January 3, 2023 by Shahriar Lahouti.
CONTENTS
- Definition
- Epidemiology
- Pathophysiology
- Clinical presentation
- Differential diagnosis
- Diagnosis
- Initial resuscitation
- Resuscitative endpoints
- Refractory shock
- Additional therapies
- RECAP
- Going further
- References
Definition
Sepsis exists on a continuum of severity ranging from infection and bacteremia to sepsis and septic shock, which can lead to multiple organ dysfunction syndrome (MODS) and death. Here the definition of sepsis and septic shock (in view of Sepsis-3)is discussed.
- Sepsis
- Definition: Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection.
- Diagnostic clinical criteria: Suspected or documented infection, plus one significant organ dysfunction or two mild organ dysfunction.
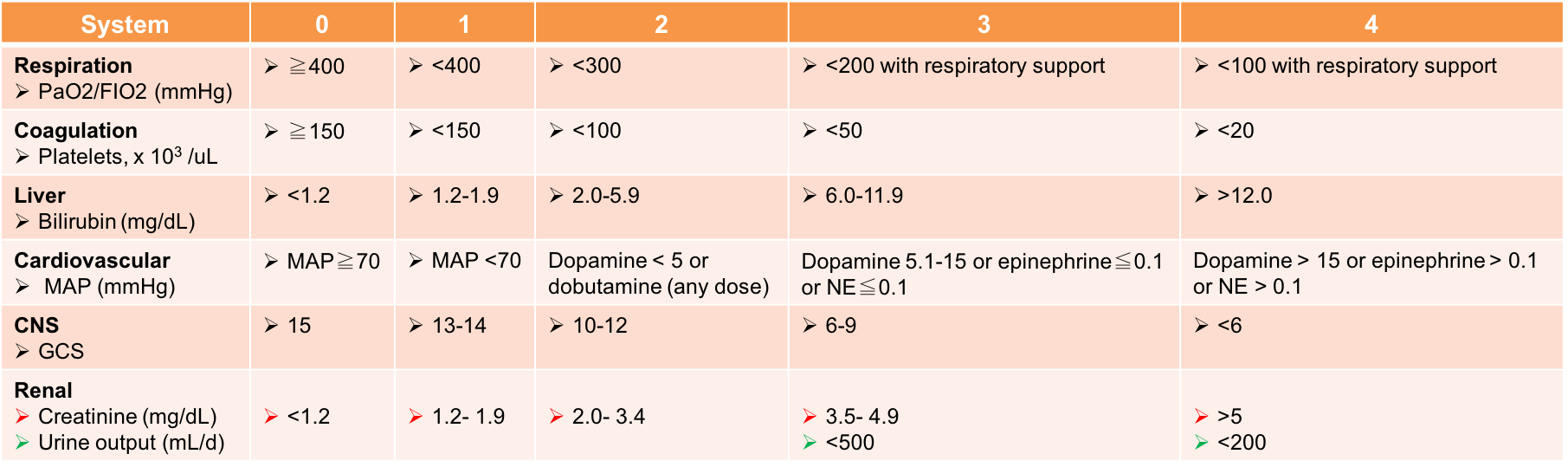
- Organ dysfunction can be identified as an acute change in total SOFA score ≥2 points *(table below).
- Organ dysfunction can be identified as an acute change in total SOFA score ≥2 points *(table below).
- Septic shock
- Definition: Septic shock is a type of vasodilatory or distributive shock. It is a subset of sepsis in which underlying circulatory and cellular/metabolic abnormalities are profound enough to substantially increase mortality.
- 👉Severe sepsis is no longer part of the definitions.
- Diagnostic clinical criteria: Patients with septic shock can be identified as *
- Clinical construction of sepsis, and
- Vasopressor requirement to maintain MAP ≥65 mm Hg (despite fluid resuscitation), and
- A serum lactate ≥ 2 mmol/L
- Definition: Septic shock is a type of vasodilatory or distributive shock. It is a subset of sepsis in which underlying circulatory and cellular/metabolic abnormalities are profound enough to substantially increase mortality.
Epidemiology
- Distributive shock (esp. septic shock) is the most common type of shock.
- Risk factors for sepsis *
- Male sex
- Black race
- Age ≥65 y
- Bacteremia
- Immunocompromised state (e.g. neoplasms, renal failure, hepatic failure, AIDS, asplenism) and immunosuppressant medications.
- Diabetes and obesity
- Malignancy
- Cancer is the most common comorbidity among the septic patients. It has been shown that cancer of all types increases the risk of developing sepsis by ~ 10-fold *.
- ICU admission and nosocomial infection
- Previous hospitalization (in particular hospitalization associated with infection)
- Community-acquired pneumonia
- Genetic defects have also been identified that may increase susceptibility to specific classes of microorganisms.
- Gram positive bacteria are the pathogens that are most commonly isolated from patients with sepsis.
Pathophysiology
The clinical syndrome of sepsis is the result of severe infection leading to systemic inflammatory response syndrome (SIRS), immune dysregulation, microcirculatory derangements; resulting to end-organ dysfunction *.
The onset and progression of sepsis center upon a dysregulation of the normal response.
- The host response to sepsis is characterized by both excessive proinflammatory and anti-inflammatory responses; leading to widespread tissue injury.
- The direction, extent, and duration of these reactions are determined by both host factors (e.g. age, coexisting illnesses, medications) and pathogen factors (e.g. microbial load and virulence).
Host response and excess pro-inflammatory mediators
- Following activation of innate immune response, a vast number of pro-inflammatory and anti-inflammatory chemokines are released.
- Large quantities of certain proinflammatory cytokines may spill into the bloodstream, and contribute to the progression of a local infection to sepsis. These include tumor necrosis factor alpha (TNF-a) and interleukin-1 (IL-1). Both cytokines can cause fever, hypotension, leukocytosis, induction of other proinflammatory cytokines, and the simultaneous activation of coagulation and fibrinolysis.
- Widespread cellular injury is the precursor to organ dysfunction. Possible mechanism of cellular injury include:
- Tissue ischemia. This can happen due to imbalances in the coagulation and fibrinolytic systems, or as a result of endothelial glycocalyx disruption *. Read more 👉 Glycocalyx in Sepsis.
- Mitochondrial dysfunction: Proinflammatory mediators and/or other products of inflammation may be the culprit. Mitochondrial dysfunction causes decreased oxygen utilization and cellular injury.
- Furthermore disruption of gut epithelium can contribute to worsening SIRS.
- The circulatory abnormalities typical of sepsis may depress the gut’s normal barrier function, allowing translocation of bacteria and endotoxin into the systemic circulation (possibly via lymphatics, rather than the portal vein) and extending the septic response *.
Organ-specific effects of sepsis
Cardiovascular
- Hypotension
- Vasodilation: ↑production of nitric oxide (NO)* and ↓compensatory secretion of antidiuretic hormone (vasopressin)* play central role in vasodilation of septic shock.
- Capillary leak and redistribution of intravascular fluid. This is a consequence of both ↑endothelial permeability and ↓ arterial vascular tone.
- The above two mechanisms have more profound effect on lowering diastolic BP (↓↓↓DBP, while ↓SBP).
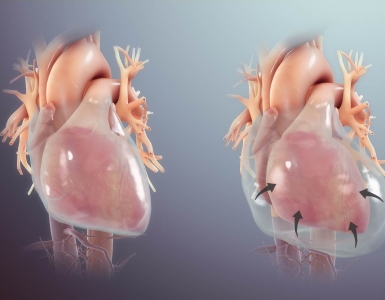
- Cardiomyopathy
- Septic cardiomyopathy
- It is a reversible form of cardiomyopathy not due to coronary disease.
- It usually occurs within 2 to 3 days of the onset of sepsis and resolves in 7 to 10 days *.
- Several pathophysiologic mechanisms are involved, e.g., downregulation of adrenergic pathways, coronary microvascular perturbation, and abnormalities in calcium handling.
- Clinical presentation
- “Cold shock” with significant drop in systolic BP, low cardiac output, cardiac arrhythmias, preload unresponsiveness, and vasopressor-refractory shock *.
- “Cold shock” with significant drop in systolic BP, low cardiac output, cardiac arrhythmias, preload unresponsiveness, and vasopressor-refractory shock *.
- Spectrum of cardiac dysfunction on echo
- Global LV systolic dysfunction (hypokinesia)
- LV dilation (diastolic dysfunction)
- However, the filling pressures remain normal to low, in contrast to cardiogenic shock, where ventricular pressures are elevated *.
- RV dysfunction
- Biventricular systolic dysfunction
- Sepsis-related Takotsubo syndrome
- It is characterized by LV apical and mid-ventricular circumferential hypokinesia and basal hypercontractility *.
- In this context, a normal or hypercontractile LV basal segment can lead to LV outflow tract obstruction (LVOTO) in takotsubo patients.
- Septic cardiomyopathy
Neurologic
- Septic encephalopathy (~40%)
- Brain dysfunction mediated by the septic inflammatory response, independent of other co-existent causes such as liver or renal dysfunction *, and is correlated with increased mortality.
- Clinical feature:
- Hyperactive or hypoactive delirium: It often occurs before the failure of other organs in septic patients * and may progress to coma.
- Other features: anorexia, malaise, myoclonus and asterixis +/- seizure.
- Cerebrospinal fluid analysis may show elevated protein levels, normal glucose levels and cell culture analysis.
- Read more 👉 Sepsis-associated encephalopathy.
Gastrointestinal
- Ileus (due to ↓mesenteric blood flow)*
- Liver dysfunction:
- In septic patients, liver dysfunction can vary from subclinical injury to overt failure.
- Sepsis-associated liver dysfunction can roughly be divided into two forms:
- Hypoxic hepatitis (shock liver)
- It is related to end-organ hypoperfusion associated with sepsis.
- Dramatic and transient increase in serum aminotransferase (e.g. > x 20 ULN) with the predominance of AST over ALT.
- Septic shock with hypoxic hepatitis can result in fulminant hepatic failure, which frequently leads to DIC and bleeding.
- Jaundice
- Much more frequent than hypoxic hepatitis in sepsis.
- Onset
- It commonly happens as a late event during sepsis in the course of multiorgan dysfunction, or
- It can happen in early stages of sepsis (bacteremic jaundice)
- It commonly happens as a late event during sepsis in the course of multiorgan dysfunction, or
- LFT: cholestatic pattern with elevation of conjugated bilirubin with disproportionately low elevations of AST, ALT, and biliary enzymes.
- Hypoxic hepatitis (shock liver)
- Management of acute liver failure 👉 here .
Respiratory
- Most common system affected, which may result in acute respiratory distress syndrome (ARDS) *.
- Endothelial injury in the pulmonary vasculature during sepsis disturbs capillary blood flow and enhances microvascular permeability, resulting in interstitial and alveolar pulmonary edema *.
- Principles of lung-protective ventilation 👉 here.
Hematologic
- Thrombocytopenia
- DIC *
Renal
- AKI is frequently common is sepsis and is associated with increased mortality.
- The most common mechanism is acute tubular necrosis (ATN).
- The old paradigm that septic AKI is due to renal hypo-perfusion and associated ATN has been changed recently by several experimental and human studies *.
- Septic kidney injury typically occurs with normal or elevated renal blood flow despite reduction in GFR. This may be due to vasodilation of capillaries which bypass the glomerulus combined with vasodilation of efferent arterioles (allowing blood to shunt through the kidney without improving the glomerular filtration rate). More on this below.
- Renoresuscitation strategies 👉 here.
- Retrospective studies have suggested that early initiation of RRT are associated with a better hemodynamic tolerance and outcomes *.
Metabolic
- Hyper-/hypoglycemia
- Adrenal insufficiency (may result in treatment-resistant shock)*
- Lactate elevation (below).
Clinical presentation
Patients with suspected or documented sepsis typically present with hypotension, tachycardia, fever, and leukocytosis. As severity worsens, signs of shock (e.g. cool skin, cyanosis) and organ dysfunction develop (e.g. delirium, oliguria, liver dysfunction).
The diagnosis of sepsis is challenging for a couple of reasons:
- The signs and symptoms of sepsis is non-specific, for example patients with non-infectious conditions such as pancreatitis or acute respiratory distress syndrome may present similarly.
- The signs and symptoms of sepsis are not sensitive.
- Some patients may lack focal signs and symptoms of infection and present with nonspecific organ dysfunction.
- The clinical findings may be modified by preexisting disease or medications. As examples, elderly, diabetic, and patients who take beta-blockers may not exhibit an appropriate tachycardia as blood pressure falls.
- The clinical presentations vary widely through the spectrum of disease.
- Young patients with vigorous physiologic reserve may show hypertension and tachycardia early in the course of disease.
In the following table common signs and symptoms of sepsis is shown.
| Signs and symptoms | Labs | |
| Infection | Non-focal signs: Temp >38.3 or <36ºC, rigors, night sweating Focal findings: – Pneumonia – Urosepsis – Cholangitis – Cellulitis – Meningitis | Non-focal: -↑CRP, ↑Procalcitonin – Positive blood cultures Focal: – Urinalysis – CSF exam – Culture draining pus |
| Physiologic stress (compensated shock) | Tachypnea Tachycardia BP (Initially may be normal/ ↑; later is ↓) Shock index(HR/SBP):↑ | ↑Lactate ↑↓Glucose ↑↓WBC count ↑Immature or bands forms Döhle bodies and toxic granulations ↑NLR |
| End-Organ dysfunction/failure | Warm shock (early sepsis) – BP: nl/ ↓ 👉↓↓↓Diastolic BP – Skin: Warm and flushed – Pulse pressure: Wide with bounding pulse Cold shock (late sepsis) – BP: ↓↓ – Skin: Cool, cyanotic, mottled. – Pulse pressure: Narrow Altered mental status Renal failure: ↓urine output Respiratory: ↑Work of breathing, ↑↓RR | Renal failure – ↑Cr, ↑BUN Liver – ↑↑↑AST/ALT – ↑ Bilirubin Hematologic – ↓PLT – DIC Respiratory – ↓PaO2/FiO2 |
Signs and symptoms of infection
- Systemic signs and symptoms of infection
- Temperature >38.3 or <36ºC
- Shaking chills (defined as involuntarily shivering such that holding a glass of water in the hand would cause the water to spill out).
- In a prospective cohort study, the estimated odd ratio of shaking chills for positive blood culture was was 5.9 (95% CI 2.05–17.17) *.
- Tachycardia, and tachypnea.
- Tachypnea is a sensitive index suggesting that something is wrong! However it is nonspecific for sepsis/septic shock.
- Localizing signs and symptoms of infection
- The common sources of septic shock with typical clinical clues and diagnostic tests are as follow.
- Pneumonia:
- Productive cough, pleuretic chest pain.
- Test: CXR, chest CT, Lung ultrasound. Note that if septic shock is due to pneumonia, there should be obvious signs of pneumonia (e.g. consolidation) in imaging. If findings are equivocal, look for alternative source of infection.
- Cholangitis
- Clues: Right upper quadrant or epigastric pain, jaundice, rigors, gram negative bacteremia. More on this here.
- Test: ↑bilirubin, ultrasound/CT shows “dilated common bile duct”.
- Urosepsis
- Clues: Frequency/urgency, dysuria, flank/back pain. More on this here.
- 👉Patients with urosepsis often rapidly improve following antibiotic and hemodynamic support (despite documented bacteremia).
- Test: Abnormal urinalysis, positive urine culture, perinephric fat stranding on CT scan.
- Clues: Frequency/urgency, dysuria, flank/back pain. More on this here.
- Vascular catheter infection
- Clues: Erythema/warmth at central line or port, dysfunctional line.
- Test: Paired blood cultures from line and peripheral cultures.
- Meningitis
- Clues: Headache, nuchal rigidity, altered mental status.
- Test: CSF analysis.
- Endocarditis
- Clues: Embolic phenomena, history of valvular disease, IV drug abuse.
- Test: Blood cultures and echo.
- Abdominal sepsis (e.g. obstruction, perforated diverticulitis/appendicitis)
- Clues: Characteristic abdominal pain, distension, nausea, vomiting, history of surgical procedure.
- Test: Ultrasonography, CT scan.
- Necrotizing fasciitis
- Streptococcal cellulitis with toxic shock
- Clues: Classic appearance of cellulitis, but patient also has sepsis. Erythroderma can be seen in some cases.
- 🚩A red flag suggesting toxic shock is a patient with an unimpressive focus of infection (e.g. a small patch of cellulitis) who is in septic shock. Most patients should not develop septic shock due to cellulitis.
- Test: Blood cultures may be positive.
- Clues: Classic appearance of cellulitis, but patient also has sepsis. Erythroderma can be seen in some cases.
- C. Difficile
- Clues: Recent antibiotic exposure, diarrhea, abdominal tenderness.
- Test: Stool for C. difficile, CT shows pancolitis.
- Pneumonia:
- 👉Keep in mind that some infections (e.g. ascending cholangitis, necrotizing fasciitis) are more likely to cause septic shock. Therefore there should be a lower threshold to diagnose septic shock and initiate aggressive management in these patients.
- The common sources of septic shock with typical clinical clues and diagnostic tests are as follow.
Signs of end-organ hypoperfusion
- Altered mental status
- Oliguria or anuria
- Warm shock (early): Warm, flushed extremities, ↓↓diastolic BP, wide pulse pressure.
- Cold shock (late): Cold, cyanotic and mottled extremities, hypotension, narrow pulse pressure.
👉Septic patients with vague presentation
- Almost 30% of septic patients present with vague presentation in ED.
- Vague presentation is defined if symptoms at the ED did not include any of the explicit symptoms including; hyperthermia or hypothermia, chills, MAP ≤ 65 mmHg, cough with productive sputum, dysuria, reported skin redness or concern for soft-tissue infection.
- Despite an apparent lower level of severity when initially assessed, those patients had an increased risk of mortality (odd ratio 2.18*) that could not be fully explained by delayed diagnosis and management of sepsis.
Laboratory findings
Laboratory features of sepsis are nonspecific too, and may be associated with abnormalities due to the underlying cause of sepsis or to tissue hypoperfusion or organ dysfunction from sepsis.
The initial investigation in patients suspected for sepsis include:
- Lactate
- A serum lactate ≥ 2 mmol/L supports the diagnosis of septic shock.
- CBC with differential
- Neutrophil-to-lymphocyte ratio (NLR) reflects physiologic stress. A NLR >10 supports the diagnosis of septic shock.
- Procalcitonin (if not definitely sepsis).
- Procalcitonin >0.5 ug/ml is consistent with a diagnosis of septic shock (but not diagnosis of it).
- CRP measurement may be preferred over procalcitonin in patients with renal failure or neutropenia ,since CRP is not affected by these situations.
- A CRP value (>100 mg/L, or >10 mg/dL) is consistent with septic shock, but not diagnostic of it.
- Complete metabolic profile
- Coagulation study
- Peripheral blood culture x2, culture of any line in place >48h.
- Urinalysis and culture.
- Imaging
- Ultrasonography
- Chest X-ray/CT.
- Consider CT abdomen/pelvis if no definite source (esp. in obtunded/elderly patients).
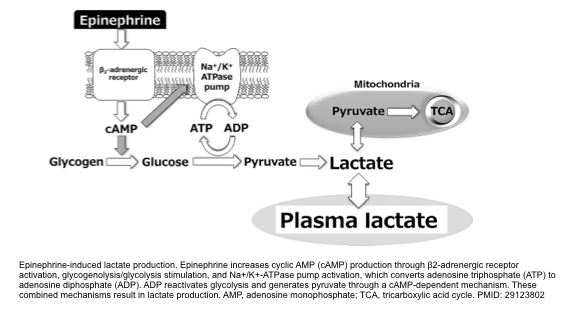
Lactate
- Lactate is produced by glycolysis and metabolized by the liver. Increased glycolysis results in increased lactate formation.
- Elevated lactate level in septic shock and other physiologic stress has been explained as:
- Endogenous epinephrine surge during physiologic stress contributes to overproduction of pyruvate derived from stimulated glycolysis (figure below).
- This pyruvate surplus is beyond the mitochondrial capacity for generating ATP (via TCA cycle). Therefore, excess pyruvate is converted into lactate *.
- Bottom line: Lactate production in septic shock is not due to anaerobic metabolism or low oxygen delivery. It is largely driven by endogenous epinephrine stimulating aerobic glycolysis via beta-2 adrenergic receptors.
- Elevated lactate
- An elevated lactate is an early indicator of shock * and is particularly useful in identification of occult shock (those who are normotensive or even hypertensive).
- However, it is very nonspecific and there are many false positives including any type of shock, liver failure, seizures, physiologic stress, beta-agonists.
- High lactate level is worrisome. This should be interpreted in appropriate clinical context. It can represent shock or some other impending disaster until proven otherwise *.
- Normal lactate
- It isn’t necessarily reassuring (can occur in shock).
Read more on lactate 👉 Sepsis-associated hyperlactatemia
Neutrophil to lymphocyte ratio (NLR)
- The NLR is the ratio of neutrophils/lymphocytes.
- The opposite changes in neutrophil and lymphocyte counts are a multifactorial dynamic process depending on finetuning and regulation of various processes such as demargination, accelerated apoptosis, influence of stress hormones (e.g. cortisol, catecholamines) *.
- During physiologic stress the endogenous cortisol and catecholamine surge will increase the neutrophil count and reduce the lymphocyte count, thereby causing ↑NLR.
- Sepsis stimulates lymphocyte apoptosis, so this may cause more dramatic elevation of the NLR compared to other forms of physiologic stress.
- The NLR reflects physiologic stress, rather than being a specific indicator of septic shock (it is not helpful to determine the precise etiology of illness).
- Pitfalls
- Exogenous steroid may increase the NLR.
- Active hematologic disorder may affect the NLR (e.g. leukemia, cytotoxic chemotherapy, granulocyte colony stimulating factor).
- Use of NLR in patients with suspected septic shock
- NLR <3
- This should cast some doubt on the diagnosis of sepsis (>95% of septic patients will have an NLR>3).
- One driver of NLR elevation is cortisol. Therefore, an unexpectedly low NLR in the context of definite septic shock may suggest the possibility of adrenal insufficiency.
- NLR 3-10 (Grey zone): NLR provides no clear information.
- NLR 10-15: Consider septic shock
- NLR > 15: Strongly suggests septic shock *.
- NLR <3
More on this👉 NLR (PulCrit)
Biologic markers
Basic concept
- Serum level of acute phase reactants (APR) is elevated in systemic inflammation and infectious process.
- Measurement of serum APR levels can be helpful to reflect the presence and intensity of an inflammatory/infectious process.
- By enlarge, elevated “APR” levels are not specific to any particular disease, nor can they distinguish infection from other causes of acute/chronic inflammation.
- C-reactive protein (CRP) and procalcitonin (PCT) are the two “APR” most commonly clinically used acute phase reactants.
- Both may take ~6-8h to elevate after onset of systemic inflammation/infection *, *.
- Generally, procalcitonin is relatively more sensitive for detection of typical bacterial infection (especially gram negative bacilli) than CRP, and is preferred biologic marker in the context of sepsis with few exceptions.
- In the following conditions, PCT can be falsely negative, and CRP measurement is recommended:
- Neutropenia
- Severe immunosuppression
- In the following conditions, PCT can be falsely negative, and CRP measurement is recommended:
- Note that neither elevated PCT, nor CRP should be used for diagnosis of sepsis and bacterial infections (both are nonspecific markers of infection, and elevated values can be seen in a variety of non-infectious conditions rheumatologic diseases and vasculitis).
- In addition, other causes of false-positives ↑PCT include:
- Renal failure
- Small-cell lung cancer
- Medullary thyroid carcinoma
- In addition, other causes of false-positives ↑PCT include:
- Procalcitonin is validated predominantly as a tool for de-escalation or discontinuation of antibiotic among medical ICU patients (with low levels supporting a decision to discontinue antibiotics).
- RCTs have demonstrated that procalcitonin use reduces antibiotic exposure and might even decrease mortality *.
Procalcitonin use in septic shock
- Procalcitonin should be measured if all of the following criteria are met:
- Patient is already started on antibiotics, based on a clinical suspicion for sepsis.
- Patient isn’t severely immunocompromised.
- Patient has not definitively been diagnosed with sepsis.
- Interpretation of procalcitonin in suspected septic shock:
- Procalcitonin >0.5 ug/ml is consistent with a diagnosis of septic shock, but not diagnostic of it *.
- Procalcitonin <0.5 ug/ml casts some doubt on the diagnosis of septic shock *:
- Consider the possibility of noninfectious sepsis mimics.
- If doubt remains, procalcitonin should be reassessed 6 to 24 hours later.
- More on PCT 👉here.
CRP use in septic shock
- CRP measurement may be preferred over procalcitonin in patients with renal failure or neutropenia since CRP is not affected by these conditions.
- Low CRP (<10-20 mg/L, or <1-2 mg/dL)
- This doesn’t exclude infection, but it casts doubt on the diagnosis of septic shock.
- Markedly elevated CRP (>100 mg/L, or >10 mg/dL)
- This is consistent with septic shock, but not diagnostic of it.
Hemodynamic evaluation
- Although septic shock has a specific hemodynamic profile on pulmonary artery catheterization (PAC), it is difficult to interpret and rarely placed in patients with suspected sepsis.
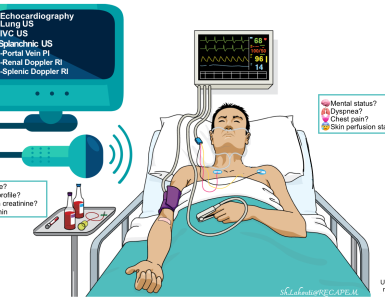
- Bedside echocardiography
- It is most helpful to identify non-distributive causes of shock (e.g. RV dysfunction, LV dysfunction, pericardial tamponade, frank hypovolemia). More on this here.
- In patients with distributive shock, echocardiography often fails to reveal any obvious cause(s) of shock. If a patient is in shock and echocardiography looks normal, then distributive shock is more likely. The most common cause of distributive shock is septic shock and it is imperative to treat empirically for sepsis.
- The clinical integregation of common echocadiographic findings in septic shock is discussed below *
- IVC size: ↓↓↓ or normal.
- Septic shock is characterized by microcirculatory dysfunction (capillary leak) resulting to relative hypovolemia.
- Unresuscitated patients with septic shock show hypovolemia with ↓IVC size. Once adequately resuscitated with fluid, IVC often appear with normal size.
- Septic shock is characterized by microcirculatory dysfunction (capillary leak) resulting to relative hypovolemia.
- Afterload: is frequently decreased; clinically manifesting as warm, flushed skin, wide pulse pressure, and low diastolic BP.
- Unresuscitated patients in early septic shock may appear with hypodynamic profile as cold and clammy skin (due to relative hypovolemia). When these patients are adequately resuscitated, the hyperdynamic profile of early sepsis will be uncovered (i.e. warm, flushed skin, wide pulse pressure, low diastolic BP).
- LVEF (pump function): Normal/hyperdynamic (early); ↓(late).
- Patients with early septic shock usually have supra-physiologic LVEF.
- However, prolonged septic shock often causes stress cardiomyopathy resulting in a hypodynamic profile with ↓cardiac output, cool, cyanotic, and mottled skin. These patients share a common hemodynamic profile to patients with cardiogenic shock.
- IVC size: ↓↓↓ or normal.
- B-lines in Lung ultrasound
- The presence of B-profile in critically ill patients is a rule rather than an exception!
- B-lines may not be a reliable sign of volume overload in the following conditions: *
- SIRS (due to various infectious or non-infectious etiologies) will result in B-profile irrespective of intravascular volume status. This will happen in the context of capillary leak.
- Lower respiratory tract infection
- Interstitial lung disease such as idiopathic pulmonary fibrosis.
- How to use B-lines in resuscitation of patients with septic shock?
- Presence of bilateral B-lines should be considered as a marker of fluid intolerance. In appropriate clinical context and in the presence of other hemodynamic signs of congestion such as engorged IVC; restrictive fluid strategy should be adopted.
Differential diagnosis
Mimics of sepsis are a common cause of misdiagnosis in the ED. All of these clinical conditions produce symptoms and signs that meet SIRS, clouding the clinical picture. Septic mimics should be strongly considered and investigated in those patients who present with non-specific signs and symptoms of “SIRS” ,especially those who also meet the following:
- Absence of clear focus of infection on initial evaluation
- Unexpectedly low procalcitonin
- Refractory shock (below)
Endocrine mimics
- DKA
- Patients with DKA are tachypneic, dehydrated from osmotic diuresis, and may be hypotensive. More on this, here.
- Clue: Large anion gap, ketoacidosis, hyperglycemia.
- Note that DKA and septic shock often coexist, so diagnosing the patient with DKA doesn’t exclude simultaneous sepsis!
- 🚩Persistent hypotension despite fluid resuscitation (2-4L) is an ominous signs, suggestive for a triggering event e.g. sepsis or profound acidosis (if pH <7.1).
- Test: Ketones in urinalysis, preferred test is elevated serum beta-hydroxybutyrate level (>3 mM).
- Adrenal crisis
- Historical clues: Recently discontinued steroid therapy, history of adrenal insufficiency.
- Clinical clues: Abdominal pain/nausea/vomiting/diarrhea (mimicking abdominal source of infection), weakness, fatigue, salt craving, and “vasopressor-refractory shock”.
- Lab clues: ↓Serum Na, ↑serum K, hypoglycemia, may have elevated eosinophil count.
- Test: Cortisol level (obtained before steroid therapy).
- Thyroid storm
- Clue: Tachycardia, tremor, altered mental status, thyromegally.
- Test: Test: TSH, free T4.
GI mimics
- Pancreatitis
- Clues: Characteristic abdominal pain, nausea/vomiting.
- Tests: Lipase level, Ultrasound/ CT of abdomen.
- Intestinal Ischemia
- Clues: Abdominal pain that often does not worsen with palpation (pain out of proportion to exam), atrial fibrillation.
- Test: CT angiography of the abdomen/pelvis.
- Decompensated cirrhosis
- Clues: Ascites, chronic hypotension, ↑INR, ↓PLT, GI bleeding. More on this, here.
- Note that even if the patient has cirrhosis this doesn’t exclude simultaneous septic shock as well.
- Tests: LFT and ultrasonography may show signs of cirrhosis.
- Clues: Ascites, chronic hypotension, ↑INR, ↓PLT, GI bleeding. More on this, here.
Toxic Ingestion/Overdose
- Sympathomimetic toxidrome and serotonin syndrome
- Clues: History of drug use, hypertension, tachycardia, hyperthermia with extreme sweating 💦, pupillary dilation 😳,hyperreflexia/clonus.
- Serotonin syndrome has a rapid onset and resolution following discontinuation of drug(s), clonus (lower extremities more than upper extremities) and nausea, vomiting, diarrhea point towards serotonin syndrome..
- Test: Infectious workup (blood & urine cultures) and CSF analysis are warranted if concern for meningitis/encephalitis.
- Clues: History of drug use, hypertension, tachycardia, hyperthermia with extreme sweating 💦, pupillary dilation 😳,hyperreflexia/clonus.
- Anticholinergic
- Clues: History of drug exposure, dilated pupils 😳, dry skin 🌵despite hyperthermia, tachycardia.
- The timing of onset of delirium may help differentiate toxin-induced causes from organic causes, as delirium from anticholinergic poisoning usually begins more abruptly than that from organic causes, such as sepsis or uremia.
- Test: ECG, physostigmine challenge
- Clues: History of drug exposure, dilated pupils 😳, dry skin 🌵despite hyperthermia, tachycardia.
- Salicylates
- Clues: History of salicylate use (without a suggestive history, salicylate intoxication can be extremely difficult to be differentiated from sepsis).
- Test: Salicylate level.
- Neuroleptic malignant syndrome
- Clues: History of rapid up-titration of neuroleptic and/or higher doses of antipsychotic, extrapyramidal symptoms most prominently rigidity (other: tremor, dystonia, dysphagia).
- Hyperthermia, autonomic dysfunction (diaphoresis, ↑HR, labile HTN, urinary incontinency) and leukocytosis up to 40.000 can be seen.
- Test: Sepsis work up as well as neuroimaging and lumbar puncture (depending on the context) might be considered.
- Clues: History of rapid up-titration of neuroleptic and/or higher doses of antipsychotic, extrapyramidal symptoms most prominently rigidity (other: tremor, dystonia, dysphagia).
- Beta blocker, calcium channel blocker and TCA intoxication
- Clues: Disproportionate bradycardia, exposure history. Typical ECG change in TCA intoxication.
- In the absence of clear exposure history, an empiric antibiotic administration is warranted (for any undifferentiated hypotensive shock).
Miscellaneous
- Anaphylaxis
- Clues: Rapid onset of reaction following exposure to trigger, Involvement of two organ systems (dermal, GI, respiratory, hemodynamic), or known allergen exposure with hypotension warrants consideration. Fever unlikely to be present. More on this, here.
- Note that patients may present with distributive shock (vasodilated and hypotensive)without dermal, GI, respiratory system involvement.
- Test: When in doubt treat for both processes i.e. sepsis and anaphylaxis with antibiotics, steroid, and epinephrine infusion. Follow the disease course over time. Patients with anaphylaxis will recover within hours (faster than septic patients) and their infectious evaluation will be negative.
- Clues: Rapid onset of reaction following exposure to trigger, Involvement of two organ systems (dermal, GI, respiratory, hemodynamic), or known allergen exposure with hypotension warrants consideration. Fever unlikely to be present. More on this, here.
Diagnosis
Identification of early sepsis
Why early diagnosis is important?
- There is a continuum of severity ranging from sepsis to occult septic shock and overt septic shock.
- The mortality rate is wide-ranging and dependent upon the population studied. It has been roughly estimated to be ≥10% (in sepsis) and ≥40% when shock is present. Therefore early diagnosis is critically important.
Why early identification is difficult?
- Signs and symptoms of infection and hypoperfusion are highly variable among the patients. Different patients present with different constellation of findings.
- No single screening tool is accurate enough for diagnosis of septic shock.
- Despite several proposed screening tools (e.g. SOFA, NEWS), they lack specificity for diagnosis of sepsis (i.e. they cannot identify patients whose organ dysfunction is truly secondary to an underlying infection).
Tools for early identification: The most commonly used scores for early identification of sepsis are briefly discussed here.
- Systemic Inflammatory Response Syndrome (SIRS): Previously a score of ≥2 was considered as a surrogate for presence of sepsis.
- SIRS is neither sensitive nor specific for sepsis. It is a common finding in patients with infections without sepsis and in patients without a suspected infection. This lack of specificity makes it impractical for triggering appropriate care.
-
Patients with sepsis may not meet the SIRS criteria for various reasons (e.g. elderly patients not mounting a febrile response, current beta blocker use, or the diagnostic futility of the WBC). This lack of sensitivity leads to missed sepsis cases when SIRS criteria are used as the sole screening tool.
- Therefore, SIRS is no longer included in the definition of sepsis.
- Sequential Organ Failure Assessment (SOFA)
- It was developed to measure organ dysfunction in the ICU patients (like the APACHE), but it was not intended to be a sepsis screening tool or guide resuscitation.
- In a retrospective validation for sepsis outside of the ICU, the SOFA was outperformed by other score *.
- Quick Sequential Organ Failure Assessment (qSOFA)
- It was derived in patients suspected of having an infection to be a prognostic indicator of mortality, but not a screening tool for identifying infection and diagnosing of sepsis.
- There has been very limited prospective validation of qSOFA, therefore it is not known if qSOFA is a reliable screening method for sepsis.
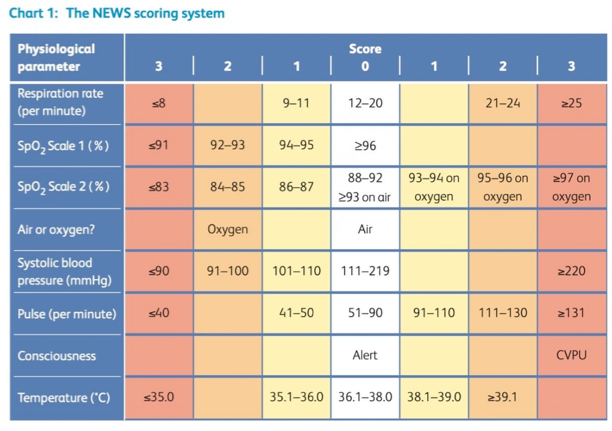
- National Early Warning system (NEWS)
- It is an aggregate scoring system derived from six physiologic parameters (table below) *.
- It can be calculated at triage without the need for blood test results.
- The aggregate score represents the risk of death from sepsis and indicates the urgency of the response.
- Aggregate score 0 to 4: Low risk
- Red score (a score of 3 in any individual parameter): Low-medium
- Aggregate score 5 to 6: Medium risk
- Aggregate score ≥7: High risk
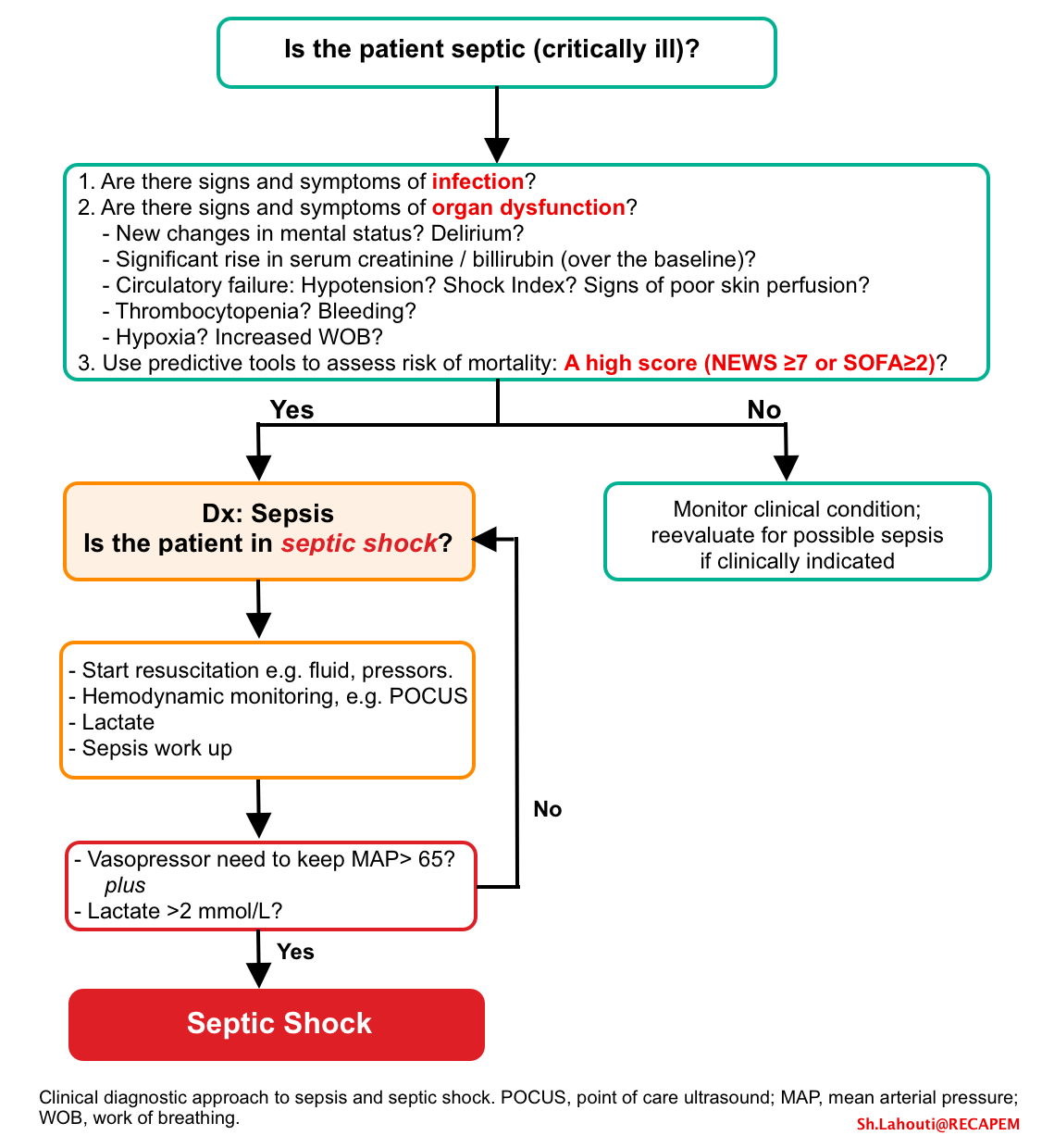
General approach to diagnosis of sepsis and septic shock
Presentation of sepsis and septic shock is highly variable among the patients and there’s no single diagnostic test that can identify patients with septic shock.
- The presumptive diagnosis should be made carefully on an individual basis, using clinical judgment and consideration of the multiple factors (rather than just using a scoring system).
The possible stepward approach to diagnosis of sepsis and occult septic shock (based on the current definition) is discussed below.
- Look for signs and symptoms of infection
- Note that some patients may not show focal signs and symptoms of infection, and may present with non-specific presentation with organ failure.
- Look for evidence of multiple organ dysfunction
- New guidelines recommend against using qSOFA as a single screening agent *. It is more helpful to look for evidence “multiple” organ dysfunction/failure.
- Use a scoring system (e.g. SOFA, NEWS) to predict risk of mortality.
- Start resuscitation, and monitor hemodynamic and clinical response.
- Blood pressure (compared to patient’s initial BP).
- Vasopressor requirement.
- Level of serum lactate rise. In appropriate context, a serum lactate >2 mmol/L suggests septic shock. A lactate level > 4mmol/L portend a significant mortality *.
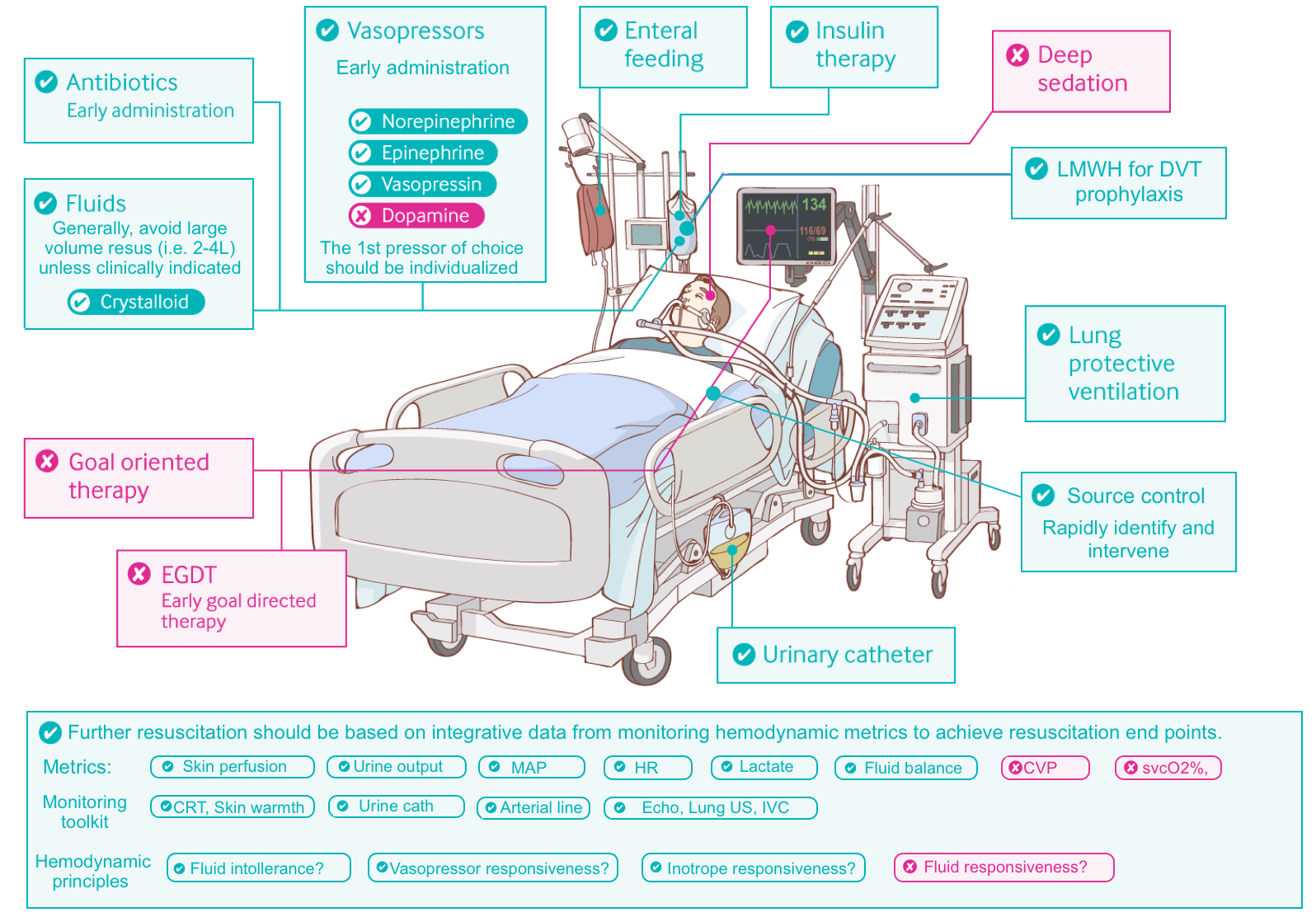
Initial resuscitation
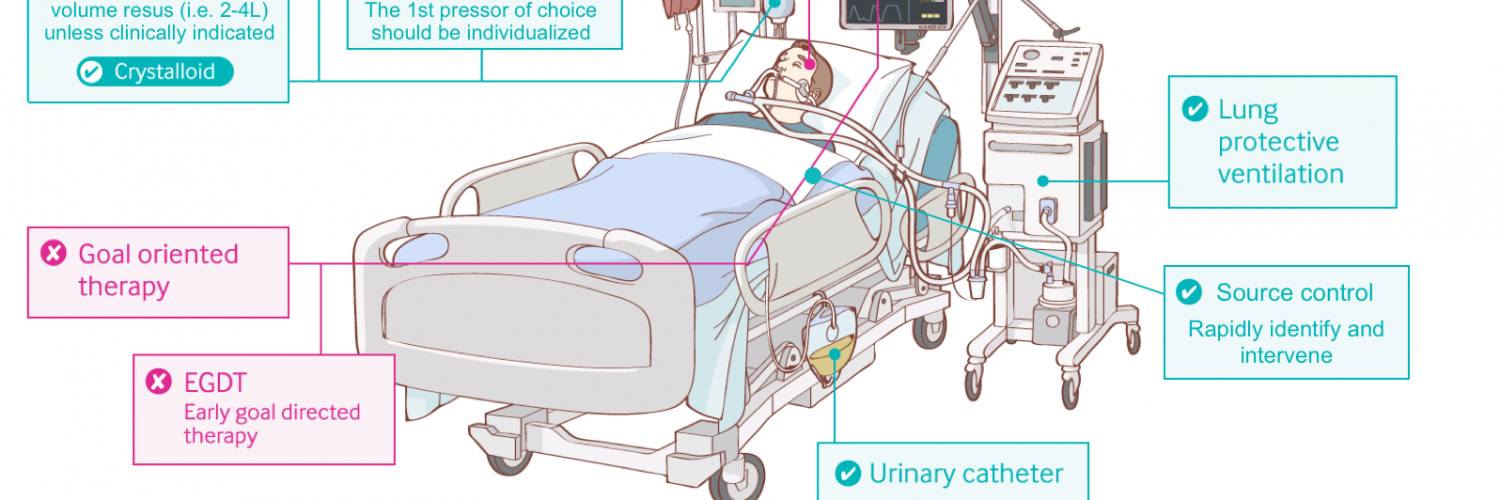
Basic principles of resuscitation in septic shock
- Time is tissue. The longer the duration of hypotension, the more will be the risk of renal failure, and multiple organ damage.
- The mortality is lower if septic shock syndrome is identified and managed promptly.
- Resuscitate proactively (support the patient and prevent further deterioration), rather than resuscitating in a reactive fashion (i.e. waiting for the patient to deteriorate further and then trying to solve the problem).
- Resuscitation should be performed individually based on patient’s physiology, hemodynamic profile. Guideline-based sequential resuscitation is flawed.
- The goal of hemodynamic resuscitation in septic shock is to improve “effective tissue perfusion”.
Hemodynamic support
Vasopressors
- The primary problem in septic shock is vasodilation and capillary leak.
- Traditional strategy of fluid over-resuscitation for hours before starting pressors is not based on pathophysiologic grounds.
- The leaking capillary system is not capable to maintain fluid intravascularly. Flooding the patient with fluid will further impair the viscous cycle of hypoxic cellular injury by worsening interstitial edema.
- Vasopressors perfectly resolve vasoplegia and improve tissue perfusion.
- 🕰Timing of administration
- Peripheral vasopressor should be started without delay to support the blood pressure and tissue perfusion *. There is no need to wait for 2-3L of crystalloid to go in!
- If the patient improves following fluid resuscitation, then pressor may be weaned off.
- If vasopressor requirements escalate, then transition to a central line may be considered.
- In a recent prospective RCT; CENSER trial; early norepinephrine was significantly associated with increased shock control by 6h.
- Peripheral vasopressor should be started without delay to support the blood pressure and tissue perfusion *. There is no need to wait for 2-3L of crystalloid to go in!
- The choice of first pressor
- There is no first line agent.
- Traditional dogma of specific sequential administration of vasopressors for all patients (beginning with norepinephrine, then with sequential addition of vasopressin, and finally epinephrine) is not supported with solid evidence *.
- First line vasopressor should be selected based on patient’s physiology (figure below) *.
- There is no first line agent.
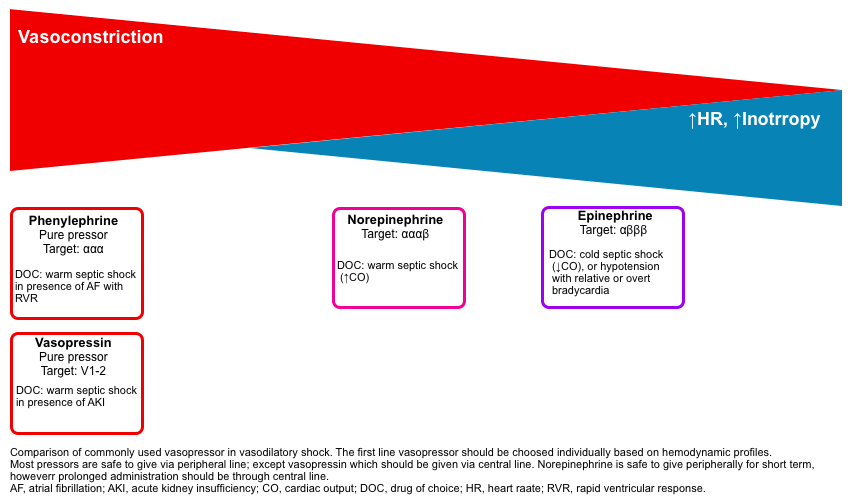
- Norepinephrine: is appropriate for most patients, esp. for vasodilatory shock with warm extremities and tachycardia *.
- Peripheral use is safe for short period of time, but for prolonged period of infusion, central access must be established.
- Weight-based dosing norepinephrine start and immidiately titrate after each 5min based on clinical end point (e.g. MAP, end-organ perfusion).
- Non-weight-based dosing (based on ~ 80kg patient): Initial: 5-15 mcg/min; titrate to goal MAP; usual dose range: 2 to 80 mcg/min. Max dose range for refractory shock: 80 to 250 mcg/min.
- Epinephrine: Most appropriate in patients with low cardiac output (e.g. late septic shock, cardiogenic shock) with cool, mottled extremities, and those hypotensive patients with inappropriately normal HR or overt bradycardia *.
- Peripheral administration is totally safe.
- Weight-based dosing epinephrine start and titrate based on clinical end point (e.g. MAP) after each 10min.
- Non-weigh-based dosing (based on ~ 80kg patient): Usual dosage range: 1 to 40 mcg/min; titrate based on clinical end point.
- Effect on blood pressure: On average, epinephrine has a relatively equivalent effect on BP compared to norepinephrine (e.g. 1 mcg/min epinephrine ~ 1 mcg/min norepinephrine) *.
- More 👉 epinephrine challenge in sepsis (PulmCrit).
- Phenylephrine: Suitable for patient with vasodilatory shock (warm extremities) and atrial fibrillation with RVR.
- Peripheral route is absolutely safe.
- Dosing. Usual dose range 40-180mcg/min.
- Vasopressin: a non-catecholamine pure vasoconstrictor (via V1-2 receptor) that may improve renal function (compared to norepinephrine).
- When should vasopressin be initiated in septic shock?
- Suitable for patients with warm extremities (vasodilatory shock) and acute kidney injury *.
- Start vasopressin when moderate doses of norepinephrine (as in 0.5 mcg/kg/min or 35 mcg/min) have been reached. This has been showed to lower risk of atrial fibrillation with the combination of vasopressin and norepinephrine compared to norepinephrine alone.
- ⚠️Avoid peripheral infusion. If vasopressin extravasates, there is no way to counteract its effect (unlike catecholamine extravasation, which can be treated with local infiltration with phentolamine).
- The combination of vasopressin plus norepinephrine can cause digital ischemia and necrosis.
- Digital ischemia is one of the most feared complications of vasopressin use *. Immediately stop vasopressin.
- Dosing in septic shock : 0.01-0.06 U/min. Titrate up by 0.005 unit/min after each 10min until target blood pressure reached.
- When should vasopressin be initiated in septic shock?
- ⚠️Dopamine: Is the only pressor which shouldn’t be used *.
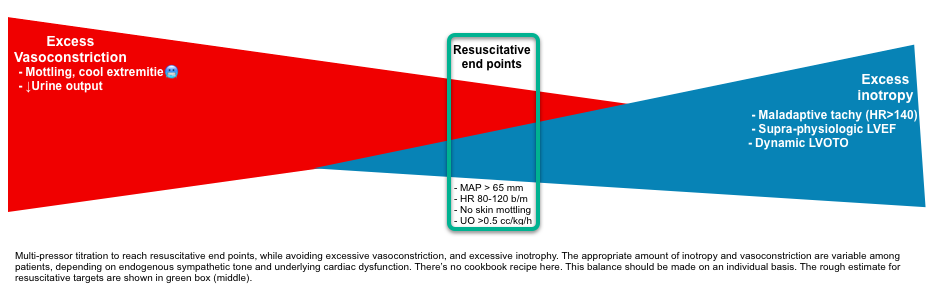
Titration of vasopressor(s) should be based on resuscitative end points while avoiding excessive vasoconstriction or excessive inotropy (shown below).
- 🎯Goal: Using the minimal vasopressor(s) enough to achieve resuscitative end points.
- There is no cookbook recipe here and the optimal balance will vary between patients, depending on catecholamine tone and underlying cardiac dysfunction.
Fluid
- Basic concept of volume management in septic shock
- Pathophysiologic culprit of septic shock is vasodilation and ↑capillary permeability.
- IV fluid cannot resolve these problems since the leaky capillary system is not capable of maintaining fluid intravascularly. In contrast, excessive fluid administration may cause interstitial edema, which can worsen the vicious cycle of cellular injury.
- Despite several studies, there is no solid evidence that fluid administration is beneficial.
- Fluid bolus therapy is not evidence based and should be avoided when possible.
- More on this👉 Myth-busting the fluid bolus (by Josh Farkas).
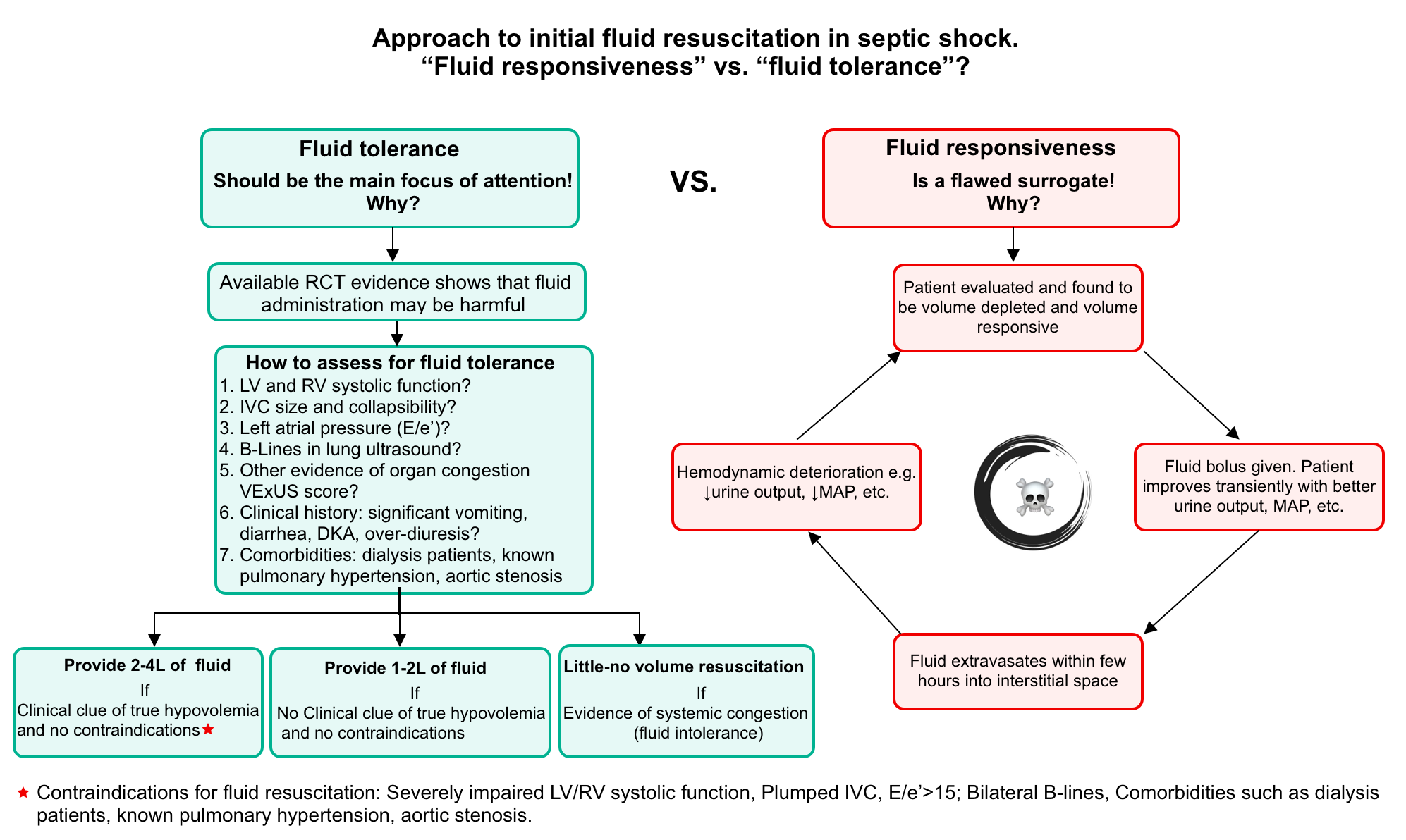
- Fluid responsiveness vs. fluid tolerance.
- Fluid responsiveness is fundamentally a flawed surrogate. Measuring fluid responsiveness has never been shown to improve clinical outcomes in septic shock. Serial assessment of fluid responsiveness may lead to a vicious cycle of perpetual fluid administration (shown below).
- Fluid tolerance is an important consideration for volume resuscitation of patients with septic shock.
- ⚠️Interpretation of IVC for volume status is misleading in septic shock.
- A collapsed IVC and slit LV in septic shock may result form vasodilation and distribution of blood out of the central veins (i.e. relative hypovolemia rather than true hypovolemia). Therefore, a collapsed IVC should not be interpreted as an indication to give fluid.
- Pathophysiologic culprit of septic shock is vasodilation and ↑capillary permeability.
- Initial fluid resuscitation
- Fluid of choice: a balanced crystalloid is a good choice (e.g. Lactated Ringers or Plasmalyte)*.
- Albumin is fluid of choice for patients with spontaneous bacterial peritonitis and/or hepatorenal syndrome.
- More on fluids: 👉 9 reasons to quit using normal saline for resuscitation (PulCrit).
- Indications: Gross volume loss (e.g. significant vomiting, diarrhea, drenching sweating, ↓oral intake, overdiuresis, DKA).
- Contraindications: Evidence of systemic congestion, pneumosepsis with risk of developing ARDS, pulmonary hypertension, dialysis patients.
- Initial fluid administration (figure below)
- In the presence of true hypovolemia and absence of contraindications: Consider large volume (2-4L) resuscitation (given slowly) with frequent hemodynamic monitoring.
- In patients without gross volume loss and no contraindications for fluid administration: Consider moderate volume resuscitation (1-2L; ~ 30mL/kg ideal body weight) slowly over several hours.
- In patients with contraindication for volume resuscitation, give little / no fluid.
- Fluid of choice: a balanced crystalloid is a good choice (e.g. Lactated Ringers or Plasmalyte)*.
- Fluid management after initial resuscitation
- Stop giving additional fluid after initial resuscitation, unless patient has ongoing fluid losses (e.g. severe diarrhea).
- Most patients will receive ~1.5 L/d of fluid along with various infusions and antibiotics. The addition of enteral nutrition will often increase this to >2-3 L/d. This fluid alone is already excessive (without the use of any additional crystalloid)!
- Available evidence supports a fluid-restrictive strategy following initial resuscitation.
- In the CLASSIC trial, additional fluid caused no improvement in hemodynamics, vasopressor dose, or urine output. In fact, a liberal fluid strategy seemed to increase the risk of kidney injury *.
- A recent multicenter RCT including 1554 adult ICU patients with septic shock found no difference in 90 day all cause mortality between patients following a restrictive IV fluid protocol (250-500cc isotonic boluses for clinical signs of hypoperfusion or replacement of documented losses) and patients following standard IV fluid therapy *.
- Monitor the net fluid balance of the patient, which includes the volume of crystalloid given, as well as the volume of antibiotics and other infusions.
- ⚠️Very high fluid balance correlates with increased mortality *. Unless there is strong evidence of pre-existing hypovolemia, avoid running the fluid balance above ~4 liters positive.
Antibiotic
Timing of antibiotic administration in septic shock
- The mortality benefit of “early” administration of antibiotics has been shown for patients with septic “shock”, but not for the cases of sepsis “without septic shock”.
- Early antibiotic administration is recommended for patients with “potential diagnosis of septic shock” (ideally within one hour of diagnosis of septic shock being made) *.
Major consideration in antibiotic selection
- Possible source(s) of infection.
- Clinical context (e.g. community- or hospital-acquired).
- Any prior culture results (especially drug-resistant organisms).
- Antibiotic allergies.
- Recent antibiotic exposures (use an agent the patient hasn’t been recently exposed to).
- Presence of invasive devices.
Antibiotic agent(s)
- A broad-spectrum beta-lactam is generally the most important antibiotic used.
- Piperacillin/tazobactam is often used for community-acquired septic shock.
- Meropenem used for nosocomial septic shock or profound beta-lactam allergy.
- Piperacillin/tazobactam is often used for community-acquired septic shock.
- MRSA coverage
- Not all patients with septic shock requires MRSA coverage *.
- MRSA isn’t a pathogen involved in urosepsis or community-acquired intra-abdominal infection. Patients with these sources of infection don’t need MRSA coverage.
- Indication for MRSA coverage (high risk of MRSA infection) *
- Endocarditis
- Pneumonia
- Soft-tissue infection
- Line infection
- Agents:
- Vancomycin
- Potential alternative include: linezolid (no risk of nephrotoxicity), or daptomycin (for non-pulmonary MRSA).
- Not all patients with septic shock requires MRSA coverage *.
Additional coverage
- For all patients with pneumonia, consider to cover atypical pathogen with azithromycin or doxycycline.
- In patients with colitis or diarrhea (especially if recent antibiotic exposure), consider to cover clostridiodes difficile with:
- Vancomycin 500mg PO q6h, and
- Metronidazole 500 mg IV q8h.
- Clindamycin may be considered for toxin suppression in patients with necrotizing fasciitis, toxic shock syndrome, or severe group A streptococcal infections (e.g. streptococcal cellulitis).
- Fungal coverage
- Empiric antifungal therapy should be considered in adults with sepsis or septic shock at high risk of fungal infection, such as those who have had total parenteral nutrition, recent broad-spectrum antibiotic exposure, perforated abdominal viscus, or immunocompromised status, or when clinical suspicion of fungal infection is high *.
- Risk factors for fungal infection in septic shock should trigger the addition of echinocandins or liposomal amphotericin B. Azoles are considered appropriate for hemodynamically stable patients.
- Empiric antifungal therapy should be considered in adults with sepsis or septic shock at high risk of fungal infection, such as those who have had total parenteral nutrition, recent broad-spectrum antibiotic exposure, perforated abdominal viscus, or immunocompromised status, or when clinical suspicion of fungal infection is high *.
Source control
Basic concept
- Source control should be undertaken in timely manner in all patients since undrained foci of infection may not respond to antibiotics alone.
- Identification
- A focused history, physical examination and POCUS* is the most valuable method for source detection.
- Following initial investigations and empiric antimicrobial therapy, further efforts aimed at identifying the source(s) of infection should be performed as promptly as is feasible. For example imaging should be performed to rule out obstruction in all patients with urosepsis.
- For patients in whom no infection is identified, aggressive imaging may be helpful to search for the source (e.g. CT abdomen/pelvis).
- Timing
- The general rule of thumb is that source control should occur as soon as possible, however, this is not always practical or feasible.
- The optimal timing of source control is unknown but guidelines suggest no more than 6 to 12 hours after diagnosis since survival is negatively impacted by inadequate source control *.
Common example of source control *
- Soft tissue infection: Debridement of necrotic tissue and drainage of discrete abscesses.
- Urinary tract infection: Drainage of abscesses, relief of obstruction, removal or changing of infected catheters.
- Catheter-related bacteremia: Removal of catheter
- Cholangitis: ERCP or percutaneous intervention for decompression.
- Peritonitis: Surgical resection, repair, or diversion of ongoing sources of contamination, drainage of abscesses.
- Septic arthritis: Joint drainage and debridement
- Prosthetic device infection: Device removal
Resuscitative endpoints
Physiologic background
- The basic physiology of cardiovascular system and key points in hemodynamic monitoring is represented in the following slides.
- Resuscitative endpoints are targets which we would ideally like our patients to reach.
- The ideal goal of resuscitation in septic shock is to improve “effective tissue perfusion”.
- Effective tissue perfusion is the interplay of macrocirculatory (systemic hemodynamic) and microcirculatory function (more on this, here).
- It was thought that optimizing macrocirculatory (systemic hemodynamic) metrics such as MAP result in improvement of effective tissue perfusion. However recent studies have debunked this concept.
- It has been shown that with progressive tissue hypoxia, the coherent between macrocirculation and microcirculation is lost. Therefore optimizing systemic hemodynamic parameters may not necessarily result in improvement of effective tissue perfusion.
Which hemodynamic metrics are targeted?
- In the absence of widely available devices that directly measure tissue perfusion, macrocirculatory indices are used as a surrogate of tissue perfusion. The commonly used resuscitative targets include *,*,*
- Perfusion target: skin perfusion, urine output, and level of consciousness.
- Basic hemodynamic target: MAP, HR
- Resuscitation target: Lactate, fluid balance, and echocardiographic indices.
- ⚠️Do not use central venous pressure (CVP) and mixed venous oxygen saturation for targeting resuscitation endpoints.
- The concept that central venous pressure reflects volume status has been thoroughly debunked *.
- Several RCTs revealed that measuring svcO2% didn’t improve outcomes compared to conventional therapy (the PROCESS, PROMISE, and ARISE trials).
Principles
- Resuscitative endpoints should be individualized. A “one-size-fits-all” approach to all situations is not appropriate.
- A MAP of 65 mmHg may be low in patients with chronic hypertension, or may be high for patients with cirrhosis (explained below).
- Use multiple parameters to assess hemodynamic status.
- No single metric is sensitive and specific enough to be used clinically. For example patients may have signs and symptoms of low perfusion (e.g. decreased urine output and cold mottled skin), despite a MAP of > 65mmHg.
Clinical application: How to use resuscitative endpoint in practice?
💡Tip: Resuscitative endpoints are most useful for titrating vasopressors and inotropes but should not be used as a trigger for fluid administration.
- Do not use resuscitative endpoints for fluid administration.
- Use resuscitative endpoints for titrating vasopressors and inotropes.
- It is almost impossible to predict exactly how the patients will respond to vasopressors or inotropes from an initial echocardiogram, which represents a snapshot of cardiac function in time.
- The optimal hemodynamic targets (e.g., MAP goal) may vary between patients.
- The best way to determine the optimal MAP goal and vasopressor dose for any specific patient may be empiric titration with close observation of the effects.
- Dynamic evaluation. Failure to meet resuscitative endpoints should prompt overall re-evaluation of the patient, for example:
- Is there a failure to detect the correct source of sepsis?
- Is the antibiotic selection correct?
The commonly used resuscitation targets are discussed below.
Resuscitation targets
Skin perfusion
Compound assessment of skin perfusion
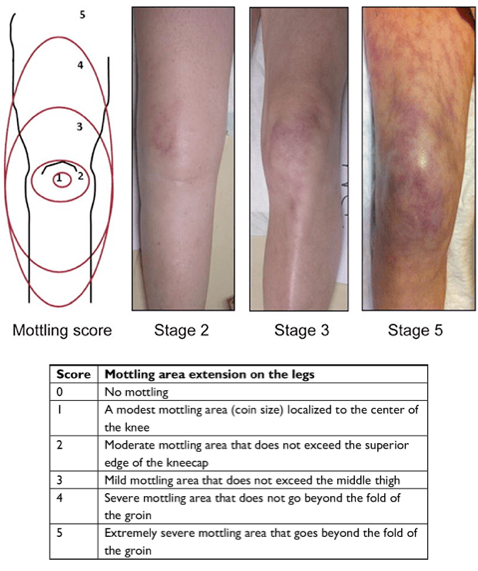
- Mottling
- It refers to patchwork appearance of skin with area of bluish-red discoloration (image below). This results from excessive vasoconstriction of cutaneous blood vessels and has been associated with ↑mortality in several studies.
- Capillary refill time (CRT)
- Normal capillary refill time is <3-4 sec, whereas >5 sec indicates delayed capillary refill.
- Skin temperature
- Warmth of the skin may be used as an indirect measurement of cardiac output.
- Early septic shock (warm shock🥵) is characterized by hyperdynamic profile due to ↓afterload and ↑cardiac output. Extremities are warm, flushed with wide pulse pressure.
- Late septic shock (cold shock🥶) is characterized with hypodynamic profile due to ↓cardiac output (secondary to sepsis induced cardiomyopathy). Extremities are cold, cyanotic, mottled with narrow pulse pressure.
- Warmth of the skin may be used as an indirect measurement of cardiac output.
Skin perfusion as a resus target
- Adequate peripheral perfusion may identify patients who won’t benefit from more aggressive resuscitation *.
- Poor skin perfusion manifests with “cold, mottled extremities” with ↓CRT.🥶
- This can happen throughout the spectrum of septic shock. Appropriate management requires interpretation of multiple hemodynamic metrics. The hemodynamic profiles associated with poor skin perfusion include:
- This can happen throughout the spectrum of septic shock. Appropriate management requires interpretation of multiple hemodynamic metrics. The hemodynamic profiles associated with poor skin perfusion include:
-
-
- Early sepsis with hypovolemia.
- 💡Tips: Hypovolemia masks hyperdynamic profile of early sepsis, causing cold mottled skin.
- Echocardiography is helpful to recognize hypovolemia (“slit” LV and RV, +/- dynamic LVOTO, virtual IVC, absence of B-lines and other evidence of congestion).
- Management: Start fluid resuscitation. The hyperdynamic nature of early sepsis will be uncovered (i.e. extremities become warm with wide pulse pressure).
- Early sepsis with excessive vasoconstriction
- Echocardiography clues: LV and RV are not small, biventricular systolic function is preserved. IVC is not virtual.
- Management: Titrating down vasopressor (e.g. norepinephrine, vasopressin).
- Late sepsis with ↓ cardiac output secondary to ↓ventricular systolic function
- Echo clues: Uni-or-biventricular systolic dysfunction with global hypokinesia.
- Management: Add inotrope e.g. epinephrine or dobutamine.
- Sepsis with ↓ cardiac output secondary to inappropriate heart rate
- See below.
- Early sepsis with hypovolemia.
-
MAP
Background
- MAP is the most useful systemic hemodynamic index for a couple of reasons:
- MAP is the mean pressure driving perfusion and perfectly correlates with tissue perfusion.
- MAP is non-invasive and reproducible (same measurement with different technique at different locations).
- Septic patients with vasodilation often have low diastolic blood pressure; so an adequate systolic blood pressure may be falsely reassuring.
The ideal target for MAP, is unknown. A reasonable goal may be to individualize targets rather than targeting one specific numeric goal.
- Conventional MAP >65 mm Hg
- In an undifferentiated patient whose baseline hemodynamic is unclear, a MAP > 65 mmHg is a reasonable place to start.
- Higher MAP (>80-85 mm Hg)
- The SEPSISPAM Trials shows that a higher MAP improved renal function in the subset of patients with chronic hypertension, but higher MAP targets were associated with increased risk of atrial fibrillation.
- In septic patients with chronic hypertension and poor urine output, a higher MAP may be targeted. If there is an improvement in urine output at higher MAP, then this strategy may be continued; otherwise, the MAP target may be decreased.
- It might be beneficial for patients
- Chronic hypertension and poor urine output.
- Cirrhosis with renal failure with a component of hepatorenal syndrome.
- Lower MAP ( e.g. > 60 mm Hg)
- Lower MAP might be appropriate in following conditions:
- Chronic hypotension (e.g. younger women, patients with cirrhosis without hepatorenal syndrome, systolic heart failure)*.
- Patients with end-stage renal failure on hemodialysis (the primary concern with low MAP is renal injury, which isn’t an issue here).
- The 65-TRIAL compared targeting a MAP >60 mm Hg vs. >65 mm Hg among patients older than 65 years old with vasodilatory shock. Targeting a lower MAP (>60 mm Hg) allowed slightly faster weaning of vasopressors, without any difference in other endpoints.
- When lowering the MAP target, ensure that the patient remains adequately perfused (monitor urine output, skin warmth and mental status).
- Lower MAP might be appropriate in following conditions:
Heart rate
Background
- Patients with septic shock show compensatory tachycardia.
- This compensatory response will improve cardiac output as: Cardiac Output = (Heart Rate)(Stroke Volume)
- The optimal HR in septic patients probably lies between 80-120 bpm.
- Spectrum of abnormal heart rate in septic patients
- Relative (HR < 80 bpm) or overt bradycardia (HR < 60 bpm).
- Relative bradycardia: A heart rate that is less than expected for the patient’s clinical condition. In a hypotensive patient with septic shock, a HR of > 80 bpm is expected. Therefore a HR < 80 is considered relative bradycardia.
- Excessive tachycardia (e.g. HR > 130-140 bpm)
- Relative (HR < 80 bpm) or overt bradycardia (HR < 60 bpm).
Management of relative bradycardia (e.g. <80 b/m) or overt bradycardia in patients with systemic hypoperfusion
- Epinephrine challenge may be used in this situation to determine if it could improve the heart rate and cardiac output.
- For patients with permanent pacemakers, pacemaker rate may be increased.
Management of excessive tachycardia (e.g. heart rate >130-140 b/m)
- Excessive tachycardia may impair diastolic filling, leading to ↓cardiac output.
- It is also associated with increase the risk of stress cardiomyopathy over time.
- Potential interventions which may be considered:
- Transition to vasopressors with less beta-adrenergic stimulation (e.g. vasopressin or phenylephrine).
- Removal of other stimuli which may be driving tachycardia (e.g. under-treated agitation, pain, or withdrawal).
- Atrial fibrillation:
- AF is the most common dysrhythmia in critically ill patients with sepsis and is independently associated with poor outcome *.
- Determine if the AF is the cause of instability (more on this here).
- Management:
- Follow the above intervention to control the rate.
- For patients with chronic AF, digoxin may be considered.
- For patients with new onset of AF and hemodynamic instability, electrical cardioversion should be considered.
- For patients with new onset of AF and stable hemodynamic; amiodarone and Mg are preferred.
Lactate
Background
- Lactate is not an indicator of perfusion, systemic oxygenation, organ failure, or anaerobic metabolism. In most cases lactate serves as an index of endogenous catecholamine surge (as explained above).
- Cycling the lactate and trying to “normalize” it isn’t evidence-based.
- Following the lactate is reasonable until it falls, but this data should be used thoughtful fashion.
The ANDROMEDA-SHOCK trial suggested that capillary refill could be superior to using lactate as a resuscitation target.
- 424 patients with septic shock compared normalization of Lactate vs normalization of capillary refill time in the resuscitation of patients with septic shock . Using lactate clearance resulted in more fluid administered, more vasopressors and more epinephrine without any significant improvement in outcomes. There was a trend toward higher 28-day mortality in the lactate clearance group (35% in the capillary refill time group vs 43% in the lactate clearance group), a difference that did not reach statistical significance. Given the lack of evidence supporting lactate as an endpoint, this trial is nearly impossible to interpret. It’s possible that this trial reveals more about the harm of chasing lactate than the benefit of chasing capillary refill.
Following lactate in patients not on epinephrine
- If the lactate is continuing to increase, this may be a sign of “missed injury.”
- Undrained source of infection.
- Inappropriate antibiotic selection.
- Incorrect diagnosis entirely.
- A rising lactate isn’t an indication to blindly give fluid or inotropes, but rather should be a sign that something is wrong and the patient needs to be re-evaluated.
Following lactate in patient on epinephrine
- Epinephrine should cause the lactate to increase (above).
- In fact, rising lactate following initiation of epinephrine is generally a positive prognostic sign *.
- However, if the lactate increases to very high levels (e.g. >10 mmol/L), then the epinephrine infusion should be titrated down (and replaced by dobutamine if an inotropic agent remains necessary).
Refractory Shock
Definition
- There is no universal consensus definition of refractory shock. Proposed definitions include failure to achieve a BP goal despite using high dose vasopressor therapy (defined as ≥0.5 mg/kg/min norepinephrine-equivalent dose *), or the need for rescue vasopressor therapy.
#1. Earlier considerations
- Check for equipment malfunction.
- Placement of a central arterial line (femoral or axillary).
- Radial arterial lines may underestimate BP.
- If patient is on sedative, shift to a more hemodynamically neutral sedative.
- Propofol or dexmedetomidine are excellent sedatives, but they do tend to reduce the blood pressure.
- In the face of refractory shock, more hemodynamically stable agents may be preferable (e.g. ketamine infusion).
- Trial of various different pressor agents (e.g. epinephrine challenge, addition of vasopressin).
- Vasopressin has been demonstrated to be safe at doses of 0.06 U/min and could likely be titrated higher.
- High-dose norepinephrine (there is no maximal dose).
- For patients with right ventricular dysfunction and hypoxemia on mechanical ventilation, inhaled pulmonary vasodilators could be trialed (e.g. inhaled nitroglycerine).
#2. Etiology and evaluation of refractory shock (septic mimics)*
- Acidosis
- Test: Blood gas, basic metabolic panel
- Rx: Reverse underlying cause, bicarbonate infusion.
- 👉A rapid significant but transient bump in BP after bicarbonate administration prove acidosis as the cause of refractoriness of shock.
- Adrenal insufficiency
- Accidence is as high as 30% in subset of patients
- Clue: Clinic, Cortisol level, ↓Na, ↑K.
- Rx: Stress dose of steroid.More on this here.
- Anaphylaxis
- Patients with anaphylaxis may present with isolated hypotension.
- Rx: Epinephrine
- Hypocalcemia
- Dx: ↓Ionized Ca, ↑QTC
- Intravenous calcium (often causes transient improvements in blood pressure which are short-lived).
- Hypothyroid
- Clues: Clinic, TSH may be false negative or delayed.
- Rx: Empiric levothyroxine
- Occult bleeding
- Is there occult bleeding e.g. retroperitoneal bleeding, ruptured EP, ruptured spleen, large vessel aneurysm?
- Test: POCUS, CTA of abdomen
- Rx: Anticoagulation reversal, transfusion, Surgery/IR.
- Toxicologic
- Occult causes, e.g. beta blocker, calcium channel blocker, TCA.
- Rx: Hyperinsulin euglycemia therapy (HIT) for BB/CCB intox, Bicarbonate for TCA intox
- Pneumothorax
- Is there pneumothorax after placement of central venous access?
- Test: POCUS
- Read more on the management of pneumothorax here.
- Second cause of shock:
- Shock often is multifactorial. Look for second causes such as pump failure or obstruction that is causing shock, e.g. cardiac tamponade in dialysis patients, pulmonary embolism, dynamic LVOTO, and aortic dissection.
- Test: POCUS
- Reducing MAP target
- If very high doses of norepinephrine are required to achieve a MAP >65 mm (e.g. >1 ug/kg/min), this may be causing more harm than good. Reducing the MAP target to >55 or >60 could potentially represent a more beneficial balance of vasopressor benefit vs harm *.
Additional therapies
Steroid
- There has been much conflicting evidence for the benefit of steroids in septic shock.
- The ADRENAL trial was an international, double-blind RCT of 3800 vented ICU patients with septic shock randomized to hydrocortisone infusion 200mg/day or placebo for 7 days. Primary outcome was 90 day mortality which was about 28% in both groups. However secondary outcomes (i.e. time on vasopressors, the duration of intubation, the length of ICU stay, and blood transfusions requirement) have been improved.
- Current recommendation of using steroid in septic shock include *:
- Consider early administration of steroid in patients who are taking steroid medications at baseline or those with concomitant adrenal suppression.
- Vasopressor refractory shock (i.e. patient remains in shock despite norepinephrine 0.5mcg/kg/min).
- Steroid dose in septic shock
- The best studied dose of steroid in septic shock is 200 mg hydrocortisone total daily.
- One small RCT demonstrated that intermittent administration of hydrocortisone (e.g. 50mg IV q6h) resulted in improved shock resolution compared to a continuous infusion *.
- If hydrocortisone is not available, any equivalent dose of steroid may be used (steroid conversion calculator). Methylprednisolone 40mg IV daily is another option.
- The best studied dose of steroid in septic shock is 200 mg hydrocortisone total daily.
DVT prophylaxis
RECAP
- Use a combination of MAP, GCS, urine output, initial lactate, compound skin perfusion assessment, POCUS to guide initial hemodynamic resuscitation, individualized to each patient.
- Excessive focus on fluid status may distract you from paying attention to other more important aspects of care.
- Do not wait for the fluid boluses to work especially if patient is in true septic shock. Start vasopressor sooner than later.
- Antibiotics and source control have been shown to improve mortality in septic shock.
- Perform advanced imaging e.g. CT of abdomen and pelvis if the infection source is not clear.
- Consider sepsis mimics and re-evaluate those patients who are not responding to initial circulatory resuscitation.
Going further
📚Landmark studies: The bottom line reviews
- Resuscitative end points
- Fluid
- Pressor
- CENSER
- CATS trial
- VASST and VANISH trials
- Steroid
- Vitamin C
The Surviving Sepsis Campaign
- Should the Surviving Sepsis Campaign Guidelines Be Retired?
- Does the Septic Shock Early Management Bundle (SEP-1) Improve Survival in Septic Adults?
- The 2018 Surviving Sepsis Campaign’s Treatment Bundle: When Guidelines Outpace the Evidence Supporting Their Use
- Challenging the one-hour sepsis bundle



Add comment