June 27, 2024, by Shahriar Lahouti.
CONTENTS
- Preface
- Pathophysiology
- Differential diagnosis
- Evaluation
- Complications
- Treatment
- Specific conditions
- Appendix
- References
Preface
Nausea and vomiting are commonly encountered symptoms in the emergency department due to multiple causes, with the most common one being GI disorder, However, symptoms may also represent disorders outside of the GI tract, such as diabetic ketoacidosis, intracranial lesions and infection, myocardial infarction, sepsis, hyperemesis gravidarum, and drug toxicities. Nausea and vomiting are also one of the most feared side effects of cancer treatment. In this post, the overall approach to patients with vomiting, therapeutic options, as well as nausea and vomiting in pregnancy (NVP), and treatment strategy for chemotherapy-induced nausea and vomiting will be covered.
Pathophysiology
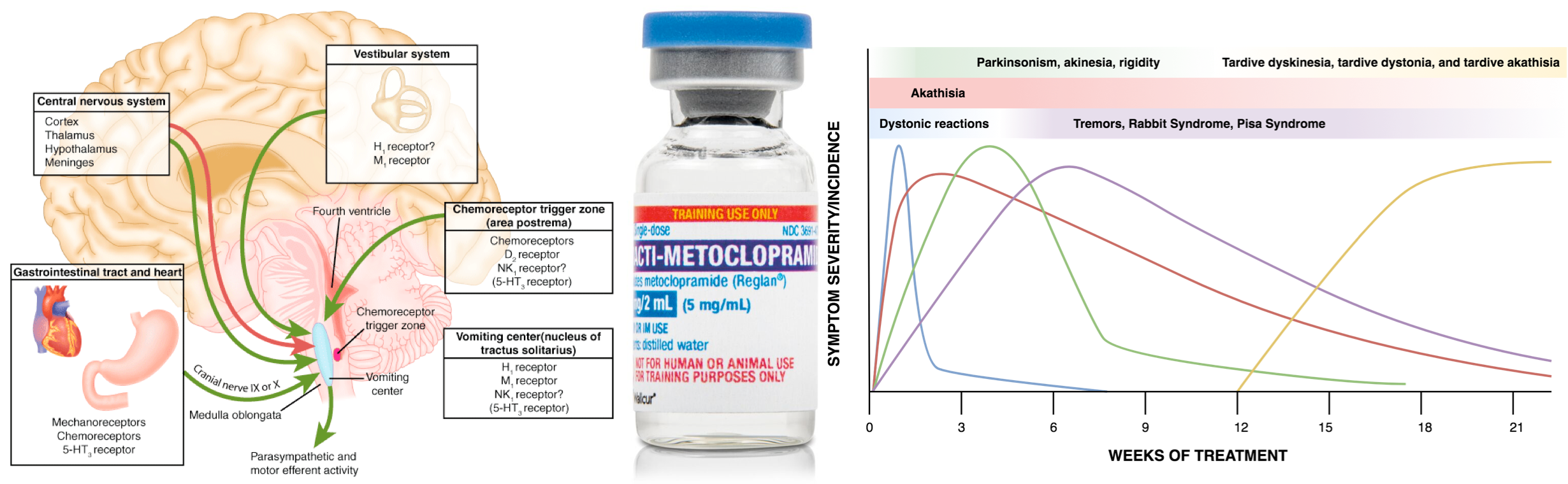
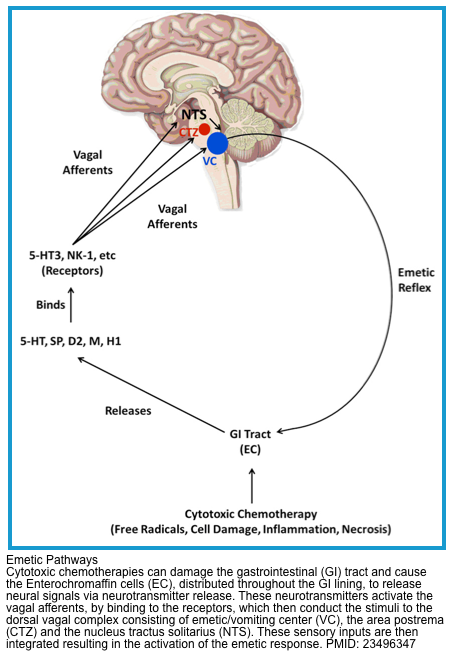
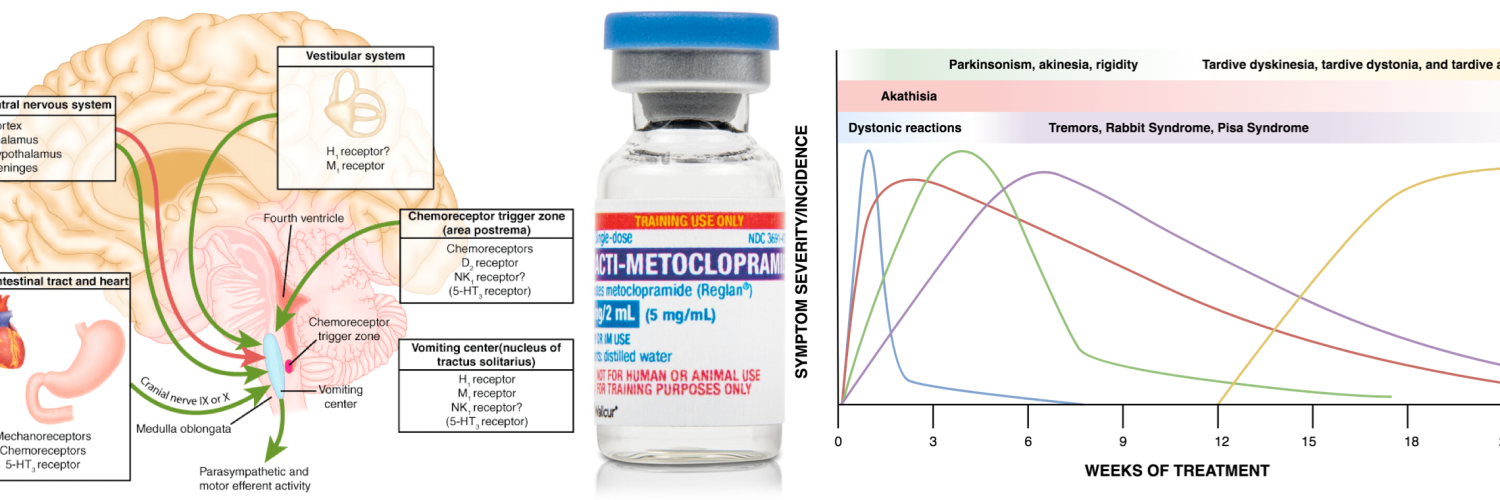
Multiple neurons in the medulla oblongata are activated sequentially to induce vomiting. The vomiting center is the chemoreceptor trigger zone (CTZ), located in the area postrema of the fourth ventricle. Chemoreceptors in this area are outside the blood-brain barrier and are stimulated by circulating medications and toxins, including dopaminergic antagonists (levodopa, bromocriptine), nicotine, digoxin, and opiate analgesics. The CTZ has neuronal connections to the vomiting center (VC), which is a collection of nuclei including the dorsal motor nucleus of the vagus and the nucleus tractus solitarius.
Another important peripheral pathway for emesis is mediated through vagal afferents. Vagal activation is triggered by direct gastric mucosal irritants (such as NSAIDs) or increased luminal distention (gastric outlet obstruction, gastroparesis). Vagus activation stimulates neurons in the area postrema and nucleus tractus solitarius.
These areas are rich in serotonin receptors and are a major site of action of antiemetic drugs, such as ondansetron. Similar receptors are found throughout the GI tract, the cortex and limbic system, the vestibular system, the heart, and genitalia *.
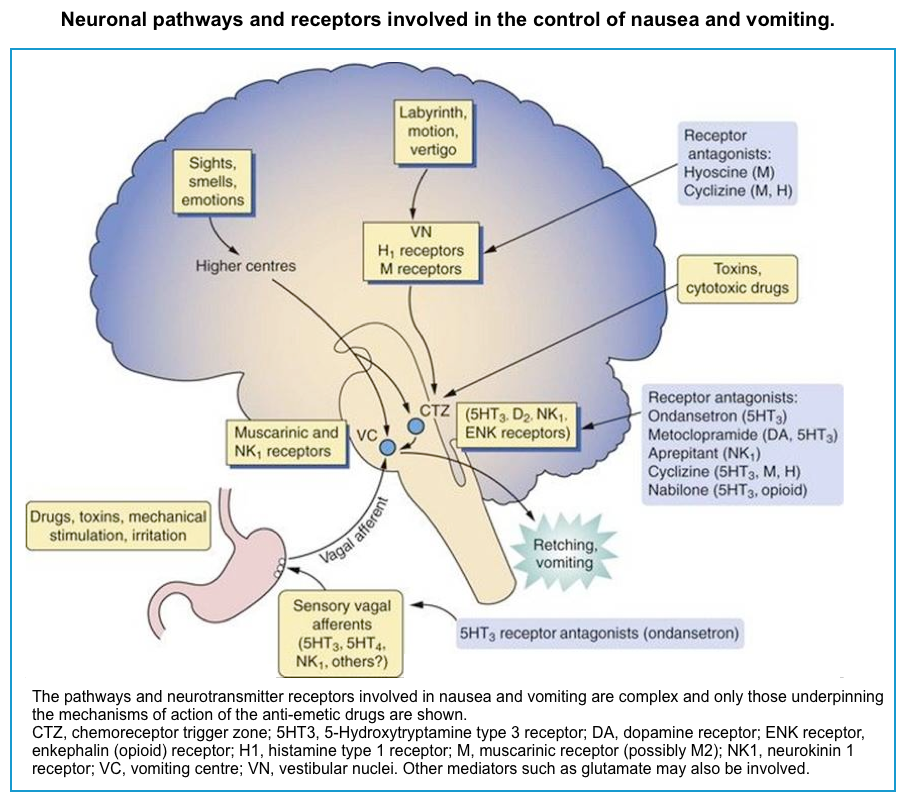
Differential diagnosis
The differential diagnosis of nausea and vomiting is broad, as the pathology of almost every organ system may lead to nausea and vomiting. A thorough history and physical examination will help guide the diagnostic approach to the patient presenting with nausea and vomiting.
- Drugs and Toxins *
- Anesthetic agents.
- Analgesics, e.g. aspirin, NSAIDs, opioids.
- Antibiotics, e.g. erythromycin, sulfonamides, acyclovir.
- Chemotherapeutics, and radiation therapy
- Cardiovascular: Antiarrhythmics, antihypertensives (beta-blockers, calcium channel blockers), digoxin, diuretics.
- Endocrine, e.g. oral contraception, oral antidiabetic agents.
- Neurology, e.g. Anticonvulsants, Parkinson’s disease medications (dopaminergic).
- Illicit drugs
- Theophylline
- Toxins, e.g. organophosphates, carbon monoxide, ricin, arsenic, alcohols, etc.
- Drug overdose, e.g. acetaminophen, salicylates, digoxin, valproic acid, theophylline intoxication.
- Drug withdrawal e.g. alcohol/opioid withdrawal.
- Neurology *
- Elevated intracranial pressure (ICH, tumor, hydrocephalus, etc).
- Meningitis.
- Migraine.
- Ear and labyrinthine disorders (often associated with vertigo):
- Vestibular neuronitis.
- Meniere’s disease.
- Gastrointestinal *
- Obstruction (e.g. intestinal obstruction, gastric outlet obstruction, intussusception, volvulus).
- Hypomotility (e.g. gastroparesis, ileus).
- Acute gastroenteritis.
- Appendicitis, cholecystitis, cholangitis, hepatitis, peritonitis, pancreatitis,
- Mesenteric ischemia.
- Functional disorders
- Psychogenic
- Irritable bowel syndrome
- Endocrine *
- Adrenal insufficiency.
- Ketoacidosis (e.g. Diabetic ketoacidosis).
- Electrolyte disorders, especially hyponatremia, and hypercalcemia.
- Hyperthyroidism.
- Fulminant hepatic failure.
- Uremia.
- Pregnancy (hyperemesis gravidarum; generally within the first nine weeks of pregnancy).
- Infectious *
- Sepsis
- Bacterial toxins
- Pneumonia
- Spontaneous bacterial peritonitis
- Urinary tract infection
- Viral infections e.g. adenovirus, rotavirus
- Miscellaneous *
- Myocardial infarction
- Acute glaucoma
- Nephrolithiasis
- Pain
- Psychiatric disorders, e.g. anorexia nervosa, bulimia, depression, conversion disorder.
- Cannabinoid hyperemesis syndrome
- Cyclic vomiting syndrome
Evaluation
History and physical examination are the highest yield investigations. In many cases, these may reveal the etiology without additional evaluation. For patients in whom the initial investigation is unrevealing, the following studies may be considered (evaluation should be tailored to the patient).
History, physical exam
▪️History
💡In acutely vomiting patients, associated symptoms, and medication history are most helpful in narrowing down the differential diagnosis.
-
- Onset and duration of the symptoms?
- The evaluation of acute vs. chronic conditions is fundamentally different (chronic symptoms are defined as those symptoms present for >1 month).
- Timing?
- Increased number of episodes in the morning, may suggest pregnancy or a CNS cause, whereas postprandial vomiting suggests a GI cause such as gastroparesis or gastric outlet obstruction.
- Associated symptoms?
- Abdominal pain? Pain preceding the nausea and vomiting is most particularly associated with an obstructive process.
- Any CNS sign, such as headache, visual changes, vertigo, or neurologic deficits, may suggest a central cause for the nausea and vomiting.
- History of recent weight loss?
- A positive history is associated with a malignancy or psychiatric component
- Past medical and travel history?
- Sick contacts or ingestion of food suspicious of a foodborne illness?
- Prior abdominal surgery is a major risk factor for bowel obstruction from adhesions.
- Review the patient’s medication list
- Onset and duration of the symptoms?
▪️Physical exams
- Vitals
- Abdominal examination, ideally using ultrasonography.
- It may estimate the size and contents of the stomach.
- Small bowel peristalsis may be assessed, to evaluate for obstruction.
- Neurologic exam: Mental status, cranial nerve findings, or neurologic deficits?
- If concerned for elevated intracranial pressure, assess this using optic nerve ultrasonography.
Labs and imaging
▪️Laboratory studies
- STAT fingerstick blood glucose.
- Chemistries (including Ca/Mg/Phos)
- Liver function tests
- Lipase, if clinical concern for pancreatitis
- Serum beta-hydroxybutyrate
- Cortisol level, if adrenal insufficiency is suspected
- TSH, if hyperthyroidism is suspected
- Pregnancy testing, as appropriate
- Serum drug levels (if indicated)
▪️Imaging studies
- Abdominal X-ray may be considered, to evaluate for obstruction or ileus.
- Neuroimaging, if history and physical examination suggest elevated intracranial pressure.
Potential complications of vomiting
- Hypovolemia and shock
- Metabolic alkalosis
- Hypokalemia
- Mallory-Weiss Tear
- Boerhaave syndrome
- Aspiration pneumonitis or pneumonia
Treatment
Principles
▪️Sniffing isopropyl alcohol pads
- This has been demonstrated to be effective in some studies *. Therefore, it may be reasonable to add this as a co-intervention with a front-line parenteral antiemetic.
▪️Initial considerations when choosing the antiemetic drug:
- Parkinson’s disease or extrapyramidal side effects from medications?
- The presence of these conditions is a relative contraindication to agents that block the D2 dopamine receptors.
- Significant QT prolongation?
- In the presence of significant QT prolongation, safe antiemetic options include *
- Olanzapine.
- Palonosetron.
- Dimenhydrinate, meclizine.
- Benzodiazepine.
- Haloperidol/droperidol: Although they slightly increase \QT interval, the amount of increase is minimal at emetic doses.
- In the presence of significant QT prolongation, safe antiemetic options include *
- Ineffective initial therapy
- If a single antiemetic agent is ineffective, then a second agent may be added to it (ideally targeting a different receptor).
- Treatment of specific causes of nausea and vomiting where a specific agent may be preferred
- Gastroenteritis
- Dopamine antagonists (e.g. metoclopramide, prochlorperazine).
- Serotonin antagonists.
- Gastroparesis
- Metoclopramide is the 1st line agent.
- Haloperidol/droperidol: are 2nd line agents.
- Olanzapine is an alternative treatment.
- Opioid-induced
- Serotonin antagonists.
- Phenothiazines.
- Metoclopramide seems to be ineffective.
- Migraine-related
- First-line agents include:
- Metoclopramide.
- Prochlorperazine or chlorpromazine.
- The addition of ondansetron may be helpful, but data are lacking.
- First-line agents include:
- Nausea and Vomiting of Pregnancy (NVP). More on this below.
- Consider checking serum electrolytes and urine ketones to exclude hyperemesis gravidarum.
- Risk of Wernicke’s encephalopathy: Consider IV thiamine.
- Safe agents seem to include:
- Metoclopramide *.
- Other dopamine antagonists are not recommended due to conflicting evidence of safety during pregnancy.
- Antihistamines (including doxylamine, and diphenhydramine)
- Metoclopramide *.
- Ondansetron is therefore not recommended as a first-line treatment.
- Ondansetron has limited safety data. A study reported no increased risk of cardiac malformation, but a slightly increased risk of oral clefts *.
- Vitamin B6 (pyridoxine), and ginger are more effective when compared to placebo.
- Gastroenteritis
-
- Vestibular disorders including motion sickness
- The pathophysiology involves H1 histamine and M1 muscarinic acetylcholine receptors. More helpful meds are:
- Anticholinergic agents.
- Antihistamine agents.
- Alternative: dopamine antagonists.
- The pathophysiology involves H1 histamine and M1 muscarinic acetylcholine receptors. More helpful meds are:
- Postoperative
- Dopamine antagonists (e.g. droperidol).
- Serotonin antagonists.
- Antihistamines.
- Neurokinin-1 antagonists.
- Dexamethasone.
- Benzodiazepines.
- Metoclopramide at 10 mg seems to be ineffective.
- Chemotherapy-induced nausea and vomiting (CINV). More on this below.
- Neurokinin-1 antagonists.
- Dexamethasone
- Serotonin antagonists.
- Dopamine antagonists (e.g. butyrophenones, olanzapine).
- Benzodiazepines.
- Less effective: antihistamines, metoclopramide, prochlorperazine.
- Radiation-induced
- Serotonin antagonists.
- Serotonin antagonists are more effective than dopamine antagonists alone or in combination with dexamethasone
- Dexamethasone
- Adding dexamethasone to a serotonin antagonist further reduces radiation-induced nausea and vomiting
- Dopamine antagonists.
- Serotonin antagonists.
- Multifactorial or unknown etiology
- Haloperidol was shown to be equivalent to etiology-guided therapies in one study, suggesting that haloperidol may have broad efficacy across different etiologies *.
- Vestibular disorders including motion sickness
Antiemetic drugs
An overview of commonly used antiemetics in practice is shown below.
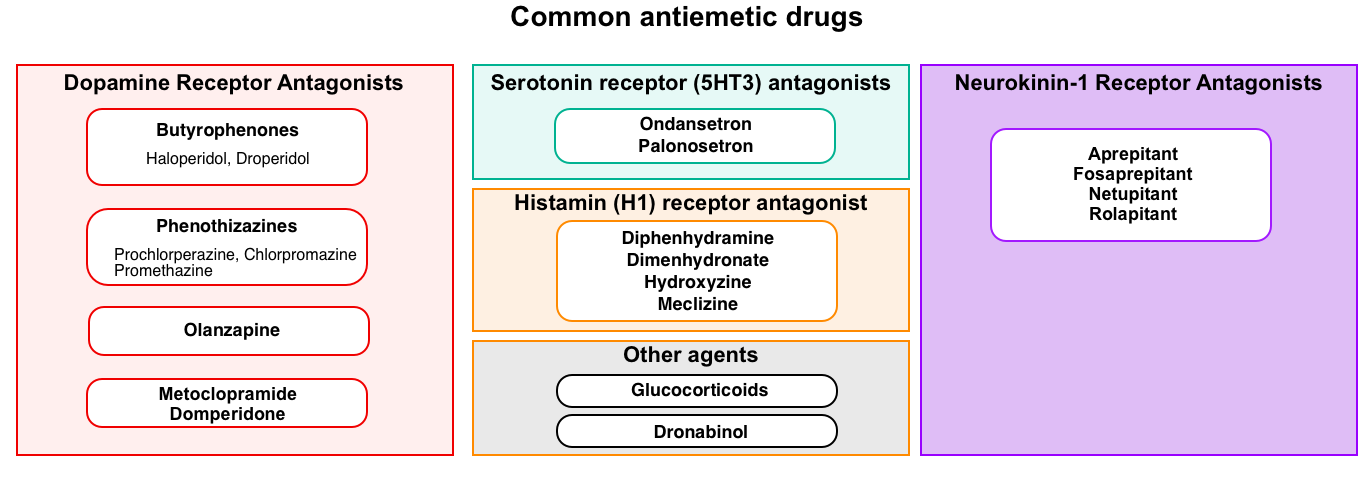
Adverse effects
QT prolongation
- The risk of QT prolongation and dysrhythmia increases with other risk factors affecting the QT interval, such as drugs, hypokalemia, and hypocalcemia.
- ⚠️Antiemetic drugs with potential for QT prolongation:
- Ondansetron (note that Palonosetron and tropisetron are not associated with QT prolongation).
- Haloperidol, droperidol.
- Phenothiazines.
- Metoclopramide can cause QT prolongation, but the effect is negligible at lower doses.
- ✅Olanzapine has no effect on the QT interval at therapeutic doses.
Extrapyramidal symptoms
There is a range of possible extrapyramidal effects including dystonia, akathisia, and parkinsonism.
- Medications with potential antagonistic effects on D2-receptor generate more of a true antipsychotic effect however, they also carry a greater side-effect profile (in terms of extrapyramidal side-effects and the risk of neuroleptic malignant syndrome). There is also an increased risk of tardive dyskinesia in patients treated with these agents for more than 12 weeks.
- For example, haloperidol, droperidol, and metoclopramide are potent D2-receptor antagonists.
- Note that domperidone is a peripheral D2-receptor antagonist (i.e. does not cross the blood-brain barrier) with less potential for extrapyramidal effects.
- ⚠️The risk of extrapyramidal side effects is greater in the following settings:
- Rapid IV administration.
- Higher doses.
- Elderly.
- Patients with underlying Parkinson’s disease or Lewy body Dementia.
- More potent D2-receptor antagonists: Haloperidol, Droperidol > Metoclopramide, Phenothiazines > Olanzapine
- Note that many antiemetics have both anti-D2 activity plus anticholinergic activity (e.g. olanzapine, prochlorperazine, promethazine).
- This intrinsic anticholinergic activity may reduce the rate of extrapyramidal symptoms caused by D2 antagonism (as Anticholinergics can be used to treat extrapyramidal symptoms).
- Agents with anti-D2 activity that lack anticholinergic activity may tend to have the highest rate of extrapyramidal symptoms (e.g. haloperidol, droperidol, and metoclopramide).
Sedation
- Sedation can be caused by antimuscarinic and antihistaminergic properties of drugs.
- Promethazine, Chlorpromazine, prochlorperazine, and other antihistamines such as doxylamine, and cyclizine are associated with sedation.
- Benzodiazepines and cannabinoids cause significant sedation which may limit their use as antiemetics.
Anticholinergic effects
- Many antiemetic drugs have anticholinergic adverse effects including confusion, delirium, hallucinations, visual disturbance, urinary retention, constipation, and tachycardia.
- ⚠️Dopamine antagonists (e.g. chlorpromazine, promethazine), antihistamines, and hyoscine are known to have anticholinergic effects.
Constipation
- Constipation is an adverse reaction to serotonin antagonists such as ondansetron and anticholinergic drugs such as hyoscine.
- Dopamine antagonists such as metoclopramide and domperidone are prokinetic so may be a better choice for patients with constipation.
Summary table
The common antiemetics pharmacologic features are summarized in the following table *.
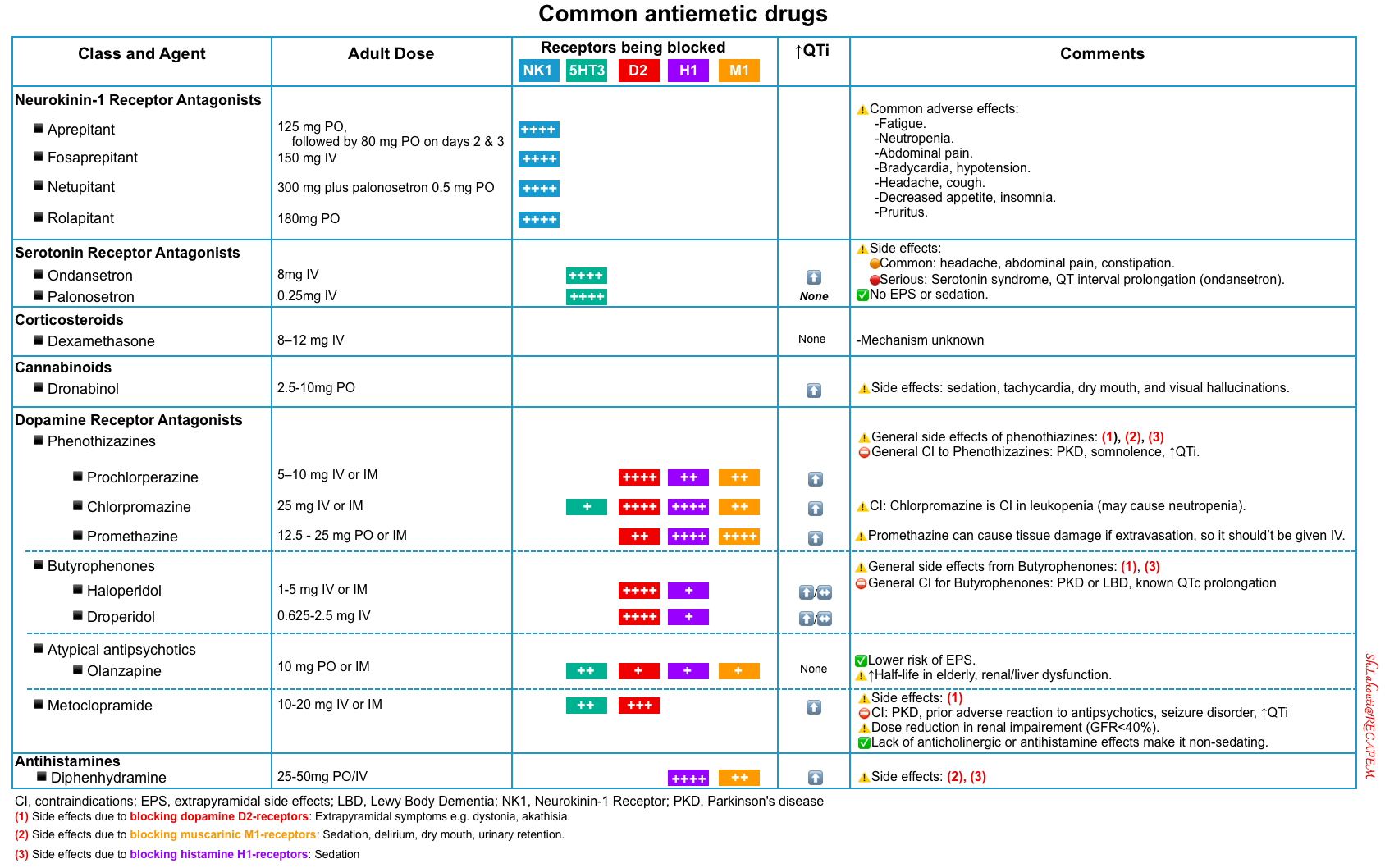
5HT3 antagonists
▪️Ondansetron
- General considerations
- Ondansetron is a broad-spectrum antiemetic with a relatively safe profile, with no sedative or extrapyramidal side effects.
- Dosing
- Ondansetron 💊
- 8 mg PO/IV q8hr PRN.
- Significant liver dysfunction (Child class C cirrhosis): max 8 mg per 24 hours.
- ⚠️Infuse over 15 minutes after further dilution with 50 mL NS/D5W or slowly push undiluted over 2-5 minutes.
- Ondansetron 💊
- Caution
- Ondansetron can prolong QT interval and cause Torsade de Pointes. Despite few reports of Tdp following the Ondansetron administration, many of these events occurred within 1-2 minutes of rapidly pushing ondansetron. Rapid IV push should be avoided.
- The most common side effects are mild headache and constipation.
- ⚠️The most serious side effects: Serotonin syndrome, and QT prolongation.
- Consider ordering an ECG before ondansetron administration for a patient with a history of cardiac disease.
▪️Palonosetron
- It has the following advantages compared to ondansetron:
- Absence of QT prolongation (perhaps the greatest advantage) *.
- Longer half-life (~40 hours).
- Superior efficacy compared to ondansetron (especially the 8-mg dosing of ondansetron).
- Dosage
Phenothiazines
▪️General considerations
- The most selective for D2-receptor antagonistic activity is prochlorperazine, resulting to
- Higher rate of extrapyramidal side effects.
- Less sedative effect than other phenothiazines.
- ⛔️Promethazine should not be given IV, since extravasation can cause tissue necrosis.
- Chlorpromazine is similar to promethazine, and it can be considered as an alternative to promethazine, especially if intravenous administration is required.
▪️Dosing
- Prochlorperazine 💊
- 5-10 mg IV q4-6 hours PRN (max 40 mg total daily).
- Chlorpromazine 💊
- 25 mg IM/IV q4-8 hours PRN.
- Promethazine 💊
- 12.5-25 mg PO/IM q4-6hr PRN (max 25 mg/dose, max 100 mg/day).
- ⚠️Avoid IV administration.
⚠️Caution
- ⛔️General contraindications to these agents:
- Parkinson’s disease
- Somnolence (with a risk of respiratory suppression)
- ⛔️Chlorpromazine should be avoided in leukopenia since it may cause neutropenia.
- ⚠️All of these agents can prolong the QT interval, and hypotension, especially in older adults with IV infusion.
Metoclopramide
▪️General considerations
- The pharmacological profile of metoclopramide can be conceptualized as a combination of haloperidol plus a little ondansetron.
- Metoclopramide has a strong D2-receptor antagonistic effect without anticholinergic activity.
- Agents with anti-D2 activity that lack anticholinergic activity (e.g. haloperidol, droperidol, and metoclopramide) may tend to have the highest rate of extrapyramidal symptoms (keep in mind that anticholinergics can be used to treat extrapyramidal symptoms).
- Advantages *
- Prokinetic properties may be beneficial for patients with gastroparesis.
- Non-sedating properties: The lack of anticholinergic or antihistamine effects makes metoclopramide a non-sedating antiemetic agent.
- May be useful for nausea and vomiting in pregnancy (NVP).
- Dosing
- Metoclopramide 💊
- Dosing for nausea: 20 mg or 25 mg IV q4-6 hours PRN.
- Slow IV infusion over 15 minutes reduces side effects (akathisia) without affecting efficacy.
- Dosing for gastroparesis: 10 mg IV q6hr-q8hr.
- Dose reduction in renal/liver impairment
- Renal adjustment:
- GFR 10-40 ml: Dose-reduce by 50%.
- GFR <10 ml/min: Dose reduced by 75%.
- Severe liver failure: Dose reduced by 50% *.
- Renal adjustment:
- Dosing for nausea: 20 mg or 25 mg IV q4-6 hours PRN.
- Metoclopramide 💊
⚠️Cautions
- Side effects:
- Extrapyramidal side-effects (acute dystonia, akathisia, parkinsonism, tardive dyskinesia).
- These are typically “dose and time-related” and rarely with 10-mg dosing.
- Extrapyramidal reactions are associated mostly with chronic use or higher doses.
- Risk factors for developing extrapyramidal reactions:
- Higher than recommended doses
- Longer durations of therapy (>12 weeks)
- Age > 60 years and pediatric patients
- Female
- Renal impairment
- Diabetes
- Concurrent use of drugs that can cause extrapyramidal symptoms, e.g. antipsychotics.
- Risk factors for developing extrapyramidal reactions:
- Extrapyramidal side-effects (acute dystonia, akathisia, parkinsonism, tardive dyskinesia).
- ⚠️Use caution in patients with moderate to severe renal or hepatic impairment.
- ⛔️Contraindications:
- Parkinson’s disease or adverse reactions to antipsychotics in the past (e.g. dystonia, neuroleptic malignant syndrome, or tardive dyskinesia).
- Seizure disorder.
- QT prolongation.
- Situations where stimulation of GI motility may be dangerous, including mechanical GI obstruction.
Other agents
Antihistamines
- These have limited use in elderly and intensive care settings due to the potential risk of generating delirium.
- ✅Clinical use:
- Vestibular abnormality
- Postoperative emesis
- Parkinson’s disease. Antihistamines may be used in such patients due to the increased risk of extrapyramidal symptoms caused by most other classes of antiemetics.
Dronabinol
- A cannabinoid receptor agonist (CBR1), is not used as an antiemetic in critical illness but could be considered as a palliative measure in patients with malignancy.
- Side effects include sedation, tachycardia, dry mouth, and visual hallucinations.
- Dose👉 Dronabinol 💊
Glucocorticoid
- The mechanism of action is unknown.
- It is primarily used for the prevention of chemotherapy-induced nausea/vomiting (CINV).
- The most commonly used agent is dexamethasone at doses of 8-16 mg.
Specific conditions
Chemotherapy-induced nausea and vomiting (CINV)
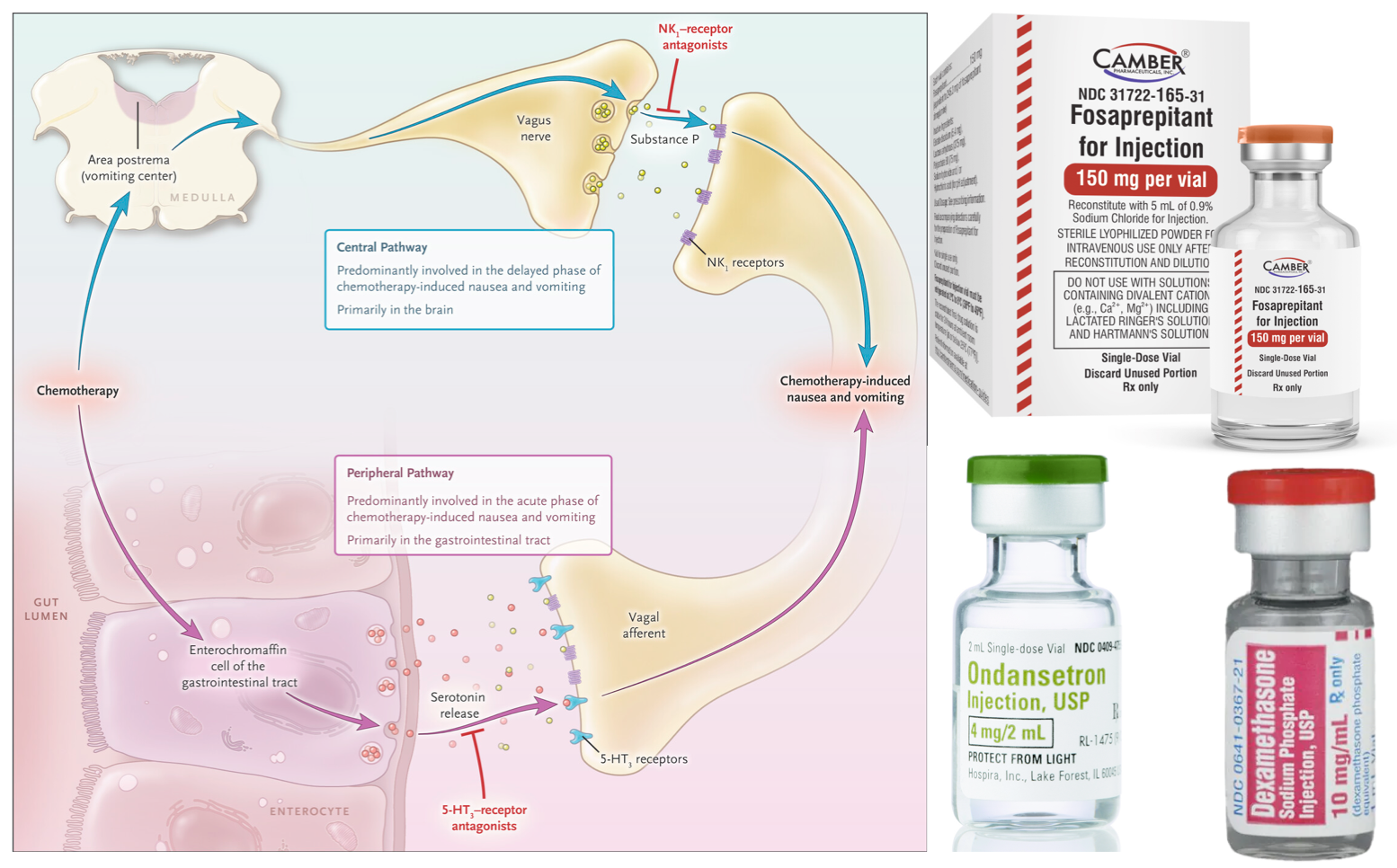
In general, chemotherapy-induced nausea (CINV) and vomiting can be classified as acute, delayed, or anticipatory, depending on the time of onset:
- Acute CINV occurs in the first 24 hours after chemotherapy administration, with a maximal intensity after 5-6 hours *.
- Delay nausea and vomiting (DNV) usually occurs 24 hours post-treatment (with maximal intensity 48-72 hours after administration) and can last up to 5-7 days.
- Anticipatory (ANV) is an expected or conditioned reflex where vomiting occurs before the administration of the chemotherapeutic agent.
- This might be triggered by factors such as tastes, odors, sights, thoughts, or anxiety associated with the treatment.
- It is usually more difficult to control than acute or delayed CINV.
▪️Pathophysiology
- The complex network of neuroanatomical and peripheral centers, neurotransmitters, and receptors that are involved in the vomiting process is explained above.
- Multiple neurotransmitters and their receptors are involved in CINV, operating via different signaling mechanisms. The three main neurotransmitters and their associated receptors are (figure below) *
- Serotonin (5-HT)
- Serotonin is the primary mediator of neural signals from the gut to the NTS, activating 5-HT3 receptors.
- Chemotherapeutic agents are toxic to the ECs lining the GI mucosa causing the generation of free radicals which in turn cause ECs to release excessive amounts of serotonin.
- Substance P (SP)
- SP primarily transmits signals from the vagus nerve to neurokinin-1 (NK-1) receptors in the CTZ.
- Like serotonin, SP release is mediated by chemotherapy but it appears to bind largely to NK-1 receptors that are centrally located (namely NTS) which then pass the signal via the vagal afferent nerves to the CTZ and then to the VC.
- Dopamine
- Dopamine receptor antagonists (e.g., promethazine, and metoclopramide) are effective in treating CINV.
- Serotonin (5-HT)
⭐️Other pathways are also involved in the induction of CINV such as gamma-aminobutyric acid (GABA), histaminic, and muscarinic receptors pathways *.
⭐️Psychological factors such as classical conditioning, and anxiety may affect both the frequency and severity of chemotherapy-induced nausea and vomiting, especially anticipatory type. Potential conditioned stimuli in the context of ANV can include the sights and smells of the clinic, nurses, treatment room, etc.
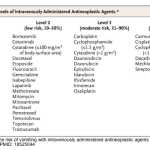
Chemotherapy regimens are classified into 4 categories according to the potential for CINV: highly emetogenic (>90%), moderately emetogenic (30–90%), low emetogenic (10–30%), and minimally emetogenic (<10%) appendix 1 *.
- Factors to be considered when treating CINV
- Patient’s expectation (anticipated NV).
- The emetogenic potential of chemotherapy drugs.
- Time of onset (acute vs. delayed CINV).
- Resistance to antiemetic treatment (breakthrough and refractory CINV).
- In general, the following principle can be applied to treatment of CINV:
- Less effective antiemetics in the treatment of CINV: Antihistamines, anticholinergics, metoclopramide, and prochlorperazine *.
- More useful antiemetics in the treatment of CINV:
- Nausea and vomiting resulting from low emetogenic chemotherapy, such as paclitaxel, can be treated with a serotonin antagonist, while highly emetogenic chemotherapy, such as cisplatin, will require a combination of antiemetic drugs (e.g. serotonin antagonist, neurokinin antagonist, and dexamethasone) *.
- Guideline recommendation *
- For patients receiving highly emetogenic chemotherapy
- Give the three-drug combination of a neurokinin-1 receptor antagonist (days 1 through 3 for aprepitant; day 1 only for fosaprepitant), a serotonin receptor antagonist (day 1 only), and dexamethasone (days 1 through 3 or 4) is recommended *.
- For moderately emetogenic chemotherapy
- Give the two-drug combination of palonosetron (day 1 only) and dexamethasone (days 1 through 3) is recommended.
- For low emetogenic agents
- Give a single dose of dexamethasone 8 mg IV before chemotherapy is suggested *.
- For delayed chemotherapy-induced vomiting,
- Give the neurokinin-1 receptor antagonists (appendix 2) *.
- For patients receiving highly emetogenic chemotherapy
Nausea and vomiting in pregnancy (NVP)
▪️Background
- NVP is defined as symptoms of nausea, vomiting, and/or dry retching commencing in the first trimester without another cause. NVP is typically a self-limited condition and not associated with adverse pregnancy outcomes.
- NVP affects ~50-80% of pregnancies
- Hyperemesis gravidarum (HG) is the term used to describe the severe end of the symptom spectrum (including weight loss >5% of pre-pregnancy body weight). Severe symptoms can negatively impact daily functioning, cause anxiety and depression, interfere with work performance, and lead some patients to consider termination of pregnancy or avoiding a future pregnancy.
- HG affects 0.2-3.6% of pregnancies.
- Risk factors for NVP & HG
- Younger, primigravid individuals.
- Nonpregnant people who experience nausea and vomiting related to estrogen-based medications, motion sickness, or migraines are more likely to experience NVP.
- Multiple gestations.
- Symptoms in a prior pregnancy.
- Hydatidiform molar pregnancy.
- Nonuse of multivitamins before six weeks of gestation or during the preconception period.
- Acid reflux or other gastrointestinal disorders.
- Genetic factors play a role in the development of HG.
▪️Clinical presentation
- Symptoms typically start at 5-6 weeks of gestation, peak at ~9 weeks, and usually subside by 16-20 weeks.
- In ~15-20% symptoms may continue until the third trimester and in ~5% it may continue until delivery.
- Almost 60% of patients are asymptomatic by 6 weeks after onset of symptoms.
- ⚠️If vomiting begins in the latter half of pregnancy and/or persists beyond a few days postpartum, other etiologies should be investigated.
🚩Red flag for diagnosis of HG
- In the presence of the following conditions, broaden your differential to include other causes of nausea and vomiting in pregnancy. These include *:
- If symptoms develop for the first time after 9 weeks of gestation.
- Abdominal pain.
- Fevers.
- Headaches.
- An abnormal neurologic exam.
- Goiter.
▪️Diagnosis
- NVP and HG are clinical diagnoses without definitive diagnostic criteria. It is generally a diagnosis of exclusion based on its first occurrence in early pregnancy with gradual resolution over weeks.
- NVP
- The diagnosis of NVP is based on the presence of nausea and/or vomiting that appears to be related to pregnancy rather than another etiology.
- Patients with the common mild form of nausea and vomiting during pregnancy maintain normal vital signs and have normal physical and laboratory examinations and pregnancy courses.
- Hyperemesis gravidarum (HG)
- It is considered the severe end of the spectrum of nausea and vomiting of pregnancy.
- The most commonly cited criteria for diagnosis of HG include:
- Persistent vomiting with weight loss that is not related to other causes
- An objective measure of acute starvation such as carbohydrate depletion, electrolyte abnormalities, and/or acid-base disturbance.
- The degree of weight loss required to meet the criteria for HG is often defined as at least 5% of pre-pregnancy weight.
- 💡Ketonuria is not an indicator of dehydration and should not be used to assess the severity of HG.
- Ketonuria is often cited as a measure of dehydration or starvation in HG, however, in a systematic review and meta-analysis, ketonuria was not found to be reliably associated with either the diagnosis or severity of HG *.
- A recently developed International consensus definition for HG (the Windsor definition) requires all of the following criteria be met *
- Symptoms start in early pregnancy <16 weeks gestation.
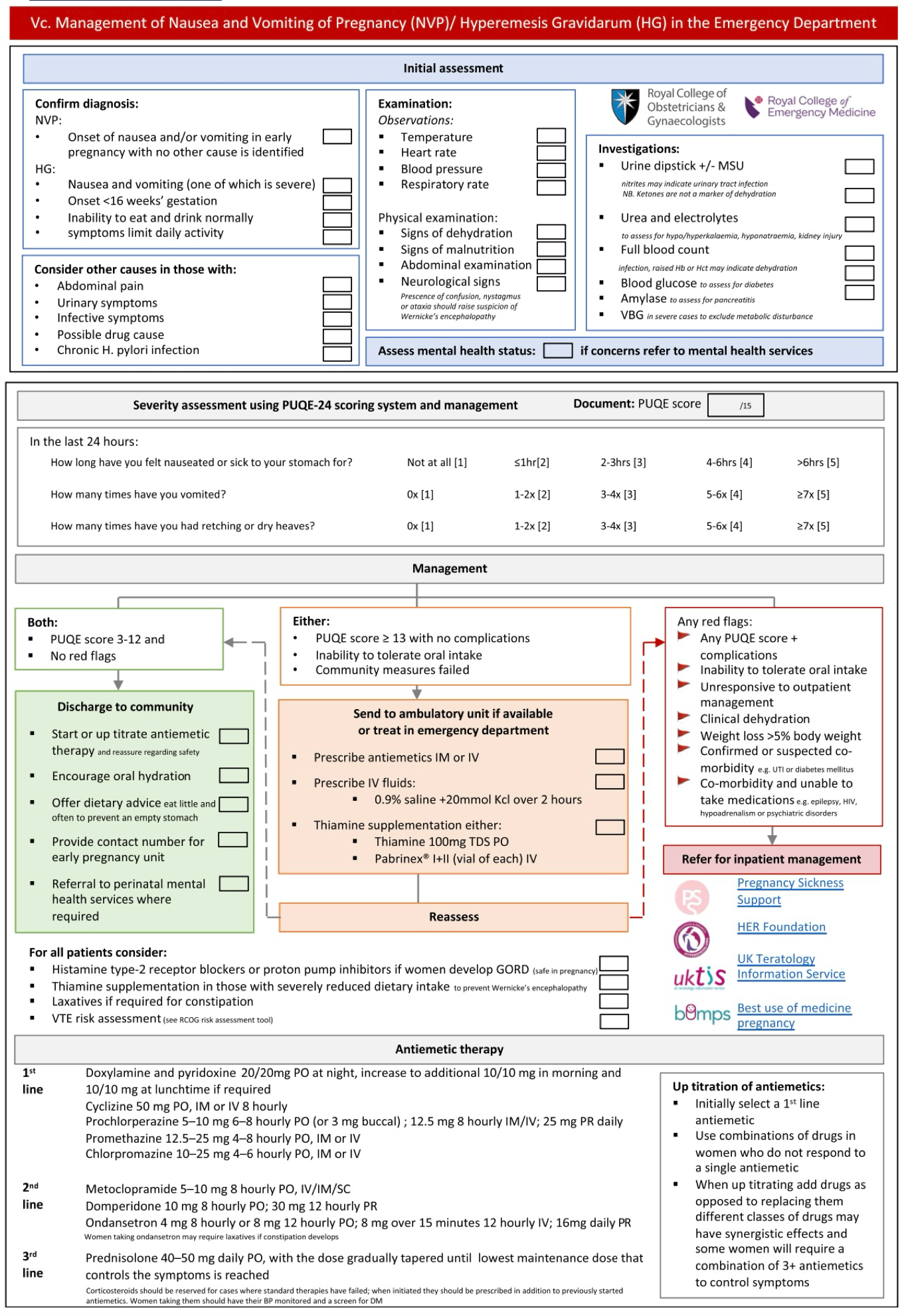
- Severe nausea and/or vomiting (PUQE-24 ≥ 13 or HELP 33-60). PUQE-24 score 🧮 / HELP score 🧮
- Inability to eat and/or drink normally.
- Strongly limiting daily activities.
- NVP
- Signs of dehydration were deemed contributory but not mandatory.
- The diagnosis of HG is not based on the PUQE-24 score *, or any other scoring system.
- Although there may be a continuum between NVP and HG, it is critical to distinguish HG from NVP as the management and potential maternal and fetal complications differ.
- Symptom scores can be used to assess NVP severity, although any scoring system will not necessarily reflect an individual’s total symptom burden.
- Scoring systems should not replace a holistic inquiry regarding the ability to eat and drink, weight, and hydration status, physical and mental functioning, and overall impact of NVP.
- Any validated scoring system can be used to guide initial treatment, but subsequent management decisions should be based on the response to treatment as per the principles of management discussed below.
▪️Differential Diagnosis of NVP in Pregnancy (*most common cause)
- Gastrointestinal
- Infectious gastroenteritis *
- Gastro-oesophageal reflux disease-Helicobacter Pylori *
- Infectious hepatitis
- Pancreatitis
- Biliary tract disease
- Peptic ulcer disease
- Bowel obstruction
- Gastroparesis
- Appendicitis
- Peritonitis
- Genitourinary
- Urinary tract infection including pyelonephritis *
- Ovarian Torsion
- Nephrolithiasis
- Metabolic/Toxic
- Drugs-including pregnancy vitamins *
- Use and/or withdrawal of cannabinoids or other illicit drugs
- Diabetic ketoacidosis
- Addison’s disease
- Thyrotoxicosis
- Non-infectious hepatitis
- Hypercalcemia
- Eating Disorders
- Central nervous system disease
- Migraine *
- Infection
- Tumors
- Raised intracranial pressure
- Vestibular system pathology: labyrinthitis, Meniere’s
▪️Investigations
- Patients with mild-moderate nausea and vomiting of pregnancy (PUQE-24 ≤12), where symptoms are not suspicious for HG or another diagnosis, do not need investigation *.
- Measurement of beta Human Chorionic Gonadotrophin (HCG) is of no practical value for the diagnosis or management of HG.
- Indications for investigations:
- Severe NVP or HG i.e. PUQE score ≥13 (repeat the following labs daily if requiring ongoing IV fluids and inadequate oral intake or significant underlying conditions e.g. Type 1 diabetes): *
- Basic metabolic profile (BMP)
- Liver function tests, amylase/lipase.
- Urinalysis
- Obstetric ultrasound to exclude multi-fetal or gestational trophoblastic disease.
- Symptoms or signs of thyrotoxicosis: Request TSH *.
- TSH < 0.25 mIU/L is suggestive of thyrotoxicosis.
- TSH should be measured in women with HG or NVP refractory to treatment or in those with milder symptoms who have signs and/ or symptoms of thyrotoxicosis.
- Women with NVP who do not meet diagnostic criteria for HG do not require TFT measurement.
- Fever or symptoms of urinary tract infection
- Midstream urine microscopy and culture, white cell count *.
- Note that White cell count is raised in pregnancy; up to 13500 in the first trimester and 14800 in the second trimester is normal.
- Severe NVP or HG i.e. PUQE score ≥13 (repeat the following labs daily if requiring ongoing IV fluids and inadequate oral intake or significant underlying conditions e.g. Type 1 diabetes): *
- History and physical examination should be directed toward the identification of alternate diagnoses.
- Temperature, weight, and assessment of percentage weight loss
- Abdomen? abdominal tenderness and signs of peritonism?
- Neck stiffness and signs of raised intracranial pressure if the history is suggestive of a CNS cause for the symptoms.
- Signs to support a diagnosis of dehydration include decreased skin turgor, dry mucous membranes, decreased urine output, concentrated urine, and postural drop in blood pressure.
- Tests to exclude alternate diagnoses should be performed only where indicated.
⭐️Considerations
- Women with HG frequently have hyponatremia, hypokalemia, hypochloremia, hypomagnesemia, and low serum urea with metabolic hypochloremic alkalosis.
- If severe, metabolic acidemia may develop.
- An elevation in serum creatinine (>70umol/L) will suggest significant dehydration. Rarely, starvation ketoacidosis may occur resulting in significant metabolic derangement.
- Liver function test
- In 15-50% of women with HG, liver enzymes are elevated with hyperemesis but are generally < x4 UNL (typically ALT rise is > AST rise) *.
- Liver dysfunction typically resolves rapidly with improvement in HG symptoms.
- DO further investigation if liver enzyme dysfunction is > 4 x UNL.
- In 15-50% of women with HG, liver enzymes are elevated with hyperemesis but are generally < x4 UNL (typically ALT rise is > AST rise) *.
- Serum amylase and lipase are elevated in 10-%15 percent of patients and may increase as much as fivefold (as opposed to a 5- to 10-fold increase in acute pancreatitis) *.
- In one study, all patients with increased serum amylase values had normal pancreatic amylase values, implying that the elevated amylase levels are most likely salivary rather than pancreatic origin.
- Although the increased serum lipase’s origin is unknown in the hyperemesis setting, serum lipase, in general, appears to be primarily of pancreatic origin. Still, other organs (such as the stomach, duodenum, small bowel, colon, and liver) may produce lipase.
- Hyperemesis gravidarum can result in thiamine (vitamin B1) deficiency, and patients may develop Wernicke’s encephalopathy (ophthalmoplegia, gait ataxia, and confusion) if not treated. This condition generally does not occur until after at least 3 weeks of persistent vomiting *.
▪️🚩Indication for hospitalization
- Severe NVP or HG (PUQE-24 score ≥13)
- Any PUQE score + complications
- NVP with an inability to tolerate oral intake after treatment with antiemetics.
- Clinical dehydration
- Comorbidities: Women with Type 1 diabetes and other high-risk conditions (e.g. short bowel syndrome ) or those requiring continuity of essential oral medications (e.g. severe epilepsy, transplant recipients) should be admitted to the hospital at least for initial management and until they are stable.
- Severe electrolyte disturbance e.g. potassium < 3.0mmol/L
- Significant renal impairment or acute kidney injury
- Malnutrition/continuing significant weight loss despite therapy or starvation ketoacidosis.
- Associated conditions requiring inpatient management e.g. infection, hematemesis, refeeding syndrome
Pharmacological Treatments
▪️The principles of holistic management of NVP and HG must include:
- Maintenance of hydration with appropriate fluid and electrolyte replacement.
- IV access and IV fluids: 0.9% sodium chloride or 5% dextrose in 0.9% normal saline. Ringer’s lactate solution may also be used.
- In extreme cases, any dextrose administration should be preceded by thiamine 100 mg IV to prevent Wernicke’s encephalopathy.
- Interventions to reduce nausea, retching, and vomiting (below).
- Management of associated gastric dysmotility i.e. gastroesophageal reflux and constipation.
- Maintenance of adequate nutrition including provision of vitamin supplements where required.
- Psychosocial assessment and support.
- Monitoring and prevention of side effects and adverse pregnancy and fetal outcomes.
- In women who are immobile or have additional risk factors for thromboembolism, thromboprophylaxis should be considered unless there are specific contraindications.
▪️Considerations for treatment choices in NVP and HG:
- Establish targets for symptom relief: the ability to eat and drink adequately without necessarily complete resolution of NVP.
- Discontinue prenatal multivitamins if they are contributing to NVP:
- Discontinue iron-containing prenatal multivitamins.
- However, continue if possible iodine (150 mcg per day) and folate (at least 400 mcg per day until 10 weeks).
- The choice of antiemetic should be individualized, based on the woman’s symptoms, previous response to treatment, and potential maternal or fetal adverse effects
- If an antiemetic is ineffective at maximal dose, discontinue before commencing an alternate agent.
- If an antiemetic is partially effective, optimize dosage and timing, and only add additional agents after maximal doses of the first agent have been trialed.
- Oral therapy is usually commenced first and parenteral or subcutaneous treatment is reserved for refractory cases.
▪️Medications for the treatment of NVP and HG
- Antiemetics: herbal/vitamin and prescribed
- Acid suppression
- Laxatives
- Steroids
- Other: e.g. supplements, antidepressants.
Antiemetic
▪️Nonpharmacologic treatment options include ginger and acupressure.
- Ginger
- Mechanism of action
- Improvement in GI motility, weak effect on cholinergic M3 receptors and serotonergic 5-HT3 and 5-HT4 receptors in the gut.
- Evidence for efficacy
- ↓Nausea but not Vomiting *
- Superior to placebo, Equal to Vitamin B6, dimenhydrinate, metoclopramide, and doxylamine.
- Recommended [maximum daily dose]
- Use standardized products rather than foods e.g. 250 mg capsule TDS-QID [1000mg] *.
- Side effects
- Heartburn
- Ginger should be avoided by any woman taking an anticoagulant, as ginger has weak anticoagulant effects *.
- Risk of teratogenesis: Not increased *
- Mechanism of action
- Acupressure with wristbands on the P6 point may reduce symptoms of NVP and HG *.
- Pyridoxine (vitamin B6)/doxylamine
- Pyridoxine alone or in combination with doxylamine is effective in reducing symptoms of mild to moderate nausea and vomiting of pregnancy.
- Mechanism of action of Vitamin B6
- Water soluble vitamin inhibits the H1 receptor, act indirectly on the vestibular system, and some inhibition of muscarinic receptors to decrease stimulation of the vomiting center.
- Evidence for efficacy
- ↓Nausea but not Vomiting
- Less effective than dimenhydrinate
- Recommended [maximum daily dose]
- The ACOG recommends pyridoxine, alone or in combination with doxylamine, as the first-line treatment for mild to moderate nausea and vomiting of pregnancy *.
- 10 to 25 mg PO 3-4 times/day [200 mg] or 37.5 mg combined with ginger 600 mg up to 2x/day.
- 10-25 mg, alone or in combination with 12.5 mg doxylamine, orally, 3 or 4 times/d *.
- Risk of teratogenesis: Not increased *
- Side effects
- Sensory neuropathy has been reported in chronic intake of pyridoxine at doses >500 mg/day.
- Practice point
- More effective when used in combination e.g. with doxylamine or dicyclomine (equivalent to metoclopramide).
- Antihistamines: Doxylamines/ Diphenhydramine/Cyclizine/ Promethazine
- Mechanism of action
- Indirectly affect the vestibular system, decreasing stimulation of the vomiting center.
- Evidence for efficacy
- Doxylamine: ↓Nausea compared with placebo with or without pyridoxine
- Recommended [maximum daily dose] Use one of the following only do not combine
- Doxylamine: 6.25-25 mg TDS [50 mg]
- Diphenhydramine: 25-50 mg TDS [150 mg] *
- Cyclizine: 12.5-50 mg TDS [150 mg]
- Promethazine: 25 mg TDS [75 mg]
- Risk of teratogenesis: Not increased *
- Side effects
- Sedation, anticholinergic effects *
- Mechanism of action
- Dopamine antagonists: Metoclopramide
- Mechanism of action
- Dopamine and serotonin receptor antagonist stimulates upper gastrointestinal motility and act on the CNS vomiting center.
- Evidence for efficacy
- Equal to ondansetron for Nausea but less effective for Vomiting.
- Recommended [maximum daily dose]
- 10 mg TDS [30 mg] PO, or IM, or IV *
- Risk of teratogenesis: Not increased *.
- Side effects
- Initial: Dystonia, Akathisia, depression.
- Prolonged use: Rare: tardive dyskinesia.
- Practice point
- Less sedation.
- To reduce the risk of extrapyramidal side effects, use for the shortest possible duration and lowest effective dose.
- Mechanism of action
- Phenothiazines: Promethazine, prochlorperazine
- Mechanism of action
- Prochlorperazine: Central and peripheral dopamine antagonists.
- Evidence for efficacy
- Superior to placebo for NVP.
- Phenothiazines have a similar efficacy to metoclopramide but increased rates of drowsiness, sedation, dry mouth, and dystonia.
- Recommended [maximum daily dose]
- Prochlorperazine: 5-10 mg TDS [30 mg] PO *
- Promethazine 12.5-25 mg PO, per rectum, IM, or IV q4-6h as needed.
- Chlorpromazine is rarely used and is reserved for severe symptoms. It can be given as 25-50 mg IV or IM q4-6h as needed, or 10-25 mg PO q4-6 h as needed.
- Risk of teratogenesis: Not increased *.
- Side effects
- Sedation, akathisia, anticholinergic effects, hypotension.
- Rare: dystonias, tardive dyskinesia with chronic use.
- Practice point
- Best reserved for evening dosing
- Mechanism of action
- Serotonin 5HT3-inhibitor: Ondansetron
- Mechanism of action
- Central (medullary vomiting center) and peripheral (small bowel) serotonin receptor blocker
- Evidence for efficacy
- Superior to a combination of doxylamine/B6 for a reduction in N and V. Superior to metoclopramide for a reduction of V but not N in HG
- Recommended [maximum daily dose]
- 4-8 mg up to TDS [16mg]
- Risk of teratogenesis
- Conflicting data. No overall risk of birth defects but a possible increased risk of cleft palate and ventricular septal defect when used in the first trimester
- Side effect
- Constipation, headache, dizziness
- Rare: QT prolongation, serotonin syndrome in combination with other serotonergic drugs.
- Practice point
- No sedation
- Expensive
- Ensure concurrent management of constipation. Bowel obstruction has been reported
- Recommended as second-line agents
- Mechanism of action
- Corticosteroids
- Mechanism of action
- Antiemetic effect on the chemoreceptor trigger zone in the brainstem
- Evidence for efficacy
- Equal to promethazine with fewer side effects.
- Superior to IV metoclopramide.
- Improved sense of well-being, appetite, and increased weight gain in HG patients. No difference in days of hospital admission or readmission rates compared to the placebo *.
- Recommended [maximum daily dose]
- Prednisone 40-50 mg/day.
- May be commenced as hydrocortisone 100 mg IV BD [200 mg]
- Methylprednisolone 16 mg PO or IV q8h, for 3 days, followed by a 2-week taper to the lowest effective dose. It should be used for no more than 6 weeks *.
- Risk of teratogenesis
- Possible increased risk of oral clefts when used < 10 weeks gestation, but data are weak *.
- Thus, methylprednisolone should be avoided as a first-line agent in the first 10 weeks of gestation.
- Side effect
- Potential Cushing’s syndrome, mood disturbance, hypertension, hyperglycemia
- Practice point
- Consider withholding until after 10 weeks gestation if alternate therapy is an option.
- Restrict to refractory cases
- Wean to 10 to 12.5 mg/day over 7 to 10 days then by 2.5 mg/day every 3 days to a minimum effective dose.
- Increase folate to 5 mg, oral, once per day if prescribing steroids in the first trimester
- Mechanism of action
Appendix
1. Emetogenic Levels of Intravenously Administered Antineoplastic Agents
2. Management of acute and delayed CINV
References
1. PMID: 24161560. Bashashati M, McCallum RW. Neurochemical mechanisms and pharmacologic strategies in managing nausea and vomiting related to cyclic vomiting syndrome and other gastrointestinal disorders. Eur J Pharmacol. 2014 Jan 5;722:79-94. doi: 10.1016/j.ejphar.2013.09.075. Epub 2013 Oct 22.
2. PMID: 26770271. Singh P, Yoon SS, Kuo B. Nausea: a review of pathophysiology and therapeutics. Therap Adv Gastroenterol. 2016 Jan;9(1):98-112. doi: 10.1177/1756283X15618131.
3. PMID: 22513952. Hines S, Steels E, Chang A, Gibbons K. Aromatherapy for treatment of postoperative nausea and vomiting. Cochrane Database Syst Rev. 2012 Apr 18;(4):CD007598. doi: 10.1002/14651858.CD007598.pub2. Update in: Cochrane Database Syst Rev. 2018 Mar 10;3:CD007598. doi: 10.1002/14651858.CD007598.pub3.
4. PMID: 32346211. Athavale A, Athavale T, Roberts DM. Antiemetic drugs: what to prescribe and when. Aust Prescr. 2020 Apr;43(2):49-56. doi: 10.18773/austprescr.2020.011. Epub 2020 Apr 1.
5. PMID: 30561479. Huybrechts KF, Hernández-Díaz S, et al. Association of Maternal First-Trimester Ondansetron Use With Cardiac Malformations and Oral Clefts in Offspring. JAMA. 2018 Dec 18;320(23):2429-2437. doi: 10.1001/jama.2018.18307.
6. PMID: 33443705. Hardy J, Davis MP. The Management of Nausea and Vomiting Not Related to Anticancer Therapy in Patients with Cancer. Curr Treat Options Oncol. 2021 Jan 14;22(2):17. doi: 10.1007/s11864-020-00813-0.
7. PMID: 34816707. Stojek M, Jasiński T. Gastroparesis in the intensive care unit. Anaesthesiol Intensive Ther. 2021;53(5):450-455. doi: 10.5114/ait.2021.110959.
8. PMID: 23496347. Janelsins MC, et al. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin Pharmacother. 2013 Apr;14(6):757-66. doi: 10.1517/14656566.2013.776541.
9. PMID: 19368415. Navari RM. Pharmacological management of chemotherapy-induced nausea and vomiting: focus on recent developments. Drugs. 2009;69(5):515-33. doi: 10.2165/00003495-200969050-00002.
10. PMID: 18525044. Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008 Jun 5;358(23):2482-94. doi: 10.1056/NEJMra0706547.
11. PMID: 28759346. Hesketh PJ, Kris MG, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017 Oct 1;35(28):3240-3261. doi: 10.1200/JCO.2017.74.4789. Epub 2017 Jul 31.
12. PMID: 28194109. Rapoport BL. Delayed Chemotherapy-Induced Nausea and Vomiting: Pathogenesis, Incidence, and Current Management. Front Pharmacol. 2017 Jan 30;8:19. doi: 10.3389/fphar.2017.00019.
13. PMID: 33045502. Koot MH, et al. Ketonuria is not associated with hyperemesis gravidarum disease severity. Eur J Obstet Gynecol Reprod Biol. 2020 Nov;254:315-320. doi: 10.1016/j.ejogrb.2020.08.014. Epub 2020 Aug 26.
14. PMID: 34555550. Jansen LAW, et al. The windsor definition for hyperemesis gravidarum: A multistakeholder international consensus definition. Eur J Obstet Gynecol Reprod Biol. 2021 Nov;266:15-22. doi: 10.1016/j.ejogrb.2021.09.004. Epub 2021 Sep 10.
15. PMID: 12011891. Koren G, et al. Motherisk-PUQE (pregnancy-unique quantification of emesis and nausea) scoring system for nausea and vomiting of pregnancy. Am J Obstet Gynecol. 2002 May;186(5 Suppl Understanding):S228-31. doi: 10.1067/mob.2002.123054.
16. PMID: 29266076. Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 189: Nausea And Vomiting Of Pregnancy. Obstet Gynecol. 2018 Jan;131(1):e15-e30. doi: 10.1097/AOG.0000000000002456.
17. PMID: 24642205. Viljoen E, Visser J, Koen N, Musekiwa A. A systematic review and meta-analysis of the effect and safety of ginger in the treatment of pregnancy-associated nausea and vomiting. Nutr J. 2014 Mar 19;13:20. doi: 10.1186/1475-2891-13-20.
18. PMID: 20942670. Niebyl JR. Clinical practice. Nausea and vomiting in pregnancy. N Engl J Med. 2010 Oct 14;363(16):1544-50. doi: 10.1056/NEJMcp1003896. Erratum in: N Engl J Med. 2010 Nov 18;363(21):2078.
19. PMID: 24748822. Nuangchamnong N, Niebyl J. Doxylamine succinate-pyridoxine hydrochloride (Diclegis) for the management of nausea and vomiting in pregnancy: an overview. Int J Womens Health. 2014 Apr 12;6:401-9. doi: 10.2147/IJWH.S46653.
20. PMID: 12011897. Magee LA, Mazzotta P, Koren G. Evidence-based view of safety and effectiveness of pharmacologic therapy for nausea and vomiting of pregnancy (NVP). Am J Obstet Gynecol. 2002 May;186(5 Suppl Understanding):S256-61. doi: 10.1067/mob.2002.122596.
21. PMID: 14662211. Yost NP, McIntire DD, Wians FH Jr, Ramin SM, Balko JA, Leveno KJ. A randomized, placebo-controlled trial of corticosteroids for hyperemesis due to pregnancy. Obstet Gynecol. 2003 Dec;102(6):1250-4. doi: 10.1016/j.obstetgynecol.2003.08.013.






Add comment