCT: It is usually the first brain imaging requested in patients with suspected stroke or acute headache but it may be helpful only in ~30% of patients with acute CVT to see the hyperdensity in the lumen 9.
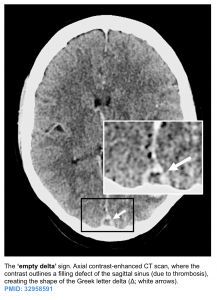
- Dense triangle sign, when thrombosis is within the superior sagittal sinus.
- Dense cord sign, when thrombosis is in a cortical or deep vein.
- CT venogram: increases performance to sensitivity of ~99% and specificity of 88%. Acute clot can appear dense (similar to contrast material). Comparison of pre- versus post-contrast images may be needed to detect the clot.
- A filling defect can be seen (e.g. empty delta sign in a dural sinus).
- MRI
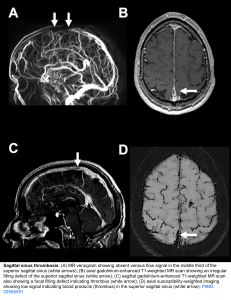
- Normally, fast flow of liquid e.g. blood will cause a hypointense signal on T2W images. Therefore for example, a normal cerebral arterial flow will appear as hypointense images.This is called “flow void”, implying that the vessel is patent.
- Absence of flow void (hyperintense signal) denotes blockage to the flow.
- Normally dural sinus can be seen as flow voids (e.g. on T2/FLAIR). Absence of these flow voids may suggest thrombosis. However, it should be emphasized that this finding is not specific for thrombosis and may be associated with transient hemodynamic conditions of slow flow, without thrombosis.
- DWI: an increased signal corresponding to the presence of intravascular clots may be depicted, even in the absence of parenchymal lesions; suggesting restriction of the movement of water molecules (i.e., restricted diffusion) at the site of occlusion 10.
- The appearance of the clot itself will vary over time👇. GRE/SWI sequences may be helpful, because susceptibility blooming artefact can cause the thrombus to be especially prominent 24 (Thrombosed veins appear appear hypointense on GRE).
- Normally, fast flow of liquid e.g. blood will cause a hypointense signal on T2W images. Therefore for example, a normal cerebral arterial flow will appear as hypointense images.This is called “flow void”, implying that the vessel is patent.
- MR venography: Gadolinium allows for direct visualization of luminal filling, similar to a CT venogram. Thus, contrast MR venography is highly accurate, on par with CT venogram. MR might be slightly superior for isolated cortical vein thrombosis or deep thromboses.
A. Acute (<5 days)
T1: Isointense to brain tissue
T2: Hypointense to brain tissue (may cause a false negative, because a hypointense clot can look like a
normal flow void.
GRE: Hypointense
B. Subacute (6-14 days)
T1: Hyperintense
T2: Hyperintense
GRE: Hypointense
C. Chronic (>14 days)
T1: Variable (may be iso-intense)
T2: Variable (iso-intense or hyperintense)
GRE: Variable (iso-intense or hypointense)
Indirect signs
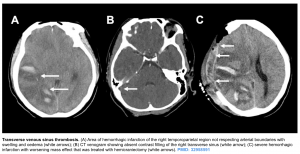
- Venous infarction: hypodense tissue may reflect ischemia. This may be differentiated from arterial occlusion if it spans multiple different arterial territories, or if it spares the cortex 11.
- Cerebral edema may be seen. This may be diffuse with sulcal effacement or may be gyral edema.
- The most commonly observed intraparanchymal finding of CVT are vasogenic and cytotoxic edema.
- Vasogenic edema is seen on diffusion-weighted MR images as hyperintense areas with high ADCs.
- Cytotoxic edema appears as lesions with low ADCs.
- Vasogenic and cytotoxic edema may coexist, so a heterogeneous pattern at diffusion weighted MRI and heterogeneous ADCs commonly are found.
- The most commonly observed intraparanchymal finding of CVT are vasogenic and cytotoxic edema.
- Hemorrhage
- This is typically parenchymal, but less often may be subarachnoid or even subdural in location. Rarely, subarachnoid hemorrhage may be the only imaging manifestation of CVT.
- Hemorrhagic infarcts do not conform to a particular arterial distribution. Some uncommon hemorrhage patterns may suggest the possibility of CVT:
- Bilateral hemorrhages, for example bilateral thalamic hemorrhages due to thrombosis of the vein of Galen or straight sinus.
- Disproportionate edema.
Laboratory tests
Aside from neuroimaging, there is no simple confirmatory laboratory test that can confidently rule out CVT in the acute phase of the disease.
D-dimer
- An elevated plasma D-dimer level supports the diagnosis of CVT, but a normal D-dimer does not exclude the diagnosis in patients with suggestive symptoms and predisposing factors 20.
- Individual assays used to measure D-dimer vary, but it is reasonable to use the same threshold levels as used in diagnostic protocols for deep venous thrombosis (eg, D-dimer >500 ng/mL of fibrinogen equivalent units).
Lumbar puncture (LP)
- It is not as part of the evaluation for suspected CVT. However, LP may be performed as part of a broader evaluation to exclude infection.
- Performing a lumbar puncture is not harmful in patients with CVT. However it is contraindicated in patients with a large lesion on CT and risk of herniation.
- In patient with elevated ICP, the opening pressure will be elevated.
- Cerebrospinal fluid may reveal nonspecific abnormalities (elevated lymphocytes, erythrocytes, and/or elevated protein) 12.
- Mild abnormalities in the cerebrospinal fluid shouldn’t lead to premature diagnostic closure with a diagnosis of “lymphocytic meningitis.”
Routine blood studies consisting of a complete blood count, ESR, chemistry panel, prothrombin time, and activated partial thromboplastin time for patients with suspected CV is recommended 13.
- The findings from these tests may suggest the presence of conditions that contribute to the development of CVT such as an underlying hypercoagulable state, infection, or inflammatory process.
Differential diagnosis
The clinical presentation of CVT can be nonspecific and the cause may not be apparent on initial routine neuroimaging studies. For patients presenting with the following sings and symptoms, the differential diagnosis encompasses as:
- Isolated intracranial hypertension (headache with or without vomiting, papilledema and visual problems)
- The main DDx are:
- Idiopathic intracranial hypertension.
- Most commonly seen in young, obese women presenting with headache and visual loss, papilledema, transient visual obscuration and pulsatile tinnitus.
- Meningitis
- Intracranial mass lesions e.g. tumor, abscess
- Idiopathic intracranial hypertension.
- 👉Diagnostic consideration: Intracranial mass lesions usually are apparent on neuroimaging with CT/MRI. If neuroimaging reveals no structural lesion, then a lumbar puncture (LP) may be indicated with measurement of opening pressure and CSF for analysis.
- The main DDx are:
- Focal neurologic syndrome (focal deficits, seizure or both). The DDx is broad and include
- Vascular etiologies e.g. ICH, ischemic stroke
- Infection e.g. meningitis, abscess
- Tumor
- Encephalopathy (altered mental status, focal deficit).
- Toxic metabolic disturbances. Specific etiologies in this context include:
- Septic encephalopathy
- Hepatic or Uremic encephalopathy
- Electrolytes: Hyper/hyponatremia, Hypo/hypercalcemia
- Glucose: Hypoglycemia, Hyperosmolar hyperglycemic state (HHS) or diabetic ketoacidosis (DKA)
- Wernicke encephalopathy
- Hypoxic-ischemic encephalopathy
- Post-transplantation encephalopathy
- Infection e.g. bacterial and viral meningoencephalitis
- Inflammation e.g. paraneoplastic and autoimmune encephalitis
- Demyelination such as acute disseminated encephalomyelitis.
- Toxic metabolic disturbances. Specific etiologies in this context include:
- Thunderclap headache (TCH). Refers to a severe headache of sudden onset that reaches its maximum intensity within one or less of onset. The key feature that differentiates TCH from other headaches is the rapidity with which it develops, extreme severity alone is insufficient 21 .
- Common etiologies of TCH include 25:
- Conditions that most commonly cause TCH
- Subarachnoid hemorrhage (SAH). See Ottawa SAH Rule
- Potential clues for aneurysmal SAH include: TCH and transient loss of consciousness or seizure, neck pain/stiffness, onset during exertion and associated strong risk factors such as hypertension, smoking, personal or family history of SAH, aneurysm or polycystic kidney disease, sympathomimetic use.
- Reversible cerebral vasoconstriction syndrome (RCVS)
- Subarachnoid hemorrhage (SAH). See Ottawa SAH Rule
- Conditions that less commonly cause TCH:
- Cervical artery dissection (carotid artery or vertebral artery dissection). Potential clues are:
- Often present after a trivial trauma such as hyperextension of the neck
- Carotid artery dissection presents as TCH and/or unilateral facial, neck with a partial Horner’s syndrome (myosis and ptosis, but not anhydrosis), and 25% of patients will have retinal or cerebral TIA within one week (neurological symptoms lag behind because it takes time to have a thrombus formed and thrown from the site of dissection)
- Vertebral artery dissection presents with posterior neck or occiput pain and posterior circulation symptoms e.g. ataxia, vertigo, dysarthria, diplopia and dysphagia.
- CVT
- Posterior reversible encephalopathy syndrome (PRES)
- Acute hypertensive crisis
- Cerebral infection (e.g. meningitis, acute complicated sinusitis)
- ICH, Acute ischemic stroke
- ⚠️Cerebellar infarction commonly presents with TCH, vertigo, cerebellar signs and cranial nerves abnormality.
- Cervical artery dissection (carotid artery or vertebral artery dissection). Potential clues are:
- Conditions that uncommonly cause TCH
- Brain tumor. Headache is worse in the morning and is aggravated by cough, Valsalva and maneuvers that increase ICP. Oftentimes history of malignancy is present.
- Pituitary apoplexy
- Colloid cyst of the third ventricle
- Aortic arch dissection
- Giant cell arteritis. Potential diagnostic clues are: age > 50 years, polymyalgia rheumatica association, jaw claudication, visual loss (mainly monocular) and fever.
- Conditions that most commonly cause TCH
- 👉Diagnostic consideration: If initial neuroimaging is nondiagnostic, patients with TCH should have a LP with measurement of opening pressure and CSF analysis to exclude SAH and meningitis. If LP is also nondiagnostic, imaging of cerebral circulation is necessary, preferably with MRA/MRV 23.
- Common etiologies of TCH include 25:
- Women during pregnancy or puerperium: Preeclampsia or eclampsia are diagnostic considerations with any of the above presentations or otherwise when presenting with ischemic stroke or intracerebral hemorrhage 23 .
Treatment
Treatment should be started as soon as the diagnosis of CVT is clearly confirmed, with rapid anticoagulant therapy, treatment of any underlying cause (eg, dehydration, sepsis, stopping any prothrombotic medications), control of seizures and management of intracranial hypertension if required.
- Heparin anticoagulation is generally the mainstay of therapy 14 .
- Presence of hemorrhagic venous infarction, ICH, or isolated SAH are not contraindications for anticoagulant treatment in CVT.
- Subcutaneous LMWH is preferred except in following conditions where unfractionated heparin should be used
- Clinically unstable patients (who are more likely to require a procedure).
- When invasive interventions such as external ventricular drain or decompressive craniectomy are planned
- Presence of other contraindications for LMWH e.g. renal failure.
- The AHA/ASA guidelines recommend anticoagulation for 3–6 months in provoked CVT, 6–12 months in unprovoked CVT, and potentially lifelong in recurrent CVT, VTE following CVT, or CVT associated with severe thrombophilia with target INR of 2–3.
Catheter-directed endovascular therapy
- This may include local administration of thrombolytics into the cerebral veins, and/or mechanical clot disruption.
- Available evidence has not shown benefit from interventional therapy.
- Seizures are frequent, affecting up to 40% of all CVT patients.
- There should be a low threshold for EEG among patients with altered mental status (who are unconscious or mechanically ventilated) or possible seizure.
- Seizure prophylaxis is generally not recommended, but it may be considered among individual patients at high risk for seizure (see above) 15.
- If seizure occurs, it should be treated aggressively.
Elevated intracranial pressure and herniation
- In the acute phase, elevated ICP may arise from single or multiple large hemorrhagic lesions, infarcts or brain edema. Elevated ICP or brain occupying lesions may cause transtentorial herniation and death.
- General supportive measures include 2
- Elevating the head of the bed.
- Hyperventilation to a target partial pressure of carbon dioxide of 30-35 mmHg.
- Osmotic therapy (mannitol or hypertonic saline).
- Acetazolamide (500-1000 mg/d) can be considered in patients with severe headaches or threat to vision.
- Acetazolamide is contraindicated in pregnancy due to a risk of teratogenicity.
- Corticosteroids should not be used except in the presence of underlying inflammatory diseases (eg, Bechet’s disease, systemic lupus erythematosus).
- Decompressive craniotomy
- In the presence of brain herniation or midline shift (‘malignant CVT’), medical therapy alone is often not sufficient to control raised intracranial pressure.
- Decompressive craniectomy allows the swelling brain to expand and could favor collateral vein drainage in CVT by reducing intracranial pressure.
- Neuroradiological features that should lead to consideration of craniectomy include 16
- Uncal herniation
- Midline shift (>5 mm)
- Herniation-induced ischemia in the territory of the posterior cerebral artery territory (which is vulnerable to local mass effect and raised intracranial pressure).
- A persistent intracranial pressure >20 cmH2O is also suggested as an indication for craniotomy.
- Communicating hydrocephalus: Impaired drainage of CSF into the venous sinuses may lead to elevated intracranial pressure, due to communicating hydrocephalus. External ventricular drain (EVD) placement may rarely be necessary. Acetazolamide could be considered to reduce CSF production.
Treat any underlying infection and inflammation
- Antibiotic treatment is mandatory whenever there is meningitis or other intracranial infection, or an infection of neighboring structure such as otitis or mastoiditis.
- Associated inflammatory disease such as lupus, vasculitis, Behcet disease; treatment with glucocorticoids may be necessary.
- Prothrombotic medications may need to be discontinued (e.g. hormonal therapy).
Neurologic deterioration
Possible cause(s) of neurological worsening include:
- Clot propagation, causing an increased amount of infarcted tissue.
- Intracranial hemorrhage (or extension of hemorrhage or edema).
- Seizure (either convulsive or non-convulsive).
- Brain herniation.
- Communicating hydrocephalus due to impaired CSF reabsorption.
In the presence of neurological worsening, following evaluation may be required:
- CT scan with CT venogram.
- EEG if seizure is a concern
- Evaluation of intracranial pressure (e.g. with ultrasonography to evaluate for papilledema).
Prognosis
CVT generally has a favorable outcome, with a complete functional recovery reported in about 75% of patients; however, about 15% die or are dependent.
In the acute setting, risk factors for a poor outcome include
- Male sex
- Older age (age > 37 years)
- GCS < 9 on admission
- Confusion or coma
- Intracranial hemorrhage
- Deep vein involvement
- Infection and malignancy
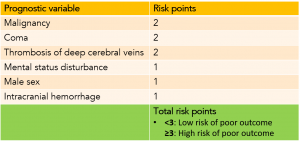
Cerebral venous thrombosis risk score
- This may be used as a rough tool to determine functional prognosis 17. (following table).
- It can also be used to avoid unnecessary or dangerous interventions (e.g. endovascular therapy) in low-risk patients, and may help to identify high-risk CVT patients.
Going further
- CVT Imaging (The Radiology Assistant)
- Radiologic Clues to Cerebral Venous Thrombosis
- CVT (CoreEM)
- Cerebral venous thrombosis (EMCrit)
- CVT (IBCC)
References
1. Ropper AH, Klein JP. Cerebral Venous Thrombosis. N Engl J Med. 2021 Jul 1;385(1):59-64. doi: 10.1056/NEJMra2106545. PMID: 34192432.
2. Ulivi L, Squitieri M, Cohen H, Cowley P, Werring DJ. Cerebral venous thrombosis: a practical guide. Pract Neurol. 2020 Oct;20(5):356-367. doi: 10.1136/practneurol-2019-002415. PMID: 32958591
3. Capecchi M, Abbattista M, Martinelli I. Cerebral venous sinus thrombosis. J Thromb Haemost. 2018 Oct;16(10):1918-1931. doi: 10.1111/jth.14210. Epub 2018 Jul 11. PMID: 29923367.4. Dmytriw AA, Song JSA, Yu E, Poon CS. Cerebral venous thrombosis: state of the art diagnosis and management. Neuroradiology. 2018 Jul;60(7):669-685. doi: 10.1007/s00234-018-2032-2. Epub 2018 May 11. PMID: 29752489.
5. Agostoni E. Headache in cerebral venous thrombosis. Neurol Sci. 2004 Oct;25 Suppl 3:S206-10. doi: 10.1007/s10072-004-0287-3. PMID: 15549538.
6. de Bruijn SF, Stam J, Kappelle LJ. Thunderclap headache as first symptom of cerebral venous sinus thrombosis. CVST Study Group. Lancet. 1996 Dec 14;348(9042):1623-5. doi: 10.1016/s0140-6736(96)07294-7. PMID: 8961993.
7. Ferro JM, Canhão P, Bousser MG, Stam J, Barinagarrementeria F; ISCVT Investigators. Early seizures in cerebral vein and dural sinus thrombosis: risk factors and role of antiepileptics. Stroke. 2008 Apr;39(4):1152-8. doi: 10.1161/STROKEAHA.107.487363. Epub 2008 Feb 28. PMID: 18309177.
8. Canedo-Antelo M, Baleato-González S, Mosqueira AJ, Casas-Martínez J, Oleaga L, Vilanova JC, Luna-Alcalá A, García-Figueiras R. Radiologic Clues to Cerebral Venous Thrombosis. Radiographics. 2019 Oct;39(6):1611-1628. doi: 10.1148/rg.2019190015. PMID: 31589585.
9. Leach JL, Fortuna RB, Jones BV, Gaskill-Shipley MF. Imaging of cerebral venous thrombosis: current techniques, spectrum of findings, and diagnostic pitfalls. Radiographics. 2006 Oct;26 Suppl 1:S19-41; discussion S42-3. doi: 10.1148/rg.26si055174. PMID: 17050515
10. Oliveira IM, Duarte JÁ, Dalaqua M, Jarry VM, Pereira FV, Reis F. Cerebral venous thrombosis: imaging patterns. Radiol Bras. 2022;55(1):54-61. doi:10.1590/0100-3984.2021.0019
11. Ge S, Wen J, Kei PL. Cerebral venous thrombosis: a spectrum of imaging findings. Singapore Med J. 2021;62(12):630-635. doi:10.11622/smedj.2021235
12. Bousser MG, Chiras J, Bories J, Castaigne P. Cerebral venous thrombosis–a review of 38 cases. Stroke. 1985 Mar-Apr;16(2):199-213. doi: 10.1161/01.str.16.2.199. PMID: 3975957.
13. Saposnik G, Barinagarrementeria F, Brown RD Jr, Bushnell CD, Cucchiara B, Cushman M, deVeber G, Ferro JM, Tsai FY; American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011 Apr;42(4):1158-92. doi: 10.1161/STR.0b013e31820a8364. Epub 2011 Feb 3. PMID: 21293023.
14. Ferro JM, Bousser MG, Canhão P, Coutinho JM, Crassard I, Dentali F, di Minno M, Maino A, Martinelli I, Masuhr F, Aguiar de Sousa D, Stam J; European Stroke Organization. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis – endorsed by the European Academy of Neurology. Eur J Neurol. 2017 Oct;24(10):1203-1213. doi: 10.1111/ene.13381. Epub 2017 Aug 20. PMID: 28833980.
15. Ferro JM, Canhão P, Bousser MG, Stam J, Barinagarrementeria F; ISCVT Investigators. Early seizures in cerebral vein and dural sinus thrombosis: risk factors and role of antiepileptics. Stroke. 2008 Apr;39(4):1152-8. doi: 10.1161/STROKEAHA.107.487363. Epub 2008 Feb 28. PMID: 18309177
16. Avanali R, Gopalakrishnan MS, Devi BI, Bhat DI, Shukla DP, Shanbhag NC. Role of Decompressive Craniectomy in the Management of Cerebral Venous Sinus Thrombosis. Front Neurol. 2019 May 15;10:511. doi: 10.3389/fneur.2019.00511. PMID: 31156540; PMCID: PMC6529953.
17. Ferro JM, Bacelar-Nicolau H, Rodrigues T, Bacelar-Nicolau L, Canhão P, Crassard I, Bousser MG, Dutra AP, Massaro A, Mackowiack-Cordiolani MA, Leys D, Fontes J, Stam J, Barinagarrementeria F; ISCVT and VENOPORT investigators. Risk score to predict the outcome of patients with cerebral vein and dural sinus thrombosis. Cerebrovasc Dis. 2009;28(1):39-44. doi: 10.1159/000215942. Epub 2009 May 6. PMID: 19420921.
18. Masuhr F, Busch M, Amberger N, Ortwein H, Weih M, Neumann K, Einhäupl K, Mehraein S. Risk and predictors of early epileptic seizures in acute cerebral venous and sinus thrombosis. Eur J Neurol. 2006 Aug;13(8):852-6. doi: 10.1111/j.1468-1331.2006.01371.x. PMID: 16879295.
19. Idiculla PS, Gurala D, Palanisamy M, Vijayakumar R, Dhandapani S, Nagarajan E. Cerebral Venous Thrombosis: A Comprehensive Review. Eur Neurol. 2020;83(4):369-379. doi: 10.1159/000509802. Epub 2020 Sep 2. PMID: 32877892.
20. Smith E, Kumar V. BET 1: Does a normal D-dimer rule out cerebral venous sinus thrombosis (CVST)? Emerg Med J. 2018 Jun;35(6):396-397. doi: 10.1136/emermed-2018-207777.1. PMID: 29784833.
21. Schwedt TJ. Thunderclap Headache. Continuum (Minneap Minn). 2015 Aug;21(4 Headache):1058-71. doi: 10.1212/CON.0000000000000201. PMID: 26252591.
22. Raam R, Tabatabai RR. Headache in the Emergency Department: Avoiding Misdiagnosis of Dangerous Secondary Causes, An Update. Emerg Med Clin North Am. 2021 Feb;39(1):67-85. doi: 10.1016/j.emc.2020.09.004. PMID: 33218663.
23. Macri E, Greene-Chandos D. Neurological Emergencies During Pregnancy. Neurol Clin. 2021 May;39(2):649-670. doi: 10.1016/j.ncl.2021.02.008. PMID: 33896537.
24. Patra A, Janu A, Sahu A. MR Imaging in Neurocritical Care. Indian J Crit Care Med. 2019 Jun;23(Suppl 2):S104-S114. doi: 10.5005/jp-journals-10071-23186. PMID: 31485117; PMCID: PMC6707490.
25. Malhotra A, Wu X, Gandhi D, Sanelli P. The Patient with Thunderclap Headache. Neuroimaging Clin N Am. 2018 Aug;28(3):335-351. doi: 10.1016/j.nic.2018.03.002. Epub 2018 Jun 8. PMID: 30007749.




Add comment