31 July 2025 by Shahriar Lahouti. Peer-reviewed by Abdolghader Pakniyat.
CONTENTS
- Introduction
- Case scenario
- Definition
- Pathophysiology
- Diagnosis
- Approach to diagnosis and management
- Appendix
- References
Introduction
Septic cardiomyopathy (SCM) is a serious complication of sepsis, characterized by global but reversible myocardial dysfunction. Despite its clinical significance, SCM lacks a clear definition and standardized criteria, complicating early recognition and treatment.
Sepsis, a systemic inflammatory response to infection, often leads to multi-organ dysfunction, including cardiac involvement. SCM describes transient cardiac impairment during sepsis that is not due to ischemia or pre-existing heart disease.
Given the key role of circulatory compromise in driving multi-organ dysfunction, a thorough understanding of cardiac dysfunction is essential. In this post, we unpack the definition, pathophysiology, and clinical approach to septic cardiomyopathy—one of the most underrecognized forms of cardiac dysfunction in critical illness.
Case Scenario
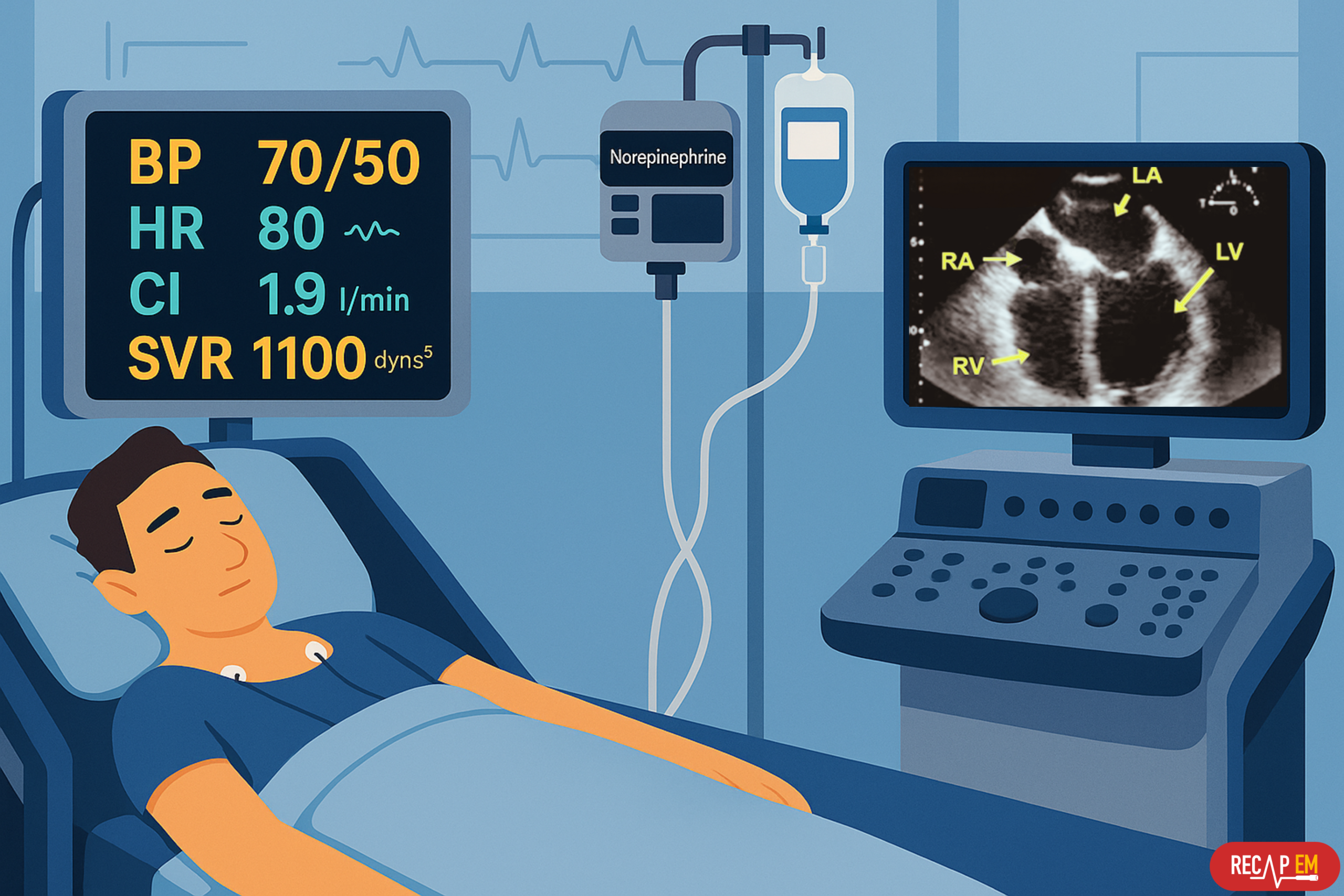
A 55-year-old male with a past medical history of HTN, CKD stage III (baseline Cr 1.8 mg/dL), presented to the ED in the morning with delirium and malaise. On arrival, he was disoriented to time and place, febrile (39.0°C), hypotensive 80/45mmHg (MAP 57) with HR 140, RR 28, SpO2 95% on RA, and CBG 200 mg/dL.
- Physicals: Delirious and disoriented, nonedematous cold extremities with sluggish CRT. Otherwise unremarkable findings.
- EKG: Sinus tachycardia, LVH.
- POCUS: Hyperdynamic underfilled LV without evidence of RV dilation, No RWMA, No systolic anterior motion of mitral valve (SAM) on M-mode noted. IVC small (virtual), and A-profile lungs.
- Lab results came back later and are shown below:
| 🧪 Initial Laboratory Results | ||
|---|---|---|
| Test | Result | Normal Range |
| Creatinine | 5 mg/dL | 0.6–1.3 |
| BUN | 90 mg/dL | 7–20 |
| Sodium | 155 mEq/L | 135–145 |
| Chloride | 120 mEq/L | 96–106 |
| Potassium | 5.0 mEq/L | 3.5–5.0 |
| Bicarbonate (HCO₃) | 15 mEq/L | 22–29 |
| Anion Gap | 20 | 8–16 |
| pH (ABG) | 7.17 | 7.35–7.45 |
| Lactate | 5 mmol/L | 0.5–2.2 |
| AST | 653 U/L | 10–40 |
| ALT | 556 U/L | 7–56 |
| ALP | 250 U/L | 44–147 |
| Total Bilirubin | 10 mg/dL | 0.1–1.2 |
| Direct Bilirubin | 5 mg/dL | 0.0–0.3 |
| LDH | 850 U/L | 140–280 |
| WBC | 19 ×10⁹/L (89% neutrophils) | 4.0–11.0 |
| Hemoglobin | 13.5 g/dL | 13.0–17.0 |
| Platelets | 80 ×10⁹/L | 150–400 |
| Procalcitonin | 23 ng/mL | < 0.1 |
| CRP | 180 mg/L | < 5 |
| cTn (Troponin) | 0.9 ng/dL | < 0.04 |
| BNP | 800 pg/mL | < 100 |
| Urinalysis | Pyuria, bacteriuria, hematuria — concerning for UTI; culture pending | |
🟥 Initial Resuscitation 🔜
- Broad-spectrum antibiotics were started immediately for presumed septic shock.
- After initial resuscitation with 4 liters of RL and norepinephrine infusion at 12 μg/min, his blood pressure was established at 95/60 mmHg, MAP 72.
- Repeat POCUS: LVEF ~ 60%, No RV dilation, IVC 1.2 cm with > 50% CI. No B-lines.
🟧 Day-1
- Late at night, the patient was found hypotensive with cold and mottled extremities, altered consciousness (GCS 10), with negligible urine output.
- SpO2 94% on NRB, BP 70/50 (MAP 57), HR 80 on norepinephrine 30 μg/min and vasopressin 0.4 U/min infusions.
- EKG no ischemic changes.
- POCUS: LVEF ~ 35%, TAPSE 12 mm, IVC 1.5cm with < 50% CI. No B-lines and effusion on lung scan.
- cTN 1.2 ng/dL, arterial lactate 10 mM.
| ❓What features in the early ED presentation support a diagnosis of septic shock rather than cardiogenic shock? ❓Despite an initial hyperdynamic heart, why did he develop low cardiac output later — even on escalating vasopressors? ⚙️What pathophysiological mechanisms explain this transition? |
Definition
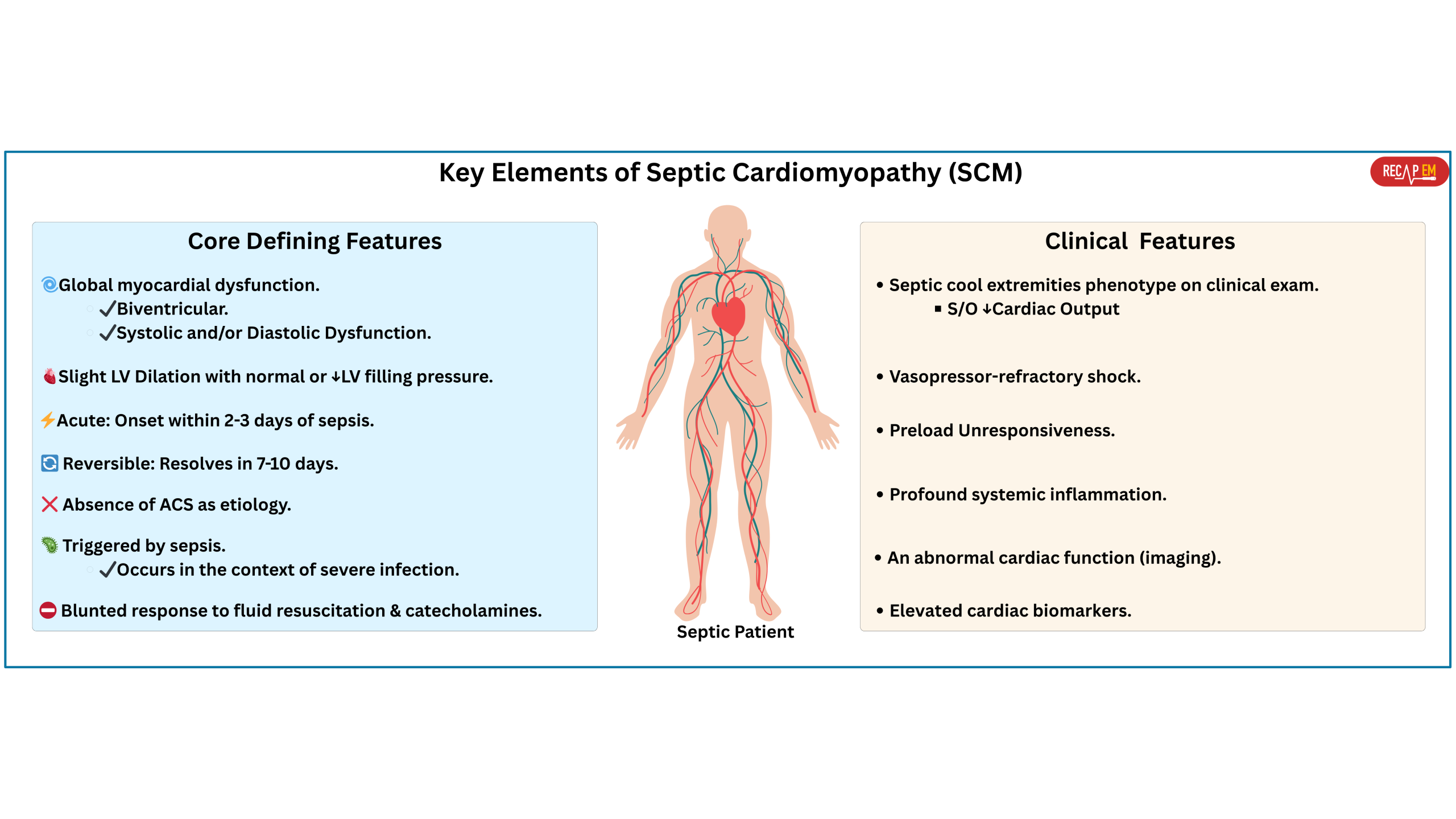
Septic cardiomyopathy remains an underrecognized and underdefined syndrome, with substantial gaps in nomenclature, definition, diagnostic criteria, epidemiology, and therapeutic strategies. These gaps stem largely from its complex, multifactorial pathophysiology, lack of reliable diagnostic tools, and the dynamic clinical environment of septic shock. Despite all controversies, there is broad agreement among experts on several key features that define the condition.
◾️Current Definition
- Global, reversible myocardial dysfunction that occurs in the setting of sepsis and is not explained by coronary artery disease or pre-existing cardiac conditions.
◾️Key defining features of SCM
- Global myocardial dysfunction
- Often biventricular (affecting both LV and RV)
- Systolic and/or diastolic dysfunction.
- Slight LV dilation with normal or low left ventricular filling pressure (LVFP) *,*.
- Acute and Reversible
- It usually occurs within 2 to 3 days of the onset of sepsis and resolves in 7 to 10 days *.
- Absence of acute coronary syndrome as etiology.
- Triggered by sepsis.
- Occurs in the context of severe infection, typically accompanied by systemic inflammation and circulatory shock.
- Blunted response to fluid resuscitation and catecholamines (e.g., abrupt vasopressor escalation without clear response)
- Reduced β-adrenergic receptor sensitivity contributes to inotropic failure despite high levels of circulating catecholamines.
Pathophysiology
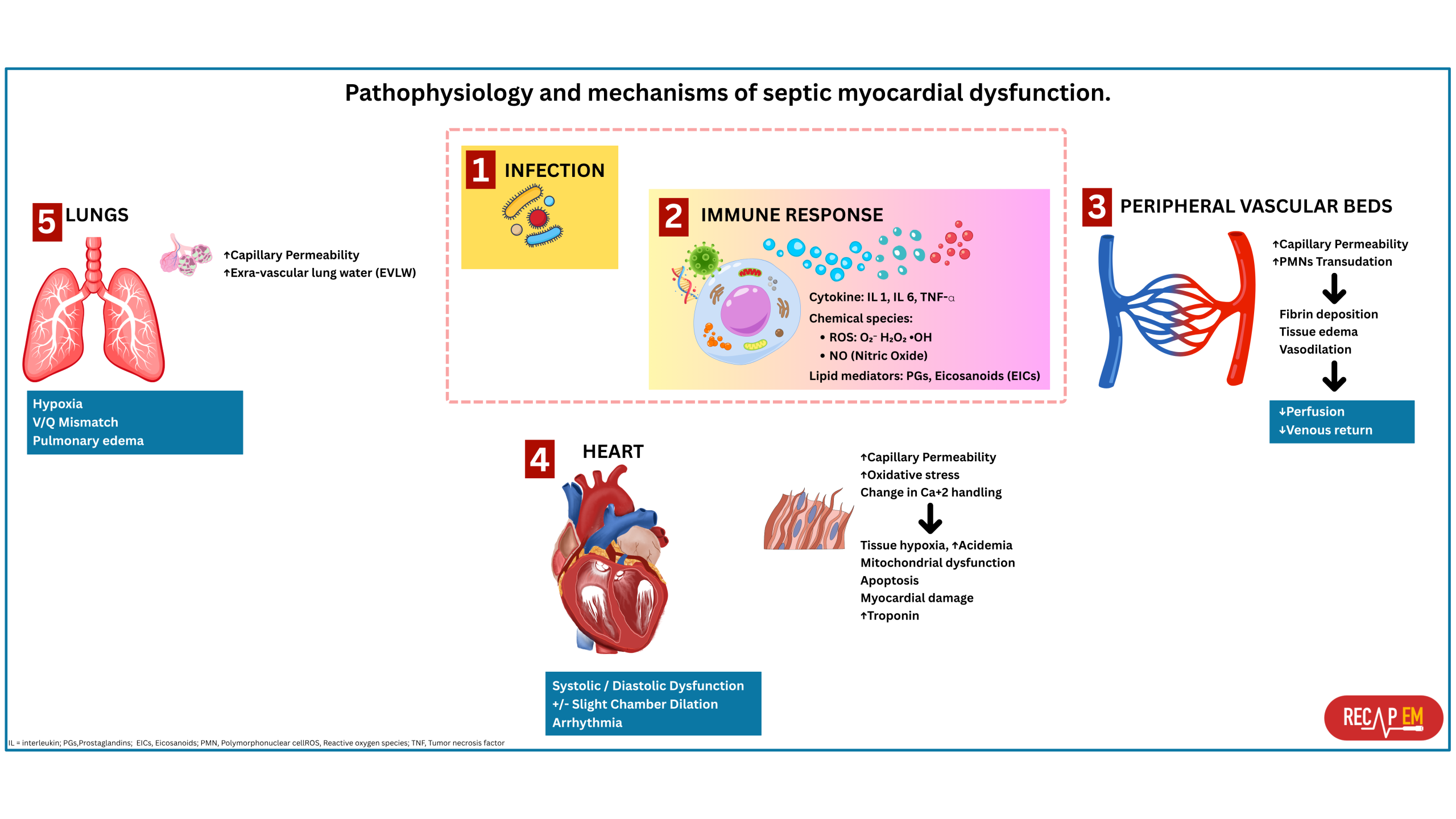
◾️The pathophysiology of SCM is multifactorial and partially understood (Figure below). Understanding these mechanisms will help you gain a clearer understanding of SCM and other cardiac dysfunctions that may occur during sepsis.
- Inflammation-induced myocardial depression
- Sepsis-induced dysregulated inflammatory response results in the transcription of proinflammatory cytokines such as IL-1, IL-6, and TNF-α that are linked to myocardial dysfunction and organ failure in septic shock.
- Excessive nitric oxide production (driven by inflammatory cytokines) also contributes to
- Myocardial depression.
- Downregulation of β-adrenergic receptors results in reduced myocyte responsiveness to adrenergic stimulation.
- Overexpression of cyclic guanosine monophosphate (cGMP) results in impaired myofilament calcium responsiveness, altering both preload and afterload.
- Mitochondrial dysfunction
- Sepsis and septic shock severely impair the “cellular power plants,” the mitochondria. Mitochondrial damage arises from inflammatory cytokines and oxidative stress. These downregulate mitochondrial protein synthesis genes during early inflammation, further impairing ATP production.
- Calcium dysregulation
- Inflammatory cytokines disrupt calcium handling in cardiomyocytes.
- Reduced calcium release affects systolic contraction.
- Impaired calcium reuptake reduces diastolic relaxation.
- Inflammatory cytokines disrupt calcium handling in cardiomyocytes.
- Adrenergic overstimulation results in deleterious effects!
- The typical compensatory response to sepsis-induced vasoplegia (↓systemic vascular resistance) is sympathetic hyperactivation (hyperadrenergic state).
- This catecholamine surge drives the typical hyperdynamic heart, seen in early sepsis.
- While this may initially help, it can later lead to heart damage by causing tachycardia, shortened diastole, and LV filling, or by its direct negative inotropic effects.
- Switching the β2-adrenoceptor from Gs (stimulatory) to Gi (inhibitory) response.
- Desensitization of β1-adrenoceptor.
- Ischemic effects secondary to a coronary microvascular dysfunction
- ☠️ Hyperdynamic LV function is linked with increased mortality *.
- The typical compensatory response to sepsis-induced vasoplegia (↓systemic vascular resistance) is sympathetic hyperactivation (hyperadrenergic state).
- Myocardial edema *
- Increased microvascular permeability and reduced lymphatic clearance lead to fluid accumulation in the myocardial interstitium.
- Even a 3.5% increase in myocardial water content can reduce cardiac output by 40% due to increased ventricular stiffness *.
Diagnosis
◾️The diagnosis of septic cardiomyopathy is usually based on the clinical and historical clues, echocardiography, biomarkers, and direct invasive and noninvasive measures of cardiac output (discussed below in detail).
Clinical features
◾️Clinical features suggestive of SCM include *
- A prior history of heart failure (septic cardiomyopathy may exacerbate pre-existing HF) *.
- Septic cool extremities phenotype on clinical exam (can suggest low cardiac output).
- Vasopressor-refractory shock *,*.
- Preload unresponsiveness *.
- Profound systemic inflammation (e.g., toxic shock syndrome or profound gram-negative septic shock).
- Elevated cardiac biomarkers.
- An abnormal cardiac function (imaging, echo).
Differential diagnosis
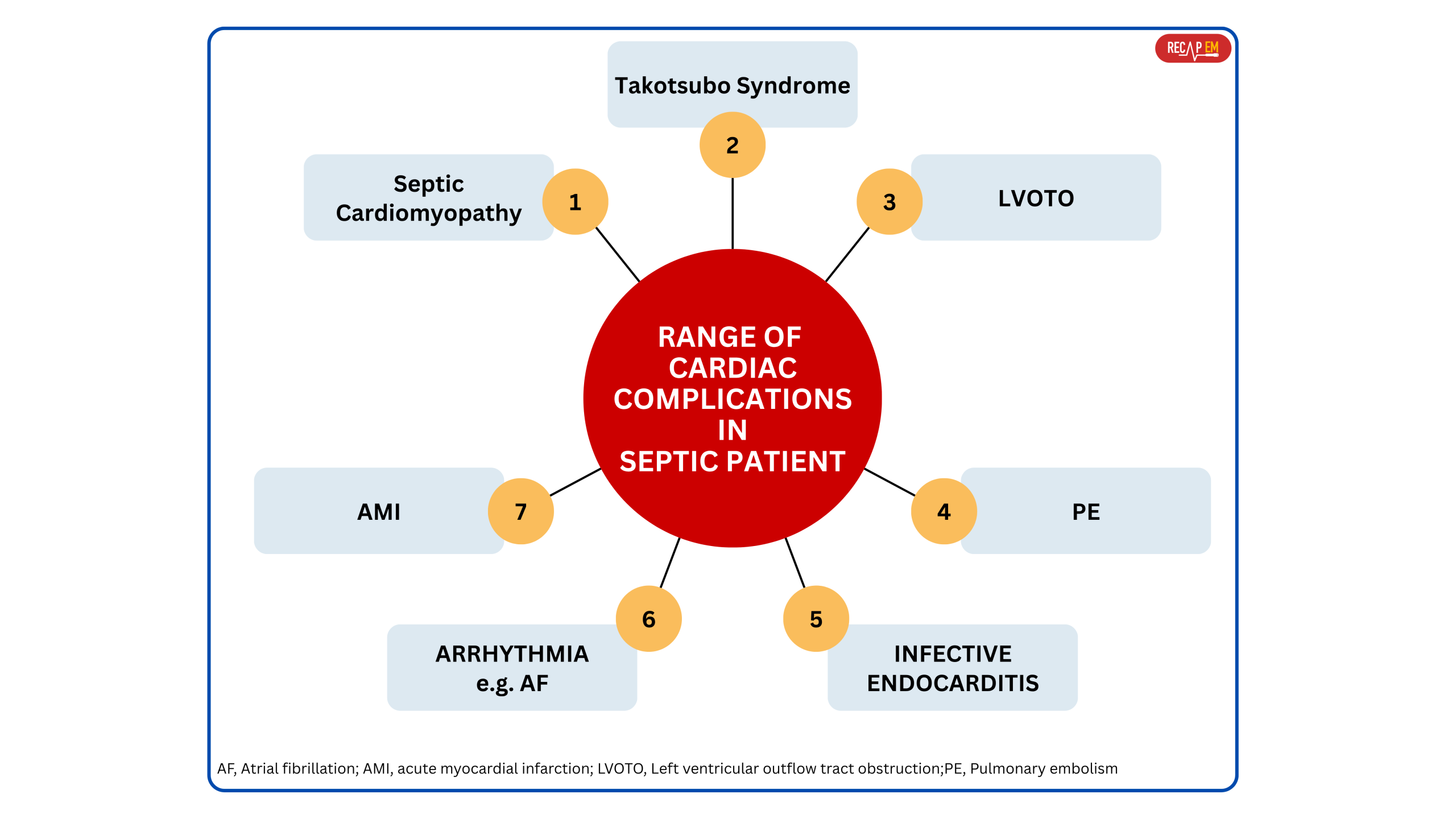
- Septic patients can develop a range of cardiac complications, not just septic cardiomyopathy (Figure below) *.
- While SCM is the most discussed in critical care, several distinct cardiac pathologies may arise in the setting of sepsis and should be differentiated for appropriate management.
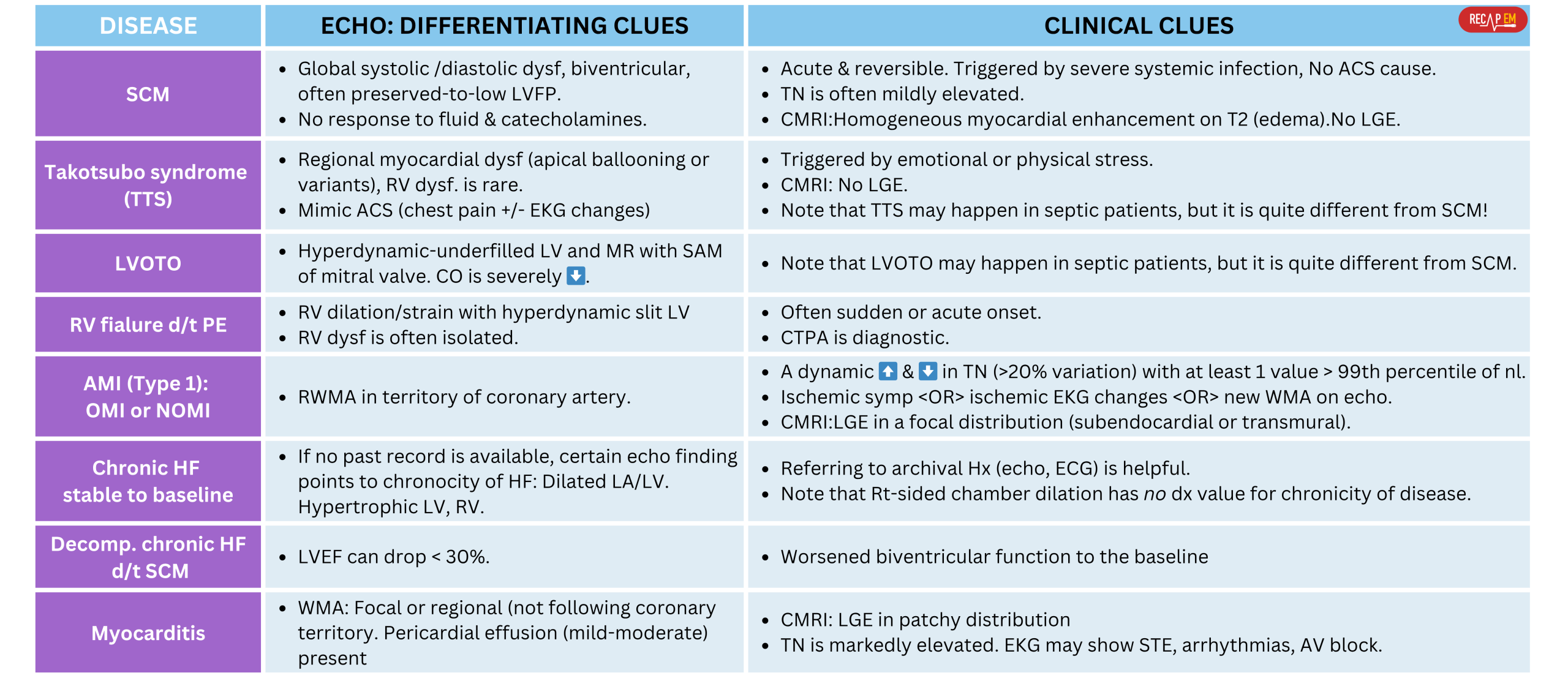
◾️Differential diagnosis of SCM includes (Below left table):
- Takotsubo Syndrome (TSS) (stress cardiomyopathy)*
- Several case series have reported TSS in patients with septic shock *. However, TSS has distinctive features *:
- Typical pattern: Regional myocardial dysfunction beyond the territory of the coronary artery: Apical ballooning or variants (mid-ventricular, basal). Usually doesn’t involve RV.
- Mechanism: Catecholamine surge (endogenous and/or exogenous) may trigger regional wall motion abnormalities.
- Mimic ACS (chest pain +/- EKG changes).
- Several case series have reported TSS in patients with septic shock *. However, TSS has distinctive features *:
- LVOTO (LV outflow tract obstruction).
- LVOTO may occur in patients with septic shock (20-30% of septic shock patients) *.
- This can happen due to high catecholamine states, inotrope administration, and hypovolemia.
- However, LVOTO should be distinguished from SCM. Echo findings in LVOTO include:
- Hyperdynamic LV, Mitral regurgitation with systolic anterior motion (SAM) of the mitral valve. More on this 👉 here.
- LVOTO may occur in patients with septic shock (20-30% of septic shock patients) *.
- Acute myocardial infarction (AMI).
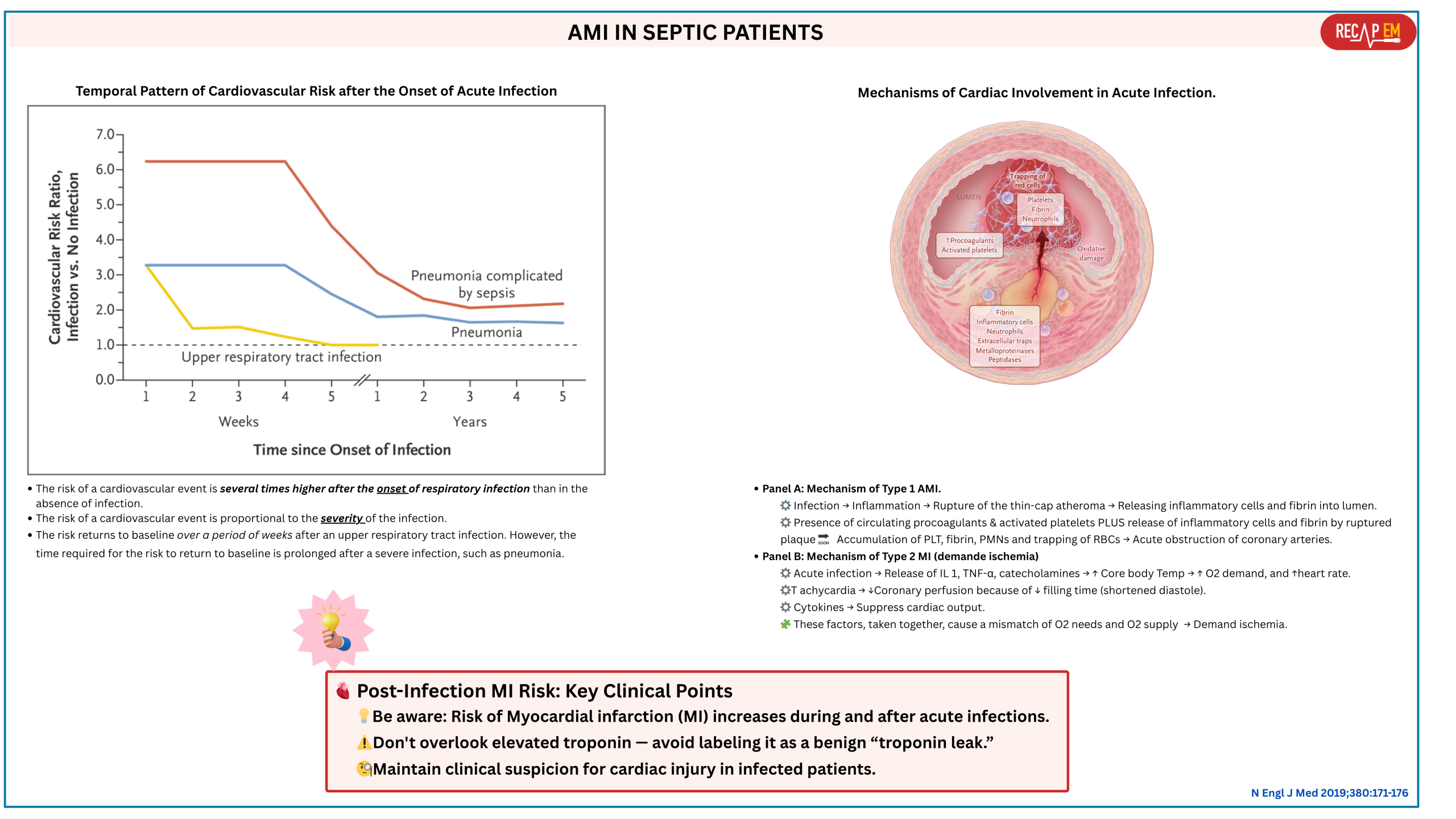
- Septic patients may develop AMI (~ 7.5%) *.
- The risk of ischemic events is associated with sepsis peak at the onset of infection and is proportional to the severity of the illness *.
- The risk of AMI persists beyond the short-term postinfection period (Below right figure).
- AMI produces regional wall motion abnormality (RWMA) in the territory of the coronary arteries, while SCM is a global dysfunction of the myocardium *.
- Septic patients may develop AMI (~ 7.5%) *.
- RV failure due to pulmonary embolism.
- Septic patients may develop PE (~ 2%) *.
- Remember that sepsis is a prothrombotic state induced by inflammatory cytokines, endothelial injury (causing micro- and macrovascular thrombosis), and platelet activation.
- Isolated massive or submassive PE causes RV dilation/strain and hyperdynamic underfilled LV. In contrast, SCM is often biventricular.
- Septic patients may develop PE (~ 2%) *.
- Myocarditis, e.g., infectious myocarditis.
- Chronic heart failure (see the diagnostic clues in the left table below):
- Stable patients compared to baseline.
- Decompensated CHF due to superimposed septic cardiomyopathy.
⎮📚Diagnostic approach to hypotensive patients with “hyperdynamic LV” see 👉 here.
💎See appendix for algorhythmic approach to patients with “hyperdynamic LV“.
Diagnostic evaluation
◾️EKG
- EKG findings are not diagnostic for septic cardiomyopathy
- There are no specific abnormalities consistent with septic cardiomyopathy.
- An EKG is essential to help exclude acute coronary syndrome.
- Sinus tachycardia and atrial fibrillation are the most common arrhythmias detected on EKG in patients with sepsis *.
◾️Cardiac biomarkers: Troponin
- Troponin elevation in sepsis is common. Overall, this correlates with increased mortality *.
- Troponin is usually only minimally elevated in septic cardiomyopathy *.
- Troponin may help exclude type-1 MI and perhaps myocarditis.
◾️Further diagnostic evaluation of sepsis, if needed. The diagnosis of SCM would be expected to occur in the context of unequivocal sepsis.
- If the diagnosis of sepsis is unclear, further investigations could include:
- Procalcitonin, CRP.
- Additional infectious evaluation.
◾️Imaging
- CT angiography to exclude PE: If RV failure is a predominant feature.
- Echocardiography (discussed here👇)
- Cardiac MRI (CMRI).
Echocardiography
Echocardiography is the primary imaging modality for assessing cardiac function in septic patients. Certain echocardiographic features seen in patients with sepsis (Figure below) are discussed here.
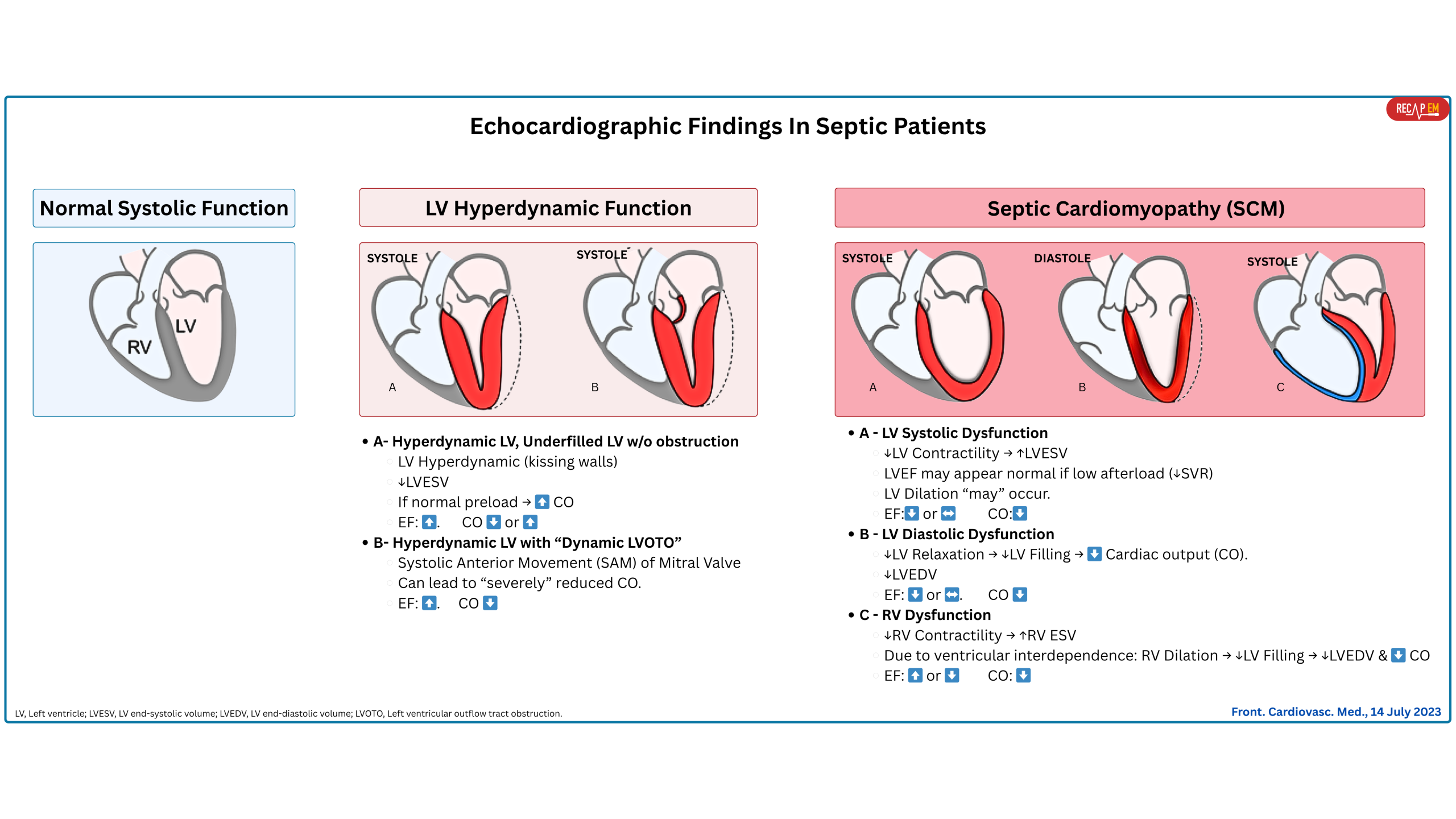
Hyperdynamic left ventricular systolic function
Basic
- Early on, septic shock often presents as a warm shock (hyperdynamic profile): ↑CO (if normal preload), ↓SVR, and tachycardia (↑HR).
- 💡In patients with underlying cardiovascular disease, early sepsis may not show up with this classic prototype.
- Later on, approximately 70% of patients present with a hypodynamic profile (↓LV contractility →↓CO) and some ventricular dilatation. When sepsis induces cardiac dysfunction without structural changes in the myocardium, it is called SCM (discussed here👇).
Pathophysiology of hyperdynamic LV
⚙️Mechanism and etiology of hyperdynamic LV
- Hyperdynamic LV as a compensatory response. This can happen in the following conditions:
- ↓ LV afterload (↓systemic vascular resistance, i.e., vasoplegia) that is typically seen in sepsis.
- ↓ LV end-diastolic volume. This can be due to:
- True volume loss, i.e., GI bleeding.
- Relative volume loss (blood vessels are leaking and cannot keep volume inside, e.g., sepsis).
- Reduced LV preload while the right-sided filling pressure is nl-high, e.g., pulmonary embolism.
- A hyperadrenergic state, either from excess endogenous or exogenous catecholamine stimulation.
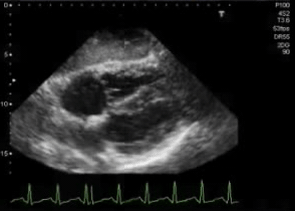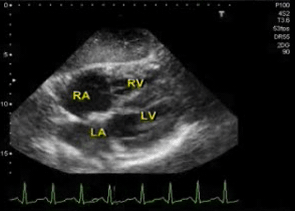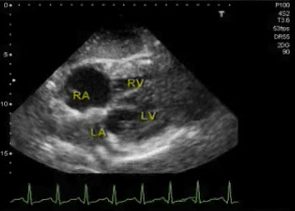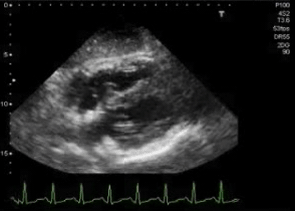
◾️Subtypes of hyperdynamic LV (Figure above)
- Hyperdynamic LV without left ventricular outflow tract obstruction (LVOTO). 📽️ Below left clip👇.
- Hyperdynamic LV with “Dynamic LVOTO” (~ 20-30% of septic shock patients) *. 🎥 Below right clip👇.
- This can happen due to high catecholamine states, inotrope administration, hypovolemia, and low SVR. 📚More on this 👉 here.
◾️Prognosis
- Hyperdynamic LV function shows increased mortality.
- In a cohort study of over one thousand patients, Chotalia and colleagues observed that individuals with supranormal LVEF (>70%) experienced worse outcomes than those with either normal or reduced LVEF *. The supranormal group also exhibited lower systemic vascular resistance (SVR), suggesting that the elevated ejection fraction may reflect persistent vasoplegia, which could have contributed to the increased mortality.
- These patients also had higher heart rates, so the hyperdynamic state may have resulted from excessive catecholamine release.
- Dynamic LVOTO has been associated with higher mortality in several studies *,*.
Depressed LV Systolic function
- Cytokine-Mediated Myocardial Depression
- Key inflammatory mediators directly impair myocardial contractility by the following mechanisms:
- Reducing β-adrenergic receptor sensitivity.
- Suppressing myofilament calcium responsiveness.
- Impairing excitation–contraction coupling.
- Key inflammatory mediators directly impair myocardial contractility by the following mechanisms:
- Mitochondrial Dysfunction
- Sepsis → Mitochondrial injury in cardiomyocytes→↓ ATP production, which is essential for actin–myosin cycling during contraction.
- Nitric Oxide (NO) and Reactive Oxygen Species (ROS)
- Excess NO (especially from inducible NOS, iNOS) decreases myocardial contractility via:
- Impaired calcium handling.
- Negative inotropic effects.
- ROS damage mitochondria, proteins, and membranes → Worsening energy failure and contractile dysfunction.
- Excess NO (especially from inducible NOS, iNOS) decreases myocardial contractility via:
- Microvascular and Endothelial Dysfunction
- Impaired coronary microcirculation leads to regional perfusion mismatch, even without large-vessel occlusion.
- Diffuse endothelial injury reduces oxygen and nutrient delivery to the myocardium.
- Autonomic Dysregulation and β-Adrenergic Desensitization
- Sepsis causes sympathetic overdrive, followed by downregulation of β-adrenergic receptors.
- This blunts the heart’s ability to respond to catecholamines (e.g., norepinephrine, epinephrine).
◾️Hemodynamic consequences of systolic dysfunction in SCM
- ↓Cardiac Output → Hypoperfusion and shock state.
- Inadequate response to catecholamines → Refractory shock despite vasopressor.
- Risk of fluid overload if LV function is severely depressed → Pulmonary edema.
◾️Clinical presentation and diagnosis
- Hypotension, despite adequate fluid resuscitation.
- Low to normal filling pressures (unlike classic cardiogenic shock).
- Echocardiography:
- Global hypokinesis. 📽️ Below clip👇.
- Reduced LVEF (but usually LVEF is > 30%, and often reversible within 7–10 days)
- Normal or low filling pressures.
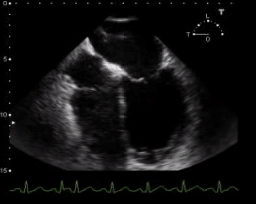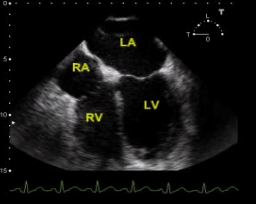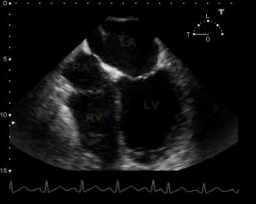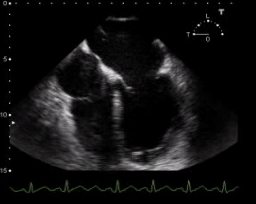
TEE. SCM with depressed LV systolic function
◾️Echocardiographic technique
- Formal echocardiographic evaluation of LV systolic function (e.g., LVEF, SV/CI, and MAPSE).
- These indices are neither sensitive nor specific for identifying ↓LV myocardial contractility.
- These are more or less “load-dependent” (affected by preload, afterload) and are also affected by LV geometry.
- Remember that the term “contractility” refers to the intrinsic property of the myocardial fiber, independent of preload and afterload.
- Advanced echocardiographic technique, e.g., global longitudinal strain (GLS).
- Reduced global longitudinal strain may be the most sensitive index of septic cardiomyopathy.
- Global longitudinal strain is less susceptible to changes in afterload.
◾️Echocardiographic techniques: Pearls & Pitfalls
- LVEF: The commonly used bedside methods for estimating LVEF (e.g., eyeballing, EPSS, FS) are discussed 👉 here.
- Stroke Volume (SV) and Cardiac Index (CI), using the left ventricular outflow tract (LVOT) diameter and the velocity time integral (VTI). See 👉 here.
- In sepsis, a low SVR can make CI appear elevated despite impaired contractility.
- MAPSE (mitral annular plane systolic excursion) measures longitudinal shortening (contraction) of LV (M-mode). See👉 here.
- GLS (global longitudinal strain)
- GLS < 20% may be consistent with septic cardiomyopathy.
- Studies on GLS as a predictor of outcome have shown more uniform results than LVEF, with a recent meta-analysis showing that lower strain (less negative
values), i.e., lower contractility, is associated with higher mortality *.
LV Diastolic Function
◾️Diastolic dysfunction is seen in ~50% of patients with sepsis *.
◾️Pathophysiology
- Myocardial Inflammation and Cytokine-Mediated Injury *
- Inflammatory cytokines → Disrupt calcium handling → Impair active myocardial relaxation (which is energy-dependent).
- Result: Impaired early diastolic relaxation and reduced ventricular compliance.
- Mitochondrial Dysfunction and ATP Depletion
- Mitochondrial dysfunction reduces ATP production, which is crucial for:
- Calcium reuptake into the sarcoplasmic reticulum.
- Active detachment of actin–myosin cross-bridges during relaxation.
- Low ATP → Delayed and incomplete diastolic relaxation.
- Mitochondrial dysfunction reduces ATP production, which is crucial for:
- Myocardial Edema and Interstitial Inflammation
- Sepsis → Capillary leak & endothelial dysfunction → Myocardial interstitial edema → ↓Myocardial compliance → ↓ Passive ventricular filling during diastole.
- Cardiac MRI sometimes shows diffuse myocardial swelling in septic patients.
- Endothelial and Microvascular Dysfunction
- Sepsis disrupts coronary microcirculation, causing regional hypoxia without obstructive coronary disease.
- Subtle myocardial ischemia and oxidative stress impair relaxation, even in the absence of necrosis.
- Autonomic Dysregulation
- Sepsis impairs baroreceptor and autonomic tone, leading to abnormal ventricular–vascular coupling.
- Altered loading conditions can exacerbate diastolic dysfunction, particularly in the setting of tachycardia.
◾️Hemodynamic consequences of diastolic dysfunction in sepsis
- Elevated left ventricular filling pressures (↑LVFP).
- Pulmonary congestion despite “normal” systolic function.
- ↓LV diastolic volume → ↓ SV and CO, particularly under stress (e.g., exercise, fever, tachycardia) → Worsening O2 delivery in a high-demand state.
◾️Echocardiographic assessment of LV diastolic dysfunction (more on this 👉 here)
- Lateral e’ wave
- E/e’ elevation
- Lateral E/e’ >13 cm/s or septal E/e’ >15 cm/s suggests septic cardiomyopathy *.
- E/e’ may be limited in patients with regional wall motion abnormalities, mitral valve disease, mechanical ventilation, age, and pericardial disease *.
- E/e’ generally correlates with LV end-diastolic pressure.
- ‼️However, in sepsis, this relationship is less robust than in congestive heart failure *.
◾️Prognostic value of echocardiographic findings
- Studies have identified a correlation between increased mortality with both reduced e′ velocities and elevated E/e′ ratios, markers suggestive of LV diastolic dysfunction*,*.
RV Dysfunction
◾️RV dysfunction (RVD) is common in patients with sepsis and septic shock (~75%) *.
- It can either be part of biventricular dysfunction or represent an isolated RV dysfunction.
◾️Pathophysiology. RV dysfunction in sepsis can be caused by:
- Primary, as part of global myocardial depression in SCM, or
- Secondary, due to increased RV afterload (e.g., ARDS, high PEEP, pulmonary vasoconstriction) *.
- Primary RV dysfunction (Direct Myocardial Depression)
- The same inflammatory and metabolic mechanisms that affect the LV also impair RV function:
- Cytokine-mediated RV depression → ↓ RV contractility.
- Mitochondrial dysfunction → ↓ ATP
- Nitric oxide and ROS → ↓Contractility.
- All in all, these result in global RV hypokinesis, typically reversible, like the LV component of SCM.
- 💡The relationship between RV dysfunction and SCM may be more straightforward because sepsis does not cause afterload reduction of the right ventricle.
- The same inflammatory and metabolic mechanisms that affect the LV also impair RV function:
- Secondary RV dysfunction ( Increased RV Afterload). Sepsis may significantly increase RV afterload through *:
- Acute Respiratory Distress Syndrome (ARDS)
- ARDS increases pulmonary vascular resistance (RV afterload) → RV strain.
- Severe hypoxemia, hypercapnia, and acidosis contribute to pulmonary vasoconstriction.
- Mechanical Ventilation / High PEEP
- High intrathoracic pressures from positive pressure ventilation increase PVR.
- It can trigger or worsen acute cor pulmonale.
- High intrathoracic pressures from positive pressure ventilation increase PVR.
- Pulmonary Microthrombi / Embolism
- Sepsis and DIC promote in situ thrombosis in pulmonary vessels.
- May contribute to acute RV dilation and failure.
- Acute Respiratory Distress Syndrome (ARDS)
◾️Hemodynamic and clinical consequences
- ↓ RV Contractility →↓ Pulmonary flow, ↓ LV preload.
- ↑ RV Afterload → RV dilation, ↓ Perfusion.
- RV Failure → ↑Venous pressures → ↓ Organ perfusion, e.g., congestive nephropathy.
- RV dilation → Interventricular septal shift to the left → Impaired LV filling, ↓ CO (ventricular interdependence).
◾️Diagnostic evaluation
- Echocardiography (below)
- IVC, VExUS (more on this, here).
- Plethoric IVC can be seen if RV failure is pronounced (and there’s no ongoing volume loss).
- Hemodynamics:
- Shock that worsens with fluids or PEEP may suggest RV dysfunction.
◾️Echocardiographic evaluation of RV dysfunction (RVD)
- Overall diagnostic and prognostic performance
- Echocardiographic diagnosis of RVD is both challenging and highly dependent on the examiner’s experience, as well as the diagnostic technique employed.
- TAPSE and RV s’ primarily assess longitudinal function.
- This limits their ability to provide a comprehensive picture of RV performance.
- RV Strain using STE may be a promising tool for detecting RV dysfunction.
- TAPSE and RV s’ primarily assess longitudinal function.
- Generally, formal echocardiographic evaluation of RVD lacks sensitivity and specificity. More advanced imaging techniques are required.
- Echocardiographic diagnosis of RVD is both challenging and highly dependent on the examiner’s experience, as well as the diagnostic technique employed.
- Echocardiographic findings in RV dysfunction
- Formal echocardiographic evaluation:
- RV/LV dilation >0.6. More on this 👉 here
- TAPSE <16 mm.
- TDI S’ wave (tricuspid systolic lateral annular velocity) < 10 cm/s.
- RV fractional area of change < 35%.
- GLS (global longitudinal strain)
- Formal echocardiographic evaluation:
◾️Prognosis
- Septic patients with right ventricular (RV) dysfunction exhibit increased mortality, as demonstrated in a study of 393 ICU patients where 28-day mortality was 31% in those with RV dysfunction, compared to 16% in those without *.
Cardiac MRI (CMRI)
- CMRI isn’t widely utilized due to logistical constraints (e.g., duration of the examination, particularly when dealing with hemodynamically unstable, mechanically ventilated patients).
- CMRI may help distinguish septic cardiomyopathy from myocarditis. It can help differentiate TSS from AMI as well.
- Late gadolinium enhancement (LGE) is a technique used in CMR for cardiac tissue characterization. Presence of LGE reflects cardiac necrosis/fibrosis *. More on this👉 here.
- Septic cardiomyopathy is marked by *:
- Homogeneous myocardial enhancement on T2 images (edema).
- No late gadolinium enhancement (LGE).
- Myocarditis typically shows late LGE in a patchy distribution.
- AMI typically shows LGE in a focal distribution (subendocardial or transmural).
- Takotsubo’s cardiomyopathy typically has no LGE.
- Septic cardiomyopathy is marked by *:
- Late gadolinium enhancement (LGE) is a technique used in CMR for cardiac tissue characterization. Presence of LGE reflects cardiac necrosis/fibrosis *. More on this👉 here.
Approach to diagnosis and management
General Principles
- Individualized approach
- Clinical manifestation of septic shock is highly variable and is influenced by several factors, such as age, premorbid condition, and source of infection.
- Therefore, conventional generalized treatments have been increasingly challenged in favor of personalized approaches tailored to the individual patient’s response.
- Just as there is heterogeneity among patients with sepsis, there is heterogeneity in the cardiac response; thus, resuscitating these patients with a single approach is likely suboptimal.
- Multidimensional approach
- Diagnosis and treatment of critical patients should be based on a multimodal approach.
- Rather than relying on a single parameter, a comprehensive hemodynamic profile (hemodynamic variables, markers of organ perfusion, and global perfusion status) should be integrated in view of the patient’s clinical context.
- Dynamic and Physiology-based approach
- Septic shock evolves through different stages. Moreover, each patient responds differently to therapeutic measures, e.g., vasopressors.
- Hemodynamic status is dynamic since patients may move from one phenotype to another according to fluid administration, correction of LV afterload, and evolution of the disease.
- Therefore, treatment of such patients requires dynamic assessment (i.e., responsiveness to treatment).
Overview of management
Robust evidence for the treatment of septic cardiomyopathy is currently lacking. The following represent conceptual approaches rather than definitive guidelines.
◾️Avoid aggravating factors
- Manage fever to reduce vasodilation and oxygen demand.
- Control agitation and shivering to minimize metabolic stress.
- Consider stress-dose corticosteroids — inflammatory cytokines may exacerbate septic cardiomyopathy.
- Intubate if necessary in cases of high work of breathing and refractory shock.
◾️Volume management
- Avoid volume overload. More on volume management in septic shock 👉 here.
- Consider diuresis in the setting of pulmonary or systemic congestion.
◾️Vasopressors
- Limit the overuse of vasoconstrictors, as they can impair cardiac output by raising afterload.
- A vasopressor-only strategy (e.g., norepinephrine + vasopressin) is generally suboptimal in myocardial depression.
◾️Inotropes
- May be necessary to ensure sufficient perfusion.
- Dobutamine or Epinephrine could be helpful.
◾️Methylene Blue in Septic Cardiomyopathy
- Elevated nitric oxide levels and mitochondrial dysfunction are believed to contribute to septic cardiomyopathy.
- Methylene blue may counteract these mechanisms by inhibiting nitric oxide synthase and improving cellular energetics.
- Some prospective studies suggest it can enhance myocardial performance in septic shock, though evidence remains limited.
◾️Mechanical Cardiovascular Support
- A limited number of case reports describe successful use of mechanical circulatory support, such as ECMO, in septic cardiomyopathy.
- It may be most beneficial in patients where vasoplegia is controlled, revealing a hemodynamic profile dominated by low cardiac output — consistent with predominant cardiogenic failure.
| Therapy | Primary Role | Mechanism | Evidence Summary | Recommendation |
|---|---|---|---|---|
| Hydrocortisone | Vasopressor-refractory septic shock | Reduces inflammation; improves vasopressor sensitivity | ADRENAL/APROCCHSS: improved shock resolution; some mortality benefit | Recommended in vasopressor-refractory shock |
| Vasopressin | Second-line vasopressor | V1 receptor agonist; vasoconstriction without tachycardia | VASST: possible benefit in less severe shock | Add to norepinephrine if MAP target unmet |
| Dobutamine | Inotrope for low CO with hypoperfusion | β1 agonist; increases inotropy | SSC: weak recommendation if ongoing hypoperfusion | Use if low CO + hypoperfusion persists |
| Levosimendan | Calcium sensitizer (experimental) | Increases contractility without ↑ O₂ demand | LeoPARDS: no benefit, increased arrhythmias | Not routinely recommended |
| Angiotensin II | Catecholamine-resistant vasoplegia | Activates angiotensin II receptors; ↑ vascular tone | ATHOS-3: ↑ MAP in refractory shock | Consider in refractory vasodilatory shock |
| Methylene Blue | Refractory vasoplegia via NO inhibition | Inhibits NO-cGMP pathway; restores vascular tone | Case reports/small trials; transient MAP benefit | Consider as salvage therapy |
◾️Additional therapies should be individualized based on the clinical phenotype, following principles similar to the management of other forms of cardiac failure patients
Appendix
◾️Diagnostic approach to hypotensive patients with “Hyperdynamic LV”
References
1. PMID: 30171861. Martin L, et al. The Septic Heart: Current Understanding of Molecular Mechanisms and Clinical Implications. Chest. 2019 Feb;155(2):427-437. doi: 10.1016/j.chest.2018.08.1037. Epub 2018 Aug 29.
2. PMID: 21906334. Vieillard-Baron A. Septic cardiomyopathy. Ann Intensive Care. 2011 Apr 13;1(1):6. doi: 10.1186/2110-5820-1-6.
3. PMID: 36893835. Lima MR, Silva D. Septic cardiomyopathy: A narrative review. Rev Port Cardiol. 2023 May;42(5):471-481. English, Portuguese. doi: 10.1016/j.repc.2021.05.020. Epub 2023 Mar 7.
4. PMID: 34605779. Chotalia M, et al. Hyperdynamic Left Ventricular Ejection Fraction in ICU Patients With Sepsis. Crit Care Med. 2022 May 1;50(5):770-779. doi: 10.1097/CCM.0000000000005315. Epub 2021 Oct 4.
5. PMID: 39663022. Hasegawa D, Sato R. Dexmedetomidine for Decatecholaminization in Septic Shock: Insights and Challenges? Chest. 2024 Dec;166(6):1264-1265. doi: 10.1016/j.chest.2024.07.141.
6. PMID: 39004217. zz Al-Regal AR, Ramzy EA, Atia AAA, Emara MM. Dexmedetomidine for Reducing Mortality in Patients With Septic Shock: A Randomized Controlled Trial (DecatSepsis). Chest. 2024 Dec;166(6):1394-1405. doi: 10.1016/j.chest.2024.06.3794. Epub 2024 Jul 14.
7. PMID: 9142032. Yu P, Boughner DR, Sibbald WJ, keys J, Dunmore J, Martin CM. Myocardial collagen changes and edema in rats with hyperdynamic sepsis. Crit Care Med. 1997 Apr;25(4):657-62. doi: 10.1097/00003246-199704000-00017.
8. PMID: 2036720. Laine GA, Allen SJ. Left ventricular myocardial edema. Lymph flow, interstitial fibrosis, and cardiac function. Circ Res. 1991 Jun;68(6):1713-21. doi: 10.1161/01.res.68.6.1713.
9. PMID: 27684877. Sato R, Kuriyama A, Takada T, Nasu M, Luthe SK. Prevalence and risk factors of sepsis-induced cardiomyopathy: A retrospective cohort study. Medicine (Baltimore). 2016 Sep;95(39):e5031. doi: 10.1097/MD.0000000000005031.
10. PMID: 29227368. Beesley SJ, Weber G, Sarge T, Nikravan S, Grissom CK, Lanspa MJ, Shahul S, Brown SM. Septic Cardiomyopathy. Crit Care Med. 2018 Apr;46(4):625-634. doi: 10.1097/CCM.0000000000002851.
11. PMID: 29724231. Ehrman RR, Sullivan AN, Favot MJ, Sherwin RL, Reynolds CA, Abidov A, Levy PD. Pathophysiology, echocardiographic evaluation, biomarker findings, and prognostic implications of septic cardiomyopathy: a review of the literature. Crit Care. 2018 May 4;22(1):112. doi: 10.1186/s13054-018-2043-8.
12. PMID: 33473203. Hollenberg SM, Singer M. Pathophysiology of sepsis-induced cardiomyopathy. Nat Rev Cardiol. 2021 Jun;18(6):424-434. doi: 10.1038/s41569-020-00492-2. Epub 2021 Jan 20.
13. PMID: 36555977. Merdji H, Siegemund M, Meziani F. Acute and Long-Term Cardiovascular Complications among Patients with Sepsis and Septic Shock. J Clin Med. 2022 Dec 12;11(24):7362. doi: 10.3390/jcm11247362.
14. PMID: 38669408. Hiraiwa H, Kasugai D, Okumura T, Murohara T. Clinical implications of septic cardiomyopathy: A narrative review. Medicine (Baltimore). 2024 Apr 26;103(17):e37940. doi: 10.1097/MD.0000000000037940.
15. PMID: 35906556. Jing C, Wang Y, Kang C, Dong D, Zong Y. Clinical features of patients with septic shock-triggered Takotsubo syndrome: a single-center 7 case series. BMC Cardiovasc Disord. 2022 Jul 29;22(1):340. doi: 10.1186/s12872-022-02787-3.
16. PMID: 36788939. Zhang H, Liu D. Sepsis-related cardiomyopathy: Not an easy task for ICU physicians. J Intensive Med. 2022 Jun 25;2(4):257-259. doi: 10.1016/j.jointm.2022.05.005.
17. PMID: 37522079. Shvilkina T, Shapiro N. Sepsis-Induced myocardial dysfunction: heterogeneity of functional effects and clinical significance. Front Cardiovasc Med. 2023 Jul 14;10:1200441. doi: 10.3389/fcvm.2023.1200441.
18. PMID: 30773249. Patel N, et al. Cardiovascular Events and Hospital Deaths Among Patients With Severe Sepsis. Am J Cardiol. 2019 May 1;123(9):1406-1413. doi: 10.1016/j.amjcard.2019.01.038. Epub 2019 Feb 7. Erratum in: Am J Cardiol. 2020 Jan 1;125(1):163-164. doi: 10.1016/j.amjcard.2019.10.001.
19. PMID: 30625066. Musher DM, Abers MS, Corrales-Medina VF. Acute Infection and Myocardial Infarction. N Engl J Med. 2019 Jan 10;380(2):171-176. doi: 10.1056/NEJMra1808137.
20. PMID: 38992133. Hasegawa D, Sato R, Lee YI, Wang HY, Nishida K, Steiger D. The prevalence, risk factors, and outcomes of acute pulmonary embolism complicating sepsis and septic shock: a national inpatient sample analysis. Sci Rep. 2024 Jul 11;14(1):16049. doi: 10.1038/s41598-024-67105-7.
21. PMID: 25112502. Mekontso Dessap A, Razazi K, Brun-Buisson C, Deux JF. Myocardial viability in human septic heart. Intensive Care Med. 2014 Nov;40(11):1746-8. doi: 10.1007/s00134-014-3428-z. Epub 2014 Aug 12.
22. PMID: 28101605. Rhodes A, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017 Mar;43(3):304-377. doi: 10.1007/s00134-017-4683-6. Epub 2017 Jan 18.
23. PMID: 34605779. Chotalia M, Ali M, Hebballi R, Singh H, Parekh D, Bangash MN, Patel JM. Hyperdynamic Left Ventricular Ejection Fraction in ICU Patients With Sepsis. Crit Care Med. 2022 May 1;50(5):770-779. doi: 10.1097/CCM.0000000000005315. Epub 2021 Oct 4.
24. PMID: 26082197. Chauvet JL, et al. Early dynamic left intraventricular obstruction is associated with hypovolemia and high mortality in septic shock patients. Crit Care. 2015 Jun 17;19(1):262. doi: 10.1186/s13054-015-0980-z.
25. PMID: 1986643. Robotham JL, Takata M, Berman M, Harasawa Y. Ejection fraction revisited. Anesthesiology. 1991 Jan;74(1):172-83. doi: 10.1097/00000542-199101000-00026.
26. PMID: 21906334. Vieillard-Baron A. Septic cardiomyopathy. Ann Intensive Care. 2011 Apr 13;1(1):6. doi: 10.1186/2110-5820-1-6.
27. PMID: 32377972. L’Heureux M, Sternberg M, Brath L, Turlington J, Kashiouris MG. Sepsis-Induced Cardiomyopathy: a Comprehensive Review. Curr Cardiol Rep. 2020 May 6;22(5):35. doi: 10.1007/s11886-020-01277-2.
28. PMID: 33388489. Ravikumar N, Sayed MA, Poonsuph CJ, Sehgal R, Shirke MM, Harky A. Septic Cardiomyopathy: From Basics to Management Choices. Curr Probl Cardiol. 2021 Apr;46(4):100767. doi: 10.1016/j.cpcardiol.2020.100767. Epub 2020 Dec 11.
29. PMID: 30501628. Havaldar AA. Evaluation of sepsis-induced cardiac dysfunction as a predictor of mortality. Cardiovasc Ultrasound. 2018 Nov 30;16(1):31. doi: 10.1186/s12947-018-0149-4.
30. PMID: 30075792. Sanfilippo F, et al. Left ventricular systolic function evaluated by strain echocardiography and relationship with mortality in patients with severe sepsis or septic shock: a systematic review and meta-analysis. Crit Care. 2018 Aug 4;22(1):183. doi: 10.1186/s13054-018-2113-y.
31. PMID: 25800584. Sanfilippo F, Corredor C, Fletcher N, Landesberg G, Benedetto U, Foex P, Cecconi M. Diastolic dysfunction and mortality in septic patients: a systematic review and meta-analysis. Intensive Care Med. 2015 Jun;41(6):1004-13. doi: 10.1007/s00134-015-3748-7. Epub 2015 Mar 24. Erratum in: Intensive Care Med. 2015 Jun;41(6):1178-9. doi: 10.1007/s00134-015-3799-9.
32. PMID: 19196490. Joulin O, M, et al. Cardiac force-frequency relationship and frequency-dependent acceleration of relaxation are impaired in LPS-treated rats. Crit Care. 2009;13(1):R14. doi: 10.1186/cc7712. Epub 2009 Feb 6.
33. PMID: 25800584. Sanfilippo F, et al. Diastolic dysfunction and mortality in septic patients: a systematic review and meta-analysis. Intensive Care Med. 2015 Jun;41(6):1004-13. doi: 10.1007/s00134-015-3748-7. Epub 2015 Mar 24. Erratum in: Intensive Care Med. 2015 Jun;41(6):1178-9. doi: 10.1007/s00134-015-3799-9.
34. PMID: 29121301. Sanfilippo F, et al. Tissue Doppler assessment of diastolic function and relationship with mortality in critically ill septic patients: a systematic review and meta-analysis. Br J Anaesth. 2017 Oct 1;119(4):583-594. doi: 10.1093/bja/aex254.
35. PMID: 32279167. Innocenti F, et al. Epidemiology of right ventricular systolic dysfunction in patients with sepsis and septic shock in the emergency department. Intern Emerg Med. 2020 Oct;15(7):1281-1289. doi: 10.1007/s11739-020-02325-z. Epub 2020 Apr 11.
36. PMID: 36789232. Boissier F, Aissaoui N. Septic cardiomyopathy: Diagnosis and management. J Intensive Med. 2021 Dec 27;2(1):8-16. doi: 10.1016/j.jointm.2021.11.004.
37. PMID: 25015102. Orde SR, et al. Outcome prediction in sepsis: speckle tracking echocardiography based assessment of myocardial function. Crit Care. 2014 Jul 11;18(4):R149. doi: 10.1186/cc13987.
38. PMID: 21771988. Eitel I, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011 Jul 20;306(3):277-86. doi: 10.1001/jama.2011.992.







Add comment