12 November 2020 by Shahriar Lahouti. Last update 15 February 2023.
CONTENTS
- Preface
- Pathophysiology
- Etiology
- Clinical findings
- Diagnosis
- Diagnosis pitfall
- Differential diagnosis
- Treatment
- Special situation
- Bradykinin-mediated angioedema
- Going further
- References
Preface
Anaphylaxis is an acute life-threatening multisystem syndrome with a wide range of clinical manifestations resulting from a sudden release of mast cell and basophil-derived mediators into the circulation. 1
- The lifetime prevalence of anaphylaxis in the U.S. is 0.05%-2%, and this incidence is on the rise.
- In a population-based study, the highest rates of anaphylaxis were seen in young males (0-4 y/o) and women 25-34 y/o.
- The mortality rate is approximately 0.7%, and most fatalities are in young adults and adolescents.
- Delayed treatment with epinephrine is a risk factor for a fatal outcome.
Pathophysiology
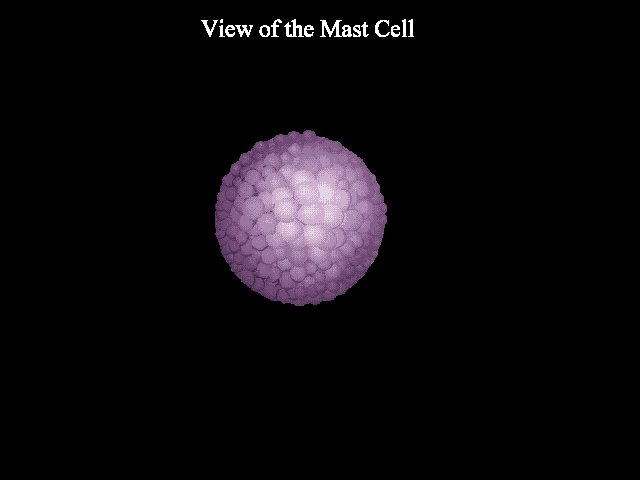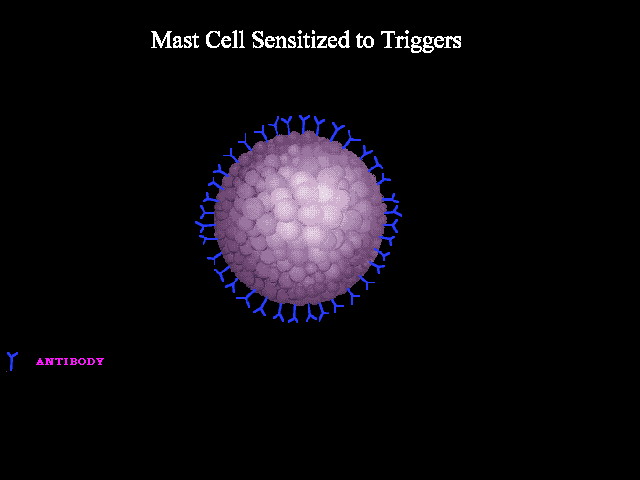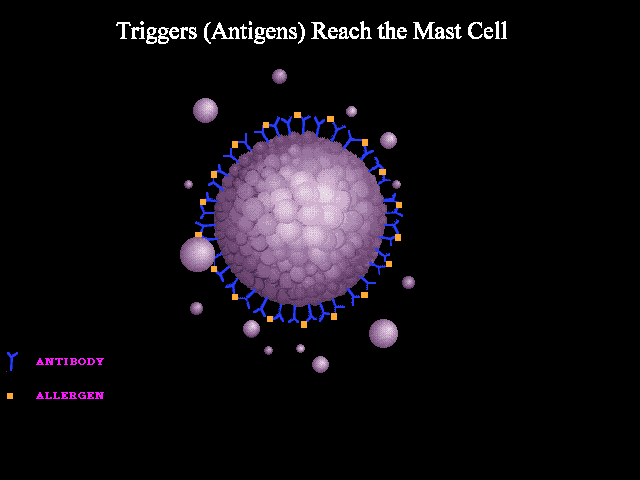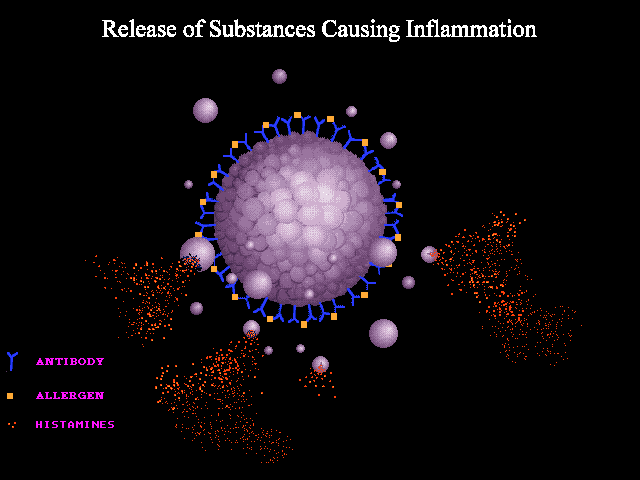
A good analogy can be made to seizure wherein abnormal neuronal discharge following a possible trigger leads to various clinical manifestations of a seizure spell. In anaphylaxis, the clinical picture is caused by the unregulated widespread release of mast cells and basophils mediators following a possible trigger i.e. allergen such as insect bite, food, etc. The mechanisms involved in the activation and degranulation of mast cells and basophils by antigen (allergen) include:
- Immunologic: Immunologic anaphylaxis is mediated by Immunoglobulin (IgE); the most classic type; or by immune-complex/complement-mediated reactions (figure 1 below).
- Non-immunologic: Certain agents or events are capable of inducing sudden mast cells or basophils degradation “directly” without the contribution of immunoglobulins.
- Some drugs can lead to direct activation of mast cells without the involvement of IgE, immune complexes, or antibodies. For example, opioids, NSAIDs, and radiocontrast agents can cause non-immunologic histamine release via direct mast cell degranulation *.
Etiology
- Insects (20%)
- Foods (31%): It is the most common trigger in children, especially peanut, tree nut, crustacean shellfish, finned fish, milk, egg
- Medications (34%)
- Antibiotics
- NSAIDs
- Radiocontrast dye
- Monoclonal antibodies (e.g. anti-TNF antibodies) infliximab
- Local anesthetics (e.g. lidocaine, benzocaine, mepivacaine)
- Paralytics
- Opioid
- Environmental, and occupational allergens (≈10%) such as natural rubber latex.
- Blood products in an IgA-deficient person
- Physical factors (Exercise, cold or heat exposure)
- Idiopathic (The precise prevalence is unknown 3)
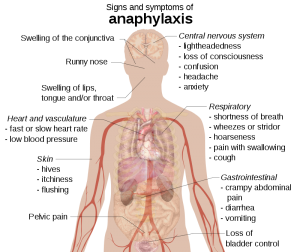
Clinical findings
- Skin and mucosa (incidence 80-90%)
- Itching
- Flushing.
- Urticaria.
- Angioedema (~80% sensitive), may involve lips, eyelids, airway, hands, feet, and genitalia.
- Conjunctivitis, conjunctival swelling, tearing.
- Nasal discharge & congestion.
- Cardiovascular (incidence 40-50%):
- Hypotension.
- Tachycardia, palpitation.
- Syncope, presyncope, urinary or fecal incontinence.
- Chest pain.
- Bradycardia, cardiac arrest.
- Respiratory (incidence 60-70%)
- Upper airway: Tongue and uvula swelling, throat tightness, voice change, stridor.
- Lower airway: Bronchospasm (wheezing), cough, dyspnea
- GI (incidence 40-50%)
- Nausea, vomiting.
- Diarrhea.
- Crampy abdominal discomfort.
- Other: Uterine cramps in women and girls
Diagnosis
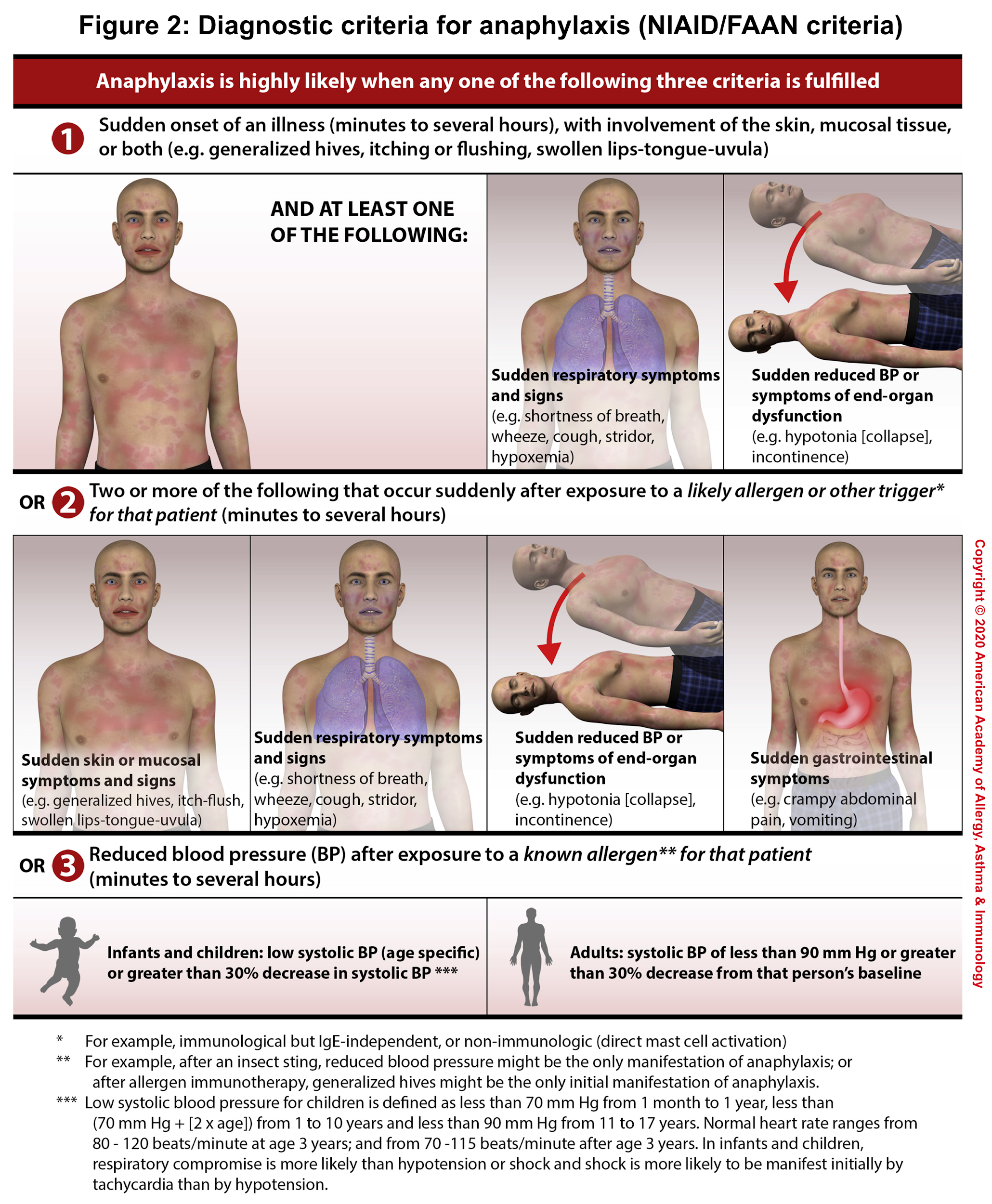
The diagnosis of anaphylaxis is primarily clinical based upon clinical symptoms and signs (number of involved organs), and a detailed history of exposure and chronicity of illness.1 There are 3 diagnostic criteria (NIAID/FAAN criteria) for anaphylaxis, each reflecting a different clinical presentation(figure 2). Anaphylaxis is highly likely when any one of the following three criteria is fulfilled 4:
- Criterion 1: Acute onset of a reaction within minutes or hours, with involvement of the skin, mucosal tissue, or both, AND at least 1 of the following:
- Respiratory compromise, OR
- Reduced blood pressure or symptoms of end-organ dysfunction
- Criterion 2: Two or more of the following that occur rapidly after exposure to a likely allergen for that patient (minutes to several hours):
- Involvement of the skin/mucosal tissue
- Respiratory compromise
- Reduced blood pressure or associated symptoms and signs of end-organ malperfusion
- Persistent gastrointestinal symptoms and signs
- Criterion 3: Reduced BP after exposure to a known allergen for that patient (minutes to several hours):
- Adults: SBP <90 mm Hg or a >30% decrease from baseline
- Infants and children: Low SBP (defined in the figure 2)1 for age or a >30% decrease from baseline
Diagnostic pitfalls
Trigger
- The absence of a trigger does not rule out anaphylaxis in an appropriate clinical context. Idiopathic anaphylaxis has been reported in different studies somewhere between 11% to 25%.
Blood Pressure
- Do Not rely on the presence of hypotension or shock for diagnosis of anaphylaxis. As implicated by diagnostic criteria 1 and 2; hypotension is not required for diagnosis of anaphylaxis accordingly.
- A patient who is suffering from a cardiovascular insult can have a normal or even high BP early in the course of illness explained by compensatory mechanisms (reflex tachycardia). It may take time for a patient to show a low BP on the monitor, depending upon his/her physiologic reserve, the severity of illness, and the time point where he is standing during his course of illness.
- Other different variables contribute to the patient’s initial blood pressure as well. These may include comorbidities, medications, or technical factors (when an inappropriately small BP cuff is used, hypotension may go undetected).
- Hypotension may go unnoticed after epinephrine administration.
- Use age-appropriate standards for normal BP for children and infants.
Skin Lesions
- Skin lesions are frequently common (90%) during an anaphylaxis event. This makes diagnostic criterion one extremely useful for diagnosis. However, in almost 10% of patients skin lesions are totally absent or unrecognized. This can be attributed to factors such as the inability to communicate to express itching (e.g. patient is anesthetized, sedated, or unconscious) ), taking antihistamines by patients, or failure to fully undress and examine the patient. In this situation, criteria 2 or 3 are helpful for diagnosing the event.
Asthma
- Anaphylaxis in a patient with asthma may be mistaken for an asthma exacerbation if accompanying skin symptoms and signs, such as itching or hives, mucosal, tongue, or lip edema, or dizziness suggestive of impending shock, are overlooked 5.
Diagnostic performance of the NIAID/FAAN criteria
- In a prospective study of 174 children and adults presenting to the emergency department with allergic reactions or suspected anaphylaxis, the NIAID/FAAN criteria diagnostic performance reported as6:
- Sensitivity: 95%
- Specificity: 71%
- Positive predictive value: 63.7%
- Negative predictive value: 96.4%
- Positive likelihood ratio: 3.26
- Negative likelihood ratio: 0.07
Pearls
- When in doubt, it’s generally wise to treat empirically for anaphylaxis while continuing to investigate other diagnostic possibilities. There will be some patients who do not neatly fit any of these criteria, but for whom epinephrine administration is appropriate. As an example, it would be appropriate to administer epinephrine to a patient with a history of severe anaphylaxis to peanut or bee sting who presents with urticaria and flushing that developed within minutes of a known or suspected ingestion of peanut 7.
Differential diagnosis
Many of the symptoms and signs associated with hypoxia and hypotension (e.g. dyspnea, stridor, wheeze, syncope) are nonspecific. The differential diagnosis of anaphylaxis includes 8:
- Dermatologic:
- Acute generalized urticaria*
& - Bradykinin-mediated angioedema *
- Acute generalized urticaria*
- Upper airway obstruction
- Vocal cord dysfunction
- Epiglottitis
- Uvulitis
- Foreign body aspiration (in children)
- Deep neck space infection
- Lower airway Disorders
- Acute asthma exacerbation*&
- Pneumothorax
- Pulmonary embolism
- Cardiac vascular
- Myocardial infarction &
- Arrhythmia
- Acute symptoms related to structural disorders (eg, aortic stenosis, hypertrophic cardiomyopathy)
- Vasovagal syncope*
- Shock: Hypovolemic, Distributive, Cardiogenic, Obstructive
- Flushing:
- Redman syndrome (vancomycin)
- Systemic mastocytosis, carcinoid syndrome
- Niacin
- Postprandial syndrome
- Scombroidosis (histamine fish)
- Food poisoning
- Pollen-food allergy syndrome
- Caustic ingestion (especially in children)
- Infectious
- Hydatid cyst
- Sepsis, Toxic shock syndrome
- Non-organic disease (psych)
- Panic attack*
- Munchausen syndrome
- Psychosomatic episode
*Common disorders
& Acute asthma symptoms, acute generalized urticaria, or myocardial infarction symptoms can also occur during an anaphylactic episode.
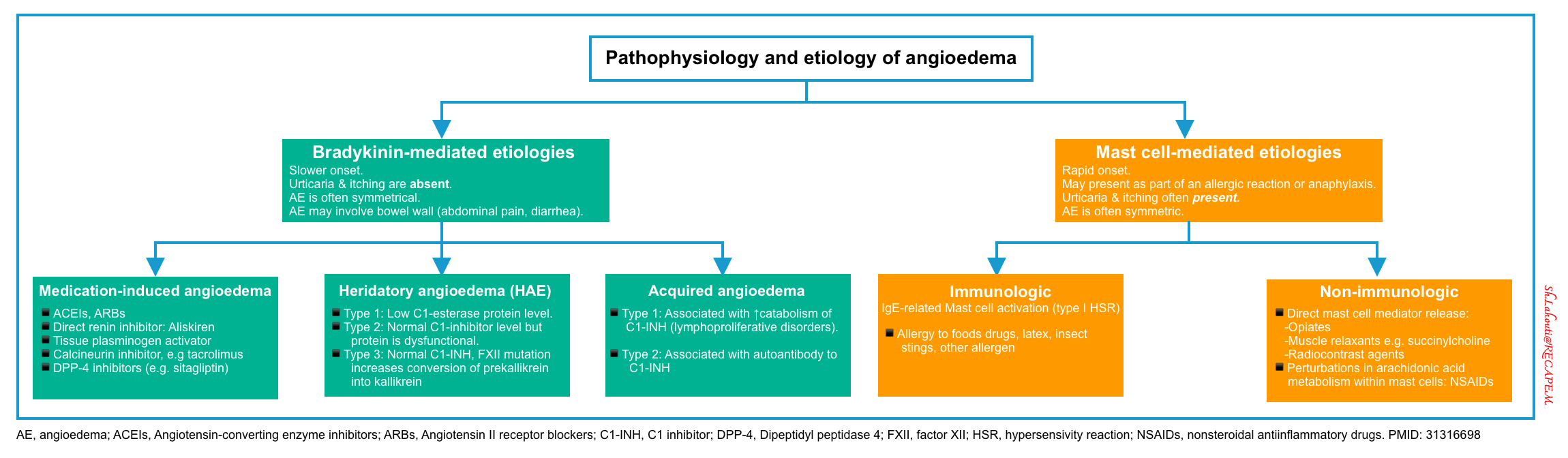
Angioedema
Angioedema Is swelling at deeper layers of skin (subcutaneous tissue) or submucosal tissue occurring either as an isolated form or as a component of anaphylaxis. It affects areas with loose connective tissue, with varying anatomic distribution such as the face, lips, mouth/throat, larynx and uvula, genitalia, and bowel wall (figure 3). The swelling of angioedema is non-pruritic and non-pitting which distinguishes angioedema from urticaria9. The most worrisome finding “stridor” is the result of upper airway involvement.10
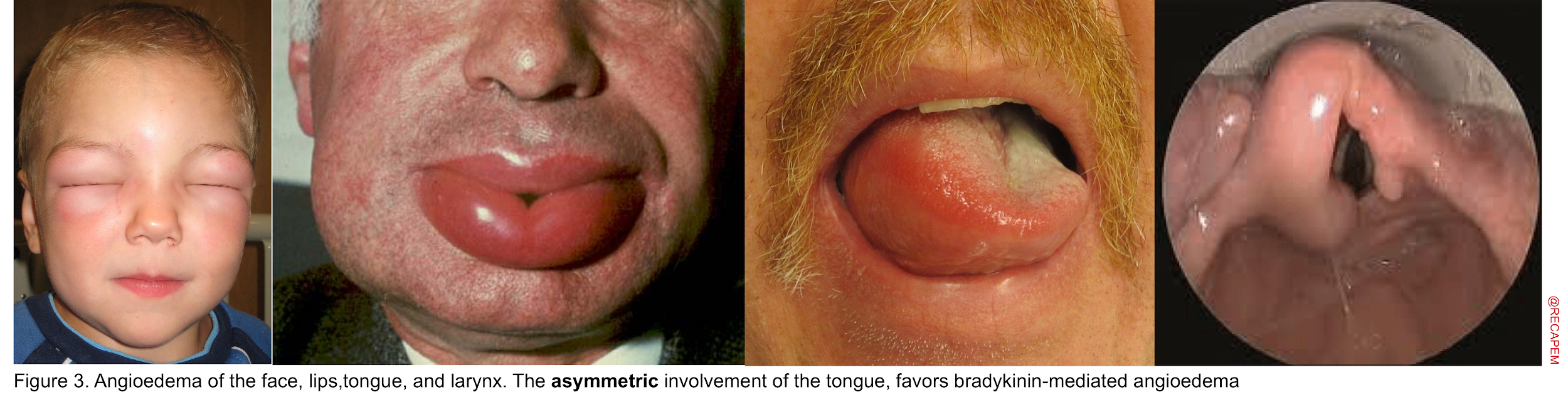
Several key points should be made here regarding the diagnosis and management of patients. First, it is essential to be familiar with the mechanisms involved in the development of angioedema since the treatment will be different for each type accordingly 11 :
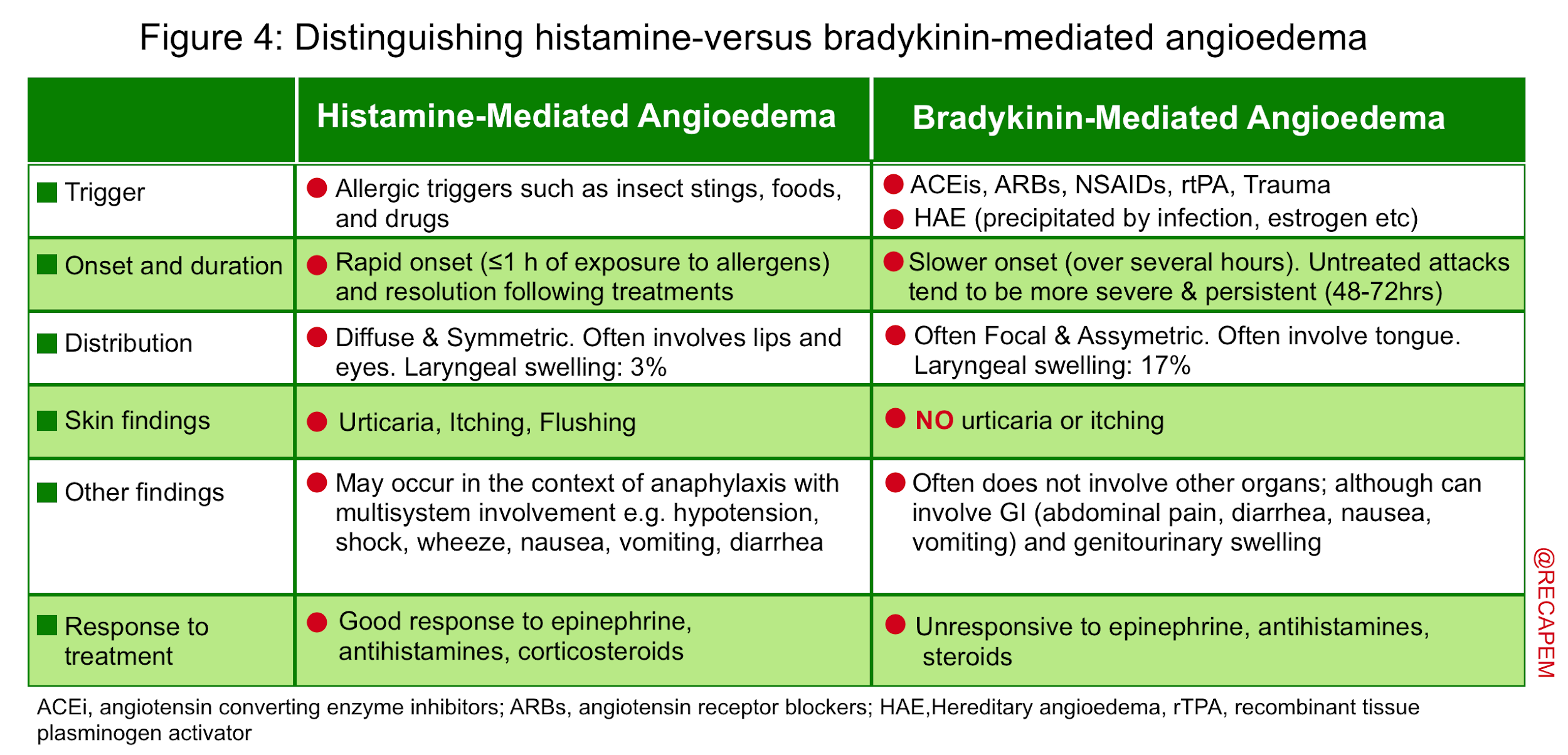
▪️Histamine-mediated angioedema
- Mechanism
- The mechanism involved in the development of allergic (histamine-mediated) angioedema is like anaphylaxis explained earlier (involves mast cell degranulation). Oftentimes it is a component of anaphylaxis with multiorgan involvement.
- Etiology and causes (discussed above)
- Clinical features 12
- Onset: Rapid following exposure to trigger.
- Resolution: Rapid following appropriate treatment.
- Distribution: Symmetric, more involving lips, face, and eyes.
- Other symptoms: Itching and urticaria are present. Other manifestations of anaphylaxis may be present as well.
- Treatment: The same as for anaphylaxis.
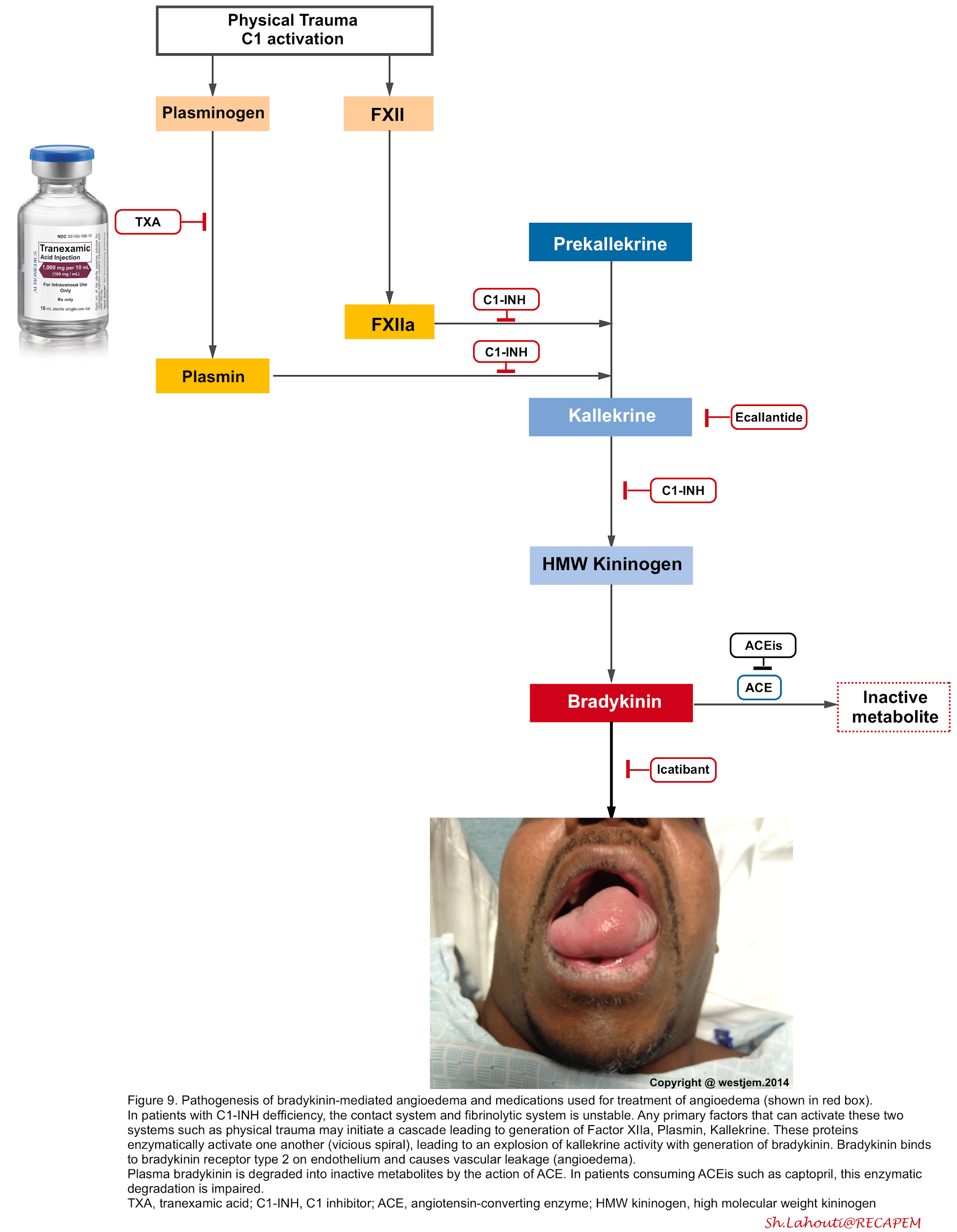
▪️Bradykinin-mediated angioedema
- Mechanism
- The mechanism is NOT related to mast cell degranulation, rather it involves a vicious cycle of inflammatory mediators generation and activation which ultimately culminates in overproduction of bradykinin (figure below).
- Causes and pathophysiology (here).
- Clinical features
- Onset: Slower pace
- Resolution: Untreated attacks may take hours to days to resolve
- Distribution: Asymmetric and focal, with a predilection for involving tongue and larynx
- Other symptoms: No itching and urticaria (since the mechanism does not involve mast cell degranulation).
- 🔴Abdominal pain, diarrhea, nausea, vomiting, and peripheral edema may be present.
- Treatment: It does not respond to medication used for anaphylaxis, therefore it is of paramount importance to identify and manage accordingly (more on this below).
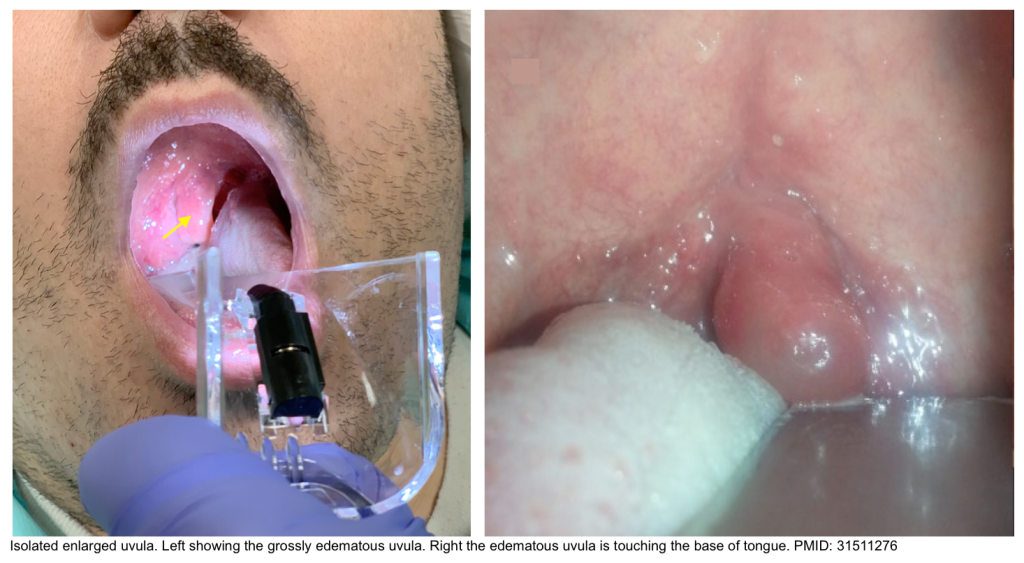
Isolated angioedema of uvula
- Isolated uvular angioedema (aka. Quincke’s disease) is a rare presentation of angioedema affecting only the uvula. This condition can compromise the airway rapidly and lead to potentially a life-threatening situation.
- Causes
- Type I immediate hypersensitivity reaction is the most common underlying etiology *.
- It is seen with atopic states and specific allergies like food allergies (peanuts, tree nuts, fish, and shellfish) *.
- Hereditary angioedema and acquired C1 esterase inhibitor deficiency.
- This should be suspected especially in patients with recurrent episodes *.
- Drugs: NSAIDs, angiotensin-converting enzyme inhibitors (ACEi) and angiotensin II receptor antagonists (ARA II)
- Substances of abuse e.g. cocaine, and cannabis *.
- Trauma (including intubation).
- Inhalation exposure, general anesthesia *.
- Infections.
- Type I immediate hypersensitivity reaction is the most common underlying etiology *.
- Clinical features
- Clinical presentation
- Foreign body sensation in his throat, drooling, gagging, and muffled voice.
- Throat pain and difficulty breathing when supine (keeping the head down may partially improve the symptoms, as the uvula will rest on the tongue and the patient can breathe freely).
- If severe, signs of upper airway obstruction.
- Absence of fever, cough, skin rash, hypotension, or tachycardia.
- Physical exam: Enlarged, swollen, congested uvula (orange-red) and somewhat translucent (uvular hydrops), without midline deviation.
- Clinical presentation
Differential diagnoses
- Epiglottitis
- Retropharyngeal abscess, and peritonsillar abscess.
Investigation
- Laboratory workups depend on the suspected etiology:
- Cell count and diff (eosinophilia may suggest an allergic reaction).
- C1 esterase and mast cell tryptase levels
- Swab cultures of the pharynx and uvula, blood cultures, latex agglutination studies looking for Haemophilus influenza type B (HIB) and Streptococcus pneumonia antigens (in both blood and urine).
- Lateral neck radiographs (to rule out epiglottic swelling).
- Toxicology of both blood and urine.
- Management
- Restoring and assuring a safe airway.
- In patients with respiratory difficulty, intubation and cricothyrotomy equipment should be at the bedside.
- Medical management
- Epinephrine
- Parenteral epinephrine should be considered in severe cases.
- Topically applying epinephrine (1:10.000 solution) with a cotton-tip applicator directly on the swollen uvula *.
- This has been shown to have a very rapid effect on markedly swollen uvula.
- Nebulized epinephrine (isomeric or racemic), or β2-mimetics like albuterol or salbutamol can be given *
- Parenteral H1 and H2 antihistamines*
- Corticosteroid
- Both parenteral and nebulized steroids are effective *.
- Plasminogen inhibitor epsilonamino-caprolic acid
- It should be considered when noninfectious uvulitis is present and the patient does not respond to the above measures, especially if a complement deficiency disorder is suspected, since this agent activates C1 from its precursor stage; however, in managing acute symptoms its value is not well established *.
- Epinephrine
- Surgical management includes uvular debulking with uvulotomy or uvulectomy.
- Restoring and assuring a safe airway.
Diagnostic work up
Diagnosis of anaphylaxis is made clinically and there’s no test to rapidly diagnose anaphylaxis. Depending on the presentation, it may be necessary to evaluate for other diagnostic possibilities. For example, a patient presents with vasodilatory shock and urticarial skin lesions. Although anaphylaxis is suspected, there is also a concern for septic shock, and therefore it is appropriate to treat the patient empirically for both conditions while an infectious workup is requested. If the infectious evaluation is negative, antibiotics are stopped; leaving the patient with a clinical diagnosis of anaphylaxis.
Therapeutic approach to anaphylaxis
Anaphylaxis is a severe, rapid systemic hypersensitivity reaction that can lead to airway and respiratory compromise, cardiovascular collapse, shock, and cardiac arrest. Unfortunately, under-recognition and undertreatment of anaphylaxis are common. Among patients who die, the time between exposure & death is13:
- 5 minutes for iatrogenic anaphylaxis (e.g. IV medication induced)
- 15 minutes for insect venom anaphylaxis
- 30 minutes for food anaphylaxis
The mortality rate is approximately 0.7%, and most fatalities are in young adults and adolescents. Delayed treatment with epinephrine is a risk factor for a fatal outcome.14
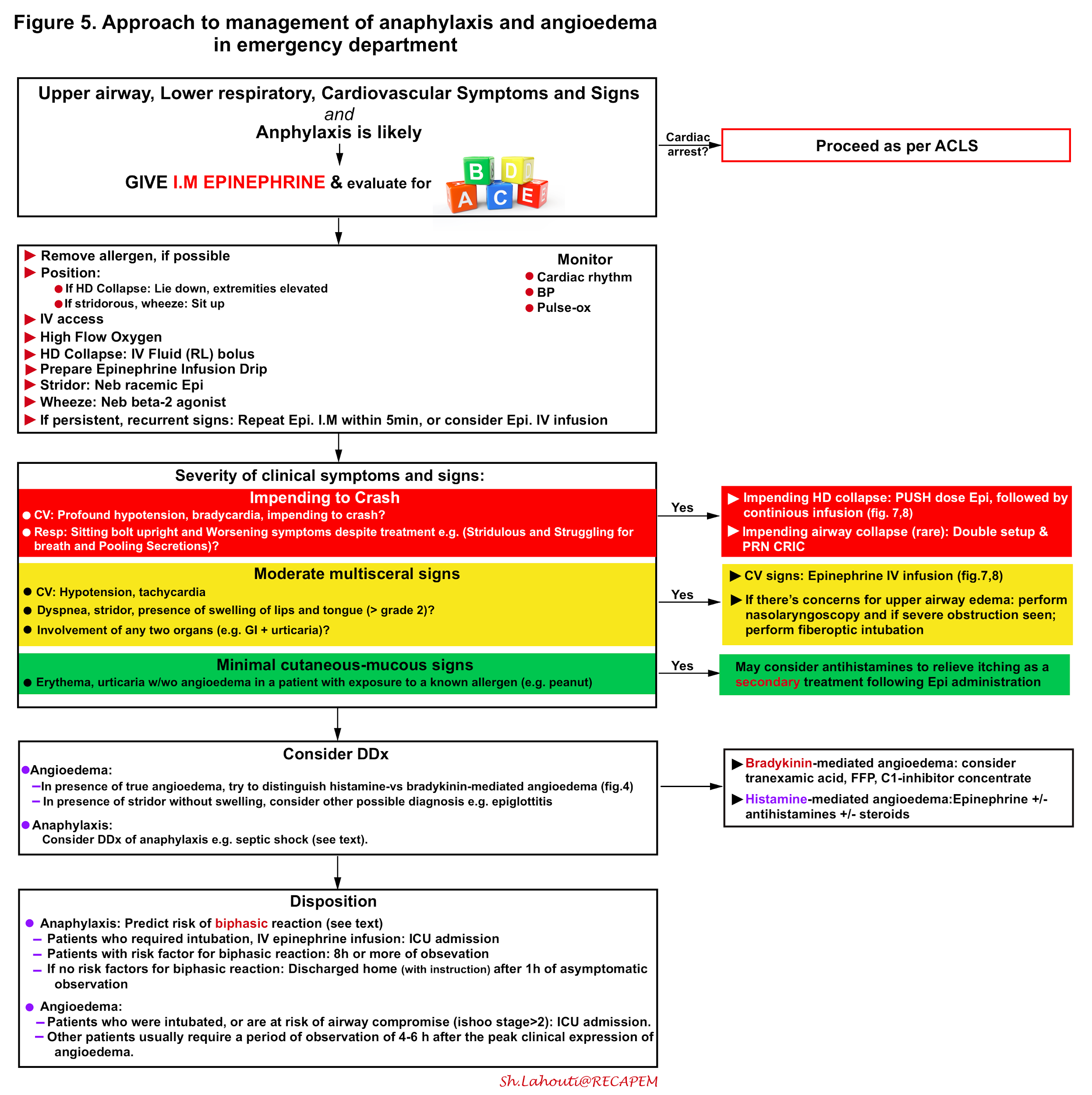
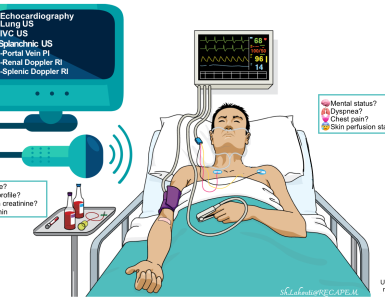
The integrated approach to critical patients with anaphylaxis and angioedema is shown below (figure 5) and involves 15 16 17
- Rapid identification
- Possible source control
- Immediate administration of epinephrine I.M.
- Evaluate Airway, Breathing, and Circulation, and appropriate intervention if deemed necessary.
- Consider the differential diagnosis of anaphylaxis and try to distinguish allergic angioedema from bradykinin-mediated angioedema (figure 4).
- Predict the risk of biphasic reaction in patients with anaphylaxis and determine the risk of developing airway compromise in patients with isolated angioedema.
Epinephrine
Physiology of Epinephrine in anaphylaxis
Epinephrine is the first-line medication in the treatment of anaphylaxis and allergic angioedema.18 Its beneficial effects include (but are not limited to)19 20:
- Decreased mediator release from mast cells and basophils via beta-2 receptor21
- Hemodynamic stabilization:
- Vasoconstriction via alpha-1 receptor
- Increased Inotropy (contractility) and chronotropy (heart rate) via beta-1 receptor
- At low doses (<8-10 mcg/kg/min), the predominant effect is an inotrope and chronotrope with a minimal vasoconstrictive effect while at higher doses (>10mcg/kg/min) the vasoconstrictive effect (alpha-agonist effects) predominates.
- Bronchodilation via beta-2 receptor
- Decreased airway edema (e.g. in the larynx) via alpha-1 receptor
Indication for Epinephrine
Epinephrine is the most important treatment for anaphylaxis and is the only medication that has been shown to be associated with a decrease in mortality. It should be administered as soon as anaphylaxis is suspected. The mortality rate of anaphylaxis is approximately 0.7%, and most fatalities are in young adults and adolescents. Delayed treatment with epinephrine is a risk factor for a fatal outcome.22
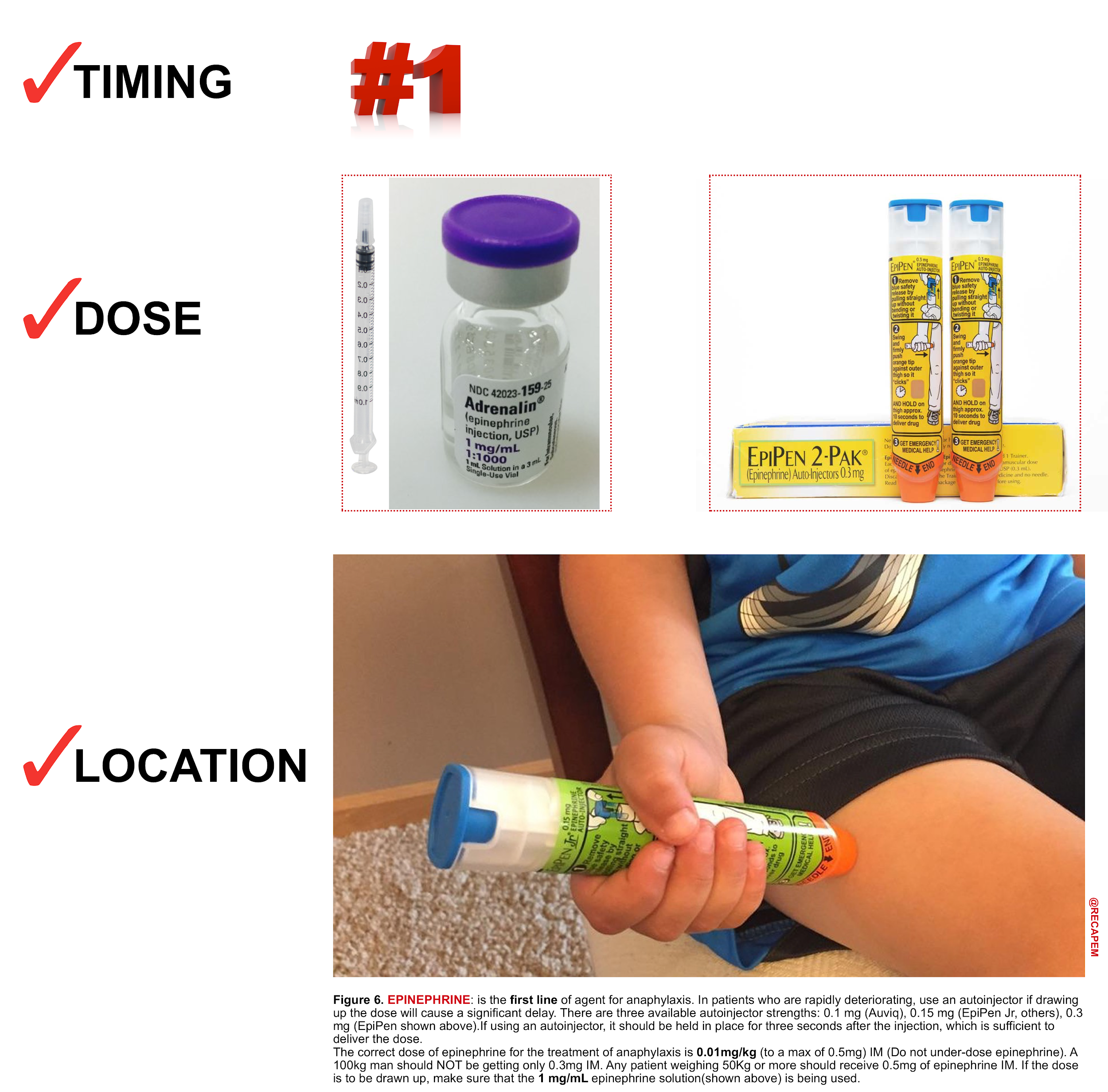
Intramuscular (IM) Epinephrine (figure 6)
Intramuscular injection is the preferred route for the initial administration of epinephrine for anaphylaxis in most settings. IM injection is recommended over subcutaneous injection because it provides a more rapid increase in the plasma and tissue concentrations of epinephrine.23
- Dose: 0.01mg/kg (of 1:1,000 formulation) to a max of 0.5mg in adults and 0.3mg in children, repeated after 5 mins if there’s no clinical improvement.
- Auto-injectors contain 0.3 mg (adult) or 0.15 mg (child) of epinephrine in a 1:1000 solution.
- Location: Inject epinephrine IM into the mid-anterolateral thigh.
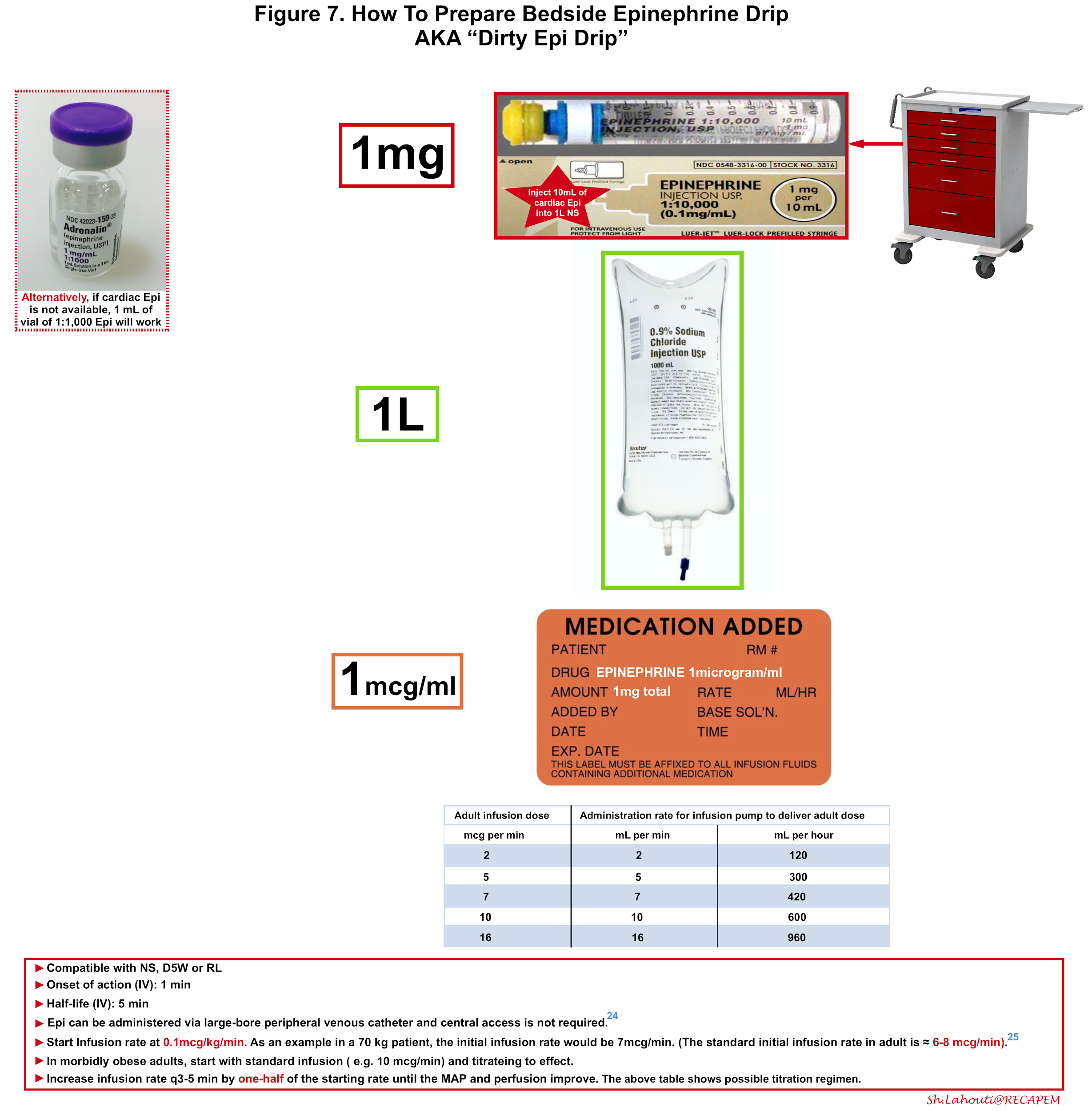
Intravenous Epinephrine (figure 7)
In patients who continue to be hypotensive after initial IM epinephrine, IV fluids should be administered. It is also prudent to begin preparing an epinephrine solution for slow, continuous infusion early, so that it is ready in case the patient fails to respond to repeated IM epinephrine and IV fluids.
Advantages of intravenous epinephrine include:
- Faster onset: In patients who are already in a shock state, peripheral perfusion is poor so the absorption of IM epinephrine is slow.
- Titration: The infusion can be given in a controlled fashion and can be weaned off or discontinued if undesired effects e.g. tachyarrhythmia encountered.
Intravenous infusion should be administered when indicated under close cardiac monitoring and great care should be exercised when preparing bedside epinephrine solution since the dosing error has been shown to be not uncommon in several studies.
Administration of epinephrine via a large-bore peripheral venous catheter is safe and the central line is not required 24. More on this here.
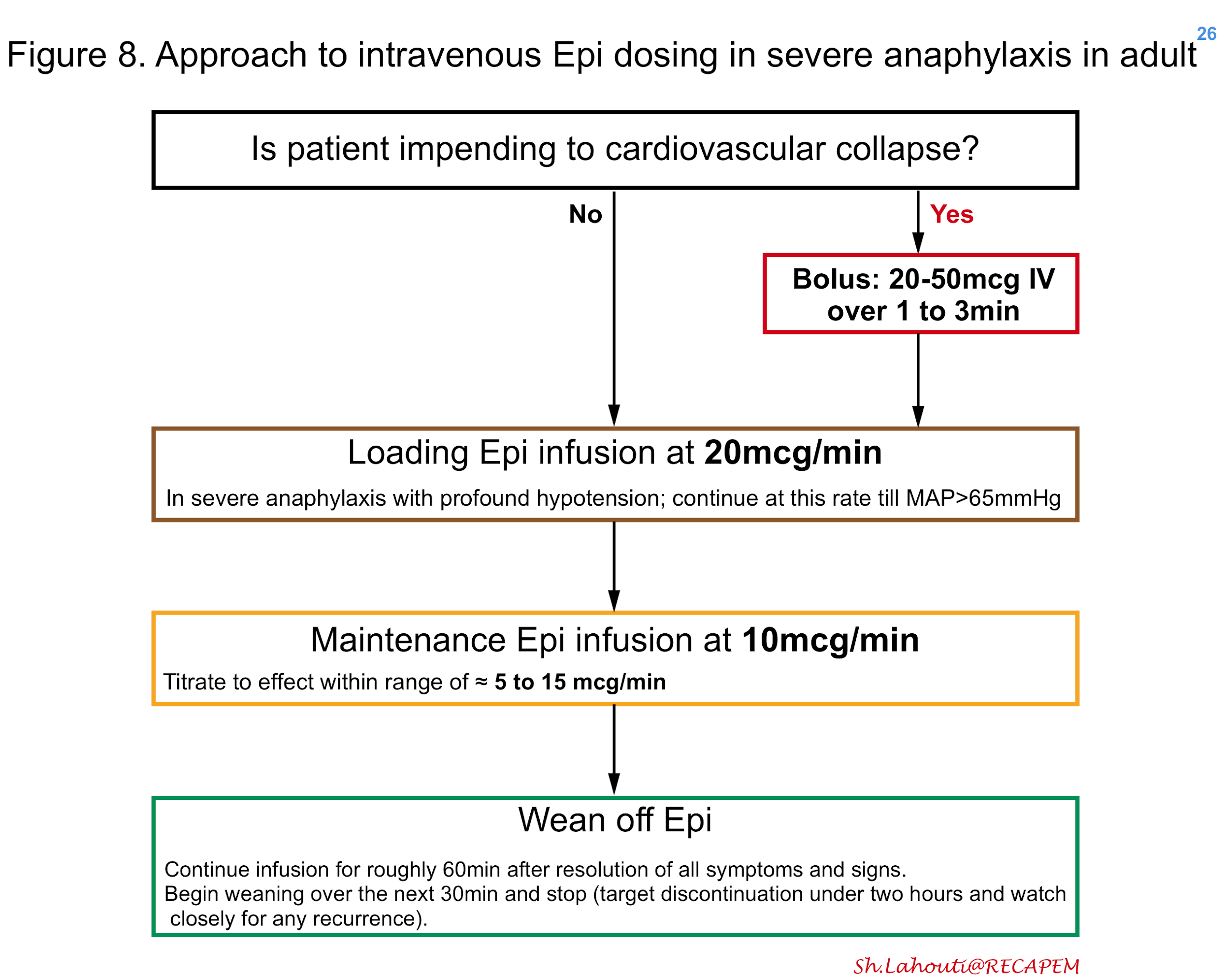
If a pre-mixed bag of epinephrine is not available, one may create as shown in figure 7. In patients with moderate anaphylaxis and hypotension, intravenous epinephrine can be started in adults at 0.1mcg/kg/min titrating every 3-5min to the effect 25 (figure 7). In patients with severe anaphylaxis and profound hypotension, the possible approach to epinephrine dosing is shown in Figure 8 26. The maintenance epinephrine infusion rate is roughly at 5 to 15 mcg/min depending on severity 27, though in practice it’s reasonable to titrate the infusion rate to the effect.
Airway management
▪️Patients with severe anaphylaxis and angioedema can be at risk of airway compromise and it is prudent to be prepared for a difficult airway, including possible cricothyroidotomy. Under these circumstances, airway management is difficult for several reasons including:
- There is a high risk of hemodynamic collapse following intubation.
- Start epinephrine & give fluid beforehand.
- Use cardiostable medications for intubation such as ketamine.
- Laryngeal edema will often preclude the use of a laryngeal mask airway *.
- Airway manipulation may worsen swelling and therefore the most experienced intubator should perform the procedure *.
- The real threat to the airway is the posterior tongue, and therefore orotracheal intubation may be simply not possible *.
▪️Indication for intubation
- While precise indications for intubation are not clear, possible situations where intubation is deemed to be necessary are:
- Worsening stridor, dyspnea, and struggling to breathe despite initial therapy.
- Significant voice change or hoarseness due to laryngeal edema.
- Pooling secretions.
- Progressive deterioration of edema.
- Nasolaryngoscopy shows significant laryngeal edema or impending closure of the posterior pharynx.
💡When in doubt, nasolaryngoscopy may help reveal whether there is significant laryngeal edema. Keep in mind that the true threat to the airway is the larynx and posterior tongue (not the lips and anterior tongue).
▪️Monitoring and classifying for severity of upper airway swelling
- Ishoo classification was proposed for monitoring the severity of upper airway swelling 28
- Stage I: Facial rash, edema, lip edema.
- Stage II: Soft palate edema.
- Stage III: Tongue edema.
- Stage IV: Laryngeal edema.
- When the airway is threatened but intubation is not indicated (Ishoo stage ≤2), one can proceed with medical treatment and perform nasolaryngoscopy, and if severe obstruction is seen, converting to fiberoptic intubation is recommended.29
▪️Intubation
- Crashing angioedema patients
- Clinical condition:
- The patient is at immediate risk of losing their airway.
- Patient is stridulous, sitting bolt upright, and struggling for breath.
- The patient may be unable to lie down.
- Management: Ketamine-dissociated cricothyrotomy
- Place the patient on 100% FiO2 using one of the following *:
- High-flow nasal cannula at 100% FiO2 and 60 liters flow.
- BiPAP mask.
- 100% Non-rebreather facemask set to flush rate (crank the flow rate well past the 15 liters/min mark).
- 100% non-rebreather facemask set to 15 liters/minute plus a nasal cannula underneath it running at 15 liters/minute.
- Give a dissociative dose of IV ketamine (e.g. 1.5-2 mg/kg) slowly over ~2min. This will dissociate the patient, without impairing the respiratory drive.
- Perform a scalpel-finger-bougie cricothyrotomy. The patient should continue breathing throughout the entire procedure, so you should be able to take your time a bit with this.
- Place the patient on 100% FiO2 using one of the following *:
- Clinical condition:
- Non-crashing angioedema patients
- Clinical condition
- The patient requires intubation but isn’t actively crashing. There is time to call for help and additional equipment.
- Possible management: The awake double setup
- Obtain an experienced intubator and someone competent at scalpel-finger-bougie cricothyrotomy.
- Nebulized racemic epinephrine can temporize while preparing for intubation.
- Perform awake fiberoptic intubation *. These patients often have tongue swelling, so the best approach is often nasotracheal intubation.
- During the intubation procedure, the second operator should be prepared to perform a cricothyrotomy if the airway is lost.
- Clinical condition
▪️Extubation
- While much has been discussed regarding intubation in several great posts, less has been talked about regarding extubation! The appropriate time for extubation can be determined by visual confirmation of the resolution of laryngeal edema rather than any arbitrary time interval. By and large the patient can be evaluated and extubated in less than 24 hours. It is reasonable to wait for the resolution of external edema if ever present (e.g. swelling of face, lips, tongue), and then proceed with further evaluation of the airway as follows 30:
- Direct laryngoscopy (video laryngoscopy is preferred) under deep sedation allows visualization of the airway including epiglottis.
- A cuff leak test may provide additional information. The absence of a leak predicted a high likelihood of upper airway obstruction (odds ratio, 18.78).
- Point of care ultrasound is a promising noninvasive method allowing visualization of the vocal cord and larynx 31.
- When in doubt, consider extubation over an airway exchange catheter as leave the airway exchange catheter in place temporarily to ensure that the airway is patent. If stridor occurs, re-intubation can be performed immediately over the exchange catheter.
Adjuvant therapies
Bronchodilators: The evidence for the use of beta-2-adrenergic agonists in anaphylaxis is extrapolated from their use in acute asthma. Bronchodilators are adjunctive treatment to epinephrine because they do not prevent or relieve mucosal edema in the upper airway or shock, for which the alpha-1-adrenergic effects of epinephrine are required. For patients with bronchospasm who are not responsive to epinephrine, inhaled bronchodilators (eg, albuterol, salbutamol) should be administered by a mouthpiece (or facemask for those whose age or condition requires) and nebulizer/compressor, as needed. Patients with milder respiratory symptoms can receive albuterol by metered-dose inhaler.
Antihistamines: ARE NOT SUBSTITUTE FOR EPINEPHRINE. These medications do not relieve upper or lower airway obstruction, hypotension, or shock, and in standard doses, do not inhibit mediator release from mast cells and basophils. Antihistamines may relieve itching and urticaria after epinephrine has been given. Possible administered medications may include:
- H1-receptor antihistamine: Diphenhydramine 50 mg IV q4-6 h, or Cetirizine 10 mg IV (the duration of action approaches 24 hours).
- H2-receptor antihistamine: Famotidine 20 mg IV q12h, or Ranitidine 50 mg IV q6h
Glucocorticoid: it has been traditionally been used for the treatment of anaphylaxis; however, there is little evidence of benefit. The onset of action of glucocorticoids takes several hours. Therefore, these medications do not relieve the initial symptoms and signs of anaphylaxis. One rationale for giving them, theoretically, is to prevent the biphasic reactions that occur in some cases of anaphylaxis. However, a recent systematic review failed to find evidence for this effect and therefore routine administration of glucocorticoid is not recommended1.
Steroids may well be beneficial for patients with severe symptoms requiring hospitalization or for those with known asthma and significant bronchospasm that persists after other anaphylaxis symptoms and signs have abated, and we do generally administer them in such situations. If given, the possible regimen may include:
- Methylprednisolone 1 to 2 mg/kg/day for one to two days is sufficient. Glucocorticoids can be stopped after that without a taper.
Specific situations
Biphasic reaction
Biphasic reactions are characterized by an initial reaction, followed by an asymptomatic period of 1 to 78 hours32 and then a subsequent return of symptoms without further exposure to antigen. A subsequent literature review designed to analyze only emergency department cases also found that the incidence was lower than in earlier reports and that many biphasic reactions were not “clinically significant”33.
Question 1. What risk factors should clinicians take into consideration in determining the likelihood of biphasic anaphylaxis?
- In a recent systematic review, the predictors for biphasic reaction were identified as:
- Severe initial reaction (odds ratio 2.11)
- Requirement for repeated epinephrine doses (i.e. >1 dose of epinephrine) with the initial presentation (odds ratio 4.82)
- Wide pulse pressure (odds ratio 2.11)
- Unknown anaphylactic trigger (odds ratio 1.63)
- Cutaneous signs and symptoms (odds ratio 2.54)
- Drug trigger in children (odds ratio 2.35)
- As indicated in an international consensus on anaphylaxis document, risk factors for severe fatal anaphylaxis include34:
- Older age
- Asthma
- Other pulmonary disease (eg, COPD, interstitial lung disease)
- Cardiovascular disease (eg, ischemic heart disease, cardiomyopathy, hypertensive vascular disease)
- Mast cells disorders
- Beta-blocker or angiotensin-converting enzyme use
- Lack of access to epinephrine or EMS
Prompt and adequate treatment of anaphylaxis appeared central to reducing biphasic anaphylaxis risk, in this review1.
Question 2. Should antihistamines or glucocorticoids be used to prevent biphasic anaphylaxis?
- Although antihistamines may relieve itching and urticaria associated with anaphylaxis, The Joint Task Force on Practice Parameter did not identify clear benefits in the prevention of biphasic anaphylaxis from antihistamines and glucocorticoids1.
- They suggest against administering glucocorticoids or antihistamines as an intervention to prevent biphasic anaphylaxis
Recommendation (from JTFPP 2020):
- They suggest that in the presence of any above risk factors for biphasic reaction, patients should be observed in a setting capable of managing anaphylaxis for 6 hours or longer.
- Administration of antihistamines and steroids have NO role in the prevention of biphasic reaction1.
Refractory anaphylaxis
Rarely patients may not respond well to maximal doses of epinephrine and fluid therapy. Such patients should be admitted to the intensive care unit and the following medications can be considered for them.
- Other vasopressors: one theory about the pathogenesis of refractory anaphylaxis proposes that the clinical manifestations may become refractory to further catecholamine administration, perhaps due to saturation or desensitization of adrenergic receptors35. The use of non-adrenergic vasopressors, such as vasopressin can be considered 36.
- Methylene blue: In patients with profound vasodilation (vasoplegia) status post cardiac surgery, methylene blue may work by inhibiting nitric oxide synthase and guanylate cyclase37. Vasoplegia may be present in some patients with refractory anaphylaxis, however the ideal dose and efficacy of methylene blue is unclear and not well-studied. In patients with cardiac surgery and vasoplegia, a single bolus of 1 to 2 mg/kg given over 20 to 60 minutes has been used. Some studies have shown promising results in the treatment of refractory anaphylaxis38. Methylene blue and other vital dyes are also rarely the cause of perioperative anaphylaxis. This drug should not be given to patients with pulmonary hypertension, underlying glucose-6-phosphate dehydrogenase deficiency, or acute lung injury. We also advise caution regarding potential drug interactions with serotonergic agents.
Anaphylaxis in patients consuming beta-blocker
The front line is epinephrine in patients taking beta-blockers, and it often works well in those taking low-moderate doses of beta-blockers. If a patient appears to be refractory to epinephrine, glucagon can be administered because its inotropic and chronotropic effects are not mediated through beta-receptors39. Glucagon can be given as:
- Start with 1-5 mg IV over five minutes.
- If there is a response, may infuse at 0.3 – 0.9 mg/hour.
- This will often elicit vomiting, so use caution if the patient has the borderline ability to protect their airway.
Bradykinin-mediated angioedema
Different causes can trigger bradykinin-mediated angioedema, however, from the standing point of pathophysiology, they all seem to share a common vicious spiral of activity. This shared pathophysiologic feature leads to similar clinical manifestations and almost similar treatment.
Key concept: Although bradykinin-mediated angioedema has different types, they all share a common pathophysiology and clinical feature, which warrant almost similar treatment. In the emergency department distinguishing various types of bradykinin-mediated angioedema has no crucial clinical implication. What matters is to distinguish histamine-mediated angioedema from bradykinin-mediated angioedema(figure 4); since treatment is different for each group11.
Pathophysiology
The pathophysiology of bradykinin-mediated angioedema involves the activation of FXII (Hageman factor), plasminogen, and kallikreins through a complex proteolytic cascade of events (in a vicious spiral fashion) so that once triggered, they enzymatically activate one another and result to over production of bradykinin (figure below) 40 41.
- The binding of bradykinin to bradykinin B2 receptors on endothelial cells induces vasodilation and increased endothelial permeability
- Bradykinin induces phosphorylation and destruction of vascular endothelial cadherin which is the major protein involved in the formation of tight junctions.
- In the absence of vascular endothelial cadherin, the loss of tight junctions leads to the movement of water from the vascular space into the surrounding tissue 42.
- Bradykinin has a very short half-life (~17 seconds) and is metabolized primarily by ACE, neutral endopeptidase (NEP), and aminopeptidase P (APP) and
secondarily by the enzymes dipeptidyl peptidase-4 (DPP-4).
Causes of bradykinin-mediated angioedema11
- Medication-induced angioedema
- Angiotensin-converting enzyme (ACE) inhibitors (~30% of all angioedema cases presented to emergency departments) *.
- ACE inhibitors impair the degradation of multiple peptides, including bradykinin and substance P.
- Angioedema can happen any time during the course of therapy from hours to years after treatment 43. The risk of angioedema with an ACE inhibitor is not related to the agent or dose.
- Some patients may experience one or more recurrent angioedema episodes in the months after the causative ACE inhibitor has been discontinued *.
- Angiotensin II receptor blockers (ARBs).
- Aliskiren (direct renin inhibitor) *
- Tissue plasminogen activator *.
- Calcineurin inhibitor tacrolimus, cyclosporine *.
- Dipeptidyl peptidase 4 (DPP-4) inhibitors (e.g. sitagliptin) *,*
- These drugs can also inhibit the degradation of bradykinin and substance P and have been associated with an increased risk of angioedema, especially when used together with immunosuppressive drugs or ACE inhibitors *.
- Estrogens (oral contraception or hormone replacement therapy can exacerbate Type 3 angioedema)
- Angiotensin-converting enzyme (ACE) inhibitors (~30% of all angioedema cases presented to emergency departments) *.
- Hereditary angioedema (HAE). Onset generally < 20 years old.
- This is due to congenital deficiency or dysfunctional complement C1-inhibitor protein.
- Type 1: Low C1-esterase protein level.
- Type 2: Normal C1-inhibitor level but the protein is dysfunctional.
- Hereditary angioedema with normal C1-inhibitor function (formerly Type 3)
- Mutation in factor XII causes an increased conversion of prekallikrein into kallikrein.
- This is due to congenital deficiency or dysfunctional complement C1-inhibitor protein.
- Acquired angioedema. This is less frequent than HAE and usually presents over the age of 40 years old.
- Type 1: Associated with increased catabolism of C1-INH (lymphoproliferative disorders e.g. chronic lymphocytic leukemia, non-Hodgkin’s lymphoma).
- Type 2: Associated with autoantibody to C1-inhibitor (may occur in lupus).
Approach to diagnosis and management
Diagnosis of angioedema as well as distinguishing allergic-versus bradykinin-mediated angioedema is made clinically. Extensive laboratory workup has a low yield in the emergency department. For patients who present with symptoms and signs of presumed angioedema, the priority in evaluation and management is applied to:
- Airway evaluation and management
- The possible indications for intubation and preferred approaches are explored earlier.
- Be prepared for a difficult airway
- Provide high-flow oxygen and correct hypoxia and hypotension if exist before trying to intubate (if times allow).
- Assess the severity of angioedema (Ishoo classification explored above)28. If the airway is threatened (e.g. moderate swelling of the lips and tongue), but there’s no potential indication for intubation (No stridor, voice change, pooling secretion, difficulty breathing); i.e. ishoo stage ≤2; perform diagnostic nasolaryngoscopy under controlled setting44. It has been shown that a significant number of patients with isolated anterior tongue swelling had asymptomatic supraglottic swelling. If severe obstruction is seen, fiberoptic intubation is performed at the same time.
- For patients with no clear indication for intubation, point-of-care ultrasound has been described as a non-invasive tool for evaluation of upper airway edema45 but further studies are required to validate its utility in emergency settings.
- Consider anaphylaxis and evaluate for its stigmata (figure 2). Evaluate breathing and circulation and employ appropriate intervention if indicated accordingly.
- As explored earlier, histamine-mediated angioedema can be a component of full-blown anaphylaxis, although the management proceeds the same as per anaphylaxis protocol.
- Distinguish histamine-versus bradykinin-mediated angioedema
- Consider personal or family history of HAE, use of angiotensin-converting enzyme inhibitors, exposure to an allergen, time of onset and rapidity of the onset of symptoms development, and finally the presence of other signs and symptoms such as itching, and urticaria (figure 4).
- If a patient does not neatly fit into either category, one can give an immediate therapeutic trial of medications for anaphylaxis. This can be used as a diagnostic/therapeutic approach to differentiating anaphylaxis versus bradykinin-mediated angioedema12.
- Treatment
- Histamine-mediated angioedema: is managed similarly to anaphylaxis
- Epinephrine: if there’s a significant threat to the airway
- Antihistamines: may relieve itching if ever exists
- Steroid: may be considered on an individual basis.
- Bradykinin-mediated angioedema
- C1-inhibitor concentrate: Inhibits FXIIa and kallikrein. It is the treatment of choice in congenital angioedema with deficient C1-inhibitor activity 46. Some studies have shown its efficacy in the treatment of ACEi-induced angioedema.Dosing of C1-INH concentrates:
- C1 esterase inhibitor, human (Berinert) 20 international units/kg IV
- C1 esterase inhibitor, human (Cinryze) 1,000 units IV over 10 min every 3 or 4 days.
- Tranexamic acid: Theoretically it inhibits the conversion of plasminogen into plasmin, which is a crucial step in amplification of kallikrein activation. Despite that the efficacy of this treatment is controversial, several studies have shown its efficacy as a front-line agent used to reverse episodes of ACEi-induced angioedema47. The possible dosing is 1 gram IV as a slow push over 10 minutes. May consider repeating if necessary, q4 hours PRN48.
- Fresh frozen plasma (FFP): In the absence of other therapeutic agents, it may be reasonable to use FFP for ACEi-induced angioedema and HAE49. Possible dosing is 2 units initially. May use an additional 2 units subsequently PRN.
- Ecallantide (kallikrein inhibitor figure 9): is approved for acute attacks of hereditary angioedema in patients ≥16 years of age. It can be administered as 30 mg SC given as 3 injections of 10 mg (1 mL).
- Icatibant (bradykinin receptor antagonist figure 9): can be given for hereditary angioedema, given as 30 mg SC in the abdominal region. Additional doses can be given within at least 6h intervals. No more than 3 doses (90 mg) should be given in a 24-hour period.
- Notice: Both the latest two medications are extremely expensive and not widely available. Several RCTs failed to show their efficacy in the treatment of ACEi-induced angioedema 50 51.
- C1-inhibitor concentrate: Inhibits FXIIa and kallikrein. It is the treatment of choice in congenital angioedema with deficient C1-inhibitor activity 46. Some studies have shown its efficacy in the treatment of ACEi-induced angioedema.Dosing of C1-INH concentrates:
- Histamine-mediated angioedema: is managed similarly to anaphylaxis
- Assess disease severity and determine patient disposition
- Retrospective studies such as those performed by Ishoo et al. describe the risk of the patient requiring ICU care and airway intervention52.
- Most patients require, at minimum, a period of observation of 4-6 hours after the peak clinical expression of angioedema to ensure that the risk of airway obstruction has resolved.
- Patients in whom the airway has been secured or in whom there is a risk of progression to requiring airway intervention (ishoo stage >2) require management in an intensive care setting.
Going further
- Anaphylaxis and anaphylactic shock (emergency medicine cases)
- Allergy, Hypersensitivity, and Anaphylaxis (EM:RAP)
- Anaphylaxis (IBCC)
- Angioedema (IBCC)
- Anaphylaxis 2020 Update(FOAMcast)
- Management of severe anaphylaxis in the emergency department(First10em)
- How to use IV epinephrine for anaphylaxis (PULMCrit)
- IV Bolus Epinephrine for Anaphylaxis(EMCrit RACC)
- Push-Dose Pressors (EMCrit)
- EMA Reviews: Angioedema(EM:RAP)
References
1. Shaker MS, Wallace DV, Golden DBK, Oppenheimer J, Bernstein JA, Campbell RL, Dinakar C, Ellis A, Greenhawt M, Khan DA, Lang DM, Lang ES, Lieberman JA, Portnoy J, Rank MA, Stukus DR, Wang J; Collaborators, Riblet N, Bobrownicki AMP, Bontrager T, Dustin J, Foley J, Frederick B, Fregene E, Hellerstedt S, Hassan F, Hess K, Horner C, Huntington K, Kasireddy P, Keeler D, Kim B, Lieberman P, Lindhorst E, McEnany F, Milbank J, Murphy H, Pando O, Patel AK, Ratliff N, Rhodes R, Robertson K, Scott H, Snell A, Sullivan R, Trivedi V, Wickham A; Chief Editors, Shaker MS, Wallace DV; Workgroup Contributors, Shaker MS, Wallace DV, Bernstein JA, Campbell RL, Dinakar C, Ellis A, Golden DBK, Greenhawt M, Lieberman JA, Rank MA, Stukus DR, Wang J; Joint Task Force on Practice Parameters Reviewers, Shaker MS, Wallace DV, Golden DBK, Bernstein JA, Dinakar C, Ellis A, Greenhawt M, Horner C, Khan DA, Lieberman JA, Oppenheimer J, Rank MA, Shaker MS, Stukus DR, Wang J. Anaphylaxis-a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020 Apr;145(4):1082-1123. doi: 10.1016/j.jaci.2020.01.017. Epub 2020 Jan 28. PMID: 32001253
2. Wood RA, Camargo CA Jr, Lieberman P, Sampson HA, Schwartz LB, Zitt M, Collins C, Tringale M, Wilkinson M, Boyle J, Simons FE. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014 Feb;133(2):461-7. doi: 10.1016/j.jaci.2013.08.016. Epub 2013 Oct 18. PMID: 24144575
3. Fenny N, Grammer LC. Idiopathic anaphylaxis. Immunol Allergy Clin North Am. 2015 May;35(2):349-62. doi: 10.1016/j.iac.2015.01.004. Epub 2015 Mar 6. PMID: 25841556
4. Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, Brown SG, Camargo CA Jr, Cydulka R, Galli SJ, Gidudu J, Gruchalla RS, Harlor AD Jr, Hepner DL, Lewis LM, Lieberman PL, Metcalfe DD, O’Connor R, Muraro A, Rudman A, Schmitt C, Scherrer D, Simons FE, Thomas S, Wood JP, Decker WW. Second symposium on the definition and management of anaphylaxis: summary report–Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006 Feb;117(2):391-7. doi: 10.1016/j.jaci.2005.12.1303. PMID: 16461139
5. Simons FE. Anaphylaxis, killer allergy: long-term management in the community. J Allergy Clin Immunol. 2006 Feb;117(2):367-77. doi: 10.1016/j.jaci.2005.12.002. PMID: 16461138
6.Loprinzi Brauer CE, Motosue MS, Li JT, Hagan JB, Bellolio MF, Lee S, Campbell RL. Prospective Validation of the NIAID/FAAN Criteria for Emergency Department Diagnosis of Anaphylaxis. J Allergy Clin Immunol Pract. 2016 Nov-Dec;4(6):1220-1226. doi: 10.1016/j.jaip.2016.06.003. Epub 2016 Jul 9. PMID: 27406968
7. Sampson HA, Muñoz-Furlong A, Bock SA, Schmitt C, Bass R, Chowdhury BA, Decker WW, Furlong TJ, Galli SJ, Golden DB, Gruchalla RS, Harlor AD Jr, Hepner DL, Howarth M, Kaplan AP, Levy JH, Lewis LM, Lieberman PL, Metcalfe DD, Murphy R, Pollart SM, Pumphrey RS, Rosenwasser LJ, Simons FE, Wood JP, Camargo CA Jr. Symposium on the definition and management of anaphylaxis: summary report. J Allergy Clin Immunol. 2005 Mar;115(3):584-91. doi: 10.1016/j.jaci.2005.01.009. PMID: 15753908
8. LoVerde D, Iweala OI, Eginli A, Krishnaswamy G. Anaphylaxis. Chest. 2018 Feb;153(2):528-543. doi: 10.1016/j.chest.2017.07.033. Epub 2017 Aug 8. PMID: 28800865; PMCID: PMC6026262
Kaplan AP, Greaves MW. Angioedema. J Am Acad Dermatol. 2005 Sep;53(3):373-88; quiz 389-92. doi: 10.1016/j.jaad.2004.09.032. PMID: 16112343
9. Caballero T, Baeza ML, Cabañas R, Campos A, Cimbollek S, Gómez-Traseira C, González-Quevedo T, Guilarte M, Jurado-Palomo GJ, Larco JI, López-Serrano MC, López-Trascasa M, Marcos C, Muñoz-Caro JM, Pedrosa M, Prior N, Rubio M, Sala-Cunill A; Spanish Study Group on Bradykinin-Induced Angioedema; Grupo Español de Estudio del Angioedema mediado por Bradicinina. Consensus statement on the diagnosis, management, and treatment of angioedema mediated by bradykinin. Part I. Classification, epidemiology, pathophysiology, genetics, clinical symptoms, and diagnosis. J Investig Allergol Clin Immunol. 2011;21(5):333-47; quiz follow 347. Erratum in: J Investig Allergol Clin Immunol. 2012;22(2):3 p following 153. PMID: 21905496
10. Bernstein JA, Cremonesi P, Hoffmann TK, Hollingsworth J. Angioedema in the emergency department: a practical guide to differential diagnosis and management. Int J Emerg Med. 2017 Dec;10(1):15. doi: 10.1186/s12245-017-0141-z. Epub 2017 Apr 13. PMID: 28405953; PMCID: PMC5389952
11. Lenschow M, Bas M, Johnson F, Wirth M, Strassen U. A score for the differential diagnosis of bradykinin- and histamine-induced head and neck swellings. Eur Arch Otorhinolaryngol. 2018 Jul;275(7):1767-1773. doi: 10.1007/s00405-018-4989-1. Epub 2018 May 2. PMID: 29721614
12. Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy. 2000 Aug;30(8):1144-50. doi: 10.1046/j.1365-2222.2000.00864.x. PMID: 10931122
13. Anagnostou K, Turner PJ. Myths, facts and controversies in the diagnosis and management of anaphylaxis. Arch Dis Child. 2019 Jan;104(1):83-90. doi: 10.1136/archdischild-2018-314867. Epub 2018 Jun 16. PMID: 29909382; PMCID: PMC6317446
14. Muraro A, Roberts G, Worm M, Bilò MB, Brockow K, Fernández Rivas M, Santos AF, Zolkipli ZQ, Bellou A, Beyer K, Bindslev-Jensen C, Cardona V, Clark AT, Demoly P, Dubois AE, DunnGalvin A, Eigenmann P, Halken S, Harada L, Lack G, Jutel M, Niggemann B, Ruëff F, Timmermans F, Vlieg-Boerstra BJ, Werfel T, Dhami S, Panesar S, Akdis CA, Sheikh A; EAACI Food Allergy and Anaphylaxis Guidelines Group. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014 Aug;69(8):1026-45. doi: 10.1111/all.12437. Epub 2014 Jun 9. PMID: 24909803
15. Campbell RL, Li JT, Nicklas RA, Sadosty AT; Members of the Joint Task Force; Practice Parameter Workgroup. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol. 2014 Dec;113(6):599-608. doi: 10.1016/j.anai.2014.10.007. PMID: 25466802
16. Soar J, Pumphrey R, Cant A, Clarke S, Corbett A, Dawson P, Ewan P, Foëx B, Gabbott D, Griffiths M, Hall J, Harper N, Jewkes F, Maconochie I, Mitchell S, Nasser S, Nolan J, Rylance G, Sheikh A, Unsworth DJ, Warrell D; Working Group of the Resuscitation Council (UK). Emergency treatment of anaphylactic reactions–guidelines for healthcare providers. Resuscitation. 2008 May;77(2):157-69. doi: 10.1016/j.resuscitation.2008.02.001. Epub 2008 Mar 20. PMID: 18358585
17. Simons FE, Ardusso LR, Bilò MB, El-Gamal YM, Ledford DK, Ring J, Sanchez-Borges M, Senna GE, Sheikh A, Thong BY; World Allergy Organization. World Allergy Organization anaphylaxis guidelines: summary. J Allergy Clin Immunol. 2011 Mar;127(3):587-93.e1-22. doi: 10.1016/j.jaci.2011.01.038. PMID: 21377030.
18. Simons FE. Pharmacologic treatment of anaphylaxis: can the evidence base be strengthened? Curr Opin Allergy Clin Immunol. 2010 Aug;10(4):384-93. doi: 10.1097/ACI.0b013e32833c2038. PMID: 20585241
19. McLean-Tooke AP, Bethune CA, Fay AC, Spickett GP. Adrenaline in the treatment of anaphylaxis: what is the evidence?. BMJ. 2003;327(7427):1332-1335. doi:10.1136/bmj.327.7427.1332
20. Vadas P, Perelman B. Effect of epinephrine on platelet-activating factor-stimulated human vascular smooth muscle cells. J Allergy Clin Immunol. 2012 May;129(5):1329-33. doi: 10.1016/j.jaci.2012.02.027. Epub 2012 Mar 27. PMID: 22460068
21. Anagnostou K, Turner PJ. Myths, facts and controversies in the diagnosis and management of anaphylaxis. Arch Dis Child. 2019 Jan;104(1):83-90. doi: 10.1136/archdischild-2018-314867. Epub 2018 Jun 16. PMID: 29909382; PMCID: PMC6317446
Simons FE, Gu X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J Allergy Clin Immunol. 2001 Nov;108(5):871-3. doi: 10.1067/mai.2001.119409. PMID: 11692118
22. Le A, Patel S. Extravasation of Noncytotoxic Drugs: A Review of the Literature. Ann Pharmacother. 2014 Jul;48(7):870-886. doi: 10.1177/1060028014527820. Epub 2014 Apr 8. PMID: 24714850
23. Lieberman P, Nicklas RA, Randolph C, Oppenheimer J, Bernstein D, Bernstein J, Ellis A, Golden DB, Greenberger P, Kemp S, Khan D, Ledford D, Lieberman J, Metcalfe D, Nowak-Węgrzyn A, Sicherer S, Wallace D, Blessing-Moore J, Lang D, Portnoy JM, Schuller D, Spector S, Tilles SA. Anaphylaxis–a practice parameter update 2015. Ann Allergy Asthma Immunol. 2015 Nov;115(5):341-84. doi: 10.1016/j.anai.2015.07.019. PMID: 26505932
24. Mali S, Jambure R. Anaphylaxis management: Current concepts. Anesth Essays Res. 2012;6(2):115-123. doi:10.4103/0259-1162.108284
25. Brown SG, Blackman KE, Stenlake V, Heddle RJ. Insect sting anaphylaxis; prospective evaluation of treatment with intravenous adrenaline and volume resuscitation. Emerg Med J. 2004 Mar;21(2):149-54. doi: 10.1136/emj.2003.009449. PMID: 14988337; PMCID: PMC1726302
26. Ishoo E, Shah UK, Grillone GA, Stram JR, Fuleihan NS. Predicting airway risk in angioedema: staging system based on presentation. Otolaryngol Head Neck Surg. 1999 Sep;121(3):263-8. doi: 10.1016/S0194-5998(99)70182-8. PMID: 10471868
27. Moellman JJ, Bernstein JA, Lindsell C, Banerji A, Busse PJ, Camargo CA Jr, Collins SP, Craig TJ, Lumry WR, Nowak R, Pines JM, Raja AS, Riedl M, Ward MJ, Zuraw BL, Diercks D, Hiestand B, Campbell RL, Schneider S, Sinert R; American College of Allergy, Asthma & Immunology (ACAAI); Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014 Apr;21(4):469-84. doi: 10.1111/acem.12341. PMID: 24730413; PMCID: PMC4100605
28. Floyd E, Goldstein NA, Jokes R, Mascaro M, Liaw C, Dickson B, Varughese D, Silverman J. An Extubation Protocol for Angioedema. OTO Open. 2017 Feb 3;1(1):2473974X17691230. doi: 10.1177/2473974X17691230. PMID: 30480175; PMCID: PMC6239052
29. Suthersan Y, Theerawit P, Hongphanut T, Kiat Boonsri C, Kiat Boonsri S. Predicting laryngeal edema in intubated patients by portable intensive care unit ultrasound. J Crit Care. 2013 Oct;28(5):675-80. doi: 10.1016/j.jcrc.2013.05.011. Epub 2013 Jun 24. PMID: 23806246
30. Pourmand A, Robinson C, Syed W, Mazer-Amirshahi M. Biphasic anaphylaxis: A review of the literature and implications for emergency management. Am J Emerg Med. 2018 Aug;36(8):1480-1485. doi: 10.1016/j.ajem.2018.05.009. Epub 2018 May 9. PMID: 29759531
31. Pourmand A, Robinson C, Syed W, Mazer-Amirshahi M. Biphasic anaphylaxis: A review of the literature and implications for emergency management. Am J Emerg Med. 2018 Aug;36(8):1480-1485. doi: 10.1016/j.ajem.2018.05.009. Epub 2018 May 9. PMID: 29759531
32. Simons FE, Ardusso LR, Bilò MB, Cardona V, Ebisawa M, El-Gamal YM, Lieberman P, Lockey RF, Muraro A, Roberts G, Sanchez-Borges M, Sheikh A, Shek LP, Wallace DV, Worm M. International consensus on (ICON) anaphylaxis. World Allergy Organ J. 2014 May 30;7(1):9. doi: 10.1186/1939-4551-7-9. PMID: 24920969; PMCID: PMC4038846
33. Dewachter P, Mouton-Faivre C, Emala CW. Anaphylaxis and anesthesia: controversies and new insights. Anesthesiology. 2009 Nov;111(5):1141-50. doi: 10.1097/ALN.0b013e3181bbd443. PMID: 19858877
34. Campbell RL, Li JT, Nicklas RA, Sadosty AT; Members of the Joint Task Force; Practice Parameter Workgroup. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol. 2014 Dec;113(6):599-608. doi: 10.1016/j.anai.2014.10.007. PMID: 25466802
35. Hosseinian L, Weiner M, Levin MA, Fischer GW. Methylene Blue: Magic Bullet for Vasoplegia? Anesth Analg. 2016 Jan;122(1):194-201. doi: 10.1213/ANE.0000000000001045. PMID: 26678471
36. Bauer CS, Vadas P, Kelly KJ. Methylene blue for the treatment of refractory anaphylaxis without hypotension. Am J Emerg Med. 2013 Jan;31(1):264.e3-5. doi: 10.1016/j.ajem.2012.03.036. Epub 2012 May 23. PMID: 22633725
37. Thomas M, Crawford I. Best evidence topic report. Glucagon infusion in refractory anaphylactic shock in patients on beta-blockers. Emerg Med J. 2005 Apr;22(4):272-3. doi: 10.1136/emj.2005.023507. PMID: 15788828; PMCID: PMC1726748
38. Caballero T, Baeza ML, Cabañas R, Campos A, Cimbollek S, Gómez-Traseira C, González-Quevedo T, Guilarte M, Jurado-Palomo GJ, Larco JI, López-Serrano MC, López-Trascasa M, Marcos C, Muñoz-Caro JM, Pedrosa M, Prior N, Rubio M, Sala-Cunill A; Spanish Study Group on Bradykinin-Induced Angioedema; Grupo Español de Estudio del Angioedema mediado por Bradicinina. Consensus statement on the diagnosis, management, and treatment of angioedema mediated by bradykinin. Part I. Classification, epidemiology, pathophysiology, genetics, clinical symptoms, and diagnosis. J Investig Allergol Clin Immunol. 2011;21(5):333-47; quiz follow 347. Erratum in: J Investig Allergol Clin Immunol. 2012;22(2):3 p following 153. PMID: 21905496
39. Campo P, Fernandez TD, Canto G, Mayorga C. Angioedema induced by angiotensin-converting enzyme inhibitors. Curr Opin Allergy Clin Immunol. 2013 Aug;13(4):337-44. doi: 10.1097/ACI.0b013e328362b835. PMID: 23743513
40. Cugno M, Nussberger J, Cicardi M, Agostoni A. Bradykinin and the pathophysiology of angioedema. Int Immunopharmacol. 2003 Mar;3(3):311-7. doi: 10.1016/S1567-5769(02)00162-5. PMID: 12639808
41. Miller DR, Oliveria SA, Berlowitz DR, Fincke BG, Stang P, Lillienfeld DE. Angioedema incidence in US veterans initiating angiotensin-converting enzyme inhibitors. Hypertension. 2008 Jun;51(6):1624-30. doi: 10.1161/HYPERTENSIONAHA.108.110270. Epub 2008 Apr 14. PMID: 18413488
42. Moellman JJ, Bernstein JA, Lindsell C, Banerji A, Busse PJ, Camargo CA Jr, Collins SP, Craig TJ, Lumry WR, Nowak R, Pines JM, Raja AS, Riedl M, Ward MJ, Zuraw BL, Diercks D, Hiestand B, Campbell RL, Schneider S, Sinert R; American College of Allergy, Asthma & Immunology (ACAAI); Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014 Apr;21(4):469-84. doi: 10.1111/acem.12341. PMID: 24730413; PMCID: PMC4100605
43. Schick M, Grether-Jones K. Point-of-Care Sonographic Findings in Acute Upper Airway Edema. West J Emerg Med. 2016 Nov;17(6):822-826. doi: 10.5811/westjem.2016.9.31528. Epub 2016 Oct 4. PMID: 27833699; PMCID: PMC5102618
44. LoVerde D, Files DC, Krishnaswamy G. Angioedema. Crit Care Med. 2017 Apr;45(4):725-735. doi: 10.1097/CCM.0000000000002281. PMID: 28291095
45. Beauchêne C, Martins-Héricher J, Denis D, Martin L, Maillard H. Intérêt de l’acide tranexamique en traitement d’urgence de première intention des crises d’angiœdème bradykinique sous IEC [Tranexamic acid as first-line emergency treatment for episodes of bradykinin-mediated angioedema induced by ACE inhibitors]. Rev Med Interne. 2018 Oct;39(10):772-776. French. doi: 10.1016/j.revmed.2018.04.014. Epub 2018 May 4. PMID: 29735174
46. Caballero T, Baeza ML, Cabañas R, Campos A, Cimbollek S, Gómez-Traseira C, González-Quevedo T, Guilarte M, Jurado-Palomo GJ, Larco JI, López-Serrano MC, López-Trascasa M, Marcos C, Muñoz-Caro JM, Pedrosa M, Prior N, Rubio M, Sala-Cunill A; Spanish Study Group on Bradykinin-Induced Angioedema; Grupo Español de Estudio del Angioedema mediado por Bradicinina. Consensus statement on the diagnosis, management, and treatment of angioedema mediated by bradykinin. Part I. Classification, epidemiology, pathophysiology, genetics, clinical symptoms, and diagnosis. J Investig Allergol Clin Immunol. 2011;21(5):333-47; quiz follow 347. Erratum in: J Investig Allergol Clin Immunol. 2012;22(2):3 p following 153. PMID: 21905496
47. Saeb A, Hagglund KH, Cigolle CT. Using Fresh Frozen Plasma for Acute Airway Angioedema to Prevent Intubation in the Emergency Department: A Retrospective Cohort Study. Emerg Med Int. 2016;2016:6091510. doi: 10.1155/2016/6091510. Epub 2016 Feb 3. PMID: 26953061; PMCID: PMC4756140
48. Lewis LM, Graffeo C, Crosley P, Klausner HA, Clark CL, Frank A, Miner J, Iarrobino R, Chyung Y. Ecallantide for the acute treatment of angiotensin-converting enzyme inhibitor-induced angioedema: a multicenter, randomized, controlled trial. Ann Emerg Med. 2015 Feb;65(2):204-13. doi: 10.1016/j.annemergmed.2014.07.014. Epub 2014 Aug 30. PMID: 25182544
49. Sinert R, Levy P, Bernstein JA, Body R, Sivilotti MLA, Moellman J, Schranz J, Baptista J, Kimura A, Nothaft W; CAMEO study group. Randomized Trial of Icatibant for Angiotensin-Converting Enzyme Inhibitor-Induced Upper Airway Angioedema. J Allergy Clin Immunol Pract. 2017 Sep-Oct;5(5):1402-1409.e3. doi: 10.1016/j.jaip.2017.03.003. Epub 2017 May 25. PMID: 28552382
50. Sinert R, Levy P, Bernstein JA, Body R, Sivilotti MLA, Moellman J, Schranz J, Baptista J, Kimura A, Nothaft W; CAMEO study group. Randomized Trial of Icatibant for Angiotensin-Converting Enzyme Inhibitor-Induced Upper Airway Angioedema. J Allergy Clin Immunol Pract. 2017 Sep-Oct;5(5):1402-1409.e3. doi: 10.1016/j.jaip.2017.03.003. Epub 2017 May 25. PMID: 28552382



Add comment