January 8, 2023 by Shahriar Lahouti.

CONTENTS
- Preface
- Pathogenesis
- Epidemiology
- Triggers
- Clinical manifestation
- Diagnosis and differentials
- Complication and prognosis
- Management
- Going further
- References
Preface
Stress cardiomyopathy (aka. apical ballooning syndrome, takotsubo cardiomyopathy, broken heart syndrome, and stress-induced cardiomyopathy) is a syndrome characterized by transient regional systolic dysfunction of the left ventricle. It can mimic myocardial infarction, but there’s no angiographic evidence of obstructive coronary artery disease or acute plaque rupture.
The name comes from the Japanese word for an octopus trap, given the appearance of the left ventricle (LV) during echo/ventriculogram (bulging out of LV apex with preserved function of the base looks like an octopus pot or “tako tsubo” in Japanese).
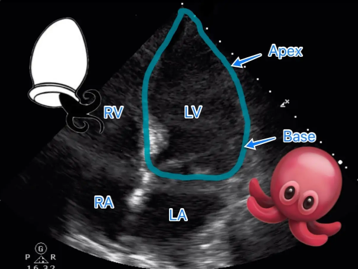
Pathogenesis
The postulated mechanisms include catecholamine excess. This may cause
- Diffuse catecholamine-induced microvascular spasm or dysfunction, resulting in myocardial stunning, or
- Catecholamine-associated myocardial toxicity
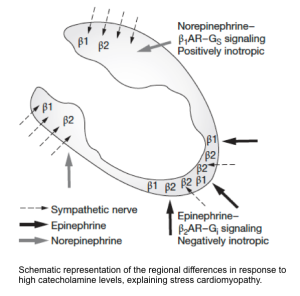
Epinephrine may have the greatest effect in apical regions of the heart where beta-adrenoreceptor density is highest at this location *.
- In a mouse model, it has been demonstrated that a high level of epinephrine is negatively inotropic due to a switch from β2 adrenoreceptor-mediated Gs protein signaling (which is positively inotropic), to Gi protein signaling (which is negatively inotropic) *.
Epidemiology
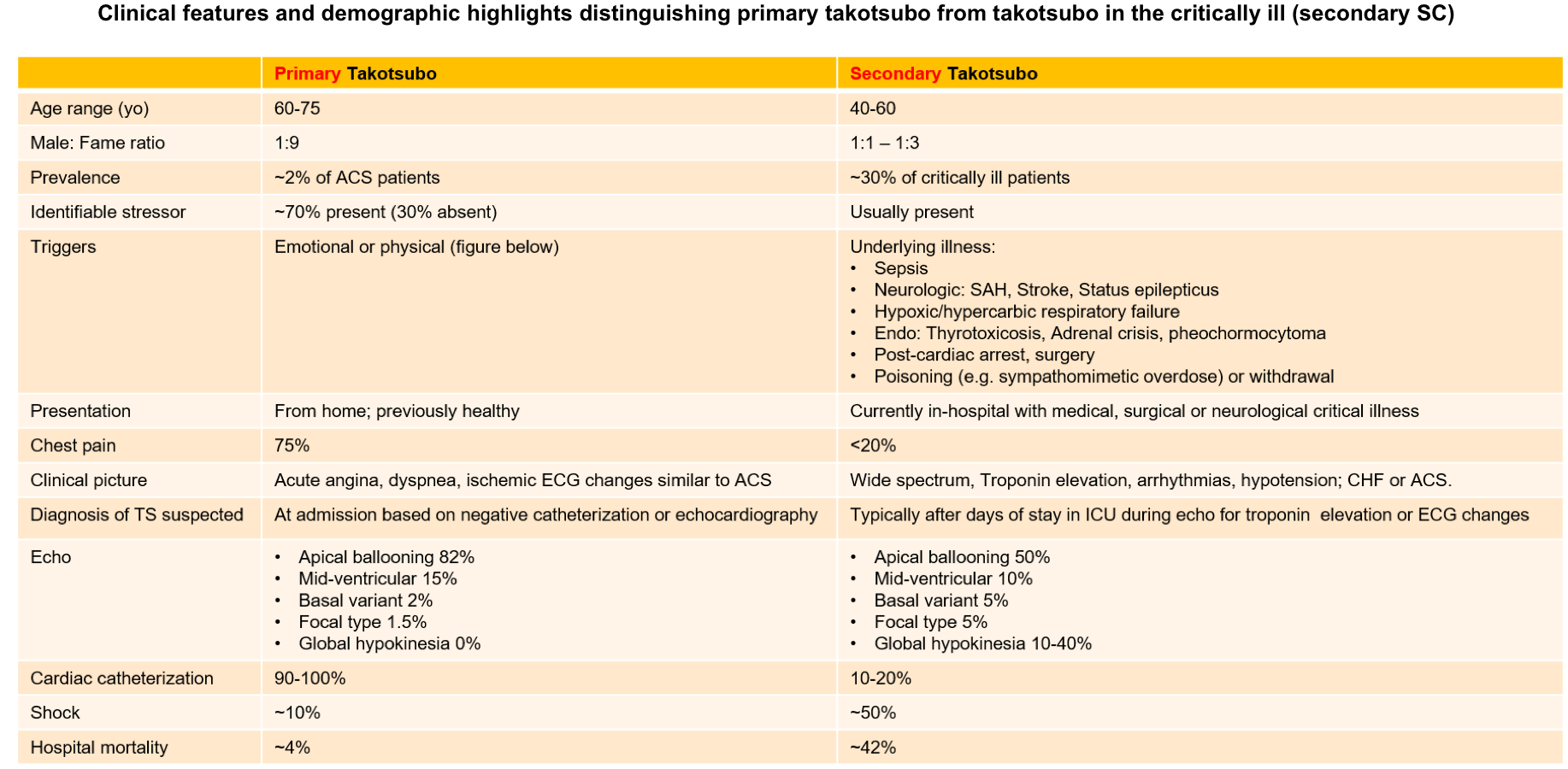
The majority of publications describing takotsubo cardiomyopathy are based on community dwelling population who presents with ACS-like picture (primary TS). Stress cardiomyopathy of the critically ill (secondary SC), is an acute cardiac dysfunction developing during the course of hospitalization with critical medical, surgical or neurological illness.
The clinical features and demographic highlight distinguishing primary from secondary takotsubo syndrome is presented in the table below.
Primary TS
- Stress cardiomyopathy accounts for ~ 2% of patients with troponin-positive chest pain *.
- ~90% of patients are women and the mean age is ~ 65 y/o *.
- Identifiable stressors (physical or emotional) are present in ~70% of patients, however, ~30% had no identifiable stressors.
- 👉Lack of an identifiable stressor doesn’t exclude Takotsubo syndrome.
- Most common clinical presentation is cardiac symptoms; with ACS-like presentation. See the table below.
Secondary TS *
- Stress cardiomyopathy has been reported in ~30% of critically ill patients admitted to ICU *. It may result from different stressors such as sepsis, respiratory failure or neurological conditions such as status epilepticus *(table below).
Comparing primary vs. secondary takotsubo cardiomyopathy
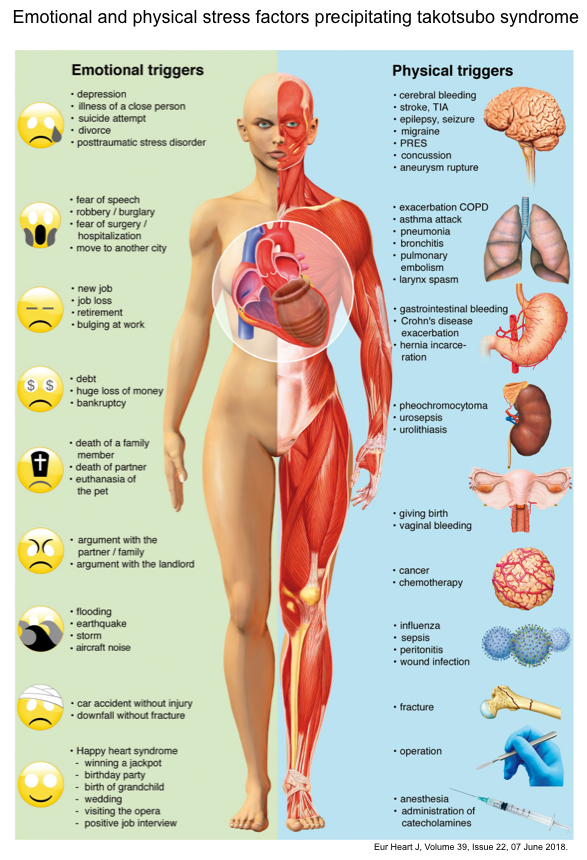
Triggers
A hallmark of TS is its association with a preceding stressful event. A systematic illustration of preceding emotional and physical stressors is shown in the figure below *.
- 👉A physical or emotional trigger is often present, but absence of triggers does not exclude takotsubo cardiomyopathy.
Drugs as risk factors for “TS”
- Antidepressants: Venlafaxine, duloxetine, maprotiline, fluoxetine.
- Chemotherapy: 5-Fluorouracil, capecitabine, trastuzumab.
- Antiarrhythmic agents: Flecainide, sotalol, amiodarone, lidocaine
Clinical manifestation
Overall, symptoms are usually more prominent with primary Takotsubo syndrome.
- Ischemic chest pain
- Syncope / cardiac arrest
- Arrhythmia (including AF, polymorphic ventricular tachycardia, VF, bradycardia, asystole).
- Dyspnea
- Pulmonary edema, cardiogenic shock.
In critically ill patients, takotsubo cardiomyopathy (secondary TS) most commonly presents with:
- Hypotension
- Requirement for inotroepes
- Troponin rise or new ECG changes
- Pulmonary edema
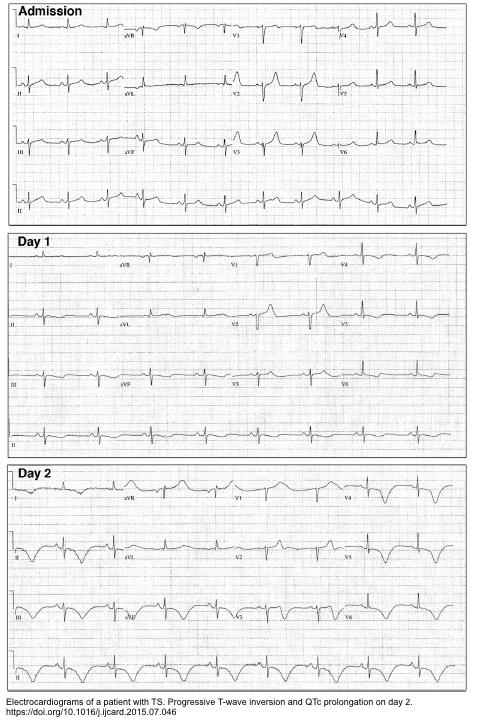
ECG
- Several ECG changes have been described for “TS”, but none of them are reliable enough to differentiate “TS” vs. occlusive MI.
- A normal ECG does not exclude the diagnosis too.
- Some ECG characteristics of TS are worth mentioning here.
Intervals
- QT prolongation is often pronounced (QTc > 500ms) and this predisposes to TdP or VF.
- LBBB may be seen.
Arrhythmia
- Atrial fibrillation can occur (although this is nonspecific).
- Malignant arrhythmia can occur (Torsade de Pointes or monomorphic ventricular tachycardia).
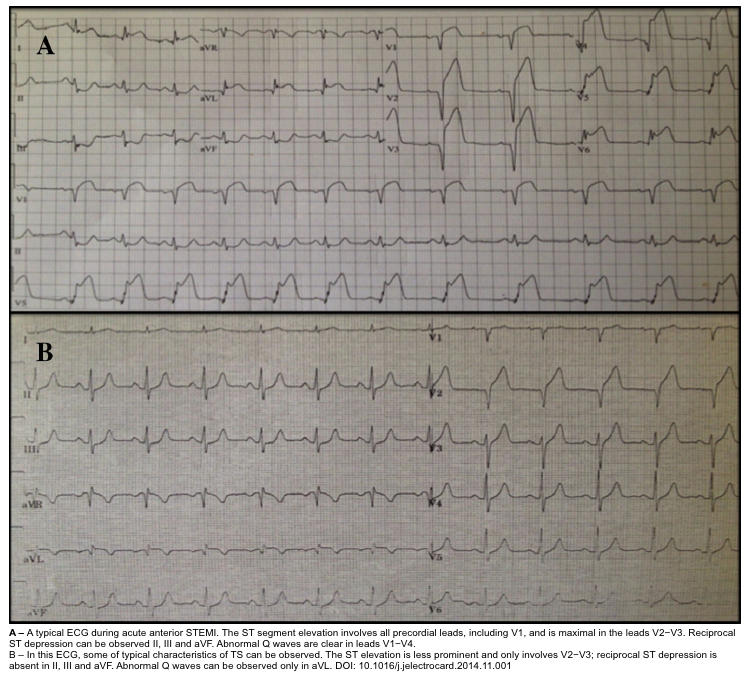
ST elevation
- Hyperacute TW may be seen.
- STE typically micks anterior occlusive MI. The STE is often most notable in V3-V6 but STE in II is also characteristic (this suggests Takotsubo, but can also result from wraparound LAD occlusion).
- Features that may support Takotsubo (rather than anterior MI) *
- Lower magnitude of STE than STE typical of anterior occlusive MI.
- Absence of pathological Q waves.
- Absence of reciprocal ST depression.
- STD in aVR and absence of STE in V1 suggests Takotsubo with sensitivity and specificity > 90% *.
T wave inversion (TWI)
- Diffuse TWI is often seen in a large number of leads (V2-V6, I, II).
- Magnitude and number of leads with TWI is typically greater than with MI.
- Takotsubo may cause bizarre, wide TWI which resembles a subarachnoid hemorrhage T-wave pattern (since both may share the same underlying physiology).
Echocardiography
- LVEF: Systolic dysfunction (with ejection fraction dropping from normal to <25-45%)*.
- WMA: LV dysfunction is identified by echocardiography or left ventriculography, which reveals regional wall motion abnormalities (RWMA) in one of the characteristic patterns (below).
- LV mural thrombus: May be identified by echo.
- Left ventricular outflow tract obstruction (LVOTO). Bedside echo is helpful for recognizing LVOTO which has therapeutic implication. More on this here.
- RV involvement
- Recent evidence show that ~ 1/3 of TS cases have both right and left ventricles involvement *.
- RV involvement is associated with a more severe impairment in LV systolic function and serves as a poor prognostic signs.
- RV involvement may be suspected by the presence of pleural effusion.
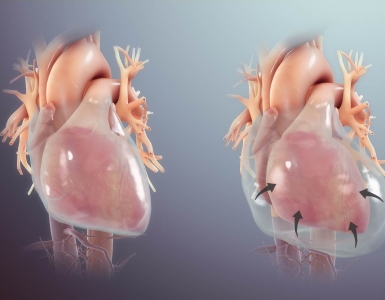
RWMA: Classic type
- There is systolic apical ballooning of the LV with hypercontractility of the base.
- It is the most common type of echocardiographic pattern in patients with primary TS.
- This may mimic LAD-occlusive MI.
- Left ventricular outflow tract obstruction (LVOTO) with systolic anterior motion of the mitral valve may occur due to basal hyperkinesis.
- This is important to recognize, as it has hemodynamic implications for management (below)
RWMA: Atypical variants
- Mid-ventricular wall variant
- Hypokinesis restricted to the mid-ventricular region with sparing of the apax.
- Basal (“Reversed”) variant
- Hypokinesis of the base with sparing of the apax.
- Focal variants
- Dysfunction of an isolated segment. Most commonly isolated anterolateral segment dysfunction of the LV (this is the closest echocardiographic mimic of an occlusive MI).
- Global hypokinesis
- Global LV dysfunction may be the most common type in patients with secondary TS *.
💡Tips
- Two patterns of TS on echocardiography are difficult to distinguish from occlusive MI:
- Traditional apical ballooning
- Focal type; if it involve a coronary distribution e.g. isolated anterolateral hypokinesia.
- Classic type with apical ballooning is the most common pattern in primary TS, however it is possible that different echocardiographic patterns evolve during different stages of Takotsubo syndrome in the same patient(video below).
Cardiac MRI (CMR)
MRI imaging is generally not indicated in patients presenting with suspected stress cardiomyopathy since most have high-risk features for ACS (including ↑troponin levels) and will require coronary angiography. Therefore MRI has the greatest value in patients whose ECGs don’t mandate urgent catheterization (e.g. presentation with a diffuse T-wave inversion pattern, rather than ST elevation).
CMR may be useful in the following conditions
- Suspected non-ST elevation ACS with low- or intermediate-risk features.
- When echocardiogram is technically suboptimal and/or there is coexistent coronary artery disease.
- CMR may assist in the differential diagnosis, e.g. differentiating myocarditis or MI.
- Delineate the full extent of ventricular abnormalities, LV /RV function.
- Identify associated complications, e.g. mural thrombus, LVOTO (see video below).
Differentiating TS vs. myocarditis or AMI
Late gadolinium enhancement (LGE) is a technique used in CMR for cardiac tissue characterization.Presence of LGE reflects cardiac necrosis/fibrosis *.
- MI typically shows late gadolinium enhancement (LGE) in a focal distribution (subendocardial or transmural).
- Myocarditis typically shows late gadolinium enhancement (LGE) in a patchy distribution.
- Takotsubo’s cardiomyopathy typically has no late gadolinium enhancement.
More on this: Late gadolinium enhancement(Radiopaedia)
Labs
Labs are not immediately helpful for diagnosis of TS.
Troponin
- Elevated in 90% of cases of Takotsubo cardiomyopathy.
- Note that the troponin bump in “TS” is disproportionately low compared to magnitude of wall motion abnormality.
BNP
- Elevated in ~80% of patients.
- The diagnostic value of BNP is questionable, since it is extremely nonspecific (esp. among critically ill patients, most of whom will have an elevated BNP).
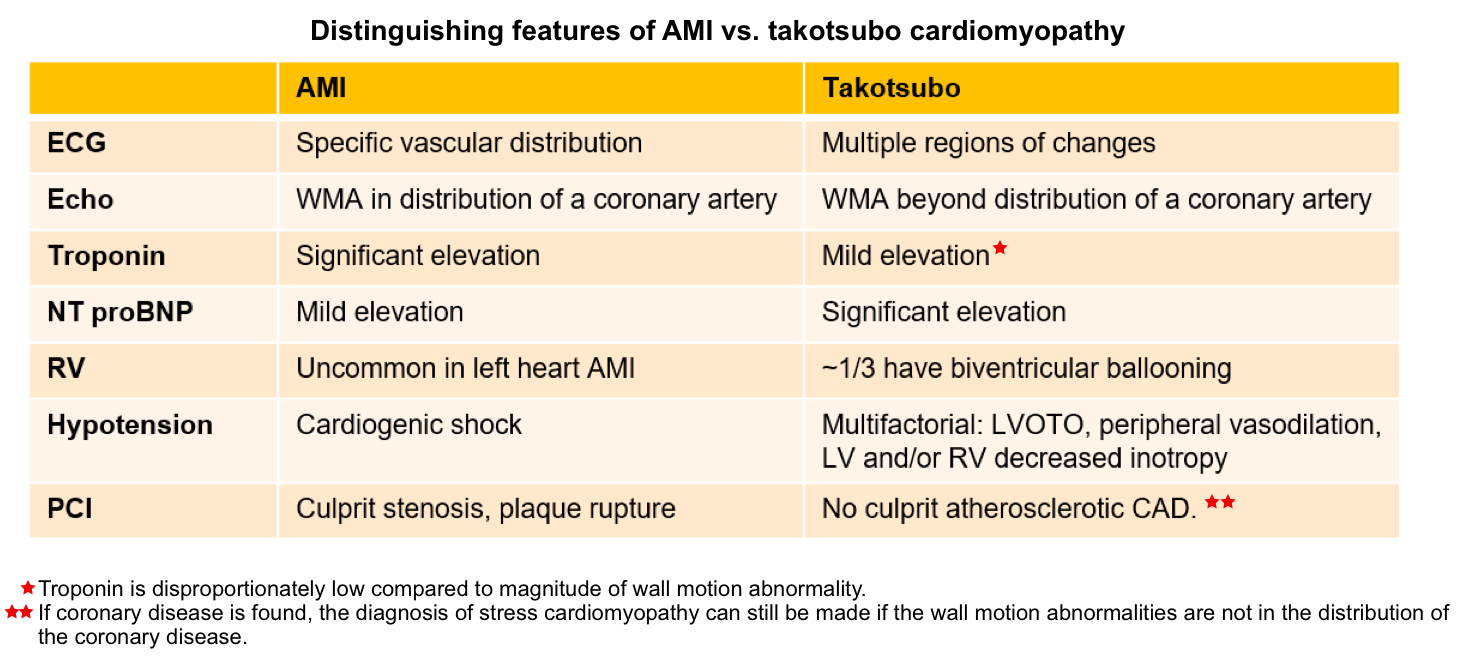
Diagnosis and differentials
Differential diagnosis
- Myocardial infarction
- Occlusive myocardial infarction (OMI).
- Non-occlusive myocardial infarction (NOMI).
- Supply-Demand mismatch (type II) myocardial infarction in the context of chronic systolic failure.
- Any other cause of acute systolic heart failure (e.g. myocarditis, postpartum cardiomyopathy, hyperthyroidism).
Definition and diagnostic criteria
The diagnosis of primary stress cardiomyopathy should be suspected in adults who present with a suspected acute coronary syndrome (ACS; with symptoms such as chest pain or dyspnea in combination with ECG changes and/or ↑troponin level), particularly when the clinical manifestations and ECG abnormalities are out of proportion to the degree of elevation in cardiac biomarkers *.
- A physical or emotional trigger is often but not always present.
We use the following proposed Mayo Clinic diagnostic criteria, all 4 of which are required for the diagnosis:
- Transient RWMA of the LV and/or RV.
- 👉This RWMA typically extends beyond a single epicardial coronary distribution; rare exceptions are the focal (within one coronary distribution) and the global type.
- New ECG abnormalities or modest elevation in cardiac troponin.
- Absence of other conditions like myocarditis that could explain the pattern of temporal LV dysfunction.
- Absence of culprit atherosclerotic CAD to explain the pattern of temporary LV dysfunction.
- 👉If coronary disease is found, the diagnosis of stress cardiomyopathy can still be made if the wall motion abnormalities are not in the distribution of the coronary disease. This exception is made since some patients with stress cardiomyopathy have concurrent coronary disease (15% in the International Takotsubo registry).
Approach to diagnosis
- Differentiation from occlusive MI or ACS: Coronary angiography
- If the ECG shows ST elevation, then cardiac catheterization is usually required to exclude occlusive MI.
- If the ECG doesn’t show features of occlusive MI, immediate catheterization isn’t necessarily required. Close observation may be reasonable, depending on the clinical scenario.
- A relatively moderate troponin elevation in the context of dramatic wall motion abnormalities would support Takotsubo cardiomyopathy (as opposed to myocardial infarction)*.
Complications and pognosis
Complications
- Hypotension and cardiogenic shock (W/WO LVOTO)
- Independent predictors of cardiogenic shock *
- Male
- Asian or Hispanic
- Comorbidities, including congestive heart failure, chronic lung condition, and chronic diabetes.
- Independent predictors of cardiogenic shock *
- Cardiac arrhythmias (e.g. AF, Tdp, Monomorphic VT, VF)
- Independent predictors of ventricular fibrillation(VF):
- Increased heart rate and blood pressure
- T-wave inversion (V4-V5)
- QTc >500 ms
- QRS duration > 105 ms *
- Left ventricular basal hypercontraction
- Independent predictors of ventricular fibrillation(VF):
- Mural thrombosis and thromboembolic complications
- Cardiac arrest
- Independent predictors of cardiac arrest *
- High peak BNP values
- ST elevation
- Atrial fibrillation
- High CRP
- Independent predictors of cardiac arrest *
👉A recent study showed that neutrophil/lymphocyte ratio (NLR) is an independent predictor of hospital-acquired complications, including pulmonary edema, cardiogenic shock, death, stroke, and LV thrombi *.
Prognosis
- Takotsubo cardiomyopathy is not a benign disease.
- The risk of severe in-hospital complications is similar to that in patients with ACS *.
- In-hospital mortality rates reported ~4%.
- Adverse outcomes may be driven by heart failure, malignant arrhythmias, and cardioembolic complications.
- LVEF generally recovers by 12 weeks, sometimes much faster. However, some patients may develop a persistent, long-term heart failure phenotype *.
Management
By enlarge the management of TS is conservative and should be focused on emotional or physical stress relief.
- Treatment should follow usual ACS algorithms. Patients with STEMI on ECG will have the diagnosis made in the catheterization suite.
-
Identify and treat the underlying cause
- Critically ill patients with secondary TS frequently have an emergent underlying cause e.g. sepsis which should be identified and treated.
-
-
Arrhythmia 👉follow the standard ACLS guideline.
-
Heart failure and shock 👉management is similar to that in the general guidelines of their management in non-TS cases except in the presence of LVOTO (see below).
-
TS without complication
The most commonly used therapy is a combination of beta blockers and angiotensin-converting enzyme (ACE) inhibitors*.
- Beta blockers
- Inhibit the Gs-protein metabolic pathway preventing recurrence of TS development as well as incidence of arrhythmias.
- Beta blockade should be avoided if LVEF is severely reduced as it may carry a risk of precipitating cardiogenic shock.
- ACE inhibitors/ ARBs
- They prevent vasospasm, consequently reducing hypertension.
- In the presence of impaired renal function, a combination of hydrazine plus isosorbide dinitrate is a more appropriate choice.
TS with rhythm disorder
- Treat arrhythmias based on usual ACLS algorithms.
- Telemetry monitoring should be carried out for at least 3 days.
- Carefully monitor QT interval. Calculate Risk score for QT prolongation.
- Avoid QT-prolonging medications, inotropes, and catecholamines (even neubulized beta-2 agonists) if possible.
- If QTc < 500 ms *
- Start beta blocker as it has protective effect against development of malignant arrhythmias.
- If QTc > 500 ms:
- Discontinue beta blocker *.
- Optimize potassium (K>3.5 mmol/L) and magnesium levels (Mg >2 mg/dL).
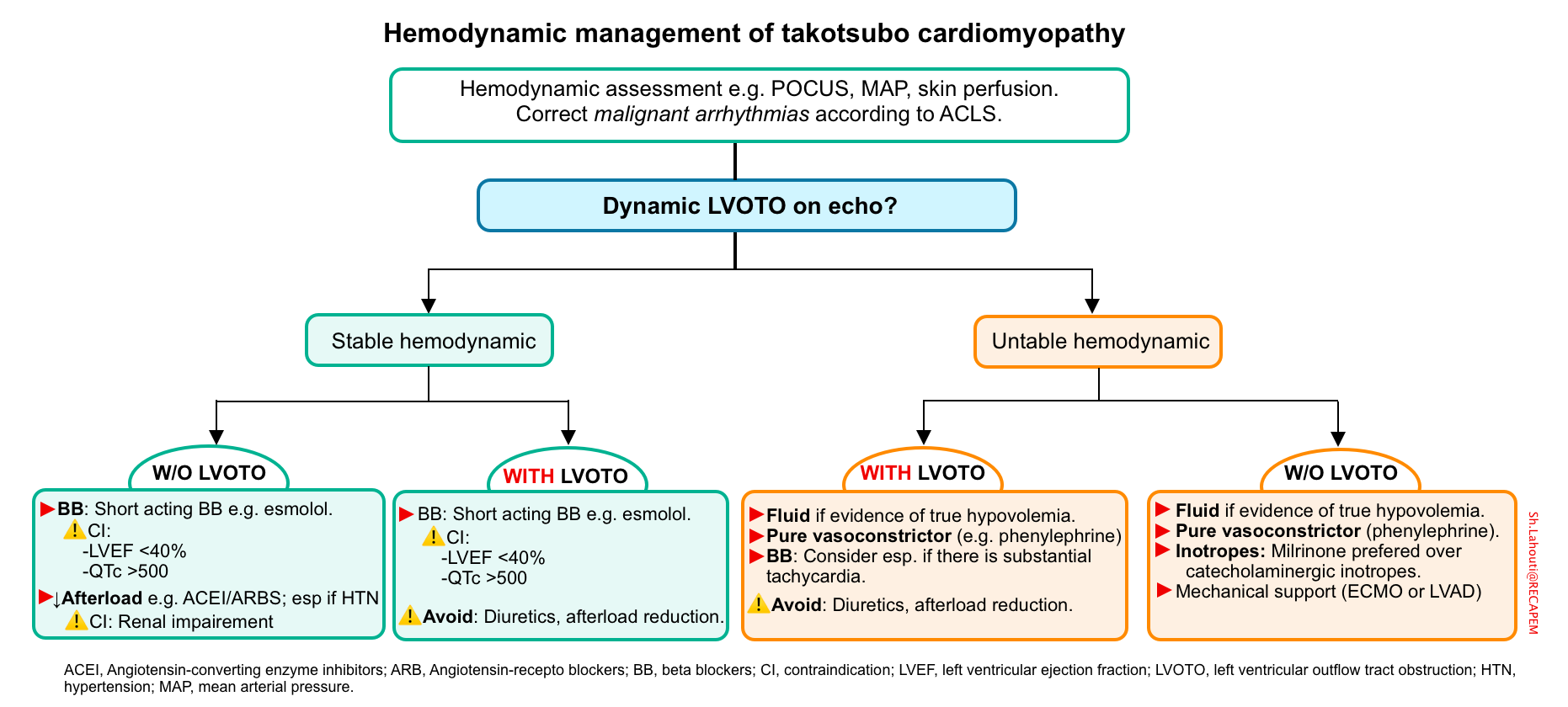
TS with cardiogenic shock
According to 2020 Mayo Clinic data, 11% of patients develop CS in the first 72 h after admission. The development of shock may not correlate with the extent of left or right ventricular systolic dysfunction. This discordance can be explained by development of LVOTO.
- LVOTO occurs in ~10% of patients with takotsubo cardiomyopathy *.
- It is important to identify LVOTO, since these patients respond differently to treatments compared to most patients with heart failure.
- ⚠️Avoid following medications in the presence of LVOTO:
- Diuretics
- Catecholaminergic inotropes e.g. dobutamine, epinephrine
- Vasodilators e.g. ACEIs, ARBs, nitrates.
Cardiogenic shock with LVOTO
- Fluid resuscitation (in the absence of moderate-severe pulmonary edema).
- Beta blockers are helpful by lowering the HR and increasing LV diastolic filling (and therefore relieving obstruction). Esmolol infusion 0.15–0.3 mg/kg/min without loading dose for 24 h, followed by bisoprolol 1.5 mg/d “PO” has been shown to relief obstruction *.
- Pure peripheral vasopressors e.g. phenylephrine to optimize MAP *.
🚩In the case of persistent hypotension despite adequate beta blockade (HR < 65) and appropriate vasopressor therapy, search for other causes of hypotension e.g. sepsis, pulmonary embolism.
Hypotensive TS without LVOTO
- Inotropes: A trial of inotropic agents such as dobutamine can serve as a temporary measure; however, these agents can cause mild LVOTO in “TS” patients *.
- Inotropes may improve cardiac output and organ perfusion. However, increased inotropy can potentially cause LVOTO physiology.
- The European Society of Cardiology guidelines suggest a preference for milrinone over catecholaminergic inotropes.
- Vasopressor e.g. phenylephrine should be started in patients with persistent hypotension or any signs of end-organ hypoperfusion.
- Mechanical support (e.g., ECMO or LVAD) may be used as a bridge to recovery in severe shock.
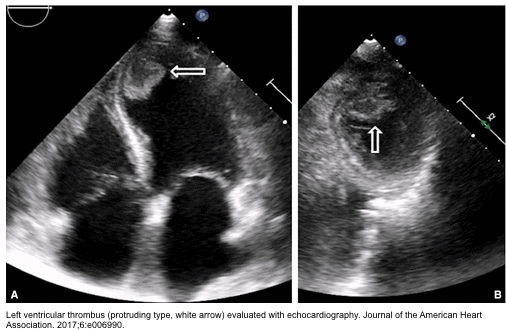
TS and thromboembolism
- Ventricular thrombus is found in 1.3% of patients with “TS” (in the International Takotsubo Registry).
- The classical apical ballooning form of stress cardiomyopathy carries a ~5% risk of mural thrombus formation within the apex.
- Assess for the potential risk of mural thrombus formation, e.g. echocardiography/CMR to evaluate extent and severity of apical ballooning.
- Scant data are available to guide anticoagulant therapy to prevent thromboembolism in patients with stress cardiomyopathy.
- Therefore prophylactic anticoagulant decisions should be individualized based on clinical judgement, the extent of echocardiographic findings, and bleeding risks.
- Mural thrombus
- If intraventricular thrombus is detected, anticoagulation with warfarin is recommended.
- The optimal duration of treatment is 3 months, but depending on the size and location of LV thrombi, and rate of recovery of cardiac function, it can be prolonged *.
- There’s no data for the role of novel oral anticoagulants, so the question of their efficacy remains unresolved.
Going further
- Takotsubo cardiomyopathy (Dr. Smith’s ECG Blog)
- Takotsubo syndrome (IBCC)
- Echo findings (CORE ULTRASOUND)
- 📚Cardiac MRI




Add comment