October 24, 2020 by Shahriar Lahouti. Last update September 13, 2023.
CONTENTS
- Preface
- Nomenclature
- Clinical presentation
- Diagnosis
- Differential diagnosis
- Initial evaluation and risk stratification
- Pathophysiology
- Initial resuscitation of high-risk PE
- Anticoagulation
- Systemic thrombolysis
- Other treatments
- Algorithm
- Special situations
- RECAP
- Appendix
- Media
- Going further
- References
Preface
Venous thromboembolism (VTE), clinically presenting as DVT or pulmonary embolism(PE), is globally the third most frequent acute cardiovascular syndrome behind myocardial infarction and stroke 1. PE is a life-threatening condition and a leading cause of morbidity and mortality. It is a heterogeneous disease. Some patients look great while others are profoundly ill. Even among the latter group, the clinical manifestation, and outcome significantly vary among the patients.
Not to mention the fact that patients can evolve rapidly in one direction or the other. Studies have shown that patients with hemodynamic instability from PE are more likely to die within the first two hours and the risk remains high for up to 72 hours after presentation, which implies the necessity for employing emergent appropriate diagnostic and therapeutic measures.
There have been many advances in the field of PE in recent years and the therapeutic options can range from anticoagulation alone, catheter-directed thrombolysis, systemic thrombolysis, catheter embolectomy, surgical embolectomy, and/or mechanical circulatory support such as extracorporeal membrane oxygenation (ECMO).
In response to increasing patient complexity and increasing therapeutic alternatives, there has been a rise in the development of multidisciplinary groups of clinicians with expertise in the diagnosis and medical, surgical, and interventional management of PE who collaborate in a novel way to improve patient care. This multidisciplinary team approach is termed the PERT (Pulmonary Embolism Response Team).
In the following discussion, the approach to the management of PE is explored (which is for the most part consistent with the PERT consortium consensus practices 2 and the recommendation from the Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology 3).
Nomenclature
PE can be classified by several parameters including; the hemodynamic status of the patient, anatomic location of the thrombus, and the temporal pattern of presentation.
The hemodynamic status (unstable vs. stable)
- This classification is the ground for risk stratifying PE into high, intermediate, and low risk (more on this below).
- Hemodynamically unstable PE is the one that causes “hypotension”; traditionally defined as systolic blood pressure (SBP) < 90 mmHg or a drop in SBP of ≥40 mmHg from baseline for a period >15 minutes or hypotension that requires vasopressor support and is not explained by other etiologies such as hypovolemia, sepsis, etc 3.
- This heterogeneous group of patients is at high risk of adverse cardiovascular events and the term “Massive” PE (MPE) is applied accordingly.
- The word ‘massive’ may seem to you a PE with a large clot, as the word massive may imply; however, it has been shown that among patients with a large clot (e.g. saddle PE) only 22% are hemodynamically unstable, with an associated mortality of 5%. 4 5
- Size Does Not Matter!
- In fact there are several other parameters in addition to the degree of mechanical obstruction (i.e. size of the clot) that may contribute to hemodynamic instability such as individual physiologic reserve and degree of pulmonary vasoconstriction mediated by platelet activation (more on this here) 6.
- The spectrum of severity and risk of death varies among individuals as some patients are dependent on high-dose vasopressor with symptoms and signs of end-organ hypoperfusion (obstructive shock ≈ crashing MPE) vs. those with low dose pressor requirement to keep their SBP above 90mmHg (non-crashing MPE) 7 8 9.
- This heterogeneous group of patients is at high risk of adverse cardiovascular events and the term “Massive” PE (MPE) is applied accordingly.
- Hemodynamically stable PE is defined as PE that does not meet the definition of hemodynamically unstable PE.
- This is a heterogeneous group with some patients presenting with right ventricle dysfunction and mild or borderline hypotension that stabilizes in response to fluid therapy (a.ka. “Submassive” or “Intermediate-risk” PE) vs. those patients presenting with small, mildly symptomatic or asymptomatic PE (also known as “low-risk PE“) 10.
- Hemodynamically unstable PE is the one that causes “hypotension”; traditionally defined as systolic blood pressure (SBP) < 90 mmHg or a drop in SBP of ≥40 mmHg from baseline for a period >15 minutes or hypotension that requires vasopressor support and is not explained by other etiologies such as hypovolemia, sepsis, etc 3.
The anatomic location
- Defines the location of the clot within the pulmonary artery (saddle, lobar, segmental, or subsegmental).
- Saddle PE
- Refers to the clots at the bifurcation of the main pulmonary artery, often extending into the right and left main pulmonary arteries (below figure).
- Traditionally saddle PE was believed to be associated with hemodynamic instability and death, but as explained earlier, several retrospective studies show that this is not true. 4 5
- Central PE
- Lobar, segmental, and subsegmental PE
- Refers to embolism that is lodged at lobar, segmental, or subsegmental branches of a pulmonary artery.
- Clot in transit (CIT)
- A clot that is “In Transit” through the right heart is often classified as a form of PE, even though the thrombus may have not yet lodged in a pulmonary artery 15.
- Saddle PE
The temporal pattern of presentation
- Patients with PE can present acutely, subacutely, or chronically.
- Acute: Patients with acute PE typically develop a rapid onset of symptoms and signs immediately after obstruction of pulmonary vessels.
- Acute onset or accelerating symptoms are worrisome.
- Subacute: Some patients with PE may also present with stable symptoms for several days or weeks following the initial event.
- Subacute presentation reduces the risk of sudden deterioration.
- Chronic: Some patients are symptomatic for > 10-14 days; suggesting a more chronic thrombus (over time, clots organize and become less responsive to thrombolysis).
- Acute: Patients with acute PE typically develop a rapid onset of symptoms and signs immediately after obstruction of pulmonary vessels.

Clinical presentation
The clinical signs and symptoms of acute PE are non-specific and highly variable from asymptomatic to full-blown cardiac arrest among the patients. Given this nonspecificity, knowledge of predisposing factors to VTE is important to determine the clinical probability of the disease. However, in 40% of patients with PE, no predisposing factors are found.16
The most common presenting signs and symptoms include 17:
- Dyspnea (73%)
- It may be acute and severe in central and extensive PE; but mild and transient in small peripheral PE.
- Syncope, presyncope (10%)
- Chest pain
- In small peripheral PE, chest pain often is pleuritic and caused by irritation of the pleura following pulmonary infarction.
- In extensive central PE; chest pain may have a typical angina character, possibly reflecting RV ischemia, and requiring differential diagnosis from an acute coronary syndrome or aortic dissection.
- Hemoptysis (13%)
- In the context of PE, hemoptysis is a result of pulmonary infarction often seen with small peripheral PE, or typically later in the course of PE with a central clot (when it breaks down and the fragments migrate distally).
- Since bleeding reflects necrosis of lung tissue, it originates from the small pulmonary capillaries and veins, hence it is often minor; unless the patient has preexisting conditions such as lung cancer, aspergilloma, cystic fibrosis, or vascular malformations.18
- 👉Keep in mind that the presence of minor hemoptysis is not a barrier to PE management (i.e. anticoagulation can be continued), unless the hemoptysis is unusually brisk.
- Silent PE
- One systematic review found that, among the 5233 patients who had a DVT, one-third also had asymptomatic PE. 19
- Sudden cardiac arrest or circulatory collapse (8%) 20 21 22
- Hypoxemia
- It is a frequent finding but <40% of patients have normal arterial oxygen saturation.23
- A normal arterial SO2 does not rule out the presence of PE.
- Tachypnea (54%)
- Tachycardia
- Generally, sinus tachycardia reflects activation of neurohormonal mechanisms (↑sympathetic discharge) in the face of ↑RV afterload.
- Sinus tachycardia is present in < 40% of patients.
- Atrial arrhythmias, most frequently atrial fibrillation, may be associated with acute PE.
- DVT (70%)
- Calf or thigh swelling, erythema, edema, tenderness, palpable cords.
Diagnosis
Definitive imaging includes computed tomographic pulmonary angiography (CTPA) and less commonly, ventilation perfusion scanning or other imaging modalities.
- For patients who are hemodynamically unstable and in whom definitive imaging is unsafe, bedside echocardiography or venous compression ultrasound may be used to obtain a presumptive diagnosis of PE to justify the administration of potentially life-saving therapies. 3
The diagnostic approach to PE will be discussed in detail separately. Here, the utility of diagnostic tools and helpful clues are briefly reviewed.
- Computed tomographic pulmonary angiography (CTPA)
- CTPA is considered diagnostic of PE if it shows PE at the segmental or more proximal level (the diagnostic imaging findings in CTPA for PE are discussed separately). See the media below.
- If there’s a high clinical suspicion for major PE in a patient with renal insufficiency, failing to get a CTPA for the fear of “contrast nephropathy” is a common mistake as such an entity probably does not exist! 52 53 (more on this here).
- Hints: Pleural effusion in PE
- In an appropriate clinical context (predisposing risk factors for VTE, pleuritic chest pain), presence of pleural effusion should raise clinical suspicion for PE 54 55. Nearly all pleural effusions due to pulmonary embolism are exudates, frequently hemorrhagic.
- Note that the presence of bloody pleural fluid is not a contraindication for the administration of anticoagulant therapy. 56
- Hints: Pleural effusion in PE
- D-dimer
- Diagnostic significance
- An elevated D-dimer alone is insufficient to make a diagnosis of PE, but a normal D-dimer can be used to rule out PE in patients with a low or intermediate probability of PE. D-dimer testing should be used in conjunction with clinical probability assessment (discussed separately).
- While D-dimer assays are highly sensitive, their specificity is low, usually between 40% and 60%.
- D-dimer results are often falsely positive, and the proportion of false positive results increases in certain clinical conditions (appendix 2).
- Adjusted D-dimer levels based on certain criteria have been proposed and may be considered as an alternative in patients with a low probability or low intermediate probability for PE. This should not be used in those with high probability or intermediate-high probability for PE.
- Age-adjusted D-dimer
- D-dimer levels rise with age such that using the traditional cutoff value of < 500 ng/mL (fibrinogen equivalent units) results in reduced specificity of D-dimer testing in older patients (>50 years), a population in whom PE is common.
- The most commonly used formula for age adjustment is Age (if over 50 years) x 10 ng/mL (or x 0.056 nmol/L).
- The reference value for D-dimer is presented in Appendix 1.
- Diagnostic significance
- Role of echocardiography in the diagnosis of PE
-
- Overall, the sensitivity of TTE for the diagnosis of PE is about 50-60% while the specificity is around 80-90%. TTE is normal in about 50% of unselected patients with acute PE, but it can provide direct and/or indirect evidence for the diagnosis.
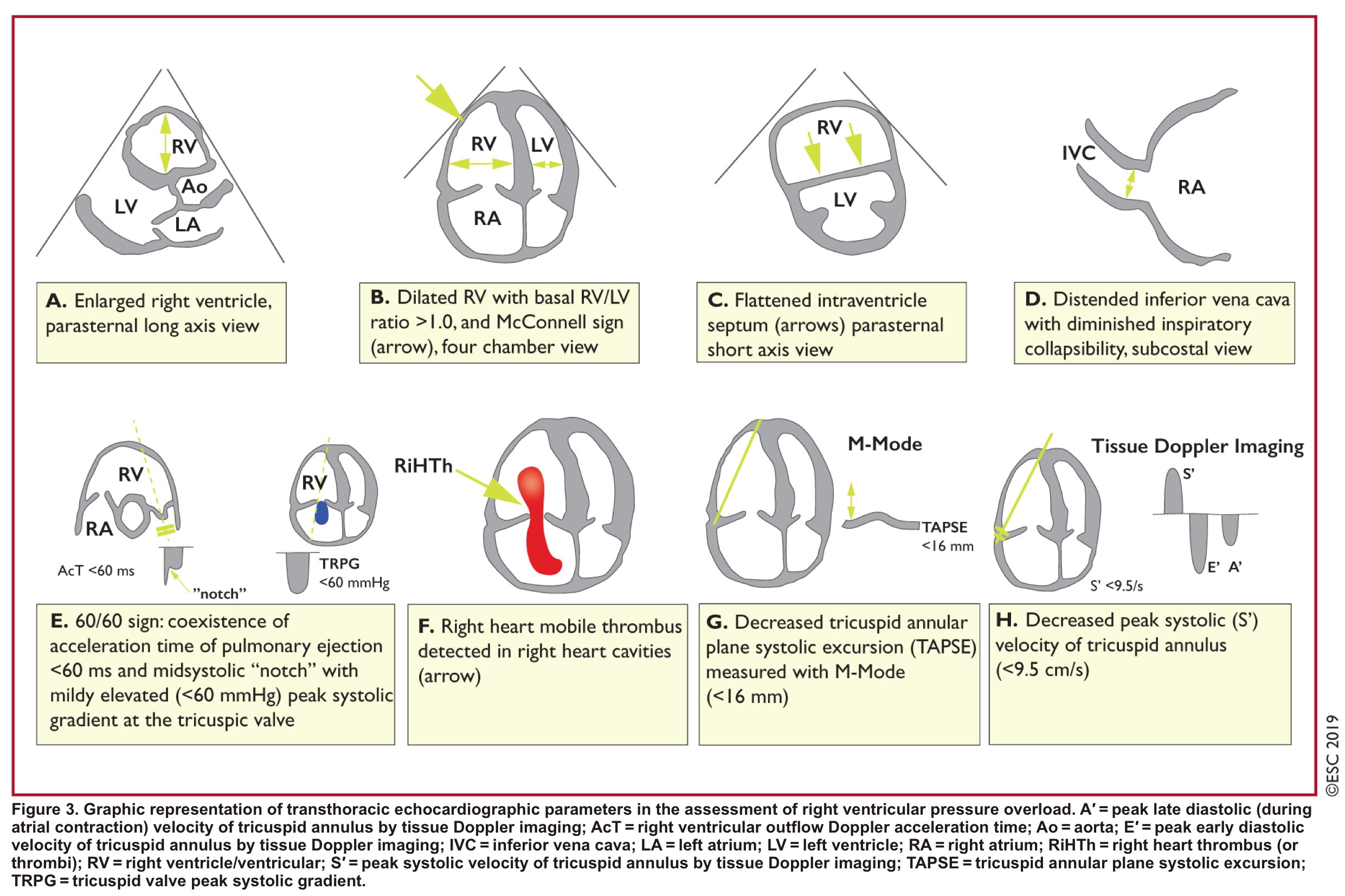
- Direct signs for PE
- Clot in transit defined as a large, mobile (i.e. unattached to any intracardiac structure), serpentine echogenicity trapped in the right heart chambers or pulmonary artery is rare, but makes the diagnosis evident.
- Indirect signs for PE (though nonspecific). Signs of RV strain
- Systolic/diastolic septal shift (D-shaped left ventricle).
- Dilatation of RA, RV (end-diastolic basal RV/LV >1.0).
- Global RV hypokinesia
- McConnell sign
- RV hypokinesia sparing the apax. It is not specific (specificity 82%) for PE and can be seen with RVMI as well. It has poor sensitivity (~45%) 3.
- Pulmonary hypertension ~ 40-50 mmHg (>60 mmHg in the case of pre-existing pulmonary hypertension). 👉See here.
- Mild to severe TR
- Secondary TR is frequent in patients with intermediate to high-risk PE allowing estimation of systolic pulmonary arterial pressure (sPAP).
- As the RV is only able to generate a sPAP of up to 60 mmHg acutely, in the acute setting the TR jet velocities are expected to be no higher than 2.5-3.5 m/s, corresponding to a sPAP of about 40-50 mmHg in acute PE.
- Conversely, a sPAP 60 mmHg may suggest a more chronic process, relating to repeated episodes of PE, or chronic pulmonary parenchymal disease and/or superimposed PE.
- Pulmonary acceleration time < 60 ms is in favor of PE.
See here.
- Pulmonary systolic notch
- Normal pulmonary systolic flow pattern has no notching. As pulmonary vascular resistance is increased, the Doppler flow wave will reflect backward to the RV during systole, which impedes RV ejection and causes Doppler envelope notching.
- Presence of early systolic notching (ESN) indicates mildly elevated mean PAP (the notch is defined early if it falls within the initial 50% of the ejection time). Moreover the presence of ‘ESN’ identifies patients with Massive or submassive PE.
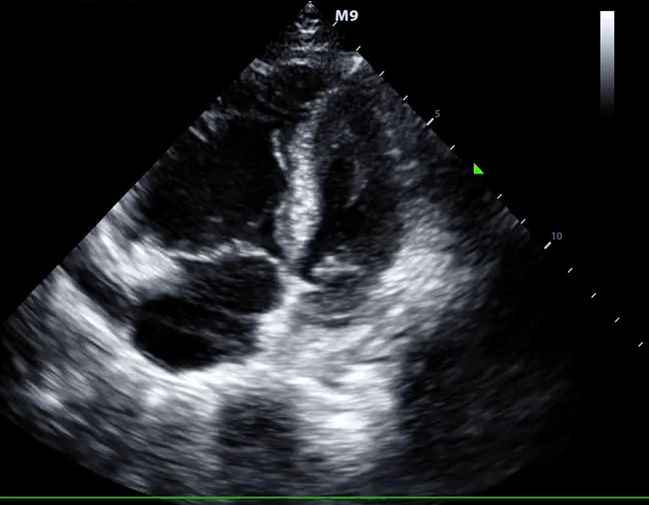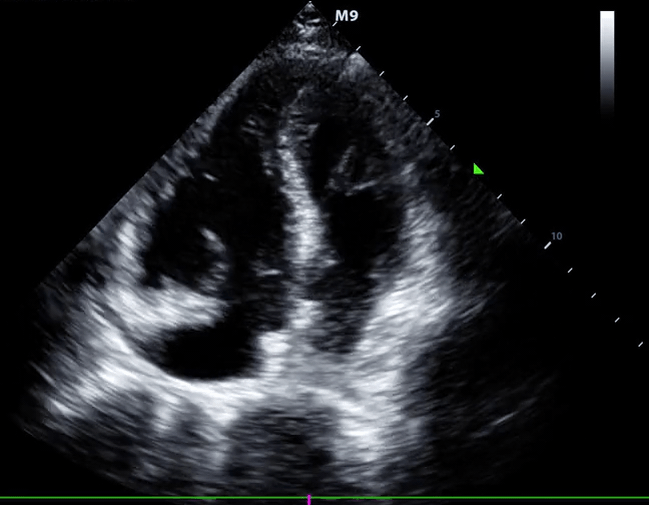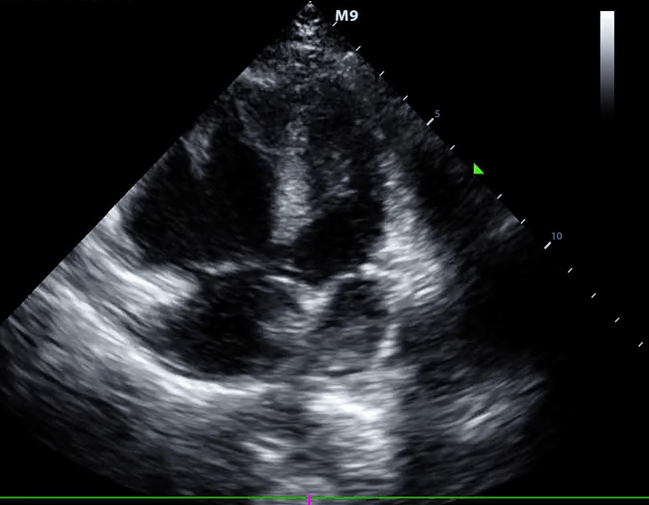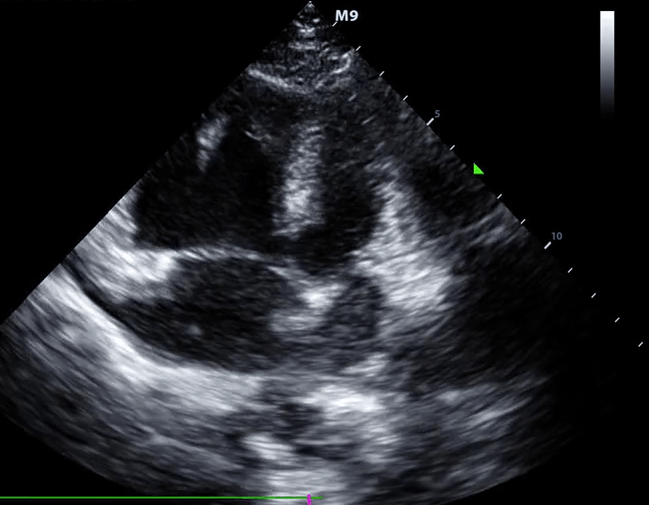
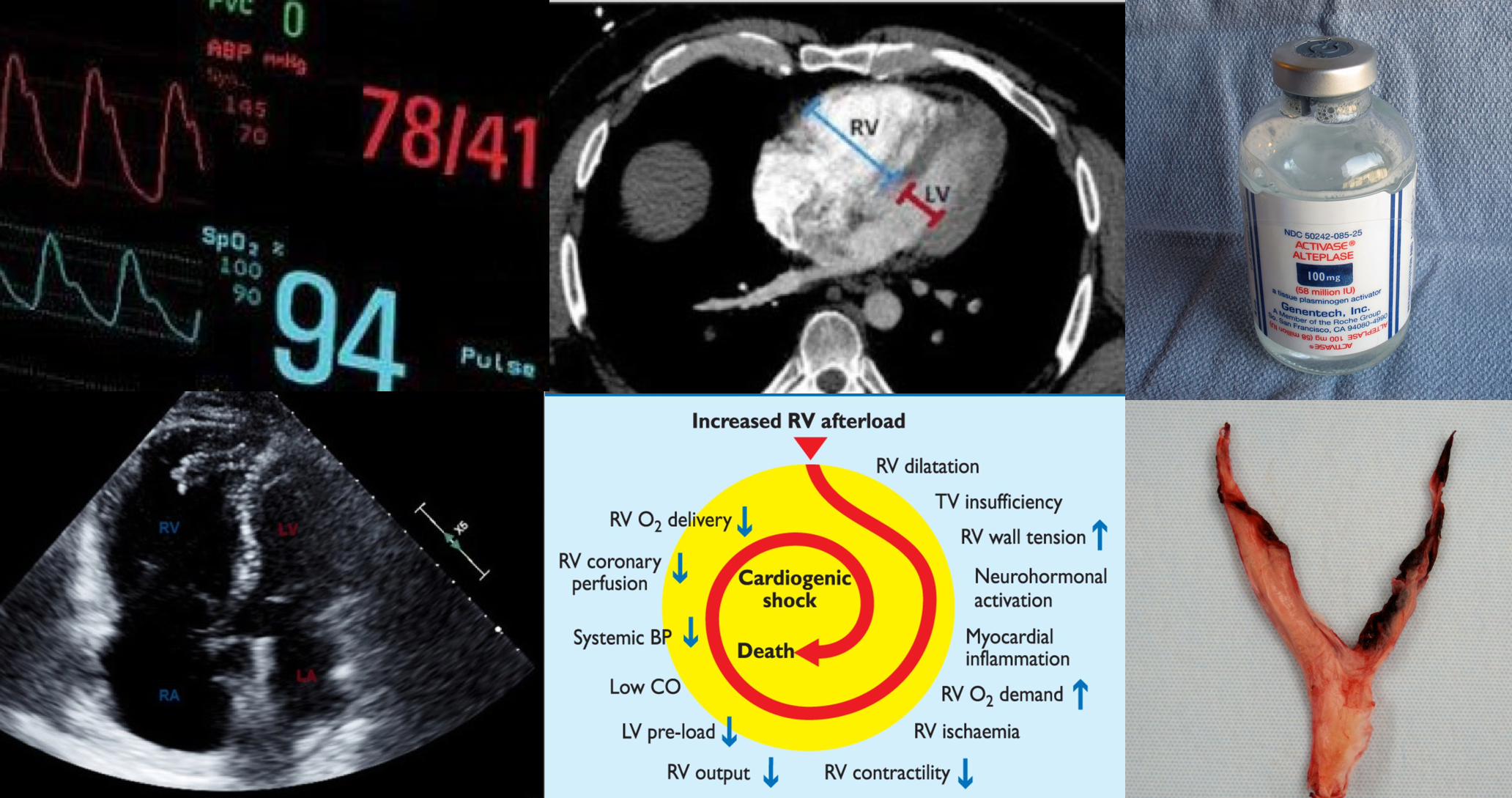
McConnell’s sign: Hypokinesis of the RV with sparing of the apex.
-
- Interpretation of findings
- Bedside echo is extremely useful in unstable patients with suspected high-risk PE. In these patients absence of echo signs of RV overload or dysfunction excludes PE as the cause of hemodynamic instability.
- In unstable patients, echocardiography may be of further help in the differential diagnosis of the cause of shock, by detecting pericardial tamponade, acute valvular dysfunction, severe global or regional LV dysfunction, aortic dissection, or hypovolemia.
- In an unstable patient with suspected PE, the presence of large clot-in-transit or unequivocal signs of RV pressure overload, especially those findings that are more specific for PE, justify emergency reperfusion treatment for PE if immediate CTPA is not feasible in a patient with high clinical probability and no other obvious causes for RV pressure overload.
- Echo findings in chronic RV dysfunction
- It may be seen in pulmonary hypertension and this should not be misinterpreted as evidence of a massive or submissive PE. Echocardiographic signs that are more suggestive of a chronic RV pressure overload may include (more on this here):
- Increased RV wall thickness (>5mm in subxiphoid view).
- A very high tricuspid regurgitant jet velocity (>3.4 m/s or a PAP of >60mmHg).
- Presence of pericardial effusion.
- In these cases, chronic thromboembolic (or other) pulmonary hypertension should be included in the differential diagnosis and if clinical suspicion for PE is high, a more comprehensive workup and imaging (CTPA, V/Q scan) is warranted.
- It may be seen in pulmonary hypertension and this should not be misinterpreted as evidence of a massive or submissive PE. Echocardiographic signs that are more suggestive of a chronic RV pressure overload may include (more on this here):
- Bedside echo is extremely useful in unstable patients with suspected high-risk PE. In these patients absence of echo signs of RV overload or dysfunction excludes PE as the cause of hemodynamic instability.
- Interpretation of findings
Differential diagnosis
Critical signs & symptoms
For patients who present with signs and symptoms of PE, the differential diagnosis depends upon the presenting signs and symptoms (e.g. dyspnea, chest pain, syncope, etc). By and large, the major competing diagnoses may include (not a comprehensive list):
- Critical DDx of chest pain
- Acute myocardial infarction, acute aortic syndromes, pericarditis, PE
- Critical DDx of dyspnea
- Heart failure, PE, pneumothorax, acute exacerbations of chronic lung disease, pneumonia, metabolic e.g. diabetic ketoacidosis.
- Critical DDx of syncope
- In appropriate clinical context, the critical conditions associated with syncope may include:
- PE
- Tamponade
- Malignant arrhythmia
- Acute aortic syndromes
- Left ventricular outflow tract obstruction
- Subarachnoid hemorrhage
- Severe volume loss (e.g. GI bleeding, ruptured ectopic pregnancy).
- In appropriate clinical context, the critical conditions associated with syncope may include:
Unstable PE
- Overview
- Most cases of obstructive shock are due to right ventricular failure from hemodynamically significant pulmonary embolism. However, in critically ill patients, shock is often multifactorial in practice for example pulmonary embolism plus sepsis (more on this here).
- In this population diagnosis and therapy are often approached simultaneously. However, diagnosis is the focus of discussion here. Accordingly, two issues should be carefully considered:
- Does this patient with hemodynamic instability have PE?
- Is this hemodynamic instability driven by PE or are other causes present (multifactorial shock)?
- Since thrombolysis is only beneficial when PE is driving the instability, it is crucially important to consider “if the PE is driving the patient’s instability”.
- Does this patient with hemodynamic instability have PE?
- Diagnostic difficulties: In unstable patients suspected of PE, sometimes performing CTPA is not feasible. In this situation, one should be able to determine the likelihood of the presence of PE by bedside diagnostic tools.
- Diagnostic tools and clues: Presence of the following findings could be helpful in making a presumptive diagnosis of PE in an appropriate clinical context 3:
- History: Presence of predisposing risk factors
- POCUS: Evidence of DVT
- In the majority of cases (70%) PE originates from DVT mostly in the lower limbs, and only rarely from the upper limb (mostly following venous catheterization).
- Bedside Echo: Direct or indirect evidence of PE
- Presence of a clot in the right heart.
- Presence of RV dysfunction and a dilated IVC (especially in the absence of other obvious causes for RV pressure overload such as pulmonary hypertension).
- Lung ultrasound or auscultation
- Clear lung fields on auscultation or absence of B-lines on ultrasound (especially in patients without a history of cardiopulmonary disease).
- Is this PE driving the hemodynamic instability?
- It is critically important to determine if the PE is driving the hemodynamic instability (figure 1 below) since it has therapeutic implications.
- Evaluation
- POCUS: Global hemodynamic assessment
- If PE is driving the instability, there should be at least a dilated IVC and dilated RV (especially in those patients without preexisting cardiopulmonary conditions). Otherwise, consider whether another process is causing the patient’s instability (e.g. hypovolemic shock plus small PE).
- In the absence of clear clinical evidence of volume loss (profound diarrhea, GI bleeding), a virtual IVC is against the diagnosis of MPE. 3
- POCUS: Global hemodynamic assessment
-
-
- CTPA: Clot burden
- In order to blame the patient’s instability on PE, there should be a moderate to large clot burden on the CT scan. If there is only a small amount of clot, PE probably is not causing the instability. For example, a patient with right ventricular dilation and small clot burden may have chronic pulmonary hypertension.
- However, the precise thrombus burden (as measured by percentage or semi-quantitative indexes such as Mastora or Qanadli) has been shown to not correlate well with the adverse outcomes 24 25 26.
- CTPA: Clot burden
-
PE in cardiac arrest
- Epidemiology
- PE has been reported to be associated with 5 to 13% of unexplained cardiac arrest 27.
- Mortality related to cardiac arrest caused by PE is high (≈95%) 28.
- Approximately 90% of episodes of cardiac arrest following PE have been reported to occur within 1-2 hours of symptom onset.
- PE has been reported in up to 63% of patients presented with pulseless electrical activity (PEA) as the initial cardiac rhythm, whereas asystole (32%) and ventricular fibrillation (5%) were responsible for the cases respectively (more on PEA👉 here) 29.
- DDx of PEA (more on this here)
- Tamponade, tension pneumothorax or hemothorax, PE.
- Acute myocardial infarction, hyperkalemia, sodium channel blocker intoxication.
- Hypovolemia (catastrophic bleeding such as aortic dissection, massive GI bleeding, etc) 36.
- Diagnosis
- The diagnosis of PE as a possible cause of unexplained cardiac arrest would be more likely in the presence of the following factors:
- Symptoms before the arrest
- In a study, the most commonly reported symptoms before cardiac arrest were syncope and acute onset of dyspnea.
- This is in contrast to cardiac arrest following coronary events or aortic dissection where the acute onset of chest pain is reported to be the most common symptom before the cardiac arrest 29.
- Presence of predisposing conditions
- The presence of underlying risk factors (e.g. major surgery and immobilization, or history of VTE, etc) will increase the probability of PE; although up to 30% of patients presenting with PE have no underlying risk factors 30.
- Pulseless electrical activity
- The initial rhythm in most patients with PE in arrest is PEA (63%).
- The presence of a clot in the right-sided heart chamber
- DVT: Ultrasonographic evidence for DVT will increase the possibility of PE, although the absence of DVT does not rule out PE 31.
- Symptoms before the arrest
- The diagnosis of PE as a possible cause of unexplained cardiac arrest would be more likely in the presence of the following factors:
- What does not indicate PE as a cause of unexplained cardiac arrest?
- While it was believed that RV enlargement during cardiac arrest on echo suggests PE, several recent studies have disproved this belief.
- In fact, RV enlargement can be seen with other possible causes of cardiac arrest such as hyperkalemia, and primary arrhythmia (VF), and this finding should Not be translated to the presence of PE 32 33 34 35.
Initial evaluation and risk stratification
Primary factors
- When a patient with suspected PE presents with hemodynamic instability, physiological-based resuscitation is started promptly (more on this below) while the decision is being contemplated for definitive therapy.
- Definitive treatment could range from full-dose systemic thrombolytic (ST), reduced-dose ST, catheter-directed thrombolysis (CDL), catheter embolectomy, and surgical embolectomy.
- To determine the line of appropriate treatment, one should assess the risk of early cardiovascular death (PE severity). The principle of risk stratification is based on the fact that “whether the hemodynamic burden of the clot is severe enough to cause RV failure and obstructive shock.
- As shown below (figure 2), risk stratification is performed by evaluating the parameters which can guide us to learn more about the hemodynamic impact of the clot.
- History
- General appearance
- Are there features of poor organ perfusion (shock state)? The worrisome findings include:
- A sense of impending doom: If the patient volunteers a sensation that they are dying, they’re often right.
- Confusion, agitation, diaphoresis
- Mottling
- Cool extremities
- Are there features of poor organ perfusion (shock state)? The worrisome findings include:
- Vital signs
- Are there findings of shock (obstructive) or impending to arrest?
- Hypotension: Traditionally, it is the pivotal parameter that defines the massive pulmonary embolism (MPE).
- SBP < 90 mmHg for 15 minutes.
- Fall in SBP by >40 mmHg for 15 minutes.
- Requirement for vasopressors to keep SBP > 90 mmHg.
- Tachycardia (HR>120/min) and shock index (SI) >1: suggest a poor hemodynamic reserve and a worse prognosis 39 40.
- Bradycardia is the most worrisome: May be a harbinger of impending brady-asystolic arrest (often how these patients die) 3.
- Tachypnea: severely elevated RR (e.g.> 30 breaths/minute) 41 42.
- Hypotension: Traditionally, it is the pivotal parameter that defines the massive pulmonary embolism (MPE).
- Are there findings of shock (obstructive) or impending to arrest?
- Imaging
- For a clot to cause hemodynamic instability, some degree of RV dysfunction should be present. “Is there evidence of right ventricle dysfunction”?
- Echocardiography
- It provides crucially important information to risk stratify presumptive PE.
- In the presence of MPE; there would be some evidence for RV dysfunction. These parameters are graphically presented above, among which the following two have been shown to be associated with unfavorable prognosis 3:
- RV/LV diameter ratio ≧ 1.0
- TAPSE <16 mm
- In addition to RV dysfunction, echo can identify the presence of a clot in the right heart (CIT, clot in transit) and patent foramen ovale (PFO), and a right-to-left shunt through PFO.
- Clot that is in transit through the heart is often classified as a form of PE, even though the thrombus has not yet lodged in a pulmonary artery 43 44 45. A large clot puts pulmonary circulation in danger as it is likely to break off at some point in the future.
- It has been reported to be associated with high mortality (odds ratio of 3 for all-cause-related mortality and 4.8 for PE-related mortality).
- Generally, the presence of a large clot in transit moves the patient’s severity classification up by one class (e.g. a patient with an otherwise low-risk submassive PE who is found to have a large CIT would be reclassified as having a high-risk submassive PE).
- PFO: In patients with major pulmonary embolism, echocardiographic detection of a PFO signifies a particularly high risk of death and arterial thromboembolic complications 46. A PFO also increases the risk of ischemic stroke due to paradoxical embolism in patients with acute PE and RV dysfunction 47.
- Echocardiography
- For a clot to cause hemodynamic instability, some degree of RV dysfunction should be present. “Is there evidence of right ventricle dysfunction”?
-
- Computed Tomographic Pulmonary Angiography (CTPA)
-
- RV dilation
- RV/LV ratio of ≧ 1.0 on CT is associated with a 2.5-fold increased risk for all-cause mortality, and with a five-fold risk for PE-related mortality 48.
- Mild RV dilation (RV/LV slightly above 0.9) on CT is a frequent finding (>50% of hemodynamically stable PE patients), and it probably has minor prognostic significance 49. Therefore if RV dilation on CT is equivocal, additional signs of RV dysfunction should be sought either on CT scan or echocardiography.
- Bowing of the RV into the LV
- This may be best observed in a coronal CT projection (as with echocardiography, evaluation of the heart in multiple planes may improve diagnostic accuracy) 3.
- Contrast reflux into the inferior vena cava and hepatic vein
- RV dilation
-
- Computed Tomographic Pulmonary Angiography (CTPA)
5. Laboratory
- Troponin
- Elevated troponin is associated with increased mortality risk (odds ratio of 5) 57.
- The cutoff values might be troponin I >0.1 ng/ml or troponin T >0.03 ng/ml 58.
- Increased circulating levels of troponins per se have relatively low specificity and positive predictive value for early mortality in normotensive patients with acute PE.
- However, when interpreted in combination with clinical and imaging findings, they may improve the identification of elevated PE-related risk and the further prognostic stratification of such patients.
- Age-adjusted high-sensitivity troponin T cut-off values (≧14 pg/mL for patients aged <75 years and ≧45 pg/mL for those ≧75 years) may further improve the negative predictive value of this biomarker 59.
- Lactate
6. ECG
- ECG signs of (sub) massive PE include RV strain pattern and RV injury pattern.
-
- RV strain pattern
- As the clot is lodged in the pulmonary artery, there will be resistance to blood flow from the right ventricle and increased pulmonary artery pressures. This causes strain on the right ventricle.
- ECG signs of RV strain may include:
- T-wave inversions in the right precordial leads (V1-4) ± the inferior leads (II, III, aVF). 👉Appendix 3.
- QR pattern in V1.
- S1Q3T3 pattern.
- Incomplete or complete right bundle branch block.
- RV injury pattern
- Impairment of RV coronary perfusion (due to the high afterload and low systemic blood pressure) can cause RV injury pattern 63.
- ECG signs of RV injury pattern may include:
- These patterns can be frequently mistaken for acute myocardial infarction. In cases of diagnostic uncertainty regarding ST elevation MI vs. PE, perform immediate bedside echocardiography (see here).
- ST elevation MI should cause regional wall motion abnormalities involving the left ventricle and dysfunction of the left ventricle.
- Massive PE will cause RV dilation and usually an under-filled left ventricle (which is vigorously contracting).
- RV strain pattern
Risk stratification
Overview
Acute PE walks through a spectrum of disease severity. It can be classified into high, intermediate, and low-risk groups. Given the disease heterogeneity, sometimes patients do not perfectly fit into any of the above categories.
High-risk PE (massive PE)
- It has been shown that high-risk PE is associated with a mortality rate of 25-65%. 66
- The characteristic feature of MPE that distinguishes this group from the others is hemodynamic instability in the presence of RV dysfunction.
- Lack of RV dysfunction argues against diagnosis of acute MPE and suggests that hemodynamic instability may be caused by another problem.
- Crashing MPE
- A subgroup of patients with MPE are at higher risk of impending cardiovascular collapse; i.e. ‘Crashing MPE’. These patients present with the typical features of patient impending to arrest including:
- Requirement for a higher dose of vasopressor (e.g. norepinephrine >>8mcg/min) to keep their SBP above 90 mmHg.
- Persistent bradycardia (e.g. HR<40) with features of shock.
- Altered, mottled, cold/clammy extremities, sensing of dying.
- Patients with PE who have survived cardiac arrest (status post cardiac arrest).
- A subgroup of patients with MPE are at higher risk of impending cardiovascular collapse; i.e. ‘Crashing MPE’. These patients present with the typical features of patient impending to arrest including:
Intermediate risk PE (Submassive PE)
- It is characterized clinically by hemodynamic stability, despite the fact that the clot burden on RV performance is significant enough to cause some degree of RV dysfunction 3. Patients with submassive PE (SMPE) can be further classified into two subgroups:
- High-risk SMPE: characterized by worrisome clinical or laboratory features such as:
- Troponin rise, or
- Elevated lactate, or
- Shock index (HR/SBP)>1, or
- Diaphoresis, severe tachypnea (e.g. RR>30), or
- Large CIT
- Low-risk SMPE: Other than evidence for RV dysfunction, they do not have worrisome laboratory findings.
- High-risk SMPE: characterized by worrisome clinical or laboratory features such as:
Low-risk PE
- Patients with clinically stable hemodynamic profile and no evidence of RV dysfunction (on echo/CT) 3.
| Keep in mind that the definition of massive and submassive PE is based on their impact on hemodynamic and RV performance. 👉The size of the clot is irrelevant. However, the absence of a large clot burden on CT is against the diagnosis of massive and submassive PE. 👉If performing CT is not possible, the presence of proximal DVT, or a large clot within the right heart is suggestive of (sub) Massive PE. |
Other prognostic factors
Other prognostic factors which are less useful are discussed below.
Brain natriuretic peptide (BNP) or NT-BNP
- Pressure overload on the heart is associated with increased myocardial stretch and leads to the release of BNP and NT-proBNP.
- However, it does not distinguish between right heart vs. left heart failure which makes it a nonspecific test for acute RV failure.
- Similar to cardiac troponins, elevated BNP or NT-proBNP concentrations possess low specificity and positive predictive value (for early mortality) in normotensive patients with PE, but low levels of BNP or NT-proBNP are capable of excluding an unfavorable early clinical outcome, with high sensitivity and a negative predictive value 69 70.
Elevated WBC count (>12.6 x 109/L)
- In a retrospective study including 14228 patients with PE, the association between the admission WBC count and 30‐day mortality and hospital readmission was examined 71.
- For patients with PE and an admission WBC count of > 12.6 ×109/L, the adjusted odds ratio for all-cause mortality was reported 2.2.
Neutrophil/lymphocyte ratio (NLR)
- The NLR is the ratio of neutrophils/lymphocytes.
- This ratio reflects occult physiologic stress because endogenous cortisol will increase the neutrophil count and reduce the lymphocyte count, thereby increasing the ratio. The NLR combines both of these changes, making it more sensitive than either alone.
- NLR may be elevated by any cause of physiologic stress. Therefore, this test will be predictive of PE-related mortality only in patients with isolated PE (no other active medical problems).
- Several meta-analyses suggest that elevated NLR may be an even stronger prognostic indicator than troponin 72 73 74.
- Studies used different cutoffs, but based on available data, NLR may be interpreted in the context of acute PE as follows:
- NLR <5.5 suggests low mortality risk (~2.7%)
- NLR >9.2 suggests high mortality risk (~26%)
- NLR in the range of 5.5-9.2 remains in the grey zone.
DVT
- The diagnosis of concomitant DVT has been identified as an adverse prognostic factor, being independently associated with death within the first 3 months after acute PE 75.
- In a meta-analysis investigating 8859 patients with PE, the presence of concomitant DVT was confirmed as a predictor of 30-day all-cause mortality (odds ratio of 1.9), although it did not predict PE-related adverse outcomes at 90 days 76.
- Thus, concomitant DVT can be regarded as an indicator of significant comorbidity in acute PE.
Clot burden on CT
- Definition
- High clot burden is poorly defined in the literature.
- In one study, an obstruction index of CTPA > 40% was defined as a large clot burden and reported to be associated with high mortality 77.
- The MOPPETT trial defined large clot burden as >70% involvement of the pulmonary vascular bed with embolism in ≥2 lobar arteries or main pulmonary arteries on CTPA or by a high probability ventilation/perfusion scan showing ventilation/perfusion mismatch in ≥2 lobes.78
- High clot burden is poorly defined in the literature.
- As explained earlier, the precise thrombus burden has not been shown to correlate well with the adverse outcomes. 24 25 26
- Bottom line
- Clot burden on CT can have diagnostic value in patients with hemodynamic instability, i.e. in unstable PE with small clot burden, one should consider other etiologies for hemodynamic instability such as hypovolemia and sepsis.
- From the standpoint of therapeutic decision-making (thrombolysis vs. anticoagulation alone) clot burden on CT per se is not useful unless other high-risk features for death from PE (e.g. hemodynamic instability, worsening clinical situation, worrisome laboratory markers) were present.
Comorbidities
- Patients with a decreased physiologic reserve and pre-existing cardiovascular and pulmonary conditions are barely tolerant to thromboemboli.
- Such patients can deteriorate and become hemodynamically unstable with even smaller clots.
- Keep in mind that the term “massive” PE does not necessarily describe the size of the PE as much as its hemodynamic effect.79
Prediction model for assessing severity of PE
There are several prognostication tools (📱 PESI, sPESI, Bova) developed for risk stratification in patients with pulmonary embolism who are hemodynamically stable. However, since none of these factors or scores definitively determines prognosis, clinician gestalt also plays an important role 2.
- One study of 11 clinical prognostic models reported that although the sensitivity of some models, including PESI and sPESI, was>89%, none had a specificity greater than 48%. 83
PESI (Pulmonary Embolism Severity Index) score & Simplified PESI score (sPESI score)
- PESI is currently the one that has been most extensively validated among the other clinical scores integrating PE severity and comorbidity to predict all-cause 30-day mortality. However, what is most deserved in the management of a patient with PE is the short-term risk of PE-related hemodynamic collapse for risk stratification and appropriate decision-making for treatment.
- Moreover, the problem arises as it emphasizes more on baseline epidemiological features of the patient rather than the patient’s acute hemodynamic status, therefore an elderly with many comorbidities but a small subsegmental PE may get a high score; while a younger adult with few comorbidities but a high-risk submissive PE incorrectly get a low score.
- According to the 2019 ESC guidelines, “Signs of RV dysfunction or elevated cardiac biomarker levels may be present, despite a calculated PESI of I-II or a sPESi of 0. Until the implications of such discrepancies for the management of PE are fully understood, these patients should be classified into the intermediate-risk category.”
| At this time, only a combination of RV dysfunction on an echo (or CTPA) with a positive cardiac troponin test has directly been tested as a guide for early therapeutic decisions (anticoagulation plus reperfusion treatment vs. anticoagulation alone) in a large RCT of PE patients presenting without hemodynamic instability. 84 |
Pathophysiology
Hemodynamic impact
- The primary cause of death in massive PE is cardiovascular collapse (but not respiratory failure) 85. Following hemodynamically significant PE, pulmonary arterial pressure (PAP) is increased which is attributed to predominantly following factors:
-
- Mechanical obstruction (Clot size)
- PAP increases if >30-50% of the total cross-sectional area of the pulmonary arterial bed is occluded by thromboembolism 86.
- Pulmonary arterial vasoconstriction
- PE-induced vasoconstriction is mediated by the platelet activation and release of thromboxane A2 and serotonin, which contributes to the initial (first 10-30 minutes) increase in pulmonary vascular resistance (PVR) after PE 87 88.
- This finding explains why the hemodynamic manifestation of PE does not depend on the size of the clot alone (figure 6)
- Hypoxic vasoconstriction
- Hypoxemia causes vasoconstriction in the affected lung area 89.
- Mechanical obstruction (Clot size)
- The right ventricle is not well-adapted to this new set of high-pressure systems, and therefore despite compensatory mechanisms, it fails to provide left ventricle preload and consequently systemic arterial pressure drops (figure 7). 90 91
- 👉The key point to understanding the PE viscous cycle is RV perfusion.
- The right ventricle is perfused by the right coronary artery during both ventricular systole and diastole.
- Perfusion pressure within the right coronary artery is equal to (aortic pressure minus pulmonary arterial pressure).
- The rising pulmonary arterial pressure initially impairs RV perfusion during diastole (causing RV ischemic injury). Later on, as systemic blood pressure falls below the critical level, RV perfusion is further insulted during ventricular systole as well (more on this here).
Respiratory interference
- Background
- Physiology of gas exchange 👉more on this here.
- Clinical findings
- Massive PE
- Due to the obstructed flow, blood cannot reach the well-ventilated alveoli. This will create units with dead space ventilation (wasted ventilation).
- The direct consequence of wasted ventilation is ↑PCO2 in pulmonary capillaries.
- Clinical pearls
- In MPE, respiratory acidosis (hypercapnia) is not corrected by measures such as increasing the respiratory rate or tidal volume, since blood flow does not even reach to alveoli for gas exchange.
- In MPE, the pulmonary artery CO2 goes up, while the alveolar CO2 goes down.
- Massive PE
-
- Subsegmental, peripheral PE
- As explained, PE causes units with dead space ventilation. The body intuitively redirects the blood flow from non-perfused units (dead space units) into well-perfused areas.
- These areas are flooded with blood flow leading to overall (↓V/Q) and hypoxemia.
- In this situation, hypoxemia responds to 100% FiO2. 95
- How unresponsive hypoxemia in PE can be explained?
- Severe unresponsive hypoxemia always reflects some sort of shunt.
- In MPE, shunt physiology can happen via the following mechanisms (100% FiO2 cannot ideally correct low arterial oxygen saturation):
- PFO: Skyrocketing right-side heart pressure can lead to an open foramen ovale thereby creating a right-to-left shunt 3.
- Atelectasis: When a saddle PE breaks off, the smaller clot fragments move distally. Such distal micro-emboli evoke inflammation which causes localized bronchoconstriction, and if severe enough, will cause atelectasia (shunt units) 96.
- Alveolar edema: As unobstructed pulmonary vascular beds receive the redirected blood from poorly perfused units, the pulmonary capillaries (and endothelial cells) within these overperfused areas are injured, and therefore alveolar edema will develop (shunt units).
- In subsegmental, peripheral PE, the development of atelectasis or pulmonary edema (as explained) can create shunt units (though uncommon).
- Subsegmental, peripheral PE
Initial resuscitation of high-risk PE
Acute RV failure with resulting low systemic output is the leading cause of death in patients with high-risk PE. Arguably definitive treatment of thromboembolic disease involves pharmacological or mechanical removal of the clot, however institution of such treatment often takes time. Meanwhile, the focus of resuscitation should be on hemodynamic support and oxygenation. The principles of acute right heart failure management have been explored previously (here). In the following discussion, the key points are reviewed 97 98.
Avoid unnecessary lines and ABG
- Patients with massive PE will often require thrombolysis. Minor vascular trauma that occurs when placing an arterial or venous line may become a real problem after giving thrombolytics. Thus, unnecessary lines or ABG sticks should be avoided. Peripheral lines are fine for short-term use of vasopressors. An ABG or VBG is exceedingly unlikely to change management.
- If lines do need to be placed, they should be inserted with extreme care by the most experienced operator with extreme care. For example, a central line should ideally be placed on the first entry into the vessel.
Avoid Intubation
- Patients with RV failure are frequently hypotensive or are highly susceptible to the development of severe hypotension during induction of anesthesia, intubation, and positive-pressure ventilation (as positive pressure within the chest reduces the preload) 3 97. Accordingly, Intubation often precipitates cardiac arrest.
- If you can’t avoid intubation, the following measures are taken to reduce the risk of cardiac arrest:
- Correct hypotension. Try to target a higher SBP in the range of 130-140mmHg usually with a push dose pressor. This will provide a safe margin if the pressure falls following intubation.
- Preoxygenate: hypoxemia causes pulmonary vasoconstriction, further worsening the RV afterload.
- Correct acidosis and hypercarbia: The hypercarbia and respiratory acidosis in patients with MPE are due to dead space ventilation. Therefore efforts to over-vigorous bag ventilation or increasing the respiratory rate and tidal volume on mechanical ventilators are futile. Inhaled pulmonary vasodilators can improve the underlying pathophysiology. Consider getting inhaled pulmonary vasopressors at the bedside and ready to be given.
- Use cardiostable sedatives (e.g. ketamine)
- Intubation: Let it be done by the most experienced operator. Employ apneic oxygenation.
- After the intubation: Avoid over-vigorous bag ventilation, set for the lowest PEEP on mechanical ventilator, and use a tidal volume of <6ml/kg lean body weight in an attempt to keep the end-inspiratory plateau pressure <30 cm H2O. Pay attention to hemodynamics and oxygenation especially within 10-15 minutes after intubation.
- More comprehensive lecture (here)
Employ fluid-conservative strategy: ‘By and large MPE isn’t a fluid-depleted state”
- In the presence of RV outflow tract obstruction (e.g. MPE), volume loading has the potential to over-distending the RV and ultimately cause a reduction in systemic cardiac output 98.
- Avoid fluid boluses unless the patient has frank clinical evidence of volume loss (e.g. watery diarrhea! GI bleeding) or a small IVC with respiratory collapsibility of > 70% is seen.
- Note that small IVC almost never occurs in the presence of MPE, unless the patient is volume-depleted otherwise the diagnosis of MPE is under question.
Pressor-aggressive strategy: “Protect right heart perfusion pressure”
- Patients with right heart failure are extremely intolerant to low systemic blood pressure.
- Right heart coronary perfusion occurs during both ventricular systole and diastole. The diastolic phase of perfusion is already impaired due to the high pulmonary arterial pressure, making the right heart perfusion more contingent upon the systolic phase.
- Keep MAP > 60-65mmHg. The agent of choice would be the one with optimal systemic vasopressor activity while having the least pulmonary vasoconstriction effect (↓PVR/SVR). Norepinephrine, epinephrine, and vasopressin administration are recommended 99 100.
Optimize oxygenation, correct hypercarbia and acidosis
- Optimizing oxygenation
- Hypoxemia has a vasospastic effect on the pulmonary vessel.
- Administration of supplemental oxygen is indicated in patients with PE and SO2<90%.
- Oxygenation techniques include high-flow oxygen (i.e. a high-flow nasal cannula) and mechanical ventilation (non-invasive or invasive) in cases of extreme instability (i.e. cardiac arrest).
- 🚩Although hypoxemia is one of the features of massive PE, a severe hypoxemia/respiratory failure that is refractory to conventional oxygen supplementation should make one consider one of the following possibilities:
- Right-to-left shunting of blood through a patent foramen ovale (PFO) or atrial septal defect. Massive PE can cause elevation of right-sided pressures contributing to right-to-left shunting of deoxygenated blood.101
- Another coexistent pulmonary process (e.g. pneumonia, pneumothorax).
- Correct hypercarbia and acidosis
- Hypercarbia and acidosis cause pulmonary vasoconstriction.
- Strategies that ↓pulmonary vascular resistance may include (inhalational agents):
- Epoprostenol at 0.05mcg/kg/min.
- Nitric Oxide at 20ppm102
- Milrinone (1mg/ml), inhale 5mg over 15 minutes103
- Nitroglycerin (1mg/ml), inhale 5mg over 15 minutes. See more on this here: PULMCrit: Nebulized nitroglycerin
- Strategies that ↓pulmonary vascular resistance may include (inhalational agents):
- Hypercarbia and acidosis cause pulmonary vasoconstriction.
Anticoagulation
Rationals
- The benefit of early therapeutic anticoagulation to improve mortality and decrease recurrence in acute PE is well-proven 104.
- Anticoagulation should be initiated even prior to the confirmed diagnosis when there is high clinical suspicion of acute PE and the bleeding risk is low, particularly if results of diagnostic tests are expected to be delayed 3.
- Keep in mind that anticoagulant prevents additional clots from forming, but it doesn’t break down the existing clots.
Agent of choice
- Selection of the initial anticoagulation agent is dependent on many factors, including risk stratification, patient clinical factors (eg, hepatic and renal function and bleeding risk), and clinician judgment.
- Unfractionated heparin (UFH)
- Most patients with intermediate and high-risk PE require advanced therapies (e.g. thrombolysis) and therefore UFH may be the preferred agent since it can be stopped if the patient begins bleeding, and also can be down-titrated in anticipation of thrombolysis or procedures.
- UFH is also recommended for patients with serious renal impairment [creatinine clearance (CrCl) ≦ 30 mL/min] or severe obesity.
- The dosing of UFH is adjusted based on the activated partial thromboplastin time (figure 10) 2 105.
- Low molecular weight heparin (LMWH)
- For patients with low-risk PE, the LMWH is preferred as it has been shown to be associated with a lower risk of major bleeding and heparin-induced thrombocytopenia 106 107.
- LMWH does not require routine monitoring of anti-Xa levels.
- If LMWH is prescribed in patients with CrCl 15-30 mL/min, an adapted dosing scheme should be used (figure 9).
- For patients with low-risk PE, the LMWH is preferred as it has been shown to be associated with a lower risk of major bleeding and heparin-induced thrombocytopenia 106 107.
- Unfractionated heparin (UFH)
Systemic thrombolysis
Background
Mechanism of action
- Thrombolytic agents activate plasminogen to form plasmin, which accelerates lysis of thromboemboli (figure 11).
- Generally, the clinical improvement is noted within the first hour of infusion. Despite the short half-life of most thrombolytic agents, fibrinolytic activity persists for approximately 1hr after the infusion is terminated.
Side effects
- Systemic thrombolytics challenge the hemostatic balance in the patients. Bleeding is a major complication with variable rates among individuals, depending on the pharmacologic properties of the thrombolytic agent used, the administration protocol, and patients’ susceptibility to bleeding.
- The small fragments generated from fibrin degradation have anticoagulant activity and these will circulate for several hours in plasma, making patients susceptible to bleeding for several hours 108.
Variability in response to thrombolytics
- The balance between fibrin generation and fibrinolysis is complex, dynamic, and unique for every patient. That is to say, some patients are highly sensitive to thrombolysis whereas other patients are resistant 109 110.
Thrombolytic in PE
- Thrombolytics were primarily and more extensively used for ischemic stroke and acute myocardial infarction, with relatively far less evidence for their application in patients with PE.
- It seems that the contraindication for the lytics and their dosage is borrowed from thrombolysis for stroke and myocardial infarction.
Why do trials on systemic thrombolytic therapy in PE show conflicting data?
- The limitations of these trials come from the following:
- Small sample size and patient crossover between the groups.
- Including variable populations of patients (heterogeneous groups, and age) with variable severity of PE (stable and unstable).
- Variable methods of administration and dosing of thrombolytic agent limits generalizability between studies.
- Studies often overlap thrombolysis and anticoagulants (e.g. heparin) in a dangerous fashion, with subsequent attribution of bleeding events to the thrombolytic agent!!
What is a consistent finding among the studies?
- Lytic in MPE is life-saving
- Thrombolytic therapy in unstable PE results in early hemodynamic improvement. It speeds up clot resolution and improvement in pulmonary obstruction, pulmonary arterial pressure, pulmonary vascular resistance, right ventricle, and pulmonary perfusion.
- These improvements are accompanied by a reduction in RV dilation on echocardiography. The use of thrombolysis for massive PE is widely accepted as the standard of care. This has been shown to reduce mortality and PE recurrence compared to anticoagulation alone 111 112 113.
- Lytic does not cut down the development of long-term complication
- Traditionally it was believed that thrombolysis would reduce the risk of chronic thromboembolic pulmonary hypertension and thereby improve long-term functional endpoints (e.g. dyspnea), however, the long-term results of the PEITHO trial convincingly disproved this 114.
- At this time, the primary reason to use thrombolysis is to reduce the risk of cardiac arrest.
Where is the most controversial area in PE management?
- There is still controversy regarding the optimal management strategy for intermediate high-risk PE.
Decision-making on institution of thrombolytic
Decisions should be made following a thorough evaluation of the risk of death from PE vs. the risk of bleeding from thrombolytics (figure 12).
- For example in a patient who is actively dying from PE and has absolute contraindication for thrombolysis, err on the side of systemic thrombolytic administration if other methods are not immediately available for clot resolution. On the other hand, in patients with a low intermediate risk of PE with relative contraindication of bleeding and in the absence of worrisome clinical findings, it might be reasonable to postpone thrombolytic therapy, pending the clinical deterioration of the patient.
- Because the decision to administer thrombolysis is difficult, most experts consider early involvement of PERT (Pulmonary embolism response team); if available.
- This involves a multidisciplinary approach that comprises a team of experts including pulmonologists, cardiologists, thoracic surgeons, interventional radiologists, emergency department physicians, and pharmacists 2.
Once the decision has been made for the administration of thrombolytic, the method of administration (e.g. catheter-directed versus systemic) and dosing (full or reduced-dose; infusion versus bolus) depends on a delicate balance between the patient’s clinical and hemodynamic status and individualized risks of bleeding, available expertise, and extent of the emboli (algorithm below).
Other treatment modalities such as interventional radiology (catheter-directed thrombolysis, percutaneous mechanical thrombectomy) and surgical embolectomy will be discussed separately.
Calculation of risk-benefit
Although the exact numbers are debatable, in the following discussion the calculation of risk-benefit is explained more.
- If we approximate the risk of intracranial hemorrhage 1% in a patient with PE whose short-term risk of cardiac arrest and death is > 5%, then patient should benefit from thrombolysis as:
Monitoring and coordinating thrombolytic and anticoagulants
During thrombolytic infusion, patients should be monitored closely for stability, clinical signs of improvement, development of neurological signs, and hemodynamic or clinical signs of bleeding.
Hints
- ⚠️Avoid unnecessary lines and ABGs if the administration of thrombolytic is contemplated.
- 🛑Preferentially stop all anticoagulants (e.g. UFH) before the institution of thrombolytic, especially in non-crashing patients.
- UFH can be reinstituted (without a bolus) after infusion of thrombolytic is completed, once aPTT results come back and is below 1.5-2.0 times the normal and ideally fibrinogen level is above 100-150 mg/dL.
Complications during thrombolytic infusion
- Minor bleeding during thrombolytic therapy is common and is not generally an indication to stop therapy.
- Minor bleeding may include bleeding at compressible sites (epistaxis or wound) or bleeding that is not hemodynamically significant (minor GI bleeding, or minor genitourinary tract bleeding) and is not at critical sites.
- Thrombolytic therapy may cause moderate bleeding in menstruating women, but it has rarely been associated with major hemorrhage. Therefore, menstruation is neither a contraindication to thrombolytic therapy nor to stop the infusion.
- Major bleeding is an indication for immediate discontinuation of infusion and treatment of bleeding.
- Bleeding is considered major if it occurs at a critical site (e.g. ICH); is not easily accessible, and/or is hemodynamically significant one which requires PRBC transfusion 117.
Thrombolytic for MPE (first or recurrent event)
- The decision to administer systemic thrombolytic in patients with unstable PE is relatively straightforward.
- Hemodynamic instability is the only widely accepted indication for full-dose systemic thrombolytic in the absence of absolute contraindications.
- In the presence of absolute contraindications, other therapeutic methods such as catheter-directed therapies (with/without thrombolysis or suction) or surgical embolectomy may be considered if available.
- However, if the patient is actively dying, and none of the alternative options are available, one may choose low-dose systemic thrombolysis as “Alteplase 50mg IV over 2hr” (Wang, Chest, 2010)118.
Thrombolysis for High-Risk Submassive PE
- Several reports have suggested that systemic thrombolysis reduces the risk of clinical decompensation compared to anticoagulation alone 119 120 121.
- At this time most experts contemplate thrombolysis for ‘high-risk SMPE’ following a thorough risk stratification and weighting benefit to the risk of treatment in addition to patient’s preferences and values.
- Reduced dose systemic thrombolysis
- Rationale for reduced-dose thrombolytic regimen
- The lungs are the only organ receiving the entire cardiac output. Therefore, in PE, the entirety of the infused dose of alteplase will go to the pulmonary arteries independent of the route of administration.
- This is in contrast to myocardial infarction, where only ~5% of the cardiac output is delivered to the coronary arteries *.
- In PE, the whole occlusion of the pulmonary artery is by the clot.
- Compare this, for example, to a coronary artery which may be partially occluded due to plaque with a moderate contribution due to acute clot formation
- The lungs are the only organ receiving the entire cardiac output. Therefore, in PE, the entirety of the infused dose of alteplase will go to the pulmonary arteries independent of the route of administration.
- There are bodies of evidence that show reduced-dose systemic thrombolysis works! the trend in the management of high-risk submassive PE is shown below.
- Rationale for reduced-dose thrombolytic regimen
-
-
- A randomized trial comparing full-dose to reduced-dose systemic thrombolytic in patients with intermediate or high-risk PE demonstrated similar improvements in a host of surrogate endpoints including metrics of RV dysfunction or PA pressure change with small differences in bleeding, favoring the reduced-dose strategy 122.
- The OPTALYSE Trial was a prospective trial comparing different regimens of alteplase administered via catheter-directed thrombolysis. There was no apparent difference in efficacy between doses of ~8 mg and ~24 mg *. This suggests that low doses of alteplase may be much more effective than we realize.
- Reduced dose systemic thrombolytic may be considered in select patients with intermediate-high risk PE and who have a low risk of bleeding.2 123
-
Thrombolysis for low-risk PE
Systemic thrombolysis is not recommended for clinically stable patients with minor RV dysfunction or minor myocardial necrosis (algorithm below) 2.
Other treatments
Percutaneous catheter-directed treatments
- Catheter-directed thrombolysis
- This involves the insertion of a catheter into the pulmonary arteries via the femoral route, to directly infuse tPA in close proximity to the clot.
- There is little theoretical or evidentiary support for why catheter-directed thrombolysis should be superior to the administration of an identical dose of tPA via peripheral circulation (all tPA infused peripherally will be transported directly to the pulmonary circulation).
- The main benefit of catheter-directed thrombolysis likely stems from the use of low-dose thrombolysis (especially in a hospital that is uncomfortable with the use of low-dose peripheral thrombolytics).
- Traditionally, the most common dose of tPA has been 0.5-1 mg/hour per catheter for a total dose of 12-24 mg delivered over a 24-hour period *.
- The optimum dosing of unfractionated heparin during catheter-directed thrombolysis is unknown. The safest approach might be a fixed low-dose infusion at ~500-1,000 units/hour *.
- Percutaneous mechanical thrombectomy
- Potential indications:
- Patients with high-risk submassive or massive PE with contraindication to thrombolysis.
- Patients in whom thrombolysis has failed to be effective.
- Potential indications:
Surgical embolectomy
- It is usually carried out with cardiopulmonary bypass, without aortic cross-clamping and cardioplegic cardiac arrest, followed by incision of the two main pulmonary arteries with the removal or suction of fresh clots.
- Possible indications
- Clot-in-transit which is lying across a patent foramen ovale (PFO). This situation carries a risk of immediate stroke if the clot breaks up and parts of it enter the systemic circulation. Therefore, surgery may be a front-line intervention in this rare scenario.
- Massive PE in a patient with absolute contraindication to thrombolysis.
- Massive PE with failure of other interventions (e.g. thrombolytic failure).
- ~10% of clots will not respond to thrombolysis (perhaps because they are chronic and have undergone organization).
- Surgical thrombectomy vs. interventional radiology clot extraction
- Currently, no high-level evidence exists comparing these modalities.
- Advances in catheter embolectomy could make interventional radiology approaches superior in many cases. However, further study is needed.
Algorithm
The algorithm for integrated risk-adapted diagnostic and management of pulmonary embolism in adult, non-pregnant patients is represented below.
Special situations
Right-heart thrombus (RHT) or clot-in-transit (CIT)
There are several discrete muscular ridges or bands in the right heart, which sometimes are mistaken for tumors or thrombi (more on this here). However, a large clot in transit is fairly unmistakable (below clip).
- A clot in transit is defined as a large, mobile (i.e. unattached to any intracardiac structure), serpentine echogenicity trapped in the right heart chambers.
- It has been shown that large CIT is associated with increased mortality and will move the patient’s severity classification up by one class 124.
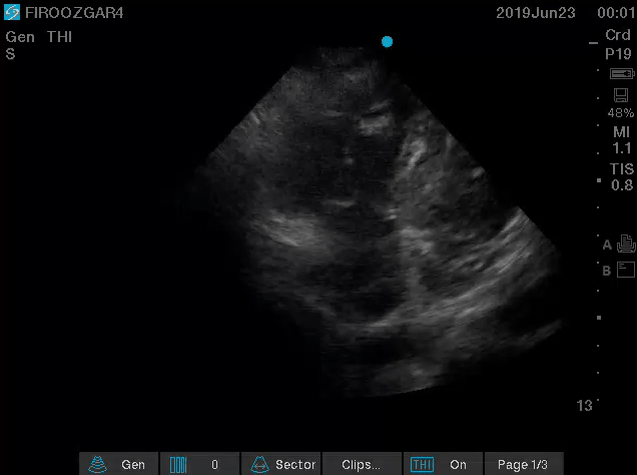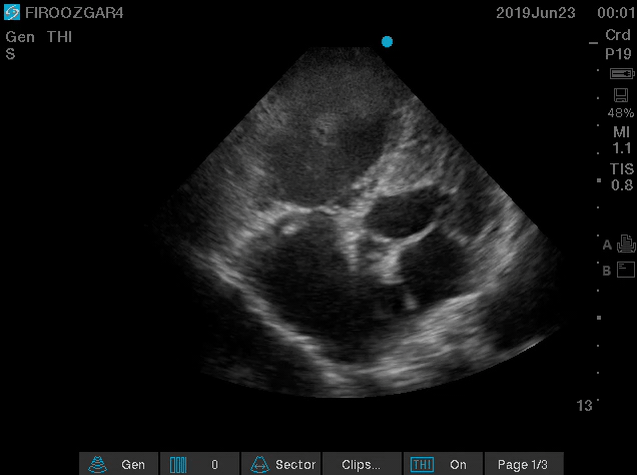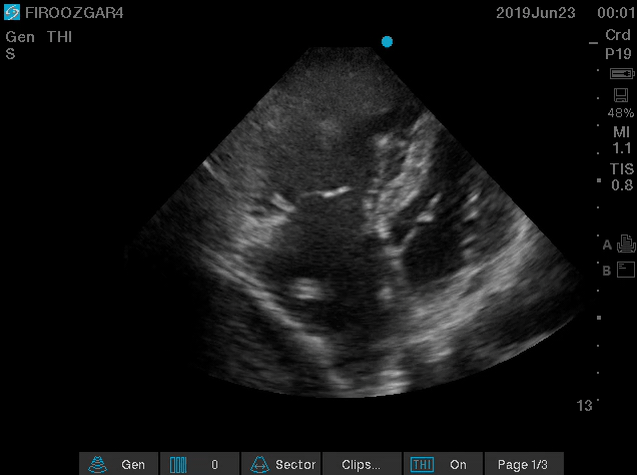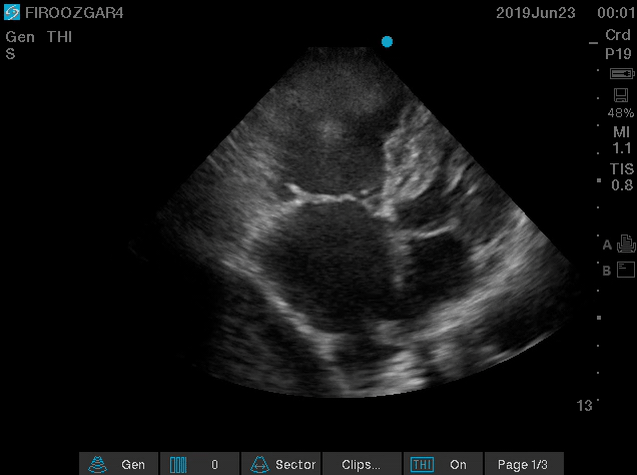
Figure 15. Large, mobile,snake-like clot in the right atrium
Management
- In the absence of a patent foramen ovale (PFO), treatment is similar to PE (with the increased severity classification taken into account) 2
- In the presence of PFO and clot-in-transit (figure 16), there’s a significant risk of paradoxical embolization into arterial circulation with a risk of stroke.
PE in cardiac arrest
Thrombolytic therapy has been reported to increase the rate of return of spontaneous circulation (ROSC) and survival (with survival >50%) 129 130 131; however, a few considerations shall be made here:
- Avoid measures that increase intrathoracic pressure
- Avoid over-aggressive bagging (explained earlier).
- Epinephrine
- Patients with PE in cardiac arrest frequently gain pulse and then re-arrest.
- If the patient regains a pulse after an epinephrine bolus, promptly consider starting a high-dose epinephrine infusion (e.g. 20 mcg/min, then titrate based on blood pressure). These patients often seem to re-arrest after the epinephrine bolus wears off.
- Thrombolysis
- Patients should receive thrombolysis regardless of their contraindications unless immediate VA-ECMO is available.
- The coding dose of alteplase is 50mg IV push over 60sec (from the “PEAPETT” Study), which may repeat after 15 minutes if ROSC is not established 129.
- Extended CPR
- Consider extended CPR (e.g. 60-90 min) to provide time for thrombolytic to circulate 132.
- Inhaled pulmonary vasodilator
- Consider administration of any pulmonary vasodilator available via the endotracheal tube (e.g. nitric oxide, epoprostenol, or milrinone) to reduce RV afterload.
Refractory hypoxemia
Severe refractory hypoxemia may rarely occur in the absence of hypotension and while such cases do not neatly fit into intermediate or high-risk PE categories, a few points should be considered here:
- Differential diagnosis
- Refractory hypoxemia (explained earlier) often reflects some degree of shunt. The differential diagnosis may include:
- Right-to-left shunt via PFO (PE increases right-sided pressure and contributes to right-to-left shunting)133.
- Coexistent pulmonary disease such as pneumonia
- Refractory hypoxemia (explained earlier) often reflects some degree of shunt. The differential diagnosis may include:
- Diagnosis
- TTE with an injection of agitated saline is the method of choice for determining right-to-left shunting.
- Chest CT may be helpful to identify other coexistent etiologies.
- Treatment of PE-related right-to-left shunting
- Oxygenation
- High-flow nasal cannula with 100% FiO2 is the first line for improving oxygenation.
- Keep in mind that intubation with positive pressure ventilation will not improve the condition and may make it worse (by increasing right-sided heart pressure, it may increase the shunt fraction).
- Inhalational pulmonary vasodilators
- Improve both hemodynamic and oxygenation via decreasing RV afterload and improving shunt fraction.
- Thrombolytic treatment
- In the absence of absolute contraindication, systemic thrombolytic treatment is potentially considered.
- Other methods such as catheter-directed thrombolysis or embolism extraction by interventional radiology are suitable options in patients with risk of bleeding.
- Oxygenation
RECAP
- PE is stratified into massive, submassive, and low-risk based on the hemodynamic profile and the presence or absence of RV dysfunction.
- Diagnostic considerations
- Findings suggestive for PE:
- Presence of VTE risk factors, ↑D-dimer (not better explained), Wells score >4
- Cardiac arrest with PEA
- DVT (esp. proximal DVT)
- Echo: RV dysfunction
- Definitive diagnosis:
- CTPA (filling defects)
- Clot-in-transit on echo (in appropriate clinical context)
- Findings suggestive for PE:
- Risk stratification: evaluation
- Syncope?
- Hypotension caused by PE (SBP <90 mm for 15 minutes, fall in SBP >40 mm for 15 minutes, requirement for vasopressors)?
- Shock index >1?
- Diaphoresis, mottling, cold extremities?
- Troponin and or lactate elevation?
- Echo: RV dilation (plus normal or dilated IVC), large clot-in-transit?
- CT: RV dilation or contrast reflux into the IVC?
- Risk stratification-approach: 👉Table above.
- Determine risk vs. benefit of thrombolytic therapy: Table above
- Management
- Massive PE in cardiac arrest
- Follow ACLS guideline
- Coding dose of thrombolytic: Alteplase 50mg IV PUSH over 2min. May repeat the dose in 15min, if ROSC is not established yet.
- Prolong CPR, if ROSC is not achieved (helping to circulate the thrombolytic).
- Massive PE (MPE)
- RV-friendly resuscitation strategy
- Fluid-conservative strategy (unless there’s overt evidence of volume loss)
- Try to avoid intubation (if possible). If intubation is required, follow hemodynamically neutral intubation.
- Keep MAP >65 mmHg. Have a low threshold to start epinephrine drip.
- Inhaled pulmonary vasodilator e.g. nitroglycerine 5mg nebulization.
- Thrombolysis
- Thrombolysis is the only evidence-based intervention that improves mortality in these patients. Do not delay thrombolysis when it is considered an option.
- No contraindication: 100 mg alteplase.
- Relative contraindication and impending to arrest (crashing PE): 50-100 mg alteplase.
- Relative contraindication & stabilized (high-risk MPE): may start with 50 mg alteplase.
- Anticoagulation
- Stop all anticoagulants before the institution of thrombolytic, especially in non-crashing patients.
- UFH is the preferred agent.
- UFH can be reinstituted (without a bolus) after infusion of thrombolytic is completed, once aPTT results come back and is below 1.5-2.0 times the normal and ideally fibrinogen level is above 100-150 mg/dL.
- RV-friendly resuscitation strategy
- Submassive PE (SMPE)
- Follow the RV-friendly resuscitation strategy (if needed).
- Thrombolysis
- High-risk SMPE & no absolute contraindication: 100mg alteplase.
- High-risk SMPE & relative contraindication: 50mg alteplase.
- Anticoagulation
- Stop all anticoagulants before the institution of thrombolytic.
- UFH is the agent of choice.
- UFH can be reinstituted (without a bolus) after infusion of thrombolytic is completed, once aPTT results come back and is below 1.5-2.0 times the normal and ideally fibrinogen level is above 100-150 mg/dL.
- Low-risk PE or low-risk SMPE
- Anticoagulation: Enoxaparin is the preferred agent. It can be given as 1 mg/kg q12 h or 1.5 mg/kg once q24 h SUBQ.
- Massive PE in cardiac arrest
Appendix 1
Appendix 2
Appendix 3
Massive PE ECG strain pattern
Media
Going further
- Diagnosis
- ECG in Pulmonary embolism (EM:RAP)
- Two EKG patterns of pulmonary embolism that mimic MI(PULMCrit)
- Pulmonary embolism (Radiopaedia)
- Pulmonary embolism (Show Me The POCUS)
- Resuscitation
- Thrombolysis
Post Peer Reviewed By: Mojtaba Chardoli. MD.
References
1. Wendelboe AM, Raskob GE. Global Burden of Thrombosis: Epidemiologic Aspects. Circ Res. 2016 Apr 29;118(9):1340-7. doi: 10.1161/CIRCRESAHA.115.306841. PMID: 27126645
2. Rivera-Lebron B, McDaniel M, Ahrar K, Alrifai A, Dudzinski DM, Fanola C, Blais D, Janicke D, Melamed R, Mohrien K, Rozycki E, Ross CB, Klein AJ, Rali P, Teman NR, Yarboro L, Ichinose E, Sharma AM, Bartos JA, Elder M, Keeling B, Palevsky H, Naydenov S, Sen P, Amoroso N, Rodriguez-Lopez JM, Davis GA, Rosovsky R, Rosenfield K, Kabrhel C, Horowitz J, Giri JS, Tapson V, Channick R; PERT Consortium. Diagnosis, Treatment and Follow Up of Acute Pulmonary Embolism: Consensus Practice from the PERT Consortium. Clin Appl Thromb Hemost. 2019 Jan-Dec;25:1076029619853037. doi: 10.1177/1076029619853037. PMID: 31185730; PMCID: PMC6714903
3. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jiménez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Ní Áinle F, Prandoni P, Pruszczyk P, Righini M, Torbicki A, Van Belle E, Zamorano JL; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603. doi: 10.1093/eurheartj/ehz405. PMID: 31504429.
4. Sardi A, Gluskin J, Guttentag A, Kotler MN, Braitman LE, Lippmann M. Saddle pulmonary embolism: is it as bad as it looks? A community hospital experience. Crit Care Med. 2011 Nov;39(11):2413-8. doi: 10.1097/CCM.0b013e31822571b2. PMID: 21705903
5. Ryu JH, Pellikka PA, Froehling DA, Peters SG, Aughenbaugh GL. Saddle pulmonary embolism diagnosed by CT angiography: frequency, clinical features and outcome. Respir Med. 2007 Jul;101(7):1537-42. doi: 10.1016/j.rmed.2006.12.010. Epub 2007 Jan 24. PMID: 17254761
6. Wood KE. Size does matter (not). Crit Care Med. 2011 Nov;39(11):2560-1. doi: 10.1097/CCM.0b013e31822a582b. PMID: 22005226
7. Mebazaa A, Tolppanen H, Mueller C, Lassus J, DiSomma S, Baksyte G, Cecconi M, Choi DJ, Cohen Solal A, Christ M, Masip J, Arrigo M, Nouira S, Ojji D, Peacock F, Richards M, Sato N, Sliwa K, Spinar J, Thiele H, Yilmaz MB, Januzzi J. Acute heart failure and cardiogenic shock: a multidisciplinary practical guidance. Intensive Care Med. 2016 Feb;42(2):147-63. doi: 10.1007/s00134-015-4041-5. Epub 2015 Sep 14. PMID: 26370690.
8. Mebazaa A, Tolppanen H, Mueller C, Lassus J, DiSomma S, Baksyte G, Cecconi M, Choi DJ, Cohen Solal A, Christ M, Masip J, Arrigo M, Nouira S, Ojji D, Peacock F, Richards M, Sato N, Sliwa K, Spinar J, Thiele H, Yilmaz MB, Januzzi J. Acute heart failure and cardiogenic shock: a multidisciplinary practical guidance. Intensive Care Med. 2016 Feb;42(2):147-63. doi: 10.1007/s00134-015-4041-5. Epub 2015 Sep 14. PMID: 26370690
9. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: Lifeline. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 2017 Oct 17;136(16):e232-e268. doi: 10.1161/CIR.0000000000000525. Epub 2017 Sep 18. PMID: 28923988
10. Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, Jenkins JS, Kline JA, Michaels AD, Thistlethwaite P, Vedantham S, White RJ, Zierler BK; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011 Apr 26;123(16):1788-830. doi: 10.1161/CIR.0b013e318214914f. Epub 2011 Mar 21. Erratum in: Circulation. 2012 Aug 14;126(7):e104. Erratum in: Circulation. 2012 Mar 20;125(11):e495. PMID: 21422387
11. Berghaus TM, Haeckel T, Behr W, Wehler M, von Scheidt W, Schwab Blair M. Central thromboembolism is a possible predictor of right heart dysfunction in normotensive patients with acute pulmonary embolism. Thromb Res. 2010 Sep;126(3):e201-5. doi: 10.1016/j.thromres.2010.06.009. Epub 2010 Jul 17. PMID: 20638710
12. Vedovati MC, Becattini C, Agnelli G, Kamphuisen PW, Masotti L, Pruszczyk P, Casazza F, Salvi A, Grifoni S, Carugati A, Konstantinides S, Schreuder M, Golebiowski M, Duranti M. Multidetector CT scan for acute pulmonary embolism: embolic burden and clinical outcome. Chest. 2012
13. Vedovati MC, Germini F, Agnelli G, Becattini C. Prognostic role of embolic burden assessed at computed tomography angiography in patients with acute pulmonary embolism: systematic review and meta-analysis. J Thromb Haemost. 2013 Dec;11(12):2092-102. doi: 10.1111/jth.12429. PMID: 24134450
14. Choi KJ, Cha SI, Shin KM, Lim JK, Yoo SS, Lee J, Lee SY, Kim CH, Park JY, Lee WK. Central emboli rather than saddle emboli predict adverse outcomes in patients with acute pulmonary embolism. Thromb Res. 2014 Nov;134(5):991-6. doi: 10.1016/j.thromres.2014.08.027. Epub 2014 Sep 8. PMID: 2522839
15. Koć M, Kostrubiec M, Elikowski W, Meneveau N, Lankeit M, Grifoni S, Kuch-Wocial A, Petris A, Zaborska B, Stefanović BS, Hugues T, Torbicki A, Konstantinides S, Pruszczyk P; RiHTER Investigators. Outcome of patients with right heart thrombi: the Right Heart Thrombi European Registry. Eur Respir J. 2016 Mar;47(3):869-75. doi: 10.1183/13993003.00819-2015. Epub 2016 Jan 21. PMID: 26797032
16. White RH. The epidemiology of venous thromboembolism. Circulation. 2003 Jun 17;107(23 Suppl 1):I4-8. doi: 10.1161/01.CIR.0000078468.11849.66. PMID: 12814979
17. Stein PD, Terrin ML, Hales CA, Palevsky HI, Saltzman HA, Thompson BT, Weg JG. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest. 1991 Sep;100(3):598-603. doi: 10.1378/chest.100.3.598. PMID: 1909617.
18. Herodotou Y, Papaioannou AI, Karakatsani A, et al. Massive hemoptysis in a patient with pulmonary embolism, a real therapeutic conundrum. Respir Med Case Rep. 2017;20:179-183. Published 2017 Feb 28. doi:10.1016/j.rmcr.2017.02.013
19. Stein PD, Matta F, Musani MH, Diaczok B. Silent pulmonary embolism in patients with deep venous thrombosis: a systematic review. Am J Med. 2010 May;123(5):426-31. doi: 10.1016/j.amjmed.2009.09.037. PMID: 20399319
20. Courtney DM, Kline JA. Prospective use of a clinical decision rule to identify pulmonary embolism as likely cause of outpatient cardiac arrest. Resuscitation. 2005 Apr;65(1):57-64. doi: 10.1016/j.resuscitation.2004.07.018. PMID: 15797276
21. Courtney DM, Sasser HC, Pincus CL, Kline JA. Pulseless electrical activity with witnessed arrest as a predictor of sudden death from massive pulmonary embolism in outpatients. Resuscitation. 2001 Jun;49(3):265-72. doi: 10.1016/s0300-9572(00)00374-9. PMID: 11719120
22. Kürkciyan I, Meron G, Sterz F, Janata K, Domanovits H, Holzer M, Berzlanovich A, Bankl HC, Laggner AN. Pulmonary embolism as a cause of cardiac arrest: presentation and outcome. Arch Intern Med. 2000 May 22;160(10):1529-35. doi: 10.1001/archinte.160.10.1529. PMID
23. Rodger MA, Carrier M, Jones GN, Rasuli P, Raymond F, Djunaedi H, Wells PS. Diagnostic value of arterial blood gas measurement in suspected pulmonary embolism. Am J Respir Crit Care Med. 2000 Dec;162(6):2105-8. doi: 10.1164/ajrccm.162.6.2004204. PMID: 11112122McIntyre KM, Sasahara AA.
24. Furlan A, Aghayev A, Chang CC, Patil A, Jeon KN, Park B, Fetzer DT, Saul M, Roberts MS, Bae KT. Short-term mortality in acute pulmonary embolism: clot burden and signs of right heart dysfunction at CT pulmonary angiography. Radiology. 2012 Oct;265(1):283-93. doi: 10.1148/radiol.12110802. PMID: 22993221; PMCID: PMC3447174
25. Subramaniam RM, Mandrekar J, Chang C, Blair D, Gilbert K, Peller PJ, Sleigh J, Karalus N. Pulmonary embolism outcome: a prospective evaluation of CT pulmonary angiographic clot burden score and ECG score. AJR Am J Roentgenol. 2008 Jun;190(6):1599-604. doi: 10.2214/AJR.07.2858. PMID: 18492912.
26. Bach AG, Nansalmaa B, Kranz J, Taute BM, Wienke A, Schramm D, Surov A. CT pulmonary angiography findings that predict 30-day mortality in patients with acute pulmonary embolism. Eur J Radiol. 2015 Feb;84(2):332-7. doi: 10.1016/j.ejrad.2014.11.023. Epub 2014 Nov 27. PMID: 25487818.
27. Summers K, Schultheis J, Raiff D, Dahhan T. Evaluation of Rescue Thrombolysis in Cardiac Arrest Secondary to Suspected or Confirmed Pulmonary Embolism. Ann Pharmacother. 2019 Jul;53(7):711-715. doi: 10.1177/1060028019828423. Epub 2019 Jan 30. PMID: 30700101
28. Laher AE, Richards G. Cardiac arrest due to pulmonary embolism. Indian Heart J. 2018 Sep-Oct;70(5):731-735. doi: 10.1016/j.ihj.2018.01.014. Epub 2018 Jan 8. PMID: 30392514; PMCID: PMC6204441
29. Kürkciyan I, Meron G, Sterz F, Janata K, Domanovits H, Holzer M, Berzlanovich A, Bankl HC, Laggner AN. Pulmonary embolism as a cause of cardiac arrest: presentation and outcome. Arch Intern Med. 2000 May 22;160(10):1529-35. doi: 10.1001/archinte.160.10.1529. PMID: 10826469
30. Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016 Jan;41(1):3-14. doi: 10.1007/s11239-015-1311-6. PMID: 26780736; PMCID: PMC4715842
31. Hull RD, Hirsh J, Carter CJ, Jay RM, Dodd PE, Ockelford PA, Coates G, Gill GJ, Turpie AG, Doyle DJ, Buller HR, Raskob GE. Pulmonary angiography, ventilation lung scanning, and venography for clinically suspected pulmonary embolism with abnormal perfusion lung scan. Ann Intern Med. 1983 Jun;98(6):891-9. doi: 10.7326/0003-4819-98-6-891. PMID: 6859705
32. Aagaard R, Caap P, Hansson NC, Bøtker MT, Granfeldt A, Løfgren B. Detection of Pulmonary Embolism During Cardiac Arrest-Ultrasonographic Findings Should Be Interpreted With Caution. Crit Care Med. 2017 Jul;45(7):e695-e702. doi: 10.1097/CCM.0000000000002334. PMID: 28403120
33. Aagaard R, Granfeldt A, Bøtker MT, Mygind-Klausen T, Kirkegaard H, Løfgren B. The Right Ventricle Is Dilated During Resuscitation From Cardiac Arrest Caused by Hypovolemia: A Porcine Ultrasound Study. Crit Care Med. 2017 Sep;45(9):e963-e970. doi: 10.1097/CCM.0000000000002464. PMID: 28430698
34. Sørensen AH, Vemmelund KB, Møller-Helgestad OK, Sloth E, Juhl-Olsen P. Asphyxia causes ultrasonographic D-shaping of the left ventricle–an experimental porcine study. Acta Anaesthesiol Scand. 2016;60(2):203-212. doi:10.1111/aas.12606
35. Berg RA, Sorrell VL, Kern KB, Hilwig RW, Altbach MI, Hayes MM, Bates KA, Ewy GA. Magnetic resonance imaging during untreated ventricular fibrillation reveals prompt right ventricular overdistention without left ventricular volume loss. Circulation. 2005 Mar 8;111(9):1136-40. doi: 10.1161/01.CIR.0000157147.26869.31. Epub 2005 Feb 21. PMID: 15723975
36. Prosen G, Križmarić M, Završnik J, Grmec S. Impact of modified treatment in echocardiographically confirmed pseudo-pulseless electrical activity in out-of-hospital cardiac arrest patients with constant end-tidal carbon dioxide pressure during compression pauses. J Int Med Res. 2010 Jul-Aug;38(4):1458-67. doi: 10.1177/147323001003800428. PMID: 20926019
37. Barco S, Ende-Verhaar YM, Becattini C, Jimenez D, Lankeit M, Huisman MV, Konstantinides SV, Klok FA. Differential impact of syncope on the prognosis of patients with acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2018 Dec 14;39(47):4186-4195. doi: 10.1093/eurheartj/ehy631. PMID: 30339253
38. Keller K, Beule J, Balzer JO, Dippold W. Typical symptoms for prediction of outcome and risk stratification in acute pulmonary embolism. Int Angiol. 2016 Apr;35(2):184-91. Epub 2015 Mar 6. PMID: 25743032
39. Freitas P, Santos AR, Ferreira AM, Oliveira A, Gonçalves M, Corte-Real A, Lameiras C, Maurício J, Ornelas É, Matos C, Faria D, Augusto J, Simões J, Ferreira I, Pedroso A, Santos AC, Gago M, Oliveira JD, Antunes RM, Correia D, Lynce A, Brito J, Aguiar C, Ferreira J, Morais C, Campos L, Raposo L, Mendes M. Derivation and external validation of the SHIeLD score for predicting outcome in normotensive pulmonary embolism. Int J Cardiol. 2019 Apr 15;281:119-124. doi: 10.1016/j.ijcard.2018.12.062. Epub 2018 Dec 28. PMID: 30638984
40. Ozsu S, Erbay M, Durmuş ZG, Ozlu T. Classification of high-risk with cardiac troponin and shock index in normotensive patients with pulmonary embolism. J Thromb Thrombolysis. 2017 Feb;43(2):179-183. doi: 10.1007/s11239-016-1443-3. PMID: 27800569
41. Cretikos MA, Bellomo R, Hillman K, Chen J, Finfer S, Flabouris A. Respiratory rate: the neglected vital sign. Med J Aust. 2008 Jun 2;188(11):657-9. PMID: 18513176
42. Paiva LV, Providencia RC, Barra SN, Faustino AC, Botelho AM, Marques AL. Cardiovascular risk assessment of pulmonary embolism with the GRACE risk score. Am J Cardiol. 2013 Feb 1;111(3):425-31. doi: 10.1016/j.amjcard.2012.10.020. Epub 2012 Nov 17. PMID: 23168283
43. Casazza F, Bongarzoni A, Centonze F, Morpurgo M. Prevalence and prognostic significance of right-sided cardiac mobile thrombi in acute massive pulmonary embolism. Am J Cardiol. 1997 May 15;79(10):1433-5. doi: 10.1016/s0002-9149(97)00162-8. PMID: 9165180
44. Barrios D, Rosa-Salazar V, Morillo R, Nieto R, Fernández S, Zamorano JL, Monreal M, Torbicki A, Yusen RD, Jiménez D. Prognostic Significance of Right Heart Thrombi in Patients With Acute Symptomatic Pulmonary Embolism: Systematic Review and Meta-analysis. Chest. 2017 Feb;151(2):409-416. doi: 10.1016/j.chest.2016.09.038. Epub 2016 Oct 14. PMID: 27746202
45. Barrios D, Rosa-Salazar V, Jiménez D, Morillo R, Muriel A, Del Toro J, López-Jiménez L, Farge-Bancel D, Yusen R, Monreal M; RIETE investigators. Right heart thrombi in pulmonary embolism. Eur Respir J. 2016 Nov;48(5):1377-1385. doi: 10.1183/13993003.01044-2016. Epub 2016 Oct 6. PMID: 27799388
46. Konstantinides S, Geibel A, Kasper W, Olschewski M, Blümel L, Just H. Patent foramen ovale is an important predictor of adverse outcome in patients with major pulmonary embolism. Circulation. 1998 May 19;97(19):1946-51. doi: 10.1161/01.cir.97.19.1946. PMID: 9609088
47. Goliszek S, Wiśniewska M, Kurnicka K, Lichodziejewska B, Ciurzyński M, Kostrubiec M, Gołębiowski M, Babiuch M, Paczynska M, Koć M, Palczewski P, Wyzgał A, Pruszczyk P. Patent foramen ovale increases the risk of acute ischemic stroke in patients with acute pulmonary embolism leading to right ventricular dysfunction. Thromb Res. 2014 Nov;134(5):1052-6. doi: 10.1016/j.thromres.2014.09.013. Epub 2014 Sep 21. PMID: 25282541
48. Meinel FG, Nance JW Jr, Schoepf UJ, Hoffmann VS, Thierfelder KM, Costello P, Goldhaber SZ, Bamberg F. Predictive Value of Computed Tomography in Acute Pulmonary Embolism: Systematic Review and Meta-analysis. Am J Med. 2015 Jul;128(7):747-59.e2. doi: 10.1016/j.amjmed.2015.01.023. Epub 2015 Feb 11. PMID: 25680885
49. Côté B, Jiménez D, Planquette B, Roche A, Mary J, Pastré J, Meyer G, Sanchez O. Prognostic value of right ventricular dilatation in patients with low-risk pulmonary embolism. Eur Respir J. 2017 Dec 14;50(6):1701611. doi: 10.1183/13993003.01611-2017. PMID: 29242264
50. Bach AG, Nansalmaa B, Kranz J, Taute BM, Wienke A, Schramm D, Surov A. CT pulmonary angiography findings that predict 30-day mortality in patients with acute pulmonary embolism. Eur J Radiol. 2015 Feb;84(2):332-7. doi: 10.1016/j.ejrad.2014.11.023. Epub 2014 Nov 27. PMID: 25487818
51. Aviram G, Cohen D, Steinvil A, Shmueli H, Keren G, Banai S, Berliner S, Rogowski O. Significance of reflux of contrast medium into the inferior vena cava on computerized tomographic pulmonary angiogram. Am J Cardiol. 2012 Feb 1;109(3):432-7. doi: 10.1016/j.amjcard.2011.09.033. Epub 2011 Nov 9. PMID: 22074991
52. Aycock RD, Westafer LM, Boxen JL, Majlesi N, Schoenfeld EM, Bannuru RR. Acute Kidney Injury After Computed Tomography: A Meta-analysis. Ann Emerg Med. 2018 Jan;71(1):44-53.e4. doi: 10.1016/j.annemergmed.2017.06.041. Epub 2017 Aug 12. PMID: 28811122
53. Gorelik Y, Yaseen H, Heyman SN, Khamaisi M. Negligible Risk of Acute Renal Failure Among Hospitalized Patients After Contrast-Enhanced Imaging With Iodinated Versus Gadolinium-Based Agents. Invest Radiol. 2019 May;54(5):312-318. doi: 10.1097/RLI.0000000000000534. PMID: 30480553.Light RW.
54. Pleural effusion in pulmonary embolism. Semin Respir Crit Care Med. 2010 Dec;31(6):716-22. doi: 10.1055/s-0030-1269832. Epub 2011 Jan 6. PMID: 21213203
55. Panjwani A, Zaid T. An interesting case of undiagnosed pleural effusion. Breathe (Sheff). 2017;13(2):e46-e52. doi:10.1183/20734735.001117
56. Findik S. Pleural effusion in pulmonary embolism. Curr Opin Pulm Med. 2012 Jul;18(4):347-54. doi: 10.1097/MCP.0b013e32835395d5. PMID: 22498732
57. Bajaj A, Saleeb M, Rathor P, Sehgal V, Kabak B, Hosur S. Prognostic value of troponins in acute non massive pulmonary embolism: A meta-analysis. Heart Lung. 2015 Jul-Aug;44(4):327-34. doi: 10.1016/j.hrtlng.2015.03.007. Epub 2015 May 11. PMID: 25976228
58. Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation. 2007 Jul 24;116(4):427-33. doi: 10.1161/CIRCULATIONAHA.106.680421. Epub 2007 Jul 2. PMID: 17606843
59. Kaeberich A, Seeber V, Jiménez D, Kostrubiec M, Dellas C, Hasenfuß G, Giannitsis E, Pruszczyk P, Konstantinides S, Lankeit M. Age-adjusted high-sensitivity troponin T cut-off value for risk stratification of pulmonary embolism. Eur Respir J. 2015 May;45(5):1323-31. doi: 10.1183/09031936.00174514. Epub 2015 Jan 22. PMID: 25614162
60. Vanni S, Viviani G, Baioni M, Pepe G, Nazerian P, Socci F, Bartolucci M, Bartolini M, Grifoni S. Prognostic value of plasma lactate levels among patients with acute pulmonary embolism: the thrombo-embolism lactate outcome study. Ann Emerg Med. 2013 Mar;61(3):330-8. doi: 10.1016/j.annemergmed.2012.10.022. Epub 2013 Jan 7. PMID: 23306454
61. Vanni S, Nazerian P, Bova C, Bondi E, Morello F, Pepe G, Paladini B, Liedl G, Cangioli E, Grifoni S, Jiménez D. Comparison of clinical scores for identification of patients with pulmonary embolism at intermediate-high risk of adverse clinical outcome: the prognostic role of plasma lactate. Intern Emerg Med. 2017 Aug;12(5):657-665. doi: 10.1007/s11739-016-1487-6. Epub 2016 Jun 28. PMID: 27350628
62. Shopp JD, Stewart LK, Emmett TW, Kline JA. Findings From 12-lead Electrocardiography That Predict Circulatory Shock From Pulmonary Embolism: Systematic Review and Meta-analysis. Acad Emerg Med. 2015 Oct;22(10):1127-37. doi: 10.1111/acem.12769. Epub 2015 Sep 22. PMID: 26394330; PMCID: PMC5306533.
63. Grant-Orser A, Ballantyne B, Haddara W. Unique ECG Findings in Acute Pulmonary Embolism: STE with Reciprocal Changes and Pathologic Q Wave. Case Rep Crit Care. 2018 Apr 3;2018:7865894. doi: 10.1155/2018/7865894. PMID: 29850272; PMCID: PMC5903203
64. Zhong-Qun Z, Chong-Quan W, Nikus KC, Sclarovsky S, Chao-Rong H. A new electrocardiogram finding for massive pulmonary embolism: ST elevation in lead aVR with ST depression in leads I and V(4) to V(6). Am J Emerg Med. 2013 Feb;31(2):456.e5-8. doi: 10.1016/j.ajem.2012.08.001. Epub 2012 Sep 20. PMID: 23000330
65. Kukla P, McIntyre WF, Fijorek K, Mirek-Bryniarska E, Bryniarski L, Krupa E, Jastrzębski M, Bryniarski KL, Zhong-qun Z, Baranchuk A. Electrocardiographic abnormalities in patients with acute pulmonary embolism complicated by cardiogenic shock. Am J Emerg Med. 2014 Jun;32(6):507-10. doi: 10.1016/j.ajem.2014.01.043. Epub 2014 Feb 3. PMID: 24602894
66. Chatterjee S, Chakraborty A, Weinberg I, Kadakia M, Wilensky RL, Sardar P, Kumbhani DJ, Mukherjee D, Jaff MR, Giri J. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA. 2014 Jun 18;311(23):2414-21. doi: 10.1001/jama.2014.5990. PMID: 24938564
67. Hariharan P, Dudzinski DM, Rosovsky R, Haddad F, MacMahon P, Parry B, Chang Y, Kabrhel C. Relation Among Clot Burden, Right-Sided Heart Strain, and Adverse Events After Acute Pulmonary Embolism. Am J Cardiol. 2016 Nov 15;118(10):1568-1573. doi: 10.1016/j.amjcard.2016.08.025. Epub 2016 Aug 24. Erratum in: Am J Cardiol. 2017 Aug 1;120(3):515. PMID: 27742425
68. Becattini C, Cohen AT, Agnelli G, Howard L, Castejón B, Trujillo-Santos J, Monreal M, Perrier A, Yusen RD, Jiménez D. Risk Stratification of Patients With Acute Symptomatic Pulmonary Embolism Based on Presence or Absence of Lower Extremity DVT: Systematic Review and Meta-analysis. Chest. 2016 Jan;149(1):192-200. doi: 10.1378/chest.15-0808. Epub 2016 Jan 6. PMID: 26204122
69. Kucher N, Goldhaber SZ. Cardiac biomarkers for risk stratification of patients with acute pulmonary embolism. Circulation. 2003 Nov 4;108(18):2191-4. doi: 10.1161/01.CIR.0000100687.99687.CE. PMID: 14597581
70. Coutance G, Cauderlier E, Ehtisham J, Hamon M, Hamon M. The prognostic value of markers of right ventricular dysfunction in pulmonary embolism: a meta-analysis. Crit Care. 2011;15(2):R103. doi:10.1186/cc10119
71. Venetz C, Labarère J, Jiménez D, Aujesky D. White blood cell count and mortality in patients with acute pulmonary embolism. Am J Hematol. 2013 Aug;88(8):677-81. doi: 10.1002/ajh.23484. Epub 2013 Jun 20. PMID: 23674436.
72. Galliazzo S, Nigro O, Bertù L, Guasti L, Grandi AM, Ageno W, Dentali F. Prognostic role of neutrophils to lymphocytes ratio in patients with acute pulmonary embolism: a systematic review and meta-analysis of the literature. Intern Emerg Med. 2018 Jun;13(4):603-608. doi: 10.1007/s11739-018-1805-2. Epub 2018 Mar 5. PMID: 29508224
73. Wang Q, Ma J, Jiang Z, Ming L. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in acute pulmonary embolism: a systematic review and meta-analysis. Int Angiol. 2018 Feb;37(1):4-11. doi: 10.23736/S0392-9590.17.03848-2. Epub 2017 May 24. PMID: 28541022.
74. Bajaj A, Saleeb M, Rathor P, Sehgal V, Kabak B, Hosur S. Prognostic value of troponins in acute non massive pulmonary embolism: A meta-analysis. Heart Lung. 2015 Jul-Aug;44(4):327-34. doi: 10.1016/j.hrtlng.2015.03.007. Epub 2015 May 11. PMID: 25976228
75. Jiménez D, Aujesky D, Díaz G, Monreal M, Otero R, Martí D, Marín E, Aracil E, Sueiro A, Yusen RD; RIETE Investigators. Prognostic significance of deep vein thrombosis in patients presenting with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2010 May 1;181(9):983-91. doi: 10.1164/rccm.200908-1204OC. Epub 2010 Jan 28. PMID: 20110556
76. Becattini C, Cohen AT, Agnelli G, Howard L, Castejón B, Trujillo-Santos J, Monreal M, Perrier A, Yusen RD, Jiménez D. Risk Stratification of Patients With Acute Symptomatic Pulmonary Embolism Based on Presence or Absence of Lower Extremity DVT: Systematic Review and Meta-analysis. Chest. 2016 Jan;149(1):192-200. doi: 10.1378/chest.15-0808. Epub 2016 Jan 6. PMID: 26204122
77. Van der Meer RW, Pattynama PM, van Strijen MJ, van den Berg-Huijsmans AA, Hartmann IJ, Putter H, de Roos A, Huisman MV. Right ventricular dysfunction and pulmonary obstruction index at helical CT: prediction of clinical outcome during 3-month follow-up in patients with acute pulmonary embolism. Radiology. 2005 Jun;235(3):798-803. doi: 10.1148/radiol.2353040593. Epub 2005 Apr 21. PMID: 15845793
78. Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol. 2013 Jan 15;111(2):273-7. doi: 10.1016/j.amjcard.2012.09.027. Epub 2012 Oct 24. PMID: 23102885.
79. Ng AC, Chow V, Yong AS, Chung T, Kritharides L. Prognostic impact of the Charlson comorbidity index on mortality following acute pulmonary embolism. Respiration. 2013;85(5):408-16. doi: 10.1159/000342024. Epub 2012 Nov 10. PMID: 23147354.
80. Aujesky D, Obrosky DS, Stone RA, Auble TE, Perrier A, Cornuz J, Roy PM, Fine MJ. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005 Oct 15;172(8):1041-6. doi: 10.1164/rccm.200506-862OC. Epub 2005 Jul 14. PMID: 16020800; PMCID: PMC2718410
81. Jiménez D, Aujesky D, Moores L, Gómez V, Lobo JL, Uresandi F, Otero R, Monreal M, Muriel A, Yusen RD; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010 Aug 9;170(15):1383-9. doi: 10.1001/archinternmed.2010.199. PMID: 20696966
82. Hobohm L, Hellenkamp K, Hasenfuß G, Münzel T, Konstantinides S, Lankeit M. Comparison of risk assessment strategies for not-high-risk pulmonary embolism. Eur Respir J. 2016 Apr;47(4):1170-8. doi: 10.1183/13993003.01605-2015. Epub 2016 Jan 7. PMID: 26743479.
83. Kohn CG, Mearns ES, Parker MW, Hernandez AV, Coleman CI. Prognostic accuracy of clinical prediction rules for early post-pulmonary embolism all-cause mortality: a bivariate meta-analysis. Chest. 2015 Apr;147(4):1043-1062. doi: 10.1378/chest.14-1888. PMID: 25317677
84. Steering Committee. Single-bolus tenecteplase plus heparin compared with heparin alone for normotensive patients with acute pulmonary embolism who have evidence of right ventricular dysfunction and myocardial injury: rationale and design of the Pulmonary Embolism Thrombolysis (PEITHO) trial. Am Heart J. 2012 Jan;163(1):33-38.e1. doi: 10.1016/j.ahj.2011.10.003. PMID: 22172434
85. Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002 Mar;121(3):877-905. doi: 10.1378/chest.121.3.877. PMID: 11888976
86. The hemodynamic response to pulmonary embolism in patients without prior cardiopulmonary disease. Am J Cardiol. 1971 Sep;28(3):288-94. doi: 10.1016/0002-9149(71)90116-0. PMID: 5155756
87. Chung T, Connor D, Joseph J, Emmett L, Mansberg R, Peters M, Ma D, Kritharides L. Platelet activation in acute pulmonary embolism. J Thromb Haemost. 2007 May;5(5):918-24. doi: 10.1111/j.1538-7836.2007.02461.x. Epub 2007 Feb 26. PMID: 17371486
88. Smulders YM. Pathophysiology and treatment of haemodynamic instability in acute pulmonary embolism: the pivotal role of pulmonary vasoconstriction. Cardiovasc Res. 2000 Oct;48(1):23-33. doi: 10.1016/s0008-6363(00)00168-1. PMID: 11033105
89. Lankhaar JW, Westerhof N, Faes TJ, Marques KM, Marcus JT, Postmus PE, Vonk-Noordegraaf A. Quantification of right ventricular afterload in patients with and without pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2006 Oct;291(4):H1731-7. doi: 10.1152/ajpheart.00336.2006. Epub 2006 May 12. PMID: 16699074
90. Bryce YC, Perez-Johnston R, Bryce EB, Homayoon B, Santos-Martin EG. Pathophysiology of right ventricular failure in acute pulmonary embolism and chronic thromboembolic pulmonary hypertension: a pictorial essay for the interventional radiologist. Insights Imaging. 2019 Feb 13;10(1):18. doi: 10.1186/s13244-019-0695-9. PMID: 30758687; PMCID: PMC637509
91. Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002 Mar;121(3):877-905. doi: 10.1378/chest.121.3.877. PMID: 11888976
92. Burrowes KS, Clark AR, Tawhai MH. Blood flow redistribution and ventilation-perfusion mismatch during embolic pulmonary arterial occlusion. Pulm Circ. 2011 Jul-Sep;1(3):365-76. doi: 10.4103/2045-8932.87302. PMID: 22140626; PMCID: PMC3224428
93. Hemnes AR, Newman AL, Rosenbaum B, Barrett TW, Zhou C, Rice TW, Newman JH. Bedside end-tidal CO2 tension as a screening tool to exclude pulmonary embolism. Eur Respir J. 2010 Apr;35(4):735-41. doi: 10.1183/09031936.00084709. Epub 2009 Aug 28. PMID: 19717480
94. Aagaard R, Løfgren B, Caap P, Mygind-Klausen T, Bøtker MT, Granfeldt A. A low end-tidal CO2/arterial CO2 ratio during cardiopulmonary resuscitation suggests pulmonary embolism. Resuscitation. 2018 Dec;133:137-140. doi: 10.1016/j.resuscitation.2018.10.008. Epub 2018 Oct 12. PMID: 30321625.
95. Fernandes CJ, Luppino Assad AP, Alves-Jr JL, Jardim C, de Souza R. Pulmonary Embolism and Gas Exchange. Respiration. 2019;98(3):253-262. doi: 10.1159/000501342. Epub 2019 Aug 7. PMID: 31390642
96. Goldhaber SZ, Elliott CG. Acute pulmonary embolism: part I: epidemiology, pathophysiology, and diagnosis. Circulation. 2003 Dec 2;108(22):2726-9. doi: 10.1161/01.CIR.0000097829.89204.0C. PMID: 14656907.
97. Lahm T, McCaslin CA, Wozniak TC, Ghumman W, Fadl YY, Obeidat OS, Schwab K, Meldrum DR. Medical and surgical treatment of acute right ventricular failure. J Am Coll Cardiol. 2010 Oct 26;56(18):1435-46. doi: 10.1016/j.jacc.2010.05.046. PMID: 20951319
98. Harjola VP, Mebazaa A, Čelutkienė J, Bettex D, Bueno H, Chioncel O, Crespo-Leiro MG, Falk V, Filippatos G, Gibbs S, Leite-Moreira A, Lassus J, Masip J, Mueller C, Mullens W, Naeije R, Noordegraaf AV, Parissis J, Riley JP, Ristic A, Rosano G, Rudiger A, Ruschitzka F, Seferovic P, Sztrymf B, Vieillard-Baron A, Yilmaz MB, Konstantinides S. Contemporary management of acute right ventricular failure: a statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur J Heart Fail. 2016 Mar;18(3):226-41. doi: 10.1002/ejhf.478. PMID: 26995592
99. Boulain T, Lanotte R, Legras A, Perrotin D. Efficacy of epinephrine therapy in shock complicating pulmonary embolism. Chest. 1993 Jul;104(1):300-2. doi: 10.1378/chest.104.1.300. PMID: 8325096
100. Gordon AC, Mason AJ, Thirunavukkarasu N, Perkins GD, Cecconi M, Cepkova M, Pogson DG, Aya HD, Anjum A, Frazier GJ, Santhakumaran S, Ashby D, Brett SJ; VANISH Investigators. Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients With Septic Shock: The VANISH Randomized Clinical Trial. JAMA. 2016 Aug 2;316(5):509-18. doi: 10.1001/jama.2016.10485. PMID: 27483065
101. Konstantinides S, Geibel A, Kasper W, Olschewski M, Blümel L, Just H. Patent foramen ovale is an important predictor of adverse outcome in patients with major pulmonary embolism. Circulation. 1998 May 19;97(19):1946-51. doi: 10.1161/01.cir.97.19.1946. PMID: 9609088
102. Kline JA, Puskarich MA, Jones AE, Mastouri RA, Hall CL, Perkins A, Gundert EE, Lahm T. Inhaled nitric oxide to treat intermediate risk pulmonary embolism: A multicenter randomized controlled trial. Nitric Oxide. 2019 Mar 1;84:60-68. doi: 10.1016/j.niox.2019.01.006. Epub 2019 Jan 8. PMID: 30633959; PMCID: PMC7047892
103. Sablotzki A, Starzmann W, Scheubel R, Grond S, Czeslick EG. Selective pulmonary vasodilation with inhaled aerosolized milrinone in heart transplant candidates. Can J Anaesth. 2005;52(10):1076-1082. doi:10.1007/BF03021608
104. Smith SB, Geske JB, Maguire JM, Zane NA, Carter RE, Morgenthaler TI. Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest. 2010 Jun;137(6):1382-90. doi: 10.1378/chest.09-0959. Epub 2010 Jan 15. PMID: 20081101; PMCID: PMC3021363
105. Raschke RA, Reilly BM, Guidry JR, Fontana JR, Srinivas S. The weight-based heparin dosing nomogram compared with a “standard care” nomogram. A randomized controlled trial. Ann Intern Med. 1993 Nov 1;119(9):874-81. doi: 10.7326/0003-4819-119-9-199311010-00002. PMID: 8214998
106. Erkens PM, Prins MH. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev. 2010 Sep 8;(9):CD001100. doi: 10.1002/14651858.CD001100.pub3. Update in: Cochrane Database Syst Rev. 2017 Feb 09;2:CD001100. PMID: 20824828
107. Prandoni P, Siragusa S, Girolami B, Fabris F; BELZONI Investigators Group. The incidence of heparin-induced thrombocytopenia in medical patients treated with low-molecular-weight heparin: a prospective cohort study. Blood. 2005 Nov 1;106(9):3049-54. doi: 10.1182/blood-2005-03-0912. Epub 2005 Jul 19. PMID: 16030191
108. Kluft C, Sidelmann JJ, Gram JB. Assessing Safety of Thrombolytic Therapy. Semin Thromb Hemost. 2017 Apr;43(3):300-310. doi: 10.1055/s-0036-1584130. Epub 2016 Jun 6. PMID: 27272963.
109. Stubblefield WB, Alves NJ, Rondina MT, Kline JA. Variable Resistance to Plasminogen Activator Initiated Fibrinolysis for Intermediate-Risk Pulmonary Embolism. PLoS One. 2016 Feb 11;11(2):e0148747. doi: 10.1371/journal.pone.0148747. PMID: 26866684; PMCID: PMC4751085
110. Longstaff C, Kolev K. Basic mechanisms and regulation of fibrinolysis. J Thromb Haemost. 2015 Jun;13 Suppl 1:S98-105. doi: 10.1111/jth.12935. PMID: 26149056
111. Chodakowski JD, Courtney DM. Pulmonary embolism critical care update: prognosis, treatment, and research gaps. Curr Opin Crit Care. 2018 Dec;24(6):540-546. doi: 10.1097/MCC.0000000000000558. PMID: 30325344
112. Wan S, Quinlan DJ, Agnelli G, Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation. 2004 Aug 10;110(6):744-9. doi: 10.1161/01.CIR.0000137826.09715.9C. Epub 2004 Jul 19. PMID: 15262836
113. Stein PD, Matta F. Thrombolytic therapy in unstable patients with acute pulmonary embolism: saves lives but underused. Am J Med. 2012 May;125(5):465-70. doi: 10.1016/j.amjmed.2011.10.015. Epub 2012 Feb 10. Erratum in: Am J Med. 2012 Jul;125(7):e13. PMID: 22325236
114. Konstantinides SV, Vicaut E, Danays T, Becattini C, Bertoletti L, Beyer-Westendorf J, Bouvaist H, Couturaud F, Dellas C, Durschmied D, Empen K, Ferrari E, Galiè N, Jiménez D, Kostrubiec M, Kozak M, Kupatt C, Lang IM, Lankeit M, Meneveau N, Palazzini M, Pruszczyk P, Rigoletto M, SalviA, Sanchez O, Schellong S, Sobkowicz B, Meyer G. Impact of Thrombolytic Therapy on the Long-Term Outcome of Intermediate-Risk Pulmonary Embolism. J Am Coll Cardiol. 2017 Mar 28;69(12):1536-1544. doi: 10.1016/j.jacc.2016.12.039. PMID: 28335835.
115. Chatterjee S, Weinberg I, Yeh RW, Chakraborty A, Sardar P, Weinberg MD, Kabrhel C, Barnes GD, Mukherjee D, Kumbhani D, Bashir R, Vaidya A, Smith A, Fuchs B, Groeneveld P, Giri J. Risk factors for intracranial haemorrhage in patients with pulmonary embolism treated with thrombolytic therapy Development of the PE-CH Score. Thromb Haemost. 2017 Jan 26;117(2):246-251. doi: 10.1160/TH16-07-0588. Epub 2016 Nov 24. PMID: 27882375
116. Fengler BT, Brady WJ. Fibrinolytic therapy in pulmonary embolism: an evidence-based treatment algorithm. Am J Emerg Med. 2009 Jan;27(1):84-95. doi: 10.1016/j.ajem.2007.10.021. PMID: 19041539
117. Sadiq I, Goldhaber SZ, Liu PY, Piazza G; Submassive and Massive Pulmonary Embolism Treatment with Ultrasound AcceleraTed ThromboLysis ThErapy (SEATTLE II) Investigators. Risk factors for major bleeding in the SEATTLE II trial. Vasc Med. 2017 Feb;22(1):44-50. doi: 10.1177/1358863X16676355. Epub 2017 Jan 31. PMID: 27913777; PMCID: PMC5347366
118. Wang C, Zhai Z, Yang Y, et al. Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: a randomized, multicenter, controlled trial. Chest. 2010;137(2):254-262. doi:10.1378/chest.09-0765
119. Riva N, Puljak L, Moja L, Ageno W, Schünemann H, Magrini N, Squizzato A. Multiple overlapping systematic reviews facilitate the origin of disputes: the case of thrombolytic therapy for pulmonary embolism. J Clin Epidemiol. 2018 May;97:1-13. doi: 10.1016/j.jclinepi.2017.11.012. Epub 2017 Nov 22. PMID: 29175415
120. Chatterjee S, Chakraborty A, Weinberg I, Kadakia M, Wilensky RL, Sardar P, Kumbhani DJ, Mukherjee D, Jaff MR, Giri J. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA. 2014 Jun 18;311(23):2414-21. doi: 10.1001/jama.2014.5990. PMID: 24938564
121. Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W; Management Strategies and Prognosis of Pulmonary Embolism-3 Trial Investigators. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002 Oct 10;347(15):1143-50. doi: 10.1056/NEJMoa021274. PMID: 12374874.
122. Wang C, Zhai Z, Yang Y, Wu Q, Cheng Z, Liang L, Dai H, Huang K, Lu W, Zhang Z, Cheng X, Shen YH; China Venous Thromboembolism (VTE) Study Group. Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: a randomized, multicenter, controlled trial. Chest. 2010 Feb;137(2):254-62. doi: 10.1378/chest.09-0765. Epub 2009 Sep 9. PMID: 19741062; PMCID: PMC7126994
123. Valerio L, Klok FA, Barco S. Immediate and late impact of reperfusion therapies in acute pulmonary embolism. Eur Heart J Suppl. 2019 Nov;21(Suppl I):I1-I13. doi: 10.1093/eurheartj/suz222. Epub 2019 Nov 21. PMID: 31777451; PMCID: PMC6868376
124. Rose PS, Punjabi NM, Pearse DB. Treatment of right heart thromboemboli. Chest. 2002 Mar;121(3):806-14. doi: 10.1378/chest.121.3.806. PMID: 11888964
125. Athappan G, Sengodan P, Chacko P, Gandhi S. Comparative efficacy of different modalities for treatment of right heart thrombi in transit: a pooled analysis. Vasc Med. 2015 Apr;20(2):131-8. doi: 10.1177/1358863X15569009. PMID: 25832601.
126. Islam M, Nesheim D, Acquah S, Kory P, Kourouni I, Ramesh N, Ehrlich M, Bajpayee G, Steiger D, Filopei J. Right Heart Thrombi: Patient Outcomes by Treatment Modality and Predictors of Mortality: A Pooled Analysis. J Intensive Care Med. 2019 Nov-Dec;34(11-12):930-937. doi: 10.1177/0885066618808193. Epub 2018 Oct 29. PMID: 30373436
127. Lu C, Li J, Wang W, Gong K, Zhao L, Fu X. Large thrombus-in-transit within a patent foramen ovale in a patient with pulmonary embolism: a case report. J Int Med Res. 2018 Oct;46(10):4332-4337. doi: 10.1177/0300060518789820. Epub 2018 Aug 8. PMID: 30088430; PMCID: PMC6166339
128. Mirijello A, D’Errico MM, Curci S, Spatuzza P, Graziano D, La Viola M, D’Alessandro V, Grilli M, Vendemiale G, Cassese M, De Cosmo S. Paradoxical embolism with thrombus stuck in a patent foramen ovale: a review of treatment strategies. Eur Rev Med Pharmacol Sci. 2018 Dec;22(24):8885-8890. doi: 10.26355/eurrev_201812_16657. PMID: 30575931
129. Sharifi M, Berger J, Beeston P, Bay C, Vajo Z, Javadpoor S; “PEAPETT” investigators. Pulseless electrical activity in pulmonary embolism treated with thrombolysis (from the “PEAPETT” study). Am J Emerg Med. 2016 Oct;34(10):1963-1967. doi: 10.1016/j.ajem.2016.06.094. Epub 2016 Jun 30. PMID: 27422214
130. Javaudin F, Lascarrou JB, Le Bastard Q, Bourry Q, Latour C, De Carvalho H, Le Conte P, Escutnaire J, Hubert H, Montassier E, Leclère B; Research Group of the French National Out-of-Hospital Cardiac Arrest Registry (GR-RéAC). Thrombolysis During Resuscitation for Out-of-Hospital Cardiac Arrest Caused by Pulmonary Embolism Increases 30-Day Survival: Findings From the French National Cardiac Arrest Registry. Chest. 2019 Dec;156(6):1167-1175. doi: 10.1016/j.chest.2019.07.015. Epub 2019 Aug 2. PMID: 31381884
131. Böttiger BW, Wetsch WA. Pulmonary Embolism Cardiac Arrest: Thrombolysis During Cardiopulmonary Resuscitation and Improved Survival. Chest. 2019 Dec;156(6):1035-1036. doi: 10.1016/j.chest.2019.08.1922. PMID: 31812186
132. Lavonas EJ, Drennan IR, Gabrielli A, Heffner AC, Hoyte CO, Orkin AM, Sawyer KN, Donnino MW. Part 10: Special Circumstances of Resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015 Nov 3;132(18 Suppl 2):S501-18. doi: 10.1161/CIR.0000000000000264. Erratum in: Circulation. 2016 Aug 30;134(9):e122. PMID: 26472998
133. Panoutsopoulos G, Ilias L, Christakopoulou I. Transient right-to-left shunt in massive pulmonary embolism. Ann Nucl Med. 2000 Jun;14(3):217-21. doi: 10.1007/BF02987863. PMID: 10921488


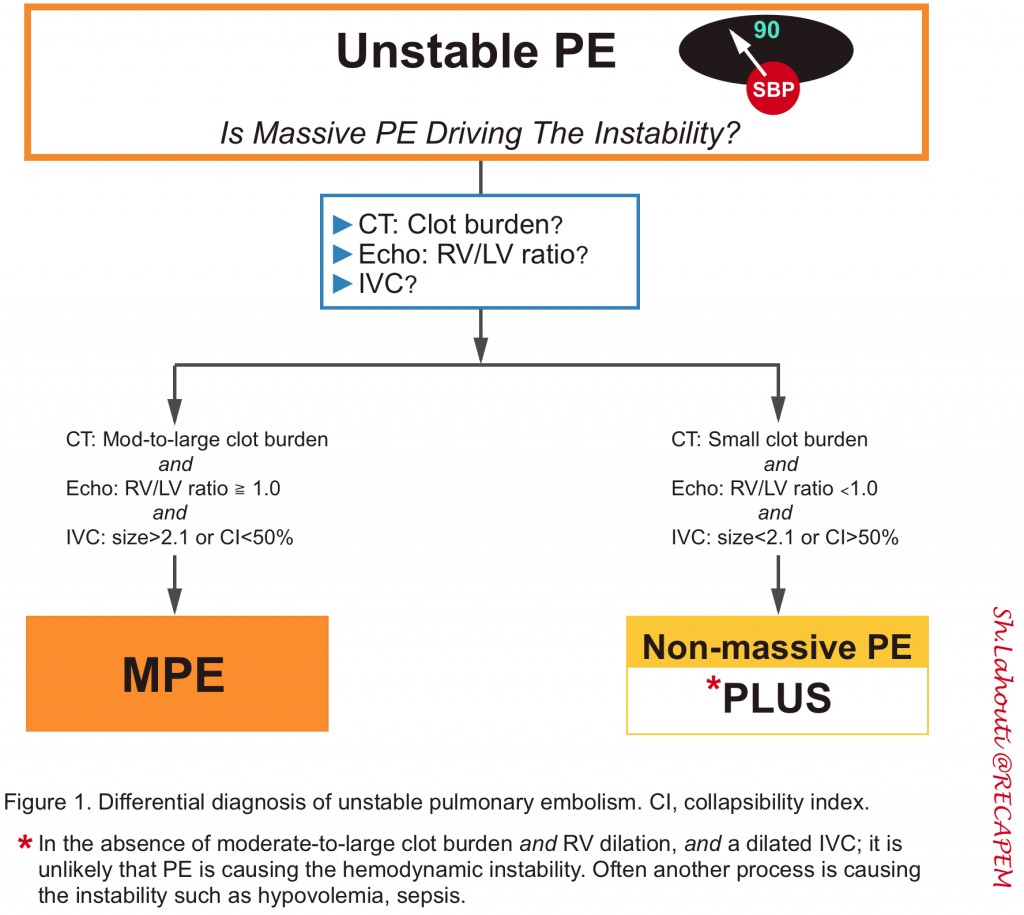
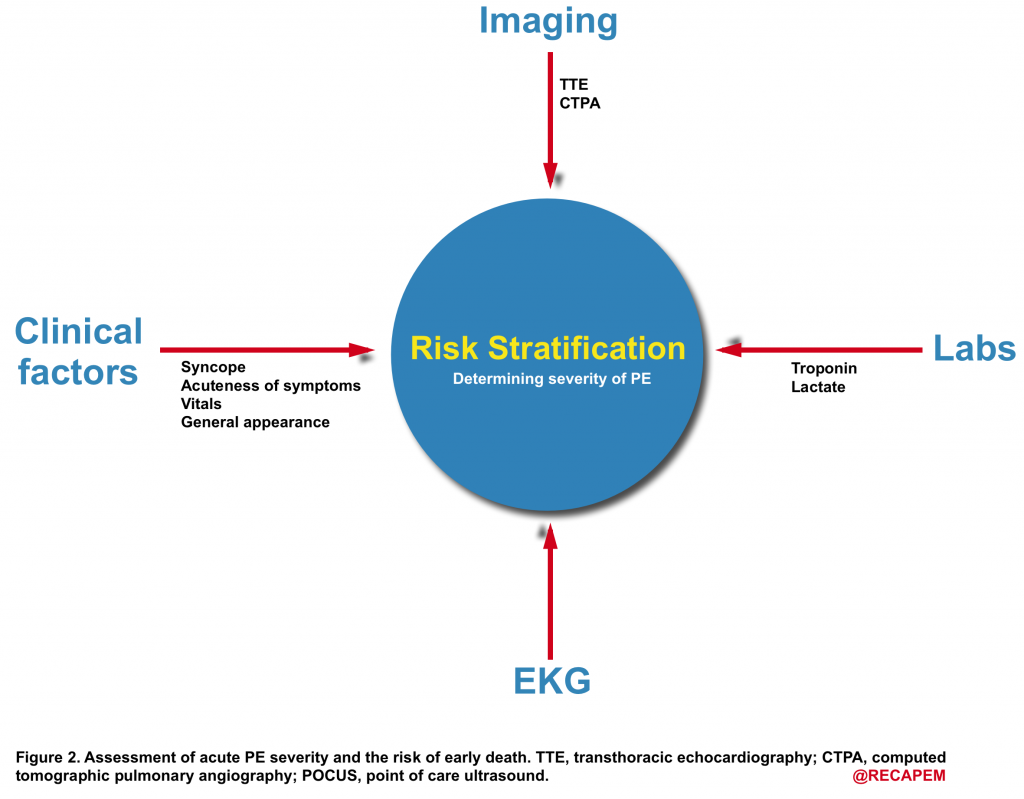
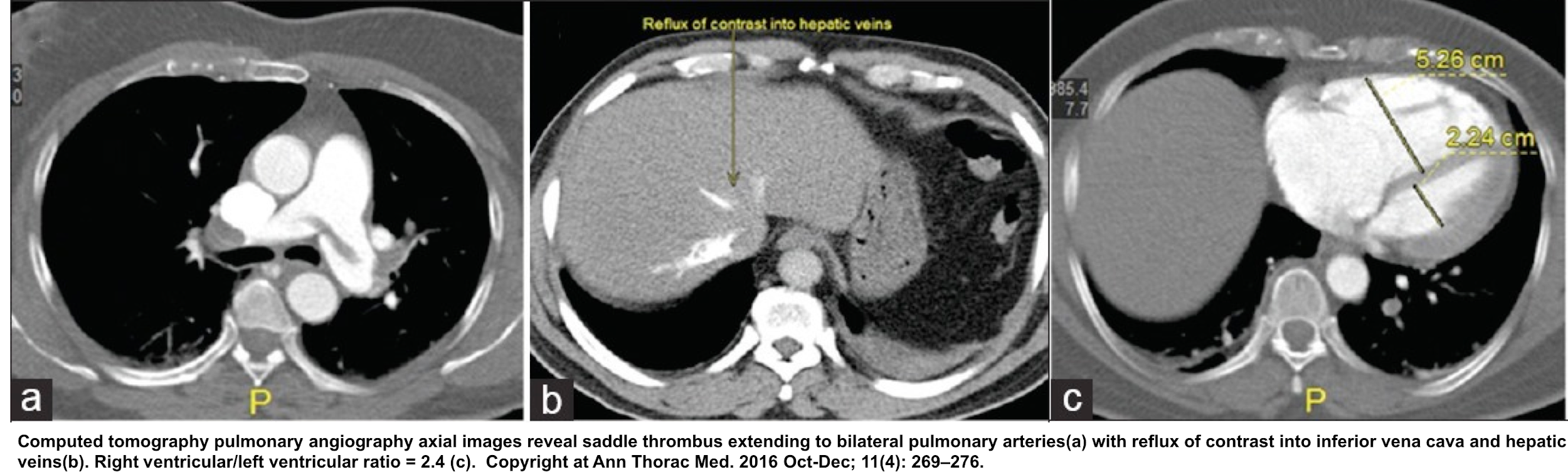
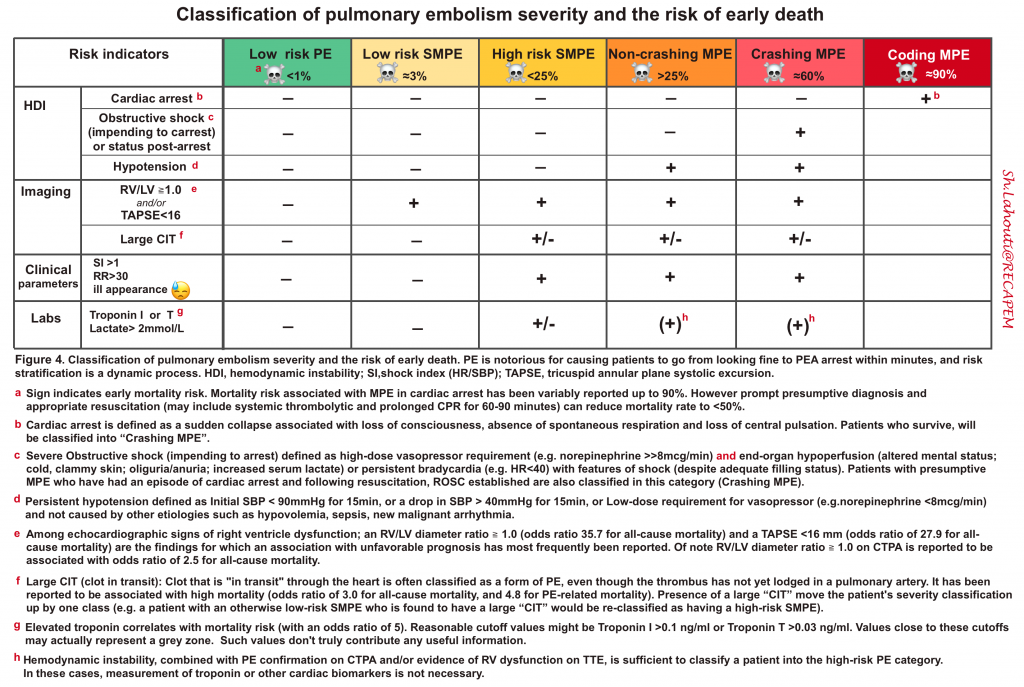
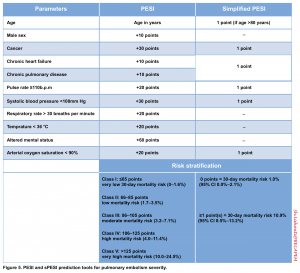
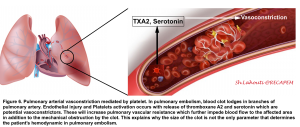
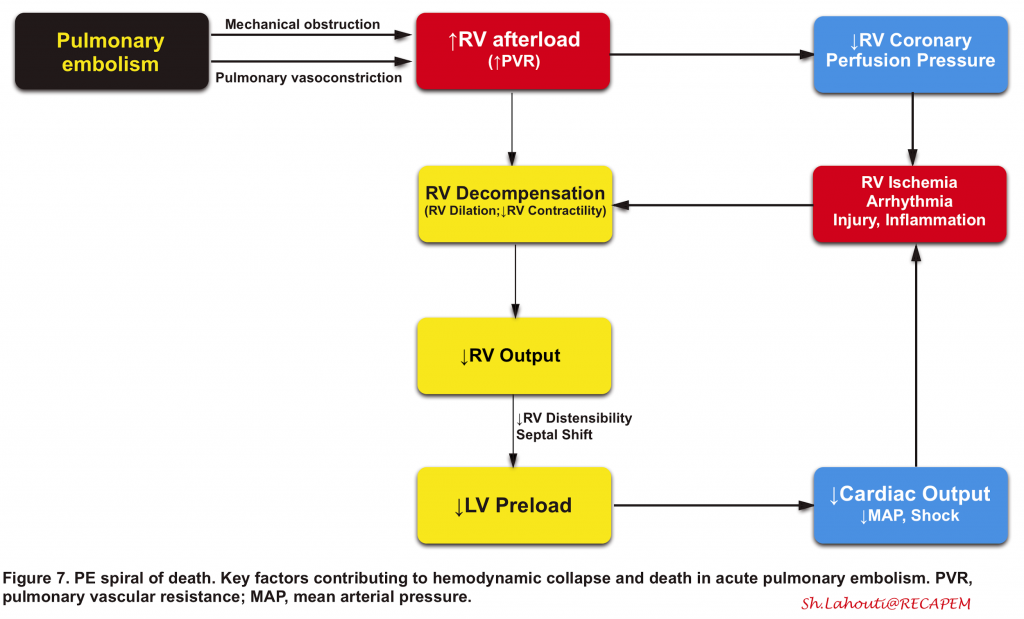
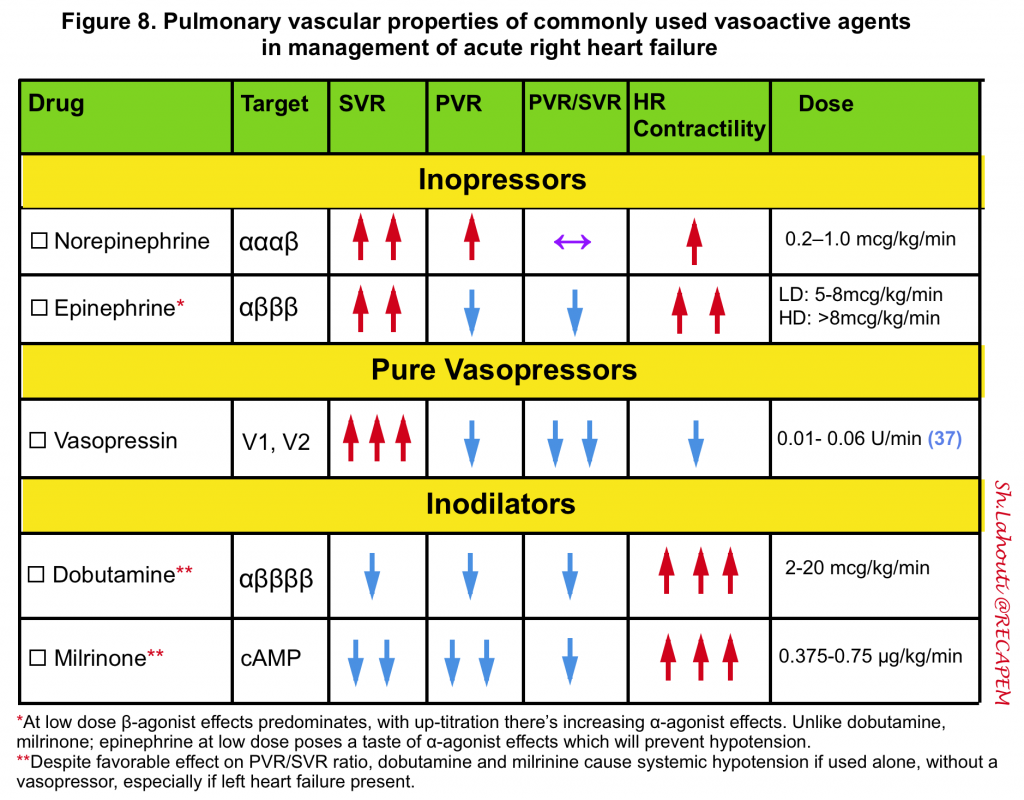
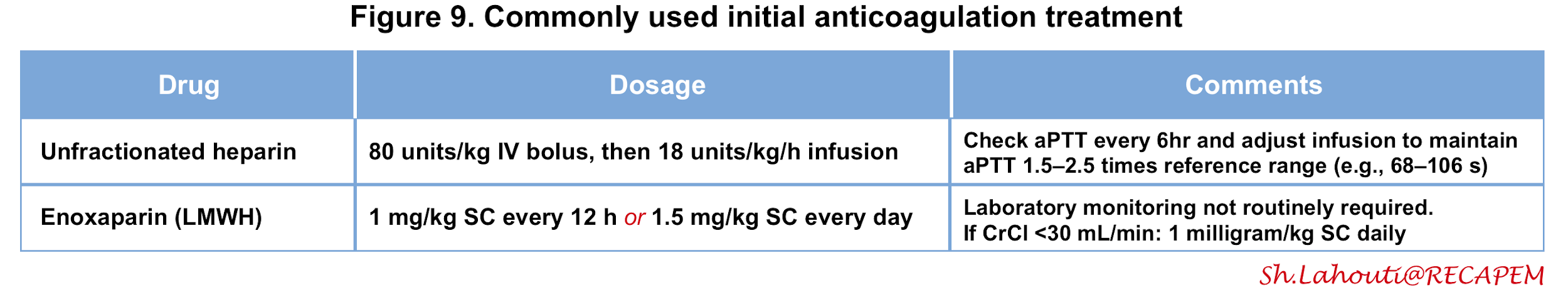
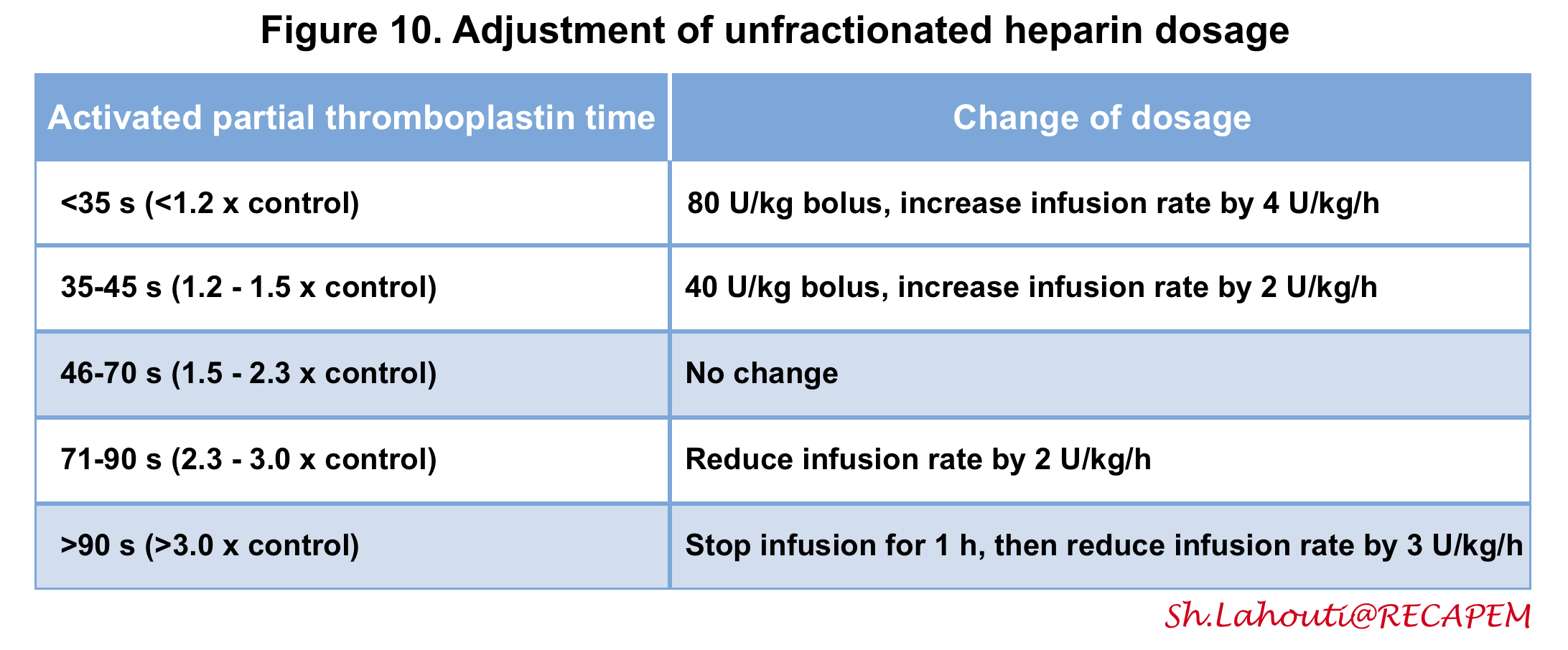
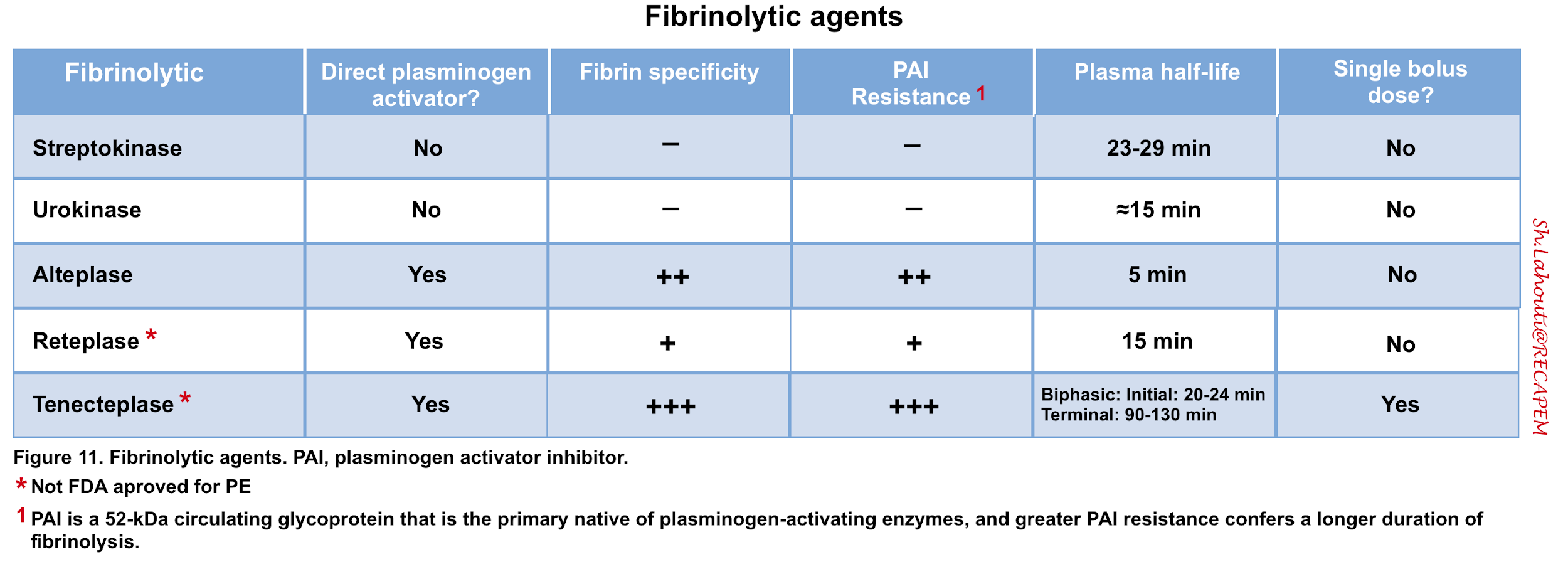
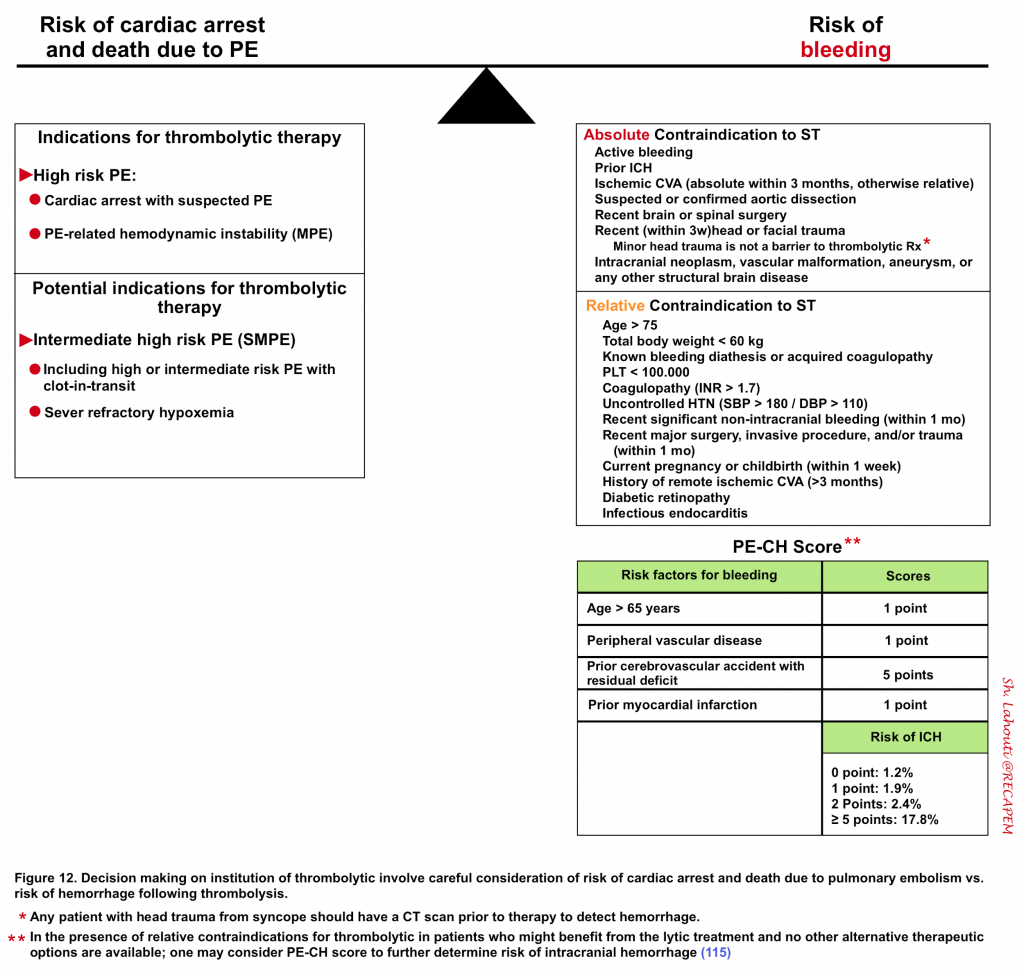
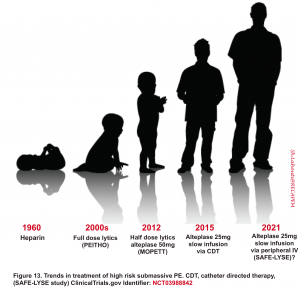
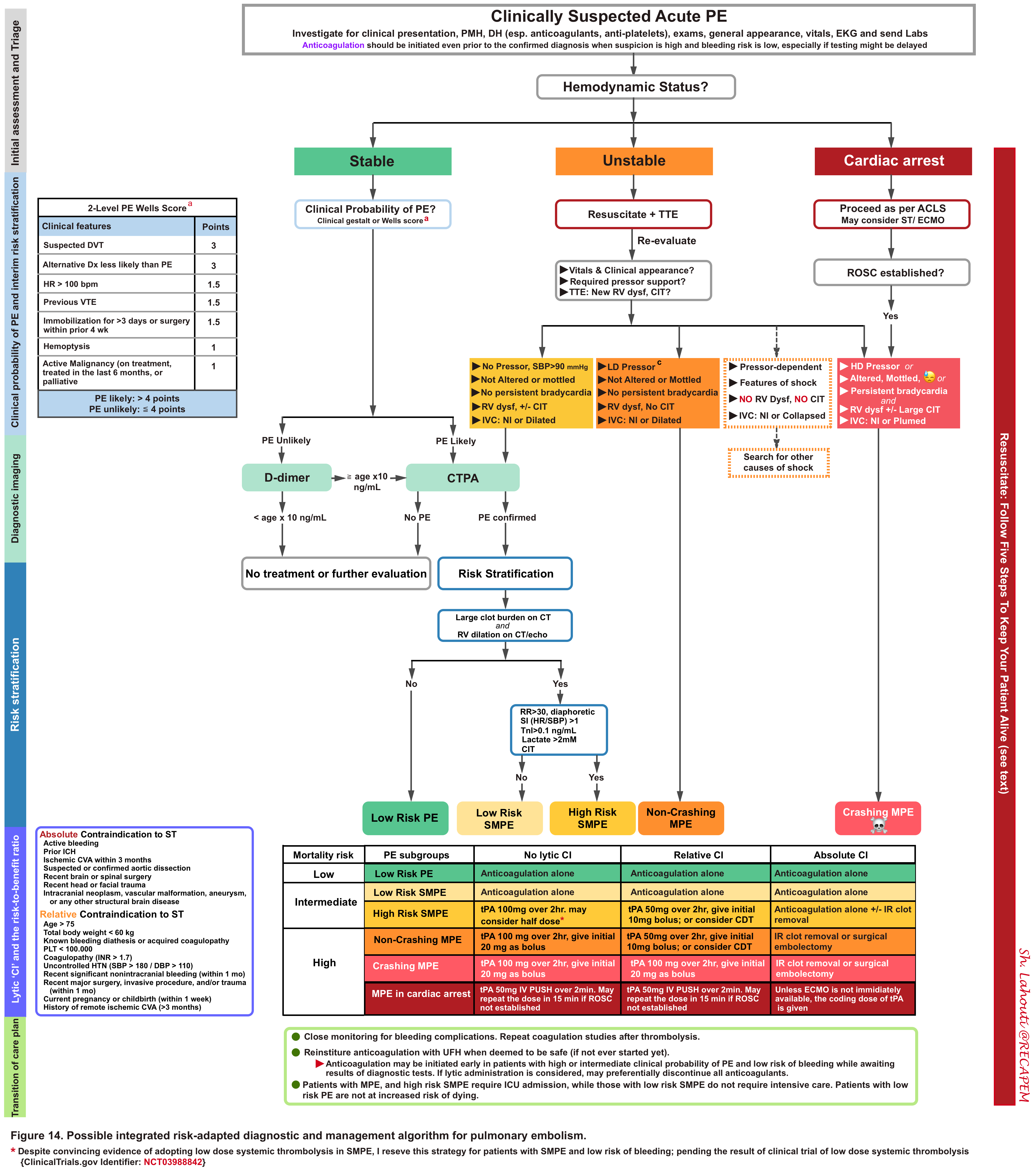
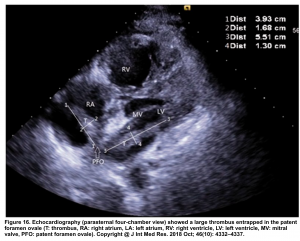
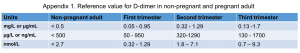
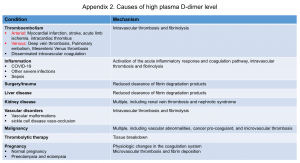
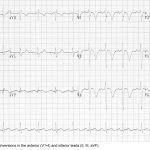

Add comment