29 January 2024, by Shahriar Lahouti.
CONTENTS
- Preface
- Definition
- Pathophysiology
- Clinical features and classification
- Diagnostic approach
- Background
- History, physical exam, initial evaluations
- Additional investigation
- Differential diagnosis
- Management
- Presumed resolved seizure
- Convulsive status epilepticus
- Nonconvulsive status epilepticus
- Antiseizure medications
- Specific conditions
- Going further
- Appendix
- References
Preface
Seizures and status epilepticus are frequent neurologic emergencies in the emergency department. Timely diagnosis and management of these life-threatening conditions are challenging. The outcome of status epilepticus depends on etiology, symptomatology, and duration of status epilepticus. Consequently, rapid and appropriate treatment to break nearly all seizures is recommended to mitigate irreversible neuronal injury and pharmacoresistance which may occur with ongoing convulsive activity. Here, the diagnosis and resuscitation of the seizing patient in the emergency setting are reviewed in light of recent recommendations and available evidence.
Definitions
Seizure
- A seizure is an episode of abnormal neurologic function caused by inappropriate excitatory electrical discharge of a group of cortical brain neurons.
- Based on the underlying cause seizures are classified as acute symptomatic seizures (i.e. provoked seizures such as seizures caused by acute metabolic
conditions, acute CNS insults, toxins, or infection) or unprovoked. - The clinical manifestation of seizures varies based on the location of the seizure in the brain and the extent of the cortex involved, including cognitive, autonomic, sensory, and motor symptoms.
- Status epilepticus (SE): Most seizures are self-limiting and typically terminate in 1-2 minutes or less. Failure of the mechanisms responsible for seizure termination leads to abnormally prolonged seizures. SE has been generally defined as enduring seizure activity that is not likely to stop spontaneously *.
- Based on the underlying cause seizures are classified as acute symptomatic seizures (i.e. provoked seizures such as seizures caused by acute metabolic
Epilepsy
- Refers to the clinical condition of a patient with recurrent, unprovoked seizures (at least two unprovoked seizures occurring more than 24 hours apart) *.
Pathophysiology
Seizures do not occur spontaneously under normal circumstances because neuronal physiology maintains the stability of neuronal membranes and prevents the rapid transfer of the synchronous discharges that initiate a seizure. Cortical neuron firing is normally controlled by excitatory and inhibitory neurotransmitters and receptors. An imbalance of excess excitation and decreased inhibition sustains a seizure *.
- Glutamate is the most common excitatory neurotransmitter and mediates the excess excitation via the N-methyl-D-aspartate (NMDA) subtype receptor.
- Gamma-aminobutyric acid (GABA) is the most common inhibitory neurotransmitter that prevents neurons from excess excitation via activation of the GABA-A subtype receptor under normal circumstances.
Why do some seizures fail to stop?
- Many seizures last 1-2 minutes before spontaneously aborting in response to endogenous seizure-terminating processes in the brain; however, when these processes fail, the seizure transforms to status epilepticus.
- The appearance of an abnormally prolonged seizure may be a manifestation of excessive excitation, a failure of inhibition, or a combination of the two processes.
- In the case of excitatory toxins, e.g. cholinergic drug overdose, substances act directly on receptors to overexcite glutamate receptors *.
- Failure of GABA inhibition during a seizure may occur, leading to SE.
- It has been shown that diazepam (a GABA receptor agonist) was less potent when administered later in the course of seizures than when given earlier. This suggests that GABA-A receptors may change in number or sensitivity during the course of SE.
- This concept is novel and has potential implications for treatment, as it implies that drugs that might be effective early in the course of status might not be effective later in the course.
- It has been shown that diazepam (a GABA receptor agonist) was less potent when administered later in the course of seizures than when given earlier. This suggests that GABA-A receptors may change in number or sensitivity during the course of SE.
Mechanism of neuronal injury in status epilepticus
- Excitotoxic mechanisms are probably the most important mediator of neuronal injury in SE.
- In normal conditions, when glutamate binds to the NMDA subtype of glutamate receptors nothing happens because the channel is blocked by a large magnesium ion stuck in the pore.
- However, under the severely depolarized conditions of SE, the magnesium ion is no longer held in the pore and the channel is not occluded. This allows calcium and other ions to flow into the neuron *. Because of the unusual properties of this channel, it does not close but instead is further activated by this influx of calcium. This leads to the pathologic accumulation of intracellular calcium, causing intracellular havoc.
- Neurons die by acute necrosis from this insult and by activation of second messenger mechanisms that lead to delayed cell death or apoptosis.
Clinical features and classification
Phases of seizures
To better understand what is happening in the brain when a seizure occurs, it is helpful to understand the phases of a seizure (figure below). Keep in mind that not everyone will have all the following phases of a seizure.
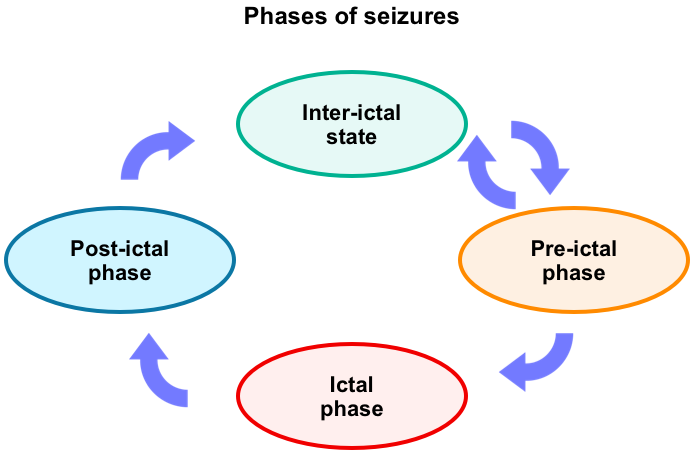
Pre-ictal (early ictal) phase: Auras
- This is commonly referred to as any premonitory subjective symptoms or sensations the patient experiences before a seizure.
- A description of the aura may provide valuable localizing information about the area of the brain involved with abnormal electrical activity.
- In actuality, auras are focal seizures that affect enough of the brain to cause symptoms, but not enough to interfere with consciousness.
Cognitive
▪️Loss of consciousness
▪️Staring
▪️Memory loss
▪️Confusion
Sensory/perception
▪️ Deja-vu, jamais vu.
▪️Dream-like or out-of-body experience, one side of the body different than the other, body size or weight alteration in perception.
▪️Unusual smells or tastes, hallucinations (olfactory, visual), racing thoughts
▪️Rising epigastric sensation (often likened to a “roller coaster” sensation)
▪️Tingling, numbness, electric shock feeling.
▪️Visual distortion, visual loss, or blurring.
▪️Spinning feeling
Motor
▪️Stiffening, falling, inability to move
▪️Dysarthria
▪️Dystonic
Automatism
▪️Aggression
▪️Eye-blinking, eyes rolling up, eyelid fluttering.
▪️Head-nodding
▪️Foot stomping
▪️Hand waving
▪️Oral-facial: Lip-smacking, chewing movements, swallowing, teeth clenching/grinding.
▪️Pedaling
▪️Pelvic thrusting
▪️Perseveration
▪️Running (cursive)
▪️Sexual
▪️Undressing
▪️Vocalization/speech
▪️Walking
Autonomic
▪️Tremors, sweating, heart racing, skin color changes.
▪️Urge to urinate or defecate, incontinence.
Ictal phase
- This phase is where signs of a seizure such as changes in behavior, consciousness, or movement abnormalities can be usually seen.
- 💪Motor (convulsive) manifestation may include *:
- Tonic refers to a sustained stiffening of muscles that commonly accompanies many seizures.
- Atonic seizures (also known as drop seizures) produce the opposite effect of tonic seizures: a sudden loss of control of the muscles, particularly of the legs, that results in collapsing to the ground and possible injuries.
- Clonic means rhythmic movements or jerking of the muscles.
- Tonic-clonic refers to a convulsion with initial stiffening of the body and extremities followed by rhythmic contractions of muscle groups.
- Myoclonic seizures consist of sudden, brief muscle contractions that may occur singly or in clusters and that can affect any group of muscles.
- Automatisms: Purposeless stereotyped repetitive behaviors such as facial grimacing, gesturing, chewing, lip smacking, or repeating words or phrases.
- Non-motor (nonconvulsive) manifestations of seizure include changes in cognition, emotion, sensory/perception, and autonomic abnormalities.
- 🧠Cognitive: Impaired awareness and consciousness, memory impairment, aphasia, mutism, reduced verbal fluency, stuttering, echolalia, and delusion.
- 😭Emotional: Agitation, anger, anxiety, fear, crying, laughing.
- 💗Autonomic: Asystole, brady/tachycardia, sweating, vomiting, piloerection, flushing, pallor, respiratory changes, erection, mydriasis, miosis, ptosis *.
- 🤡Perceptual/sensory: Hallucinations (olfactory, gustatory, auditory, visual), blindness, sensory disturbance, and pain (including headache).
- 💪Motor (convulsive) manifestation may include *:
Post-ictal phase
- Definition: Following the end of a seizure, there is a period of transition from the ictal state back to the pre-seizure baseline level of awareness and function, referred to as the postictal period *.
- Duration: Postictal period duration is variable.
- Patients can have an immediate recovery in some seizures such as absence seizures, while others may take the patient several minutes to return to baseline.
- Duration is variable depending on several factors:
- Spectrum of clinical manifestation: Transient behaviors that may follow a seizure may include *:
- Confusion and suppressed alertness, exhaustion, sleep.
- Memory loss, difficulty talking, aphasia.
- Hemianopsia
- Depression, fear, frustration, embarrassment, loneliness.
- Perceptual alterations, psychosis.
- Pain, headache.
- Weakness.
- Todd paralysis (postictal paresis): This refers to transient postictal paralysis that occurs in some patients, most commonly following a seizure that is confined to one hemisphere *.
- The classic example of postictal paresis is the weakness of a hand, arm, or leg that appears following a focal motor seizure involving one side of the body. The degree of weakness is usually moderate but can be severe.
Types of seizure
Most seizures can be classified as either focal or generalized (according to whether the onset of electrical activity involves a focal region of the brain or both sides of the brain simultaneously), or unknown (if seizure onset is either missed or obscured).
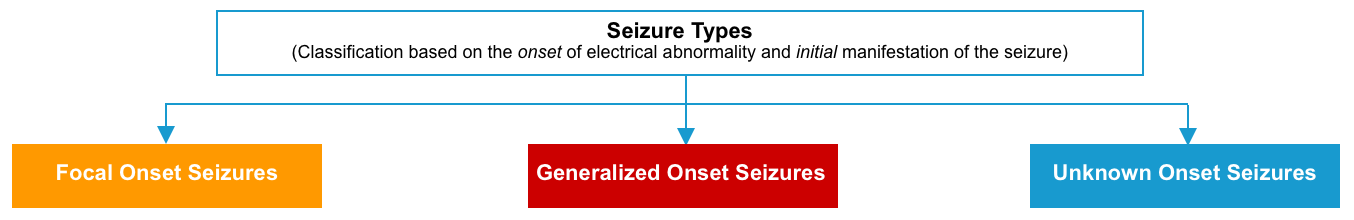
Focal seizures
- Pathophysiology
- These are due to electrical discharges beginning in a localized region of the cerebral cortex.
- Focal seizures are more likely caused by a localized structural lesion of the brain *.
- The discharge may remain localized (formerly called simple partial seizure) or may spread, involving nearby cortical regions (complex partial seizure) or the entire cortex (partial seizure with secondary generalization).
- Of note, the terms simple partial, complex partial, and secondarily generalized have been eliminated since they were difficult to define pragmatically and were often used incorrectly.
- The term “focal to bilateral tonic-clonic” is used to describe a seizure that begins focally but then spreads to engage bilateral networks *.
- These are due to electrical discharges beginning in a localized region of the cerebral cortex.
- Classification
- Focal seizures are further classified according to whether consciousness is altered or not during the event.
- Moreover, focal seizures are subgrouped into motor and nonmotor seizures, based on signs and symptoms at onset.
- Additional descriptors for focal seizures may be added based on specific motor or nonmotor symptoms. Focal seizures can also be more clearly described based on their elemental features (e.g. presence or absence of automatism, emotional and cognitive impairment).
Generalized seizures
- Pathophysiology
- These are caused by near-simultaneous activation of the entire cerebral cortex. The attacks begin with the abrupt loss of consciousness *.
- Metabolic disorders and diffuse brain injury will usually have a generalized onset (whereas a discrete lesion in the brain tends to lead to partial-onset seizures).
- These are caused by near-simultaneous activation of the entire cerebral cortex. The attacks begin with the abrupt loss of consciousness *.
- Classification
- Generalized seizures are further broken down into motor and nonmotor (absence) seizures.
- Additional descriptors of generalized seizures may be added based on specific motor or nonmotor symptoms.
- Generalized tonic-clonic seizures are the most common type of generalized seizures.
- Other subtypes of generalized seizures are absence seizures, more often seen in childhood in association with generalized epilepsy syndromes, and clonic, myoclonic, tonic, and atonic seizures *.
- Generalized seizures are further broken down into motor and nonmotor (absence) seizures.
Duration of ictal phase
- Most seizures are self-terminating and will generally stop within < 5 minutes (~1-3 min duration).
- Status epilepticus (SE) has been generally defined as ongoing seizure activity that is not likely to stop spontaneously.
- The duration of a prolonged seizure that meets the definition of SE varies depending on the type of seizure (explained below).
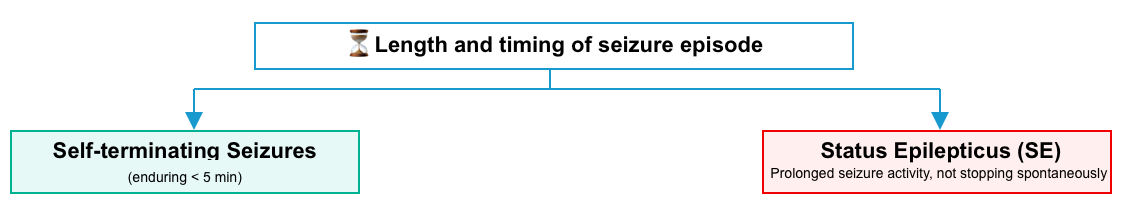
Status epilepticus
- Status epilepticus is a collective term for a group of conditions that result in prolonged or recurrent cerebral electrical discharges.
- All seizure types can develop to status epilepticus.
- The two main semiological forms of status epilepticus are convulsive SE and non-convulsive SE (NCSE).
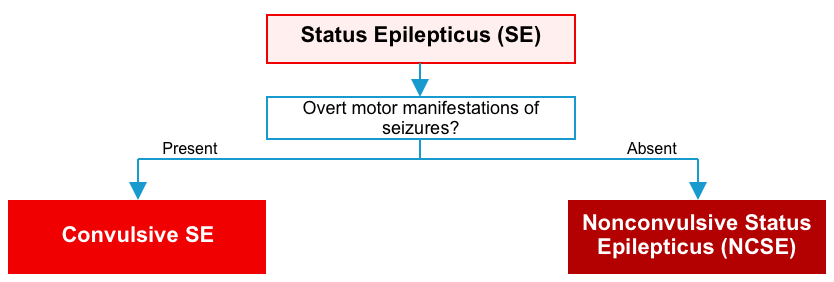
Convulsive status epilepticus
- Any following is considered convulsive status epilepticus *:
- Epilepsia partialis continua refers to ongoing simple, focal seizures (e.g. twitching of a single extremity without alteration of consciousness).
- Note that this is generally not life-threatening, as it does not affect consciousness or airway protection.
Clinical course of status epilepticus
- It can be divided into four stages *:
- Early SE (impending SE): Developing seizures leading up to status epilepticus.
- Established SE: Persisting (i.e. >5 minutes convulsive status epilepticus, or nonconvulsive/focal status epilepticus >10 min) despite first-line benzodiazepine administration (aka. benzodiazepine-refractory SE).
- Refractory SE: Persisting epilepticus despite treatment with first-and second-line antiseizure medications in different drug classes (e.g. a benzodiazepine + levetiracetam) *.
- Super-refractory SE: Persisting epilepticus for 24 hours despite treatment with two antiseizure medications and general anesthesia, or when seizures reemerge during an attempted anesthetic wean.
- Prolonged super-refractory status epilepticus: Super-refractory status epilepticus which lasts for >7 days, including ongoing need for anesthetics *.
Nonconvulsive ES (NCSE)
NCSE is defined as status epilepticus without a prominent (clinically obvious) motor component. NCSE may have no motor components or may have subtle movements (e.g. subtle facial twitching) *.
- Nonconvulsive status epilepticus can be defined as a condition of ongoing or intermittent seizure activity without convulsions, and without recovery of consciousness between the attacks *. The duration of seizure activity should meet any of the following:
- Continuous seizure activity lasting > 10 min.
- Intermittent seizure activity occurring for >20% of the time over the span of an hour *.
- More on the diagnosis, below.
Clinical presentation
- NCSE presents with a persistent alteration in behavior or consciousness without convulsive activity, but the range of possible symptoms is broad (panel below) *.
Impaired mental status
▪️Confusion, obtundation, or coma.
▪️Unexplained fluctuations in mental status.
Psychiatric/emotional disturbance
▪️Psychosis.
▪️Mood disturbance, fear, and agitation
Movement
▪️Subtle rhythmic twitching (especially involving the face or eyes).
▪️Automatisms (e.g., lip smacking, chewing).
▪️Catatonia
▪️Gaze deviation.
▪️Limb paralysis.
Perceptual and sensory disturbance
▪️Hallucinations (olfactory, gustatory, auditory, visual).
▪️Blindness.
▪️Sensory disturbance and pain (including headache).
Speech and language abnormality Aphasia, paraphasic errors.
▪️Mutism, reduced verbal fluency.
▪️Echolalia.
▪️Stuttering.
Classification of status epilepticus
Based on clinical manifestation, status epilepticus is classified into two main taxonomic criteria (figure below) *
- The presence or absence of prominent motor symptoms.
- The degree of consciousness impairment.
It is noteworthy that the symptoms and signs of SE change dynamically over the course of SE; e.g. bilateral convulsive status epilepticus frequently evolves into a coma state with subtle if any, convulsive movements. This condition is traditionally called subtle SE *.
- If the patient presents to the hospital in this stage, the semiology of SE might be classified as NCSE with coma.
Regarding the therapeutic approach as well as proper classification of SE, however, it is more appropriate to classify SE according to the true initial semiology of seizures, which can only be presumed in some cases. If NCSE with coma is presumed to have evolved from CSE, it is recommended that the patient be treated for the latter *.
Etiology
It is important to identify the underlying cause(s) of seizures as these have therapeutic implications. Sometimes multiple factors may combine to lower the patient’s seizure threshold. These should be addressed ideally to reduce the likelihood of recurrent seizures.
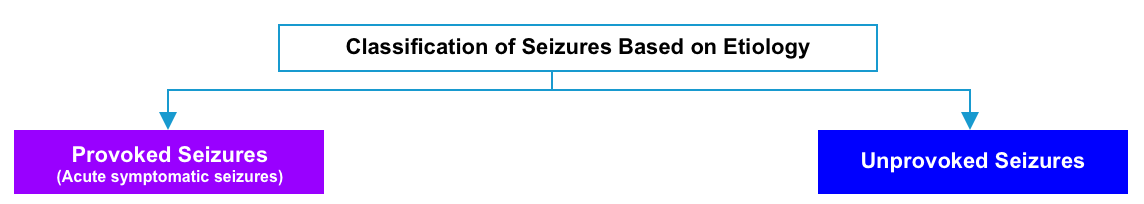
Seizures are classified as acute symptomatic seizures (i.e. provoked) or unprovoked based on their cause.
- Acute symptomatic seizure
- Refers to a seizure that occurs at the time of a systemic insult (e.g. systemic toxic-metabolic insult, drug or alcohol withdrawal) or in close temporal association with a documented brain insult (e.g. stroke, encephalitis, or acute head injury).
- Acute symptomatic seizures may recur during the index illness but generally carry a low risk for future epilepsy compared with unprovoked seizures.
- Having said that, some patients will go on to develop remote symptomatic seizures or epilepsy related to a prior stroke, hemorrhage, or head injury.
- Unprovoked seizure
- Refers to a seizure of unknown etiology and one that occurs due to a preexisting brain lesion or progressive nervous system disorder.
- Unprovoked seizures that are determined to be due to an underlying brain lesion or disorder are also referred to as remote symptomatic seizures.
- Unprovoked seizures carry a higher risk of future epilepsy compared with acute symptomatic seizures *.
Diagnostic approach
Background
Paroxysmal neurological disorder encompasses a heterogeneous disorder with abrupt onset of relatively transient neurologic findings (see differential diagnosis).
- Nature of symptoms
- Refers to whether the signs and symptoms of the spell are negative (i.e. loss of neurological function such as loss of vision, hearing, sensation, or ability to move a part of the body); or positive suggesting active discharge from the CNS including visual (e.g. bright lines, shapes, objects), auditory (e.g. tinnitus), somatosensory (paresthesia), or motor (e.g. jerking movements).
- Focal symptoms
- Denotes dysfunction at anatomically localized brain area. A focal cortical dysfunction cannot cause bilateral motor or sensory dysfunction or an altered level of consciousness.
- Focal signs and symptoms of neurologic dysfunction can happen within a distinct distribution within a vascular territory (e.g. as in a transient ischemic attack) or do not follow a distinct vascular distribution.
- In focal seizures, initial symptoms depend on the location of primary epileptogenic foci in the brain (figure below).
- Onset of neurologic dysfunction
- The onset of dysfunction is rapid in TIA and seizures, and more gradual in migraine aura.
- Course and progression
- The neurologic dysfunction may involve one or more modalities or functions (e.g. motor +/- sensory).
- TIA may result in simultaneous loss of one or more neurologic functions depending on the involved vascular territory.
- Migraine aura
- The symptoms typically progress from one modality to another (e.g. after the visual symptoms clear, paresthesia begins. When paresthesia is clear, aphasia or other cortical function abnormalities may develop).
- Focal seizures
- A focal seizure affecting the motor cortex may result in rhythmic jerking movements of the face, arm, or leg on the side of the body opposite the involved cortex (anatomic march, aka. Jacksonian seizure *).
- Some focal seizures spread to engage bilateral networks, causing generalized tonic-clonic seizures.
History
The goals of the history are to characterize the event as a seizure and rule out alternative diagnoses, determine whether similar events have happened in the past, and evaluate for underlying risk factors for seizures in the past medical history, family history, and medications.
Description of the event: ask about the circumstances leading up to the seizure, the ictal behaviors, and the postictal state.
- Ictal behaviors
- Loss of consciousness? Motor vs. non-motor manifestations? Focal symptoms with/without subsequent generalization?
- Note that loss of consciousness is not necessary to diagnose seizures and is only seen in generalized or focal seizures with impaired awareness.
- In patients with known epilepsy, ask about any change in frequency or pattern of ictal behavior.
- Loss of consciousness? Motor vs. non-motor manifestations? Focal symptoms with/without subsequent generalization?
- Recall of the event
- Patients without impairment of consciousness (e.g. focal seizures) can typically provide a complete description of the event.
- Patients with impairment of consciousness (generalized seizures, or focal seizures with impaired awareness) typically cannot, or can only remember the early stages of the seizure.
- Course of the event
- Most seizures, whether focal or generalized, have a clear and abrupt clinical onset and rapid progression of symptoms over seconds.
- The pace of progression is more rapid than migraine aura, for example, which increases in intensity throughout 5 to 10 minutes.
- Most seizures, whether focal or generalized, have a clear and abrupt clinical onset and rapid progression of symptoms over seconds.
- Duration of seizure episode
- The majority of seizures end spontaneously within 2-3 minutes.
- Differentiate seizure from status epilepticus.
- More prolonged symptoms may be a clue to alternative conditions such as migraine, transient ischemic attack, or psychogenic nonepileptic seizure.
- Postictal period
- Most patients begin to recover responsiveness and alertness within 10 to 20 minutes of a generalized seizure and show gradual, consistent improvement in postictal symptoms as time elapses.
- 🔴Patients with prolonged postictal symptoms should be evaluated for ongoing subclinical seizure activity with electroencephalography (EEG) and should undergo neuroimaging and lumbar puncture, as clinically indicated.
- In some cases, the postictal symptoms may be the presenting clinical feature, when the seizure itself is very brief, unwitnessed, or occurs during sleep. Particularly when the event is a first-time seizure, the clinical syndrome in these cases can be indistinguishable from that of acute stroke.
- Most patients begin to recover responsiveness and alertness within 10 to 20 minutes of a generalized seizure and show gradual, consistent improvement in postictal symptoms as time elapses.
- If the patient has a known seizure disorder, ask about the time since the last seizure, and typical ictal behavior.
- A history of prior status epilepticus increases the risk of future status epilepticus.
- Several medical conditions can increase the likelihood of provoked seizures:
- Diabetes as a cause of hypoglycemia.
- Renal failure as a cause of hypocalcemia.
- Cancer or an immunocompromised state, increases the likelihood of a structural CNS lesion.
Preceding events or illnesses
- Ask about any recent trauma or a history of head trauma:
- Did trauma precede the seizure?
- Was there trauma as a result of the seizure?
- Any febrile illness or other complaints.
Review of the medication list
- Patients on antiseizure medications: Missed doses? recent adjustment in medications? drug-drug interaction that may affect the therapeutic level of antiepileptic drugs?
- Review medications and look for other drugs that affect the seizure threshold.
Use of alcohol or other substances
Physical exams
Vital signs
-
Hypertension and tachycardia are common secondary to the catecholamine surge.
-
Hyperthermia may be due to the seizure itself or underlying infectious etiology.
-
Patients may have altered mental status during the seizure (for generalized tonic-clonic seizures or complex focal seizures) or maintain a normal mental status.
-
Patients may present in the postictal period.
-
It is important to distinguish an altered level of consciousness due to a postictal period versus a transition of seizures to nonconvulsive status epilepticus.
Focal neurological deficits
-
Papilledema may indicate increased intracranial pressure due to an intracranial mass or infection.
-
Lateralizing signs may indicate a structural brain abnormality.
- Immediately following the seizure, patients may have focal neurologic defects in areas involved in the seizure (e.g. Todd’s paralysis, focal hyperreflexia, or aphasia). Prompt examination is important, as findings may disappear over time.
- A transient, focal neurologic deficit is important for 2 reasons:
- This supports that the event was truly a seizure.
- Focality suggests a focal neurologic lesion as the trigger of the seizure. In the presence of focal neurologic deficit, neuroimaging should be performed.
- Signs of head trauma
- Tongue biting
- Look for tongue biting, especially lateral tongue biting as this is highly specific (96%-100%) for seizure *.
- Incontinence (only 38% sensitive and 57% specific) *.
Initial evaluation
Lab tests
- Point-of-care blood glucose measurement in all patients.
- Electrolyte panel (especially sodium and calcium), renal function, and liver function tests (including ammonia level).
- Creatinine kinase (to evaluate for rhabdomyolysis due to seizures).
- Pregnancy test if relevant.
- Complete blood count.
- Coagulation studies (INR, PTT).
- Urinalysis.
- Toxicology workup as indicated (e.g. salicylate level, urine toxicology screen including cocaine and methamphetamine).
- Antiseizure medication levels:
- In patients with breakthrough seizures, antiepileptic drug levels should be determined despite apparent compliance, if applicable.
- Valproic acid, phenobarbital, and phenytoin levels.
- These are often available locally, with a reasonably rapid turnaround time.
- Other antiseizure medication levels are typically sent out tests (e.g. levetiracetam, carbamazepine, lacosamide).
- These may remain useful later on, to establish compliance.
- Valproic acid, phenobarbital, and phenytoin levels.
- In patients with breakthrough seizures, antiepileptic drug levels should be determined despite apparent compliance, if applicable.
ECG
- Obtain an ECG to exclude an arrhythmia causing syncope.
- 💡Keep in mind that cardiogenic syncope may cause brief convulsive movements including myoclonus, head/eye deviation, or automatism.
Additional investigation
Neuroimaging
A neuroimaging study should be performed in all adults with a first-time seizure and all patients with status epilepticus to evaluate for a culprit structural brain abnormality.
- In general, MRI is preferred over CT because it has a superior sensitivity for detecting a variety of acute and remote causes of seizure and epilepsy (e.g. infarcts, tumors, etc.).
- Alternatively, CT is preferred urgently in those patients suspected to have intracranial lesions.
- It is used to exclude acute neurologic problems that require urgent intervention *.
- Indications for non-contrast head CT *,*
- First-time seizure.
- Status epilepticus.
- Persistent focal deficit or lack of return to the neurologic baseline.
- Prolonged postictal state (expert opinion: 30 minutes = prolonged).
- New focal-onset seizure.
- Patients with a change in established seizure patterns.
- Fever.
- Persistent headache.
- History of acute head trauma.
- History of malignancy, immunocompromised, bleeding disorder/anticoagulation, shunt, neurocutaneous disorder, chronic alcohol abuse.
- A non-contrast head CT is often the most appropriate initial study. CT can identify hemorrhages, gross structural malformations, large tumors, and calcified lesions.
- CT angiography may be considered if there is suspicion of stroke or vascular malformations such as arteriovenous malformations or arteriovenous fistulas.
MRI
- The purpose is to identify the underlying pathology *.
- Urgent MRI should be considered for acute patients with negative CT findings but for whom there is an ongoing concern for a structural cause for seizure *.
- Outpatient MRI is reasonable in patients evaluated in the ED who have returned to a normal baseline and have a normal neurologic examination, particularly if an initial head CT is normal *.
- Importantly, while structural abnormalities on brain MRI or CT usually suggest symptomatic seizures, these findings should not be interpreted in isolation *.
- Many MRI findings are nonspecific and may be incidental to the index event.
- MRI abnormalities may take several weeks to resolve, so these don’t necessarily represent active seizure activity.
Lumbar puncture
Lumbar puncture should be performed if the clinical presentation is suggestive of an acute infectious process that involves the central nervous system or if neuroimaging studies raise concern for an alternative meningeal process such as leptomeningeal cancer or chronic meningitis.
- Potential indications for lumbar puncture include *:
- Fever.
- Infectious symptoms before seizure onset.
- Known immunosuppression.
- MRI reveals leptomeningeal enhancement.
- No apparent cause for the patient’s status epilepticus.
- When indicated, LP should be performed after obtaining a CT scan to rule out mass effects.
- Note that prolonged convulsive seizure itself can cause transient, mild cerebrospinal fluid pleocytosis (but not above ~80-100/mm3) *.
EEG
Indications
- Suspicion for nonconvulsive status epilepticus (NCSE)
- Persistently abnormal mental status following any clinically evident seizures *.
- Fluctuating mental status or unexplained alteration of mental status *.
- Prolonged focal deficits that are unexplained (by a structural cause on neuroimaging).
- Acute supratentorial brain injury with altered mental status *.
- Comatose patients following cardiac arrest *.
- Status epilepticus (SE)
- EEG is usually indicated in SE unless the patient’s mental status returns to normal.
- The usefulness of EEG in status epilepticus:
- To identify continued seizure activity in intubated patients with status epilepticus.
- Assessment of treatment efficacy in patients with known status epilepticus (e.g. titration of antiepileptic infusions to target absence of seizures or burst-suppression).
- Paroxysmal clinical events that are suspected to be possible seizures *
- Patients at risk for seizures which may be masked by the requirement for pharmacologic paralysis *
Outpatient
- An EEG should be obtained in all patients presenting with first-time seizures who are back to baseline with no clear precipitant.
- In patients who have returned to baseline, outpatient EEG is appropriate and may be more representative of a patient’s underlying risk for seizure recurrence than EEG performed immediately after a seizure, which may be confounded by medication effects and acute postictal changes *.
Duration of EEG monitoring
A single “spot” EEG recording over an hour may have only ~50% sensitivity for detecting a seizure, as compared to ongoing continuous EEG monitoring *.
- Increasing monitoring for 24-48 hours further increases sensitivity.
- However, beyond a certain duration, the value of ongoing EEG monitoring becomes unclear. For example, if the patient is having a subclinical seizure every 30 hours, does that truly matter? Would treating such an abnormality lead to more benefit than harm?
Continuous EEG vs. spot EEG (aka. screening EEG)
- As a general rule of thumb, if the following criteria are met, a short EEG recording may be sufficient to exclude seizures with reasonable sensitivity *:
- Lack of prior seizure history.
- No ongoing coma.
- EEG recording reveals no worrisome findings (e.g. lateralized periodic or rhythmic delta activity).
Differential diagnosis
Mimics of convulsive seizures
Convulsive seizure is primarily a clinical diagnosis, and accurate diagnosis requires differentiating from other common clinical events that can mimic convulsive seizure (box below).
| Psychogenic nonepileptic seizure (PNES). Cerebral Amyloid Angiopathy spells Hyperkinetic movement disorders Other paroxysmal neurologic events ▪️Syncope (convulsive syncope) ▪️Migraine aura. ▪️Transient ischemic attack (TIA) |
Psychogenic nonepileptic seizures
- Psychogenic nonepileptic seizures (PNES) are paroxysmal behaviors that resemble epileptic seizures.
- PNES can occur in patients with epilepsy (~10%), i.e. patients can have episodes of both true seizures and pseudoseizures! *.
- PNES is often related to psychological stress exceeding an individual’s coping capacity
Differentiating PNES from true seizure
- This is sometimes challenging. Some clinical features can help distinguish PNES from epileptic seizures. However, diagnostic errors often result from an overreliance on specific clinical features *.
- Note that no single feature is pathognomonic for PNES. When typical seizures can be recorded, video-EEG is the diagnostic gold standard for PNES.
- Clinical clues suggesting PNES (table below)*,*
- Unusual movement
- Very irregular movements
- Asynchronous movement of limbs (in an epileptic seizure, the limbs generally move synchronously).
- Discontinuous motor patterns (i.e. changing side and region of body abruptly, without a “marchlike” propagation)
- Side-to-side head movements or body turning (jacitation)
- Pelvic thrusting or overarching of trunk (opisthotonic posturing)
- Complex movements such as writhing, flailing, and whole-body thrashing
- Retained consciousness
- Movement of all extremities with preservation of consciousness (e.g. speaking or following commands).
- Responsiveness to noxious stimuli (e.g. nasal swab for influenza).
- Facial features and injuries
- In PNES, the eyes are typically closed at the onset of convulsive PNES (while in convulsive epileptic seizures, a patient’s eyes tend to be open).
- Although eye closure can be observed during epileptic seizures, eye closure throughout the entire ictal episode is considered a characteristic feature of PNES.
- Forced eye closure: Resistance to the eyes being pulled open by an examiner is also a sign of PNES.
- Irregular eye movements (unliked consistent gaze deviation that may be seen in seizure).
- Optokinetic nystagmus: viewing an optokinetic drum or video will elicit nystagmus.
- The mouth is clenched shut during PNES, while during the tonic phase of a convulsive epileptic seizure, a patient’s mouth is often widely open.
- Tongue bites: Patients with PNES may experience tip-of-the-tongue, lip, or buccal bites, while tongue bites are often located laterally in patients with epileptic seizures
- In PNES, the eyes are typically closed at the onset of convulsive PNES (while in convulsive epileptic seizures, a patient’s eyes tend to be open).
- Absence of postictal period in PNES
- Complete and abrupt recovery is more often observed following PNES.
- Postictal confusion can be seen in both PNES and convulsive epileptic seizures, but the ability to recall events that occurred during a seizure supports a diagnosis of PNES over a diagnosis of epilepsy.
- Unusual movement
- Other suggestive clues of epileptic seizure
Diagnosis and management
- When in doubt, video EEG capture can help make these distinctions.
- If a diagnosis can’t be made with certainty, the safest approach can be to treat these as genuine seizures (e.g. with benzodiazepine, airway protection).
Cerebral Amyloid Angiopathy (CAA) spells
- Cerebral amyloid angiopathy (CAA) is characterized by amyloid beta-peptide deposits within small- to medium-sized blood vessels of the brain and leptomeninges.
- The incidence of CAA is strongly age-dependent. CAA-related symptoms are uncommon at ages <60 years.
- The predominant clinical manifestation is lobar intracerebral hemorrhage (ICH). More on this here.
- A less common clinical manifestation of CAA is transient focal neurologic episodes (TFNE). These have also been called “amyloid spells” *.
- Clinical features of TFNE
- Recurrent, brief (enduring for several minutes), stereotyped spells of either negative (weakness, numbness, paresthesias, vision loss) or positive neurological symptoms (positive visual aura, limb jerking), that can spread smoothly over contiguous body parts over several minutes *.
- Clinical features more suggestive of TFNE over other diagnoses include the smooth spread of the symptoms over minutes and the stereotypic recurrence of symptoms over time.
- Symptoms of TFNE tend to localize to the site of a cortical SAH, prior lobar hemorrhage, or focus of cortical superficial siderosis *.
- Clinical features of TFNE
Hyperkinetic movement disorders
- Hyperkinetic movement disorders are characterized by excessive movements and include chorea, athetosis, ballism, dystonia, tics, tremors, and myoclonus *.
- Movement disorders may be confused with seizures if the motor activity is classic in appearance (e.g. isolated dystonic posturing may be confused with focal
epileptic seizures). These may also be confused with epilepsia partialis continua if they are sustained rather than episodic *.
- 💡Helpful distinguishing clues
- Movement disorders do not cause impaired consciousness.
- Some patients with dystonia and other movement disorders may obtain symptomatic relief from sensory tricks, such as lightly touching their faces to ameliorate cervical dystonia *.
- 💡Helpful distinguishing clues
- Movement disorders may be confused with seizures if the motor activity is classic in appearance (e.g. isolated dystonic posturing may be confused with focal
- The diagnosis of these disorders is based in large part on history; causes include genetic variants, infections, metabolic derangements, structural brain malformations, and malignancies. A video-electroencephalography (EEG) recording of events is invaluable in difficult cases *.
Other paroxysmal neurologic events
Mimics of nonconvulsive status epilepticus
Alteration in “behavior” or “level of consciousness” is the predominant presentation of NCSE. Common differential diagnoses of NCSE are mentioned here (box below) *.
| ▪️Porlonged postictal state ▪️Delirium and acute toxic-metabolic encephalopathy (TME) ▪️Psychogenic non-epileptic status ▪️Drug-associated states (e.g. neuroleptic malignant syndrome, serotonin syndrome, and anticholinergic toxicity) |
Nonconvulsive status epilepticus
- The diagnosis of NCSE is suspected based on the presence of risk factors, clinical features suggestive of NCSE, and EEG (table below)*, *, *.
- For a general schema to show a unified clinical approach to diagnose nonconvulsive status epilepticus refer to the appendix.
- NCSE vs. postictal state
- Postictal state can last minutes to hours in some patients, and it is difficult to know the extent to which it might represent ongoing seizure activity (particularly when occurring at depth).
- EEG is indicated for patients not returning to baseline within an hour.
- 💡In postictal states, EEG should show background slowing or suppression *.
- NCSE vs. toxic-metabolic encephalopathy (TME)
- NCSE and toxic/metabolic encephalopathy may appear with similar clinical manifestations, and may also simultaneously coexist *. Even with complete EEG, laboratory, and imaging evaluation, it may sometimes be challenging to determine precisely what is going on.
- 💡EEG findings in encephalopathy include diffuse slowing or a burst-suppression pattern *. Alternatively, EEG findings revealing seizures or ictal-interictal continuum abnormalities suggest NCSE.
- Psychogenic non-epileptic status
- Up to 25% of patients with non-epileptic attack disorder can develop psychogenic non-epileptic status. They often have a diagnosis of epilepsy and take antiepileptic medication.
- 💡In psychogenic non-epileptic status, EEG shows a normal waking rhythm.
- Drug-associated states
- Neuroleptic malignant syndrome, serotonin syndrome, and anticholinergic toxicity have typical clinical profiles and can be associated with reduced consciousness. There will be a history of relevant drug exposure, with improvement after withdrawing the offending drug.
- Neuroleptic malignant syndrome when associated with a catatonic state may resemble NCSE but with an elevated serum creatine kinase.
- Serotonin syndrome may present with clonus that should not be confused with the myoclonus of NCSE. EEG should resolve residual concerns of NCSE.
Management
Presumed resolved seizure
Overview
- ABCDEFG (ABC’s and Don’t Ever Forget the Glucose)
- Establish IV access (for medication delivery if recurrent seizure in the ED).
- Distinguish between seizure vs seizure mimics (explored above).
- Distinguish between first-time seizure vs. recurrent seizure.
- This distinction has therapeutic implications since the medical management will change depending on which it is.
- Ask specifically about subtle motor jerking, positive sensory phenomena, staring spells, or hallucinations in the weeks and months before presenting to the ED that may suggest an underlying subtle chronic seizure disorder.
- In one observational study, 28% of patients who presented with a presumed first-time generalized tonic-clonic seizure had a history of minor epileptic symptoms such as myoclonus or simple partial seizures.
- Acute repetitive seizures (seizure clusters)?
- Refers to ≥3 seizures within 24 hours in patients whose mental status is relatively preserved between seizures *.
- This typically occurs in the following conditions *:
- Patients with epilepsy whose habitual seizure frequency is < 3 seizures per day. This commonly is seen in the context of frontal lobe epilepsy.
- Acute symptomatic seizures (provoked seizures), especially in critically ill patients.
- Note that in critically ill patients, whose mental status might be affected by the underlying cause of the seizure, it might be challenging to determine if the patient would return to baseline between the seizures.
- Using an approach as aggressive as that used in status epilepticus may be associated with unnecessary morbidity *.
- Intubation and anesthetic management of these patients may obscure the clinical examination that is important in understanding the patient’s response to treatment.
- Categorize the seizure.
- Identify the underlying cause of the seizure (above).
- Assess for complications of seizure (e.g. traumatic brain injury, neurogenic pulmonary edema). More on this below.
- Assess anti-seizure drug levels
- Anti-seizure drug levels are recommended for any patient with known epilepsy/seizure disorder who has a breakthrough seizure (especially with a change in their seizure pattern).
Indication for hospitalization
- Patients with status epilepticus (both convulsive and nonconvulsive).
- Patients with prolonged postictal state.
- Patients with altered mental status (especially a fluctuating LOC).
- Patients with focal neurologic dysfunction that is unexplained by a structural lesion.
- All provoked seizures *.
Indication to start antiseizure drugs in ED
The decision to initiate an antiseizure drug in the ED is complex. It involves an assessment of the underlying cause of the seizure, the likelihood of seizure recurrence, and the impact of medication on quality of life *.
- The decision to start antiepileptic drugs should be made in conjunction with a neurologist.
- Antiepileptic drugs are used for the prevention of seizures caused by CNS processes that are not completely reversible.
Status epilepticus
- Any patient who seizes for >5 min should receive an antiseizure drug, even if benzodiazepine is successful in stopping the seizure *.
Breakthrough seizure
- Send antiseizure drug (ASD) serum level and identify possible causes or triggers.
- If the serum level of ASD is inadequate: Start a loading dose of antiepileptic drug in ED, preferentially via IV route.
- If ASD serum level is not available and the patient has a missed dose: Give the usual dose of the drug in ED.
- If the serum level is adequate and the seizure frequency and pattern fall within the expected range for the patient: No need for further dose or adjustment.
- If the serum level is adequate, but the frequency or pattern of seizure is changed: May consider giving additional agents.
Patients with first-time seizures who return to their baseline mental status
- Unprovoked seizure
- Assess for the risk of seizure recurrence. The risk of recurrence will be increased in the presence of the following risk factors *:
- Remote symptomatic cause.
- Epileptiform abnormality on interictal EEG.
- Abnormal neuro exam beyond the postictal period including focal finding & intellectual disability.
- Significant brain imaging abnormality.
- A first seizure that occurs during sleep.
- Likely the first presentation of epilepsy syndrome.
- Antiseizure drugs do not need to be started in the ED for a first unprovoked seizure in patients with a low risk of recurrence (<50%) * (see following algorithm).
- Assess for the risk of seizure recurrence. The risk of recurrence will be increased in the presence of the following risk factors *:
- Provoked seizure
- The antiseizure drugs need to be started in the ED if any of the following conditions are met *:
- Convulsive seizure > 3min.
- Repetitive seizures ( ≥3 seizures within 24h)
- Active underlying condition or critical illness causing provoked seizure(s)
- Traumatic brain injury, acute ischemic stroke, intracranial hemorrhage.
- The antiseizure drugs need to be started in the ED if any of the following conditions are met *:
- For 2 or more seizures >24 hours apart
- Antiepileptic drugs should be started in the ED in consultation with a neurologist.
Convulsive status epilepticus
Overview
🔴Examination, identification of potential cause, application of the ABC (airway, breathing, circulation), and treatment should all begin simultaneously.
🔴The main goals of immediate supportive care are:
- Establish and maintain adequate airway, breathing, and circulation.
- Direct a focused history and physical examination toward possible causes and subsequent injuries.
- Look for signs of head trauma, sepsis, anisocoria, or meningitis.
- Identify and treat life-threatening causes of status epilepticus, such as trauma, sepsis, meningitis, encephalitis, or structural brain lesions.
- Stop the seizure and thereby prevent brain injury.
- The goal of treatment is seizure control as soon as possible since mortality in SE correlates with time to treatment and duration of seizure activity.
-
- Treatment of generalized SE should begin at 5 minutes (T1 timepoint), whereas it is typically recommended to begin treatment for focal SE at 10 minutes, and absence SE at 10 to 15 minutes *.
-
- The goal of treatment is seizure control as soon as possible since mortality in SE correlates with time to treatment and duration of seizure activity.
🔴The duration of time in which a patient can be in convulsive status epilepticus before brain damage occurs is unknown.
- Clinical data indicate that permanent neuronal damage may occur around 30 minutes of epileptic activity *.
- However, irreversible neuronal injury and pharmacoresistance may occur before this traditionally defined time parameter, and spontaneous cessation of epileptic activity is unlikely to occur after 5 minutes of ongoing convulsive activity *.
- Therefore, it is reasonable to employ an appropriate strategy to break nearly all seizures within 30 minutes. This requires rapid escalation to intubation.
- Having said that, the following approach to the management of convulsive SE in adults is outlined.
Initial approach
🔴Airway and breathing
- Maintain an open airway through positioning maneuvers and airway devices as needed.
- Administer 100% O2 and assess for cyanosis by visual appearance and pulse oximetry.
- For patients with transient apnea or hypoxemia, the clinician may use bag-mask ventilation as long as the airway can be maintained and spontaneous breathing with adequate oxygenation resumes within a short period of time.
- Rapid sequence endotracheal intubation (RSI) is indicated in patients with any one of the following conditions:
- Unprotected or unmaintainable airway.
- Apnea or inadequate ventilation.
- Hypoxemia.
- Status epilepticus lasting ≥20-30 minutes.
- Need to protect airway for urgent brain imaging (e.g. in a patient with preceding trauma or signs of basilar territory stroke).
🔴Circulation and vascular access
- Establish 2 large-bore IV access.
- Hemodynamic support
- 💡Status epilepticus does not independently cause hypotension, at least early in its course.
- Hypotension or evidence of circulatory shock in SE should prompt consideration of an underlying systemic condition such as cardiogenic cause, traumatic hemorrhage, or sepsis.
- Hypotension may also result from benzodiazepines, barbiturates, and sedative/anesthetic agents used to treat SE.
- Give IV fluid followed by vasopressors in patients with suspected shock.
- 💡Status epilepticus does not independently cause hypotension, at least early in its course.
🔴Initial studies: Point-of-care blood glucose, and labs (see above)
🔴Identify and treat potential underlying cause
- Consider immediate life threats that require immediate and specific treatments (figure below).
- More on toxicologic seizures below.
🔴Emergency antiseizure treatment (discussed below)
🔴Additional investigation (e.g. head CT, LP, EEG).
| Benzodiazepine: First-line treatment |
Benzodiazepines are used in patients with continuous or very frequent seizures to temporarily control the seizures until more specific agents can be given.
Indication to give benzodiazepines
- Give a benzodiazepine for any seizure lasting ≥5 minutes *.
- Most seizures are self-limited. Often this will involve a tonic phase, followed by a clonic phase, and finally a post-ictal phase. If the patient is still in a tonic phase after 3 minutes, it is unlikely that their seizure will break spontaneously.
Dosing
- ⚠️Avoid benzodiazepine underdosing!
- The risk of respiratory compromise secondary to the proper use of benzodiazepines is lower than that related to status epilepticus *.
- Prompt and appropriately dosed benzodiazepine treatment is particularly important, as SE tends to become self-sustaining and pharmacoresistant as it continues * (see pathophysiology, above).
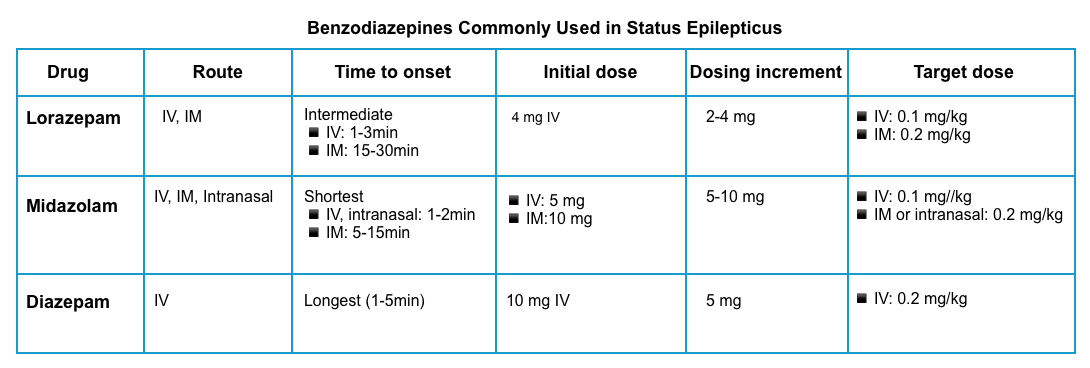
- Lorazepam
- It is preferred over other benzodiazepines based on pharmacokinetic properties.
- The typical loading dose of lorazepam is 0.1 mg/kg IV, infused at a maximum rate of 2 mg/min, allowing 3-5 min to assess the effect before deciding whether additional doses are necessary. An alternative is a fixed dose of lorazepam (4 mg), repeated if the patient is still seizing.
- For patients without IV access, give 10 mg of intramuscular midazolam👇.
- Midazolam
- Initial dosing: 0.2 mg/kg IM, up to 10 mg per dose *.
- Diazepam
- Initial dosing 10 mg IV, may repeat q5-10 min to a maximum cumulative dose of 30 mg.
- Diazepam should not be given intramuscularly due to erratic absorption.
- ⚠️Note that diazepam has a rapid redistribution from the brain into the peripheral tissues *. This will shorten its duration of action and may increase the risk of recurrent seizures as the diazepam wears off.
- Initial dosing 10 mg IV, may repeat q5-10 min to a maximum cumulative dose of 30 mg.
| Conventional antiseizure drugs for status epilepticus |
Conventional antiseizure drugs used in SE include levetiracetam, valproic acid, lacosamide, fosphenytoin, phenytoin, and phenobarbital.
Indications
- Any patient who seizes for >5 min should receive an antiseizure drug, even if benzodiazepine is successful in stopping the seizure *.
- This is especially important if diazepam or midazolam is used to stop seizures, as they have a short duration of action.
- Exceptions to the need for a nonbenzodiazepine antiseizure medication may include patients who have seizures that stop with lorazepam treatment and have a rapidly reversible cause of generalized convulsive status epilepticus that has been definitively corrected, such as severe hypoglycemia.
- The antiseizure medication should be ordered and given as early as possible.
- One of these antiepileptic agents should be started within 20 min of diagnosis.
- The most updated consensus guidelines do not currently recommend one second-line agent over another.
Agent of choice
- The choice among antiseizure medications can be made according to individual patient factors such as comorbid conditions and potential adverse effects, as well as local availability and clinician experience.
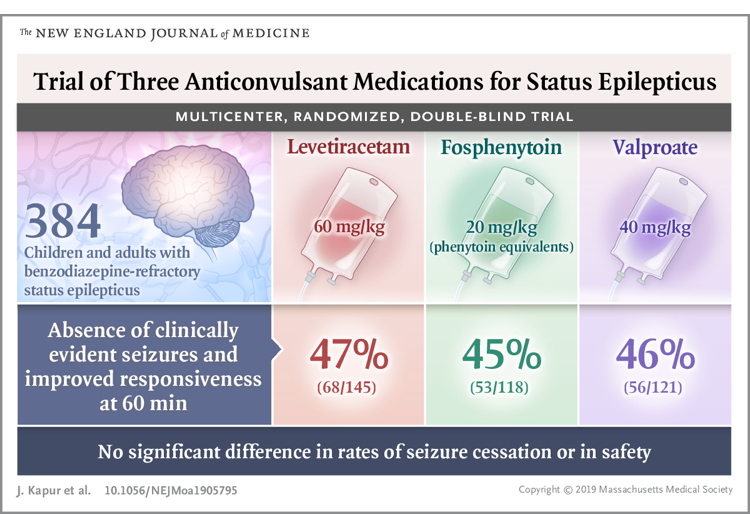
- Efficacy among the IV antiseizure drugs is equal and the adverse effects occur at similar rates *.
- High-quality evidence from the Established Status Epilepticus Treatment Trial (ESETT) trial that fosphenytoin, valproic acid, and levetiracetam are equally effective.
Dosing
- ⚠️Avoid underdosing of antiseizure medications. It is important to calculate the weight-based dose.
Conventional antiseizure medication for status epilepticus
- Levetiracetam
- It is commonly used as a first-line antiseizure medication.
- Although the efficacy of levetiracetam is equal to other agents (e.g. fosphenytoin or valproic acid), it has a superior safety profile due to the lack of hepatic metabolism, medication interaction, and effect on blood pressure.
- Levetiracetam has no contraindications, so this is a convenient agent to order for patients who present with status epilepticus (without any additional information known about the patient).
- Loading dose: 60 mg/kg up to a max of 4.5g, infused over 10 min. (Any patient >69 kg should receive 4500 mg.) *
- If still seizing, may give an additional 20 mg/kg IV (max 1500 mg) over 5 minutes.
- It is commonly used as a first-line antiseizure medication.
- Valproic acid
- Loading dose: 40 mg/kg and infused at a rate of 10 mg/kg/min in adults (max 3000 mg) *, thus, the full dose can be given in 4 min.
- If still seizing may add 20 mg/kg up to 2000 mg over 5 min.
- Loading dose: 40 mg/kg and infused at a rate of 10 mg/kg/min in adults (max 3000 mg) *, thus, the full dose can be given in 4 min.
- Lacosamide
- Loading dose: 10 mg/kg IV (max 500 mg IV over 5-10 min)
- If still seizing, give an additional 5mg/kg (max 250 mg IV) over 5min.
- Loading dose: 10 mg/kg IV (max 500 mg IV over 5-10 min)
- Fosphenytoin
- Loading dose: 20 mg/kg phenytoin equivalents as either slow IV push or IV infusion at a rate not to exceed 150 mg/min (max 1500 mg) *.
- If still seizing may give an additional 5 mg/kg (max 500 mg).
- Phenobarbital
- Phenobarbital is an effective antiseizure medication, but it is difficult to titrate and may lead to somnolence. Thus, for most patients, phenobarbital isn’t a front-line agent. However, for patients with alcohol withdrawal seizures, phenobarbital is the preferred antiseizure therapy.
- Loading dose: 20 mg/kg infused at a rate of 30 to 50 mg/minute.
| Intubation |
- Predicted clinical course of prolonged seizure with respiratory depression with the use of escalating doses of anesthetics.
🔴Indications
- If aspirating or apneic.
- If no response to the first adequate dose of benzodiazepine.
- Target intubation by ~20 minutes after seizure initiation, regardless of whether or not the conventional antiseizure medication has arrived.
- For a patient with active convulsive seizures, don’t delay intubation while waiting for the antiseizure medication to arrive from the pharmacy.
- The antiseizure medication should be ordered ASAP and given as early as possible.
- Intubation shouldn’t be delayed while waiting to see if the second-line antiseizure medication agent will work.
- If the anti-epileptic arrives from the pharmacy and breaks the seizure within 20 minutes then intubation is not necessary. However, in most scenarios, this is logistically impossible.
🔴Method: Rapid Sequence Induction (RSI).
🔴Preoxygenation
- Status epilepticus precludes adequate preoxygenation and denitrogenation, and patients are at a high risk of rapid desaturation with high O2 consumption rates.
- Place nasal trumpets and non-rebreather facemask to provide apneic oxygenation.
- Consider bagging the patient with BVM until laryngoscopy.
🔴Induction agent
- Propofol or propofol + ketamine (may have a synergistic effect through modulating GABA and NMDA receptors).
- Propofol
- It is generally the best induction drug here, given its potent anti-epileptic activity.
- The only exception would be a patient with severe hypotension (in whom midazolam provides more hemodynamic stability)
- Dose: IV Propofol 1.5-2 mg/kg.
- It is generally the best induction drug here, given its potent anti-epileptic activity.
- Ketamine
- Midazolam
- Used for patients in shock.
- Dose: 0.2 mg/kg IV push over 1-2min. May use 0.1 mg/kg in hemodynamically unstable patients.
- Propofol
🔴Have on-hand rescue vasopressors as needed (e.g. have a norepinephrine infusion and/or push-dose epinephrine ready). The combination of sedation, vasodilation from propofol, and positive pressure ventilation may decrease the blood pressure.
🔴Paralytic agents
- The rationale to use
- Maximizing the chance of first-pass success in status epilepticus.
- Note that muscular paralysis won’t prevent brain damage from ongoing seizure activity.
- The goal is always to control the seizure itself, not to mask it using paralytics.
- Long-term neuromuscular blockade should be avoided so that clinicians can monitor for ongoing seizure activity and perform serial neurological exams until EEG monitoring is available.
- Agent of choice
- Depends on patient factors, duration of seizure activity, and access to the rocuronium reversal agent, sugammadex 💉.
- Succinylcholine
- 🚫Contraindicated in prolonged status epilepticus, as this may lead to rhabdomyolysis and hyperkalemia.
- If there are no clear contraindications for using succinylcholine and the patient has been seizing for < 20-25 min, it is reasonable to use succinylcholine given its short duration of action *.
- Dose: 1 to 1.5 mg/kg IV.
- Rocuronium
- It causes prolonged paralysis, which may be problematic because it can mask ongoing seizure activity *.
- Rocuronium can be considered if sugammadex can be used following intubation to reverse paralysis and determine if there is residual seizure activity (if EEG isn’t readily available).
- Dose: 1 to 1.2 mg/kg IV.
- Placement of a tourniquet on an extremity before paralysis may prevent the paralytic from entering that extremity, thereby allowing you to determine if the seizure has been terminated.
- Succinylcholine
- Depends on patient factors, duration of seizure activity, and access to the rocuronium reversal agent, sugammadex 💉.
🔴Start sedative infusion
- Anesthetic infusion provides a dual purpose, breaking the seizure and providing anesthesia following intubation.
- Propofol (+/- ketamine) will generally break the seizure. However, an ongoing infusion of propofol is still needed to prevent seizure recurrence.
- If possible, propofol should be infused at a moderate-high rate (e.g. 50-80 mcg/kg/min).
- A low vasopressor dose may be needed to allow for propofol administration (e.g. phenylephrine or norepinephrine).
- If possible, propofol should be infused at a moderate-high rate (e.g. 50-80 mcg/kg/min).
- For severely hypotensive patients, a midazolam infusion may be used instead of propofol.
- The main drawback of midazolam is that it accumulates and wears off slowly, delaying extubation.
- Propofol (+/- ketamine) will generally break the seizure. However, an ongoing infusion of propofol is still needed to prevent seizure recurrence.
| Anesthetic agents (continuous infusion) |
- SE that is not controlled by the initial two anticonvulsant treatments is considered to be refractory and requires treatment with general anesthesia *.
- It was recently found that early use of anesthesia and thereby therapeutic coma (TC) induction after unsuccessful first-line treatment was associated with shorter SE duration as well as intensive care unit/hospital stay, than when a second-line ASM was applied first *.
🟢Consideration
- For continuous anesthetic infusion, patients should be intubated (avoid continuous anesthetic infusion if unable to intubate).
- Continuous EEG monitoring should be performed if continuous anesthetic infusion is being used.
🟢Dosing and duration of treatment
- Therapeutic target: Electrographic seizure cessation or burst suppression.
- Dosing
- Before initiating continuous anesthetic infusion, repeat boluses until seizure cessation.
- For refractory seizure, re-bolus and increase infusion rate *.
- Escalate both antiseizure medications and anesthetic infusions simultaneously until seizure control is achieved.
- A common mistake is to focus excessively on anesthetic infusions while forgetting to escalate antiseizure medications.
- Maximize the dose of each antiseizure medication. For status epilepticus it’s generally preferable to start with high doses to achieve seizure control.
- Before initiating continuous anesthetic infusion, repeat boluses until seizure cessation.
- Duration and intensity of treatment
- Once seizure control is achieved, therapies are typically continued for about one day *.
- Subsequently, the anesthetic agent may be gradually weaned off. The optimal rate of weaning off anesthetic infusions is not known.
- Ideally, this will result in the patient awakening without recurrent seizures.
- It is essential to continue maintenance of antiseizure medications throughout this period and beyond.
🟢Anesthetic agents
- First-line agent: Propofol or midazolam
- Propofol
- Propofol has the advantage that it is more easily titratable. For many patients, utilizing propofol may allow for rapid seizure lysis followed by extubation within <24 hours.
- Loading dose: 2 mg/kg IV bolus (up to 200 mg). May repeat q3-5 minutes if hemodynamically tolerated, to a total dose of 10 mg/kg.
- Maintenance infusion dose: Initial 30 mcg/kg/min. Maintenance 30-200 mcg/kg/min until the seizure stops.
- ⚠️Avoid propofol in patients on a ketogenic diet (as this may increase the risk of propofol infusion syndrome) *.
- Consider early initiation of enteral nutrition to reduce the risk of propofol infusion syndrome *.
- Midazolam
- High-dose midazolam infusions often take a long time to wear off so they may delay extubation.
- Loading dose: 0.2mg/kg IV (push over 1-2 min), max 20 mg. Repeat 0.2-0.4 mg/kg boluses q5-10min until the seizure stops, with a max total dose of 2mg/kg.
- Maintenance infusion dose: Initial 0.1 mg/kg/h; 0.1 to 2.9 mg/kg/h titrate to seizure suppression.
- Propofol
- Second-line agent: Ketamine
- Ketamine inhibits NMDA receptors, so it may function synergistically with either propofol or midazolam (which work via the GABA receptor).
- Ketamine is highly effective, with a superior side-effect profile compared to pentobarbital coma.
- During an episode of ongoing status epilepticus, there is a reduction in the number of inhibitory GABA receptors and an increase in the number of stimulatory glutamate receptors (e.g. NMDA receptors). Thus, the efficacy of agents targeting GABA receptors may decrease over time, whereas the efficacy of agents targeting glutamate receptors may be preserved over time. This provides a rationale for the use of ketamine in super-refractory status epilepticus (since it functions as an NMDA receptor).
- Loading dose: 1.5mg/kg IV (push over 3-5min), max 150 mg. Repeat until seizure cessation, max total dose of 4.5 mg/kg.
- Maintenance infusion dose: 1.2 mg/kg/h. Maintenance 0.3 to 7.5 mg/kg/h, titrate to seizure suppression.
- Ketamine inhibits NMDA receptors, so it may function synergistically with either propofol or midazolam (which work via the GABA receptor).
- Third-line agent: Pentobarbital coma
- Pentobarbital coma is the treatment of last resort.
- Pentobarbital coma is highly effective, but also fairly morbid.
- Due to the long half-life of pentobarbital, a high-dose pentobarbital coma obligates patients to spend a long time on mechanical ventilation.
- Pentobarbital also causes substantial hypotension and ileus.
- Loading dose: 5-15 mg/kg at 50 mg/min. May provide additional doses if seizures continue, up to a maximal total loading dose of 25 mg/kg.
- Maintenance infusion dose: Start at 1 mg/kg/hr, then titrate between 0.5-5 mg/kg/hr.
Subsequent management
Super-Refractory Status Epilepticus
Definition
- It is defined as persisting epilepticus for > 24 hours despite two antiseizure drugs and anesthetic agents, or when seizures reemerge on attempted anesthetic wean.
Management
- The following treatment may be used as adjunctive therapy (low level of evidence):
- Ketamine infusion *
- Ketamine is emerging as a preferred agent to control super-refractory status epilepticus *.
- Ketogenic diet *
- Hypothermia
- Inhaled anesthetic drugs
- Perampanel (a novel selective, non-competitive AMPA-type glutamate receptor antagonist) *
- Ketamine infusion *
New-onset refractory status epilepticus (NORSE)
Definition
- It is defined as refractory status epilepticus occurring in the context of new-onset status epilepticus in a patient with no history of seizure and no clear cause of seizure *.
Common causes
- Infectious (20%)
- Autoimmune (40%) such as anti-NMDA encephalitis.
- Paraneoplastic (30%)
Management: Consider early empiric therapy with high-dose steroids, plasma exchange, and/or IV immunoglobulins *.
Management of complications
The major complications of prolonged status are listed below. The propofol infusion syndrome will be explored here.
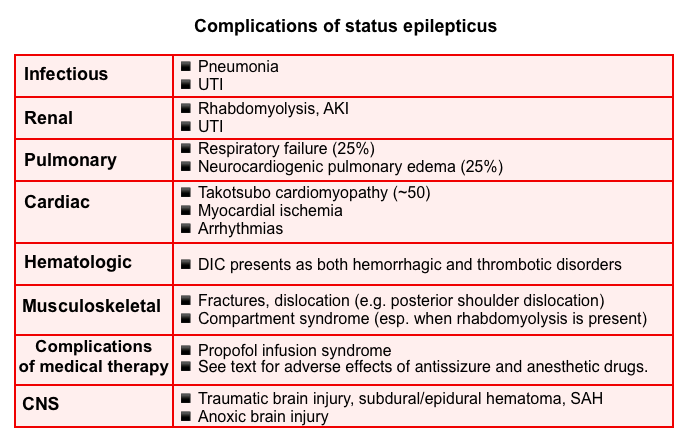
Propofol infusion syndrome (PRIS)
- Mechanism: Direct mitochondrial respiratory chain inhibition, impaired mitochondrial fatty acid metabolism.
- Diagnosis: It is a life-threatening condition characterized by acute refractory bradycardia progressing to asystole and one or more of the following *
- Metabolic acidosis
- Rhabdomyolysis
- Hyperlipidaemia
- Renal failure
- Enlarged or fatty liver
- Epidemiology: Although its incidence is low (around 1%), its mortality is high at 30% *
- Risk factors *
- Propofol infusion >4mg/kg/hr for 48 hours (large dose, long time); but can occur at lower doses.
- Younger age.
- Acute neurological injury, critical illnesses.
- Catecholamine or corticosteroid infusion.
- Low carbohydrate, high-fat diet, ketogenic diet *.
- Evaluation
- ECG: Brugada-like pattern, RBBB, arrhythmia, heart block.
- Blood gas: unexplained lactic acidosis; hyperkalemia (if rhabdomyolysis or renal failure).
- Management: Monitoring and supportive care.
- Monitor for early warning signs (↑lactate, ↑CK, urinary myoglobin, ECG)
- Discontinue propofol immediately.
- Adequate carbohydrate intake (6-8mg/kg/min) +/- carnitine supplementation.
Nonconvulsive status epilepticus
Overview
- Little agreement on diagnostic criteria, clinical forms, consequences, and treatment.
- No clinical and EEG findings are 100% specific *.
- There is no high level of evidence regarding the management of NCSE.
- Seizures may cause harm in different ways:
- Nonconvulsive status epilepticus could delay awakening from mechanical ventilation, and thereby extend ICU and hospital length of stay, leading to iatrogenic harm due accumulation of nosocomial complications.
- Among patients with severe brain injury, seizures may exacerbate abnormal physiology (e.g. causing further elevation of intracranial pressure).
- Overdiagnosis and aggressive use of anticonvulsants can contribute to morbidity and mortality.
- The most aggressive therapies involve continuous anesthetic infusions, which may require prolonged intubation. This exposes patients to risks involved with ongoing intubation.
- Individualized approach
- The approach to any individual patient may depend on a careful weighing of risks vs. benefits for individual therapies.
- It’s important to recognize that merely the presence of NCSE does not necessarily mandate very aggressive therapy (unlike convulsive status epilepticus).
Approach
- Identify and treat underlying causes and life threats.
- Generally, nonconvulsive status epilepticus should be treated similarly to status epilepticus, escalating the medical management until seizures are controlled.
- However, treatment escalation may be less aggressive in certain patients depending on the clinical circumstances. For example:
- Patients with generalized convulsive status epilepticus who subsequently transition into NCSE should generally be treated in an aggressive fashion (similar to treatment of ongoing convulsive status epilepticus).
- Patients with absence seizures or preserved consciousness may often be treated less aggressively.
- However, treatment escalation may be less aggressive in certain patients depending on the clinical circumstances. For example:
- Use cEEG monitoring if available to detect and diagnose NCSE and to assess response to treatment.
Antiseizure medications
Principles of pharmacotherapy
🔵Mechanism of action
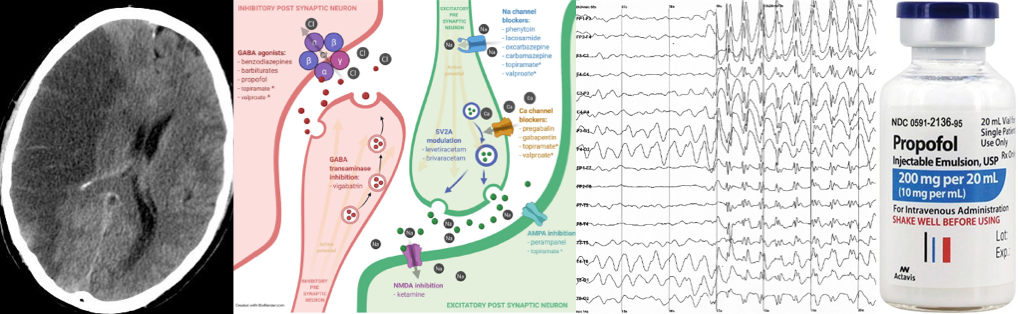
- Antiseizure and anesthetic agents work through modulation of three major channels including voltage-gated ion channels, enhancement of GABA-mediated inhibitory neurotransmission, and reduction of glutamate-mediated excitatory neurotransmission.
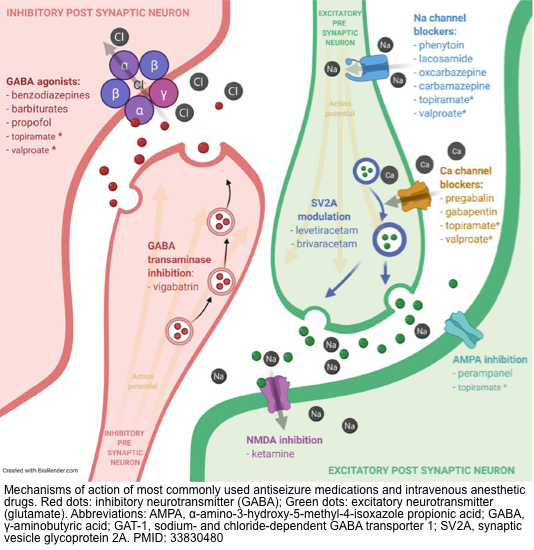
🔵Selection of medication
- Antiseizure drugs often have similar efficacy when compared to one another *.
- Therefore, selecting agents mostly based on safety profile and availability may be reasonable. Optimal selection of pharmacotherapy interventions is essential
to achieve adequate efficacy while minimizing drug interactions and adverse effects (appendix).- Check for drug-drug interactions (following tables) or use the Medscape Drug Interaction Checker.
- Selecting the antiepileptic drug (loading dose) for patients on chronic antiseizure medication therapy
- All patients with status epilepticus should be loaded with an antiseizure medication (usually levetiracetam, fosphenytoin, or valproate).
- For patients on one of these drugs previously:
- If the patient is believed to be adherent with therapy, it is reasonable to load with a different drug. For example, a valproate load could be used in a patient on chronic levetiracetam.
- If the patient is non-adherent, then re-loading with the patient’s chronic antiseizure drug could make sense.
- When in doubt, levetiracetam is often a good choice here (because supra-therapeutic levels are reasonably safe).
- Combination therapy
- It’s logical to combine agents with different mechanisms of action, when possible.
- Note that a combination of two sodium-channel blockers may cause increased adverse effects (e.g. phenytoin plus lacosamide).
- Avoid the combination of phenytoin and valproic acid.
- Antiseizure medications may have pharmacological interactions with each other (see appendix).
- It’s logical to combine agents with different mechanisms of action, when possible.
🔵Dosing
- Initial loading doses for emergent and urgent therapy in SE are provided in the table below.
- Most agents are dosed aggressively to quickly suppress seizure activity without inducing major adverse effects and there is usually no need for lower dosing.
- Maintenance doses should be initiated within 12 hours after a loading dose is given.
- Lower dosing is recommended for certain agents in selected patients (e.g. dose adjustment of levetiracetam in patients with renal impairment).
- Certain drug interactions with hepatic enzyme inducers or inhibitors will also require increases or decreases in daily dosing, respectively.
🔵Therapeutic drug monitoring (TDM)
- Monitoring of drug levels is generally available for valproic acid, phenytoin, and phenobarbital.
- As a rule of thumb, concentration should be measured at a steady state. The exceptions to this are *
- Patients with a diagnosis of status epilepticus
- Suspected toxicity
- Overdose
- Generally, the trough level (obtained before a dose) is most informative, as this reflects the minimal drug concentration achieved (corresponding to the highest risk of breakthrough seizure) *.
- If this concentration is subtherapeutic, seizure control may be compromised and a loading dose may be administered to quickly achieve adequate concentrations.
- Free concentrations for phenytoin and valproic acid (if available) are more useful compared with total levels since they are not affected by protein binding or drug interactions and represent the actual concentration of the agent in the CNS.
Table: Antiseizure agents
The pharmacologic properties, loading and maintenance doses as well as common antiseizure drug adverse effects are summarized below *,*.
Specific conditions
Seizures in intoxicated patients
🔴Common causes of toxicologic seizures *
- Sodium channel blocker intoxication
- Tricyclic antidepressants: amitriptyline, clomipramine, desipramine, imipramine, nortriptyline.
- Other antidepressants: bupropion, venlafaxine, citalopram, fluoxetine.
- Type Ia antiarrhythmics: disopyramide, procainamide, quinidine.
- Type Ic antiarrhythmics: flecainide, propafenone.
- Beta-blockers: acebutolol, betaxolol, propranolol.
- Antipsychotics: thioridazine, loxapine.
- Antihistamines: chlorpheniramine, diphenhydramine, doxepin.
- Antiepileptics: carbamazepine, lacosamide, lamotrigine, phenytoin.
- Antimalarials: chloroquine, hydroxychloroquine, quinine.
- Miscellaneous: Amantadine, cocaine, tramadol, topiramate.
- Isoniazid
- Hypoglycemic agents: Insulin, sulfonylurea agents.
- Sympathomimetic (e.g. amphetamines, cocaine, MDMA).
- Local anesthetic systemic toxicity *
- Salicylate
- Lithium
- Tramadol
- Cholinergic (e.g. organophosphate and carbamate insecticides, physostigmine, edrophonium).
- Carbon monoxide poisoning, heavy metals.
- Withdrawal states (e.g. chronic alcohol, benzodiazepine, barbiturate, baclofen, gabapentin, or antiseizure medications).
🔴Acute management of convulsive status epilepticus
- First-line treatment: Benzodiazepines *
- Lorazepam 0.1 mg/kg IV.
- Midazolam 10 mg IV/IM.
- Correct hypoglycemia, and electrolyte disturbances such as hyponatremia.
- Second-line treatment: Intubation followed by continuous anesthetic infusion (for the dual purpose of sedation and seizure management) should be considered for ongoing convulsive status epilepticus. A conventional antiseizure medication should be added as well👇.
- Conventional antiseizure medication
- Not every patient with toxicological seizures requires antiseizure medication.
- For example, if the cause of the seizure is being treated, that may be adequate (e.g. hypertonic bicarbonate administered for sodium channel blocker intoxication).
- Agent of choice
- The primary choice is usually between phenobarbital vs. levetiracetam.
- Phenobarbital is a good option, especially for seizures due to the withdrawal of ethanol, benzodiazepines, or barbiturates.
- A dose of ~15-18 mg/kg should provide antiepileptic activity, while simultaneously treating the withdrawal syndrome.
- Levetiracetam is a reasonable choice of antiepileptic agent *.
- Levetiracetam appears to be a safe choice, but its efficacy for toxicology-related seizures remains difficult to prove.
- Phenobarbital is a good option, especially for seizures due to the withdrawal of ethanol, benzodiazepines, or barbiturates.
- The primary choice is usually between phenobarbital vs. levetiracetam.
- Not every patient with toxicological seizures requires antiseizure medication.
- ⚠️Avoid phenytoin in toxicologic seizures since it has class Ib antiarrhythmic activity, which may aggravate the sodium-blocking effect of some intoxications *.
- Conventional antiseizure medication
🔴Resuscitation antidotes are given under special conditions
- Hypertonic bicarbonate (100mEq or 2mEq/kg) for sodium channel blocker poisoning, e.g. TCA intoxication.
- High-dose IV pyridoxine (e.g. 5g) for isoniazid poisoning *.
- Atropine and pralidoxime for organophosphate poisoning *.
- Urgent hemodialysis should be considered for lithium, theophylline, and salicylate intoxication *.
Going further
- Toxic seizures (LIFE IN THE FASTLANE)
- Status Epilepticus: Emergency Management by Justin Morgenstern
- Resuscitationist’s guide to status epilepticus (PulmCrit)
- Status epilepticus (WikEM)
- Status Epilepticus (IBCC)
- Emergency Management of Status Epilepticus (Emergency medicine Cases)
- Nonconvulsive Status Epilepticus (IBCC)
- EEG interpretation and ictal-interictal continuum (IBCC)
Appendix
Antiseizure and anesthetic drug adverse effects
Antiepileptic drug interactions
References
1. PMID: 21109098. Huff JS, Fountain NB. Pathophysiology and definitions of seizures and status epilepticus. Emerg Med Clin North Am. 2011 Feb;29(1):1-13. doi: 10.1016/j.emc.2010.08.001.
2. PMID: 15562299. Devinsky O. Effects of Seizures on Autonomic and Cardiovascular Function. Epilepsy Curr. 2004 Mar;4(2):43-46. doi: 10.1111/j.1535-7597.2004.42001.x.
3. PMID: 20724220. Theodore WH. The postictal state: effects of age and underlying brain dysfunction. Epilepsy Behav. 2010 Oct;19(2):118-20. doi: 10.1016/j.yebeh.2010.06.031. Epub 2010 Aug 17.
4. PMID: 28276060. Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, Lagae L, Moshé SL, Peltola J, Roulet Perez E, Scheffer IE, Zuberi SM. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017 Apr;58(4):522-530. doi: 10.1111/epi.13670. Epub 2017 Mar 8.
5. PMID: 35393970. Trinka E, Leitinger M. Management of Status Epilepticus, Refractory Status Epilepticus, and Super-refractory Status Epilepticus. Continuum (Minneap Minn). 2022 Apr 1;28(2):559-602. doi: 10.1212/CON.0000000000001103.
6. PMID: 35605066. Czuczwar SJ, editor. Epilepsy [Internet]. Brisbane (AU): Exon Publications; 2022 Apr 2.
7. PMID: 29650639. Kinney MO, Craig JJ, Kaplan PW. Non-convulsive status epilepticus: mimics and chameleons. Pract Neurol. 2018 Aug;18(4):291-305. doi: 10.1136/Pract Neurol-2017-001796. Epub 2018 Apr 12.
8. PMID: 31557430. Baker AM, Yasavolian MA, Arandi NR. Nonconvulsive status epilepticus: overlooked and undertreated. Emerg Med Pract. 2019 Oct;21(10):1-24. Epub 2019 Oct 1.
9. PMID: 33475321. Hirsch LJ, Fong MWK, Leitinger M, LaRoche SM, Beniczky S, Abend NS, Lee JW, Wusthoff CJ, Hahn CD, Westover MB, Gerard EE, Herman ST, Haider HA, Osman G, Rodriguez-Ruiz A, Maciel CB, Gilmore EJ, Fernandez A, Rosenthal ES, Claassen J, Husain AM, Yoo JY, So EL, Kaplan PW, Nuwer MR, van Putten M, Sutter R, Drislane FW, Trinka E, Gaspard N. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2021 Version. J Clin Neurophysiol. 2021 Jan 1;38(1):1-29. doi: 10.1097/WNP.0000000000000806.
10. PMID: 33480193. Kim D, Kim JM, Cho YW, Yang KI, Kim DW, Lee ST, No YJ, Seo JG, Byun JI, Kang KW, Kim KT; Drug Committee of Korean Epilepsy Society. Antiepileptic Drug Therapy for Status Epilepticus. J Clin Neurol. 2021 Jan;17(1):11-19. doi: 10.3988/jcn.2021.17.1.11
11. PMID: 24655445. Huff JS, Melnick ER, Tomaszewski CA, Thiessen ME, Jagoda AS, Fesmire FM; American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with seizures. Ann Emerg Med. 2014 Apr;63(4):437-47.e15. doi: 10.1016/j.annemergmed.2014.01.018. Erratum in: Ann Emerg Med. 2017 Nov;70(5):758.
12. PMID: 23961297. Najafi MR, Chitsaz A, Najafi MA. Jacksonian seizure as the relapse symptom of multiple sclerosis. J Res Med Sci. 2013 Mar;18(Suppl 1):S89-92.
13. PMID: 23041172. Brigo F, Storti M, Lochner P, Tezzon F, Fiaschi A, Bongiovanni LG, Nardone R. Tongue biting in epileptic seizures and psychogenic events: an evidence-based perspective. Epilepsy Behav. 2012 Oct;25(2):251-5. doi: 10.1016/j.yebeh.2012.06.020. Epub 2012 Oct 2.
14. PMID: 23142708. Brigo F, Nardone R, Ausserer H, Storti M, Tezzon F, Manganotti P, Bongiovanni LG. The diagnostic value of urinary incontinence in the differential diagnosis of seizures. Seizure. 2013 Mar;22(2):85-90. doi: 10.1016/j.seizure.2012.10.011. Epub 2012 Nov 9.
15. PMID: 30299997. Tranvinh E, Lanzman B, Provenzale J, Wintermark M. Imaging Evaluation of the Adult Presenting With New-Onset Seizure. AJR Am J Roentgenol. 2019 Jan;212(1):15-25. doi: 10.2214/AJR.18.20202. Epub 2018 Oct 9.
16. PMID: 19054416. Hope OA, Zeber JE, Kressin NR, Bokhour BG, Vancott AC, Cramer JA, Amuan ME, Knoefel JE, Pugh MJ. New-onset geriatric epilepsy care: Race, setting of diagnosis, and choice of antiepileptic drug. Epilepsia. 2009 May;50(5):1085-93. doi: 10.1111/j.1528-1167.2008.01892.x. Epub 2008 Nov 17.
17. PMID: 23925763. Hakami T, McIntosh A, Todaro M, Lui E, Yerra R, Tan KM, French C, Li S, Desmond P, Matkovic Z, O’Brien TJ. MRI-identified pathology in adults with new-onset seizures. Neurology. 2013 Sep 3;81(10):920-7. doi: 10.1212/WNL.0b013e3182a35193. Epub 2013 Aug 7.
18. PMID: 34618762. Rosenthal ES. Seizures, Status Epilepticus, and Continuous EEG in the Intensive Care Unit. Continuum (Minneap Minn). 2021 Oct 1;27(5):1321-1343. doi: 10.1212/CON.0000000000001012.
19. PMID: 18025394. Krumholz A, Wiebe S, Gronseth G, Shinnar S, Levisohn P, Ting T, Hopp J, Shafer P, Morris H, Seiden L, Barkley G, French J; Quality Standards Subcommittee of the American Academy of Neurology; American Epilepsy Society. Practice Parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2007 Nov 20;69(21):1996-2007. doi: 10.1212/01.wnl.0000285084.93652.43.
20. PMID: 15159471. Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004 May 25;62(10):1743-8. doi: 10.1212/01.wnl.0000125184.88797.62.
21. PMID: 31886441. Rossetti AO, Hirsch LJ, Drislane FW. Nonconvulsive seizures and nonconvulsive status epilepticus in the neuro ICU should or should not be treated aggressively: A debate. Clin Neurophysiol Pract. 2019 Aug 9;4:170-177. doi: 10.1016/j.cnp.2019.07.001
22. PMID: 21386814. Devinsky O, Gazzola D, LaFrance WC Jr. Differentiating between nonepileptic and epileptic seizures. Nat Rev Neurol. 2011 Apr;7(4):210-20. doi: 10.1038/nrneurol.2011.24. Epub 2011 Mar 8.
23. PMID: 23468561. Stone J, Reuber M, Carson A. Functional symptoms in neurology: mimics and chameleons. Pract Neurol. 2013 Apr;13(2):104-13. doi: 10.1136/Pract Neurol-2012-000422.
24. PMID: 31703811. Charidimou A. Cerebral amyloid angiopathy-related transient focal neurological episodes (CAA-TFNEs): A well-defined clinical-radiological syndrome. J Neurol Sci. 2019 Nov 15;406:116496. doi: 10.1016/j.jns.2019.116496. Epub 2019 Nov 6.
25. PMID: 34779831. Sanchez-Caro JM, de Lorenzo Martínez de Ubago I, de Celis Ruiz E, Arribas AB, Calviere L, Raposo N, Blancart RG, Fuentes B, Diez-Tejedor E, Rodriguez-Pardo J. Transient Focal Neurological Events in Cerebral Amyloid Angiopathy and the Long-term Risk of Intracerebral Hemorrhage and Death: A Systematic Review and Meta-analysis. JAMA Neurol. 2022 Jan 1;79(1):38-47. doi: 10.1001/jamaneurol.2021.3989.
26. PMID: 33896535. Apetauerova D, Patel PA, Burns JD, Lerner DP. Movement Disorder Emergencies. Neurol Clin. 2021 May;39(2):615-630. doi: 10.1016/j.ncl.2021.01.005. Epub 2021 Mar 31.
27. PMID: 30743298. Rajan S, Kaas B, Moukheiber E. Movement Disorders Emergencies. Semin Neurol. 2019 Feb;39(1):125-136. doi: 10.1055/s-0038-1677050. Epub 2019 Feb 11
28. PMID: 11499637. Müller J, Wissel J, Masuhr F, Ebersbach G, Wenning GK, Poewe W. Clinical characteristics of the geste antagoniste in cervical dystonia. J Neurol. 2001 Jun;248(6):478-82. doi: 10.1007/s004150170156.
29. PMID: 28168537. Cossu G, Colosimo C. Hyperkinetic Movement Disorder Emergencies. Curr Neurol Neurosci Rep. 2017 Jan;17(1):6. doi: 10.1007/s11910-017-0712-7.
30. PMID: 17362837. Meierkord H, Holtkamp M. Non-convulsive status epilepticus in adults: clinical forms and treatment. Lancet Neurol. 2007 Apr;6(4):329-39. doi: 10.1016/S1474-4422(07)70074-1.
31. PMID: 12531946. Husain AM, Horn GJ, Jacobson MP. Non-convulsive status epilepticus: usefulness of clinical features in selecting patients for urgent EEG. J Neurol Neurosurg Psychiatry. 2003 Feb;74(2):189-91. doi: 10.1136/jnnp.74.2.189.
32. PMID: 27571047. Schmitt SE. Utility of Clinical Features for the Diagnosis of Seizures in the Intensive Care Unit. J Clin Neurophysiol. 2017 Mar;34(2):158-161. doi: 10.1097/WNP.0000000000000335.
33. PMID: 33475321. Hirsch LJ, Fong MWK, Leitinger M, LaRoche SM, Beniczky S, Abend NS, Lee JW, Wusthoff CJ, Hahn CD, Westover MB, Gerard EE, Herman ST, Haider HA, Osman G, Rodriguez-Ruiz A, Maciel CB, Gilmore EJ, Fernandez A, Rosenthal ES, Claassen J, Husain AM, Yoo JY, So EL, Kaplan PW, Nuwer MR, van Putten M, Sutter R, Drislane FW, Trinka E, Gaspard N. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2021 Version. J Clin Neurophysiol. 2021 Jan 1;38(1):1-29. doi: 10.1097/WNP.0000000000000806.
34. PMID: 24084180. Drislane FW. Overlap of encephalopathies and epileptic seizures. J Clin Neurophysiol. 2013 Oct;30(5):468-76. doi: 10.1097/WNP.0b013e3182a73bfa.
35. PMID: 30921018. VanHaerents S, Gerard EE. Epilepsy Emergencies: Status Epilepticus, Acute Repetitive Seizures, and Autoimmune Encephalitis. Continuum (Minneap Minn). 2019 Apr;25(2):454-476. doi: 10.1212/CON.0000000000000716.
36. PMID: 22528274. Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, Laroche SM, Riviello JJ Jr, Shutter L, Sperling MR, Treiman DM, Vespa PM; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012 Aug;17(1):3-23. doi: 10.1007/s12028-012-9695-z.
37. PMID: 28027373. Gavvala JR, Schuele SU. New-Onset Seizure in Adults and Adolescents: A Review. JAMA. 2016 Dec 27;316(24):2657-2668. doi: 10.1001/jama.2016.18625.
38. PMID: 35393970. Trinka E, Leitinger M. Management of Status Epilepticus, Refractory Status Epilepticus, and Super-refractory Status Epilepticus. Continuum (Minneap Minn). 2022 Apr 1;28(2):559-602. doi: 10.1212/CON.0000000000001103.
39. PMID: 37469676. Byun JI. Management of convulsive status epilepticus: recent updates. Encephalitis. 2023 Apr;3(2):39-43. doi: 10.47936/encephalitis.2022.00087. Epub 2023 Jan 6.
40. PMID: 11547716. Alldredge BK, Gelb AM, Isaacs SM, Corry MD, Allen F, Ulrich S, Gottwald MD, O’Neil N, Neuhaus JM, Segal MR, Lowenstein DH. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001 Aug 30;345(9):631-7. doi: 10.1056/NEJMoa002141. Erratum in: N Engl J Med 2001 Dec 20;345(25):1860.
41. PMID: 33664203. Rossetti AO, Alvarez V. Update on the management of status epilepticus. Curr Opin Neurol. 2021 Apr 1;34(2):172-181. doi: 10.1097/WCO.0000000000000899.
42. PMID: 33176370. Der-Nigoghossian C, Tesoro EP, Strein M, Brophy GM. Principles of Pharmacotherapy of Seizures and Status Epilepticus. Semin Neurol. 2020 Dec;40(6):681-695. doi: 10.1055/s-0040-1718721. Epub 2020 Nov 11.
43. PMID: 31774955. Kapur J, Elm J, Chamberlain JM, Barsan W, Cloyd J, Lowenstein D, Shinnar S, Conwit R, Meinzer C, Cock H, Fountain N, Connor JT, Silbergleit R; NETT and PECARN Investigators. Randomized Trial of Three Anticonvulsant Medications for Status Epilepticus. N Engl J Med. 2019 Nov 28;381(22):2103-2113. doi: 10.1056/NEJMoa1905795.
44. PMID: 34300194. Alolayan YS, McKinley K, Bhatia R, Alkhachroum A. Review and Updates on the Treatment of Refractory and Super Refractory Status Epilepticus. J Clin Med. 2021 Jul 7;10(14):3028. doi: 10.3390/jcm10143028.
45. PMID: 35605086. Heuser K, Olsen KB, Ulvin LB, Gjerstad L, Taubøll E. Modern Treatment of Status Epilepticus in Adults. In: Czuczwar SJ, editor. Epilepsy [Internet]. Brisbane (AU): Exon Publications; 2022 Apr 2. Chapter 5.
46. PMID: 34045273. De Stefano P, Baumann SM, Semmlack S, Rüegg S, Marsch S, Seeck M, Sutter R. Safety and Efficacy of Coma Induction Following First-Line Treatment in Status Epilepticus: A 2-Center Study. Neurology. 2021 Aug 10;97(6):e564-e576. doi: 10.1212/WNL.0000000000012292. Epub 2021 May 27.
47. PMID: 33480193. Kim D, Kim JM, Cho YW, Yang KI, Kim DW, Lee ST, No YJ, Seo JG, Byun JI, Kang KW, Kim KT; Drug Committee of Korean Epilepsy Society. Antiepileptic Drug Therapy for Status Epilepticus. J Clin Neurol. 2021 Jan;17(1):11-19. doi: 10.3988/jcn.2021.17.1.11
48. PMID: 32140640. Mahmoud SH, Ho-Huang E, Buhler J. Systematic review of ketogenic diet use in adult patients with status epilepticus. Epilepsia Open. 2019 Nov 24;5(1):10-21. doi: 10.1002/epi4.12370.
50. PMID: 35151187. Perez DQ, Espiritu AI, Jamora RDG. Perampanel in achieving status epilepticus cessation: A systematic review. Epilepsy Behav. 2022 Mar;128:108583. doi: 10.1016/j.yebeh.2022.108583. Epub 2022 Feb 9
51. PMID: 17567345. Kam PC, Cardone D. Propofol infusion syndrome. Anaesthesia. 2007 Jul;62(7):690-701. doi: 10.1111/j.1365-2044.2007.05055.x.
52. PMID: 21464061. Diedrich DA, Brown DR. Analytic reviews: propofol infusion syndrome in the ICU. J Intensive Care Med. 2011 Mar-Apr;26(2):59-72. doi: 10.1177/0885066610384195.
53. PMID: 18664783. Fong JJ, Sylvia L, Ruthazer R, Schumaker G, Kcomt M, Devlin JW. Predictors of mortality in patients with suspected propofol infusion syndrome. Crit Care Med. 2008 Aug;36(8):2281-7. doi: 10.1097/CCM.0b013e318180c1eb.
54. PMID: 29957667. Patsalos PN, Spencer EP, Berry DJ. Therapeutic Drug Monitoring of Antiepileptic Drugs in Epilepsy: A 2018 Update. Ther Drug Monit. 2018 Oct;40(5):526-548. doi: 10.1097/FTD.0000000000000546.
55. PMID: 16227064. Wills B, Erickson T. Drug- and toxin-associated seizures. Med Clin North Am. 2005 Nov;89(6):1297-321. doi: 10.1016/j.mcna.2005.06.004.
56. PMID: 31461049. Gitman M, Fettiplace MR, Weinberg GL, Neal JM, Barrington MJ. Local Anesthetic Systemic Toxicity: A Narrative Literature Review and Clinical Update on Prevention, Diagnosis, and Management. Plast Reconstr Surg. 2019 Sep;144(3):783-795. doi: 10.1097/PRS.0000000000005989.
57. PMID: 28753046. Lee T, Warrick BJ, Sarangarm P, Alunday RL, Bussmann S, Smolinske SC, Seifert SA. Levetiracetam in toxic seizures. Clin Toxicol (Phila). 2018 Mar;56(3):175-181. doi: 10.1080/15563650.2017.1355056. Epub 2017 Jul 28


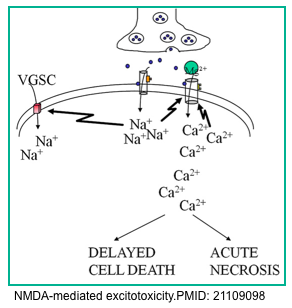
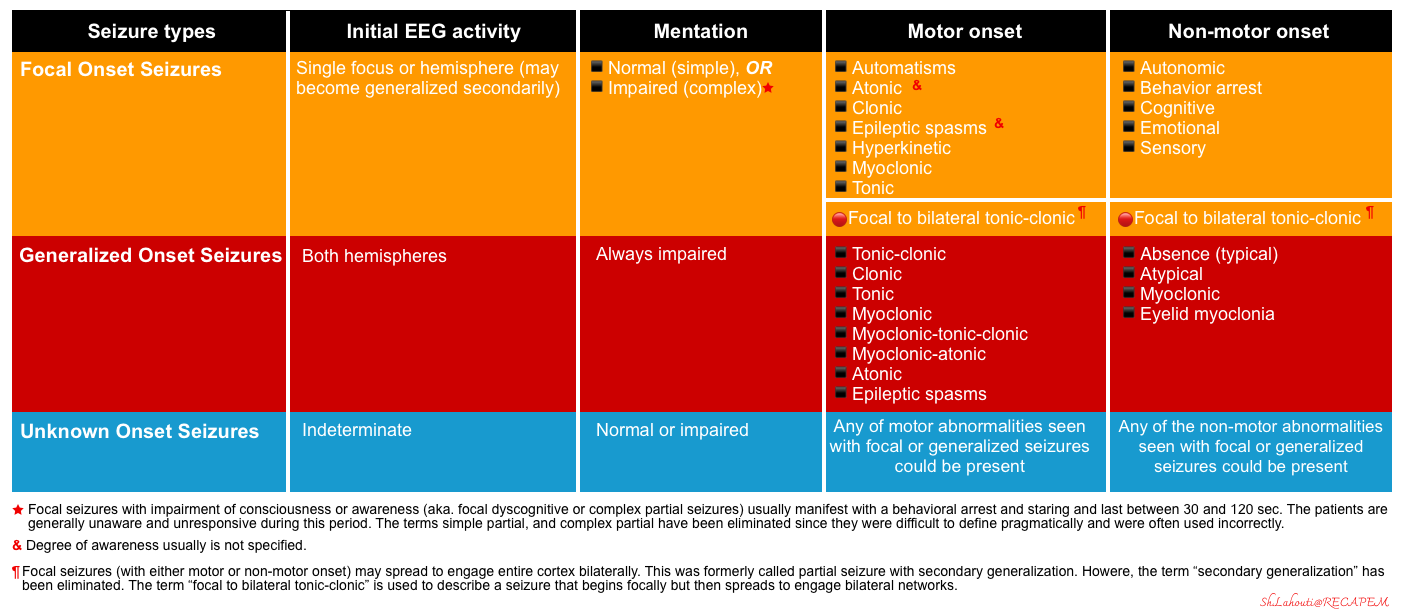
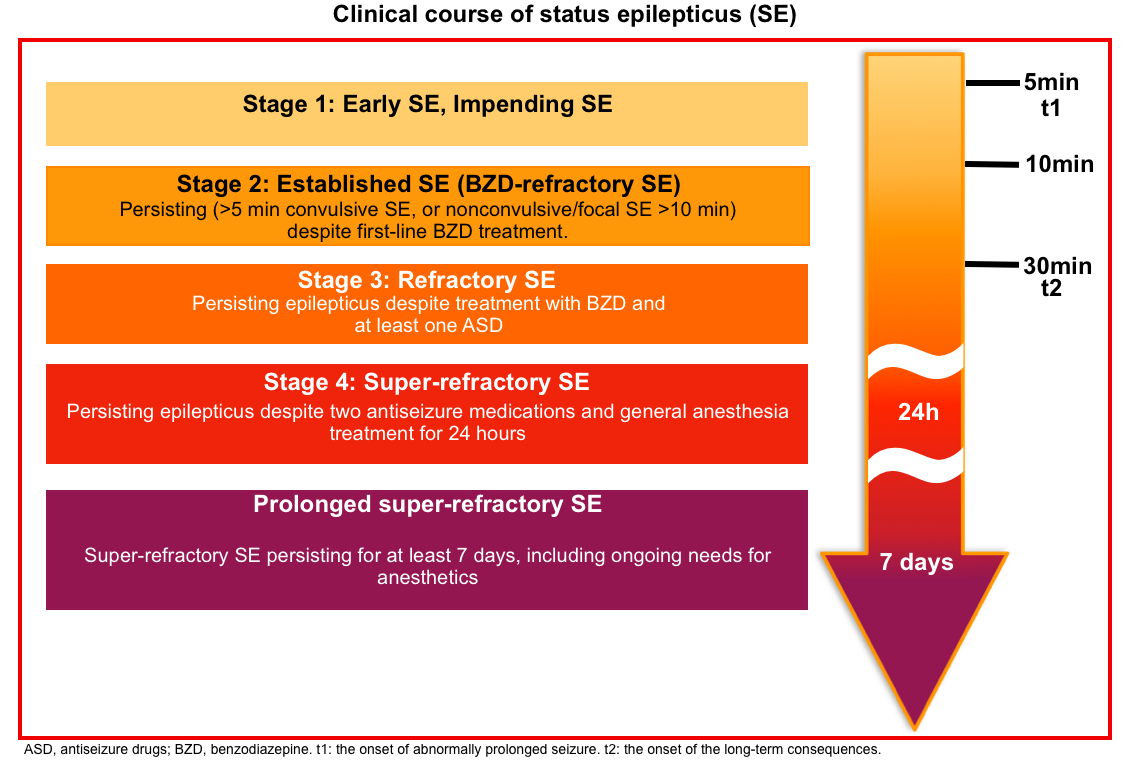
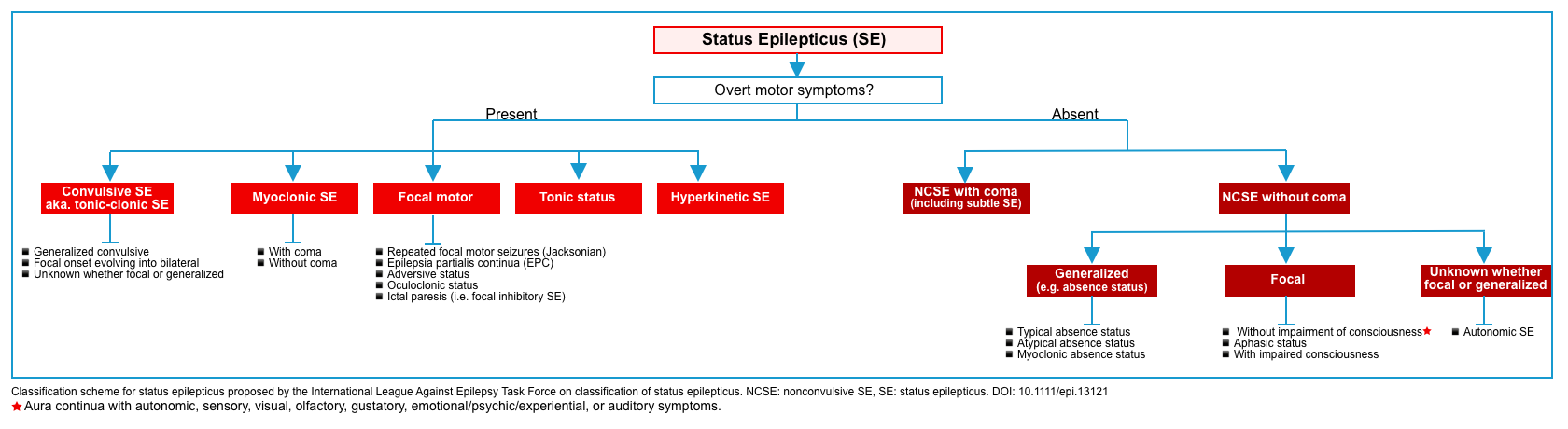
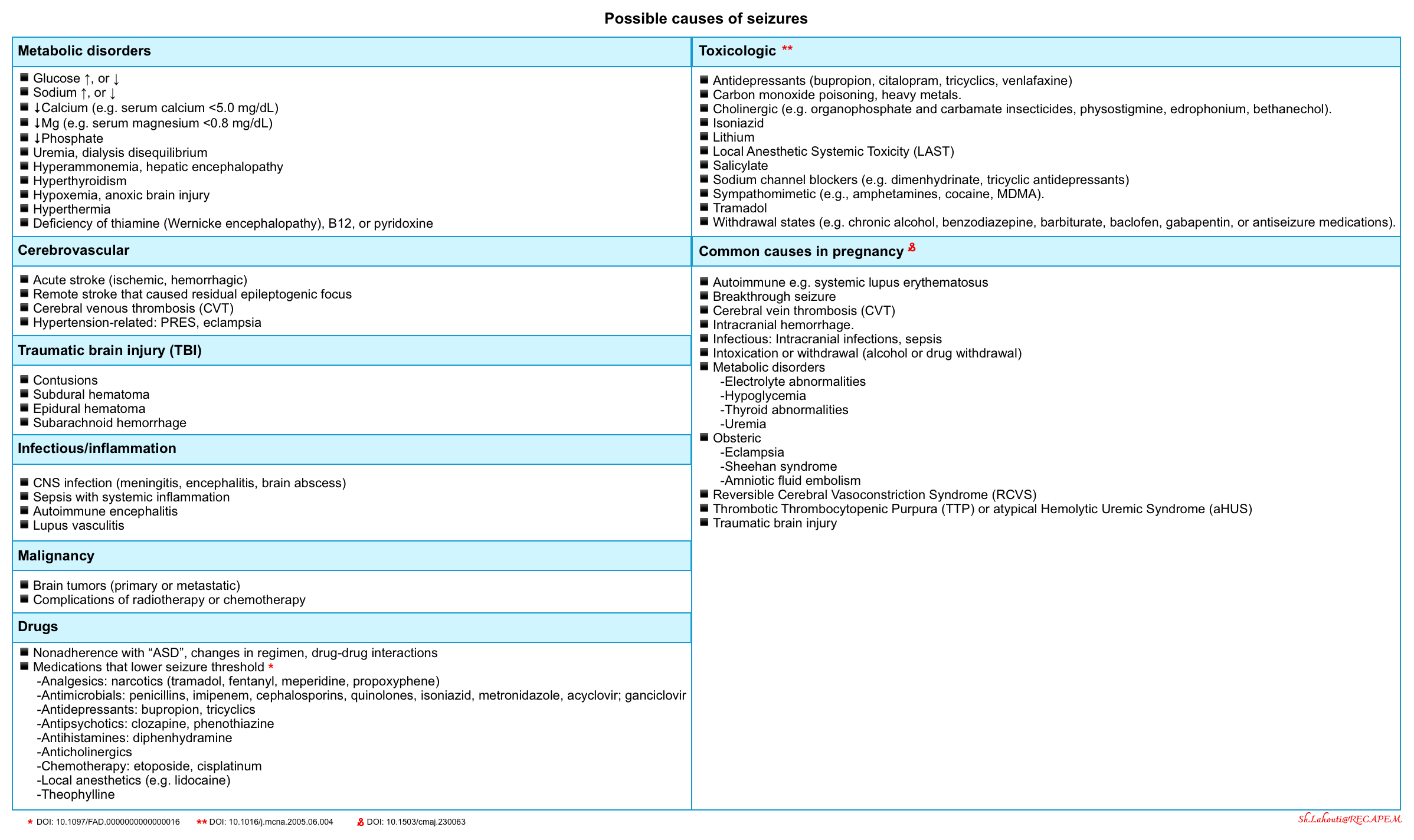
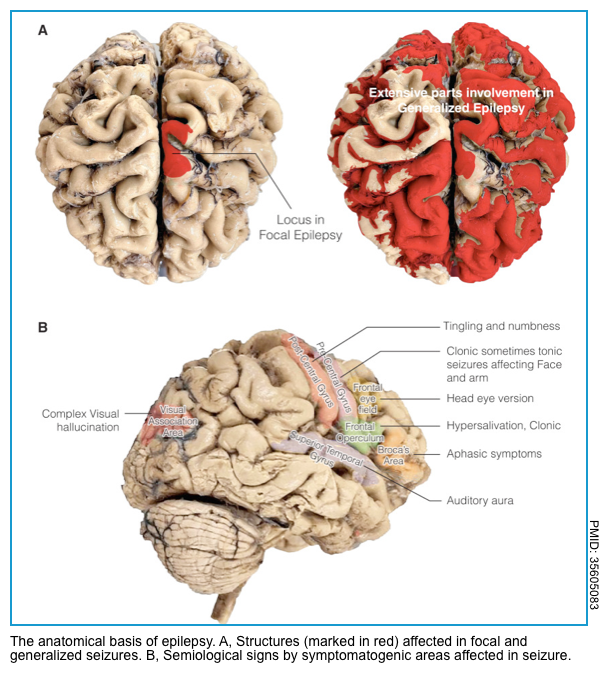
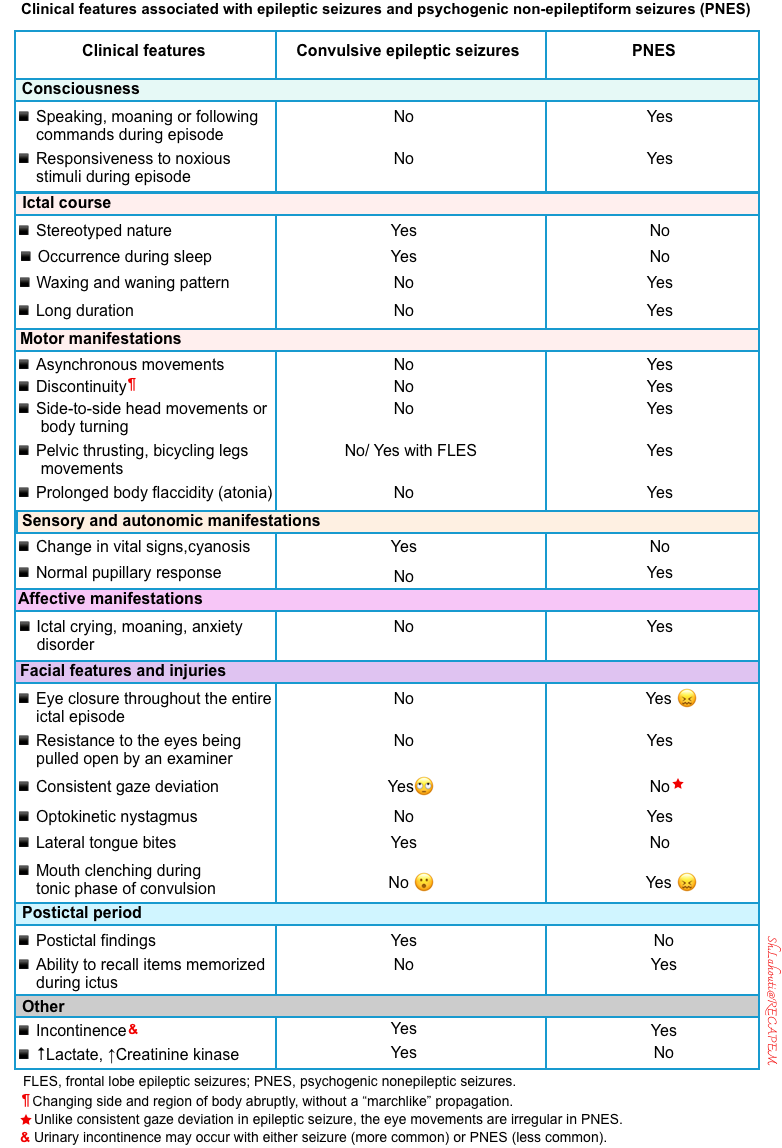
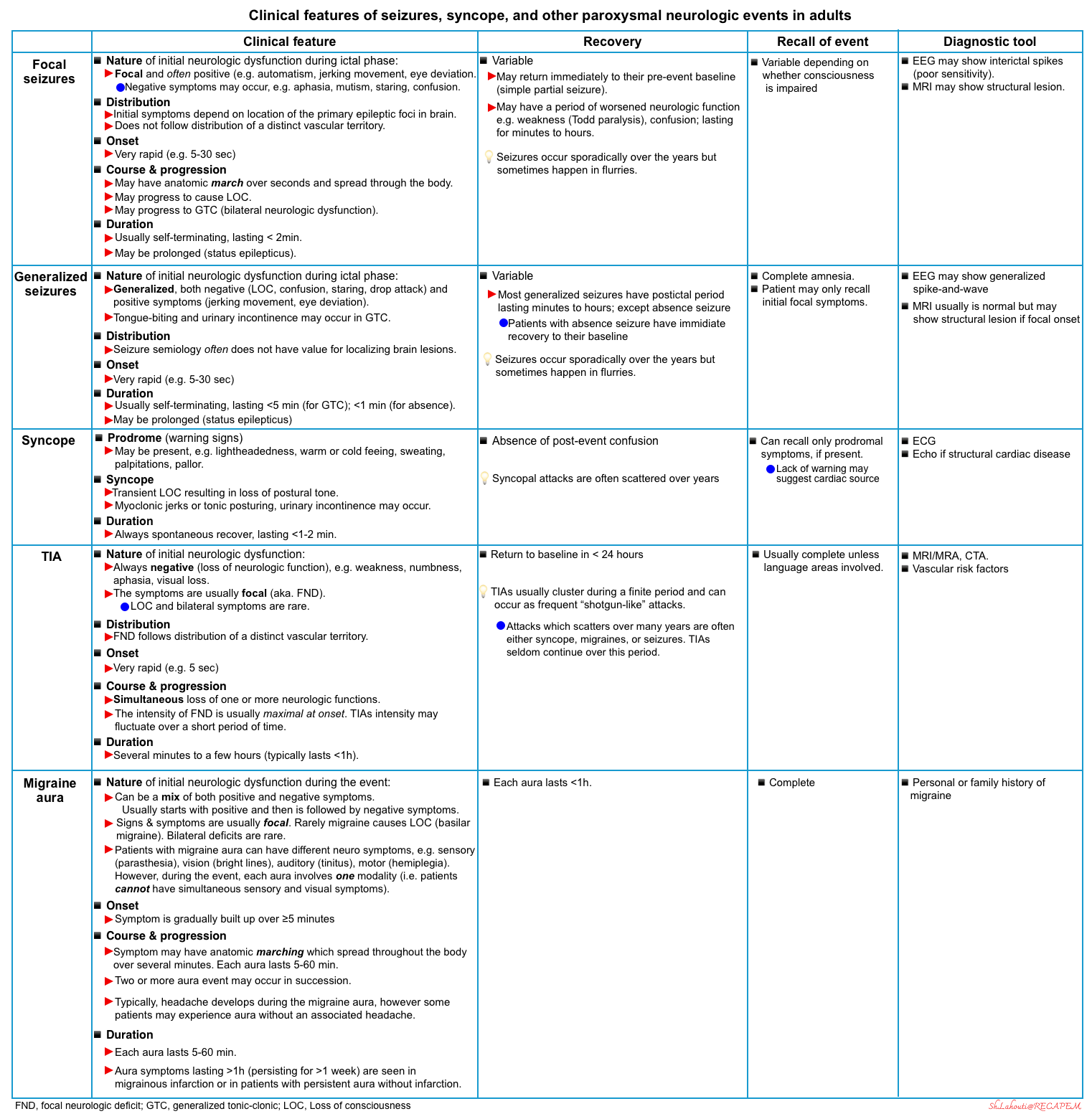
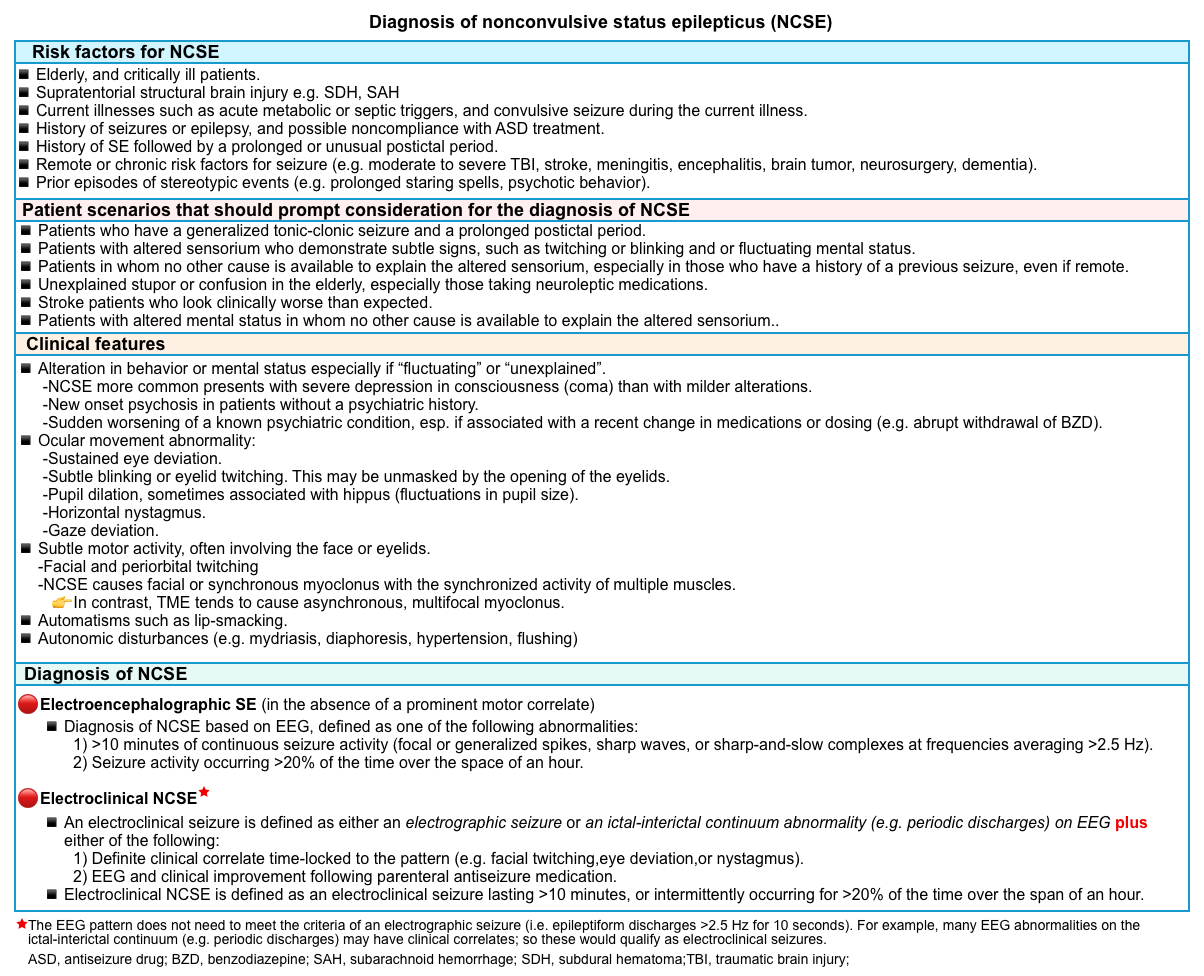
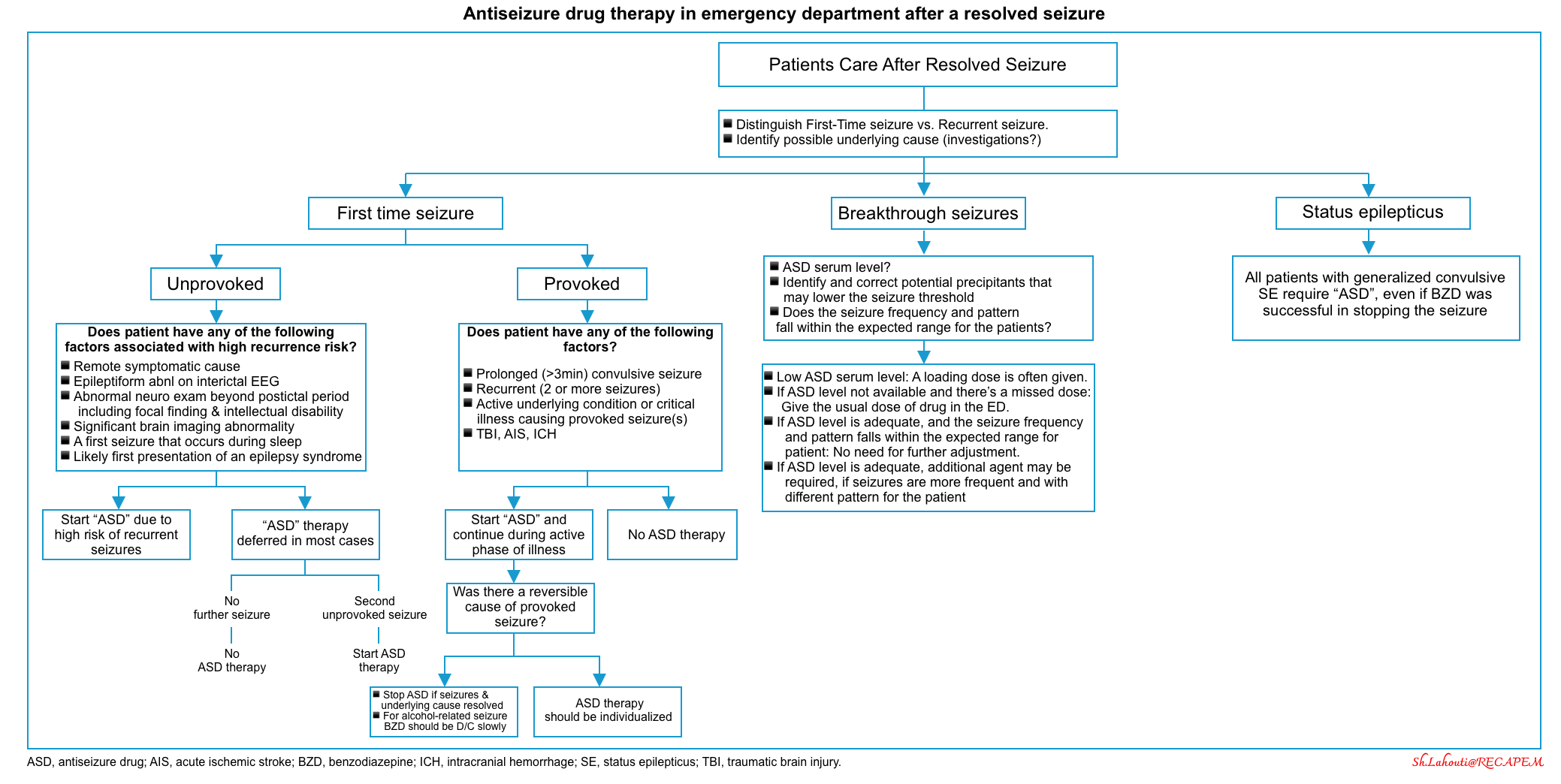
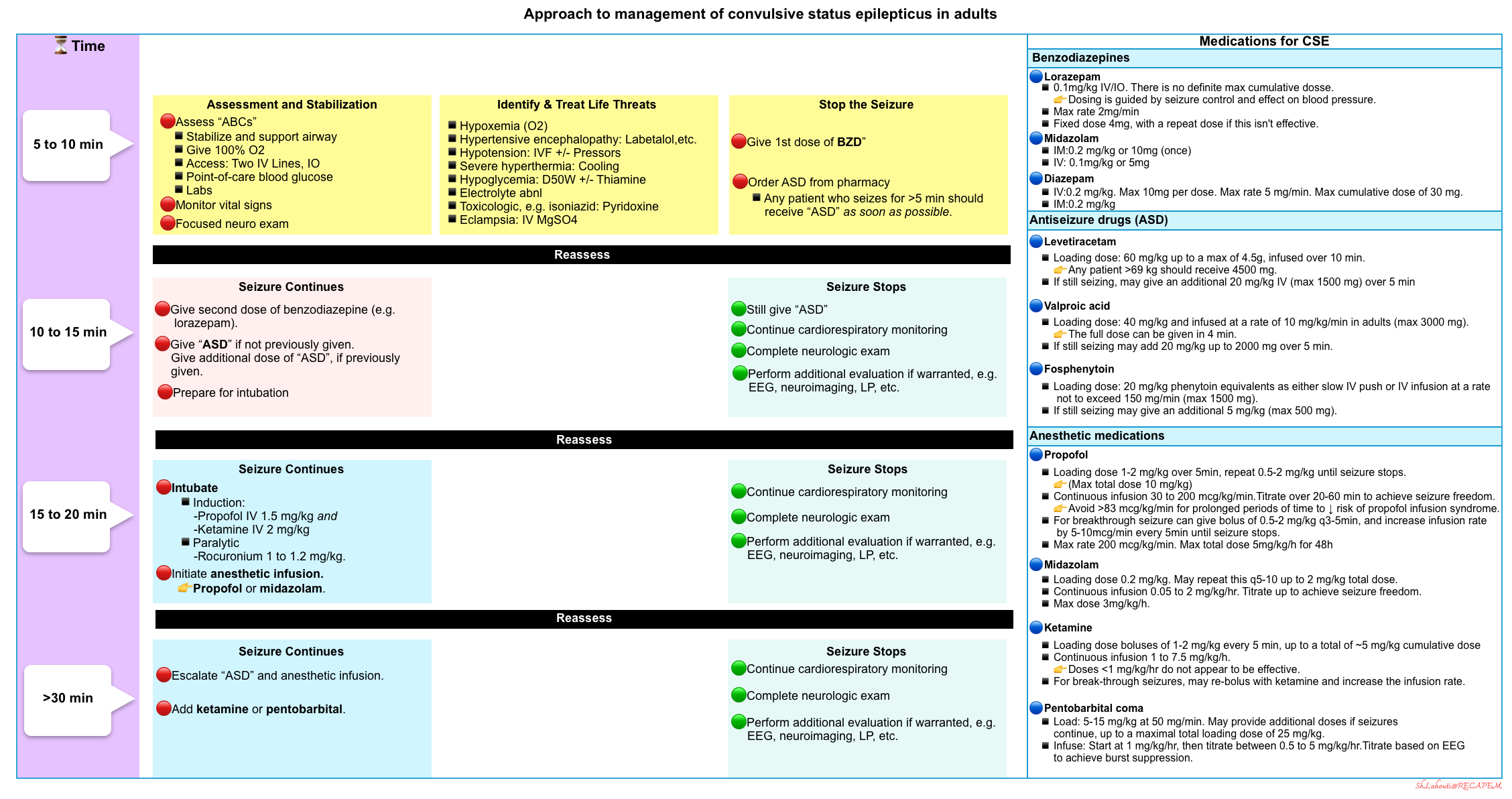
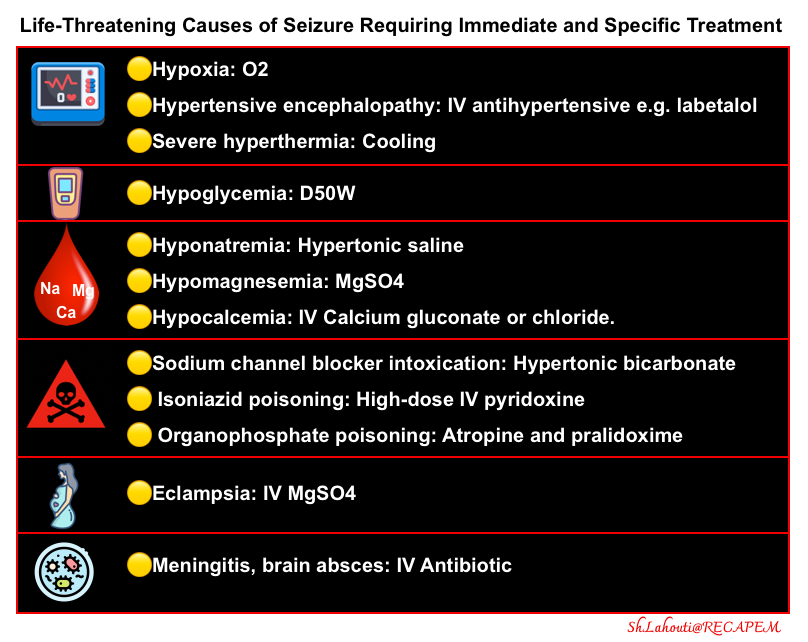
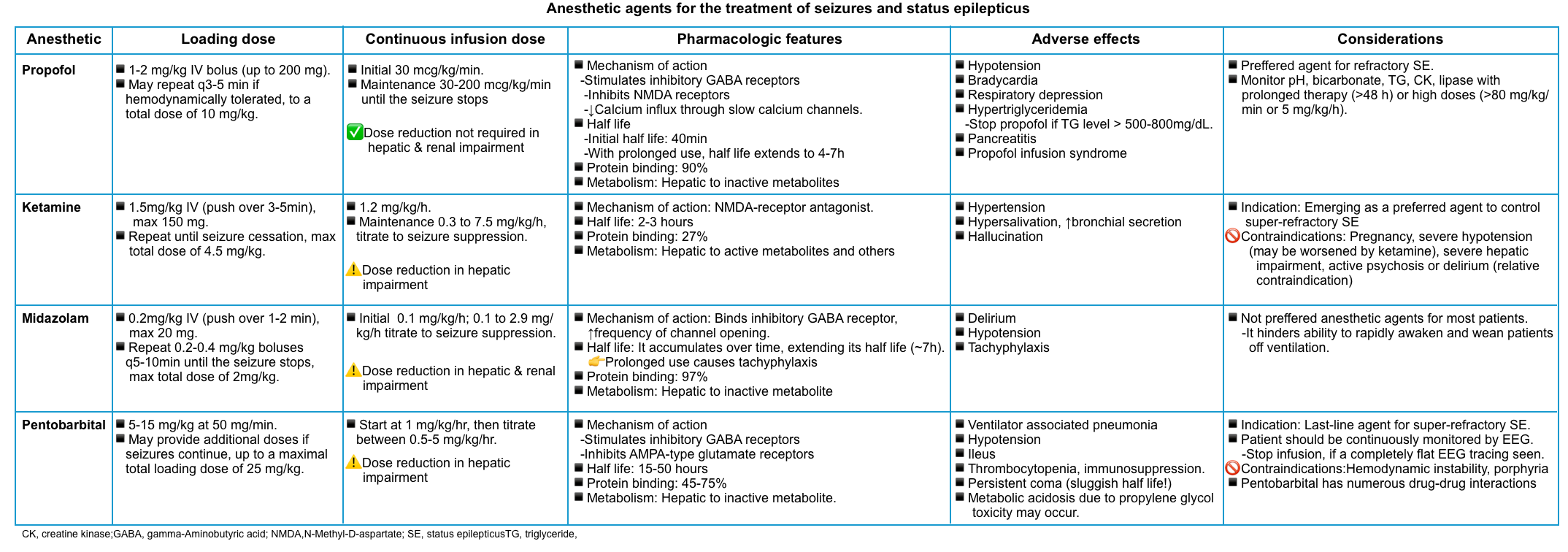
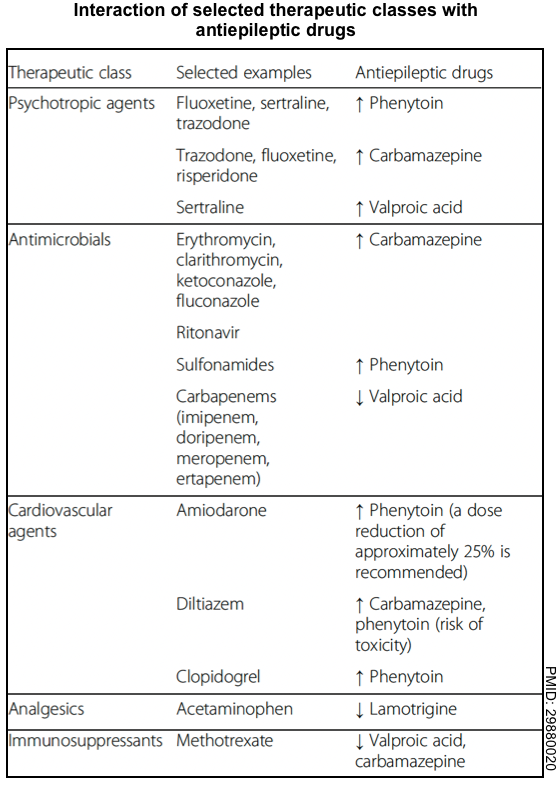
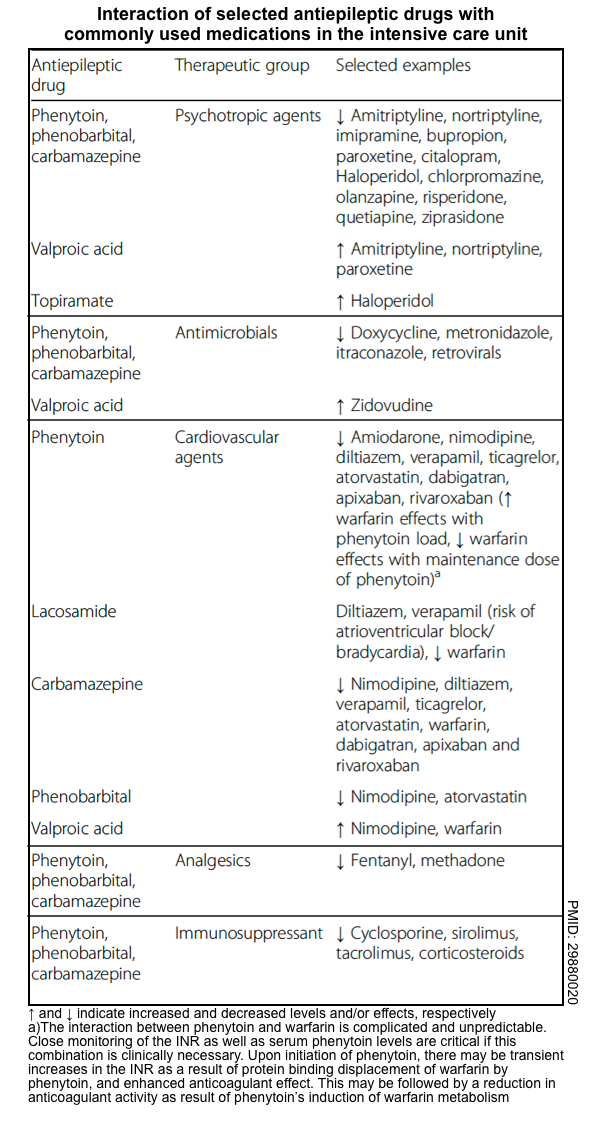
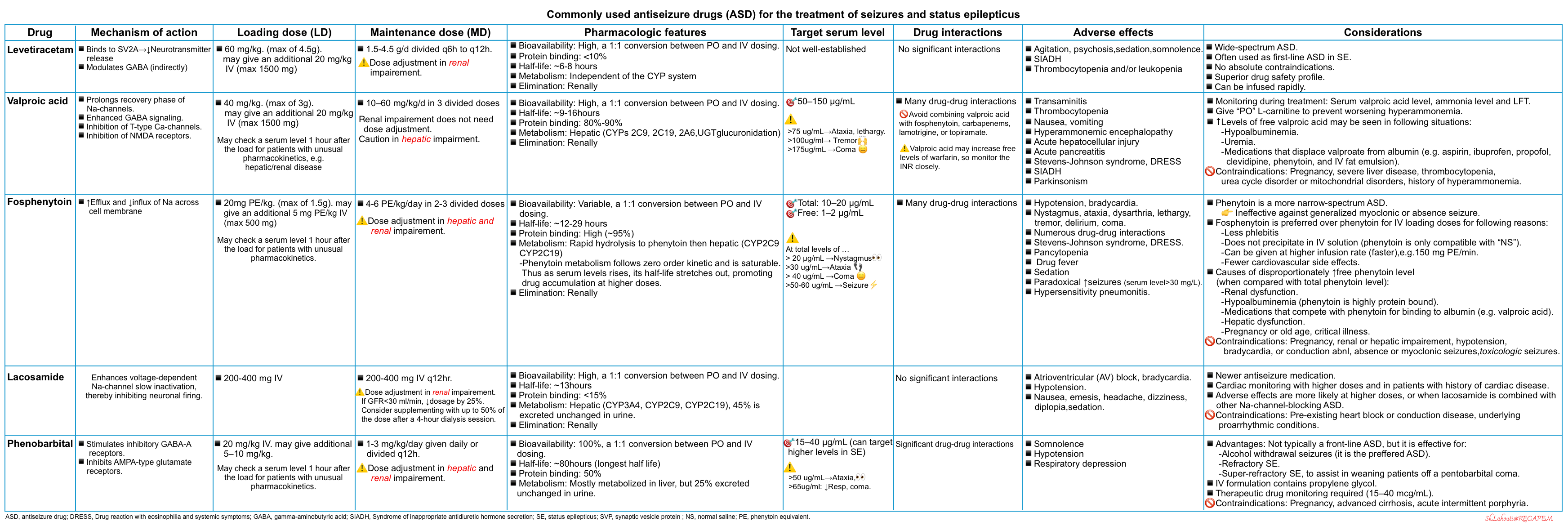
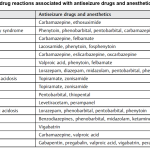

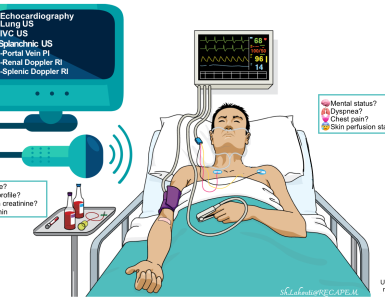

Add comment