September 24, 2021 via Shahriar Lahouti
CONTENTS
- Preface
- Pathophysiology of stroke
- Clinical presentation
- Anatomy of stroke
- Physical exam
- Differential diagnosis
- Approach to diagnosis and management
- Initial Management of Acute Ischemic Stroke
- Subsequent Management
- Disposition
- Media
- Going further
- References
AIS, acute ischemic stroke
CAA, cerebral amyloid angiopathy
CVA, cerebrovascular accident
CVT, cerebral vein thrombosis
DWI, diffusion-weighted imaging
EVT, endovascular therapy
FLAIR, fluid-attenuated inversion recovery
ICH, intracranial hemorrhage
IV-tPA; intravenous tissue plasminogen activator (alteplase in our discussion)
NCCT, noncontrast computed tomography
CTA, CT angiography
LSW, Last seen well
LVO, large vessel occlusion
MRI, magnetic resonance imaging
MRA, MR angiography
MT, mechanical thrombectomy;
Preface
Cerebrovascular disease encompasses a variety of medical conditions that affect the cerebral circulation and brain function. Stroke is generally defined as any disease process that interrupts blood flow to the brain. In the following discussion acute ischemic stroke and transient ischemic attack are reviewed in the light of recent guidelines.
Pathophysiology of stroke
The significance of grasping pathophysiologic classification (figure 1) is that each type of stroke has a different presentation, treatment and prognosis. Acute cerebrovascular syndrome (ACVS) is a constellation of signs and symptoms that are caused by any acute vascular pathology (arterial or venous) that injures the central nervous system tissues. Arterial CVA is classified broadly into two major types; ischemic (≈68%) and hemorrhagic (≈32%) 1. Ischemic stroke (the main focus of this discussion) has three main subtypes; thrombotic, embolic and hypoperfusion.
Clinical Presentation
Acute ischemic stroke and transient ischemic attack (TIA) are medical emergencies that occur as a result of disruption in blood flow to the brain, and originate from a similar pathophysiologic process along the spectrum of acute cerebrovascular syndrome. The core feature of ischemic cerebrovascular accidents is abrupt onset of focal neurologic deficit (FND). However the reverse is not true, that is to say not all neurological signs and symptoms with sudden onset of FND are ischemic (see DDx below).
NATURE OF SYMPTOMS: The symptoms and signs in ischemic attack are ‘negative’ in nature (i.e. loss of normal brain function) hence called “Neurologic Deficit”; as opposed to positive or irritative phenomena, which usually have a non-vascular cause (e.g. migraine, seizure, radiculopathy, etc).
- Negative symptoms may include loss of vision, hearing, feeling, or ability to move a part of the body
- Positive symptoms suggest active discharge from the central nervous system including visual (eg, bright lines, shapes, objects), auditory (eg, tinnitus), somatosensory (eg, paresthesia), or motor (eg, jerking movements).
FOCAL SYMPTOMS: Denotes dysfunction at anatomically localized brain area. In the context of ischemic events the focal signs and symptoms are negative in nature; called ‘FND’ such as unilateral motor or sensory deficit. For example a focal ischemic insult at the frontal lobe cannot cause bilateral motor deficit or any sensory deficit. Examples for non-focal deficit may include altered level of consciousness, bilateral motor or sensory deficit, amnesia2.
- Since focal neurologic deficit can be caused by different pathophysiologic processes (ischemic stroke, hemorrhage, local effect of a mass lesions such as tumor or an enlarging cerebral artery aneurysm, herniation syndromes etc.) it is more helpful to consider ‘FND’ in correlation with a vascular territory.
ONSET: Thrombotic stroke has a gradual onset while embolic stroke and SAH have an abrupt onset. Most patients with ‘ICH’ have gradual onset (sometimes they may have abrupt onset with maximal deficit severity at the very beginning).
The negative symptoms in ischemic vascular accidents may involve one or more modality or function (e.g. motor +/- sensory). The key feature is that it all happens simultaneously at the very onset of attack (while for example in migraine aura the symptoms typically progresses from one modality to another; i.e. after the visual symptoms clear, paresthesia begins. when paresthesia is clear, aphasia or other cortical function abnormalities may develop).
COURSE & PROGRESSION: The neurologic deficits in thrombotic stroke have stuttering (wax and waning) patterns. ‘ICH’ has progressive patterns and severity of signs and symptoms progress over time. Deficits in patients with embolic stroke may suddenly resolve (since endogenous plasminogen activator may lyse the clot).
DURATION: The duration of neurologic deficit has traditionally been used to separate ‘TIA’ from infarction.
Typically the signs and symptoms of TIA last less than 1-2 hours (by classic ‘time-based’ definition < 24 hours). However with the advent of neuroimaging it has been shown that time is not reliable to distinguish ‘TIA’ from stroke as 33% of patients whose neurologic symptoms have lasted for less than 24 hours and have been resolved by the time of evaluation shown to have evidence of brain tissue infarction in diffusion-weighted MRI. These patients are at a higher risk of developing full blown ischemic infarction.
In the ‘tissue-based’ definition, TIA is a transient episode of neurologic dysfunction caused by focal brain, spinal cord, or retinal ischemia; without acute infarction 3. The clinical significance of the ‘tissue-based” definition relies in two facts:
- All patients suspected of having acute cerebrovascular syndrome should have early neuroimaging 4.
- Patients with acute cerebrovascular syndrome (brief and spontaneously resolving) whose neuroimaging shows evidence of infarction have higher risk of developing ischemic CVA within the next 48hours.
ASSOCIATED SYMPTOMS: Certain associated symptoms can suggest specific stroke subtypes:
- Altered level of consciousness: Despite that differential diagnosis of altered level of consciousness is broad, When other clinical presentations are suggestive for stroke, reduced alertness can be caused by the following process:
- Elevated ICP secondary to ICH, SAH, CVT, massive embolic or thrombotic stroke (a large hemispheric infarct are typically followed by edema that can progress to coma).
- Posterior circulation large arteries thrombotic or embolic stroke (In particular, ischemia involving the tegmentum of the pons can cause loss of consciousness)
- Thalamic or pontine hemorrhage
👉Patients with signs and symptoms of systemic hypoperfusion (i.e. shock state) can present with altered level of consciousness, pallor, sweating, tachycardia or severe bradycardia, and low blood pressure. The neurologic signs are typically bilateral, although they may be asymmetric when there is preexisting cerebral vascular occlusive disease.
- Seizure: occurrence of seizure within the acute phase of stroke suggests a cortical lesion. It is most commonly seen with SAH, ICH (lobar ICH), CVT and rarely may be seen with ischemic stroke (brain embolism).
- Headache: Presence of headache is often more consistent with other diagnosis (ICH, SAH, CVT) rather than ischemic stroke (rarely some patients may have headaches in the prodromal period before thrombotic strokes).
- Vomiting: It is more suggestive for elevated intracranial pressure (ICH, SAH, CVT) or may be seen in patients with posterior circulation large artery ischemia.
- Fever: Raise the possibility of endocarditis and embolic stroke.
- Chest pain: Patients with aortic dissection (type A) may develop acute ischemic stroke which is preceded by sudden onset of chest pain. Rarely patients with extensive atherosclerosis may develop ‘AIS’ and acute myocardial infarction simultaneously!
- Neck pain: It may suggest cervicocerebral arterial dissection.
- Elevated ICP: Global symptoms of elevated ICP include headache, depressed global consciousness and vomiting. Focal symptoms of elevated ICP may be caused by local effects in patients with mass lesions or by herniation syndromes (e.g. subfalcine, central transtentorial, uncal transtentorial, upward cerebellar, cerebellar tonsillar/foramen magnum, and transcalvarial).
- Signs of elevated ICP include CN VI palsies, papilledema.
- Nowadays bedside ultrasound is widely available, and elevated ICP can be easily identified. Ocular sonography can provide a noninvasive measure of optic nerve sheath diameter, which has been found to correlate with ICP. A number of studies have found that diameters of 5 to 6 mm have the ability to discriminate between normal and elevated ICP in patients with intracranial hemorrhage and traumatic brain injury (more on this here).
- The etiologies of elevated ‘ICP’ is discussed here. For the purpose of this discussion, presence of intracranial hypertension may suggest hemorrhagic infarction or a massive ischemic infarct.
Other distinguishing features of ischemic stroke
One may distinguish ischemic vascular accidents from other disease processes by investigating certain historical and clinical features (figure 4).
Anatomy of stroke
The negative symptoms of ischemic stroke should usually correlate with a specific vascular territory. The cornerstone of diagnosis and appropriate care of patients with acute stroke is to recognize location and extent of the lesion.
The brain neuroanatomy and relevant vascular supply is shown below (see following slides).
Arterial territory
The arterial territories of anterior circulation (ACA, MCA) and posterior circulation (PCA, vertebrobasilar artery) are shown below (figure 2).
Territorial signs and symptoms
- Findings of acute ischemic stroke in anterior and posterior circulation are summarized in the following table (figure 3a). Schematic presentation of occlusion in ACA, MCA shown in figure 3b.
- Note that the degree of collateral circulation may cause variations in the specific clinical symptoms and their severity.
Ischemic stroke can be classified to acute infarction due to large vessels occlusion or blockage of “penetrating branches” of the vessels (lacunar stroke).
Large vessel occlusion (LVO)
LVO stroke (“cortical stroke”) is invariably defined as severe stroke associated with blockages of the proximal intracranial anterior or posterior circulation.
- It is the most severe form of acute ischemic stroke accounting for ~10% of acute ischemic strokes and is associated with poor outcome if not treated.
- Inclusive definition of LVO 45
- Anterior circulation: Intracranial ICA, M1, A1
- Posterior circulation: Basilar artery, P1
- An NIHSS ≥6 has traditionally been used to predict a cortical stroke; however there’re several caveats here:
- Approximately 5% of large vessel occlusions presenting within 3 h of the time last known well will have a National Institutes of Health Stroke Scale score <4.
- NIHS scoring is complicated and time consuming in ED.
- VAN tool (a mnemonic for Vision, Aphasia and Neglect): A more recent tool called the VAN tool has been shown to have a 100% sensitivity and 90% specificity, 74% PPV and 100% NPV for large vessel stroke 46 ,47. Patient must have weakness plus one or all of the V, A, or N to be VAN positive.
- Step 1- Weakness: ask the patient to raise both arms up for 10 seconds and assess for drift, weakness or paralysis; if any of these are present proceed to step 2.
- Step 2- V or A or N
- Visual disturbance: Field cut, diplopia or blindness
- Aphasia: Expressive (repeat and name 2 objects) or receptive (unable to follow commands, close eyes or make a fist)
- Neglect: Inability to track to one side, ignoring one side, unable to feel both sides at the same time or unable to identify own arm
- CTA is the preferred method for the assessment of “vessel status” in patients with acute cerebrovascular syndrome. Advances in the endovascular treatment of LVO stroke reinforce the need to determine the “vessel status” of suspected stroke patients early.
LVO syndromes
Anterior cerebral artery syndrome
- Either side
- Contralateral leg weakness and sensory loss.
- Frontal lobe dysfunction (poor judgement, flat affect, apraxia, abulia = apathy and reduced speech, incontinence).
- Alien hand sign: one hand acts involuntarily
- Non-dominant hemisphere: Acute confusion and contralateral hemineglect.
- Dominant hemisphere: Transcortical motor aphasia (due to involvement of the supplementary motor area). This is similar to Broca’s aphasia, but with preservation of repetition.
Proximal MCA syndromes (occlusion near the base of M1)
- Either side:
- Contralateral hemiplegia involving the face and arm > leg.
- Contralateral hemisensory loss.
- Contralateral hemianopia.
- Ipsilateral conjugate eye deviation.
- Nondominant hemisphere: Hemispatial neglect (generally of the left side) and anosognosia.
- Dominant hemisphere: Global aphasia.
Posterior cerebral artery syndromes
- Either side:
- Hemianopia or superior quadrantanopia.
- Thalamic involvement may cause contralateral sensory loss (ventroposterolateral nuclei) or reduced arousal.
- Uncommonly, may cause contralateral hemiplegia (due to involvement of the internal capsule).
- Dominant hemisphere:
- Alexia without agraphia (patients are able to write but not read). This results from infarction of the splenium of the corpus callosum, thereby cutting off visual information from the language processing centers.
- Difficulty naming objects (transcortical sensory aphasia).
- Visual agnosia (inability to describe what an object is used for).
- Altered memory (anterograde amnesia), if the medial temporal lobes are involved.
- Nondominant hemisphere:
- Prosopagnosia (inability to recognize faces).
- Bilateral infarctions: Cortical blindness (Anton syndrome).
Proximal basilar artery syndrome (base of the basilar artery)
- Altered level of consciousness (ranging from somnolence to coma).
- Quadriplegia. May also have “crossed” paralysis (e.g. left face and right limbs).
- Oculomotor abnormalities, which may include horizontal gaze palsy (bilateral or unilateral), internuclear ophthalmoplegia (unilateral or bilateral), one and a half syndrome, skew deviation, gaze paretic nystagmus, or bilateral ptosis.
- Pinpoint pupils.
- Bulbar symptoms, which may include: Facial weakness, dysphagia, dysarthria, palatal myoclonus.
- Pseudobulbar affect.
- Sensory loss to light touch.
Mid basilar artery (Locked-in syndrome)
- Locked-in state
- Quadriplegia and facial paralysis
- Horizontal gaze palsy (vertical gaze remain intact).
- Dysphagia.
- Vertigo.
- Hearing loss can occur.
Top of the basilar syndrome
- This results in ischemia of the midbrain, thalamus, and occipital lobes (but not the pons). The cerebellum may also be involved via the superior cerebellar artery.
- Altered level of consciousness (ranging from somnolence to coma).
- Pupillary abnormalities (may involve afferents to Edinger-Westphal nucleus, CN3 nucleus, or descending sympathetic system). Pupils may be dilated, mid-position, or small.
- Vertical gaze impairment, internuclear ophthalmoplegia, or skew deviation.
- Hemianopsia or complete cortical blindness.
- Amnesia, agitation, and hallucinations (may involve colors and objects).
- Ataxia, tremor, dysarthria (if the cerebellum is involved).
- Homonymous hemianopsia.
Lacunar stroke
Lacunar infarcts are small (2 to 15 mm in diameter) noncortical infarcts caused by occlusion of a single penetrating branch of a large cerebral artery.
Pure motor hemiparesis, Dysarthria-clumsy hand syndrome, Ataxic hemiparesis
- Symptoms:
- Pure motor: Isolated contralateral face/arm/leg weakness. No sensory signs. Dysarthria and dysphagia may be present.
- Dysarthria-clumsy hand: Dysarthria, facial weakness, slight weakness/clumsiness of the contralateral hand.
- Ataxic hemiparesis: Ipsilateral hemi-body weakness and limb ataxia (that is disproportionate to the weakness).
- Localization:
- Corona radiata (small MCA branches).
- Posterior limb of internal capsule (lenticulostriate arteries, anterior choroidal artery, or perforators from the posterior cerebral artery).
- Cerebral peduncle (small proximal posterior cerebral artery branches).
- Anterior pons (basilar perforators).
Pure sensory stroke (thalamic lacunar stroke)
- Symptoms: Unilateral sensory loss of all modalities in face, arm and leg without motor deficit.
- Localization: Infarction of the ventral posterior lateral (VPL) and ventral medial nuclei (VPM), supplied by thalamo-perforators from the posterior cerebral artery.
Sensorimotor stroke (thalamocapsular lacunar stroke)
- Symptoms: Combination of thalamic lacune plus pure motor hemiparesis.
- Localization: Posterior limb of the internal capsule plus either thalamic VPL/VPM or thalamic somatosensory radiation. May result from infarction of the thalamoperforator branches of the posterior cerebral artery, or lenticulostriate arteries.
Physical exam
Assessment of airway, breathing, and circulation is the top priority. Next, the goals of examination are to confirm the diagnosis of stroke, exclude stroke mimics, and identify comorbidities. In cases where time-sensitive treatment decisions must be made, the initial assessment may be very brief and performed in parallel to other interventions.
NIHSS: National Institutes of Health Stroke Scale is widely used to assess neurological deficits in stroke patients in a structured manner. It provides a general measure of neurologic deficits using 11 items to produce a score of 0 to 42. Higher scores predict a larger lesion size, greater stroke severity, and worsened short- and long-term outcomes. All patients should have the ‘NIHSS’ calculated, as it is a widely accepted measure of stroke severity. Generally, this should be performed after initial stabilization and neuroimaging.
Notably, the ‘NIHSS’ is a tool designed for research and is good at revealing the potential for stroke in the anterior circulation but is not as reliable for the posterior circulation.
Level of consciousness
0 = Alert.
1 = Drowsy.
2 = Obtunded.
3 = Unresponsive.
Level of consciousness questions: (What month is it? Your age?)
0 = Correct.
1 = 1 mistake-or-unable to speak because of endotracheal intubation, orotracheal trauma or severe dysarthria from any cause.
2 = 2 mistakes -or- aphasic.
Ability to follow two simple commands: (ask to open and close the eyes and then to grip and release the non-paretic hand).
0 = Correct.
1 = 1 failure.
2 = 2 failures.
Gaze palsy: (follow finger with your eyes)
0 = Normal.
1 = Partial gaze palsy.
2 = Complete gaze palsy.
Visual fields: (if nonverbal, test with visual threat)
0 = Normal.
1 = Partial hemianopia (e.g. quadrantanopia).
2 = Complete hemianopia.
3 = Bilateral hemianopia.
Facial palsy:
0 = None.
1 = Minor paralysis (e.g. nasolabial flattening).
2 = Partial paralysis (e.g. lower face).
3 = Complete unilateral paralysis.
Limb strength: (repeat for each limb)
0 = Normal.
1 = Drift.
In arms: Score if arm drifts down before 10 seconds.
In legs: Score if leg drifts down before 5 seconds.
2 = Some effort against gravity.
3 = No effort against gravity.
4 = No movement.
Limb ataxia: (finger-nose & heel-shin)
0 = None.
1 = One limb.
2 = Two or more limbs.
Sensory:
0 = Normal.
1 = Mild-moderate sensory loss.
2 = Severe sensory loss.
Best language: (name items)
0 = No aphasia.
1 = Mild aphasia. Some obvious loss of fluency or facility of comprehension, without significant limitation on ideas expressed or form of expression.
2 = Severe aphasia. All communication is through fragmentary expression; great need for inference.
3 = Mute.
Dysarthria: (ask to repeat words)
0 = Normal articulation.
1 = Mild dysarthria. Slurs at least some words and, at worst, can be understood with some difficulty.
2 = Severe dysarthria. Speech is so slurred as to be unintelligible in the absence of or out of proportion to any dysphasia, or is mute.
👉Remember that dysarthria is impairment of the motor function (CN IX, X, XII) necessary for speaking.
Extinction and inattention: (e.g. double simultaneous tactile extinction)
0 = Normal.
1 = Visual, tactile, auditory, spatial, or personal inattention.
2 = Profound hemi-inattention or extinction to more than one modality.
A. Mental status
1. Level of alertness
2. Language: Comprehension, repetition, fluency, naming, reading, writing.
3. Memory: Immediate, short-term, long-term
4. Calculation
5. Construction
6. Abstraction
B. Cranial nerves (CN)
1. Olfaction (CN I)
2. Vision (CN II). Visual field and acuity.
3. Pupillary light reflex ( CNs II, III)
4. Eye movement (CNs III, IV, VI)
5. Fascial sensation (CN V)
6. Fascial strength (CN V, VII)
7. Hearing and vestibular function (CN VIII)
8. Palatal movement (CNs IX, X)
9. Dysarthria (CN IX, X, XII)
10. Head rotation (CN XI)
11. Shoulder elevation (CN XI)
12. Tongue movements (CN XII)
C. Motor
1. Gait
2. Coordination
3. Involuntary movements
4. Pronator drift
5. Individual muscles (strength, bulk, tone)
D. Reflexes
1. Tendon reflexes
2. Plantar reflex
3. Superficial reflexes
E. Sensory
1. Light touch
2. Pain and temperature
3. Joint position sense
4. Vibration
5. Double simultaneous stimulation
Differential Diagnosis
The history and physical examination should be used to distinguish between other disorders in the differential diagnosis of stroke (figure 5) . As examples, seizures, syncope, migraine, and hypoglycemia can mimic acute ischemia. The most difficult cases involve patients with focal signs and altered level of consciousness. It is important to ask the patient or a relative whether the patient takes insulin or oral hypoglycemic agents, has a history of a seizure disorder or drug overdose or abuse, medications on admission, recent trauma, or hysteria.
Delineating the duration of attack is helpful to distinguish ischemic accidents from certain other diseases with paroxysmal nature (e.g. migraine, seizure).
A scoring system (FABS) is proposed for screening and stratifying stroke mimics from acute cerebral ischemia and to identify patients who may require magnetic resonance imaging to confirm or refute a diagnosis of stroke in the emergency setting 5. This scoring system includes 6 variables with 1 point for each variable, if present:
- Absence of Facial droop
- Negative history of Atrial fibrillation
- Age <50 years
- Systolic Blood pressure <150 mm Hg at presentation
- History of Seizures
- Isolated Sensory symptoms without weakness at presentation
FABS score ≥3 could identify patients with stroke mimic with sensitivity of 90% and specificity of 91% The negative predictive value and positive predictive value were reported as 93% and 87% respectively.
It seems to be reliable in stratifying stroke mimics from acute cerebral ischemia cases among patients in whom the head CT was negative for any acute findings. It can help clinicians consider advanced imaging for further diagnosis.
Approach To Diagnosis and Management
The classic mantra, “time is brain” explains the current AHA/ASA stroke guidelines recommendation to enact “an organized protocol for the emergency evaluation of patients with suspected stroke”. A door-to-needle time of ≤60 minutes is the benchmark for achieving rapid treatment with intravenous alteplase 6. The in-hospital timeline is shown below (figure 6).
Medical Stabilization
AIRWAY: All patients with suspected acute stroke should be assessed immediately upon arrival for airway compromise 6. Patients who are unable to clear oral secretions or maintain airway stability should be immediately intubated ( neurocritical care intubation).
BREATHING: Provide supplemental O2 if oxygen saturation is <94%. Supplemental O2 is not recommended in non-hypoxic patients with ‘AIS’ 6.
CIRCULATION: All patients with suspected acute stroke should be assessed immediately upon arrival for hemodynamic stability 6.
- Hypotension with evidence of poor perfusion (shock state) can mimic stroke especially in elderly patients and should be appropriately managed.
- Hypertension: Patients with cerebrovascular accidents frequently have high blood pressure. The approach to blood pressure management in ‘AIS’ is inherently different from the approach in acute hemorrhagic stroke. For this reason, a neuroimaging study (CT or MRI) is critical to help guide blood pressure therapy in patients with acute stroke.
- IV Access: Obtain peripheral intravenous (IV) access and avoid unnecessary lines, and ABG since minor vascular trauma in patients with ischemic CVA who are deemed to be candidates for thrombolysis may become a real problem.
- Initiate labwork
DISABILITY: Perform a focused neurological exam and obtain a point-of-care glucose.
- The focused exam is structured around relevant data gathered during the medical history and is catered to the differential diagnosis. The examiner should be focused on determining whether (1) there is a lesion and (2) where the lesion is localized.
👉ECG: Baseline ECG assessment is recommended in patients presenting with “AIS” but should not delay initiation of IV alteplase in selected patients.
👉Nothing by mouth (NPO) to protect against aspiration
- Dysphagia is common in patients with acute stroke and is associated with development of aspiration pneumonia.
- Dysphagia related to stroke is more precisely characterized by oropharyngeal dysphagia, defined by swallowing impairment of the upper digestive tract.
- Cranial nerves involved in swallowing include: sensory components of CN V, IX, X and motor components of CN V, VII, X, XI and XII.
- All patients should receive a bedside swallowing screening examination prior to initiation of a diet (e.g. supervised ability to drink a glass of water by a bedside nurse or speech and language therapist). The test is passed if the patient is able to drink the entire volume of water (in a single or sequential swallows) without coughing, gurgling or chocking during or immediately (e.g. within one minute) after swallowing.
- It is important to keep the patient ‘NPO’ (nothing by mouth) to protect against aspiration until the swallowing function is evaluated 6.
👉Nonessential testing and procedures should not delay performing brain imaging within 25 minutes of the patient’s arrival 6.
Brain imaging
All patients suspected for ‘AIS’ and ‘TIA’ (determined to be stable) should be taken immediately for neuroimaging (Non-contrast head CT +/- other modalities) 6. Brain and neurovascular imaging plays a crucial role in acute stroke by 7 8:
- Differentiating ischemia from hemorrhage (Is there an ICH at NCCT that is a contraindication to IV-tPA or EVT, or is there a large well-established hypoattenuating infarct?)
- Excluding stroke mimics, such as tumor
- Assessing the status of large cervical and intracranial arteries (Is there a proximal LVO seen at CTA that can be treated with EVT?)
- Estimating the volume of brain tissue that is irreversibly infarcted (ie, infarction core)
- Estimating the extent of potentially salvageable brain tissue that is at risk for infarction (ie, ischemic penumbra)
The approach to imaging may differ according to individual patient characteristics (eg, time from stroke onset, potential candidate for reperfusion therapies) and local availability of stroke expertise and imaging capabilities.
- For example in patients suspected of ‘TIA’ who are asymptomatic (time is not brain) by the time of presentation to the hospital it may be reasonable to obtain brain MRI initially if available.
However, when a patient is a potential candidate for IV-tPA based on clinical characteristics and initial head CT, do not delay thrombolytic administration while waiting to perform more advanced imaging such as CTA.
NONCONTRAST HEAD CT: It is the most common imaging modality used for triage of ‘AIS’; since it is widely available, rapid, and can easily detect ‘ICH’. Most ‘AIS’ are not visualized by a non-contrast brain CT in the early hours of a stroke.Therefore, the utility of the first brain CT is primarily to exclude ‘ICH’, abscess, tumor, and other stroke mimics, as well as to detect current contraindications to thrombolytics (e.g. extensive regions of clear hypoattenuation)7.
👉Minor ischemic changes (i.e., early signs of infarction) on CT are not a contraindication to treatment; these include subtle or small areas of hypodensity, loss of gray-white distinction, obscuration of the lentiform nucleus, or the presence of a hyperdense artery sign (figure 7). In patients with a hyperdense MCA sign, IV-tPA can be beneficial.
👉IV-tPA is not beneficial in the presence of extensive regions of obvious hypodensity consistent with irreversible injury on initial head CT and is not recommended 6 (figure 7). Severe hypoattenuation is defined as obvious hypodensity which represents irreversible injury 6. These patients have a poor prognosis despite IV-tPA.
Brain MRI
Standard brain MRI protocols that include conventional T1-weighted, T2-weighted, FLAIR, DWI and the apparent diffusion coefficient (ADC) map; can reliably diagnose both ‘AIS’ and acute hemorrhagic stroke in emergency settings.
Major drawbacks of MRI are that it is not readily available and its use may be limited by contraindications (e.g. metal implant or pacemakers) or patient intolerance (i.e. claustrophobia). Newer ultrafast MRI protocols can reduce acquisition times from the 15 to 20 min required for conventional MRI to 5 min or less.
👉Brain MRI with DWI is superior to NCCT for the detection of acute infarction 9. It is the diagnostic gold standard in acute cerebrovascular syndrome to differentiate TIA from infarction and non-ischemic mimics. Up to 30% of suspected TIA patients with clinical resolution of symptoms will show a rule-in infarction on MRI (figure 9).
Moreover DWI can provide prognostic information in patients with ‘TIA’ 10 11 (more on this below).
DWI-ADC
Diffusion-weighted imaging (DWI) is a MRI technique in which contrast within the image is based on microscopic motion of water. Thus, it is more sensitive to early changes of cytotoxic or vasogenic damage at cellular level than traditional MRI measurements such as T1 or T2 relaxation rates.
- DWI is inheretently a T2 weight sequence.
- Structures with increased diffusion such as CSF will appear dark on DWI images.
- Lesions with decreased diffusion will appear bright (hyperintense signal).
- ADC maps provide pure information on diffusion without any T2 weighting.
- Structures with increased diffusion such as CSF will appear bright on ADC maps.
- Lesions with decreased diffusion will appear dark (hypointense signal).
Causes of decreased diffusion
- Failure of Na/K-ATPase: Acute ischemic stroke (see image below), necrotizing infections e.g. HSV, hypo-hyperglycemia, drug-induced encephalopathies like methotrexate
- Tissue vacuolization, spongiform changes: demyelination, dysmyelination, diffuse axonal injury.
- High protein concentration or increased viscosity: Pyogenic infection, hemorrhage
- Dense cell packing: neoplasm such as lymphoma, high grade glioma, small cell lung cancer metastases.
Ischemic changes on MRI
Hyperacute (0-6hrs)
- DWI-ADC: Within minutes of infarction, DWI demonstrates ↑signal (bright) and ↓ADC value (dark).
- Other MRI sequences: the affected parenchyma appears normal, although changes in flow will be detected (occlusion on MRA) and the thromboembolism may be detected (e.g. on SWI). Slow or stagnant flow in vessels may also be detected as a loss of normal flow void and high signal on T2/FLAIR.
Late hyperacute (6-24hrs)
- DWI-ADC: as described above.
- T2/FLAIR: Generally, after 6 hours, high T2 signal will be detected, initially more easily seen on FLAIR than conventional T2.
- T1: hypointensity is only seen after 16 hours and persists.
Acute (6-72hrs)
- DWI-ADC: the infarcted parenchyma continues to demonstrate ↑DWI signal (bright) and ↓ADC signal (dark).
- T2/FLAIR: remains hyperintense on T2 and FLAIR, with T2 signal progressively increasing during the first 5 days.
- T1 signal remains low.
Subacute (3 days-21 days)
- DWI-ADC: DWI remains elevated (bright) due to persistent high T2/FLAIR signal (T2 shine-through). In contrast ADC demonstrates pseudonormalization (becomes less dark) typically occurring between 5-14 days.
- T2 remains high and T1 signal remains low.
- Gadolinium-based MRI: After day 5 the cortex usually demonstrates contrast enhancement on T1 C+.
- Less common patterns of enhancement include arterial enhancement, encountered in approximately half of strokes and becomes evident after 3 days, and meningeal enhancement which is uncommon and is usually seen between 2 and 6 days.
Chronic (beyond 3 weeks)
- DWI-ADC: DWI become less intess and ultimately normalizes (isointense). The ADC becomes less dark and ultimately shows increased diffusivity (bright)
- T2 signal remains high and T1 signal remains low.
- Gadolinium-based MRI: Cortical contrast enhancement usually persists for 2 to 4 months. Importantly if parenchymal enhancement persists for more than 12 weeks the presence of an underlying lesion should be considered.
DWI-FLAIR mismatch: It refers to evidence of a hyperintense lesion on DWI consistent with acute infarction but no corresponding signal abnormality on the FLAIR images (figure 10). This mismatch indicates that the stroke is relatively acute (i.e. within 4.5 h), since insufficient time has passed for development of hyperintense signal on FLAIR, a sign of vasogenic edema. In some trials this DWI-FLAIR mismatch has been used to select patients for treatment with IV-tPA when the time of stroke onset is unwitnessed or unknown 12.
Susceptibility-weighted Imaging (SWI)
SWI is particularly sensitive to compounds which distort the local magnetic field. therefore it’s useful in detecting blood products, calcium, etc. SWI is the most sensitive sequences for depicting hemorrhagic transformation in patients with ischemic stroke.
Hemorrhagic transformation demonstrates a spectrum of findings ranging from small petechial areas of micro bleeding to large parenchymal hematoma.
- Micro bleeding is present in one-half to the majority of patients with ischemic stroke and is seen around 48 hours after onset of symptoms and are not associated with worse outcomes. Guidelines state that the presence of fewer than five areas of micro bleeding on initial MR images does not contraindicate thrombolysis because they are not associated with increased adverse outcomes.
- Parenchymal hematoma is a rarer type of hemorrhagic transformation that results from vessel wall rupture caused by high reperfusion pressure. It is more common with cardioembolic events, is associated with hyperglycemia, most commonly occurs in the basal ganglia, and confers a much worse prognosis.
👉Hemorrhagic transformation is rare in the first 12 hours after stroke onset (the hyperacute stage), particularly within the first 6 hours. When it occurs, it is usually within the first 24–48 hours and, in almost all cases, is present 4–5 days after stroke.
Vascular imaging: CTA/MRA
With the advent of endovascular therapies, identifying the presence of intracranial large-vessel occlusion is important for therapeutic decisions. CTA or MRA can detect these lesions 7 (figure 11).
- If a patient is a possible candidate for EVT, vascular imaging is recommended concurrently with the initial head CT; however, these additional studies should not delay thrombolytic administration.
- Practically speaking, if the clinical suspicion is high, and the resources are available, neurology and pharmacy can accompany the patient to the scanner, mix tPA, recheck blood pressure and administer thrombolysis as soon as the head CT rules out a bleed. Then, while tPA is running proceed with CTA head and neck.
- It is important to realize that in patients with no history of renal insufficiency, it is not necessary to have a serum creatinine result prior to performing contrasted studies for stroke, because these studies are not associated with significantly increased risk of acute kidney injury.
Who needs CTA?
If resources are available and patients meet the general criteria for mechanical thrombectomy, CTA should be performed
- All patients presenting within 0-4.5h of LSW.
- For patients presenting within 4.5 – 24h of LSW and NIHSS ≥6 or VAN positive or true LVO stroke syndrome described above.
Perfusion study
In acute ischemic stroke, the area of irreversible brain infarct (core) is surrounded by ischemic tissue (penumbra) that may potentially be salvageable (figure 12), regardless of the time of onset of symptoms.
- The infarct core refers to tissue which has already been damaged. Even if the vessel could be immediately opened, the core infarct would not recover.
- Radiological definition of core infarct is based on the development of cytotoxic edema, reflective of neuronal cells swelling. This cytotoxic edema causes a diffusion restriction on MRI, which is the reference standard for defining the core infarct.
- Ischemic penumbra is tissue which surrounds the core infarct. The ischemic penumbra is malperfused and nonfunctional, but the tissue is still potentially viable if blood supply can be restored. The ischemic penumbra is often maintained by a thin and slow flow of blood supplied via collateral circulation.
- The entire purpose of revascularization is to revive the ischemic penumbra. Alternatively, if the ischemic penumbra is small, then there is little more tissue to salvage; the stroke has already completed.
Perfusion study (Multimodal CT or MRI) can provide crucial information about the volume of tissue that is irreversibly damaged (infarction core) and the volume of tissue that is critically hypoperfused but potentially salvageable with reperfusion (ischemic penumbra) 7. This will guide further therapy for patients who fall outside the time ranges for thrombolysis or where the time of symptom onset is unclear.
- The mantra of stroke is that “time is brain.” However, this is only partially true, because different patients progress at different speeds over time. The pace of ischemic penumbra necrosis varies widely among patients and depends on degree of collateral circulation.
- Some patients may rapidly complete an infarction within hours; while others may continue to have large ischemic penumbra for many hours. The latter group may remain a good candidate for revascularization therapy beyond the traditional tPA window. Thus, 4.5 hours could have an entirely different meaning among different group of patients.
Moving from “time threshold” to “tissue threshold”
- With the advent of modern neuroimaging, it is possible to rapidly determine the amount of salvageable tissue. This may largely replace time cutoffs when considering candidacy for interventions.
CT-perfusion parameters
Cerebral blood flow (CBF) and time to maximum enhancement are among the most accurate values for use in acute stroke evaluation.
- Cerebral blood flow <30% is used to identify the core infarct.
- Maximal transit time (Tmax) relates to maximal time blood spends in a given region. Elevated maximal transit time using a cutoff of 6 seconds identifies both the core infarct and ischemic penumbra.
The key issue is the degree of mismatch between the two images:
- If the two images match up, then the volume of ischemic penumbra is small (completed infarct).
- If the ischemic core is much smaller, then the volume of ischemic penumbra must be large (implying significant salvageable brain tissue). The mismatch ratio of penumbra/core (ratios >1.8 suggest benefit from endovascular therapy).
Reperfusion Therapy
Ischemic CVA is a time-sensitive diagnosis (time is brain⏳) and the most important factor in successful reperfusion therapy of ‘AIS’ is early reperfusion for appropriate candidates.
The immediate goal of reperfusion treatment for ‘AIS’ is to salvage regions of the brain that are ischemic but not yet infarcted. The long term goal is to reduce stroke-related disability and mortality. The options for reperfusion therapy are intravenous thrombolytics and mechanical thrombectomy.
Intravenous Alteplase (tPA)
At the current time, alteplase is the only approved thrombolytic agent for ‘AIS’ treatment (if patients meet eligibility criteria).
BENEFIT: Several studies have shown the potential benefit of IV-tPA if administered within 4.5 hours of ischemic stroke symptom onset 13 14. It has been shown that earlier thrombolytic treatment was associated with reduced mortality and symptomatic intracranial hemorrhage and higher rates of independent ambulation at discharge.
RISK OF ICH: Using the National Institute of Neurological Disorders and Stroke (NINDS) definition for symptomatic intracranial hemorrhage (any hemorrhagic transformation temporally related to any neurologic worsening 15); IV-tPA is associated with increased risk of symptomatic intracranial hemorrhage (5-7%) 14. However this definition of ICH may be overly inclusive because it captures even small petechial hemorrhages associated with minimal neurologic deterioration that are unlikely to have altered long-term functional outcome.
In contrast by using a more clinically sensible definition of symptomatic intracerebral hemorrhage (including only those hemorrhages which are associated with clinical worsening of ≥4 points on NIHSS score 16), the rate of symptomatic intracranial hemorrhage was reported as 1.8% in other studies 17 .
Although several variables can affect the outcome (e.g. size and site of the clot 18 19, clot age and composition 20, age, sex, stroke severity 21 22), the only factor that has been shown to be independently associated with response to treatment is ‘TIME-TO-TREATMENT’. 13 14 23
DECISION MAKING
All adult patients with a clinical diagnosis of ‘AIS’ should be rapidly screened for treatment with IV-tPA. Simultaneously, patients with suspected ‘AIS’ involving the anterior circulation should be evaluated for mechanical thrombectomy.
BENCHMARK: A door-to-needle time of ≤60 minutes is the benchmark for achieving rapid treatment with IV-tPA (figure 6).
CONTRAINDICATION FOR THROMBOLYTIC: Assess for presence of contraindication for thrombolytic (figure 14)6.
The potential risks should be discussed during IV-tPA eligibility deliberation and weighed against the anticipated benefits during decision making.
A number of clinical issues may complicate the decision to use reperfusion therapy for ’AIS’. These are discussed here.
Considerations
Hematologic considerations 6
In most cases, the results of routine laboratory tests including coagulation parameters and platelet count are not required to proceed with intravenous alteplase treatment. IV-tPA should not be delayed while results are pending unless one of the following conditions is present:
- Clinical suspicion of a bleeding abnormality or thrombocytopenia
- Current or recent use of anticoagulants (eg, heparin, warfarin, direct oral anticoagulants [DOACs])
- Use of anticoagulants is not known
Alteplase could be considered when appropriate laboratory tests such as aPTT, INR, clotting time, thrombin time, or direct factor Xa activity assays are normal or when the patient has not taken a dose of these ACs for >48 h and renal function is normal.
The treatment should be discontinued if the result of the laboratory results were:
- PLT< 100.000
- INR>1.7
- PTT>40s
👉In otherwise eligible patients who were taking antiplatelet drug monotherapy or combination therapy before the stroke, IV alteplase is recommended.
Rapidly improving stroke symptoms: It should be considered an exclusion for reperfusion therapy only for patients who improve to the degree that any remaining deficits are non-disabling 24.
Severity of stroke: Large infarcts are defined as those that involve more than one third of the ‘MCA’ territory or more than one half of the ‘PCA’ territory based upon neuroimaging with CT or MRI. Though less reliable, large infarct size can also be defined clinically (eg, NIHSS score >15). It is worth mentioning here that although the severity of neurologic deficit on ‘NIHSS score’ is associated with intracranial hemorrhage 25; stroke severity alone cannot be used to select or exclude patients for intravenous thrombolysis 14 6 .
- Minor stroke: is defined as low points on NIHSS scoring system (NIHSS ≦5 score) in the absence of persistent disabling neurologic deficit. Any of the following should be considered disabling deficits
- Complete hemianopia: ≥2 on the National Institutes of Health Stroke Scale (NIHSS) question 3
- Severe aphasia: ≥2 on NIHSS question 9
- Visual extinction: ≥1 on NIHSS question 11
- Any weakness limiting sustained effort against gravity: ≥2 on NIHSS question 5 or 6
- Any deficits that lead to a total NIHSS >5
- Any remaining deficit considered potentially disabling by the patient, family, or the treating practitioner
- For patients with an NIHSS score of 0 to 5, a clearly disabling deficit has also been defined as one that would prevent the patient from performing basic activities of daily living (ie, bathing, walking, toileting, and eating) or returning to work 26
Imaging: Minor ischemic changes (ie, early signs of infarction) on CT are not a contraindication to treatment; these include subtle or small areas of hypodensity, loss of gray-white distinction, obscuration of the lentiform nucleus, or the presence of a hyperdense artery sign. In the presence of extensive regions of obvious hypodensity consistent with irreversible injury on initial head CT intravenous thrombolytic is not recommended.
Seizure at onset: IV alteplase is reasonable in patients with a seizure at the time of onset of acute stroke if evidence suggests that residual impairments are secondary to stroke and not a postictal phase.
Dural puncture: IV alteplase may be considered for patients who present with AIS, even in instances when they may have undergone a lumbar dural puncture in the preceding 7 days.
Specific situations
Timing
- Unwitnessed stroke onset and “wake-up” stroke: When the exact time of stroke onset is not known, it is defined as the last time the patient was known to be normal. For patients whose stroke symptoms are first noted upon awakening from sleep, the last time known to be normal may be the time they went to bed (if the patient can report this reliably) or the last time seen normal by a friend or family member. Such patients are not eligible for alteplase treatment unless the time last known to be normal is less than 4.5 hours. Imaging-based selection of patients with unknown stroke onset time for intravenous alteplase treatment is under investigation.When there is an ischemic parenchymal brain lesion on magnetic resonance imaging (MRI) diffusion-weighted imaging but no corresponding hyperintensity on fluid-attenuated inversion recovery (FLAIR), an imaging mismatch correlates with a stroke onset time of 4.5 hours or less 27.
- Although several small studies have shown promising results for imaging-based selection of patients for intravenous alteplase administration when time is not known, additional trials are needed to determine the efficacy and safety of this treatment 28.
- 3-4.5 hours: For otherwise eligible patients, the benefit of IV alteplase extends to 4.5 hours.However there are additional exclusion criteria for this time window (age >80 years old, an NIHSS score >25, a combination of previous stroke and diabetes, and oral anticoagulant use regardless of INR). However, these are not considered as absolute contraindications to intravenous alteplase treatment in the 3- to 4.5-hour time window, and alteplase is still beneficial in these patients.
- 4.5-6 hours: Patients within 4.5 to 6 hours from stroke symptom onset should not receive intravenous alteplase because harm may exceed benefit, but they should be evaluated to determine if they are candidates for mechanical thrombectomy.
- Beyond 6 hours: IV alteplase is not considered as a therapeutic option. However, mechanical thrombectomy using imaging-based selection (CTA, MRA) of patients with anterior circulation stroke who have were last known to be normal or at neurologic baseline 6 to 24 hours before treatment
- Beyond 24 hours: Patients beyond 24 hours from ischemic CVA symptoms are not eligible for either IV thrombolytic or mechanical thrombectomy.
Age ≧80 years: They still benefit from IV-tPA treatment despite a higher mortality rate compared with younger patients. For otherwise eligible patients, age is not a contraindication for treatment. However age ≧80 years is a relative contraindication in the 3-to 4.5-hour time window.
Concurrent acute myocardial infarction: For patients presenting with concurrent “AIS” and acute MI, treatment with IV-tPA at the dose appropriate for cerebral ischemia, followed by percutaneous coronary angioplasty and stenting if indicated, is reasonable.
Preexisting disability: Preexisting disability does not seem to independently increase the risk of ICH after IV alteplase, but it may be associated with less neurological improvement and higher mortality. Therapy with IV alteplase for acute stroke patients with preexisting disability (mRS score ≥2) may be reasonable, but decisions should take into account relevant factors, including quality of life, social support, place of residence, need for a caregiver, patients’ and families’ preferences, and goals of care.
Posterior circulation stroke: If otherwise eligible for IV-tPA, they should receive the treatment. Mechanical thrombectomy is beneficial for select patients with ‘AIS’ caused by a proximal intracranial arterial occlusion in the anterior circulation, but trials that established the benefit of mechanical thrombectomy largely excluded patients with posterior circulation infarcts. However, endovascular interventions for vertebrobasilar occlusions, including mechanical thrombectomy, may be treatment options for stroke centers with appropriate expertise.
Arterial dissection: Patients with cervicocephalic arterial dissection will benefit from reperfusion therapy (IV-tPA, and/or MT) if otherwise eligible 29. This does not include aortic dissection wherein tPA is absolutely contraindicated.
- Patients with cervicocerebral arterial dissection who are not candidates for reperfusion therapy, benefit from antiplatelet or antithrombotic agents, though the superiority of antithrombotic drugs over antiplatelet has not been shown 30.
Mechanical Thrombectomy (MT)
Patients with ‘AIS’ due to proximal large artery occlusion may not respond well to IV-tPA and eligibility for ‘MT’ should be considered simultaneously while evaluating for IV-tPA treatment. It is indicated for patients with ‘AIS’ due to a large artery occlusion in the anterior circulation who can be treated within 24 hours of the time last known to be well (ie, at neurologic baseline), regardless of whether they receive IV-tPA for the same ischemic stroke event.
Patients with ischemic stroke from large vessel occlusion should receive IV-tPA without delay, if eligible, even if ‘MT’ is being considered 6 . ‘MT’ treatment should then be started as quickly as possible 31, and should not be delayed to assess the response to IV-tPA.
Patient selection
General criteria: All patients should meet the general criteria for ‘MT’ which include:
- ‘MT’ can be performed within 24h of the symptom onset at a stroke center with appropriate expertise in the use of stent retrievers
- Vascular imaging(e.g. CTA) shows proximal large artery occlusion in the anterior circulation
- Persistent disabling neurologic deficit
- A small infarct core (i.e. limited signs of early ischemic change) on neuroimaging and absence of hemorrhage
Specific criteria: For patient selection within a certain time window certain eligibility criteria should be met.
- Within the 6 hours: For patients who can be treated within 6 hours of the symptom onset, the following criteria for ‘MT’ is adapted and modified from MR CLEAN trial 32:
- Age ≧18 y
- Pre-stroke disability score {modified Rankin Scale (mRS) score of ≤1}
- NIHSS score ≧ 6 or or any persistent neurologic deficit that is potentially disabling
- ASPECTS score ≥6 on noncontrast brain CT or DWI-MRI (figure 16)
- Intracranial arterial occlusion of the distal intracranial ICA, or the M1 or M2 segments of the MCA, or the A1 or A2 segments of ACA, demonstrated with CTA, MRA.
- 6 to 24 hours: The The DEFUSE 3 33 and DAWN 34 trials selected patients for treatment beyond 6 hours using imaging-based criteria which are mentioned in the following figure (figure 15).
Algorithm
Initial Management of Acute Ischemic Stroke
IV alteplase administration
“Time is brain”- The sooner intravenous alteplase treatment is initiated after ischemic stroke (in eligible patients), the more likely it is to be beneficial. Before initiating IV-tPA make sure that the following conditions are met 6:
- The diagnosis is acute ischemic stroke
- Treatment is commencing within the required 4.5 hour time window after the onset of symptoms, defined as the time last seen normal or at baseline
- There is a persistent, measurable neurologic deficit
- Eligibility criteria are met
- Serum glucose must be checked to rule out hypoglycemia as a cause of neurologic deficit.
- The noncontrast head CT or brain MRI is without hemorrhage or other contraindication
- Blood pressure parameters are met
- Two intravenous lines, preferably large bore, are in place
- Accurate body weight has been determined
All patients who have received IV alteplase for acute ischemic stroke should be admitted to an intensive care unit or dedicated stroke unit for at least 24 hours of close neurologic and cardiac monitoring 6.
Blood pressure management
The approach to blood pressure management in acute ischemic stroke is inherently different from hemorrhagic stroke (see here). The blood pressure targets 6 for patients with acute ischemic stroke and therapeutic options are shown in figure below.
In patients with acute ischemic stroke who are otherwise eligible for reperfusion therapy, the blood pressure should be acutely controlled to the level below 185/110 mm Hg (otherwise the patient cannot receive reperfusion therapy) 6. The blood pressure should be closely monitored during the treatment and within 24hours post-treatment (figure 7).
👉Generally reversible and titratable agents are best option, since they allow rapid and safe titration to the goal blood pressure (e.g. nicardipine, clevidipine, labetalol). However in patients who have comorbid conditions (e.g. aortic dissection, acute myocardial infarction, preeclampsia/eclampsia) different therapeutic regimens may be considered.
In patients who are not candidate for reperfusion therapy, the blood pressure should not be treated acutely unless the hypertension is extreme (SBP>220 or DBP>120 mmHg), or the patient has active ischemic coronary disease, heart failure, aortic dissection, hypertensive encephalopathy, or pre-eclampsia/eclampsia. When treatment is indicated, cautious lowering of blood pressure by approximately 15% during the first 24 hours after stroke onset is suggested.
👉Pain and anxiety both can cause elevated blood pressure. Before initiating antihypertensive medications make sure that pain and anxiety have been adequately treated especially among intubated patients.
Management of complications associated with thrombolytic
In patients undergoing fibrinolytic therapy, physicians should be prepared to treat potential emergent adverse effects, including bleeding complications and angioedema that may cause partial airway obstruction 6.
- Early neurological worsening: In patients treated with thrombolytics, the most feared complication is tPA related hemorrhage. However other possibilities for worsening neurologic function should be considered. This include
- Stroke related seizure
- Stuttering lacunar infarct
- Re-occlusion of a vessel
- Symptomatic intracerebral hemorrhage should be suspected in any patient who develops sudden neurologic deterioration, a decline in level of consciousness, new headache, nausea and vomiting, or a sudden rise in blood pressure after thrombolytic therapy is administered, especially within the first 24 hours of treatment. The management of patients with symptomatic ICH is summarized below (figure 19). More on management of intracerebral hemorrhage here.
- Blood pressure dependence
- Patient requires a threshold BP to maintain collateral perfusion; and BP falls below that threshold.
- If the head CT does not show a bleed, then it is important to determine if the patient has a BP-dependent exam. This can be accomplished by lowering the head of the bed and bolusing fluids and/or administering pressors. The goal is usually to achieving the last MAP documented before the patient had worsened.
- Systemic bleeding: Mild systemic bleeding usually occurs in the form of oozing from intravenous catheter sites, ecchymoses (especially under automated blood pressure cuffs), and gum bleeding; these complications do not require cessation of treatment 6.
- More serious bleeding, such as from the gastrointestinal or genitourinary system, may require discontinuation of alteplase depending on the severity.
- Rarely, patients who suffer stroke after a recent myocardial infarction can develop bleeding into the pericardium, resulting in life-threatening tamponade. Consequently, patients who become hypotensive after alteplase should be evaluated with urgent echocardiography 6.
- Angioedema: Rarely it may happen following tPA administration (~2%).
- This appears to be a form of bradykinin-mediated angioedema. More on this here.
- Management is summarized below (figure 20).
Other therapeutic measures
Antiplatelet therapy: Administration of aspirin (dose 160 to 300 mg) 6 is recommended in patients with AIS within 24 to 48 hours after onset. For those treated with IV alteplase, aspirin administration is generally delayed until 24 hours later. Note that aspirin, if and when indicated, can be given rectally if swallowing assessment is not performed or the patient has dysphagia
- Dual antiplatelet therapy (DAPT): In patients presenting with minor non-cardioembolic ischemic stroke (NIHSS score ≤5) who did not receive IV-tPA; treatment with dual antiplatelet therapy (aspirin and clopidogrel) started within 24 hours after symptom onset and continued for 21 days is effective in reducing recurrent ischemic stroke for a period of up to 90 days from symptom onset 35.
- Dual antiplatelet therapy is not generally recommended for patients with moderate or large strokes.
Anticoagulation for cardioembolic infarction in context of atrial fibrillation
- Anticoagulation may increase the risk of hemorrhagic transformation.
- General recommendations are to start oral anticoagulation with warfarin 4-14 days after the stroke 6 .
- Warfarin may be preferable to a direct oral anticoagulant (DOAC), since warfarin can be rapidly reversed if hemorrhagic transformation occurs. Bridging with heparin is not generally beneficial.
- For larger strokes with a higher risk of hemorrhagic transformation, it makes sense to delay anticoagulation initiation towards closer to the ~14 day mark.
Statin therapy: The utility of statin therapy during the acute phase of ischemic stroke is less well-studied; however several studies have shown that long-term intensive statin therapy is associated with a reduced risk of recurrent ischemic stroke and cardiovascular events. Therefore for patients with acute ischemic stroke starting statin treatment as soon oral medications can be used safely seems reasonable 36.
- High-intensity statin (e.g. atorvastatin 80 mg) is often recommended.
Fever: Hyperthermia (temperature > 38C) contributes to neuronal injury 6 in patients with acute stroke via several different mechanisms (more on this here). Besides treatment of fever, make sure to search for and exclude certain conditions including meningitis, subdural empyema, brain abscess, infective endocarditis and more common problems such as urinary tract infection, pneumonia.
Hypo/hyperglycemia: Both conditions (hypoglycemia and hyperglycemia) can mimic cerebrovascular accidents and also can cause neuronal injury through multiple mechanisms 6.
- Hypoglycemia ( blood sugar <60 mg/dL) should be corrected.
- Hyperglycemia is common in patients with acute stroke and is associated with poor functional outcome. It may be due to preexisting diabetes, newly diagnosed diabetes or stress hyperglycemia. Recent guidelines suggest to keep serum glucose in the range of 140 to 180 mg/dL. However several studies have shown that tight control of glucose with intravenous insulin does not improve functional outcome in patients with acute ischemic stroke.
Prophylaxis for deep vein thrombosis: VTE prophylaxis with thigh-length intermittent pneumatic compression (IPC), starting at admission, for patients within 72 hours of acute ischemic stroke onset who have restricted mobility is recommended in addition to routine care (hydration and aspirin) 6.
- Pharmacologic VTE prophylaxis can be administered for select patients within 48 hours of acute ischemic stroke onset who have restricted mobility. Exceptions include:
- DAPT- Patients with TIA or minor stroke who are being treated with dual antiplatelet therapy (DAPT)
- Patients receiving full-dose heparin or oral anticoagulation for another indication.
- Delay chemical DVT prophylaxis for 24 hours in patients treated with thrombolytics or successful endovascular thrombectomy.
- Option may include subcutaneous LMWH (e.g. enoxaparin 40mg daily) or subcutaneous low-dose unfractionated heparin (5000 units two to three times daily)
Seizure management
- Seizure prophylaxis is not recommended in acute ischemic stroke 6 . In “AIS” seizure is often due to hemorrhagic transformation.
- If seizure happens, it will be treated in usual fashion.
Determining The Stroke Subtypes
Treatment of patients with ‘AIS’ involves several phases. The initial phase of management includes medical stabilization and reperfusion therapy (IV-tPA and/or MT) if eligible.
The next phase of management includes determining the pathophysiology of the ‘AIS’ via performing a more comprehensive evaluation, so that secondary prevention of stroke could be implemented as soon as possible.
As already discussed (figure 4), the pathophysiology of the “AIS” and “ TIA”can be classified into (TOAST classification system 37):
- Large artery atherosclerosis
- Cardioembolism
- Small-vessel occlusion
- Stroke of other determined etiology
- Stroke of undetermined etiology
- Two or more causes identified
- Negative evaluation
- Incomplete evaluation
Determining the pathophysiology of ischemic stroke and “TIA” is typically accomplished by performing a comprehensive evaluation including:
- Cardiac monitoring for possible occult atrial fibrillation
- Echocardiography (TTE/TEE) to identify the possible source of cardioembolism (figure 4).
- Vascular study (Duplex ultrasound of neck, CTA or MRA of neck and head arteries) to determine the status of major cerebral vessels.
- Blood test including hypercoagulable studies in select patients.
However sometimes despite a thorough evaluation, the etiology of ischemic stroke is not well-defined; hence called cryptogenic stroke. It is defined as brain infarction that is not attributable to a source of definite cardioembolism, large artery atherosclerosis, or small artery disease despite a thorough vascular, cardiac, and serologic evaluation 38.
- The pathophysiology of cryptogenic stroke is likely heterogeneous. Proposed mechanisms include cardiac embolism secondary to occult paroxysmal atrial fibrillation, aortic atheromatous disease or other cardiac sources, paradoxical embolism from atrial septal abnormalities such as patent foramen ovale, hypercoagulable states, and preclinical or subclinical cerebrovascular disease.
- It has been shown that a significant proportion of cryptogenic strokes adhere to embolic infarct topography on brain imaging.
- Cryptogenic stroke is a diagnosis of exclusion.
- The acute management of cryptogenic stroke is similar to that of other ischemic stroke subtypes.
Secondary Prevention
Once the pathophysiology of the ischemic event is identified, then decision on treatment regimen for secondary prevention of ischemic attack can be made which include medical treatment (antiplatelets or antithrombotic agents) or vascular intervention (i.e. carotid revascularization). The general approach for secondary prevention of ischemic events in patients with ‘AIS’ and ‘TIA’ is provided in the following algorithm (figure 21). Keep in mind that this algorithm is intended to provide a general picture of using antiplatelet and antithrombotic drugs and the outlined steps may vary among patients. Refer to guidelines for the chosen drug and dosage in the selected patient 6 38.
Disposition
Admission
Almost all ‘AIS’ patients with persistent neurologic deficits, in addition to high-risk TIA patients, should be admitted to the hospital 6.
Patients receiving reperfusion therapies should be admitted to institutions with neurosurgical capabilities in the event of (HT) warranting emergency intervention.
Patients in extremis or with signs of increased intracranial pressure (hydrocephalus, midline shift, altered mental status, seizures) should be cared for in an intensive care unit (preferably one with neurocritical care expertise).
Discharge
Some patients with ‘TIA’ may be discharged home if certain criteria are met. As discussed earlier, the definition of ‘TIA’ has evolved to ‘tissue-based’ and virtually all patients who have a suspected TIA require urgent evaluation due to the high stroke risk associated with TIA as defined by this new definition.
The appropriate initial evaluation of ‘TIA’ may include basic laboratory studies that are suggested by the history and physical examination,EKG, brain imaging, and neurovascular imaging.
The nature and tempo of the symptoms and signs and a negative brain imaging (DWI-MRI) for infarction can suggest the brain ischemia i.e. ‘TIA’ as the cause of the patient’s signs and symptoms. However many other non-vascular etiologies can mimic ‘TIA’ which have been discussed earlier.
Upon clinical diagnosis of ‘TIA’, risk stratification should be performed to identify those patients who are at higher risk of developing ischemic stroke and therefore benefit from hospital admission and possible urgent intervention or treatment. It has been shown that the most clinically used prediction tool for risk stratification (ABCD2 score 39 ) does not provide an accurate estimate of stroke risk and clinical decisions based on an ABCD2 score cut-off are subject to significant misclassification error. Clinical judgment and integrating certain clinical and imaging features are more accurate in predicting risk of cerebrovascular events 40.
Symptom-based risk stratification identifies patients with high or moderate risk as:
- High risk symptoms: Unilateral weakness (face, arm, leg), slurred speech
- Moderate risk symptoms: Presence of any of the following symptoms and (absence of motor or language disturbance): Hemi-sensory deficits, monocular visual loss, hemi-visual field deficit, or presence of posterior circulation signs and symptoms(e.g. Dysarthria, diplopia, ataxia)
Time from symptom onset to health care presentation risk stratification 41
- High risk: < 48h
- Moderate risk: 48 hours to 2 weeks
- Low risk: more than 2 weeks
Other clinical features associated with high risk ‘TIA’ include:
- Known significant carotid stenosis
- Patients with diabetes
- Crescendo TIA
- Large ischemic stroke in the past
- Suspected embolic source (e.g. AF, DCM, valvular heart disease, recent acute myocardial infarction)
- Positive initial DWI-MRI imaging for infarction 42 43 44
After comprehensive evaluation, some patients without high risk feature who meet all of the following criteria may be safely discharged home :
- The patient’s symptoms are completely resolved and they have not been stuttering or crescendo-like, suggesting an unstable process.
- Neuroimaging is reassuring.
- Vessel imaging of the head and neck does not reveal new significant/critical large vessel stenosis or occlusion warranting immediate intervention.
- The patient does not have evidence of a clear and unaddressed cardioembolic etiology (such as atrial fibrillation).
- Early specialty follow-up (preferably with a vascular neurologist) can be arranged.
Media
Imaging of acute ischemic stroke
Evolution from acute to chronic
Going further
- TIA Update (Emergency medicine case)
- ED stroke management in the age of endovascular therapy (Emergency medicine cases)
- NeuroEMCrit- Acute ischemic stroke part I
- Ischemic Stroke (EM:RAP)
References
1. Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson LM, Truelsen T, O’Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C; Global Burden of Diseases, Injuries, Risk Factors Study 2010 (GBD 2010); GBD Stroke Experts Group. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013 Nov;1(5):e259-81. doi: 10.1016/S2214-109X(13)70089-5. Epub 2013 Oct 24. PMID: 25104492; PMCID: PMC4181351
2. Ishihara T, Sato S, Uehara T, Ohara T, Hayakawa M, Kimura K, Okada Y, Hasegawa Y, Tanahashi N, Suzuki A, Nakagawara J, Arii K, Nagahiro S, Ogasawara K, Uchiyama S, Matsumoto M, Iihara K, Toyoda K, Minematsu K; PROMISE-TIA Study Investigators. Significance of Nonfocal Symptoms in Patients With Transient Ischemic Attack. Stroke. 2018 Aug;49(8):1893-1898. doi: 10.1161/STROKEAHA.118.022009. PMID: 30012818
3. Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, Hatsukami TS, Higashida RT, Johnston SC, Kidwell CS, Lutsep HL, Miller E, Sacco RL; American Heart Association; American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Interdisciplinary Council on Peripheral Vascular Disease. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009 Jun;40(6):2276-93. doi: 10.1161/STROKEAHA.108.192218. Epub 2009 May 7. PMID: 19423857
4. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013 Jul;44(7):2064-89. doi: 10.1161/STR.0b013e318296aeca. Epub 2013 May 7. Erratum in: Stroke. 2019 Aug;50(8):e239. PMID: 23652265.
5. Goyal N, Tsivgoulis G, Male S, Metter EJ, Iftikhar S, Kerro A, Chang JJ, Frey JL, Triantafyllou S, Papadimitropoulos G, Abedi V, Alexandrov AW, Alexandrov AV, Zand R. FABS: An Intuitive Tool for Screening of Stroke Mimics in the Emergency Department. Stroke. 2016
6. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-e418. doi: 10.1161/STR.0000000000000211. Epub 2019 Oct 30. Erratum in: Stroke. 2019 Dec;50(12):e440-e441. PMID: 31662037
7. Potter CA, Vagal AS, Goyal M, Nunez DB, Leslie-Mazwi TM, Lev MH. CT for Treatment Selection in Acute Ischemic Stroke: A Code Stroke Primer. Radiographics. 2019 Oct;39(6):1717-1738. doi: 10.1148/rg.2019190142. PMID: 31589578
8. Kamalian S, Lev MH. Stroke Imaging. Radiol Clin North Am. 2019 Jul;57(4):717-732. doi: 10.1016/j.rcl.2019.02.001. Epub 2019 Apr 8. PMID: 31076028.
9. Schellinger PD, Bryan RN, Caplan LR, Detre JA, Edelman RR, Jaigobin C, Kidwell CS, Mohr JP, Sloan M, Sorensen AG, Warach S; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Evidence-based guideline: The role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010 Jul 13;75(2):177-85. doi: 10.1212/WNL.0b013e3181e7c9dd. Erratum in: Neurology. 2010 Sep 7;75(10):938. PMID: 20625171; PMCID: PMC2905927
10. Wen HM, Lam WW, Rainer T, Fan YH, Leung TW, Chan YL, Wong KS. Multiple acute cerebral infarcts on diffusion-weighted imaging and risk of recurrent stroke. Neurology. 2004 Oct 12;63(7):1317-9. doi: 10.1212/01.wnl.0000140490.22251.b6. PMID: 15477564.
11. Sylaja PN, Coutts SB, Subramaniam S, Hill MD, Eliasziw M, Demchuk AM; VISION Study Group. Acute ischemic lesions of varying ages predict risk of ischemic events in stroke/TIA patients. Neurology. 2007 Feb 6;68(6):415-9. doi: 10.1212/01.wnl.0000252938.76188.52. PMID: 17283315
12. Thomalla G, Fiebach JB, Østergaard L, Pedraza S, Thijs V, Nighoghossian N, Roy P, Muir KW, Ebinger M, Cheng B, Galinovic I, Cho TH, Puig J, Boutitie F, Simonsen CZ, Endres M, Fiehler J, Gerloff C; WAKE-UP investigators. A multicenter, randomized, double-blind, placebo-controlled trial to test efficacy and safety of magnetic resonance imaging-based thrombolysis in wake-up stroke (WAKE-UP). Int J Stroke. 2014 Aug;9(6):829-36. doi: 10.1111/ijs.12011. Epub 2013 Mar 12. PMID: 23490032
13. Saver JL, Fonarow GC, Smith EE, Reeves MJ, Grau-Sepulveda MV, Pan W, Olson DM, Hernandez AF, Peterson ED, Schwamm LH. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013 Jun 19;309(23):2480-8. doi: 10.1001/jama.2013.6959. PMID: 23780461
14. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, Grotta J, Howard G, Kaste M, Koga M, von Kummer R, Lansberg M, Lindley RI, Murray G, Olivot JM, Parsons M, Tilley B, Toni D, Toyoda K, Wahlgren N, Wardlaw J, Whiteley W, del Zoppo GJ, Baigent C, Sandercock P, Hacke W; Stroke Thrombolysis Trialists’ Collaborative Group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014 Nov 29;384(9958):1929-35. doi: 10.1016/S0140-6736(14)60584-5. Epub 2014 Aug 5. PMID: 25106063; PMCID: PMC4441266
15. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995 Dec 14;333(24):1581-7. doi: 10.1056/NEJM199512143332401. PMID: 7477192
16. Mazya M, Egido JA, Ford GA, Lees KR, Mikulik R, Toni D, Wahlgren N, Ahmed N; SITS Investigators. Predicting the risk of symptomatic intracerebral hemorrhage in ischemic stroke treated with intravenous alteplase: safe Implementation of Treatments in Stroke (SITS) symptomatic intracerebral hemorrhage risk score. Stroke. 2012 Jun;43(6):1524-31. doi: 10.1161/STROKEAHA.111.644815. Epub 2012 Mar 22. Erratum in: Stroke. 2012 Sep;43(9):e102. PMID: 22442178
17. Mazya M, Egido JA, Ford GA, Lees KR, Mikulik R, Toni D, Wahlgren N, Ahmed N; SITS Investigators. Predicting the risk of symptomatic intracerebral hemorrhage in ischemic stroke treated with intravenous alteplase: safe Implementation of Treatments in Stroke (SITS) symptomatic intracerebral hemorrhage risk score. Stroke. 2012 Jun;43(6):1524-31. doi: 10.1161/STROKEAHA.111.644815. Epub 2012 Mar 22. Erratum in: Stroke. 2012 Sep;43(9):e102. PMID: 22442178
18. Seners P, Turc G, Maïer B, Mas JL, Oppenheim C, Baron JC. Incidence and Predictors of Early Recanalization After Intravenous Thrombolysis: A Systematic Review and Meta-Analysis. Stroke. 2016 Sep;47(9):2409-12. doi: 10.1161/STROKEAHA.116.014181. Epub 2016 Jul 26. PMID: 27462117
19. Molina CA, Montaner J, Arenillas JF, Ribo M, Rubiera M, Alvarez-Sabín J. Differential pattern of tissue plasminogen activator-induced proximal middle cerebral artery recanalization among stroke subtypes. Stroke. 2004 Feb;35(2):486-90. doi: 10.1161/01.STR.0000110219.67054.BF. Epub 2004 Jan 5. PMID: 14707233
20. Overgaard K. Thrombolytic therapy in experimental embolic stroke. Cerebrovasc Brain Metab Rev. 1994 Fall;6(3):257-86. PMID: 7811566.
21. Whiteley WN, Emberson J, Lees KR, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, Grotta J, Howard G, Kaste M, Koga M, von Kummer R, Lansberg MG, Lindley RI, Lyden P, Olivot JM, Parsons M, Toni D, Toyoda K, Wahlgren N, Wardlaw J, Del Zoppo GJ, Sandercock P, Hacke W, Baigent C; Stroke Thrombolysis Trialists’ Collaboration. Risk of intracerebral haemorrhage with alteplase after acute ischaemic stroke: a secondary analysis of an individual patient data meta-analysis. Lancet Neurol. 2016 Aug;15(9):925-933. doi: 10.1016/S1474-4422(16)30076-X. Epub 2016 Jun 8. PMID: 27289487
22. Whiteley WN, Slot KB, Fernandes P, Sandercock P, Wardlaw J. Risk factors for intracranial hemorrhage in acute ischemic stroke patients treated with recombinant tissue plasminogen activator: a systematic review and meta-analysis of 55 studies. Stroke. 2012 Nov;43(11):2904-9. doi: 10.1161/STROKEAHA.112.665331. Epub 2012 Sep 20. PMID: 22996959
23. Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: a systematic review. JAMA. 2015 Apr 14;313(14):1451-62. doi: 10.1001/jama.2015.3058. PMID: 25871671
24. Re-examining Acute Eligibility for Thrombolysis (TREAT) Task Force:, Levine SR, Khatri P, Broderick JP, Grotta JC, Kasner SE, Kim D, Meyer BC, Panagos P, Romano J, Scott P; NINDS rt-PA Stroke Trial Investigators. Review, historical context, and clarifications of the NINDS rt-PA stroke trials exclusion criteria: Part 1: rapidly improving stroke symptoms. Stroke. 2013 Sep;44(9):2500-5. doi: 10.1161/STROKEAHA.113.000878. Epub 2013 Jul 11. Erratum in: Stroke. 2014 Mar;45(3):e52. PMID: 23847249; PMCID: PMC3789593
25. Whiteley WN, Emberson J, Lees KR, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, Grotta J, Howard G, Kaste M, Koga M, von Kummer R, Lansberg MG, Lindley RI, Lyden P, Olivot JM, Parsons M, Toni D, Toyoda K, Wahlgren N, Wardlaw J, Del Zoppo GJ, Sandercock P, Hacke W, Baigent C; Stroke Thrombolysis Trialists’ Collaboration. Risk of intracerebral haemorrhage with alteplase after acute ischaemic stroke: a secondary analysis of an individual patient data meta-analysis. Lancet Neurol. 2016 Aug;15(9):925-933. doi: 10.1016/S1474-4422(16)30076-X. Epub 2016 Jun 8. PMID: 27289487
26. Khatri P, Kleindorfer DO, Devlin T, Sawyer RN Jr, Starr M, Mejilla J, Broderick J, Chatterjee A, Jauch EC, Levine SR, Romano JG, Saver JL, Vagal A, Purdon B, Devenport J, Pavlov A, Yeatts SD; PRISMS Investigators. Effect of Alteplase vs Aspirin on Functional Outcome for Patients With Acute Ischemic Stroke and Minor Nondisabling Neurological Deficits: The PRISMS Randomized Clinical Trial. JAMA. 2018 Jul 10;320(2):156-166. doi: 10.1001/jama.2018.8496. PMID: 29998337; PMCID: PMC6583516
27. Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, Cheripelli B, Cho TH, Fazekas F, Fiehler J, Ford I, Galinovic I, Gellissen S, Golsari A, Gregori J, Günther M, Guibernau J, Häusler KG, Hennerici M, Kemmling A, Marstrand J, Modrau B, Neeb L, Perez de la Ossa N, Puig J, Ringleb P, Roy P, Scheel E, Schonewille W, Serena J, Sunaert S, Villringer K, Wouters A, Thijs V, Ebinger M, Endres M, Fiebach JB, Lemmens R, Muir KW, Nighoghossian N, Pedraza S, Gerloff C; WAKE-UP Investigators. MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset. N Engl J Med. 2018 Aug 16;379(7):611-622. doi: 10.1056/NEJMoa1804355. Epub 2018 May 16. PMID: 29766770
28. Marshall RS. Image-Guided Intravenous Alteplase for Stroke – Shattering a Time Window. N Engl J Med. 2019 May 9;380(19):1865-1866. doi: 10.1056/NEJMe1904791. PMID: 31067378
29. Engelter ST, Dallongeville J, Kloss M, Metso TM, Leys D, Brandt T, Samson Y, Caso V, Pezzini A, Sessa M, Beretta S, Debette S, Grond-Ginsbach C, Metso AJ, Thijs V, Lamy C, Medeiros E, Martin JJ, Bersano A, Tatlisumak T, Touzé E, Lyrer PA; Cervical Artery Dissection and Ischaemic Stroke Patients-Study Group. Thrombolysis in cervical artery dissection–data from the Cervical Artery Dissection and Ischaemic Stroke Patients (CADISP) database. Eur J Neurol. 2012 Sep;19(9):1199-206. doi: 10.1111/j.1468-1331.2012.03704.x. Epub 2012 Mar 26. PMID: 22448957
30. Chowdhury MM, Sabbagh CN, Jackson D, Coughlin PA, Ghosh J. Antithrombotic treatment for acute extracranial carotid artery dissections: a meta-analysis. Eur J Vasc Endovasc Surg. 2015 Aug;50(2):148-56. doi: 10.1016/j.ejvs.2015.04.034. Epub 2015 Jun 21. PMID: 26109428.
31. ahan R, Saver JL, Schwamm LH, Fonarow GC, Liang L, Matsouaka RA, Xian Y, Holmes DN, Peterson ED, Yavagal D, Smith EE. Association Between Time to Treatment With Endovascular Reperfusion Therapy and Outcomes in Patients With Acute Ischemic Stroke Treated in Clinical Practice. JAMA. 2019 Jul 16;322(3):252-263. doi: 10.1001/jama.2019.8286. PMID: 31310296; PMCID: PMC6635908
32. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama à Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg-Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015 Jan 1;372(1):11-20. doi: 10.1056/NEJMoa1411587. Epub 2014 Dec 17. Erratum in: N Engl J Med. 2015 Jan 22;372(4):394. PMID: 25517348
33. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG; DEFUSE 3 Investigators. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018 Feb 22;378(8):708-718. doi: 10.1056/NEJMoa1713973. Epub 2018 Jan 24. PMID: 29364767; PMCID: PMC6590673.
34. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG; DAWN Trial Investigators. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018 Jan 4;378(1):11-21. doi: 10.1056/NEJMoa1706442. Epub 2017 Nov 11. PMID: 29129157.
35. Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, Kim AS, Lindblad AS, Palesch YY; Clinical Research Collaboration, Neurological Emergencies Treatment Trials Network, and the POINT Investigators. Clopidogrel and Aspirin in Acute Ischemic Stroke and High-Risk TIA. N Engl J Med. 2018 Jul 19;379(3):215-225. doi: 10.1056/NEJMoa1800410. Epub 2018 May 16. PMID: 29766750; PMCID: PMC6193486
36. Cappellari M, Bovi P, Moretto G, Zini A, Nencini P, Sessa M, Furlan M, Pezzini A, Orlandi G, Paciaroni M, Tassinari T, Procaccianti G, Di Lazzaro V, Bettoni L, Gandolfo C, Silvestrelli G, Rasura M, Martini G, Melis M, Calloni MV, Chiodo-Grandi F, Beretta S, Guarino M, Altavista MC, Marcheselli S, Galletti G, Adobbati L, Del Sette M, Mancini A, Orrico D, Monaco S, Cavallini A, Sciolla R, Federico F, Scoditti U, Brusaferri F, Grassa C, Specchio L, Bongioanni MR, Sparaco M, Zampolini M, Greco G, Colombo R, Passarella B, Adami A, Consoli D, Toni D. The THRombolysis and STatins (THRaST) study. Neurology. 2013 Feb 12;80(7):655-61. doi: 10.1212/WNL.0b013e318281cc83. Epub 2013 Jan 23. PMID: 23345634; PMCID: PMC3590058.
37. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993 Jan;24(1):35-41. doi: 10.1161/01.str.24.1.35. PMID: 7678184
38. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL; American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018 Mar;49(3):e46-e110. doi: 10.1161/STR.0000000000000158. Epub 2018 Jan 24. Erratum in: Stroke. 2018 Mar;49(3):e138. Erratum in: Stroke. 2018 Apr 18;: PMID: 29367334
39. Johnston SC, Rothwell PM, Nguyen-Huynh MN, Giles MF, Elkins JS, Bernstein AL, Sidney S. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007 Jan 27;369(9558):283-92. doi: 10.1016/S0140-6736(07)60150-0. PMID: 17258668
40. Wardlaw JM, Brazzelli M, Chappell FM, Miranda H, Shuler K, Sandercock PA, Dennis MS. ABCD2 score and secondary stroke prevention: meta-analysis and effect per 1,000 patients triaged. Neurology. 2015 Jul 28;85(4):373-80. doi: 10.1212/WNL.0000000000001780. Epub 2015 Jul 1. PMID: 26136519; PMCID: PMC4520819
41. Lin K, Lindsay P, Shams T, Smith E, Boulanger JM, Butcher K, Gubitz G, Lang E. A summary of the Canadian Stroke Best Practice Recommendations, Sixth Edition (2018): Updates relevant to prehospital and emergency medicine providers. CJEM. 2018 Sep;20(5):685-692. doi: 10.1017/cem.2018.438. PMID: 30990157
42. Calvet D, Touzé E, Oppenheim C, Turc G, Meder JF, Mas JL. DWI lesions and TIA etiology improve the prediction of stroke after TIA. Stroke. 2009 Jan;40(1):187-92. doi: 10.1161/STROKEAHA.108.515817. Epub 2008 Nov 6. PMID: 18988917
43. Crisostomo RA, Garcia MM, Tong DC. Detection of diffusion-weighted MRI abnormalities in patients with transient ischemic attack: correlation with clinical characteristics. Stroke. 2003 Apr;34(4):932-7. doi: 10.1161/01.STR.0000061496.00669.5E. Epub 2003 Mar 13. PMID: 12637695
44. Ay H, Oliveira-Filho J, Buonanno FS, Schaefer PW, Furie KL, Chang YC, Rordorf G, Schwamm LH, Gonzalez RG, Koroshetz WJ. ‘Footprints’ of transient ischemic attacks: a diffusion-weighted MRI study. Cerebrovasc Dis. 2002;14(3-4):177-86. doi: 10.1159/000065682. PMID: 12403950.
45. Rennert RC, Wali AR, Steinberg JA, et al. Epidemiology, Natural History, and Clinical Presentation of Large Vessel Ischemic Stroke. Neurosurgery. 2019;85(suppl_1):S4-S8. doi:10.1093/neuros/nyz042
46. Teleb MS, Ver Hage A, Carter J, Jayaraman MV, McTaggart RA. Stroke vision, aphasia, neglect (VAN) assessment-a novel emergent large vessel occlusion screening tool: pilot study and comparison with current clinical severity indices. J Neurointerv Surg. 2017 Feb;9(2):122-126. doi: 10.1136/neurintsurg-2015-012131. Epub 2016 Feb 17. PMID: 26891627; PMCID: PMC5284468.
47. Navalkele D, Vahidy F, Kendrick S, Traylor A, Haydel M, Drury S, Martin-Schild S. Vision, Aphasia, Neglect Assessment for Large Vessel Occlusion Stroke. J Stroke Cerebrovasc Dis. 2020 Jan;29(1):104478. doi: 10.1016/j.jstrokecerebrovasdis.2019.104478. Epub 2019 Nov 6. PMID: 31704124.



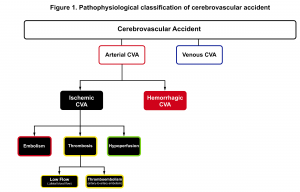
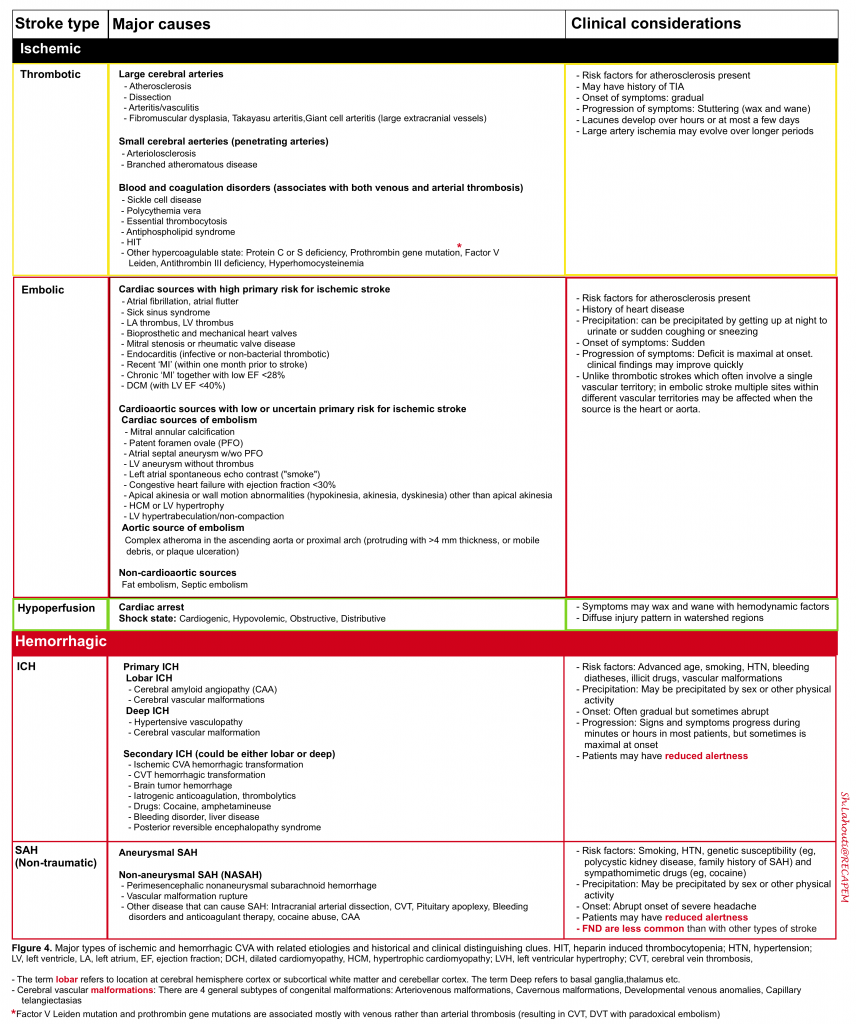
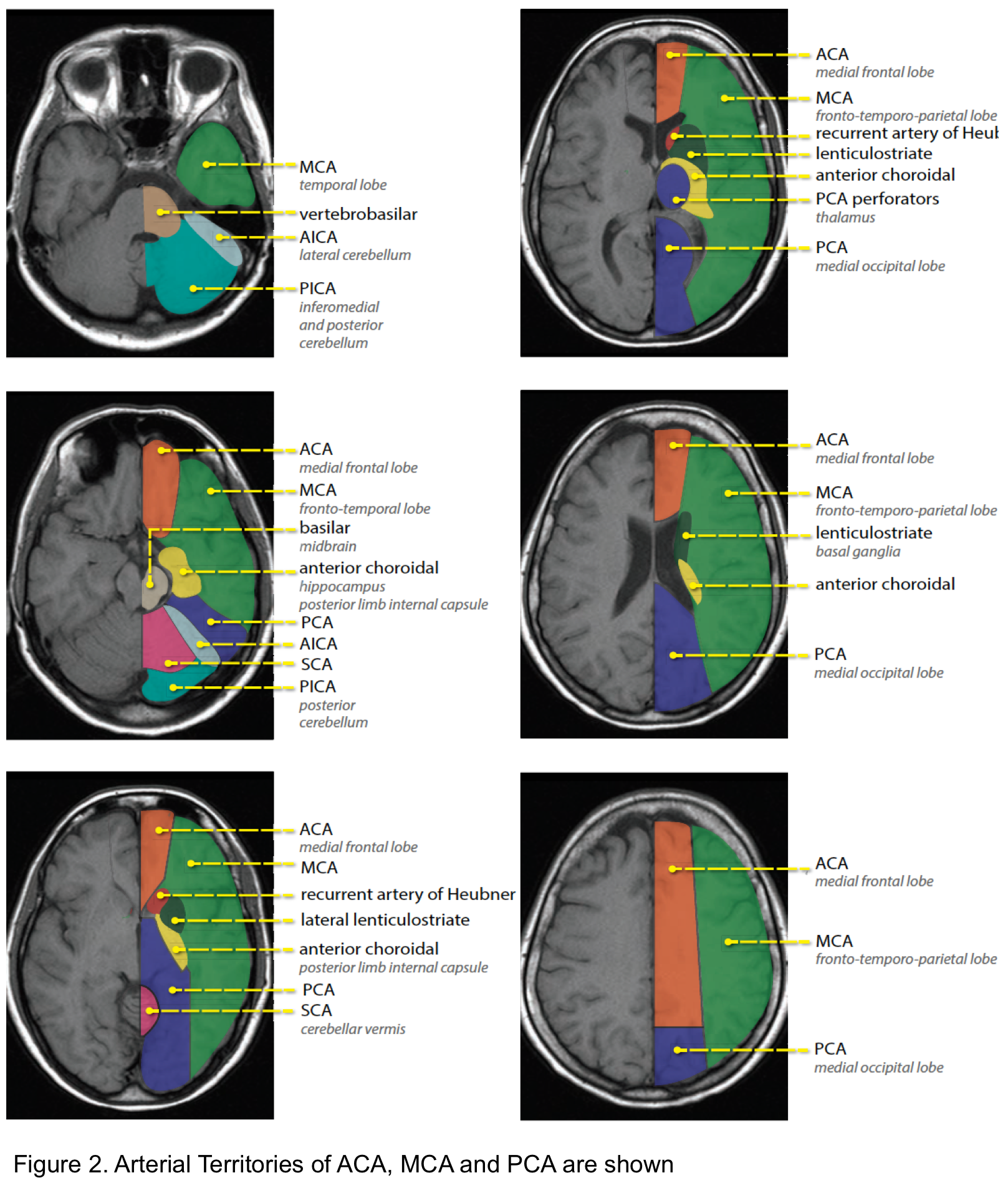
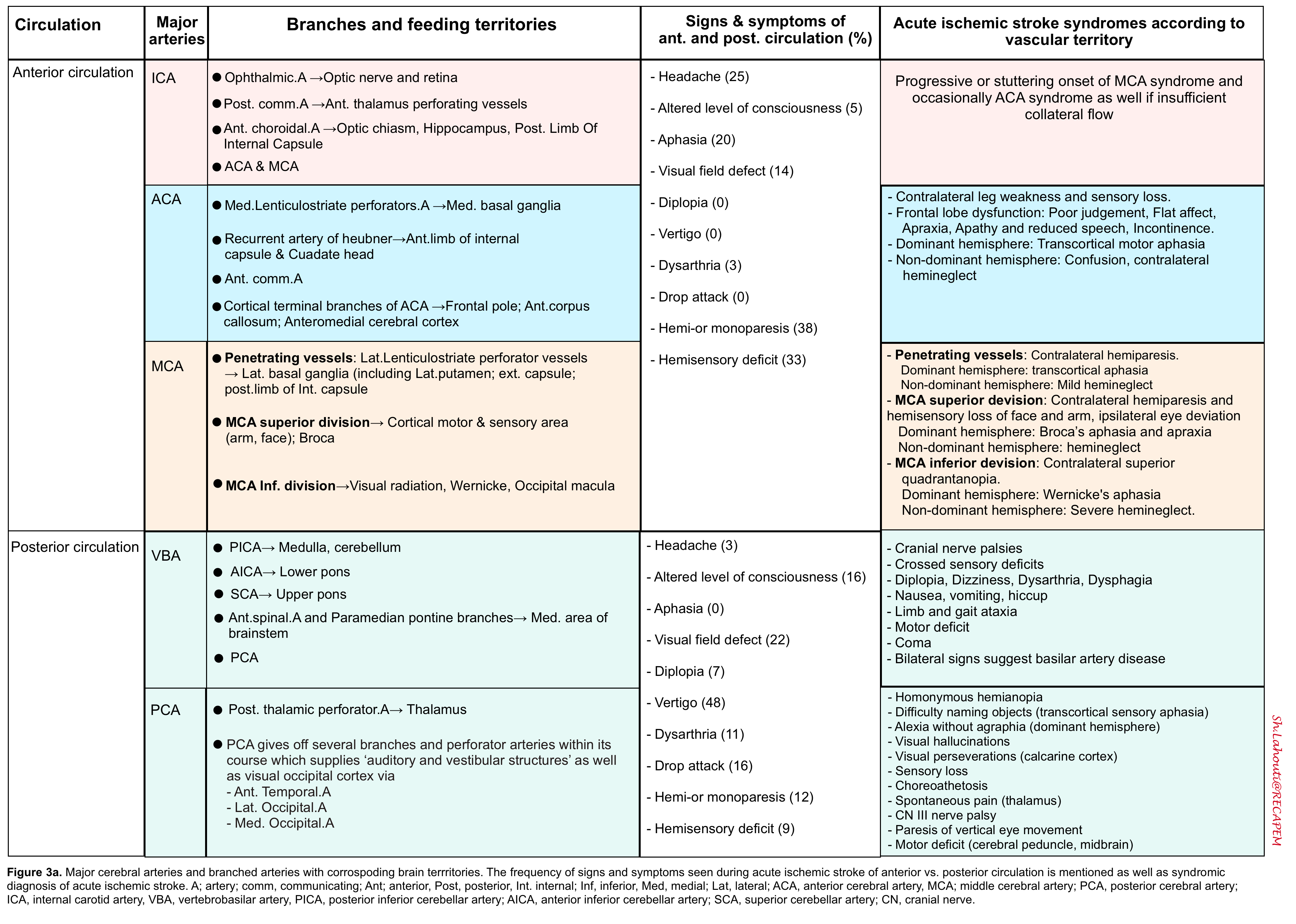
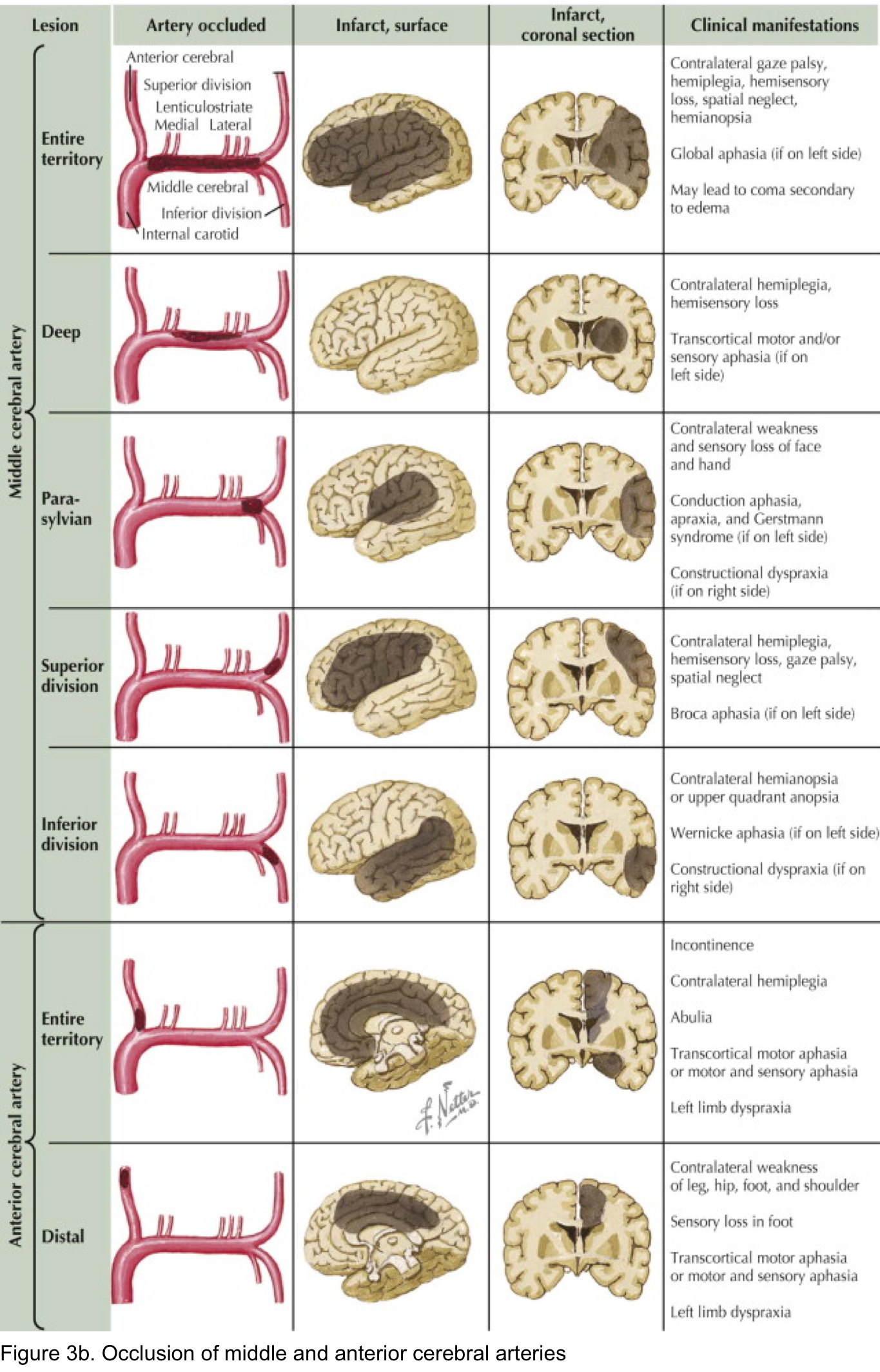
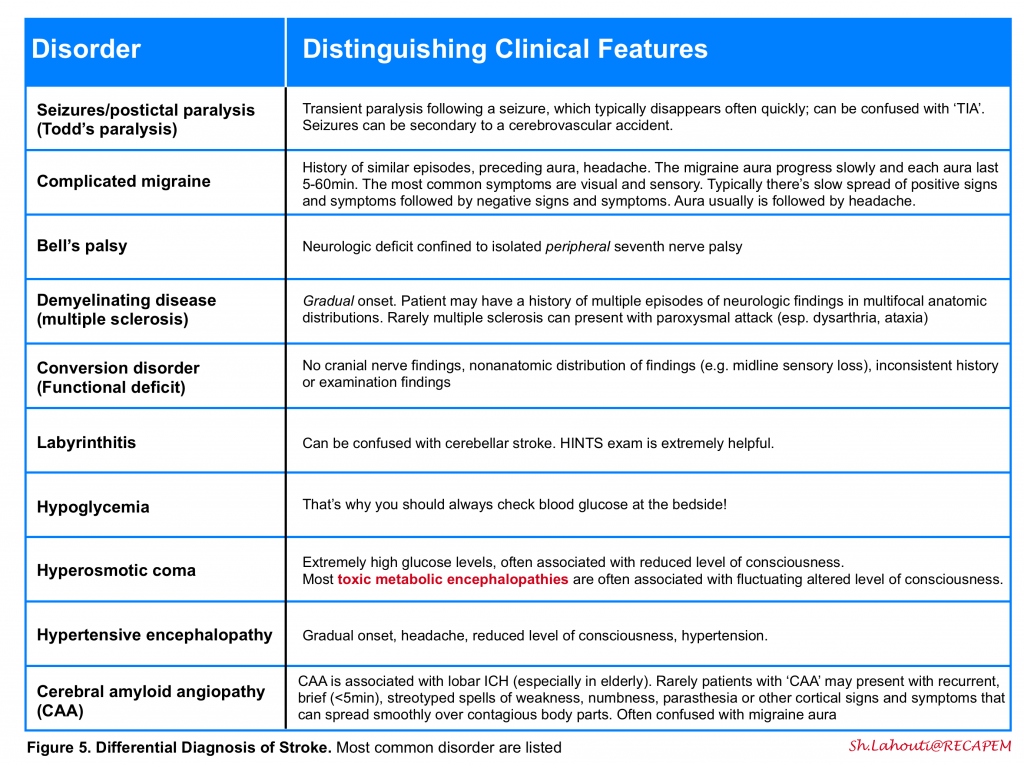
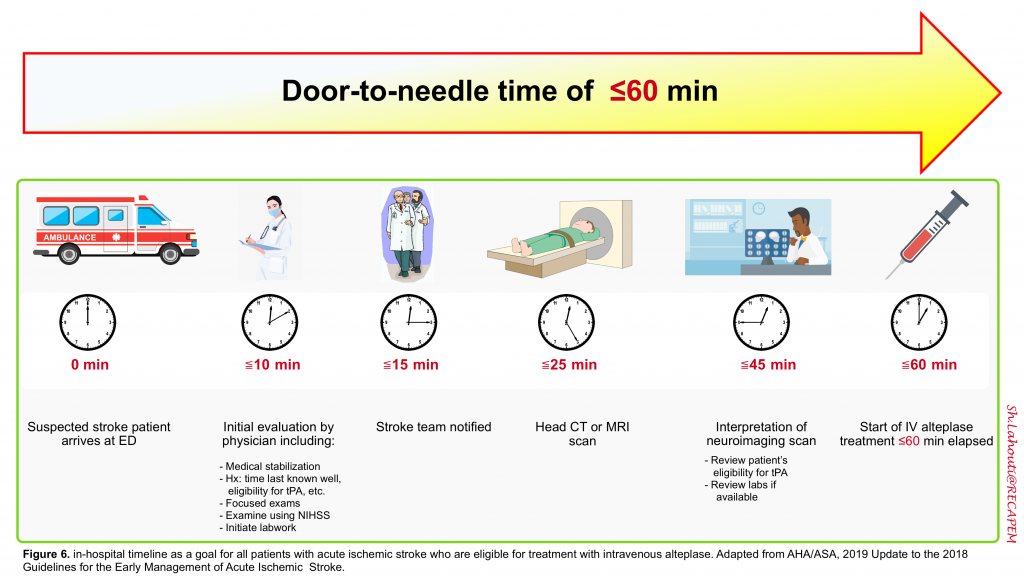
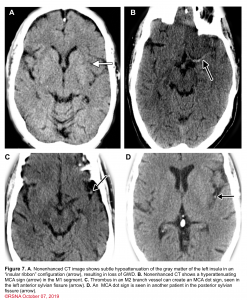
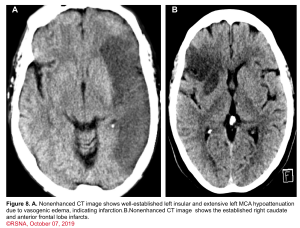
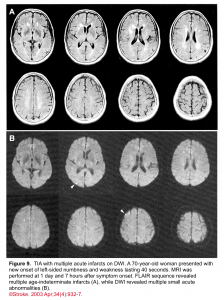
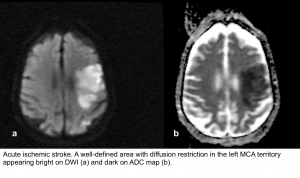
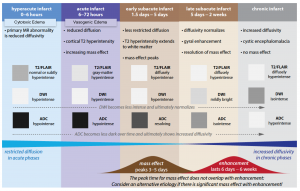
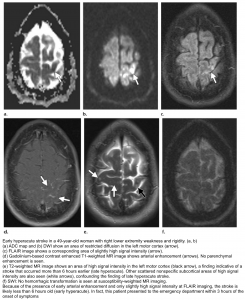
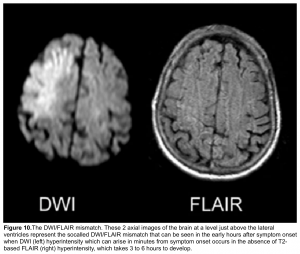
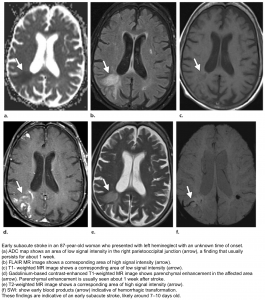
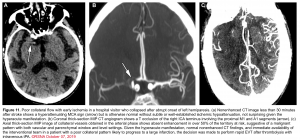
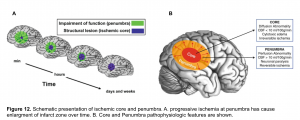
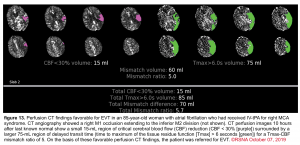
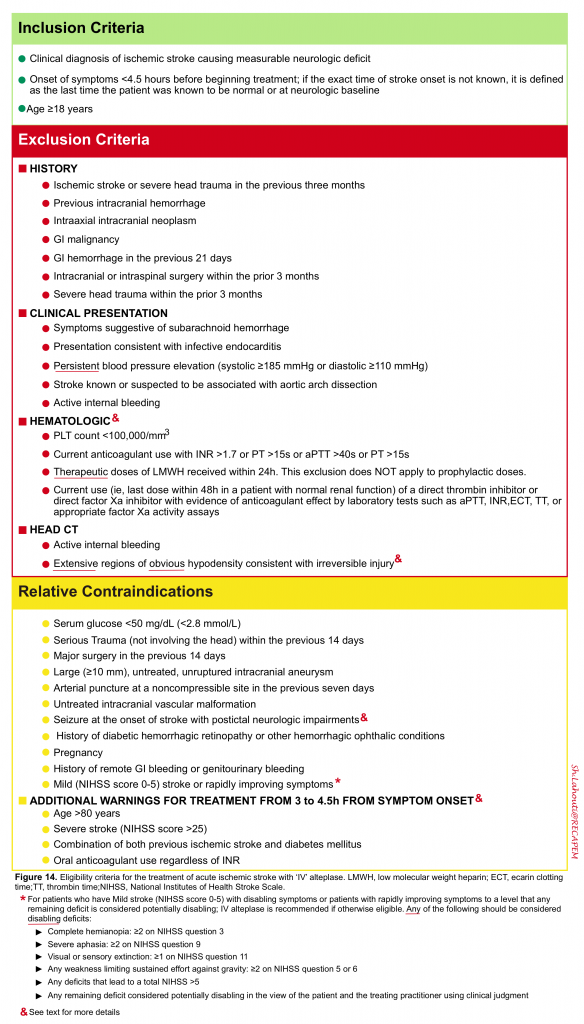
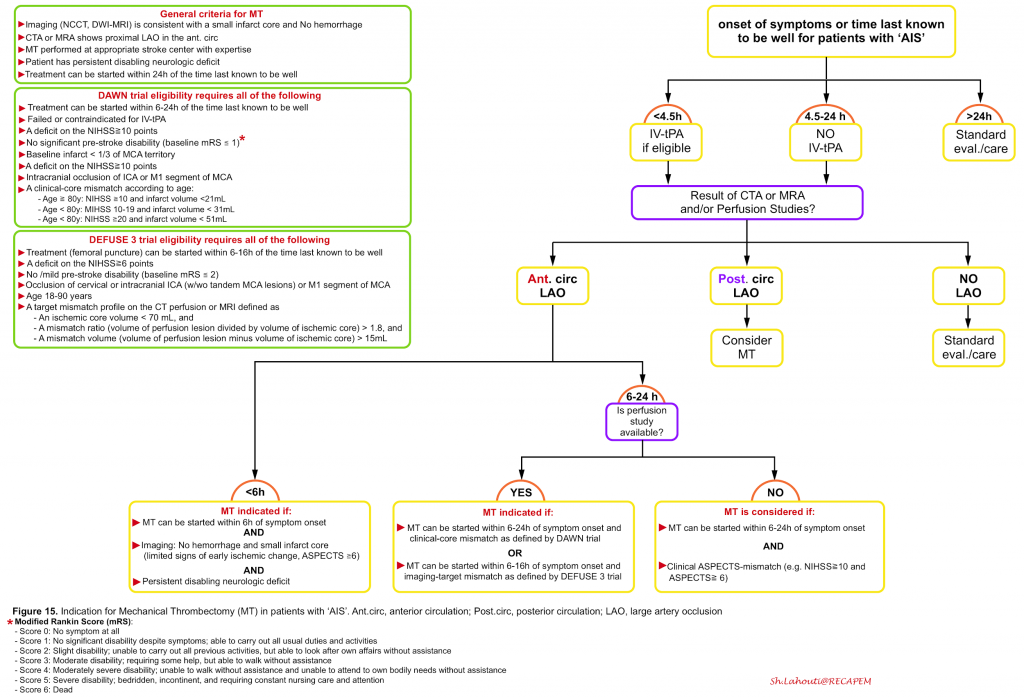
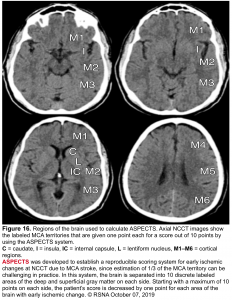
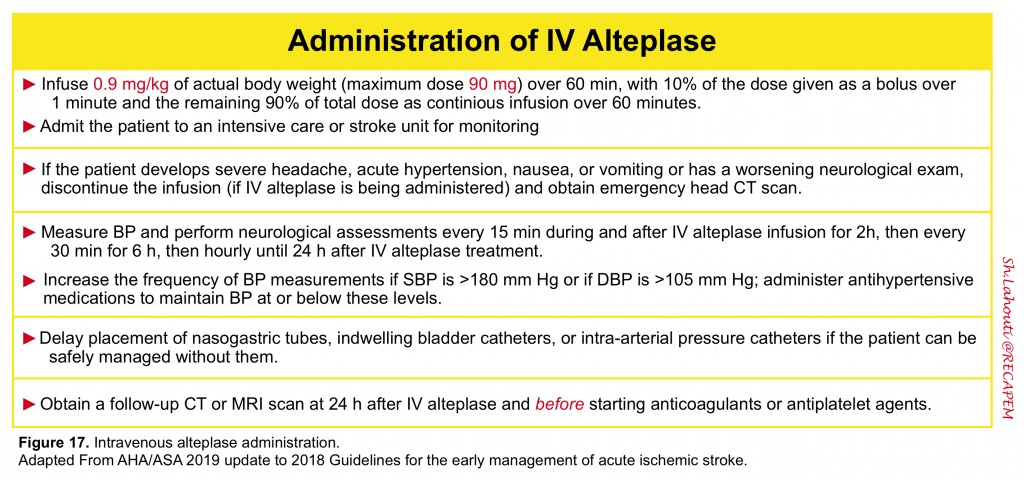

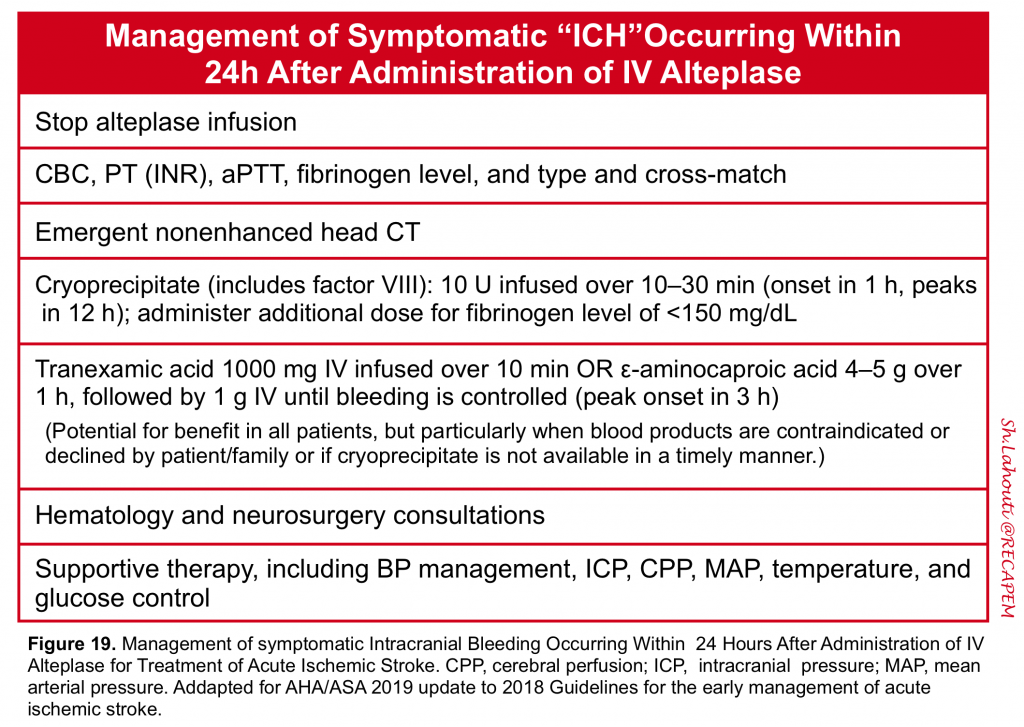
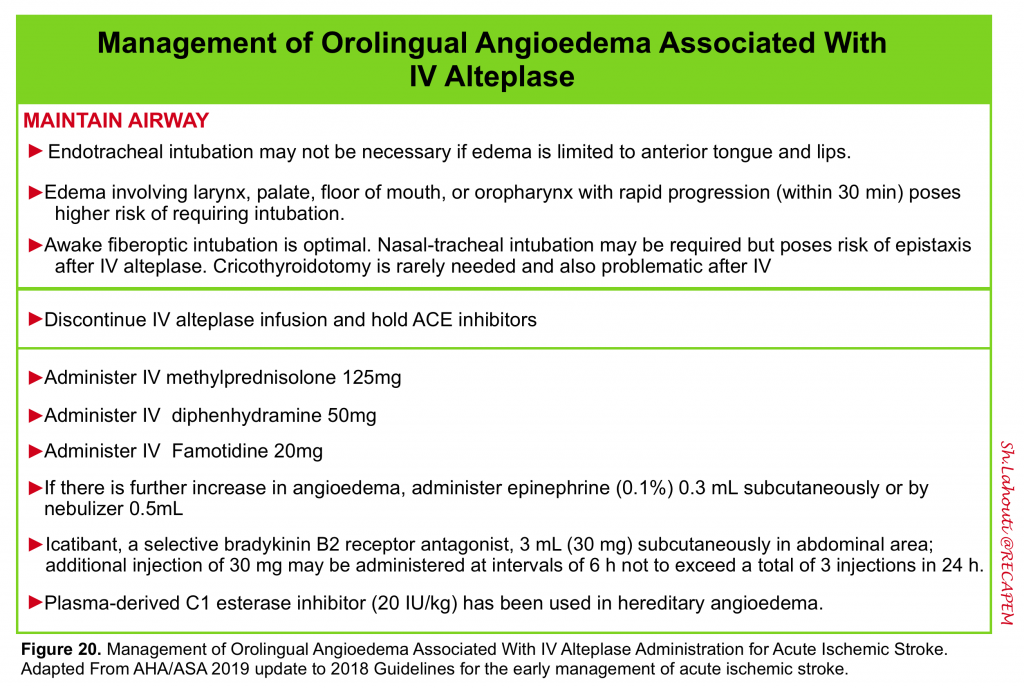
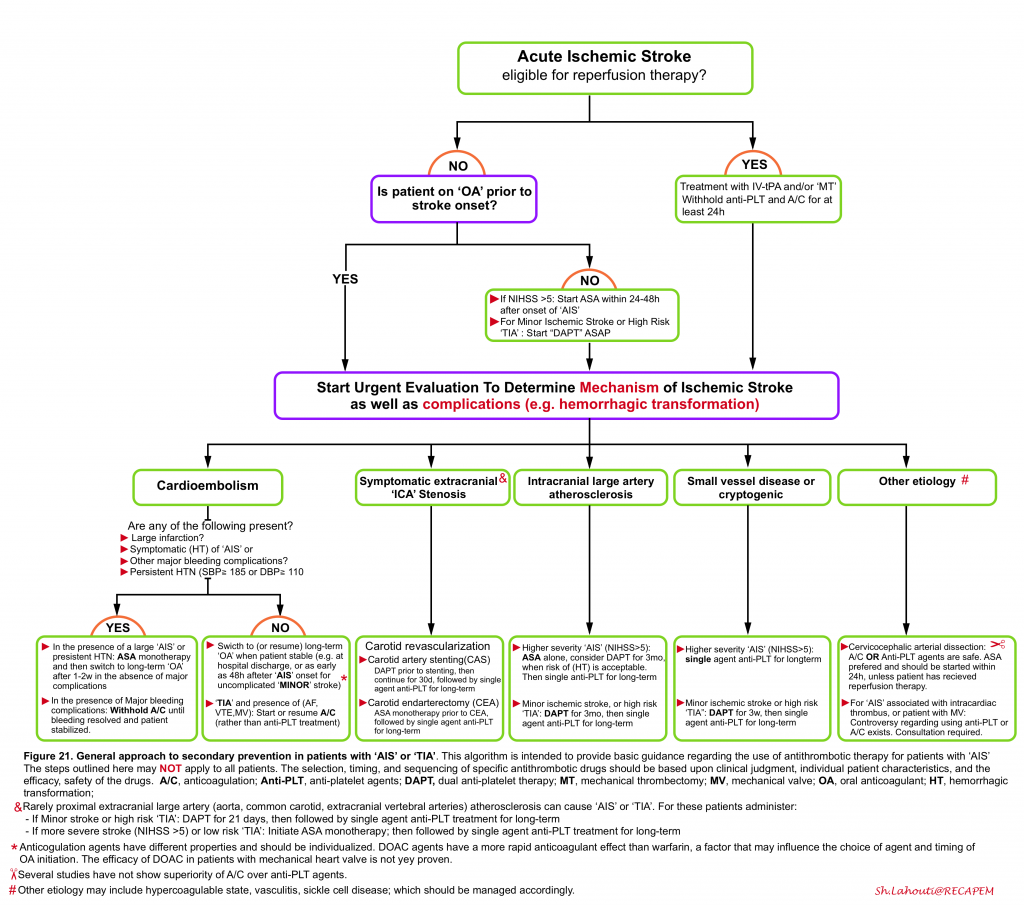



Add comment