May 2, 2021, via Sahriar Lahouti. Last update July 14, 2024
CONTENTS
- Preface
- Definition
- Principles of management of tachydysrhythmias
- Wide Complex Tachyarrhythmias: Challenges
- Definition and Classification of VT
- Prognosis for SCD
- Monomorphic VT
- Electrical storm
- Polymorphic VT
- RECAP
- Going Further
- Appendix
- References
ACLS: Advanced Cardiovascular Life Support
AF: atrial fibrillation
AFL: atrial flutter
AIVR: Accelerated idioventricular rhythm
AMI: acute myocardial infarction
AVCR: atrioventricular conduction ratio
AVNRT: AV node re-entry tachycardia
AANRT: antidromic AV re-entry tachycardia
BB: beta blockers
BBB: bundle branch block
CCB: calcium channel blocker
CMP: cardiomyopathy
CPVT: Catecholaminergic polymorphic VT
EAD: early after depolarization
DAD: delayed after depolarization
Focal AT: focal atrial tachycardia
HCMP: hypertrophic cardiomyopathy
HD: hemodynamic
ICD: Implantable Cardioverter Defibrillator
IHD: ischemic heart disease
LBBB: left bundle branch block
MAT: multifocal atrial tachycardia
MVT: monomorphic ventricular tachycardia
NCT: narrow complex tachycardia
PMVT: polymorphic ventricular tachycardia
PMT: pace maker mediated tachycardia
pVT: pulseless ventricular tachycardia
PSVT: paroxysmal supraventricular tachycardia
OAVRT:orthodromic AV re-entry tachycardia
OHCA: Out-of-hospital cardiac arrest
RBBB: right bundle branch block
RWMA: regional wall motion abnormality
RVOT VT: right ventricular outflow tract VT
SCA: sudden cardiac arrest
SCD: sudden cardiac death
ST: sinus tachycardia
SVT: supraventricular tachycardia
Tdp: torsade de pointes
VA: ventricular arrhythmia
VT: ventricular tachycardia
VF: ventricular fibrillation
WCT: wide complex tachycardia
WPW: Wolff-Parkinson-White syndrome
Preface
Poor perfusion in critical patients with hemodynamic instability stems from problems with either cardiac pump function; pipe, tank, or a complex of multiple overlapping problems. Cardiac function (cardiac output) is primarily determined by heart rate and contractility and is severely impacted by heart rates at extreme (more on this here). When there are multiple overlapping perfusion problems in a patient, oftentimes the priority is given to correction of heart rate and rhythm with few exceptions. The “primacy of the heart rate” simply states that the more abnormal the heart rate, the more likely it is the primary cause of clinical instability. That’s why intervention to correct the abnormal heart rate has priority in resuscitation. In this post, tachyarrhythmias are explored with a focus on wide-complex tachycardia. For commonly used abbreviations in the text, refer to the above panel.
Definition
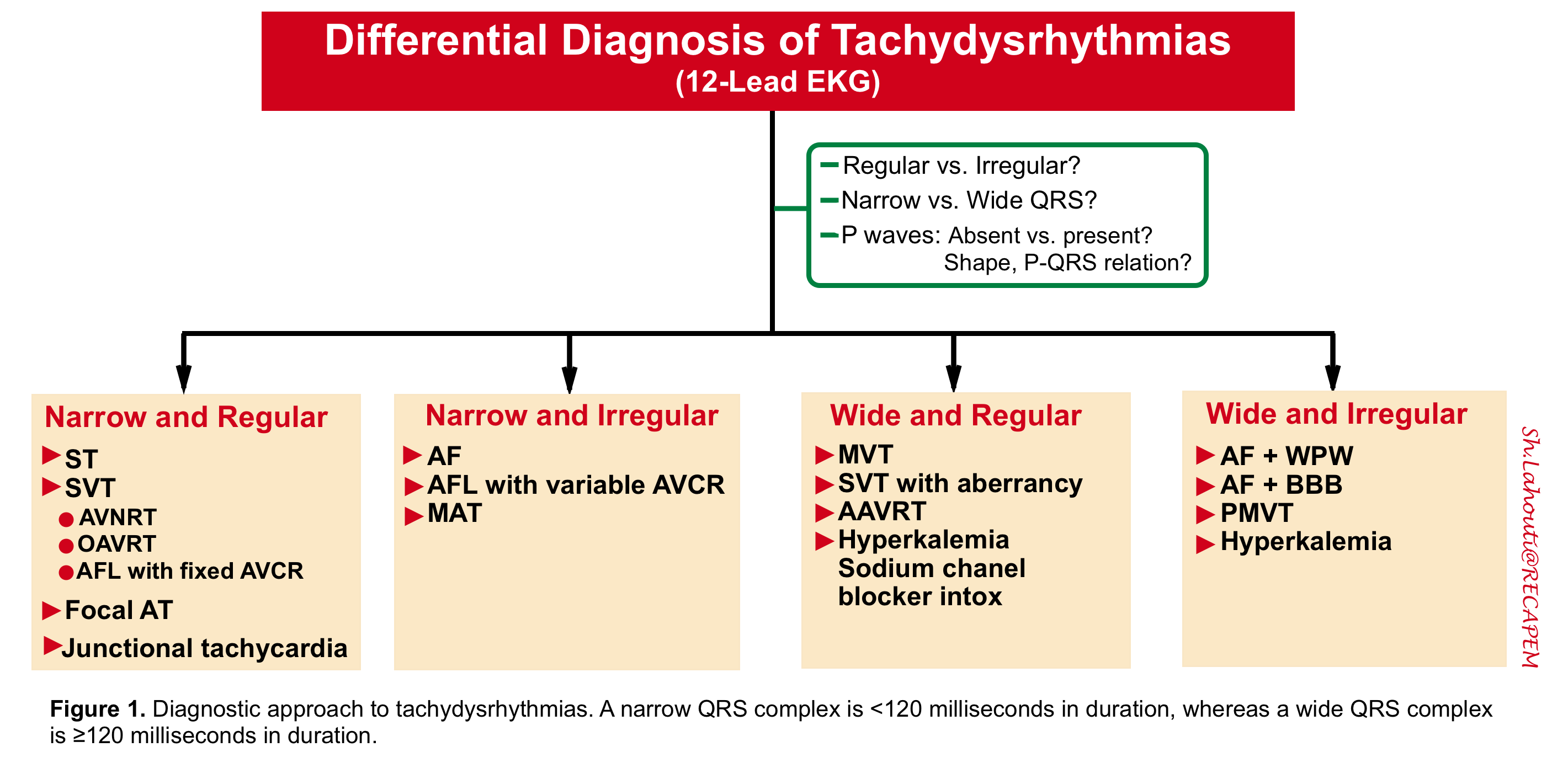
Tachyarrhythmias, are defined as abnormal heart rhythms with a ventricular rate of 100 or more beats per minute. From the diagnostic (and therapeutic) perspective, it is helpful to broadly classify tachyarrhythmias into 4 categories (figure 1) based on the width, morphology, and regularity of the QRS complex.
Principles of Management of Tachydysrhythmias
In patients who present with any tachyarrhythmias, two fundamental issues should be considered in the ED:
- The priority when evaluating a patient with tachyarrhythmias is an assessment of the patient’s hemodynamic stability 1. Unstable patients and those in cardiac arrest should be treated immediately (before an extensive diagnostic evaluation). Unstable conditions confer an imminent risk of further deterioration, irreversible organ injury, or cardiac arrest and include 2 3 4:
- Acute altered mental status
- SBP < 90 mmHg (MAP<65 mmHg). 👉Remember that cardiac output can be dangerously low while the patient maintains a “normal” BP. Blood pressure ≠ cardiac output.
- Ischemic chest pain
- Dyspnea from pulmonary congestion
- Extremely rapid ventricular rates approaching >220-240/min in adults, as seen with PMVT and AF with ventricular pre-excitation
- Clinicians should judge whether the heart rate is the primary cause of the clinical symptoms or a compensatory response to another pathology. The “Primacy of the Heart Rate” implies that heart rate at extreme should be considered for intervention in unstable patients with multiple overlapping perfusion problems; though there are some exceptions (see below). Although this is a tough decision, by and large, abnormal heart rate might be assumed as a primary cause of clinical instability when:
- Non-sinus tachycardia at > 170 bpm
- AF with rapid ventricular response > 130-150 bpm
- HR < 30 bpm (discussed separately)
💡Generally Heart rates less than 150 bpm are unlikely to be the cause of unstable symptoms unless the patient has abnormal myocardium at baseline due to valvular disease, cardiomyopathy, etc. If sinus tachycardia or AF with rapid ventricular response is present, the provider should identify and treat the underlying cause (i.e. hemorrhage, pulmonary embolism, volume depletion, sepsis, or cardiac tamponade,).
👉AF with rapid ventricular response (in the range of 110-150) is often an innocent bystander, especially in patients with chronic AF. In such patients, one should investigate for other causes of instability such as blood loss, sepsis etc (more on this here ).
Basic evaluation
1. ECG
Obtain a 12-lead ECG as it plays a pivotal role in the diagnosis of arrhythmias, acute ischemia, some electrolyte disturbances as well as certain drug intoxication. Categorization of the QRS as wide vs. narrow and the rate as regular vs. irregular offers an immediate approach to differential diagnosis (figure 1 above).
2. POCUS
Bedside ultrasound provides critical information virtually in any patient (through a wide range of clinical spectrum). It can help providers make decisions whether the tachycardia is the primary cause of instability or is a compensatory response to another pathology. For example, evidence of volume overload often implies that the rapid response is primary, whereas evidence of volume depletion may point toward a compensatory response (e.g., hemorrhage, sepsis). Moreover, ultrasound (echo) can also provide useful information for common causes of a patient’s hemodynamic deterioration such as:
- Cardiac tamponade
- Pulmonary embolism (more on this here and here)
- Decompensated heart failure
- AMI (visualizing RWMA)
- Devastating conditions such as aortic dissection
- Valvular emergencies such as acute mitral valve regurgitation and aortic regurgitation
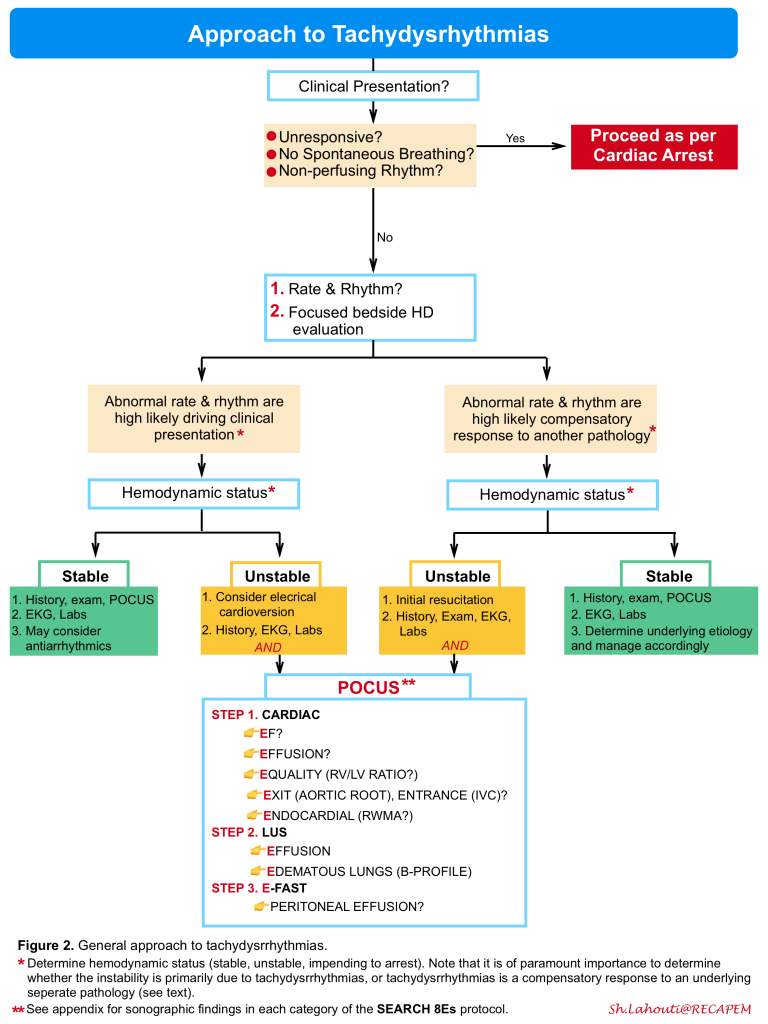
There are several ultrasound protocols for the evaluation of patients with signs and symptoms of poor perfusion in ED (e.g., Rapid Ultrasound in Shock(RUSH)30 Abdominal and Cardiac Evaluation in Shock (ACES)31 and Search 8Es 32, etc). The latter is incorporated in our diagnostic approach to patients with tachydysrhythmias below (figure 2). For sonographic findings in each category of the SEARCH 8Es protocol see appendix.
The application of bedside ultrasound during cardiac arrest has gained favor and several studies have shown promising results 33. This will be discussed separately.
Patient with OHCA whose cardiac monitoring revealed asystole, while echo shows quivering mitral valves. This is fine VF and the patient should be defibrillated immediately.
3. Labs
Order CBC, electrolytes, and renal function. Use clinical context to decide on requesting other labs:
- Troponin: It is reasonable to request troponin for ventricular tachycardia. Note that most patients with PSVT do not require troponin.
- TSH: may be requested in selected patients.
- Other labs may be requested based on underlying conditions and comorbidities.
Algorithm
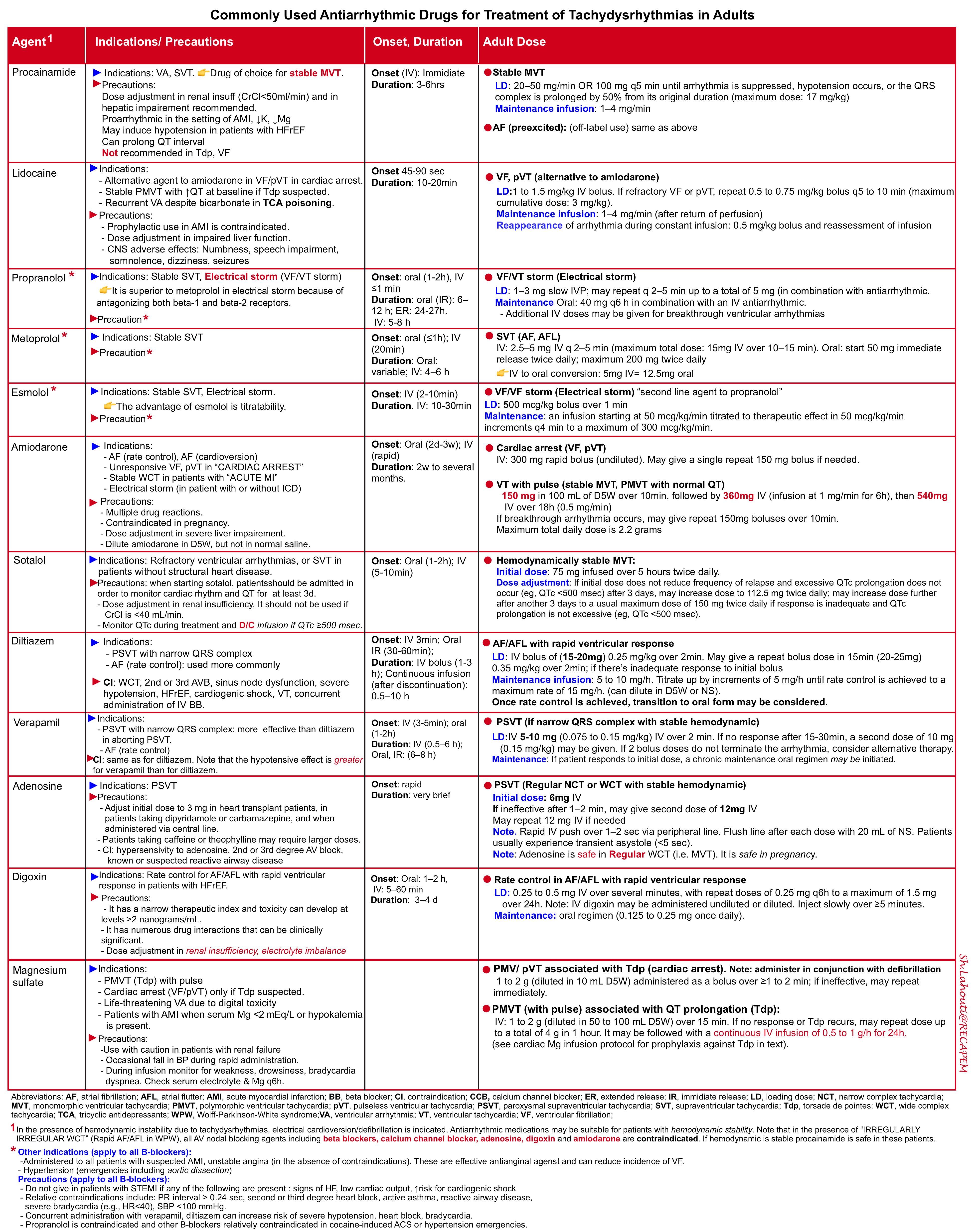
Antiarrhythmic medications
The most commonly used antiarrhythmic medications in the ED for select patients with hemodynamic stability are summarized in the following table (figure 3). For more on the mechanism of antiarrhythmic drugs see appendix.
👉Stable NCT due to AVNRT can be terminated by adenosine, BBs, CCBs, and amiodarone. Selecting the first line of pharmacological treatment depends on the patient’s comorbidities and drug side effect profile. However, verapamil is strongly effective in the termination of AVNRT.
👉For ventricular rate control in the setting of AF, and AFL; medications such as BBs, CCBs, amiodarone, and digoxin can be selected based on patients’ comorbidities and drug side effects. Diltiazem is the most frequently used drug in patients with relatively normal LVEF. Hypotension is less common than verapamil.
👉AF + WPW (EKG: irregular WCT): Electrical cardioversion is often selected. All AV nodal blocking agents including beta-blockers, calcium channel blockers, adenosine, digoxin, and amiodarone are contraindicated. Procainamide is safe.
Electrical treatment
Generally, it is a more safe and effective treatment than any antiarrhythmic medication if performed in an appropriate setting. Do not be afraid of electricity!
Synchronized cardioversion is indicated for all unstable tachycardia (HR > 150 bpm). In critical condition go immediately to unsynchronized shock. To deliver sync shock; engage sync mode before each attempt and look for sync markers on the R wave. Ensure that the device is not syncing to the T-wave (to avoid the R-on-T phenomenon) 5 .
- Regular NCT: Start with 50-100 J. If it fails, increase it in a stepwise fashion.
- Irregular NCT: Start with 200j monophasic or 120-200j biphasic and then increase in a stepwise fashion.
- Regular WCT: Start with 100j, and if fails; increase in a stepwise fashion.
- Irregular WCT: Unsynchronized 360j monophasic, or biphasic device-specific defibrillation dose
Other treatments
▪️If there is any concern for hyperkalemia as the cause of WCT, immediate treatment with CaCl 1g IV bolus should be given, regardless of patient stability, followed by reassessment of the EKG and laboratory confirmation.
▪️If sodium channel blocker intoxication is suspected to be the cause of WCT; immediate treatment with sodium bicarbonate 150 mEq IV bolus should be started and response to treatment be assessed.
Wide Complex Tachyarrhythmias: Challenges
Wide complex tachycardia (QRS duration ≧ 120 msec) 6 represents a unique clinical challenge for two reasons:
#1. Diagnosing the arrhythmia is difficult: Although most WCTs are due to VT, the differential diagnosis includes a variety of SVTs (figure 1). Diagnostic algorithms to differentiate these two etiologies are complex and imperfect 7 8 . (see below)
#2. Urgent therapy is often required – Patients may be unstable at the onset of the arrhythmia or deteriorate rapidly at any time, particularly if the WCT is VT or SVT at an extremely rapid rate (e.g., >200 beats per minute).
Definition and Classification of VT
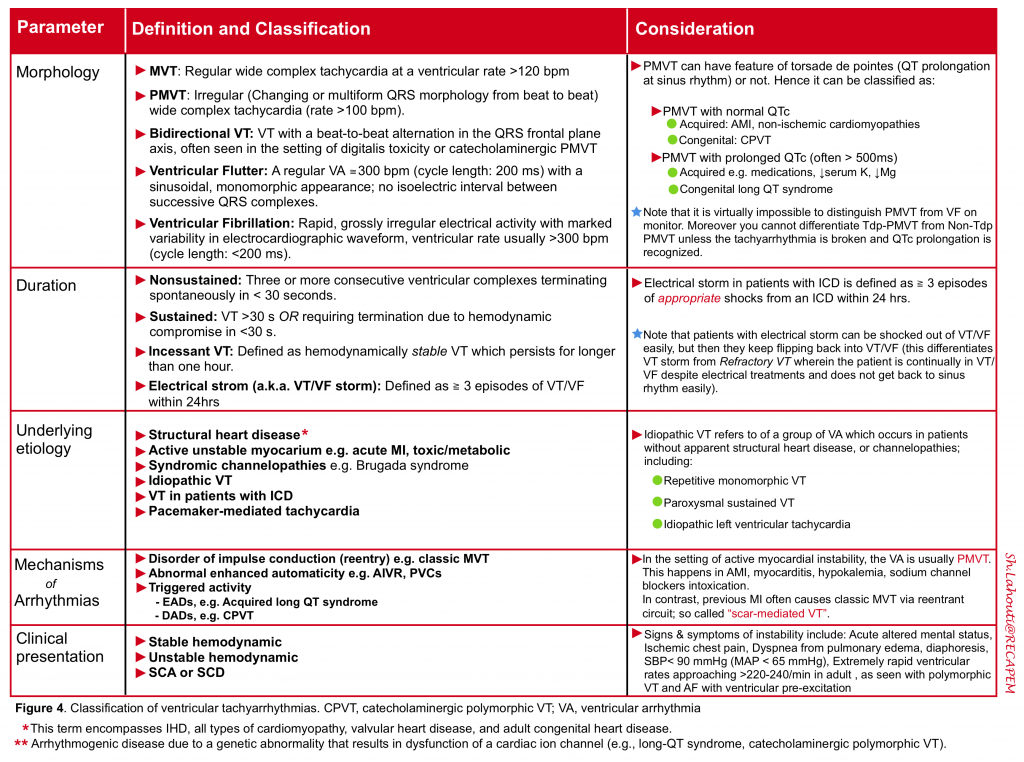
Ventricular Tachycardia (VT) is a wide complex tachycardia originating in the ventricles. VT can be classified based on the following parameters 9 (figure 4).
Prognosis for SCD
The prognosis of patients presenting with VT depends on several parameters that have been associated with risk for sudden cardiac death (SCD). Indices for poor prognosis may include:
- Sustained VT
- Polymorphic VT/VF
- Clinical presentation with syncope, hemodynamic instability, SCD
- Structural heart disease (e.g. Previous MI, IHD, CMP, HCMP) and syndromic channelopathies
Patients at high risk of “SCD” may be candidates for secondary prophylaxis with ICD or catheter ablation following electrophysiologic studies 38.
Monomorphic VT (MVT)
Classic Monomorphic VT
Wide and regular ventricular tachyarrhythmia at rate > 120 bpm is assumed VT until proven otherwise.
Among the ventricular tachydysrhythmias, the “classic” VT (monomorphic VT) is overwhelmingly the most common and most important etiology encountered.
Pathophysiology
The mechanism of “classic VT” is commonly reentrant, with perhaps the most common etiology being “scar-mediated” reentrant circuit VT (e.g. previous MI) 10.
👉Keep in mind that primary VF and polymorphic VT (PMVT) without the presence of QT prolongation on the baseline ECG most commonly occur in the setting of acute myocardial ischemia or infarction. Most patients with monomorphic VT (MVT) have underlying structural heart disease (e.g., previous MI, HF). Acute ischemia is not associated with MVT, rather it may facilitate the conversion of MVT to VF.
Clinical Presentation
Symptoms during VT depend on the ventricular rate, duration of the tachycardia, and the presence and extent of the underlying heart disease and peripheral vascular disease. VT can occur in several forms including; short, asymptomatic, nonsustained episodes; sustained, hemodynamically stable events (generally occurring at slower rates or in otherwise normal hearts); or unstable runs, often resulting in hemodynamic collapse and degenerating into VF. VTs are initially nonsustained but can later become sustained.
Differential diagnosis
- Regular SVT (e.g. ST) with aberrancy
- Antidromic AVRT
- Hyperkalemia: HR usually is < 120 bpm
- Sodium channel blocker intoxication (often very wide QRS > 200 ms)
- Accelerated Idioventricular Rhythm (AIVR): It is a regular wide complex rhythm with ventricular response at 50-110 bpm. This is a reperfusion rhythm often seen post-lytics for STEMI; Think of “slow VT”. The treatment is observation, not medication. 👉Pitfall: Mistaking AIVR (post-lytics for STEMI) for VT and treating with lidocaine may cause cardiovascular collapse.
- Pacemaker mediated tachycardia
In the absence of hyperkalemia or other toxicologic/metabolic causes of QRS widening, the differential of a wide regular tachycardia is VT vs. supraventricular tachycardia (SVT) with aberrancy.
👉👉It is safest to assume regular WCT as VT until proven otherwise (for the following reasons):
- VT is far more common than SVT, by a factor of four in unselected populations and by as much as 10-fold in patients with prior myocardial infarction.
- Among patients with WCT, VT is much more common than SVT with aberrant conduction.
- More importantly, inadvertently treating VT as though it were SVT could precipitate hemodynamic collapse and/or cardiac arrest, while “mistreating” SVT as though it were VT will does not cause a clinically significant adverse effect.
- Despite multiple ECG algorithms and rules to distinguish VT from SVT with aberrancy (Brugada, Wellens,etc) none are better than 90% specific to identify SVT with aberrancy. No feature or combination of ECG features is 100% specific for SVT with aberrancy. Hence, using an algorithm/rule, there is a 10% chance that you will label VT as SVT with aberrancy erroneously and if you treat the patient with AV nodal blockers, cardiovascular collapse may result.
👉Useful parameters in history and clinical presentation favoring the diagnosis of VT may include:
- Prior MI, history of CABG, stent in place
- Heart failure
- Recent angina
- Cardiomyopathy
- Advanced age: although advanced age makes a wide complex tachycardia being VT much more likely than SVT with aberrancy, up to 50% of patients under 40 years of age who present with a wide complex regular tachycardia have VT. So VT is also common in the young age group!
- Family history of SCD: suggesting conditions such as HOCM, congenital long QT syndrome, Brugada syndrome, or arrhythmogenic right ventricular dysplasia that are associated with episodes of VT.
👉Response to adenosine does not rule out VT.
👉Clinical stability does not differentiate between VT and SVT with aberrancy either. Some patients with monomorphic VT are stable!
👉The presence of hemodynamic stability should not be regarded as diagnostic of SVT.
A general approach to management:
Management decisions can be stratified into those involved in acute management (or termination of VT) and those involved in long-term therapy (or prevention of recurrence or SCD)
Acute management: The first priority when evaluating a patient with a WCT is the assessment of patient stability.
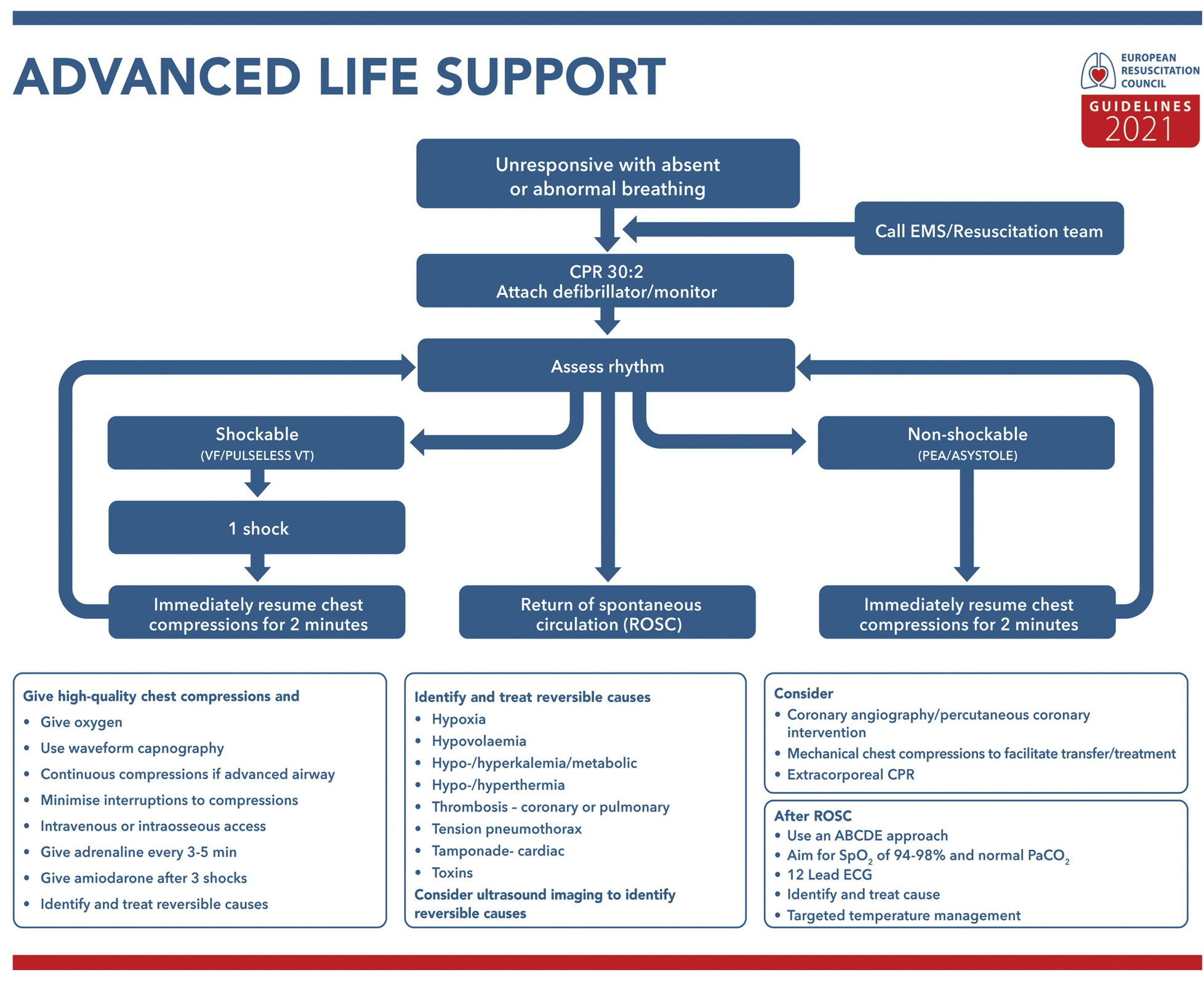
Patient in cardiac arrest: Patients who become unresponsive or pulseless are considered to have a cardiac arrest and are treated according to standard ACLS algorithms (figure 5).
👉Antiarrhythmic drug for refractory VF/VT in cardiac arrest: Amiodarone is the initial drug of choice 11.
Based on a single controlled trial with a survival to-hospital admission endpoint, IV amiodarone emerged as the initial treatment of choice. Lidocaine may be given intravenously for patients in whom amiodarone is unsuccessful and possibly for those who have an acute transmural MI as the triggering mechanism for cardiac arrest. IV procainamide is rarely used in this setting.
A recent study, randomizing IV amiodarone or lidocaine against a placebo, showed no difference between either drug or placebo for survival to discharge outcomes or survival with favorable neurologic status 12. However, survival to hospital admission was significantly better with both active drugs compared to placebo.
👉Double sequential defibrillation for VF/VT in cardiac arrest? Although some case reports have shown good outcomes, a 2020 ILCOR systematic review found no evidence to support double sequential defibrillation and recommended against its routine use. A recent pilot RCT suggests that changing the direction of the defibrillation current by repositioning the pads may be as effective as double sequential defibrillation while avoiding the risks of harm from increased energy and damage to defibrillators. Based on current evidence, it is not known whether double sequential defibrillation is beneficial 13.
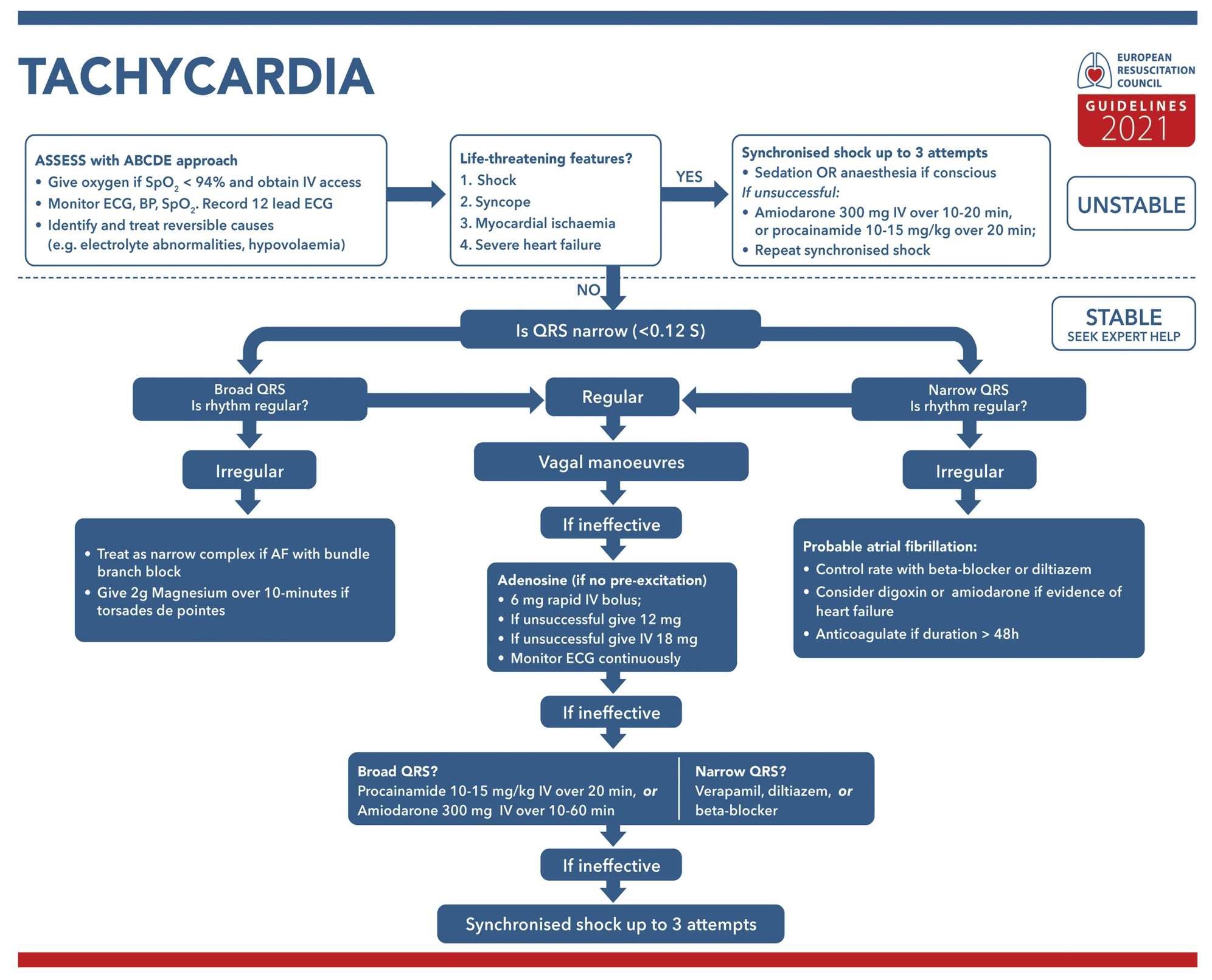
Unstable patients: In this setting, emergency synchronized cardioversion (after intravenous sedation, whenever possible) is the treatment of choice regardless of the mechanism of the arrhythmia. This should be done immediately, before an extensive diagnostic evaluation (figure 6).
Stable patients: A stable patient with WCT shows no evidence of hemodynamic compromise despite a sustained rapid heart rate. Such patients should have continuous monitoring and frequent reevaluations due to the potential for rapid deterioration as long as the WCT persists (figure 6).
- MVT (regular WCT) that does not cause hemodynamic decompensation can be treated medically to achieve acute termination by IV administration of adenosine, amiodarone, or procainamide.
- If the arrhythmia does not respond to medical therapy, electrical direct-current (DC) cardioversion can be used.
💌Remember that hanging your hat on mean arterial pressure for determining hemodynamic stability is not reasonable, since tissue perfusion correlates well with cardiac output, but not blood pressure (see here). Many patients with known heart disease and VT may not be considered “unstable” according to ACLS guidelines but nonetheless may have poor cardiac output and be unable to tolerate antidysrhythmic medications. 👉In patients with known structural heart disease and VT, even with normal BP, consider incipient shock and immediate cardioversion.
Initial evaluation in stable patients: The primary goals of the initial evaluation of a stable patient with WCT are to determine the etiology of the WCT and to elucidate any underlying conditions related to the event (e.g. heart failure, myocardial ischemia, drug reaction, or electrolyte abnormalities). In a stable patient, a focused clinical evaluation should include the following:
History: Age, history of heart disease, presence of an implantable cardioverter-defibrillator (ICD), presence of a pacemaker, medications and comorbidities such as renal insufficiency.
Exam: In addition to vital signs, pay specific attention to signs of poor perfusion such as acute confusion, ↑capillary refill time, diaphoresis, and pulmonary edema. The presence of a fistula should raise suspicion of hyperkalemia contributing to the arrhythmia.
EKG, Labs, and POCUS (see above)
Pharmacotherapy
For stable WCT, “Procainamide” is the drug of choice 14.Amiodarone and lidocaine are less effective 9 15 16.
Scar-mediated MVT is the classic VT seen in older patients with a cardiac history (e.g. past MI). The drug of choice for these patients is Procainamide 14.
Adenosine may be used for undifferentiated Regular Monomorphic Wide Complex Tachycardia 17.
If hyperkalemia is suspected as the cause of VT, immediate treatment with IV CaCl should be given regardless of patient stability, followed by reassessment of the EKG and laboratory confirmation.
If sodium channel blocker intoxication is suspected to be the cause of VT; immediate treatment with sodium bicarbonate IV bolus should be started and response to treatment be assessed.
Electrical treatment
Despite hemodynamic stability synchronized cardioversion is frequently used as the first option for patients with scar-mediated MVT. If pharmacotherapy fails, cardioversion is selected for appropriate candidates (with procedural sedation when feasible).
Special conditions
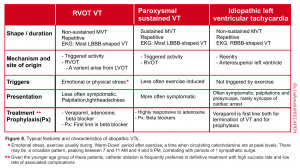
Idiopathic VT
It refers to a group of ventricular tachycardia that occurs in patients without apparent structural heart disease, or channelopathies. The basic difference between classic and idiopathic VT is presented in the table below (figure 7).
Three commonly recognized syndromes are repetitive monomorphic VT (a.k.a. right ventricular outflow tract “RVOT” VT), paroxysmal sustained VT and idiopathic left ventricular tachycardia. The characteristic features of each are summarized in the following table (figure 8).
Diagnosis of idiopathic VT may require electrophysiologic study and expert consultation is recommended. oftentimes these patients are young and less symptomatic.
👉Do not administer verapamil or beta blocker to patients with WCT whose clinical presentation may fit into a group of idiopathic VT. Expert consultation is warranted.
VT in patients with ICD
Patients with ICD represent a specific high-risk population in whom WCT is much more likely to be VT. Patients who have ICD for primary prevention are felt to be at high risk for developing life-threatening ventricular arrhythmia, whereas those patients with ICD for secondary prevention have experienced malignant ventricular arrhythmia or sudden cardiac arrest. Therefore any WCT in patients with ICD should be assumed “VT”. ICD patients with recent electrical shock(s) should be under close observation and continuous cardiac monitoring.
ICD patients can present to ED for several related complaints; broadly classified into:
- Appropriately delivered shock by ICD: The patient had a true episode(s) of concerning arrhythmia and ICD was shocked appropriately.
- Inappropriately delivered shock by ICD: Patient had no arrhythmia on device interrogation and no concerning clinical features. In this situation, ICD has delivered shock inappropriately. The application of a magnet can put the device into VVI mode (pacing preserved, shocking disabled).
- Inappropriately not delivered shock by ICD: The patient presents with concerning clinical features (palpitation, pre-syncope) but the ICD did not detect the events.
The systemic approach to ICD patients with recent shock(s) or concerning clinical features is presented below (figure 9)
VT in patients with ICD can happen due to the following reasons 36 40
- When the VT has a slower rate e.g. < 175 bpm (which is below the device detection threshold which is usually set at > 175 bpm). In other words, VT is too slow for ICD to recognize. Manage VT just like any other VTs.
- When recurrent episodes of VT happen (frequent VT happens above the detection threshold e.g. > 175 bpm). In this situation, consider
- Possible causes such as severe HF, electrolyte imbalance, ICD malfunction
- Prophylactic measures are taken to minimize the frequency and recurrence of VTs including administration of amiodarone, beta blocker, and sedation 36.
Pacemaker-mediated Tachycardia (PMT)
It occurs in the setting of a dual-chamber pacemaker. Pacemaker-mediated tachycardia can be caused via two mechanisms 18:
- In a patient who has a functional AV node, the PM-mediated tachycardia is initiated by a PVC, which causes a retrograde action potential up the AV node followed by retrograde atrial depolarization. The atrial lead of the dual-chamber pacemaker is designed to detect inherent atrial activity; thus, it detects the retrograde P-wave and triggers an impulse via the ventricular lead. The resulting ventricular action potential starts from the ventricular lead and arrives at the AV node after it has repolarized and is ready to accept another retrograde action potential, thereby continuing the reentrant loop
- Another mechanism of pacemaker-mediated tachycardia occurs when a patient with a dual-chamber pacemaker develops any atrial tachycardia that is sensed by the atrial lead, triggering a matching ventricular-paced beat up to the maximal ventricular output rate setting of the pacemaker
⭐️Treatment: The application of a magnet turns off the anti-tachycardia function (including defibrillation and synchronized cardioversion) of an implanted cardiac device, as well as the sensing function of a pacemaker (rendering the device simply a pacemaker at a set rate regardless of the patient’s intrinsic function).
Electrical storm
Definition: Generally defined as three or more episodes of VF or sustained VT within 24 hours.
Patients can be shocked out of VT/VF easily, but then they keep flipping back into VT/VF (this differentiates VT storm from refractory VT wherein the patient is continually in VT/VF and never goes back to sinus rhythm) 19.
👉The problem with VT Storm isn’t generally breaking any individual episode of VT. Rather, the problem is often that the VT keeps on coming back – so it’s difficult to keep patients out of VT for a prolonged period of time.
Clinical presentation
- If a patient has an ICD (implanted cardiac defibrillator), this may present with recurrent ICD firing.
- If a patient doesn’t have an ICD, this may cause recurrent symptoms. Depending on the heart rate and cardiac function, symptoms may range from palpitations to recurrent cardiac arrest.
Pathophysiology
- The electrical storm is a vicious cycle.
- VT/VF increases intracellular calcium levels, which may be pro-arrhythmic 39.
- Shocks and episodes of cardiac arrest (e.g. treated with epinephrine) may cause myocardial injury.
- Myocardial injury & pain stimulate an outpouring of endogenous catecholamines 37, promoting recurrent arrhythmia.
- That’s why medications that suppress sympathetic surge play a key role in management. These may include providing a generous amount of analgesia/sedation, beta-blockers e.g. propranolol, and antiarrhythmic with beta-blocker properties i.e. amiodarone.
- 💡Key point: The worst approach is watchful waiting since the natural history of VT storms is generally to deteriorate (even despite the use of standard ACLS algorithms to interrupt each individual episode of VT). Aggressive therapy is needed to stop this process.
- Remember that the therapeutic approach to Tdp storm is different from the VT storm (see Figure 10).
Etiologies
- Most patients have structural heart disease (commonly ischemic cardiomyopathy)20. If the patient has a structurally normal heart, this suggests a rare form of arrhythmia requiring specific management (e.g. Brugada syndrome, catecholaminergic polymorphic VT). However, careful assessment is required as some of the known triggers are reversible, including 21 40
- Drug toxicity
- Electrolyte disturbances (i.e, hypokalemia and hypomagnesemia)
- New or worsened heart failure
- Acute myocardial ischemia
- Thyrotoxicosis
- QT prolongation (which may be related to drug toxicity, electrolyte imbalance, or an underlying syndrome such as long QT syndrome)
- Heart failure exacerbation, volume overload
- Medication toxicity, substance abuse (e.g. sympathomimetics)
- Medication non-adherence
- Sepsis 19
Management
- The initial treatment approach for patients with electrical storms or incessant VT is based on hemodynamic stability or instability. Patients with cardiac arrest or hemodynamically unstable ventricular arrhythmias should initially undergo electrical cardioversion according to advanced cardiac life support protocol.
- Patients with electrical storms who are hemodynamically stable should be treated with both intravenous (IV) antiarrhythmic therapy and a beta blocker in addition to correcting possible electrolyte abnormalities, especially hypokalemia and hypomagnesemia (figure 10):
- Antiarrhythmic medication: The first line antiarrhythmic drug is “Amiodarone” (rather than lidocaine and procainamide). Amiodarone should be given as 150 mg IV over 10 minutes, followed by 1 mg/minute IV infusion for 6 hours, followed by 0.5 mg/minute IV infusion for 18 additional hours.
- Beta-blocker: Non-selective beta blocker “Propranolol” is superior to metoprolol because of antagonizes both beta-1 and beta-2 receptors 22. As an alternative, IV esmolol infusion is supported by evidence as well 23.
- Propranolol: IV regimen: Bolus 0.15 mg/kg IV over 10 minutes, then 3-5 mg IV Q6hr 24. Oral regimen: Propranolol 40 mg PO q6hrs has been proven superior to metoprolol (50 mg q6hr) 22.
-
Esmolol: IV Loading dose is 0.3-0.5 mg/kg IV (~30 mg). Start infusion at 0.050 mg/kg/min (~3 mg/min). May re-load & up-titrate infusion in increments of 0.05 mg/kg/min every 10 minutes. The maximum dose is 0.3 mg/kg/min. The advantage of esmolol is titratability. From a mechanistic standpoint, it may be less effective than propranolol because it lacks efficacy at beta-2 receptors.
Sedation
- It can break the VT storm if provided adequately. Propofol or benzodiazepine (if cannot intolerable to propofol) may be choosen.
Stellate ganglion block
- A systematic review and meta-analysis of 22 unique case series with a total of 35 patients has shown a dramatic reduction in arrhythmia burden 25. Other studies have shown its safety and efficacy in the termination of VT storm 26. For performing the procedure, you can see here and here for ultrasound-guided approach.
Treatment of the causes of electrical storm
- Revascularization & therapy for myocardial infarction: AMI is an important cause of VT storm. In an appropriate setting where active ischemia is felt to be a contributing factor, additional therapies for ACS should also be considered (e.g. aspirin, P2Y12 inhibitor) and emergent coronary revascularization should be pursued.
- ICD optimization: Any patient with an ICD requires device interrogation. Ensure that the device is truly detecting VT (rather than over-responding to artifact). Anti-tachycardia overdrive pacing may be optimized to break episodes of VT without requiring shocks 36.
- Catheter ablation of VT/VF: Once the patient is stabilized, ablation may be considered especially for monomorphic VT storm
- Decompensated heart failure: beta-blockers, ACE inhibitors, and diuretics are considered and titrated to maximally tolerated doses
- Correction of any identified inciting factors should occur. This may include the removal of any offending drugs (eg, prescription or illicit drugs which prolong the QT interval) and correction of any electrolyte disturbances (ie, hypokalemia, hypomagnesemia, etc).
Polymorphic VT (PMVT)
▪️Background
- Irregular Wide QRS Tachycardia with rapidly changing QRS morphology and shape from beat to beat.
- Polymorphic VT may be caused by several etiologies. PMVT is often equated to torsade de pointes, but it’s not the same thing.
- Torsades de pointes is polymorphic VT that occurs in the setting of a long-QT interval and is characterized by a waxing and waning QRS amplitude.
- It commonly happens in the setting of underlying bradycardia.
- Torsades de pointes is polymorphic VT that occurs in the setting of a long-QT interval and is characterized by a waxing and waning QRS amplitude.
- PMVT is usually a non-perfusing rhythm; hence patients are hemodynamically unstable.
- Immediate treatment is unsynchronized cardioversion. However, treatment for intermittent or recurrent PMVT depends on the patient’s presentation and the QTc interval during the intermittent sinus rhythm.
▪️Pathophysiology
- There is often an underlying “Dynamic Unstable” myocardium e.g. AMI, myocarditis, and dynamic changes in QTc.
Causes
- J-wave syndrome: QTi is generally normal but can be prolonged in MI, Takotsubo, or hypothermia. Etiologies may include:
- Genetic channelopathy:
- Brugada syndrome.
- Early repolarization.
- Acute myocardial ischemia.
- Takotsubo cardiomyopathy (acute phase).
- Hypothermia.
- Genetic channelopathy:
- Torsade de pointes (QT prolongation is the primary abnormality)
- Acquired: Medications that cause prolongation of QT, ↓K, ↓Mg. Refer here for a list of QT-prolonging drugs.
- Congenital: Congenital long QT syndromes (LQTS).
- Catecholaminergic polymorphic VT (CPVT)
- This is a genetic disorder presenting in familial and sporadic forms.
- Patients are either asymptomatic (identified by familial screening) or they may present with syncope or stress-induced (emotional or physical stress)27 cardiac arrest precipitated by VT/VF.
- ECG is unremarkable during normal sinus rhythm. However, during the episode of PMVT, the tachydysrhythmia has two major features:
- The PMVT is not associated with prolonged QTc.
- Bidirectional VT: Once thought to be pathognomonic for digital intoxication; it can happen in CPVT 28.
- This happens due to intracellular calcium overload which in turn leads to DADs and triggered activity.
- Diagnosis is confirmed by an exercise stress test or epinephrine infusion test; upon which increasing frequency of PVCs with increasing exercise severity is noticed, although both sustained and non-sustained VT may happen.
Differential diagnosis of irregular WCT
- PMVT
- AF + WPW
- Characterized by irregularly irregular polymorphic wide complex tachycardia with a characteristically high rate (>200-250 bpm) and changing QRS shape, width, and amplitude from beat to beat.
- Management: The first line treatment regardless of hemodynamic stability is synchronized cardioversion. Otherwise “Procainamide” is recommended in this situation.
- 👉👉All AV nodal blocking agents (e.g. BB, CCBs, amiodarone, digoxin, adenosin) are contraindicated in this situation.
- AF + BBB
- The irregular WCT has the characteristic of RBBB-shaped or LBBB-shaped. If diagnosed definitively, treatment with any AV nodal blocking agent is suitable and safe.
- Hyperkalemia (sine wave)
- Coarse ventricular fibrillation.
- It is practically impossible to differentiate PMVT from VF on the monitor.
- Several studies have shown that pulseless rhythm appeared to be Tdp (twisting around a point) turned out to be VF.
- This distinction does not imply immediate management (since both will receive defibrillation), however, it has implications for preventing further dysrhythmia.
- It is practically impossible to differentiate PMVT from VF on the monitor.
Initial Approach to PMVT
▪️Background
- Despite that unsynchronized cardioversion is the first therapeutic measure for most patients with irregular WCT; diagnosis and management of irregular WCT is challenging for two reasons:
- AF + WPW: Failure to recognize this pattern is unforgiving. In this condition, all AV nodal blocking agents including amiodarone are absolutely contraindicated. All these medications may lead to degenerating dysrhythmia into VF. The only possible safe antiarrhythmic medication is “Procainamide”.
- PMVT: Failure to identify Tdp (as a subtype of PMVT), since the principles of therapeutic approach for Tdp is different from PMVT with normal QTc.
- Identifying PMVT with normal QTc signals for a thorough evaluation for possible underlying ischemia, and cardiomyopathy.
- The antiarrhythmic medications that are allowed in this setting including amiodarone and beta-blockers are contraindicated in patients with Tdp.
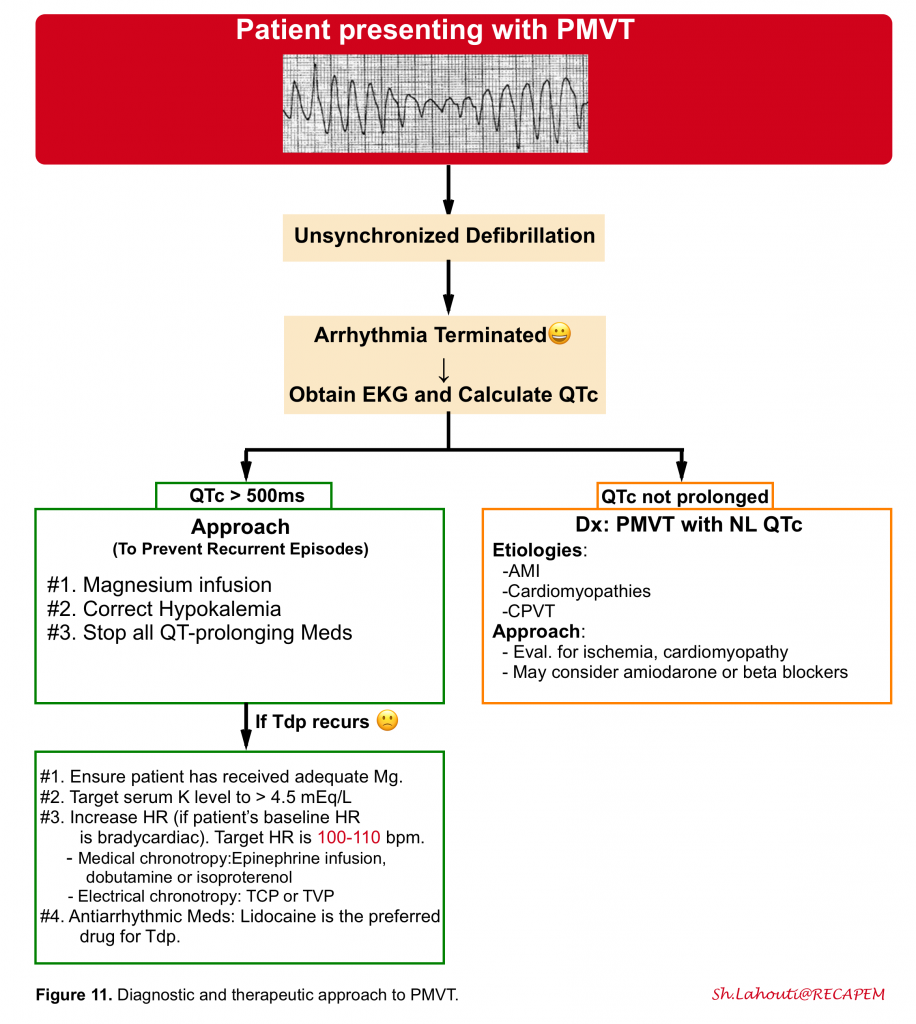
▪️The initial approach to PMVT is shown below (figure 11).
- Convert into sinus rhythm
- Evaluate the ECG in sinus rhythm
- Long-term management
- Convert into sinus rhythm
- PMVT is inherently a non-perfusing rhythm.
- This is a short-lived transitional state that usually flips back into sinus rhythm, or less commonly degenerates into ventricular fibrillation.
- The best approach to PMVT is probably immediate defibrillation (unsynchronized defibrillation)
- PMVT is inherently a non-perfusing rhythm.
- Evaluate the ECG in sinus rhythm
- Once the patient has converted into sinus rhythm, obtain an ECG. Focus on the following key points:
- QTc interval (QT prolongation suggests torsade de pointes).
- Any signs of active myocardial ischemia.
- Any signs of J-waves or J-wave syndromes.
- Once the patient has converted into sinus rhythm, obtain an ECG. Focus on the following key points:
- Long-term management
- In stable patients, acute suppression of recurrent PMVT is achieved by “propranolol”.
- If it fails, CCBs such as verapamil or flecainide are helpful 29.
- Long-term management of patients with sustained PMVT involves ICD placement and administration of beta-blockers to minimize arrhythmia and ICD shock delivery.
- In stable patients, acute suppression of recurrent PMVT is achieved by “propranolol”.
QT prolongation
▪️Recognizing QT prolongation
- Rule of thumb: if QT is >1/2 the RR interval, then the QT is probably prolonged.
- Tachycardia: this will over-call QT prolongation.
- Bradycardia: This will under-call QT prolongation.
▪️QTc calculation and interpretation
- MDCalc online calculator: QTc calculator
- Interpretation:
- QTc > ~475 ms is statistically elevated (above the 99th percentile).
- Torsade is unusual unless QTc is >500 ms.
- QRS prolongation (e.g. bundle branch blocks) will prolong the QT without increasing the risk of torsade *. Interpretation of QT intervals here is challenging.
- A common approach is to subtract the excessive duration of the QRS interval (e.g. QRS – 120 ms) from the QT interval. This may be done using the Mayo Clinic calculator here.
Causes of QTc prolongation
- Electrolyte abnormality:
- ⬇️Ca (prolongation of the ST segment duration).
- ⬇️K
- ⬇️Mg
- Hypothermia.
- Hypothyroidism.
- Takotsubo cardiomyopathy
- Myocarditis.
- Septic cardiomyopathy.
- Congenital long QT syndromes
- Prolonged QTc secondary to conduction abnormality and prolonged QRS complex (e.g. bundle branch block).
- Medications *. For a complete list of QT drugs see the Arizona CERT database at www.qtdrugs.org.
- Antiarrhythmics: TdP risk is often highest with antiarrhythmic medications that prolong the QT interval (with a notable exception of amiodarone, which doesn’t tend to cause TdP).
- Class IA: Quinidine, disopyramide, procainamide.
- Class IC: Flecainide.
- Class III: Dofetilide, ibutilide, sotalol, dronedarone.
- Antimicrobials
- Clarithromycin, erythromycin.
- Fluoroquinolones.
- Fluconazole, itraconazole, voriconazole, posaconazole.
- Pentamidine.
- Quinine
- Amantadine
- Anaesthetics
- Sevoflurane
- Propofol
- Psychotropic
- Haloperidol, droperidol, chlorpromazine, pimozide.
- Tricyclic antidepressants.
- Citalopram, escitalopram.
- Other
- Methadone.
- Cocaine, loperamide (massive doses).
- Ondansetron (when pushed rapidly).
- Propofol.
- Arsenic trioxide, sunitinib, vandetanib.
- Antiarrhythmics: TdP risk is often highest with antiarrhythmic medications that prolong the QT interval (with a notable exception of amiodarone, which doesn’t tend to cause TdP).
Torsade de pointes (TdP)
▪️Background
- Torsades de pointes is polymorphic VT that occurs in the setting of a long-QT interval.
- It commonly happens in the setting of underlying bradycardia (including high-grade AV block) that leads to a long-short sequence initiating Tdp.
- The twisting of the points, although characteristic, may not always be seen, especially if the episode is nonsustained or if only a limited number of leads are available.
- Therefore the morphology of Tdp (twisting around a point) is not reliable and sufficient enough to distinguish PMVT with normal QT vs. PMVT with prolonged QT (Tdp).
- The diagnosis of Tdp is only made when tachyarrhythmia is broken and you can appreciate the prolonged QTc during normal sinus rhythm.
▪️Risk factors for TdP
- Female sex
- Baseline QT prolongation.
- Bradycardia.
- Electrolyte disturbances: ⬇️K, ⬇️Mg,
- Recent cardioversion from atrial fibrillation (especially with a QT-prolonging medication).
- Heart failure.
- Digoxin use.
Principles of management of Tdp
- Immediate defibrillation if the patient has ongoing PMVT (unsynchronized initially started at 200 J biphasic).
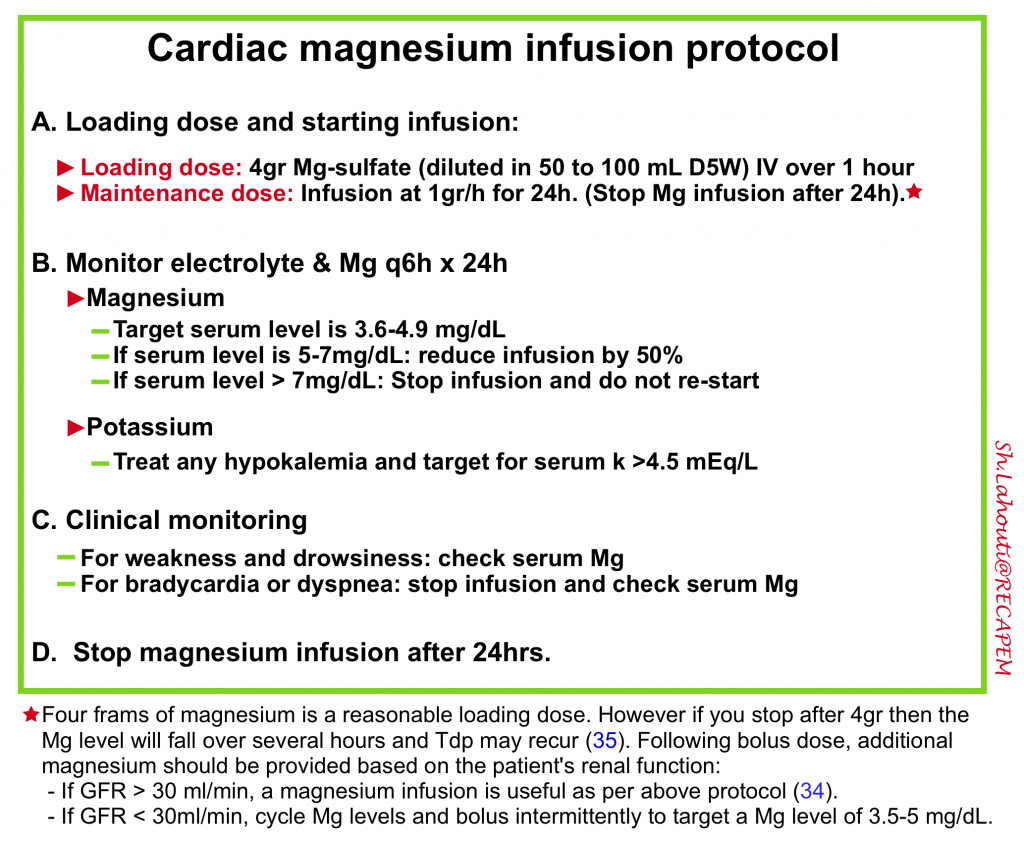
- Once PMVT is broken and QTc prolongation is noted in sinus rhythm, then all attempts should be focused on administration of magnesium (even if serum Mg is normal) as per following protocol 34 35 and correction of hypokalemia. More on the rationale for magnesium infusion here.
- Stop all medications with QT-prolonging effects.
- If despite all measures, Tdp recurs, first defibrilate it (as mentioned). Then employ the following steps for prevention of Tdp recurrence:
- Make sure that the patient has received an adequate amount of magnesium.
- One may consider re-loading Mg with 2-4 gr IV immediately if the patient was bolused with Mg a few hours ago without an infusion.
- Target serum potasium level to > 4.5 mEq/L.
- For some patients with baseline bradycardia, speeding up the heart rate with a target HR > 100-110 bpm may seem reasonable except for a few patients with some forms of congenital Tdp). This can be achieved by medical or electrical treatment:
- Medical chronotropy: This is the easiest and fastest approach to stabilize the patient. The ideal chronotrope depends on the patient’s hemodynamics and baseline blood pressure:
- Baseline severe hypotension: Epinephrine infusion.
- Baseline normotension or mild hypotension: Dobutamine or isoproterenol infusion.
- Electrical chronotropy may be used if medical chronotropy fails:
- Transcutaneous pacing may work, but this is painful for conscious patients.
- Transvenous pacing is more comfortable, but this is more invasive and takes a bit longer to achieve.
- Patients with a pacemaker may have the device rate increased.
- Medical chronotropy: This is the easiest and fastest approach to stabilize the patient. The ideal chronotrope depends on the patient’s hemodynamics and baseline blood pressure:
- Make sure that the patient has received an adequate amount of magnesium.
- If all the above measures were ineffective, one may consider the administration of antiarrhythmic.
- The drug of choice is “Lidocaine” (since it has no QT-prolonging effect).
- ⚠️Avoid amiodarone, procainamide, beta-blockers, or most other antiarrhythmics.
- If a patient fails to respond and Tdp recurs, then one may consider an alternative diagnosis such as PMVT due to ischemia or catecholaminergic ventricular tachycardia.
RECAP
- Two fundamental principles in the initial evaluation of patients with tachyarrhythmias include determining hemodynamic stability and recognizing whether the arrhythmia is the cause of clinical presentation.
- By and large abnormal HR might be assumed as a primary cause of clinical instability when; non-sinus tachycardia at > 170 bpm or AF with rapid ventricular response > 130-150 bpm.
- AF with rapid ventricular response (in the range of 110-150) is often an innocent bystander, especially in patients with chronic AF. In such patients, one should investigate for other causes of instability such as blood loss, sepsis etc.
- Generally, tachyarrhythmia in patients with hemodynamic instability calls for electrical cardioversion/defibrillation while patients who are stable can be treated by antiarrhythmic medications.
- Electrical cardioversion/defibrillation is the easiest and fastest approach to stabilize the patients if indicated and practiced appropriately. It is safer than any other antiarrhythmic medication.
- It is safest to assume regular WCT as VT until proven otherwise.
- PMVT can not be distinguished from Tdp unless the rhythm is broken and QT prolongation is noted on baseline EKG.
- The worst approach to patients with VT/VF storm (electrical storm) is watchful waiting. Electrical storm is a vicious cycle and VT/CF will recur.
- If Tdp is recognized, appropriate administration of magnesium and correction of hypokalemia (if exists) play pivotal roles in the prevention of recurrence of Tdp. Failure to administer magnesium infusion often leads to the recurrence of the tachydysrhythmia events.
- Procainamide is the drug of choice for most stable patients with WCT. It is also safe in patients with (AF +WPW). However, there are still certain conditions in the setting of VT; where “Amiodarone” is superior to procainamide. These include:
- VT/VF in cardiac arrest
- Electrical storm
- ICD patients with VT above detection rate
- Acute MI with PMVT (👌keep in mind that patients with AMI benefit from the beta-antagonist effect of a medication. Amiodarone has a beta-antagonist effect in addition to Na, K channel blockade properties)
- Avoid giving medication with AV nodal blocking properties such as BBs, CCBs, adenosine, amiodarone, and digoxin to patients with Irregularly irregular WCT (at very fast rates usually > 250-300).
- Avoid giving procainamide, BBs, and amiodarone to patients with Tdp. These medications will further prolong QTc. If a decision is made to give an antiarrhythmic medication as a last-ditch attempt, “Lidocaine” is preferred over other drugs.
Going further
- Tachydysrhythmias with Amal Mattu (emergency medicine cases)
- ECG Course: The Paced ECG (EM:RAP)
- Non-torsade VT/VF storm (IBCC)
- Torsades de Pointes
Appendix
References
1. Brugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomström-Lundqvist C, Calkins H, Corrado D, Deftereos SG, Diller GP, Gomez-Doblas JJ, Gorenek B, Grace A, Ho SY, Kaski JC, Kuck KH, Lambiase PD, Sacher F, Sarquella-Brugada G, Suwalski P, Zaza A; ESC Scientific Document Group. 2019 ESC Guidelines for the management of patients with supraventricular tachycardiaThe Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur Heart J. 2020 Feb 1;41(5):655-720. doi: 10.1093/eurheartj/ehz467. Erratum in: Eur Heart J. 2020 Nov 21;41(44):4258. PMID: 31504425
2. Neumar RW, Otto CW, Link MS, Kronick SL, Shuster M, Callaway CW, Kudenchuk PJ, Ornato JP, McNally B, Silvers SM, Passman RS, White RD, Hess EP, Tang W, Davis D, Sinz E, Morrison LJ. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010 Nov 2;122(18 Suppl 3):S729-67. doi: 10.1161/CIRCULATIONAHA.110.970988. Erratum in: Circulation. 2011 Feb 15;123(6):e236. Erratum in: Circulation. 2013 Dec 24;128(25):e480. PMID: 20956224.
3. Soar J, Böttiger BW, Carli P, Couper K, Deakin CD, Djärv T, Lott C, Olasveengen T, Paal P, Pellis T, Perkins GD, Sandroni C, Nolan JP. European Resuscitation Council Guidelines 2021: Adult advanced life support. Resuscitation. 2021 Apr;161:115-151. doi: 10.1016/j.resuscitation.2021.02.010. Epub 2021 Mar 24. PMID: 33773825
4. Panchal, A. R., Bartos, J. A., Cabañas, J. G., Donnino, M. W., Drennan, I. R., Hirsch, K. G., Kudenchuk, P. J., Kurz, M. C., Lavonas, E. J., Morley, P. T., O’Neil, B. J., Peberdy, M. A., Rittenberger, J. C., Rodriguez, A. J., Sawyer, K. N., & Berg, K. M. (2020). Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. In Circulation (Vol. 142, Issue 16 2). https://doi.org/10.1161/CIR.0000000000000916.
5. Link MS, Atkins DL, Passman RS, Halperin HR, Samson RA, White RD, Cudnik MT, Berg MD, Kudenchuk PJ, Kerber RE. Part 6: electrical therapies: automated external defibrillators, defibrillation, cardioversion, and pacing: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010 Nov 2;122(18 Suppl 3):S706-19. doi: 10.1161/CIRCULATIONAHA.110.970954. Erratum in: Circulation. 2011 Feb 15;123(6):e235. PMID: 20956222.
6. Hollowell H, Mattu A, Perron AD, Holstege C, Brady WJ. Wide-complex tachycardia: beyond the traditional differential diagnosis of ventricular tachycardia vs supraventricular tachycardia with aberrant conduction. Am J Emerg Med. 2005 Nov;23(7):876-89. doi: 10.1016/j.ajem.2005.04.015. PMID: 16291445.
7. Jastrzebski M, Kukla P, Czarnecka D, Kawecka-Jaszcz K. Comparison of five electrocardiographic methods for differentiation of wide QRS-complex tachycardias. Europace. 2012 Aug;14(8):1165-71. doi: 10.1093/europace/eus015. Epub 2012 Feb 14. PMID: 22333239
8. Dancy M, Camm AJ, Ward D. Misdiagnosis of chronic recurrent ventricular tachycardia. Lancet. 1985 Aug 10;2(8450):320-3. doi: 10.1016/s0140-6736(85)90363-0. PMID: 2862480
9. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018 Oct 2;72(14):e91-e220. doi: 10.1016/j.jacc.2017.10.054. Epub 2018 Aug 16. Erratum in: J Am Coll Cardiol. 2018 Oct 2;72(14):1760. PMID: 29097296.
10. Ulus T, Kudaiberdieva G, Gorenek B. The onset mechanisms of ventricular tachycardia. Int J Cardiol. 2013 Aug 10;167(3):619-23. doi: 10.1016/j.ijcard.2012.09.034. Epub 2012 Oct 3. PMID: 23040077
11. Field JM, Hazinski MF, Sayre MR, Chameides L, Schexnayder SM, Hemphill R, Samson RA, Kattwinkel J, Berg RA, Bhanji F, Cave DM, Jauch EC, Kudenchuk PJ, Neumar RW, Peberdy MA, Perlman JM, Sinz E, Travers AH, Berg MD, Billi JE, Eigel B, Hickey RW, Kleinman ME, Link MS, Morrison LJ, O’Connor RE, Shuster M, Callaway CW, Cucchiara B, Ferguson JD, Rea TD, Vanden Hoek TL. Part 1: executive summary: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010 Nov 2;122(18 Suppl 3):S640-56. doi: 10.1161/CIRCULATIONAHA.110.970889. PMID: 20956217
12. Kudenchuk PJ, Brown SP, Daya M, Rea T, Nichol G, Morrison LJ, Leroux B, Vaillancourt C, Wittwer L, Callaway CW, Christenson J, Egan D, Ornato JP, Weisfeldt ML, Stiell IG, Idris AH, Aufderheide TP, Dunford JV, Colella MR, Vilke GM, Brienza AM, Desvigne-Nickens P, Gray PC, Gray R, Seals N, Straight R, Dorian P; Resuscitation Outcomes Consortium Investigators. Amiodarone, Lidocaine, or Placebo in Out-of-Hospital Cardiac Arrest. N Engl J Med. 2016 May 5;374(18):1711-22. doi: 10.1056/NEJMoa1514204. Epub 2016 Apr 4. PMID: 27043165
13. Nurul, mas’ud waqiah. (2020). Highlights of the 2020 American Heart Association Guidelines For CPR and ECC. American Journal of Heart Association, 53(9), 1689–1699.
14. Ortiz M, Martín A, Arribas F, Coll-Vinent B, Del Arco C, Peinado R, Almendral J; PROCAMIO Study Investigators. Randomized comparison of intravenous procainamide vs. intravenous amiodarone for the acute treatment of tolerated wide QRS tachycardia: the PROCAMIO study. Eur Heart J. 2017 May 1;38(17):1329-1335. doi: 10.1093/eurheartj/ehw230. PMID: 27354046; PMCID: PMC5410924
15. deSouza IS, Martindale JL, Sinert R. Antidysrhythmic drug therapy for the termination of stable, monomorphic ventricular tachycardia: a systematic review. Emergency Medicine Journal 2015;32:161-167.
16. Long B, Koyfman A. Best Clinical Practice: Emergency Medicine Management of Stable Monomorphic Ventricular Tachycardia. J Emerg Med. 2017 Apr;52(4):484-492. doi: 10.1016/j.jemermed.2016.09.010. Epub 2016 Oct 15. PMID: 27751700
17. Marill KA, Wolfram S, Desouza IS, Nishijima DK, Kay D, Setnik GS, Stair TO, Ellinor PT. Adenosine for wide-complex tachycardia: efficacy and safety. Crit Care Med. 2009 Sep;37(9):2512-8. doi: 10.1097/CCM.0b013e3181a93661. PMID: 19623049
18. Martindale J, deSouza IS. Managing pacemaker-related complications and malfunctions in the emergency department. Emerg Med Pract. 2014 Sep;16(9):1-21; quiz 21-2. PMID: 25436255
19. Geraghty L, Santangeli P, Tedrow UB, Shivkumar K, Kumar S. Contemporary Management of Electrical Storm. Heart Lung Circ. 2019 Jan;28(1):123-133. doi: 10.1016/j.hlc.2018.10.005. Epub 2018 Oct 15. PMID: 30554598
20. Muser D, Santangeli P, Liang JJ. Management of ventricular tachycardia storm in patients with structural heart disease. World J Cardiol. 2017 Jun 26;9(6):521-530. doi: 10.4330/wjc.v9.i6.521. PMID: 28706587; PMCID: PMC5491469
21. Haegeli LM, Della Bella P, Brunckhorst CB. Management of a Patient With Electrical Storm: Role of Epicardial Catheter Ablation. Circulation. 2016 Feb 16;133(7):672-6. doi: 10.1161/CIRCULATIONAHA.115.016336. PMID: 26884622
22. Chatzidou S, Kontogiannis C, Tsilimigras DI, Georgiopoulos G, Kosmopoulos M, Papadopoulou E, Vasilopoulos G, Rokas S. Propranolol Versus Metoprolol for Treatment of Electrical Storm in Patients With Implantable Cardioverter-Defibrillator. J Am Coll Cardiol. 2018 May 1;71(17):1897-1906. doi: 10.1016/j.jacc.2018.02.056. PMID: 29699616
23. Driver BE, Debaty G, Plummer DW, Smith SW. Use of esmolol after failure of standard cardiopulmonary resuscitation to treat patients with refractory ventricular fibrillation. Resuscitation. 2014 Oct;85(10):1337-41. doi: 10.1016/j.resuscitation.2014.06.032. Epub 2014 Jul 14. PMID: 25033747.
24. Fudim M, Boortz-Marx R, Ganesh A, Waldron NH, Qadri YJ, Patel CB, Milano CA, Sun AY, Mathew JP, Piccini JP. Stellate ganglion blockade for the treatment of refractory ventricular arrhythmias: A systematic review and meta-analysis. J Cardiovasc Electrophysiol. 2017 Dec;28(12):1460-1467. doi: 10.1111/jce.13324. Epub 2017 Sep 1. PMID: 28833780
25. Sorajja D, Munger TM, Shen WK. Optimal antiarrhythmic drug therapy for electrical storms. J Biomed Res. 2015 Jan;29(1):20-34. doi: 10.7555/JBR.29.20140147. Epub 2015 Jan 15. PMID: 25745472; PMCID: PMC4342432
26. Tian Y, Wittwer ED, Kapa S, McLeod CJ, Xiao P, Noseworthy PA, Mulpuru SK, Deshmukh AJ, Lee HC, Ackerman MJ, Asirvatham SJ, Munger TM, Liu XP, Friedman PA, Cha YM. Effective Use of Percutaneous Stellate Ganglion Blockade in Patients With Electrical Storm. Circ Arrhythm Electrophysiol. 2019 Sep;12(9):e007118. doi: 10.1161/CIRCEP.118.007118. Epub 2019 Sep 13. PMID: 31514529
27. Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, DeSimone L, Coltorti F, Bloise R, Keegan R, Cruz Filho FE, Vignati G, Benatar A, DeLogu A. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002 Jul 2;106(1):69-74. doi: 10.1161/01.cir.0000020013.73106.d8. PMID: 12093772.
28. Nof E, Lahat H, Constantini N, Luria D, Rosenfeld G, Eldar M, Pras E, Glikson M. A novel form of familial bidirectional ventricular tachycardia. Am J Cardiol. 2004 Jan 15;93(2):231-4. doi: 10.1016/j.amjcard.2003.09.049. PMID: 14715357
29. Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang CE, Huikuri H, Kannankeril P, Krahn A, Leenhardt A, Moss A, Schwartz PJ, Shimizu W, Tomaselli G, Tracy C. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013 Dec;10(12):1932-63. doi: 10.1016/j.hrthm.2013.05.014. Epub 2013 Aug 30. PMID: 24011539
30. Seif D, Perera P, Mailhot T, Riley D, Mandavia D. Bedside ultrasound in resuscitation and the rapid ultrasound in shock protocol. Crit Care Res Pract. 2012;2012:503254. doi: 10.1155/2012/503254. Epub 2012 Oct 24. PMID: 23133747; PMCID: PMC3485910
31. Atkinson PRT, McAuley DJ, Kendall RJ, et al. Abdominal and Cardiac Evaluation with Sonography in Shock (ACES): an approach by emergency physicians for the use of ultrasound in patients with undifferentiated hypotension Emergency Medicine Journal 2009;26:87-91.
32. Ahn JH, Jeon J, Toh HC, Noble VE, Kim JS, Kim YS, Do HH, Ha YR. SEARCH 8Es: A novel point of care ultrasound protocol for patients with chest pain, dyspnea or symptomatic hypotension in the emergency department. PLoS One. 2017 Mar 29;12(3):e0174581. doi: 10.1371/journal.pone.0174581. PMID: 28355246; PMCID: PMC5371336
33. Gaspari R, Weekes A, Adhikari S, Noble V, Nomura JT, Theodoro D, Woo M, Atkinson P, Blehar D, Brown S, Caffery T, Douglass E, Fraser J, Haines C, Lam S, Lanspa M, Lewis M, Liebmann O, Limkakeng A, Lopez F, Platz E, Mendoza M, Minnigan H, Moore C, Novik J, Rang L, Scruggs W, Raio C. A retrospective study of pulseless electrical activity, bedside ultrasound identifies interventions during resuscitation associated with improved survival to hospital admission. A REASON Study. Resuscitation. 2017 Nov;120:103-107. doi: 10.1016/j.resuscitation.2017.09.008. Epub 2017 Sep 13. PMID: 28916478.
34. Sleeswijk ME, Tulleken JE, Van Noord T, Meertens JH, Ligtenberg JJ, Zijlstra JG. Efficacy of magnesium-amiodarone step-up scheme in critically ill patients with new-onset atrial fibrillation: a prospective observational study. J Intensive Care Med. 2008 Jan-Feb;23(1):61-6. doi: 10.1177/0885066607310181. PMID: 18320707.
35. Biesenbach P, Mårtensson J, Lucchetta L, Bangia R, Fairley J, Jansen I, Matalanis G, Bellomo R. Pharmacokinetics of Magnesium Bolus Therapy in Cardiothoracic Surgery. J Cardiothorac Vasc Anesth. 2018 Jun;32(3):1289-1294. doi: 10.1053/j.jvca.2017.08.049. Epub 2017 Sep 1. PMID: 29169799.
36. Braunschweig F, Boriani G, Bauer A, Hatala R, Herrmann-Lingen C, Kautzner J, Pedersen SS, Pehrson S, Ricci R, Schalij MJ. Management of patients receiving implantable cardiac defibrillator shocks: recommendations for acute and long-term patient management. Europace. 2010 Dec;12(12):1673-90. doi: 10.1093/europace/euq316. Epub 2010 Oct 25. PMID: 20974757.
37. Nademanee K, Taylor R, Bailey WE, Rieders DE, Kosar EM. Treating electrical storm : sympathetic blockade versus advanced cardiac life support-guided therapy. Circulation. 2000 Aug 15;102(7):742-7. doi: 10.1161/01.cir.102.7.742. PMID: 10942741.
38. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ; ESC Scientific Document Group. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015 Nov 1;36(41):2793-2867. doi: 10.1093/eurheartj/ehv316. Epub 2015 Aug 29. PMID: 26320108.
39. Tsuji Y, Heijman J, Nattel S, Dobrev D. Electrical storm: recent pathophysiological insights and therapeutic consequences. Basic Res Cardiol. 2013 Mar;108(2):336. doi: 10.1007/s00395-013-0336-2. Epub 2013 Feb 21. PMID: 23429935.
40. Iftikhar S, Mattu A, Brady W. ED evaluation and management of implantable cardiac defibrillator electrical shocks. Am J Emerg Med. 2016 Jun;34(6):1140-7. doi: 10.1016/j.ajem.2016.02.060. Epub 2016 Mar 3. PMID: 26993075







Thanks, this was great and very useful.
i dont think you talked about WPW in the classification, as i reached this site googling if it was another name for AVRT or a specific type of AVRT.
but ended up getting a great review instead!