25 March, 2022 by Shahriar Lahouti.
CONTENTS
- Preface
- Etiology
- Clinical presentation
- Differential diagnosis
- Noncontrast head CT
- Lumbar puncture
- CT angiography (CTA)
- MRI/MRA
- Diagnostic algorithm
- SAH grading scales
- Initial resuscitation
- Etiologies of neurologic worsening in SAH
- Neurocritical Management
- Other therapeutic consideration
- Further going
- References
Preface
Non-traumatic subarachnoid hemorrhage (SAH) is a type of hemorrhagic stroke and is a neurologic emergency with substantial morbidity and mortality. Here the most essential approach to diagnosis and management of patients with aneurysmal subarachnoid hemorrhage is reviewed.
Etiology
- Primary SAH (This will cause diffuse or basilar SAH)
- Aneurysmal SAH
- Approximately 85% of primary SAHs are due to aneurysmal rupture 1.
- The mean age of patients with aneurysmal SAH is 60 years.
- Stronger risk factors for aneurysmal SAH include 2:
- Hypertension, smoking, alcoholism.
- Sympathomimetic use (e.g. cocaine).
- Genetic diseases (polycystic kidney disease, Ehlers-Danlos and Marfan syndrome, fibromuscular dysplasia).
- Personal or family history of SAH or another aneurysm.
- Cerebral aneurysms of more than 7 mm in diameter.
- Increasing age (peak in fifth and sixth decade).
- Female sex (female to male ratio of 1.6:1).
- Perimesencephalic hemorrhage (a.k.a. prepontine SAH). See below
- Other vascular anomalies
- Almost 5% of SAHs are due to other vascular malformations (e.g. AVM, dural arteriovenous fistula, arterial dissection).
- Aneurysmal SAH
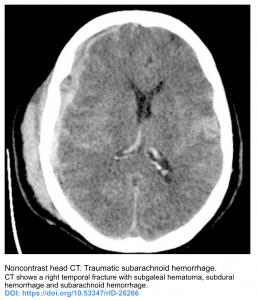
- Secondary SAH (This will cause SAH in cerebral convexity, rather than basal cistern)
- Trauma
- Coagulopathy
- Cerebral amyloid angiopathy (CAA)
- Primary intracranial hemorrhage with secondary extension to the subarachnoid space
- Cerebral venous sinus thrombosis (CVT)
- Reversible cerebral vasoconstriction syndrome (RCVS)
- Posterior reversible encephalopathy syndrome (PRES)
- Cerebral vasculitis
- Tumor
- Sympathomimetic abuse
- Vertebral artery dissection
Clinical presentation
Headache
- The classic presentation of SAH is thunderclap headache, referring to a severe headache of sudden onset that reaches its maximum intensity within one or less of onset. The key feature that differentiates TCH from other headaches is the rapidity with which it develops, extreme severity alone is insufficient 4.
- 10-20% of patients may have had a milder sentinel headache which occurred days or weeks earlier.
- Other features accompanied with thunderclap headache that are suggestive for SAH are:
- Headache may begin during exertion
- Transient loss of consciousness or seizure
- Presence of strong risk factors for “SAH” (see above)
- See Ottawa SAH Rule
- Other causes of thunderclap headache is mentioned in the panel below 3,4.
Conditions that most commonly cause TCH
1.Subarachnoid hemorrhage (SAH)
2.Reversible cerebral vasoconstriction syndrome (RCVS)
Conditions that less commonly cause TCH:
1.Cervical artery dissection (carotid artery or vertebral artery dissection).
2. CVT
3. Posterior reversible encephalopathy syndrome (PRES)
4. Acute hypertensive crisis
5. Cerebral infection (e.g. meningitis, acute complicated sinusitis)
6. ICH, Acute ischemic stroke
Conditions that uncommonly cause TCH
1. Brain tumor.
2. Pituitary apoplexy
3. Colloid cyst of the third ventricle
4. Aortic arch dissection
5. Giant cell arteritis.
Other presentations 2
- Transient elevation of ICP can cause nausea, vomiting and syncope as well as cardiopulmonary complications such as neurogenic pulmonary edema and cardiac arrhythmias.
- More sever and sustained ICP elevation is associated with coma and/or sudden death.
Noncontrast head CT
When a clinical suspicion for SAH exists, non-contrast head CT is the first diagnostic tool. It is also valuable in excluding other pathologies such as intracranial hemorrhage, malignancy, or abscess.
- Timing of NCCT: At the onset of the bleed, subarachnoid blood is most readily visible on the CT, but it becomes more difficult to appreciate as red blood cell (RBC) degradation progresses. The sensitivity is nearly 100% within six hours of headache onset, after which time blood starts looking a bit more grey, so the sensitivity may decrease slightly.
- Location of the blood:
- All aneurysmal SAH predominantly involve basal cisterns. Additional location of blood in aneurysmal SAH include the sylvian fissures, interhemispheric fissures, interpeduncular fissure and suprasellar, ambient, and quadrigeminal cisterns 5 ,6. A less typical presentation of aneurysmal SAH may include
- Intracerebral extension of SAH 20-40%
- Intraventricular hemorrhage 15-35%
- Subdural hematoma without associated head trauma 2-5% 2.
- Does location of the blood predict site of aneurysm?
- Location of the blood on CT has been shown to be a poor predictor of the site of the aneurysm except in patients with ruptured anterior cerebral artery (ACA) or anterior communicating artery aneurysm and in some patients with a parenchymal hematoma as 7:
- Blood predominantly in interhemispheric fissure is more suggestive for ACA or anterior communicating artery.
- Focal frontal lobe intracerebral hematoma is seen more commonly with ACA or anterior communicating artery aneurysm rupture.
- Focal anterior temporal lobe intracerebral hematoma may suggest middle cerebral artery aneurysm rupture.
- Location of the blood on CT has been shown to be a poor predictor of the site of the aneurysm except in patients with ruptured anterior cerebral artery (ACA) or anterior communicating artery aneurysm and in some patients with a parenchymal hematoma as 7:
- All aneurysmal SAH predominantly involve basal cisterns. Additional location of blood in aneurysmal SAH include the sylvian fissures, interhemispheric fissures, interpeduncular fissure and suprasellar, ambient, and quadrigeminal cisterns 5 ,6. A less typical presentation of aneurysmal SAH may include
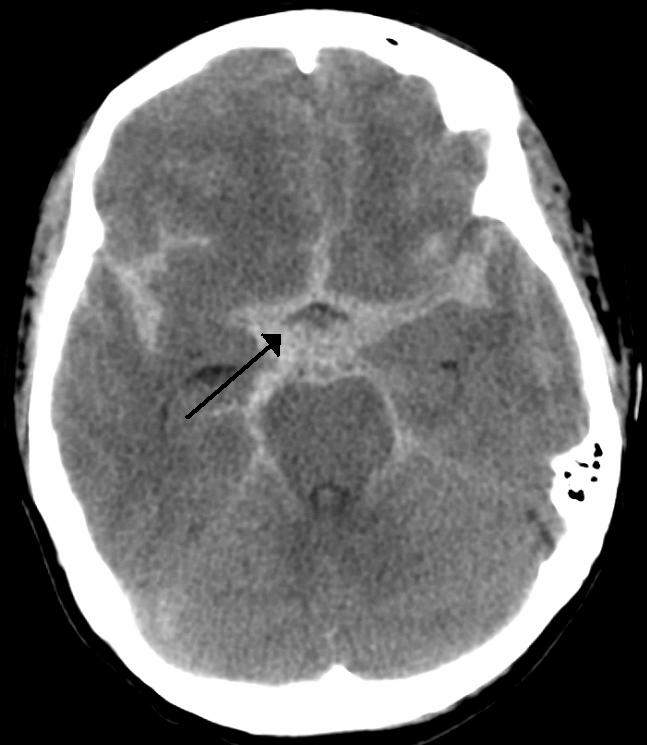
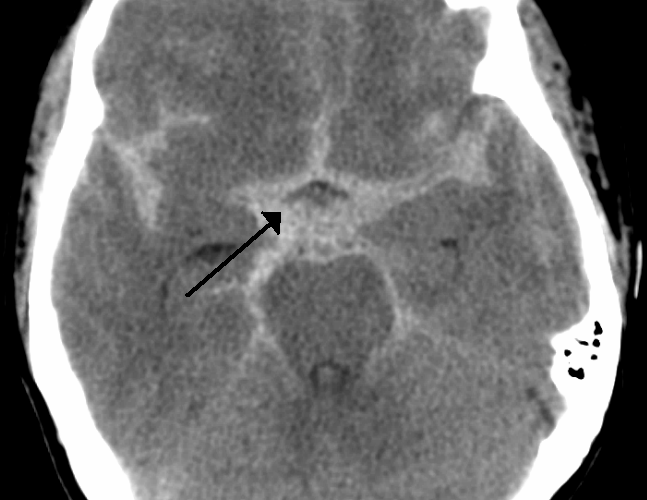
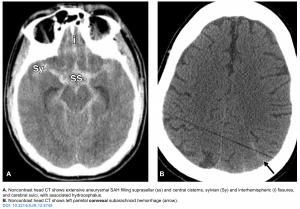
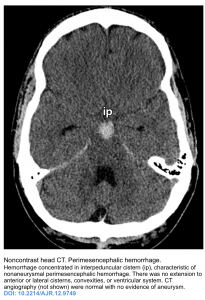
- Distribution of the blood: Generally distribution of the blood has implications about the whether or not the cause of the SAH is aneurysm. Three patterns of distribution have been recognized (see the following figures) 6:
- Suprasellar cisterns with diffuse peripheral extension: This pattern is characteristic of aneurysmal rupture.
- Isolated cerebral convexity without basal cistern or ventricular involvement. This is caused by a heterogeneous group of diseases (see above). There are two main clinical presentations of convexal SAH, roughly stratified by patient age:
- Age ≤60 years: They are more likely to have reversible cerebral vasoconstriction syndrome (RCVS).
- Age > 60 years: more likely to have cerebral amyloid angiopathy (CAA).
- Basal cistern without peripheral extension. This is suggestive for perimesencephalic hemorrhage (PMH).
-
PMH constitute 10% of all SAH. This results from bleeding from venous origin, which is centered in the perimesencephalic area.
- Findings on noncontrast head CT 6
-
Blood should be centered immediately anterior to the midbrain or pons (may variably involve the interpeduncular, crural, ambient, quadrigeminal, prepontine, or carotid cisterns).
-
Blood may thinly extend into the suprasellar cistern and the basal portions of the sylvian and interhemispheric fissures, but should not extend into the distal portions of the sylvian or interhemispheric fissures.
-
Small amounts of blood may sediment in the occipital horns of the lateral ventricles, but frank intraventricular hemorrhage should not be seen.
- No paranchymal hematoma should occur.
-
- Aneurysm should be excluded with CTA and/or digital subtraction angiography.
-
Lumbar puncture
Background
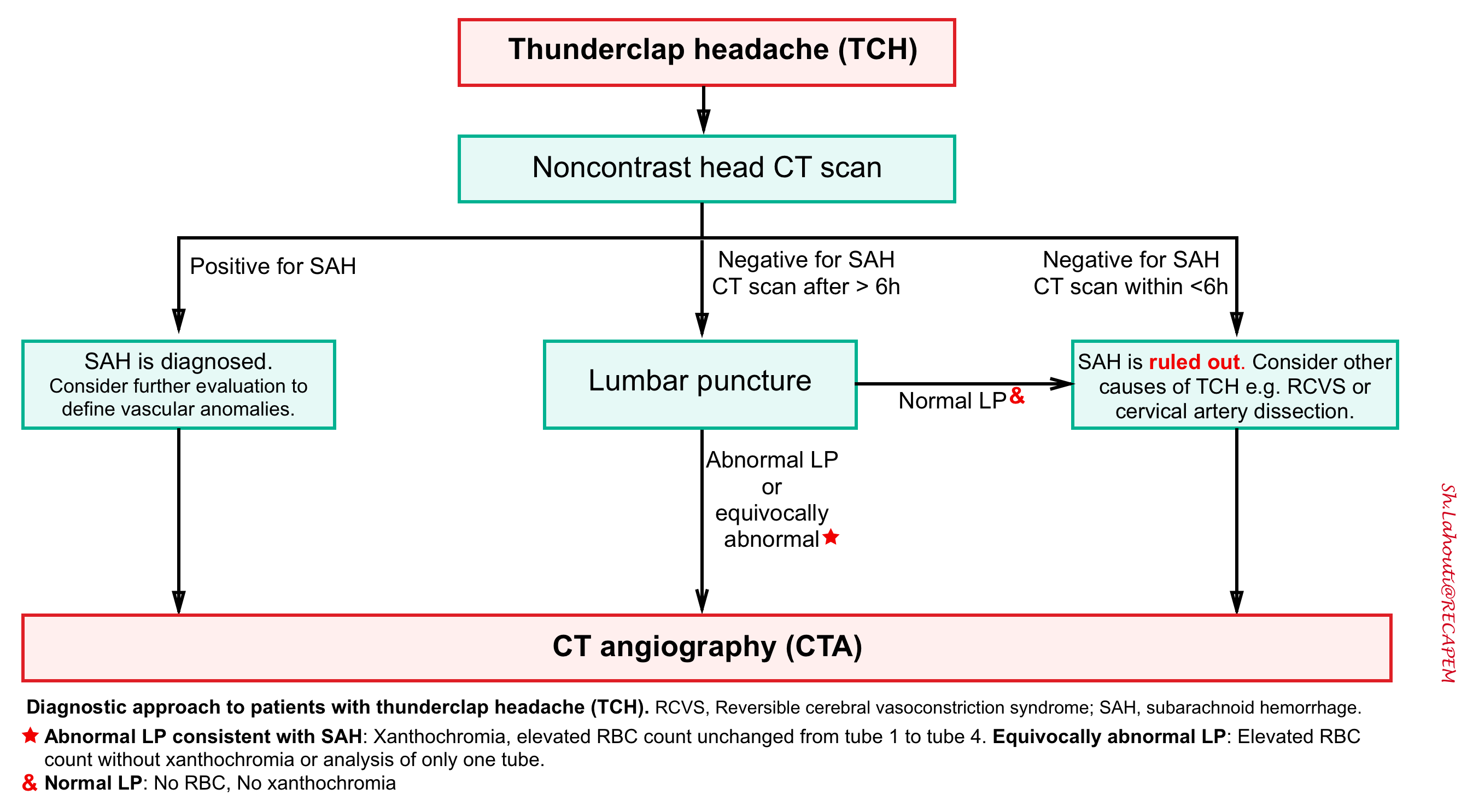
- Traditionally, LP has been used to evaluate for SAH in patients with negative noncontrast head CT scan who have high suspicion for SAH (primarily if the CT scan was obtained >6 hours after headache onset).
- If LP is the chosen follow-up test, it is performed to assess for two findings consistent with SAH:
- RBCs: There’s no absolute cut off that can reliably differentiate SAH from traumatic tap, however recent literature suggests that the RBC count in all patients with subarachnoid hemorrhage is 2,000 × 106/L or greater in the final tube 8:
- Note that this cut-off point does not exclude all instances of traumatic tap but significantly decreases the likelihood of traumatic tap and improves the LP test characteristics.
- Xanthochromia refers to the yellow hue noted on CSF that occurs due to RBC breakdown. It develops within 2-4 hours after the acute hemorrhage and should be present after 12 hours.
- This can be very helpful to make the diagnosis of SAH when the RBC count has downtrended between the first and last tube.
- RBCs: There’s no absolute cut off that can reliably differentiate SAH from traumatic tap, however recent literature suggests that the RBC count in all patients with subarachnoid hemorrhage is 2,000 × 106/L or greater in the final tube 8:
- The only way an LP can be entirely reassuring is if there are zero RBCs and there is no xanthochromia.
Potential drawback of LP
- LP is not a perfect test, with sensitivity and specificity in the 85% range.
- It can be difficult to execute the procedure for several reasons, including the patient’s body habitus, previous history of spinal procedures, and the fact that it is time-consuming in some cases 9.
- LP is an invasive procedure with risks of infection and hematoma. Moreover, LP is contraindicated in patients with coagulopathy or those taking antiplatelet agents or therapeutic anticoagulation.
- Traumatic LPs, occurring in 10% of all ED LPs, can often obscure results leading to a misdiagnosis or requiring the physician to discard the results altogether.
- It’s unclear how to optimally differentiate between SAH and a traumatic LP (falling numbers of RBCs from tube #1 to tube #4 can be seen with both a traumatic tap and a SAH; various articles disagree about optimal RBC cutoffs for tube #4).
- Moreover most clinical laboratories do not have validated spectrophotometry 10 , rendering differentiation of traumatic tap from SAH more doubtful.
Due to uncertainty in the interpretation of LP results, LP for the diagnosis of SAH is controversial 11. Recent studies challenge LPs’ value in this setting, given the improvement in third-generation CT scanners and increased utility of CT angiographies 9.
CT angiography (CTA)
CTA is highly sensitive and specific (~95%) for aneurysm detection. Some experts recommends performance of CTA after non-contrast head CT (rather than standard performance of LP after non-contrast head CT). The potential advantages of CTA may include:
- For patients who do not have a SAH, CTA is useful to evaluate for the possibility of reversible cerebral vasoconstriction syndrome (RCVS) or cervical artery dissection.
- There is increasing recognition that many patients with thunderclap headache have RCVS, a diagnosis that requires CT angiography. Thus, patients presenting with thunderclap headache may benefit from CT angiography even if the LP is negative. Such patients may be better served by receiving both a CT and CTA immediately. If both the CT and CTA are negative, then SAH can generally be safely excluded12 .
- For patients who have a SAH, CTA is essential to evaluate for underlying vascular anomalies (e.g. aneurysms, AVM).
- For patients with a thunderclap headache and possible SAH, the finding of an aneurysm may indicate the need for further diagnostic testing (e.g. with lumbar puncture).
A potential drawback of CTA is that it may reveal asymptomatic unruptured aneurysms in ~2% of the population. Thus, it must be born in mind that detecting an aneurysm without subarachnoid blood is not diagnostic of an aneurysmal SAH. However, detecting unruptured aneurysms may provide an opportunity to advise patients regarding practices that could reduce their risk of aneurysmal rupture in the future (e.g. BP control, tobacco cessation, avoidance of excessive alcohol use, and avoidance of sympathomimetics).
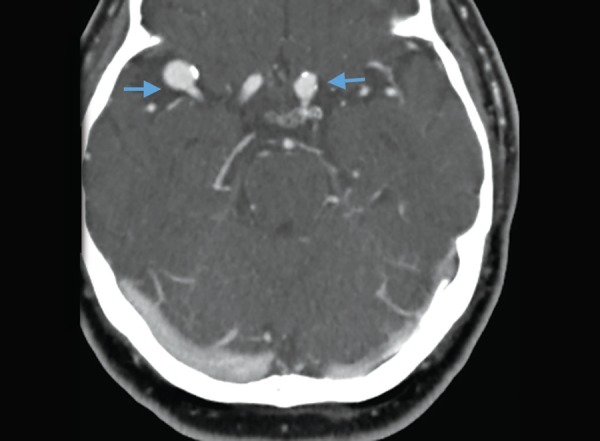
Aneurysms on CT angiography. Two contrast filled out pouchings (arrows) along right MCA and left supraclinoid ICA. PMID: 31485116
MRI/MRI
Head CT and MRI are considered to be equally sensitive in detecting SAH in the first 2 days, except in the hyperacute first 6 hours after SAH, during which head CT may miss a small proportion of SAHs and MRI may be slightly superior. However due to logistic barriers, head CT remains the diagnostic modality of choice for early SAH.
- Diagnosis of SAH.
- FLAIR is sensitive in the acute phase of SAH, showing sulcal hyperintensity 13.
- Hemosiderin-sensitive MRI sequences (gradient recalled echo [GRE] and susceptibility-weighted imaging [SWI]) or fluid-attenuated inversion recovery (FLAIR) sequences have superior sensitivity to detect subacute or chronic SAH compared to head CT, rendering MRI a potentially viable option for patients who present late and have a negative CT scan.
- Detection of underlying pathology
- MRI may be helpful in differentiating alternative pathologies, such as arteriovenous malformations and inflammatory, infectious, and neoplastic etiologies 14 .
Diagnostic algorithm
Patients with acute thunderclap headache have a differential diagnosis beyond subarachnoid hemorrhage with a number of dangerous secondary causes of headaches that are life, limb, brain, or vision threatening 15. Therefore the traditional approach to TCH which has narrowly focused on ruling out SAH is horribly insufficient.
With ongoing improvements in CT scanning technology, a CT/CTA strategy may offer patients a rapid evaluation for numerous vascular pathologies.
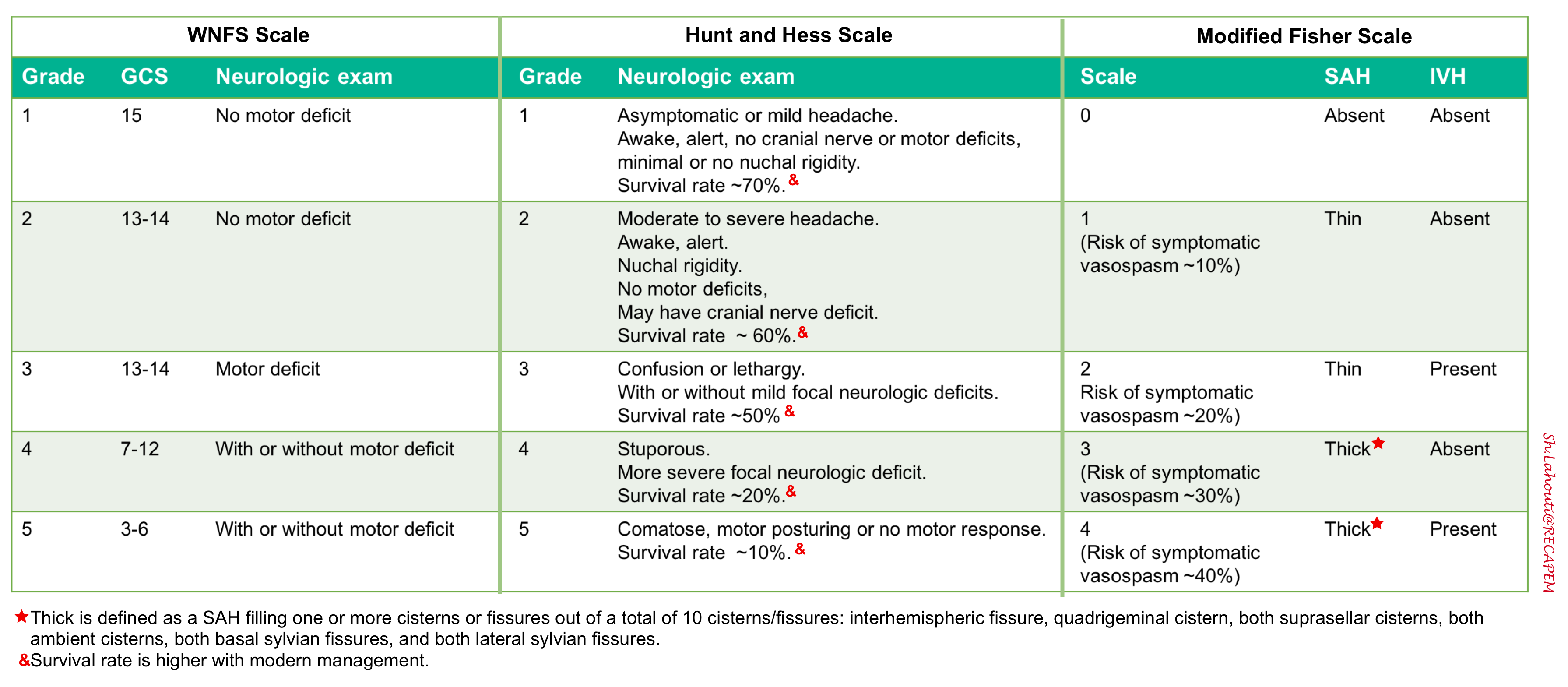
SAH grading scales
A number of grading systems are used in practice to standardize the clinical classification of patients and expressing severity of SAH.
- The grading system proposed by Hunt and Hess is more easier to use and more utilized in practice. The classifications range from one to five, and each is associated with a mortality percentage 16.
- The World Federation of Neurological Surgeons (WFNS) incorporates the Glasgow Coma Scale combined with the presence of motor deficit.
- The Fisher grade is an index of vasospasm risk based upon a CT-defined hemorrhage pattern and the Claassen grading system (also known as modified Fisher scale) is a similar index of the risk of delayed cerebral ischemia due to vasospasm. The grading system is from zero to four, with a high score indicating an elevated incidence of vasospasm 17.
SAH grading scales are helpful to determine the urgency of further treatment and predict possible clinical worsening. Conventionally, clinical grades 1-3 are often referred to as “low grade” SAH, whereas grades 4-5 are referred to as “high grade” SAH.
- A potential weakness of these scores is that the patient’s mental status may be initially be obtunded by hydrocephalus, followed by rapid improvement after insertion of a ventricular drain.
Initial resuscitation
The emergency evaluation and management of patients with SAH should focus on the airway, breathing, and circulation (the ABCDs). The general overview is provided below:
- A: Assess airway upon arrival
- B: Assess breathing. Provide supplemental O2 if SaO2 is <94%.
- C: Assess hemodynamic stability upon arrival. Obtain peripheral intravenous (IV) access. Consider BP control.
- D: Monitor the patient’s Glasgow Coma Scale score and be vigilant for impending loss of airway protection and need for intubation.
- Stat noncontrast head CT.
- Once a SAH is suspected, a CT of the head with the assistance of the Ottawa SAH Rule should be utilized as an initial diagnostic measure. If further investigation is needed, a CTA of the head or a LP can be considered 18 .
- Anticoagulation reversal
- Seizure prophylaxis
- Aggressively treat pain, vomiting to avoid Valsalva effect, fever and urinary retention.
- Neurosurgical consultation to discuss coiling or clipping.
-
Identify and treat hydrocephalus.
- Stat noncontrast head CT.
Airway management
-
Patients who are unable to protect their airway should be immediately intubated. Other potential indications for intubation include:
-
Impending respiratory failure
-
Refractory agitation
-
Rapidly deteriorating mental status.
-
Status epilepticus.
- Expected decompensation during transport within hospital or to another hospital.
-
- Airway management should be performed with efforts to avoid elevating the intracranial pressure or causing hemodynamic instability.
-
Aim for a target systolic blood pressure (SBP) <160 mm Hg.
-
Elevate head of bed 30°.
-
Ensure adequate post-intubation sedation to avoid sudden spikes in MAP or ICP.
-
Blood pressure control
- Adequate blood pressure to perfuse the brain is needed (with hypotension being particularly dangerous).
- Excess blood pressure could potentially encourage aneurysmal rebleeding.
- Rebleeding should be considered the first and most dire complication of SAH in the ED setting.
- Evidence is lacking to support a specific BP target for aneurysmal SAH, but it is generally believed that hypertension should be avoided and treated if present.
- Guidelines recommend typically targeting a SBP <160 (American Heart Association) or MAP <110 mm Hg (Neurocritical Care Society), however it may be personalized, based on baseline blood pressure.
- Management:
- Treat pain immediately, before starting an antihypertensive.
- Arterial line to monitor BP continuously.
- Between the time of SAH symptom onset and aneurysm obliteration, blood pressure should be controlled with a titratable agent to balance the risk of stroke, hypertension-related rebleeding, and maintenance of cerebral perfusion pressure.
- Start an infusion if indicated 12:
- Nicardipine 5 mg/h IV, increase by 2.5 mg/h q10min (max 15 mg/h) until the target blood pressure is achieved and then immediately titrate down to maintenance infusion of 3-5 mg/hr. If BP falls below target, decrease infusion to 2.5 mg/hr or stop entirely.
- Clevidipine: Start at 1-2 mg/hour. Double every 2 minutes until BP begins approaching target, then titrate by smaller increments every 5-10 minutes. Dose range is 1-32 mg/hour.
- ⚠️Avoid nitroprusside and nitroglycerin due to their significant vasodilatory effect and the risk of increasing intracranial pressure (ICP).
Anticoagulation reversal.
- Aggressively manage any endogenous or exogenous coagulopathy (see the table below).
- Information necessary for appropriate reversal will be the current INR in patients taking vitamin K antagonists and the time from last dose in patients on direct thrombin inhibitors or Xa inhibitors. If information is not available, full reversal dosing is recommended 9.
- 👉Note: Platelets should only be given if levels are low (<100,000 per μL) or to prepare for surgery 9.
Seizure prophylaxis.
- Consider seizure prophylaxis for all patients.
- Levetiracetam (15-20mg/kg over 30 minutes) is generally the preferred agent for seizure prophylaxis 19.
- Phenytoin has been associated with poor outcomes and should be avoided 20.
Neurological worsening in patients with known SAH
There are several causes of neurological decompensation including 2, 21:
- Aneurysm rebleeding.
- Repeat noncontrast head CT is diagnostic.
- Seizure.
- EEG is diagnostic especially in nonconvulsive seizures and status epilepticus in comatose patients.
- Vasospasm and delayed cerebral ischemia.
- CTA and CTP are diagnostic.
- Worsening hydrocephalus
- Repeat non-contrast head CT is diagnostic
- Hyponatremia
- High fever (e.g. central fever).
- Cardiopulmonary causes (Takotsubo cardiomyopathy, neurogenic pulmonary edema).
- Echocardiography and coronary angiography is diagnostic.
- Medication effect (e.g. sedation).
- Infection (e.g. ventriculitis following ventricular drain placement).
Refer to media below.
Neurocritical management
Rebleeding
- Rebleeding occurs in ~15% of patients and is associated with high mortality rate (20-60%).
- It has its highest rate (8% to 23%) within the first 72 hours after SAH, with the majority of rebleeding (50% to 90%) occurring within the first 6 hours.
- If the aneurysm is not secured, the risk of rebleeding will continue to gradually increase over time (~1% daily increase over the first month). After the first month, rebleeding rates are low, at 3% per year.
- Risk factors for rebleeding 2:
- Poor-grade SAH
- History of hypertension, a diastolic blood pressure >90 mmHg on admission.
- A large aneurysm
- Coagulopathy or use of antiplatelet medications.
- Longer duration of time to aneurysm treatment.
- Neurologic deterioration is often rapid and dramatic.
- Diagnosis: Repeat noncontrast head CT is helpful.
- Effective strategy to reduce risk of rebleeding
- Blood pressure control (see above)
- Anticoagulation reversal (table above)
- Treatment of the aneurysm: The best measure to reduce the risk of rebleeding is the prompt treatment of the unsecured, ruptured aneurysm (preferably within 24 hours, and immediately if rebleeding does occur).
- The ISAT trials showed that endovascular coiling generally yields superior outcomes compared to surgical clipping after SAH 22. Currently, endovascular coiling is preferred over surgical clipping whenever possible. However many aneurysms are not suited for endovascular coiling. The choice of treatment depends on the patient’s age as well as the aneurysm location, morphology, and relationship to adjacent vessels.
- Surgical clipping may be preferred for aneurysm with wide neck-to-body ratio, crucial arteries arising from aneurysm dome, middle cerebral artery aneurysm, aneurysm with large parenchymal hematoma.
- Endovascular coiling is suitable for patients with older age, poor clinical grade, multiple comorbidities, top of the basilar aneurysm, high surgical risk.
- If the optimal management modality is equivocal, endovascular coiling is usually preferred.
- The ISAT trials showed that endovascular coiling generally yields superior outcomes compared to surgical clipping after SAH 22. Currently, endovascular coiling is preferred over surgical clipping whenever possible. However many aneurysms are not suited for endovascular coiling. The choice of treatment depends on the patient’s age as well as the aneurysm location, morphology, and relationship to adjacent vessels.
- Antifibrinolytics (tranexamic acid)
- A large, multicenter RCT study showed that early short tranexamic acid treatment did not improve clinical outcome at 6 months, as measured by the modified Rankin Scale 23.
- Tranexamic acid may be considered for occasional patients with challenging coagulopathies (e.g. hyperfibrinolysis revealed on thromboelastography) or in those whose aneurysm management needs to be delayed (e.g. patients requiring long distance inter-hospital transfer)24.
Seizure and seizure prophylaxis
- Approximately 20% of patients with SAH suffer a seizure prior to arrival and another 5–10% experience a seizure after admission.
- Early seizures may increase the risk of aneurysm re-rupture and elevated intracranial pressure (ICP).
- Nonconvulsive seizures or status epilepticus may occur in ~10% of patients, especially comatose patients and has been associated with delayed cerebral ischemia and worse outcomes.
- Risk factors for seizure include 2:
- >65 years old.
- Focal pathology:
- Thick SAH on CT scan.
- Intraparenchymal hemorrhage.
- Cerebral infarct.
- Rebleeding.
- Aneurysm in middle cerebral artery
- Surgical aneurysm clipping.
- Management
- Continuous EEG monitoring should be considered in patients with high-grade SAH or those with persistent coma after initial resuscitation, or evidence of seizure 2.
- Medication
- Use of a short course (3 to 7 days) of prophylactic anti-epileptic in the immediate post-hemorrhage period is recommended by both American Heart Association (AHA) and Neurocritical Care Society (NCS).
- Levetiracetam is generally the preferred agent for seizure prophylaxis, based on its safety profile 19.
- Duration
- For patients who don’t have a seizure, prophylaxis can be discontinued after seizure has been excluded (either via EEG or clinical examination) and the aneurysm has been secured.
- Patients with seizure should be treated in the usual fashion, including longer durations of antiepileptic therapy (e.g. several months of maintenance therapy).
Delayed cerebral ischemia
- Definition
- Delayed cerebral ischemia is defined as any neurologic deterioration that persists for more than 1 hour and cannot be explained by any other neurologic or systemic condition.
- Delayed cerebral ischemia occurs on average 3 to 14 days after SAH with a peak risk around days 7-10. It generally resolves by day 21.
- It is the leading cause for morbidity in patients who survive the initial SAH.
- Pathophysiology
- Historically, delayed cerebral ischemia was thought to be caused by cerebral vasospasm. However, evidence now indicates that the pathophysiology of delayed cerebral ischemia includes an interaction of early brain injury, microthrombosis, cortical spreading depolarizations, related ischemia, and cerebral vasospasm 2.
- Risk factors for delayed cerebral ischemia 2:
- Younger age
- Greater SAH thickness (More severe SAH)
- Intraventricular hemorrhage, as demonstrated by the modified Fisher Scale.
- Additional risk factors include
- Poor clinical grade
- Loss of consciousness at ictus
- Cigarette smoking
- Cocaine use
- SIRS
- Hyperglycemia
- Hydrocephalus,
- Nonconvulsive seizures
- Prophylaxis against Delayed Cerebral ischemia
- Nimodipine (60 mg every 4 hours for 21 days).
- A calcium channel blocker shown to improve outcomes, is recommended and can be started in the ED if the patient can take oral medications (ideally 60mg PO q4h) 25.
- Maintenance of normal intravascular volume status
- Nimodipine (60 mg every 4 hours for 21 days).
- Monitoring for delayed cerebral ischemia
- Serial neurological examination every 1 to 2 hours
- Head CT: Expert recommendation implies that all patients with SAH undergo a head CT at 24 to 48 hours after aneurysm treatment to establish any treatment related infarctions. Any subsequent new hypodensities not attributable to EVD insertion or intraparenchymal hematoma should be regarded as cerebral infarctions from delayed cerebral ischemia regardless of the clinical signs.
- Monitoring ICP and cerebral perfusion pressure
- Transcranial Doppler
- Continuous EEG
- CTA and CT perfusion (CTP)
- Angiographic vasospasm vs. clinical symptomatic vasospasm
- It is very important to differentiate between angiographic vasospasm and clinical symptomatic vasospasm 2.
- Radiologic vasospasm (detected on CTA/TCD) happens in most patients (~70%) with SAH and is not associated with poor outcome.
- Only ~30% of patients with SAH with vasospasm have tissue ischemia with neurological deterioration (termed symptomatic vasospasm or delayed cerebral ischemia).
- If vasospasm does not cause neurological deterioration, then it can often be observed without treatment.
- It is very important to differentiate between angiographic vasospasm and clinical symptomatic vasospasm 2.
- Diagnosis:
- New onset of focal or global neurologic deficits or a decrease of ≥2 points on the GCS that lasts for at least 1 hour.
- Exclude other neurologic or systemic conditions that can cause neurologic deterioration such as fever, seizures, hydrocephalus, sepsis, hypoxemia, sedation, and other metabolic causes.
- CTA: Vasospasm can be identified on CTA. Simultaneously CTA can be helpful to exclude other alternative causes.
- Neurological improvement following augmentation of BP may support the diagnosis of symptomatic vasospasm.
- Management of symptomatic cerebral vasospasm
- Correct hypovolemia
- Induce hypertension
- Target
- A mean arterial pressure (MAP) of ~ 20 mm Hg above the baseline MAP is set as the first goal (often MAP >90 mm Hg).
- Agents
- α1 receptor agonists (norepinephrine or phenylephrine) continuous infusion 2.
- This group of drugs are the vasopressors of choice in SAH, as the brain vessels lack α1 receptors, and therefore only systemic but not brain vasoconstriction is achieved.
- Monitoring
- Blood pressure augmentation should progress in a stepwise fashion with frequent neurologic assessments at each step.
- If the clinical examination has returned to the prior baseline examination, no further blood pressure elevations are necessary.
- If clinical examination further deteriorates at this BP goal, In the latter case, increases in the blood pressure goal should be attempted.
- Target
- Endovascular therapy using intraarterial vasodilators (nicardipine, milrinone, verapamil) or angioplasty may be helpful if induced hypertension therapy fails.
Hydrocephalus
- Acute symptomatic hydrocephalus occurs in 20% of patients with SAH, usually within minutes to days after SAH onset.
- Causes of hydrocephalus may include bleeding into the ventricular system, obstruction of the fourth ventricle by blood, or impaired reabsorption of CSF at the arachnoid granulations.
- Hydrocephalus can resolve spontaneously in 30% of patients but can also rapidly worsen.
- Clinical presentation of hydrocephalus in the context of SAH is progressive deterioration in level of consciousness, impaired upgaze, hypertension, and delirium 19.
- Risk factor for development of hydrocephalus include 28:
- Older age
- IVH
- Posterior circulation aneurysms
- Treatment with antifibrinolytic agents
- Low GCS on presentation
- The diagnosis is made by serial head CT and clinical symptoms. Ocular ultrasound is helpful as well.
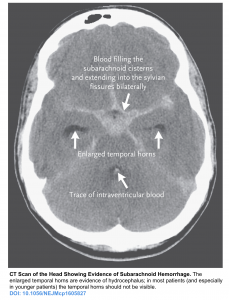
- Head CT: Dilatation of the ventricles accompanied by subendymal CSF ooze and mass effect indicates hydrocephalus. Assessment of the ventricular dilation is very subjective but the presence of following signs can be helpful
- Enlarged temporal horns (in most patients and especially in younger patients, the temporal horns should not be visible). See the image below.
- Rounding frontal horns and outward bowing of the lateral walls of the third ventricle.
- Head CT: Dilatation of the ventricles accompanied by subendymal CSF ooze and mass effect indicates hydrocephalus. Assessment of the ventricular dilation is very subjective but the presence of following signs can be helpful
- Management
- Symptomatic hydrocephalus (e.g. obtundation, coma) should be immediately treated 19.
- External ventricular drain (EVD) is the treatment of choice 2.
- A rapid weaning of the EVD is recommended after aneurysm obliteration or within 48 hours of insertion if the patient is neurologically stable.
- In those for whom weaning is unsuccessful (approximately 40%), placement of a chronic ventriculoperitoneal shunt may be required.
- Lumbar drain insertion is another option for the management if the patient has communicating hydrocephalus.
- In patients with obstructive hydrocephalus (due to obstruction of the ventricular system by blood, or a parenchymal hemorrhage), lumbar drainage is contraindicated.
Hyponatremia
- Hyponatremia is the most common electrolyte disorder after SAH and can occur in up to 30% of patients.
- Etiologies of hyponatremia after SAH
- Pseudohyponatremia
- Cerebral salt wasting: Caused by excessive secretion of brain natriuretic peptide (BNP) with subsequent suppression of aldosterone synthesis 26.
- Syndrome of inappropriate secretion of antidiuretic hormone (SIADH)
- Multifactorial
- It is difficult to differentiate cerebral salt wasting from SIADH, since laboratory findings are fairly similar in both.
- In both cerebral salt wasting and SIADH, the laboratory findings are similar as:
- Low serum sodium (<134 mEq/mL)
- Low serum osmolality (<274 mmol/L)
- High urine sodium (>20 mmol/L)
- High urine osmolality (>100 mmol/L).
- The only differentiating finding is the patient’s intravascular volume status:
- Cerebral salt wasting is a hypovolemic state.
- SIADH is an euvolemic or even hypervolemic state.
- In both cerebral salt wasting and SIADH, the laboratory findings are similar as:
- Investigation
- Evaluate volume status.
- Check serum osmolality, urine sodium and urine osmolality.
- Evaluate thyroid and adrenal functions (cortisol level).
- Serum glucose and triglyceride levels.
- Management
- Hyponatremia in subarachnoid hemorrhage is treated with hypertonic saline e.g. 3% saline (regardless to the underlying etiology) 27.
- Remember that treatment of asymptomatic hyponatremia of SIADH in other settings usually consists of fluid restriction. However this is so dangerous in SAH, since it increases the risk symptomatic cerebral vasospasm.
- Hypovolemia may be corrected with administration of isotonic fluid.
- Fludrocortisone (0.1-0.3 mg PO BID) is added If there’s ongoing salt wasting in an effort to limit natriuresis. Note that fludrocortisone can cause hypokalemia, which may require appropriate potassium repletion.
- Treat other identifiable condition (e.g. adrenal insufficiency).
- Hyponatremia in subarachnoid hemorrhage is treated with hypertonic saline e.g. 3% saline (regardless to the underlying etiology) 27.
For Na level <133 mEq/L or a decrease of 6 mEq/L in 24 to 48 hours
1. NaCl tabs 3g PO/NGT every 6 hours.
2. Initiate 3% NaCl infusion at 20 mL/hour IV.
3. Check serum Na every 6 hours.
a. If Na <130 mEq/L:
-Increase rate by 20 mL/hour (max rate = 80 mL/hour)
-If on hold at present, initiate 3 percent NaCl infusion at 20 mL/hour IV
b. If Na = 130 to 135 mEq/L:
-Increase rate by 10 mL/hour (max rate = 80 mL/hour)
-If on hold at present, initiate 3 percent NaCl infusion at 10 mL/hour IV
c. If Na = 136 to 140 mEq/L: No change
d. If Na ≥140 mEq/L: Hold infusion
Fever
- Following SAH, both infectious and non-infectious fever (a.k.a. central, neurogenic fever) can happen and it has been associated with delayed cerebral ischemia and worse clinical outcome 2.
- Central fever (neurogenic) is the most common medical complication after SAH, occurring in up to 70% of patients and is more likely to occur in patients with high-grade SAH and poor neurologic status.
- The cause of central or neurogenic fever in the setting of SAH is thought to involve hypothalamic irritation from blood products 19.
- Central fever remains a diagnosis of exclusion, following evaluation for infection. Evaluation may include cultures of blood, sputum, and cerebrospinal fluid (for patients with external ventricular drains).
- Regardless of the etiology, fever should always be treated aggressively to reduce secondary brain injury (acetaminophen and cooling blankets).
Other therapeutic considerations
Analgesia
- SAH may be painful, with ongoing meningeal irritation causing headache.
- Management
- ⚠️NSAIDs should generally be avoided due to risks of coagulopathy and renal dysfunction (at least until the aneurysm is secured)19.
- Scheduled acetaminophen may be helpful (e.g. 1 gram q6hrs).
- Dexamethasone: Meningeal chemical irritation from the SAH often responds to one or several single doses of dexamethasone (2 mg to 10 mg) 2.
- Opioid should be minimized as they are not effective for meningeal pain (e.g. by using PRN boluses for breakthrough pain only). Short-acting opiates (e.g. fentanyl) are preferred 9.
Hyperglycemia
- Glycemic dysfunction is very common after SAH because of stress and has been associated with delayed cerebral ischemia and poor clinical outcome. However, it remains unclear whether this is merely an association or causative.
- Hypoglycemia can lead to brain metabolic crisis and must be vigilantly avoided.
- In the absence of clinical trials of glucose control in patients with SAH, current recommendations are to maintain a blood glucose level between 80 mg/dL and 200 mg/dL.
DVT prophylaxis
- Deep vein thrombosis after SAH is common, with rates between 2% and 20% depending on the screening method.
- Prior to aneurysm protection
- Chemical DVT prophylaxis is contraindicated.
- Pneumatic compression devices should be used for DVT prophylaxis.
- After aneurysm protection
- When to initiate DVT prophylaxis:
- After aneurysm coiling: chemical DVT prophylaxis may usually be started immediately (but confirm this with the neurointerventional team).
- After surgical clipping: chemical DVT prophylaxis may usually be started after >24 hours (but confirm this with the neurosurgery team).
- Agent:
- Heparin-induced thrombocytopenia is relatively common in this context, with some series reporting rates of 6%. The use of low-molecular-weight heparin may be preferable in patients with adequate renal function, because low-molecular-weight heparin causes less heparin-induced thrombocytopenia than unfractionated heparin 2.
- When to initiate DVT prophylaxis:
Cardiopulmonary complications
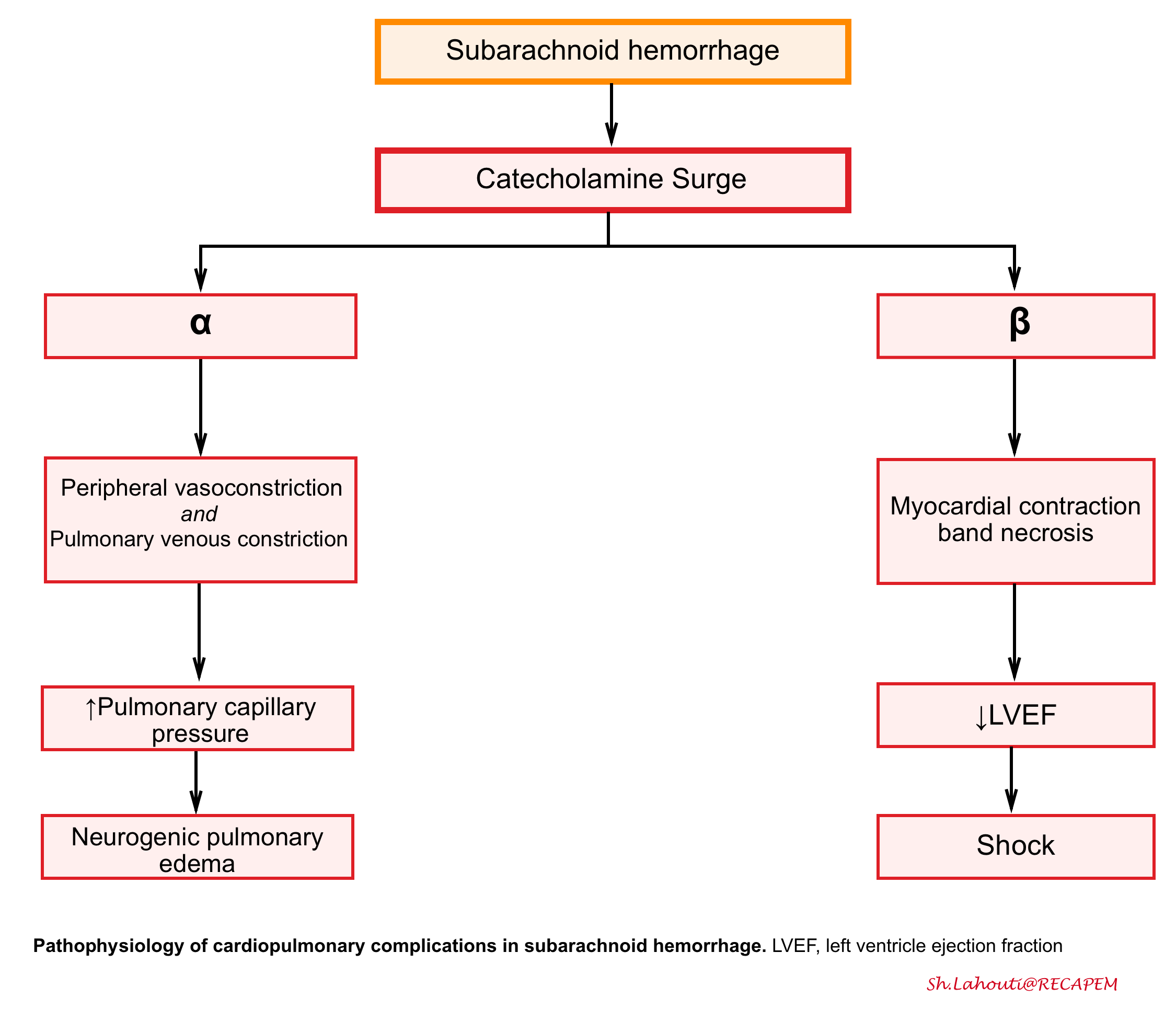
- Cardiopulmonary dysfunction is a well-known complication of SAH and can range from minor ECG changes to severe stress cardiomyopathy (Takotsubo cardiomyopathy) and neurogenic pulmonary edema (see the following figure).
- The severity of SAH is an independent predictor of cardiopulmonary injury, suggesting that the cardiopulmonary injury is neurally mediated.
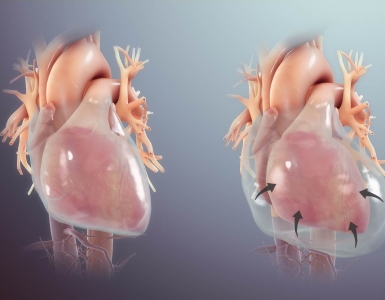
- Stress cardiomyopathy (Takotsubo cardiomyopathy)
- The catecholamine surge during aneurysm rupture results in direct myocardial injury (contraction band necrosis), leading to decreased inotropy and an increase in cardiac preload due to venous constriction, as well as increased cardiac afterload due to peripheral arterial constriction.
- Definition of Takotsubo cardiomyopathy involve several criteria which involve 29:
- Transient regional wall motion abnormalities (RWMAs) of the LV and/or RV after a stressful trigger e.g. emotional.
- RWMAs extend beyond the distribution of a single coronary artery.
- New and reversible ECG abnormalities e.g. ST elevation, diffuse T-wave inversion (note that ECG can’t reliably differentiate Takotsubo cardiomyopathy versus occlusive MI).
- Elevated BNP (~80% of patients).
- Troponin elevation (relatively small rise compared to magnitude of wall motion abnormality).
- Echocardiography may show classic pattern of akinesia of the apex, with hypercontractility of the base.
- LV outflow tract obstruction (LVOTO) may occur when the base of the heart contracts vigorously, causing the turbulent flow of blood in the aortic outflow tract to pull the anterior leaflet of the mitral valve anteriorly (causing mitral regurgitation) 30. This is important to recognize because it has hemodynamic implications for management.
- Management of unstable patients with Takotsubo cardiomyopathy: treatment depends on whether there is an associated left ventricular outflow tract obstruction (LVOT) 31.
- Unstable patients without LVOT: vasopressors, inotropes (if required, give the lowest possible dose), or in severe cases, mechanical circulatory support.
- Unstable patients with LVOT: Treatment is different compared to most patients with heart failure as:
- Preload and afterload reduction (e.g. nitroglycerin) will make LVOT worse and should be avoided.
- Beta-blockade and fluid loading may improve LVOTO.
- Hypotension in the context of LVOTO may respond to a pure vasoconstrictor (e.g. phenylephrine).
- Inotropic agents (e.g. dobutamine) may exacerbate LVOTO.
- Neurogenic pulmonary edema
- Pulmonary edema may occur either as a result of the acute left ventricular dysfunction or independently as neurogenic pulmonary edema from substantial increases in pulmonary capillary pressures from the sympathetic surge.
- Neurogenic pulmonary edema should be on differential diagnosis if there is unexplained early hypoxemia in a patient with severe, high-grade SAH.
- Etiology
- Intracranial hemorrhage (especially SAH, intraparenchymal hemorrhage)
- Severe TBI with elevated ICP
- Status epilepticus
- Encephalitis
- Guillain-Barre syndrome (GBS) with dysautonomia
- Acute hydrocephalus
- Ischemic stroke (especially posterior strokes involving the medulla, or anterior strokes involving the insular cortex)*
- Differential diagnosis of neurogenic pulmonary edema 32:
- Iatrogenic volume overload
- Cardiogenic pulmonary edema primarily due to Takotsubo cardiomyopathy.
- Aspiration pneumonitis or pneumonia.
- Treatment of neurogenic pulmonary edema is supportive with lung protective therapy according to current
ARDS management strategies 19.- Avoid aggressive diuresis, as this may increase the risk of cerebral vasospasm.
Further going
- Guidelines:
- Podcasts:
- Imaging:
aSAH 4: Complications from Oliver Flower on Vimeo.
aSAH 3: Vasospasm from Oliver Flower on Vimeo.
References
1: Schwedt TJ, Matharu MS, Dodick DW. Thunderclap headache. Lancet Neurol. 2006 Jul;5(7):621-31. doi: 10.1016/S1474-4422(06)70497-5. PMID: 16781992.
2: Muehlschlegel S. Subarachnoid Hemorrhage. Continuum (Minneap Minn). 2018 Dec;24(6):1623-1657. doi: 10.1212/CON.0000000000000679. PMID: 30516599.
3: Malhotra A, Wu X, Gandhi D, Sanelli P. The Patient with Thunderclap Headache. Neuroimaging Clin N Am. 2018 Aug;28(3):335-351. doi: 10.1016/j.nic.2018.03.002. Epub 2018 Jun 8. PMID: 30007749
4: Schwedt TJ, Matharu MS, Dodick DW. Thunderclap headache. Lancet Neurol. 2006 Jul;5(7):621-31. doi: 10.1016/S1474-4422(06)70497-5. PMID: 16781992
5: Fink KR, Benjert JL. Imaging of Nontraumatic Neuroradiology Emergencies. Radiol Clin North Am. 2015 Jul;53(4):871-90, x. doi: 10.1016/j.rcl.2015.02.004. PMID: 26046515.
6: Marder CP, Narla V, Fink JR, Tozer Fink KR. Subarachnoid hemorrhage: beyond aneurysms. AJR Am J Roentgenol. 2014 Jan;202(1):25-37. doi: 10.2214/AJR.12.9749. PMID: 24370126.
7: van der Jagt M, Hasan D, Bijvoet HW, Pieterman H, Dippel DW, Vermeij FH, Avezaat CJ. Validity of prediction of the site of ruptured intracranial aneurysms with CT. Neurology. 1999 Jan 1;52(1):34-9. doi: 10.1212/wnl.52.1.34. PMID: 9921845.
8: Perry JJ, Alyahya B, Sivilotti ML, Bullard MJ, Émond M, Sutherland J, Worster A, Hohl C, Lee JS, Eisenhauer MA, Pauls M, Lesiuk H, Wells GA, Stiell IG. Differentiation between traumatic tap and aneurysmal subarachnoid hemorrhage: prospective cohort study. BMJ. 2015 Feb 18;350:h568. doi: 10.1136/bmj.h568. PMID: 25694274; PMCID: PMC4353280.
9: Patel S, Parikh A, Okorie ON. Subarachnoid hemorrhage in the emergency department. Int J Emerg Med. 2021 May 12;14(1):31. doi: 10.1186/s12245-021-00353-w. PMID: 33980142; PMCID: PMC8117305.
10: Maher M, Schweizer TA, Macdonald RL. Treatment of Spontaneous Subarachnoid Hemorrhage: Guidelines and Gaps. Stroke. 2020 Apr;51(4):1326-1332. doi: 10.1161/STROKEAHA.119.025997. Epub 2020 Jan 22. PMID: 31964292.
11: Gottlieb M, Morgenstern J. Lumbar Puncture Should Not Be Routinely Performed For Subarachnoid Hemorrhage After A Negative Head Ct. Ann Emerg Med. 2021 Jun;77(6):643-645. doi: 10.1016/j.annemergmed.2020.11.018. PMID: 34030777.
12: Marcolini E, Hine J. Approach to the Diagnosis and Management of Subarachnoid Hemorrhage. West J Emerg Med. 2019 Mar;20(2):203-211. doi: 10.5811/westjem.2019.1.37352. Epub 2019 Feb 28. PMID: 30881537; PMCID: PMC6404699.
13: Patra A, Janu A, Sahu A. MR Imaging in Neurocritical Care. Indian J Crit Care Med. 2019 Jun;23(Suppl 2):S104-S114. doi: 10.5005/jp-journals-10071-23186. PMID: 31485117; PMCID: PMC6707490.
14: de Oliveira Manoel AL, Mansur A, Murphy A, Turkel-Parrella D, Macdonald M, Macdonald RL, Montanera W, Marotta TR, Bharatha A, Effendi K, Schweizer TA. Aneurysmal subarachnoid hemorrhage from a neuroimaging perspective. Crit Care. 2014 Nov 13;18(6):557. doi: 10.1186/s13054-014-0557-2. PMID: 25673429; PMCID: PMC4331293.
15: Tabatabai RR, Swadron SP. Headache in the Emergency Department: Avoiding Misdiagnosis of Dangerous Secondary Causes. Emerg Med Clin North Am. 2016 Nov;34(4):695-716. doi: 10.1016/j.emc.2016.06.003. Epub 2016 Sep 3. PMID: 27741984.
16: Lantigua H, Ortega-Gutierrez S, Schmidt JM, et al. Subarachnoid hemorrhage: who dies, and why?. Crit Care. 2015;19(1):309. Published 2015 Aug 31. doi:10.1186/s13054-015-1036-0
17: Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES Jr, MacDonald RL, Mayer SA. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery. 2006 Jul;59(1):21-7; discussion 21-7. doi: 10.1227/01.NEU.0000218821.34014.1B. PMID: 16823296.
18: Patel S, Parikh A, Okorie ON. Subarachnoid hemorrhage in the emergency department. Int J Emerg Med. 2021 May 12;14(1):31. doi: 10.1186/s12245-021-00353-w. PMID: 33980142; PMCID: PMC8117305.
19: Chung DY, Abdalkader M, Nguyen TN. Aneurysmal Subarachnoid Hemorrhage. Neurol Clin. 2021 May;39(2):419-442. doi: 10.1016/j.ncl.2021.02.006. Epub 2021 Mar 31. PMID: 33896527; PMCID: PMC8147706.
20: Diringer MN, Bleck TP, Claude Hemphill J 3rd, Menon D, Shutter L, Vespa P, Bruder N, Connolly ES Jr, Citerio G, Gress D, Hänggi D, Hoh BL, Lanzino G, Le Roux P, Rabinstein A, Schmutzhard E, Stocchetti N, Suarez JI, Treggiari M, Tseng MY, Vergouwen MD, Wolf S, Zipfel G; Neurocritical Care Society. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care. 2011 Sep;15(2):211-40. doi: 10.1007/s12028-011-9605-9. PMID: 21773873.
21: John, S.M., W.; Qualls, S.; Edlow, B. , Emergency neurological life support
subarachnoid hemorrhage protocol version 4.0, in Neurocritical Care Society. 2019. Page 20.
22: Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, Sandercock P; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005 Sep 3-9;366(9488):809-17. Doi: 10.1016/S0140-6736(05)67214-5. PMID: 16139655.
23: Post R, Germans MR, Tjerkstra MA, Vergouwen MDI, Jellema K, Koot RW, Kruyt ND, Willems PWA, Wolfs JFC, de Beer FC, Kieft H, Nanda D, van der Pol B, Roks G, de Beer F, Halkes PHA, Reichman LJA, Brouwers PJAM, van den Berg-Vos RM, Kwa VIH, van der Ree TC, Bronner I, van de Vlekkert J, Bienfait HP, Boogaarts HD, Klijn CJM, van den Berg R, Coert BA, Horn J, Majoie CBLM, Rinkel GJE, Roos YBWEM, Vandertop WP, Verbaan D; ULTRA Investigators. Ultra-early tranexamic acid after subarachnoid haemorrhage (ULTRA): a randomised controlled trial. Lancet. 2021 Jan 9;397(10269):112-118. doi: 10.1016/S0140-6736(20)32518-6. Epub 2020 Dec 23. PMID: 33357465.
24: Lawton MT, Vates GE. Subarachnoid Hemorrhage. N Engl J Med. 2017 Jul 20;377(3):257-266. doi: 10.1056/NEJMcp1605827. PMID: 28723321.
25: Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, Patel AB, Thompson BG, Vespa P; American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012 Jun;43(6):1711-37. doi: 10.1161/STR.0b013e3182587839. Epub 2012 May 3. PMID: 22556195.
26: Berendes E, Walter M, Cullen P, Prien T, Van Aken H, Horsthemke J, Schulte M, von Wild K, Scherer R. Secretion of brain natriuretic peptide in patients with aneurysmal subarachnoid haemorrhage. Lancet. 1997 Jan 25;349(9047):245-9. doi: 10.1016/s0140-6736(96)08093-2. PMID: 9014912.
27: Sterns RH, Silver SM. Cerebral salt wasting versus SIADH: what difference? J Am Soc Nephrol. 2008 Feb;19(2):194-6. doi: 10.1681/ASN.2007101118. Epub 2008 Jan 23. PMID: 18216309.
28: Xie Z, Hu X, Zan X, Lin S, Li H, You C. Predictors of Shunt-dependent Hydrocephalus After Aneurysmal Subarachnoid Hemorrhage? A Systematic Review and Meta-Analysis. World Neurosurg. 2017 Oct;106:844-860.e6. doi: 10.1016/j.wneu.2017.06.119. Epub 2017 Jun 23. PMID: 28652120.
29: Keramida K, Backs J, Bossone E, Citro R, Dawson D, Omerovic E, Parodi G, Schneider B, Ghadri JR, Van Laake LW, Lyon AR. Takotsubo syndrome in Heart Failure and World Congress on Acute Heart Failure 2019: highlights from the experts. ESC Heart Fail. 2020 Apr;7(2):400-406. doi: 10.1002/ehf2.12603. Epub 2020 Jan 29. PMID: 31994355; PMCID: PMC7160490.
30: Di Vece D, Silverio A, Bellino M, Galasso G, Vecchione C, La Canna G, Citro R. Dynamic Left Intraventricular Obstruction Phenotype in Takotsubo Syndrome. J Clin Med. 2021 Jul 22;10(15):3235. doi: 10.3390/jcm10153235. PMID: 34362020; PMCID: PMC8347696.
31: Medina de Chazal H, Del Buono MG, Keyser-Marcus L, Ma L, Moeller FG, Berrocal D, Abbate A. Stress Cardiomyopathy Diagnosis and Treatment: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018 Oct 16;72(16):1955-1971. doi: 10.1016/j.jacc.2018.07.072. PMID: 30309474; PMCID: PMC7058348.
32: Davison DL, Terek M, Chawla LS. Neurogenic pulmonary edema. Crit Care. 2012 Dec 12;16(2):212. doi: 10.1186/cc11226. PMID: 22429697; PMCID: PMC3681357
32: Davison DL, Terek M, Chawla LS. Neurogenic pulmonary edema. Crit Care. 2012 Dec 12;16(2):212. doi: 10.1186/cc11226. PMID: 22429697; PMCID: PMC3681357.




Add comment