March 30, 2022 by Shahriar Lahouti
CONTENTS
- Introduction
- Terminology
- Pathogenesis
- Clinical setting and risk factors
- Imaging findings
- Lumbar puncture
- Diagnosis
- Management
- Prognosis
- RECAP
- Going further
- Appendix
- References
Introduction
Reversible cerebral vasoconstriction syndrome (RCVS) and posterior reversible encephalopathy syndrome (PRES) have become increasingly recognized, mainly due to the spreading of brain magnetic resonance (MRI) and clinical awareness. PRES and RCVS are descriptive terms, each bringing together conditions with similar clinical-imaging manifestations. The underlying pathophysiology in both is related to reversible dysregulation of the cerebral vasculature. Here the causes, clinical and imaging features, management, and prognosis of PRES and RCVS are described.
Terminology
RCVCS
- Reversible cerebral vasoconstriction syndrome represents a group of conditions that show identical clinical, imaging (reversible multifocal narrowing of the cerebral arteries) and prognostic features regardless to the associated condition. RCVS has been variably termed as:
- Migrainous vasospasm or migraine angiitis.
- Thunderclap headache-associated vasospasm
- Postpartum cerebral angiopathy
- Drug induced cerebral arteritis
PRES
- Posterior reversible encephalopathy syndrome is a clinicoradiographic syndrome and is often referred to as
- Posterior leukoencephalopathy syndrome
- Hyperperfusion encephalopathy
- Brain capillary leak syndrome
- However none of these names are satisfactory as:
- Brain injury is not always reversible.
- Involvement is not necessarily confined to the white matter nor to the posterior regions of the brain.
Pathogenesis
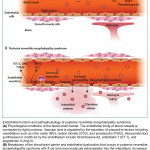
Several pathophysiological mechanisms have been proposed for both syndromes but pathogenesis remains unclear. Abnormal cerebrovascular tone and endothelial dysfunction may explain both syndromes. In both syndromes, a blood flow dysregulation has been suggested to have a causative role but other mechanisms as immune system dysregulation or endothelium dysfunction may play a role in pathogenesis 1.
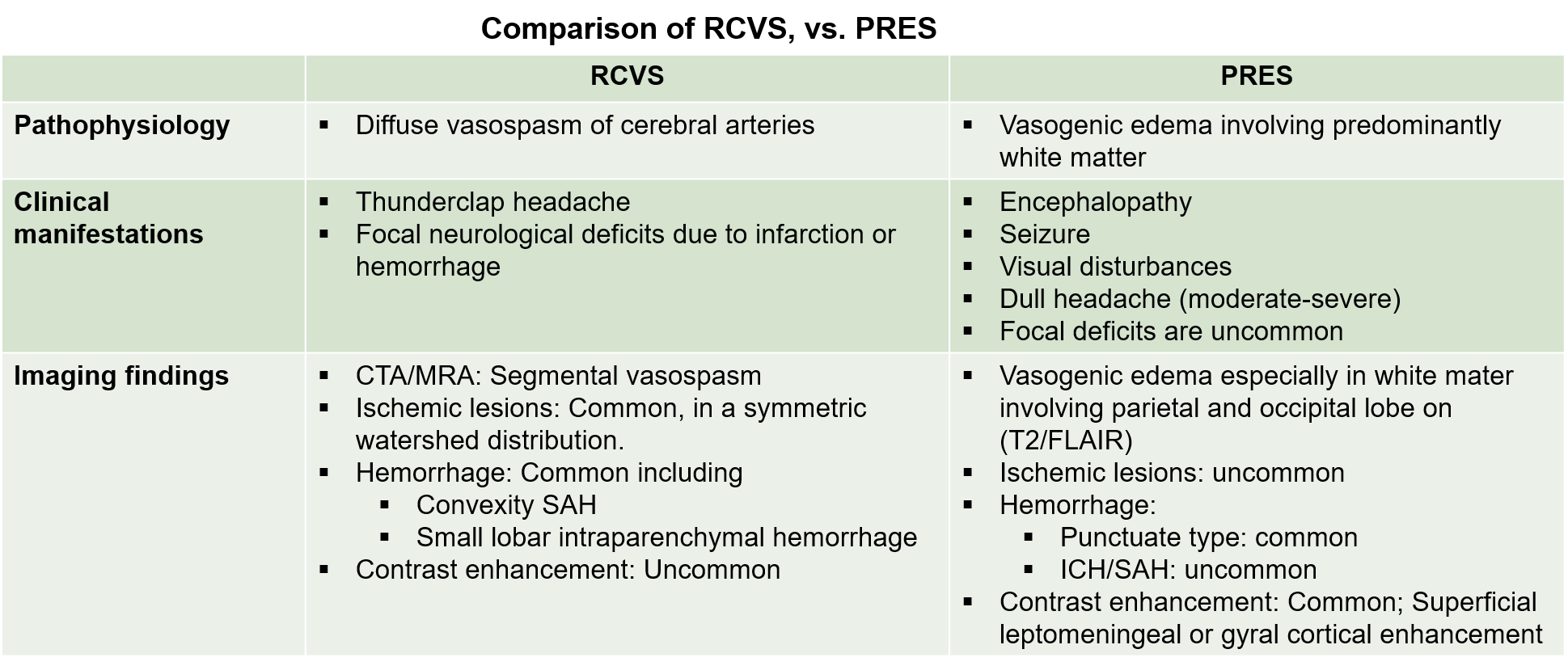
Although dysregulation of cerebral vasculature is culprit in both PRES and RCVS, these two syndromes differ as:
- RCVS involves excessive vasospasm, causing inadequate blood flow through the arterioles.
- PRES involves failure of autoregulation, with excess blood flow through the arterioles.
RCVS
- It is the most common cause of thunderclap headache in patients younger than 60 years old without aneurysmal SAH and is the most common cause of recurrent thunderclap headaches 2.
- Women are more commonly affected than men (with ratio 2:1).
- The average age is ~40 years old. RCVS occurs predominantly between the ages of 20-50, being reported up to 76 years old.
- The primary problem seems to be diffuse, multifocal vasospasm of intracranial arteries. The exact process is unknown and is believed to relate to autonomic over-activity, endothelial dysfunction, and oxidative stress 2.
- The pathogenesis of the thunderclap headache in RCVS may results from a positive feed-forward cycle involving increasing pain, increasing sympathetic tone, and increasing vasospasm due to innervation of arteries via the first division of the trigeminal nerve 2.
PRES
- Patients in all age groups appear susceptible (reported cases as young as 2 years and as old as 90 years).
- PRES is more common among women, even when patients with eclampsia are excluded.
- Pathogenesis: because of heterogenous nature of this disorder, different mechanisms may be involved in different clinical situations
- Failure of autoregulation and hypertension:
- In cases where hypertension is a key feature, hyperperfusion is thought to play a critical role. Normally, the cerebral arterioles will vasoconstrict in the context of hypertension, thereby brain tissue is protected from experiencing hypertension. At extremely high blood pressures, autoregulation may fail, causing the brain tissue to experience very high blood pressures. Hypertension may lead to fluid exudation and tissue edema 3.
- However both the absolute BP and the rate of BP rise are important. Patients with chronic hypertension can tolerate extremely high blood pressures without developing PRES. Alternatively, patients with baseline hypotension or highly labile blood pressures may be more likely to develop PRES 4.
- The posterior brain regions can be particularly susceptible to hyperperfusion because little sympathetic innervation exists in the posterior fossa 3.
- Endothelial dysfunction:
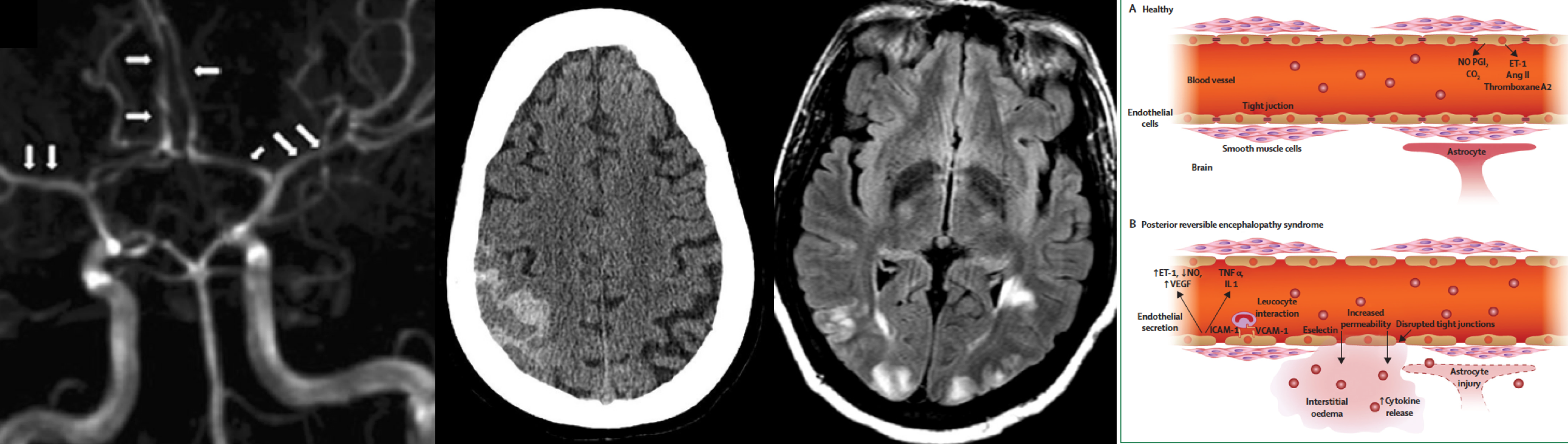
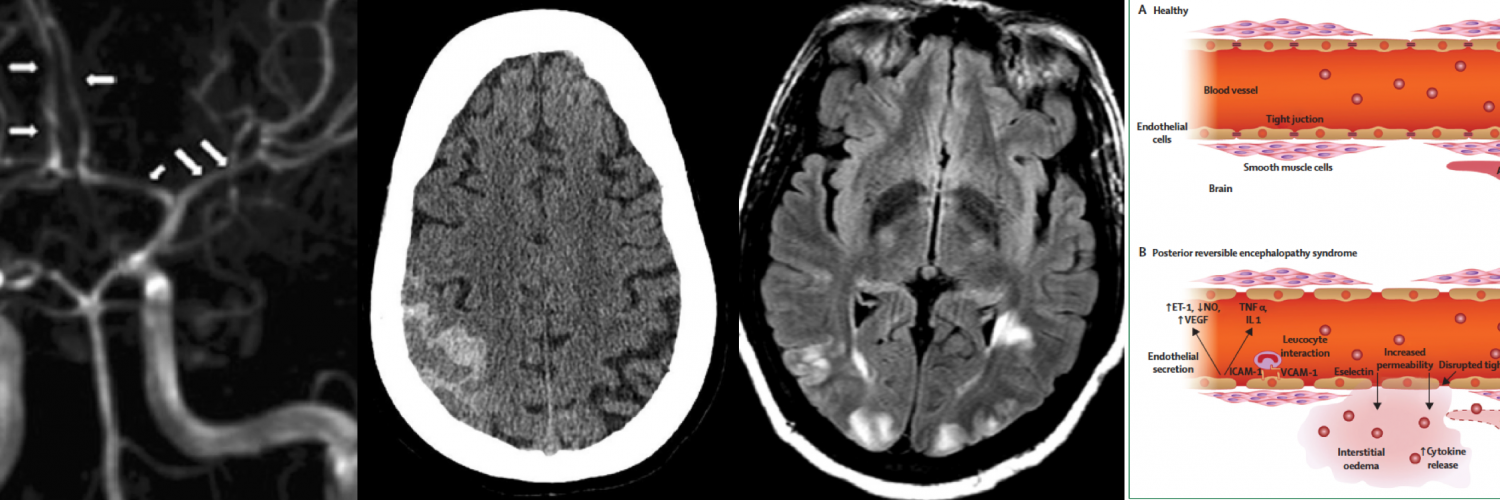
- Dysfunction of the vascular endothelium may promote exudation of fluid from the vasculature and tissue edema 5. (See appendix 1)
- Endothelial dysfunction may be especially relevant in the context of cytotoxic chemotherapies or preeclampsia.
- Focal vasoconstriction and cerebral ischemia:
- In occasional patients, dysfunctional attempts at autoregulation may result in reactive focal vasoconstriction. This may lead to focal hypoperfusion and infarction 4.
- When focal vasoconstriction occurs, this may represent a combined syndrome involving both PRES and reversible cerebral vasoconstriction syndrome (RCVS).
- Other mechanisms:
- Failure of autoregulation and hypertension:
RCVS-PRES Overlap
- The two disorders commonly coexist 6,18.
- The most common form of PRES-RCVS overlap appears to occur in patients who initially present with PRES. Over time, cerebral vasculature responds to hypertension and endothelial damage with vasospasm, thereby causing RCVS to be superimposed on top of PRES. RCVS might complicate the majority of patients with PRES.
- Less commonly, ~20% of RCVS may be complicated by the subsequent occurrence of PRES. In these patients, the primary insult is RCVS, which leads to subsequent hypertension and sympathetic activation. This hypertension may overwhelm autoregulation in posterior areas of the brain which aren’t experiencing vasoconstriction, leading to PRES. Essentially, the body is trying to overcome the cerebral vasoconstriction of RCVS, but this leads to an excessive blood pressure.
Clinical manifestations and risk factors
RCVS and PRES are intracranial vascular manifestation of a wide variety of diseases. Headache, visual symptoms, seizures, and confusion occur in both syndromes. RCVS is usually heralded by recurrent thunderclap headaches, whereas encephalopathy and seizures are typical in PRES.
RCVS
- Clinical manifestations
- Headache: 90% of patients experience thunderclap headache (TCH) with only less than 10% of patients presenting with a subacute or less severe headaches. The absence of headache at onset is exceptional.
- Associated features are often present and may include nausea and vomiting, photophobia, phonophobia, and visual changes 8.
- Most patients have triggering factors e.g. exertion, emotion, orgasm, swimming, bathing/showering, or Valsalva maneuvers (coughing, sneezing, defecation) 2.
- The TCH in RCVS improves within 1-3 hours, which helps differentiate this from subarachnoid hemorrhage 2.
- Headaches in RCVS tend to recur over a span of days to weeks. In one study, patients reported an average of four recurrences.
- A history of multiple thunderclap headaches recurring over several days is nearly pathognomonic for the diagnosis of RCVS.
- 🚩Co-existing neck pain warrants investigation for cervical artery dissection ( a prospective French study found an association of RCVS and cervical artery dissection in 12% of their patients with RCVS and 7% of patients who presented with cervical artery dissection) 9.
- Differential diagnosis of thunderclap headache 10:
- SAH
- Cervical artery dissection (carotid artery or vertebral artery dissection)
- CVT
- Acute hypertensive crisis
- ICH
- Acute ischemic stroke
- Pituitary apoplexy
- Colloid cyst of the third ventricle
- Aortic arch dissection
- Giant cell arteritis
- Blood pressure: The initial BP can be elevated with RCVS due to severe headache pain, the disease itself, or the associated condition (e.g. eclampsia, cocaine exposure).
- Neurological sequelae. More than 90% of RCVS patients ultimately have a benign course. However, approximately 75% of admitted patients eventually develop parenchymal lesions. These typically evolve within 1-2 weeks of the initial headache, as vasospasm continues to intensify. Clinical symptoms will vary widely, depending on which sequelae occur (e.g. ischemic strokes may cause focal neurological deficits). Hemorrhages and seizures usually occur within a week of onset, whereas ischemic complications occur after 2-3 weeks. The reported parenchymal lesions may include any of the following (or any combination of following lesions):
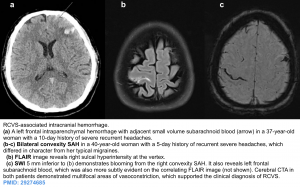
- Convexity subarachnoid hemorrhage (~ 30-38%) 11
- This is the most common neurologic complication of RCVS.
- Several studies have shown that RCVS is the most frequent cause of cortical (convexity) SAH in individual below age 60 years.
- Convexity SAH tend to be early features seen in the first week after onset of TCHs in the context of RCVS.
- These hemorrhages are typically small and self-limited, located over the frontal and parietal lobes, near the vertex of the head. However, there is a risk that hemorrhage could cause thrombosis of nearby cortical veins.
- In some cases, a convexity SAH provides a key clue which helps reveal the diagnosis of RCVS.
- Intraparenchymal hemorrhages (~12%).
- These are usually small and lobar. These can develop a few days after onset, suggesting a mechanistic role for reperfusion injury.
- Generally hemorrhagic complications tend to be early features seen in the first week after onset of TCHs.
- Hemorrhagic complications are more frequent in older female patients and do not necessarily adversely impact hospital length of stay or outcome.
- Subdural hemorrhage (2%) 11
- Watershed ischemic strokes (6-39%) 8
- It is the second most common neurologic complication of RCVS.
- These most often occur in cortical/subcortical regions in a bilateral and symmetric distribution along arterial watershed areas, between the anterior and posterior circulation. Larger infarcts are often wedge shaped.
- If infarcts are seen within the anterior or posterior cerebral artery territories, this may suggest a concomitant cervical artery dissection which warrants additional investigation.
- Ischemic strokes generally occur at the end of the second week often after resolution of headaches presumably reflecting the delay in resolution of cerebral vasoconstriction 27(later than hemorrhagic complications).
- PRES (10-36%)
- Brain edema (up to 38%)
- Seizures (up to 20%).
- Generalized tonic clonic seizures are reported in up to 20% at presentation but are rarely recurrent in the absence of ischemic or hemorrhagic brain injury.
- Coma and death (<5%)
- Less than 5% of patients develop coma or death secondary to progressive arterial vasoconstriction causing massive brain edema, large ischemic stroke, and or intraparenchymal hemorrhage.
- Convexity subarachnoid hemorrhage (~ 30-38%) 11
- Headache: 90% of patients experience thunderclap headache (TCH) with only less than 10% of patients presenting with a subacute or less severe headaches. The absence of headache at onset is exceptional.
- Risk factors and triggers 8 ,11 ,
1. Change in estrogen- progeterone levels: Pregnancy, preeclampsia/eclampsia, oral contraceptives medications
2. Headache disorder: Migraine, exertional headache
3. Vasoconstrictive medications
– Antimigraine agents: triptans, ergot tartrate
– Antidepressants: SSRIs, SNRIs
– Adrenergic agents: epinephrine, pseudoephedrine, nasal decongestants, midodrine, phenylephrine,
amphetamine derivatives.
– Bromocriptine, Cabergoline
– Chemotherapeutics: Cyclophosphamide, tacrolimus.
– Erythropoietin
– NSAIDs
– Blood transfusion, intravenous immune globulin (IVIG).
4. Secondary complication of other cerebrovascular disease
– Cerebral venous thrombosis (CVT).
– Carotid or vertebral artery dissection.
– Posterior reversible encephalopathy syndrome (PRES).
– Cerebral Hyperperfusion Syndrome status post carotid endarterectomy.
– Spinal subdural hematoma
5. Other risk factors
– Metabolic: Hypercalcemia, porphyria
– Environmental exposure, trauma: High altitude, cold water exposure, head trauma, neurosurgery
– Tumors: Pheochromocytoma, Carcinoid, Carotid paraganglioma
– Systemic lupus erythematosus
– Thrombotic thrombocytopenic purpura (TTP)
PRES
- Clinical manifestations 12
- Headache (50%): Typically constant, nonlocalized, moderate to severe and unresponsive to analgesia.
- Seizure (66%): Seizure are often the presenting manifestation and often recur. Only a minority of patients with milder disease are seizure free.
- Variety of types of seizures may occur (e.g., generalized tonic-clonic seizures, complex partial seizures, status epilepticus)
- Hypertension (70%): Absence of hypertension does not simply exclude PRES.
- Encephalopathy (40%): Varies from somnolence to coma.
- Visual disturbances (30%): Auras, blurred vision, diplopia, visual hallucinations (Anton syndrome), visual field deficits, cortical blindness.
- Focal neurological deficits (10%): Vary depending on the site of involvement. May include hemiparesis, aphasia/dysarthria, or ataxia.
- Complications associated with PRES
- Superimposed infarction
- Intracranial hemorrhage
- RCVS
- Risk factors and triggers 4, 12
1. Hypertension
– Hypertensive emergency
– Preeclampsia, eclampsia (toxemia of pregnancy)
– Renal failure
– Dysautonomia ( Guillain Barre syndrome, spinal cord injury).
– Induced hypertension (e.g., treatment for vasospasm in aneurysmal subarachnoid hemorrhage)
– Sympathomimetics (therapeutic or illicit)
2. Endothelial dysfunction and systemic inflammation
– Autoimmune diseases, especially vasculitis (e.g. lupus, scleroderma, Sjogren’s disease, rheumatoid arthritis).
– Sepsis
– Thrombotic microangiopathies ( e.g. hemolytic uremic syndrome, thrombotic thrombocytopenic purpura).
3. Medications that cause hypertension and/or endothelial dysfunction
– Intravenous immunoglobulin
– Calcineurin inhibitors (tacrolimus, sirolimus, cyclosporine).
– High-dose corticosteroids.
– Filgrastim
– Chemotherapy
4. Other risk factors
– Metabolic: Hypercalcemia, Hypomagnesemia, Porphyria
– Renal failure: promotes endothelial dysfunction & volume overload
– Fluid overload
– Sickle cell disease
– Solid organ transplantation
– Bone marrow or stem cell transplantation
RCVS-PRES Overlap
Teasing apart the features of PRES vs. RCVS is often impossible. The table below shows more classic features of PRES and RCVS. Patients with substantial symptomatology and imaging features of both syndromes may have PRES-RCVS overlap 1.
Imaging findings
RCVS
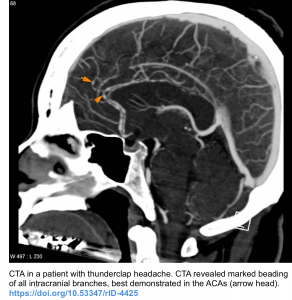
Abnormal cerebral angiography is the primary diagnostic feature of RCVS 8.
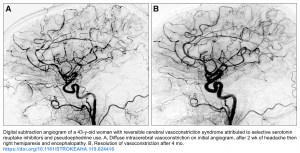
- These neurovascular abnormalities (vasoconstriction) seen on CTA/MRA/DSA are dynamic and progress proximally along the circle of Willis arteries and their branches.
- The typical vasoconstriction is widespread and bilateral.
- These are characteristically seen on neurovascular imaging as smooth, tapered narrowing involving large to medium-sized arteries followed by abnormally dilated segments of second-order and third-order branches is the most characteristic finding. This dilatation gives the typical “beaded or sausage-shaped” appearance of cerebral arteries.
- Normalization of the angiographic findings is usually seen within 8-12 weeks 2.
- The differential diagnosis of beaded vessels includes:
- Vasospasm following SAH: Notably, it involves longer segments of more proximal branches without alternating areas of narrowing 17.
- Primary angiitis of the central nervous system
- Intracranial atherosclerosis. It is seen in 7.5-30% of the asymptomatic population thus potentially complexifying evaluation on DSA.
Diagnostic modalities (neurovascular imaging)
- CT angiography (CTA) has a sensitivity of 80% when compared to invasive angiography.
- Initial CTA can be normal (21%) because the condition starts distally in the vessels that are not visualized
- In patients with high degree of clinical suspicion for RCVS, a follow up CTA (or MRA) performed after 3-5 days is often revealing. Vasoconstriction is often maximal about two weeks after the initial symptoms.
- MRA has a similar performance to CTA.
- MRA may have the added benefit of evaluating for cervical artery dissection, which may occur simultaneously with RCVS. However, this requires specifically ordering a cervical MRA and protocoling the study appropriately. Cervical MRA may often require intravenous contrast, unlike an isolated MRA of the brain (which may often be done without intravenous contrast, by using time-of-flight MRI techniques to detect flowing blood).
- DSA; Digital subtraction angiography (invasive angiography)
- DSA is the gold standard diagnostic test 14, but is invasive and is less commonly used in clinical practice. In the context of RCVS, angiography may carry a 9% risk of provoking ischemia.
- It is generally unnecessary to immediately reach diagnostic certainty regarding RCVS (because >90% of patients will improve spontaneously and because there are no proven therapies for RCVS). Thus, it may be safer to tolerate some diagnostic uncertainty and perform a repeat CTA or MRA (rather than proceeding immediately to invasive angiography).
- Indications for invasive angiography might include progressive clinical deterioration despite conservative management, or an inability to exclude aneurysmal SAH.
Noncontrast CT is often initially normal in RCVS. It may be used to detect complications associated with RCVS including 15
- Lobar ICH
- Convexity SAH. Remember that RCVS is the most common cause of cortical surface (convexity) subarachnoid hemorrhage in patients <60 years old.
- Ischemic infarction 16
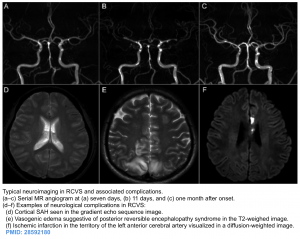
MRI: similar to non-contrast CT, MRI is often initially normal in RCVS (50%). It may be used to detect complications associated with RCVS (see above).
- FLAIR.
- Sulcal hyperintensitiesmay reflect convexity SAH.
- Vasogenic edema (T2/FLAIR hyperintensity) that is symmetrically distributed in a watershed distribution.
- DWI: watershed infarction can be seen.
- MRA: vascular narrowings can be seen.
PRES
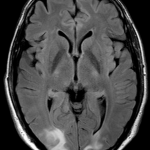
The hallmark imaging finding in PRES is vasogenic edema
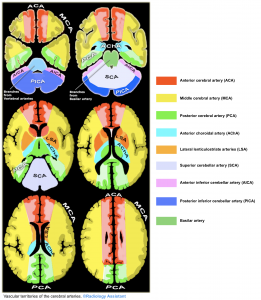
- In typical lesions, edema tends to occur in a bilateral pattern within the white matter, centered at the watershed areas between vascular territories (i.e. the distribution of edema is usually not confined to a single vascular territory 26). It commonly involve the posterior cerebral hemispheres, particularly the parieto-occipital regions (though several variation in location can occur. see patterns of white matter vasogenic edema below) 12.
- CT scan may be normal, but in more severe cases the edema will also be visible on CT scanning as hypodense areas within the white matter.
- The characteristic vasogenic edema is best detected on MRI as focal or confluent areas of increased signal on T2/FLAIR images.
- FLAIR sequences can improve sensitivity and detect subtle peripheral lesions, showing cortical lesions to be more common than once thought by T2 imaging.
- MRI with gadolinium may show gyriform, patchy, subcortical nodular enhancement, reflecting disruption of the blood-brain barrier.
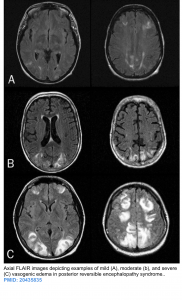
- The vasogenic edema severity is variable as shown in figure below.
- Importantly, neither the patterns themselves nor the severity of vasogenic edema are related to severity of clinical symptoms 12, whereas extensive edema has been shown to be associated with poor outcome.
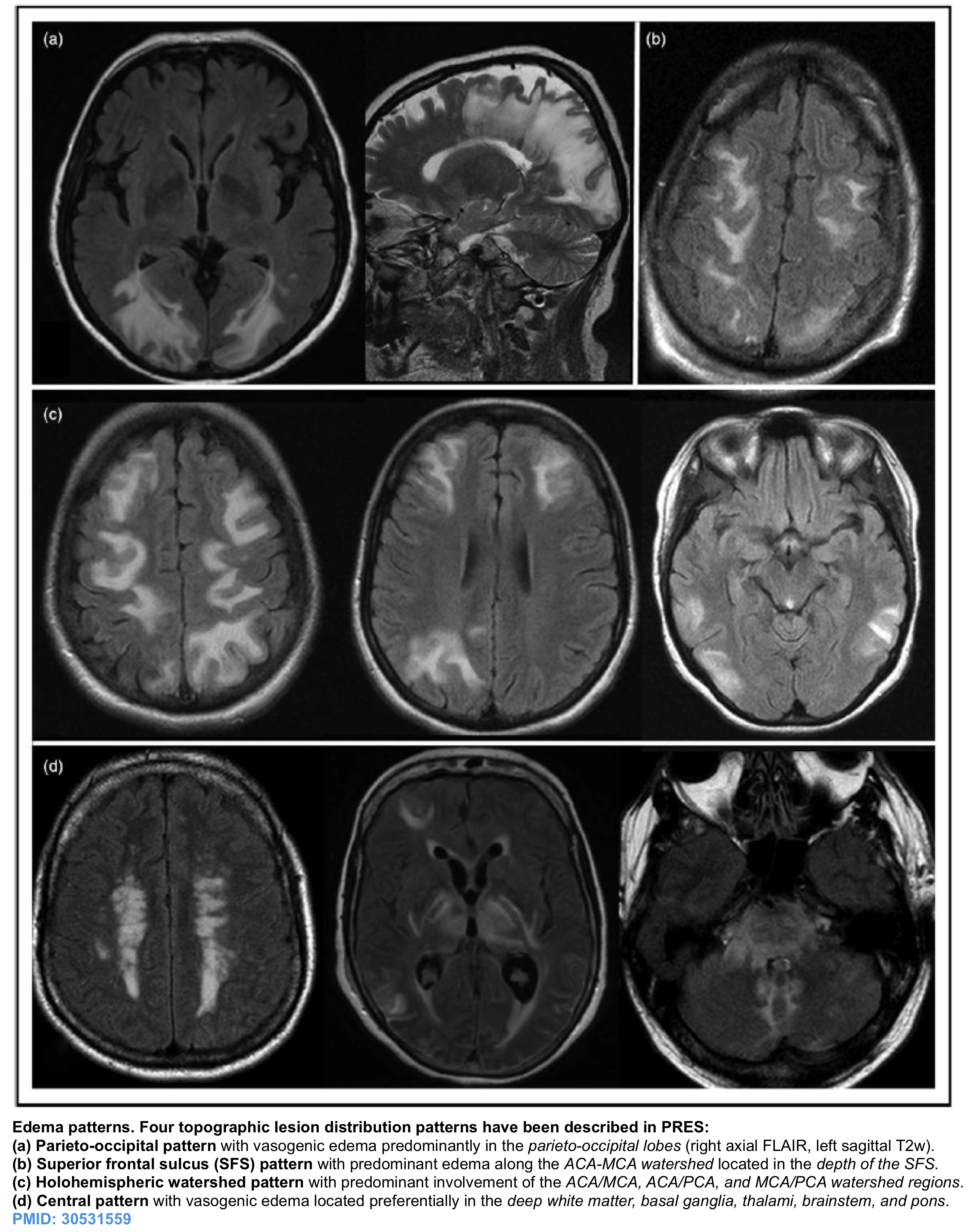
The four most common patterns of white matter vasogenic edema have been described 12. Edema tends to occur in a bilateral pattern within the white matter, centered at the watershed areas between vascular territories (the figure below).
- Parieto-occipital pattern (50%). Edema is predominantly along the MCA-PCA watershed, located within the parietal and occipital lobes.
- Factors that help to distinguish PRES from bilateral posterior cerebral artery infarctions:
- The calcarine and paramedian parts of the occipital lobe are usually spared in PRES.
- Relative sparing of the cortical gray matter in PRES is also a distinguishing clue.
- Factors that help to distinguish PRES from bilateral posterior cerebral artery infarctions:
- Superior frontal sulcus pattern (25%). Edema is predominantly along the ACA-MCA watershed, located in the depth of the superior frontal sulcus.
- Holohemispheric watershed pattern (25%). Edema is located along anterior, posterior, medial, and lateral watershed zones.
- Central pattern (10%). Edema is in the deep white matter, basal ganglia, thalami, brainstem, and pons.
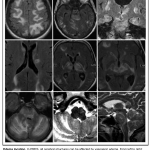
Note that edema lesions can occur in atypical regions, such as frontal lobe, thalamus, basal ganglia or cerebellum (appendix 2). These lesions can be accompanied by more classic manifestations as detailed above, for example typical parieto-occipital lesions.
Radiologic differential diagnosis of PRES with helpful clinical distinguishing clues (in parenthesis) are mentioned below 7
- CNS infection. (Presence of fever, CSF pleocytosis. Imaging findings can be unilateral)
- Progressive multifocal leukoencephalopathy (PML). (subacute-to-chronic clinical presentation)
- Autoimmune or paraneoplastic encephalitis (History of malignancy or tumor, presence of neural specific antibody in serum or CSF; CSF pleocytosis, unilateral findings on MRI with predominance of limbic system involvement).
- Cerebral vasculitis. (subacute clinical presentation, CSF pleocytosis, cytotoxic edema not in topographic distribution typical of PRES).
- Toxic (e.g. heroin) encephalopathy. (history of substance abuse or positive drug screen test, presence of characteristic toxidrome, MRI abnormalities tend to be symmetric).
Complications of PRES. may be demonstrated on MRI
- Superimposed infarction:
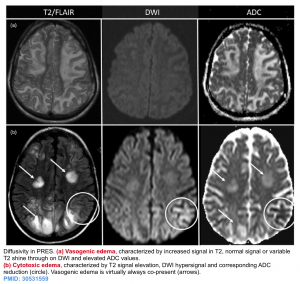
- Note that PRES typically causes vasogenic edema, with a characteristic appearance on different MRI sequences (shown in the top row below)
- T2: hyperintense in affected regions.
- DWI: usually normal, sometimes hyperintense due to edema (T2 shine through).
- ADC: usually increased signal due to increased diffusion.
- In ~20% of patients with PRES, small areas of brain tissue may become ischemic. This causes cytotoxic edema, which is marked on MRI by hyperintensity on DWI and hypointensity on ADC (shown in the bottom row below). Areas of hypointensity on ADC have greater specificity for ischemia that will progress to tissue infarction. This is a poor prognostic sign 12.
- Note that PRES typically causes vasogenic edema, with a characteristic appearance on different MRI sequences (shown in the top row below)
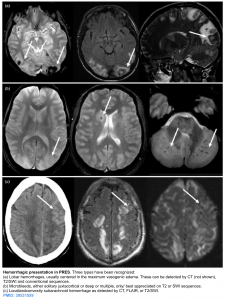
- Intracranial hemorrhage
-
- Petechial or large paranchymal hemorrhage may occur as well as convexal SAH. These can be detected by CT, T2/SWI sequences
- These are more common in patients with coagulopathy or thrombocytopenia.
Lumbar puncture
Lumbar puncture (LP) is not required for diagnosis of RCVS and PRES, however if it may be required to exclude alternative diagnosis.
- RCVS: By enlarge, LP is normal (e.g. protein level < 60mg/dL, ≤5 WBC). Although RCVS can cause small subarachnoid hemorrhages along the cortical surface, RCVS doesn’t cause the lumbar puncture findings which are seen in aneurysmal subarachnoid hemorrhage (e.g., xanthochromia and elevated erythrocytes).
- PRES: Common findings in PRES include mildly elevated protein (~50-100 mg/dL) with a normal cell count. Protein elevation may correlate with edema, as a marker of blood-brain barrier dysfunction 21.
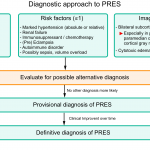
Diagnosis
Both syndrome are clinicoradiologic diagnosis.
RCVS
The diagnosis of RCVS is based upon the characteristic clinical, brain imaging features. Key components of diagnosis are
- Single or recurrent thunderclap headache (the presence of multiple TCH recurring over a few days has nearly 100% sensitivity and specificity for diagnosis of RCVS).
- Typical brain imaging
- Absence of aneurysmal SAH
Proposed diagnostic criteria for RCVS (adapted from the International Headache Society diagnostic criteria for ‘‘acute reversible cerebral angiopathy’’ and the criteria proposed in 2007):
- Acute and severe headache (often thunderclap headache) with or without focal neurological deficits or seizures
- Monophasic course without new symptoms more than 1 month after clinical onset
- Segmental vasoconstriction of cerebral arteries demonstrated by angiography (MRA, CTA or catheter)
- Exclusion of subarachnoid hemorrhage due to a ruptured aneurysm
- Normal or near normal CSF (protein ,1 g/l, white cells ,15/mm3 , normal glucose)
- Complete or marked normalization of arteries demonstrated by a repeat angiogram (MRA, CTA or catheter) after 12 weeks, although they may be normal earlier.
PRES
- There’s no specific diagnostic criteria for diagnosis of PRES.
- No single diagnostic test proves PRES (although MRI may be strongly suggestive)
- Keep in mind that
- Headache attributed to hypertensive encephalopathy and PRES are not equivalent diagnoses.
- About 30% of PRES cases are associated with normal or only mildly elevated blood pressure values.⚠️A normal baseline BP does not exclude PRES.
- Many of these PRES cases end up being attributed to other known associations including immunosuppressive and cytotoxic medications, sepsis, autoimmune disorders, and renal failure.
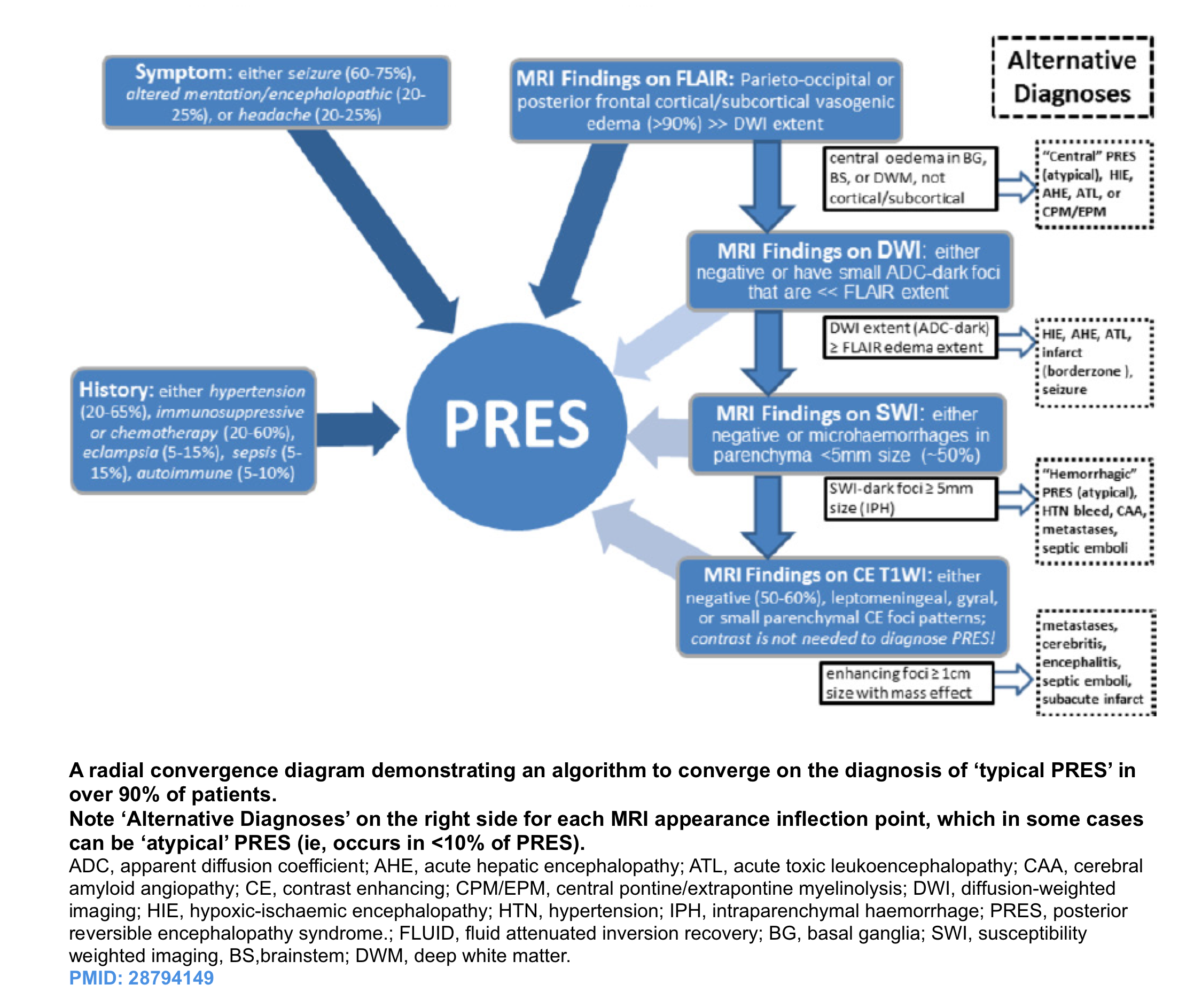
- The diagnosis of PRES is often approached, using a combination of clinical features, supportive evidence, and exclusion of other possibilities 3. This approach is helpful in most cases of PRES which show typical clinicoradiologic picture (~90% of cases) see appendix 3:
- Presence of at least one acute neurological symptoms (seizure, altered mental state, headache, visual disturbances)
- More or equal than one risk factor (severe hypertension, renal failure, immunosuppressant drugs or chemotherapy, eclampsia, autoimmune disorder)
- Brain imaging with bilateral vasogenic edema, cytotoxic edema with patterns of PRES or normal brain imaging
- No other alternative diagnosis.
- Clinical improvement over time
- A preliminary diagnostic algorithm that combines clinical and radiological features for the vast majority of patients using a ‘90% rule’, has been proposed 24. This approach can detect PRES in many cases even with a paucity of clinical data. However ‘atypical’ PRES can be a diagnostic dilemma that at times may necessitate a thorough review of past records and sequential imaging.
- The consternation in diagnosis often arises in obtunded patients with frank encephalopathy (i.e. not able to provide additional history) that exhibit atypical or severe appearances, whether ‘central’ PRES, frank >5 mm-sized hemorrhages or unilateral (‘tumefactive’) PRES 25 .
Management
Management of these syndromes includes the removal of identified triggers, symptomatic treatment of headache or seizures, and moderate blood pressure control 19.
Evaluation & removal of any identified triggers
- See the list of potential causes of RCVS and PRES above.
- Maintain euvolemia. Treat hypervolemia with diuresis or dialysis and hypovolemia with a fluid challenge.
- Treat preeclampsia as recommended.
- Treatment of other acute medical condition (e.g. sepsis, renal failure) may also hasten the patient’s recovery.
- Treatment of TTP with plasmapheresis or immunoglobulin has resulted in resolution of PRES.
- Magnesium:
- Magnesium has vasodilatory and anticonvulsant effects.
- In the context of eclampsia and PRES, hypomagnesemia should be avoided and serum levels be maintained in the high normal range 28. However, it remains unclear whether magnesium is beneficial among patients with other types of PRES.
- Treatment for RCVS in pregnancy is similar to treatment of preeclampsia/eclampsia, including blood pressure control and IV magnesium. For other patients with RCVS, some reported cases suggest a beneficial effect of magnesium for the treatment of refractory headaches (especially among patients with low magnesium level) 29.
Analgesia
- Opioid and/or acetaminophen should be strongly considered.
- ⚠️NSAIDs may be undesirable in the context of a risk of intracranial hemorrhage. Furthermore, NSAIDs have been implicated in causing RCVS 8.
- ⚠️Triptans and ergot alkaloid are contraindicated in RCVS because of their vasoconstrictive actions.
Blood pressure management
- Hypotension:
- Hypotension: is detrimental in RCVS, as this might increase the risk of ischemic stroke. Management should involve ensuring a state of euvolemia (e.g. intravenous fluid).
- ⚠️Note that vasopressors could exacerbate RCVS, so these may not be desirable.
- Hypotension: is detrimental in RCVS, as this might increase the risk of ischemic stroke. Management should involve ensuring a state of euvolemia (e.g. intravenous fluid).
- Among patients with hypertension, blood pressure should be promptly but carefully controlled.
- Ensure that pain is adequately treated, as this may be a contributing factor to hypertension.
- Calcium channel blocker (e.g. nicardipine or clevidipine infusion, with eventual transition to oral nifedipine ER) might be the agents of choice, given some possibility that this may improve vasospasm of the smaller vessels.
- ⚠️Nitroglycerine should be avoided, as this may aggravate PRES 20 .
Seizure management
- Seizure prophylaxis isn’t indicated.
- Seizures should be treated as per usual regimens 19 .
- For seizure in the context of eclampsia, magnesium infusion is the treatment of choice for preventing seizure recurrence.
- Have a low threshold for EEG in patients with persistent and unexplained alteration in mental status, to evaluate for nonconvulsive status epilepticus.
Prognosis
Both syndromes are self-limited, with clinical recovery occurring within days to weeks. Long-term deficits and mortality are uncommon.
RCVS
- Most patients will do well. Cerebral vasoconstriction will resolve over time (hence the name reversible cerebral vasoconstriction syndrome). However, parenchymal damage due to ischemia or hemorrhage may not always resolve. In other words, the vasospasm may be reversible but its consequences may not be.
- Progressive vasoconstriction can occur in <5% of patients (leading to large ischemic strokes). This may be more common among postpartum women. Retrospective data suggest that baseline infarction and glucocorticoid exposure are predictors of poor outcome 23.
- Following resolution, recurrent RCVS is very uncommon (5%).
PRES
- As a general rule, patients with PRES can look awful initially (e.g., due to brainstem involvement), yet subsequently make excellent recoveries. Recovery can take several days, so patience is required. Unfortunately, PRES can occasionally cause irreversible brain injury.
- Factors associated with worse clinical outcomes 22:
- Extensive vasogenic edema
- Secondary intracranial hemorrhage in addition to PRES.
- Restricted diffusion on MRI, suggestive of cerebral infarction.
RECAP
- RCVS and RRES are thought to share a common pathophysiology of dysregulation of cerebral arterial tone. Vasoactive drugs and hypertension are often implicated as a trigger in RCVS and PRES accordingly.
- PRES involves leaky vessels and more gradual onset headaches whereas RVCS involves constricted vessels and is usually a thunderclap presentation. There is overlap between the two and may exist on a spectrum
- Thunderclap headache is the core clinical picture of RCVS.
- Unlike migraine headache, the RCVS cause thunderclap headache which is quite distinguishable. Misdiagnosis of migraine can lead to inappropriate treatment with antimigraine agents such as triptans, which can exacerbate vasoconstriction and stroke risk.
- Unlike SAH, the headache in RCVS usually improve within 1-3 hours.
- Neck pain: RCVS may coexist with cervical artery dissection, so patients with RCVS and neck pain require further evaluation for cervical artery dissection.
- Seizure, encephalopathy and visual disturbances are common clinical findings in PRES.
- 20% of patients with PRES do not have hypertension. Do not assume that because a patient is normotensive he does not have PRES.
Going further
Appendix 1
Appendix 2
Appendix 3
References
1: Pilato F, Distefano M, Calandrelli R. Posterior Reversible Encephalopathy Syndrome and Reversible Cerebral Vasoconstriction Syndrome: Clinical and Radiological Considerations. Front Neurol. 2020 Feb 14;11:34. doi: 10.3389/fneur.2020.00034. PMID: 32117007; PMCID: PMC7033494.
2: Arrigan MT, Heran MKS, Shewchuk JR. Reversible cerebral vasoconstriction syndrome: an important and common cause of thunderclap and recurrent headaches. Clin Radiol. 2018 May;73(5):417-427. doi: 10.1016/j.crad.2017.11.017. Epub 2017 Dec 21. PMID: 29274685.
3: Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015 Sep;14(9):914-925. doi: 10.1016/S1474-4422(15)00111-8. Epub 2015 Jul 13. Erratum in: Lancet Neurol. 2015 Sep;14(9):874. PMID: 26184985.
4: Tetsuka S, Ogawa T. Posterior reversible encephalopathy syndrome: A review with emphasis on neuroimaging characteristics. J Neurol Sci. 2019 Sep 15;404:72-79. doi: 10.1016/j.jns.2019.07.018. Epub 2019 Jul 17. PMID: 31349066
5: Gewirtz AN, Gao V, Parauda SC, Robbins MS. Posterior Reversible Encephalopathy Syndrome. Curr Pain Headache Rep. 2021;25(3):19. Published 2021 Feb 25. doi:10.1007/s11916-020-00932-1.
6: Levitt A, Zampolin R, Burns J, Bello JA, Slasky SE. Posterior Reversible Encephalopathy Syndrome and Reversible Cerebral Vasoconstriction Syndrome: Distinct Clinical Entities with Overlapping Pathophysiology. Radiol Clin North Am. 2019 Nov;57(6):1133-1146. doi: 10.1016/j.rcl.2019.07.001. Epub 2019 Aug 16. PMID: 31582040.
7: Toledano M, Fugate JE. Posterior reversible encephalopathy in the intensive care unit. Handb Clin Neurol. 2017;141:467-483. doi: 10.1016/B978-0-444-63599-0.00026-0. PMID: 28190431.
8: Cappelen-Smith C, Calic Z, Cordato D. Reversible Cerebral Vasoconstriction Syndrome: Recognition and Treatment. Curr Treat Options Neurol. 2017 Jun;19(6):21. doi: 10.1007/s11940-017-0460-7. PMID: 28456915.
9: Mawet J, Boukobza M, Franc J, Sarov M, Arnold M, Bousser MG, Ducros A. Reversible cerebral vasoconstriction syndrome and cervical artery dissection in 20 patients. Neurology. 2013 Aug 27;81(9):821-4. doi: 10.1212/WNL.0b013e3182a2cbe2. Epub 2013 Jul 24. PMID: 23884040.
10: Malhotra A, Wu X, Gandhi D, Sanelli P. The Patient with Thunderclap Headache. Neuroimaging Clin N Am. 2018 Aug;28(3):335-351. doi: 10.1016/j.nic.2018.03.002. Epub 2018 Jun 8. PMID: 30007749.
11: Chen SP, Fuh JL, Wang SJ. Reversible cerebral vasoconstriction syndrome: current and future perspectives. Expert Rev Neurother. 2011 Sep;11(9):1265-76. doi: 10.1586/ern.11.112. PMID: 21864073.
12: Liman TG, Siebert E, Endres M. Posterior reversible encephalopathy syndrome. Curr Opin Neurol. 2019 Feb;32(1):25-35. doi: 10.1097/WCO.0000000000000640. PMID: 30531559.
13: Macri E, Greene-Chandos D. Neurological Emergencies During Pregnancy. Neurol Clin. 2021 May;39(2):649-670. doi: 10.1016/j.ncl.2021.02.008. PMID: 33896537.
14: Burton TM, Bushnell CD. Reversible Cerebral Vasoconstriction Syndrome. Stroke. 2019 Aug;50(8):2253-2258. doi: 10.1161/STROKEAHA.119.024416. Epub 2019 Jul 5. PMID: 31272323.
15: Singhal AB, Hajj-Ali RA, Topcuoglu MA, Fok J, Bena J, Yang D, Calabrese LH. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol. 2011 Aug;68(8):1005-12. doi: 10.1001/archneurol.2011.68. Epub 2011 Apr 11. PMID: 21482916.
16: Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol. 2012 Oct;11(10):906-17. doi: 10.1016/S1474-4422(12)70135-7. PMID: 22995694.
17: Miller TR, Shivashankar R, Mossa-Basha M, Gandhi D. Reversible Cerebral Vasoconstriction Syndrome, Part 2: Diagnostic Work-Up, Imaging Evaluation, and Differential Diagnosis. AJNR Am J Neuroradiol. 2015 Sep;36(9):1580-8. doi: 10.3174/ajnr.A4215. Epub 2015 Jan 22. PMID: 25614476; PMCID: PMC7968777.
18: Miller TR, Shivashankar R, Mossa-Basha M, Gandhi D. Reversible Cerebral Vasoconstriction Syndrome, Part 1: Epidemiology, Pathogenesis, and Clinical Course. AJNR Am J Neuroradiol. 2015 Aug;36(8):1392-9. doi: 10.3174/ajnr.A4214. Epub 2015 Jan 15. PMID: 25593203; PMCID: PMC7964694.
19: Staykov D, Schwab S. Posterior reversible encephalopathy syndrome. J Intensive Care Med. 2012 Feb;27(1):11-24. doi: 10.1177/0885066610393634. Epub 2011 Jan 21. PMID: 21257628.
20: Finsterer J, Schlager T, Kopsa W, Wild E. Nitroglycerin-aggravated pre-eclamptic posterior reversible encephalopathy syndrome (PRES). Neurology. 2003 Sep 9;61(5):715-6. doi: 10.1212/01.wnl.0000080369.87484.06. PMID: 12963776.
21: Neeb L, Hoekstra J, Endres M, Siegerink B, Siebert E, Liman TG. Spectrum of cerebral spinal fluid findings in patients with posterior reversible encephalopathy syndrome. J Neurol. 2016 Jan;263(1):30-4. doi: 10.1007/s00415-015-7928-8. PMID: 26477022.
22: Schweitzer AD, Parikh NS, Askin G, Nemade A, Lyo J, Karimi S, Knobel A, Navi BB, Young RJ, Gupta A. Imaging characteristics associated with clinical outcomes in posterior reversible encephalopathy syndrome. Neuroradiology. 2017 Apr;59(4):379-386. doi: 10.1007/s00234-017-1815-1. Epub 2017 Mar 13. PMID: 28289809; PMCID: PMC5565839.
23: Singhal AB, Topcuoglu MA. Glucocorticoid-associated worsening in reversible cerebral vasoconstriction syndrome. Neurology. 2017 Jan 17;88(3):228-236. doi: 10.1212/WNL.0000000000003510. Epub 2016 Dec 9. PMID: 27940651; PMCID: PMC5272793.
24: Gao B, Lyu C, Lerner A, McKinney AM. Controversy of posterior reversible encephalopathy syndrome: what have we learnt in the last 20 years? J Neurol Neurosurg Psychiatry. 2018 Jan;89(1):14-20. doi: 10.1136/jnnp-2017-316225. Epub 2017 Aug 9. PMID: 28794149
25: McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS, Teksam M. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol. 2007 Oct;189(4):904-12. doi: 10.2214/AJR.07.2024. PMID: 17885064.
26: Jamieson DG, McVige JW. Imaging of Neurologic Disorders in Pregnancy. Neurol Clin. 2020 Feb;38(1):37-64. doi: 10.1016/j.ncl.2019.09.001. PMID: 31761061.
27: Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007 Dec;130(Pt 12):3091-101. doi: 10.1093/brain/awm256. Epub 2007 Nov 19. PMID: 18025032.
28: Fischer M, Schmutzhard E. Posterior reversible encephalopathy syndrome. J Neurol. 2017 Aug;264(8):1608-1616. doi: 10.1007/s00415-016-8377-8. Epub 2017 Jan 4. PMID: 28054130; PMCID: PMC5533845.
29: Mijalski C, Dakay K, Miller-Patterson C, Saad A, Silver B, Khan M. Magnesium for Treatment of Reversible Cerebral Vasoconstriction Syndrome: Case Series. Neurohospitalist. 2016 Jul;6(3):111-3. doi: 10.1177/1941874415613834. Epub 2015 Oct 30. PMID: 27366294; PMCID: PMC4906552.



Add comment