1 June, 2023 by Shahriar Lahouti.
CONTENTS
- Preface
- Physiology of pleural fluid formation
- Pathophysiology of pleural effusion
- Clinical presentation
- Diagnosis
- Differential diagnosis
- Diagnostic considerations
- General approach to management
- Treatment
- Specific clinical conditions
- Appendix
- Media
- References
Preface
The prevalence of pleural effusion is high among critically ill patients with rates of up to 50%-60% in some studies. It usually causes a diagnostic dilemma with a long list of differential diagnoses. Moreover, pleural effusions can cause respiratory or hemodynamic compromise in certain groups of patients. This warrants immediate pleural drainage even before the directed treatment of underlying cause has been implemented. This post emphasizes the importance of pleural effusion diagnosis and management in critically ill patients.
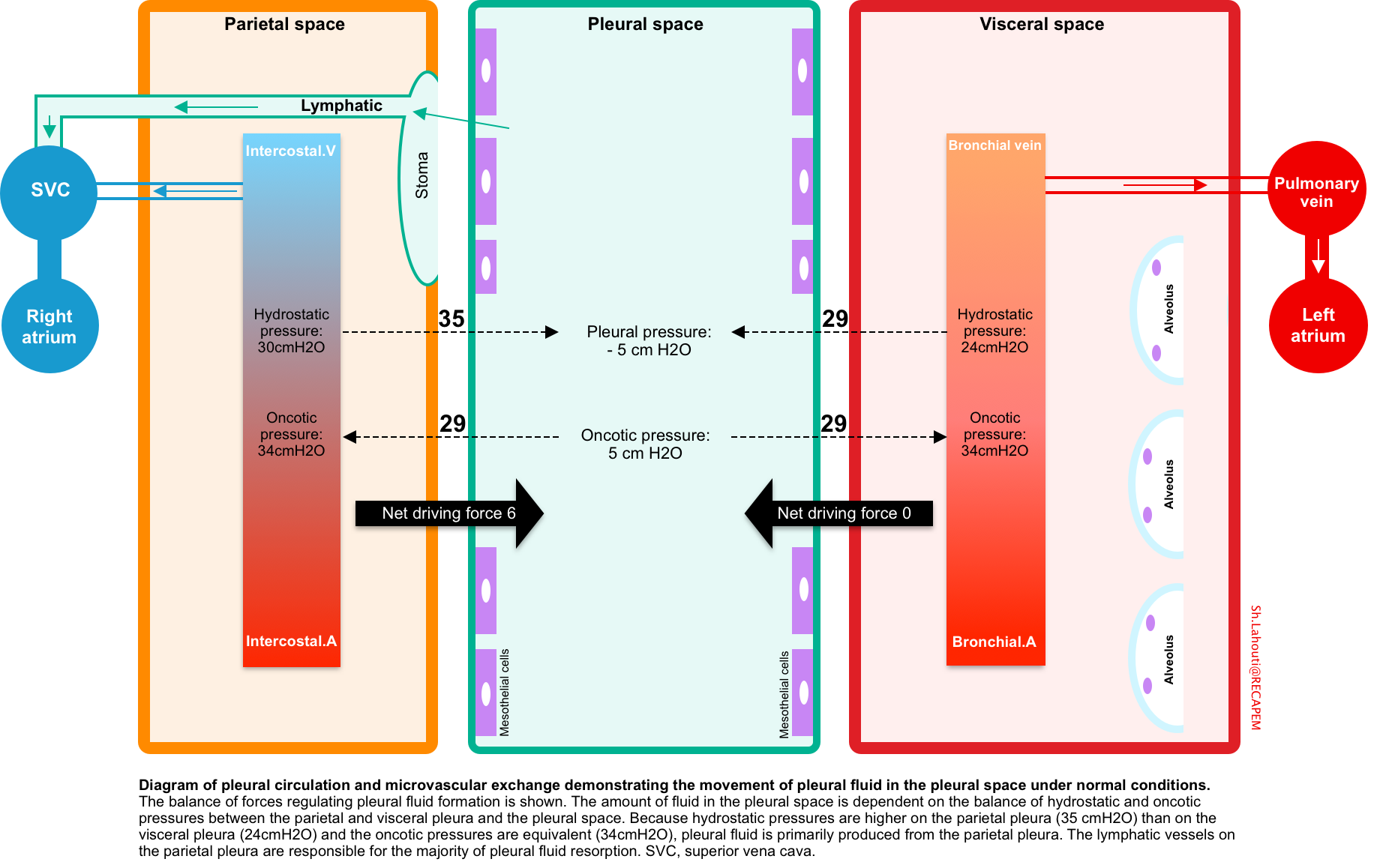
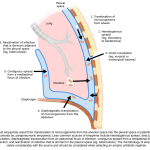
Physiology of pleural fluid formation
Normally a small amount of fluid (~ 0.01 ml/kg/h) constantly enters the pleural space, all of which is removed by lymphatics of parietal pleura. Parietal pleura has the capacity to remove up to 0.2ml/kg/h of pleural fluid *.
Pleural fluid is produced and absorbed primarily on the parietal surface and is dependent on the balance of hydrostatic and oncotic pressure differences between the systemic and bronchial circulations and the pleural space (figure below).
- Hydrostatic pressure
- Both parietal and visceral pleura are supplied by systemic arteries, however the hydrostatic pressure is relatively higher in parietal pleura. This accounts for formation of pleural fluid from parietal surface.
- Note that parietal pleura is fed by intercostal arteries which have a higher pressure relative to bronchial arteries which supply the visceral pleura.
- Oncotic pressure
- The protein level in the systemic capillary beds of pleural surface as well as pulmonary interstitium is ~ 4.5 g/dL.
- The parietal capillary beds are relatively impermeable to proteins, so the fluid that diffuses to the pleural space has a lower total protein (~1.5 g/dL contributing to osmotic pressure of ~ 5cmH2O in the pleural space) *, *.
- In physiologic state, the oncotic pressure gradient is ~ 29 cmH2O across both parietal and visceral surface.
- Net driving pressure
- The net driving pressure (hydrostatic-oncotic pressure) across the parietal is 6 cmH2O, while it is zero over the visceral pleura.
- This positive driving pressure across the parietal pleura is thought to drive the interstitial fluid into the pleural space and hence it has a lower protein content than serum.
- Absorption of fluid
- Only parietal pleura has lymphatic vessels. These are responsible for pleural fluid resorption, and the flow rate of these vessels can increase by a factor of ~20 in response to increases in pleural liquid formation.
- Pleural lymphatic vessels spill to superior vena cava.
Pathophysiology of pleural effusions
Accumulation of excess fluid can occur if there is excessive production or decreased absorption or both, overwhelming the normal homeostatic mechanism *.
- Increased pleural fluid formation
- Increased interstitial fluid of the lung
- This is the most common cause of pleural effusions.
- Whenever the interstitial lung edema exceeds to > 5g/gram of dry lung weight, it will spill into pleural space. Pleural fluid accumulates irrespective of protein level of edema. Most common causes include:
- Heart failure (LV failure, biventricular failure)
- Parapneumonic effusions
- Pulmonary embolism (1)
- Non-cardiogenic pulmonary edema (ARDS)
- Lung transplantation
- Increased hydrostatic pressure gradient
- If the hydrostatic pressure gradient (intravascular pressure – pleural space pressure) is increased, pleural fluid formation will be increased. This can happen through the following mechanisms:
- Increased systemic hydrostatic pressure
- Heart failure (RV or LV failure)
- Superior vena cava syndrome (SVC syndrome)
- Pericardial effusion (2)
- Increased negative pressure in the pleural space
- Atelectasis e.g. secondary to bronchial obstruction
- Trapped lung
- Increased systemic hydrostatic pressure
- If the hydrostatic pressure gradient (intravascular pressure – pleural space pressure) is increased, pleural fluid formation will be increased. This can happen through the following mechanisms:
- Increased pleural capillary permeability
- Inflammation of pleural surface e.g. tuberculosis
- Pleural metastasis
- Pulmonary embolism (1)
- Pericardial disease (2)
- Passage of fluid from other cavities
- Hepatic hydrothorax
- Pericardial disease (2)
- Decreased oncotic pressure gradient
- Hypoalbuminemia (typical serum albumin, <1.5 mg/dl) *
- Rupture/compression of thoracic duct
- Chylothorax
- Vascular rupture
- Hemothorax
- Increased interstitial fluid of the lung
- Decreased pleural fluid absorption
- Obstruction of the lymphatics draining the parietal pleura
- Mediastinal masses
- Elevated systemic venous pressure
- Superior vena cava syndrome
- Isolated right ventricular failure (RVF) e.g. pulmonary arterial hypertension
- Increased systemic venous pressure in RVF →↑Pleural fluid formation from parietal pleural capillaries and ↓lymphatic clearance →Pleural effusions
- Obstruction of the lymphatics draining the parietal pleura
Different pathophysiologic mechanisms may contribute to formation of pleural effusions in a single disease entity.
- (1) In patients with pulmonary embolism (PE), pleural effusion can form through the following mechanism:
- Increased capillaries permeability in PE: This is mediated through release of inflammatory mediators from the platelets-rich-thrombi.
- PE causes increased pulmonary arterial pressure (PAP) and consequently leaads to an increased right atrial pressure (RAP). The elevated RAP causes systemic venous congestion leading to decreased parietal venous and lyphatic drainage and formation of transudative pleural effusion.
- (2) Pleural effusions in patients with pericardial disease can happen by the following mechanisms:
- Increased systemic and pulmonary venous pressure caused by pericardial disease can lead to elevated systemic hydrostatic pressure and contribute to transudative pleural effusion.
- Inflammation of pericardium causes inflammation of the pleura covering the pericardium. This leads to exudative pleural effusion.
- Direct passage of fluid from pericardial space to pleural space (most pleural effusion in the context of pericardial effusion are left sided)
Clinical presentation
A pleural effusion should be considered a manifestation of another disease and not a unique diagnostic entity. It is often difficult to determine whether the patient’s symptoms are secondary to the pleural effusion or to the underlying disease process.
Signs and symptoms
- The most relevant manifestation of pleural effusion may include
- Respiratory: Clinical features of respiratory distress (e.g. dyspnea, tachypnea, hypoxemia).
- Hemodynamic compromise (hypotension, tachycardia, signs and symptoms of end-organ hypoperfusion)
- Pleuritic chest pain is often caused by inflammation of the parietal pleura and is most likely associated with an exudative pleural effusion.
Clinical presentation of pleural effusion presents depends on several factors such as size of the effusion, underlying respiratory reserve, comorbidities and rate of fluid accumulation.
- Size of effusion
- Size of the pleural effusion can be roughly estimated based on ultrasound and/or CT scan.
- Massive PLEF: involving the entire hemithorax
- Large: PLEF occupying >50% hemithorax (volume > ~1L)
- Moderate: PLEF of 25-50% of hemothorax
- Small: PLEF of < 25% of hemithorax.
- Generally, larger effusions are more likely to cause respiratory distress * as well as hemodynamics compromise *.
- Size of the pleural effusion can be roughly estimated based on ultrasound and/or CT scan.
- Respiratory reserve
- Patients with underlying poor respiratory reserve, e.g. interstitial lung disease or those with multiple comorbidities may rapidly deteriorate with lesser amount of effusion.
Diagnosis
Detection of pleural effusions and the creation of an initial differential diagnosis are highly dependent upon imaging of the pleural space.
- It is more sensitive than CXR in diagnosing pleural effusions (92%-94%) and can detect as little as 5-20 mL of fluid.
- Technique: see media below.
- Pleural ultrasound can also give better insight into the volume and character of an effusion (explained below).
- Volume of effusion
- The simplest way to estimate pleural effusion volume is to use the formula by Balik et al.
- Patient supine with mild (15°) trunk elevation, transducer perpendicular to the dorsolateral chest wall, measurements taken at end-expiration
- Measurement: measures the maximal effusion diameter (in millimeters) between the diaphragm and base of the lungVolume of PLEF
- Pleural effusion volume (mL) = (measured distance in mm) x 20
- The simplest way to estimate pleural effusion volume is to use the formula by Balik et al.
- Volume of effusion
- CT scanning is excellent at detecting small amounts of fluid and is also often able to identify the underlying intrathoracic causes.
- CT is not able to differentiate between a transudative or exudative pleural effusion with similar fluid densities and non-differentiating rates of loculation and pleural thickening. However, CT can help distinguish between pleural effusion and empyema.
- Precise volume of the effusion can be calculated using proper volumetry (Pleural effusion volume calculator (CT).
Differential diagnosis
Oftentimes, the associated clinical presentations, historical features and POCUS findings can aid in discerning the underlying etiology. Diagnostic thoracentesis can be helpful (if indicated) to diagnose only certain causes of pleural effusions (more on this below).
- Sick contact: Pneumonia
- Asbestos exposure: Mesothelioma, benign asbestos pleural effusion
- Alcohol abuse or pancreatic disease: Pancreatic effusions
- Abdominal surgical procedures: Postoperative pleural effusion, subphrenic abscess, pulmonary embolism
- Smoking, Cancer: Malignancy
- Risk factors for venous thromboembolism: Pulmonary embolism
- Risk factors for aortic dissection: Acute aortic syndromes
- Cardiac surgery or myocardial injury: Pleural effusion secondary to coronary artery bypass graft surgery or Dressler’s syndrome
- Chronic hemodialysis: Heart failure, uremic pleuritis
- Cirrhosis: Hepatic hydrothorax, spontaneous bacterial empyema
- Esophageal dilatation or endoscopy: Pleural effusion secondary to esophageal perforation
- Medication use: Medication-induced pleural disease.
- Nitrofurantoin, dantrolene, methysergide, dasatinib, amiodarone, Interleukin-2, procarbazine, methotrexate, clozapine, phenytoin, β-blockers, ergot drugs.
- Remote inflammatory pleural process: Trapped lung
- Trauma: Hemothorax, chylothorax, duropleural fistula
- Systemic lupus erythematosus: Lupus pleuritis, pneumonia, pulmonary embolism
Associated clinical signs and symptoms
- Fever: Pneumonia, empyema, tuberculosis
- Hemoptysis: Lung cancer, pulmonary embolism, tuberculosis
- Weight loss: Malignancy, tuberculosis, anaerobic bacterial/fungal pneumonia.
- Sudden onset of pleuretic chest pain: Pulmonary embolism
- Sudden onset of severe stabbing chest pain with maximal intensity at onset: Aortic dissection
- Ascites: Hepatic hydrothorax, ovarian cancer, Meigs’ syndrome
- Dyspnea on exertion, orthopnea, peripheral edema: Heart failure, constrictive pericarditis.
- Unilateral lower extremity swelling: Pulmonary embolism
- Background
- The evaluation of underlying disease relies upon a careful history and examination but will frequently necessitate imaging and pleural fluid analysis in order to narrow the differential diagnosis *.
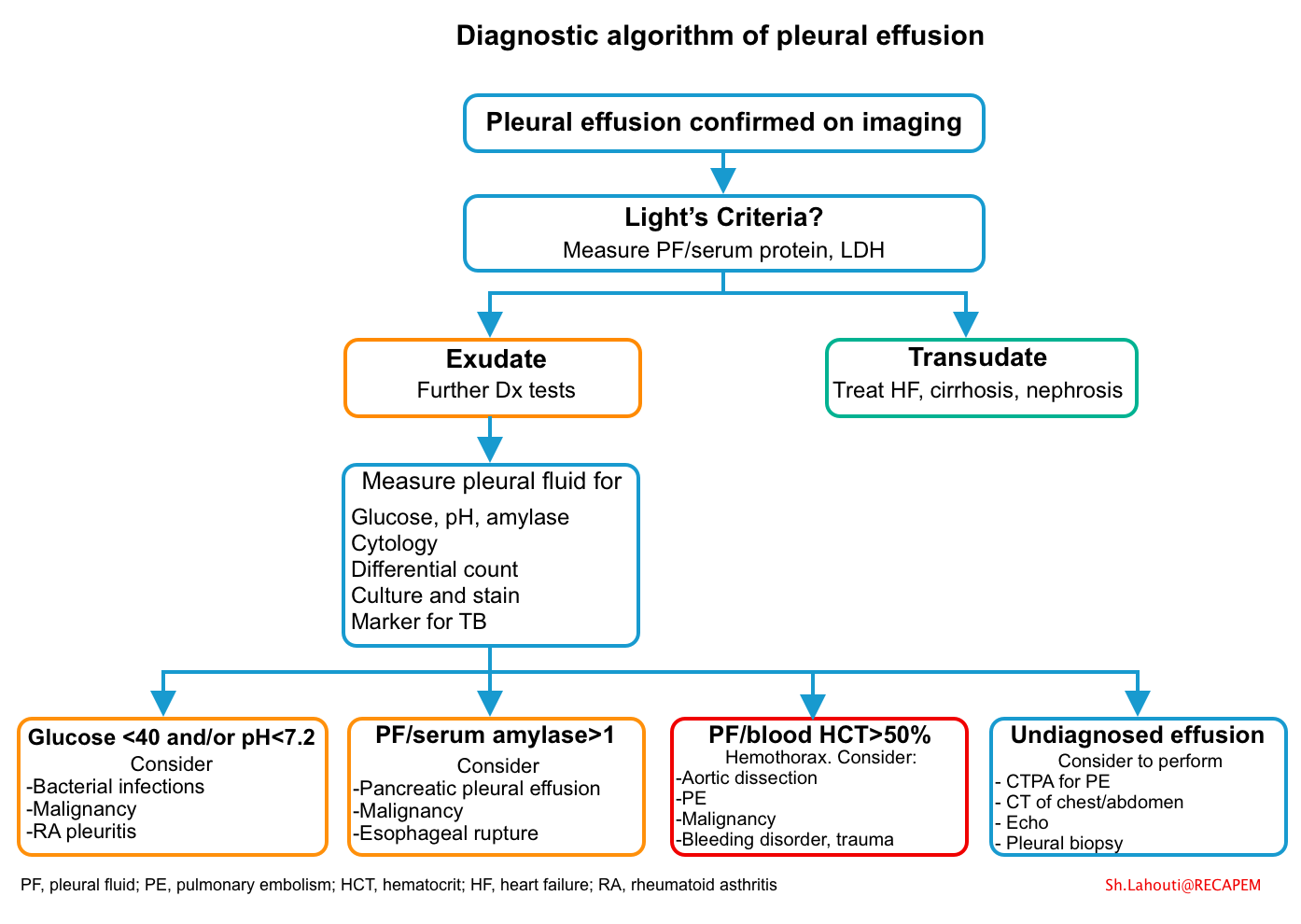
- Traditionally, effusions are divided into transudative and exudative based on Light’s criteria (table below).
- Transudates
- They usually result from imbalances in hydrostatic and oncotic pressures in the chest.
- Transudates have a limited number of diagnostic possibilities that can usually be discerned from the patient’s clinical presentation.
- Exudates
- Increased mesothelial and capillary permeability or impaired lymphatic drainage usually causes exudates.
- Exudates can also result from movement of fluid from the peritoneal space, as seen with acute or chronic pancreatitis, chylous ascites, and peritoneal carcinomatosis.
- Transudates
- Pleural fluid assay
- Standard pleural fluid assays: Differential cell count, protein, glucose, LDH *.
- Pleural fluid protein and LDH are used for Light’s criteria.
- Pleural fluid glucose (and/or pH plus LDH) is helpful to identify complicated infectious pleural effusion (see below).
- Cell count: pleural fluid differential cell counts is virtually never diagnostic but sometimes may provide useful information in some settings:
- Counts >50,000/microL are usually found only in complicated parapneumonic effusions, including empyema.
- Prapneumonic effusions due to bacterial pneumonia, acute pancreatitis, and lupus pleuritis usually have total nucleated cell counts >10,000/microL.
- Chronic exudates, e.g. TB pleurisy and malignancy, typically have nucleated cell counts < 5000/microL.
- Generally, the timing of thoracentesis in relation to the acute pleural injury determines the predominant cell type.
- The early cellular response to pleural injury is neutrophilic.
- As the time from the acute insult lengthens, if the pleural injury is not ongoing, the effusion develops a lymphocyte predominance
- Advanced analysis: Amylase, triglycerides, cholesterol, pH, microscopy for chylomicrons, adenosine deaminase, creatinine, NT-proBNP, gram and acid-fast bacillus (AFB) stain, bacterial and AFB culture, and cytology.
- Standard pleural fluid assays: Differential cell count, protein, glucose, LDH *.
- Diagnostic criteria
- Light’s criteria
- It can be used to determine whether the fluid is transudative or exudative.
- According to the traditional Light’s Criteria Rule, if at least one of the following three criteria (i.e component tests of the rule) is fulfilled, the fluid is defined as an exudate (calculator)
- Pleural fluid protein/serum protein ratio >0.5, or
- Pleural fluid LDH/serum LDH ratio > 0.6, or
- Pleural fluid LDH >2/3 upper limit of the normal serum LDH *
- The Light’s criteria is 98% sensitive and 83% specific for diagnosis of exudate effusion.
- Because of lower specificity, some transudative effusion is mislabeled as exudative effusion.
- If Light’s criteria is suggestive for exudative effusion but clinical picture is more suggestive for transudative effusion, apply following tests which are more specific for diagnosis of exudate pleural effusions
- Serum to pleural fluid albumin gradient (the difference between the serum and pleural values) < 1.2 g/dL
- Serum to pleural fluid protein gradient (the difference between the serum and pleural values) < 3.1 g/dL
- If Light’s criteria is suggestive for exudative effusion but clinical picture is more suggestive for transudative effusion, apply following tests which are more specific for diagnosis of exudate pleural effusions
- Hemothorax
- The diagnosis of a hemothorax is established if the pleural fluid/blood hematocrit ratio is >50% *.
- It is important to distinguish a hemothorax from bloody (hemorrhagic) pleural effusions, because of the different causes. Pleural effusions with a hematocrit level greater than 5% may appear bloody and are often mistaken as a hemothorax, unless the hematocrit is measured.
- Etiology: Trauma, aortic dissection, malignancy, pulmonary embolism, bleeding disorder, iatrogenic.
- Light’s criteria
| Transudative pleural effusion | Exudative pleural effusion |
| 🛑Heart failure 🛑Hepatic hydrothorax 🛑Nephrotic syndrome 🛑Serum albumin, <1.5 mg/dl) 🛑Malignancyc(~10%) 🛑Atelectasis, trapped lung 🛑Superior vena caval obstruction 🛑Peritoneal dialysis 🛑Myxedema 🛑Pulmonary arterial hypertension 🛑Pulmonary embolism (25%) 🛑Pericardial disease 🛑CSF leak or ventriculopleural shunt 🛑Urinothorax 🛑Extravascular migration of central venous catheter | 🛑Parapneumonic effusions and empyema 🛑Tuberculous pleurisy 🛑Malignancy (~90%) 🛑Pulmonary embolism (~75%) 🛑Post CABG 🛑Intra-abdominal processes: pancreatitis; subphrenic/hepatic abscess 🛑Collagen vascular disease: Lupus pleuritis, rheumatoid pleurisy 🛑Iatrogenic: Esophageal perforation 🛑Uremic pleural effusion 🛑Drug-induced pleural disease (below panel) 🛑Gynecologic: ovarian hyperstimulation, Meigs’ syndrome, postpartum complications 🛑Hemothorax: Trauma, aortic dissection, malignancy, pulmonary embolism, bleeding disorder, iatrogenic 🛑Chylothorax: Trauma or in patients with lymphoma. 🛑Cholesterol effusions: TB, rheumatoid effusions, and any other chronic pleural effusion. 🛑Miscellaneous: benign asbestos pleural effusion, drowning, electrical burns, capillary leak syndromes. |
Diagnostic consideration
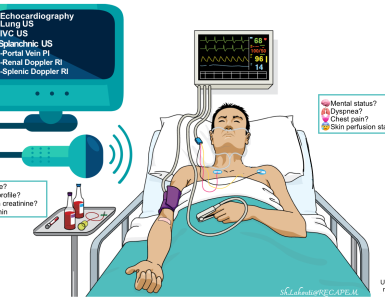
- In critically ill patient bedside ultrasound can rapidly identify hemodynamically significant pleural effusion (which may warrant emergent drainage).
- POCUS and echo gives additional diagnostic clues for underlying life-threatening cause(s) of pleural effusion such as
- RV failure
- Pulmonary embolism
- Decompensated chronic pulmonary hypertension
- Acute aortic syndromes
- Tamponade
- Differentiating ADHF from non-cardiac causes of respiratory distress.
- Bedside ultrasound can provide clues for underlying abdominal cuase(s) of pleural effusion, e.g. cirrhotic ascites, pancreatitis, etc.
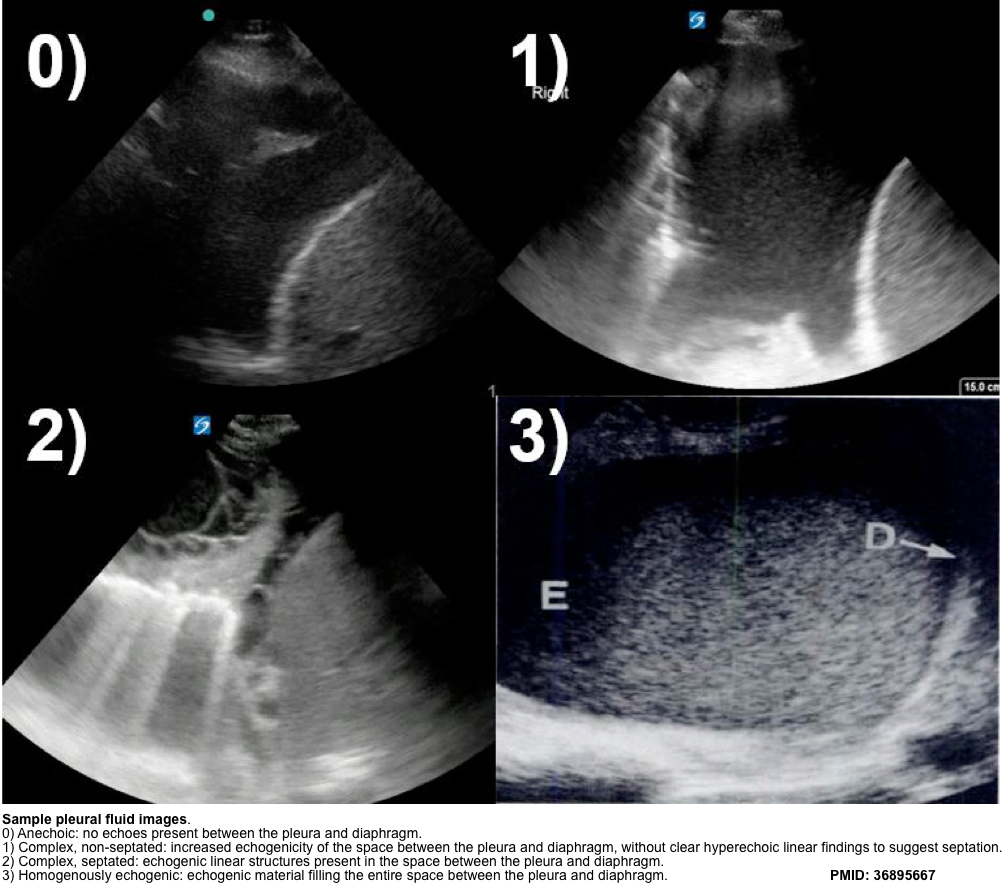
- Pleural ultrasound can also give better insight into the character of an effusion.
- Transudative vs. exudative effusions
- Homogeneously anechoic effusions may be either transudates or exudates, but any degree of heterogeneity is pathognomonic of a complex effusion (non-transudative).
- Exudative effusions often demonstrate punctate, hyperechoic foci floating within the effusion, referred to as the plankton sign.
- Homogeneously echogenic effusions tend to occur in hemothorax and empyema.
- Transudative vs. exudative effusions
- Chest CT is helpful in characterizing empyema and malignant pleural effusions. CT scans also help determine the presence of mediastinal adenopathies or pulmonary lesions.
- CT findings in empyema
- Loculations, often lenticular in shape, may be appreciated by tapered borders and obtuse angles between the fluid and the chest wall with an absence of the meniscus sign.
- Parietal pleural thickening and pleural enhancement is seen in patients with empyema.
- Thickening of the visceral and parietal pleura is suggestive of empyema when associated with significant (usually >30 mm) separation of the pleural surfaces (split pleura sign).
- Increased attenuation of extracostal fat.
- Air within the pleural fluid (e.g. pockets or bubbles of air, gas-liquid level). The DDx include
- Associated pneumothorax
- Bronchopleural fistula
- Air introduced during thoracentesis
- A nonexpandable lung after pleural drainage
- Gas-producing anaerobic organisms.
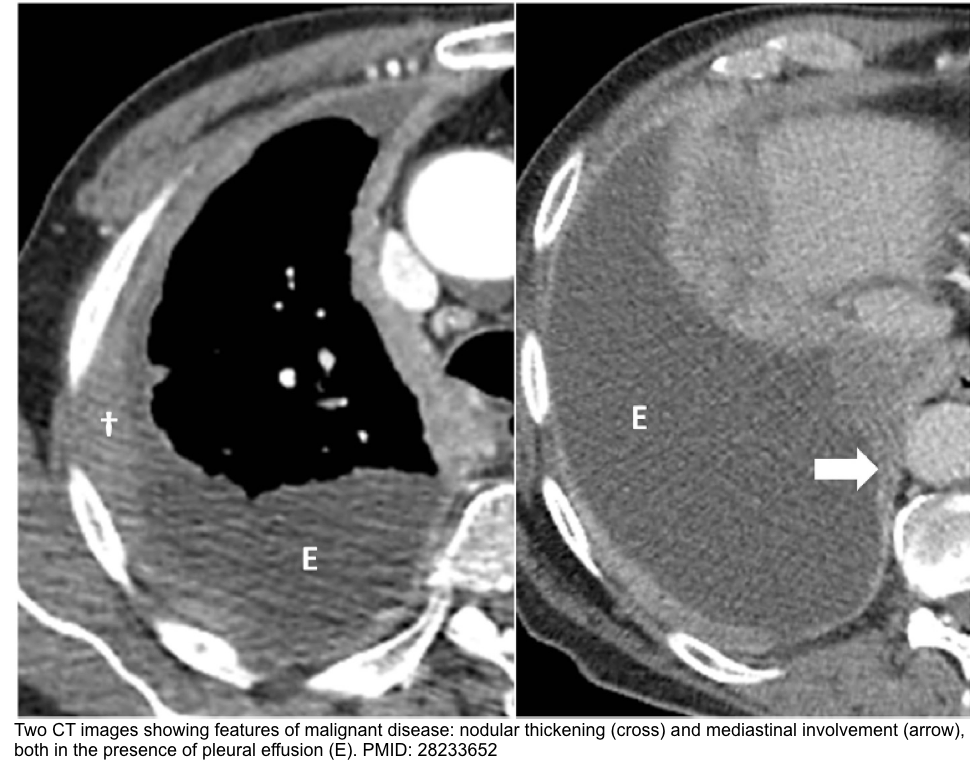
- CT finding in malignant pleural effusion
- Circumferential pleural thickening
- Nodular pleural thickening
- Parietal pleural thickening >1 cm
- Mediastinal pleural involvement.

Thoracentesis
- Indications
- Any new effusions that measure greater than 1mm on decubitus CXR, CT, or US require diagnostic thoracentesis, unless there is a clear clinical diagnosis
(e.g. HF) and no evidence for superimposed pleural space infection.
- Any new effusions that measure greater than 1mm on decubitus CXR, CT, or US require diagnostic thoracentesis, unless there is a clear clinical diagnosis
- Pleural fluid analysis (above)
- The diagnostic algorithm of pleural effusion based on pleural fluid analysis is shown below.
- Diagnostic significance of pleural fluid analysis
-
- Only a select number of diagnoses can be established definitively by pleural fluid analysis (table below).
| Disease | Diagnostic pleural tests |
| Empyema | Observation (pus, putrid odor), positive culture |
| Malignancy | Positive cytology |
| Tuberculous pleurisy | Positive AFB stain, culture |
| Fungal-related effusions | Positive fungal stain, culture |
| Chylothorax | Triglycerides >110 mg/dL, chylomicrons by lipoprotein electrophoresis |
| Cholesterol effusion | Cholesterol >200 mg/dL with a cholesterol to triglyceride ratio >1, cholesterol crystals under polarizing light |
| Hemothorax | Ratio of pleural fluid to blood hematocrit >0.5 |
| Cerebrospinal fluid leakage into pleural space | Detection of beta-2 transferrin |
| Urinothorax | Pleural fluid creatinine to serum ratio always >1 but diagnostic if >1.7 |
| Peritoneal dialysis | Protein <0.5 mg/dL and pleural fluid to serum glucose ratio >1 in peritoneal dialysis patient |
| Esophageal rupture | High salivary isoenzyme form of amylase, low pH (often as low as 6), ingested vegetable or meat fragments |
| Extravascular migration/misplacement of a CVC | Pleural fluid to serum glucose ratio >1, pleural fluid gross appearance mirrors infusate (eg, milky white if lipids infused) |
| Rheumatoid pleurisy | Cytologic evidence of elongated macrophages and distinctive multinucleated giant cells (tadpole cells) in a background of amorphous debris |
-
Complete blood count can be helpful to determine potential infection or acute blood loss from a hemothorax.
-
Check chemistry to evaluate for renal dysfunction.
- Transaminases, international normalized ratio, and albumin may be helpful if cirrhosis is suspected.
-
Check serum lipase if pancreatitis is suspected.
-
Urinalysis for protein may be helpful if nephrotic syndrome is suspected.
-
Check coagulation markers if hemothorax is suspected.
-
Serum protein and lactate dehydrogenase (LDH) are needed if diagnostic thoracentesis is being performed.
- Other labs may be indicated based on suspected underlying cause of pleural effusion, e.g. autoimmune profile, work up for malignancy including tumor markers
Pleural biopsy
- For exudative pleural effusion cases that are still undiagnosed after thoracentesis, pleural biopsy provides a reasonable diagnostic value.
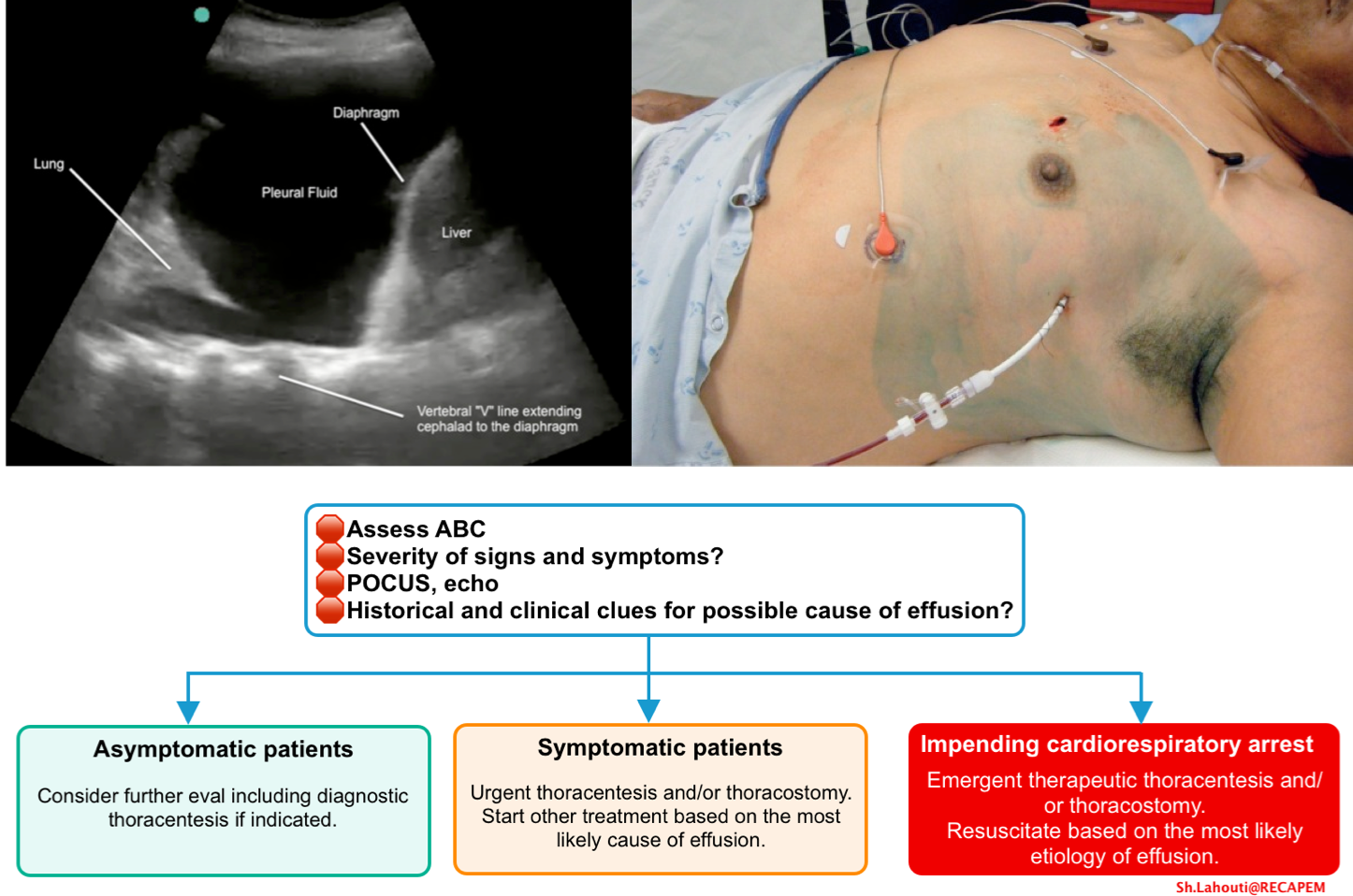
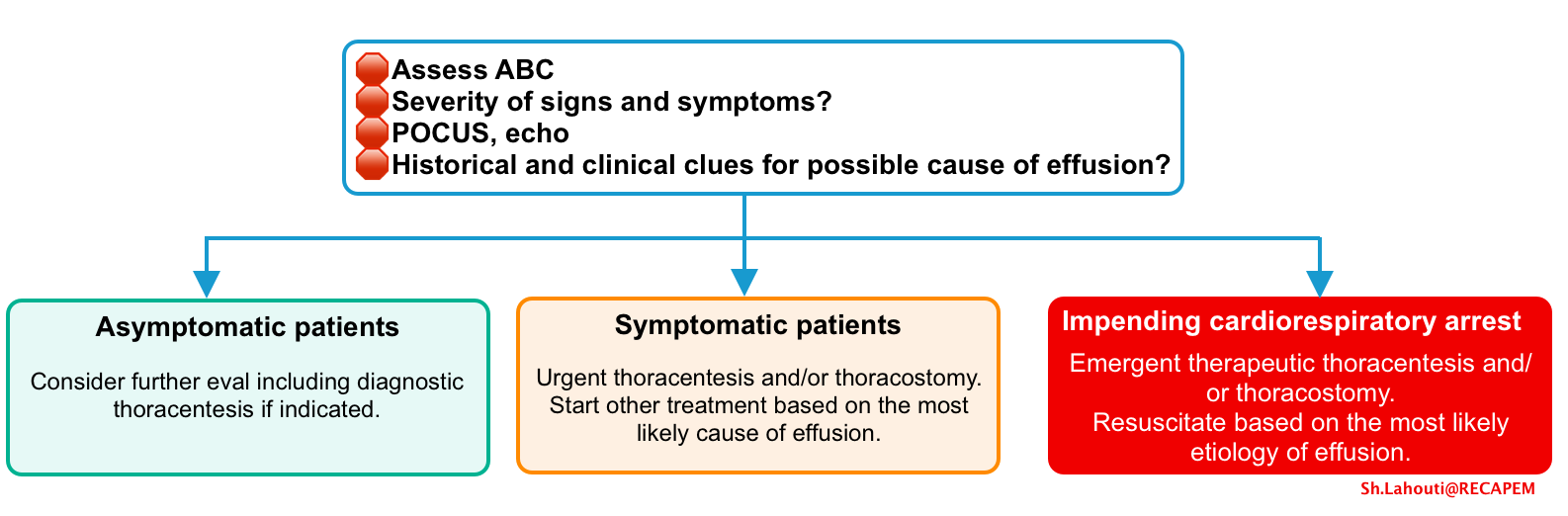
General approach to management
- Assess airway, breathing, circulation and resuscitate the patients based on the most likely etiology for the effusion.
- Patients with impending cardiorespiratory arrest require emergent pleural drainage.
Treatment
Initial treatment of patients with a symptomatic non-malignant pleural effusion involves treating the primary disorder and draining the pleural effusion.
Overview of drainage procedure
- Techniques
- Thoracentesis
- Thoracentesis is a percutaneous procedure where pleural fluid is removed either through a needle (typically for small volumes e.g. <30 mL), needle over catheter system, or a small bore catheter (see media here).
- Thoracostomy
- Placement of a thoracostomy tube (diameter ≥16 Fr) or thoracostomy catheter (diameter ≤14 Fr) through the chest wall into pleural space.
- Tube size varries from 12 to 42 Fr.
- Placement of a thoracostomy tube (diameter ≥16 Fr) or thoracostomy catheter (diameter ≤14 Fr) through the chest wall into pleural space.
- Thoracentesis
- Choosing the drainage technique
- The suitable drainage procedure depends on
- Effusion size
- Effusion character (loculated vs. free-flowing)
- Need for complete drainage
- Recurrent or persistent pleural effusion: may require indwelling pleural catheter (IPC)
- Institutional factors, operator preference, and availability of expertise
- The suitable drainage procedure depends on
Therapeutic thoracentesis/thoracostomy
- Indications
- It is considered for moderate-large pleural effusion with any following characteristics:
- Exudative pleural effusion
- Symptomatic effusions (e.g. dyspnea, pleuritic chest pain, hypoxemia, or impending to cardiorespiratory collapse).
- Recurrent symptomatic pleural effusion
- Isolated symptomatic pleural effusion
- In the absence of simultaneous total body volume overload (peripheral edema), large volume diuresis frequently leads to hypovolemia. Therefore thoracentesis should be considered.
- Deteriorating oxygenation and ventilation in patients on invasive or non-invasive ventilation, despite optimizing respiratory parameters.
- For example patients with cardiogenic or non-cardiogenic pulmonary edema who require higher PEEP to maintain oxygenation. Drainage of pleural effusion will improve respiratory function.
- Complicated parapneumonic effusions and empyema (see below).
- Hemothorax
- It is considered for moderate-large pleural effusion with any following characteristics:
- Volume of fluid removal
- Traditionally, fluid removal in one session was limited to 1.5 L to avoid re-expansion pulmonary edema *.
- Large volume thoracentesis (LVT) refers to the removal of >1.5 L of pleural fluid during a therapeutic thoracentesis. It is indicated in the presence of dyspnea due to a moderate to large pleural effusion *.
- ⚠️Contraindication for LVT:
- Hepatic hydrothorax
- Effusion associated with nonexpendable lung (NEL) *.
- NEL refers to the inability of the lung to fully re-expand after thoracentesis.
- It occurs most commonly following treatment of an underlying inflammatory or infectious condition of the pleura.
- Diagnosis is suggested when a therapeutic thoracentesis does not improve dyspnea and when fluid is replaced by air after thoracentesis on chest imaging (“pneumothorax ex vacuo”).
- ⚠️Contraindication for LVT:
- Complications
- Pneumothorax
- Infection
- Intercostal nerve or artery injury
- Organ injury (e.g. lung, diaphragm, heart, liver, or spleen)
- Pulmonary edema related to re-expansion pulmonary edema (RPE)
Removal
- Criteria
- Once the following criteria are met, thoracostomy tube or catheter removal are recommended to minimize the risk of infectious complications.
- The lung is adequately expanded.
- Fluid drainage < ~150cc over 24h ( ~ <2 mL/kg body weight per day) *
- In patients with empyema, if the majority of fluid pockets have been drained and the patient clinical condition is improved (e.g. afebrile, no leukocytosis), and pleural-pleural apposition has occurred as documented by imaging, the remainder of the pleural abnormality is likely to resolve on antibiotics alone.
- Once the following criteria are met, thoracostomy tube or catheter removal are recommended to minimize the risk of infectious complications.
- Removal technique
- Preparation
- All supplies in the room should be readily available including a vaseline gauze, dry dressings, pre-cut tapes (see media below)
- Inform the patient that this procedure is invariably painful otherwise it may cause an unexpected sudden gasp/inspiration.
- The thoracostomy tube or catheter should be removed at end-inspiration. Explain the technique to the patients and ask them to hold the breath and bear down during the removal procedure.
- Ask the patient to perform the planned technique before the procedure.
- Technique
- The sutures that secured the tube in place should be removed prior to attempting to remove the tube.
- Hold the dressing near its insertion site with the nondominant hand, ask the patient to perform the planned technique.
- At the appointed time, remove the tube or catheter quickly with the dominant hand while placing a dry sterile dressing with a vaseline gauze *.
- During placement, some operators provide a suture at the exit site to tie at the time of tube removal. If this approach is used, then two operators may be preferred at the time of removal to avoid air entrainment during the process: one to pull the tube or catheter and occlude the site with a dressing and the other to tie the suture.
- Fullow up
- Get a CXR to evaluate for recurrence of pneumothorax and/or re-accumulation of fluid.
- An additional chest imaging the next day is not necessary unless clinically indicated.
- Preparation
Specific clinical conditions
Recurrent or persistent pleural effusion
- Etiology
- Heart failure
- Hepatic hydrothorax
- Nonexpandable lung
- Pleural infection
- Recurrent pulmonary embolism
- Connective tissue disease
- Malignancy
- Clinical re-evaluation and additional testing
- Take another thorough history and physical exams.
- Evaluate the risk factors for or symptoms of chronic liver disease, heart failure, and connective tissue disease, and pulmonary embolism.
- Routine lab tests and repeat pleural fluid analysis and/or thoracoscopic biopsy. Other tests may be indicated based on historical and clinical findings.
- Contrast enhanced chest CT, CTPA (if PE is suspected)
- Echocardiography
- Abdominal ultrasound/ CT scan
- Management
- Repeat thoracentesis
- Repeat thoracentesis is best suited to patients who experienced relief from drainage on presentation, patients with non-malignant pleural effusion that re-accumulate slowly (e.g. once every 4 weeks or more), and patients with a grim prognosis (e.g. < 2 weeks).
- In general, multiple thoracenteses (>2-3 times) should be avoided, since it has been associated with following complications:
- Infection
- Inducing thickened visceral pleura or non-expandable lung.
- Maximize primary therapy
- Optimize diuretic medications for patients with extensive fluid overload from long standing heart failure with chronic renal insufficiency, and advanced liver disease and hepatic hydrothorax.
- Refractory effusions
- Recurrent symptomatic pleural effusion despite repeated thoracentesis (e.g. >2 to 3) and optimal medical therapy.
- Management
- Indwelling pleural catheter (IPC) placement
- Suitable for both expandible and non-expandible symptomatic recurrent pleural effusion.
- IPC is preferred over pleurodesis when the pleural fluid output is too high (e.g. > 300mL/d)
- IPC is not suitable for patients who do not have the ability to perform intermittent drainage or patients in whom long-term drainage is anticipated (e.g. longer that 6 months)
- Pleurodesis
- Suitable for only expandible lung with symptomatic recurrent pleural effusion
- Pleurodesis is preferred over IPC when the risk of pleural fluid infection is considerably high (e.g. hepatic hydrothorax)
- Indwelling pleural catheter (IPC) placement
- Repeat thoracentesis
Heart failure
- Heart failure is the most common cause of transudative pleural effusion.
- In left-sided or biventricular heart failure, pleural effusion is bilateral in ~ 80% of paatients. However, a unilateral pleural effusion does not exclude heart failure.
- Although more commonly associated with left-sided failure, pleural effusion also occurs in isolated right-sided heart failure.
- Almost ~13% of patients with idiopathic or familial pulmonary artery hypertension have pleural effusions.
- Usually these effusions are transudate and small.
- They appear to be most commonly right sided (58%) or bilateral (26%) *.
- If clinical history is compatible, the cardiological cause of a pleural effusion should be confirmed by methods other than thoracentesis.
- Indication for diagnostic thoracocentesis:
- Unilateral effusion
- Fever
- Bilateral effusion which is not comparible in size
- Pleuretic chest pain
- Lack of radiologic response to appropriate dose of diuretics
- Diuretics in management of ADHF 👉see here.
Pearl
- Patients with cardiac effusions treated with diuretics are reported to have an increased pleural fluid protein level relative to its blood level, resulting in the effusion being classified as an exudate by Light’s criteria.
- In patients with an exudative effusion suspected of having a cardiac cause, correlation with echocardiography or NT-proBNP measurement may be helpful to clarify the aetiology; care should be taken to exclude other relevant potential causes, such as malignancy or pulmonary emboli.
Hepatic hydrothorax
- Definition
- Hepatic hydrothorax (HH) refers to the presence of a transudative pleural effusion (usually >500 mL) in a patient with cirrhosis (portal hypertension) who does not have other reasons to have a pleural effusion (e.g. cardiac, pulmonary, or pleural disease).
- Epidemiology
- It occurs in approximately 5-15% of patients with cirrhosis.
- Patients who develop hepatic hydrothorax are more likely to have ascites, hepatic encephalopathy, acute kidney injury (AKI), and increased risk of mortality.
- Pathogenesis
- This condition is the result of a pressure gradient between the peritoneal and pleural spaces (negative pressures), which favors the passage of abdominal fluid to the thorax. This could explain why some patients with hepatic hydrothorax do not have apparent ascites.
- This is also promoted by diaphragmatic defects most frequently found in the tendinous portion of the right diaphragm.
- Spontaneous bacterial empyema (SBEM):
- Similar to spontaneous bacterial peritonitis (SBP) in patients with ascites, patients with hepatic hydrothorax may develop SBEM.
- It is thought to occur due to seeding of the pleural effusion with bacteria that spread directly from the abdominal cavity in a patient with SBP or from bacteremia.
- This condition is the result of a pressure gradient between the peritoneal and pleural spaces (negative pressures), which favors the passage of abdominal fluid to the thorax. This could explain why some patients with hepatic hydrothorax do not have apparent ascites.
- Clinical presentation
- Dyspnea
- Nonproductive cough
- Pleuritic chest pain
- Fatigue due to hypoxemia
- Abdominal distension (ascites)
- Severe dyspnea and hypotension (caused by acute tension hydrothorax) *
- Fever, new or worsening pleuritic chest pain, encephalopathy, or an unexplained worsening of renal function are suggestive for spontaneous bacterial empyema (SBEM) *.
- Diagnosis
- Diagnosis should be considered in patients with cirrhotic portal hypertension who present with pleural effusion confirmed on radiographic imaging.
- Hepatic hydrothorax is a diagnosis of exclusion and it should be made by documentation of a pleural effusion and exclusion of alternative causes for the effusion.
- Imaging
- Ultrasound to confirm presence of pleural effusion.
- Chest CT : To confirm the diagnosis and to exclude infection or an alternate cause of a pleural effusion such as mediastinal, pulmonary, or pleural lesions or malignancies.
- Echo: to assess cardiac function and rule cardiac causes of pleural effusion.
- Abdominal ultrasound: To evaluate for hepatic mass lesions, hepatic and portal venous flow, and ascites.
- Thoracentesis and fluid analysis in all suspected cases of hepatic hydrothorax.
- Pleural effusions deriving from portal hypertension are transudative in nature and therefore similar to the ascitic fluid, with a low protein concentration (<2.5 g/dL) and with a serum-to-pleural fluid albumin gradient >1.1 g/dL *.
- Total protein and albumin levels may be marginally higher in pleural fluid than in ascitic fluid due to greater efficiency of water absorption by the pleural surface.
- Total protein and albumin levels may be marginally higher in pleural fluid than in ascitic fluid due to greater efficiency of water absorption by the pleural surface.
- In uncomplicated hepatic hydrothorax, the polymorphonuclear cell (PMN) count is low (<250 cells/mm3), whereas in the setting of SBEM the PMN count is elevated. The diagnostic criteria for SBEM are *
- Positive pleural fluid culture and a PMN cell count >250 cells/mm3
- Negative pleural fluid culture and a PMN cell count >500 cells/mm3
- No evidence of pneumonia on a chest imaging study
- When the etiology of HH is uncertain, other analyses of pleural fluid such as PCR for mycobacterium, amylase concentration for pancreatic pleural effusion, cytology to rule out malignancy, and triglyceride level to assess chylothorax can be considered *.
- Pleural effusions deriving from portal hypertension are transudative in nature and therefore similar to the ascitic fluid, with a low protein concentration (<2.5 g/dL) and with a serum-to-pleural fluid albumin gradient >1.1 g/dL *.
- Imaging
- Management
- Mildly to moderately symptomatic patients
- Sodium-restricted diet and diuretics (start with furosemide 40mg and spironolactone 100mg daily).
- Serial thoracentesis: For patients who are refractory to sodium restriction and diuretics *.
- Other therapeutic consideration for such patients include transjugular intrahepatic portosystemic shunt (TIPS) placement, pleurodesis, thoracoscopic surgery to repair diaphragmatic defects, and liver transplantation.
- Severely symptomatic patients
- Therapeutic thoracentesis (followed by management with a sodium-restricted diet and diuretics to prevent reaccumulation of fluid).
- It is the most effective way to reduce large effusions (>1.5 L).
- As a general rule, no more than 2 L of fluid should be removed because of the risk of pulmonary edema and hypotension *. However, this “2 L rule” was developed in patients with causes other than cirrhosis for their effusions; patients with cirrhosis may be able to tolerate the removal of larger volumes of fluid.
- Therapeutic thoracentesis (followed by management with a sodium-restricted diet and diuretics to prevent reaccumulation of fluid).
- Refractory hydrothorax: Persistent pleural effusion despite successful treatment of ascites *.
- Therapeutic thoracentesis is required to provide symptomatic relief from dyspnea but the effect is transient.
- TIPSS can be considered either as a definitive treatment or as a bridge to transplantation in patients with refractory hydrothorax.
- Mildly to moderately symptomatic patients
⚠️Avoid chest tube insertion for hepatic hydrothorax since it can result in massive protein and electrolyte depletion, infection, renal failure, and bleeding *.
- Exception include patients with spontaneous bacterial empyema (SBEM) and frank pus or in patients undergoing pleurodesis, who require chest tube placement.
Pulmonary embolism
- Epidemiology
- In a systematic review and meta-analysis, the pooled incidence of pleural effusion in PE was up to 41% *.
- In most PE patients with pleural effusion, the effusion is small and usually unilateral, however bilateral effusion can be present.
- It has been shown that there was no relationship between the sidedness of the PE and the pleural effusions *.
- Although pleural effusions are typically present on the same side as the lung affected by PE, they may be unilateral despite bilateral embolic disease or bilateral when the pulmonary embolism is unilateral. Surprisingly, the effusion is also present unilaterally and contralateral to the embolism in ∼7.5% of patients with effusions due to pulmonary embolism.
- Pathogenesis: here
- Diagnosis
- PE should be strongly considered in patients with pleural effusion, when one of the following suggestive clues are present *:
- Risk factors for VTE
- Sudden onset of pleuritic chest pain
- A PLEF occupying <1/3 of the hemithorax
- Dyspnea disproportionate to pleural effusion size
- Any undiagnosed pleural effusion
- Diagnosis is made by CT angiography, as pleural fluid analysis has a low diagnostic yield.
- PE should be strongly considered in patients with pleural effusion, when one of the following suggestive clues are present *:
- Treatment
- Complications
- In patients with PE and pleural effusion, if the pleural effusion increases in size or appears on the contralateral side, one of the following complications should be considered *:
- Pleural cavity infection
- Hemothorax
- Recurrent thromboembolism
- The treatment of hemothorax complicating the anticoagulation or thrombolytic treatment involves immediate discontinuation of anti-thrombotic therapy and insertion of chest tube.
- If brisk bleeding persists, a thoracotomy with resection of infarcted lobe may be warranted.
- In patients with PE and pleural effusion, if the pleural effusion increases in size or appears on the contralateral side, one of the following complications should be considered *:
Infectious pleural effusions (IPE)
- Infectious pleural effusion is secondary to bacterial pneumonia, a lung abscess or infected bronchiectasis.
- Classification of IPE
- Non complicated (parapneumonic)
- It is a reactive pleuritis due to underlying pneumonia.
- This type of pleural effusions are exudative, sterile and free-flowing.
- Imaging
- Small to moderate size (<100 mL on imaging) and free-flowing (i.e. no loculation)
- Fluid analysis
- Is not required, but if sampled, there is no evidence of infection by culture or chemistry.
- A neutrophilic exudate (an elevated protein level >0.5 percent of serum and/or a LDH level >0.6 that in the serum), a normal pH, a normal glucose level, and does not contain microorganisms.
- Is not required, but if sampled, there is no evidence of infection by culture or chemistry.
- These are associated with a favourable evolution with antibiotic therapy alone.
- Complicated parapneumonic effusion
- Refers to an effusion that has been infected with bacteria or other microorganisms (e.g. positive Gram stain or biochemical evidence of marked inflammation).
- These types of effusions are in the fibrinopurulent stage *.
- Imaging
- Large free-flowing effusion (e.g. > half the hemithorax or volume > 1L)
- Loculated effusions (presence of internal septations)
- Effusions associated with thickened parietal pleura
- Air bubbles within the effusion
- Chemistry: Evidence of infection by culture or chemistry are present (pH < 7.2 or glucose < 40 mg/dL and LDH>1000 IU/L)
- These will require thoracic drainage or surgery to resolve.
- Empyemas
- Refers to presence of frank pus in the pleural space or a positive culture for pleural fluid.
- For sources of empyema, see appendix
- These effusions are in the organized stage and always needs to be drained.
- Refers to presence of frank pus in the pleural space or a positive culture for pleural fluid.
- Non complicated (parapneumonic)
- Treatment
- Drainage
- Chest tube or catheter thoracostomy drainage is the least invasive option for drainage of infected pleural fluid in patients with a complicated parapneumonic effusion or empyema.
- A small-bores chest tubes (10 to 14 Fr), or a 14 Fr pigtail catheter placed with the modified Seldinger technique.
- Antibiotic regimen
- Generally, empiric antibiotic regimens should include an antibiotic that targets anaerobic bacteria, which are common causes of complicated parapneumonic effusions and empyema.
- Additional antibiotics should be selected based on the site of acquisition (eg, community- versus hospital-acquired), mode of acquisition (eg, aspiration, trauma), and local epidemiology.
- Evaluate response to treatment
- Failure to improve or worsening after antibiotics and tube thoracostomy drainage (eg, the effusion worsens, fever persists or new fever develops, persistent or worsening leukocytosis) may indicate that antibiotic coverage and/or drainage is inadequate.
- If inadequate drainage is suspected, administer intrapleural tPA/DNase and consider to place a second (or third) chest tube or catheter before resorting to video-assisted thoracoscopic surgery (VATS) *.
- Inadequate drainage is suspected when any of the following is present:
- Low volume of drainage
- Minimally changed or unchanged effusion size
- Undrained loculation on imaging
- Lung not re-expanding
- Inadequate drainage is suspected when any of the following is present:
- If inadequate drainage is suspected, administer intrapleural tPA/DNase and consider to place a second (or third) chest tube or catheter before resorting to video-assisted thoracoscopic surgery (VATS) *.
- If good clinical response is seen (improved symptoms, fever, leukocytosis)
- Continue treatment
- Once drainage is <100mL/d, remove the tube.
- Change to oral antibiotic if clinical improvement is satisfactory.
- Failure to improve or worsening after antibiotics and tube thoracostomy drainage (eg, the effusion worsens, fever persists or new fever develops, persistent or worsening leukocytosis) may indicate that antibiotic coverage and/or drainage is inadequate.
- Drainage
Malignant pleural effusions
- Epidemiology
- Approximately 15% of all patients with cancer develop malignant pleural effusions.
- The presence of an malignant pleural effusion usually portends a poor prognosis (with rare exception of lymphoma).
- Although malignant pleural effusion can have any size, malignant effusions are the most common cause of massive pleural effusions.
- Definitions
- Malignant pleural effusions (MPE)
- In MPEs, infiltration of cancer cells into pleural tissue is observed; pleural tissue invasion by malignant cells may be seen on pleural biopsy and may result in positive fluid cytology.
- Paramalignant effusions
- These result from tumor effects that indirectly act on the pleural space such as by bronchial obstruction, mediastinal lymph node infiltration, thromboembolism, or superior vena cava syndrome * .
- In paramalignant pleural effusions, pleural fluid cytology and pleural biopsy are negative because cancer cells have not invaded pleural membranes.
- Paramalignant effusions are managed according to the underlying etiology.
- Malignant pleural effusions (MPE)
- Management
- Goal
- Palliative treatment to provide symptom relief and improvement of life quality.
- Candidate for pleural interventions
- Symptomatic patients with malignant pleural effusions.
- Initial treatment
- Therapeutic thoracentesis is usually indicated for symptomatic patients with malignant pleural effusion * .
- As much fluid as is tolerated up to a limit of 1.5 L because of concern that volumes removed above this amount increases the risk for serious procedure-related complications.
- Chest tube or small bore catheter drainage (ie, the tube or catheter is left in place)
- It is an alternative to large volume thoracentesis and may be performed in those who need especially large volumes of fluid removed slowly (over hours or days) for symptom relief.
- Therapeutic thoracentesis is usually indicated for symptomatic patients with malignant pleural effusion * .
- Treatment of recurrent pleural effusion
- More than 50% of malignant pleural effusion recur and among those, up to two-thirds recur rapidly within 1 month while the remainder recur more slowly.
- Treatment option include *
- Repeat thoracentesis
- Indwelling pleural catheter (IPC) drainage
- Pleurodesis by various techniques (bedside via chest tube or catheter, thoracoscopic)
- Combination therapies
- Complete or partial pleurectomy with decortication
- Pleuroperitoneal shunt
- Goal
Trapped lung
Trapped lung is a type of nonexpandable lung and happens when the visceral pleura becomes encased with a fibrotic rind that prevents re-expansion of the lung.
- Nonexpandable lung is present when the lung is unable to expand to the chest wall and achieve visceral and parietal pleural apposition.
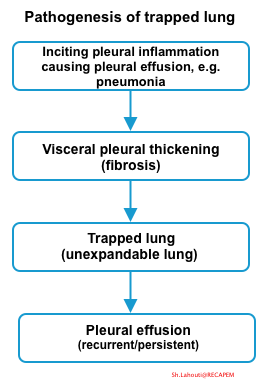
Pathogenesis
- The inciting event is any etiology associated with pleural inflammation. This will cause pleural effusion and triger the fibrosis of visceral pleura.
- Visceral pleura fibrosis prevents complete lung expansion (even if pleural fluid is drained).
- Once fibrosis becomes organsided, the visceral pleura is encased with a fibrotic rind and trapped lung develops.
- Pathogenesis of pleural effusion in the trapped lung:
- Trapped lung causes a greater negative pleural pressure, caused by visceral pleural thickening preventing lung expansion.
- This negative pleural pressure facilitates movement from pleural capillaries into the pleural space *.
- Visceral pleural thickening leads to trapped lung which perpetuates the effusion. Eventually, a steady state of fluid formation is established, leading to persistence of pleural effusion of constant volume. However active inflammation is usually absent at this stage *.
Etiology
- Most common causes of pleural inflammation that eventually lead to trapped lung include *
- Pneumonia with parapneumonic effusion (bacterial and mycobacterial)
- Hemothorax
- Spontaneous pneumothorax
- Thoracic operations including coronary artery bypass surgery
- Uremia
- Rheumatoid pleuritis
Clinical feature and diagnosis
- History
- Symptoms of pleural inflammation e.g. pleuretic chest pain, fever in the remote past.
- A history of past pleural infection, thoracic surgery, rheumatoid pleural disease, thoracic radiation or a hemothorax
- Signs and symptoms
- Most patients with trapped lung do not have symptoms referable to the pleural effusion.
- Rather they will typically develop discomfort throughout the thoracentesis due to further reductions in intrathoracic pressure.
- If thoracentesis is performed, the effusion rapidly recurs.
- The pleural fluid is transudative.
- Typically, the pleural fluid white blood cell count is relatively low and primarily consists of mononuclear cells.
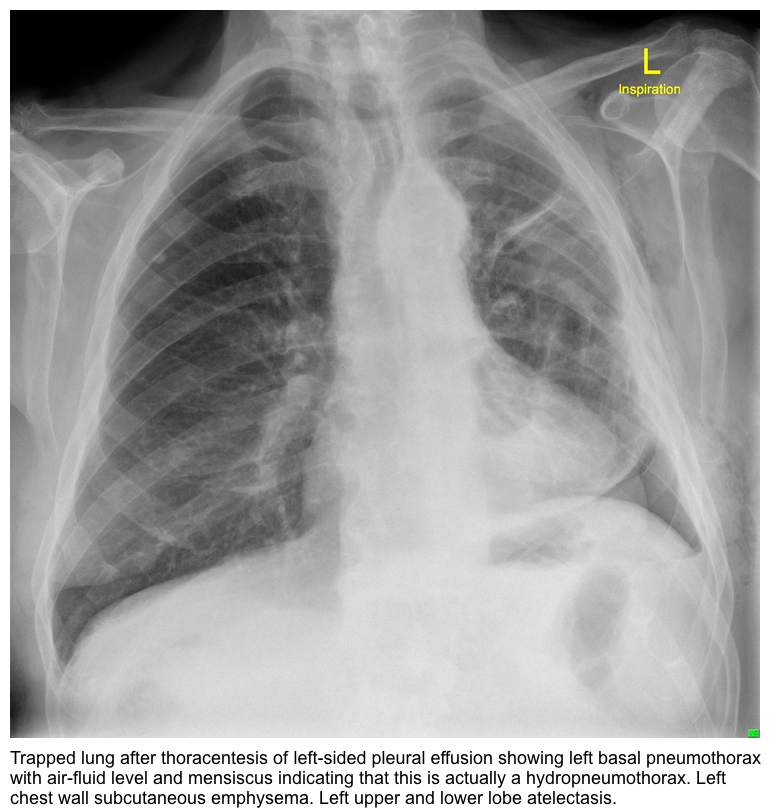
- Following thoracentesis radiograph can show air in the pleural space *.
- In the setting of trapped lung, the air does not represent a complication of thoracentesis, but replacement of the fluid by air which has entered due to the low pressure, probably along the catheter tract or due to a minor break in technique.
- Imaging
- CXR may show reduced size of the hemithorax with the effusion, indicating that the pleural pressure on the side with the effusion is more negative than that on the contralateral side.
- Lobar atelectasis
- CECT: reveals loculation and pleural thickening, although sometimes pleural thickening is only apparent on an air contrast CT.
Diagnosis
- Confirmation of visceral pleural thickening (a pleural rind) requires air contrast CT or direct visualization via video-assisted thoracoscopy.
- Air contrast CT: To improve visualization of the pleural rind, an air contrast CT can be performed by injecting 200 to 400 mL of air at the termination of a therapeutic thoracentesis and obtaining a chest CT.
Management
- For patients who have asymptomatic trapped lung, pleural drainage procedures are NOT indicated, because the pleural fluid will reaccumulate due to the negative pleural pressure *.
- Decortication of the pleural rind on the visceral pleura is suitable for patients without malignant pleural effusion who are surgical candidates and have disabling shortness of breath due to trapped lung.
Differential diagnosis of nonexpandable lung
- Nonexpandable lung can be developed by
- Pleural disease
- Trapped lung
- Lung entrapment (explained below)
- Extrapleural processes such as
- Endobronchial obstruction
- Chronic lung atelectasis
- Processes that will increase the elastic recoil of the lung (ie, interstitial lung disease or lymphangitic carcinomatosis).
- Pleural disease
- Lung entrapment
- Is a type of non-expandable lung in which the lung cannot expand fully because of an active disease, such as pleural malignancy or infection that restricts expansion of the lung and/or visceral pleura.
- The key features of nonexpandable lung due to pleural disease include
- Development of chest discomfort (dyspnea, chest pain) during thoracentesis procedure, leading to incomplete drainage of a pleural effusion.
- It is thought that the chest discomfort is related to the negative pleural pressure created in the pleural space when the lung does not re-expand to fill the space created by fluid removal.
- Development of hydropneumothorax after pleural drainage procedure.
- After a thoracentesis, nonexpandable lung should be suspected if a post-thoracentesis radiograph shows air in the pleural space, especially when it appears at the base of the lung *.
- Lung does not fully expand after a thoracentesis.
- Development of chest discomfort (dyspnea, chest pain) during thoracentesis procedure, leading to incomplete drainage of a pleural effusion.
- Key features of lung entrapment which differentiate it from trapped lung include:
- Improvement of dyspnea after thoracentesis, despite that the lung does not fully expand radiographically after draining the pleural fluid.
- The pleural fluid analysis is usually exudative (in contrast to trapped lung which is transudative).
- CXR: Lung entrapment may show contralateral mediastinal shift, whereas in trapped lung there may be ipsilateral mediastinal shift.
- CT or ultrasound:
- The visceral pleural surface in lung entrapment may appear nodular, thickened, or hyperechoic and the pleural fluid is often hyperechoic and/or loculated
- Treatment
- In general, the treatment of lung entrapment is focused on treating the underlying disease process (eg, infection, inflammation, malignancy).
- If patients experience an improvement in dyspnea, even if the lung does not re-expand, they can achieve significant pleural palliation by placement of indwelling pleural catheters (IPCs).
Superior vena cava (SVC) syndrome
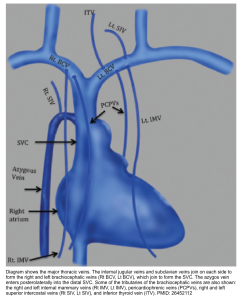
Anatomy
- The SVC is formed by the confluence of the right and left brachiocephalic veins which provide venous drainage of the head, neck, and upper limbs. It courses along the right middle mediastinum, with the trachea and ascending aorta on its left, and drains into the right atrium.
- The mean length of SVC in adult is ~ 7cm, and its maximum diameter in adults is ~ 2.1 cm *.
- Tributory veins:
- The azygos vein is the major tributary which drains posteriorly into the distal SVC.
- A few mediastinal, esophageal, and pericardial veins may drain directly into the SVC (shown below), and become more prominent on imaging in the presence of SVC obstruction.
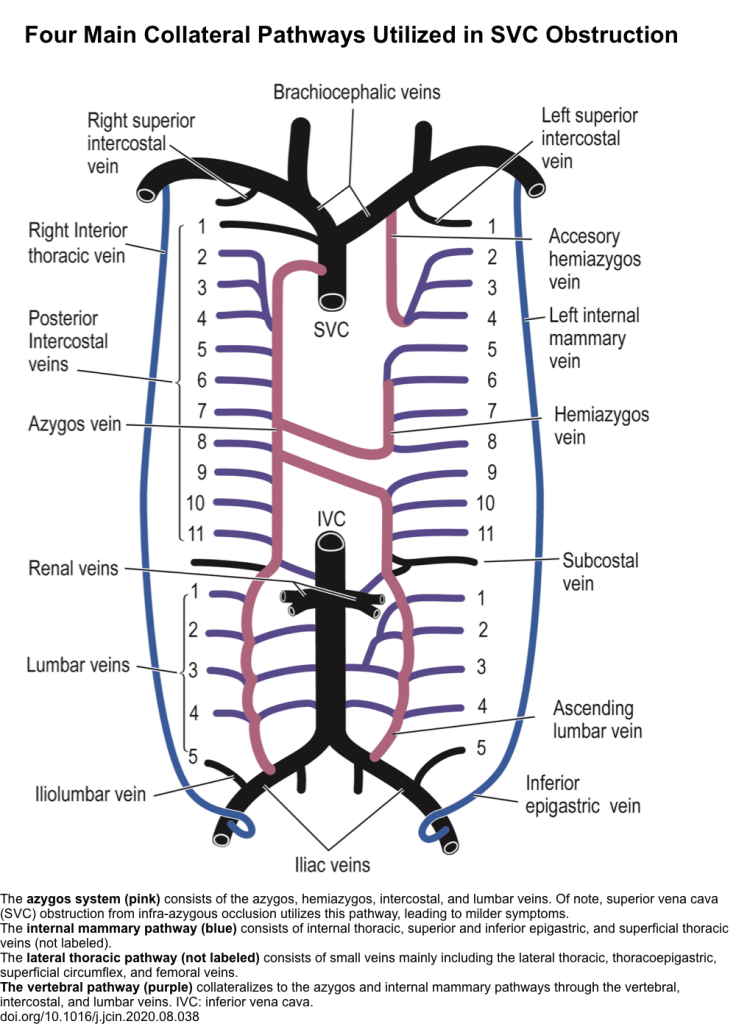
- SVC syndrome results from any condition that leads to obstruction of blood flow through the SVC. As a result, upper body venous pressure is markedly elevated initially but decreases over time when the venous collaterals form as alternative pathways for the return of venous blood to the right atrium.
- There are 4 main collateral pathways (figure below) which become identifiable at imaging in cases of SVC obstruction.
- Azygous venous system (this is the largest and consists of azygos, hemiazygos, intercostal, and lumbar veins)
- Internal mammary pathway
- Lateral thoracic pathway
- Vertebral venous pathway
- The severity of presentation of SVC syndrome is inversely related to the development of these collateral veins and the rapidity.
- There are 4 main collateral pathways (figure below) which become identifiable at imaging in cases of SVC obstruction.
- Cardiac output may be diminished transiently by acute SVC obstruction, but within a few hours, blood return is reestablished by increased venous pressure and collaterals.
Etiology
- Malignant Causes (70%)
- Lung cancer
- Lymphoma
- Thymoma
- Primary mediastinal germ cell neoplasms
- Mesothelioma
- Solid tumors with mediastinal lymph node metastases (e.g. breast cancer)
- Benign Causes (30%)
- Thrombosis (bland thrombus) in vicinity of indwelling intravascular devices e.g. central venous catheters, pacemakers, defibrillator, indwelling hemodialysis catheters.
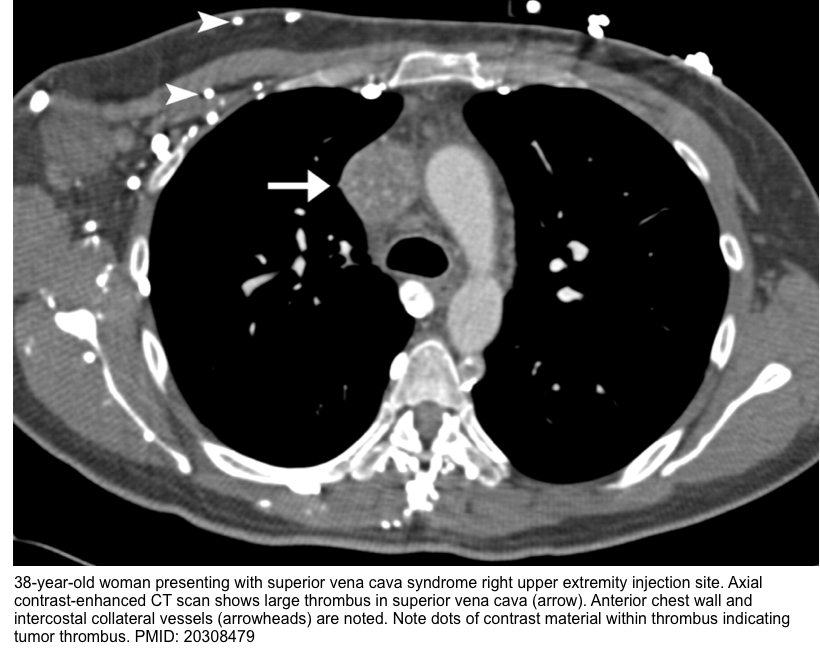
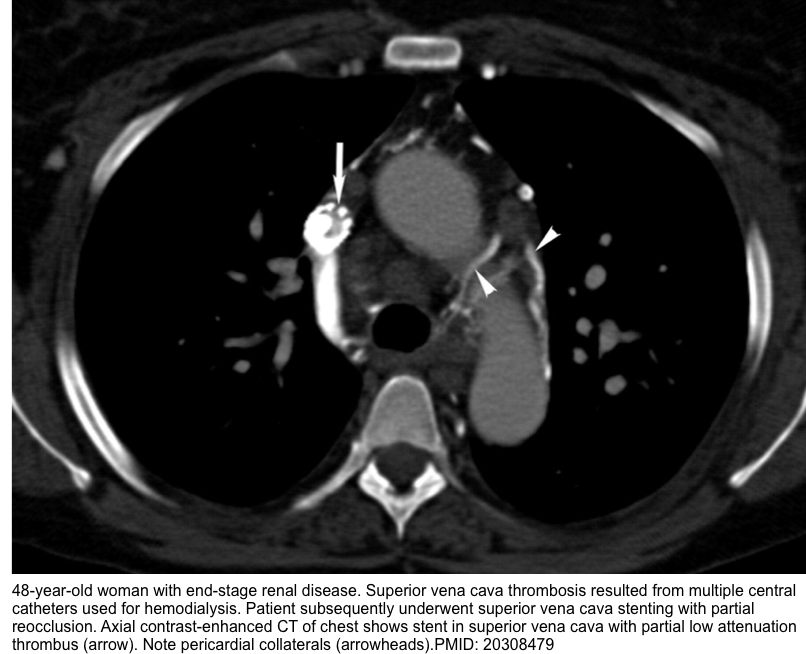
- Remember that SVC thrombus can be bland or tumor thrombus (see imaging below).
- Fibrosing mediastinitis: infectious (e.g. histoplasmosis, TB), postradiation fibrosis.Clinical presentation varies depending on the severity, location, and rapidity of onset of obstruction and establishment of collateral veins.
- Thrombosis (bland thrombus) in vicinity of indwelling intravascular devices e.g. central venous catheters, pacemakers, defibrillator, indwelling hemodialysis catheters.
Clinical manifestation
- Clinical presentation varies depending on the severity, location, and rapidity of onset of obstruction and establishment of collateral veins.
-
- Swelling: Face, head fullness, neck, arms, chest wall
- Upper airway edema (nasal passage, pharynx, larynx): dyspnea, stridor, cough, hoarseness, and dysphagia
- Respiratory distress can happen as a result of:
- Chest wall edema causing pulmonary restriction
- Pleural effusion
- Airway obstruction
- Cerebral edema: Headaches, confusion, or visual/auditory disturbances. Cerebral edema can lead to brainstem herniation, and possibly death.
| Pearl: Major causes of instability in cancer patients with SVC syndrome are: 🛑Airway obstruction 🛑Pleural 🛑Pericardial effusions |
Grading severity of symptoms *
- Grade 0 (asymptomatic)
- Asymptomatic but radiographically SVC obstruction is present.
- Grade 1 (mild)
- Edema in head or neck (vascular distention) +/- cyanosis, plethora
- Grade 2 (moderate)
- Edema in head or neck with functional impairment such as
- Mild dysphagia
- Cough
- Mild or moderate impairment of head, jaw, or eyelid movements
- Visual disturbances caused by ocular edema
- Edema in head or neck with functional impairment such as
- Grade 3 (severe)
- Mild or moderate cerebral edema (headache, dizziness)
- Mild/moderate laryngeal edema
- Diminished cardiac reserve (syncope after bending)
- Grade 4 (life-threatening)
- Significant cerebral edema (confusion, obtundation)
- Significant laryngeal edema (stridor)
- Significant hemodynamic compromise (syncope without precipitating factors, hypotension, renal insufficiency)
- Grade 5 (fatal): Death
Anatomic classification of SVC obstruction based on location
- Obstruction of brachiocephalic veins
- Multiple collateral veins will be formed and may include any of the pathway mentioned below (below figure A).
- Obstruction of upper SVC proximal to the azygos vein
- An obstruction cephalad to the azygos vein causes the blood to return to right atrium through the ayzgos system and intercostal veins into the SVC (below figure B).
- An obstruction cephalad to the azygos vein causes the blood to return to right atrium through the ayzgos system and intercostal veins into the SVC (below figure B).
- Obstruction at the level of the azygos vein
- In SVC obstruction at the level of azygos, blood cannot re-enter the SVC through the azygos system and is forced to utilize other collateral veins, leading into the inferior vena cava (IVC) and from there into the right atrium (below figure C).
- The resulting symptoms are usually severe.
- Obstruction distal to the azygos vein
- In SVC obstruction below the level of azygos vein, blood will be redirected via a robust azygos and hemi-azygos system in a retrograde manner ultimately to the IVC, hence causing less severe symptoms (below figure D).
- This will lead to milder symptoms.
Diagnosis
- SVC syndrome may be suspected based on characteristic symptoms and signs of thoracic central venous obstruction.
- Diagnosis is confirmed by imaging which shows thoracic central venous obstruction.
Initial evaluation
- Imaging
- Contrast enhanced CT
- Advantage
- Optimal visualization of the SVC
- Localizing the extent of venous blockage
- Differentiation of thrombosis from extrinsic compression
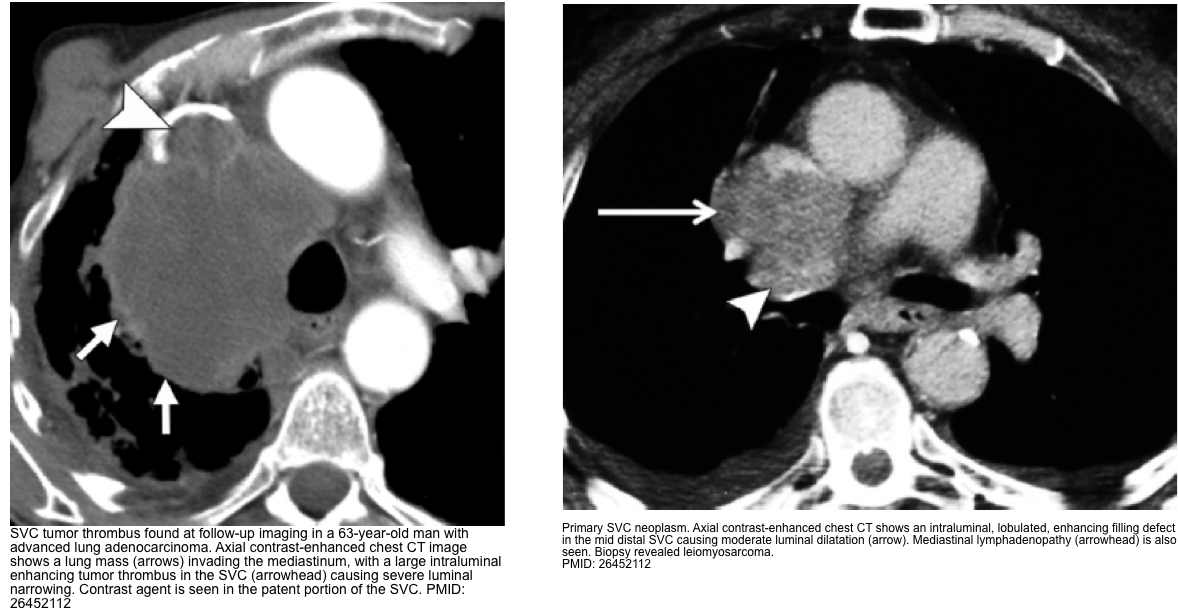
- Bland thrombus appears as central or eccentric nonenhancing filling defect in the SVC, usually in relation to a CVC or pacer lead
- Tumor thrombus often shows heterogeneous contrast enhancement, is generally larger and lobulated. It is associated with an adjacent mass or wall invasion.
- Identification of collateral pathway.
- Identifying the underlying associated primary etiology
- CT findings include *
- Lack of opacification of SVC
- Presence of an intraluminal filling defect or narrowing of the SVC
- Visualization of the collateral pathway
- Note that the presence of collateral vessels on contrast-enhanced CT is a very accurate predictor of clinically relevant and symptomatic SVC syndrome, while delineation of SVC obstruction alone is a less specific predictor *.
- Indication: CECT is the diagnostic modality of choice in following conditions
- Patients with grade 3 and 4 SCV syndrome.
- Grade 1-2 patients who have a known malignancy that is associated with SCV syndrome.
- Advantage
- Contrast enhanced CT
-
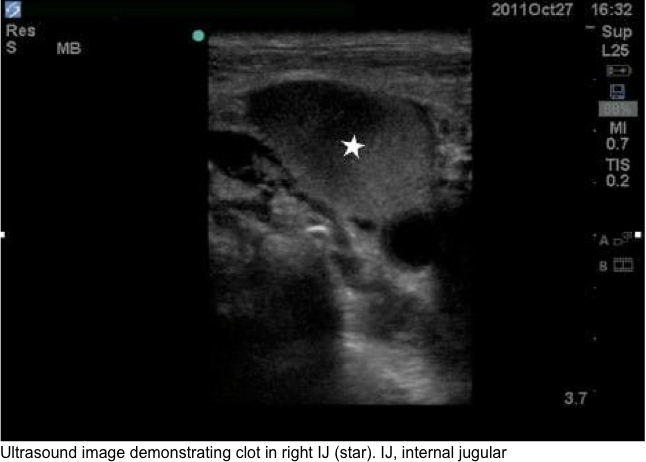
- Duplex ultrasound
- Advantage
- Showing thrombus in the jugular, subclavian, and the axillary veins.
- Identifying indwelling device-associated thrombi
- Identifying the upper extremity access site for venography and endovascular intervention.
- Disadvatage
- Duplex is not helpful to show SVC and brachiocephalic veins.
- Duplex is not helpful to show SVC and brachiocephalic veins.
- Diagnostic findings
- Direct findings:presence of clot in a vein (image below).
- Indirect evidence of central venous obstruction is shown by monophasic waveforms or lack of normal respiratory phasicity in the brachiocephalic, internal jugular, or subclavian veins *.
- Indications
- For grade 1 and 2 SVC syndrome, Duplex is useful for excluding thrombus in the subclavian, axillary, and brachiocephalic veins.
- It is the initial imaging study for patients with mild symptoms who have an indwelling device or a malignancy at low risk to cause SVC syndrome who present with extremity swelling.
- Advantage
- Conventional catheter-based digital subtraction venography
- It is the gold standard for evaluation of SVC obstruction, including the presence of thrombus.
- Disadvantage
- Not helpful to evaluate the specific cause of extrinsic SVC compression.
- Magnetic resonance venography
- It is equally sensitive and specific, when compared with conventional venography, in identifying SVC obstruction.
- Advantage
- Can be performed in patients who have contrast dye allergy, or in cases where venous access cannot be obtained.
- Duplex ultrasound
- Labs: Coag profile, renal and liver profile.
General approach to management
- Assess “ABCD” and determine the severity of symptoms (as this has therapeutic implication)
- Be prepared for difficult airway intubation (due to neck, larynx, tracheal edema)
- Be prepared for hemodynamic instability secondary to pleural effusion, and cardiac tamponade *.
- Cerebral edema can cause brain herniation and coma.
- Head should remain elevated.
- ⚠️Avoid upper extremity IM drug administration.
- ⚠️Avoid overhydration. Diuretic can be used if needed to maintain euvolemia.
- Steroid may be helpful in just two situations:
- Patients receiving radiotherapy on an emergency basis for severe airway obstruction that is not amenable to stenting, a short course of high-dose corticosteroids to minimize the risk of central airway obstruction secondary to edema.
- Symptomatic SVC syndrome caused by steroid-responsive malignancies, such as lymphoma or thymoma.
- Systemic anticoagulation is required (if not contraindicated) in patients with SVC syndrome and thrombosis.
Initial resuscitation and treatment
- The proposed management algorithm of SVC syndrome based on severity of symptoms is represented below.
- General principles involve:
- Grade 4 (life-threatening symptoms) represents a true medical emergency
- Secure airway, breathing and circulation.
- Immediate endovenous recanalization with SVC stent placement is required to decrease the risk of sudden respiratory failure and death * .
- If extensive thrombosis has been shown, direct thrombolytic or thrombectomy is warranted.
- Symptomatic patients without life-threatening symptoms
- Endovascular stent placement should be considered. This will resolve the SVC symptoms rapidly.
- For patients with proven chemo-sensitive tumors, chemotherapy is required
- For patient with radio-sensitive tumor, RT should be strongly considered.
- Grade 4 (life-threatening symptoms) represents a true medical emergency
| Pearl 🛑The management of patients with life-threatening SVC syndrome is evolving from radiation therapy to endovascular therapy (ET) as the first-line treatment. |

Appendix
Media
Detection of pleural effusion: Ultrasound
Pleural effusion volume: Ultrasound methods
Theraapeutic thoracentesis: US guidance for pleural catheter placement
References
1. Lai-Fook SJ. Pleural mechanics and fluid exchange. Physiol Rev. 2004 Apr;84(2):385-410. doi: 10.1152/physrev.00026.2003. PMID: 15044678.
2. PMID: 31640432. Ferreiro L, Toubes ME, San José ME, Suárez-Antelo J, Golpe A, Valdés L. Advances in pleural effusion diagnostics. Expert Rev Respir Med. 2020 Jan;14(1):51-66. doi: 10.1080/17476348.2020.1684266. Epub 2019 Nov 5.
3. PMID: 36874438. Bediwy AS, Al-Biltagi M, Saeed NK, Bediwy HA, Elbeltagi R. Pleural effusion in critically ill patients and intensive care setting. World J Clin Cases. 2023 Feb 16;11(5):989-999. doi: 10.12998/wjcc.v11.i5.989.
4. PMID: 29466146. Feller-Kopman D, Light R. Pleural Disease. N Engl J Med. 2018 Feb 22;378(8):740-751. doi: 10.1056/NEJMra1403503.
5. PMID: 20646244. Walden AP, Garrard CS, Salmon J. Sustained effects of thoracocentesis on oxygenation in mechanically ventilated patients. Respirology. 2010 Aug;15(6):986-92. doi: 10.1111/j.1440-1843.2010.01810.x. Epub 2010 Jul 20.
6. PMID: 29699542. Wang Z, Cai QZ, Ban CJ, Chen D, Xu LL, Wang XJ, Wang Z, Yang Y, Lv XZ, Shi HZ. Improved heart hemodynamics after draining large-volume pleural effusion: a prospective cohort study. BMC Pulm Med. 2018 Apr 25;18(1):62. doi: 10.1186/s12890-018-0625-5.
7. PMID: 11779540. Traylor JJ, Chan K, Wong I, Roxas JN, Chandraratna PA. Large pleural effusions producing signs of cardiac tamponade resolved by thoracentesis. Am J Cardiol. 2002 Jan 1;89(1):106-8. doi: 10.1016/s0002-9149(01)02180-4.
8. PMID: 1638726. Vaska K, Wann LS, Sagar K, Klopfenstein HS. Pleural effusion as a cause of right ventricular diastolic collapse. Circulation. 1992 Aug;86(2):609-17. doi: 10.1161/01.cir.86.2.609.
9.PMID: 24407535. Chidambaram S, Sangareddi V, Ganesan G, Dhandapani VE, Ravi MS, Meenakshi K, Muthukumar D, Swaminathan N, Ravishankar G. An echocardiographic assessment of cardiovascular hemodynamics in patients with large pleural effusion. Indian Heart J. 2013 Dec;65(6):666-70. doi: 10.1016/j.ihj.2013.10.013. Epub 2013 Nov 9.
10. PMID: 31092513. Mercer RM, Corcoran JP, Porcel JM, Rahman NM, Psallidas I. Interpreting pleural fluid results . Clin Med (Lond). 2019 May;19(3):213-217. doi: 10.7861/clinmedicine.19-3-213.
11. PMID: 23206715.Thomas R, Lee YC. Causes and management of common benign pleural effusions. Thorac Surg Clin. 2013 Feb;23(1):25-42, v-vi. doi: 10.1016/j.thorsurg.2012.10.004.
12. PMID: 21757129. Brunelli A, Beretta E, Cassivi SD, Cerfolio RJ, Detterbeck F, Kiefer T, Miserocchi G, Shrager J, Singhal S, Van Raemdonck D, Varela G. Consensus definitions to promote an evidence-based approach to management of the pleural space. A collaborative proposal by ESTS, AATS, STS, and GTSC. Eur J Cardiothorac Surg. 2011 Aug;40(2):291-7. doi: 10.1016/j.ejcts.2011.05.020.
13. PMID: 17218577. Huggins JT, Sahn SA, Heidecker J, Ravenel JG, Doelken P. Characteristics of trapped lung: pleural fluid analysis, manometry, and air-contrast chest CT. Chest. 2007 Jan;131(1):206-13. doi: 10.1378/chest.06-0430.
14. PMID: 21281436. Chen CH, Shih CM, Chou JW, Liu YH, Hang LW, Hsia TC, Hsu WH, Tu CY. Outcome predictors of cirrhotic patients with spontaneous bacterial empyema. Liver Int. 2011 Mar;31(3):417-24. doi: 10.1111/j.1478-3231.2010.02447.x. Epub 2011 Jan 14.
15. PMID: 32585037. Banini BA, Alwatari Y, Stovall M, Ogden N, Gershman E, Shah RD, Strife BJ, Shojaee S, Sterling RK. Multidisciplinary Management of Hepatic Hydrothorax in 2020: An Evidence-Based Review and Guidance. Hepatology. 2020 Nov;72(5):1851-1863. doi: 10.1002/hep.31434. Epub 2020 Oct 22.
16. PMID: 12554036. Sherman SC. Reexpansion pulmonary edema: a case report and review of the current literature. J Emerg Med. 2003 Jan;24(1):23-7. doi: 10.1016/s0736-4679(02)00663-7.
17. PMID: 29653741. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018 Aug;69(2):406-460. doi: 10.1016/j.jhep.2018.03.024. Epub 2018 Apr 10. Erratum in: J Hepatol. 2018 Nov;69(5):1207.
18.PMID: 36983315. Li P, An J, Wang S, Hu X, Zeng T, Wan C, Shen Y, Wang T. Incidence and Prognostic Role of Pleural Effusion in Patients with Pulmonary Embolism: A Systematic Review and Meta-Analysis. J Clin Med. 2023 Mar 16;12(6):2315. doi: 10.3390/jcm12062315.
19. PMID: 33425521. Jackson K, Aujayeb A. Pleural Effusions in Pulmonary Emboli: A Single Centre Experience. Cureus. 2020 Dec 6;12(12):e11942. doi: 10.7759/cureus.11942.
20. PMID: 34527360. Hu K, Chopra A, Kurman J, Huggins JT. Management of complex pleural disease in the critically ill patient. J Thorac Dis. 2021 Aug;13(8):5205-5222. doi: 10.21037/jtd-2021-31.
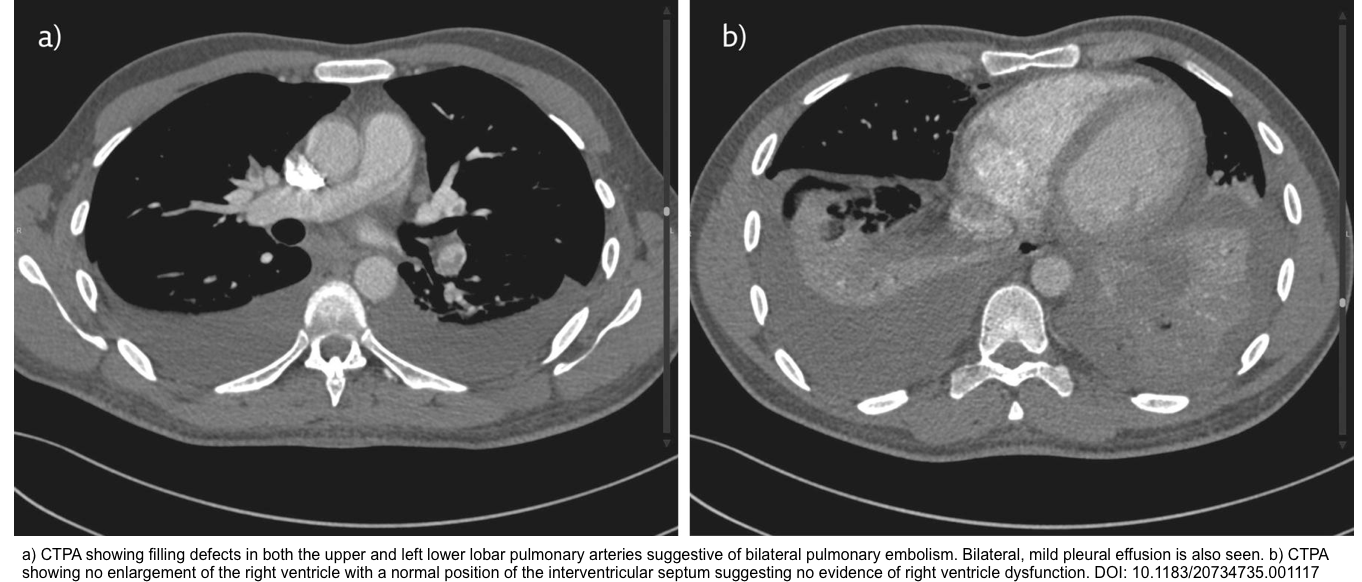
21. PMID: 29158780. Panjwani A, Zaid T. An interesting case of undiagnosed pleural effusion. Breathe (Sheff). 2017 Jun;13(2):e46-e52. doi: 10.1183/20734735.001117.
22. PMID: 34884236. Kulandaisamy PC, Kulandaisamy S, Kramer D, Mcgrath C. Malignant Pleural Effusions-A Review of Current Guidelines and Practices. J Clin Med. 2021 Nov 26;10(23):5535. doi: 10.3390/jcm10235535.
23. PMID: 28836006. Morin S, Grateau A, Reuter D, de Kerviler E, de Margerie-Mellon C, de Bazelaire C, Zafrani L, Schlemmer B, Azoulay E, Canet E. Management of superior vena cava syndrome in critically ill cancer patients. Support Care Cancer. 2018 Feb;26(2):521-528. doi: 10.1007/s00520-017-3860-z. Epub 2017 Aug 24.
24. PMID: 33357528. Azizi AH, Shafi I, Shah N, Rosenfield K, Schainfeld R, Sista A, Bashir R. Superior Vena Cava Syndrome. JACC Cardiovasc Interv. 2020 Dec 28;13(24):2896-2910. doi: 10.1016/j.jcin.2020.08.038.



Add comment