Sep 8, 2021 via Shahriar Lahouti
CONTENTS
- Clinical manifestation
- Laboratory diagnosis
- ECG diagnosis
- Risk factors
- Severity and risk stratification
- Treatment
- Hyperkalemic cardiac arrest or peri-arrest
- Causes of hyperkalemia
- Investigation
- Media
- Going further
- Reference
Clinical Manifestation
Hyperkalemia is a potentially life-threatening disorder. Symptoms and physical examination findings are often absent. When present, the signs and symptoms are quite non-specific and may include:
- Cardiac: Bradycardia, ventricular tachycardia/fibrillation, sudden cardiac death
- Neuromuscular: Weakness, paresthesias, areflexia, ascending paralysis
- GI: Nausea, vomiting, diarrhea 1
Laboratory diagnosis
Hyperkalemia is defined as a measured serum K of > 5.5 mEq/L or >5.0 mM, depending on the source (serum or plasma measurement is acceptable, however serum K is 0.1 to 0.7 mM/L higher).
Pseudohyperkalemia refers to artificially elevated serum potassium secondary to
- Hemolysis
- Severe polycythemias causing potassium release during clotting (e.g. platelets > 1 million or WBC count > 50,000). This can be avoided by point-of-care testing or measuring labs in a heparinized tube.
- Prolonged tourniquet application.
The first response to a lab report of hyperkalemia should be to obtain an ECG.
- If the ECG shows features of hyperkalemia, this confirms the diagnosis.
- If the lab reports severe hyperkalemia but the ECG is normal, repeat the lab.
ECG diagnosis
An ECG is not a sensitive method of detecting hyperkalemia and should not be relied upon to rule it out. However, the ECG has a high specificity for detecting hyperkalemia and could be used as a rule in test 20.
- Mean sensitivity ~ 20%. In severe hyperkalemia, the mean sensitivity improves to ~30%.
- Mean specificity ~97%. In severe hyperkalemia, the mean sensitivity is decreased to ~95%.
Hyperkalemia is a great imitator and can cause a variety of ECG changes. In critically ill patients, the ECG can be the most immediately available diagnostic tool in identifying patients with potentially lethal hyperkalemia.
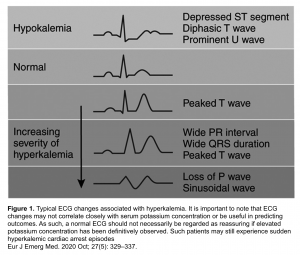
The ECG manifestations of hyperkalemia relative to potassium concentration have been described; however this ECG sequence of changes often does not occur in a stepwise fashion and their evolution may be unpredictable 2 (figure 1). More on this below.
The following patterns are highly suggestive of hyperkalemia (see following slides):
- Peaked T-waves: Narrow, pointy, prominent T-waves.
- In patients with inverted TW, hyperkalemia may manifest with pseudonormalization of inverted TW.
- Bradydysrhythmias: sinus bradycardia, high-grade AV block with slow junctional and ventricular escape rhythms, slow AF
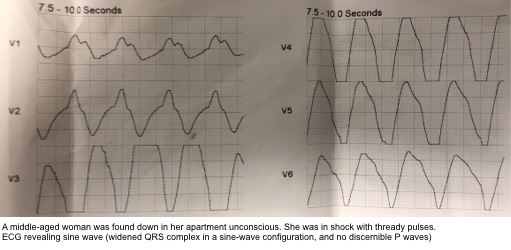
- Sine-wave pattern: profound widening of QRS complex and peaked T-waves mimics a sine wave.
- The sine wave pattern depicts worsening cardiac conduction delay caused by the elevated level of extracellular K. The morphology of this sinusoidal pattern on ECG results from the fusion of wide QRS complexes with T waves. There are no discernible P waves.
- Ventricular tachycardia mimic: Hyperkalemia causes QRS widening and P wave flattening. If the patient is tachycardic, it may look like ventricular tachycardia.
- Useful clue for diagnosis: compared to ventricular tachycardia, the QRS is more wide (> 1 big box) and the rate is slower (e.g. ≈ 120).
Presence of any ECG changes associated with hyperkalemia warrant emergency treatment (see below). In one study of 188 patients with severe hyperkalemia presence of bradycardia, QRS widening or junctional rhythm were associated with increased likelihood of short-term adverse event 3.
👉In an unstable patient with bradycardia, QRS widening or sine-wave pattern; it may be reasonable to give IV calcium while awaiting a potassium level.
Risk factors
Factors associated with an increased likelihood of the development of hyperkalemia include 4 :
- Chronic kidney disease G4-G5 (estimated GFR < 30 ml/min)
- Acute kidney injury
- Heart failure
- Diabetes mellitus
- Gout
- Severe tissue breakdown
- Coronary arterial disease, Peripheral vascular disease
- Metabolic acidosis (non-organic)
- Malignancy
- Drugs: renin-angiotensin-aldosterone system inhibitors, NSAIDs,potassium-sparing diuretics, beta blockers, digitalis.
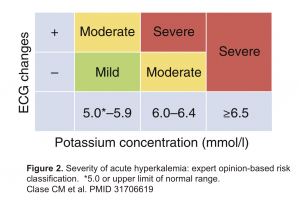
Severity and Risk Stratification
Hyperkalemia can be classified according to serum potassium level, however the severity of clinical manifestations depend not only on the serum K level but also on the rapidity of onset, presence of concomitant electrolyte abnormalities, medications and other comorbidities.
No evidence-based definition for “severe” hyperkalemia exists. Clinical judgement is needed, with attention to the following risk factors associated with life-threatening (severe) hyperkalemia :
- Serum Level: Potassium > 6.5 mM (regardless of the presence or absence of any ECG changes).
- Acuity: Acute hyperkalemia is worrisome while chronic hyperkalemia is more well-tolerated.
- Ongoing potassium release (e.g. by tumor lysis syndrome or rhabdomyolysis) or ongoing potassium absorption (e.g. significant GI bleeding) increase the likelihood of deterioration.
- Acute significant renal impairment
The following figure shows a consensus definition of severe hyperkalemia from a KDIGO conference 5
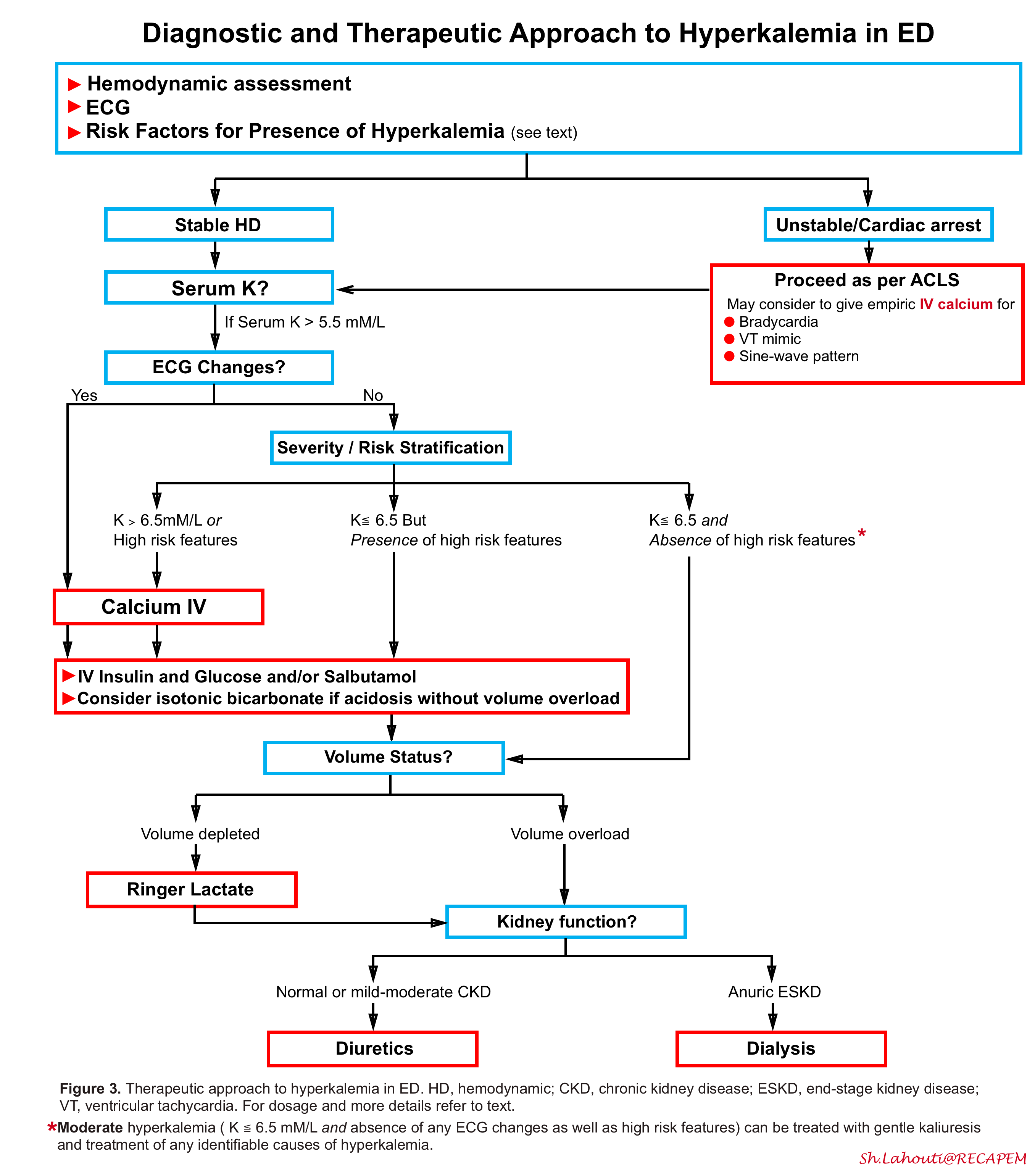
Treatment
The first step of treatment is to determine whether hyperkalemia is life-threatening (severe). Once identified, the entire clinical picture must be taken into account, including an assessment of hemodynamic stability, the presence of other electrolyte abnormalities, and an ECG evaluation.
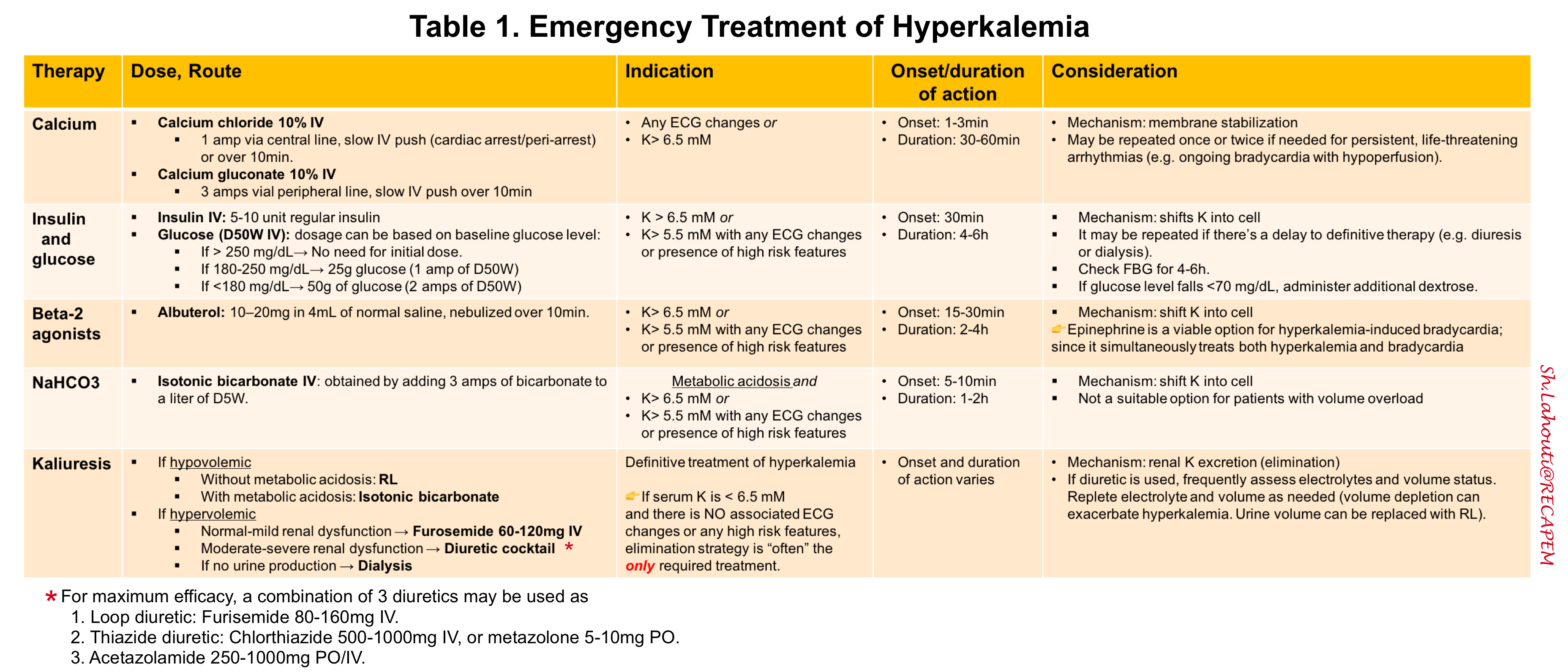
The approach to management of hyperkalemia in the ED (figure 3 below) and emergency therapeutic options (table 1 below) summarized here.
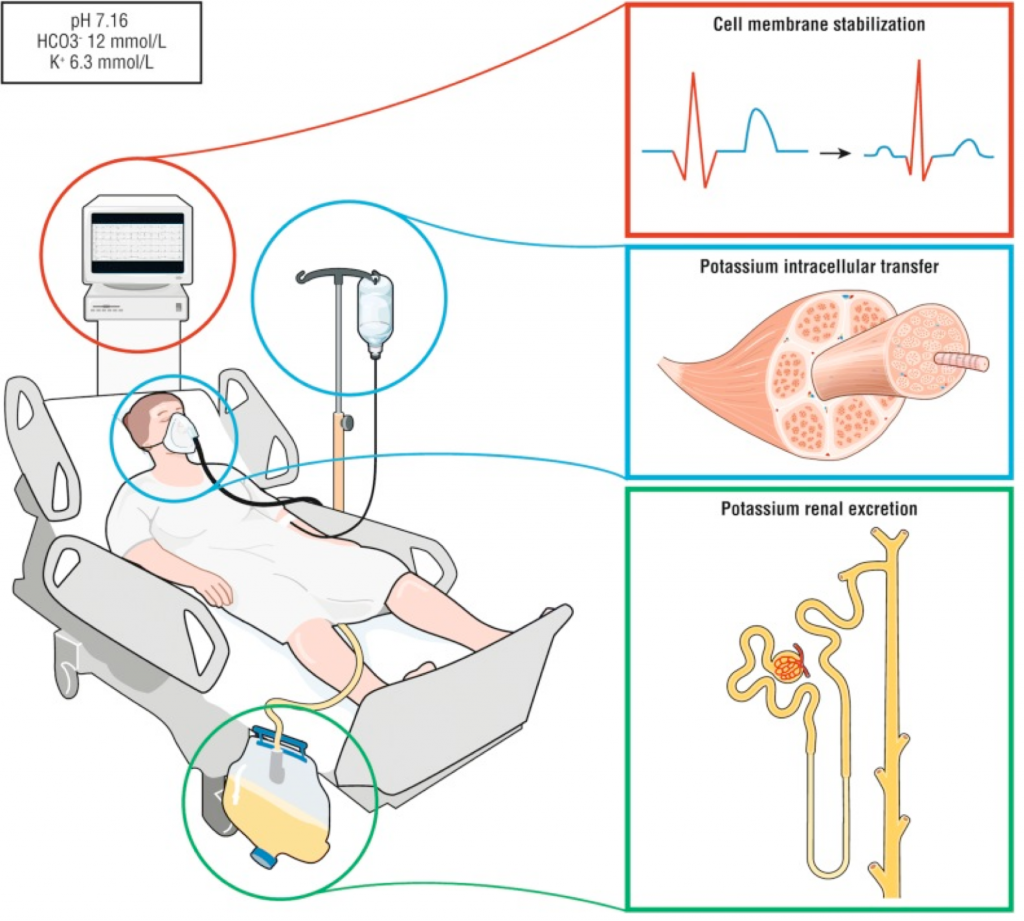
IV Calcium
Calcium acts rapidly, stabilizing the myocardium within 1-3 min, with overall effects lasting 30-60 minutes, but it does not alter the plasma potassium concentration.
Indications:
- Any ECG changes
- Serum K > 6.5 mM/L regardless to ECG changes
- Serum K ≦ 6.5mM/L but presence of high risk features (above)
Classically, calcium is contraindicated in the setting of hyperkalemia in digoxin toxicity, although recent studies and literature reviews suggest that the risk of calcium-induced arrhythmia in hyperkalemic digoxin toxicity is overstated and may be based on outdated case reports rather than empirical data. One study found ‘NO’ increase in mortality between a cohort of digoxin toxic patients who received calcium compared with those who did not 6.
Initial dose:
- Peripheral access: 3 grams IV calcium gluconate over 10 minutes.
- Central access: 1 gram IV calcium chloride over 10 minutes or slow IV push (peripheral IV administration may be used in cardiac arrest).
Re-dosing once or twice may be indicated if needed. If a point-of-care electrolyte monitor is available, check calcium levels and avoid pushing the ionized calcium >3 mM.
IV insulin plus dextrose
Insulin binds to the insulin receptor on skeletal muscle, activates the sodium–potassium adenosine triphosphatase, and leads to potassium transfer from the extracellular to intracellular space 7. The potassium shift lasts for several hours, but may need to be redosed periodically if there is a delay to definitive therapy (e.g. diuresis or dialysis).
Indications:
- Serum K > 6.5 mM/L
- Serum K ≦ 6.5mM/L with any ECG changes or presence of high risk features
Insulin dose: 5-10 units regular insulin (must be given IV)
- In patients with renal insufficiency and hyperkalemia, 5 units of insulin reduced serum potassium to the same extent as 10 units of insulin, but with a lower rate of hypoglycemia 8 .
Dextrose dose:
- Traditionally, 2 ampules of D50W have often been used (100 ml total, providing 50 grams of dextrose). 👉Only one ampule of D50W may be inadequate to prevent hypoglycemia.
- Dextrose administration may be omitted if the baseline glucose level is already >250 mg/dL 9. If the baseline glucose is high (e.g. 180-250 mg/dL) then the dose of dextrose might be cut in half, to 25 grams.
Monitor for hypoglycemia: Follow the finger stick glucose level for 4-6 hours. If glucose level falls below 70 mg/dL, preemptively administer additional dextrose 10 .
Beta-2 agonist
Albuterol: Causes a small shift of potassium into cells.
-
Requires a lot of albuterol (10-20 mg, equal to about 4-8 nebulizer treatments back-to-back). Logistically, the best way to achieve this dose is to provide albuterol as a continuous nebulized therapy.
IV epinephrine: It should not be used solely for hyperkalemia. However, if the patient does require a vasopressor, then epinephrine may be a sensible choice.
Epinephrine works perfectly in hyperkalemia-induced bradycardia, because it simultaneously treats both the hyperkalemia and the bradycardia.
Bicarbonate
Hypertonic bicarbonate: It has been proven to be ineffective in RCTs 11 12. The hypertonic nature of the fluid pulls potassium out of the cells due to osmotic shifts (‘solvent drag’) 13. This counteracts the effect of increasing the pH, with an overall neutral effect on the potassium.
Isotonic bicarbonate: It works but only in patients with metabolic acidosis 14 15. Isotonic bicarbonate decreases the potassium in three ways; dilution, shifting of potassium into muscle cells and prompted renal potassium excretion by alkalosis 16.
- Isotonic bicarbonate is generally obtained by adding three 50-mEq amps of bicarbonate to a liter of D5W (this creates a 150 mM solution of bicarbonate). However this requires giving 1-2 liters of fluid and therefore is not a viable treatment for patients with volume overload.
Potassium-removing strategies
Most patients will require elimination of excess potassium from the body eventually. This may be achieved either via the kidneys (kaliuresis) or via dialysis. Patients with end-stage renal disease on chronic dialysis will require emergent dialysis, however for most other patients, kaliuresis should be attempted prior to emergent dialysis.
The overall approach to volume management and potassium elimination strategy in hypperkalemic patients is represented below.
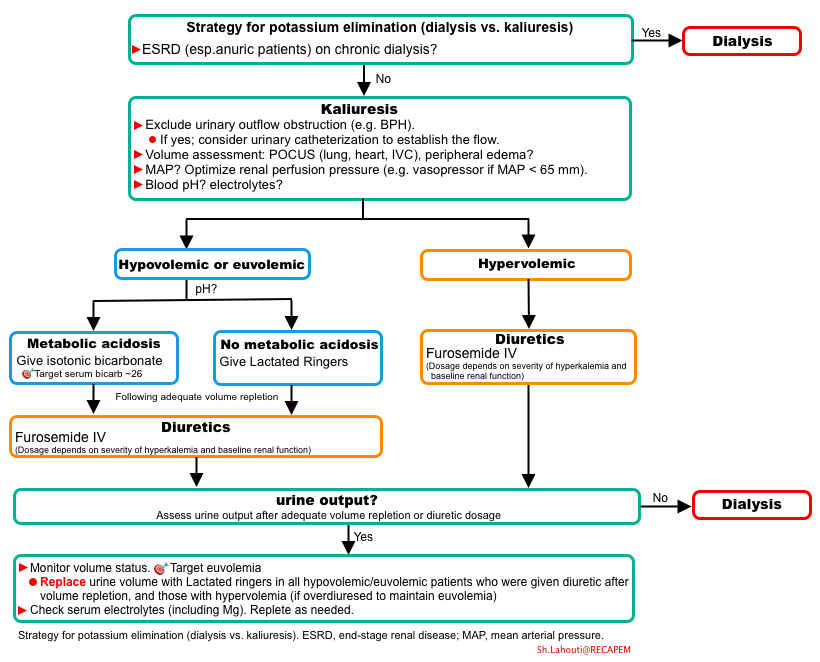
Volume depleted patients
The first step in management of patients with hypovolemia is volume resuscitation. This can be achieved by administration of ringer lactate or isotonic bicarbonate depending on the presence or absence of metabolic acidosis:
- In the absence of metabolic acidosis, ringer lactate is preferred as the resuscitative fluid.
- In the presence of metabolic acidosis (excluding lactic acidosis or ketoacidosis), isotonic bicarbonate should be given.
- Isotonic bicarbonate: Prepared by adding three 50-mEq amps of bicarbonate to a liter of D5W (this creates a 150 mM solution of bicarbonate).
- Therapeutic Target: Serum bicarbonate 24-28 mM. If the patient remains hypovolemic after receiving enough sodium bicarbonate to normalize the serum bicarbonate level, then residual hypovolemia can be treated with ringer lactate.
- Calculate the bicarbonate deficit (MDCalc).
- Volume of Isotonic bicarbonate required: Divide the bicarbonate deficit by 150 to estimate the number of liters of isotonic bicarbonate required. The dose is usually 1-2 liters.
- Infusion rate: It should be infused rapidly for patients with hypovolemia and severe hyperkalemia (e.g. 500-1,000 ml/hour).
Volume overload
👉Patients with normal renal function or mild renal dysfunction: IV loop diuretic alone may be sufficient (e.g. 60-120 mg IV furosemide).
👉Patients with moderate/severe renal dysfunction who might need emergent dialysis: Aggressive attempt to stave off dialysis with multiple diuretics:
- Loop diuretic (e.g. 80- 160 mg IV furosemide). Onset time is 30-60 min. Best used in fluid-overloaded patients with functioning kidneys. No effect in anuric patients.
- Thiazide (500-1,000 mg IV chlorothiazide, or 5-10 mg metolazone).
- +/- Acetazolamide (250-1,000 mg IV/PO).
- +/- Fludrocortisone 0.2 mg PO (esp. patients on ACEi/ARB). It may help stimulate the kidneys to secrete potassium. Indications (more on this here):
- Fludrocortisone is primarily useful in patients with mineralocorticoid insufficiency (for example patients on ACEi/ARB or NSAIDs).
- May consider fludrocortisone if the patient starts producing lots of urine, but the potassium level isn’t falling.
Replace urine losses with crystalloid to avoid hypovolemia. Use isotonic bicarbonate or balanced crystalloid as described above on volume resuscitation.
👉Dialysis is indicated for patients with anuric ESKD, chronic hemodialysis, or patients who don’t respond to the diuretic.
GI potassium elimination
GI potassium binding agents have slow onset of action. This limits their utility in the ED though their use may assist in further management beyond ED care. Neither sodium polystyrene sulfonate (e.g. kayexalate) nor patiromer has been proven to lower potassium acutely 17 18.
Sodium zirconium cyclosilicate is an oral potassium binder which is safe and somewhat effective. It has a small effect size and causes about 0.2 mM reduction in potassium within 4 hours and 0.4 mM reduction within 24 hours. Whether this is clinically beneficial depends on the context (more on this here)
Hyperkalemic cardiac arrest or peri-arrest
Hyperkalemia should be strongly suspected in patients with cardiac arrest especially when the risk factors associated with development of hyperkalemia is present.
- Have a low threshold to administer calcium in patients with cardiac arrest. Note that CaCl can be given via peripheral line as well.
The following approach is recommended for patients with suspected hyperkalemia (based on patient history and rhythm strip) or confirmed hyperkalemia (based on a point of care blood gas) in cardiac arrest in addition to usual ACLS measures:
- Calcium
- CaCl: Push 1 amp (1 gram) of 10% calcium chloride through a large bore well-running peripheral IV or central line (preferable). Repeat as needed to achieve QRS <100ms and p waves re-appear.
- Calcium gluconate: Routine use of calcium gluconate in cardiac arrest is not recommended due to the lack of improved survival.
- However, if it is not available, calcium gluconate is considered as an alternative, and given as 30 mL of calcium gluconate (10%) as a rapid bolus. Repeat as needed to achieve QRS <100ms and p waves re-appear.
- 👉It is importnat to know that it takes 3 grams of calcium gluconate to equal the amount of calcium in 1 gram of Ca Cl.
- Epinephrine
- Epinephrine has the potential to treat hyperkalemia, bradycardia, and maintain coronary arterial perfusion pressure.
- Dosage: Bolus dose is given at 20-50 mcg IV q2-5 min (followed by continuous infusion at 2-10 mcg/min).
- For vasopressors in PEA, see here.
- Sodium bicarbonate
- If severe acidosis is present consider to give 50 mEq of sodium bicarbonate.
- 👉Do NOT stop CPR until serum potassium level is normalized.
- CPR in patients with cardiac arrest due to hyperkalemia may take longer time than usual.
- When ROSC is achieved, it will be primarily due to the effects of calcium rather than decreased potassium levels.
- Since the stabilizing effects of calcium will wear off in 20-30min, shifting the potassium (e.g. IV insulin and dextrose) and enhancing its elimination (as described above) should be promptly considered.
- Consider repeating the calcium bolus if there are any worsening ECG changes.
- Intra-arrest dialysis
- In cardiac arrest, case reports have demonstrated successful ROSC and good neurologic outcomes despite prolonged arrest when dialysis is initiated during CPR to correct hyperkalemia 19.
Cardiac ultrasound showing poor function and low volume. More cases of severe hyperkalemia 👉 Dr. Smith’s ECG Blog
Causes of hyperkalemia
- Pseudohyperkalemia: tourniquet use, hemolysis (in vitro), leukocytosis, thrombocytosis
- Cellular lysis: hemolysis, hematoma, rhabdomyolysis, tumor lysis syndrome, GI bleeding
- Renal failure: primary if
- Oliguria
- GFR <15 ml/min
- Iatrogenic:
- Drugs: ACEi/ARBS, potassium-sparing diuretics, NSAIDs, Beta-blockers, antibiotics (trimethoprim, pentamidine, ketoconazole, IV penicillin G-potassium), calcineurin inhibitors (cyclosporine, tacrolimus), digoxin toxicity, succinylcholine
- Diabetic ketoacidosis, hyperglycemic hyperosmolar nonketotic syndrome
- Dysfunction of the renin-angiotensin-aldosterone system (Type IV renal tubular acidosis)
- Hyperkalemic periodic paralysis
- Transfusion of aged blood
- Aldosterone deficiency
- Obstructive uropathy
Investigations
- Review medication list and consider clinical context. Consider digoxin levels in patients taking digoxin.
- Full panel of serum electrolytes
- BUN, creatinine
- Consider evaluation for cellular lysis (e.g. CK, LDH levels)
- Consider evaluation for adrenal insufficiency (e.g. random cortisol, ACTH stimulation test).
- May consider measurement of renin and aldosterone levels if no clear cause of hyperkalemia is identified.
Media
Hyperkalemic ECG manifestation
Going further
- Emergency Management of Hyperkalemia (Emergency Medicine Cases)
- Treatment of Severe Hyperkalemia (EMCrit)
- Management of Severe Hyperkalemia (PulmCrit)
Top image credit: Ann intensive care, published online 2019 Feb 28.
References
1. Pepin J, Shields C. Advances in diagnosis and management of hypokalemic and hyperkalemic emergencies. Emerg Med Pract. 2012 Feb;14(2):1-17; quiz 17-8. PMID: 22413702
2. Campese VM, Adenuga G. Electrophysiological and clinical consequences of hyperkalemia. Kidney Int Suppl (2011). 2016;6(1):16-19. doi:10.1016/j.kisu.2016.01.003
3. Durfey N, Lehnhof B, Bergeson A, Durfey SNM, Leytin V, McAteer K, Schwam E, Valiquet J. Severe Hyperkalemia: Can the Electrocardiogram Risk Stratify for Short-term Adverse Events? West J Emerg Med. 2017 Aug;18(5):963-971. doi: 10.5811/westjem.2017.6.33033. Epub 2017 Jul 10. PMID: 28874951; PMCID: PMC5576635
4. Lindner G, Burdmann EA, Clase CM, et al. Acute hyperkalemia in the emergency department: a summary from a Kidney Disease: Improving Global Outcomes conference. Eur J Emerg Med. 2020;27(5):329-337. doi:10.1097/MEJ.0000000000000691
5. Clase CM, Carrero JJ, Ellison DH, Grams ME, Hemmelgarn BR, Jardine MJ, Kovesdy CP, Kline GA, Lindner G, Obrador GT, Palmer BF, Cheung M, Wheeler DC, Winkelmayer WC, Pecoits-Filho R; Conference Participants. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020 Jan;97(1):42-61. doi: 10.1016/j.kint.2019.09.018. Epub 2019 Oct 10. PMID: 31706619
6. Levine M, Nikkanen H, Pallin DJ. The effects of intravenous calcium in patients with digoxin toxicity. J Emerg Med. 2011 Jan;40(1):41-6. doi: 10.1016/j.jemermed.2008.09.027. Epub 2009 Feb 6. PMID: 19201134.
7. Ho K. A critically swift response: insulin-stimulated potassium and glucose transport in skeletal muscle. Clin J Am Soc Nephrol. 2011 Jul;6(7):1513-6. doi: 10.2215/CJN.04540511. Epub 2011 Jun 23. PMID: 21700825.
8. LaRue HA, Peksa GD, Shah SC. A Comparison of Insulin Doses for the Treatment of Hyperkalemia in Patients with Renal Insufficiency. Pharmacotherapy. 2017 Dec;37(12):1516-1522. doi: 10.1002/phar.2038. Epub 2017 Nov 27. PMID: 28976587
9. Harel Z, Kamel KS. Optimal Dose and Method of Administration of Intravenous Insulin in the Management of Emergency Hyperkalemia: A Systematic Review. PLoS One. 2016 May 5;11(5):e0154963. doi: 10.1371/journal.pone.0154963. PMID: 27148740; PMCID: PMC4857926
10. Moussavi K, Fitter S, Gabrielson SW, Koyfman A, Long B. Management of Hyperkalemia With Insulin and Glucose: Pearls for the Emergency Clinician. J Emerg Med. 2019 Jul;57(1):36-42. doi: 10.1016/j.jemermed.2019.03.043. Epub 2019 May 11. PMID: 31084947.
11. Kim HJ. Acute therapy for hyperkalemia with the combined regimen of bicarbonate and beta(2)-adrenergic agonist (salbutamol) in chronic renal failure patients. J Korean Med Sci. 1997 Apr;12(2):111-6. doi: 10.3346/jkms.1997.12.2.111. PMID: 9170015; PMCID: PMC3054237.
12. Blumberg A, Weidmann P, Shaw S, Gnädinger M. Effect of various therapeutic approaches on plasma potassium and major regulating factors in terminal renal failure. Am J Med. 1988 Oct;85(4):507-12. doi: 10.1016/s0002-9343(88)80086-x. PMID: 3052050.
13. Conte G, Dal Canton A, Imperatore P, De Nicola L, Gigliotti G, Pisanti N, Memoli B, Fuiano G, Esposito C, Andreucci VE. Acute increase in plasma osmolality as a cause of hyperkalemia in patients with renal failure. Kidney Int. 1990 Aug;38(2):301-7. doi: 10.1038/ki.1990.200. PMID: 2402122.
14. Blumberg A, Weidmann P, Ferrari P. Effect of prolonged bicarbonate administration on plasma potassium in terminal renal failure. Kidney Int. 1992 Feb;41(2):369-74. doi: 10.1038/ki.1992.51. PMID: 1552710.
15. Gutierrez R, Schlessinger F, Oster JR, Rietberg B, Perez GO. Effect of hypertonic versus isotonic sodium bicarbonate on plasma potassium concentration in patients with end-stage renal disease. Miner Electrolyte Metab. 1991;17(5):297-302. PMID: 1668124.
16. Weisberg LS. Management of severe hyperkalemia. Crit Care Med. 2008 Dec;36(12):3246-51. doi: 10.1097/CCM.0b013e31818f222b. PMID: 18936701.
17. Long B, Warix JR, Koyfman A. Controversies in Management of Hyperkalemia. J Emerg Med. 2018 Aug;55(2):192-205. doi: 10.1016/j.jemermed.2018.04.004. Epub 2018 May 3. PMID: 29731287
18. Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010 May;21(5):733-5. doi: 10.1681/ASN.2010010079. Epub 2010 Feb 18. PMID: 20167700.
19. Kose N, Bilgin F. Successful Treatment of a Patient with Cardiac Arrest Due to Hyperkalemia by Prolonged Cardiopulmonary Resuscitation along with Hemodialysis: A Case Report and Review of the Literature. Medicina (Kaunas). 2021 Aug 7;57(8):810. doi: 10.3390/medicina57080810. PMID: 34441016; PMCID: PMC8400023
20. Rafique Z, Aceves J, Espina I, Peacock F, Sheikh-Hamad D, Kuo D. Can physicians detect hyperkalemia based on the electrocardiogram? Am J Emerg Med. 2020 Jan;38(1):105-108. doi: 10.1016/j.ajem.2019.04.036. Epub 2019 Apr 22. PMID: 31047740


Add comment