November 29, 2022 by Shahriar Lahouti. Last update June 24, 2024.
CONTENTS
- Preface
- Case scenario
- Pathophysiology
- Predisposing factors
- Etiology
- Clinical presentation
- Echocardiography
- Management
- Case revisited
- Media
- Going further
- References
Preface
Left ventricular outflow tract obstruction (LVOTO) is the third most frequent cause of unexplained hypotension, and it should be considered in any hypotensive critically ill patients *. The current trend in treating hypotension in critical settings is to place a greater emphasis on inotropes and vasopressor support and less on fluid resuscitation in order to limit the potential harm from fluid overload. This combination may trigger LVOTO in susceptible patients. In fact, LVOTO will often respond to various interventions in the opposite fashion than might be expected for most patients in the shock state. It has been shown that dynamic LVOTO has been frequently underdiagnosed in critical care settings *. Unrecognized LVOTO may lead to a spiral of inappropriate vasopressor/inotropic use resulting in severe hypotension, collapse, and death.
Case scenario
A 69-year-old obese man with a history of hypertension, diabetes, and stage III chronic kidney disease was admitted to the hospital for urosepsis. Two days into his admission, he was found to be unresponsive, with a heart rate of 122 bpm, and a blood pressure of 60/35 mm Hg. He rapidly progressed to pulseless electrical activity (PEA). CPR was performed with the return of spontaneous circulation. He was emergently intubated and norepinephrine was started with dose escalation rapidly over the next hour due to continued hypotension.
- Bedside echo: A small, underfilled, hyperdynamic left ventricle, but no evidence of RV strain.
- Lung ultrasound: Bilateral diffuse B-lines.
- IVC: poor window.
👉How will you evaluate the refractory shock in critical patients? (see below)
Pathophysiology of LVOT obstruction
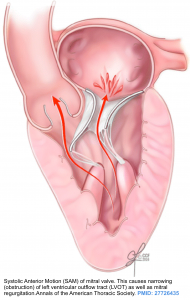
In patients with a small left ventricular chamber size (e.g. due to hypertrophy), the LV outflow tract is narrowed and the blood flowing through this narrowed segment has a high velocity. Fast-flowing blood through the LV outflow tract will pull the mitral valve leaflets toward the septum (anteriorly) due to the Venturi effect *. This is known as as Systolic Anterior Motion (SAM) of the mitral valve and contributes to dynamic obstruction of LVOT.
- SAM is defined as the anterior excursion of one or both mitral valve leaflets into the LVOT during systole (figure below).
- This has two hemodynamic consequences:
- Left Ventricular Outflow Tract Obstruction (LVOTO)
- Anterior motion of the mitral valve during systole can cause an obstruction of the LV outflow tract. This will impair systolic ejection of the blood into the aorta and will reduce cardiac output; which can potentially cause cardiogenic shock.
- Mitral Regurgitation (MR)
- The “systolic anterior motion of mitral valve” causes mitral regurgitation as the mitral valve is opened during systole.
- Opening of mitral valve leaflets (due to “SAM” of leaflets) will cause a posteriorly directed mitral regurgitation (MR).
- MR will cause elevated left atrial pressure and can contribute to cardiogenic pulmonary edema.
- The “systolic anterior motion of mitral valve” causes mitral regurgitation as the mitral valve is opened during systole.
- Left Ventricular Outflow Tract Obstruction (LVOTO)
Predisposing factors
Dynamic LVOTO is more common in the elderly, females, and patients with hypertension, diabetes, and chronic vascular disease. This results from the interplay of multiple factors including anatomic and physiologic conditions *.
▪️Anatomic predisposing factors
- Left ventricular hypertrophy (esp. anterobasal hypertrophy), small left ventricle size, sigmoid septum.
- Elongated/floppy mitral valve leaflets *.
- A combination of apical hypokinesis and hypercontractility of the base of the heart will predispose to the generation of dynamic LVOTO. This condition can be seen in patients with a classic form of takotsubo cardiomyopathy and LAD artery ischemia (see below).
▪️Physiologic predisposing factors
- Generally, LVOTO is a dynamic phenomenon and varies according to the loading conditions. Any factors that can cause the left ventricle to be hyperkinetic and underfilled (thereby increasing the velocity of blood ejected through the LV outflow tract and reducing the end-systolic chamber size) can perpetuate LVOTO.
- Factors that increase LVOTO include *
- ↓Preload: Anemia, dehydration, sepsis (relative hypovolemia), diuretics, vasodilators e.g. nitrates.
- ↓Afterload: vasodilators, sepsis, and some anesthetic medications.
- ↑LV inotropy (contractility): High endogenous sympathetic tone, exogenous inotropes *.
- Tachycardia (reduced diastolic filling time).
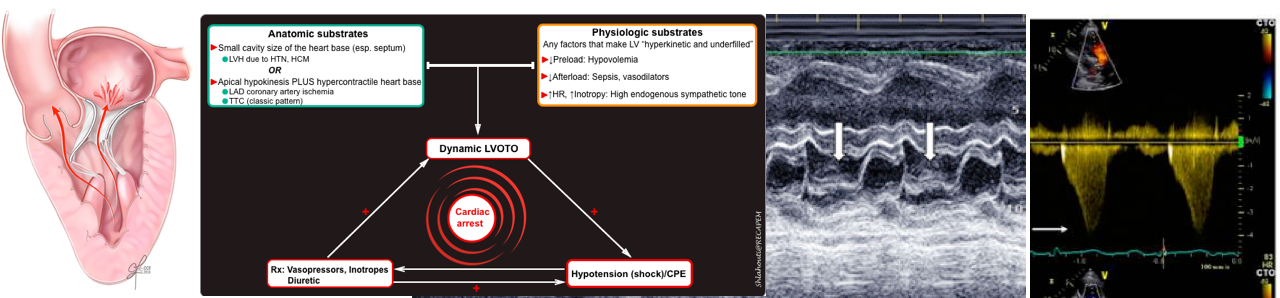
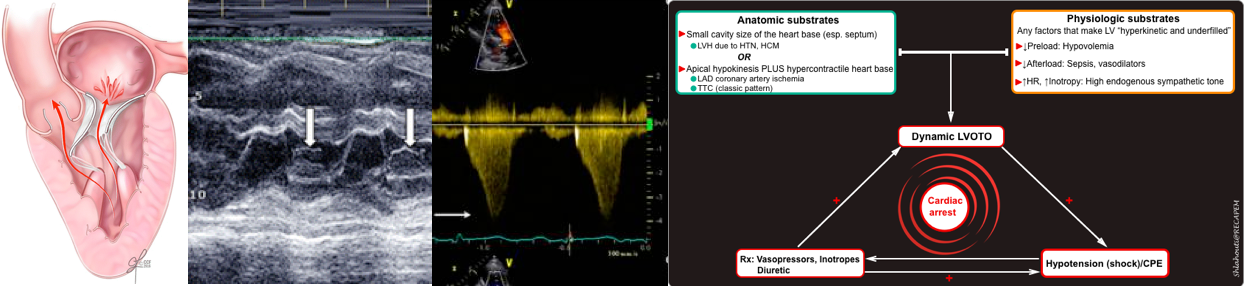
Vicious spirals of dynamic LVOTO
Dynamic LVOTO once established can cause deteriorating hemodynamic status to pulseless electrical activity.
- If unrecognized, high doses of vasopressor use in patients with (resistant) shock, can lead to worsening hemodynamics.
- Vasopressors and inotropes make the left ventricle hyperkinetic and underfilled (esp. in the presence of hypovolemia), thereby increasing the velocity of blood ejected through the LVOT and reducing the end-systolic chamber size).
- Worsening LVOTO severity promotes further hemodynamics derangement leading to pulseless electrical activity and cardiac arrest.
Etiology
LVOTO has been classically described in patients with hypertrophic cardiomyopathy, however, it has been reported in other conditions.
- Hypertensive cardiomyopathy *
- Hypertrophic cardiomyopathy (HCM)
- Dynamic LVOTO is typically observed in the setting of hypertrophic cardiomyopathy *.
- Approximately one-third of patients with HCM have LVOT obstruction at rest.
- One-third of patients have provokable obstruction via maneuvers that change loading conditions (e.g. amyl nitrite, dobutamine, or the Valsalva maneuver *).
- The remaining third have left ventricular hypertrophy with neither baseline nor provokable obstruction *,*.
- Dynamic LVOTO is typically observed in the setting of hypertrophic cardiomyopathy *.
- Takotsubo cardiomyopathy (TTC)
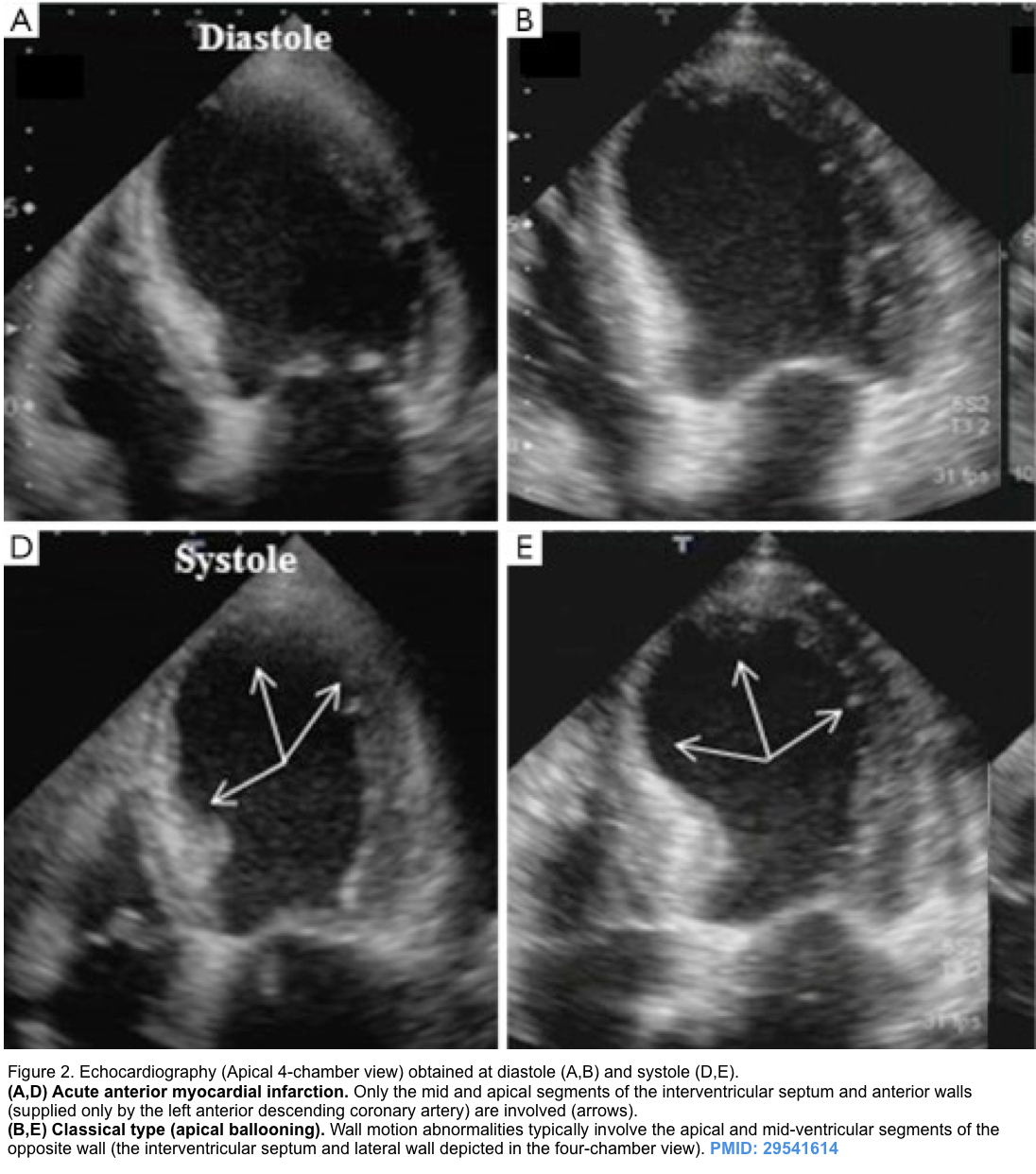
- LVOTO can occur in the classic form of Takotsubo cardiomyopathy with apical hypokinesis (apical ballooning) and hypercontractility of the base of the heart (figure 2 below) *,*.
- Apical hypokinesis causes septal angulation which, combined with basal hyperkinesis, causes LVOTO.
- Takotsubo cardiomyopathy is complicated by LVOTO in approximately 20% of cases *,*. Associated risk factors include:
- Older age, septal bulging, SAM-induced mitral regurgitation, and hemodynamic instability.
- LVOTO can occur in the classic form of Takotsubo cardiomyopathy with apical hypokinesis (apical ballooning) and hypercontractility of the base of the heart (figure 2 below) *,*.
- Left anterior descending (LAD) coronary artery ischemia with apical hypokinesis
- Distributive shock with volume depletion
- A combination of profuse vasodilation and high sympathetic tone can cause dynamic LVOTO, especially in patients with septic shock *,*,*.
Clinical presentation
- Syncope, dyspnea, hypotension, and cardiogenic shock.
- Cardiac arrest {pulseless electrical activity (PEA)}
- Vasopressor refractory shock
- Inotropes increase the velocity of the blood flow through the LVOT, which will worsen the anterior mitral motion.
- Diuretic refractory cardiogenic pulmonary edema (CPE)
- Diuresis reduces the diastolic volume of the left ventricle. This moves the mitral valve closer to the LVOT, worsening obstruction.
Echocardiography
Diagnosis of dynamic LVOTO should be suspected in an appropriate clinical setting. Bedside echo plays an essential role in confirming the diagnosis and excluding other possibilities of hemodynamic deterioration in critical patients.
- Left ventricular hypertrophy with small chamber size.
- Hyperkinetic LV with near obliteration of the left ventricular cavity during systole (i.e. a pathologically elevated ejection fraction). More on hyperdynamic LV here.
- Mitral regurgitation (MR)
- MR is generally present, and it is typically directed into the posterolateral left atrium.
- In contrast to posteriorly directed MR, the presence of a central or anteriorly directed MR jet suggests the presence of intrinsic mitral valve disease.
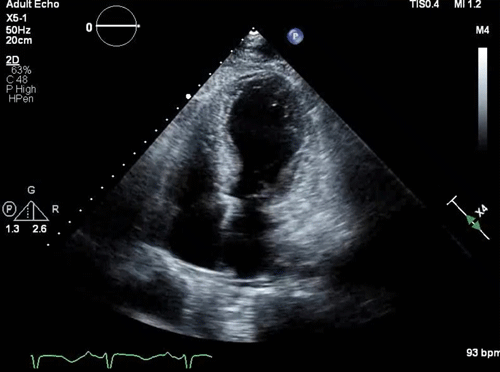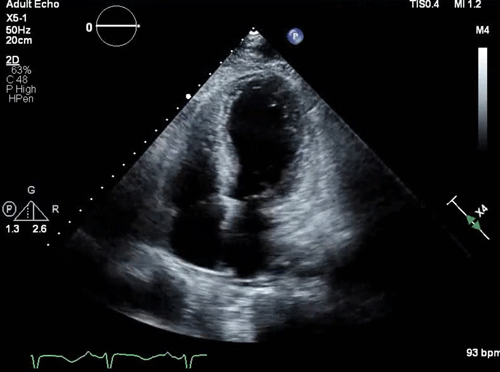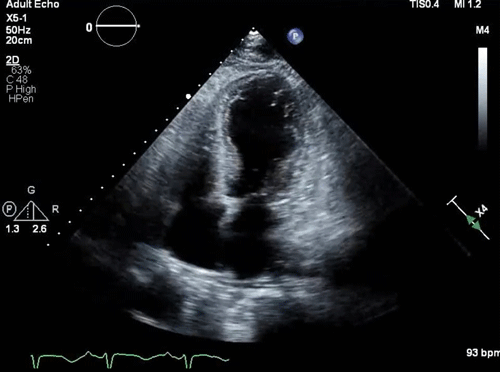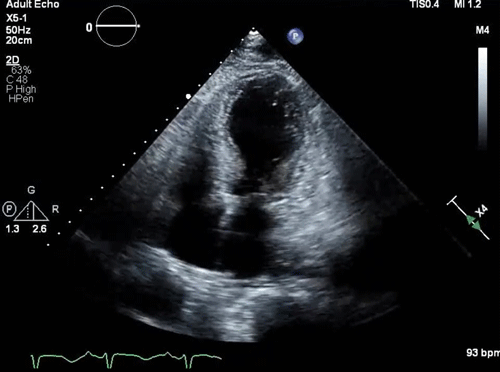
- Systolic anterior motion of the mitral valve
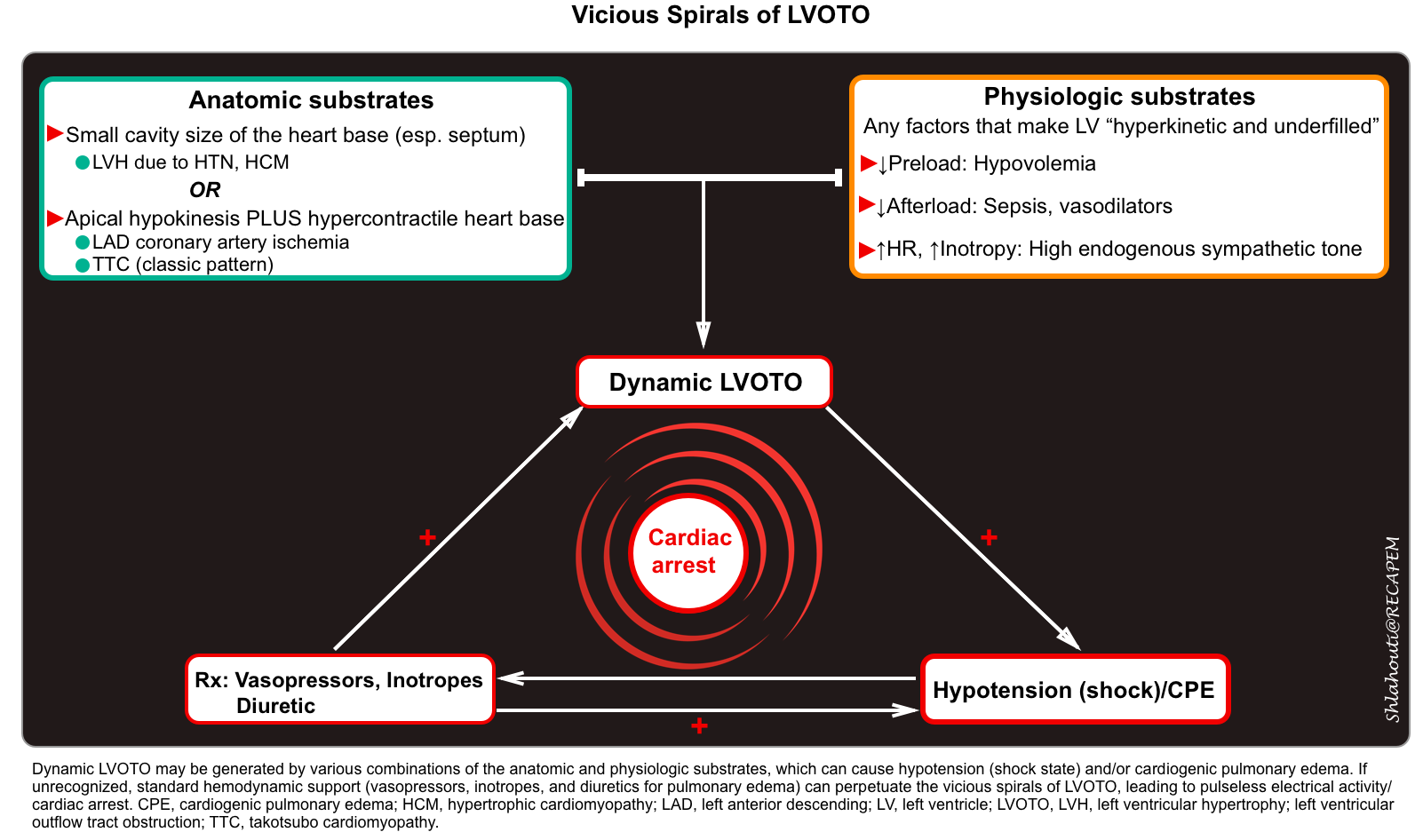
- During systole, the mitral valve leaflets are tugged toward the septum (video clip below).
- M-mode parasternal long-axis echo can confirm the LVOT obstruction (figure 3 below).
- Takotsubo pattern (apical dilation with basal hyperkinesis, shown below).
Parasternal long-axis view: a small, underfilled, hyperdynamic left ventricle with systolic anterior motion (SAM) of the anterior mitral valve leaflet into the left ventricle outflow tract (LVOT) during systole (a zoom-in view shows the “SAM” of the anterior mitral valve leaflet).
Takotsubo pattern (apical dilation with basal hyperkinesis)
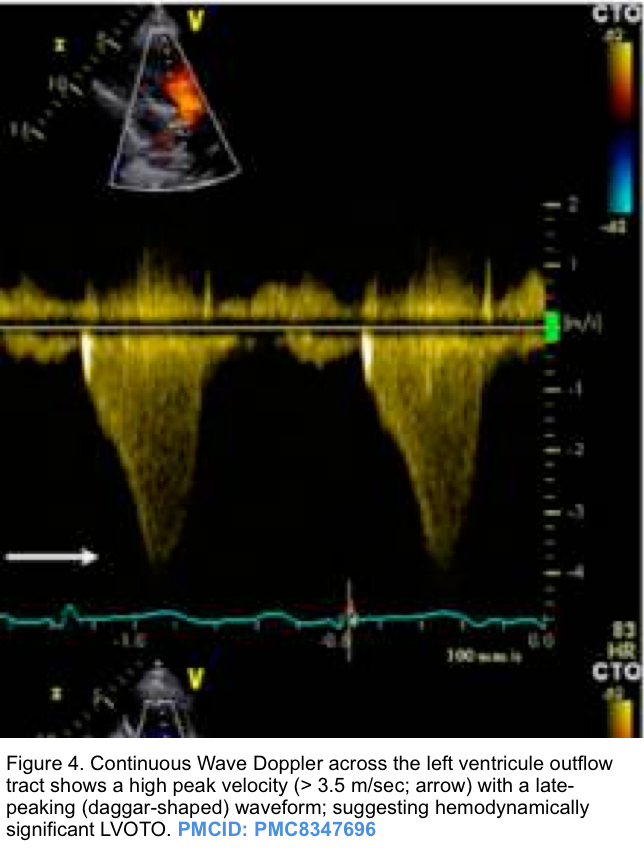
- The hallmark of dynamic LVOTO is a high-velocity, late-peaking continuous-wave Doppler signal on echocardiographic examination through the LV outflow tract (described as “dagger-shaped”, figure 4 below).
- The late-peaking nature may help differentiate this from aortic stenosis, which increases velocity with a more symmetric appearance.
- The maximal pressure gradient can be calculated based on the modified Bernoulli equation (∆P = 4V2).
- LVOT gradient >30 mm Hg (2.7 m/s) is considered pathologically elevated *.
- LVOT gradient >50 mm Hg (3.5 m/s) is severely elevated.
Management
Principles of management
▪️Medication/Intervention to Avoid
- ⚠️Vasodilators
- Dihydropyridine calcium channel blockers (e.g. amlodipine), nitrates, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers *.
- These can produce a fall in peripheral resistance with an increase in LVOTO and filling pressures, thereby resulting in hypotension and/or worsening of heart failure symptoms.
- Dihydropyridine calcium channel blockers (e.g. amlodipine), nitrates, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers *.
- ⚠️Inotropes: Dobutamine, epinephrine.
- ⚠️Diuretics
- By reducing preload, diuretics can result in reduced LV filling, a smaller LV chamber, and therefore greater LVOT obstruction.
- ⚠️Intra-aortic balloon pump (which reduces afterload).
Management of LVOTO in patients with hypotension/cardiogenic shock
- Pure vasoconstrictors (phenylephrine, vasopressin)*
- Vasoconstriction reduces LVOTO via several mechanisms, including ↑afterload, ↑preload, and mild reflexive bradycardia, which improves ventricular filling and increases ventricular volume.
- Phenylephrine may selectively improve vascular tone and reduce LVOTO *. It is more convenient, since it has a shorter half-life, allowing it to be more titratable.
- Norepinephrine primarily functions as a vasoconstrictor, so it shouldn’t be a strong inducer of LVOTO. However, case reports describe norepinephrine causing LVOTO *.
- Fluid administration
- Fluid resuscitation will improve preload and ventricular filling in hypovolemic patients.
- Using vasopressors before addressing intravascular volume deficits in critically ill patients can increase contractility in a volume-depleted and small left ventricle, stimulating the onset of LVOT obstruction.
- Fluid resuscitation will improve preload and ventricular filling in hypovolemic patients.
- Beta-blockers
- Beta-blockers may decrease LVOTO, thereby improving cardiac output and blood pressure by several mechanisms including ↓heart rates (permitting improved LV filling) and a negative inotropic effect both of which alleviate LVOTO.
- In hemodynamically unstable patients, it may be safest to use a short-acting agent e.g. an esmolol infusion, with gradual up-titration and careful monitoring of hemodynamic effects *.
Case revisited
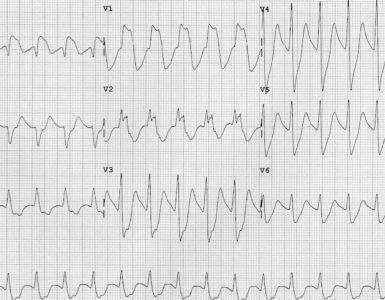
▪️Bedside echo is an essential tool to assess the underlying cause(s) of shock (more on this 👉 📖).
▪️In patients with refractory shock, evaluation should focus on identifying the primary cause and reversible secondary contributors, such as hypovolemia, pump failure, or obstruction causing shock * (more on this here).
- In this patient, a small, underfilled hyperdynamic LV without evidence of RV strain was seen. The algorithmic approach to patients with hyperdynamic LV in patients with shock is shown in the figure below (more on this 👉 📖).
-
- Echocardiography using M-mode in parasternal long axis view confirmed LVOTO in our patient.
▪️Patient hemodynamics stabilized following discontinuation of norepinephrine and initiation of vasopressin and esmolol infusion.
Media
Going further
- Approach to hyperdynamic left ventricle in patients in shock state
- Transient ‘SAM’ of the Mitral Valve Leaflet in a Critical Care Patient with a Structurally Normal Heart
- Dynamic left ventricular outflow tract obstruction (IBCC)
References
1. Sobczyk D. Dynamic left ventricular outflow tract obstruction: underestimated cause of hypotension and hemodynamic instability. J Ultrason. 2014 Dec;14(59):421-7. doi: 10.15557/JoU.2014.0044. Epub 2014 Dec 30.
2. Chockalingam A, Dorairajan S, Bhalla M, Dellsperger KC. Unexplained hypotension: the spectrum of dynamic left ventricular outflow tract obstruction in critical care settings. Crit Care Med. 2009 Feb;37(2):729-34. doi: 10.1097/CCM.0b013e3181958710.
3. Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy. Lancet. 2017 Mar 25;389(10075):1253-1267. doi: 10.1016/S0140-6736(16)31321-6. Epub 2016 Nov 30. Erratum in: Lancet. 2017 Mar 25;389(10075):1194.
4. Manabe S, Kasegawa H, Arai H, Takanashi S. Management of systolic anterior motion of the mitral valve: a mechanism-based approach. Gen Thorac Cardiovasc Surg. 2018 Jul;66(7):379-389. doi: 10.1007/s11748-018-0915-0. Epub 2018 Apr 3.
5. Slama M, Tribouilloy C, Maizel J. Left ventricular outflow tract obstruction in ICU patients. Curr Opin Crit Care. 2016 Jun;22(3):260-6. doi: 10.1097/MCC.0000000000000304.
6. Mingo S, Benedicto A, Jimenez MC, Pérez MA, Montero M. Dynamic left ventricular outflow tract obstruction secondary to catecholamine excess in a normal ventricle. Int J Cardiol. 2006 Oct 10;112(3):393-6. doi: 10.1016/j.ijcard.2005.07.075. Epub 2005 Nov 14.
8. Kuroda K, Kato TS, Amano A. Hypertensive cardiomyopathy: A clinical approach and literature review. World J Hypertens 2015; 5(2): 41-52 [DOI: 10.5494/wjh.v5.i2.41]
9. Yoshida N, Miyoshi T, Ninomaru T, Nagamatsu Y, Tamada N, Hiranuma N, Sasaki Y, Kitamura A, Kanda G, Kobayashi N, Nakagiri K, Fujii T. Left ventricular outflow tract obstruction caused by massive mitral annular calcification in a patient with hypertensive heart disease. J Cardiol Cases. 2015 Jun 12;12(3):87-90. doi: 10.1016/j.jccase.2015.05.008.
10. Kelshiker MA, Mayet J, Unsworth B, Okonko DO. Basal septal hypertrophy. Curr Cardiol Rev. 2013 Nov;9(4):325-30. doi: 10.2174/1573403×09666131202125424.
11. Authors/Task Force members, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014 Oct 14;35(39):2733-79. doi: 10.1093/eurheartj/ehu284. Epub 2014 Aug 29.
12. Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013 Jan 19;381(9862):242-55. doi: 10.1016/S0140-6736(12)60397-3. Epub 2012 Aug 6.
13. Marian AJ, Braunwald E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ Res. 2017 Sep 15;121(7):749-770. doi: 10.1161/CIRCRESAHA.117.311059.
14. Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002 Mar 13;287(10):1308-20. doi: 10.1001/jama.287.10.1308.
15.Di Vece D, Silverio A, Bellino M, Galasso G, Vecchione C, La Canna G, Citro R. Dynamic Left Intraventricular Obstruction Phenotype in Takotsubo Syndrome. J Clin Med. 2021 Jul 22;10(15):3235. doi: 10.3390/jcm10153235.
16. Conradi PM, van Loon RB, Handoko ML. Dynamic left ventricular outflow tract obstruction in Takotsubo cardiomyopathy resulting in cardiogenic shock. BMJ Case Rep. 2021 Mar 24;14(3):e240010. doi: 10.1136/bcr-2020-240010.
17. De Backer O, Debonnaire P, Gevaert S, Missault L, Gheeraert P, Muyldermans L. Prevalence, associated factors and management implications of left ventricular outflow tract obstruction in takotsubo cardiomyopathy: a two-year, two-center experience. BMC Cardiovasc Disord. 2014 Oct 22;14:147. doi: 10.1186/1471-2261-14-147.
18. attar Y, Siew KSW, Connerney M, Ullah W, Alraies MC. Management of Takotsubo Syndrome: A Comprehensive Review. Cureus. 2020 Jan 3;12(1):e6556. doi: 10.7759/cureus.6556.
19. hockalingam A, Tejwani L, Aggarwal K, Dellsperger KC. Dynamic left ventricular outflow tract obstruction in acute myocardial infarction with shock: cause, effect, and coincidence. Circulation. 2007 Jul 31;116(5):e110-3. doi: 10.1161/CIRCULATIONAHA.107.711697.
20. Ozaki K, Okubo T, Hagiya K, Kubota N, Tsuchida K, Takahashi K, Oda H, Minamino T. Unstable angina complicated with dynamic left ventricular outflow tract obstruction. J Cardiol Cases. 2021 Feb 12;23(4):181-188. doi: 10.1016/j.jccase.2021.01.013.
21. Villareal RP, Achari A, Wilansky S, Wilson JM. Anteroapical stunning and left ventricular outflow tract obstruction. Mayo Clin Proc. 2001 Jan;76(1):79-83. doi: 10.4065/76.1.79.
22. Dugar S, Latifi M, Moghekar A, Duggal A. All Shock States Are Not the Same. Systolic Anterior Motion of Mitral Valve Causing Left Ventricular Outflow Tract Obstruction in Septic Shock. Ann Am Thorac Soc. 2016 Oct;13(10):1851-1855. doi: 10.1513/AnnalsATS.201604-288CC.
23. Balik M, Novotny A, Suk D, Matousek V, Maly M, Brozek T, Tavazzi G. Vasopressin in Patients with Septic Shock and Dynamic Left Ventricular Outflow Tract Obstruction. Cardiovasc Drugs Ther. 2020 Oct;34(5):685-688. doi: 10.1007/s10557-020-06998-8.
24. Slama M, Tribouilloy C, Maizel J. Left ventricular outflow tract obstruction in ICU patients. Curr Opin Crit Care. 2016 Jun;22(3):260-6. doi: 10.1097/MCC.0000000000000304.
25. Afonso LC, Bernal J, Bax JJ, Abraham TP. Echocardiography in hypertrophic cardiomyopathy: the role of conventional and emerging technologies. JACC Cardiovasc Imaging. 2008 Nov;1(6):787-800. doi: 10.1016/j.jcmg.2008.09.002. Epub 2008 Nov 18.
26. Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, Kimmelstiel C, Kittleson M, Link MS, Maron MS, Martinez MW, Miyake CY, Schaff HV, Semsarian C, Sorajja P. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020 Dec 22;142(25):e558-e631. doi: 10.1161/CIR.0000000000000937. Epub 2020 Nov 20. Erratum in: Circulation. 2020 Dec 22;142(25):e633.
27. Chockalingam A, Tejwani L, Aggarwal K, Dellsperger KC. Dynamic left ventricular outflow tract obstruction in acute myocardial infarction with shock: cause, effect, and coincidence. Circulation. 2007 Jul 31;116(5):e110-3. doi: 10.1161/CIRCULATIONAHA.107.711697.
28. Pablo CR, David AO, Carlos V, Álvaro A, Williams H, Carolina I, Marta M, Leonor NM, Ignacio A, San Román A. Beta Blockers as Salvage Treatment in Refractory Septic Shock Complicated With Dynamic Left Ventricular Outflow Tract Obstruction: A Rare Case Presentation. J Investig Med High Impact Case Rep. 2021 Jan-Dec;9:23247096211056491. doi: 10.1177/23247096211056491.




Add comment