13 July 2023 by Shahriar Lahouti.
CONTENTS
- Preface
- Etiology
- Pathophysiology
- Diagnosis
- Transthoracic echocardiography
- Discordant grading of AS
- Evaluation severity of AS
- Approach to management in ED
- Definitive treatment
- Multiple and Mixed Valvular Heart Disease
- Appendix
- Going further
- Media
- References
Preface
Aortic stenosis is one of the most common forms of valvular heart disease with increasing prevalence with age. Left ventricular outflow tract obstruction (aortic stenosis) can happen at different levels, namely sub-valvular, valvular, or supra-valvular obstruction.
Severe aortic stenosis can cause heart failure and cardiogenic shock. This post will cover severe (critical) valvular aortic stenosis.
Case scenario
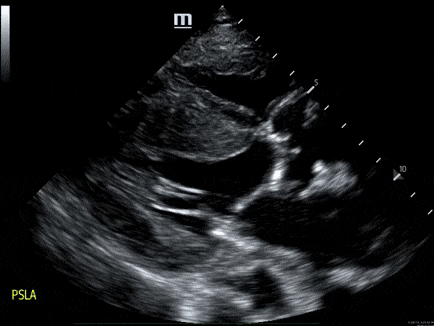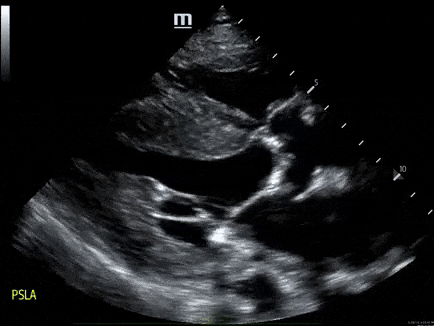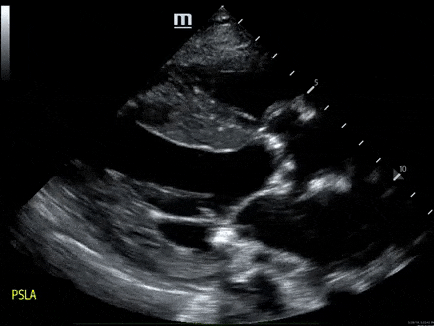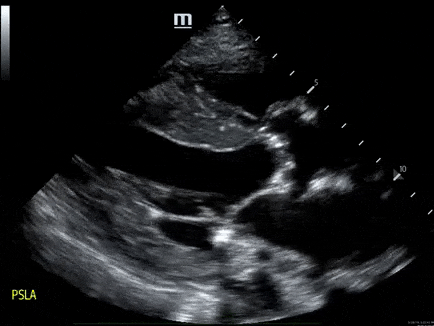
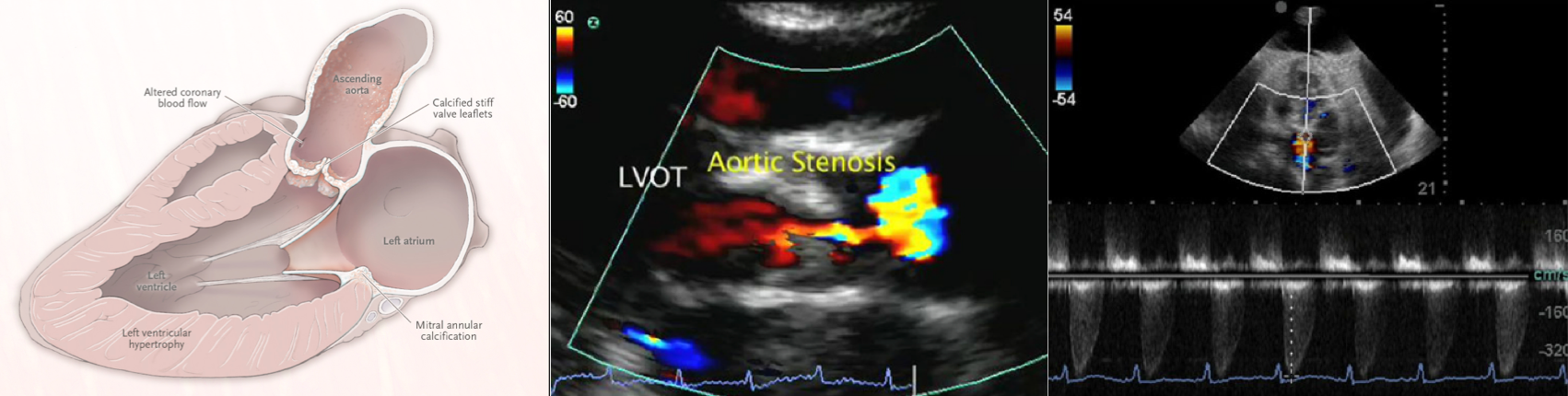
A 79-year-old male with a past medical history of HTN, and CKD, presents via EMS after a syncopal episode. He reports multiple episodes of syncope within the past two weeks. He also has dyspnea that has been worsening over several weeks.
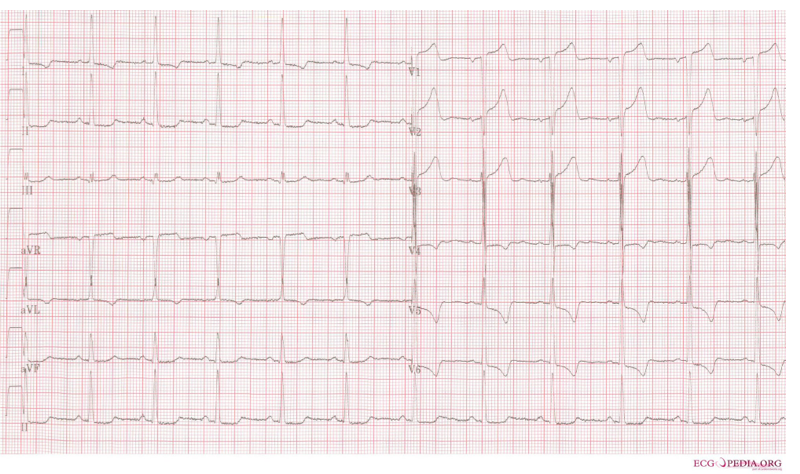
- His vital signs are as follows: HR 123, BP 80/60, RR 28, SpO2 85% on room air. ECG: LVH
- The echo is shown below. Bedside ultrasound reveals diffuse B lines consistent with pulmonary edema, and he is placed on non-invasive positive pressure ventilation.
- How would you manage this patient? see here
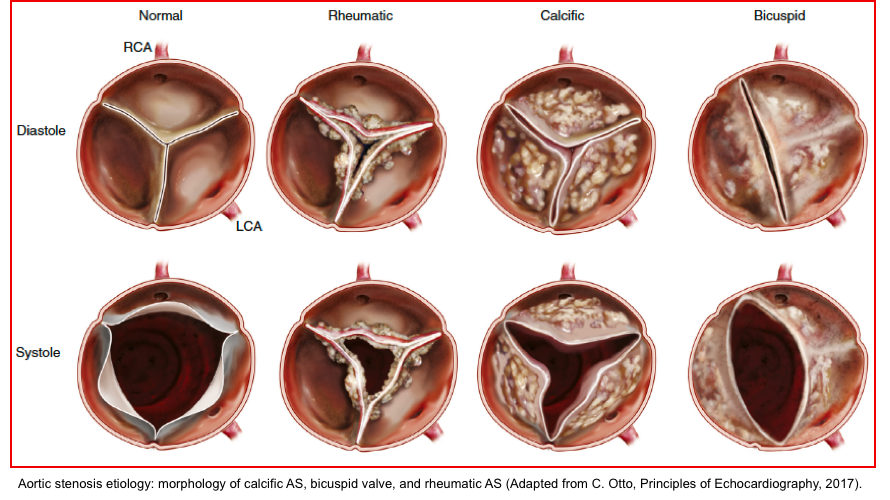
Etiology
Valvular aortic stenosis (is the center of focus in this post).
- Rheumatic valve disease
- Degenerative disease
- Calcific disease of a trileaflet aortic valve (associated with advanced age).
- Superimposed calcification of a congenitally abnormal valve (bicuspid aortic valve).
- Valve thrombosis
Sub-valvular AS
- Fixed obstruction e.g. a discrete fibrous subaortic membrane *.
- Dynamic obstruction (dynamic LVOTO)
Supra-valvular AS
- It is a rare condition caused by systemic elastin arteriopathy. The most frequently described variant is the so-called “hourglass”, given by pronounced aortic thickening with a resulting annular ridge at the superior margin of the Valsalva sinuses *.
Pathophysiology
In individuals with normal aortic valves, the effective area of valve opening equals the cross-sectional area of the left ventricular outflow tract, which is approximately 3 to 4 cm2 in adults.
- A normally opening aortic valve is not obstructive to blood flow.
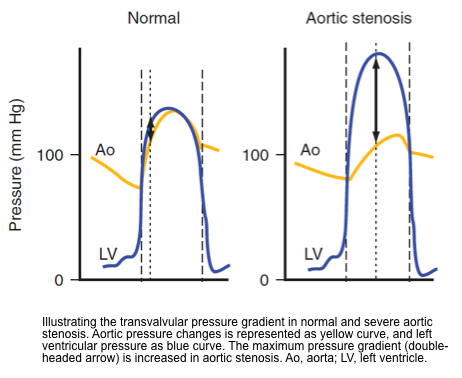
- In a normal cardiac cycle, left ventricular (LV) pressure increases till it reaches aortic pressure, the AV then opens with the ejection of blood, and the LV and aortic pressures are relatively identical. When pressure in LV falls below aortic pressure the AV closes ending systole.
- The transvalvular flow is laminar with a peak velocity < 2 m/s.
- In AS (small stenotic orifice) there is obstructive to blood flow.
- The LV needs to exert more pressure to eject blood through the valve leading to increased LV systolic pressure.
- The transvalvular gradient is built up with LV pressure higher than aortic pressure.
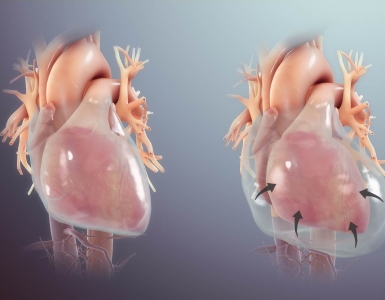
- Narrowing of the AV orifice results in an increased resistance to left ventricular ejection ( ⬆️ LV afterload), with rising systolic pressure in the LV.
- Progressive stenosis of the AV leads to an increasing transvalvular systolic pressure gradient.
- Adaptive response
- The stenotic process of AS is often gradual in onset and progression, resulting in adaptive changes in the left ventricle *.
- The progressively increasing systolic pressure in the LV leads to concentric hypertrophy (as a compensatory adaptive mechanism to maintain cardiac output).
- Concentric LV hypertrophy accommodates the afterload, thereby maintaining ejection fraction and stroke volume.
- This process of compensatory LV hypertrophy holds LV end-diastolic volume and systolic ejection fraction within the normal range for a prolonged time.
- Maladaptive response
- Progressive LV hypertrophy to compensate for worsening AS can become maladaptive over time, leading to left ventricular dysfunction.
- Diastolic LV dysfunction
- With progressively increasing LV wall thickness, the LV becomes less compliant, and left ventricular end-diastolic pressure tends to increase (LV diastolic dysfunction).
- This excessive hypertrophy with impaired diastolic filling makes patients with critical AS very preload dependent and they will often require higher filling pressures to maintain adequate preload.
- LV remodeling puts the patients at risk for the following maladaptive changes *
- Secondary mitral regurgitation *
- Elevated PCWP
- Post-capillary pulmonary hypertension (Type II pHTN)
- Right ventricular dysfunction.
- With progressively increasing LV wall thickness, the LV becomes less compliant, and left ventricular end-diastolic pressure tends to increase (LV diastolic dysfunction).
- Systolic LV dysfunction
- Eventually, LV systolic performance deteriorates as a result of several contributing factors *
- LV wall fibrosis
- Subendocardial ischemia
- Regional wall motion abnormalities
- Eventually, LV systolic performance deteriorates as a result of several contributing factors *
Diagnosis
Aortic stenosis typically represents a chronic condition that leads to ADHF or CS due to progressive valve and myocardial dysfunction or more often a superimposed secondary disease process.
Diagnostic challenge
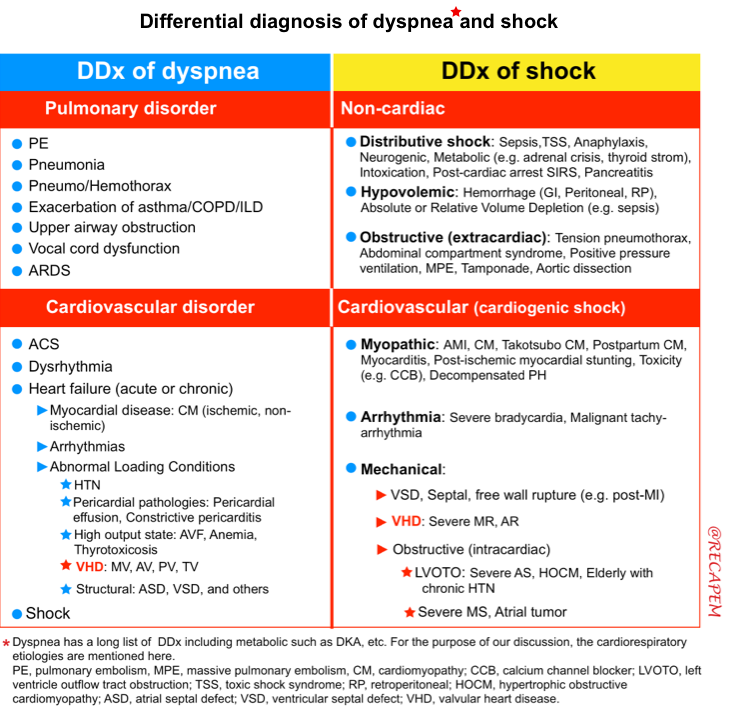
- In unstable patients with ADHF, and CS; it is a challenging consideration to identify the forefront cause of instability. For example, in a patient with severe AS in CS, one should consider
- Is the patient instability due to the severe AS? or
- Is the AS an incidental finding or a complicating factor?
👉The major concern in ED is to rapidly identify life-threatening valvular lesions. Three essential questions should be kept in mind when screening for valvular emergencies in ED:
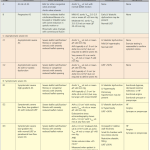
| 1. Is there a “severe” valvular dysfunction? 2. Does this valvular lesion explain my patient’s clinical presentation? 3. How can this finding change my management plan? |
Clinical manifestation
Triggers
- Many adults with severe symptomatic AS have relatively stable symptoms with decompensation triggered by an intercurrent illness, such as
- Acute myocardial infarction
- Arrhythmia esp. atrial fibrillation (with rapid ventricular rate)
- Systemic infection, sepsis.
- Hypovolemia, GI bleeding.
- Uncontrolled hypertension.
- Infective endocarditis.
- Pulmonary embolism.
Signs and symptoms
- The range of clinical presentation of patients with severe AS is shown below.
- Patients with chronic symptomatic AS can have exertional dyspnea, presyncope or syncope, and chest pain.
- Angina in patients with AS without significant obstructive coronary artery disease can cause myocardial ischemia by several mechanisms:
- Reduced coronary perfusion pressure is severe AS (CPP= Aortic DBP – LVEDP). This is due to
- ⬆️LVEDP
- ⬇️Diastolic coronary perfusion time during tachycardia
- ⬇️Coronary flow reserve related to myocardial and vascular factors.
- ⬆️Total LV oxygen demand as a result of ↑LV mass; oxygen consumption per gram of myocardium is normal.
- Reduced coronary perfusion pressure is severe AS (CPP= Aortic DBP – LVEDP). This is due to
- Angina in patients with AS without significant obstructive coronary artery disease can cause myocardial ischemia by several mechanisms:
- With more severe decompensated AS, patients present with signs and symptoms of heart failure with cardiogenic shock.
Differential diagnosis
Hemodynamically significant AS must be on the differential in the undifferentiated patient presenting with acute pulmonary edema, syncope, or cardiogenic shock, particularly if they are elderly.
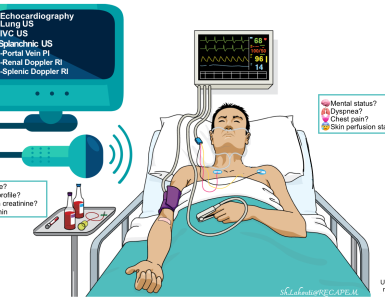
Diagnostic evaluation
- Bedside US/echo
- Diagnosis of MS is based on echocardiography (discussed below).
- Bedside US/echo also plays a key role in the evaluation of patients with suspected cardiopulmonary disease and helps to narrow down the differential diagnosis.
- ECG
- In severe AS, ECG may show LVH with a strain pattern (figure below).
- ECG may also reveal conditions that contributed to the decompensation of AS e.g. myocardial infarction, AF, etc.
- Labs
- Requested based on suspected diagnosis and also to evaluate the possible triggers or comorbidities
- CBC, electrolytes including Ca/Mg/Phos.
- Hemolytic anemia is a common complication of aortic stenosis.
- Hemoglobin and lactate dehydrogenase (LDH) are helpful for screening for this condition *.
- Hemolytic anemia is a common complication of aortic stenosis.
- Troponin only if ACS is suspected.
- Lactate level.
- Liver function tests (marked transaminase elevation suggests shock liver with poor cardiac output).
- Brain natriuretic peptide (BNP) levels are unhelpful (cardiopulmonary ultrasonography is a superior test).
- CBC, electrolytes including Ca/Mg/Phos.
- Requested based on suspected diagnosis and also to evaluate the possible triggers or comorbidities
Transthoracic echocardiography
Bedside echocardiography plays a key role in prompt diagnosis and appropriate management of critically ill patients. Echocardiographic assessment of aortic stenosis (🎥media below) is discussed here.
| Objectives ✅When suspect aortic stenosis? ✅Identifying acute severe aortic stenosis |
When suspect to AS?
In the appropriate clinical context (historical clues, signs and symptoms), the presence of ‘AS’ is suspected based on 2D echo findings.
- 2D evaluation
- Leaflet mobility, extent and pattern of calcification, patterns of leaflet motion.
- Assess LVEF
- Visualize the aortic root.
- Look for LV hypertrophy (LVH).
| ↑Brightness/thickness of AV, ↓leaflet motion, LVH in appropriate clinical context are highly suggestive for severe AS. Diagnostic echo study is recommended *. |
| Thin AV, normal systolic excursion, a normal aortic root, and normal LV wall thickness and systolic function suggest absence of significant AS |
Clip illustrating AV calcification with restricted AV leaflet mobility.
Identifying severe aortic stenosis
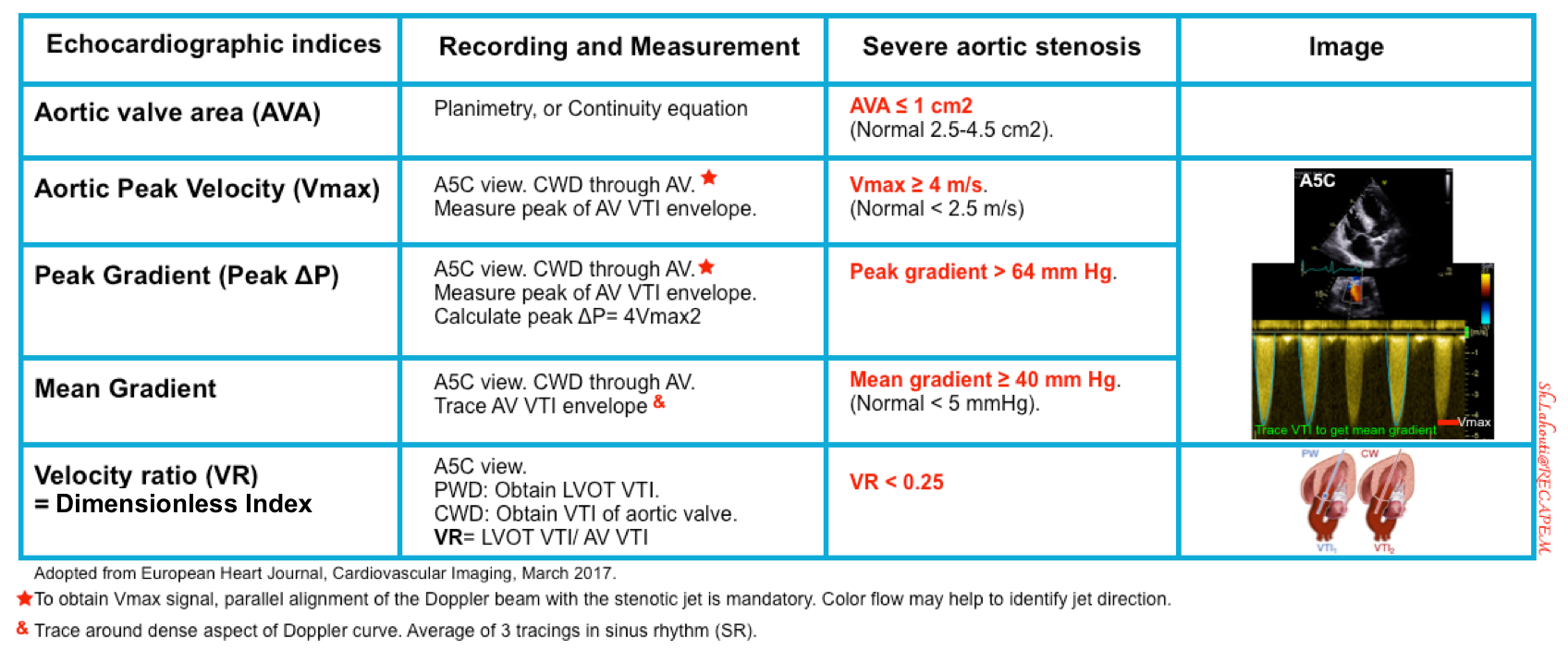
Several spectral Doppler measurements are used to define severe AS (table below).
- Basic principles
- Spectral Doppler allows a graphic display of velocity over time. This is used to assess the velocity of blood through an orifice e.g. cardiac valves.
- Narrowing of an orifice leads to increased velocity of flow.
- Velocities are used to calculate pressure gradients across an orifice by using the modified Bernoulli equation (pressure gradient = 4Velocity2).
- The pressure gradient across an orifice is used in the calculation of valve area.
- One can use pulse wave Doppler (Low flow velocity, e.g. LVOT VTI) and continuous wave Doppler (High flow velocity, e.g. AV VTI).
- Technical aspects
- Flow TOWARDS the probe is ABOVE the baseline, flow AWAY the probe is BELOW the baseline.
- It is important to maintain ultrasound beam parallel to flow to ensure accurate measurement of blood flow velocity.
- Spectral Doppler allows a graphic display of velocity over time. This is used to assess the velocity of blood through an orifice e.g. cardiac valves.
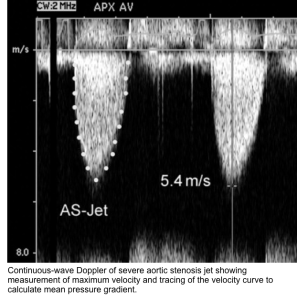
Aortic peak velocity and mean pressure gradient
- Get an apical 5C view. Place continuous wave (CW) Doppler through the aortic valve.
- Note it is important to use CWD through AV due to the higher velocities when compared to the lower LVOT velocities which are captured with PWD.
- Measurements
- Aortic Peak Velocity (Vmax)
- Peak Transvalvular Pressure Gradient can be calculated by modified Bernoulli equation as pressure gradient = 4xVmax²
- Mean Transvalvular Pressure Gradient
- Tracing the AV VTI envelope will allow the US to automatically calculate the mean gradient.
- Aortic Peak Velocity (Vmax)
Mean gradient caveats
By and large, the pressure gradient is a flow-dependent measure and is not an accurate index for the severity of AS in the following conditions (i.e. the pressure gradient may underestimate the severity of AS) *
- Reduced LVEF.
- The weak left ventricle is unable to generate enough flow (stroke volume) to create an elevated flow velocity or pressure gradient despite a severely stenotic valve. This causes a “low flow-low gradient” condition with the risk of underestimating the severity of AS *.
- Mitral regurgitation (MR).
- In the presence of MR, a portion of LV stroke volume is directed into the low-pressure left atrium. This causes a reduction in effective LV forward flow *.
- Mitral stenosis.
- In severe MS, left ventricular preload is reduced (underfilled LV), therefore LV outflow is reduced consequently.
- RV dysfunction, and tricuspid regurgitation.
- Virtually, any cause(s) of RV dysfunction leads to reduced LV preload (underfilled LV).
- Development of TR in the context of RV dysfunction, perpetuates the spirals of RV dysfunction, resulting in worsening LV underfilling.
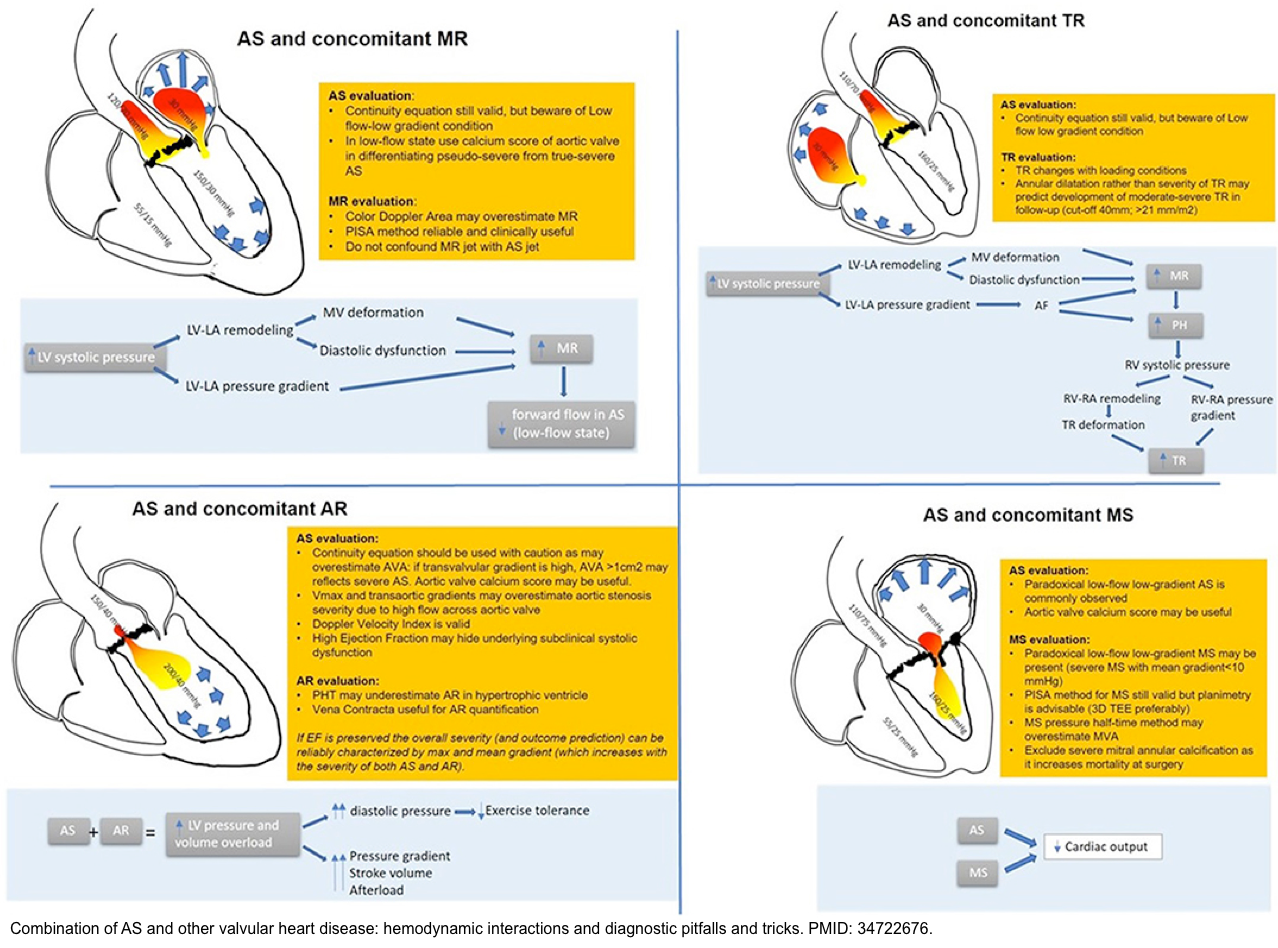
- Hemodynamic interactions of concomitant valvulopathies in the presence of aortic stenosis are shown below *.
| 🔴Pressure gradient is a “flow-dependent” parameter. 🔴Pressure gradient may underestimate AS severity in the condition of “low LV outflow state”. Low flow is defined as a stroke volume index <35 mL/m2 🔴Remember that stroke volume depends on contractility, preload, and afterload. Therefore, a low flow state (SVi <35mL/m2) can be caused by: 1. ↓Contractility (↓EF): Cardiomyopathy e.g. IHD 2. ↓Preload: Small LV chamber size (LVH), MS, severe RV dysfunction, and TR. 3. ↑Afterload: Systolic arterial hypertension 4. Stroke volume is also reduced in the presence of mitral regurgitation since the forward flow through LVOT is reduced (a portion of LV outflow is back to LA). |
Aortic valve area (AVA)
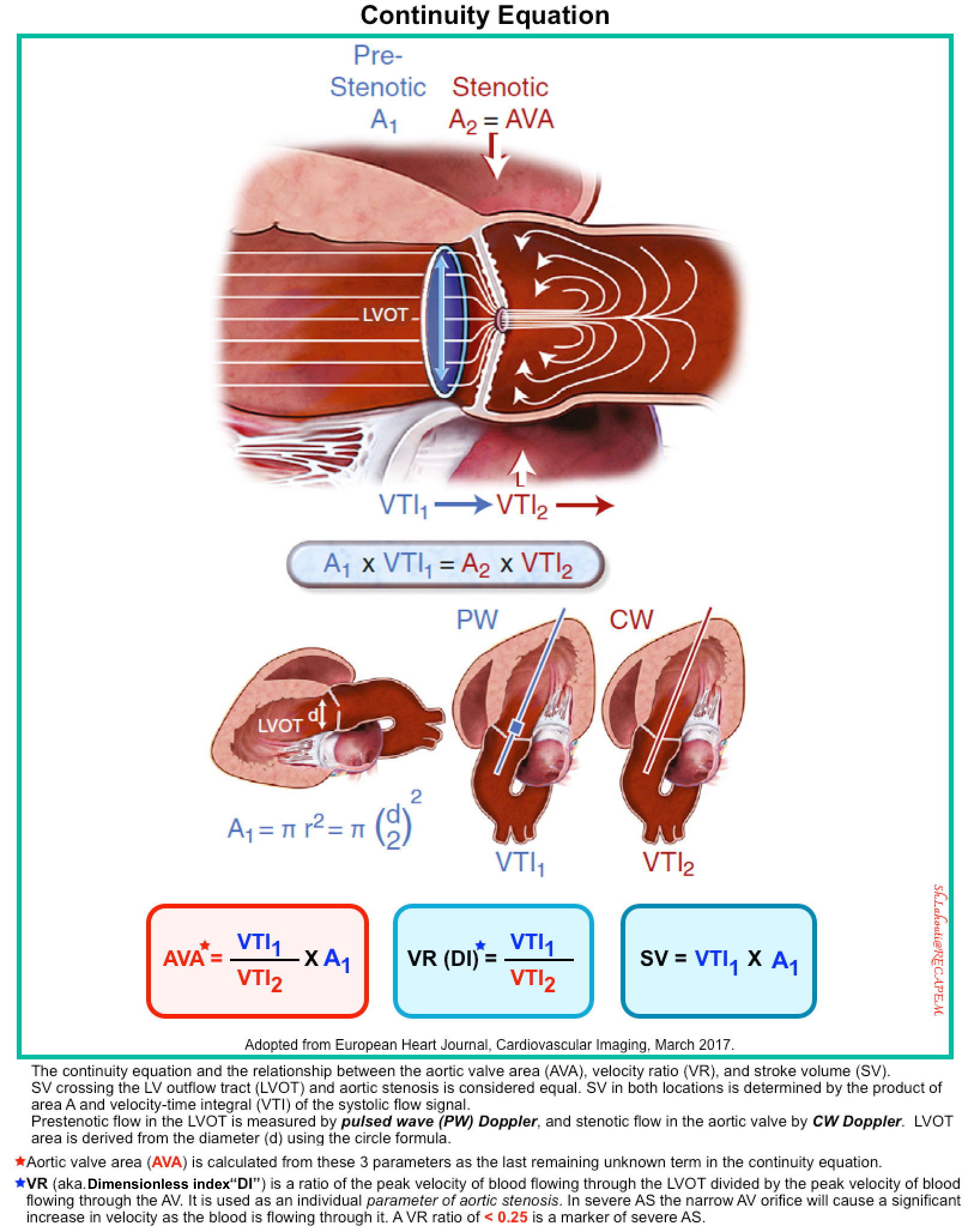
AVA can be measured by planimetry or alternatively by the continuity equation. The latter is discussed here.
- Continuity Equation
- If LV function and output are reduced, less flow-dependent parameters to define AS severity (i.e. continuity equation) are required *.
- The aortic valve area (AVA) is calculated from the continuity equation based on the consideration that the same stroke volume SV) passes through the LV outflow tract (LVOT) and the aortic valve (figure below) *.
- Stroke volume is measured as the product of velocity-time integral (VTI) of flow and cross-sectional area; therefore the equation is: A1 × VTI1=A2 × VTI2
- A1 is the LVOT area; VTI1 is the prestenotic velocity-time integral of flow within the LVOT (measured by PWD).
- LVOT area is calculated as A1 = π r2 (r is the radius, or diameter/2).
- A2 is the AVA, and VTI2 is the velocity-time integral of the stenotic jet through the aortic valve (measured by CWD).
- A1 is the LVOT area; VTI1 is the prestenotic velocity-time integral of flow within the LVOT (measured by PWD).
- Aortic valve area (AVA) can be calculated as AVA=A2= VTI1/VTI2 x A1
- Severe AS: AVA<1cm2
⚠️Pitfalls: Source of error in AVA measurement
- Error in LVOT diameter measurement
- Note that a 1mm change in measurement of the LVOT diameter results in a 0.1 cm² difference in AVA calculation.
- Severe calcification and poor echogenicity are important challenges to measuring the LVOT diameter accurately.
- Solution: Measure LVOT diameter in the PLAXview, using the zoom mode, in mid-systole, inner edge, and repeat at least 3-5 times.
- At which level should LVOT diameter be measured? Measured at the level of (leaflet insertion, i.e. aortic annulus) or more apically, i.e. 5 to 10 mm below the annulus?
- Answer: Measurement of the LVOT at the annulus level provides higher measurement reproducibility and ensures that diameter and pulse Doppler are measured at the same anatomical level *.
- At which level should LVOT diameter be measured? Measured at the level of (leaflet insertion, i.e. aortic annulus) or more apically, i.e. 5 to 10 mm below the annulus?
- Error in measurement of aortic valve VTI
- The highest aortic velocity should be measured.
- Record at multiple sites: apical view, right parasternal view, suprasternal view, and the subcostal view.
- Obtain a parallel alignment between the aortic jet and the ultrasound beam
- Error in the measurement of LVOT VTI
- Obtain a parallel alignment between the aortic jet and the ultrasound beam
- Problem: If the Doppler sample is positioned too far from the aortic orifice, it will lead to an overestimation of AS severity.
- Problem: If the Doppler sample is positioned too close to the aortic orifice (within the flow acceleration), it will cause an underestimation of AS severity.
- Solution: Move the Doppler sample up and down in order to obtain a nice Doppler trace with a closure click (possibly missing in very severe AS) without the opening click.
- AVA should be adjusted for patients with small BSA (AVAi = AVA/BSA)
- An AVA <1 cm² may also be observed in small-sized patients. In these circumstances, AVA should be adjusted for BSA, with the threshold being 0.6 cm²/m².
- Solution: Indexed AVA (AVAi) is required in patients with small body size (BSA <1.6 m2)
- Note: Avoid indexing AVA in large and obese patients, since it will cause an overestimate of AS severity.
Echocardiographic definition of severe AS *
- Severe AS is defined by aortic valve area (AVA) ≤1 cm2, aortic peak velocity ≥4 m/s, and/or mean gradient ≥40 mm Hg *.
Discordant grading of aortic stenosis
Background
- The parameters above mentioned allow the description of different grades of aortic stenosis:
- Mild AS: A valve area >1.5 cm2, a peak velocity of 2.6-2.9 m/s, a mean gradient <20 mm Hg.
- Moderate AS: A valve area between 1.5-1 cm2, a peak velocity between 3-4 m/s, or a mean gradient between 20-40 mm Hg.
- Severe AS: A peak velocity ≥ 4 m/s, a mean gradient ≥ 40 mmHg and an AVA ≤ 1 cm2 (or AVAi ≤ 0.6 cm2/m2) *.
Definition of discordant AS
- Discordant grading is defined when one parameter suggests a moderate AS while the other suggests a severe AS *. For example when:
- AVA <1 cm² while the mean gradient is ≥40 mmHg and Vmax ≥4 m/sec. This condition is typically called “High Gradient” Severe AS.
- AVA ≥1 cm² while mean gradient ≥40 mmHg and Vmax ≥4 m/sec. This can be seen in the following high-flow states as
- Aortic regurgitation
- Hyperthyroidism
- Anemia
- AVA ≤ 1 cm2, Vmax<4 m/sec, and a mean pressure gradient (MPG) < 40 mm Hg.
🧐Incidence and diagnostic significance
- The above-mentioned criteria for grading severity of AS is not always consistent and the proportion of patients with discordant parameters is not negligible *.
- For example only 50% of patients with severe AS (defined as AVA ≤ 1 cm2) have a mean pressure gradient ≥ 40 mm Hg. This type of severe AS is called “High Gradient Severe AS“.
- In contrast more than 50% of patients with severe AS (defined as AVA ≤ 1 cm2) have a mean gradient < 40 mm Hg.
- This AVA-gradient discrepancy raises uncertainty about the actual stenosis severity!
- Identifying severe AS among these patients is important as it has therapeutic implications.
Low gradient aortic stenosis (LG AS)
Subtypes
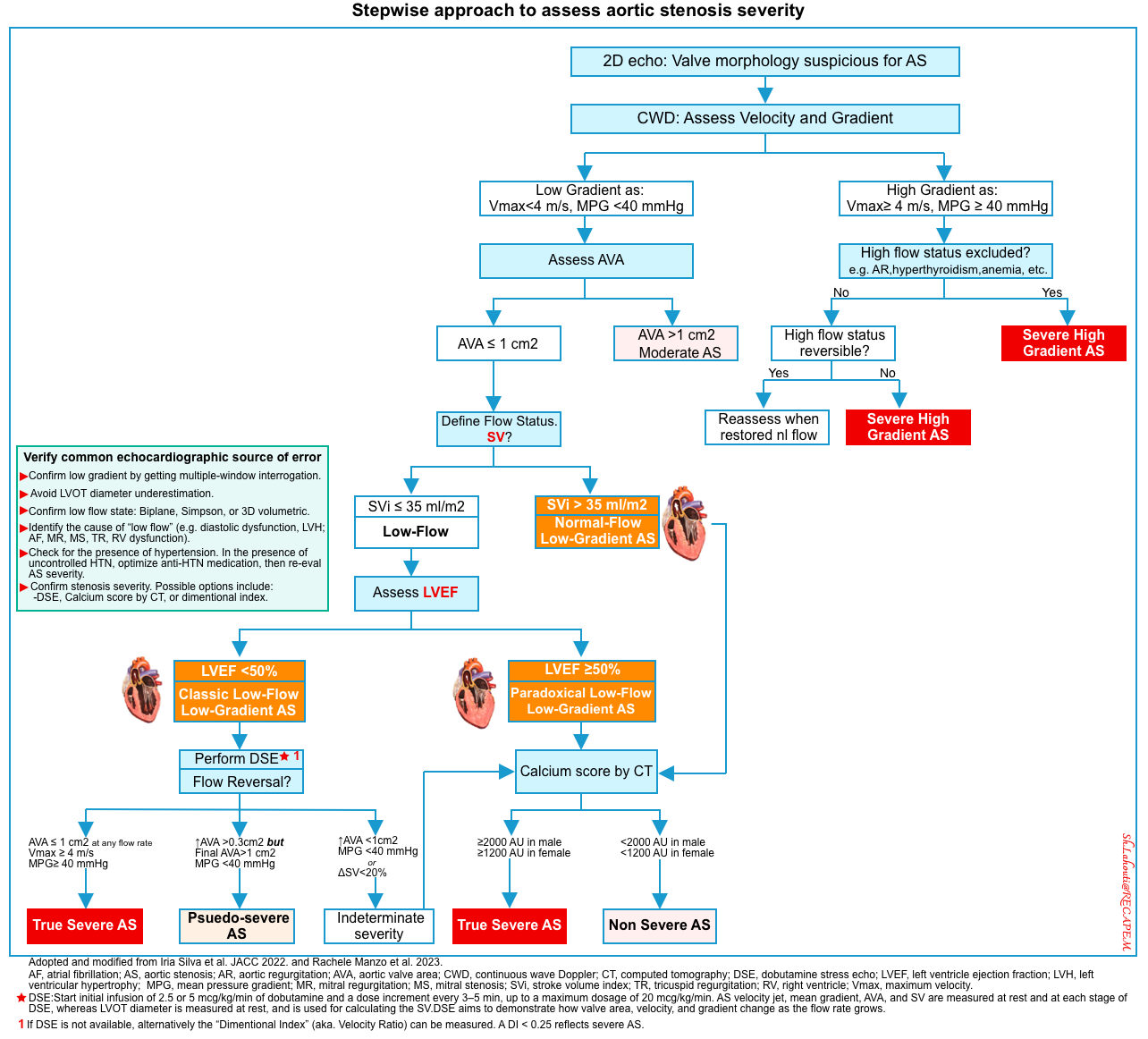
Three main subtypes of LG AS (Vmax <4 m/sec and MPG <40 mm Hg) can be identified depending on the flow status of SVi (stroke volume index), and LVEF *. A stepwise approach to the assessment of the severity of aortic stenosis is provided below.
- Classic low flow, low gradient (LF-LG) aortic stenosis.
- Prevalence: 5-10% of severe AS.
- Definition
- Low gradient (≤40 mm Hg).
- AVA ≤ 1 cm2
- Low flow: Low stroke volume index (SVi ≤35 mL/m2 ). Measurement of stroke volume (🎥media below).
- Reduced LVEF (<50%).
- Pathophysiology
- The low flow state (SVi < 35 ml/m2) with an LVEF <50% is predominantly caused by LV systolic dysfunction *.
- The cause of the low-flow state is likely systolic dysfunction, caused by concurrent ischemic cardiomyopathy, or AS-induced myocardial ischemia.
- In this context (SVi < 35 ml/m2 and LVEF <50%), the AVA ≤ 1 cm2 may happen due to either:
- A truly stenotic valve.
- LV systolic dysfunction (e.g. prior myocardial infarction), impairing the aortic valve to completely open, despite the presence of a merely mildly or moderately stenotic valve (pseudo-severe AS).
- LF-LG aortic stenosis shares several pathophysiological and clinical similarities with HFrEF.
- The low flow state (SVi < 35 ml/m2) with an LVEF <50% is predominantly caused by LV systolic dysfunction *.
- Paradoxical LF-LG aortic stenosis
- Prevalence: 5-25% of severe AS
- Definition
- Low gradient (≤40 mm Hg).
- AVA ≤ 1 cm2
- Low flow: Low stroke volume index (SVi ≤35 mL/m2 )
- Paradoxical, referring to the preserved LVEF (≥50%).
- Pathophysiology
- Despite preserved LVEF, the left ventricle fails to produce enough forward flow (forward stroke volume) to create an elevated pressure gradient. The possible causes of low forward stroke volume (wherein low flow is not caused by LV systolic dysfunction) are *:
- Diastolic LV dysfunction (underfilled LV)
- Severe concentric LV hypertrophy with a small LV chamber size results in decreased LV diastolic filling and reduced stroke volume.
- Atrial fibrillation
- Tachycardia, esp AF is poorly tolerated in patients with diastolic dysfunction, leading to worsening LV underfilling and thus ↓LV outflow.
- Mitral regurgitation (mechanism: ↓LV effective forward flow)
- Mitral stenosis (mechanism: Underfilled LV)
- RV dysfunction and tricuspid regurgitation (mechanism: Underfilled LV)
- Elevated LV afterload caused by severe, uncontrolled, long-standing hypertension.
- Diastolic LV dysfunction (underfilled LV)
- In fact, paradoxical LF-LG AS shares many pathophysiological and clinical similarities with HFpEF.
- These entities are often associated with older age, female sex, and systemic hypertension.
- Despite preserved LVEF, the left ventricle fails to produce enough forward flow (forward stroke volume) to create an elevated pressure gradient. The possible causes of low forward stroke volume (wherein low flow is not caused by LV systolic dysfunction) are *:
- Prevalence: 5-25% of severe AS
- Normal Flow, Low Gradient (NF-LG) AS
- Prevalence: 15-40% of severe AS
- Definition
- Low gradient (≤40 mm Hg).
- AVA ≤ 1 cm2
- Normal flow: Stroke volume index (SVi > 35mL/m2 )
- Preserved LVEF (≥50%).
- Pathophysiology
- The reduction of the gradient despite a normal SVi can depend on a low transvalvular flow rate, calculated as SVi/LV ejection time.
- If the latter is prolonged, such as during bradycardia or because of systemic hypertension, the flow rate and consequently, the mean gradient will be lower.
- The reduction of the gradient despite a normal SVi can depend on a low transvalvular flow rate, calculated as SVi/LV ejection time.
- Prevalence: 15-40% of severe AS
Evaluation of severity of AS
As explained above, in the presence of low flow (low stroke volume), Vmax and mean gradient are invalid methods for identifying severe AS. Diagnosis of LFLG severe AS is important since it carries a poor prognosis.
- When to suspect low gradient AS?
- Low gradient (LG) severe AS is suspected when the echocardiogram reveals an aortic valve area (AVA) ≤1 cm (with AVA indexed to body surface area ≤0.6 cm /m ) associated with a transvalvular mean pressure gradient ≤40 mmHg *.
- A diagnostic approach for assessing AS in different flow-gradient scenarios (see algorithm,👉 here).
- Step #1: Rule out measurement errors *
- Confirm low gradient by getting multiple-window interrogation.
- Avoid LOVT diameter underestimation. For the pitfalls of LVOT VTI 👉 media below.
- Step #2: Confirm low flow state and identify the cause of “LF” (e.g. diastolic dysfunction, AF, MR, MS, TR, RV dysfunction).
- Step #3: Check for the presence of hypertension.
- In the presence of uncontrolled HTN, optimize anti-HTN medication, then re-assess AS severity.
- Step #4: Confirm stenosis severity. Possible options include:
- Low-dose dobutamine stress echocardiography (DSE) if feasible.
- The main purpose of DSE is patient therapeutic management.
- Patients with pseudo-severe AS may not benefit from aortic valve replacement (AVR) and heart failure therapy should be considered in these patients.
- Patients with truly severe AS may benefit from intervention.
- The main purpose of DSE is patient therapeutic management.
- Assess aortic valve calcification by multi-detector computed tomography (MDCT).
- Assess “ Dimensionless Index” (aka. Velocity Ratio).
- It can be used at the bedside in critical patients when other sophisticated tests (e.g. dobutamine stress echo) are not feasible *.
- It can be thought of as a ratio of the peak velocity of blood flowing through the left ventricular outflow tract (LVOT) divided by the peak velocity of blood flowing through the AV (🎥media below).
- PWD through LVOT. Measure LVOT VTI.
- CWD through AV. Measure AV VTI
- Calculate velocity ratio: LVOT VTI / AV VTI.
- A ratio of < 0.25 is concerning for severe AS.
- In severe AS the narrow AV orifice will cause a significant increase in velocity as the blood flows through it compared to the wider LVOT.
- Low-dose dobutamine stress echocardiography (DSE) if feasible.
- Step #1: Rule out measurement errors *
🟠Stepwise approach to assess the severity of aortic stenosis is provided here.
Approach to Management in ED
Critical aortic stenosis (AS) is the single most challenging valvular disease encountered in the ED.
- Patients with critical AS have a fixed cardiac output and cannot meaningfully increase cardiac output to meet the physiologic demands of critical illness.
- The cornerstone of resuscitation include
- Avoiding systemic hypotension
- Maintaining sinus rhythm
- Avoiding excessive tachycardia
Basic principles
- Medical therapy
- Patients with critical valvular disease usually fail to respond to medical therapy alone *.
- The goal of medical therapy and mechanical circulatory support (MCS) is stabilization prior to definitive correction of the lesion.
- Generally, medical stabilization prior to definitive intervention differs substantially between stenotic lesions (e.g. LVOTO) and regurgitant lesions (aortic regurgitation, mitral regurgitation).
- Patients with regurgitant lesions
- These patients are highly afterload-sensitive, especially with reduced LVEF.
- They typically require aggressive afterload reduction and inotropic support *.
- Patients with stenotic lesions
- These patients are preload sensitive, especially with LV diastolic dysfunction
- They may paradoxically require B-blockade and vasoconstrictors *.
- Patients with regurgitant lesions
- The effect of positive pressure ventilation (PPV) among patients with valvular emergencies is variable with harmful effects in critical aortic stenosis.
- Mechanical circulatory device (MCS)
- When MCS is indicated, early application is preferred to reduce the need for vasopressors and inotropes, which may have unfavorable effects on cardiac loading conditions.
- Possible options include: aortic balloon pumps, percutaneous centrifugal pumps, and full veno-arterial ECMO
- Consultation and referral
- Cardiac surgeon consultation is warranted early for valvular emergencies.
- By and large, novel percutaneous intervention may be effective for selected patients. Therefore, consider referral to a comprehensive valve center with proficiency in both surgical and percutaneous interventions for valvular heart diseases *.
- Hemodynamic monitoring
- Patients with valvular emergencies (especially multiple or mixed valvular lesions) often have a very narrow window for hemodynamic optimization warranting close monitoring *.
- Hemodynamic assessment can generally be made non-invasively (bedside ultrasound/echo/doppler, A-line BP monitoring; physical exams).
- Pulmonary arterial catheterization (PAC) might be considered for critical valvular emergencies if:
- Multiple or mixed valvulae lesions
- Unresponsiveness to initial therapy
- Poor transthoracic echocardiographic windows and inability to perform a transesophageal echocardiogram.
Medical management
The following discussion is focused on severe AS with hypotension and cardiogenic shock.
| Protect coronary perfusion pressure 👉Coronary perfusion pressure = Aortic diastolic BP – LVEDP |
- Patients with AS in CS state have a high risk of myocardial ischemia for the following reasons
- Most patients are hypotensive, and aortic diastolic BP is reduced.
- LVEDP is increased secondary to the stenotic valve with pressure overload/LVH.
- Goal
- Maintain adequate CPP while avoiding excessive afterload *.
- Vasopressor of choice
- If LVEF is preserved (classic high flow, high gradient AS): Phenylephrine
- Phenylephrine is a purse vasopressor. It maintains MAP and CPP, and perhaps even causes reflexive bradycardia leading to increased diastolic filling time.
- If LVEF is reduced: Norepinephrine
- It has mild inotropic effects as well as vasopressor effects and is less likely to cause significant tachycardia such as epinephrine
- If LVEF is preserved (classic high flow, high gradient AS): Phenylephrine
| Volume management: Optimizing preload |
- Patients with severe AS are preload-dependent.
- Diastolic dysfunction due to LVH makes the ventricle dependent on an increased filling pressure to maintain cardiac output (“preload sensitive”) *.
- Volume management
- Fluid administration:
- Indication: Severe AS in CS without evidence of central congestion (e.g. B-lines on POUCS)
- It is reasonable to consider a 250 ml crystalloid bolus followed by reassessment *.
- Remember while these patients are dependent on higher filling pressures, elevated left-sided pressures predispose them to functional MR, elevated PCWP, post-capillary pulmonary hypertension, and subsequent RV failure thus fluid administration must be judicious with a constant reassessment of volume status.
- ⚠️Avoid diuretics or venodilators
- Fluid administration:
| Avoid tachycardia |
- Tachycardia impairs diastolic filling time as well as decreases the time for ejection through the severely stenotic aortic valve.
- Goal HR is slow to normal in the setting of AS with severe LVH and diastolic dysfunction.
| Support contractility |
- By and large, inotropes can cause excessive chronotropy further impairing diastolic filling time thus inotropic agents must be utilized with care.
- Indication: The classic LFLG AS in CS
- Agent: Consider low-dose inotropes that have less effect on chronotropy, e.g. dobutamine *.
- ⚠️Avoid BB or CCB that impair contractility
| Identify and treat reversible causes |
- Patients with chronic AS can have an acute decompensation in the setting of a systemic illness or stressor thus it is important to identify these stressors and treat any reversible causes.
- For severe AS in CS, consider
- AF with RVR: Consider cardioversion in unstable patients.
- Sepsis: Support MAP and start appropriate antibiotics
- Hypovolemia/ GI bleeding can cause acute decompensation as these patients are preload dependent. Resuscitate as needed.
| Avoid intubation (if possible) |
- Intubation can cause hypotension (secondary to induction agents, decreased sympathetic response, PPV) thus impairing CPP leading to myocardial ischemia, worsening CS, and even cardiac arrest *.
- If intubation is unavoidable resuscitate first:
- Correct HR before intubation. If you are preparing to intubate a hemodynamically tenuous patient in new atrial fibrillation (or any SVT for that matter), consider cardioversion prior to induction.
- Correct hypotension. Have vasopressors such as phenylephrine or norepinephrine inline (or infusing)
- Optimize preload. Consider a small fluid bolus (if there is any uncertainty regarding volume status, err on the side of fluid administration).
- Use hemodynamically stable induction agents (consider awake intubation)
- ✅Etomidate is the RSI induction agent of choice in patients with “AS” because it is both hemodynamically stable and comes with a generally favorable side effect profile.
- 🛑Propofol is a profound vasodilator and can acutely drop preload and promote hemodynamic collapse.
- 🛑Ketamine promotes tachycardia (an unfavorable property in patients with AS).
- Minimize PEEP as it can decrease the preload.
Mechanical circulatory support & interventions
- Many patients with CS secondary to severe AS may fail to stabilize with medical therapy alone and will require mechanical circulatory support (MCS) and/or temporizing balloon aortic valvuloplasty (BAV) as a bridge to definitive interventional or surgical treatment.
- Mechanical support
- IABP: It can reduce LV afterload, increase mean arterial pressure, and increase coronary perfusion pressure in patients with AS.
- Impella: It can also be used in patients with severe AS, although placement can be difficult due to the need to cross the stenotic AV
- TandemHeart or peripheral ECMO can be used as a bridge to definitive therapy
- Intervention
- Balloon aortic valvuloplasty (BAV)
- BAV can be performed as a bridge to transcatheter or surgical aortic valve replacement (AVR) in hemodynamically unstable patients at high risk for morbidity and mortality with AVR.
- Balloon aortic valvuloplasty (BAV)
🔴Summary of principles of hemodynamic resuscitation in valvular emergencies
- Patients with critical valvular disease usually fail to respond to medical therapy alone *.
- The goal of medical therapy and mechanical circulatory support (MCS) is stabilization prior to definitive correction of the lesion.
- Generally, patients with regurgitant lesions require aggressive afterload reduction and inotropic support *, while those with stenotic lesions may paradoxically require β-blockade and vasoconstrictors (table below) *.
Definitive treatment
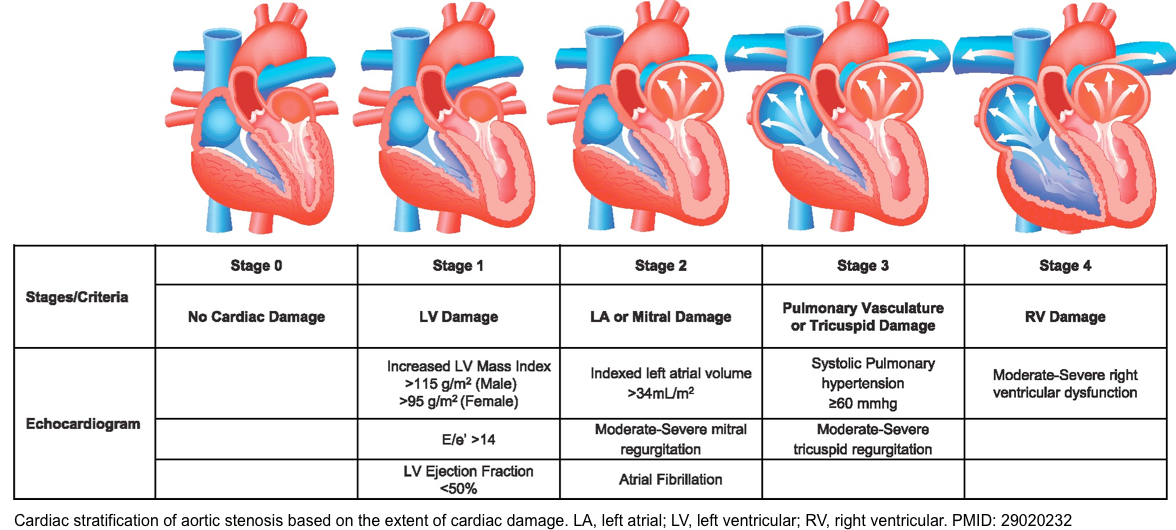
Staging: Current guidelines recommend the classification of AS by disease stage based on the integration of patient symptoms, valve morphology, stenosis severity, and LV function (see appendix).
Definitive treatment
- Surgical aortic valve replacement is often considered the gold standard for severe AS treatment. However, patients with severe AS presenting with CS have high surgical risk, and thus transcatheter aortic valve implementation (TAVI) is now the preferred definitive treatment in this population *.
Case conclusion
- The patient was placed on a phenylephrine infusion, and preload was optimized prior to intubation.
- After intubation, he was admitted to the CCU, where he was diuresed throughout the next week. He responded well to medical management and was extubated.
- Ultimately, he was transferred to another facility for TAVR and had a full recovery.
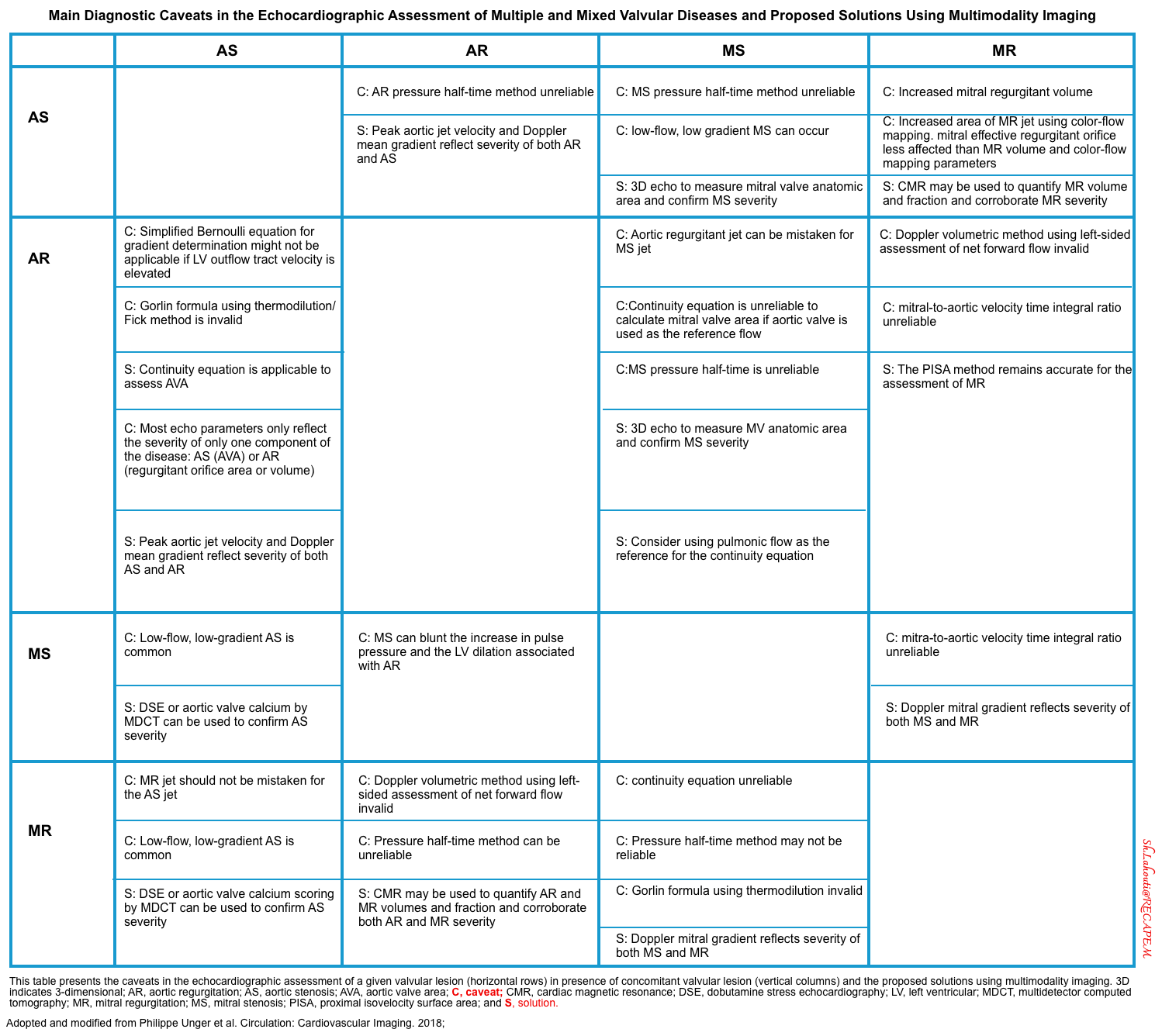
Multiple and Mixed Valvular Heart Diseases
- Multiple VHD refers to the combination of stenotic or regurgitant lesions occurring on ≥2 cardiac valves.
- Mixed VHD refers to the combination of stenotic and regurgitant lesions on the same valve *.
- The hemodynamic and clinical consequences of any given valvular lesion may be modulated by the concomitant presence of mixed or multiple VHD. These hemodynamic interactions can promote, exacerbate, or, in contrast, blunt the clinical expression of each singular lesion *.
- Conditions that reduce the effective forward flow in patients with aortic stenosis:
- Increasing LV volume loading: Concomitant AR
- Decreasing LV preload: Concomitant MS
- Concurrent MR
- ↑Afterload e.g. HTN
- ↓Inotrope
- Hypotension→ ↓Coronary perfusion pressure
- Tachycardia
- The main diagnostic caveats in the echocardiographic assessment of multiple and mixed valvular diseases are summarized in the following table.
Appendix
Going further
- Measuring cardiac output (POCUS 101)
- POCUS for aortic stenosis
- Aortic stenosis: Breaking down the continuity equation
- Reference (normal) values for echocardiography
Media
Aortic stenosis vs. aortic sclerosis. Basic echo measurements
Stroke volume determination (LVOT VTI)
References
1. PMID: 33914604. Boskovski MT, Gleason TG. Current Therapeutic Options in Aortic Stenosis. Circ Res. 2021 Apr 30;128(9):1398-1417. doi: 10.1161/CIRCRESAHA.121.318040. Epub 2021 Apr 29.
2. PMID: 29020232. Généreux P, Pibarot P, Redfors B, Mack MJ, Makkar RR, Jaber WA, Svensson LG, Kapadia S, Tuzcu EM, Thourani VH, Babaliaros V, Herrmann HC, Szeto WY, Cohen DJ, Lindman BR, McAndrew T, Alu MC, Douglas PS, Hahn RT, Kodali SK, Smith CR, Miller DC, Webb JG, Leon MB. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur Heart J. 2017 Dec 1;38(45):3351-3358. doi: 10.1093/eurheartj/ehx381.
3.Bombace, S.; Meucci, M.C.; Fortuni, F.; Ilardi, F.; Manzo, R.; Canciello, G.; Esposito, G.; Grayburn, P.A.; Losi, M.A.; Sannino, A. Beyond Aortic Stenosis: Addressing the Challenges of Multivalvular Disease Assessment. Diagnostics 2023, 13, 2102. https://doi.org/10.3390/diagnostics13122102
4. PMID: 33588124. Abecasis J, Gomes Pinto D, Ramos S, Masci PG, Cardim N, Gil V, Félix A. Left Ventricular Remodeling in Degenerative Aortic Valve Stenosis. Curr Probl Cardiol. 2021 May;46(5):100801. doi: 10.1016/j.cpcardiol.2021.100801. Epub 2021 Jan 24.
5. PMID: 29525246. Gottlieb M, Long B, Koyfman A. Evaluation and Management of Aortic Stenosis for the Emergency Clinician: An Evidence-Based Review of the Literature. J Emerg Med. 2018 Jul;55(1):34-41. doi: 10.1016/j.jemermed.2018.01.026. Epub 2018 Mar 7.
6. PMID: 33332149. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O’Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021 Feb 2;143(5):e35-e71. doi: 10.1161/CIR.0000000000000932. Epub 2020 Dec 17. Erratum in: Circulation. 2021 Feb 2;143(5):e228. Erratum in: Circulation. 2021 Mar 9;143(10):e784.
7. PMID: 28385280. Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, Lancellotti P, LeFevre M, Miller F Jr, Otto CM. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017 Apr;30(4):372-392. doi: 10.1016/j.echo.2017.02.009.
8. PMID: 29158019. Lee PH, Hong JA, Sun BJ, Han S, Park S, Jang JY, Kim DH, Kang DH, Song JK, Song JM. Impact of Significant Mitral Regurgitation on Assessing the Severity of Aortic Stenosis. J Am Soc Echocardiogr. 2018 Jan;31(1):26-33. doi: 10.1016/j.echo.2017.09.012. Epub 2017 Nov 20.
9. PMID: 34722676. Mantovani F, Fanti D, Tafciu E, Fezzi S, Setti M, Rossi A, Ribichini F, Benfari G. When Aortic Stenosis Is Not Alone: Epidemiology, Pathophysiology, Diagnosis and Management in Mixed and Combined Valvular Disease. Front Cardiovasc Med. 2021 Oct 15;8:744497. doi: 10.3389/fcvm.2021.744497.
10. PMID: 27190103. Clavel MA, Magne J, Pibarot P. Low-gradient aortic stenosis. Eur Heart J. 2016 Sep 7;37(34):2645-57. doi: 10.1093/eurheartj/ehw096. Epub 2016 Mar 31.
11. PMID: 35199011. Silva I, Salaun E, Côté N, Pibarot P. Confirmation of Aortic Stenosis Severity in Case of Discordance Between Aortic Valve Area and Gradient. JACC Case Rep. 2022 Feb 2;4(3):170-177. doi: 10.1016/j.jaccas.2021.11.009.
12. PMID: 33076698. Altes A, Thellier N, Rusinaru D, Marsou W, Bohbot Y, Chadha G, Leman B, Paquet P, Ennezat PV, Tribouilloy C, Maréchaux S. Dimensionless Index in Patients With Low-Gradient Severe Aortic Stenosis and Preserved Ejection Fraction. Circ Cardiovasc Imaging. 2020 Oct;13(10):e010925. doi: 10.1161/CIRCIMAGING.120.010925. Epub 2020 Oct 20.
13. PMID: 31492536. Akodad M, Schurtz G, Adda J, Leclercq F, Roubille F. Management of valvulopathies with acute severe heart failure and cardiogenic shock. Arch Cardiovasc Dis. 2019 Dec;112(12):773-780. doi: 10.1016/j.acvd.2019.06.009. Epub 2019 Sep 3.
14. PMID: 32314662. Jentzer JC, Ternus B, Eleid M, Rihal C. Structural Heart Disease Emergencies. J Intensive Care Med. 2021 Sep;36(9):975-988. doi: 10.1177/0885066620918776. Epub 2020 Apr 21.
15. PMID: 21178291. Spaccarotella C, Mongiardo A, Indolfi C. Pathophysiology of aortic stenosis and approach to treatment with percutaneous valve implantation. Circ J. 2011;75(1):11-19. doi: 10.1253/circj.cj-10-1105.
16. PMID: 35579641. UK TAVI Trial Investigators; Toff WD, Hildick-Smith D, Kovac J, Mullen MJ, Wendler O, Mansouri A, Rombach I, Abrams KR, Conroy SP, Flather MD, Gray AM, MacCarthy P, Monaghan MJ, Prendergast B, Ray S, Young CP, Crossman DC, Cleland JGF, de Belder MA, Ludman PF, Jones S, Densem CG, Tsui S, Kuduvalli M, Mills JD, Banning AP, Sayeed R, Hasan R, Fraser DGW, Trivedi U, Davies SW, Duncan A, Curzen N, Ohri SK, Malkin CJ, Kaul P, Muir DF, Owens WA, Uren NG, Pessotto R, Kennon S, Awad WI, Khogali SS, Matuszewski M, Edwards RJ, Ramesh BC, Dalby M, Raja SG, Mariscalco G, Lloyd C, Cox ID, Redwood SR, Gunning MG, Ridley PD. Effect of Transcatheter Aortic Valve Implantation vs Surgical Aortic Valve Replacement on All-Cause Mortality in Patients With Aortic Stenosis: A Randomized Clinical Trial. JAMA. 2022 May 17;327(19):1875-1887. doi: 10.1001/jama.2022.5776.






Add comment