27 August, 2024, by Shahriar Lahouti.
CONTENTS
- Preface
- Background & epidemiology
- Pharmacokinetics
- Toxicokinetics
- Clinical manifestation
- Differential diagnosis
- Investigations
- Diagnosis
- Risk assessment
- Management
- RECAP
- Going further
- References
Preface
Salicylate toxicity is a complex problem that may develop with either acute or chronic exposure to salicylates. Patients with salicylate poisoning may have involvement of multiple organ systems particularly the central nervous system, GI, and pulmonary system. Salicylate toxicity is common and is under-represented in poison center data because it is often not recognized. The in-hospital mortality for unrecognized chronic aspirin poisoning is three times higher than if the diagnosis is made in the emergency department *. The mortality rate among patients with severe salicylate toxicity is ~5%. A delay in diagnosis and initiation of treatment in such patients is associated with a 15% mortality rate *.
In this post, the sources of salicylates, pathophysiological effects, clinical presentation during the various stages of acute and chronic poisoning, and treatment strategies for salicylate toxicity will be reviewed.
Background and epidemiology
▪️Background
- Salicylates have analgesic, antipyretic, and anti-inflammatory properties, all of which are mediated via the inhibition of cyclooxygenase enzymes *.
- Sources
- Salicylates are found in a myriad of prescription and over-the-counter medicinal preparations, including acetylsalicylic acid tablets and analgesic mixtures.
- Methyl salicylate (oil of wintergreen), found in topical liniments and solutions used in hot vapors, is the most concentrated form of salicylate: 1 ml of a 98% solution contains 1400 mg of salicylate. One teaspoon (5 mL) provides the equivalent of 7000 mg of salicylic acid.
- A large amount of salicylate (8.7 mg/ml) is also found in bismuth subsalicylate, which is sold as a generic medication under the brand name Pepto-Bismol.

▪️Epidemiology
- In 2018, the U.S. National Poison Data System recorded approximately 18,460 exposures related to salicylates. Over 200 were noted to have serious outcomes, with 28 fatalities *.
Pharmacokinetics
- Acetylsalicylic acid is rapidly absorbed from the stomach, duodenum, and jejunum.
- After ingestion of therapeutic doses of immediate-release acetylsalicylate, therapeutic serum concentrations are achieved in 30 minutes, and maximum concentrations
are often attained in less than 1 hour. - Acetylsalicylic acid is a prodrug with a short half-life (~20 minutes) because of rapid hydrolysis of salicylic acid by serine esterases in the intestinal wall, erythrocytes, and liver *.
- The apparent half-life for salicylate is about 2-3 hours at antiplatelet doses and increases to 12 hours at antiinflammatory doses demonstrating dose-dependent elimination.
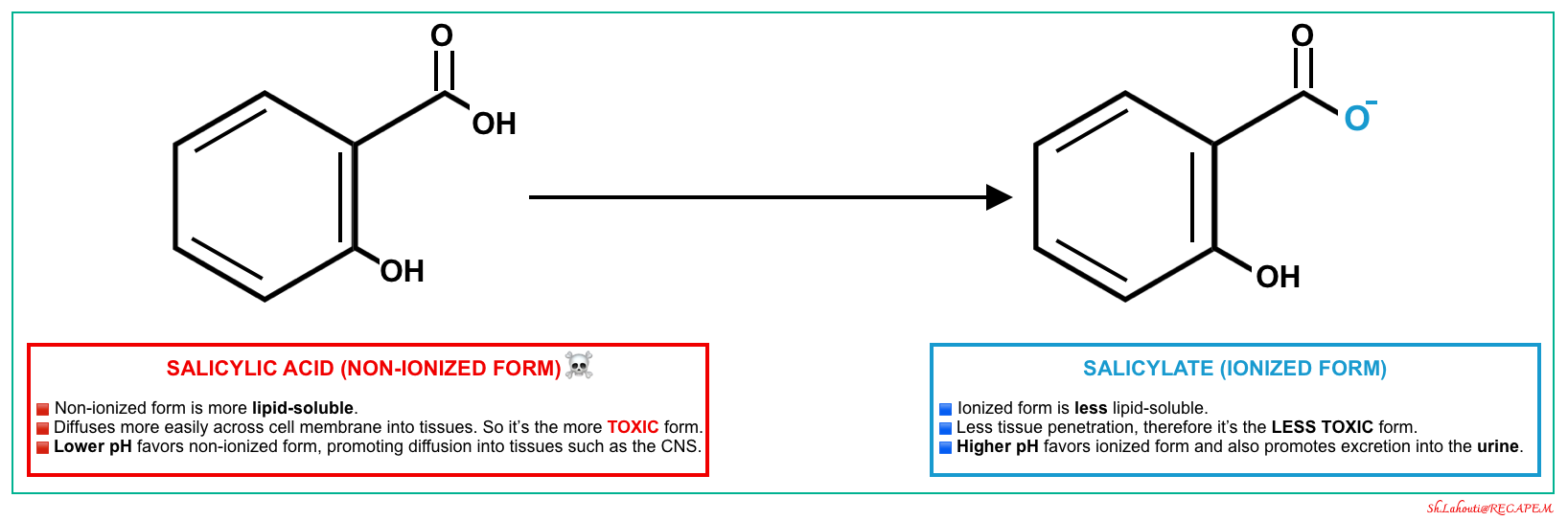
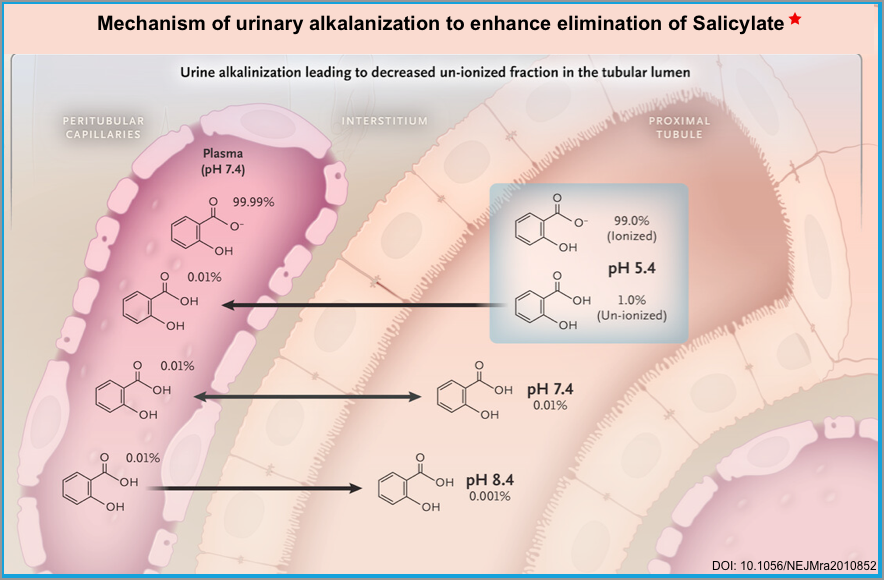
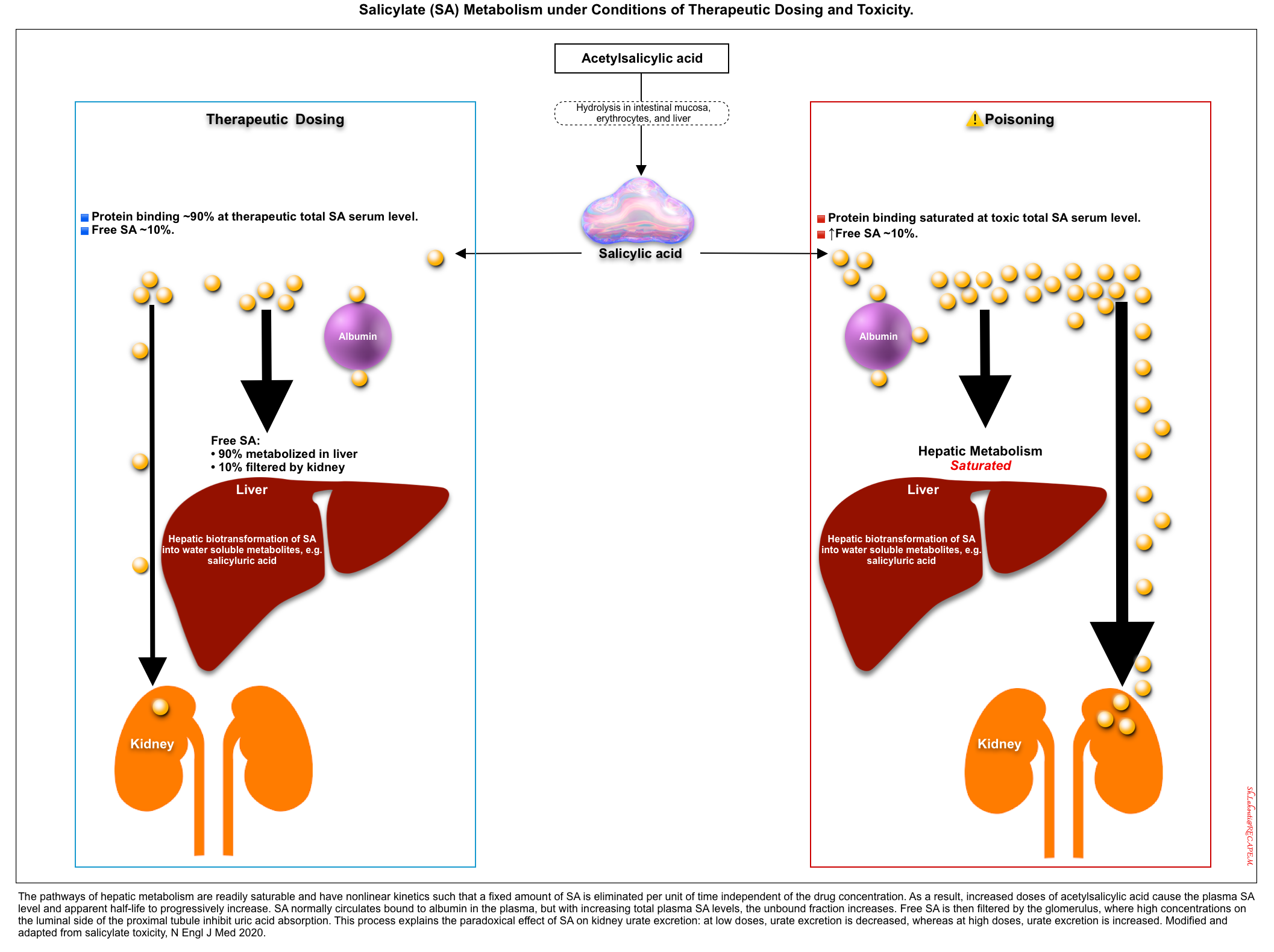
- At therapeutic concentrations, 90% of salicylate is protein-bound (primarily to albumin) which remains in the intravascular compartment, and almost 10% is free in plasma. In solutions, this latter free fraction of SA exists in two forms depending on pH, “non-ionized form (SA)” and “ionized form (salicylate)” (left figure below).
- Non-ionized form is more lipid soluble and more toxic ☠️.
- More acidic pH favors the formation of a non-ionized form *.
- It more easily crosses the cell membrane and enters the tissues such as the CNS.
- It is filtered through renal glomeruli and can be reabsorbed by renal tubules back into the plasma.
- Ionized form is less toxic as it has less penetration into the tissue.
- More alkaline pH favors the formation of an ionized form.
- 💡Alkalinization of serum can be used to keep salicylate in the plasma compartment and out of tissues *.
- 💡Alkalinization of urine entraps the salicylate in urine and increases the renal elimination of salicylate *.
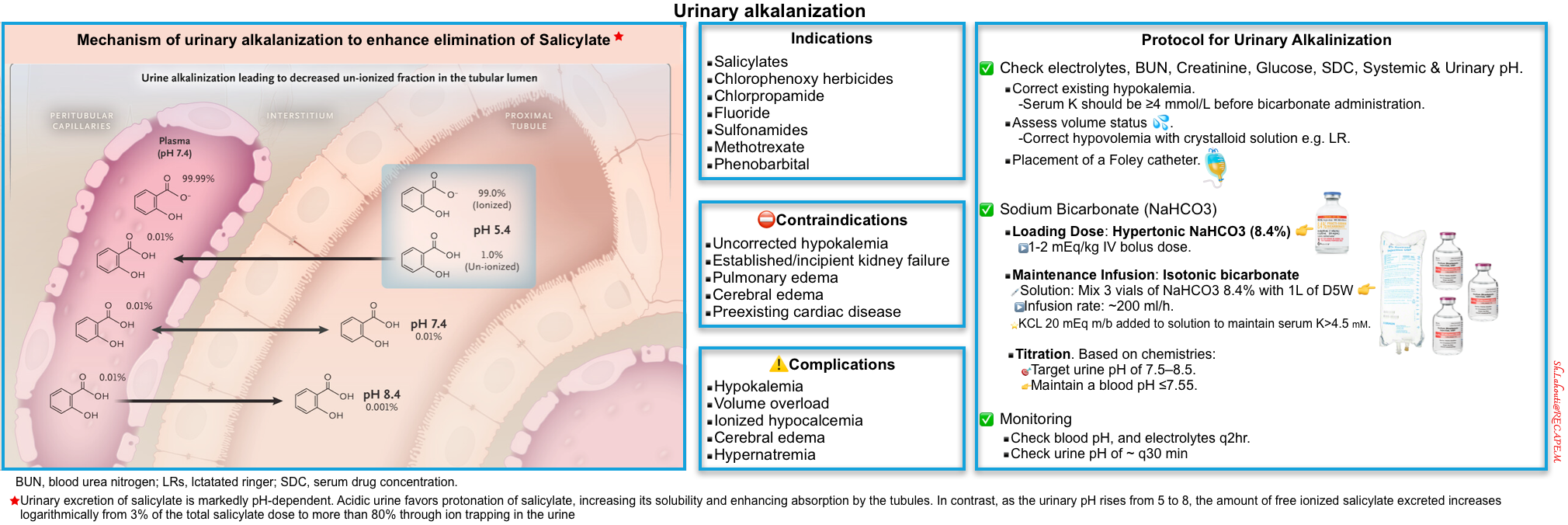
- If the urinary pH rises from 5 to 8, the amount of free ionized salicylate excreted increases logarithmically from 3% of the total salicylate dose to more than 80% through ion trapping in the urine (right figure below) *.
- Non-ionized form is more lipid soluble and more toxic ☠️.
- Aspirin undergoes biotransformation in the liver and is then eliminated by the kidneys.
- Salicylate is metabolized via hepatic glucuronidation, oxidation, and glycine conjugation.
- The major metabolite, salicyluric acid, is less toxic and more rapidly excreted than salicylate.
- The kidneys excrete salicyluric acid and other metabolites *.
- The hepatic metabolism of salicylate rapidly becomes saturated in the overdose situation following zero-order kinetics (i.e. a set amount of salicylate is eliminated per unit of time) *.
- This can explain the prolonged apparent half-lives of salicylate at toxic concentrations (~20 hours) compared to half-lives at therapeutic concentrations (~3 hours).
- Moreover, patients on chronic daily treatment of salicylate can develop intoxication with even a small change in dosage. This is explained by the fact that in chronic daily therapy with salicylate, metabolic enzymes are saturated and a minor increase in dosage can trigger intoxication.
- Salicylate is metabolized via hepatic glucuronidation, oxidation, and glycine conjugation.
Toxicokinetics
In overdose, several factors contribute to variable pharmacokinetics and present very challenging obstacles to effectively managing patients poisoned with salicylates. The important factors that contribute to the acuity of salicylism, magnitude, and duration of toxicity include:
- Dose of ingestion.
- Formulation (e.g. immediate-release, enteric-coated) influences the absorption rate.
- Therapeutic doses of enteric-coated tablets produce peak serum concentrations at 4-6 hours after ingestion, and in overdose, the reported peak is delayed up to 24 hours after ingestion.
- Ingestion of methyl salicylate or other liquid formulations may have much more rapid absorption and achieve peak levels more rapidly.
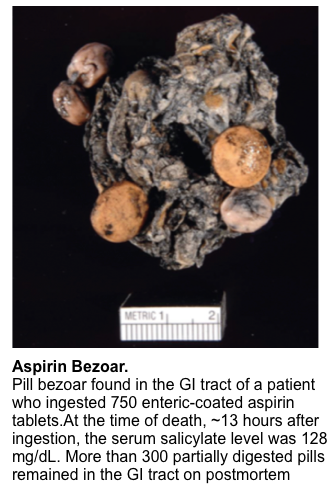
- There is potential for the formation of gastric bezoars in overdose with enteric-coated salicylate (figure below). This exhibits erratic absorption and can serve as a source of delayed and ongoing absorption *.
- Chronocity.
- When administered chronically, a small increase in dosage or a small decrease in metabolism or elimination results in substantial increases in serum salicylate concentrations and the risk of toxicity.
- Hepatic and renal function.
- Acute Kidney Injury (AKI) can lead to salicylate overdose even with therapeutic doses; this is especially true with the elderly.
- Serum and urine pH (explained above).
Clinical manifestations
- Clinical manifestations of salicylate toxicity depend on the dose ingested, duration of exposure, and age and comorbidities of the patient.
- Clinical symptoms will be variable if the patient ingested more than one drug or the ingested aspirin formulation contains a CNS depressant, which might blunt the expected hyperventilation and respiratory alkalosis.
- High clinical suspicion and a detailed history should be used to diagnose salicylate intoxication. Identifying salicylate toxicity is difficult for the following reasons:
- Patients who present early in the course of salicylate poisoning may have modest symptoms *.
- Hyperventilation and tachycardia may be mistaken for emotional excitation or anxiety.
- GI irritation may or may not be present, and tinnitus or other symptoms of ototoxicity may be overlooked.
- Fever generally will be absent.
- Patients who present in the late phases of salicylate toxicity often are misdiagnosed as sepsis *. Without a suggestive history, it can be extremely difficult to diagnose.
- Patients with chronic salicylate poisoning often present with neurologic disorders which may be nonspecific and often misleading.
- Patients who present early in the course of salicylate poisoning may have modest symptoms *.
Acute salicylate intoxication in adults
The acute form of salicylate intoxication generally occurs in young adults who have a psychiatric history or who have had a previous overdose. Such persons tend to ingest
salicylate alone or in combination with other drugs in a suicide attempt *.
- Tachypnea/hyperpnea.
- Observe the respiratory pattern rather than relying on respiratory rate. Patients may hyperventilate with a normal respiratory rate by increasing tidal volume (hyperpnea) *.
- Tachycardia.
- Diaphoresis 😓.
- Hyperthermia 🥵.
- Acid-base disturbances (see below).
▪️GI
- Nausea, vomiting 🤮.
- Vomiting can lead to hypokalemia and metabolic alkalosis.
- Gastritis, gastric perforation, and GI bleeding.
- Salicylate causes local gastric irritation. Gastric perforation has been reported after a significant acute aspirin ingestion *.
- Diarrhea.
▪️Otic
- Tinnitus, vertigo, hearing loss.
- Ototoxicity is thought to be secondary to interference with chloride channels in the cochlear hair cells that transmit sound waves *.
▪️Neurologic
- Delirium, agitation, lethargy, confusion, seizure, coma, ataxia, hallucinations, psychosis, cerebral edema.
- The CNS effects are the most visible and most consequential clinical effects in salicylate-poisoned patients.
- Pathophysiology of CNS effects include:
- Neuronal energy depletion (salicylate uncouples neuronal and glial oxidative phosphorylation) results in severe cellular acidosis.
- Neuroglycopenia.
- Salicylate poisoning can cause decreased CSF glucose concentration despite a normal serum glucose level *. Altered glucose metabolism and transport play a role in the deleterious neurologic effects of neuroglycopenia.
⚠️CNS dysfunction leading to cerebral edema is an ominous development and a sign of severe toxicity, requiring rapid and aggressive treatment.
▪️Pulmonary
- Non-cardiogenic pulmonary edema (NCPE).
- The onset of pulmonary edema might be acute, delayed, or during recovery *.
- Risk factors *:
- Older age
- Cigarette smoking
- Chronic salicylate ingestion
- Patients who have neurological symptoms and metabolic acidosis on admission
- Acute lung injury occurs in the setting of salicylate toxicity due to increased pulmonary capillary permeability and exudation of protein edema fluid into the interstitial or alveolar space.
- Inhibition of prostacyclin synthesis in alveolar membranes and coincident lipoxygenase accumulation results in increased permeability in alveolar capillaries *.
▪️Hematology
- Increased prothrombin time
- At supratherapeutic doses, salicylate decreases the plasma concentration of the γ-carboxyglutamate containing coagulation factors. The result of this interruption of vitamin K cycling is similar to that of warfarin *.
- Platelet dysfunction
- Although antiplatelet activity is a well-known and often desired effect of aspirin (but not other salicylate products), hemorrhage is a rare complication of acute (even massive) overdose.
Acid-base disturbances
The classic pattern of findings is as follows:
- This develops early, as a result of direct stimulation of the medullary respiratory center by salicylate.
- ⭐️Co-ingestion or administration of CNS depressants, e.g. opioids, may blunt this initial respiratory stimulation.
- Salicylate-induced respiratory alkalosis is almost universally associated with a bicarbonate deficit and paradoxical aciduria, limiting salicylate excretion *.
2️⃣High anion-gap metabolic acidosis
- Later on, an anion gap metabolic acidosis develops creating a mixed acid-base picture.
- Metabolic acidosis reflects increases in ketoacid, salicylic acid, and lactic acid.
- Most of the elevated anion gap is not due to salicylate itself (<5 mM contribution) *.
- Salicylates uncouple oxidative phosphorylation in mitochondria, induce lipolysis, stimulate skeletal muscle metabolism, and inhibit portions of the Krebs cycle, which leads to a build-up of metabolic products such as lactate, pyruvate, and ketoacids *.
👉The reduction in serum bicarbonate is caused both by concomitant metabolic acidosis and by an initial respiratory alkalosis-induced bicarbonaturia *.
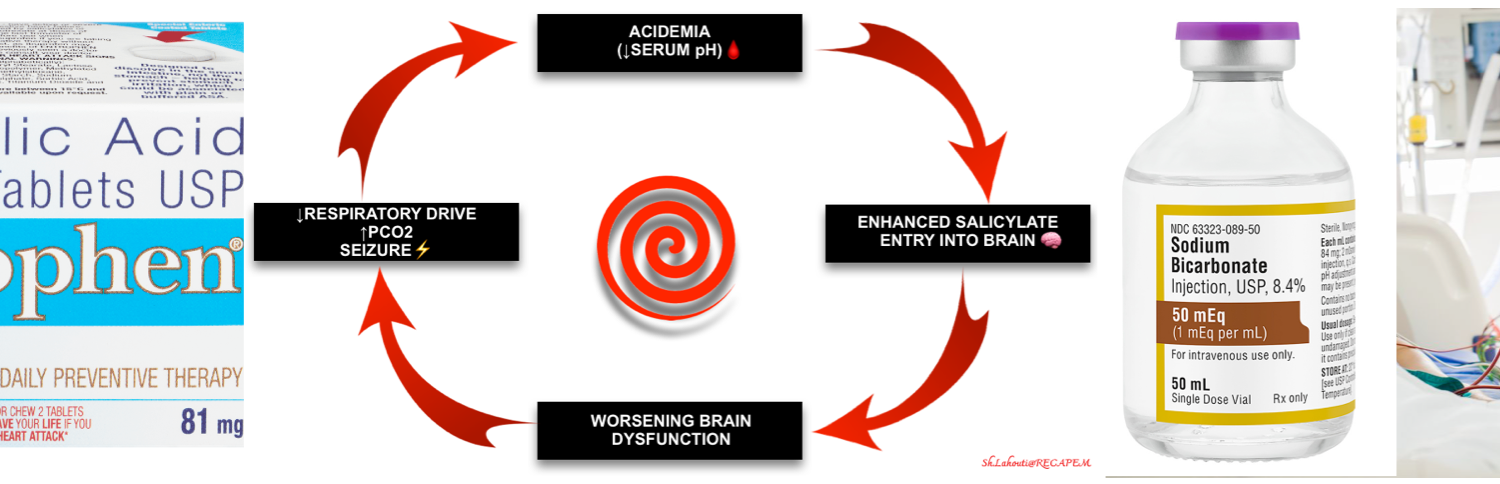
3️⃣Respiratory acidosis
- This is often a late finding reflecting respiratory exhaustion, ARDS, or severe CNS dysfunction.
- Note that decreased ventilation can also be related to co-ingestions e.g. opioids or iatrogenic medication.
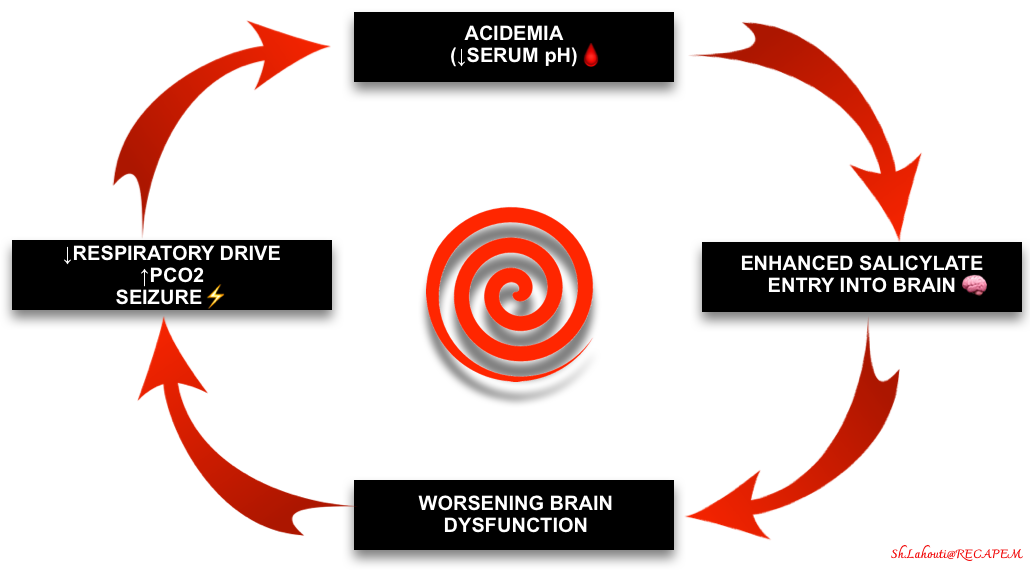
- Regardless of the etiology, this is a very ominous finding because these patients are not defending their pH. Acidemia promotes the entry of salicylate into the brain, worsening the intoxication. This creates the possibility of a death spiral involving hypercapnia, acidosis, and CNS dysfunction (figure below).
Chronic salicylate intoxication
A chronic form of intoxication may occur in patients who are ingesting acetylsalicylic acid therapeutically and then have an inadvertent overdose. Toxicity can develop even with small increases in doses due to saturable kinetics *.
- Generally, nausea and vomiting are less common than in acute salicylate toxicity.
- Neurologic abnormalities are the most common manifestation of chronic salicylate intoxication, including
- Lethargy, altered mental status, irritability, hallucinations, paranoia, bizarre behavior, memory deficits, and tremors.
- Neurologic abnormalities in chronic salicylate poisoning may be nonspecific and often misleading.
- Chronic salicylism should be considered in a patient with unexplained neurologic or behavioral dysfunction, especially in the presence of a mixed acid-base disturbance, tachypnea, dyspnea, or unexplained pulmonary edema.
- Chronic salicylism may develop in patients taking carbonic anhydrase inhibitors for the treatment of glaucoma.
- The normal anion gap (hyperchloremic) metabolic acidosis produced by carbonic anhydrase inhibitors increases the volume of distribution for salicylate and facilitates its entry into the CNS, causing toxicity at a “therapeutic” serum salicylate concentration.
Differential diagnosis
Because salicylism can mimic several other disorders, a key step in the differential diagnosis is to measure a serum salicylate concentration in any patient with metabolic acidosis without a clear etiology, even with a normal anion gap.
🟥Infection
- Sepsis
- Meningitis
- Pneumonia
🟥Endocrine
- Diabetes Ketoacidosis
- Clues: The clinical history (eg, polydipsia, polyuria) combined with hyperglycemia. Note that salicylism does not cause significant hyperglycemia and is more likely to cause hypoglycemia.
- Euglycemic DKA
- Measure a serum salicylate concentration when the distinction cannot be made clinically.
🟥Organic psychosis *
🟥Toxicologic *
- Sympathomimetic toxicity
- In contrast to salicylate intoxication, patients with sympathomimetic toxicity commonly present with hypertension, while vomiting is rare.
- Toxic alcohol poisoning/ Alcohol withdrawal
- Acute iron intoxication
- Clues: Compared with salicylism, iron intoxication is more likely to cause bloody vomiting and delayed (ie, 6 to 24 hours) development of acidosis.
- Test: Serum iron concentration.
Click on the image to enlarge
Investigations
- Fingerstick blood glucose.
- Cell count with differential, comprehensive metabolic profile.
- Venous/arterial blood pH, urinalysis with urine pH determination.
- Serum lactate
- Coagulation studies
- Creatinine kinase (rhabdomyolysis can occur)
- Serum drug screen to evaluate for coingestions, such as acetaminophen and tricyclic antidepressants.
- Salicylate concentration
- Cultures and inflammatory markers (if suspected sepsis)
- Lumbar puncture (if suspected of CNS infection).
- ECG
- Bedside ultrasound and echo, especially in patients with:
- Hypotension
- Hypoxemia, respiratory distress (to differentiate cardiac vs. non-cardiac pulmonary edema, see here.)
- Brain CT/MRI in a patient with altered mental status or focal neurological deficits that are not clearly attributable to a noncerebral cause (eg, hypoglycemia).
- EEG in altered patients suspected of nonconvulsive status epilepticus.
Diagnosis
The diagnosis of salicylate toxicity is suspected from a history of ingestion or characteristic signs, symptoms, and acid-base findings. The diagnosis is confirmed by a supratherapeutic serum salicylate concentration (i.e. >30 mg/dL).
- Salicylate poisoning is suspected in patients with any of the following:
- History of a single acute overdose.
- Repeated ingestions of therapeutic doses.
- Unexplained metabolic acidosis.
- Unexplained confusion and fever (in older patients).
- Other findings compatible with sepsis (e.g. fever, hypoxia, noncardiogenic pulmonary edema, dehydration, hypotension).
Diagnostic pitfalls
🟥History & physical exam
- Although history is the source of the most helpful information for identifying the etiology of poisoning, it is notoriously unreliable when provided by a patient following intentional ingestion *.
- Salicylate toxicity has no unique symptoms or physical exam findings. Many of the clinical findings are not specific, including:
- Tachycardia, tachypnea/hyperpnea, hypotension, hyperthermia (late, severe toxicity).
- Vomiting 📖
- Seizures can be caused by different etiologies. For a full discussion, see here.
- Delirium. This has a broad differential, discussed here.
- Non-cardiogenic pulmonary edema. The etiologies of pulmonary edema are discussed here.
🟥Elevated anion gap
- A normal anion gap does not exclude salicylate toxicity in patients with an unknown ingestion. The “classic” metabolic disturbances of salicylate toxicity can be altered in the following conditions:
- Mixed ingestions that include aspirin and respiratory depressants e.g. opioids
- The timing of exposure
- A negative anion gap metabolic acidosis may be noted in salicylate toxicity due to aberrant reading of salicylate ions as chloride ions by analyzer electrodes *.
- In chronic toxicity, a normal anion-gap acidosis can occur because of compensatory renal bicarbonate loss and chloride retention in salicylate-induced respiratory alkalosis *.
🟥Salicylate level
- A supratherapeutic serum salicylate concentration (i.e. >30 mg/dL) in an appropriate clinical context can be confirmatory.
- The specificity of the commercially available tests for serum salicylate concentration is very high.
- The only reported significant interference has been with diflunisal (an NSAID), which produces false-positive results for significantly high serum salicylate concentrations.
- Caveats
- Always treat patients based on clinical status, as there can be a poor correlation between salicylate concentration and toxicity.
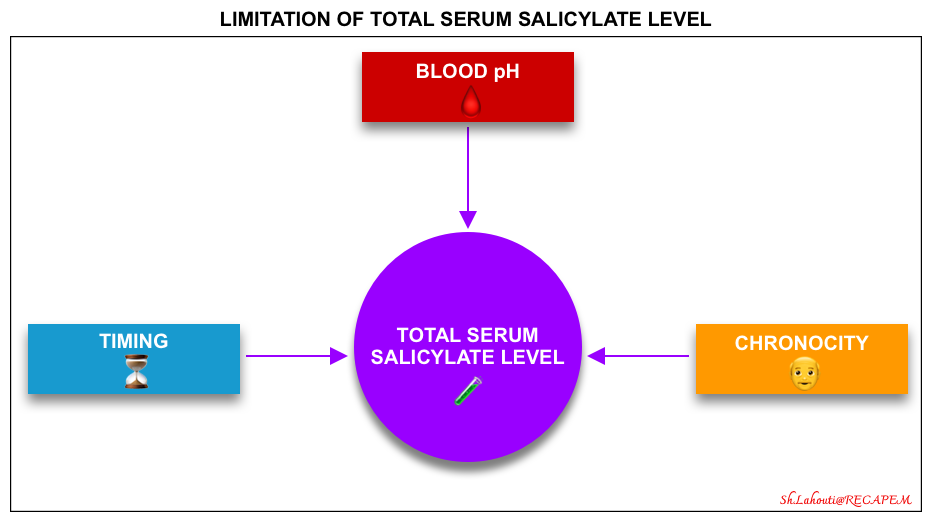
- There are certain limitations in the interpretation of serum salicylate levels (figure below).
-
- Timing (time since ingestion)⏳
- Changes in serum salicylate concentration lag behind clinical signs by several hours.
- Significant toxicity has been reported to develop even in cases in which salicylates were undetectable on an initial assay ~4 hours after ingestion *.
- Levels usually peak around 6 hours after ingestion. However, the peak may be delayed for >12 hours in patients who have ingested enteric-coated tablets.
- A pitfall in treating salicylate toxicity is reliance on a single serum level *.
- For example, a patient with lethargy and hyperpnea with a serum concentration of 50 mg/dL should be considered to have severe toxicity; their concentration in 4 hours may well be >90 mg/dL.
- 💡Hints
- Do not exclude the diagnosis or underestimate the degree of toxicity in a patient with clinical salicylism (eg, history of ingestion with classic signs).
- In an appropriate clinical context, treatment for salicylate intoxication should be started promptly. Do not delay treatment for the initial result of salicylate level.
- Obtain an initial serum salicylate level, and a repeat serum salicylate level at 2 hours to determine if the concentration is increasing or decreasing.
- Changes in serum salicylate concentration lag behind clinical signs by several hours.
- Variability in serum pH
- Tissue toxicity depends on the non-ionized form of salicylic acid.
- The non-ionized salicylate level depends on both the total salicylate level and the pH (non-ionized salicylate level is increased by academia).
- For example, a patient with a pH of 7.2 and a total salicylate level of 46 mg/dL has the same level of nonionized salicylate as another patient with a pH of 7.55 and a total salicylate level of 100 mg/dL.
- Always obtain pH in addition to salicylate concentrations. If both are decreasing, this can herald a worsening clinical status as the salicylate moves into tissues.
- Tissue toxicity depends on the non-ionized form of salicylic acid.
- Chronicity: Acute vs. chronic intoxication
- In chronic intoxication, the serum levels may underestimate the severity of the intoxication, because salicylate has already accumulated in the brain.
- Some patients with chronic toxicity may even have a salicylate concentration within the therapeutic range.
- Timing (time since ingestion)⏳
Risk assessment
Intoxication is a dynamic process. Following the initial resuscitation of critical poisoning patients (discussed below), assess the risk of severe toxicity (which can change over time) to further guide the lines of treatment.
- The following general principles to determine the severity of toxicity can be used (figure below) *.
- Dose of ingestion and formulation?
- Patient’s symptoms, signs, and acid-base status?
- Trajectory of serum salicylate level?
- Note that in chronic toxicity, clinical findings are a better indicator of severity than serum salicylate level.
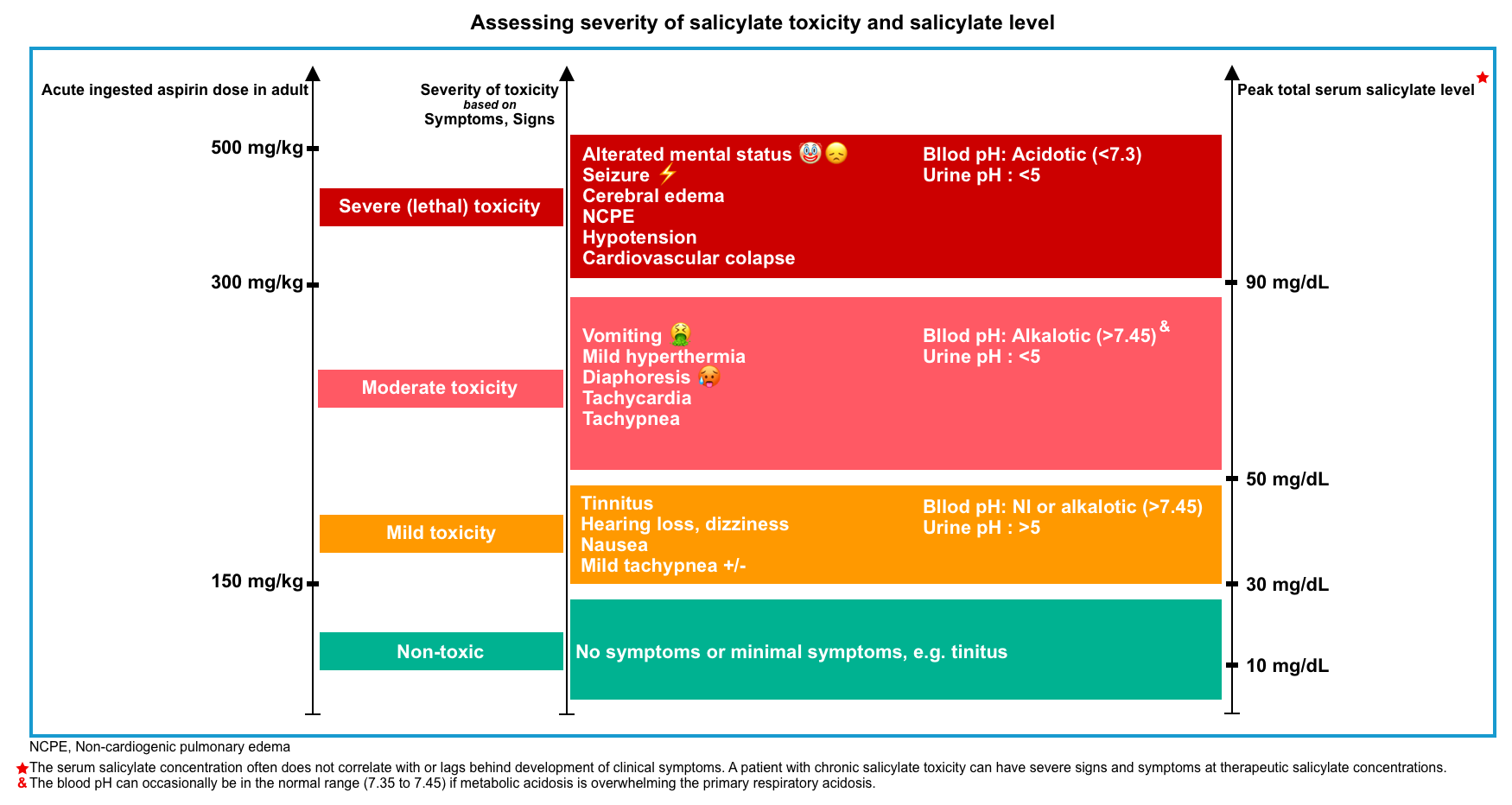
🟥The Done nomogram is misleading and can grossly underestimate toxicity; it should not be used *.
- The Done nomogram was developed to predict the severity of toxicity based on the serum salicylate concentration and time elapsed after ingestion. However, subsequent studies found it has inadequate accuracy, likely because of delayed absorption kinetics of enteric-coated aspirin, modeling errors in the early publication, and changing terminology regarding the grading of toxicity since the 1960s.
Management
Resuscitation
| Airway |
⛔️Avoid Intubation
- Avoid (or delay) intubation and sedation if possible *,*.
- ⚠️Intubation is extremely dangerous for patients with combined metabolic acidosis and respiratory alkalosis.
- In this condition, even brief pauses or reductions in respiratory rate can further decrease pH by eliminating the compensatory hyperventilation response.
- Hypoventilation causes a further drop in pH (↑non-ionized form of SA)and results in exponential increases in CSF salicylate levels. This may push patients into the death spiral involving hypercapnia, acidosis, and CNS dysfunction.
- ⚠️Intubation is extremely dangerous for patients with combined metabolic acidosis and respiratory alkalosis.
▪️Indication of intubation
- Loss of airway protection e.g. active vomiting in patients with altered mental status, ongoing seizures, and deteriorating mental status *.
- Hypoventilation (when respiratory efforts are faltering* and PCO2 is rising, and hypoventilation is not attributable to reversible causes e.g. coingested opioids).
- In these patients, intubation should improve pH, unlike most hyperventilating patients.
▪️Considerations to reduce the risk of hemodynamic deterioration, if intubation is necessary:
- Pre-intubation
- Maintain or exceed minute ventilation requirements.
- Give 2 mEq/kg bolus of hypertonic bicarbonate before intubation *.
- This should be done > 5-10 minutes before paralysis so that the patient has time to blow off excess CO2 from the infused bicarbonate.
- Bag valve mask hyperventilation should be employed in any patient who is acidemic with a spontaneously ventilating PCO2 of <20 mm Hg *.
- Consider giving 1 ampule of D50% IV immediately before sedation (to treat unrecognized neuroglycopenia, a sinister complication of salicylate toxicity).
- Intubation
- Awake intubation (delayed-sequence intubation with ketamine) is the first choice.
- Standard rapid-sequence intubation medications may be used if delayed-sequence intubation is not possible.
- Post-intubation
- After intubation, immediately connect the patient to a ventilator and adjust settings to maximize minute ventilation (e.g. 8-10 cc/kg breaths, increase rate as high as possible without causing autoPEEP).
- ⚠️Do not use standard “lung-protective” ventilator settings.
- Once the metabolic acidosis is controlled (over several hours), ventilator settings should be titrated down.
- After intubation, immediately connect the patient to a ventilator and adjust settings to maximize minute ventilation (e.g. 8-10 cc/kg breaths, increase rate as high as possible without causing autoPEEP).
| Breathing |
Oxygenation
- Administer supplemental oxygen as needed.
- A patient with pulmonary edema may ultimately develop high oxygen requirements.
- For refractory hypoxemia, noninvasive ventilation is a temporizing measure, but tracheal intubation with PEEP is typically required to prevent hypoventilation.
| Ciculation |
- Patients are generally substantially volume-depleted for profound sensible (eg, vomiting) and insensible (eg, diaphoresis, hyperthermia, respiratory effort) losses.
- Total body fluid deficit is often ~ 4-6 liters *, however, echocardiography evaluation and confirmation are warranted.
- The target of volume repletion should be a state of euvolemia *.
- IV Fluid of choice:
- If pH <7.5: Isotonic bicarbonate may be used for both volume resuscitation and alkalization.
- If pH>7.5: Lactated ringers (LR) or plasmalyte may be used for volume resuscitation.
- ⛔️Avoid normal saline as it can cause normal anion-gap acidosis and lower pH *.
- Vasopressor should be started, if blood pressure does not respond to fluids.
| Disability |
▪️Glucose
- CNS glucose levels are often lower than the blood level.
- Indication of glucose administration:
- Any neurologic deficit, including altered mental status or seizures (even if their serum glucose levels are normal) *.
- Target the serum glucose in patients with moderate-severe intoxication in the high normal range (~100 to 140 mg/dL).
- In a patient who is not able to eat, this can be accomplished using IV boluses of dextrose (50 to 100 mL of 50% dextrose) or by adding 50 to 100 g of dextrose to each liter of maintenance fluid.
Decontamination
- AC 📖
- Consider activated charcoal if there are no contraindications.
- MDAC 📖
- Consider multiple doses of activated charcoal if salicylate concentrations are rising, as this finding indicates ongoing, prolonged systemic absorption (e.g. bezoars) *.
- Whole-bowel irrigation(WBI) 📖
- Do not routinely perform WBI for salicylate toxicity. Studies have not demonstrated a clinical benefit in patients treated with whole-bowel irrigation.
- WBI might be considered in the rare circumstance of an alert and cooperative patient after massive ingestion (eg, an entire bottle of tablets) of a delayed-release preparation (but first discuss the risks and benefits with a poison control center or medical toxicologist).
Alkalinization
▪️Background
- Alkalinization is the mainstay of treatment for both acute and chronic salicylate intoxication, for two reasons
- Reduce penetration of salicylate into the tissues (especially the brain).
- This is still beneficial, even in patients with anuric renal failure.
- Increase excretion of salicylate in the urine.
- Reduce penetration of salicylate into the tissues (especially the brain).
- When indicated, alkalization therapy should be administered in the emergency department because it can be initiated immediately *.
▪️Indication
- Salicylate level >40 mg/dL.
- All symptomatic patients.
▪️Protocol for Urinary Alkalinization in Adults
- Check baseline electrolytes, BUN, serum creatinine, glucose, serum drug concentrations, as well as systemic and urinary pH.
- Serum potassium should be ≥4 mmol/L before buffer administration *.
- Assess volume status.
- Correct hypovolemia with crystalloid solution e.g. RL.
- Sodium bicarbonate
- Loading dose: Hypertonic NaHCO3 (8.4%) 1-2 mEq/kg IV bolus dose *.
- Maintenance infusion: Isotonic bicarbonate at ~200 ml/h (this can be prepared by mixing 3 vials of NaHCO3 8.4% with 1L of D5W).
- Duration of treatment
- Serum alkalization can be stopped when the following criteria are met:
- Two levels show that the salicylate level is consistently decreasing.
- Salicylate level <40 mg/dL *.
- The patient is asymptomatic with a normal respiratory rate.
- Following discontinuation of alkalinization, it may be reasonable to follow labs for 2-4 additional hours to ensure that acidosis doesn’t recur (especially in chronic toxicity).
- Serum alkalization can be stopped when the following criteria are met:
- Monitoring
- Check blood pH q2hr.
- Maintain a blood pH of ~7.55.
- Check urine pH of ~ q30 min.
- 🎯Target urine pH of > 7.5.
- A further IV bolus of 1 mEq/kg of sodium bicarbonate may be necessary if sufficient alkalinization of the urine is not achieved.
- 🎯Target potassium level >4.5 mEq/L.
- Check blood pH q2hr.
- ⚠️Avoid acetazolamide to alkalinize the urine. Acetazolamide raises urine pH and lowers systemic pH, which may cause clinical deterioration in some cases *.
Extracorporeal Treatment
▪️Background
- Hemodialysis is the most efficient way to eliminate salicylate and normalize salicylate-induced acid-base and electrolyte imbalances *.
- In severe salicylate intoxication, dialysis is life-saving *.
- Dialysis often takes several hours to set up. Therefore, for patients who are deteriorating clinically, it is often wise to initiate dialysis early.
- Risk-benefit assessment
- The risks of performing hemodialysis early (under controlled settings) are far lower than the risks of severe salicylate intoxication (ICU mortality of 15%).
- Clinical status is more important than the absolute salicylate concentration, and both must be weighed regarding optimal treatment.
- Any severe signs and symptoms of salicylate poisoning should be considered for hemodialysis.
- Levels alone are poor indicators for hemodialysis as patients can be severely poisoned with levels lower than traditional teaching below.
- When in doubt, careful attention to the patient with serial evaluations may be helpful.
- Serial neurologic examination may be the best single reflection of real-time salicylate levels in the tissue (“end-organ” effect).
▪️Indications (EXTRIP guideline*)
- Altered mental status.
- Noncardiogenic pulmonary edema requiring supplemental oxygen.
- Salicylate level
- >100 mg/dL
- >90 mg/dL with renal dysfunction
- >90 mg/dL despite supportive care (e.g. fluid resuscitation)
- > 80 mg/dL despite supportive care and in the context of renal dysfunction
- pH < 7.2 despite supportive care (e.g. bicarbonate)
- Volume overload which prevents adequate alkalization is a potential indication for dialysis.
▪️Choice of ECRT therapy
- Intermittent hemodialysis is the preferred modality for patients with salicylate intoxication.
- This is preferred over continuous renal replacement because it is more efficient.
- Intermittent hemodialysis is recommended even in the context of hypotension. Since fluid isn’t being removed, there is no evidence that hemodialysis causes more hemodynamic instability than continuous renal replacement therapy.
▪️ECRT Session
- ECRT cessation is indicated if
- Clinical improvement is apparent, and
- Salicylate level < 19mg/dL, or
- ECRT has been performed for a period of 4-6 hours when salicylate level is not readily available.
👉It is indicated to continue IV bicarbonate therapy between ECRT sessions.
▪️Rebounding salicylate levels
- Hemodialysis will typically drop the blood salicylate levels dramatically. However, levels may rebound following cessation of dialysis.
- Follow salicylate levels and clinical symptoms following dialysis. In some cases, multiple dialysis runs may be necessary.
Monitoring & observation
🟧Careful observation of the patient (e.g. serial neurologic, and respiratory assessment), correlation of the serum salicylate concentrations with blood pH, and repeat determinations of serum salicylate concentrations every 2 to 4 hours are essential until the patient is clinically improving and has a low serum salicylate concentration in the presence of a normal or high blood pH.
- Analyses should be obtained more frequently (i.e. every 2 hours) when managing seriously ill patients to assess treatment efficacy and the possible need for HD. These include:
- Labs (glucose, electrolytes, CK, blood gas, urinalysis, serum salicylate level) at least q2hr.
- 🎯Glucose: Target serum glucose > 100-140mg/dl.
- 🎯Target potassium > 4.5 mEq/L.
- Alkalinization if the patient has symptoms or a salicylate level >40 mg/dL
- 🎯Serum pH target: ~ 7.55
- 🎯Urine pH target: >7.5
RECAP
- Pitfalls in the emergency department management of salicylate–poisoned patients:
- Failure to recognize the presence of salicylate toxicity.
- 💡Salicylates should be considered in the differential diagnosis of an adult patient with acid-base abnormalities of uncertain cause, especially when there are concurrent neurologic symptoms.
- Reliance on one or two serum levels of salicylate that may not describe a trend of decreasing the total body burden of aspirin clearly.
- Misinterpretation of low serum salicylate levels as nontoxic and failure to comprehend the changing acid-base status of the patient.
- Waiting until serum salicylate levels are determined before beginning urinary alkalinization.
- Failure to recognize the emergent need for definitive therapy (hemodialysis) based on impending end-organ injury.
- Inappropriately or prematurely initiating intubation and mechanical ventilation without hyperventilation and simultaneous hemodialysis.
- Failure to recognize the presence of salicylate toxicity.
- There is no specific antidote for salicylate poisoning. Good supportive care and attention to the following specific issues are important:
- Acidemia should be avoided.
- Alkalinization of serum and urine should be started early in the ED for all symptomatic patients.
- Euvolemia should be achieved.
- Avoid giving normal saline (may lower the pH, worsening intoxication).
- Electrolyte balance should be restored. Particular attention should be paid to glucose and potassium supplementation.
- Avoid intubation if possible.
- In severe salicylate intoxication, dialysis may be life-saving.
- When in doubt, it may be best to err on the side of early dialysis.
- Call nephrology early and often. For patients who are deteriorating, advocate for early dialysis.
- When in doubt, it may be best to err on the side of early dialysis.
- Acidemia should be avoided.
Going further
- Pearls and Pitfalls of Salicylate Toxicity in the Emergency Department (emDocs)
- Salicylate intoxication (IBCC)
- Salicylate Poisoning (Emergency Medicine Cases)





Add comment