August 18, 2024, by Shahriar Lahouti. Last Update 15 September 2025.
CONTENTS
- Preface
- Diagnosis
- Resuscitation
- General principles
- Airway management
- Neurologic toxicity
- Respiratory failure
- Cardiovascular toxicity
- Hyperthermia
- Resuscitation antidote
- Considerations
- Risk assessment
- General Management
- Appendix
- Going further
- References
Preface
Critical poisoning refers to intoxicated patients in cardiac arrest, refractory shock, or other conditions posing an imminent threat of cardiac arrest *. Importantly, drug poisoning can produce a wide range of clinical presentations and may occur in isolation or with other pathology (e.g., trauma, infection). In general, diagnosis and treatment of intoxicated critical patients should proceed simultaneously while resuscitation is ongoing. Further management should be directed to providing supportive care, which is the cornerstone of the treatment of the poisoned patient, preventing poison absorption, and, when applicable, using antidotes and enhanced elimination techniques.
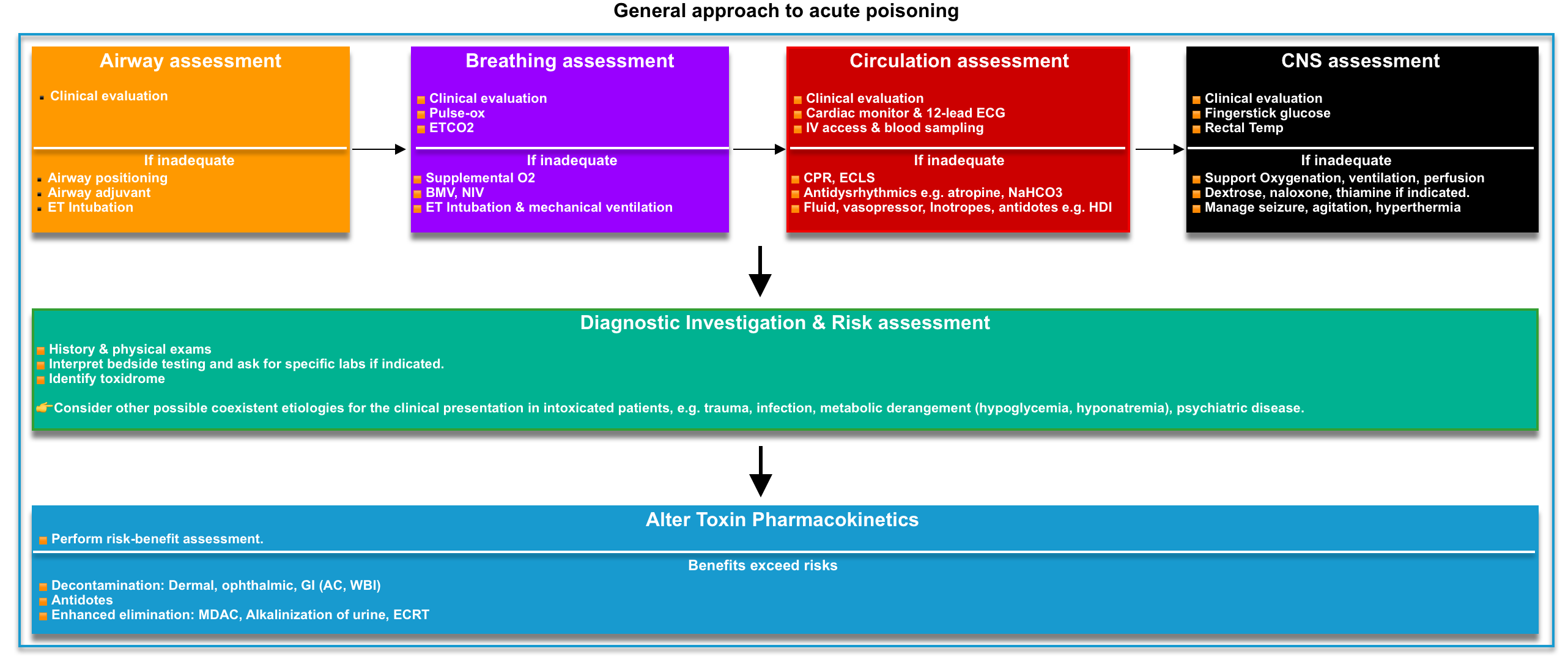
Acute poisoning is a dynamic medical illness, and these patients are a heterogeneous group. Management of an individual patient requires more than an understanding of the agent ingested. A highly organized approach (figure below) is essential if the emergency physician is to ensure the effective delivery of time-critical interventions while at the same time devising a management plan tailored to the individual patient’s needs *.
Diagnosis
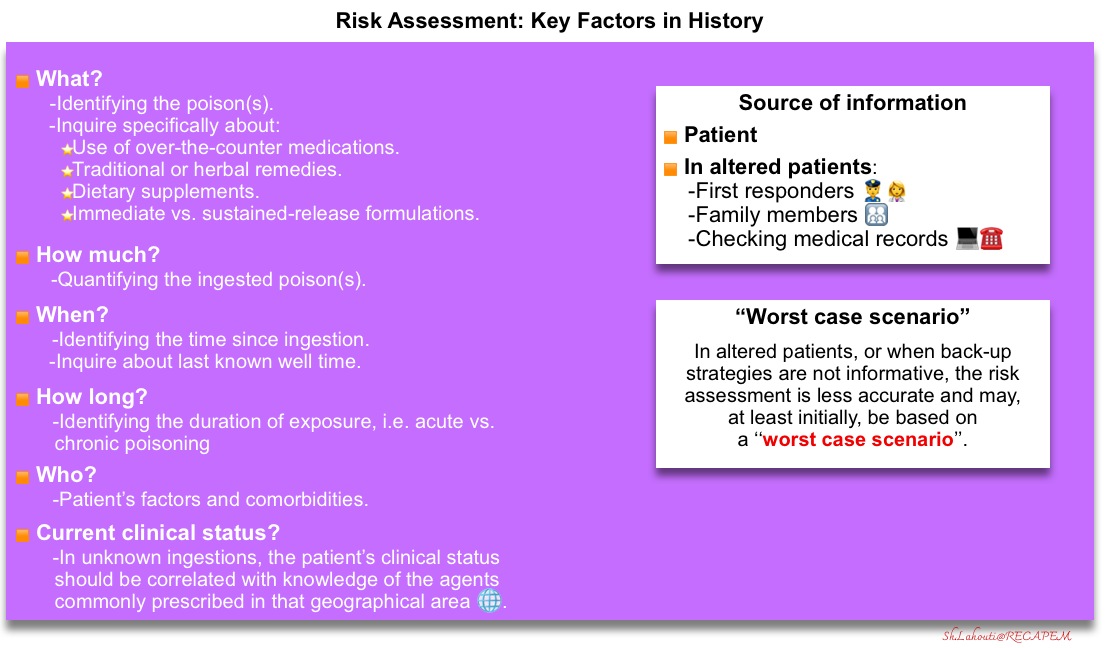
A brief history, initial screening examination, and investigations should be performed on all patients to identify immediate measures required to stabilize the patients with critical poisoning and prevent deterioration of these patients (more on Risk Assessment below).
History & physical exams

- Although history is the source of the most helpful information for identifying the etiology of poisoning, it is notoriously unreliable when provided by a patient following intentional ingestion *.
- The key elements of history include:
- Identifying the poison (what?).
- Look for medication lists and medical records.
- Inquire specifically about the use of over-the-counter medications, traditional or herbal remedies, and dietary supplements.
- Immediate vs. sustained-release formulations?
- Look for medication lists and medical records.
- Timing (when?)
- The duration (how long?), i.e., acute vs. chronic poisoning.
- The amount of exposure (how much?).
- Patient factors (who?)
- Identifying the poison (what?).

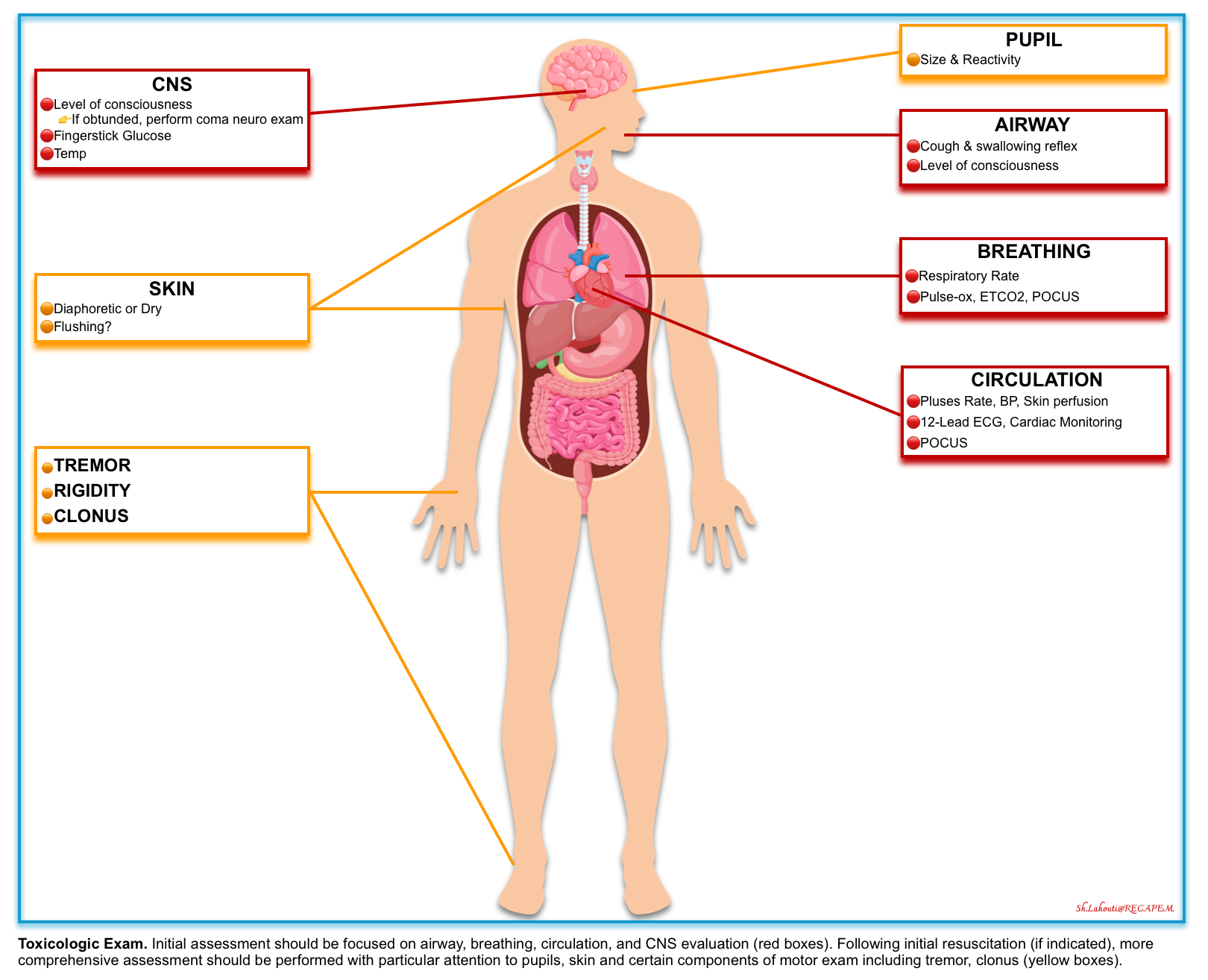
- A brief initial screening examination should be performed on all patients to identify immediate measures required to stabilize and prevent deterioration of the patient. These focused assessments (figure below) include:
- Airway
- Mental status, pupil size, and reactivity.
- Vital signs
- Skin temperature and moisture
- Expose the patient completely and look for signs of trauma, drug use (e.g., needle tracks), infection, or extremity swelling
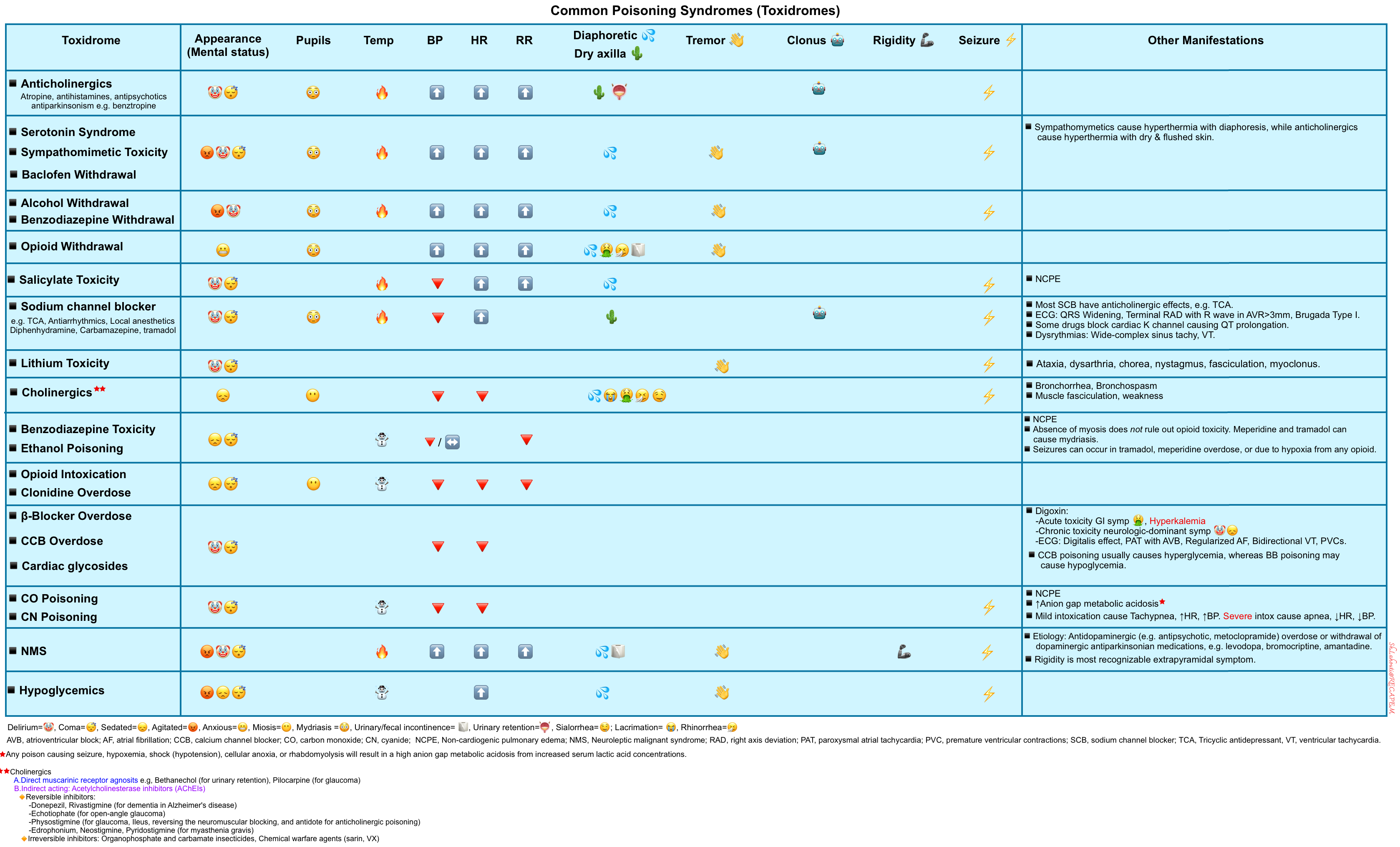
Toxidromes

- Toxidrome refers to a constellation of findings to define a particular toxic syndrome (table below)*,*,*.
- This can be sought after the initial evaluation and stabilization of patients, helping to narrow the potential etiologies of poisoning.
- Identifying the toxidromes can provide a provisional diagnosis that allows proceeding to treatment while collateral information is gathered *.

- The sensitivity and specificity of any given toxidrome are often unknown *.
- Note that a particular patient may not manifest all the symptoms or findings typically associated with a given toxidrome *.
- For example, despite being anticholinergic, tricyclic antidepressants may cause miosis due to competing effects from the activation of muscarinic and alpha receptors.
- In complex polysubstance intoxications, toxidromes can be muddled up.
- For example, if a patient overdosed on mixed heroin and methamphetamine, pupils will likely be midrange rather than pinpoint or dilated. Even in the case of a pure overdose, anoxic injury may obscure findings once the drug has been metabolized *.

- Trauma
- Infection
- Metabolic derangement (eg, hypoglycemia, hyponatremia)
- Psychiatric disease
Investigation
For critically ill intoxicated patients, history is often unreliable, and a comprehensive investigation is warranted.

- Fingerstick glucose (in all altered patients).
- Complete blood count with differential.
- Electrolytes, renal, and liver function tests.
- Serum lactate.
- Coagulation studies.
- Creatine kinase (if rhabdomyolysis is a consideration due to prolonged coma or seizures).
- Pregnancy test if appropriate.
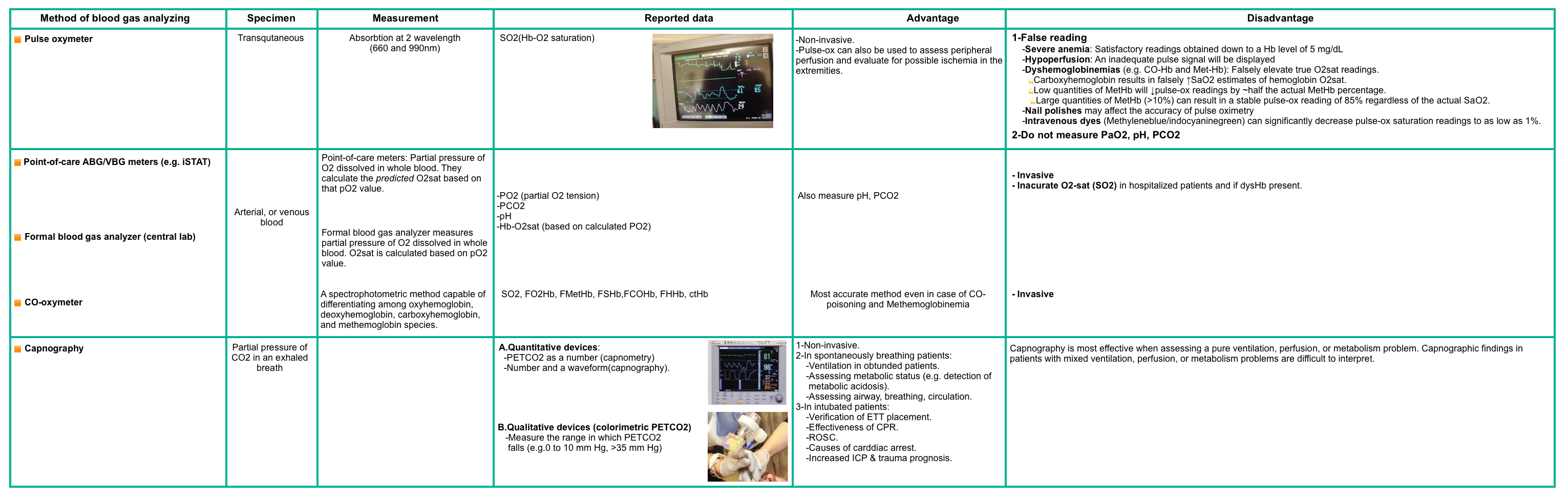
- Blood gas, carboxyhemoglobin, and methemoglobin levels in appropriate circumstances (e.g., smoke inhalation or cyanosis not improving with supplemental oxygen, respectively). Different methods of analysis are compared in the following table.
- Note that oxygen saturation reading by pulse oximetry is falsely normal despite severe hypoxia in patients with carbon monoxide, cyanide, and hydrogen sulfide poisoning.
- In cases of methemoglobinemia, the pulse oximeter may read around 85% despite visible cyanosis (more on this Appendix 2).

- In addition to a complete evaluation of the ECG, pay special attention to the following aspects:
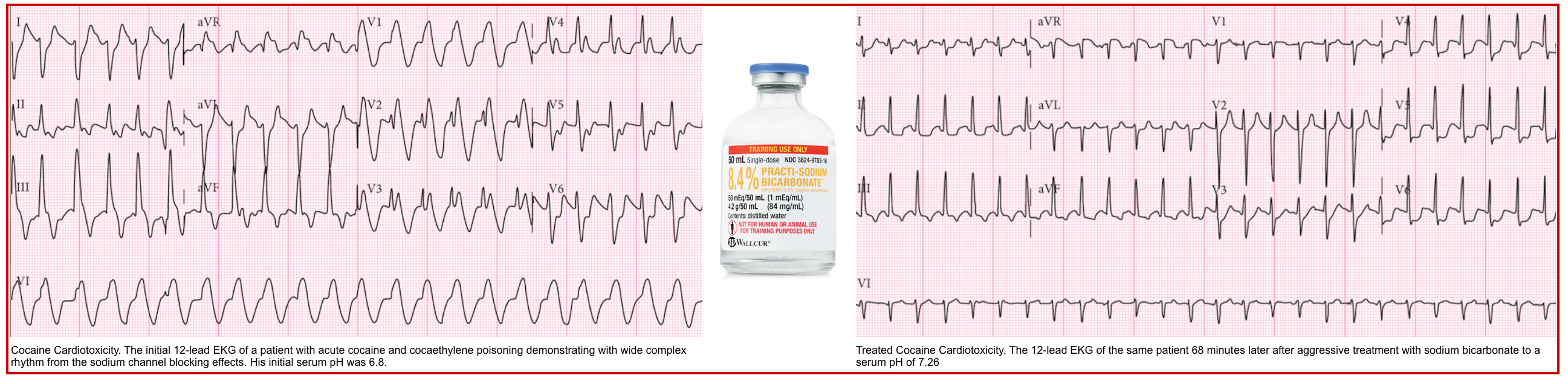
- Sodium channel blockade may be revealed by QRS widening, a tall R-wave in aVR, and occasionally a Brugada pattern in V1 (more on these, here).
- Improvement in the EKG with hypertonic bicarbonate supports the diagnosis of sodium channel blocker intoxication.
- Potassium channel blockade may be revealed by the predominant widening of the QTc.
- A QTc interval >~500 ms may increase the risk of torsade de pointed.
- For ECG signs of Digoxin poisoning, see here.
- Sodium channel blockade may be revealed by QRS widening, a tall R-wave in aVR, and occasionally a Brugada pattern in V1 (more on these, here).

- Point-of-care ultrasound
- POCUS to assess hemodynamic status and guide the appropriate resuscitative option (more on this, here).
- Head CT in patients with altered mental status of unknown etiology or if trauma is suspected.
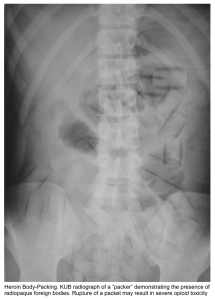
- An abdominal X-ray (or CT scan) may be useful in several situations.
- Plain film radiographs may visualize certain radiopaque toxins (e.g., heavy metals)
- Ingested drug packets of “body packers” or “body stuffers” may be seen on imaging

- Serum acetaminophen and salicylate levels.
- These are highly recommended routine screening tests in poisoned patients because early poisoning is often asymptomatic and does not have a readily identifiable toxidrome at the time when antidotal treatment is most efficacious.
- These are especially important in patients presenting with altered mental status or self-harm ingestion, for whom an accurate history may not be available.
- Urine toxicology panel for “drugs of abuse”.
- Diagnosis should not be relied on these tests due to high rates of false positive and false negative results. A positive urine toxicology result should be submitted for confirmatory testing (e.g. using gas chromatography/mass spectroscopy). For a summary of urine drug immunoassay, see appendix 3.
- False positive results
- Prescription pharmaceuticals may cross-react with the urine toxicology panel, generating false-positive results. As an example, diphenhydramine can cause a false-positive result for tricyclic antidepressants.
- A positive test may be due to recent exposure, but that drug is not responsible for the acute toxicity experienced by the patient. As an example, a patient may have used cocaine 3 days prior but presents comatose and apneic following an acute fentanyl ingestion. In this case, the cocaine screen would likely be positive, but the opioid screen would be negative.
- False-negative results
- It may reflect a drug concentration below the threshold for detection when the specimen is obtained. As an example, a patient may be comatose and apneic from fentanyl despite a urine drug screen negative for opiates.
- False positive results
- Diagnosis should not be relied on these tests due to high rates of false positive and false negative results. A positive urine toxicology result should be submitted for confirmatory testing (e.g. using gas chromatography/mass spectroscopy). For a summary of urine drug immunoassay, see appendix 3.
- Select quantitative assays
- They are useful in guiding the management of certain intoxications when interpreted in conjunction with clinical status and the timing of poisoning.
Resuscitation
General Principles
- Treatment and stabilization of critically poisoned patients often must be performed before the involved poison is known *.
- Resuscitation and Supportive Care are the core of management.
- Timely and effective airway management, hemodynamic support, and correction of critical vital signs and metabolic derangements are essential to the care of the poisoned patient and take priority over the identification of the toxicant and antidotal therapy.
- Prolonged resuscitation is in general indicated in poisoned patients with cardiac arrest, as patients are often young with minimal preexisting organ dysfunction *.
- Management of patients with critical poisoning often differs from standard resuscitation.
- For example, hypotension in patients with β-blocker or calcium channel blocker toxicity may not respond to standard vasopressors or cardiac pacing but is amenable to targeted therapies such as high-dose insulin.
- Venoarterial extracorporeal membrane oxygenation can be lifesaving for patients with cardiogenic shock or dysrhythmias that are refractory to other treatment measures. Because venoarterial extracorporeal membrane oxygenation implementation takes time, the process should be started early in patients who are not responding well to other therapies *.
Airway management
▪️Assessment of airway protection
- Reflexes
- The gag reflex is not a reliable indicator and should not be used to assess whether patients can protect their airways.
- Airway protection is best evaluated by the swallowing and cough reflexes.
- Level of consciousness
- The decision regarding intubation is much more complicated than an arbitrary GCS cutoff.
- A low GCS in intoxicated patients can be caused by reversible conditions (e.g., hypoglycemia, opioid toxicity).
- No published study is available to support that a low GCS (i.e. <8) should be used as a cutoff for intubating an intoxicated patient *.
- The Glasgow Coma Score does not predict loss of swallowing * or cough reflexes *.
- Although the risk of aspiration increases with a decreasing level of consciousness *, a GCS >8 in the poisoned patients also does not rule out the possibility of aspiration pneumonia. Other factors also appear to be associated with the risk of aspiration, such as
- Using solely a GCS≤8 to identify the need for tracheal intubation in an unresponsive, poisoned patient who is protecting their airway, maintaining adequate oxygen saturation, hemodynamically stable, and expected not to deteriorate based on the suspected ingestion (eg, short-acting sedative such as ethanol) is not evidence-based *.
▪️Indication for intubation
- Intubation is always a clinical decision that should be made at the bedside in intoxicated patients.
- Key factors to consider when deciding on intubation:
- Reversible conditions and availability of an antidote
- Response to an antidote may occasionally abolish the need for intubation.
- Dextrose IV: 0.5-1g/kg, if hypoglycemia is known or suspected.
- Naloxone should be administered if opioid intoxication is suspected. More on this, here.
- Response to an antidote may occasionally abolish the need for intubation.
- Inadequate airway protection (evidenced by pooling secretion and absence of cough reflex).
- Failure of oxygenation or ventilation
- Severe failure of oxygenation and/or ventilation, in the absence of an immediately reversible cause, is an indication for intubation in the context of an intoxicated patient.
- 💡 Mild hypercapneic acidosis may be clinically observed if the patient is protecting their airway and otherwise doing well clinically. This isn’t necessarily an indication for either intubation or BiPAP. Treat the patient, not the blood gas!
- Clinical trajectory
- When intubation is not obviously needed, a wise approach may be to wait and watch carefully. If the patient’s mental status is gradually clearing, then ongoing observation is best. Alternatively, if the mental status is progressively deteriorating over time, then intubation may be wise.
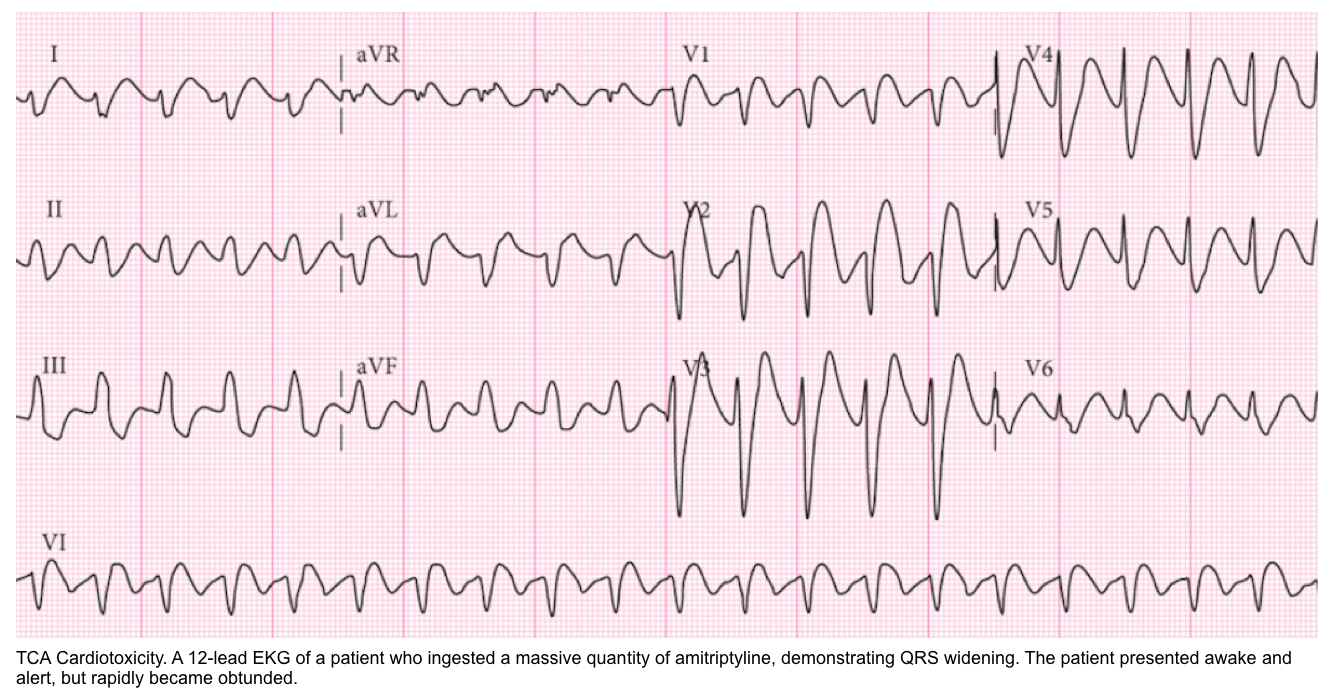
- In some intoxications (e.g., tricyclic antidepressants), patients may deteriorate rapidly and unexpectedly, so it may be rational to pursue airway control earlier than usual (figure below).
- Multiple organ failure (MOF)
- The number of failing organs can be used as a rough gauge for how likely the patient is to deteriorate and require intubation.
- The presence of multiorgan failure increases the likelihood that the patient will deteriorate and favors intubation (e.g. poor mental status plus worsening hypoxemia plus deteriorating blood pressure).
- Emergent requirement for performing additional testing or transportation.
- Reversible conditions and availability of an antidote
▪️Special consideration before intubation
- Correct physiologic killer pre-intubation HOp Killers (Credit to Scott Weingart at emcrit.org)
- Hypotension. Support hemodynamics with a vasopressor, if needed.
- Hypoxemia
- Among critically ill patients undergoing intubation, hypoxemia increases the risk of cardiac arrest and death *.
- Preoxygenation during intubation reduces the risk of death *.
- In a multicenter, randomized trial conducted at 24 emergency departments and ICUs in the United States, preoxygenation with noninvasive ventilation resulted in a lower incidence of hypoxemia during intubation than preoxygenation with an oxygen mask *.
- Acidosis (pH)
- Profound metabolic acidosis in patients requiring intubation, if done incorrectly, can lead to severe cardiac dysrhythmias.
- Bicarbonate Therapy
- NaHCO3 can increase HCO3 and correct metabolic acidosis, only if CO2 can be blown off.
- However, patients with severe toxicologic metabolic acidosis (e.g., methanol, ethylene glycol) are already tachypneic to blow off CO2, so any further CO2 or whenever the ventilation rate slows (eg, due to seizures, fatigue, or iatrogenic sedation or paralysis) the arterial pH falls abruptly, sometimes well below 7.0. and lead to cardiac dysrhythmias.
- Bicarbonate infusion is not effective for severe respiratory acidosis (e.g, hypoventilation, benzodiazepine or opioid overdose).
- Bicarbonate therapy can be helpful in specific conditions when enough time is allowed to blow off the added CO2 from the infused bicarbonate. These might include:
- Hypoventilation with respiratory acidosis due to TCA intoxication (as this will worsen TCA intoxication, generating a vicious spiral).
- Profound toxicologic metabolic acidosis (e.g., methanol, ethylene glycol, salicylates *) with signs of impending respiratory failure (e.g. a seemingly normal PaCO of 30-40 mmHg, or when respiratory efforts are faltering).
- Procedure
- Give 1 to 3 vials of 50 mL sodium bicarbonate 8.4%, IV bolus before and after intubation.
- Consider Ventilator-Assisted Pre-Oxygenation (VAPOX).
- NaHCO3 can increase HCO3 and correct metabolic acidosis, only if CO2 can be blown off.
▪️Paralytic agent
- Rocuronium is preferred over succinylcholine in the following conditions:
- Suspected organophosphate poisoning
- In organophosphate poisoning, succinylcholine may have a markedly prolonged duration of action, as the cholinesterases that degrade it are inactivated by the toxin.
- Acute digoxin toxicity.
- Digoxin poisoning may produce hyperkalemia, a contraindication to succinylcholine use.
- Suspected organophosphate poisoning
Respiratory failure
▪️Etiology
- Central hypoventilation
- Opioids
- Sedating agents, e.g., benzodiazepines
- Neuromuscular weakness
- Organophosphate and carbamate
- Respiratory insufficiency can result from muscle weakness, decreased central drive, increased secretions, and bronchospasm
- Organophosphate and carbamate
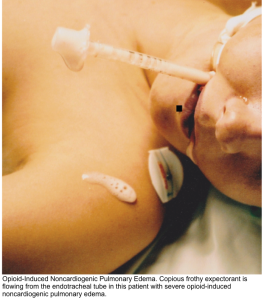
- Pulmonary edema
▪️Management
- Supplemental oxygen as needed to all critically ill patients.
-
Assess for an opioid toxidrome; if present, give naloxone immediately, as discussed here.
-
Note that naloxone is helpful in the reversal of life-threatening respiratory depression, e.g., apnea, severe bradypnea (e.g., RR<5), hypoxia, and/or severe hypercapnia.
-
- Evaluate for cholinergic toxidrome. If critical cholinergic poisoning is present, give atropine, as discussed above.
- All causes of respiratory failure are initially managed supportively with endotracheal intubation and lung-protective mechanical ventilation strategies.
Neurologic toxicity
Obtundation
▪️Background
- Have a low threshold to obtain a head CT in altered patients of unknown etiology or if trauma is suspected.
- For etiologies of altered mental status, see here.
▪️Initial approach
- IV Dextrose
- Hypoglycemia should be treated or excluded.
- Check a finger stick glucose to rule out hypoglycemia.
- If a glucometer is not immediately available or if the finger stick glucose is borderline, administer IV glucose empirically.
- Hypoglycemia should be treated or excluded.
- Naloxone trial
- Naloxone can be considered especially if there is suspicion of opioid intoxication (e.g., low respiratory rate or miotic pupils) in obtunded patients (P on AVPU). More on this here.
- Naloxone administration is not recommended for altered mental status alone in patients with adequate respiration (RR >~8) as it may precipitate acute opioid withdrawal or unmask another toxidrome, e.g., sympathomimetic.
- Thiamine (500mg IV)
⚠️Flumazenil should not be used, even when benzodiazepine toxicity is suspected, because it can precipitate benzodiazepine withdrawal, causing seizures.
Agitation
▪️ Background
- Evaluate the causes of agitation (discussed here).
- Try to eliminate and treat the cause(s) of agitation, e.g., pain, hypoxia, hypoglycemia (discussed here).
- Avoid physical restraints
- Although this may be needed initially, to protect the patient and staff from harm, the goal is always to calm the patient chemically by discontinuing restraints as early as possible.
▪️Medical treatment
- Benzodiazepine
- For toxicologic agitation, titrated doses of benzodiazepines can be considered (explored here).
- Large doses may be required and are appropriate in monitored settings where advanced airway interventions are available if needed.
- For toxicologic agitation, titrated doses of benzodiazepines can be considered (explored here).
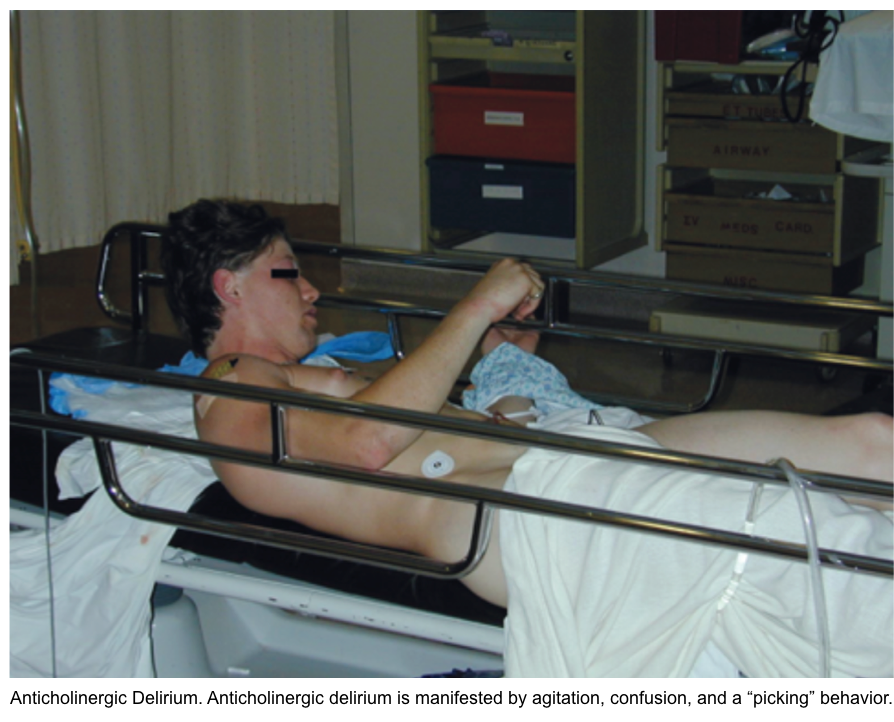
- Physostigmine (an antidote for anticholinergic toxidrome)
- Indication
- Patients who manifest both peripheral AND moderate central (moderate to severe agitation/delirium) anticholinergic toxicity, without contraindications to physostigmine, should be treated with this medication.
- ⛔️Contraindication
- If a condition other than a purely anticholinergic poisoning is suspected (e.g., TCA overdose).
- Epilepsy.
- Cardiac conduction abnormality.
- GI obstruction.
- Reactive airway disease.
- Administration
- Before physostigmine is given, the patient should be placed on a cardiac monitor, and atropine and resuscitative equipment should be available at the bedside.
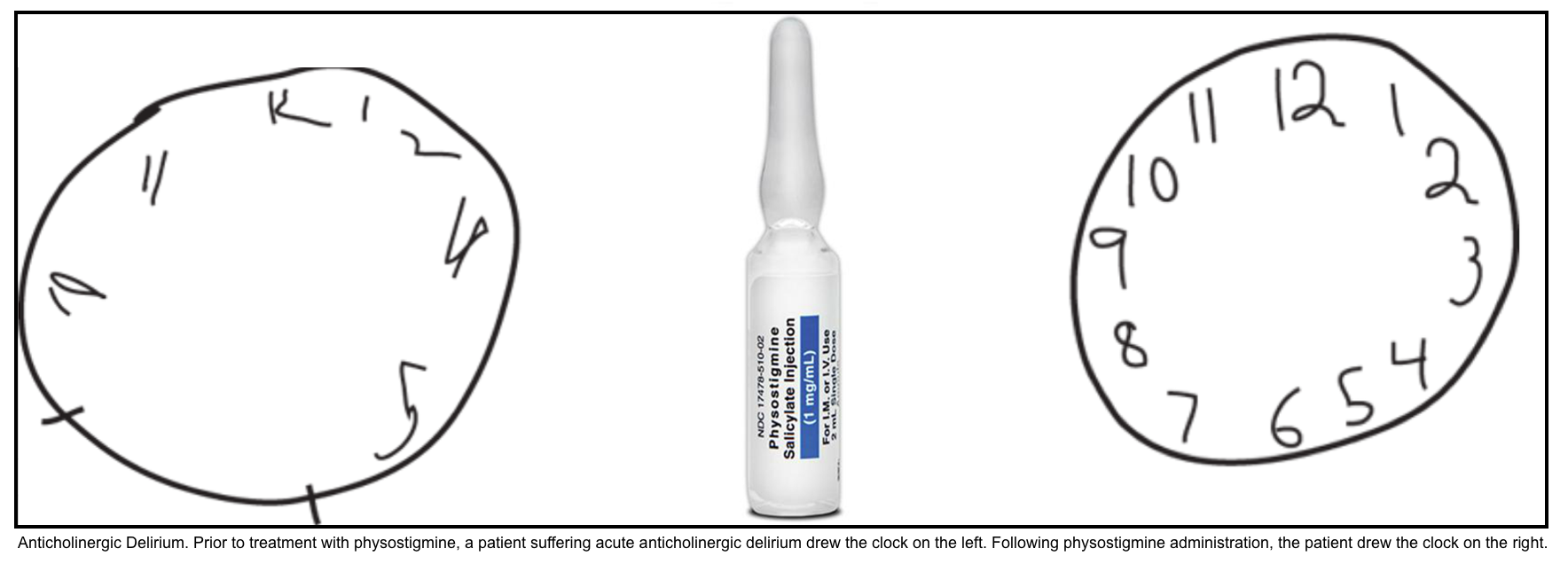
- In adults, the recommended dose of physostigmine is 0.5 to 2 mg, slow IV push over 5 min.
- Overly rapid administration may result in cholinergic symptoms or seizures.
- The half-life of physostigmine is ~15 minutes, but its effects often last significantly longer. Additionally, smaller doses may be repeated after 30 minutes if delirium recurs.
- Indication
Seizure
Background
- Toxicologic seizures are common, responsible for ~9% of status epilepticus cases and ~6% of new-onset seizures *.
- Compared with non-drug-induced seizures, toxicologic seizures have a higher rate of complications (e.g., hypoxia, hypercapnia, rhabdomyolysis, metabolic acidosis, elevated lactate, and brain injury from excessive metabolic demand) and mortality.
- Pathophysiology
- Unlike most epilepsy, toxicologic seizures begin as a generalized brain process often resulting from an acute imbalance in inhibitory (GABA) and excitatory (acetylcholine, glutamate, dopamine, norepinephrine, and serotonin) transmission, favoring neuroexcitatory over neuroinhibitory tone *.
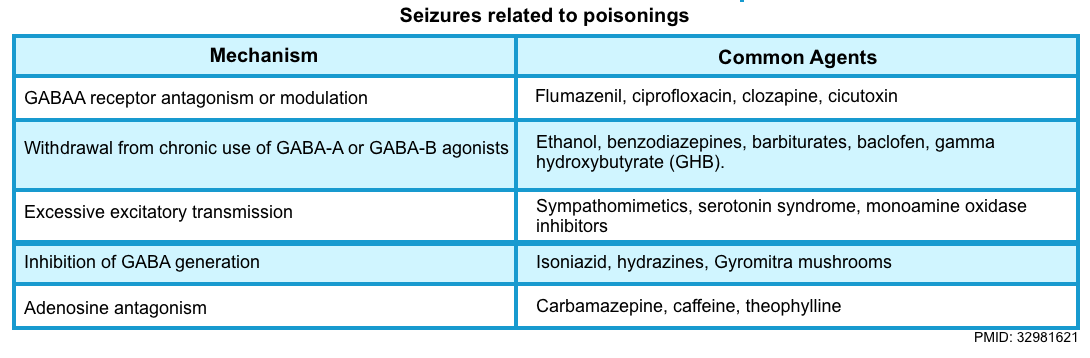

- Sodium channel blocker
- Sympathomimetics (e.g., amphetamines, cocaine, MDMA)
- Cholinergics e.g., organophosphate and carbamate insecticides, physostigmine, and bethanechol.
- CO poisoning, heavy metals.
- Antidepressants (bupropion, citalopram, tricyclics, venlafaxine)
- Local anesthetic systemic toxicity (LAST)
- Lithium
- Tramadol.
- Salicylate.
- Hydrazines (isoniazid or Gyromitra mushrooms).
- Withdrawal (e.g., ethanol, benzodiazepine, baclofen, antiepileptic medications).

- Nontoxic causes of seizure disorders
- Metabolic/electrolyte disorder (hypoxemia, hypoglycemia, uremia,
/
Na,
Ca,
Mg).
- CNS: Stroke (hemorrhagic, non-hemorrhagic); subarachnoid hemorrhage, traumatic brain injury, intracranial neoplasm, intracranial infection (abscess, meningitis, encephalitis).
- Metabolic/electrolyte disorder (hypoxemia, hypoglycemia, uremia,
- Seizure mimics (toxic-induced neuromuscular disorders that may be mistaken for seizures)
- Serotonin syndrome
- Although the neurologic presentation of serotonin syndrome is commonly limited to muscular rigidity and myoclonus, it also may include more clinically overt seizure activity.
- Acute dystonia (butyrophenones)
- Chorea (cocaine)
- Akathisia (phenothiazines)
- Strychnine, Tetanus
- Strychnine-and-tetanus-induced convulsions are mediated primarily by effects at the level of the spinal cord (hence the use of the term spinal convulsions to describe the seizure-like posturing and movements observed in the former) and are not considered true seizures *.
- Neurotoxic scorpion envenomation (Centruroides sculpturatus).
- Serotonin syndrome
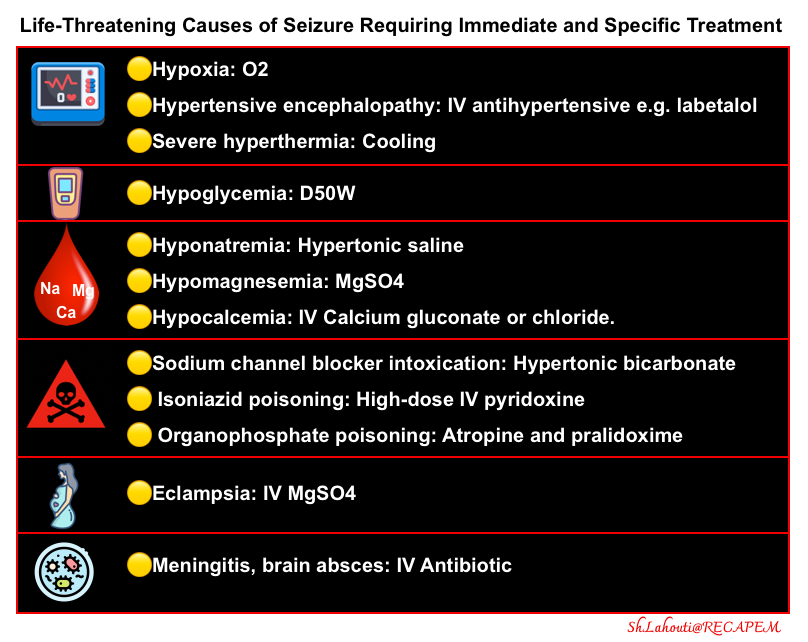

- Benzodiazepine is the front-line treatment.
- Hypoglycemia or hyponatremia should be treated immediately if present.
- Second-line agents should be used if the patient remains in status epilepticus.
- Second-line agents include induction of anesthesia with any of the following agents, followed by intubation.
- After intubation, a propofol infusion may be used to provide sedation and prevent seizure recurrence (discussed here ).
- Antiepileptic agent
- An antiepileptic agent should generally be added as well. However, this is not necessary for every patient with a toxicological seizure.
- For example, if the cause of the seizure is being treated, that may be adequate (e.g., hypertonic bicarbonate administered for sodium channel blocker intoxication).
- Phenobarbital is the antiepileptic of choice in toxicologic seizures *.
Avoid phenytoin in toxicological seizures *.
- A small study provided evidence for the safety of levetiracetam for the treatment of toxicologic seizures; however, larger studies are required to evaluate the efficacy *.
- An antiepileptic agent should generally be added as well. However, this is not necessary for every patient with a toxicological seizure.

- Hypertonic sodium bicarbonate should be given to patients with sodium channel blocker poisoning in addition to benzodiazepine.
- High-dose IV pyridoxine (25 mg/kg IV over 15–30 min, up to 5 g) should be given if isoniazid or hydrazine (i.e., Gyromitra esculenta mushroom poisoning) is suspected.

- Send urine drug screen, acetaminophen, and salicylate levels.
- Send antiepileptic drug levels as indicated.
- Obtain a head CT scan if:
- Partial seizure
- Status epilepticus
- Head trauma
- Prolonged postictal state
- Obtain continuous EEG monitoring if status epilepticus.
- Regardless of the use of neuromuscular blockers for intubation, patients who presented with status epilepticus should undergo continuous electroencephalography monitoring, as 14%of treated generalized convulsive status epilepticus may evolve into nonconvulsive status epilepticus.
Cardiovascular toxicity
Dysrhythmias

- Sinus tachycardia *
- Sympathomimetics, e.g., cocaine, methylphenidate, theophylline.
- Anticholinergics, e.g., antihistamines, TCA, phenothiazines, clozapine, and atropine.
- Carbon monoxide.
- Thyroid hormone.
- Drug withdrawal states.
- Bradycardia/heart block. More on this, here.
- CCB, BB, Cardiac glycosides, e.g., digoxin.
- Cholinergic agents.
- Central alpha-2 agonists (e.g., clonidine, guanfacine, dexmedetomidine, oxymetazoline, tetrahydrozoline).
- Magnesium.
- Wide-complex tachycardia
- Stimulants (e.g., cocaine).
- Cardiac glycosides.
- Sodium channel blockers, e.g., TCAs, phenothiazines. More on this here.
- Chlorinated hydrocarbons, e.g., chloral hydrate, solvents.
- Torsades de pointes (causing QTi prolongation), discussed here *.
- For a complete list of QT drugs, see the Arizona CERT database at www.qtdrugs.org.

- Toxicologic wide-complex tachycardia.
- Wide-complex sinus tachycardia
- Sodium channel blockers may prolong the QRS interval, leading to a wide-complex sinus tachycardia.
- If this is suspected, Hypertonic sodium bicarbonate should be administered.
- True monomorphic ventricular tachycardia (VT)
- Treatment of toxicologic VT is different from non-toxicologic patients; for example, except for lidocaine, most antiarrhythmic agents, such as procainamide and amiodarone, are contraindicated.
- Helpful treatments for toxicologic VT include:
- Hypertonic sodium bicarbonate
- Lidocaine
- Intravenous lipid emulsion (ILE)
- Wide-complex sinus tachycardia
- Toxicological bradycardia
- This is often refractory to standard Advanced Circulatory Life Support (ACLS) protocols.
- Treatment strategy
- Digoxin-specific antibody fragments (DSFab). This should be considered for bradycardia due to digoxin toxicity, as suggested by digitalis effect on ECG, known exposure to digoxin or plants containing cardiac glycosides. More on this here.
- When in doubt, consider empirically treating the patient as if they have a calcium channel or beta-blocker intoxication, as discussed here.
Cardiogenic and Vasodilatory shock
Vasopressors and Inotropes

- Cardiac monitoring, bedside echo, and clinical exam (e.g., skin temperature, mottling, etc) can guide appropriate resuscitation.
- Point-of-care ultrasound should be employed to distinguish between vasodilatory, cardiogenic, and mixed shock. This will guide therapy concerning fluid tolerance, vasopressors, and inotropes.
- For example, calcium channel blockers may initially cause vasodilatory shock, which may respond to calcium and vasopressors alone. However, at highly toxic doses (where receptor specificity is lost), they may produce cardiogenic shock with myocardial depression, favoring the use of inotropes (discussed here).

- IV fluids and vasopressors are commonly used in the initial resuscitation of patients with hypotension or shock of unknown cause, including patients with drug-induced shock.
- Avoid excessive IV fluid administration in the absence of evidence of hypovolemia.
- Volume repletion (if indicated) can also optimize kidney function and elimination of some drugs (e.g., lithium, dabigatran, baclofen, digoxin) *.
- A vasopressor such as norepinephrine (see Appendix 1) should be started early for hypotensive patients. Unusually high doses may be required.
- Avoid excessive IV fluid administration in the absence of evidence of hypovolemia.
- Inotrope choice is guided by knowledge of the toxin’s toxicodynamic properties and assessment of circulatory status (e.g., cardiac pump failure vs. vasodilatory shock).
- Extracorporeal membrane oxygenation should be considered for cases of cardiovascular failure refractory to other treatment modalities.
Hyperthermia

- Hyperthermia can be defined as a core temperature > ~40 °C (104°F) due to failure of thermoregulation.
- In contrast to fever, the setting of the thermoregulatory center during hyperthermia remains unchanged (i.e., at normothermic levels), while body temperature increases in an uncontrolled fashion and overrides the ability to lose heat (heat-dissipating mechanisms failure) *.
- Exogenous heat exposure and endogenous heat production are two mechanisms by which hyperthermia can result in dangerously high internal temperatures.

- Environmental
- Heat cramp/ syncope/exhaustion.
- Heat strokes:
- Exertional heat stroke is caused by exertion in hot weather (e.g., marathoners, military recruits).
- Non-exertional heat stroke results from a poor heat-dissipation mechanism during exposure to environmental heat *.
- Drug & Toxicologic
- Neuroleptic malignant syndrome
- Serotonin syndrome
- Malignant hyperthermia
- Lethal catatonia
- Withdrawal syndromes: alcohol, benzodiazepine, and baclofen withdrawal
- Sympathomimetic overdose.
- Salicylate intoxication.
- Anticholinergic intoxication.
- Endocrine
- Thyroid storm.
- Pheochromocytoma.
- Adrenal crisis.
- Neurologic *
- Status epilepticus
- Hypothalamic disease (e.g., CVA or hemorrhage).
- Cerebral hemorrhage
- Infection
- Infection usually doesn’t cause profound temperature elevation, but this can occur, especially in the following situations:
- CNS infection (meningitis/encephalitis).
- Sepsis + other factors limiting heat loss (e.g., anticholinergics, phenothiazines).
- Infection usually doesn’t cause profound temperature elevation, but this can occur, especially in the following situations:

- Hyperthermia can promote seizure, rhabdomyolysis, organ failure, and disseminated intravascular coagulation. Failure to promptly cool hyperthermic patients results in thermal organ damage.
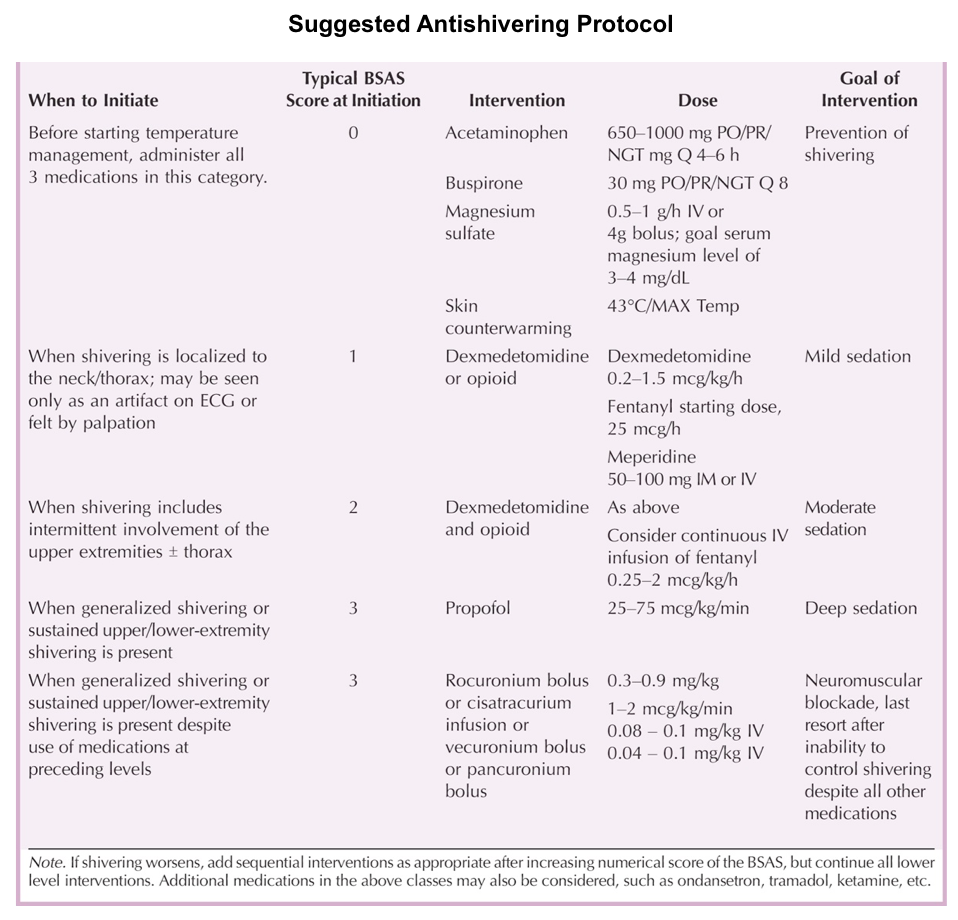
- A common adverse effect of targeted temperature management is shivering, occurring in up to 40% of patients *.
- Clinical assessment of shivering can be measured using the Bedside Shivering Assessment Scale (BSAS).
- Treatment
- Physical cooling
- This is essential in controlling hyperthermia, and may include one or more of the following (depending on available resources and the severity of hyperthermia):
- Internal cooling, e.g., with chilled crystalloid (at 0-4 °C)
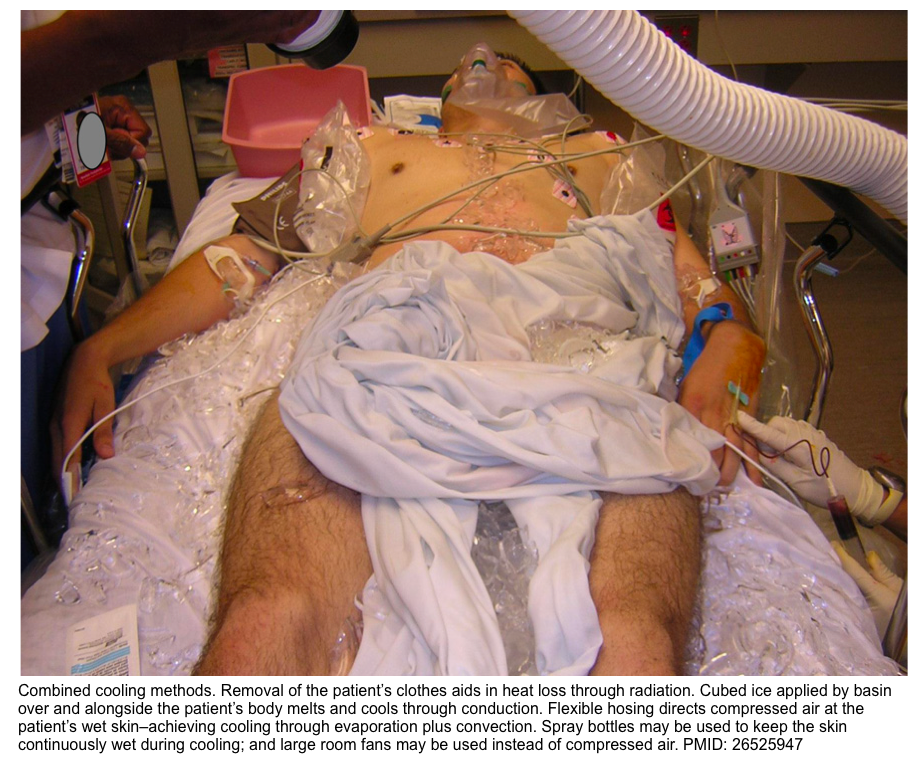
- Surface cooling methods, e.g., ice packs, immersive ice baths, or evaporative cooling (spray lukewarm water and fan the unclothed patient’s body) *.
- With all cooling methods, the goal is to reduce the core temperature to ~39°C (102.2°F) and to avoid overshoot hypothermia *.
- This is essential in controlling hyperthermia, and may include one or more of the following (depending on available resources and the severity of hyperthermia):
- Controlling agitation & shivering
- Rationals
- Ongoing agitation can increase heat generation, impairing the ability to control hyperthermia.
- Shivering should be controlled during the acute cooling phase as it will impair temperature management.
- Agitation treatment
- IV Benzodiazepine
- Especially useful in hyperthermia that is due to intoxication (e.g., sympathomimetic).
- Beneficial properties include muscle relaxation and anti-seizure effects.
- Opioid
- These can control sources of pain appropriately (especially if pain is the driver of agitation).
- If serotonin syndrome is possible, then fentanyl should be avoided.
- Propofol
- Useful among intubated patients.
- Advantages include anti-seizure activity and the ability to lift sedation to re-evaluate mental status.
- Dexmedetomidine
- It can be considered for non-intubated patients, since it is titratable and doesn’t suppress respiration.
- Logistically challenging to initiate rapidly (loading bolus should be avoided given the potential for bradycardia).
- Sympatholytic properties may assist with the control of shivering.
- IV Benzodiazepine
- Shivering (see table below for suggested antishivering protocol).
- α-Agonists: IV Dexmedetomidine
- Anesthetic and sedatives: IV Benzodiazepines, Propofol
- Opioids: IV Fentanyl (if there is no suspicion of serotonin syndrome).
- IV magnesium.
- Mg may work by reducing smooth muscle tone and subsequent vasodilation, leading to a reduced incidence of shivering *.
- IV ondansetron has some anti-shivering effects *.
- Buspirone 30 mg enterally.
- Rationals
- Intubation and sedation
- In patients with severe hyperthermia (e.g., seizure, coma, severe rigidity interfering with mechanical ventilation or temperature control, and refractory agitation), sedation and intubation with neuromuscular paralysis are required, combined with physical cooling.
- Physical cooling

Resuscitation Antidote
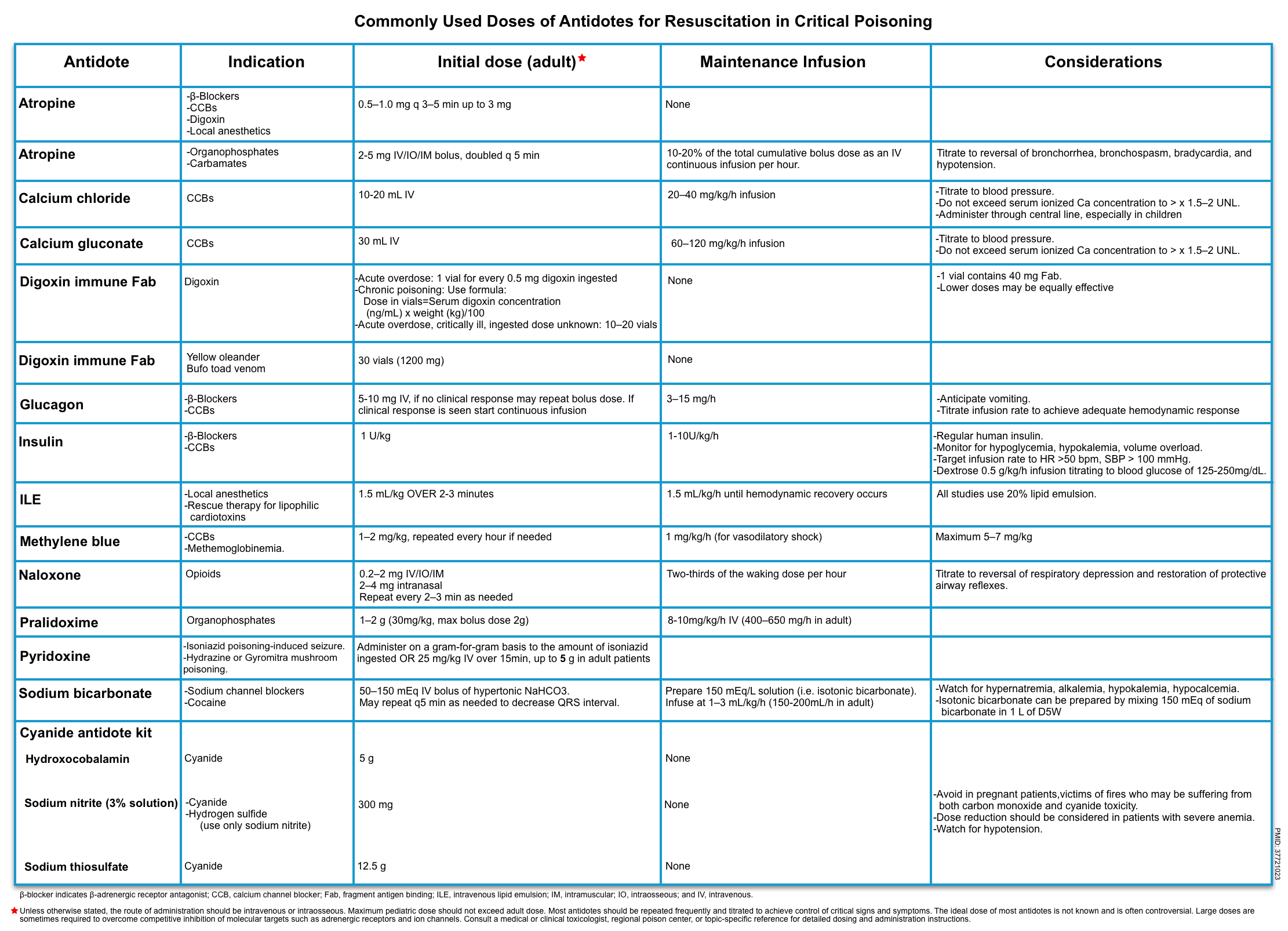
Antidotes reduce or reverse poison effects by a variety of means. They may prevent absorption, bind and neutralize poisons directly, antagonize end-organ effects, or inhibit conversion to more toxic metabolites.
- Antidote administration is appropriate when the actual or predicted severity of poisoning warrants its use, and the expected benefits of therapy outweigh its associated risks.
- There are instances in which prompt administration of a specific antidote is potentially lifesaving during the initial resuscitation of patients with critical poisoning (table below) *.
Consideration
| Flumazenil |
- Clinical features of benzodiazepine toxicity
- The classic presentation of a patient with an isolated BZD overdose consists of CNS depression with unremarkable vital signs (coma with normal vitals).
- Respiratory depression is uncommon with isolated oral ingestions but may occur with a sedative co-ingestion or following rapid IV administration.
- Sedative co-investments, such as an opioids or ethanol, increase the risk for dangerous complications such as synergistic respiratory depression.
- Flumazenil
- It is a nonspecific competitive antagonist of the BZD receptor. It only binds to the BZD site on GABA-A receptors. The onset of action is ~2min with the peak effect of ~8min. Resedation may occur by ~1 hour.
- Flumazenil administration may cause
seizures.
Contraindications
- As part of a “coma cocktail” routinely in all patients with undifferentiated obtundation *.
- Altered mental status with a witnessed or suspected seizure.
- Any suspicion for proconvulsant co-ingestion (eg, TCA, bupropion).
- Patients taking a BZD for a seizure disorder.
- Iatrogenic BZD overdose caused by treatment of status epilepticus.
- Indication
- Reversing procedural sedation in patients who do not use BZDs chronically, and do not have the above risk factors, is a commonly accepted indication for flumazenil administration.
| Atropine use in toxicology |
- Bradycardic peri-arrest
- Effectiveness
- It is only effective for bradycardias mediated by excess vagal tone, such as:
- Organophosphate and carbamate poisoning.
- Bradycardia due to inferior MI.
- Digoxin toxicity (a vagotonic state)
- It will predictably fail in the following conditions:
- High-degree AV block.
- BB and CCB toxicity.
- It is only effective for bradycardias mediated by excess vagal tone, such as:
- Contraindication
- Atropine is contraindicated in patients who have had cardiac transplantation, in whom it may precipitate asystole.
- Dosing
- Start at 1 mg of atropine; additional doses can be given to a maximal dose of ~3 mg.
- In patients with cholinergic poisoning, larger doses may be needed.
- Effectiveness
- Organophosphate & carbamate poisoning
- For patients with life-threatening organophosphate or carbamate poisoning, including cardiac arrest, bradycardia, hypotension, bronchorrhea, or bronchospasm, and respiratory depression, early atropine administration improved survival in a clinical trial *.
- Dosing
- Much larger doses of atropine are often required for this indication than for typical bradycardia.
- Initial bolus dose
- 2-5 mg IV/IM/IO bolus (0.05 mg/kg IV in children).
- Escalate (double) dose q3-5 min until bronchial secretions and wheezing stop (until full atropinization is achieved i.e. clear chest on auscultation, HR>80 bpm, systolic blood pressure >80 mmHg). This may require several vials of atropine (figure below).
- Maintenance infusion
- Give 10-20% of the total cumulative bolus dose as an IV continuous infusion per hour.
- Adjust the infusion rate as needed to maintain adequate response without causing atropine toxicity (e.g. confusion, pyrexia, absent bowel sounds, urinary retention).
- When atropine toxicity occurs, the infusion should be held until toxicity resolves and restarted at ~80% of the previous infusion rate.
- Initial bolus dose
Note that tachycardia and mydriasis are neither appropriate markers for therapeutic improvement (as they may indicate continued hypoxia, hypovolemia, or sympathetic stimulation) nor contraindication to atropine use.
- Much larger doses of atropine are often required for this indication than for typical bradycardia.
- Diagnostic evaluation of acute toxicity with organophosphate & carbamate
- If the diagnosis of cholinergic toxicity is in doubt, an atropine challenge test (1 mg IV in adults, 0.01 to 0.02 mg/kg in children) can be performed.
- In non-intoxicated individuals, the heart rate will rise above the baseline by >20%, and anticholinergic signs (mydriasis, decreased bowel sounds, dry skin) will develop.
- The absence of anticholinergic signs (tachycardia, mydriasis, decreased bowel sounds, dry skin) strongly suggests poisoning with organophosphate or carbamate.
- If the diagnosis of cholinergic toxicity is in doubt, an atropine challenge test (1 mg IV in adults, 0.01 to 0.02 mg/kg in children) can be performed.

Risk assessment
- The toxicological risk assessment is a cognitive process performed by the clinician to predict the clinical course for a specific exposure. The historical component of the risk assessment is discussed above.
- Risk assessment will guide the triaging of patients and initiation of therapy, which should be conducted concurrently with resuscitation and supportive care *.
- Risk assessment should be adjusted according to new information from history and/or investigations and clinical progression.
▪️Interpretation
- This information should be interpreted with knowledge of the poisoning being treated, including:
- Agents with delayed clinical toxicity (Toxic bombs, figure below).
- The onset of poisoning is usually rapid (e.g., within 2–4 hours) after acute intentional poisoning, but there are exceptions, including ingestion of a sustained-release xenobiotic or one that is metabolized to a more toxic compound (e.g., acetaminophen, methanol) or cellular poisons (e.g., bromoxynil, salicylate, 2,4-dinitrophenol) *.
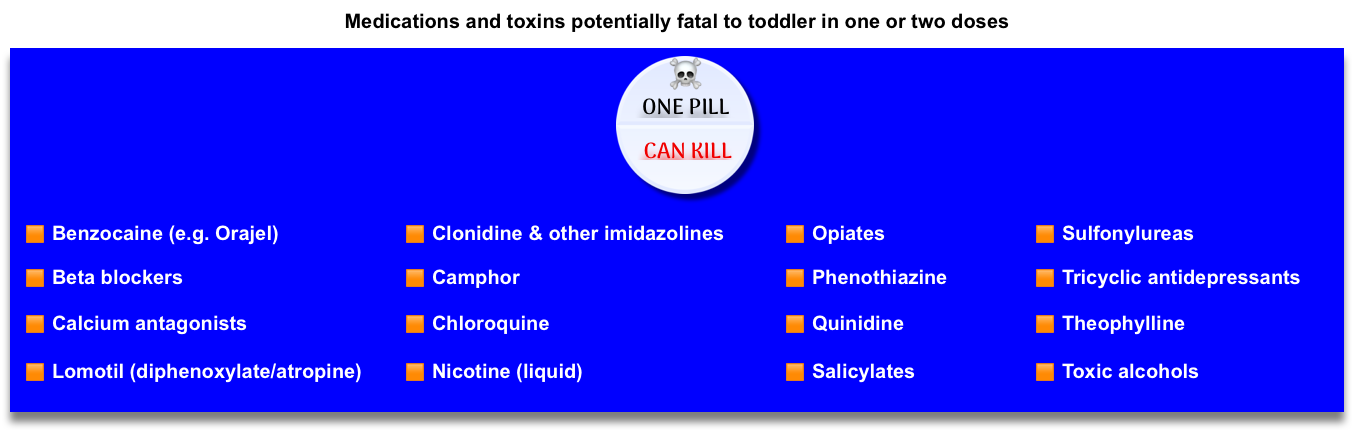
- One pill can kill (figure below) *, *
- It is critically important to know which substances are lethal for children, even in low doses, as the majority of reported toxic exposures occur in children less than 6 years old.
- Other parameters should be considered in certain acute exposures that are associated with more severe outcomes.
- Chronic poisoning (e.g., over weeks) may be associated with persistent toxicity, due to intercurrent illness with AKI or drug-drug interactions, which cause the accumulation of a therapeutic drug over time.
- Here, clinical toxicity may be severe (e.g., neurotoxicity including confusion and seizures from lithium or complete heart block from digoxin) despite relatively lower plasma concentrations than are observed after acute poisoning *.
- Agents with delayed clinical toxicity (Toxic bombs, figure below).
Severe toxicity
Following initial risk assessment, severe toxicity can be suggested in the presence of certain conditions (figure below).

GI Decontamination
▪️In general, GI decontamination may be considered in individual patients after a three-question risk-benefit analysis:
- Is this exposure likely to cause significant toxicity?
- Is GI decontamination likely to change clinical outcomes?
- Is it possible that GI decontamination will cause more harm than good?
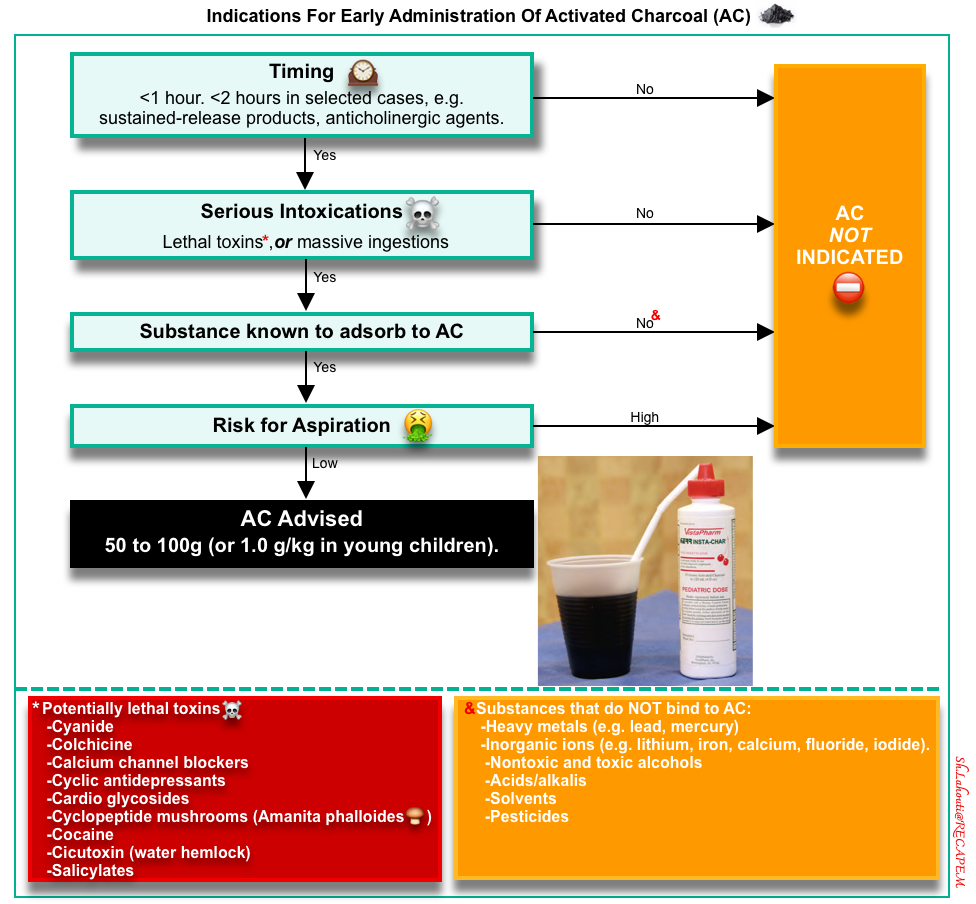
Single-Dose Activated Charcoal (AC)
▪️Mechanism of action
- AC is made from superheated carbonaceous material, producing a highly porous substance with a high surface area covered by a carbon-based network. This adsorbs chemicals within minutes of contact, preventing GI absorption and subsequent toxicity *,*.
- ⛔️AC does NOT bind to the following agents and the administration of AC is contraindicated *:
- Heavy metals (e.g., lead, mercury).
- Inorganic ions (e.g., lithium, iron, calcium, fluoride, iodide).
- Nontoxic and toxic alcohols.
- Acids/alkalis.
- Solvents.
- Pesticides.
▪️Indication
- The decision to give AC requires individual patient risk assessment. The benefits of AC are more likely to occur if:
▪️⛔️Contraindication
- If the ingested drug has low toxicity (e.g. ibuprofen, diazepam).
- If the risk of aspiration is high *:
- Unprotected airway.
- AC should not be given to a non-intubated patient with an altered mental status or with another instability that suggests a risk of requiring imminent intubation.
- Nausea and vomiting.
- Unprotected airway.
▪️Complications
- Vomiting.
- Aspiration of the activated charcoal.
- Impaired absorption of orally administered antidotes.
▪️Administration
- AC is available as a powder mixed with water to form a slurry that is gritty and poorly palatable.
- AC is also commercially available as a suspension with thickening agents, such as sorbitol, which may help improve palatability and additionally act as a cathartic.
- If vomiting is anticipated, an intravenous antiemetic is recommended.
▪️Considerations
- Intubation should never be performed solely to facilitate the administration of charcoal.
- It is reasonable to give a dose of AC to any patient who requires intubation due to intoxication (e.g., immediately following intubation) via NG tube.
- A nasogastric tube should not be placed solely to administer activated charcoal because of the risk of aspiration.
- If an OG or NG tube is used, time should be allowed for the last dose to pass through the stomach before the tube is removed. Suctioning the tube before removal may prevent subsequent AC aspiration.
Whole bowel irrigation (WBI)

- Administration of osmotically balanced polyethylene glycol electrolyte solution (PEG-ES) will induce liquid stool and mechanically flush pills, tablets, or drug packets from the GI tract.
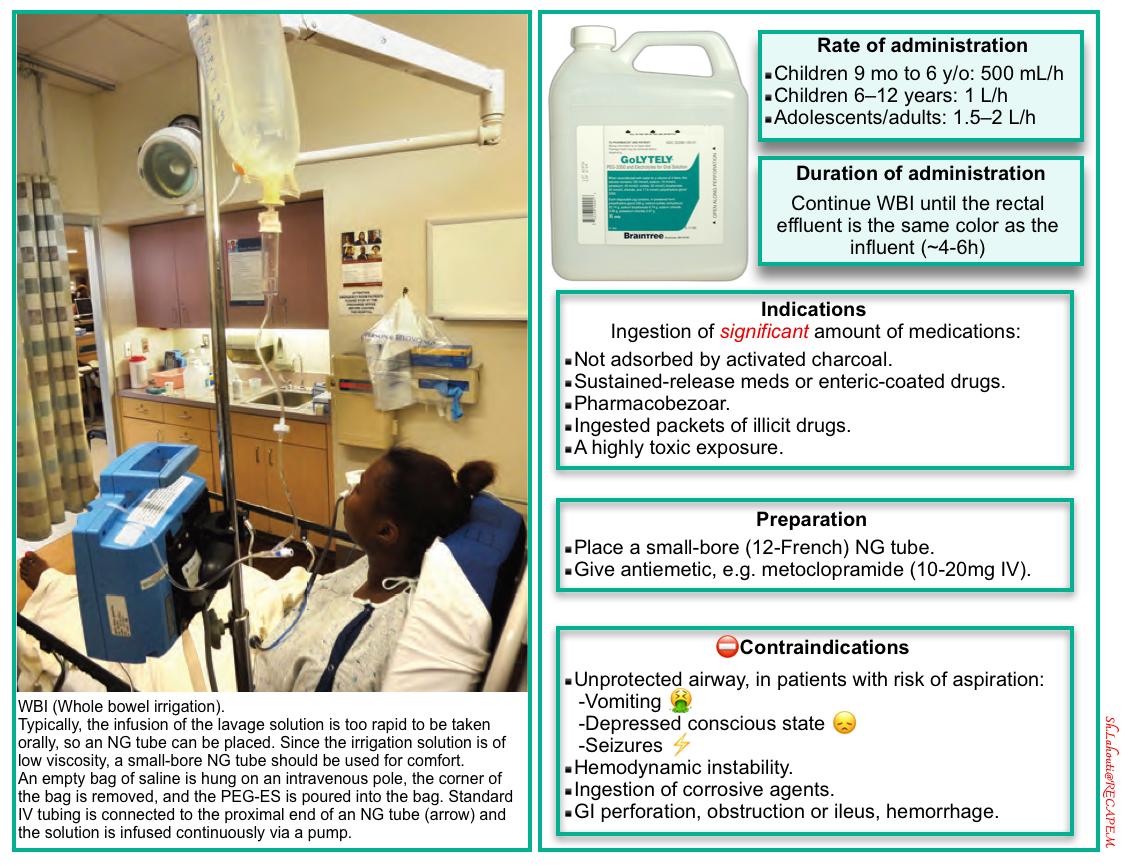

- Whole-bowel irrigation (WBI) should not be used routinely in the management of the poisoned patient (because there is no clinical proof it will change clinical outcome).
- Ingestion of a significant amount of medications *:
- Not adsorbed by activated charcoal.
- Sustained-release medications or enteric-coated drugs (particularly when patients present > 2 hours after ingestion and therefore are not likely to benefit from charcoal administration).
- Pharmacobezoar.
- Ingested packets of illicit drugs.

- Unprotected airway.
- Hemodynamic instability (concern for sequestration of bowel and worsening of shock).
- Intractable vomiting.
- Ingestion of corrosive agents.
- GI perforation, obstruction or ileus, hemorrhage.

- Procedure
- An NG tube is required because most patients will not drink the PEG-ES at the necessary rate.
- Place a small-bore (12-French) NG tube to a sufficient distance that the tip lies in the central portion of the stomach.
- Since the irrigation solution is low viscosity, a small-bore NG tube should be used for comfort.
- Confirm NG placement with a radiograph.
- Attach the tube to the reservoir bag of PEG-ES and hang it from an elevated site (an extended intravenous pole).
- Antiemetic, e.g., metoclopramide (10-20mg IV), may be coadministered to decrease vomiting and facilitate gastric passage.
- Dosage
- Polyethylene glycol is marketed as a powder. Add tap water to make a total volume of 4L. The recommended rate of administration is as follows:
- Children 9 months to 6 years: 500 mL/h
- Children 6–12 years: 1000 mL/h
- Adolescents/adults: 1500–2000 mL/h
- Continue WBI until the rectal effluent is the same color as the influent (i.e., clear), usually between 4 and 6 h.
- Radiographic studies may be useful in some circumstances (eg, iron ingestion, body packing) to confirm the absence of residual toxins.
- Polyethylene glycol is marketed as a powder. Add tap water to make a total volume of 4L. The recommended rate of administration is as follows:
Other methods of GI decontamination
⚠️Other forms of GI decontamination, such as gastric lavage and forced emesis, are rarely recommended, as they are of low efficacy given that most patients present many hours after poisoning and are poorly tolerated *.
- Lavage requires a large-bore orogastric tube, thereby requiring intubation.
- Electrolyte and water imbalances have been reported, and both delay the time to administration of the more effective activated charcoal.
Antidote
After resuscitation, a structured risk assessment is used to identify patients who may benefit from an antidote, decontamination, or enhanced elimination techniques. For a comprehensive list of antidotes, see here.
| Toxin | Antidote | Dosing |
| Acetaminophen | N-acetylcysteine (NAC) | 21-hour regimen: Consists of 3 doses; total dose delivered: 300 mg/kg. ▪️Loading dose: 150 mg/kg (maximum: 15 g) infused over 1 hour. ▪️Second dose: 50 mg/kg (maximum: 5 g) infused over 4 hours. ▪️Third dose: 100 mg/kg (maximum: 10 g) infused over 16 hours. |
| Anticholinergics | Physostigmine | IV: 0.5-2 mg over 5 min in adolescents and adults, may repeat q1-2h PRN. The effects are transient (30–60 min). The lowest effective dose may be repeated when symptoms return. |
| Ethylene glycol, methanol | Fomepizole | IV: 15 mg/kg IV over 30 min and then 10 mg/kg IV over 30 min every 12 h |
| Iron | Deferoxamine | IV: start 5 mg/kg/h continuous infusion and titrate to 15 mg/kg/h as tolerated, a total daily dose of 6–8 g *. 100 mg of deferoxamine binds 8.5 mg of iron. |
| Sulfonylureas | Octreotide | SC, IV: 50-100 mg q6-12h in adults |
| Salicylates | Bicarbonate | |
| Valproic acid | L-Carnitine | Clinically ill: 100 mg/kg IV over 30 min (max dose 6 g), followed by 50 mg/kg IV (max dose 3 g) q8h *. Clinically well: PO: 100 mg/kg per d (max 3 g) divided q6h *. |
| Dabigatran (Pradaxa) | Idarucizumab | 5 g IV, provided as 2 separate vials each containing 2.5 g/50 mL. Limited data support administration of an additional 5 g. |
| Warfarin | Vitamin K | For dosing, see here. |
| Methotrexate | Folinic acid (leucovorin) | IV: 100 mg/m2 over 15–30 min q3–6h for several days with absence/resolution of bone marrow toxicity. More on dosing, here. |
▪️Considerations
- There are specific considerations relevant to nephrologists when prescribing antidotes, including:
- Antidote removal by extracorporeal treatments, which prompts dose up-titration (e.g., ethanol or fomepizole)
- Antidote accumulation in patients with advanced kidney disease, which prompts dose down-titration (e.g., EDTA)
- Persistent or recurrent toxicity in patients with advanced kidney disease requiring repeated doses of antidotes (e.g,. dabigatran, digoxin) *.
Enhanced elimination
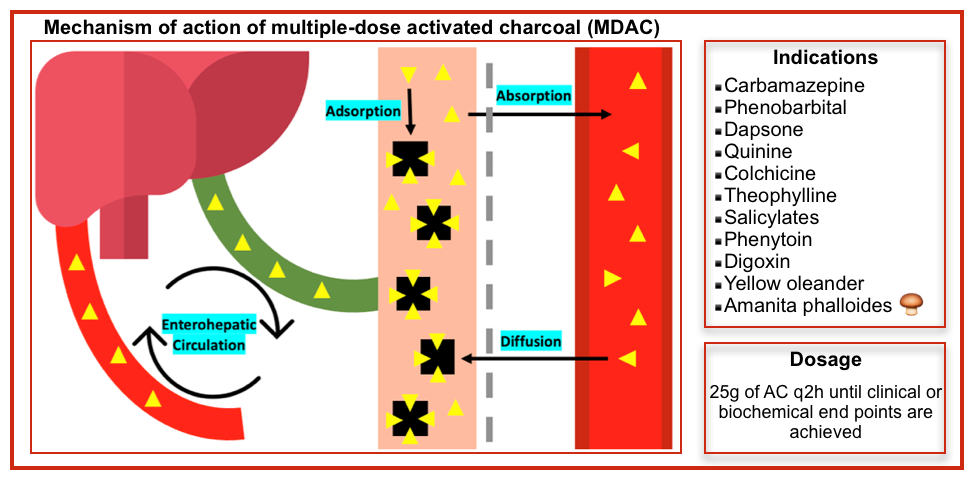
Multiple-dose activated charcoal (MDAC)
▪️Mechanism of action
- Interruption of enterohepatic recirculation.
- Facilitation of transluminal diffusion from the body into the bowel lumen (“gut dialysis”), followed by excretion.
- Reduced absorption of extended or delayed release formulations.
- The molecular weight may pass down a concentration gradient between the intravascular space and activated charcoal in the gut lumen. Multidose-activated charcoal may also adsorb residual intraluminal toxins; this is more likely for substances that slow gastric motility or form bezoars.
▪️Indication *
- Carbamazepine coma (reduces duration of coma)
- Phenobarbital coma (reduces duration of coma)
- Dapsone toxicity with significant methemoglobinemia
- Quinine overdose
- Colchicine
- Theophylline overdose if hemodialysis/hemoperfusion is unavailable
- Salicylates
- Phenytoin
- Digoxin
- Yellow oleander
- Amanita phalloides 🍄🟫 *
▪️⛔️Contraindications
- Unprotected airway
- Bowel obstruction
- Caution in ingestions resulting in reduced GI motility
▪️Administration
- This is similar to that of AC, but the concurrent use of a cathartic, such as sorbitol, is not recommended.
- This is of particular importance to young children, in whom the development of diarrhea may lead to dangerous fluid shifts and electrolyte imbalance.
- Dosage
- Adults: 25 g of AC q2 hours until clinical or biochemical endpoints are achieved (~24–48 h) *.
- Children (<12 y old): 0.25–0.5 g/kg every 2–4 h or rate of 0.2 g/kg/h for 24–48 h.
Urinary alkalinization
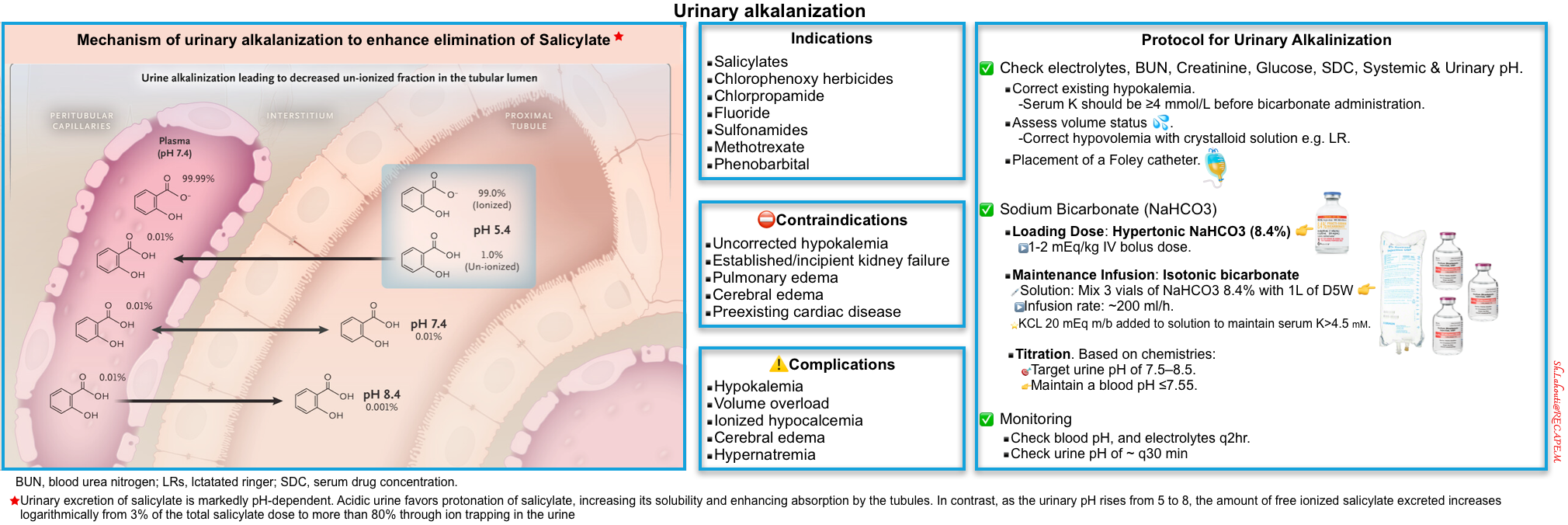
▪️Mechanism of action
- Increasing urine pH can increase the solubility of the poison or
- Increasing the proportion of the ionized form of a weak acid *.
- Ionized poisons are less easily reabsorbed through kidney tubules and more readily eliminated in urine (see figure below).
▪️Indication *
- Salicylate toxicity (moderate-severe), when the criteria for hemodialysis are not met *.
- Barbiturates: Phenobarbital, primidone.
- Fluoride
- Chlorpropamide
- Chlorophenoxy herbicides
- Methotrexate
- Sulfonamides
▪️⛔️Contraindication
- Established or incipient kidney failure
- Uncorrected hypokalemia
- Pulmonary edema
- Cerebral edema
- Preexisting cardiac disease *
▪️Complication
- Hypokalemia
- Volume overload
- Hypernatremia
- Ionized hypocalcemia (resulting from increased protein binding of calcium) *. This can lead to tetany, prolonged QTi, and cardiac dysrhythmias.
▪️Protocol for Urinary Alkalinization in Adults With Normal Renal Function
- Check baseline electrolytes, BUN, serum creatinine, glucose, serum drug concentrations, as well as systemic and urinary pH.
- Serum potassium should be ≥4 mmol/L before buffer administration *. Correct existing hypokalemia for the following reasons :
- When the kidney retains potassium, it does this by secreting acid into the urine.
- Isotonic bicarbonate tends to drop the potassium, so preemptive potassium repletion helps stay ahead of this.
- Serum potassium should be ≥4 mmol/L before buffer administration *. Correct existing hypokalemia for the following reasons :
- Assess volume status.
- Correct hypovolemia with crystalloid solution e.g., RL (since hypovolemia will tend to cause increased renal bicarbonate reabsorption).
- Placement of a Foley catheter to accurately measure urine output.
- Sodium bicarbonate
- Loading dose: Hypertonic NaHCO3 (8.4%) 1-2 mEq/kg IV bolus dose *.
- Maintenance infusion: Isotonic bicarbonate at ~200 ml/h (this can be prepared by mixing 3 vials of NaHCO3 8.4% with 1L of D5W).
- Potassium chloride 20 mEq may be added to the solution to maintain the serum K level > 4.5 mM. KCL 40 mEq can be given to treat hypokalemia in the absence of AKI and oliguria *.
- Titrate infusion to keep a urine pH of 7.5-8.5 while maintaining a serum pH of < ~7.55 *.
- Duration of treatment: Stop alkalinization when drug levels fall below toxic levels (e.g., salicylate levels <40 mg/dL *).
- Monitoring
- Check blood pH q2hr.
- Maintain a blood pH of ~7.55.
- Check urine pH every 30 min.
- 🎯Target urine pH of > 7.5.
- A further IV bolus of 1 mEq/kg of sodium bicarbonate may be necessary if sufficient alkalinization of the urine is not achieved.
- 🎯Target potassium level >4.5 mM.
- Check blood pH q2hr.
- ⚠️Avoid acetazolamide to alkalinize the urine. Acetazolamide raises urine pH and lowers systemic pH, which may cause clinical deterioration in some cases *.
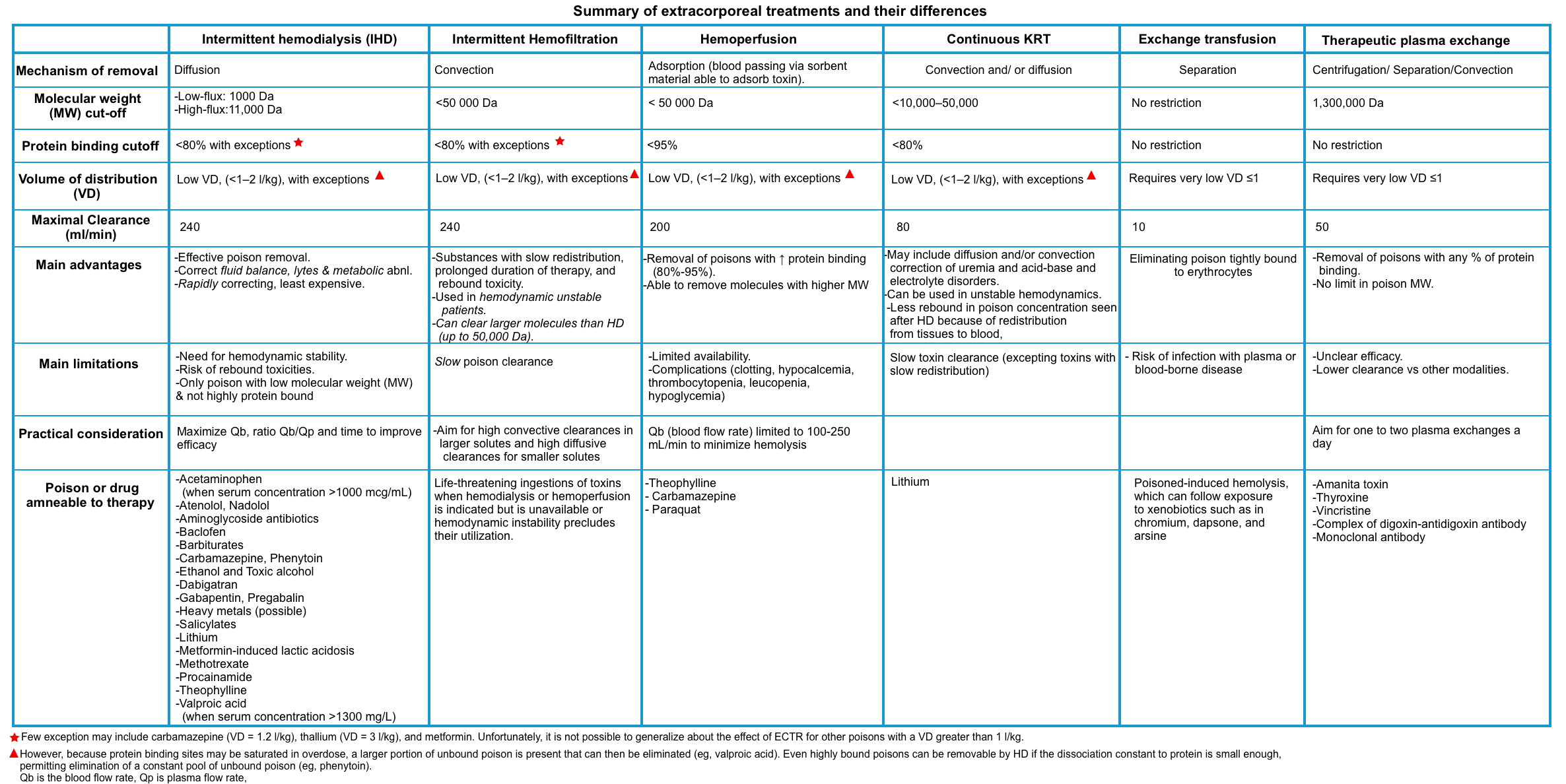
Extracorporeal removal treatment (ECTR)
After the initial stabilization of a poisoned patient, a comprehensive risk assessment must be performed to determine whether or not to use ECTR and if so, which type. The stepwise approach for the initiation of extracorporeal techniques for enhanced elimination in poisoned patients is illustrated below *.
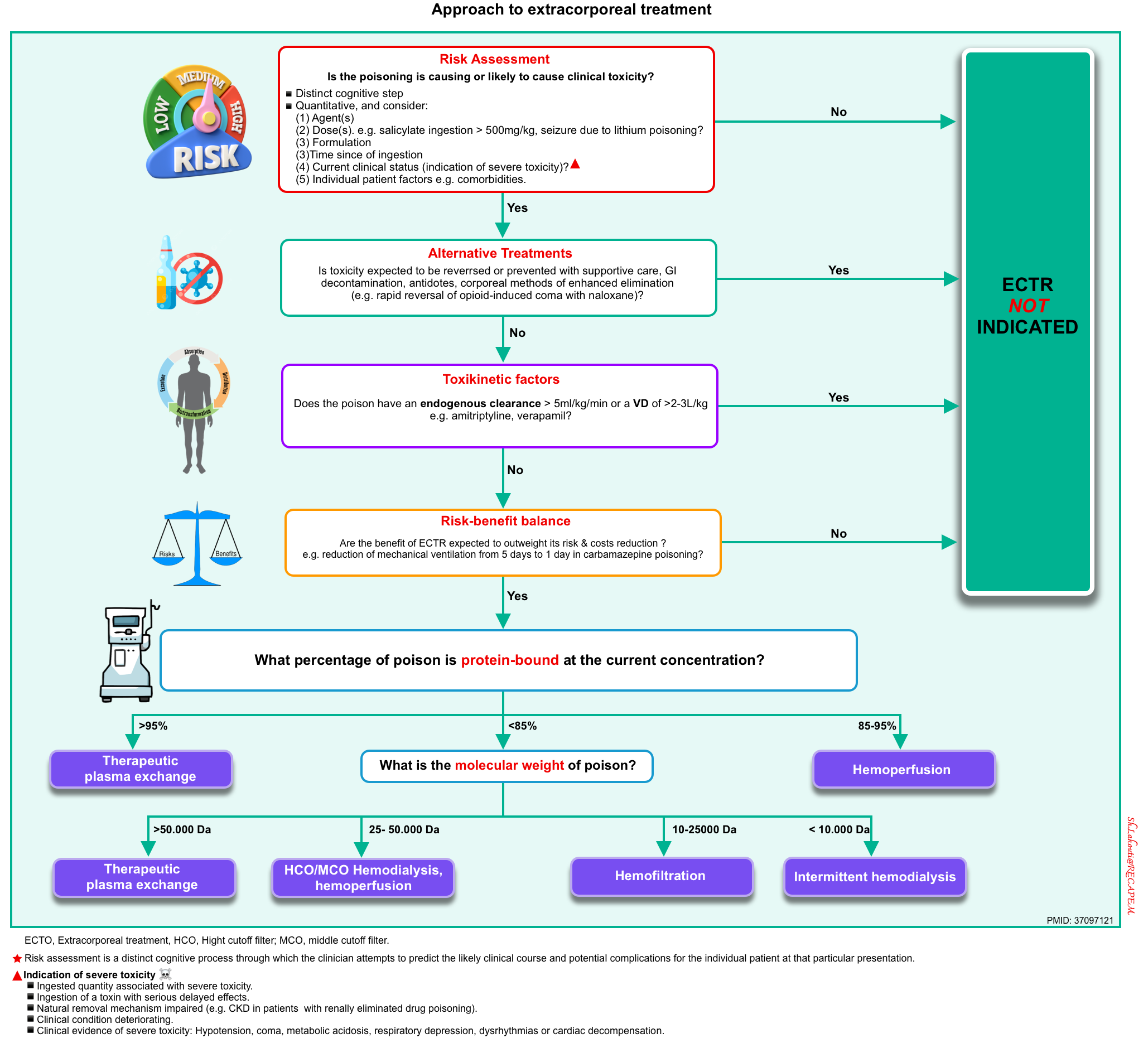
▪️Indication for ECTR
- The use of extracorporeal techniques to remove toxins is justified if
- There is an indication of severe toxicity (discussed above) and
- Absence of alternative treatment, reversible cause, and
- If the total body elimination of the toxin can be increased by 30% or more by using an extracorporeal technique.
▪️Whether extracorporeal removal is possible depends on the following factors:
- Characteristics of the toxin itself (figure below) and
- The elimination technique used (see the table below).
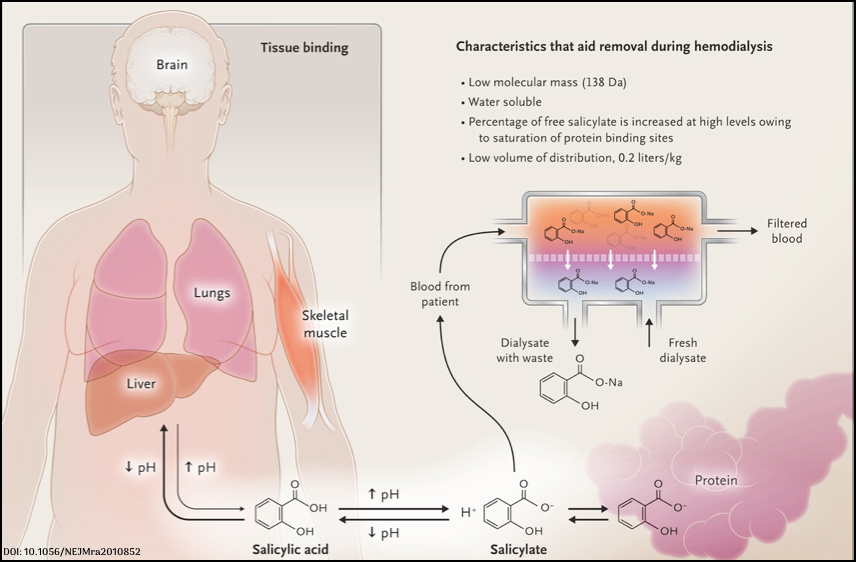
Salicylate poisoning. Characteristics that help removal during hemodialysis
Toxins characteristics
▪️Hemodialysis is most useful in removing toxins with the following characteristics:
- Low molecular weight
- A poison can be removed by an extracorporeal treatment only if it can pass through the pores of the membrane.
- Most encountered poisons have a molecular weight of 2000 Da.
- Most encountered poisons have a molecular weight of 2000 Da.
- Typically, membranes used for intermittent HD have a molecular cutoff of 10,000 Da, whereas membranes used for hemofiltration can remove poisons with a molecular weight of up to 50,000 Da.
- Poisons with very high molecular weights (.100,000 Da, e.g. monoclonal antibodies) can only be removed by techniques such as exchange transfusion or therapeutic plasma exchange.
- A poison can be removed by an extracorporeal treatment only if it can pass through the pores of the membrane.
- Small volume of distribution (<1 L/kg)
- The Vd relates to the concentration of the xenobiotic in the blood or serum as a proportion of the total body burden. The larger the Vd, the less the xenobiotic is present in the blood compartment for elimination.
- A xenobiotic with a Vd <1 L/kg is usually considered amenable to ECTR, e.g. alcohols, phenobarbital, lithium, salicylates, valproic acid, bromide, fluoride ions, and theophylline.
- High water solubility
- Water soluble xenobiotics usually have a lower Vd.
- In contrast, lipid-soluble xenobiotics have large Vd (≥1 L/kg of body weight) and are not significantly removed by hemodialysis, e.g. digoxin, many β-blockers (with the possible exception of atenolol), diazepam, organic phosphorus compounds, antipsychotics, quinidine, and TCAs.
- Low degree of protein-binding
- Xenobiotics with low protein binding are amenable to hemodialysis. Generally, a poison is not considered removable by HD or hemofiltration if the protein binding is > 80%, but there are exceptions:
- Some poisons (salicylates, valproic acid) are highly protein-bound at therapeutic concentrations, but at toxic concentrations, protein-binding sites are saturated, leading to a larger percentage of poison in unbound form.
- Some poisons have a high dissociation quotient (phenytoin), meaning they do not bind tightly to albumin, and once the unbound poison is removed, the bound poison quickly dissociates from serum proteins, replenishing the pool of removable poison.
- Certain disease states, such as hypoalbuminemia or CKD, can influence the percentage of poison that is bound and/or reduce protein-binding sites.
- Xenobiotics with low protein binding are amenable to hemodialysis. Generally, a poison is not considered removable by HD or hemofiltration if the protein binding is > 80%, but there are exceptions:
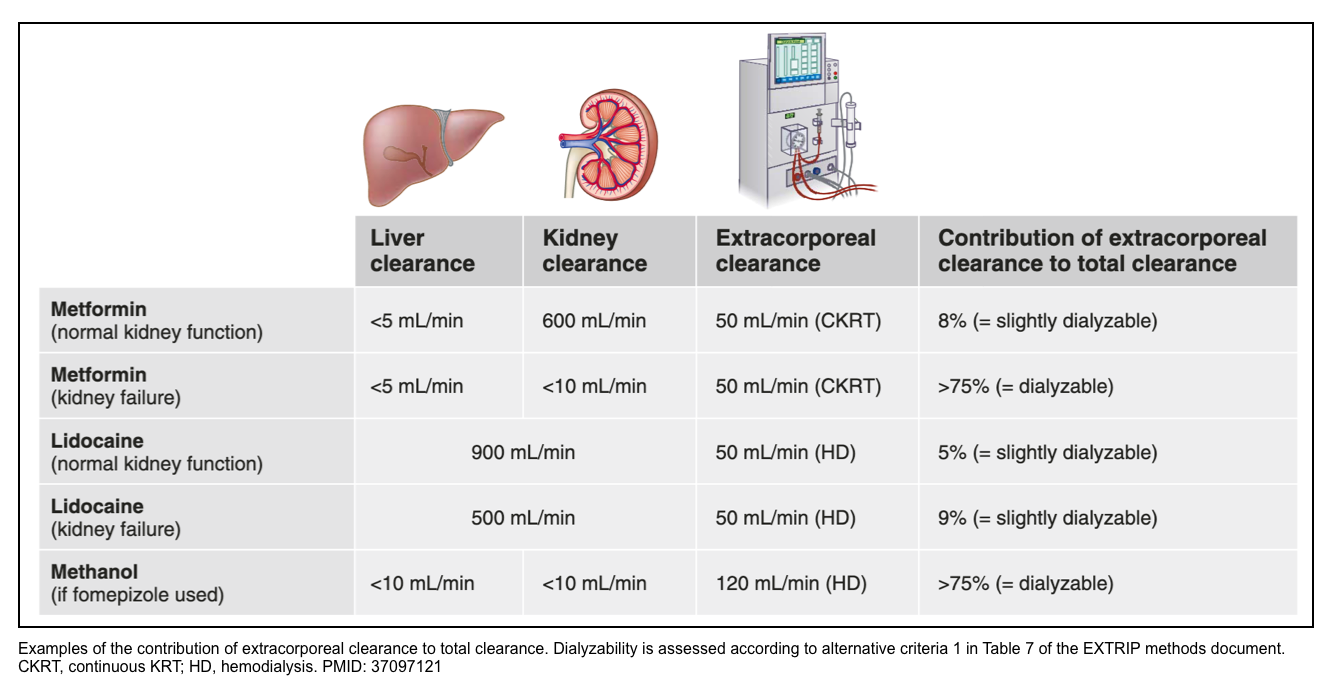
- High dialysis clearance relative to total body clearance
- An extracorporeal treatment must contribute substantially to the total clearance of that poison. For example, if liver clearance exceeds 1000 ml/min, extracorporeal clearance (which cannot realistically exceed 200 ml/min) will be inconsequential to increase total clearance.
- A generally accepted principle is that the extracorporeal treatment is worthwhile only if a large portion of the total body drug burden can be eliminated or if total body clearance of the xenobiotic is increased by a factor of at least 2.
- This substantial increase is easier to achieve when the xenobiotic has a low endogenous clearance.
- Examples of xenobiotics with low endogenous clearances (<4 mL/min/kg) include valproic acid, lithium, paraquat, phenytoin, salicylate, and theophylline.
- Xenobiotics with high endogenous clearances include many β-adrenergic antagonists, lidocaine, opioids, nicotine, and CAs.
- In poisons that are predominantly eliminated by the kidneys, the presence of AKI will increase the relative contribution of extracorporeal clearance to total clearance. For example, the endogenous metformin clearance is 600 ml/min; assuming a clearance of metformin using CKRT of 50 ml/min, CKRT will contribute at most 10% of total clearance, whereas it will contribute almost completely to total clearance in patients with anuric AKI.
- By contrast, HD will contribute little to total clearance regardless of kidney function for lidocaine elimination, because of extensive liver metabolism, whereas HD will greatly contribute to overall methanol clearance once a patient receives fomepizole.
- An extracorporeal treatment must contribute substantially to the total clearance of that poison. For example, if liver clearance exceeds 1000 ml/min, extracorporeal clearance (which cannot realistically exceed 200 ml/min) will be inconsequential to increase total clearance.
Extracorporeal Elimination Techniques
▪️The following table provides a summary of elimination techniques. Assessment of risk-benefit ratio and consultation with a nephrologist in specific conditions is recommended.
▪️Risks of extracorporeal treatments include:
- Those associated with catheter insertion
- Bleeding from anticoagulation
- Antidote removal (e.g., N-acetylcysteine, ethanol, fomepizole, and pyridoxine) *.
Hemodialysis (HD)
▪️Background
- During HD, poison is removed from the blood by diffusion, across a semipermeable membrane, passively down a concentration gradient *.
- Major advantages of HD over other ECTR techniques include *
- Widely available.
- It is the least expensive and the quickest to implement.
- Fluid removal (although rarely required in intoxicated patients).
- Correction of metabolic abnormalities.
- Replacement of kidney function.
- Highest achievable clearance among all extracorporeal treatments.
▪️Operational factors that will enhance poison clearance during hemodialysis *
- Larger surface area of dialysis membrane
- High blood and dialysate flows
- Increased ultrafiltration rate (with replacement solution)
- Increased duration of treatment
- Reduced recirculation from the vascular access
- Two distinct extracorporeal circuits in parallel
💡In poisoning, a filter with the largest surface area and maximal blood and dialysate flow rates should be used unless a contraindication is present (e.g., concern for disequilibrium syndrome, as can be seen in severe azotemia) *.
Hemoperfusion
▪️Background
- Refers to the circulation of blood through an extracorporeal circuit containing an adsorbent such as activated charcoal or polystyrene resin.
- In contrast to hemodialysis circuits, hemoperfusion devices use a charcoal (or other adsorbent) filter, which comes into direct contact with blood, partially overcoming molecular weight and protein-binding limitations
- Clearance rates are higher with hemoperfusion than hemodialysis if the adsorbent binds the ingested toxin; the extraction ratio for hemoperfusion approximates 1.0 for some poisons, and drug clearance rates approach the rate of blood flow through the hemoperfusion circuit.
- HP can remove small- and large-sized poisons (including those that are highly protein-bound).
- Toxin requirements:
- Low volume of distribution
- Low endogenous clearance
- Bound by activated charcoal *.
▪️Indication
- Life-threatening poisoning caused by:
- Theophylline (high-flux hemodialysis is an alternative)
- Carbamazepine (multidose activated charcoal or high-efficiency hemodialysis is also effective)
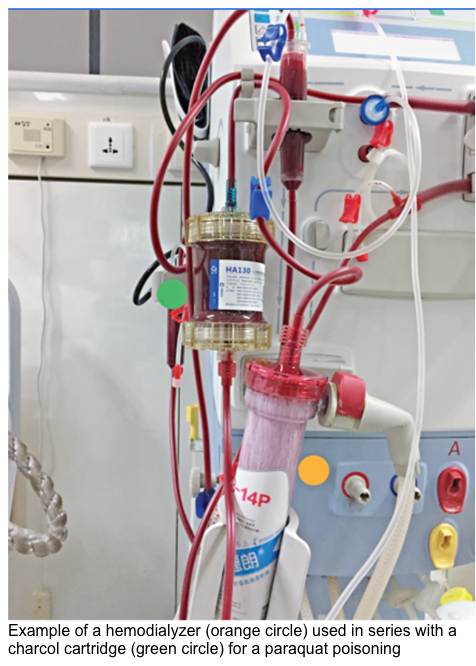
- Paraquat (theoretical benefit only if instituted early after exposure)
▪️⛔️Contraindication
- Hemodynamic instability
- Infants (generally)
- Poor vascular access
- Significant coagulopathy
- Toxin not bound to activated charcoal
Hemofiltration
- During hemofiltration, solute and solvent in the blood are removed by convection or solvent drag and replaced by a physiological solution.
- Hemofiltration has similar advantages to HD but can eliminate larger poisons, up to 50,000 Da, and it can be used in patients with unstable hemodynamics.
Continuous kidney replacement therapy
▪️Background
- CKRT includes 4 methods of continuous hemofiltration:
- Continuous veno-venous hemofiltration (CVVH)
- Continuous veno-venous hemodiafiltration (CVVHD)
- Continuous arterio-venous hemofiltration (CAVH)
- Continuous arterio-venous hemodiafiltration (CAVHD)
- Comparative schematic layouts of hemodialysis (HD), continuous venovenous hemofiltration (CVVHDF), continuous venovenous hemofiltration with dialysis (CVVHD), and hemoperfusion (HP) are shown below.
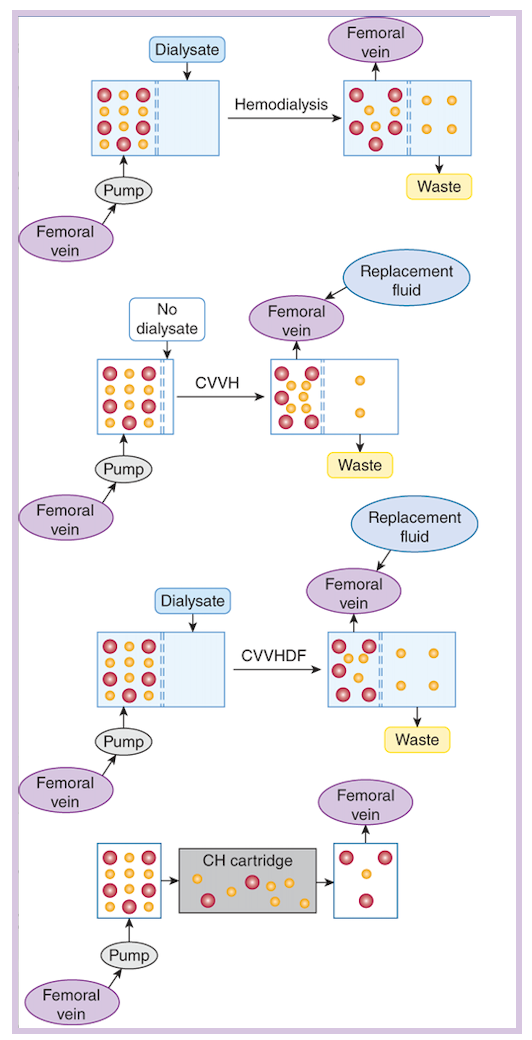
Red circles are high molecular weight (MW) xenobiotics, such as methotrexate, whose high MW makes them too large to be removed by HD. Yellow circles are low MW diffusible solutes such as urea or methanol. In dialysis, solute moves across a semipermeable membrane (dashed lines) from a solution in which it is present in a high concentration (blood) to one in which it is at a low concentration (dialysate). In CVVH and CVVHDF, plasma moves across a similar membrane in response to hydrostatic pressures; replacement fluid must be provided. The latter also uses a dialysate to augment clearance. The availability of blood pumps has made arteriovenous modalities nearly obsolete. Charcoal hemoperfusion (CH) requires blood movement through a sorbent-containing cartridge and does not include dialysis or hemofiltration.
- To perform CKRT, blood is pumped by the patient’s arterial (CAVH) or venous (CVVH) pressure, or by a hemodialysis machine entrained in the circuit (CAVHD, CVVHD). Blood that enters the hemofiltration circuit passes through filters (sheet membrane or hollow fiber) with large pores, and an ultrafiltrate forms, which drags solutes with molecular weights up to 50,000 daltons (depending upon hemofilter pore size). Cells and solutes larger than the pore size remain in the blood and return to circulation.
- In general, CKRT has lower clearance rates than conventional hemodialysis.
- The advantages of CKRT are its applicability in hemodynamically unstable patients and its ability to be set up and delivered by regular intensive care unit staff.
▪️Indication
- Life-threatening ingestions of toxins when hemodialysis or hemoperfusion is indicated but is unavailable or hemodynamic instability precludes their utilization.
Exchange transfusion
▪️It refers to the removal of a quantity of blood from a poisoned patient and its replacement with an identical quantity of whole blood; the process is usually repeated 2-3 times.
▪️Indication
- Exchange transfusions are rarely indicated but may be useful in the following conditions:
- Massive hemolysis (e.g., due to arsine or sodium chlorate poisoning)
- Severe methemoglobinemia
- Severe sulfhemoglobinemia (e.g., secondary to hydrogen sulfide exposure)
- Neonatal drug toxicity.
▪️Complications
- Transfusion reactions
- Ionized hypocalcemia
- Hypothermia
Recommendation of ECTR in poisoning
▪️Extracorporeal Treatment for Individual Toxins
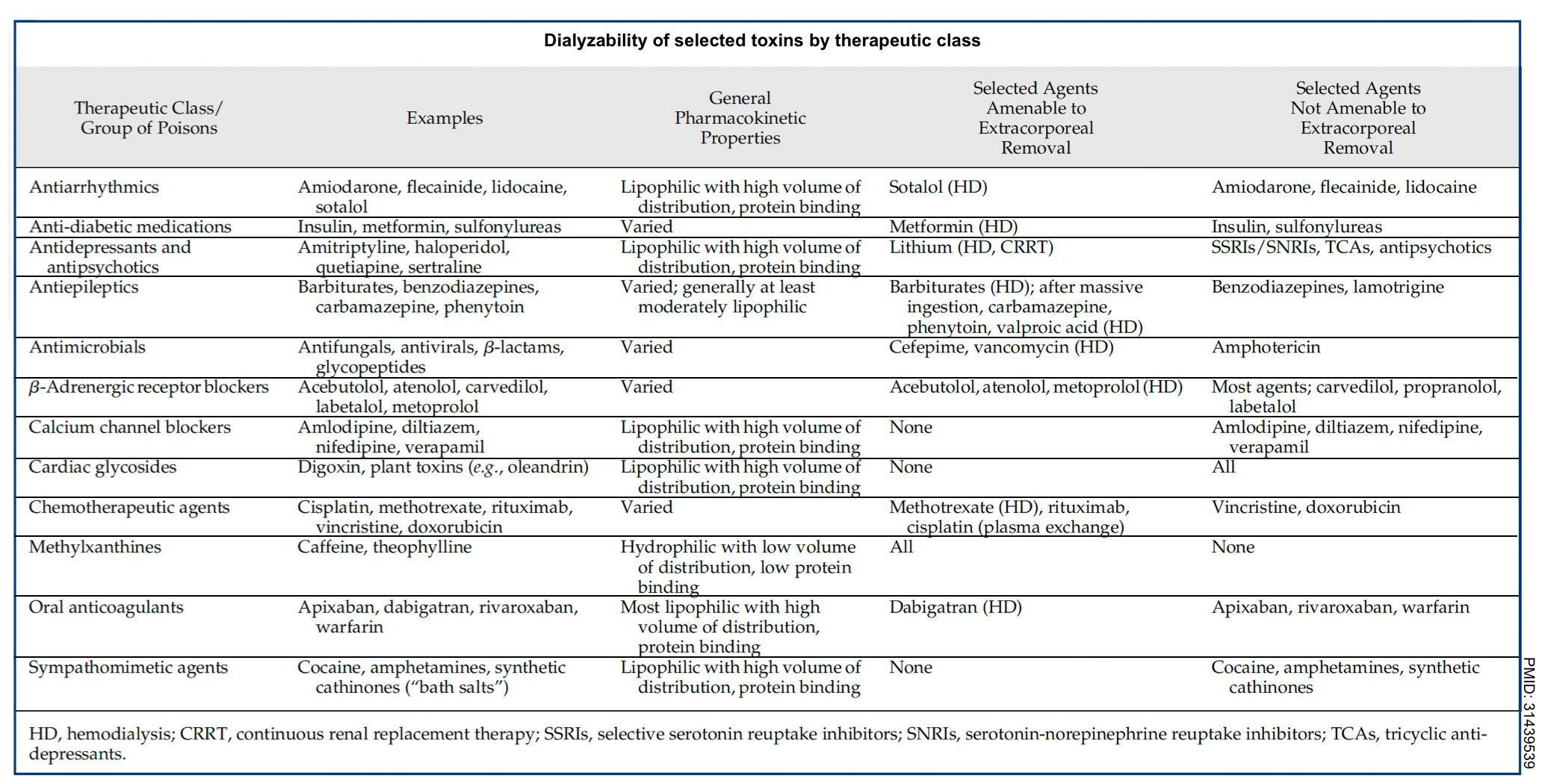
- Across different Western countries, ethylene glycol, lithium, and salicylates have consistently comprised roughly two-thirds of poisonings treated via extracorporeal therapies. The dialyzability of selected toxins by therapeutic class is summarized below.
▪️Recommendations
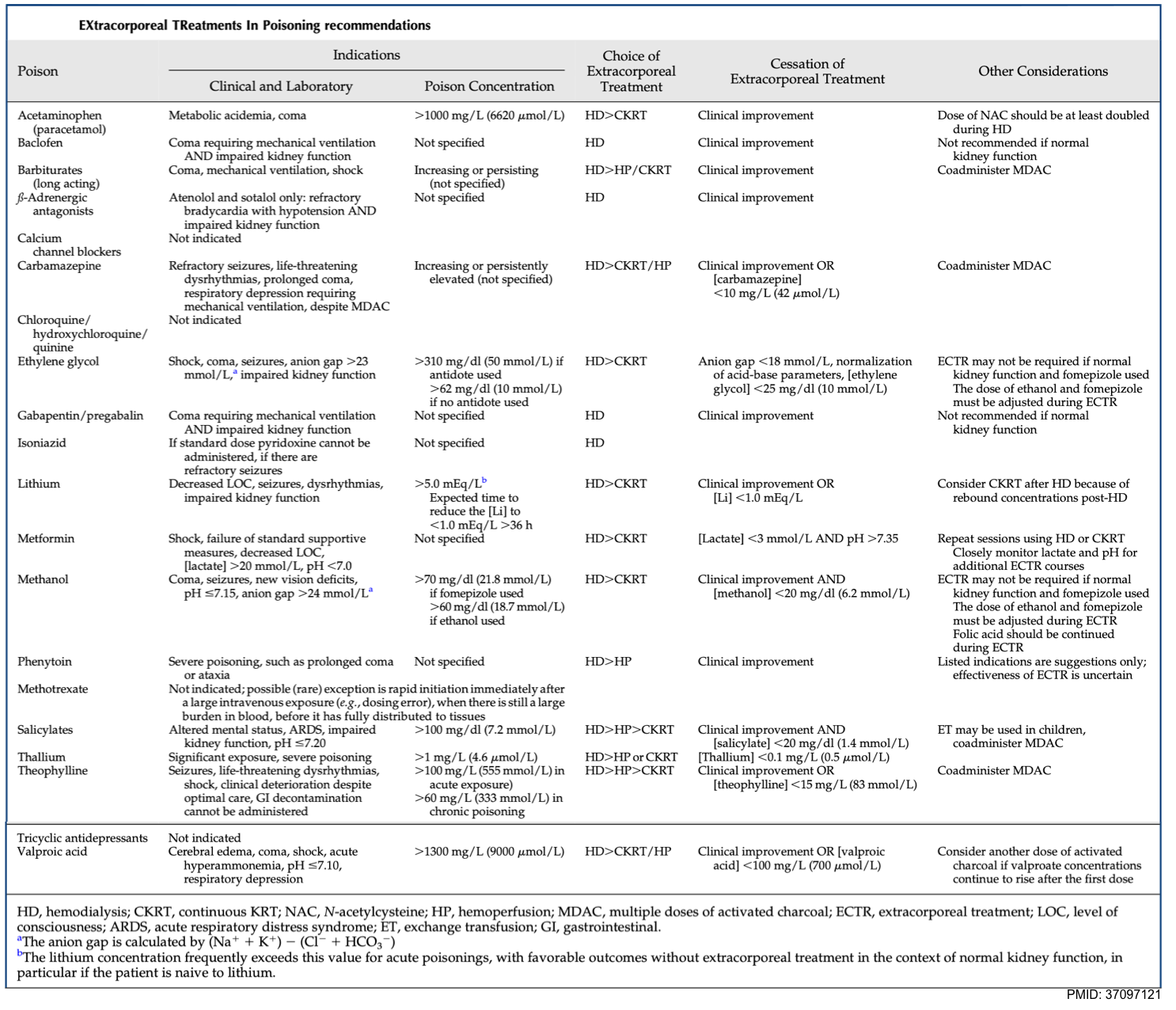
- Evidence-based consensus recommendations for the use of extracorporeal treatments for managing poisoning have been published by the EXtracorporeal Treatments in Poisoning (EXTRIP) workgroup, a large multinational multidisciplinary group. More on this, here.
⭐️In general, decision-making occurs on a case-by-case basis. For example, AKI may be an indication for extracorporeal treatments in some poisonings (e.g. metformin, baclofen), but not others.
Poison control consultation
- Discussing complicated cases with poison control or a local toxicologist is generally advisable.
- A good time to do this is generally once the dust has settled. A secondary review of the case may reveal issues that were overlooked initially.
- ☎️Contact information:
- United States: 1-800-222-1222
- Phone numbers for international poison centers are here.









Add comment