18 December 2024, by Shahriar Lahouti.
CONTENTS
- Introduction
- Physiologic effects
- Choosing noninvasive respiratory support
- General principles
- Noninvasive positive pressure ventilation
- High-flow oxygen via nasal cannula
- RECAP
- Media🎥
- References
Introduction
Noninvasive respiratory support (NIRS) refers to supplying oxygenation and ventilatory assistance without an invasive artificial airway. Different methods of noninvasive respiratory support are used in the acute care setting for patients with acute respiratory failure. The three main methods are high-flow oxygen therapy, and noninvasive positive pressure ventilation (NPPV) either as continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BPAP). Non-invasive respiratory support is the mainstay for treating patients with acute or acute-on-chronic forms of respiratory failure. The basic principles and practical aspects of using non-invasive respiratory support in adult patients with acute respiratory failure are discussed here.
Physiologic effects
High-Flow Oxygen Therapy
High-flow oxygen via nasal cannula (HFNC) is a technique in which heated and humidified oxygen is delivered to the nose at high flow rates (~60 L/M). Patients can usually tolerate high flow rates because the gas is heated and humidified (otherwise this is very uncomfortable).
◾️Essentional concept
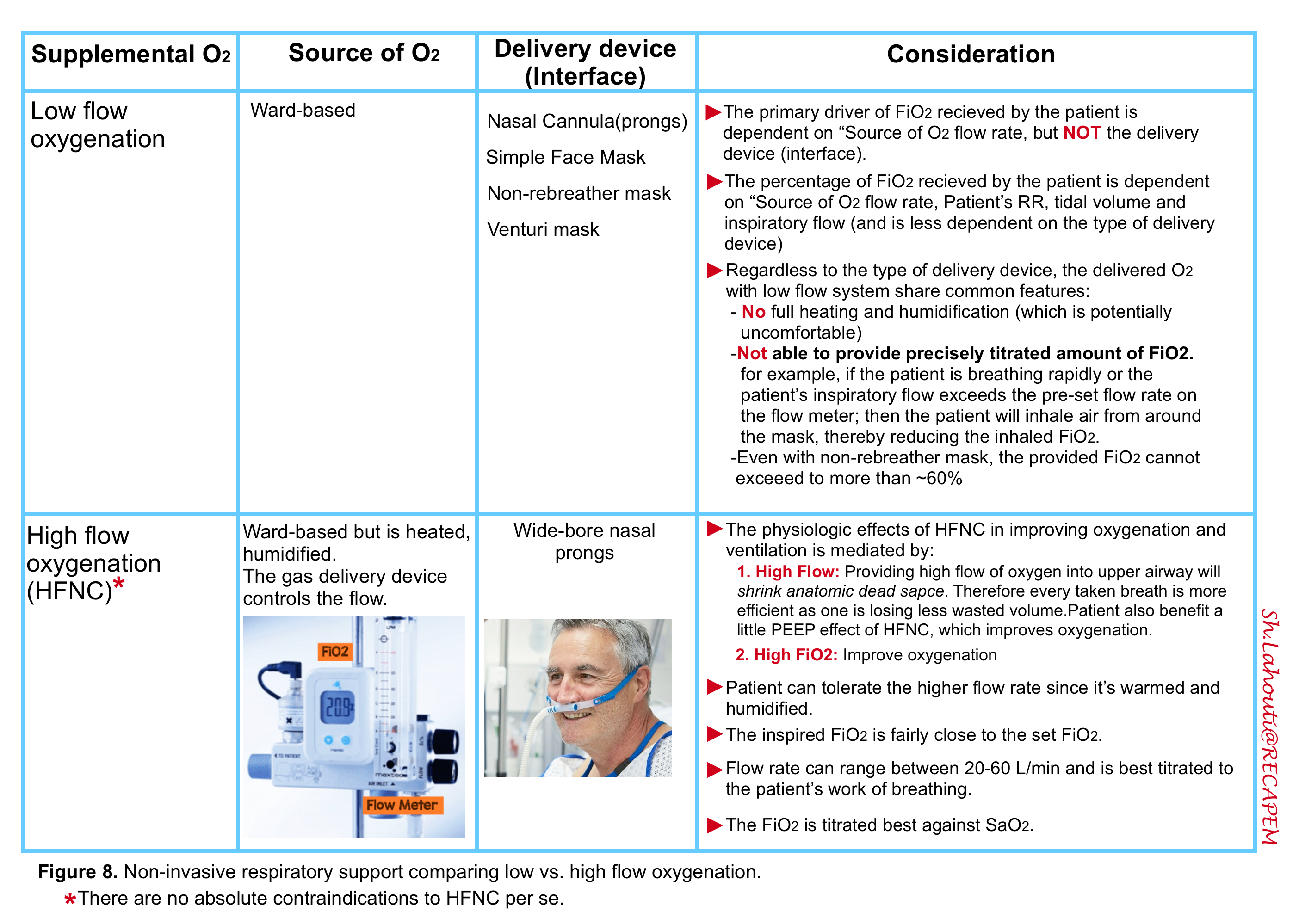
- The oxygen content of each inhaled breath (FiO2) is not dependent on the O2 delivery device. Rather it is the flow rate of the oxygen source that is the primary driver of the FiO2 received by the patient *.
- For example, the traditional teaching was that the maximal FiO2 that could be delivered by a simple face mask is ~50% when an oxygen flow rate of 15 L/min is used. However, FiO2 values of 90% and higher can be delivered by a simple face mask when the source oxygen flow rate is set to a higher level (≥40 L/min).
◾️Mechanisms of clinical benefit: HFNC physiologically works to improve oxygenation and ventilation through the following mechanisms (media below 🎥) *:
-
- A high flow rate has the following beneficial effects:
- Minimal entrainment of room air. This results in more efficient delivery of oxygen leading to a higher FiO2 received by the patient.
- Patients in respiratory distress generate high inspiratory flow rates that exceed the flow rates of standard oxygen equipment, resulting in the entrainment of room air and a reduction in the delivery of the set FiO2. The rate of flow in HFNC generally exceeds that of the patient, entraining very little room air and resulting in a FiO2 that is more reliably delivered *.
- Washout of nasopharyngeal dead space tends to improve ventilation (i.e. increasing tidal volume & decreasing respiratory rate) and oxygen delivery.
- Dead space is the volume of gas that is moved in and out of the airway but doesn’t participate in CO2 clearance from the blood. Therefore, dead space is detrimental to the patient because the patient has to expend work to move this gas, but it provides no effective ventilation.
- HFNC jets fresh gas into the nose and upper pharynx, which reduces the anatomic dead space (media below 🎥).
- Normally the anatomic dead space is made up of the nose, trachea, bronchi, and terminal bronchioles.
- With HFNC, the anatomic dead space extends from the respiratory bronchioles to only the mid-tracheal level (as the fresh gas is being pumped into the upper airway).
- By shrinking the anatomic dead space, HFNC makes ventilation more efficient. This allows the patient to achieve the same amount of CO2 clearance while moving less gas into their lungs, which reduces the work of breathing.
- Modest positive end-expiratory (PEEP) effect
- HFNC increases nasopharyngeal airway pressure that peaks at the end of expiration (i.e. “PEEP effect”).
- Generally, the mean airway pressure is increased by ~1 cm H2O per 10 L/minute of flow *).
- This “PEEP effect” can potentially decrease the work of breathing, unload auto-PEEP (if present), and enhance oxygenation in patients with alveolar filling diseases such as cardiogenic pulmonary edema or acute respiratory distress syndrome.
- HFNC increases nasopharyngeal airway pressure that peaks at the end of expiration (i.e. “PEEP effect”).
- Minimal entrainment of room air. This results in more efficient delivery of oxygen leading to a higher FiO2 received by the patient.
- A high flow rate has the following beneficial effects:
- Humidified and heated (to core temperature) oxygen results in increasing water content in mucous. This can facilitate secretion removal and may also decrease the work of breathing.
Noninvasive Positive Pressure Ventilation (NPPV)
◾️Noninvasive positive pressure ventilation (NPPV) is mechanical ventilation without an artificial airway. Compared to invasive mechanical ventilatory support requiring endotracheal intubation, patients require little to no sedation and benefit from reduced risk of airway injury and nosocomial pneumonia. NPPV supports oxygenation and ventilation through a noninvasive interface, with the added benefits of enhanced patient comfort and preservation of patient interaction, including speech, swallowing, and airway defense mechanisms.
◾️The physiological benefits of NPPV include:
- Decreasing the respiratory effort and the work of breathing *
- Employing supra-atmospheric pressure intermittently to the airways elevates transpulmonary pressure, i.e. the pressure outside the lungs is greater than the pressure inside the lungs. This causes air to be forced into the lungs (down the pressure gradient), resulting in unloading the inspiratory muscle. Consequently, the respiratory effort and the work of breathing are reduced.
- NPPV alleviates dyspnea and decreases respiratory rate and CO2 retention.
- In patients with severe COPD exacerbations, the combination of PEEP with inspiratory pressure support further reducing breathing effort by counteracting the effects of auto-PEEP *.
- Improving oxygenation and ventilation
- NPPV helps to keep the chest and lungs expanding by increasing the functional residual capacity (the amount of air remaining in the lungs after expiration) after normal (tidal) expiration; this is the air in the alveoli available for gas exchange.
- Positive pressure inside the airways also helps open or “splint” the airways, reducing atelectasis, improving alveolar ventilation, and reducing ventilation/perfusion (V/Q) mismatch.
- Higher pressure in the alveoli increases the solubility of oxygen, which increases its ability to cross the alveolocapillary membrane and enter the bloodstream.
- Improving preload and afterload in cardiogenic pulmonary edema *.
- NPPV increases the intrathoracic pressure, which causes preload to decrease due to a diminished venous return, and also reduces the transmural pressure, thereby decreasing the afterload. This is especially important in acute cardiogenic pulmonary edema.
Choosing noninvasive respiratory support (NIRS)
General principles
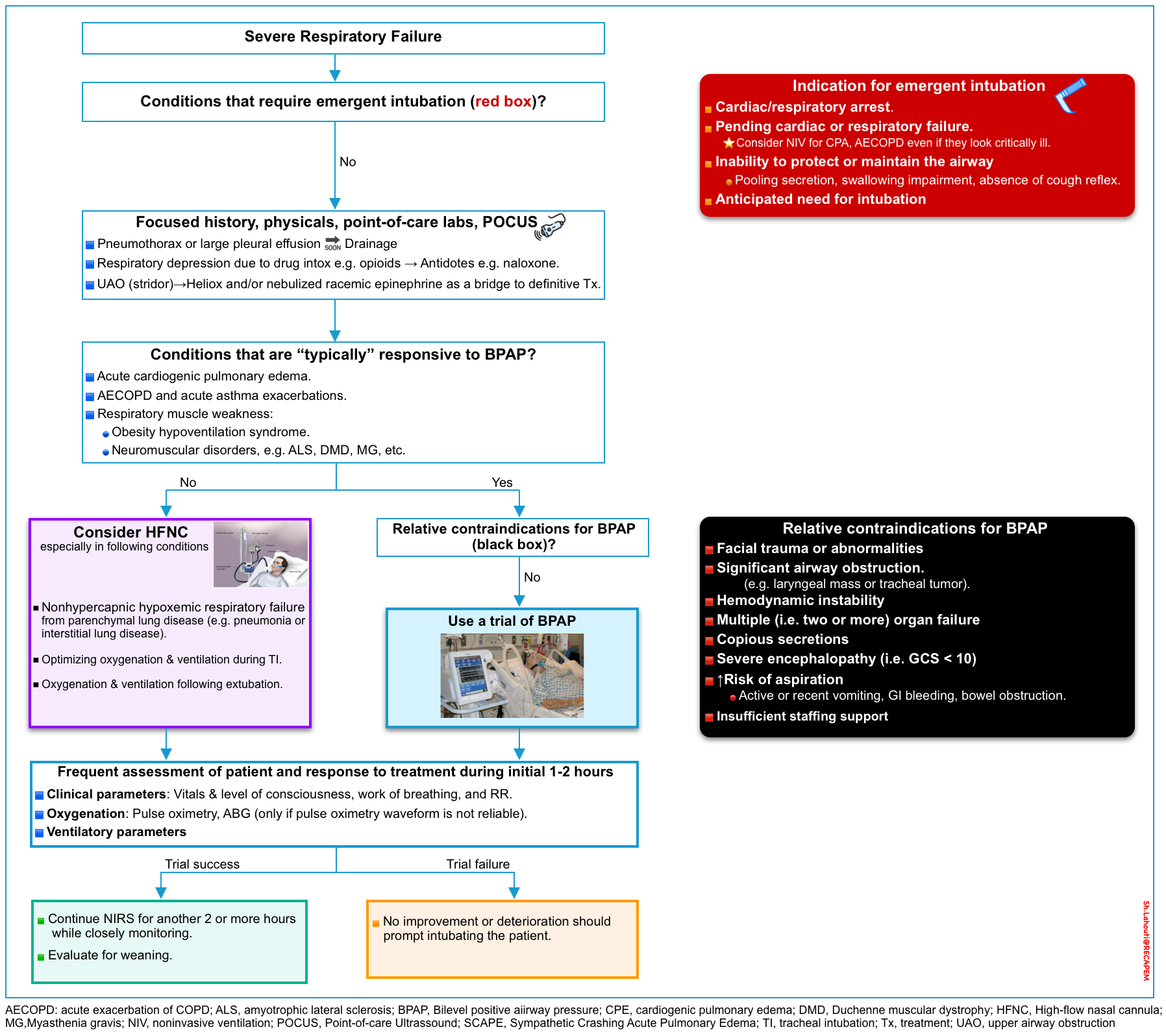
◾️The keys to successfully using NIRS on an emergency basis are patient selection and aggressive therapy to reverse the disease inciting respiratory failure.
- When assessing patients for noninvasive respiratory support, the clinician should evaluate for the presence of contraindications (including the emergent need for intubation), and identify whether the condition responsible for acute respiratory failure is typically responsive to NIRS.
- The method of choice for providing NIRS depends on the underlying diagnosis rather than blood gas values!
- Patients with the following conditions may present with frank respiratory failure but NIRS will not be effective at all.
- Avoid NIRS for primary CNS disorders, e.g. sedative overdose, encephalitis, etc.
- Patients with upper airway obstruction (e.g. stridor) do not benefit from NIRS too.
◾️The major benefit of NIRS is the avoidance of intubation and risks of invasive mechanical ventilation.
- Since delayed mechanical ventilation is associated with a poor outcome, trials of noninvasive respiratory support should be short (eg, two hours or less) and patients should be monitored and reassessed frequently to ensure rapid intubation when needed.
◾️An overall view of using noninvasive respiratory support for adult patients with respiratory failure in an acute care setting is shown in the figure below.
🟦When to consider noninvasive ventilation?
- To minimize the risk of failure or complications, every patient should be properly assessed for suitability to receive NIV safely.
- Indications for NIV use include *
- Clinical signs of respiratory failure
- Dyspnea (moderate to severe)
- Signs of increased work of breathing, accessory muscle use, and abdominal paradox
- Tachypnea (>24 bpm in obstructive, >30 bpm in restrictive)
- Gas exchange
- Acute or acute on chronic ventilatory failure (best indication), PaCO2 >45 mm Hg, pH<7·35
- Hypoxemia (use with caution), PaO2/FIO2 ratio<200
- Clinical signs of respiratory failure
- Patients should be closely monitored during the first 24 hours after initiating NIV, as this is the period with the highest rate of treatment failure.
- Although data points at presentation such as a high RR (respiratory rate), low arterial pH values, or low PaO2/FiO2 can help predict failure, the most robust predictor of treatment failure during this period is failing to show an improvement in these parameters at 1‑2 h after initiating NIV treatment *.
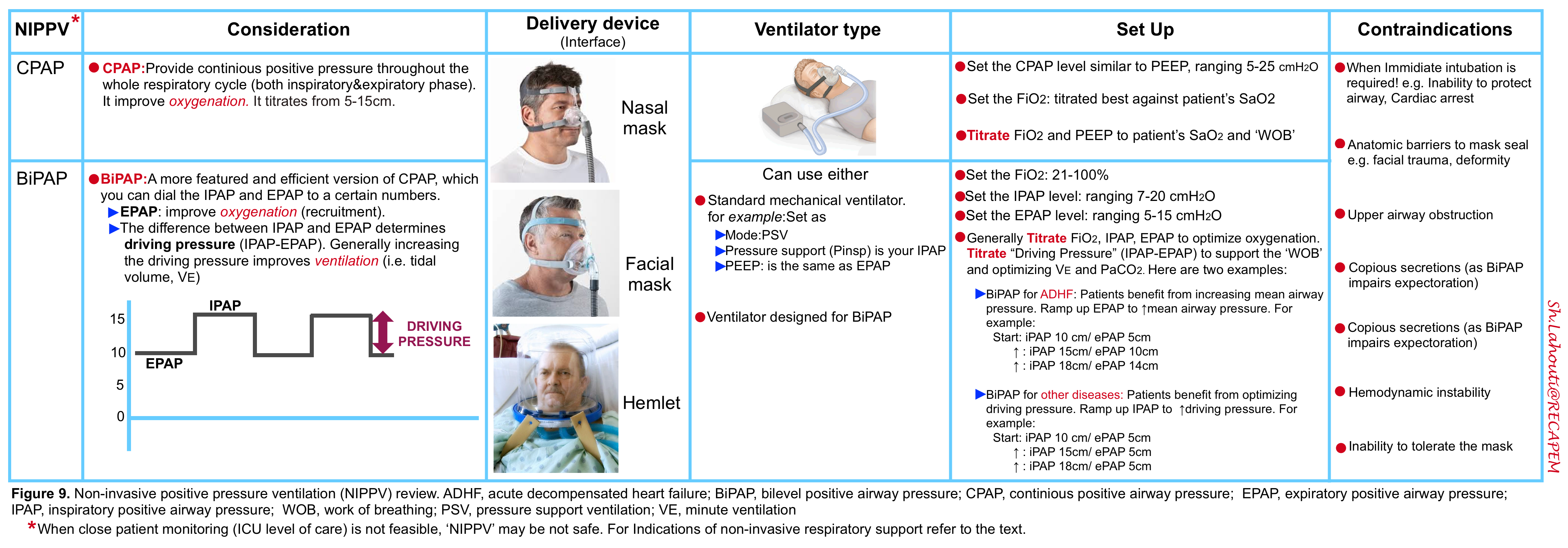
🟦Contraindications to noninvasive positive pressure ventilation
- Absolute: ⚠️The need for emergent intubation *
- Inability to protect or maintain the airway (evidenced by pooling secretion, swallowing impairment, and absence of cough reflex).
- Pulmonary gas exchange requires an unobstructed oropharyngeal inlet, which is maintained in awake patients by the upper airway musculature.
- The level of alertness needed to maintain airway tone is the same as required to maintain brisk protective reflexes to prevent aspiration of oral and gastric fluids.
- Cardiac or respiratory arrest.
- Pending cardiac (e.g. unstable cardiac arrhythmia) or respiratory failure.
- Note that in certain clinical situations, patients may look critically ill, yet do well without intubation. Clinical judgment is advised.
- Acute cardiogenic pulmonary edema (may respond rapidly with BiPAP and high-dose nitroglycerine infusion).
- Bronchospasm (asthma or COPD).
- When in doubt, a reasonable approach is often to support the patient on BiPAP while simultaneously preparing for intubation.
- If the patient responds well to BiPAP, then BiPAP may be continued.
- If the patient requires intubation, then the BiPAP will help pre-oxygenate before intubation.
- Note that in certain clinical situations, patients may look critically ill, yet do well without intubation. Clinical judgment is advised.
- Anticipated need for intubation, for example, patients with the potential for respiratory decompensation who must be transported out of the emergency department (e.g. for scans/procedures) often require intubation.
- Inability to protect or maintain the airway (evidenced by pooling secretion, swallowing impairment, and absence of cough reflex).
- Relative. BiPAP is generally avoided in the following conditions, but exceptions exist! *
- Anatomical abnormalities that prevent proper mask fit, e.g. severe facial trauma and/or deformities (helmet interface may be able to be used) *.
- Significant upper airway obstruction (e.g. laryngeal mass or tracheal tumor).
- Inability to cooperate (although sedation can sometimes be helpful).
- Hemodynamic instability *
- Severe encephalopathy (i.e. Glasgow coma score < 10)
- Severe multi-organ (i.e. two or more) organ failure
- For patients with severe multi-organ (ie, two or more) organ failure, these techniques are less likely to be successful (unless the cause of respiratory failure is very rapidly reversible) *.
- Copious respiratory secretions
- In general, BiPAP provides positive airway pressure and reduces the pressure gradient which forces secretions out of the airway.
- Patients with COPD and a mild amount of secretion may do well on BiPAP (especially if BiPAP is alternated with HFNC to allow for coughing and clearing secretions). However, BiPAP should be avoided for patients with copious secretions. Such patients may initially improve on BiPAP but eventually develop mucus plugging with subsequent deterioration.
- Patients at high risk of aspiration (contraindication only for fascial mask)
- The risk of aspiration is related to mental status and risk of vomiting:
- Mental status: Patients with depressed mental status are at increased risk of aspirating. While most patients with severely impaired consciousness should not receive BiPAP, reduced consciousness due to hypercapnic encephalopathy may be an exception to the rule. This is because BiPAP is a treatment for acute hypercapnia; thus, mental status should improve with NIV *.
- The likelihood that hypercapnic encephalopathy will respond to NIV is inversely related to the severity of the hypercapnia.
- Such patients should be closely monitored (eg, in intensive care or high-dependency unit); improved consciousness should be apparent within one to two hours after initiating NIV. Patients who deteriorate or fail to improve should be promptly intubated.
- Risk of vomiting:
- Mental status: Patients with depressed mental status are at increased risk of aspirating. While most patients with severely impaired consciousness should not receive BiPAP, reduced consciousness due to hypercapnic encephalopathy may be an exception to the rule. This is because BiPAP is a treatment for acute hypercapnia; thus, mental status should improve with NIV *.
- The risk of aspiration is related to mental status and risk of vomiting:
- Prolonged duration of ventilation is anticipated
- Clinical judgment is required when anticipating the duration of ventilation. Nonetheless, for patients with acute respiratory failure, NPPV is generally considered a short-term form of ventilation (eg, one to three days) while therapy for the underlying disorder is being treated.
- If it is anticipated that ventilation will be required for a more prolonged period (e.g. ≥4 days), NPPV is less appealing and patients should probably be intubated (e.g. severe Guillain Barre syndrome).
- Clinical judgment is required when anticipating the duration of ventilation. Nonetheless, for patients with acute respiratory failure, NPPV is generally considered a short-term form of ventilation (eg, one to three days) while therapy for the underlying disorder is being treated.
- Insufficient staffing support
🟦What are the goals and benefits of using NIV?
- Keep in mind that, the goal of NIRS is essentially to support the patient long enough for other therapies to work (e.g. vasodilators and diuretics for pulmonary
edema, bronchodilator aerosols, and corticosteroids for reactive airway disease). - The goals and benefits of NIV in the acute care setting are as follows:
- Improve gas exchange
- Avoid intubation
- Decrease mortality
- Decrease the length of time on the ventilator
- Decrease the duration of hospitalization
- Decrease the incidence of ventilator‑associated pneumonia
- Relieve symptoms of respiratory distress
- Improve patient‑ventilator synchrony
- Maximize patient comfort
- Serial clinical evaluation should show that the patient’s condition is stable or improving over a period of time (see below).
- Note that establishing the above goals may take a bit of time.
- Do not expect the patient to improve immediately.
- Do not assume that the patient can be weaned off noninvasive respiratory support within a few hours.
- Do not expect that the ABG/VBG must immediately improve, or some arbitrary blood gas targets should be met.
Noninvasive positive pressure ventilation
- Patients most likely to benefit from noninvasive positive pressure ventilation in acute care setting are:
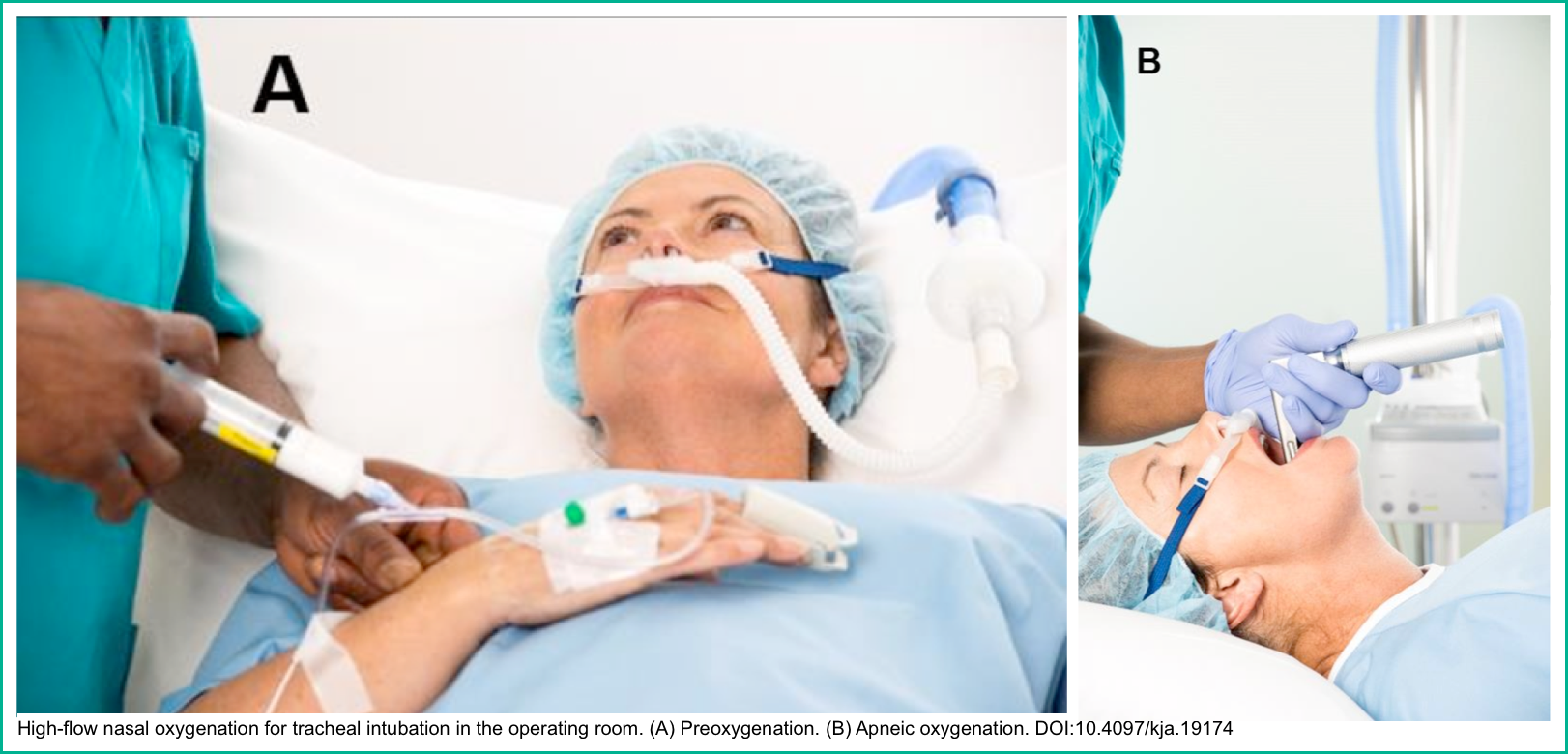
⭐️In addition to treating acute respiratory failure in critically ill inpatients, NIV has also been used in other clinical settings, e.g. oxygenation before and during intubation (media below) *.
How to start a patient on NIV?
- Education
- Plan adequate training for all staff with a calibrated protocol
- Environment
- Choose an appropriate setting (monitoring) for starting NIV according to the severity of ARF. At a minimum, continuous pulse oximetry should be provided.
- Patients requiring NIRS are generally quite sick, so it is imperative to make sure your team is aware of the situation and prepared as well.
- Ensure that you have intubation equipment and airway adjuncts ready and available at the bedside in case the initial non-invasive procedures fail.
- Consider whether the patient may need medication to provide anxiolysis or provide very judicious amounts of sedation.
- Indication
- Select patients according to the team’s experience, location, availability of intubation, do‑not‑intubate status, and likelihood of success.
- Information
- Explain the technique to competent patients to improve their compliance.
- Equipment:
- Choose the interface(s) that best fits the facial anatomy; also consider rotating different interfaces to enhance comfort.
- Select the appropriate interface designed for use with the ventilator type that you are going to use.
- Noninvasive ventilators: Interfaces with entrainment valves are designed for noninvasive ventilators that use a single‑limb circuit.
- The entrainment valve is required to prevent asphyxia if the ventilator fails or the tubing becomes disconnected.
- Masks with leak ports should only be used with single‑limb circuit ventilators.
- Invasive (aka. full mode, standard) ventilators: Masks without leak ports should only be used.
- Noninvasive ventilators: Interfaces with entrainment valves are designed for noninvasive ventilators that use a single‑limb circuit.
- Choose a ventilator with a good air-leak compensation that displays flow/pressure/volume curves.
- Starting ventilation.
- Choose a pressure mode (i.e. pressure support) with PEEP (if invasive ventilator is chosen). Start with low pressures, then increase gradually depending on comfort.
- Set adequate FiO2 and essential alarms.
- Tighten the straps of the interface enough to avoid leaks, without making them too tight
- Monitoring ventilation.
- Check clinical status, and monitor SpO2. Reset the ventilator according to patient‑ventilator synchrony, comfort, and leaks.
- Prevent skin lesions (i.e. protective devices, rotating interfaces).
- Consider humidification.
- Carefully consider sedation.
- Consider management of secretions, if required.
- Be prepared to reassess these patients frequently to properly up-or-down-titrate their respiratory support.
Equipment
Ventilatory type
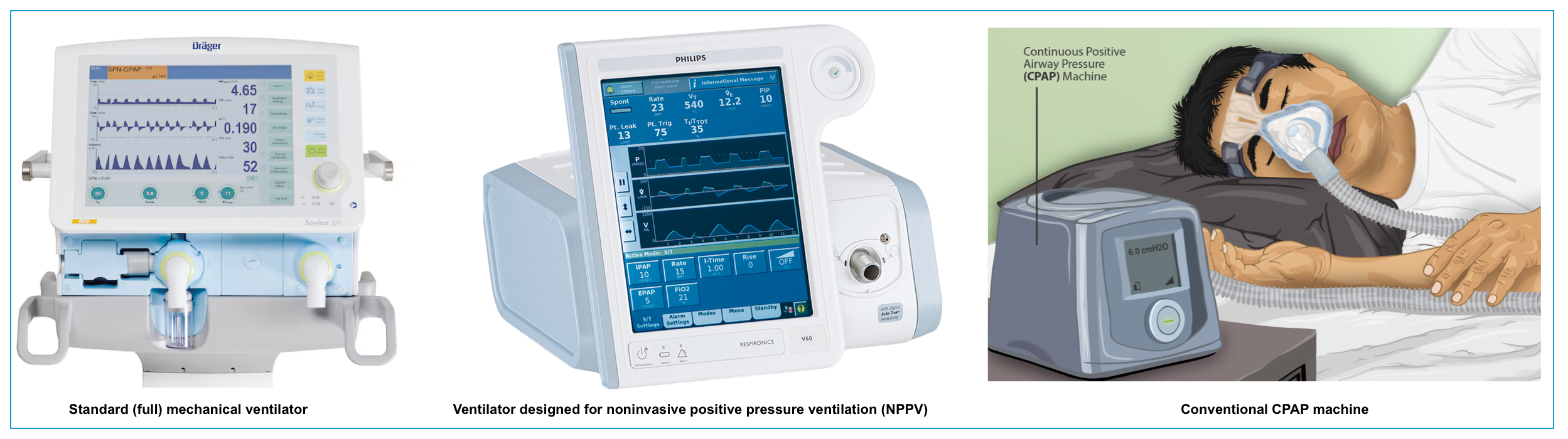
NPPV can be delivered via a standard ventilator or dedicated devices for the NPPV (aka. BiPAP).
Most standard ventilators provide an NPPV mode, but many centers deploy dedicated NPPV devices with characteristics that are distinct from standard invasive mechanical ventilators.
- Standard mechanical ventilator
- These are standard mechanical ventilators, designed for use with intubated patients.
- Depending on the device, they may not compensate well for gas leaks around the mask.
- Ventilator designed for NPPV
- These are smaller portable pressure ventilators with reduced air compressor size.
- Most have a single limb circuit that delivers oxygen to the patient and allows for exhalation.
- To prevent the accumulation of CO2, the circuit is continuously flushed with supplemental oxygen during the expiratory phase with exhaled gas released through an exhalation port near the mask.
- In contrast to traditional ventilators, NPPV devices continuously monitor for air leaks and attempt to compensate for this loss of volume.
- As such, NPPV units are designed to tolerate some degree of air leak and compensate by maintaining airway pressure.
- These devices are designed specifically to optimize both breath triggering and leak compensation.
Interface
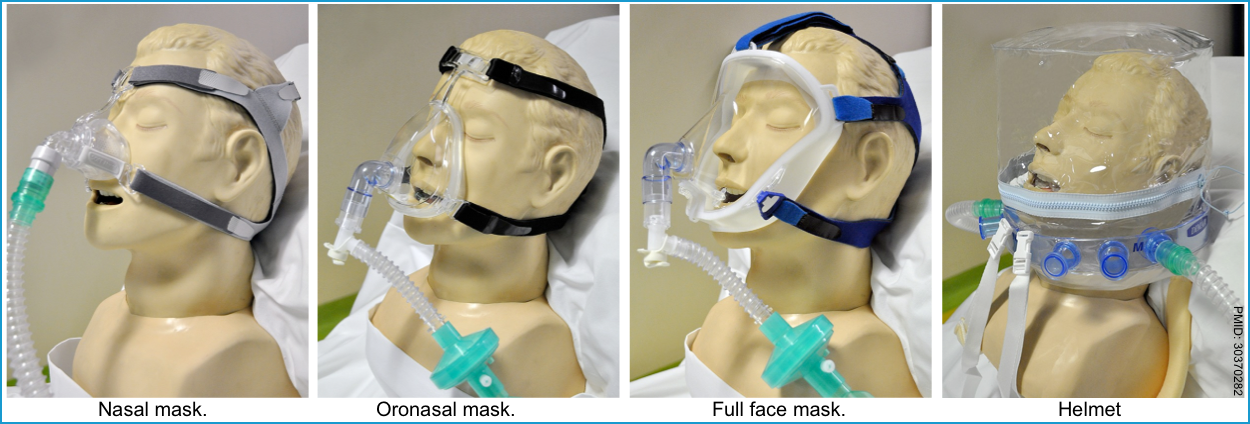
- Various types of interface, including mouthpiece, oral (lip-seal mouthpiece) mask, nasal pillows, nasal mask, oronasal mask, total face mask, and helmet (table below), are available for NIV treatment. The features of commonly used interfaces are summarized in the following table *.
- Oronasal mask (aka. fascial mask) is more commonly used in critical care settings.
- Factors that should be considered when choosing the interface for NIV include *:
- Air leakage. A significant amount of air leakage can disturb NIV treatment due to patient-ventilator asynchrony.
- Risk of skin ulceration. Contact pressure caused by wearing a poorly fitting face mask can lead to discomfort, rashes, and pressure injuries.
- Risk of aspiration, if the patient vomits.
| Advantages | Disadvantages | |
| Total face mask covers mouth, nose, and eyes 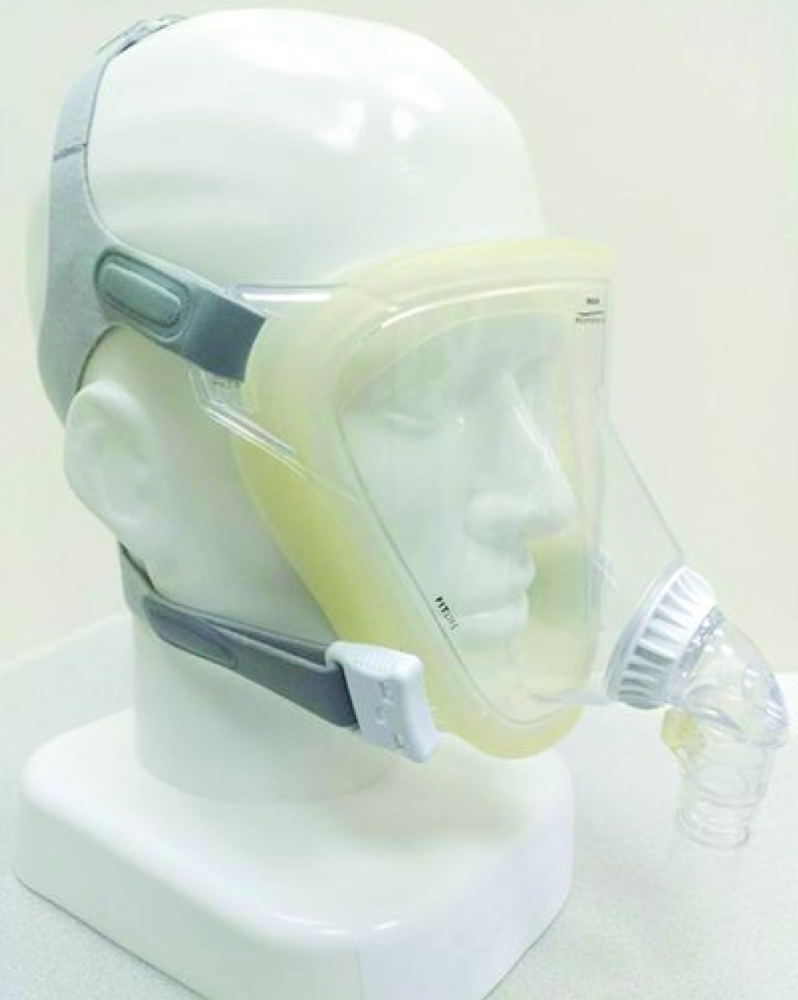 | -Minimum air leaks * -Little cooperation is required. -Easy fitting and application. | -Risk of aspiration, if the patient vomits * -Claustrophobia. -Speaking is difficult. |
| Fascial (or oronasal) mask covers mouth and nose 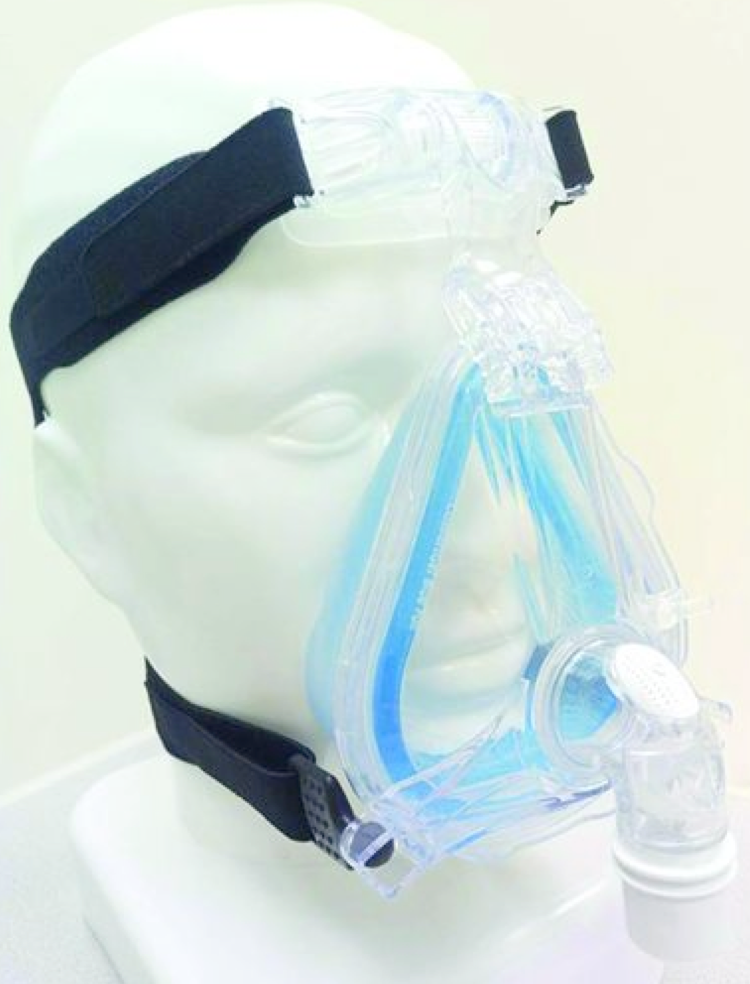 | -Few air leaks. -Little cooperation is required. -Can be adjusted for comfort. | -Risk of aspiration, if the patient vomits. -Possible nasal skin damage -Speaking and coughing difficult |
| Nasal mask covers the nose and not the mouth  | -Eliminate aspiration risk. -Possible nasal skin damage. -Speaking and coughing difficult. | -Air leaks if mouth opens. -Possible nasal skin damage. -Needs patent nasal passages. |
| Helmet covers the whole head and all or part of the neck (no contact with face) 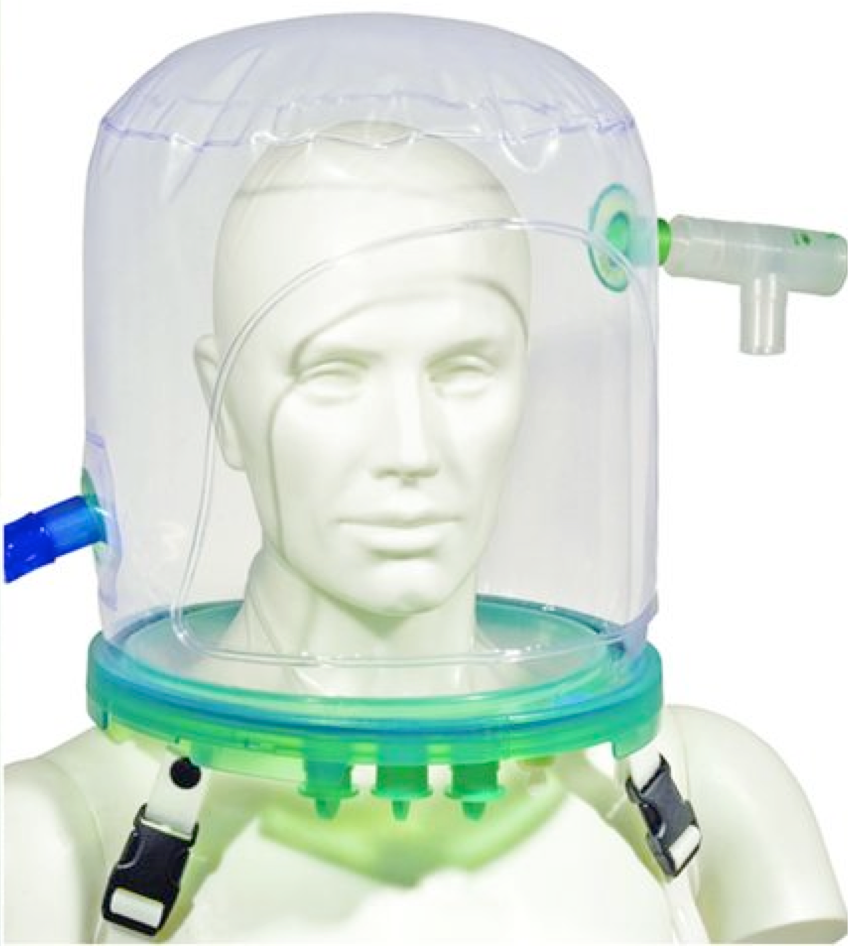 | -↓Risk of skin ulceration & nasal skin damage * -Easier to perceive facial expression & speak with patients. -↓Aspiration risk | -Rebreathing * -Noisy. -Delayed triggering of ventilator may ↓mechanical support of breaths. -Discomfort of axilla (from straps). |
NIV Modes
🟦Continuous positive airway pressure (CPAP)
- Definition
- CPAP refers to the delivery of a continuous level of positive airway pressure throughout the respiratory cycle. No additional pressure above the level of CPAP is provided, and patients must initiate all breaths.
- Unlike BIPAP, continuous positive airway pressure (CPAP) does not deliver ventilation per se because it does not assist inspiration *.
- CPAP is typically more effective at improving oxygenation than ventilation.
- CPAP is functionally similar to positive end-expiratory pressure (PEEP).
- It provides a number of physiologic functions, including increased mean airway pressure, alveolar recruitment to reduce shunt fraction, and attenuation of dynamic airway collapse. It also counteracts the excessive intrinsic PEEP seen in obstructive lung conditions such as COPD.
- Although the spontaneous tidal volume is augmented with CPAP, the tidal volume cannot be titrated as effectively as bilevel positive pressure ventilation; thus it is not the optimal mode for treating disorders that require increased alveolar ventilation (i.e. disorders associated with acute hypercapnia).
- CPAP is functionally similar to positive end-expiratory pressure (PEEP).
- CPAP refers to the delivery of a continuous level of positive airway pressure throughout the respiratory cycle. No additional pressure above the level of CPAP is provided, and patients must initiate all breaths.
- Indications in the acute care setting
- Acute cardiogenic pulmonary edema *.
- Reminder
- In the devices designed for noninvasive ventilation, if ΔPsupport /∆Pinsp is set to zero, the ventilator functions like a conventional CPAP system.
- Setting and titration *
- Start CPAP level at 5 to 8 cm H2O.
- Gradually increase CPAP level by 2 to 3 cm H2O increments every 5 to 10 minutes as tolerated (up to 20 cm H2O) to achieve improvement in dyspnea and reduction in respiratory rate.
- Provide O2 supplementation as needed to keep O2 saturation >90%.
🟦Bilevel positive airway pressure (BiPAP)
- Definition
- BiPAP is the most commonly used mode of NPPV in patients with acute respiratory failure.
- It aims to deliver two levels of positive airway pressure support. The lower level is similar to CPAP; however, it is more commonly called positive end‑expiratory airway pressure (PEEP) as it is present only at the expiratory phase of breathing.
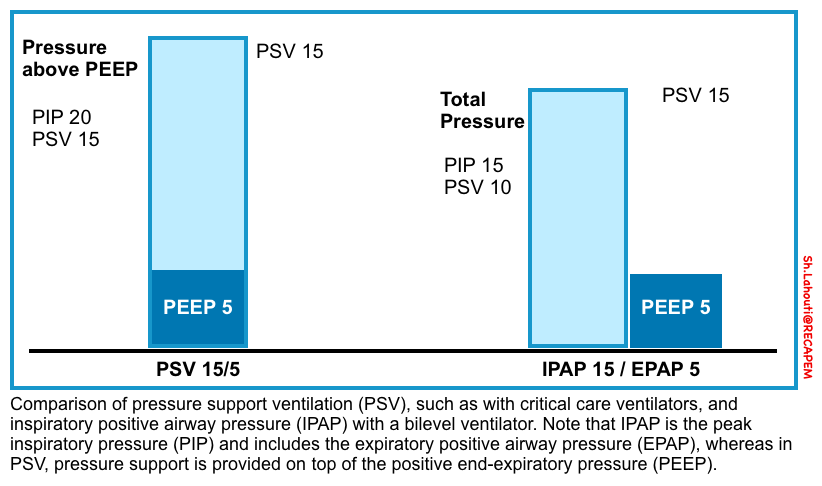
- BiPAP is CPAP with the addition of inspiratory pressure support (PS).
- It provides positive airway pressure at two different levels:
- IPAP: Refers to pressure during the inspiratory phase when the patient triggers a breath. It is always set higher than the EPAP, typically by a minimum of 5 cm H2O.
- EPAP: Refering to a baseline level of positive pressure at all times.
- This will improve oxygenation (recruitment).
- EPAP can counteract the intrinsic PEEP seen in COPD.
- It also reduces preload and afterload (same as CPAP, effective in the treatment of cardiogenic pulmonary edema).
- Delta PAP or driving pressure: This is the difference between IPAP and EPAP.
- This determines and directly correlates with the tidal volume delivered.
- A higher driving pressure will increase the patient’s tidal volume.
- Generally, increasing the driving pressure will improve ventilation (assuming that the respiratory rate is constant).
- This determines and directly correlates with the tidal volume delivered.
-
- Indications in the acute care setting
🟦Pressure‑support mode versus CPAP/BPAP
- Pressure support is generally considered to be the same as CPAP/BIPAP, except that pressure support is delivered by a ventilator and BIPAP through a noninvasive ventilator.
- In noninvasive ventilation, the level of pressure support is applied as pressure above baseline PEEP. However, the approach is different with bilevel ventilators where IPAP (inspiratory positive airway pressure) and EPAP (expiratory positive airway pressure) are set. In this configuration, the difference between IPAP and EPAP is the level of pressure support.
Uncommon modes
◾️Background
- Occasionally, modes may be used which include ventilator-triggered breaths (sometimes referred to as a “backup rate”).
- Ventilator-triggered breaths may be volume-cycled or pressure-cycled (as with an invasive ventilator).
- Not all portable NPPV ventilators have these modes. So, if volume-limited or pressure-supported ventilation is required, switching to a standard ventilator may be necessary.
◾️Indications
- The use of ventilator-triggered breaths in acute respiratory failure is somewhat questionable. If the patient is so sick that they aren’t generating a reliable respiratory rate, they probably ought to be intubated.
- Potential indications to use ventilator-triggered breaths:
- Very sick patients who are unwilling to be intubated (DNI).
- Patients with central sleep apnea, who benefit from chronic use of nocturnal noninvasive ventilation with ventilator-triggered breaths.
- Ventilator-triggered breaths are excellent for supporting respiration during the apneic period of rapid sequence intubation (media below 🎥) *.
Initial setting
◾️Principles
- IPAP
- IPAP is generally set higher than EPAP by at least 5 cm H2O.
- Increase IPAP based on dyspnea, respiratory rate, tidal volume, and patient-ventilator synchrony.
- In general, increasing the IPAP may increase the risk of gas insufflation into the gastrointestinal tract, increasing the risk of aspiration.
- The exact pressure at which aspiration risk increases is unclear, but this probably occurs around 20 cm H2O.
- Consequently, EPAP should generally not exceed 20 cm H2O.
- EPAP
- Increase expiratory positive airway pressure if hypoxemia continues or in cases of morbid obesity.
- A high level of EPAP can impair exhalation and also reduce the driving pressure (as the maximal IPAP is limited to 20 cm H2O).
- Increase expiratory positive airway pressure if hypoxemia continues or in cases of morbid obesity.
- Driving pressure (IPAP – EPAP)
-
Increase driving pressure or delta if the patient remains hypercapnic.
-
Remember that to decrease the partial pressure of CO2, it is necessary to increase the delivered tidal volumes, which is done by increasing the driving pressure.
-
-
- Generally, the recommended initial settings for BiPAP follow as:
- IPAP of 8 cm H2O and EPAP of 3 cm H2O (providing PS {i.e. driving pressure} of 5 cm H2O).
- EPAP and IPAP are titrated to optimize respiratory assistance in 3 to 5 cm H2O steps, allowing a brief trial period at each level *.
- Similarly, tidal volume augmentation is best achieved by serial increases of IPAP in 3 to 5 cm H2O increments, keeping EPAP at the target level.
◾️Setting BiPAP for COPD/asthma
- BiPAP achieves two things for these patients:
- The EPAP balances out AutoPEEP (positive pressure due to gas trapping in the patient’s lungs). This makes it easier for patients to take each breath.
- The driving pressure (IPAP-EPAP) provides support for each new breath.
- Titration
- The best approach is titration at the bedside, depending on the patient’s comfort and tidal volumes.
- Example:
- Start at 10 cm inspiratory pressure / 5 cm expiratory pressure.
- Increase to 15 cm inspiratory pressure / 8 cm expiratory pressure.
- Increase to 18cm inspiratory pressure / 8 cm expiratory pressure.
◾️Setting BiPAP for cardiogenic pulmonary edema
- These patients benefit from a higher EPAP.
- A high EPAP will maintain high intrathoracic pressures throughout the respiratory cycle, which will offload the heart.
- Note that CPAP is also suitable and is equally effective.
- Titration
- Example:
- Start at 10 cm inspiratory pressure / 5 cm expiratory pressure (allowing the patient to get used to the mask).
- Increase to 15 cm inspiratory pressure / 10 cm expiratory pressure.
- Increase to 18 cm inspiratory pressure / 15 cm expiratory pressure.
- Example:
◾️Setting BiPAP for neuromuscular weakness
- These patients benefit from a higher “driving pressure” which provides mechanical support for each breath.
- Titration
- Example:
- Start at 10 cm inspiratory pressure / 5 cm expiratory pressure.
- Increase to 15 cm inspiratory pressure / 5 cm expiratory pressure.
- Increase to 18 cm inspiratory pressure / 5 cm expiratory pressure.
- Example:
Post-procedural care
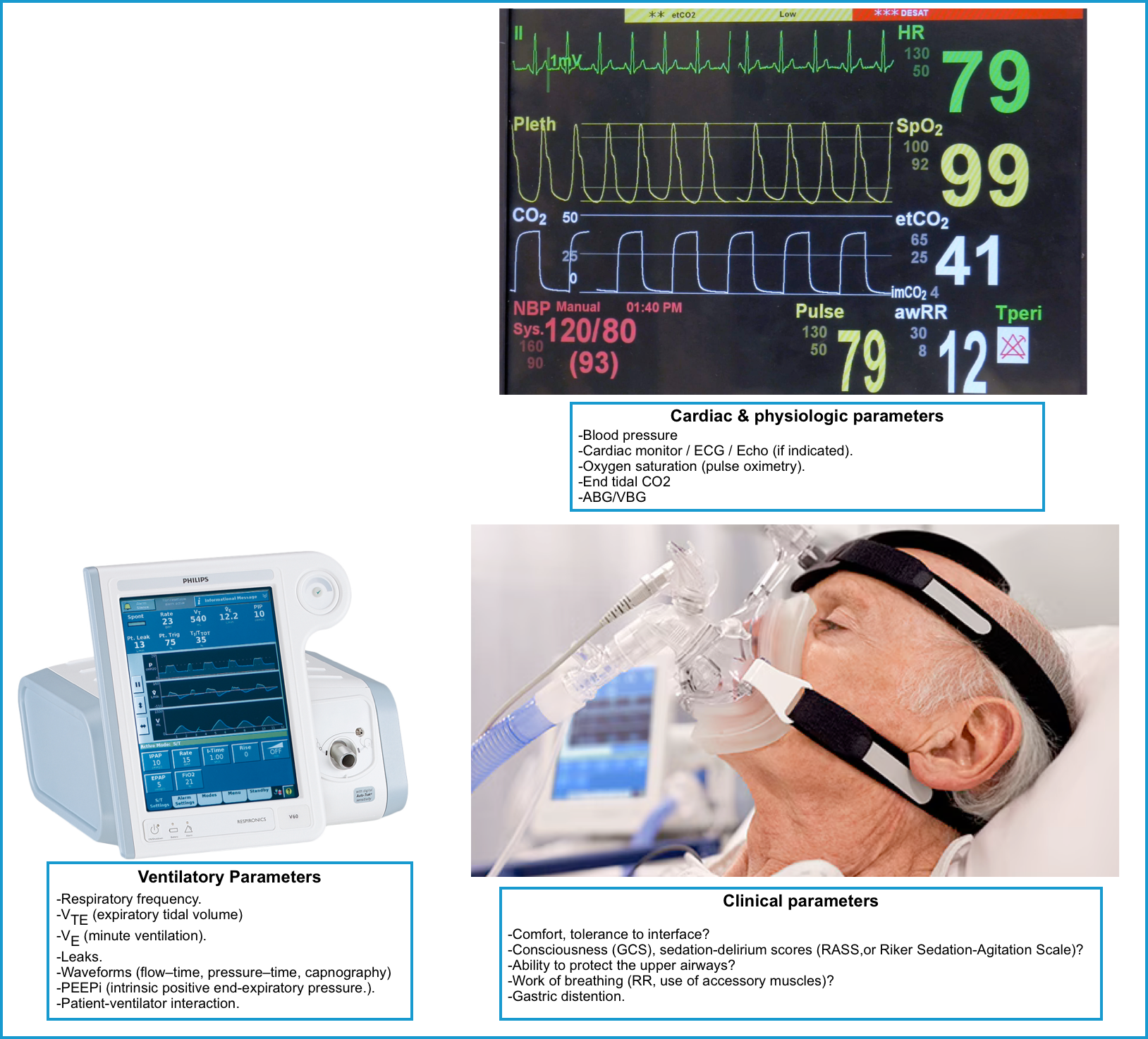
The goal of NIRS is to improve pulmonary gas exchange and alleviate respiratory distress and work of breathing. Patients on NIRS must be monitored closely. Tolerance of and
improvement with NPPV and HFNO is generally recognizable within the first to second hours of application.
- Reassess frequently for tolerance and efficacy of NIV (at least every 30 min) for the first 1 to 2 hours.
◾️Monitor patients for signs of respiratory worsening or failure, including:
- Clinical parameters
- General appearance (including mentation)
- How does the patient look?
- Comfort, tolerance to interface?
- Consciousness-sensorium (GCS 🧮), sedation-delirium scores (RASS 🧮,or Riker Sedation-Agitation Scale)
- As long as the patient is mentating normally, their PaCO2 is not profoundly elevated.
- When in doubt, frequent re-assessment will often clarify the patient’s trajectory.
- Ability to protect the upper airways, and the presence of an effective cough reflex?
- How does the patient look?
- Respiratory evaluation
- Respiratory rate trends are extremely useful.
- Work of breathing (retractions, tri-podding, the ability to speak in sentences, and the patient’s perception of their breathing, use of accessory muscles).
- Look for side effects of NPPV:
- NPPV is generally safe. Most complications are local and related to a tightly fitting mask:
- Skin irritation.
- Nasal bridge pain.
- Mucosal dryness, sinus pain, congestion, or epistaxis.
- Eye irritation.
- Mild gastric distention.
- Complications related to positive pressure ventilation (rarely, e.g. barotrauma, hemodynamic instability).
- Decreased cardiac output
- Anxiety, fear, and claustrophobia *
- NPPV is generally safe. Most complications are local and related to a tightly fitting mask:
- General appearance (including mentation)
- Physiologic parameters
- Oxygenation should be adequate (e.g. >88%).
- If the patient has a reliable pulse oximetry waveform, this is the preferred assessment of oxygenation.
- There are generally no advantages to using serial ABGs to assess oxygenation.
- ABGs should only be used if pulse oximetry is unreliable (e.g. due to a poor waveform or hemoglobinopathy).
- Blood gas evaluation?
- If the other parameters are reassuring (i.e. oxygenation, work of breathing, appearance, mentation), then an ABG/VBG is unnecessary.
- Alternatively, if the patient is deteriorating clinically, then a normal ABG shouldn’t be reassuring.
- The main indication for blood gas analysis is if the patient’s mental status is abnormal.
- However, it’s unclear whether mental status is altered due to hypercapnia or medication (e.g. patients receiving sedation to tolerate the BiPAP).
- VBG (if indicated) is entirely adequate in nearly all cases (unless the VBG oxygen saturation is extremely low).
- If the other parameters are reassuring (i.e. oxygenation, work of breathing, appearance, mentation), then an ABG/VBG is unnecessary.
- Oxygenation should be adequate (e.g. >88%).
- Cardiac parameters
- Blood pressure
- Closely monitor BP around the initiation of NPPV as positive pressure can worsen hemodynamics.
- If worsening hypotension, perform a point-of-care ultrasound and evaluate for pneumothorax.
- ECG.
- Echocardiography (only in selected patients when needed).
- Blood pressure
- Radiological evaluation
- Chest radiography, computed tomography, lung and diaphragm ultrasonography (only in selected patients when needed).
- Ventilatory parameters
- In the presence of a good mask seal, the ventilator provides real-time information regarding tidal volume, minute ventilation, and respiratory rate. Look for:
- Respiratory frequency.
- VTE (expiratory tidal volume)
- VE (minute ventilation).
- Leaks.
-
Feel around the mask with an ungloved hand.
-
Check tidal volumes and/or leakage percentage on a ventilator.
-
- Waveforms (flow–time, pressure–time, capnography)
- PEEPi (intrinsic positive end-expiratory pressure.).
- Patient-ventilator interaction.
- Absolute and trends of values can ensure the appropriate ventilation.
- In the presence of a good mask seal, the ventilator provides real-time information regarding tidal volume, minute ventilation, and respiratory rate. Look for:
◾️Monitoring failure during noninvasive ventilation
- Failure of NIV is usually defined as a need for intubation due to a lack of improvement in clinical parameters, arterial blood gases, or death.
- It is very important to identify patients who are at risk of failing NIV, because an inappropriate delay in intubation may cause an increase in morbidity and mortality
- ⭐️Consider reversible causes of distress (e.g. equipment failure, reversal of medications) before intubation, when clinically appropriate.
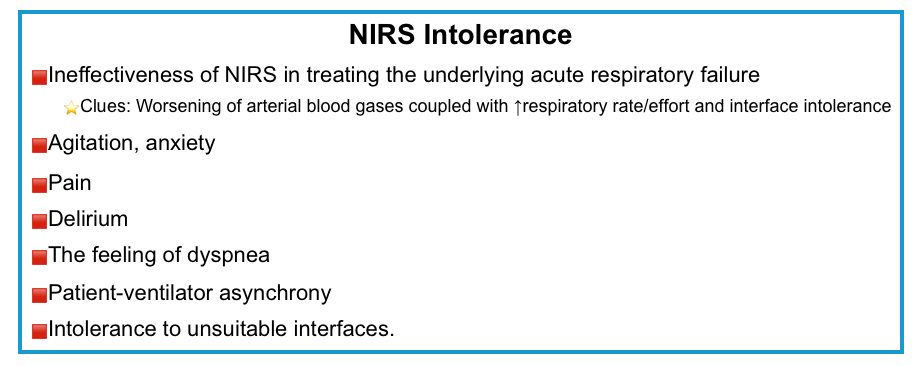
Noninvasive respiratory support intolerance
- NIRS intolerance is multifactorial. Common causes are summarized below.
Patient-ventilator asynchrony (PVA)
Many patients do not initially tolerate NPPV due to patient discomfort or ventilator asynchrony (ie, the phases of ventilator breath do not match that of the patient).
Patient-ventilator asynchrony leads to unnecessary imposed muscle loading and may appear clinically as a patient who is fighting the ventilator (patient discomfort). This may lead to unnecessary administration of sedation, NIV failure, need for invasive mechanical ventilation, and ICU stay *.
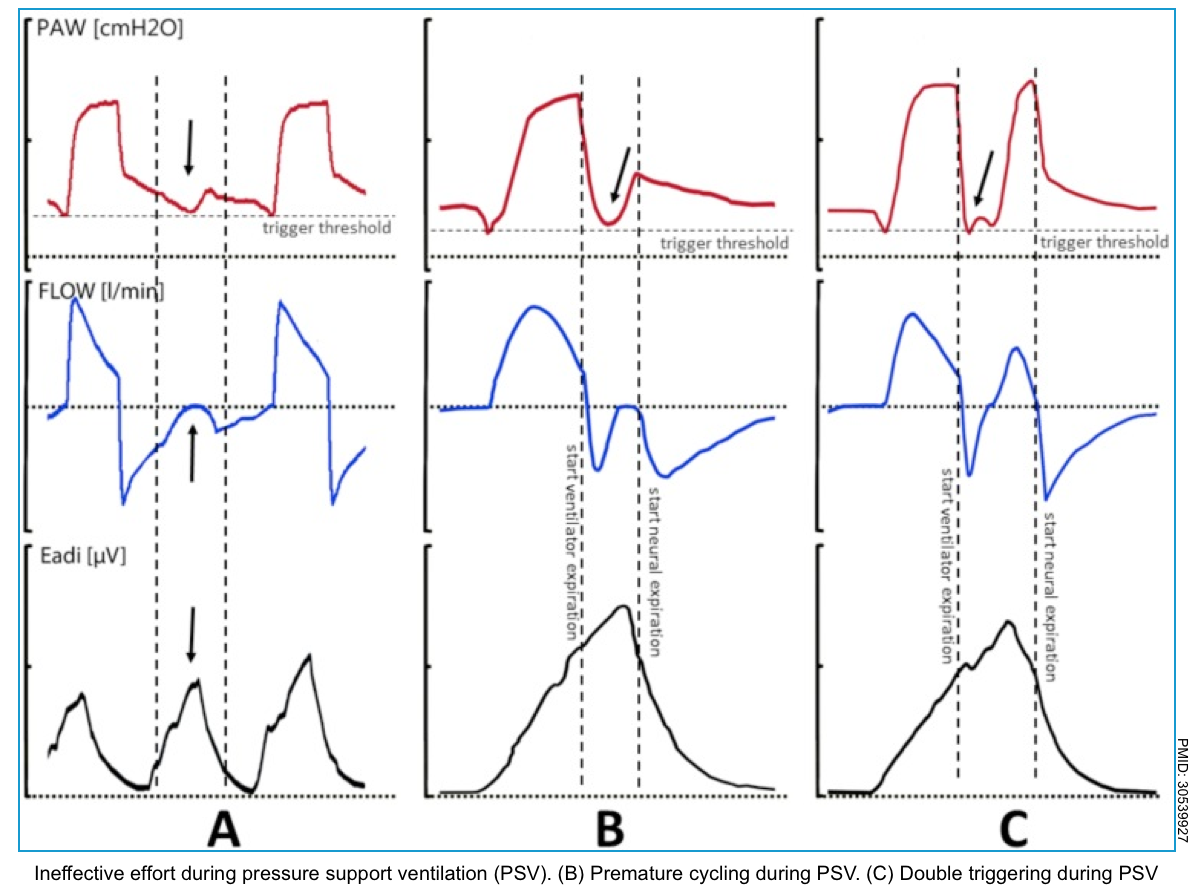
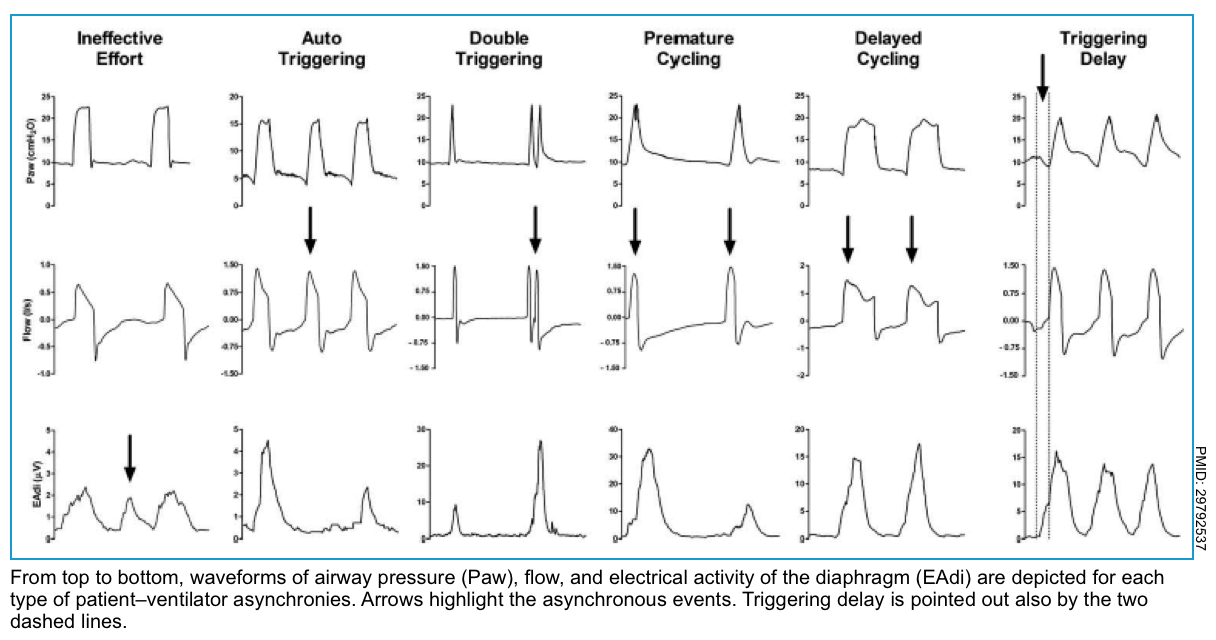
- Ineffective triggering (aka. “missed triggering” or “wasted effort”).
- This is the most frequent asynchrony and it develops when the patient’s effort is not recognized by the ventilator.
- Ventilator graphics show a downward concavity of the flow/time waveform with a simultaneous upward concavity of the pressure/time waveform *.
- Causes *
- Weak inspiratory effort, for example when the inspiratory efforts are reduced by ventilator over-assistance and excessive sedation levels.
- High intrinsic positive end-expiratory pressure (PEEPi).
- Excessively low ventilator trigger sensitivity setting
- Management strategy
- Avoid overassistance. Increase the patient respiratory drive by diminishing the IPAP to decrease the rate of ineffective triggering.
- Reduce tidal volume and minute ventilation mostly in COPD patients to minimize PEEPi, a determinant for ineffective triggering.
- Inspiratory trigger sensitivity should be optimized because a low inspiratory trigger threshold may cause auto-triggering, in contrast, a high inspiratory trigger
threshold may result in ineffective triggering. - Avoid deep sedation and long-lasting sedative drugs, mostly during NIV.
- Correct choosing of the right interface during NIV to avoid air leaks.
- Triggering delay
- The patient manages to activate the trigger after a considerable time.
- In pressure waveform before ventilator support there is a pressure drop caused by the inspiratory effort of the patient.
- Causes: Same as for ineffective triggering.
- Double triggering
- In this type of asynchrony, two consecutive ventilator cycles occur (triggered by the patient) separated by an expiratory time lower than one-half of the mean expiratory time. The patient’s effort is not completed at the end of the first ventilator cycle and triggers a second ventilator cycle.
- The ventilator graphic shows double triggering as one respiratory phase has ended, and the next one starts immediately.
- Hyperinflation may occur as tidal volume (TV) is twice dispensed.
- Causes:
- The patient’s high ventilatory requirements
- Inappropriate inspiratory time (low TV, low pressure support, ventilator set inspiratory time too short).
- It is frequent in patients with severe lung injury and increased respiratory drive.
- Auto-triggering
- It is defined as a cycle delivered by the ventilator without a prior airway pressure decrease, which indicates that the ventilator delivered a breath that was not triggered by the patient.
- Causes
- Airway leaks or strong cardiac oscillations
- Since a drop in airway pressure or a flow signal is used to trigger the ventilator, airway leaks or strong cardiac oscillations can reach the triggering threshold of the ventilator in the absence of the patient’s effort and provoke the insufflation of repeated extra breaths. More than one breath occurs in a single patient effort.
- Airway leaks or strong cardiac oscillations
- Premature cycling
- The ventilator’s set inspiratory time is too short for the patient. The patient’s inspiratory effort can still be happening even though the ventilator’s inspiration is already over.
- Premature cycling produces a decrease in airway pressure, which can be seen immediately after the end of the inspiratory phase
- Delayed cycling
- The ventilator’s set inspiratory time exceeds the patient’s neural inspiratory time. The patient ends inspiration before the ventilator does. It can activate expiratory muscles when the ventilator continues dispensing volume during inspiration.
- Causes
- Inappropriate timing cycling setting
- Air leaks.
- COPD and asthma are risk factors for delayed cycling.
- Delayed cycling is evidenced in the pressure waveform as an increase in airway pressure near the end of the inspiratory phase.
Troubleshooting patient-ventilator asynchrony (PVA)
Different strategies can reduce the incidence of PVA both during invasive and non-invasive ventilation, including *:
- Auto-PEEP (if present) should be treated.
- Depending on the ventilator used, this may involve:
- Reducing respiratory rate and/or tidal volume.
- Raising the inspiratory flow rate.
- Treating the underlying airflow obstruction, when present.
- Application of extrinsic PEEP.
- Depending on the ventilator used, this may involve:
- Reduce an interface leak.
- A mask leak can increase the time required for the ventilator to achieve its pressure target, prolonging inspiration and causing discomfort.
- To reduce the interface leak, the following measures can be tried:
- Tightening the straps.
- Changing the mask.
- Changing the mode of ventilation.
- For example, pressure-regulated volume-controlled ventilation (rather than flow-limited) may help (although only select standard ventilators are typically capable of delivering time-limited ventilatory modes).
- Discomfort and anxiety should be treated.
- Discomfort can be triggered by agitation, anxiety, pain, delirium, the feeling of dyspnea, and intolerance to unsuitable interfaces.
- The following treatment steps can be employed to improve patient comfort and reduce anxiety:
- Careful explanation and reassurance. This can help to alleviate fears and anxieties associated with NIV.
- Switching to an alternate interface may improve comfort and tolerance if the source of discomfort is ill-fitting masks or local discomfort associated with the interface.
- For example, patients who feel a sense of pressure or suffocation with an oronasal mask may do better on a nasal mask or pillows with or without chin straps.
- Lowering the setting or changing the mode (when the above simple adjustments fail).
- Note that, when a “mode change” is planned for comfort or asynchrony, pressure support ventilation (PSV) is preferred.
- PSV allows the patient to trigger each breath and independently regulate the depth and pattern of breathing. It is reasonable to expect that BiPAP provides a similar level of comfort, although this has not been studied.
- Note that, when a “mode change” is planned for comfort or asynchrony, pressure support ventilation (PSV) is preferred.
- Sedation
Sedation
Background
- Poor acceptance of noninvasive respiratory support (NIRS) is multifactorial.
- Discomfort associated with NIRS can be managed with appropriate sedative and analgesic medications, provided that concomitant optimization of the ventilatory setting and limitation of other factors related to respiratory disease severity are not neglected.
- First address any potential easily reversible issues such as mask fit and attempt verbal calming and coaching (discussed above).
- Several studies have shown how administering sedative and analgesic medications during NIV can reduce the risk of tracheal intubation, delirium, and hospital length of stay in different categories of patients with ARF *.
- Data are limited on the best approach to sedation-assisted NIRS.
- The risk-benefit ratio of sedatives and analgesics for patients with acute respiratory failure on NPPV is poorly studied.
- One prospective observational study reported that, compared with the administration of either a sedative or an analgesic, the combined use of both agents was associated with a five-fold increase in NIV failure (due to worsening respiratory failure) *.
- Single-agent therapy was not associated with adverse outcomes. However, methodologic flaws prohibit firm conclusions from this study.
- The risk-benefit ratio of sedatives and analgesics for patients with acute respiratory failure on NPPV is poorly studied.
- There are no national or international guidelines defining what sedative and analgesic medications should be given in patients with hypoxemic or hypercapnic acute respiratory failure treated with NRS when discomfort might jeopardize the efficacy of ventilatory support.
- The following recommendation is based on the recent clinical practice guideline from the Italian Society of Anesthesia, Analgesia, and Intensive Care (SIAARTI)📖.
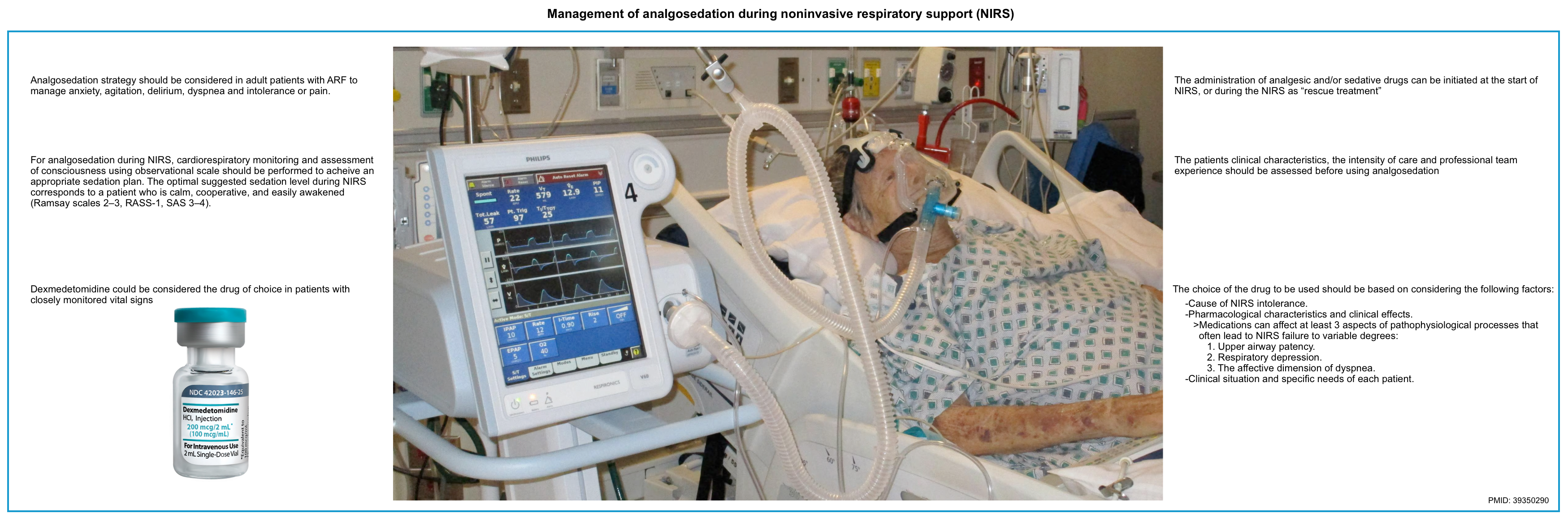
Analgosedation strategy
◾️Which patients could benefit from analgosedation during NIRS treatment?
- During NIRS, the use of an analgosedation strategy should be considered in adult patients with hypoxemic or hypercapnic ARF of various etiologies who need to be managed for anxiety, agitation, delirium, dyspnea, and intolerance or pain.
- Make sure that NIRS intolerance is not related to the condition of respiratory failure (severity or evolution) or inappropriate settings before giving sedative/analgesic medications.
- These strategies can be implemented when there are no signs of deterioration, lack of response to NRS, and contraindications to the used pharmacological agents.
◾️Goal
- The goal of analgosedation in NIRS should include:
- Comfort
- The ideal target would be to have a patient alert, conscious, and responsive to verbal stimuli.
- Reduction of dyspnea (in terms of respiratory drive and rate)
- Maintenance of sleep-wake rhythm
- Hemodynamic stability.
- Comfort
- The doses should be titrated to achieve NIRS tolerance while avoiding adverse events.
◾️Medications
- Factors that should be considered when choosing an analgesic/sedative medication:
- Cause of NIRS intolerance.
- Pharmacological characteristics and clinical effects.
- Medications can affect at least three aspects of pathophysiological processes that often lead to NIRS failure to variable degrees:
- Upper airway patency.
- Respiratory depression.
- The affective dimension of dyspnea.
- Medications can affect at least three aspects of pathophysiological processes that often lead to NIRS failure to variable degrees:
- Clinical situation and specific needs of each patient.
- ⚠️Note that sedation may cause “intubation delay” especially in “de novo” hypoxemic ARF patients.
- First line of treatment
- Dexmedetomidine 💉
- Currently, dexmedetomidine has emerged as the potential best medication for this purpose but is also one of the most widely studied *,*,*.
- In a meta-analysis of 12 studies, the use of dexmedetomidine reduced the risk of intubation (relative risk [RR] 0.54, 95% CI 0.41-0.71; moderate certainty), delirium (RR 0.34, 95% CI 0.22-0.54, moderate certainty), and intensive care unit length of stay (mean difference -2.40 days, 95% CI -3.51 to -1.29 days; low certainty) *.
-
Dose: 0.2-0.5 μg/kg/h IV up to 1.5 μg/kg/h.
-
⚠️Bolus should generally be avoided due to the risks of bradycardia and hypotension *.
-
- Advantages: Titratable agent, doesn’t suppress respiratory drive.
- Disadvantages:
- Gradual onset (a loading bolus is generally avoided, as this may cause marked hemodynamic instability).
- May cause bradycardia and hypotension.
- Limited availability.
- Currently, dexmedetomidine has emerged as the potential best medication for this purpose but is also one of the most widely studied *,*,*.
- Dexmedetomidine 💉
- Other medications (see the following table)
- Opioids. Among commercially available opioids, Remifentanil 💉has several unique pharmacokinetic characteristics and seems to give the most promising results.
- Advantages of ramifentanil
- Easily titratable given its very short half-life, low accumulation, and organ-independent metabolism.
- Disadvantage of opioids
- Difficulty in clinical assessment of the patients.
- If an opioid is given, a reduction in respiratory rate is not necessarily indicative of clinical improvement, so this makes it harder to assess the patient.
- Difficulty in clinical assessment of the patients.
- Opioids are possibly useful in the following situations:
- Asthma or COPD with marked tachypnea (may reduce respiratory rate, allowing for more effective exhalation).
- Pneumonia with marked tachypnea (reduction in respiratory rate may prevent the patient from tiring out).
- Any situation where the patient has severe air hunger that is leading to dyssynchrony or flow starvation on noninvasive ventilation (the BiPAP or CPAP cannot provide a fast enough flow to satisfy the patient).
- ⚠️Cautions
- At high doses, remifentanil may give progressive inhibition of spontaneous respiratory activity up to apnea.
- Some modes of ventilatory monitoring should be employed in patients receiving opioids (e.g. monitoring of tidal volumes and minute ventilation on the BiPAP machine).
- Advantages of ramifentanil
- Ketamine 💉 (dissociate dose, e.g. > 1-2 mg/kg IV bolus)
- Possibly useful for patients who are truly crashing (and thus unable to wait long enough to use another agent). This isn’t a long-term sedation solution, but rather a bridge to another strategy, for example:
- Ketamine dissociation, the patient improves on BiPAP → Re-evaluate, and consider initiation of dexmedetomidine or droperidol as needed.
- Ketamine dissociation, the patient fails to respond to BiPAP → Intubation.
- Possibly useful for patients who are truly crashing (and thus unable to wait long enough to use another agent). This isn’t a long-term sedation solution, but rather a bridge to another strategy, for example:
- Opioids. Among commercially available opioids, Remifentanil 💉has several unique pharmacokinetic characteristics and seems to give the most promising results.
| Analgosedative effect | Level of sedation | Advantages | Disadvantages | Considerations | |
| Dexmedetomidine 💉 | ◾️Sedation > analgesia ◾️Anxiolytic, sedative, and some analgesic effects. | ◾️No respiratory drive suppression ◾️↓Salivary secretion ◾️Diuretic effects ◾️Pulmonary protective effect ◾️Titratable agent | ◾️Bradycardia ◾️Hypotension ◾️Delayed onset of action | ⚠️Avoid loading bolus (risk of hemodynamic instability) | |
| Ketamine 💉 | ◾️Low dose: Pure analgesic ◾️High dose: Sedation > analgesia | Dissociative | ◾️No respiratory drive suppression ◾️Rapid onset of action ◾️Hemodynamically stable. ◾️Bronchodilation. ◾️Antidepressant effects | ◾️Short-term solution (patients will wake up within ~30-60 minutes). ◾️Hypersalivation ◾️Hallucination/agitation (low dose) ◾️Recovery agitation ◾️Laryngospasm ◾️Risk of emesis as patients emerge from sedation. | ◾️Hypersalivation is problematic with a BiPAP mask interface ◾️ketamine is useful for patients who are truly crashing |
| Opioids Remifentanil 💉 | Analgesia > Sedation | Minimal | ◾️No respiratory drive suppression | ◾️Hypoventilation (↓RR) ◾️Hypercapnia | maybe the first choice when the main goal is pain control |
| Benzodiazepines Midazolam 💉 | Pure sedative | Minimal/moderate | ◾️Respiratory depression ◾️Risk of delirium ◾️Risk of UAW collapse | Avoid during NIRS because of unpredictable dose-response curve. | |
| Propofol💉 | Pure sedative | Moderate/deep | Respiratory drive suppression ↓Inspiratory effort | propofol dose-concentration dependently →↑UAW collapsibility |
◾️What is the most appropriate timing to start or end analgesic and/or sedative drugs during NIRS?
- These medications can be initiated at two different times *:
- At the start of treatment to improve patient comfort and prevent the onset of patient intolerance.
- During the NIRS as “rescue treatment” at the onset of intolerance and refusal of NIRS.
- In the case of NIRS intolerance, analgesic and/or sedative drugs may reduce the incidence of tracheal intubation. However, it should only be used as a last resort after having excluded all the other causes of discomfort and after attempting non-pharmacological measures (such as interface replacement, improving ventilator synchrony, noise reduction, and humidification).
- Nevertheless, analgosedation should never delay tracheal intubation, potentially masking patient discomfort due to NIRS ineffectiveness *.
- Duration of treatment
- Analgesic and/or sedative drugs should be continued as long as the reasons for which it was prescribed persist or as long as no related adverse events (respiratory, hemodynamic, or other) occur *.
- Nevertheless, analgosedation should be always titrated using validated scores:
- The most commonly used scores in the ICU are Ramsay Sedation Scale, Richmond Agitation Sedation Scale (RASS 🧮), and Riker Sedation Agitation Scale (SAS).
- The optimal suggested sedation level during NIRS corresponds to a patient who is calm, cooperative, and easily awakened.
- (Ramsay scales 2–3, RASS-1, SAS 3–4) *.
Trial duration and reassessment
◾️Trial duration and predictors of success
- Most initial trials of NPPV should target one to two hours unless the patient acutely deteriorates during that period *.
- However, in practice, a significant amount of clinical judgment is required when assessing the response to NPPV.
- There have been efforts to develop prediction tools for NPPV failure.
- Two scoring systems that can help predict NPPV failure include:
- Ratio of Oxygen Saturation Index🧮
- HACOR Score 🧮 (heart rate, acidosis, consciousness, oxygenation, and respiratory rate score) *.
- A higher score is predictive of NPPV failure with good predictive power after one hour of NIV (area under the receiver operating curve [AUC] 0.88)
- Physiologic parameters and predictive scores may be integrated into management based on the clinical judgment of the providers at the bedside.
◾️Trial success
- A trial is considered successful when both clinical and gas exchange criteria are improved.
- In such cases, it is reasonable to persist with NIV and monitor for another two hours or more to ensure continued success.
- Success predictors:
- Increase in arterial oxygenation.
- Decrease in respiratory rate.
- Decrease in dyspnea.
- Significant rise in PaO2/FiO2 (Partial pressure of oxygen/Fraction of inspired oxygen).
- Continued improvement should prompt weaning while deterioration at any point should prompt intubation and mechanical ventilation.
◾️Trial failure
- If a patient does not improve or deteriorate following a one to two-hour trial of NPPV, the patient should be considered to have failed NPPV and be promptly intubated.
- Clinical judgment is imperative as no explicit physiologic thresholds are indicative of the need for intubation and invasive mechanical ventilation *.
- Clinical criteria suggesting failure include:
- Hemodynamic instability
- Worsening encephalopathy or agitation
- Worsening gas exchange
- Increasing respiratory rate (e.g. persistent tachypnea >30 bpm)
- Inability to clear secretions
- Inability to tolerate any of the interfaces
◾️Weaning
- A patient is deemed ready to wean when gas exchange and clinical parameters of acute respiratory failure have improved dramatically and the cause of respiratory failure
has improved. - Generally, there is no universal protocol for weaning. Therefore, weaning should be individualized and dependent upon the patient’s response to decreased support.
- Physiologic parameter: While there are no universal physiologic parameters that support readiness for weaning from NIV, the following parameters can reasonably suggest weaning readiness:
- A respiratory rate ≥12 and ≤22 bpm.
- Peripheral oxygen saturation (SpO2 ) ≥90% on ≤60% FiO2 or predicted needs can be met with oxygen delivered via high-flow nasal cannulae (HFNC) or low-flow oxygen.
- Hemodynamic stability (preferably off or on low-dose vasopressors and heart rate ≥50 and ≤120 bpm).
- The pH is preferably >7.25 and the patient should ideally be afebrile, awake and alert, or easily arousable.
- Minimal NPPV settings (e.g. BiPAP of 10/5 cm H2O or CPAP of ≤10 cm H2O).
- Procedure of weaning
- Weaning NPPV and HFNC support is performed in a similar stepwise approach to the initiation and titration of the therapy.
- Weaning from NPPV may be accomplished by progressively decreasing the amount of positive airway pressure, permitting the patient to be disconnected from the NPPV for progressively longer durations, or a combination of both.
-
Flow generator
-
3 types: air-oxygen blender (most common), built-in flow generator, and air entrainment system.
-
-
Active humidifier
-
Non-humidified gas can have adverse effects such as mucociliary dysfunction, epithelial damage, mucus plugging, and ulceration of mucosa.
-
-
Heated circuit
-
Heat in the circuit helps prevent condensation and promotes maximum delivery of medical gas to the patient.
-
-
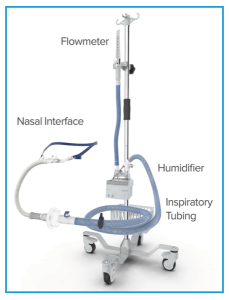
Nasal cannula
- Oxygen delivered through high-flow nasal cannulae (HFNC) has been successfully used in several settings, including *:
- Patients with severe nonhypercapnic hypoxemic respiratory failure from parenchymal lung disease (e.g. pneumonia or interstitial lung disease) *.
- Optimizing oxygenation and ventilation during tracheal intubation *
- Oxygenation and ventilation following extubation *
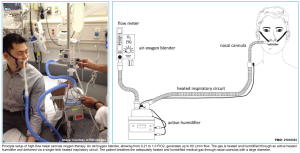
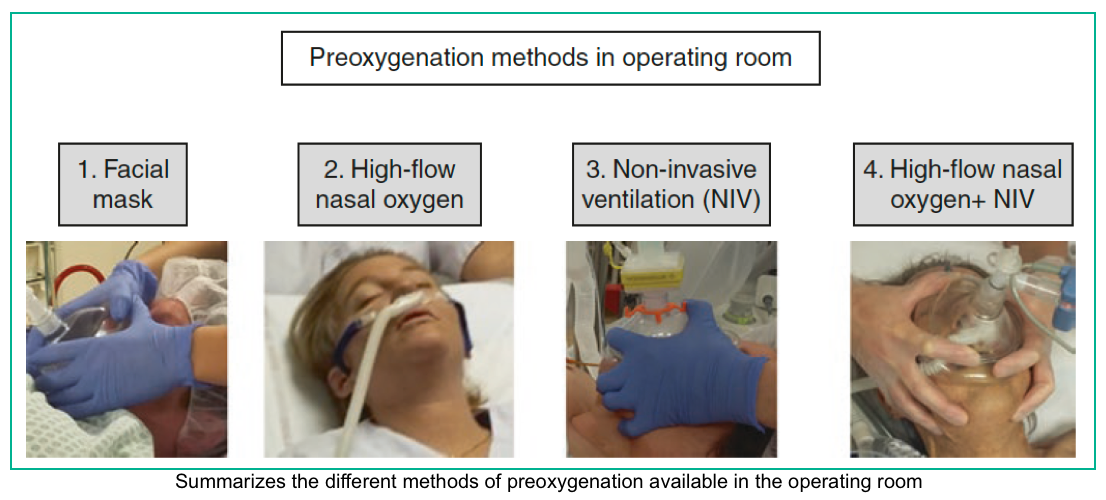
- Pre-and Apnoeic high flow oxygenation for rapid sequence intubation (different methods of preoxygenation shown in the right figure below) *,*,*.
- HFNC is generally well tolerated.
- There are no significant contraindications (other than obvious ones, such as bilateral nasal packing). This allows HFNC to be used in situations where BiPAP may be contraindicated (e.g. patients at high risk of emesis).
- No real risks (aside from potentially delaying intubation).
- Can be continued for a prolonged time (unlike BiPAP, which eventually causes nasal ulceration).
- Allows patients to eat.
- Allows unimpaired ability to communicate (facilitating patient assessment).
- Provides powerful support of oxygenation (with up to 100% FiO2 and some PEEP) *.
- Provides some support of ventilation (due to washout of dead space).
Set up
The combination of flow and FIO2 supports oxygenation, while the flow itself clears dead space and reduces the work of breathing needed for strict ventilatory problems.
◾️Flow rate
- Physiologic benefit of higher flow rate:
- Greater dead space washout which may translate into a reduction in the work of breathing.
- Increased PEEP (but this is a fairly minimal effect).
- The usual range of flow rate is ~ 20-60 L/min.
- Titrating the flow rate *:
- Flow rate is titrated predominantly against the patient’s work of breathing (e.g. respiratory rate).
- For patients with refractory hypoxemia, increasing the flow could theoretically increase the oxygenation a little bit (due to PEEP).
- Initiate flow at 20 L/min and quickly titrate up to flows of 30 to 60 L/min as required to achieve the desired physiologic effect and patient tolerance.
- For patients who are in acute respiratory failure and doing poorly, it may be helpful to increase the flow rate as high as the patient will tolerate (e.g. 60 L/minute) *.
◾️FiO2
- May be adjusted between 21%-100%.
- This is titrated against oxygen saturation *.
◾️Weaning
- If the patient can be weaned down to 20 L/min flow at 50% FiO2, then they may be ready to tolerate a nasal cannula at 6 L/min.
- When in doubt, empirically employ a trial of the low-flow nasal cannula.
- Note that a standard nasal cannula at 6 L/min can provide ~ 40-50% FiO2, so patients may be ready to transition to a low-flow cannula earlier than may be obvious.
RECAP
- The essential goal of noninvasive respiratory support (NIRS) is to support the patient long enough for other medical therapies to work while maintaining oxygenation and preventing respiratory muscle fatigue by boosting ventilation and oxygenation.
- To be effective, NIRS must be applied early in the course of respiratory failure (before the patient has developed severe diaphragmatic fatigue).
- For employing NIRS, patient selection must consider the overall condition of the patient, tolerance of the interface, and the anticipated reversibility of the underlying insult.
- To decide whether to intubate or to use NIRS, use clinical judgment rather than relying on any physiologic numbers. Clinical context matters!
- Patients with acute cardiogenic pulmonary edema or COPD may look quite ill, yet do well without intubation.
- Patients with reduced consciousness due to hypercapnic encephalopathy may do well on NIRS, while severely impaired consciousness is considered a contraindication for BiPAP.
- Do not use BiPAP blindly in any patient with hypercarbia. The underlying diagnosis is more important than blood gas values in determining the line of treatment.
- The goal of NIRS is not necessarily to improve the blood gas values, it is to reduce the work of breathing and improve patient comfort (thereby avoiding respiratory exhaustion). Focus on the patient, not the blood gas numbers!
- Be prepared for prompt intubation (i.e. difficult airway assessment completed along with drugs, equipment, and plan established) in case NIRS fails.
- NIRS response is typically recognized within 1-2 hours of initiation. Early intubation should be considered for patients who fail to improve quickly to avoid complicated emergency intubation amid deterioration on NIRS.
Media
- Wash out anatomic dead space with high-flow oxygen
-
In this short video watch the clearance of radioactive tracer, as flow increases, from an upper airway model. This is visualized using a gamma camera superimposed on a CT scan.
-
References
Robert F. Reardon, et al. Principles of Peri-intubation Oxygenation, the WALLS manual of emergency airway management sixth edition, Calvin A. Brown III, MD. Wolters Kluwer 2023, PP 71-84.






Add comment