April 21, 2021, via Shahriar Lahouti. Last updated: Dec 22, 2021

CONTENTS
- Introduction
- Clinical Scenario
- Misconceptions
- Definition and clinical classification of AHFS
- Framework for pathophysiology of AHFS
- Clinical picture and diagnosis
- Investigation
- Differential diagnosis
- General approach to AHFS
- RECAP
- Appendix
- Going further
- References
Introduction
Acute Heart Failure Syndrome (AHFS) has a broad clinical and hemodynamic spectrum resulting from a multitude pathophysiologic disease processes. Some patients are critically ill and emergent identification and resuscitation are crucial. Appropriate management of these patients depends on pathophysiology of the ‘AHFS” which is notably different among various phenotypes of heart failure (see here).
Flash pulmonary edema (aka Hypertensive cardiogenic pulmonary edema, crashing pulmonary edema , acute hypertensive heart failure etc) is a general clinical term used to describe a particularly dramatic form of acute decompensated heart failure 1. Due to the central role of sympathetic overdrive in the pathophysiology of this subset of patients, sympathetic crashing acute pulmonary edema (SCAPE) 2 is a better terminology for understanding the syndrome of rapid onset, life-threatening pulmonary edema 3.
In this post, the conceptual framework for pathophysiology of AHFS is briefly discussed first and then SCAPE is explored in detail. This post is not about:
- Cardiogenic shock
- Mild-moderate decompensated heart failure
- Less common types of heart failure with unique physiology such as
- Acute valvular regurgitation (Mitral regurgitation, aortic regurgitation)
- Mitral stenosis
- Aortic stenosis
- Dynamic LV outflow tract obstruction
Clinical scenario
A 60-year-old female with a past history of hypertension and heart failure presented to the ED with acute dyspnea and chest tightness. She appeared anxious and diaphoretic, sitting bolt upright. Her BP was 195/89 mm Hg, PR 143 bpm, RR 32/min, SPO2 88% on HFNC, and temperature 36.5 C. Bilateral basal rales noted. ECG showed sinus tachycardia with nonspecific ST-T changes. Findings in bedside ultrasound were supportive for diagnosis of acute pulmonary edema. How emergent management should proceed with this patient?
Misconceptions
#1: Patients with Sympathetic Crashing Acute Pulmonary Edema (SCAPE) are universally fluid-overloaded!
#2: Diuretics play a pivotal role in amelioration of disease processes!
#3: Initiating NTG @ 10-40 mcg/min (low dose) is effective!
Definition and Classification of AHFS
Acute heart failure is not a single disease but rather a heterogeneous clinical syndrome with complex pathophysiologic processes and different overlapping pathologic mechanism 4; hence called “Acute Heart Failure Syndrome” in this post ( a variety of terms have been used in literature e.g. acute decompensated heart failure and so on). By enlarge, AHFS can be defined as the new onset or recurrence of symptoms and signs of HF requiring urgent or emergent therapy 5.
The cardinal clinical manifestation of “AHFS” include:
- Clinical Congestion: manifested as pulmonary congestion (pulmonary edema) or systemic congestion (extremities edema)
- End-organ Hypoperfusion: manifested as renal or hepatic dysfunction
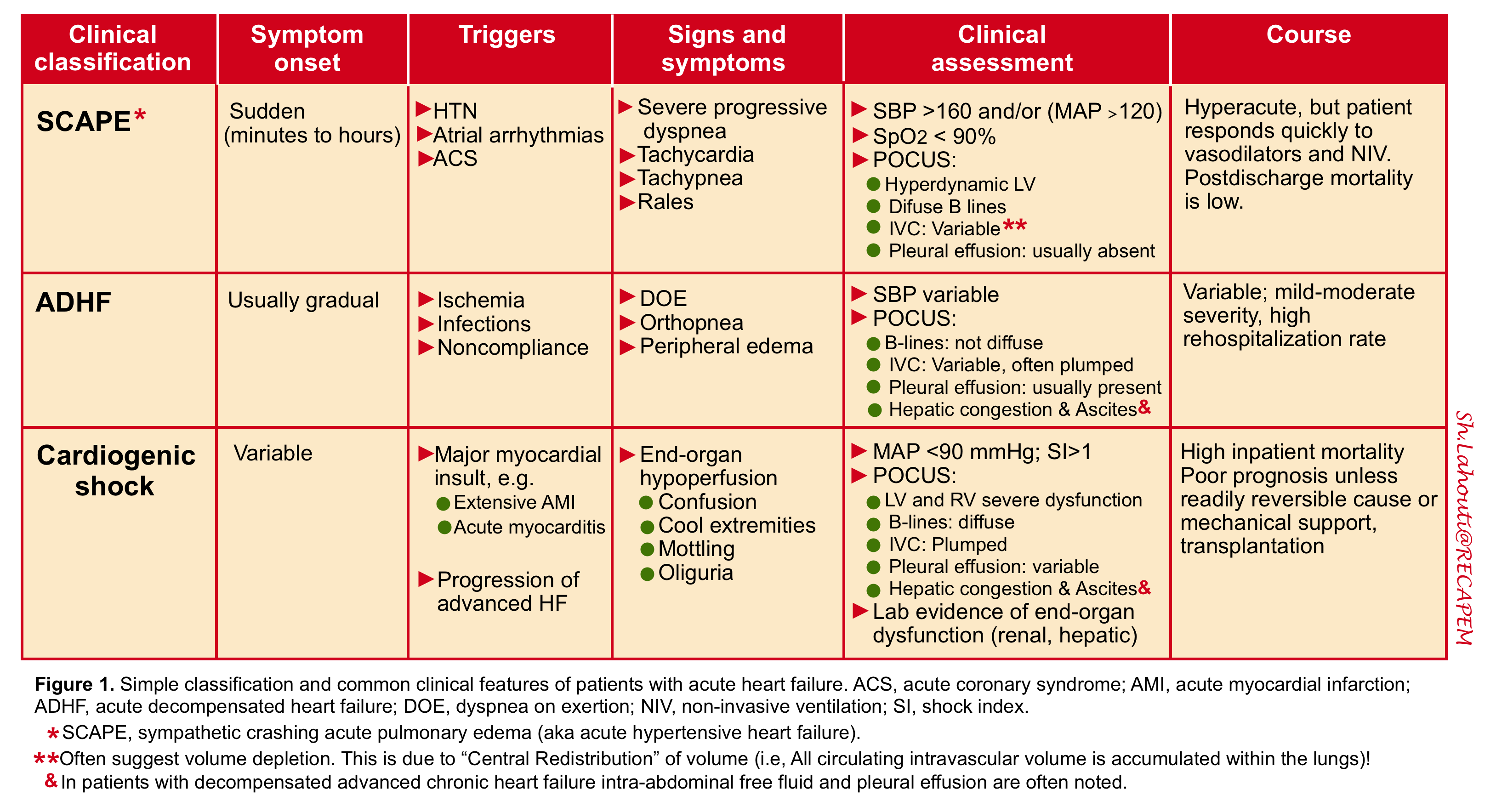
While the following clinical classification system (figure 1) does not fully encompass some less common clinical scenarios (e.g. isolated right HF or high output HF), it usefully captures the vast majority of AHFS patients likely to be seen in routine clinical practice. A simplified classification scheme defines three general groups of AHF patients 6.
- Such phenotypes can occur de novo or in patients with pre-existing chronic heart failure {whether EF is preserved (HFpEF) or reduced (HFrEF)}.
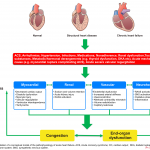
- From the hemodynamic perspective, the unique feature common to all phenotypes of “AHFS” is elevated left ventricular diastolic pressure (LVEDP).
Framework for pathophysiology of AHFS and SCAPE
One potentially useful framework for understanding the pathophysiology of AHF is to consider it as the result of the interaction of underlying substrate, triggers, and amplifying mechanisms; all of which contribute to a common set of clinical signs and symptoms (primarily related to congestion, end-organ dysfunction, or both) that define AHFS 7 (figure 2).
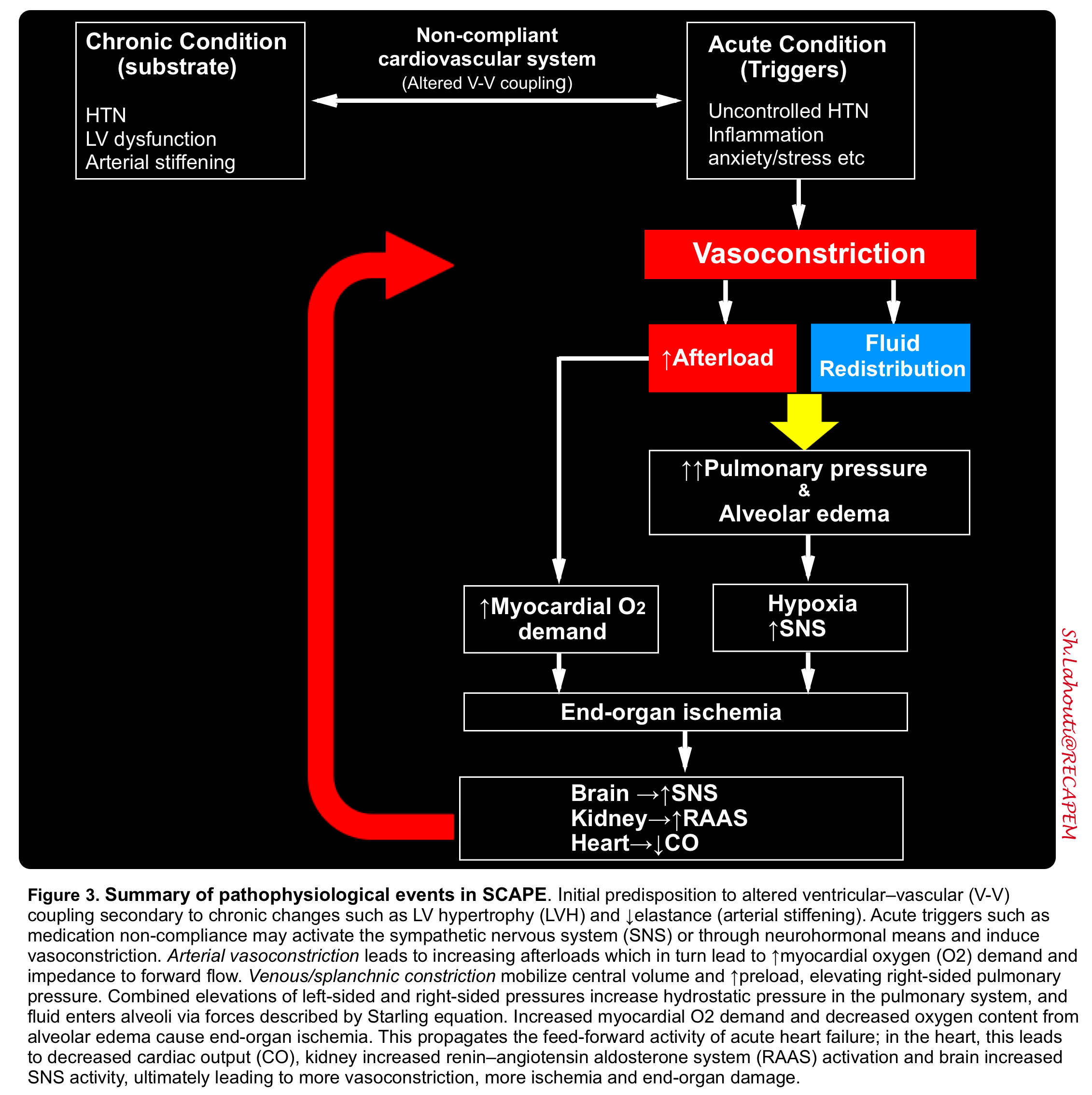
- SCAPE is the most dramatic form of AHFS. The underlying pathophysiologic mechanisms are similar to other phenotypes of “AHFS”. However there are notable differences between these two. Important distinguishing features of SCAPE are abnormal vasoconstriction secondary to sympathetic activation, exaggerated degree of pulmonary RAAS, and decline in NO production (figure 3).
Substrate: substrate refers to underlying cardiac structure and function. That is to say whether the patient has a pre-existing cardiac dysfunction or not.
- Most patients with “SCAPE” have underlying chronic left ventricular dysfunction (whether systolic or most commonly diastolic dysfunction secondary to hypertension).
- However, the underlying substrate may be one of normal ventricular function, for example, patients without a prior history of HF who develop AHFS because of sudden changes in ventricular function from an acute insult such as extensive MI or acute myocarditis.
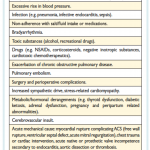
Triggers: Initiating mechanisms vary according to, and interact with, the underlying substrate and may be cardiac or extracardiac (appendix 1).
- Principles:
- For patients with normal substrate (normal myocardium), a substantial insult to cardiac performance (e.g. acute myocarditis) is required to lead to the clinical presentation of AHF.
- For patients with abnormal substrate at baseline (asymptomatic LV dysfunction), smaller perturbations (e.g. poorly controlled hypertension, AF, or ischemia) may precipitate an AHF episode.
- Acute triggers for “SCAPE” include
- Sympathetic overdrive
- Noncompliance with antihypertensives medications.
- Exertion, stress, or anxiety.
- Sympathomimetic substance abuse.
- Withdrawal (e.g. from clonidine).
- Acute MI.
- Volume overload (e.g. missed hemodialysis sessions, nonadherence with diuretics).
- Sympathetic overdrive
Amplifying mechanisms: these perpetuate and contribute to the episode of decompensation. Ongoing LV dysfunction (with increasing LVEDP), alveolar flooding and hypoxemia; all can perpetuate the vicious cycle of sympathetic surge contributing to worsening clinical condition.
- Neurohormonal (e.g. RAAS) and inflammatory activation
- Ongoing myocardial injury with progressive myocardial dysfunction
- Worsening renal function
- Peripheral vascular dysfunction (e.g. peripheral vasoconstriction)
For more detailed schema see appendix 2.
Misconception and pitfalls:
Congestion is NOT equal to volume overload. Overemphasis on the role of volume overload in pathogenesis of AHFS leads to mismanagement of SCAPE subtype. There are two distinct pathways that can result in pulmonary congestion in acute decompensated heart failure:
#1. Cardiac failure: Progressive worsening of cardiac function results in activation of neurohormonal mechanisms (e.g. RAAS) where excess volume can accumulate gradually and contributes to decompensated heart failure.
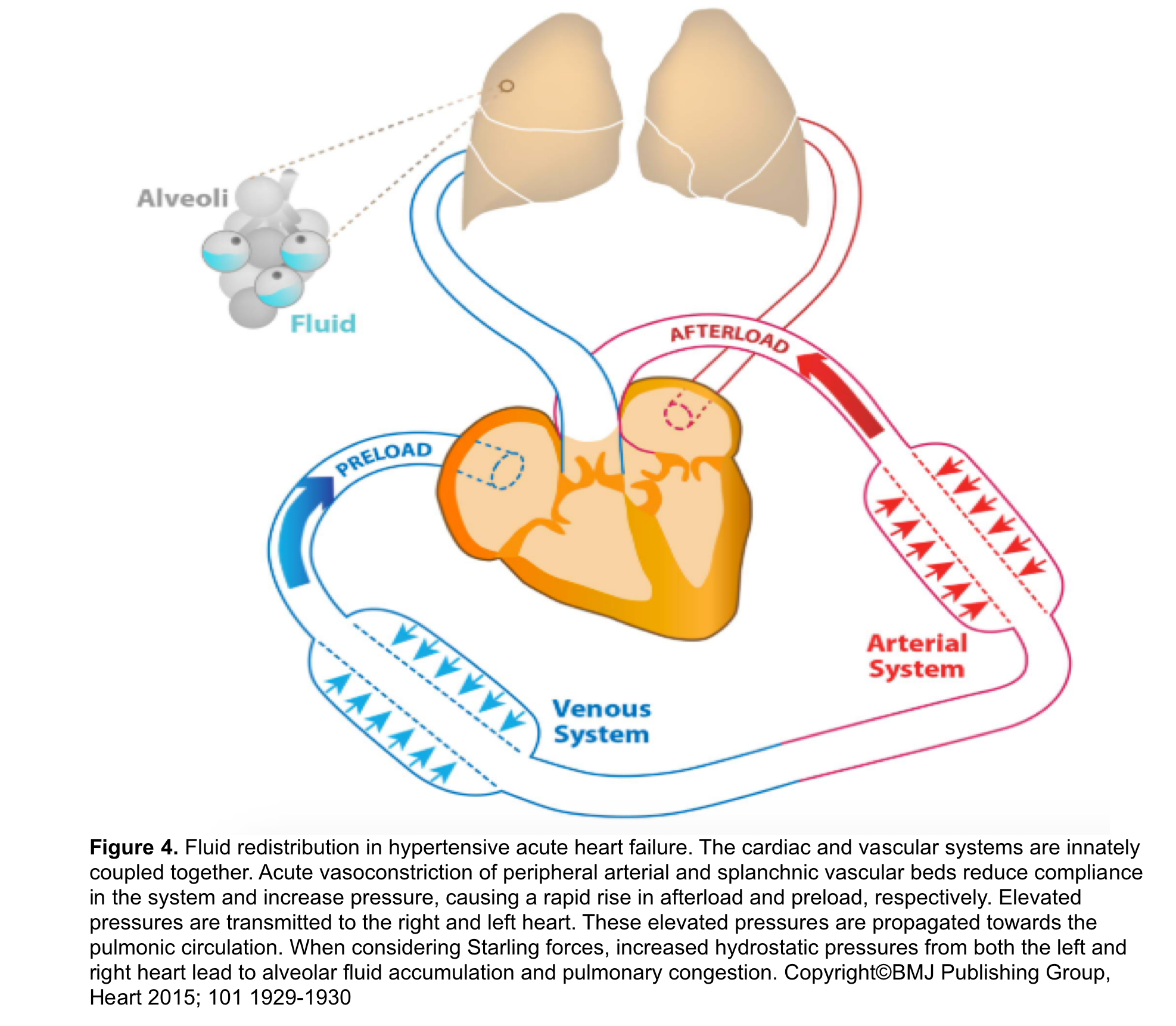
#2. Vascular failure: Results from an abrupt redistribution of fluid from the splanchnic and central circulation into the pulmonary vasculature rather than an accumulation of volume. This is the principal mechanism of pulmonary congestion in “SCAPE” (figure 4):
- Recent studies have shown that LVEDP can rise without precipitous change in body weight 8 9. Several vascular abnormalities including arterial stiffness, abnormal vascular compliance and peripheral vasoconstriction (high SNS tone 13 and dysregulated vascular tone due to ↓nitric oxide production) contribute to central “Redistribution of Volume”. This results in pulmonary venous congestion and edema 10 11 12.
The clinical observation that vasodilator treatment can improve dyspnea in SCAPE without significant diuresis has led to the concept that vascular mechanisms play pivotal role in pathophysiology of the disease and can lead to increased diastolic filling pressures in the setting of minimal total body volume changes.
Clinical feature and diagnosis
SCAPE is the extreme end of the spectrum of acute pulmonary edema. Rapid “Volume Redistribution” (from peripheral vascular system to pulmonary circulation) associated with sympathetic surge causes dramatic onset and rapid progression of dyspnea (minutes to hours) to life-threatening pulmonary edema, giving the emergency physicians a narrow time window to intervene and improve patient outcome.
History
The patient may or may not have a previous diagnosis of HF. Oftentimes patients have a history of poorly controlled hypertension 14 and poor adherence to medication. This condition may less often be triggered by atrial arrhythmias and ACS 6.
- History of recurrent episodes of SCAPE may be present.
Symptoms and signs
The core clinical findings include 22
- Acute onset of dyspnea (often ≦6 h).
- Hypoxemia (SpO2 ≤90%)
- Tachypnea (RR ≧30/min) and marked dyspnea.
- Hypertension (BP>160/100 and/or MAP>120 mmHg).
- Clinical features of sympathetic activation
- Diaphoresis 😓, pallor.
- Tachycardia.
- Agitation.
- Extensive bilateral rales on auscultation 3.
- POCUS shows diffuse B-lines.
Other classic symptoms and signs of AHFS may not be present (e.g. cardiomegaly, lower extremity edema, worsening exercise intolerance, weight gain, hepatic congestion, jugular venous distention) 15.
Diagnosis
Diagnosis of “SCAPE” is clinical and is not often a dilemma. However, keep in mind that any form of severe respiratory failure (see below) may lead to distress and hypertension. Hallmarks of SCAPE is rapid improvement with therapy.
- In uncertain situations, it is reasonable to initiate treatment for SCAPE while simultaneously investigating for other problems.
Investigation
ECG: Evidence of left atrial enlargement and LV hypertrophy is sensitive (non-specific) for chronic LV dysfunction.
- May suggest an acute tachydysrhythmia as the cause of HF or aid in the diagnosis of acute MI or likelihood of a prior MI.
- The presence of a left bundle branch block is a marker for diminished LV systolic function.
Bedside ultrasound (echocardiography): Bedside ultrasound will show diffuse B lines on exam. Cardiac evaluation often reveals diastolic dysfunction, and “IVC” may actually show fluid depletion.
- 👉Keep in mind that due to sympathetic surge and severe vasoconstriction, intravascular volume is redistributed centrally and accumulated within the lung interstitium and alveoli.
Labs
- CBC, comprehensive metabolic panel (poor perfusion can lead to electrolyte abnormalities and acute renal or hepatic insufficiency).
- Troponin: High values can lead to the diagnosis of an MI as a triggering event; note that more sensitive troponin assays will frequently be positive at the baseline in many patients with chronic HF 16 (more on this here).
- TSH if thyroid disease suspected.
- BNP levels are unhelpful (POCUS is a superior test 17) and it should be interpreted with caution in acute HF presentations. It can be elevated in a variety of other conditions.
Differential diagnosis
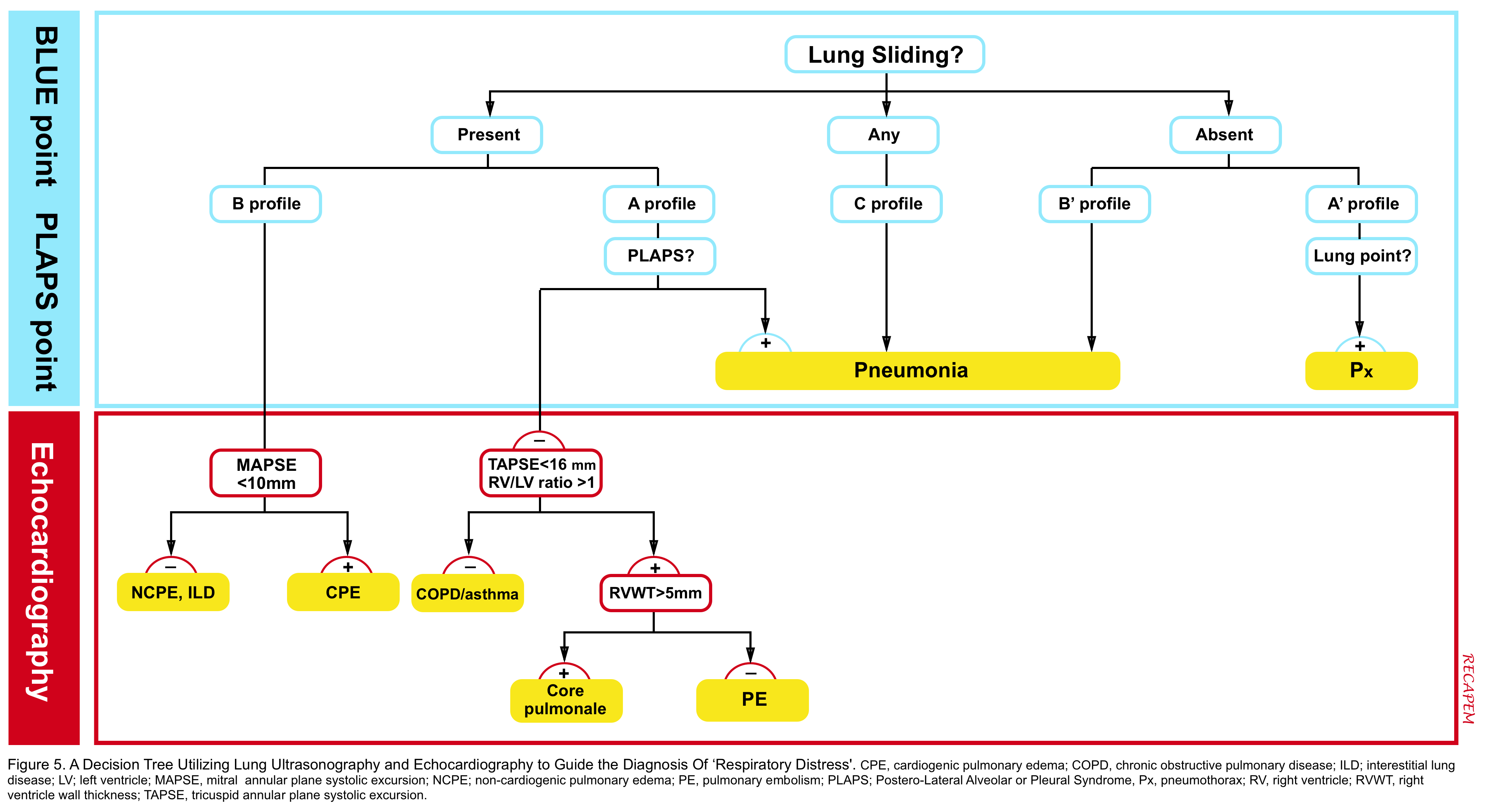
Patients with SCAPE present with undifferentiated respiratory distress (more on this here). However diagnosis is straightforward when history, clinical signs and symptoms are integrated and supported by other diagnostic findings in POCUS (see figure 5) and EKG.
Despite critically ill appearance of the patients and severe respiratory distress; they often respond rapidly to appropriate vasodilator medication and noninvasive ventilatory support.
Other conditions rarely may mimic clinical presentation of SCAPE and they must be excluded:
- Pulmonary embolism: Pre-test probability for thromboembolic disease and POCUS can be helpful (more on this here).
- Noncardiogenic pulmonary edema (NCPE): It is invariably associated with an underlying disease, which may or may not be readily apparent. The diagnosis of NCPE often depends on pretest probabilities: acute respiratory distress in a patient with possible source of infection (i.e. UTI) or pancreatitis should raise the strong possibility that the respiratory failure is due to NCPE.
- Cardiogenic pulmonary edema: Echocardiography can support diagnosis of CPE, if mitral annular plane systolic excursion (MAPSE) is < 10mm.
- Pneumonia: It has a more subacute course. More importantly systolic blood pressure is not usually skyrocketed.
- Asthma: Lung ultrasound is potentially helpful as it reveals absence of B-lines in asthma.
General approach to AHFS
General initial stabilization and management of patients with acute heart failure (AHFS) is shown below (figure 6).
Principles of management in patients with SCAPE
The hallmark of SCAPE is rapid deterioration, however aggressive treatment can rapidly reverse this process and will avoid intubation, ICU admission, and morbidity 18. The key principles of management strategy include
- Promptly apply positive pressure noninvasive ventilation.
- Aggressive control of hypertension (control the afterload).
- Close monitoring of patient’s clinical status.
1.Noninvasive ventilation
Immediately apply BiPAP. The provided end-expiratory positive pressure (EPAP) will reduce preload and afterload, exerting a physiologic effect to vasodilators and also improve oxygenation (recruitment).
- Start at a low pressure (e.g. IP 12 cm, EP 6 cm). Up-titrate IP/EP by 2cm H2O as tolerated, with a goal of increasing the expiratory pressure (e.g. titrate up to ~18 cm/15 cm).
- The maximal safe pressure is not precisely defined, but pressures above ~20 cm may increase the risk of gastric insufflation.
2. Aggressive control of hypertension
The keystone of management involves reducing the afterload (see pathophysiology of SCAPE above).
Blood pressure target: There is no well-defined BP goal, but a common target may be to reduce the systolic BP to <140-160 mmHg rapidly. However, the MAP or diastolic BP may actually be superior targets, since they are more closely correlated with systemic vascular resistance 23.
Agents: Vasodilators
- The most commonly used vasodilators are nitrate. Nitrates are the first line, with nitroglycerin being the most common. At moderate doses, nitroglycerin mostly affects the preload, but at high IV infusion doses ( >250 μg/min), arteriolar dilation occurs, which works to reduce afterload 19. Appropriately dosed nitro drip will turn off sympathetic surge of SCAPE patients.
- It is a safe agent with a short half-life (<10 minute), which makes it easy to titrate.
- Caution is warranted for the use of nitrates with low SBP, bradycardia, recent use of phosphodiesterase type 5 inhibitors (eg, sildenafil, tadalafil) within the past 48hrs and elevated intracranial pressure.
- Most importantly, nitroglycerin at these high doses should be titrated with the physician at the bedside. In cases of hypotension, stop or decrease the dose, because hypotension is usually transient.
- In case of refractory hypertension, other medications that can be used are
- Clevidipine: Start at 1-2 mg/h. Double q2min until BP begins approaching target, then titrate by smaller increments q5-10min. Dose range is 1-32 mg/h. Clevidipine is the preferred agent, due to its shorter half-life and stronger evidentiary basis in acute heart failure.
- Nicardipine: Start at 5 mg/hr. If BP is above target, increase by 2.5 mg/hr every 15-30 min, to a maximum rate of 15 mg/hour. Nicardipine has a longer half-life, so there is a risk of causing overshoot hypotension.
- ACE inhibitors and ARBs: can be used in refractory hypertension, if renal function allows. The main drawback is nephrotoxicity. These agents may not be ideal for patients with acute kidney injury, or multimorbid patients with numerous active medical problems.
- Enalaprilat IV (1.25 mg, may repeat q15min to a maximal dose of 5 mg) can be an option.
- ⚠️Contraindicated agent: Beta-blockers. Remember that beta-blockers will impair pump function, so they are generally contraindicated in the context of acute decompensated cardiogenic pulmonary edema (including SCAPE).
👉Nitroglycerin administration- High dose nitroglycerin regimen
- Rational: High dose nitroglycerin for SCAPE patients has been shown to be associated with lower rates of mechanical ventilation, improvement in blood pressure, shorter length of stay, and lower rates of ICU admission (moderate quality evidence) 20, although exact protocol may vary.
- Administration
- Preparation: Standard nitroglycerin mix is 200 mcg/ml (premixed solutions available), or you can mix 50mg of nitroglycerin with 250mL of 0.9% NS or 5% Dextrose. In order to give for example 400mcg/min for 2 minute, you need 4cc of this solution and give it over 2 minute.
- Loading dose: Start with an initial loading dose of IV nitroglycerine of ~600-1000 mcg (based on initial BP) 22. This may be provided in one of two ways:
- Maintenance infusion: Infusion may be initiated at a rate of ~100mcg/min 22.
- Titration: If BP isn’t controlled promptly (remains the same or increasing): up-titrate the infusion rate by 20mcg/minevery 10min till SBP starts decreasing. Note that very high doses (e.g. 800 mcg/min) may be required for limited periods of time, to break the cycle of progressive hypertension.
- Continue the appropriate dose of nitroglyceine until the resolution of symptms (any of two) 22:
- Decrease in RR by 25% of initial RR.
- RR <24/min
- SBP <140-160
- Subjective improvement of symptoms
- Refractory hypertension: If hypertension is refractory to 800 mcg/min nitroglycerine, then consider the addition of nicardipine, clevidipine, IV enalaprilat, captopril, and/or fentanyl.
- Weaning off nitroglycerine: Generally the SCAPE will start resolving rapidly (within minutes to a couple of hours), causing the BP to decrease. Watch the blood pressure closely and down-titrate the nitroglycerine infusion accordingly, to avoid hypotension.
- Reduce the NTG infusion rate by 20mcg/min every 10min, and stop.
💊Remember that sublingual nitroglycerin takes time to peak and has a bioavailability of around 30%-60%; which makes it unsuitable for such an emergent condition.
Diuretics
- Diuretics may have a role (if subsequent hemodynamic evaluation reveals volume overload) but are usually not necessary during the initial stage of resuscitation.
- Remember that SCAPE patient are not volume overloaded (often volume depleted) despite significant life-threatening pulmonary edema.
- Diuretic is not effective in initial phase of resuscitation for two reasons:
- First, severe vasoconstriction and poor renal perfusion hamper diuretic access to renal tubules for exerting its effects.
- Second, patients with SCAPE are often volume depleted. Overdiuresis can further contribute to volume depletion, and volume depletion can cause stress response and sympathetic surge.
- If subsequent hemodynamic evaluation (based on history, physical exam and POCUS) suggests volume overload, one can administer furosemide 40-80 mg IV.
3.Close monitoring of clinical status 👁🗨
If SCAPE is treated appropriately, it will break rapidly. The vicious spiral will stop and the patient will improve. The most obvious way to tell this is happening is often when the BP starts to drop precipitously. Management involves:
- Rapidly down-titrate the nitroglycerine infusion and BiPAP pressures (while watching for recurrent SCAPE).
- Consider any triggers of SCAPE and treat them (e.g. nonadherence to antihypertensives, volume overload).
Algorithm
RECAP:
- SCAPE patients frequently end up to be intubated and admitted to the ICU, if not appropriately managed. This requires understanding the pathophysiology of the disease.
- Congestion does not equal volume overload. Despite dramatic pulmonary edema, oftentimes they are volume depleted. The key pathophysiologic mechanism for pulmonary congestion is sympathetic overdrive and consequent central redistribution of volume.
- These patients respond rapidly to BiPAP and High dose nitroglycerin.
- Low-moderate dose nitroglycerin as well as diuretics do NOT work in these patients.
- High dose nitroglycerin turns off sympathetic surge and perfectly works in reducing afterload. Be prompt and aggressive and do not leave the room untill the patient becomes stabilized.
Going Further:
- Sympathetic Crashing Acute Pulmonary Edema (SCAPE) EMCrit
- Flash Pulmonary Edema (EM:RAP)
- Emergencies with a Side of Hypertension (EMCrit)
- Hypertensive emergency(IBCC)
- SCAPE (FOAMcast)
- Morning Report Pearls (CORE EM)
- HF, APE
Post peer reviewed by Mojtaba Chardoli.
The copyright of the header image: Eur Heart J Suppl, Volume 18,December 2016.
References
1. Rimoldi SF, Yuzefpolskaya M, Allemann Y, Messerli F. Flash pulmonary edema. Prog Cardiovasc Dis. 2009 Nov-Dec;52(3):249-59. doi: 10.1016/j.pcad.2009.10.002. PMID: 19917337
2.Weingart S. Emcrit Podcast 1, Sympathetic Crashing Acute Pulmonary Edema. [Last accessed on 2016 Oct 30]. Available from: http://www.emcrit.org.
3. Agrawal N, Kumar A, Aggarwal P, Jamshed N. Sympathetic crashing acute pulmonary edema. Indian J Crit Care Med. 2016;20(12):719-723. doi:10.4103/0972-5229.195710
4. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016 Aug;18(8):891-975. doi: 10.1002/ejhf.592. Epub 2016 May 20. PMID: 27207191
5. Arrigo M, Jessup M, Mullens W, et al. Acute heart failure. Nat Rev Dis Primers. 2020;6(1):16. Published 2020 Mar 5.
6. G. Micheal Felker and John R. Teerlink. Diagnosis and Management of Acute Heart Failure. In: Braunwald’s Heart Disease, A textbook of cardiovascular medicine. Eleventh edition. Philadelphia, PA: Elsevier
7. Sabbah HN. Pathophysiology of acute heart failure syndrome: a knowledge gap. Heart Fail Rev. 2017 Nov;22(6):621-639. doi: 10.1007/s10741-017-9651-2. PMID: 28952056
8. Zile MR, Bennett TD, St John Sutton M, Cho YK, Adamson PB, Aaron MF, Aranda JM Jr, Abraham WT, Smart FW, Stevenson LW, Kueffer FJ, Bourge RC. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008 Sep 30;118(14):1433-41. doi: 10.1161/CIRCULATIONAHA.108.783910. Epub 2008 Sep 15. PMID: 18794390
9. Fudim M, Hernandez AF, Felker GM. Role of Volume Redistribution in the Congestion of Heart Failure. J Am Heart Assoc. 2017 Aug 17;6(8):e006817. doi: 10.1161/JAHA.117.006817. PMID: 28862947; PMCID: PMC5586477
10. Viau DM, Sala-Mercado JA, Spranger MD, O’Leary DS, Levy PD. The pathophysiology of hypertensive acute heart failure. Heart. 2015 Dec;101(23):1861-7. doi: 10.1136/heartjnl-2015-307461. Epub 2015 Jun 29. PMID: 26123135
11.Cotter G, Metra M, Milo-Cotter O, Dittrich HC, Gheorghiade M. Fluid overload in acute heart failure–re-distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail. 2008 Feb;10(2):165-9. doi: 10.1016/j.ejheart.2008.01.007. PMID: 18279771
12. Miller WL. Fluid Volume Overload and Congestion in Heart Failure: Time to Reconsider Pathophysiology and How Volume Is Assessed. Circ Heart Fail. 2016 Aug;9(8):e002922. doi: 10.1161/CIRCHEARTFAILURE.115.002922. PMID: 2743683. doi:10.1038/s41572-020-0151-7.
13. Mattia Arrigo, John T. Parissis, Eiichi Akiyama, Alexandre Mebazaa, Understanding acute heart failure: pathophysiology and diagnosis, European Heart Journal Supplements, Volume 18, Issue suppl_G, December 2016, Pages G11–G18, https://doi.org/10.1093/eurheartj/suw044
14. Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med. 2006 Dec;119(12 Suppl 1):S3-S10. doi: 10.1016/j.amjmed.2006.09.011. PMID: 17113398.
15. Bistola V, Polyzogopoulos E, Ikonomidis I, Parissis J. Congestion in acute heart failure with reduced vs. preserved left ventricular ejection fraction: differences, similarities and remaining gaps. Eur J Heart Fail. 2018 Apr;20(4):748-750. doi: 10.1002/ejhf.1115. Epub 2017 Dec 18. PMID: 29251810
16. Kristian Thygesen, Joseph S Alpert, Allan S Jaffe, Bernard R Chaitman, Jeroen J Bax, David A Morrow, Harvey D White, ESC Scientific Document Group, Fourth universal definition of myocardial infarction (2018), European Heart Journal, Volume 40, Issue 3, 14 January 2019, Pages 237–269, https://doi.org/10.1093/eurheartj/ehy462
17. Pivetta E, Goffi A, Nazerian P, Castagno D, Tozzetti C, Tizzani P, Tizzani M, Porrino G, Ferreri E, Busso V, Morello F, Paglieri C, Masoero M, Cassine E, Bovaro F, Grifoni S, Maule MM, Lupia E; Study Group on Lung Ultrasound from the Molinette and Careggi Hospitals. Lung ultrasound integrated with clinical assessment for the diagnosis of acute decompensated heart failure in the emergency department: a randomized controlled trial. Eur J Heart Fail. 2019 Jun;21(6):754-766. doi: 10.1002/ejhf.1379. Epub 2019 Jan 28. PMID: 30690825.
18. Mallick, Piyush; Upadhyay, Surjya; Senthilnathan, TS; Waleed, El Matit; Weingart, Scott 258: A PROTOCOL OF BOLUS-DOSE NITROGLYCERIN AND NON-INVASIVE VENTILATION TO AVERT INTUBATION IN EMERGENCY DEPARTMENT ACUTE PULMONARY EDEMA, Critical Care Medicine: December 2011 – Volume 39 – Issue 12 – p 67 doi: 10.1097/01.ccm.0000408627.24229.88
19. Levy P, Compton S, Welch R, Delgado G, Jennett A, Penugonda N, Dunne R, Zalenski R. Treatment of severe decompensated heart failure with high-dose intravenous nitroglycerin: a feasibility and outcome analysis. Ann Emerg Med. 2007 Aug;50(2):144-52. doi: 10.1016/j.annemergmed.2007.02.022. Epub 2007 May 23. PMID: 17509731
20. Wang K, Samai K. Role of high-dose intravenous nitrates in hypertensive acute heart failure. Am J Emerg Med. 2020 Jan;38(1):132-137. doi: 10.1016/j.ajem.2019.06.046. Epub 2019 Jun 25. PMID: 31327485.
21. Wilson SS, Kwiatkowski GM, Millis SR, Purakal JD, Mahajan AP, Levy PD. Use of nitroglycerin by bolus prevents intensive care unit admission in patients with acute hypertensive heart failure. Am J Emerg Med. 2017 Jan;35(1):126-131. doi: 10.1016/j.ajem.2016.10.038. Epub 2016 Oct 18. PMID: 27825693.
22. Mathew R, Kumar A, Sahu A, Wali S, Aggarwal P. High-Dose Nitroglycerin Bolus for Sympathetic Crashing Acute Pulmonary Edema: A Prospective Observational Pilot Study. J Emerg Med. 2021 Sep;61(3):271-277. doi: 10.1016/j.jemermed.2021.05.011. Epub 2021 Jun 30. PMID: 34215472.
23. Harrison N, Pang P, Collins S, Levy P. Blood Pressure Reduction in Hypertensive Acute Heart Failure. Curr Hypertens Rep. 2021 Feb 20;23(2):11. doi: 10.1007/s11906-021-01127-8. PMID: 33611627





Add comment