5 October 2023 by Shahriar Lahouti.
CONTENTS
- Introduction
- Epidemiology
- Definition
- Basic cardiovascular physiology
- Pathophysiology
- Etiology and precipitating factors
- Approach to diagnosis of CS
- Hemodynamic evaluation
- Management
- Media
- Going further
- Appendix
- References
Introduction
Cardiogenic shock (CS) is the most severe form of acute heart failure. It is a potentially complex, and hemodynamically diverse state of end-organ hypoperfusion that is frequently associated with multisystem organ failure. Despite improving survival in recent years, patient morbidity and mortality still remain high. This review will cover recent updates regarding the definition, underlying causes, pathophysiology, and management in light of recent literature with a focus on left ventricular failure.
Epidemiology
- The prevalence of cardiogenic shock (CS) varies according to the definition of CS, clinical setting care, and the era of data collection.
- The prevalence of CS is ~15% in the intensive care unit (ICU)/ICCU *.
- In-hospital mortality varies between 30% and 60% *. However, recent datasets reported lower mortality rates of ~37.5% *,*.
- The epidemiology of CS has shifted from ACS-CS to decompensated HF (HF-CS) in the contemporary advanced CICUs.
- While a decade ago, ACS accounted for ~80% of CS *, recent studies show that only 30% of CS cases are related to ACS *, and the remaining cases are associated with decompensated HF.
- It is important to realize that there are differences between ACS-CS and HF-CS in terms of manifestation, management strategies, and prognosis.
- The manifestation of HF-CS is different from those patients without preexisting HF who develop CS (e.g. some patients with AMI-CS) *.
- Patients with chronic advanced HF may have compensatory adaptations that allow them to tolerate a degree of hemodynamic compromise that a patient without preexisting HF might not.
- Non-ACS-CS patients have a greater burden of disease (more severe pre-existent HF, pulmonary hypertension, arrhythmias), but in-hospital survival is significantly better than ACS-related CS *, *.
- In contrast, ACS has been shown to be a predictor of worse outcomes in patients with CS *.
- The manifestation of HF-CS is different from those patients without preexisting HF who develop CS (e.g. some patients with AMI-CS) *.
Definition
Cardiogenic shock (CS) is a clinical diagnosis and refers to a complex clinical syndrome caused by an insufficient cardiac output resulting in a state of tissue hypoperfusion which, depending on its severity, may result in multi-organ dysfunction and death *.
- The clinical triad of CS includes *:
- Ineffective cardiac output (CO)
- Tissue and organ hypoperfusion
- Normal or elevated cardiac filling pressures (congestion)
Shock is a state of hypoperfusion (defined by clinical and biochemical signs of hypoperfusion). Hypotension is not required for the definition of shock state *.
- Patients in an early shock state can have normal-high BP (through a compensatory mechanism) while tissue perfusion may be significantly impaired *,*,*. Early recognition of hypoperfusion signs identifies ‘high-risk’ patients regardless of hypotension *.
CS is a clinical diagnosis and hemodynamic parameters {e.g. reduced cardiac index (CI), elevated pulmonary capillary wedge pressure (PCWP)}, are not mandatory in clinical practice *,*.
Basic cardiovascular physiology
Cardiac output (CO) is the amount of blood pumped by the heart per minute and is affected by HR and stroke volume (SV).
- CO = (SV x HR)/1000.
- Cardiac output (L/min); stroke volume (mL/beat).
- Cardiac output is dependent on heart rate and stroke volume.
- Clinical hint💡
- Bradycardia can directly pull down cardiac output and cause hemodynamic instability.
- Severe bradycardia (e.g. HR< 30 bpm) should always raise concern for shock even in normotensive patients *.
- Mild-moderate tachycardia will increase the cardiac output.
- ⚠️Do not suppress a sinus tachycardia. This is a compensatory mechanism in early circulatory derangement that may be keeping the patient alive.
- Bradycardia can directly pull down cardiac output and cause hemodynamic instability.
Stroke volume (SV) is defined as the volume of blood pumped out of the left ventricle during each systolic cardiac contraction. SV is affected by preload, myocardial contractility, and afterload.
- Preload
- Starling’s law of the heart or the Frank-Starling mechanism is shown in Figure 1A.
- In a healthy physiologic state, increasing the length of myofibril (before contraction, i.e. preload) up to a certain limit will tend to increase cardiac contraction. This is referred to as preload responsive, i.e. increasing the preload results in increasing cardiac output.
- Patients with congestive heart failure maintain their stroke volume close to normal at the expense of elevated left ventricular filling pressure (hypervolemia). Increased preload works here to keep stroke volume and cardiac index at a sub-optimal level (figure 1B).
- Starling’s law of the heart or the Frank-Starling mechanism is shown in Figure 1A.
- Contractility and afterload
- Stroke volume and cardiac output depend on contractility and afterload as well. Increasing contractility or decreasing the afterload leads to increasing the stroke volume (figure 2).
Arterial blood pressure is the inflow pressure for “organ perfusion”, therefore it provides an indirect measure of blood flow to the organs.
- Mean arterial pressure (MAP) more closely reflects the entry pressure for perfusion of most vital organs *.
- The relationship of MAP, CO, and systemic vascular resistance (SVR) is expressed mathematically as MAP = CO x SVR
- Clinical hint 💡
- Vasopressors raise MAP by increasing SVR. In a failing heart, this excessive afterload (↑SVR) is poorly tolerated, thereby “CO” can progressively drop.
Systemic vascular resistance (SVR) is the amount of force exerted on circulating blood by the vasculature of the body.
- SVR ≈ LV afterload.
- SVR can provide valuable information for distinguishing the classes of shock from each other. It is decreased in early distributive shock and is often increased in most other classes of shock (classes of shock,👉 here).
- SVR= 80 x (MAP-RAP)/CO
- Wherein SVR (dynes. sec/cm5 ); mean arterial pressure “MAP” (mm Hg), right atrial pressure “RAP” (mm Hg), and cardiac output “CO” (L/min).
Pathophysiology
Although etiologies of CS vary widely, the pathophysiology of CS comprises several unique but overlapping components to be considered.
The following mechanisms may happen separately at different stages of CS, or each may occur simultaneously. The magnitude of the initial cardiac insult and/or early application of interventions may either mask or delay some of these stages.
- An initial cardiac insult that decreases cardiac output (CO)
- Left and/or right ventricular dysfunction causes a drop in LV cardiac output and stroke volume, resulting in:
- Hypotension
- Elevated LV end-diastolic pressure *.
- Left and/or right ventricular dysfunction causes a drop in LV cardiac output and stroke volume, resulting in:
- Central congestion (elevated LV and/or RV filling pressures)
- Vasoconstriction (including venoconstriction) will develop as a compensatory mechanism for hypotension. This will cause redistribution of blood volume into the circulating compartment, leading to elevated CVP and/or PCWP *.
- Vasoconstriction causes elevation in afterload too.
- Although vasoconstriction may superficially normalize the blood pressure, eventually the failing heart cannot handle the excessive preload and afterload. Therefore the LV output will drop further.
- Microcirculatory dysfunction
- It is present early in CS, and may precede central hemodynamic congestion * (see appendix).
- Mechanisms of microcirculatory dysfunction include:
- Reduced cardiac output and increased vascular tone.
- The microcirculatory network is “flow-dependent”. Reduced “CO” and vasospasm are potential contributing factors.
- The microcirculatory network is “flow-dependent”. Reduced “CO” and vasospasm are potential contributing factors.
- Activation of systemic inflammatory response.
- The cytokines and reactive oxygen species can cause diminished regional autoregulation leading to impaired microcirculation *.
- Reduced cardiac output and increased vascular tone.
- Microcirculatory dysfunction results in reduced responsiveness to cellular metabolic demand, leading to tissue hypoxia *.
- Systemic inflammatory response syndrome (SIRS)
- Clinically overt inflammation is seen in ~30% of CS patients by day 2 post-CS onset and may result in an initially reduced SVR *.
- Mechanism of systemic inflammatory response activation:
- Primary cardiac insult can trigger a noninfectious inflammatory response.
- Concurrent infection: Approximately 25% of CS cases develop infection complications *. Risks for bloodstream infection include:
- Vascular access
- Hypoperfusion-related damage to the gut mucosal barrier and resulting bacterial translocation (BTL).
- Activation of the inflammatory cascade is associated with further myocardial depression and interferes with catecholamine secretion *.
- Multi-organ dysfunction (MOD)
- The gut appears to be among the first organs involved in shock.
- Microcirculatory injury in the intestinal barrier leads to increased bacterial translocation (BTL) *.
- BTL results in the release of bacterial gram-negative lipopolysaccharides or endotoxins into the systemic circulation, which further perpetuates spirals of SIRS.
💡More on Coronary Perfusion Pressure
- Progressive reduction in coronary perfusion pressure (CPP) will happen due to the above mechanisms (#1-5). This causes a double-hit impact on myocardial performance, resulting in worsening myocardial function and progressive end-organ dysfunction.
- Physiologic background
- The left ventricle is perfused mainly in diastole.
- Coronary perfusion pressure (CPP) of LV is defined as (DBP – LVEDP) *.
- Systemic hypotension and elevated LVEDP have a cumulative impact on reducing LV perfusion.
- Coronary perfusion pressure (CPP) of LV is defined as (DBP – LVEDP) *.
- The right ventricle is perfused in both systole and diastole.
- CPP of RV in diastole is defined as (MAP– MPAP); where MAP, is mean arterial blood pressure; MPAP, is mean pulmonary artery pressure) *.
- The left ventricle is perfused mainly in diastole.
- Clinical significance
- These equations underscore the significance of raising CPP in hypotensive CS to prevent further myocardial damage. This should be done before the administration of inotropic medications.
- Inotropic medications increase cardiac contractility, hence increasing O2 consumption and metabolic demands. Obviously, the blood flow should be optimized first (by raising CPP) before any attempts to optimize contractility.
Etiology and precipitating factors of CS
- Acute reduction in LV pump function
- ACS
- Tachymyopathy
- Cardiomyopathies *
- Ischemic and hypertrophic cardiomyopathy, Takotsubo cardiomyopathy.
- Myocarditis (e.g. viral and bacterial infections, reactions to medications, and underlying autoimmune diseases).
- Stunned myocardium from prolonged ischemia (e.g. cardiac arrest, hypotension, cardiopulmonary bypass).
- Pregnancy-associated heart disease *
- Peri-partum cardiomyopathy
- Coronary artery dissection
- ACS
- Arrhythmia
- Tachyarrhythmia (most often new-onset atrial fibrillation) *.
- Bradyarrhythmia.
- RV dysfunction. These can happen alone or in association with left-sided pathologies (preexisting LV dysfunction, or concurrent LV dysfunction) *
- RV dysfunction secondary to RV pump failure: RV infarction, progressive cardiomyopathy, septic cardiomyopathy *.
- RV dysfunction secondary to pulmonary vascular disease: Worsening pulmonary hypertension, acute pulmonary embolism.
- Valvular or mechanical dysfunction
- Native valve dysfunction (e.g. endocarditis, ruptured papillary muscle)
- Prosthetic valve dysfunction (e.g. thrombosis)
- LV outflow tract obstruction (LVOTO)
- Ventricular septal defect or free wall rupture.
- Intracardiac mass (e.g. left atrial myxoma causing obstruction to LV filling) *, thrombi, tumor metastasis.
- Pericardial disease *
- Tamponade
- Progressive pericardial constriction
- Volume alteration
- Non-adherence with HF medications *
- Iatrogenic
- Cardio-depressant sedatives (e.g. propofol)
- Excessive use of diuretics
- Excessive volume loading in RV shock
- Other
- Uncontrolled hypertension
- Thyroid disease (both hyperthyroidism and hypothyroidism)
- Toxidromes e.g. β-blockers, calcium channel blockers, digoxin, antiarrhythmics toxicity *.
- Checkpoint inhibitors
Approach to diagnosis of CS
The initial diagnostic approach and medical stabilization are usually started in the ED. The framework for the diagnosis of CS involves:
- Is this patient in a hypoperfusion state (shock state)?
- Does this patient with hypoperfusion have cardiogenic shock?
- How severe is this cardiogenic shock?
- Is this patient with low cardiac output in cardiogenic shock?
| Is this patient in a hypoperfusion state (shock)? |
Evaluate for evidence of hypoperfusion
The clinical and biochemical evidence of hypoperfusion is mentioned here. However, these are not universally present through different stages of CS and also are not highly specific for hemodynamic derangement *.
Skin perfusion status: Cool/clammy/mottled extremities and sluggish CRT are suggestive of low CO.
- Cool, clammy skin is due to compensatory peripheral vasoconstriction that redirects blood centrally, to maintain vital organ perfusion *.
- Caveats:
- A cool, clammy, or cyanotic skin may also be due to the underlying peripheral arterial disease.
- A warm, hyperemic skin does not exclude the absence of shock because such an appearance may be present in early distributive shock (pathologic vasodilation, vasoplegia).
- Caveats:
- Mottling is less sensitive, but more specific for hypoperfusion and increased mortality *,*,*.
- A cyanotic, mottled appearance is a late and worrisome feature of shock.
Brain: Altered level of consciousness (LOC) and mentation.
- Altered LOC and delirium are not specific symptoms of cerebral hypoperfusion *.
- This can happen due to intracranial pathologies or more commonly secondary to toxic and metabolic disturbances.
- Normal mentation doesn’t prove that perfusion is adequate.
- For example, some patients in occult CS may have normal mentation despite profoundly low cardiac output.
Kidney: Doubling creatinine over baseline and urine output <30 mL/h.
- Approximately 25% of patients with CS develop AKI *.
- AKI has diverse causes with several non-hemodynamic contributors such as infection, iatrogenic damage (e.g. contrast media, nephrotoxic medication), and elevated intra-abdominal pressure *.
Liver: Transaminitis
- Only 25% of patients with CS have an abnormal liver function test in the acute phase *.
- Derangement in liver function tests is not specific for hypoperfusion and the differential diagnosis is diverse.
- In the setting of CS, abnormal liver function test results would be observed under both reduced perfusion (ischemic hepatitis, aka shock liver) and venous congestion (congestive hepatopathy):
- Congestive hepatopathy (due to elevated RAP)
- Mild-moderate transaminitis with elevated ALP x2-3 UNL.
- Elevated direct and indirect bilirubin with total bilirubin level often < 3 mg/dL *.
- Shock liver (ischemic hepatitis)
- Severely elevated AST (> x10 UNL) *.
- Severe hyperbilirubinemia with a total bilirubin level as high as 15-20 mg/dL *.
- Markedly ↑LDH. Increased LDH tends to be massive and an ALT/LDH ratio of <1.5 helps distinguish ischemic injury from other forms of acute hepatitis *.
- Mild-moderate ↑ALP is also reported.
- Congestive hepatopathy (due to elevated RAP)
Blood pressure: SBP <90 or MAP <60 or >30 mmHg drop from baseline.
- Shock is a state of hypoperfusion. However, hypoperfusion is not synonymous with hypotension.
- For example, in patients with normotensive CS, SBP is >90 mm Hg despite the presence of clinical and biochemical evidence of hypoperfusion.
Tachycardia (a PR >100 bpm) *.
- Generally, both pulmonary edema and systemic hypoperfusion are hyperadrenergic states.
- Tachycardia is a compensatory response to underlying hemodynamic derangement. However, components of compensatory response may be blunted in patients who are using cardio-depressant medications, e.g. β-blockers.
Bradycardia
- Marked bradycardia (e.g. HR<40) should always raise concern for shock even in normotensive patients.
- Relative bradycardia.
- In cardiogenic shock, a HR in the range of 90-110 bpm is expected. HR < 80 is called relative bradycardia in patients with CS and may provide diagnostic clues for the underlying cause(s), such as:
- Hyperkalemia
- Inferior AMI
- Intoxications: β-blockers, calcium channel blockers, digoxin *.
- Conduction abnormalities
- In cardiogenic shock, a HR in the range of 90-110 bpm is expected. HR < 80 is called relative bradycardia in patients with CS and may provide diagnostic clues for the underlying cause(s), such as:
Shock Index >>0.8 can suggest hemodynamic instability.
- Shock index may be insensitive in the presence of negative chronotropic medications (e.g. beta-blockers), conduction system disease, and inferior AMI.
Arterial lactate ≥ 2mmol/L
- Lactate elevation is not very sensitive for diagnosis of shock.
- For example, lactate can be normal in shocked patients who have inadequate sympathetic nervous function.
- Elevated lactate is very nonspecific and has many false positives such as liver failure, seizures, and β-2 receptor stimulation (e.g. epinephrine, albuterol administration) *,*. More on this, here.
Diagnostic pitfalls
- There is no sign, symptom, or lab test that is entirely sensitive or specific for shock (explained above).
- Diverse clinical presentation
- The clinical presentation of shock is not uniform. Shock is a physiologic continuum that can progress through several stages with different clinical presentations. These clinical stages include pre-shock (occult shock), overt (established) shock, and refractory shock * (figure below).
- Shock is often multifactorial
- Many patients with shock have a combination of more than one form of shock. For example, a patient with cardiogenic shock who develops septic complications.
- Overlapping pictures
- Certain forms of shock share overlapping features (e.g. late septic shock may look quite similar to advanced cardiogenic shock).
| Does this patient with hypoperfusion have cardiogenic shock? |
As explored above signs and symptoms of hypoperfusion might not help to identify the cardiogenic cause of shock. In the next few sentences, the distinguishing features of cardiogenic shock are discussed.
Diagnostic clues for cardiogenic cause of shock
1.Low cardiac Index
- The core feature of CS is ineffective cardiac output.
- Pay attention to pulse pressure. A narrow PP (PP< SBP/4) may indicate a low CO *.
- Perform echocardiography
- The presence of a hyperdynamic heart 📖 will rule out CS.
- Cardiac output can be measured by echocardiography.
- A low CI (e.g. < 2.2 L/min/m2) is the key finding in CS with different hemodynamic phenotypes.
-
Caveat: Although CI thresholds <1.8 to 2.2 L/min/m2 have been proposed for CS, absolute cutoffs are likely impractical given that end-organ hypoperfusion with higher CIs has been documented *.
-
- A low CI (e.g. < 2.2 L/min/m2) is the key finding in CS with different hemodynamic phenotypes.
2.Elevated left atrial pressure (pulmonary congestion)
- Elevated left atrial pressure reflects the wet (pulmonary edema) feature of cardiogenic shock.
- Clinical clues for elevated LAP: Dyspnea, orthopnea, and rales in lung fields.
- Biochemical markers: ⬆️BNP.
- A low BNP level argues against CS in the setting of hypotension; however, an elevated BNP level is not helpful in establishing the diagnosis of CS *.
- Echocardiography: Assessment of mitral inflow velocities
- In patients without systolic dysfunction, a septal E/e’ >15 is highly suggestive of elevated LAP; while an E/e'<8 reflects normal LAP *.
- E/e’ between 8-15. This range can be used as a spectrum. A value closer to 8 suggests a lower LAP, and a value closer to 15 is suggestive of a higher LAP.
- In patients with systolic dysfunction (↓LVEF), use E/A ratio. An E/A > 2 is suggestive of elevated LAP.
- In patients without systolic dysfunction, a septal E/e’ >15 is highly suggestive of elevated LAP; while an E/e'<8 reflects normal LAP *.
- Lung ultrasound (LUS): Diffuse bilateral B-lines on LUS may suggest ↑LAP.
3.Hypervolemia (systemic congestion)
- Patients with chronic heart failure often have systemic congestion. Elevated right-sided filling pressure may reflect volume overload.
- Clinical clues: Jugular venous distension (JVD), peripheral edema, ascites, pleural effusion, and pericardial effusion.
- Biochemical markers: ⬆️BNP, abnormal renal and liver function test (congestive hepatopathy, nephropathy).
- Enlarged IVC size with decreased respiratory collapsibility.
- A higher grade on the VExUS score. More on this👉 here.
Diagnostic approach to CS
Collect and collaborate data (multidimensional evaluation)
- Obtain history, physicals (e.g. vitals, signs and symptoms of hypoperfusion and congestion), labs, and bedside ultrasound/echo information.
- Labs:
- CBC, electrolytes, LFTs, lactate, TSH (if thyroid disease is suspected), and digoxin levels (for patients on digoxin).
- Troponin, BNP.
- 12-lead ECG.
- Imaging: Echocardiography, IVC, ultrasound of the lung/abdomen.
- Echocardiography should be used to determine the underlying diagnosis, guide interventions, and monitor response to therapies (figure below).
- The first-line echo should be performed in ED as per FoCUS protocols (FoCUS protocols and challenges )
- More comprehensive echocardiography should be performed as early as possible. More on this,👉 here.
- Echocardiography should be used to determine the underlying diagnosis, guide interventions, and monitor response to therapies (figure below).
- Labs:
Summary of in-hospital monitoring and investigations *
Differential diagnosis
DDx of shock
- Determine the physiologic class of shock, based on integrated findings (table below).
- Keep in mind that patients with multiple chronic problems or multifactorial shock may defy classification.
- Exclude the following conditions which require a different set of treatments.
- Obstructive shock (e.g. pulmonary embolism, tamponade).
- Structural abnormalities: Valvular emergencies, dynamic left ventricular outflow tract obstruction (LVOTO), intracardiac mass?
- Aortic dissection. Is the aortic root enlarged? Look for signs of aortic direction including direct signs (intimal flap) and indirect signs (e.g. aortic regurgitation, pericardial effusion, and pleural effusion). More on this, here.
DDx of pulmonary edema
- The etiologies of pulmonary edema (based on its pathophysiology) are represented below. The two predominant pathophysiologic mechanisms that can cause pulmonary edema include:
- Perform echo and lung ultrasound to distinguish these two subtypes of pulmonary edema.
- Echo: Generally, the most helpful variable that can differentiate these two subtypes of pulmonary edema is the left atrial pressure (LAP).
- Elevated LAP is the core feature of hydrostatic pulmonary edema. A septal E/e’ >15 is highly suggestive of hydrostatic pulmonary edema. More on this,👉 here.
- Lung ultrasound: The presence of B-lines might suggest pulmonary edema *. Helpful clues to differentiate hydrostatic vs. permeability pulmonary edema on lung ultrasound are discussed here.
- Hydrostatic pulmonary edema
- The B-lines are bilateral and symmetric.
- Asymmetric B-lines sometimes are present in cardiogenic pulmonary edema (2.1%) *.
- This will cause an A/B profile on lung ultrasound (slides below).
- It can be seen in severe “mitral regurgitation” *.
- Usually, it is right-sided pulmonary edema, but rarely left-sided pulmonary edema can happen (figure below).
- Unilateral pulmonary edema is related to an independently increased risk of mortality and should be promptly recognized to avoid delays in treatment *.
- Asymmetric B-lines sometimes are present in cardiogenic pulmonary edema (2.1%) *.
- Presence of lung sliding.
- Thin and regular pleural lines.
- Pleural effusion(s), usually bilateral.
- Generally, pleural effusion denotes chronicity of heart failure syndrome.
- Effusion is transudative. The absence of plankton sign/hematocrit sign is typical for transudative effusions.
- The B-lines are bilateral and symmetric.
- Hydrostatic pulmonary edema
-
- Increased permeability pulmonary edema
- The B-lines could be bilateral or unilateral.
- Lung sliding is present but may be diminished due to inflammation.
- Pleural lines are thick and irregular with or without subpleural consolidation.
- Pleural effusion(s), if present, is exudative.
- Mechanism: Increased vascular permeability from inflammation/infection/malignancy
- Lung ultrasound appearance: The presence of plankton sign/hematocrit sign is suggestive of exudative effusion.
- Increased permeability pulmonary edema
| How severe is this cardiogenic shock? |
Five evolutionary stages of CS have been described by the Society for Cardiovascular Angiography and Interventions (SCAI) *.
- This classification can be applied rapidly bedside upon patient presentation, across all clinical settings.
- The SCAI classification establishes a unifying system for grading the severity of CS.
- It uses bedside clinical assessment of hypoperfusion, measurement of lactate level, and hemodynamic evaluation.
- Patients with acute cardiovascular disease are stratified into 5 SCAI shock stages of escalating severity*.
- SCAI classification perfectly classifies normotensive hypoperfusion as stage C cardiogenic shock.
A: At risk
- A hemodynamically stable patient who is not currently experiencing signs or symptoms of CS, but is at risk for its development (e.g. large AMI, decompensated heart failure).
- Exam: Normal mentation, warm and well-perfused extremities with strong distal pulses, clear lung sound, and normal JVP.
- Labs: Normal lactate, normal (or at baseline) renal function.
- Hemodynamic: Normotensive (SBP ≥100 mmHg or at baseline).
- If invasive hemodynamics are assessed:
- CI ≥2.5 L/min/m2 (if acute).
- CVP ≤10 mmHg.
- PCWP ≤15 mmHg.
- PA saturation ≥65%
- If invasive hemodynamics are assessed:
B: Beginning (pre-shock=occult shock)
- Unstable hemodynamic (such as hypotension or compensatory tachycardia) WITHOUT hypoperfusion (Hypotensive Normoperfusion).
- Patients with preserved perfusion despite significantly abnormal invasive hemodynamics (such as reduced CI) are also classified as SCAI stage B.
- Exam: Normal mentation, warm and well-perfused extremities with strong distal pulses, rales in lung fields, JVD (mild volume overload).
- Labs: Normal lactate, minimal acute renal function impairment, ⬆️ BNP.
- Hemodynamic: Hypotension (SBP<90, or MAP<60, or > 30mm Hg drop from baseline), OR Tachycardia (pulse ≥100) *.
- If hemodynamics are done:
- CI ≥2.2 L/min/m2 (if acute)
- PA saturation ≥65%
- If hemodynamics are done:
C: Clasic CS
- A patient who manifests with hypoperfusion and who requires an initial set of interventions (inotropes, pressor, mechanical support including ECMO) beyond volume resuscitation. These patients typically present with relative hypotension (but hypotension is not required, therefore normotensive CS will be classified as stage C).
- Exam: Looks unwell, delirious, acute alteration in mental status, cold and clammy extremities, sweating, mottling may be present, volume overload, extensive rales.
- Labs: Lactate ≥2 mmol/L, ⬆️Creatinine to 1.5 x baseline (or 0.3mg/dL) or > 50% drop in GFR, ⬆️LFTs, ⬆️BNP.
- Lactate is commonly used to detect hypoperfusion but may be dissociated from hemodynamics in cases such as chronic HF where patients may have a normal lactate level with a depressed cardiac index.
- Generally, arterial lactate >2 mmol/L is consistent with at least SCAI SHOCK stage C, although some patients may demonstrate other manifestations of end-organ hypoperfusion with a normal lactate level, and there are also causes of an elevated lactate level other than shock, such as mesenteric ischemia or compartment syndrome
- Hemodynamic: May include any of the following:
- SBP <90 (or MAP <60, or >30mm Hg drop from baseline), OR Tachycardia; AND drugs/devices used to maintain BP above these targets.
- If invasive hemodynamics are assessed (strongly recommended) *:
- Note that patients with established CS can be further subcategorized as LV-dominant, RV-dominant, or biventricular shock. These will produce different hemodynamic profiles (shock profile). This is discussed more in Forrester classification for hemodynamic phenotyping. A summary of the hemodynamic phenotype (shock profile) is provided below.
- The distinction between SCAI SHOCK stages B and C is critical.
- Patients with hypoperfusion in the absence of hypotension (normotensive CS; classified as stage C in SCAI) are at higher risk of dying than patients with hypotension and preserved perfusion (hypotensive normoperfusion; classified as stage B in SCAI) *.
- The stage B (pre-shock: Hypotensive Normoperfusion“) may occur in severe AHF.
- Hemodynamic: SBP < 90 mm Hg, CI ≥2.2, SVR 800–1600 dynes-s/cm –5.
- Absence of overt clinical signs and symptoms of hypoperfusion.
- Lactate levels and labs are normal.
- The stage B (pre-shock: Hypotensive Normoperfusion“) may occur in severe AHF.
- Patients with hypoperfusion in the absence of hypotension (normotensive CS; classified as stage C in SCAI) are at higher risk of dying than patients with hypotension and preserved perfusion (hypotensive normoperfusion; classified as stage B in SCAI) *.
D: Deteriorating
- A patient who has failed to stabilize despite intense initial efforts.
- Classification in this stage requires that a patient meet the following conditions *:
- The patient has had some degree of appropriate treatment/medical stabilization.
- At least 30 min have elapsed, but the patient has not responded with hypotension or end-organ hypoperfusion resolution.
- The patient requires further escalation (escalation is an increase in the number or intensity of IV therapies to address hypoperfusion or the addition of MCS) to maintain perfusion.
- Exam: Any of stage C and worsening (or not improving) signs/ symptoms of hypoperfusion despite the initial therapy.
- Labs: Any of stage C and lactate rising and persistently >2 mmol/L, deteriorating renal function, worsening LFTs, rising BNP.
- Hemodynamic: Any of stage C AND requiring escalating doses OR increasing numbers of pressors or the addition of a mechanical circulatory support device to maintain perfusion.
E: Extremis
- A patient who meets any of the following conditions:
- If perfusion cannot be restored using multiple simultaneous vasoactive drugs and/or MCS devices (refractory Shock), or
- Patients with refractory shock impending circulatory collapse, or
- Cardiac arrest with ongoing cardiopulmonary resuscitation (CPR), or
- Refractory cardiac arrest with ongoing CPR.
- Exam: Typically unconscious, near pulselessness, cardiac collapse, defibrillator used, mechanical ventilation.
- Stage E prospectively is a patient with cardiovascular collapse or ongoing CPR *.
- Labs: Lactate ≥8 mmol/L, CPR (A-modifier), severe acidosis (e.g. pH <7.2) , base deficit > 10 mEq/L *.
- Hemodynamic: Hypotension despite maximal support, no SBP without resuscitation, PEA or refractory VT/VF, need for bolus doses of vasopressors.
- The SCAI classification is validated in a large cohort of unselected intensive cardiac care unit (ICCU) patients providing robust mortality risk stratification regardless of CS etiology, in a manner that was amplified by the presence of CA *.
- Additional prognostic variables (besides classification staging) *
- Age
- The trajectory of SCAI. This classification is inherently dynamic, and a rising or persistently elevated SCAI Shock stage portends worse outcomes *.
- Duration of time spent in higher SCAI Shock stages *,*.
- Cardiac arrest
- However, cardiac arrest events are heterogeneous, and single defibrillation for a brief ventricular arrhythmia without CPR and normal neurologic function does not change the prognosis of a patient with CS *. Instead, two aspects of cardiac arrest can change the prognosis:
- Neurologic status (awake or comatose) and
- Physiologic impact of the arrest, as prolonged CA may fundamentally change the patient trajectory if ischemia-reperfusion heralds multiorgan
failure.
- It has been suggested that cardiac arrest can portend worsening of prognosis only if the patients sustained an anoxic brain injury, evidenced by a GCS <9; alternatively, the absence of a motor response to voice (i.e. not following commands) is a helpful definition *.
- However, cardiac arrest events are heterogeneous, and single defibrillation for a brief ventricular arrhythmia without CPR and normal neurologic function does not change the prognosis of a patient with CS *. Instead, two aspects of cardiac arrest can change the prognosis:
| Phenotyping the CS |
- Background
- Most patients with advanced HFrEF have a low cardiac index (CI< 2.2 L/min/m2); however, they do not have CS.
- Patients with chronic compensated heart failure may be doing well clinically with elevated systemic vascular resistance.
- In contrast, patients with a higher CI (CI>2.2 L/min/m2) can have signs and symptoms of hypoperfusion *.
- Moreover, there are different hemodynamic phenotypes for CS * determined by:
- Most patients with advanced HFrEF have a low cardiac index (CI< 2.2 L/min/m2); however, they do not have CS.
- Use Forrester classification for hemodynamic phenotyping of CS (figure below).
- These formal definitions of hemodynamic shock phenotypes may help guide therapy and improve outcomes *.
- Hemodynamic phenotyping (shock profile) can be performed based on the cardiac index and pulmonary capillary wedge pressure. Patients may be categorized as shown in the following figure.
- Assess for clinical signs and symptoms of hypoperfusion, and congestion.
- Perform echocardiography and lung ultrasound to evaluate the left and right ventricular function, and evidence of congestion (invasive hemodynamic evaluation might be required for a certain group of patients).
- Note that CS has a complex and dynamic pathophysiology. The patient may go from one category to another.
- The common physiological characteristic is a low cardiac index (CI), but pulmonary capillary wedge pressure (PCWP), central venous pressure (CVP), and systemic vascular resistance (SVR) may vary *.
- There are two other uncommon but hemodynamically distinct entities overlaid on this framework: Normotensive CS and right ventricular CS (which will be covered in the following discussion).
| Classic CS |
- Most common reported CS phenotype in AMI-CS (~70%) *.
- Hemodynamic profile: ↓CI {systemic hypoperfusion (cold)}, ↑PCWP (wet lung)}.
- Cardiogenic shock patients may look misleadingly fine, but they are indeed critically ill.
- Patients in classic CS cannot be managed with volume administration or removal (figure below).
- Giving volume will worsen their pulmonary congestion (making them more wet).
- Removing volume will worsen their systemic hypoperfusion (making them more cold).
- Therefore, management of these patients usually requires interventions to improve cardiac function (e.g. inotropes, revascularization, or mechanical support).
- Early diagnosis facilitates appropriate critical management.
- Patients with unrecognized CS will generally fail to respond to non-intensive therapy. Undiagnosed patients often run in vicious circles (the patient is initially managed with fluid removal, then develops worsening renal failure, then is given fluid back, and then develops pulmonary edema).
| Vasodilatory CS or Mixed Shock |
- 20% of patients in the SHOCK trial had low SVR at the onset of CS *.
- In patients with AMI-CS, mixed CS appeared to be associated with higher mortality and morbidity *.
- Hemodynamic profile: Concomitant ↓SVR and ↓CI *.
- Pathophysiologic processes that can lead to vasodilatory CS *
- A noninfectious inflammatory response.
- Concurrent infection.
- Metabolic derangements such as multiorgan failure and acidosis (sometimes termed hemometabolic CS).
- Vasoplegia * after cardiac arrest (CA) or cardiac surgery.
- Vasodilatory medications.
- Interpatient differences in the immune response to acute insults.
- As explained above, activation of systemic inflammatory response commonly occurs in CS.
- In essence, the final common pathway of all types of shock is micro-circulatory alteration mediated by activation of systemic inflammatory cascade *.
- This also accounts for the fact that different classes of shock can not be differentiated in later stages *.
- Keep in mind that some patients with septic shock may develop a septic cardiomyopathy. Read more on this 📖.
- Patients who are warm/wet may often be managed with diuresis and/or vasodilation to reduce their afterload (vasodilation shifts fluid out of the lungs without affecting the total body volume).
| Euvolemic (or hypovolemic) CS |
- It is most commonly seen in diuretic-responsive patients with chronic HF and subacute decompensation. However, it also represents 28% of patients with AMI-CS *,*.
- Hemodynamic profile: ↓CI (hypoperfused=cold) and ↑SVR.
- Patients with compensated CHF maintain their stroke volume at the expense of a higher preload (hypervolemia). Overdiuresis can lead to decompensation by pulling down the stroke volume and “CO” (figure 1B).
- Compared with patients with classic CS, those with euvolemic CS were less likely to have had a previous MI or CKD and had significantly lower PCWP *.
- Patients who are cold/dry may often respond to fluid administration by increasing stroke volume (figure above) *.
| Normotensive CS |
- 5.2% of CS in the SHOCK trial registry *.
- Hemodynamic profile
- They have a “comparable” CI, PWCP but a higher SVR compared with hypotensive patients with CS (accounting for relatively higher blood pressure).
- Typically, ↓CI (hypoperfused), ↑/⬌PCWP.
- SBP is kept >90 mm Hg at the expense of an elevated SVR.
- Lactate ≥ 2mmol/L
- Clinical significance: There is a risk of potential hypoperfusion without profound hypotension *. See media below.
- SCAI classification of CS nicely classifies these patients at stage C and distinguishes them from “Hypotensive Normoperfusion” patients (SCAI stage B).
| Biventricular or RV-predominant CS |
- 5.3% of patients with MI-induced CS *.
- Biventricular dysfunction (either as RV-predominant or LV-predominant CS) is associated with worse outcomes compared with CS due to “isolated” LV failure *,*.
- Hemodynamic profile: They may present as ‘wet-cold’ or ‘wet-warm’*.
- Relatively higher C, and LVEF, with NO differences in CI or PCWP.
- Different values of SVR according to the extent of systemic inflammatory response.
- Pulmonary artery pressure *:
- If the cause of biventricular CS is predominantly RV pump failure (e.g. RV myocardial infarction): Pulmonary artery pressure is usually low or normal.
- If the cause of biventricular failure is predominantly elevated RV afterload (e.g. pulmonary embolism, Type 1 PH): Pulmonary artery pressure is elevated.
- If the cause of biventricular CS is predominantly LV failure (e.g. Type 2 pulmonary hypertension= PVH): Pulmonary artery pressure is elevated.
- The severity of the RV instability can be assessed by calculating the pulmonary artery pulsatility index (PAPI).
- PAPI= (PASP-PADP)/RAP.
- Where PASP, is pulmonary artery systolic pressure; PADP is pulmonary artery diastolic pressure; RAP, is right atrial pressure.
- A PAPI ≤1 identifies patients with severe RV dysfunction *.
- PAPI= (PASP-PADP)/RAP.
Hemodynamic evaluation
Principles
- No single hemodynamic variable is sensitive and/or specific enough to be used for identifying cardiovascular insufficiency *. Multidimensional assessment can circumvent this pitfall.
- All hemodynamic variables should be interpreted in a “clinical context”.
- For example, a patient with chronic compensated heart failure may be doing well clinically with an elevated systemic vascular resistance (SVR). The elevated systemic vascular resistance works here as a compensatory mechanism, allowing the maintenance of adequate blood pressure.
- The current concept of functional hemodynamic monitoring encourages dynamic assessment of hemodynamic variables in response to treatment *.
- For a complete discussion, refer👉here.
Non-invasive hemodynamic assessment
- It is essential for the initial diagnosis and management of patients with CS *.
- Note that a complete hemodynamic assessment and the adequacy of end-organ and tissue perfusion in response to individualized targets can be made by integrating serial markers of systemic perfusion,* echocardiography, and ultrasound of the abdomen and lung *. More on this, 👉here.
Pulmonary artery catheterization (PAC)
- Routine use of PA catheterization is not recommended by AHA guidelines, even in CS *.
- PAC is an invasive procedure with the associated risk of complications (table 1👉 📖).
- With the advent of non-invasive monitoring, i.e. echocardiography, medical staff are less skilled at insertion and troubleshooting of PA catheters. For technical aspects of accurate PAC hemodynamics (table 2 👉📖).
- PAC always reveals abnormal numbers, but it’s unknown what should be done with this data (read this fantastic article; “Alice in intensiveland”) *.
- For example, there is no defined goal for cardiac output or systemic vascular resistance. A cardiac index that may be adequate for one patient will leave another patient in cardiogenic shock.
- Possible indications for PAC
- Unresponsiveness to initial therapy.
- Documentation of hemodynamics to determine candidacy for cardiac transplantation or ventricular assist device.
- Uncertain nature of shock (mixed shock) with no alternative source of hemodynamic information (e.g. poor TTE windows and inability to perform a TEE) *.
- Contraindications to PAC
- Bundle branch block (especially left bundle branch block), since PA catheterization may cause right bundle branch block *.
- Arrhythmia or hyperkalemia (insertion may trigger arrhythmias).
- Risk of displacing other devices (e.g. transvenous pacemaker).
- Prosthetic or stenotic tricuspid/pulmonic valve.
Normal value of hemodynamic variables (pulmonary artery catheter data): See appendix *.
- Directly measured parameters by PAC include CVP; pulmonary artery systolic pressure (PASP); pulmonary artery diastolic pressure (PADP); PCWP, which is also commonly referred to as pulmonary arterial occlusion pressure; cardiac output by thermodilution and mixed venous oxygen saturation (SvO2).
- Derived parameters: In addition to directly measured parameters, derived parameters (e.g. systemic vascular resistance and pulmonary vascular resistance) can be calculated. Some of these derived parameters that have prognostic significance and can be used to tailor therapy for patients with CS are discussed here *.
- Markers of cardiogenic shock
- Cardiac Power Index (CPI)
- CPI is an indicator of significant ventricular dysfunction and has proven to be a strong hemodynamic predictor of poor outcome at CPI < 0.4 W/m2 *.
- Cardiac Power Index (CPI) = MAP x CI/451 (Watts/m2). Normal: 0.5-0.7 Watts/m2
- Cardiac Power Index (CPI)
- Markers of RV function
- Pulmonary artery pulsatility index (PAPI)
- RAP/PCWP ratio
- Pulmonary artery pulsatility index (PAPI)
The hemodynamic profiles of cardiogenic shock subtypes are summarized below.
Management
Chain of survival
The chain of survival of cardiogenic shock is shown below. For optimal outcomes, all links in the chain are optimally required including:
- Early diagnosis.
- Early cardiovascular and hemodynamic support (medical treatment and mechanical circulatory support, to buy time for definitive treatment).
- Early intervention (e.g. early revascularization for AMI-CS) to reverse the cause.
System of care
- A model analogous to primary PCI pathways has been proposed by the American Heart Association to facilitate optimal care coordination and minimize time delay *.
- Level I Center (cardiogenic shock center) is capable of PCI and implementation of MCS (short-term or long-term), and available on-site operating rooms with cardiothoracic surgery and a highly experienced multidisciplinary team (MDT).
- Level II Centers have PCI available but usually do not have MCS.
- All patients with CS should be rapidly transferred to a tertiary care center which has the service of cardiac catheterization, and a dedicated ICU/ICCU with availability of short-term MCS *.
Medical Treatment
The center of focus in this review is the management of florid cardiogenic shock typical of LV failure or biventricular failure. Medical treatment of CS in specific clinical conditions is discussed separately as:
- Takotsubo Syndrome 📖, Dynamic LVOTO 📖.
- SCAPE (Sympathetic Crashing Acute Pulmonary Edema) 📖.
- RV failure: Principles of hemodynamic management 📖.
- Valvular Emergencies: AS 📖, AR📖,MS 📖, MR📖.
The overall view of the medical management of florid cardiogenic shock typical of LV failure or biventricular failure is shown below.
Principles
- Both RV and LV functions have to be improved since the heart consists of two pumps connected in series *.
- A dynamic personalized therapeutic strategy should be employed *.
- A “one-size-fits-all” approach to treating patients with CS does not account for individual treatment responsiveness and is not appropriate *.
- There are 4 primary objectives in hemodynamic support of patients with CS, all of which should be achieved in a timely manner *.
- Circulatory support
- Adequate circulatory support is defined by an increase in MAP and enhanced microvascular organ perfusion *.
- Target: Generally, an initial MAP of 65-70 mmHg should be targeted *.
- Monitoring the response to treatment: Improving functional markers of organ perfusion e.g. creatinine, urine output, and mental status is reassuring *. Down-trending lactate should be interpreted in the clinical context. More on this, here.
- Ventricular unloading
- Defined as a reduction in myocardial work and wall stress, which is best achieved by reducing ventricular pressure and volume *.
- Target: A 90 <SBP<120 mmHg; PCWP 10-18 *.
- Monitoring the response to treatment: Resolving tachycardia (in the presence of improvement in organ perfusion), and down-trending BNP are reassuring *. Improving echocardiographic markers of (E/A, E/e’ ratio).
- Myocardial perfusion
- See coronary perfusion pressure above.
- Improving LV perfusion pressure (DBP-LVEDP) is achieved by increasing DBP and reducing LVEDP (LV preload).
- Improving RV perfusion (MAP – MPAP) is achieved by raising MAP and reducing mean pulmonary artery pressure.
- This is often associated with successful circulatory and ventricular support.
- Monitoring the response to treatment: A down-trending cardiac troponin and resolving ST-T changes are reassuring *.
- See coronary perfusion pressure above.
- Decongestion
- Refers to a reduction in total body volume and elevated venous filling pressures, which is commonly associated with worsening renal function, hepatic failure, bowel edema, and subsequent sepsis *.
- Target: A RAP 5-10 mm Hg, RAP/PCWP <0.86 *.
- Monitoring the response to treatment: A down-trending serum creatinine, improving LFTs, coagulation profile *, decreasing IVC size, and increasing collapsibility are reassuring.
- Circulatory support
- Limit the dose and the duration of vasoactive medications to the lowest possible dose when tissue perfusion is restored *.
- Vasoactive medications may restore hemodynamics but they have significant shortcomings in many CS patients that render them inadequate. They will solve one part of the equation but at the cost of another, for example:
- Vasopressors increase MAP but do not improve microvascular organ perfusion.
- Increased MAP leads to increased LV afterload, resulting in increased myocardial work and wall stress promoting myocardial ischemia, impairing cardiac function, and increasing cardiac filling pressures.
- In other words, vasopressors can increase BP, but typically at a cost of decreased CO and increased myocardial oxygen needs.
- Inotropes have similar physiology which directly increase myocardial work and increase the likelihood of myocardial ischemia.
- Vasopressors increase MAP but do not improve microvascular organ perfusion.
- Vasoactive medications may restore hemodynamics but they have significant shortcomings in many CS patients that render them inadequate. They will solve one part of the equation but at the cost of another, for example:
- Mechanical circulatory support (MCS): The use of MCS has been increasingly advocated to circumvent the above shortcomings.
Optimizing Lung Function
Intubation
- It is often required for frank CS (SCAI stage C) and multiorgan failure (e.g. delirium, renal failure).
- It provides full support for the work of breathing, which may allow the shunting of blood away from the diaphragm and towards vital organs *.
- The effects of positive pressure ventilation can vary (figure below) *.
- ⚠️Intubation in CS carries risks of hypotension/arrest. It’s often safer to resuscitate them before intubation *.
BiPAP (non-invasive ventilation)
- It has been shown that patients with respiratory distress due to heart failure respond well to BiPAP. This can be applied to patients with less severe forms of CS without change in mental status or organ failure (e.g. SCAI stage B).
- The most important parameter is the expiratory pressure, which should be ramped up rapidly if possible.
Pleural effusion drainage
- Drainage of pleural effusion is indicated in patients with significant respiratory distress or hypoxemia who have large effusion(s). More on this, here.
Inhaled pulmonary vasodilators
- Inhaled epoprostenol or nitric oxide may be considered for an intubated patient with biventricular failure or severe hypoxemia. Physiological benefits include:
- Reduction in RV afterload may improve cardiac output among patients with right ventricular failure.
- Inhaled pulmonary vasodilators will improve “perfusion: ventilation matching” and thereby improve oxygen saturation.
- Administration of an inhaled pulmonary vasodilator can worsen cardiogenic pulmonary edema in patients with biventricular failure as improving RV output will result in receiving more blood in the left ventricle thereby increasing the pulmonary capillary wedge pressure. However, in the intubated patient this generally isn’t a major problem.
Optimize MAP and HR
In the presence of multiple hemodynamic derangements, it is crucially important to identify whether the abnormal heart rate is the cause of instability (i.e. if the abnormal heart rate is the cause of hypoperfusion).
Arrhythmia-CS
- Identify if the abnormal HR is the cause of CS. This could be present if the heart rate lies in the extremes, such as
- Non-sinus tachycardia > 170 bpm.
- Atrial fibrillation with ventricular response > 150 bpm.
- Bradycardia: e.g. HR <30 bpm.
- If shock is caused by new-onset tachyarrhythmia (e.g. atrial fibrillation with ventricular response >>150), then reversion to sinus rhythm may be beneficial.
- However, if the heart rate isn’t very high (e.g. AF with RVR <150) then be careful. Slowing down the heart rate may lead to deterioration of clinical condition.
- If shock is caused or aggravated by severe bradycardia, this should be treated accordingly.
Optimizing The Mean Arterial Pressure (MAP
Hypotension impairs coronary and end-organ perfusion and should be corrected promptly.
- Optimal MAP
- The optimal MAP differs from patient to patient, and the risks of hypoperfusion with lower MAPs must be balanced (and individualized) with the potentially deleterious impact of vasoactive agents on myocardial oxygen demand, ischemia, and arrhythmia associated with higher MAP targets *.
- Often an ideal blood pressure will be in the low-normal range (e.g., MAP 60-65 mm Hg).
- A higher MAP may be beneficial in previously hypertensive patients *.
- First-line vasopressor: It should be selected based on the patient’s physiology.
- Norepinephrine is widely recommended as a front-line agent for CS.
- Norepinephrine will improve blood pressure, but there is a risk that excessive afterload drops the cardiac output.
- Epinephrine (low dose) could be a reasonable choice for a patient with reduced ejection fraction, hypotension, and poor cardiac output.
- Epinephrine can be a therapeutic alternative to the combination of dobutamine and norepinephrine *.
- At a very low dose (e.g. 0-5 mcg/min) epinephrine acts as an inotrope with a neutral effect on systemic vascular resistance (SVR). However, unlike dobutamine, epinephrine doesn’t cause vasodilation.
- At low doses (e.g. 5-10 mcg/min) epinephrine works predominantly as an inotrope with minimal increase in SVR.
- Epinephrine can be a therapeutic alternative to the combination of dobutamine and norepinephrine *.
- Vasopressin
- It is a non-sympathomimetic vasoconstrictor agent that increases SVR and MAP but does not affect pulmonary vascular resistance.
- Vasopressin increases systemic arterial pressure by specifically inhibiting the same intracellular enzymes responsible for the vasodilator action of milrinone and may be used to counteract vasodilatation caused by milrinone *.
- Vasopressin may be helpful in patients with tachycardia or pulmonary hypertension.
- In combination with milrinone, administration of vasopressin at low doses increased systolic pressure and allowed discontinuation or a decrease in catecholamine vasopressors *.
- Norepinephrine is widely recommended as a front-line agent for CS.
- ⚠️Dopamine: Should be avoided.
- In the SOAP II trial, the pre-defined subgroup analysis of CS patients showed that dopamine was associated with higher 28-day mortality and increased arrhythmia burden, compared with norepinephrine *.
- In a propensity-matching-score analysis from the ESC HF Long-Term registry, dopamine was associated with worse short- and long-term outcomes compared with other inotropes and vasopressors *.
Hypertension (or high-normal BPs) should be managed with afterload reduction.
- In patients with evidence of poor perfusion who are normotensive or hypertensive, afterload reduction may improve cardiac output, decongest the lungs, and reduce the myocardial workload *.
- Agent of choice: In the acute phase, a high-dose nitroglycerine infusion is the safest vasodilator.
- High doses may be needed to achieve arterial vasodilation 👉 📖.
- This should be titrated against the patient’s blood pressure. See appendix.
Optimize volume status
Fluid administration
- Consider giving a fluid challenge (e.g. Ringer’s lactate 250 mL over 15–30 min) if the following conditions are met:
- No evidence of pulmonary congestion (e.g. no B-lines on lung ultrasound).
- The multidimensional hemodynamic assessment suggests true hypovolemia (no evidence of systemic congestion).
- Fluid should be given in boluses of 250-500 ml fluid challenges, with careful determination of the effect on the patient. Avoid giving further fluid, if it is not causing clinical improvement.
- Static hemodynamic parameters (e.g., CVP, PCWP) do not predict fluid responsiveness and should not be used as the primary determinant of fluid administration.
Diuresis
- Diuresis should be considered if all the following conditions are met:
- Presence of significant pulmonary and/or systemic congestion *.
- Multidimensional hemodynamic assessment suggests total body fluid overload.
- Loop diuretics remain the cornerstone for the treatment of congestion in patients with heart failure. If patient is not responding well to furosemide, consider adding a thiazide diuretic (e.g., metolazone 5 mg q12hr-q24hr) *. More on this, 👉 here.
Optimize contractility
- The addition of an inotrope (dobutamine) is recommended with a class IIb/C recommendation, reflecting the paucity of data in this setting *.
- Inotropes will cause a short-term improvement in hemodynamics. However, available evidence indicates that inotrope use is associated with worse outcomes *.
- Inotropes should be used only if necessary, for the following indication:
- Hypoperfusion with low-normal blood pressure (e.g. acute kidney injury with poor urine output despite all above resuscitative measures).
- Hypotensive patients with cardiogenic pulmonary edema: In such patients, inotropes may be used with the goal of reducing the PCWP and decongesting the lungs.
- Dobutamine or milrinone?
- An RCT comparing dobutamine vs. milrinone in patients with cardiogenic shock found no difference between the two agents *.
- Both agents may cause hypotension, so they shouldn’t be used in profoundly hypotensive patients. Thus, it’s generally preferable to raise the MAP first.
- Dobutamine has a shorter half-life which makes it more easily titratable, which arguably makes it the preferred agent.
- Milrinone has a longer half-life (2.3 hours) making rapid titration impossible. Moreover, milrinone is renally cleared so it may exhibit erratic pharmacokinetics in shocked patients (e.g., accumulating unexpectedly if there is a reduction in renal function).
- Levosimendan
- The inotropic effect of levosimendan is the result of a combined effect from both calcium sensitization and selective and potent phosphodiesterase 3 inhibition.
- It also reduces peripheral vascular resistance by inhibiting K + channels in vascular smooth muscle, inducing arterial and venous vasodilatation *.
- It may be used in particular CS with
Considerations
- Blood transfusion is indicated to standard target >7 mg/dL or, in a patient with evidence of active myocardial ischemia a target of >8 mg/dL is warranted.
- ⚠️β-blockers should be avoided in CS and should be held in patients with CS who were previously taking them *.
- ⚠️Avoid using medications with negative inotropic effects (e.g. non-dihydropyridine calcium channel blockers) in patients with CS *.
- ⚠️Avoid using nephrotoxic medications in CS.
- ⚠️Avoid vasodilators (e.g. ACEIs, ARBs) in hypotensive patients with CS.
Mechanical Circulatory Support (MCS)
- Background
- Temporary MCS (tMCS) has an emerging role in CS.
- Early use of MCS in patients with CS refractory to fluid load and inotropes/vasopressors is recommended (IIb/C).
- However, MCS is associated with significant complications, requires specialist multidisciplinary expertise for implantation and management, and high-quality evidence regarding outcomes is largely absent.
- Role of tMCS. Depending on the context, MCS may play a variety of different roles *
- Bridge to recovery.
- Bridge to re-evaluation, ideally following resolution of multi-organ failure (“bridge to bridge”).
- Bridge to a more durable platform (LVAD or transplantation)
- Temporary MCS (tMCS) has an emerging role in CS.
- Options include intra-aortic balloon pumps (IABP), percutaneous centrifugal pumps, and full veno-arterial ECMO (characteristic of MCS 👉appendix).
- Factors involved in determining mechanical support device
- Absolute contra-indications for all types of temporary MCS (tMCS) *
- Severe irreversible noncardiac organ failure limiting survival (e.g. severe anoxic brain injury or metastatic cancer).
- Irreversible cardiac failure if transplantation or long-term ventricular assist device (VAD) is not an option.
- Relative contra-indications for all types of tMCS *
- Moderate to severe aortic regurgitation.
- Severe coagulopathy or contraindication to anticoagulation, including advanced liver disease.
- Limited vascular access.
- Aortic dissection, aneurysm, or severe atherosclerosis.
- Advanced age.
- Prolonged CPR >45 min.
Intra-aortic balloon pumps (IABP)
- The most popular devices overall and the most thoroughly investigated. However, RCTs fail to show improvement in patient-centered outcomes *,*.
- The IABP produces a modest increase in CO of 0.5–1 L/min and may have even less benefit in patients with tachycardia and irregular rhythms *.
- Contraindications *
- Aortic dissection
- Abdominal aortic aneurysm
- Tachyarrhythmias
Impella
- Impella is a microaxial pump giving only left-sided support that unloads the left ventricle by expelling blood flow from the LV into the aorta and may provide up to >5 L/min of blood flow depending on the device used and depending on afterload.
- Although it provides superior hemodynamic support compared to IABP, Impella failed to show any difference when compared to an intra-aortic balloon pump AMI-CS *.
- A recent retrospective registry study compared matched patients treated with Impella vs. IABP showed that patients treated with Impella had some improvement in renal function, more bleeding, and more peripheral vascular complications, but no difference in mortality *.
- Contraindications *
- LV thrombus.
- Severe aortic stenosis is a consideration, but if the valve can be crossed with a wire is not an absolute contraindication.
Mechanical aortic valve.
Aortic dissection. - Ventricular septal defect (VSD) *
TandemHeart
- It provides a continuous flow (4 L/min) via a centrifugal pump.
- In two randomized studies, including AMI-CS patients, TandemHeart significantly improved hemodynamic indices as compared to IABP, but 30-day mortality did not differ between the two groups *,*.
- Contra-indication: Intra-atrial thrombus *
Venous-arterial ECMO
- It provides cardiopulmonary support by draining venous blood from the right atrium and returning it after oxygenation to the ascending aorta (central cannulation) or to the iliac artery (peripheral cannulation).
- V-A ECMO provides high levels of biventricular cardiac (V-A) and respiratory support (V-V) in a large spectrum of clinical settings, including CS patients with malignant arrhythmias and CA *.
- Contra-indication: Aortic dissection (if peripheral cannulation) *
Post-tMCS monitoring
- The principles of in-hospital patient evaluation and monitoring were discussed earlier.
- Of note, echocardiography plays a potential role in monitoring patients with transient mechanical circulatory support. See appendix.
Etiologic treatment
Early identification and treatment of the underlying etiology of CS is potentially beneficial in improving outcomes *. The general principles of CS management and Heart Team activation are presented in the following figure *.
- Non-ACS etiologies of CS. The treatment is discussed separately (for a summary of treatment principles of patients with non-ACS CS, see the appendix).
- AMI-CS
- In patients with ACS in cardiogenic shock, early revascularization strategy is the cornerstone in the management *,*. More on this, here.
- Revascularization is essential. This is beneficial even at delayed time points.
- Thrombolysis works poorly in CS. PCI or CABG is generally necessary.
- PCI strategy: Early culprit vessel revascularization is recommended.
- Patients with acute myocardial infarction (AMI) with cardiogenic shock frequently have multiple vessel disease.
- The CULPRIT-SHOCK trail Among patients who had multivessel coronary artery disease and AMI with cardiogenic shock, the 30-day risk of a composite of death or severe renal failure leading to renal-replacement therapy was lower among those who initially underwent PCI of the culprit lesion only than among those who underwent immediate multivessel PCI *.
- In patients with ACS in cardiogenic shock, early revascularization strategy is the cornerstone in the management *,*. More on this, here.
Media
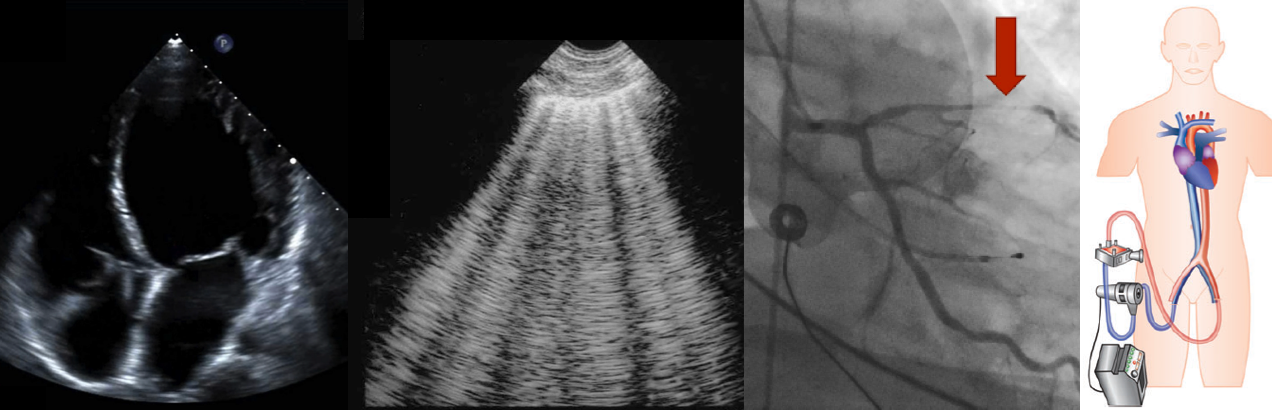
Normotensive Cardiogenic Shock
Going further
- Cardiogenic Shock (emDocs)
- A Tale of Two Shocks: A Cardiogenic Shock Interview With The Heart Failure Specialists
- Cardiogenic Shock Simplified (Emergency Medicine Cases)
- Cardiogenic shock & severe LV failure (IBCC)
- Cardiogenic Shock — The Next Level & Mechanical Circulatory Support (EMCrit)
Appendix
Pathophysiology of cardiogenic shock with staged abnormalities of clinic examination
Potential challenges in using echocardiography to determine the underlying cause of AHF
Proposed initial focused cardiac and lung ultrasonography assessment for patients with suspected AHF in acute care settings
Pulmonary artery catheter hemodynamic data
Characteristics of mechanical circulatory supports
Echocardiography for acute mechanical circulatory support
Management of non-acute coronary syndrome etiologies of cardiogenic shock
References
1. Berg DD, Bohula EA, van Diepen S, Katz JN, Alviar CL, Baird-Zars VM, Barnett CF, Barsness GW, Burke JA, Cremer PC, Cruz J, Daniels LB, DeFilippis AP, Haleem A, Hollenberg SM, Horowitz JM, Keller N, Kontos MC, Lawler PR, Menon V, Metkus TS, Ng J, Orgel R, Overgaard CB, Park JG, Phreaner N, Roswell RO, Schulman SP, Jeffrey Snell R, Solomon MA, Ternus B, Tymchak W, Vikram F, Morrow DA. Epidemiology of Shock in Contemporary Cardiac Intensive Care Units. Circ Cardiovasc Qual Outcomes. 2019 Mar;12(3):e005618. doi: 10.1161/CIRCOUTCOMES.119.005618. PMID: 30879324
2.Mebazaa A, Tolppanen H, Mueller C, Lassus J, DiSomma S, Baksyte G, Cecconi M, Choi DJ, Cohen Solal A, Christ M, Masip J, Arrigo M, Nouira S, Ojji D, Peacock F, Richards M, Sato N, Sliwa K, Spinar J, Thiele H, Yilmaz MB, Januzzi J. Acute heart failure and cardiogenic shock: multidisciplinary practical guidance. Intensive Care Med. 2016 Feb;42(2):147-63. doi: 10.1007/s00134-015-4041-5. Epub 2015 Sep 14. PMID: 26370690
3.Vallabhajosyula S, Dunlay SM, Murphree DH Jr, Barsness GW, Sandhu GS, Lerman A, Prasad A. Cardiogenic Shock in Takotsubo Cardiomyopathy Versus Acute Myocardial Infarction: An 8-Year National Perspective on Clinical Characteristics, Management, and Outcomes. JACC Heart Fail. 2019 Jun;7(6):469-476. doi: 10.1016/j.jchf.2018.12.007. Epub 2019 May 8. PMID: 31078481
4.Hernandez GA, Lemor A, Blumer V, Rueda CA, Zalawadiya S, Stevenson LW, Lindenfeld J. Trends in Utilization and Outcomes of Pulmonary Artery Catheterization in Heart Failure With and Without Cardiogenic Shock. J Card Fail. 2019 May;25(5):364-371. doi: 10.1016/j.cardfail.2019.03.004. Epub 2019 Mar 8. PMID: 30858119
5.Harjola VP, Lassus J, Sionis A, Køber L, Tarvasmäki T, Spinar J, Parissis J, Banaszewski M, Silva-Cardoso J, Carubelli V, Di Somma S, Tolppanen H, Zeymer U, Thiele H, Nieminen MS, Mebazaa A; CardShock Study Investigators; GREAT network. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail. 2015 May;17(5):501-9. doi: 10.1002/ejhf.260. Epub 2015 Mar 28. Erratum in: Eur J Heart Fail. 2015 Sep;17(9):984. PMID: 25820680
6. Bhatt AS et al. . De Novo vs Acute-on-Chronic Presentations of Heart Failure-Related Cardiogenic Shock: Insights from the Critical Care Cardiology Trials Network Registry. J Card Fail. 2021 Oct;27(10):1073-1081. doi: 10.1016/j.cardfail.2021.08.014. PMID: 34625127; PMCID: PMC8514080.
7. Chioncel et al. 2020. Epidemiology, pathophysiology and contemporary management of cardiogenic shock – a position statement from the Heart Failure Association of the European Society of Cardiology. European Journal of Heart Failure, 22(8), 1315–1341. https://doi.org/10.1002/ejhf.1922
8. Jentzer, J. C., Rayfield, C., Soussi, S., Berg, D. D., Kennedy, J. N., Sinha, S. S., Baran, D. A., Brant, E., Mebazaa, A., Billia, F., Kapur, N. K., Henry, T. D., & Lawler, P. R. (2022). Advances in the Staging and Phenotyping of Cardiogenic Shock. JACC: Advances, 1(4), 100120. https://doi.org/10.1016/j.jacadv.2022.100120
9. Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, Jaeschke R, Mebazaa A, Pinsky MR, Teboul JL, Vincent JL, Rhodes A. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014 Dec;40(12):1795-815. doi: 10.1007/s00134-014-3525-z. Epub 2014 Nov 13. PMID: 25392034; PMCID: PMC4239778
10. Menon V, Slater JN, White HD, Sleeper LA, Cocke T, Hochman JS. Acute myocardial infarction complicated by systemic hypoperfusion without hypotension: report of the SHOCK trial registry. Am J Med. 2000 Apr 1;108(5):374-80. doi: 10.1016/s0002-9343(00)00310-7. PMID: 10759093
11. Seferović et al. 2019. Heart failure in cardiomyopathies: a position paper from the Heart Failure Association of the European Society of Cardiology. European Journal of Heart Failure, 21(5), 553–576. https://doi.org/10.1002/ejhf.1461
12. Ponikowski et al. Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016 Jul 14;37(27):2129-2200. doi: 10.1093/eurheartj/ehw128. Epub 2016 May 20. Erratum in: Eur Heart J. 2016 Dec 30;: PMID: 27206819
13. van Diepen, S., Katz, J. N., Albert, N. M., Henry, T. D., Jacobs, A. K., Kapur, N. K., Kilic, A., Menon, V., Ohman, E. M., Sweitzer, N. K., Thiele, H., Washam, J. B., & Cohen, M. G. (2017). Contemporary Management of Cardiogenic Shock: A Scientific Statement from the American Heart Association. In Circulation (Vol. 136, Issue 16, pp. e232–e268). Lippincott Williams and Wilkins. https://doi.org/10.1161/CIR.0000000000000525
14. Wung SF. Bradyarrhythmias: Clinical Presentation, Diagnosis, and Management. Crit Care Nurs Clin North Am. 2016 Sep;28(3):297-308. doi: 10.1016/j.cnc.2016.04.003. Epub 2016 Jun 22. PMID: 27484658
15. Legrand M, Zarbock A. Ten tips to optimize vasopressors use in the critically ill patient with hypotension. Intensive Care Med. 2022 Jun;48(6):736-739. doi: 10.1007/s00134-022-06708-y. Epub 2022 May 3. PMID: 35504977
16. Furer A, Wessler J, Burkhoff D. Hemodynamics of Cardiogenic Shock. Interv Cardiol Clin. 2017 Jul;6(3):359-371. doi: 10.1016/j.iccl.2017.03.006. PMID: 28600090
De Backer D, Creteur J, Dubois MJ, Sakr Y, Vincent JL. Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am Heart J. 2004 Jan;147(1):91-9. doi: 10.1016/j.ahj.2003.07.006. PMID: 14691425
17. Bellumkonda L, Gul B, Masri SC. Evolving Concepts in Diagnosis and Management of Cardiogenic Shock. Am J Cardiol. 2018 Sep 15;122(6):1104-1110. doi: 10.1016/j.amjcard.2018.05.040. Epub 2018 Jun 22. PMID: 30072134
18. Kirschenbaum LA, Astiz ME, Rackow EC, Saha DC, Lin R. Microvascular response in patients with cardiogenic shock. Crit Care Med. 2000 May;28(5):1290-4. doi: 10.1097/00003246-200005000-00005. PMID: 10834667
19. Kohsaka S, Menon V, Lowe AM, Lange M, Dzavik V, Sleeper LA, Hochman JS; SHOCK Investigators. Systemic inflammatory response syndrome after acute myocardial infarction complicated by cardiogenic shock. Arch Intern Med. 2005 Jul 25;165(14):1643-50. doi: 10.1001/archinte.165.14.1643. PMID: 16043684
20. Hochman JS. Cardiogenic shock complicating acute myocardial infarction: expanding the paradigm. Circulation. 2003 Jun 24;107(24):2998-3002. doi: 10.1161/01.CIR.0000075927.67673.F2. PMID: 12821585
21. Parenica J, Jarkovsky J, Malaska J, Mebazaa A, Gottwaldova J, Helanova K, Litzman J, Dastych M, Tomandl J, Spinar J, Dostalova L, Lokaj P, Tomandlova M, Pavkova MG, Sevcik P, Legrand M; GREAT Network. Infectious Complications and Immune/Inflammatory Response in Cardiogenic Shock Patients: A Prospective Observational Study. Shock. 2017 Feb;47(2):165-174. doi: 10.1097/SHK.0000000000000756. PMID: 27749762; PMCID: PMC5266423
22. Bellumkonda L, Gul B, Masri SC. Evolving Concepts in Diagnosis and Management of Cardiogenic Shock. Am J Cardiol. 2018 Sep 15;122(6):1104-1110. doi: 10.1016/j.amjcard.2018.05.040. Epub 2018 Jun 22. PMID: 30072134
23. Harjola, V. et al. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). In European Journal of Heart Failure (Vol. 19, Issue 7, pp. 821–836). John Wiley and Sons Ltd. https://doi.org/10.1002/ejhf.872
24. Moore JP, Dyson A, Singer M, Fraser J. Microcirculatory dysfunction and resuscitation: why, when, and how. Br J Anaesth. 2015 Sep;115(3):366-75. doi: 10.1093/bja/aev163. PMID: 26269467.
25. Nagatomo Y, Tang WH. Intersections Between Microbiome and Heart Failure: Revisiting the Gut Hypothesis. J Card Fail. 2015 Dec;21(12):973-80. doi: 10.1016/j.cardfail.2015.09.017. Epub 2015 Oct 3. PMID: 26435097; PMCID: PMC4666782
26. Nguyen T, Do H, Pham T, Vu LT, Zuin M, Rigatelli G. Left ventricular dysfunction causing ischemia in patients with patent coronary arteries. Perfusion. 2018 Mar;33(2):115-122. doi: 10.1177/0267659117727826. Epub 2017 Aug 21. PMID: 28823216
27. Price LC, Wort SJ, Finney SJ, Marino PS, Brett SJ. Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review. Crit Care. 2010;14(5):R169. doi: 10.1186/cc9264. Epub 2010 Sep 21. PMID: 20858239; PMCID: PMC3219266.
28. Reyentovich A, Barghash MH, Hochman JS. Management of refractory cardiogenic shock. Nat Rev Cardiol. 2016 Aug;13(8):481-92. doi: 10.1038/nrcardio.2016.96. Epub 2016 Jun 30. PMID: 27356877
29. Wilcox SR. Nonischemic Causes of Cardiogenic Shock. Emerg Med Clin North Am. 2019 Aug;37(3):493-509. doi: 10.1016/j.emc.2019.03.007. Epub 2019 May 14. PMID: 31262417
30. Tehrani, B. N., Truesdell, A. G., Psotka, M. A., Rosner, C., Singh, R., Sinha, S. S., Damluji, A. A., & Batchelor, W. B. (2020). A Standardized and Comprehensive Approach to the Management of Cardiogenic Shock. In JACC: Heart Failure (Vol. 8, Issue 11, pp. 879–891). Elsevier Inc. https://doi.org/10.1016/j.jchf.2020.09.005
Arrigo M, Tolppanen H, Sadoune M, Feliot E, Teixeira A, Laribi S, Plaisance P, Nouira S, Yilmaz MB, Gayat E, Mebazaa A; GREAT Network. Effect of precipitating factors of acute heart failure on readmission and long-term mortality. ESC Heart Fail. 2016 Jun;3(2):115-121. doi: 10.1002/ehf2.12083. Epub 2016 Jan 21. PMID: 27812386; PMCID: PMC5066631.
31. Platz E, Jhund PS, Claggett BL, Pfeffer MA, Swedberg K, Granger CB, Yusuf S, Solomon SD, McMurray JJ. Prevalence and prognostic importance of precipitating factors leading to heart failure hospitalization: recurrent hospitalizations and mortality. Eur J Heart Fail. 2018 Feb;20(2):295-303. doi: 10.1002/ejhf.901. Epub 2017 Sep 4. PMID: 28872259; PMCID: PMC5826811
32. Galbois A, Bigé N, Pichereau C, Boëlle PY, Baudel JL, Bourcier S, Maury E, Guidet B, Ait-Oufella H. Exploration of skin perfusion in cirrhotic patients with septic shock. J Hepatol. 2015 Mar;62(3):549-55. doi: 10.1016/j.jhep.2014.10.012. Epub 2014 Oct 19. PMID: 25457199
33. Dumas G, Lavillegrand JR, Joffre J, Bigé N, de-Moura EB, Baudel JL, Chevret S, Guidet B, Maury E, Amorim F, Ait-Oufella H. Mottling score is a strong predictor of 14-day mortality in septic patients whatever vasopressor doses and other tissue perfusion parameters. Crit Care. 2019 Jun 10;23(1):211. doi: 10.1186/s13054-019-2496-4. PMID: 31182133; PMCID: PMC6558704
34. Hiemstra B, Eck RJ, Wiersema R, Kaufmann T, Koster G, Scheeren TWL, Snieder H, Perner A, Pettilä V, Wetterslev J, Keus F, van der Horst ICC; SICS Study Group. Clinical Examination for the Prediction of Mortality in the Critically Ill: The Simple Intensive Care Studies-I. Crit Care Med. 2019 Oct;47(10):1301-1309. doi: 10.1097/CCM.0000000000003897. PMID: 31356472; PMCID: PMC6750157.
35. Noitz M, Szasz J, Dünser MW. Regional perfusion monitoring in shock. Curr Opin Crit Care. 2020 Jun;26(3):281-288. doi: 10.1097/MCC.0000000000000716. PMID: 32348094
36. Tarvasmäki T, Haapio M, Mebazaa A, Sionis A, Silva-Cardoso J, Tolppanen H, Lindholm MG, Pulkki K, Parissis J, Harjola VP, Lassus J; CardShock Study Investigators. Acute kidney injury in cardiogenic shock: definitions, incidence, hemodynamic alterations, and mortality. Eur J Heart Fail. 2018 Mar;20(3):572-581. doi: 10.1002/ejhf.958. Epub 2017 Sep 27. PMID: 28960633
37. Jäntti T, Tarvasmäki T, Harjola VP, Parissis J, Pulkki K, Sionis A, Silva-Cardoso J, Køber L, Banaszewski M, Spinar J, Fuhrmann V, Tolonen J, Carubelli V, diSomma S, Mebazaa A, Lassus J; CardShock investigators. Frequency and Prognostic Significance of Abnormal Liver Function Tests in Patients With Cardiogenic Shock. Am J Cardiol. 2017 Oct 1;120(7):1090-1097. doi: 10.1016/j.amjcard.2017.06.049. Epub 2017 Jul 14. PMID: 28821350
38. Alvarez AM, Mukherjee D. Liver abnormalities in cardiac diseases and heart failure. Int J Angiol. 2011 Sep;20(3):135-42. doi: 10.1055/s-0031-1284434. PMID: 22942628; PMCID: PMC3331650.
39. Lattell J, Upadhyay GA. Bradyarrhythmias and Physiologic Pacing in the ICU. J Intensive Care Med. 2022 May;37(5):595-610. doi: 10.1177/0885066621992740. Epub 2021 Apr 5. PMID: 33813926
40. Kraut JA, Madias NE. Lactic acidosis. N Engl J Med. 2014 Dec 11;371(24):2309-19. doi: 10.1056/NEJMra1309483. PMID: 25494270
Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013 Oct;88(10):1127-40. doi: 10.1016/j.mayocp.2013.06.012. PMID: 24079682; PMCID: PMC3975915
41. Jozwiak M, Monnet X, Teboul JL. Less or more hemodynamic monitoring in critically ill patients. Curr Opin Crit Care. 2018 Aug;24(4):309-315. doi: 10.1097/MCC.0000000000000516. PMID: 29889132
42. Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation. 2008 Feb 5;117(5):686-97. doi: 10.1161/CIRCULATIONAHA.106.613596. PMID: 18250279
43. Naidu, S. et al. SCAI SHOCK Stage Classification Expert Consensus Update: A Review and Incorporation of Validation Studies. Journal of the Society for Cardiovascular Angiography & Interventions, 1(1), 100008. https://doi.org/10.1016/j.jscai.2021.100008
44. Sanfilippo F, Scolletta S, Morelli A, Vieillard-Baron A. Practical approach to diastolic dysfunction in light of the new guidelines and clinical applications in the operating room and in the intensive care. Ann Intensive Care. 2018 Oct 29;8(1):100. doi: 10.1186/s13613-018-0447-x. Erratum in: Ann Intensive Care. 2018 Nov 6;8(1):106. PMID: 30374644; PMCID: PMC6206316
45. Saxena A, Garan AR, Kapur NK, O’Neill WW, Lindenfeld J, Pinney SP, Uriel N, Burkhoff D, Kern M. Value of Hemodynamic Monitoring in Patients With Cardiogenic Shock Undergoing Mechanical Circulatory Support. Circulation. 2020 Apr 7;141(14):1184-1197. doi: 10.1161/CIRCULATIONAHA.119.043080. Epub 2020 Apr 6. PMID: 32250695
46. Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med. 2005 Dec 29;353(26):2788-96. doi: 10.1056/NEJMcp052699. PMID: 16382065
Huppert LA, Matthay MA, Ware LB. Pathogenesis of Acute Respiratory Distress Syndrome. Semin Respir Crit Care Med. 2019 Feb;40(1):31-39. doi: 10.1055/s-0039-1683996. Epub 2019 May 6. PMID: 31060086; PMCID: PMC7060969.
47. Volpicelli G, et al. International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012 Apr;38(4):577-91. doi: 10.1007/s00134-012-2513-4. Epub 2012 Mar 6. PMID: 22392031
48. Attias D, Mansencal N, Auvert B, Vieillard-Baron A, Delos A, Lacombe P, N’Guetta R, Jardin F, Dubourg O. Prevalence, characteristics, and outcomes of patients presenting with cardiogenic unilateral pulmonary edema. Circulation. 2010 Sep 14;122(11):1109-15. doi: 10.1161/CIRCULATIONAHA.109.934950. Epub 2010 Aug 30. PMID: 20805429
49. Jentzer JC, Burstein B, Van Diepen S, Murphy J, Holmes DR Jr, Bell MR, Barsness GW, Henry TD, Menon V, Rihal CS, Naidu SS, Baran DA. Defining Shock and Preshock for Mortality Risk Stratification in Cardiac Intensive Care Unit Patients. Circ Heart Fail. 2021 Jan;14(1):e007678. doi: 10.1161/CIRCHEARTFAILURE.120.007678. Epub 2021 Jan 19. PMID: 33464952
50. Naidu SS, Baran DA, Jentzer JC et al. SCAI SHOCK Stage Classification Expert Consensus Update: A Review and Incorporation of Validation Studies: This statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association (AHA), European Society of Cardiology (ESC) Association for Acute Cardiovascular Care (ACVC), International Society for Heart and Lung Transplantation (ISHLT), Society of Critical Care Medicine (SCCM), and Society of Thoracic Surgeons (STS) in December 2021. J Am Coll Cardiol. 2022 Mar 8;79(9):933-946. doi: 10.1016/j.jacc.2022.01.018. Epub 2022 Jan 31. PMID: 35115207
51. Jentzer JC, van Diepen S, Barsness GW, Henry TD, Menon V, Rihal CS, Naidu SS, Baran DA. Cardiogenic Shock Classification to Predict Mortality in the Cardiac Intensive Care Unit. J Am Coll Cardiol. 2019 Oct 29;74(17):2117-2128. doi: 10.1016/j.jacc.2019.07.077. Epub 2019 Sep 20. PMID: 31548097.
52. Baran DA, Long A, Badiye AP, Stelling K. Prospective validation of the SCAI shock classification: Single center analysis. Catheter Cardiovasc Interv. 2020 Dec;96(7):1339-1347. doi: 10.1002/ccd.29319. Epub 2020 Oct 7. PMID: 33026155; PMCID: PMC7821022
53. Hanson ID, Tagami T, Mando R, Kara Balla A, Dixon SR, Timmis S, Almany S, Naidu SS, Baran D, Lemor A, Gorgis S, O’Neill W, Basir MB; National Cardiogenic Shock Investigators. SCAI shock classification in acute myocardial infarction: Insights from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv. 2020 Nov;96(6):1137-1142. doi: 10.1002/ccd.29139. Epub 2020 Jul 16. PMID: 32672388
54. Baran DA, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019 Jul 1;94(1):29-37. doi: 10.1002/ccd.28329. Epub 2019 May 19. PMID: 31104355.
55. Jacobs AK, Leopold JA, Bates E, Mendes LA, Sleeper LA, White H, Davidoff R, Boland J, Modur S, Forman R, Hochman JS. Cardiogenic shock caused by right ventricular infarction: a report from the SHOCK registry. J Am Coll Cardiol. 2003 Apr 16;41(8):1273-9. doi: 10.1016/s0735-1097(03)00120-7. PMID: 12706920.
56. Saxena A, Garan AR, Kapur NK, O’Neill WW, Lindenfeld J, Pinney SP, Uriel N, Burkhoff D, Kern M. Value of Hemodynamic Monitoring in Patients With Cardiogenic Shock Undergoing Mechanical Circulatory Support. Circulation. 2020 Apr 7;141(14):1184-1197. doi: 10.1161/CIRCULATIONAHA.119.043080. Epub 2020 Apr 6. PMID: 32250695
57. Lim N, Dubois MJ, De Backer D, Vincent JL. Do all nonsurvivors of cardiogenic shock die with a low cardiac index? Chest. 2003 Nov;124(5):1885-91. doi: 10.1378/chest.124.5.1885. PMID: 14605064
58. Kohsaka S, Menon V, Lowe AM, Lange M, Dzavik V, Sleeper LA, Hochman JS; SHOCK Investigators. Systemic inflammatory response syndrome after acute myocardial infarction complicated by cardiogenic shock. Arch Intern Med. 2005 Jul 25;165(14):1643-50. doi: 10.1001/archinte.165.14.1643. PMID: 16043684
59. Menon V, White H, LeJemtel T, Webb JG, Sleeper LA, Hochman JS. The clinical profile of patients with suspected cardiogenic shock due to predominant left ventricular failure: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries in cardiogenic shock? J Am Coll Cardiol. 2000 Sep;36(3 Suppl A):1071-6. doi: 10.1016/s0735-1097(00)00874-3. PMID: 10985707
60. Jentzer JC, Bhat AG, Patlolla SH, Sinha SS, Miller PE, Lawler PR, van Diepen S, Khanna AK, Zhao DX, Vallabhajosyula S. Concomitant Sepsis Diagnoses in Acute Myocardial Infarction-Cardiogenic Shock: 15-Year National Temporal Trends, Management, and Outcomes. Crit Care Explor. 2022 Feb 4;4(2):e0637. doi: 10.1097/CCE.0000000000000637. PMID: 35141527; PMCID: PMC8820909.
61. Jentzer JC, Ahmed AM, Vallabhajosyula S, Burstein B, Tabi M, Barsness GW, Murphy JG, Best PJ, Bell MR. Shock in the cardiac intensive care unit: Changes in epidemiology and prognosis over time. Am Heart J. 2021 Feb;232:94-104. doi: 10.1016/j.ahj.2020.10.054. Epub 2020 Oct 24. PMID: 33257304
62. van Diepen S, Alemayehu WG, Zheng Y, Theroux P, Newby LK, Mahaffey KW, Granger CB, Armstrong PW. Temporal changes in biomarkers and their relationships to reperfusion and to clinical outcomes among patients with ST segment elevation myocardial infarction. J Thromb Thrombolysis. 2016 Oct;42(3):376-85. doi: 10.1007/s11239-016-1390-z. PMID: 27324144
63. Levy B, Fritz C, Tahon E, Jacquot A, Auchet T, Kimmoun A. Vasoplegia treatments: the past, the present, and the future. Crit Care. 2018 Feb 27;22(1):52. doi: 10.1186/s13054-018-1967-3. PMID: 29486781; PMCID: PMC6389278
64. Lim HS. Cardiogenic Shock: Failure of Oxygen Delivery and Oxygen Utilization. Clin Cardiol. 2016 Aug;39(8):477-83. doi: 10.1002/clc.22564. Epub 2016 Aug 10. PMID: 27509355; PMCID: PMC6490770
65. Squara P, Hollenberg S, Payen D. Reconsidering Vasopressors for Cardiogenic Shock: Everything Should Be Made as Simple as Possible, but Not Simpler. Chest. 2019 Aug;156(2):392-401. doi: 10.1016/j.chest.2019.03.020. Epub 2019 Mar 29. PMID: 30935893
66. Menon V, White H, LeJemtel T, Webb JG, Sleeper LA, Hochman JS. The clinical profile of patients with suspected cardiogenic shock due to predominant left ventricular failure: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries in cardiogenic shock? J Am Coll Cardiol. 2000 Sep;36(3 Suppl A):1071-6. doi: 10.1016/s0735-1097(00)00874-3. PMID: 10985707
67. Stevenson LW, Pagani FD, Young JB, Jessup M, Miller L, Kormos RL, Naftel DC, Ulisney K, Desvigne-Nickens P, Kirklin JK. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant. 2009 Jun;28(6):535-41. doi: 10.1016/j.healun.2009.02.015. PMID: 19481012
68. Stevenson LW, Pagani FD, Young JB, Jessup M, Miller L, Kormos RL, Naftel DC, Ulisney K, Desvigne-Nickens P, Kirklin JK. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant. 2009 Jun;28(6):535-41. doi: 10.1016/j.healun.2009.02.015. PMID: 19481012
69. Jacobs AK et al. Cardiogenic shock caused by right ventricular infarction: a report from the SHOCK registry. J Am Coll Cardiol. 2003 Apr 16;41(8):1273-9. doi: 10.1016/s0735-1097(03)00120-7. PMID: 12706920
70. Jain P et al. Right Ventricular Dysfunction Is Common and Identifies Patients at Risk of Dying in Cardiogenic Shock. J Card Fail. 2021 Oct;27(10):1061-1072. doi: 10.1016/j.cardfail.2021.07.013. PMID: 34625126
71. Burstein B, van Diepen S, Wiley BM, Anavekar NS, Jentzer JC. Biventricular Function and Shock Severity Predict Mortality in Cardiac ICU Patients. Chest. 2022 Mar;161(3):697-709. doi: 10.1016/j.chest.2021.09.032. Epub 2021 Oct 2. PMID: 34610345.
72. Korabathina R, Heffernan KS, Paruchuri V, Patel AR, Mudd JO, Prutkin JM, Orr NM, Weintraub A, Kimmelstiel CD, Kapur NK. The pulmonary artery pulsatility index identifies severe right ventricular dysfunction in acute inferior myocardial infarction. Catheter Cardiovasc Interv. 2012 Oct 1;80(4):593-600. doi: 10.1002/ccd.23309. Epub 2012 Jan 10. PMID: 21954053
73. Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, Jaeschke R, Mebazaa A, Pinsky MR, Teboul JL, Vincent JL, Rhodes A. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014 Dec;40(12):1795-815. doi: 10.1007/s00134-014-3525-z. Epub 2014 Nov 13. PMID: 25392034; PMCID: PMC4239778
74. Pinsky MR. Functional hemodynamic monitoring. Crit Care Clin. 2015 Jan;31(1):89-111. doi: 10.1016/j.ccc.2014.08.005. PMID: 25435480; PMCID: PMC4250574.
75. Miller A et al. FUSIC HD. Comprehensive hemodynamic assessment with ultrasound. J Intensive Care Soc. 2022 Aug;23(3):325-333. doi: 10.1177/17511437211010032. Epub 2021 Apr 23. PMID: 36033241; PMCID: PMC9411780
76. Bartlett RH. Alice in intensiveland. Being an essay on nonsense and common sense in the ICU, after the manner of Lewis Carroll. Chest. 1995 Oct;108(4):1129-39. doi: 10.1378/chest.108.4.1129. PMID: 7555127
77. Vahdatpour C, Collins D, Goldberg S. Cardiogenic Shock. J Am Heart Assoc. 2019 Apr 16;8(8):e011991. doi: 10.1161/JAHA.119.011991. PMID: 30947630; PMCID: PMC6507212
78. Geller, B et al. 2022. Escalating and De-escalating Temporary Mechanical Circulatory Support in Cardiogenic Shock: A Scientific Statement From the American Heart Association. In Circulation (Vol. 146, Issue 6, pp. E50–E68). Lippincott Williams and Wilkins. https://doi.org/10.1161/CIR.0000000000001076
79. Squara P, Hollenberg S, Payen D. Reconsidering Vasopressors for Cardiogenic Shock: Everything Should Be Made as Simple as Possible, but Not Simpler. Chest. 2019 Aug;156(2):392-401. doi: 10.1016/j.chest.2019.03.020. Epub 2019 Mar 29. PMID: 30935893
80. Esposito ML, Kapur NK. Acute mechanical circulatory support for cardiogenic shock: the “door to support” time. F1000Res. 2017 May 22;6:737. doi: 10.12688/f1000research.11150.1. PMID: 28580136; PMCID: PMC5443341
81. Lescroart M, Pequignot B, Janah D, Levy B. The medical treatment of cardiogenic shock. J Intensive Med. 2023 Jan 19;3(2):114-123. doi: 10.1016/j.jointm.2022.12.001. PMID: 37188116; PMCID: PMC10175741
82. Lescroart M, Pequignot B, Janah D, Levy B. The medical treatment of cardiogenic shock. J Intensive Med. 2023 Jan 19;3(2):114-123. doi: 10.1016/j.jointm.2022.12.001. PMID: 37188116; PMCID: PMC10175741
83. Wilcox SR. Nonischemic Causes of Cardiogenic Shock. Emerg Med Clin North Am. 2019 Aug;37(3):493-509. doi: 10.1016/j.emc.2019.03.007. Epub 2019 May 14. PMID: 31262417.
84. Squara P, Hollenberg S, Payen D. Reconsidering Vasopressors for Cardiogenic Shock: Everything Should Be Made as Simple as Possible, but Not Simpler. Chest. 2019 Aug;156(2):392-401. doi: 10.1016/j.chest.2019.03.020. Epub 2019 Mar 29. PMID: 30935893
85. Levy B et al. Experts’ recommendations for the management of adult patients with cardiogenic shock. Ann Intensive Care. 2015 Dec;5(1):52. doi: 10.1186/s13613-015-0052-1. Epub 2015 Jul 1. Erratum in: Ann Intensive Care. 2015 Dec;5(1):26. Benjelid, Karim [corrected to Karim, Bendjelid]; Splaulding, Christian [corrected to Spaulding, Christian]. PMID: 26152849; PMCID: PMC4495097.
86. Demiselle J, Fage N, Radermacher P, Asfar P. Vasopressin and its analogues in shock states: a review. Ann Intensive Care. 2020 Jan 22;10(1):9. doi: 10.1186/s13613-020-0628-2. PMID: 31970567; PMCID: PMC6975768
87. Gold JA, Cullinane S, Chen J, Oz MC, Oliver JA, Landry DW. Vasopressin as an alternative to norepinephrine in the treatment of milrinone-induced hypotension. Crit Care Med. 2000 Jan;28(1):249-52. doi: 10.1097/00003246-200001000-00043. PMID: 10667533
88. De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, Brasseur A, Defrance P, Gottignies P, Vincent JL; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010 Mar 4;362(9):779-89. doi: 10.1056/NEJMoa0907118. PMID: 20200382.
89. Mebazaa A et al. ESC Heart Failure Long-Term Registry Investigators. Long-term safety of intravenous cardiovascular agents in acute heart failure: results from the European Society of Cardiology Heart Failure Long-Term Registry. Eur J Heart Fail. 2018 Feb;20(2):332-341. doi: 10.1002/ejhf.991. Epub 2017 Oct 8. PMID: 28990358
90. Dooley DJ, Lam PH, Ahmed A, Aronow WS. The Role of Positive Inotropic Drugs in the Treatment of Older Adults with Heart Failure and Reduced Ejection Fraction. Heart Fail Clin. 2017 Jul;13(3):527-534. doi: 10.1016/j.hfc.2017.02.008. Epub 2017 May 2. PMID: 28602370
91. Mathew R, Di Santo P, Jung RG, Marbach JA, Hutson J, Simard T, Ramirez FD, Harnett DT, Merdad A, Almufleh A, Weng W, Abdel-Razek O, Fernando SM, Kyeremanteng K, Bernick J, Wells GA, Chan V, Froeschl M, Labinaz M, Le May MR, Russo JJ, Hibbert B. Milrinone as Compared with Dobutamine in the Treatment of Cardiogenic Shock. N Engl J Med. 2021 Aug 5;385(6):516-525. doi: 10.1056/NEJMoa2026845. PMID: 34347952
92. Mebazaa A, Combes A, van Diepen S, Hollinger A, Katz JN, Landoni G, Hajjar LA, Lassus J, Lebreton G, Montalescot G, Park JJ, Price S, Sionis A, Yannopolos D, Harjola VP, Levy B, Thiele H. Management of cardiogenic shock complicating myocardial infarction. Intensive Care Med. 2018 Jun;44(6):760-773. doi: 10.1007/s00134-018-5214-9. Epub 2018 May 16. PMID: 29767322.
93. Jones TL, Nakamura K, McCabe JM. Cardiogenic shock: evolving definitions and future directions in management. Open Heart. 2019 May 8;6(1):e000960. doi: 10.1136/openhrt-2018-000960. PMID: 31168376; PMCID: PMC6519403
94. Combes A, Price S, Slutsky AS, Brodie D. Temporary circulatory support for cardiogenic shock. Lancet. 2020 Jul 18;396(10245):199-212. doi: 10.1016/S0140-6736(20)31047-3. PMID: 32682486.
95. Fryer ML, Balsam LB. Mechanical Circulatory Support for Cardiogenic Shock in the Critically Ill. Chest. 2019 Nov;156(5):1008-1021. doi: 10.1016/j.chest.2019.07.009. Epub 2019 Jul 30. PMID: 31374209
96. Thiele H, Sick P, Boudriot E, Diederich KW, Hambrecht R, Niebauer J, Schuler G. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005 Jul;26(13):1276-83. doi: 10.1093/eurheartj/ehi161. Epub 2005 Feb 25. PMID: 15734771
97. Burkhoff D, Cohen H, Brunckhorst C, O’Neill WW; TandemHeart Investigators Group. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006 Sep;152(3):469.e1-8. doi: 10.1016/j.ahj.2006.05.031. PMID: 16923414.
98. Keebler ME, Haddad EV, Choi CW, McGrane S, Zalawadiya S, Schlendorf KH, Brinkley DM, Danter MR, Wigger M, Menachem JN, Shah A, Lindenfeld J. Venoarterial Extracorporeal Membrane Oxygenation in Cardiogenic Shock. JACC Heart Fail. 2018 Jun;6(6):503-516. doi: 10.1016/j.jchf.2017.11.017. Epub 2018 Apr 11. PMID: 29655828
99. Bangalore S, Gupta N, Guo Y, Lala A, Balsam L, Roswell RO, Reyentovich A, Hochman JS. Outcomes with invasive vs conservative management of cardiogenic shock complicating acute myocardial infarction. Am J Med. 2015 Jun;128(6):601-8. doi: 10.1016/j.amjmed.2014.12.009. Epub 2014 Dec 30. PMID: 25554376
100. Hochman JS, Sleeper LA, Godfrey E, McKinlay SM, Sanborn T, Col J, LeJemtel T. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK: an international randomized trial of emergency PTCA/CABG-trial design. The SHOCK Trial Study Group. Am Heart J. 1999 Feb;137(2):313-21. doi: 10.1053/hj.1999.v137.95352. PMID: 9924166
101. Thiele H et al. CULPRIT-SHOCK Investigators. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N Engl J Med. 2017 Dec 21;377(25):2419-2432. doi: 10.1056/NEJMoa1710261. Epub 2017 Oct 30. PMID: 29083953.


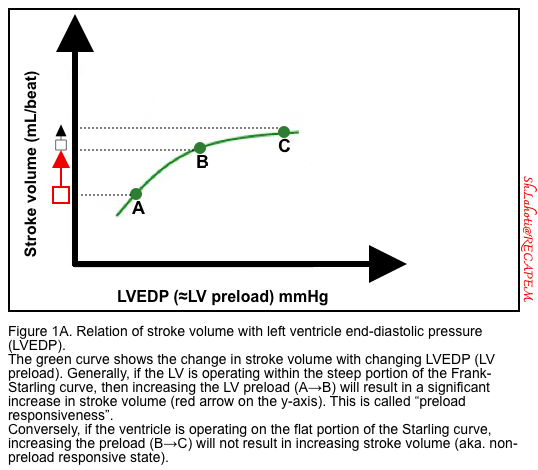
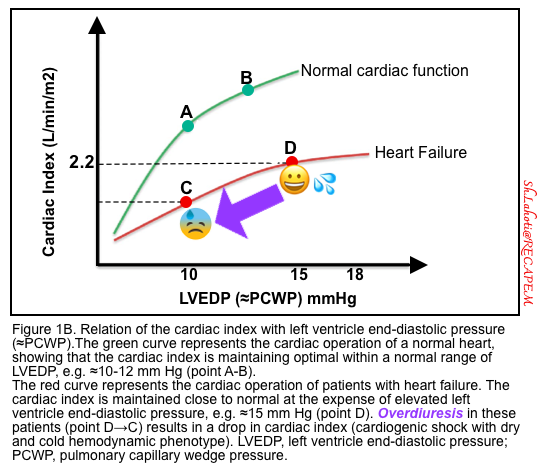
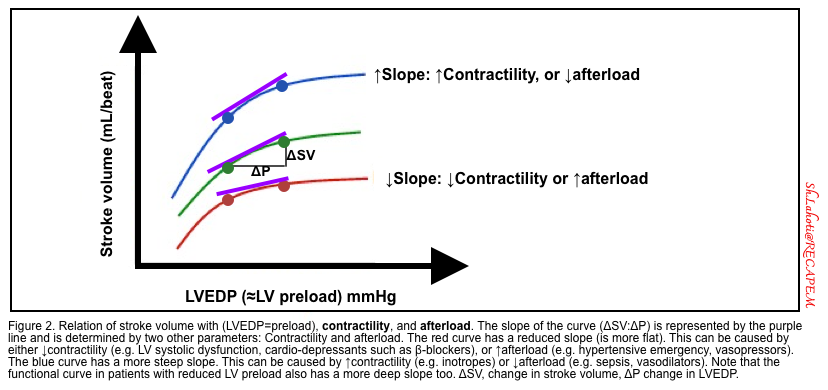
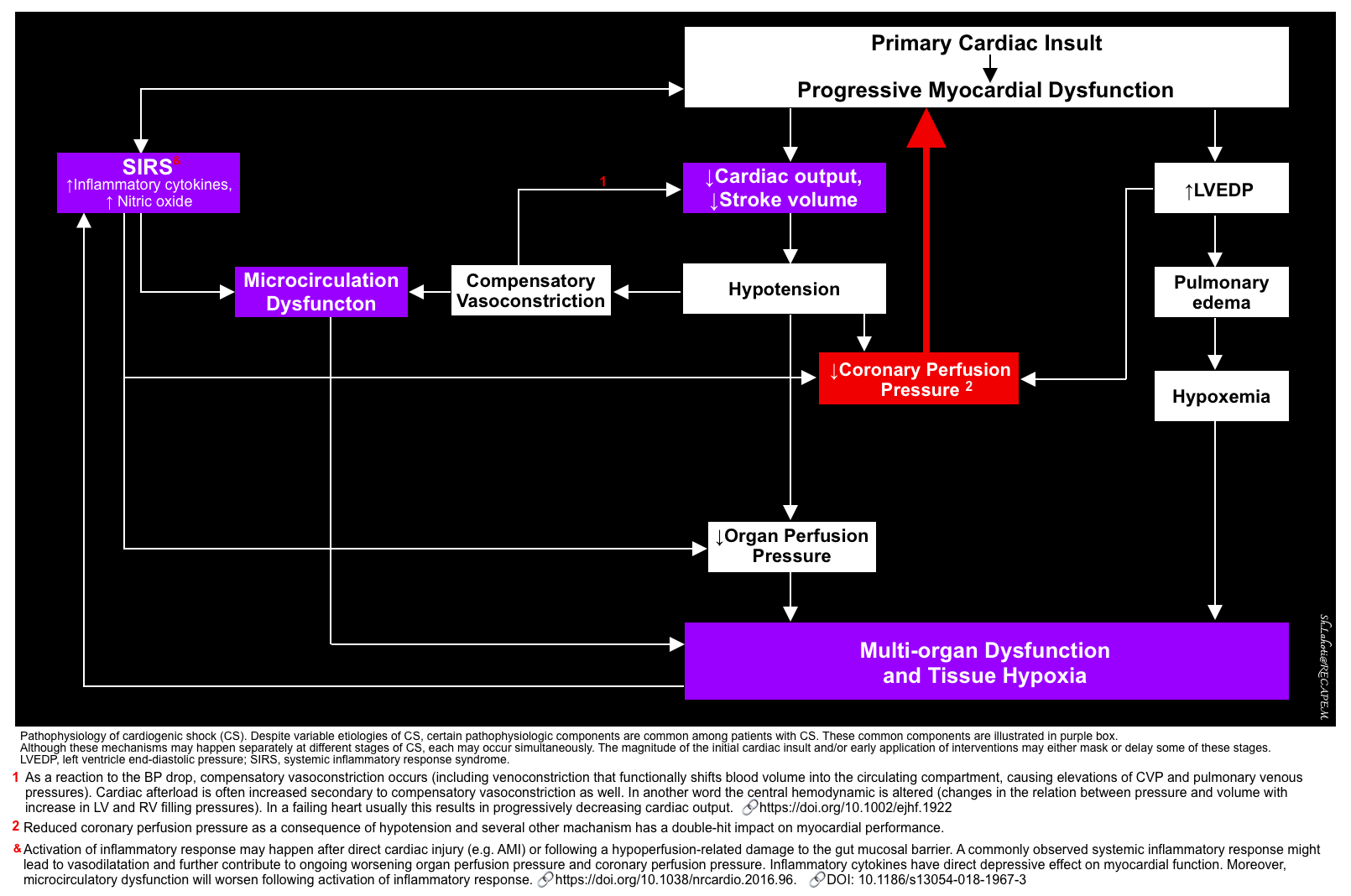
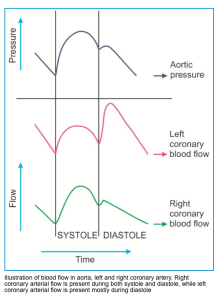
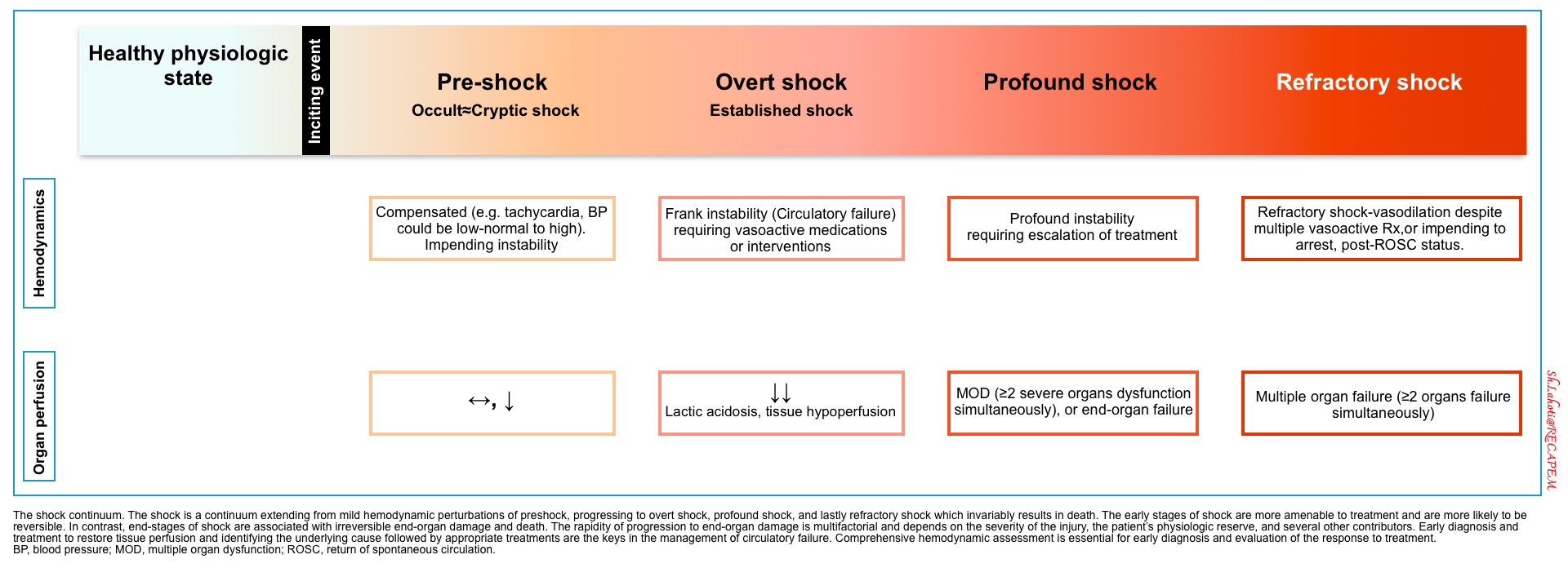
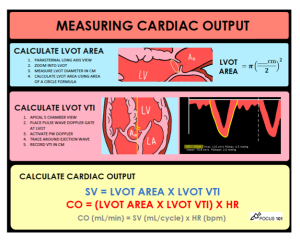
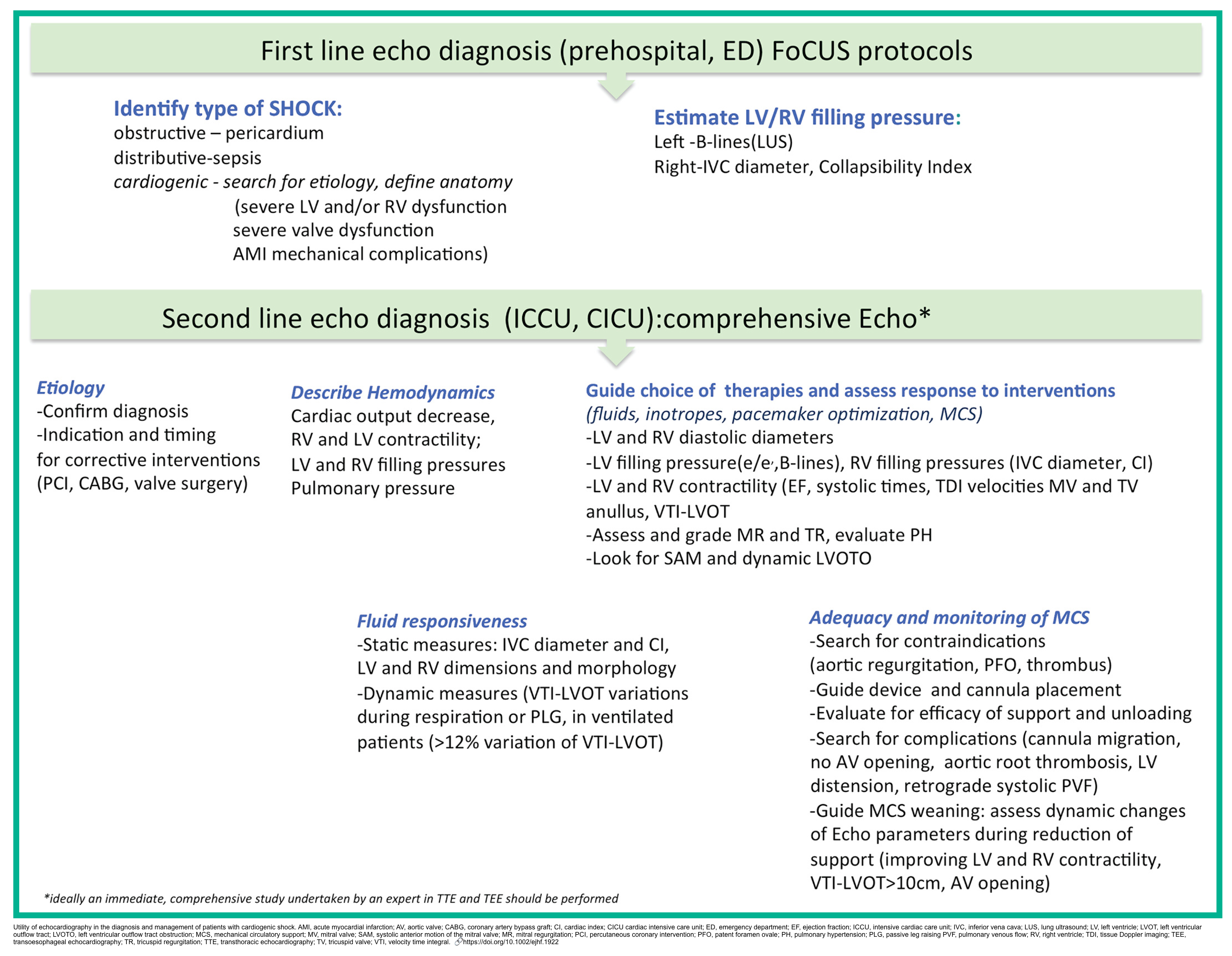
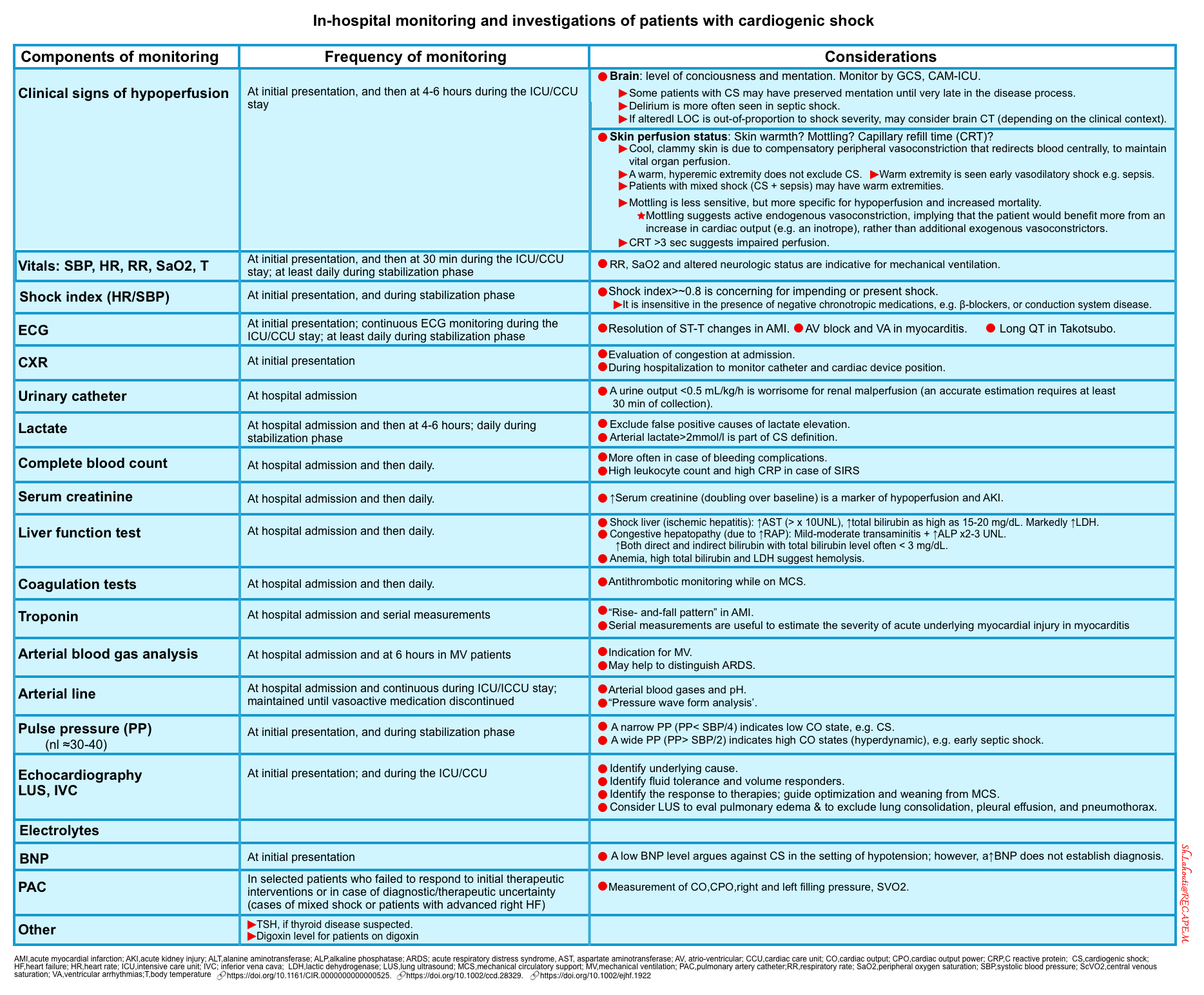
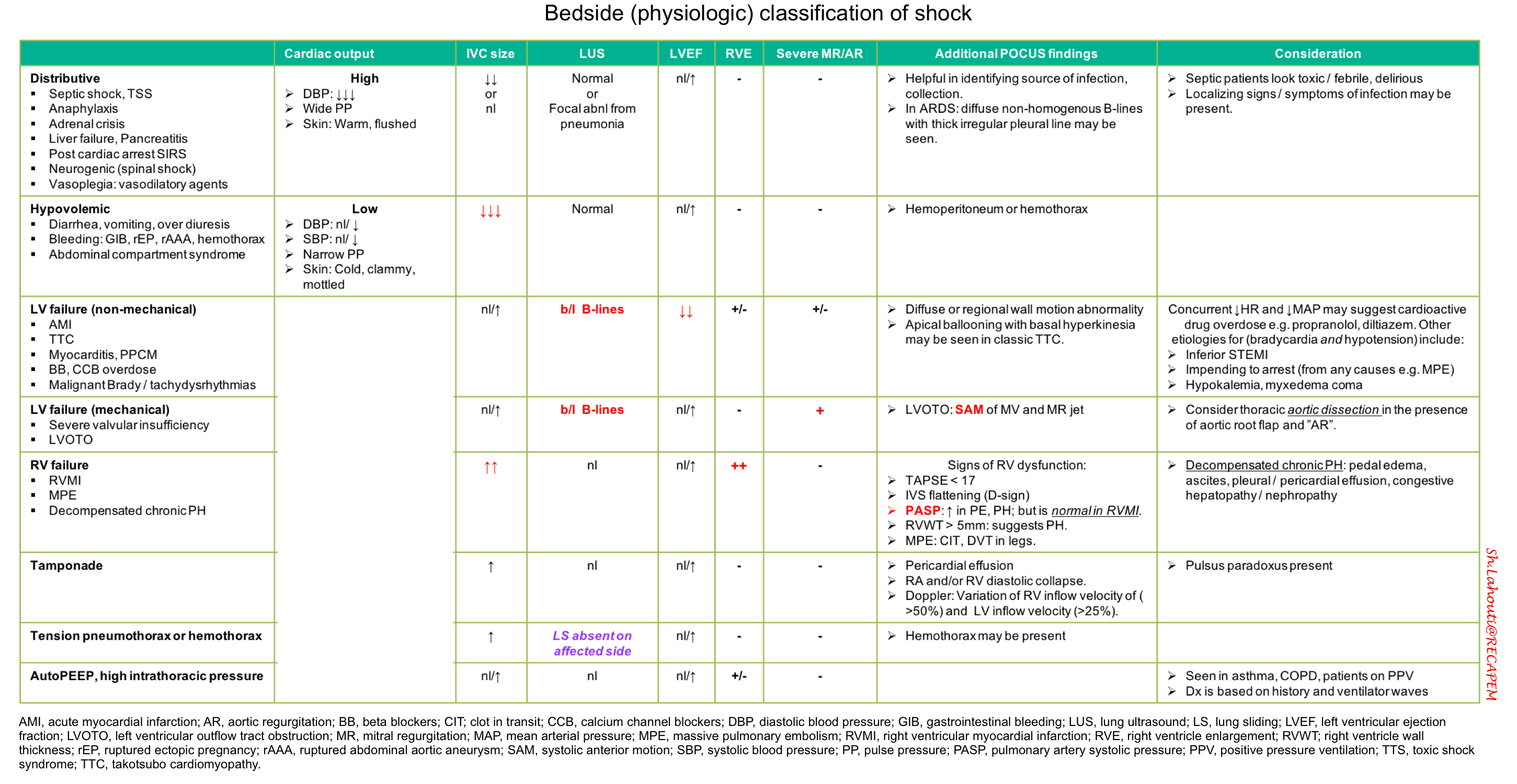
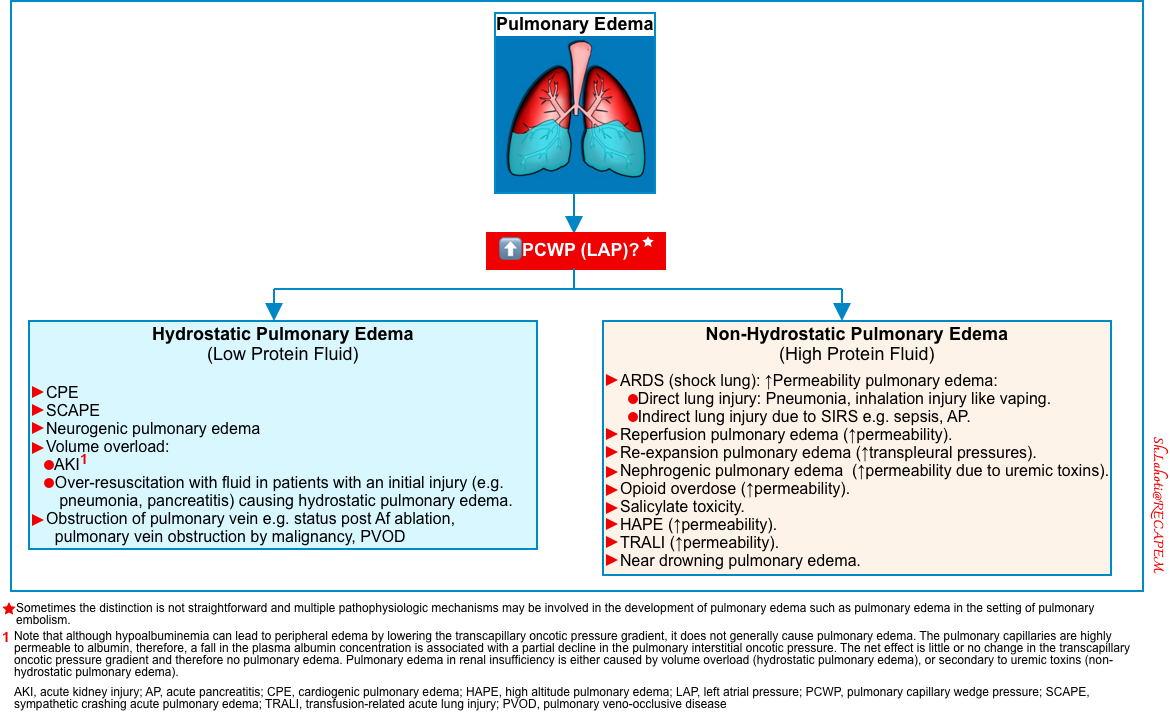
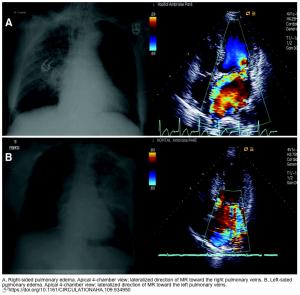
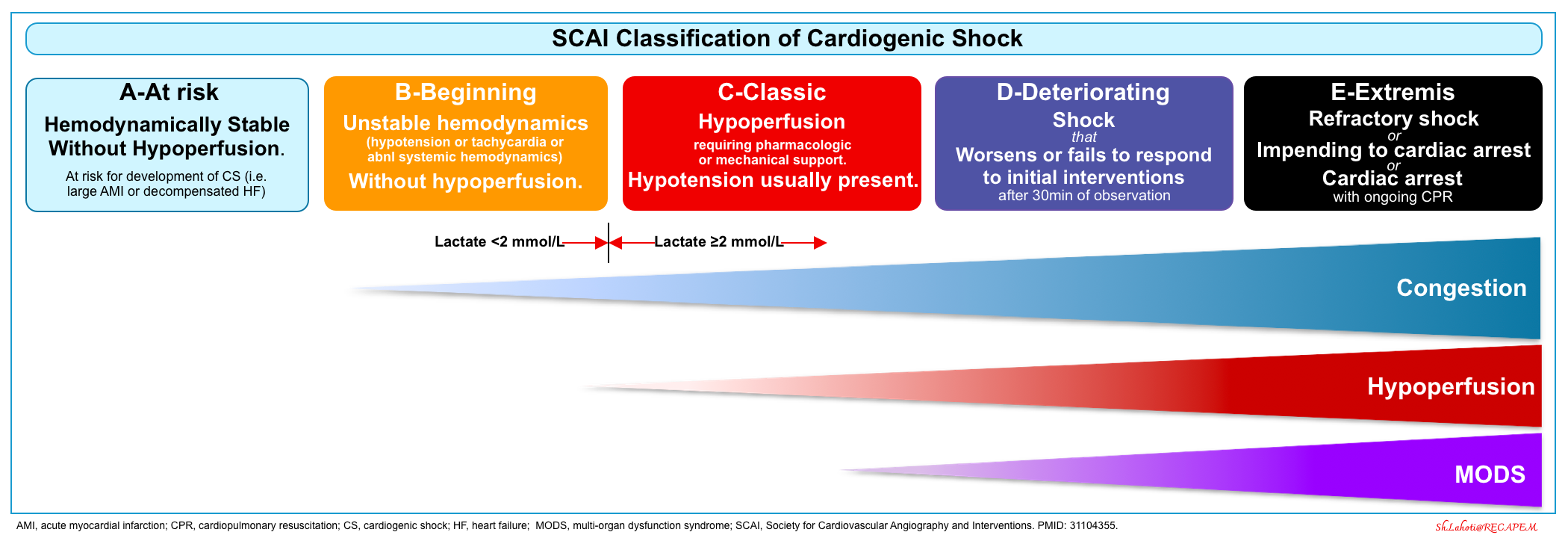
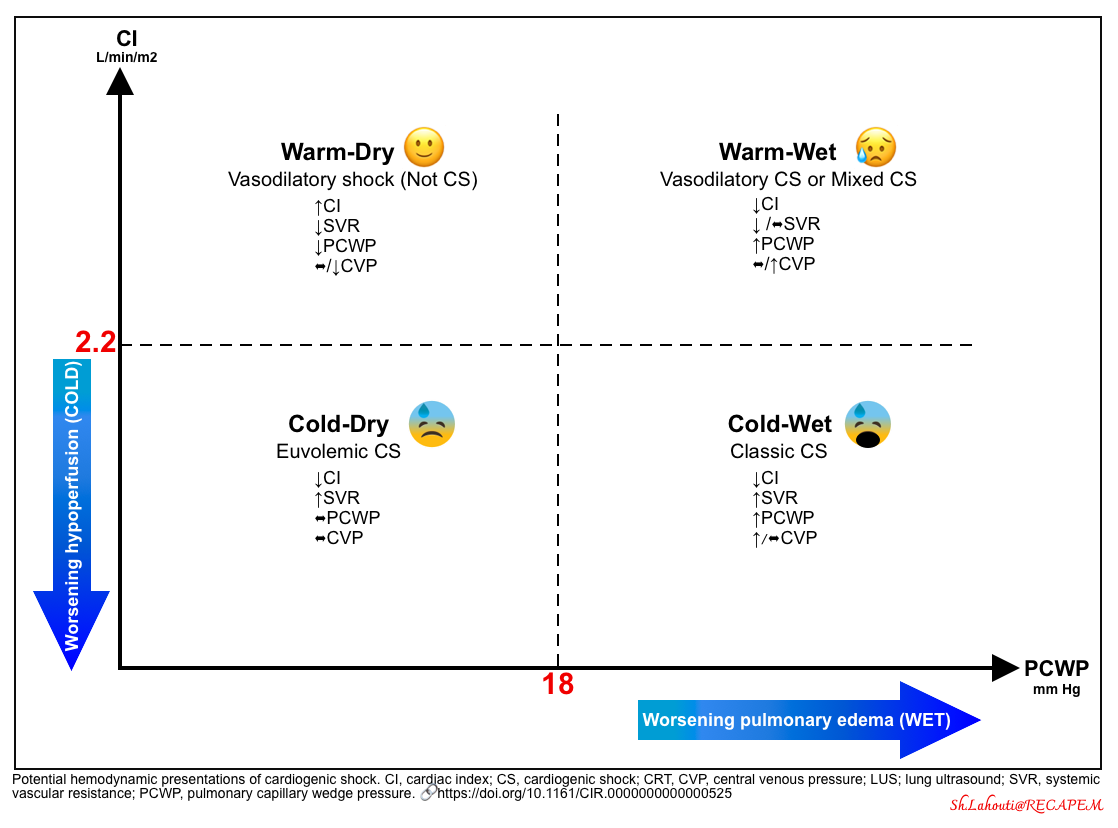
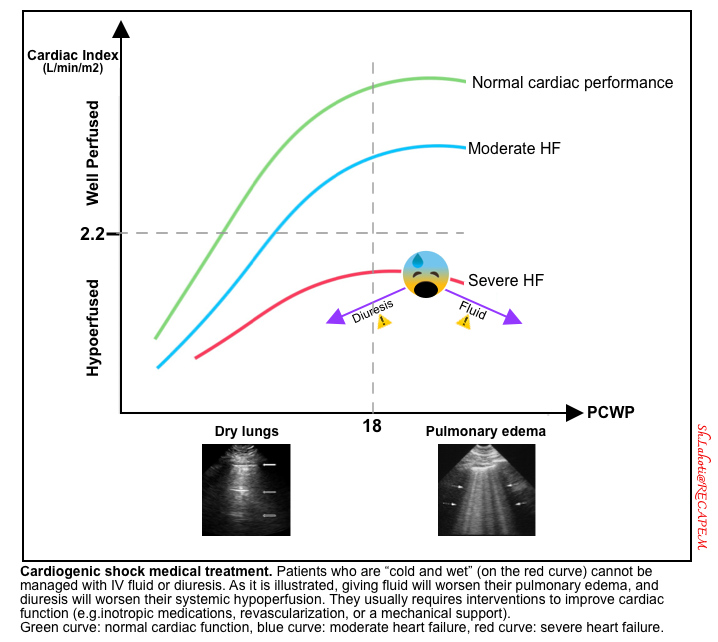
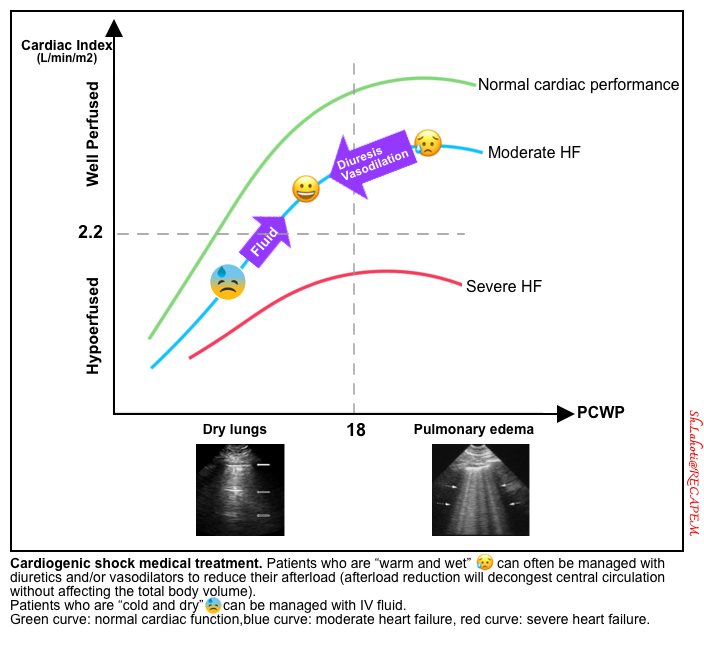
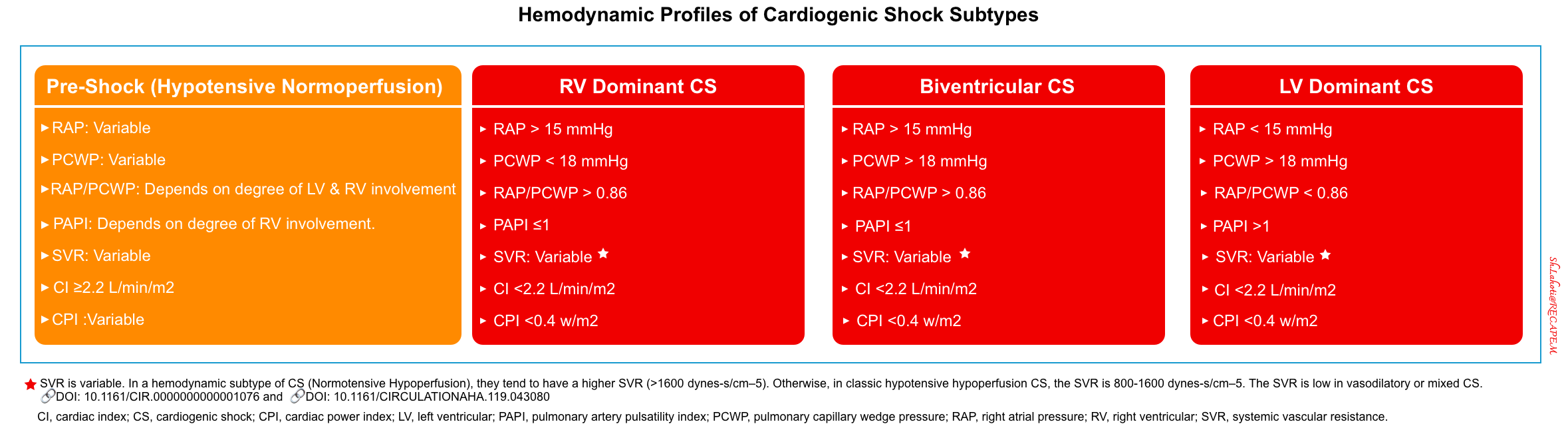
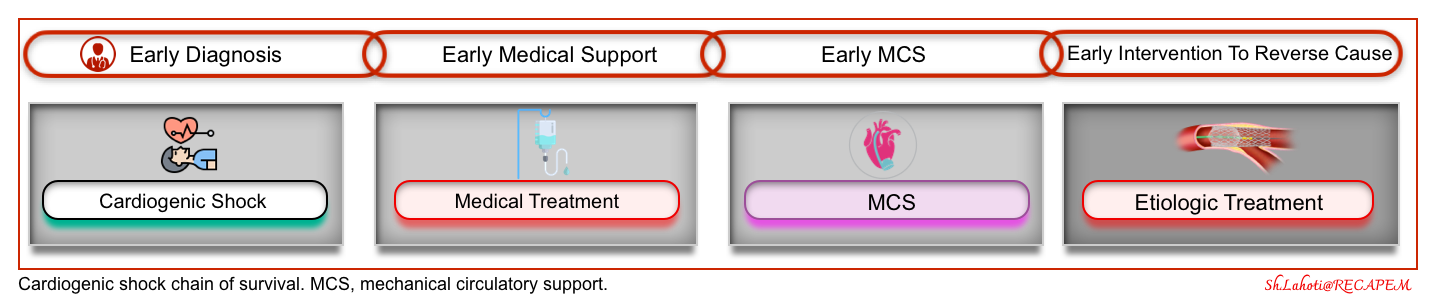
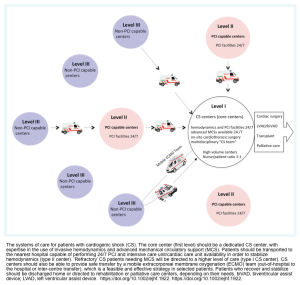
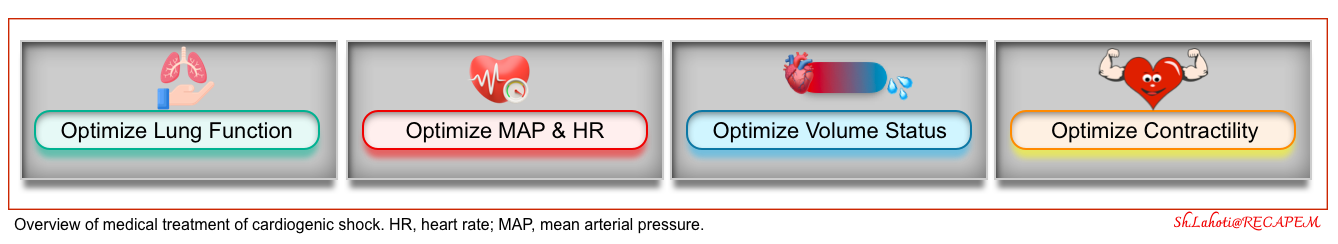
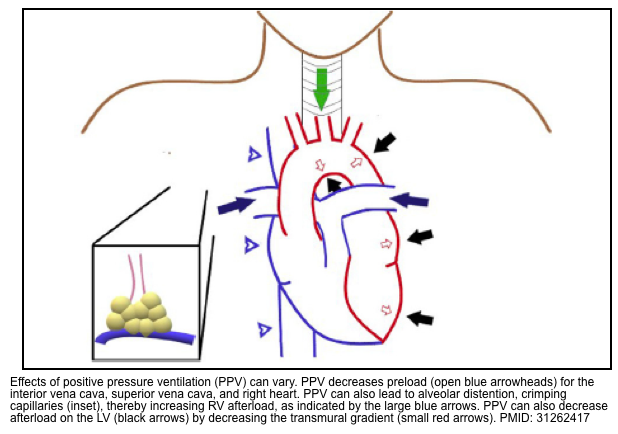
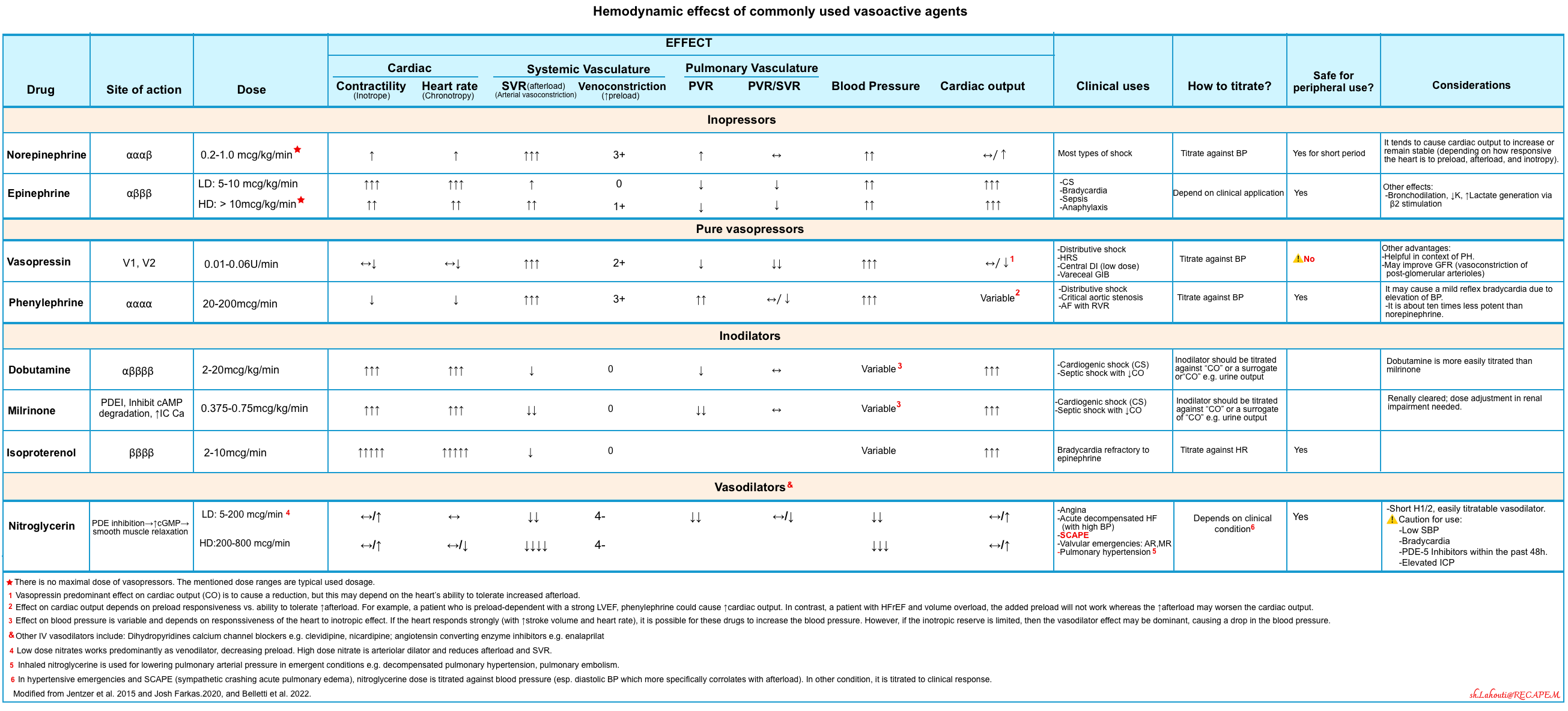
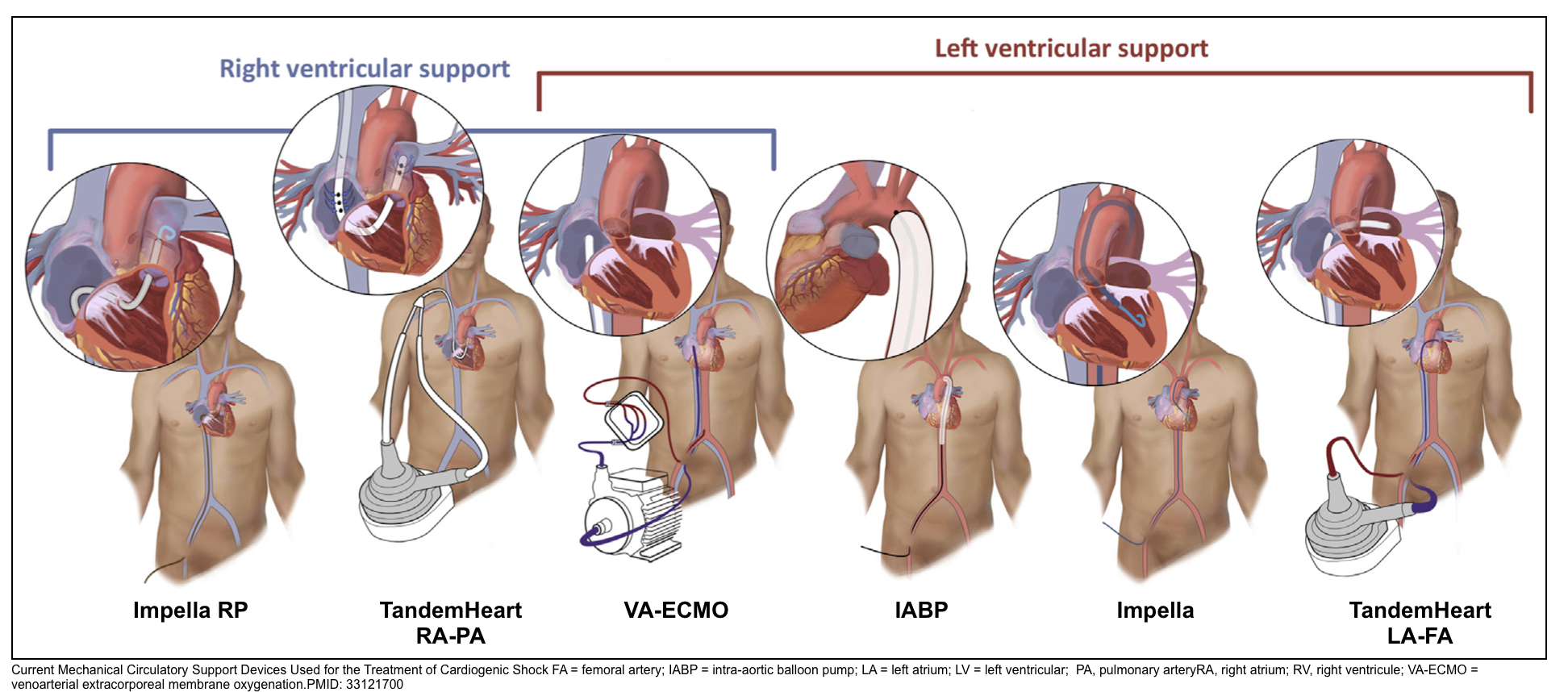
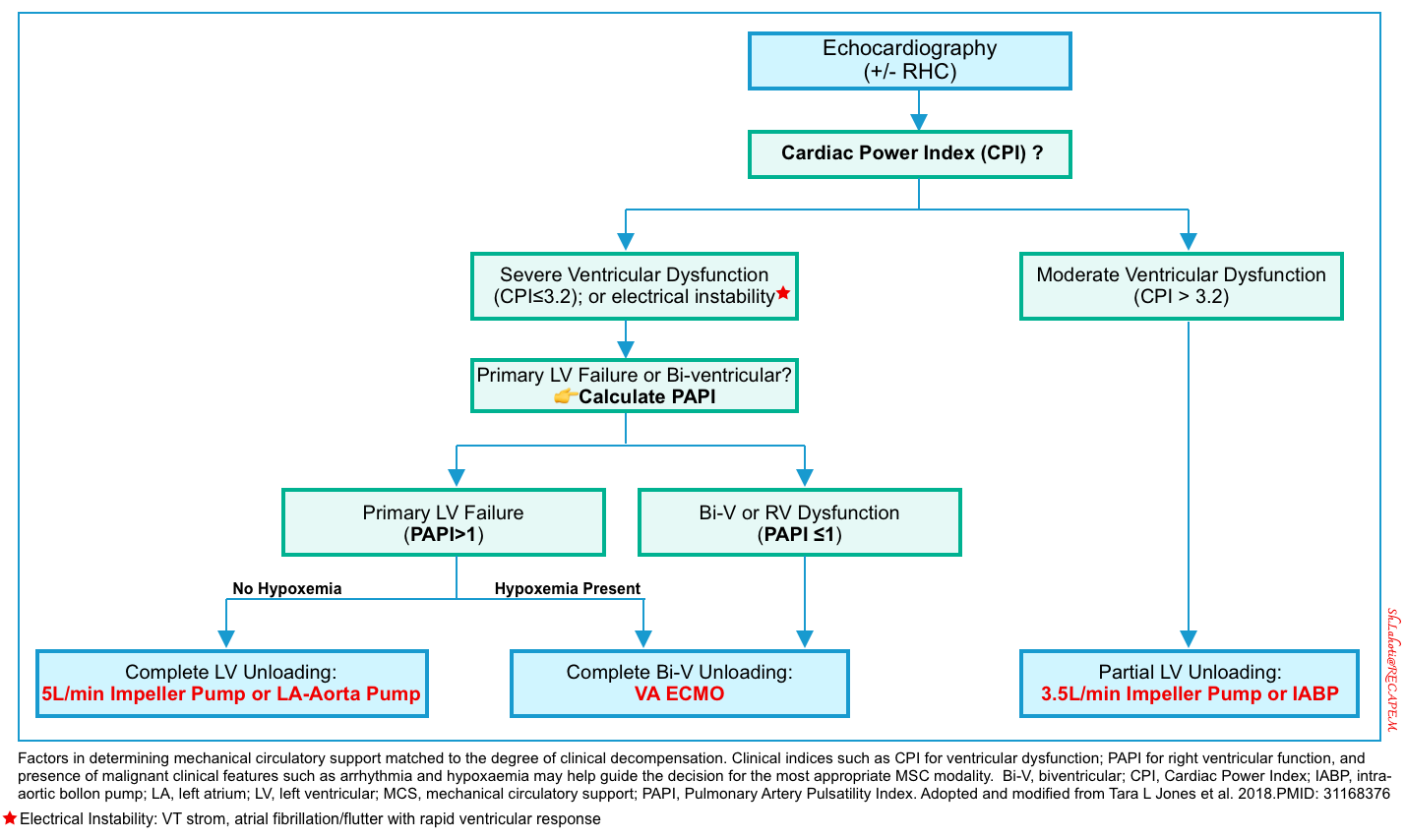
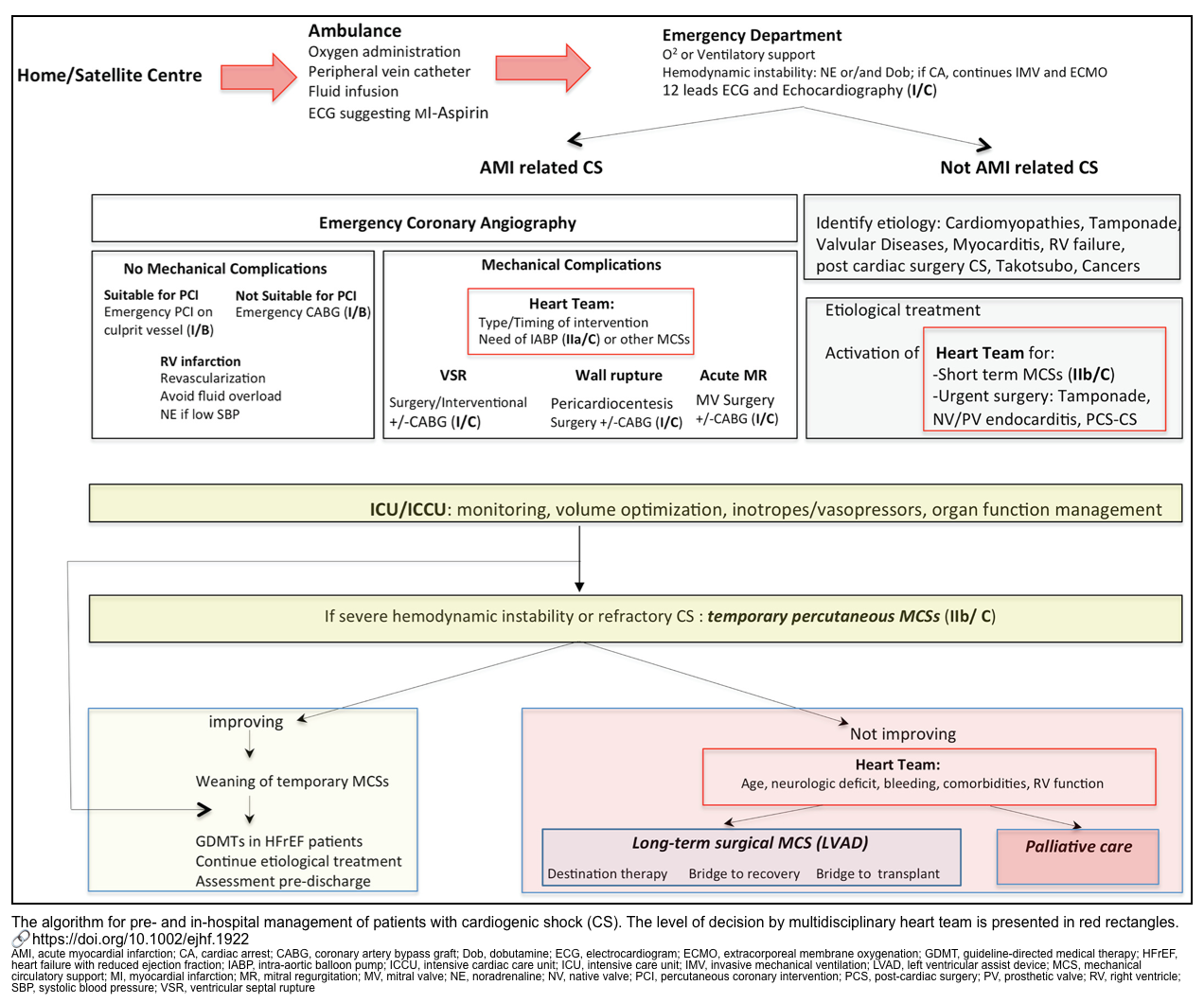
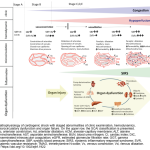
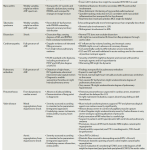
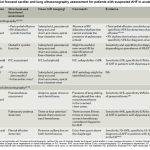
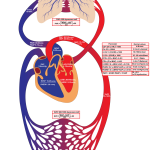
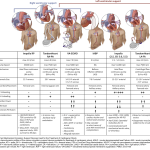
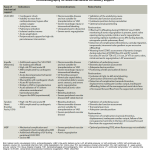

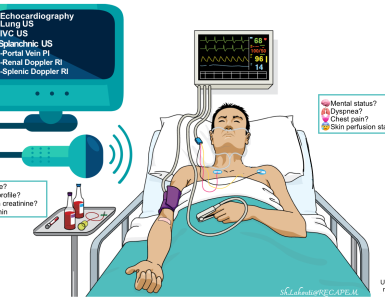

Add comment