3 August 2025 by Shahriar Lahouti. Peer-reviewed by Abdolghader Pakniyat.

CONTENTS
- Introduction
- Cardiovascular system
- Hemodynamic assessment
- Modern hemodynamic evaluation at the bedside
- RECAP
- Appendix
- References
Introduction
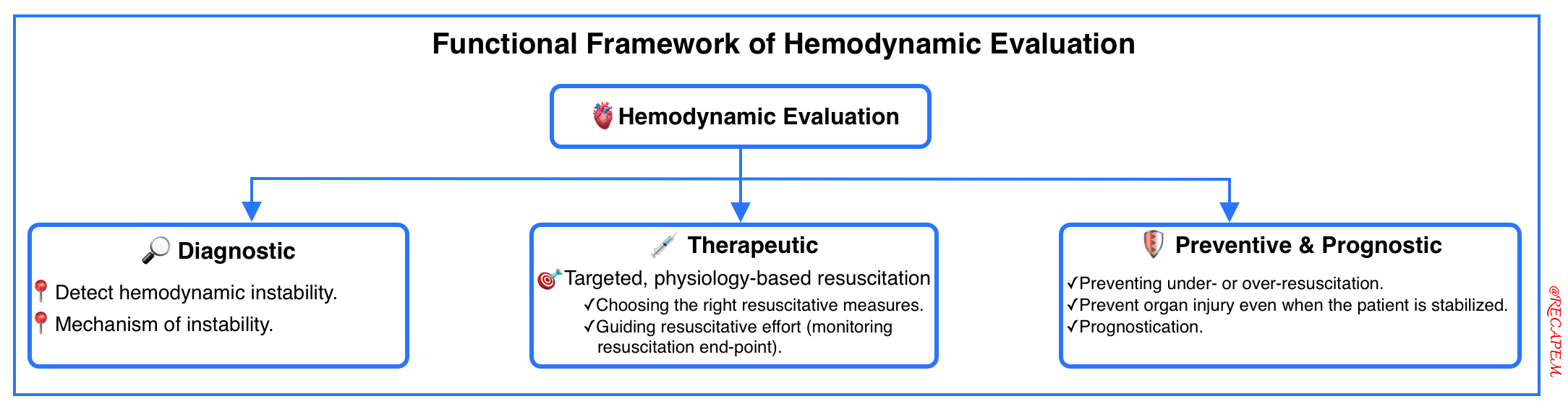
Hemodynamic evaluation refers to assessing the cardiovascular system’s ability to deliver oxygen and nutrients to tissues, clear metabolic waste, and maintain organ perfusion and cellular function. This is a key component of the evaluation of critically ill patients and has diagnostic, therapeutic, and preventive utility *.
⎮Hemodynamic assessment calls for a structured, physiology-informed approach, one that moves beyond single-parameter thinking.
The conceptual framework of modern hemodynamic evaluation is based on the following key pillars (below left figure):
- Multimodal assessment, not single-modality.
- Physiology-based (not protocol-bound):
- Contextual & Individualized approach (not one-size-fits-all).
- Perfusion-centered mindset (not number-chasing, e.g., BP alone)
- Understanding the interplay of variables (not parameter tunnel vision).
- Mechanistically informed (not purely descriptive labeling shock)
- Dynamic evaluation (not single-point snapshots).
In this series of posts, “Hemodynamic Insight”, we’ll explore how to apply fundamental physiologic principles to critically ill patients, bridging evidence with bedside sense.
This first article offers a brief overview of circulatory failure and the principles that shape a structured approach to hemodynamic evaluation.
Cardiovascular system
Anatomic backbone
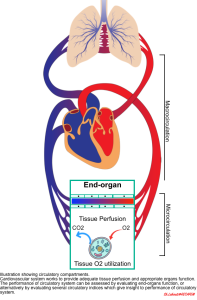
◾️The function of the cardiovascular (CV) system is to provide optimal conditions for the body’s organs by establishing “adequate tissue perfusion”. However, perfusion alone is just “delivery”! To be more precise, the essential function of the CV system is to maintain “effective tissue perfusion“.
◾️Effective tissue perfusion = Delivery + Utilization.
- It’s defined as adequate blood flow that meets the metabolic demands of tissue (adequate DO₂ and O₂ extraction).
- ⚠️ As will be discussed more, you can have flow without function (e.g., warm extremities but rising lactate), which means perfusion is not yet effective.
- 🎯 Improving “effective tissue perfusion” is the ideal goal of resuscitation in critically ill patients.
- Effective tissue perfusion is the interplay of upstream and downstream circulatory compartments (figure below):
- Upstream (macro-circulation)
- The heart, vena cava, pulmonary artery, and aorta provide blood flow and pressure gradients through the organs.
- Downstream (micro-circulation)
- The small vessels and capillary system operate at the tissue level, contributing to the distribution of blood flow, the supply of oxygen and nutrients, and the elimination of waste products, such as carbon dioxide.
- Upstream (macro-circulation)
Overview of Circulatory Failure
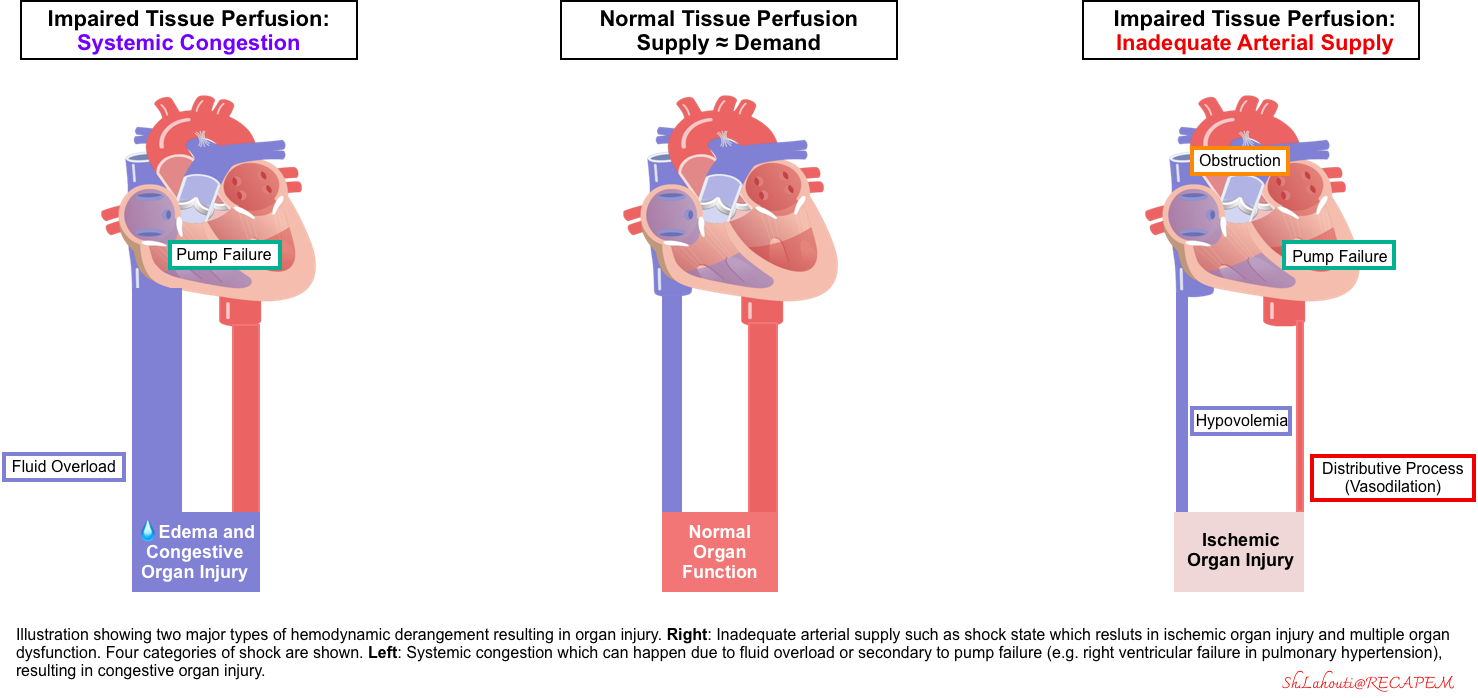
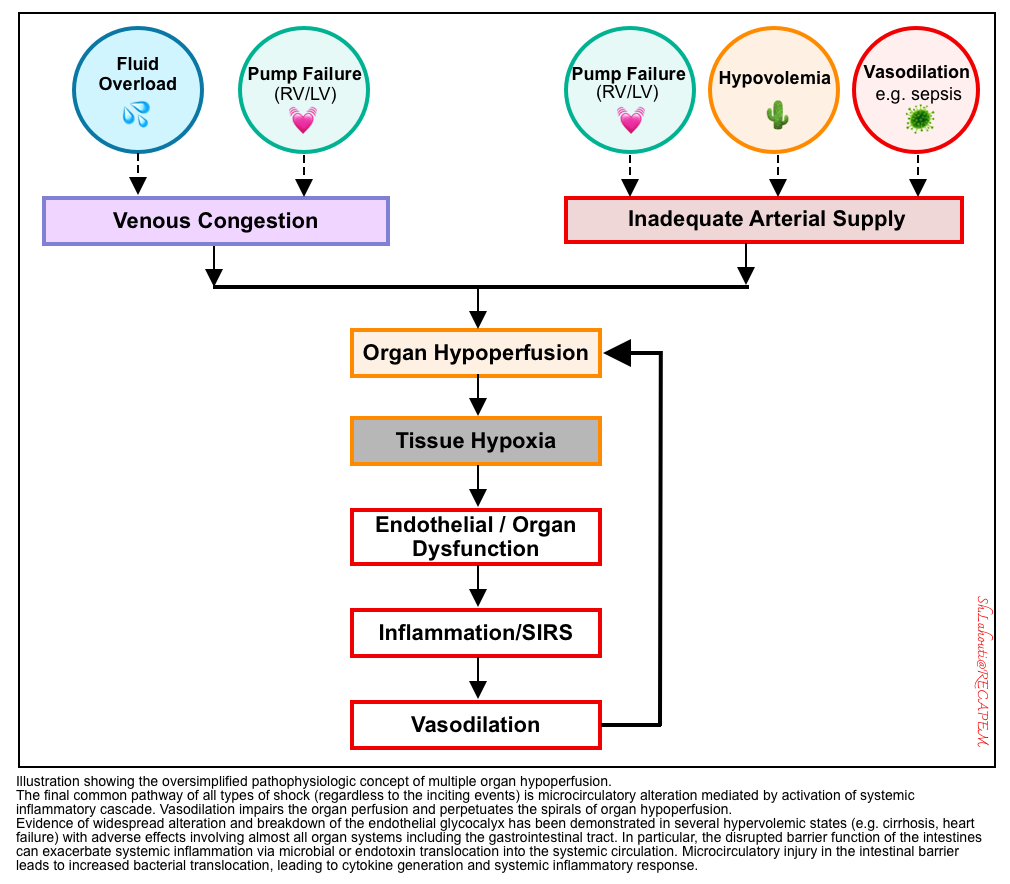
◾️Various inciting events (e.g., pump failure, hemorrhage, infection, etc) can cause hemodynamic instability (circulatory failure), resulting in “reduced organ perfusion” that leads to “end-organ injury“
◾️Organ hypoperfusion is not just because of inadequate forward flow (left figure below). Rather, organ hypoperfusion can be either due to:
- Impaired forward flow (shock) resulting in “ischemic organ injury“, OR
- Increased backward pressure (e.g., fluid overload or elevated right atrial pressure) leading to systemic congestion and congestive organ injury such as congestive hepatopathy *.
- ⎮⚠️ Shock is the most urgent, rapidly lethal type of hemodynamic instability.
- Shock has been traditionally taught as a prototype framework of hemodynamic instability. But that doesn’t mean it’s the only concern!
- 💡Hemodynamic instability could be more appropriately referred to as any pathophysiologic condition leading to a state of “organ hypoperfusion“, whether from “inadequate forward flow” or “pathologic backward pressure“.
- Shock has been traditionally taught as a prototype framework of hemodynamic instability. But that doesn’t mean it’s the only concern!
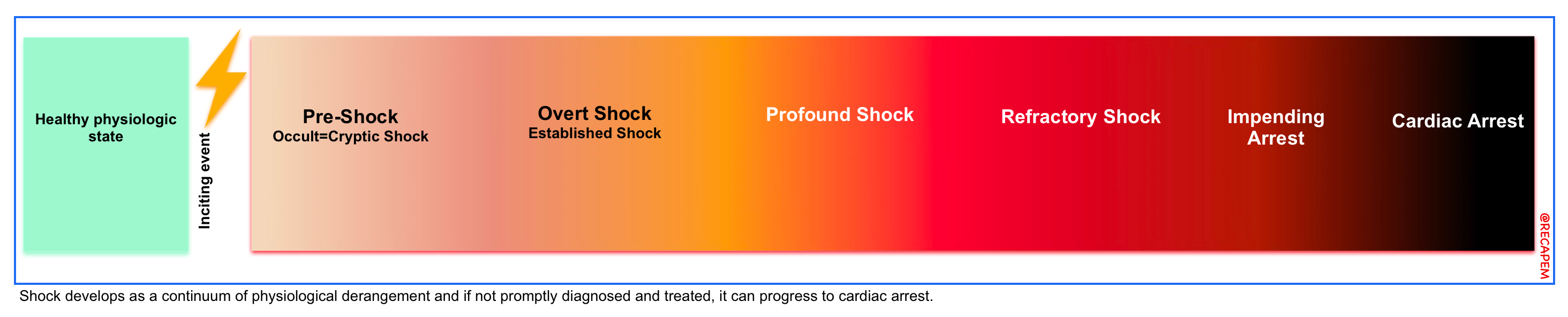
◾️Spectrum of circulatory failure
- Patients with circulatory failure can present with a wide range of presentations, regardless of the underlying mechanism of circulatory failure.
- During the initial phase of the disease, alteration of hemodynamic markers often precedes overt clinical manifestations of end-organ dysfunction. For example:
- In the setting of fluid overload, elevated right atrial pressure can be detected sooner than the overt manifestation of congestive organ failure.
- The same principle is working in the shock state.
- Shock is a physiologic continuum; beginning with an inciting event (e.g., focus of infection), triggering pathophysiological changes that can progress through several stages (right figure below) *.
- During the occult phase of shock, tachycardia and other compensatory responses take place before the overt manifestation of end-organ dysfunction (e.g., altered level of consciousness, ischemic hepatitis).
- This principle underscores the hemodynamic evaluation in critically ill patients, which allows early diagnosis and prompt correction of the underlying cause of circulatory failure.
- During the initial phase of the disease, alteration of hemodynamic markers often precedes overt clinical manifestations of end-organ dysfunction. For example:
◾️Pathophysiology of Circulatory Failure ⎯ Core feature
- The final biochemical & metabolic pathway of circulatory failure leads to micro-circulatory dysfunction, regardless of the inciting trigger (hypo/hypervolemia, pump failure, or vasodilatory process).
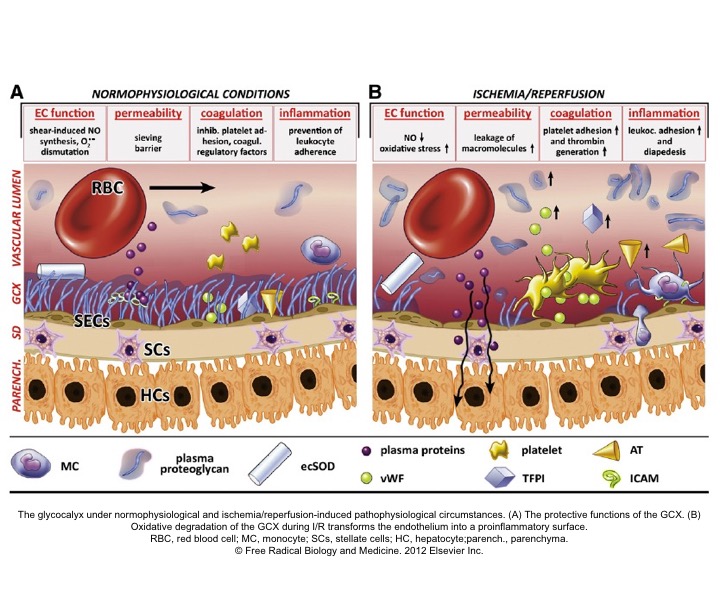
- Micro-circulation has been the focus of attention recently in maintaining “effective tissue perfusion” *,*.
- Healthy endothelial function and glycocalyx (GC) molecules are essential factors among the list to maintain effective tissue perfusion.
- Endothelial disruption, loss of GC integrity (right below figure), and generation of systemic inflammatory response are the final common pathways of end-organ hypoperfusion and congestion (left below figure).
- 🔄 These will cause vasodilation, which further perpetuates the spirals of organ hypoperfusion.
- Endothelial disruption, loss of GC integrity (right below figure), and generation of systemic inflammatory response are the final common pathways of end-organ hypoperfusion and congestion (left below figure).
- Healthy endothelial function and glycocalyx (GC) molecules are essential factors among the list to maintain effective tissue perfusion.
- Micro-circulatory alteration in shock
- Traditionally, shock has been classified based on systemic hemodynamic profile (e.g., hypovolemic, cardiogenic, obstructive, and distributive) *.
- However, this mechanistic classification of shock is an oversimplification *.
- The final common pathway of all types of shock is micro-circulatory alteration (mediated by activation of the systemic inflammatory cascade) *.
- This also accounts for the fact that different classes of shock can’t be differentiated in later stages *.
- Traditionally, shock has been classified based on systemic hemodynamic profile (e.g., hypovolemic, cardiogenic, obstructive, and distributive) *.
- Microcirculatory alteration in venous congestion
Hemodynamic assessment
Multimodal toolkit
🧰 Hemodynamic evaluation is grounded in a multimodal toolkit including:
- Hemodynamic variables
- Markers of organ perfusion
- Global markers of hypoperfusion
#1. Hemodynamic variables
◾️Definition
- Hemodynamic variables are parameters that directly describe or quantify the flow and pressure of blood within the circulatory system.
- They are typically related to blood pressure, flow, resistance, and cardiac function.
◾️Measured or calculated (derived) variables
- Measured variables → obtained using sensors or monitors, e.g., arterial blood pressure, CVP, HR.
- Derived (calculated) variables → obtained by applying formulas using one or more measured parameters, e.g., shock index, pulse pressure, CO/CI, SVR.
◾️Static vs. dynamic variables
- Static variables
- These are single, snapshot values that reflect pressure or flow at a fixed point in time, without accounting for changes in response to physiologic stimuli (e.g., MAP, HR, CO).
- Dynamic variables
- These are real-time, responsive parameters that reflect how the cardiovascular system reacts to changes in preload or pressure, especially used to predict fluid responsiveness *.
- They determine “where the heart stands on the Frank-Starling curve”.
- Dynamic variables that might be useful for the prediction of fluid responsiveness, such as pulse pressure variation, heart rate variability * will be discussed separately.
#2. Markers of organ perfusion
◾️Definition
- These are clinical signs and laboratory values indicating that one or more vital organs are not receiving adequate blood flow or oxygen, or are failing to extract/utilize oxygen efficiently.
- Note that these are the consequences of organ hypoperfusion (not an early sign), so while they may help to confirm organ hypoperfusion.
- ⎮⚠️ They are often too delayed to rely on for early recognition or resuscitation guidance.
◾️Markers of organ hypoperfusion include:
- Altered mental status → cerebral hypoperfusion?
- Low urine output and rising serum creatinine → renal hypoperfusion?
- Elevated liver enzymes → hepatic hypoperfusion?
- Elevated troponin → cardiac ischemia?
#3. Global markers of hypoperfusion
- These markers reflect microcirculatory function; however, they don’t directly assess microcirculation and include:
- Lactate
- Skin perfusion status (CRT, mottling, skin temperature).
- Tissue oxygen saturation measured by near-infrared spectroscopy (NIRS).
Principles of Hemodynamic Assessment
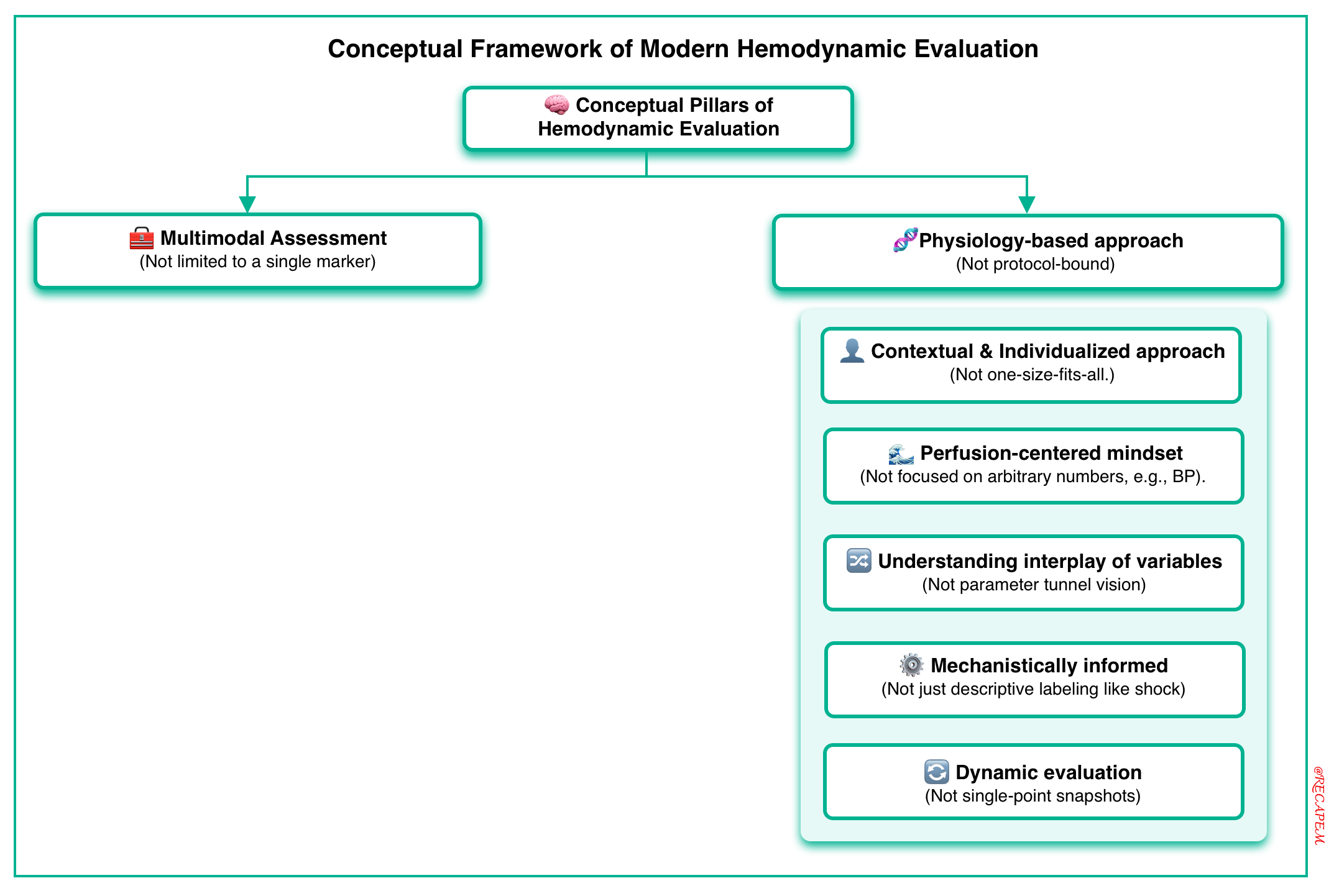
Modern hemodynamic evaluation rests on a small number of essential principles, key elements that anchor a nuanced, physiology-based, and clinically adaptable approach (figure below).
#1. Multimodal assessment
◾️Rationale
- Hemodynamics are complex and interrelated.
- No single marker is sensitive or specific enough for diagnosing hemodynamic instability and guiding the resuscitation (see appendix).
- The following examples lay the ground for multimodal evaluation:
- Blood Pressure (MAP) ⎯ is an arbitrary cutoff for MAP below 65 mmHg; diagnostic for shock ⁉️
- A low BP is not sensitive to exclude organ hypoperfusion.
- BP can be maintained by vasoconstriction despite poor organ perfusion.
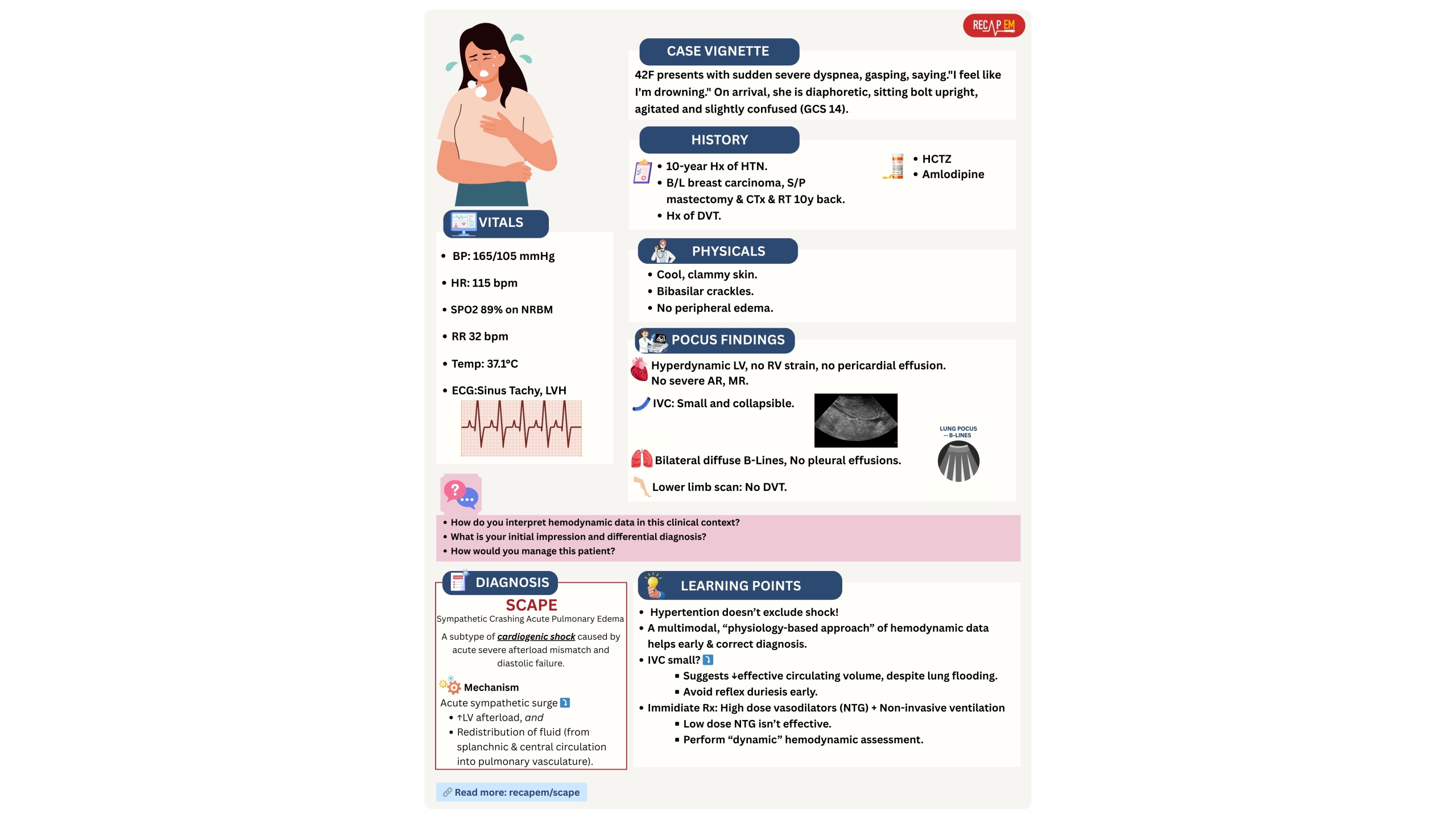
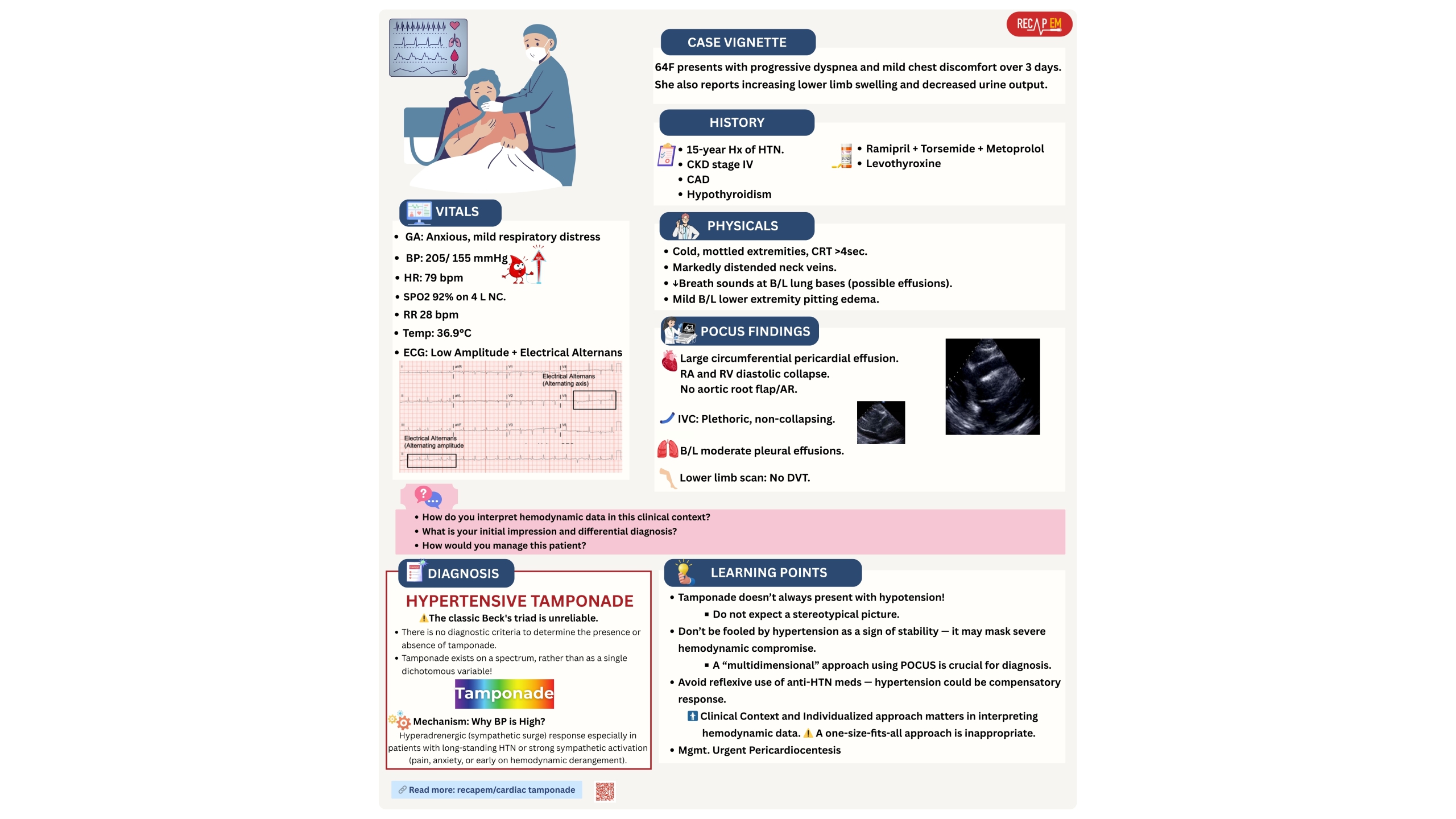
- Patients in early stages of shock or specific types of shock can present with hypertension (see below vignette #1).
- The compensatory mechanism (sympathetic surge) can keep BP normal or even high in early shock.
- BP can be maintained by vasoconstriction despite poor organ perfusion.
- A lower MAP is not specific for shock. Patients with advanced cirrhosis have a lower baseline BP.
- A low BP is not sensitive to exclude organ hypoperfusion.
- Tachycardia
- Tachycardia is not sensitive to identifying early shock stages (compensatory response).
- Medications (e.g., β-blockers) and physiologic reserve (e.g., young healthy vs. elderly frail) can alter the compensatory HR response to shock; therefore, tachycardia may be blunted in beta-blocked or elderly patients.
- Tachycardia isn’t specific for shock.
- Several conditions can cause tachycardia, e.g., fever, pain, and anxiety.
- Tachycardia is not sensitive to identifying early shock stages (compensatory response).
- Urine output (UOP) ⎯ UOP < 0.5 cc/kg/h is worrisome for renal hypoperfusion ⁉️
- A patient with chronic renal disease may have low urine output regardless of perfusion.
- Lactate ⎯ Is an arbitrary lactate level of > 2 mmol/L diagnostic for circulatory insufficiency ⁉️
- Not sensitive for identifying shock state.
- Lactate can be normal in shocked patients who have inadequate sympathetic nervous function. 📚Read more on lactate👉 here.
- Not specific either.
- Patients with liver failure could have an elevated lactate in the absence of a shock state.
- Not sensitive for identifying shock state.
- Cool extremities ⎯ are this diagnostic for shock ⁉️
- Not sensitive.
- Patients with early distributive shock can present with warm extremities.
- Not specific.
- Patients with peripheral vascular disease, Raynaud’s phenomenon, high sympathetic tone (pain, anxiety, certain medications like vasopressors) can have cool extremities at their baseline.
- Patients with severe heart failure can have cool extremities while maintaining core perfusion at their baseline.
- Not sensitive.
- Delirium ⎯ is an altered mental status diagnostic for shock and cerebral hypoperfusion ⁉️
- Not sensitive.
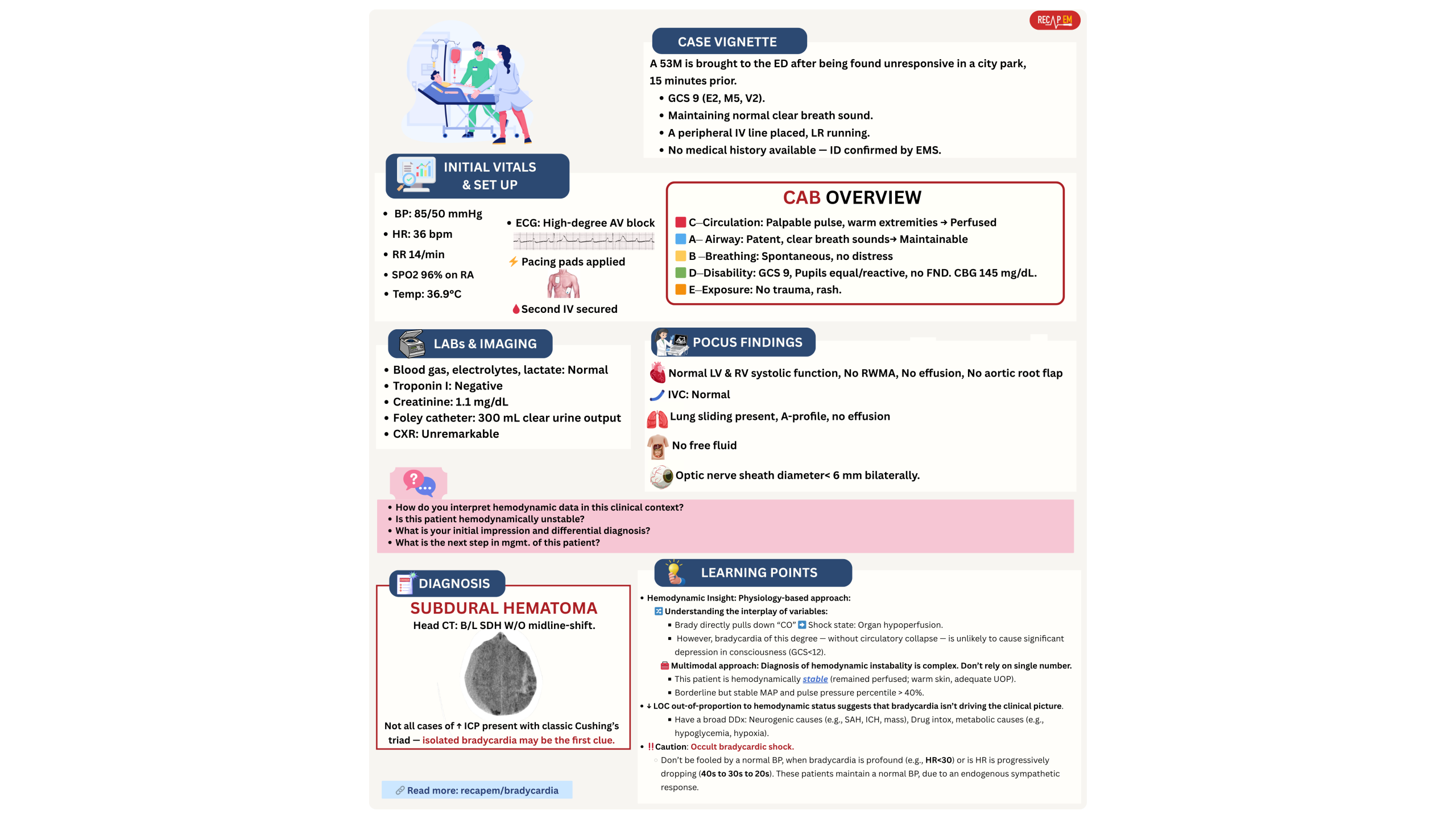
- Patients with cardiogenic shock may look OK, indecisively (delirium can be a late presentation in cardiogenic shock). Below vignette #2.
- Not specific.
- Patients with hepatic or uremic encephalopathy can have delirium at their baseline. Read more on etiologies of altered mentation here.
- Not sensitive.
- LV Ejection fraction (LVEF) ⎯ does a low LVEF reflect acute circulatory failure (organ hypoperfusion) ⁉️
- Not sensitive.
- Patients with early distributive shock or specific types of shock (e.g., SCAPE, vignette #1) can present with normal or supra-normal LVEF.
- Not specific.
- Patients with underlying HFrEF have a low EF at their baseline, but are doing fine!
- Not sensitive.
- Blood Pressure (MAP) ⎯ is an arbitrary cutoff for MAP below 65 mmHg; diagnostic for shock ⁉️
- Rule: Do not rely on any single hemodynamic marker.
- Collect data to create a comprehensive hemodynamic profile *.
- Value of multimodal approach:
- Enhancing physiologic understanding.
- Do the data make physiological sense together? For example, do the HR, BP, CVP, cardiac function, and clinical exam tell a coherent story?
- Early diagnosis of circulatory failure and reduce diagnostic errors.
- Giving key clues for making a mechanistic diagnosis (e.g., cardiogenic vs. distributive shock).
- Enhancing physiologic understanding.
◾️ Bedside assessment
- 🧰 Combine different tools and markers:
- Vitals: BP, HR?
- Physical exam: Skin perfusion status, level of consciousness, urine output?
- POCUS: LVEF, RAP, RV systolic function.
- Lab markers (lactate, serum creatinine, LFTs)
- Invasive monitoring is used if needed.
◾️Pitfalls
- 🚫 Avoid tunnel vision. Don’t equate findings with the disease without considering the underlying modifier. For example:
- Hypotension is not equal to hypoperfusion (i.e., shock state).
- Confusion alone is not equal to brain hypoperfusion or septic encephalopathy.
#2. Physiology-based approach
A physiology-based approach to hemodynamic evaluation and management means that clinical decisions are guided by a deep understanding of cardiovascular physiology, rather than relying solely on fixed protocols, rigid targets, or “one-size-fits-all” thresholds.
- Avoids blindly chasing “magic numbers” (like CVP = 12, or MAP = 65 in every patient).
- Instead, seeks to understand what each number means in the physiological context of the individual patient.
◾️Physiology-based approach involves:
- Contextual & Individualized approach (not one-size-fits-all).
- Interpretation in clinical context: Numbers only make sense when interpreted within the patient’s overall clinical picture.
- Perfusion-centered mindset: Prioritize tissue and organ perfusion over arbitrary targets.
- Understanding the interplay of variables: Integrate preload, afterload, contractility, heart rate, and vascular tone.
- Mechanistically informed, not just descriptive.
- Dynamic evaluation
◾️Value of a physiology-centered mindset
- Physiologic reasoning helps interpret data coherently. Do the HR, BP, CVP, cardiac function, and clinical exam all fit together?
- This clarifies unexpected findings and guides what you should anticipate. For example:
- In chronic RV failure, a plethoric IVC is expected — a small IVC should prompt you to consider hidden issues like hypovolemia (e.g., GI bleeding). See vignette #7.
- A moderate bradycardia can’t cause an isolated severe drop in GCS (~ <10), while other hemodynamic variables and markers of organ perfusion are maintained. In such patients, bradycardia is not driving the clinical picture (below vignette #5).
- This clarifies unexpected findings and guides what you should anticipate. For example:
- Physiologic insight prevents errors in interpreting complex findings.
- Do not expect a stereotypical picture (i.e., a clean, textbook pattern) to identify hemodynamic instability. The clinical presentation is not uniform among patients in a shock state. For example:
- A patient with cardiogenic shock can still “look okay”, mentating, talking, while other organs are quietly failing. See below vignette #2.
- Cardiac tamponade doesn’t always present with Beck’s triad (hypotension, JVD, muffled heart sound). See below vignette #3.
- A plethoric IVC isn’t always present in tamponade (below vignette #4).
- A physiology-based strategy reveals key clues to the cause of instability.
- Identifying the mechanism improves treatment accuracy, avoids harmful interventions, and directs targeted, life-saving therapy.
| Clinical Vignette |
A. Contextual & Individualized approach
◾️Rationale
- The state of circulatory failure (shock) is not homogeneous. No two patients are the same.
- The interplay of multiple parameters makes its clinical presentation and outcome variable (discussed above).
- The same value can indicate very different things depending on:
- The patient’s baseline physiology.
- The acute pathophysiologic changes, e.g., vasodilation (distributive shock) vs. pump failure (cardiogenic shock).
- The dynamic trends (is it rising or falling?)
- Organ perfusion markers (mental status, urine output, lactate).
- The following examples will lay the ground for an interpretation in a clinical context:
- A MAP of 65 mm Hg may be low in patients with chronic hypertension or may be normal-high in patients with cirrhosis.
- IVC size and collapsibility index (CI) ⎯ does a flat and collapsible IVC call for tons of IV fluid administration ⁉️
- Patients with septic shock can have flat IVC due to vasodilation. Giving too much fluid is harmful! 📚More on IVC👉 here.
- Patients with Sympathetic Crashing Acute Pulmonary Edema (SCAPE) can have a flat IVC while their lungs are flooding with fluid! (above vignette #1👆).
⎮⚠️”Avoid one-size-fits-all” *.
- Analyzing hemodynamic data and management of patients should be contextualized & individualized. For example:
- Instead of giving uniform fluids or targeting a fixed MAP (e.g., 65 mmHg), you individualize therapy based on each patient’s physiological response.
B. Perfusion-centered mindset
The heart of modern hemodynamic assessment lies in shifting our focus away from chasing isolated numbers like BP, CVP, toward evaluating whether tissues and organs are being well-perfused. This is the essence of perfusion-centered thinking.
◾️Rationale: A perfusion-centered mindset shifts the focus from numbers to function and outcomes *.
- How❓
- The circulatory system works to provide adequate organ/tissue perfusion, thereby maintaining organ function.
- Preserving organ function reflects the synergy of macro- and microcirculatory components.
- The traditional approach focused on reaching MAP or CVP targets (macrocirculatory markers), but didn’t ensure effective tissue oxygenation. A patient may have normal MAP yet still be in shock if microcirculatory perfusion fails.
- Loss of coherence between macro- and microcirculation can result in organ dysfunction despite seemingly normal macro values. More on this here.
- 🎯 Therefore, the ultimate goal of resuscitation is not just to raise the blood pressure, but rather to restore effective tissue perfusion and organ function.
◾️Pearls & pitfalls
- ⚠️Markers of organ function (organ perfusion) are often delayed or indirect, not always apparent, and early.
- For example, abnormal mental status could be a late finding in cardiogenic shock.
- 💡Perfusion-centered resuscitation should not be aimed at “normal numbers,” but at restoring effective perfusion that sustains organ function.
- In practice, this means:
- Don’t treat any single value (e.g., HR, MAP, or CVP) in isolation.
- Look for improving organ function, lactate, and tissue-level signs of perfusion.
- In practice, this means:
- ⚠️Be cautious of normal appearing hemodynamics values that mask ongoing hypoperfusion‼️
◾️Bedside assessment
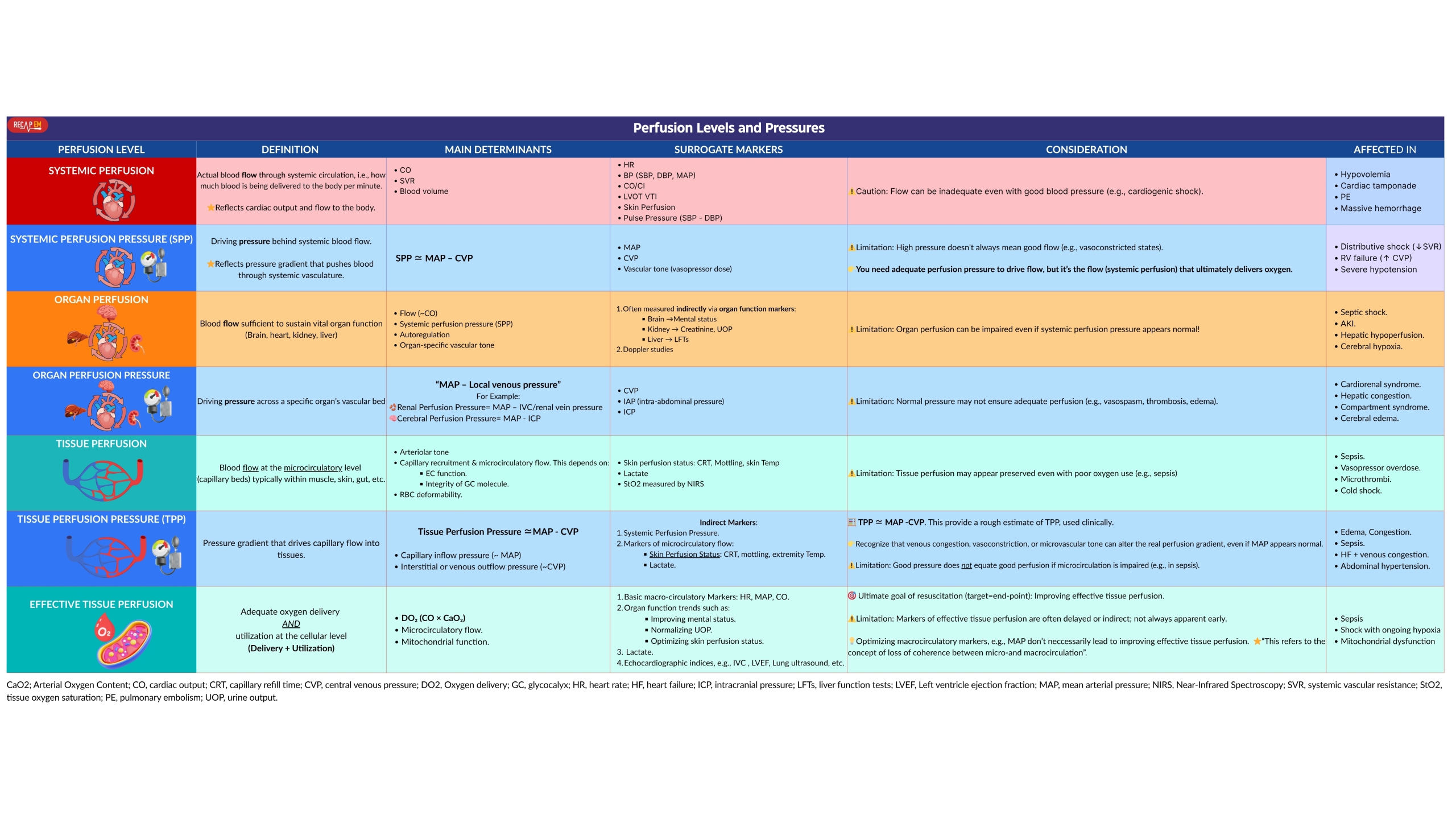
- To apply perfusion-centered thinking effectively, it’s crucial to distinguish between systemic perfusion, organ perfusion, and tissue perfusion.
- Perfusion exists on multiple levels (table below).
-
- Systemic perfusion vs. systemic perfusion pressure
- Systemic perfusion refers to blood flow through the systemic circulation (i.e., the amount of blood being delivered to the body per minute).
- Reflects cardiac output and flow to the body.
- Systemic perfusion pressure refers to the driving pressure behind systemic blood flow, usually estimated as “MAP – CVP“.
- Reflects a pressure gradient that pushes the blood through the systemic vasculature.
- 🧠 Clinical analogy:
- Think of the circulatory system like a garden hose:
- Systemic perfusion is the actual flow of water coming out of the nozzle.
- Systemic perfusion pressure is the pressure in the hose.
- You can have:
- High pressure, but poor flow (kinked hose = vasoconstriction, low CO).
- Normal pressure, but inadequate tissue delivery (septic vasodilation, microvascular failure).
- Think of the circulatory system like a garden hose:
- 🔍 Key clinical insight:
- You need adequate perfusion pressure to drive flow, but it’s the flow (systemic perfusion) that ultimately delivers oxygen.
- Systemic perfusion refers to blood flow through the systemic circulation (i.e., the amount of blood being delivered to the body per minute).
- Organ perfusion vs. organ perfusion pressure
- Organ perfusion refers to the blood flow that reaches and circulates within a specific organ (e.g., kidney, brain, liver).
- It reflects oxygen and nutrient delivery to sustain organ function.
- Organ perfusion pressure is defined as the driving pressure for blood to flow through an organ’s vasculature.
- Pressure gradient across the organ, often estimated as “MAP – local venous pressure“ (e.g., MAP – IVC pressure for the kidney).
- 🧠 Clinical analogy: Think of an organ like a sponge that’s irrigated by a faucet:
- Organ perfusion is how well the sponge is getting soaked.
- Organ perfusion pressure is the water pressure coming in.
- You can have:
- Normal pressure but poor soaking, if the sponge is stiff, blocked, or compressed (e.g., increased ICP, abdominal compartment syndrome).
- Low pressure and poor soaking (e.g., classic hypoperfusion or ischemia).
- Good soaking (perfusion) despite lower pressure, if autoregulation is intact (e.g., healthy kidneys tolerating MAP ~65).
- 🔍Clinical insight:
- Organ perfusion pressure is a tool, not the ultimate goal.
- What truly matters is actual organ perfusion — is the organ receiving blood and working?
- This distinction is crucial when interpreting:
- Renal function in septic patients with high CVP.
- Cerebral perfusion in TBI (MAP – ICP).
- Liver function in shock with venous congestion.
- Organ perfusion refers to the blood flow that reaches and circulates within a specific organ (e.g., kidney, brain, liver).
- Tissue perfusion vs. tissue perfusion pressure and effective tissue perfusion
- Tissue perfusion is defined as the blood flow at the capillary level in tissues.
- It reflects microcirculatory circulation, delivering oxygen and nutrients.
- Tissue perfusion pressure refers to the driving pressure across the capillary bed, usually approximated by “MAP – CVP“.
- It reflects the gradient pushing blood through tissues.
- Effective tissue perfusion refers to the net oxygen delivery and utilization at the cellular level.
- It reflects the final goal, which is that oxygen gets to cells and is metabolized.
- 🧠 Clinical analogy:
- Imagine watering a garden bed with soil and roots:
- Tissue perfusion pressure: The water pressure at the hose nozzle, which is pushing water into the bed.
- Tissue perfusion: The amount of water reaching the soil, i.e., how much is getting through the surface.
- Effective tissue perfusion: The roots absorbing and using the water, i.e., are the cells metabolizing oxygen?
- Imagine watering a garden bed with soil and roots:
- 🔍Clinical insight:
- A patient can have normal tissue perfusion pressure (e.g., MAP 70, and a CVP of <10 mmHg) but poor capillary flow due to vasoconstriction or sepsis.
- A patient can have normal tissue perfusion (e.g., warm skin, good CRT) but ineffective perfusion at the cellular level, reflected by rising lactate, or organ failure.
- Effective tissue perfusion is the true endpoint of resuscitation.
- Tissue perfusion is defined as the blood flow at the capillary level in tissues.
- Systemic perfusion vs. systemic perfusion pressure
C. Understanding the interplay of variables
- Considers preload, afterload, contractility, SVR, HR, venous return, and how they interact to determine cardiac output and ultimately tissue oxygen delivery.
- These variables are tightly linked: a change in one affects the others.
- For example, if LV preload drops (e.g., hypovolemia), cardiac output may decrease unless compensated by increased HR or contractility.
- Conversely, increased afterload (e.g., hypertension) can reduce stroke volume if contractility is impaired.
- These variables are tightly linked: a change in one affects the others.
- The value of recognizing how variables interact
- This integrated view helps avoid misinterpreting isolated parameters and guides targeted, effective treatment to improve tissue oxygen delivery (below vignette #6).
4. Mechanistically informed
- It focuses on understanding the precise underlying mechanisms of hemodynamic instability or shock.
- It often involves “breaking down” the cardiovascular system into components (preload, afterload, contractility, HR) and analyzing each.
- Mechanistic diagnosis guides the right questions: “What is the cause of circulatory failure”?
- The mechanism of circulatory failure should guide the therapy.
- ⎮💡Treating the cause, not just the number is the key.
- For example, in bradycardic shock, fixing the HR won’t help if the cause of bradycardia is AMI, poisoning,↑ICP, hypoxia, or electrolyte abnormalities.
- 💡“Identify & Treat The Reversible Cause(s)”.
5. Dynamic evaluation
- Hemodynamic instability evolves, walks through a spectrum of severity.
- Patients with septic shock can develop cardiogenic shock later (Septic Cardiomyopathy)
- Hemodynamic responses to vasoactive agents and fluid are not uniform among patients.
- Vasopressor responses often deviate from textbook expectations.
- These support the need for continuous reassessment and real-time physiologic understanding, not static protocols.
◾️Dynamic evaluation
- Referring to ongoing, repeated clinical evaluation of a patient’s hemodynamic status over time or in response to interventions. For example:
- Reassess the patient after a change (e.g., after a fluid bolus, vasopressor titration, or intubation) by your hemodynamic toolkit (e.g., vitals, organ perfusion markers, POCUS).
- Goal
- Detecting deterioration or improvement.
- Are serial changes in mentation, urine output, lactate, skin signs, and vitals matching expectations?
- Are you just treating the monitor or the actual physiology?
- Did fluids/inotropes/pressors improve clinical endpoints?
- Did you cause any harm, e.g., signs of new congestion, arrhythmia, or vasopressor-induced ischemia?
- Refining the diagnosis (e.g., early distributive → late cardiogenic physiology). See below vignette #7.
- Adjusting the treatment (e.g., fluids, vasopressors, inotropes) accordingly.
- Should you escalate, de-escalate, or change the treatment?
- Is further resuscitation needed, or has the patient entered a no-flow, no-recruitable state?
- Detecting deterioration or improvement.
◾️Bedside dynamic reassessment (see above).
⚠️Pitfalls
- Avoid defaulting to fixed algorithms instead of tailoring to the specific patient physiology.
- A fixed, protocol-driven mindset is wrong! For example, “Let’s just follow the sepsis protocol” ‼️
- Avoid resuscitative measures without reassessing the patients thereafter.
- For example, giving fluids reflexively, with no check on response or risk of congestion.
Modern hemodynamic evaluation at the bedside
◾️Data Collection (multimodal) and Integration → Interpretation (analyzing, physiology-based) → Mechanism of instability? → Action? → Dynamic evaluation
- 🧰 Collect all relevant data (multimodal assessment, explained above).
- 🧐 Integrate the whole clinical picture: comorbidities, syndrome, and trajectory.
- 💻Referring to archived data (old echo, CT, labs, ECGs) helps clarify the condition and make a physiologic narrative from hemodynamic data.
- Do not anchor too early on one abnormal finding, without integrating other findings or understanding the baseline (e.g., “echo says LVEF is low, so this is cardiogenic shock”).
- Experts don’t start with lactate! They start with “Who is this patient? What’s their baseline? What just happened?”
- 💻Referring to archived data (old echo, CT, labs, ECGs) helps clarify the condition and make a physiologic narrative from hemodynamic data.
- 🧠 Interpret (analyze) the data, making sense of that picture to diagnose correctly and guide the therapy.
- Do the heart rate, blood pressure, venous pressure, cardiac function, and clinical exam tell a coherent story?
- Are there contradictions that suggest a hidden pathology? e.g., a small IVC in a patient with underlying RV failure can suggest ongoing volume loss.
- Thinking that every patient can fit into one clean shock box, rather than exploring mixed or evolving forms (e.g., septic + cardiogenic).
- 🔎 Identify the mechanism of instability, rather than labeling shock.
- 🎯 Resuscitative measures targeted to preserve organ perfusion and function.
- 🔄 Dynamic evaluation. Re-evaluate the whole clinical picture after therapeutic/supportive measures.
| First: Hemodynamic Data Integration | Second: Hemodynamic Data Interpretation | |
| Definition | The process of gathering and combining multiple data points from various sources | The process of analyzing, reasoning, and drawing conclusions from the integrated data |
| Focus | Assembling a coherent picture from scattered values | 🧐Making sense of that picture to guide decisions |
| Tools involved | Clinical context Vitals Echo Labs (lactate, creatinine, LFTs) Pressures Perfusion signs | Clinical reasoning Physiology knowledge Trajectory trends |
| Example | Noting: HR 120, MAP 65, IVC flat, lactate 5.0, CRT delayed, EF normal | Concluding: likely distributive shock with preserved cardiac function and inadequate perfusion |
| Risk if poor | Fragmented or misleading picture; missing key variables | Misdiagnosis, inappropriate therapy (e.g., fluids in RV failure) |
| Analogy | 🧩Like assembling all the puzzle pieces | 🧠Like seeing the image that the puzzle creates |
Appendix
Diagnostic performance of certain hemodynamic markers in cardiogenic shock.
Bedside classification of shock
Gutentor Simple Text









Add comment