4 October 2020, by Shahriar Lahouti. Updated 27 May 2024.
CONTENTS
- Preface
- Etiology
- Evaluation
- Differential diagnosis
- Risk stratification
- Management
- Bleeding in cirrhosis
- Approach to lower GI bleeding
- Aortoenteric fistula
- RECAP
- Media
- Checklist
- Going further
- References
Preface
Gastrointestinal bleeding (GIB) is a common problem encountered in the emergency department. Patients with GIB may present with blood loss that is visible to the patient or clinician; hence the name overt GIB, or may have no visible evidence of blood loss, (occult blood loss) 1.
The accurate diagnosis of GI bleeding relies on prompt resuscitation, initial risk evaluation, and provisional clinical diagnosis followed by appropriate definitive investigation which enables specific interventions.
In this post, the main focus of the discussion is overt GI bleeding with hemodynamic instability. Usually, the diagnosis is straightforward, however critical patients often have multiple causes for hemodynamic instability (multifactorial shock). The problem arises when the clinician does not consider other coexisting etiologies for shock, or when resuscitative efforts are delayed or misguided.
Etiology
The annual incidence of hospitalization for acute UGIB in the United States is approximately 65 per 100,000 individuals and is more common than lower gastrointestinal (GI) bleeding 2.
The hospitalization rate for UGIB is estimated to be six-fold higher than for lower GI bleeding.
Acute GIB is a common problem, occurring in the upper GI tract of 100–200 per 100.000 persons annually and in the lower GI tract of 20.5–27.0 per 100.000 persons annually. Although 80%–85% of cases of GI bleeding resolve spontaneously, it can result in massive hemorrhage and death. The most common causes of upper and lower GIB are shown below {34}.
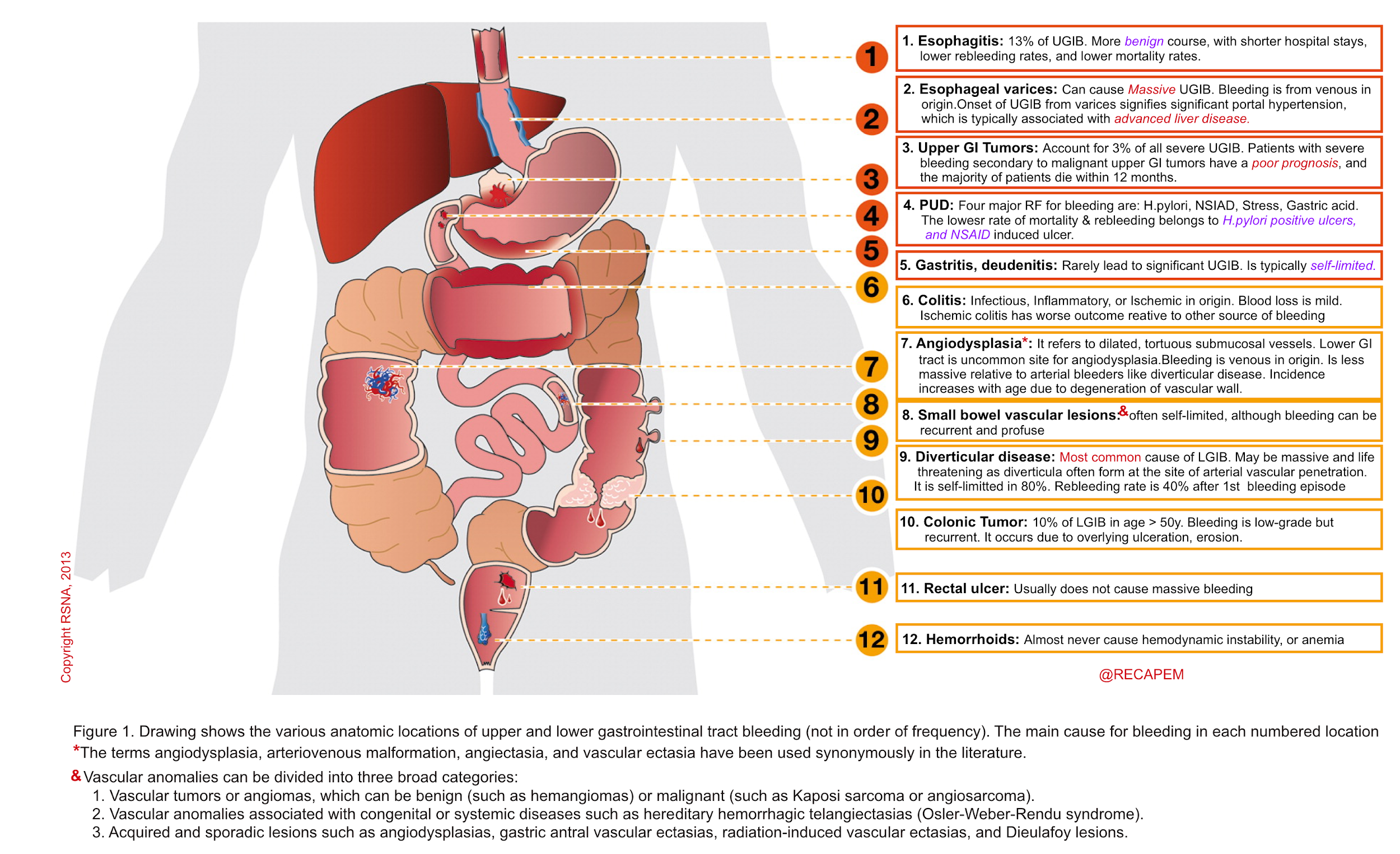
Evaluation
Introduction with a clinical scenario

The initial evaluation of the patients suspected of significant GIB includes history, physical exam, monitoring, laboratories, point of care ultrasound, and possibly nasogastric (NG) lavage. The information obtained is used to guide decisions regarding initial resuscitation, medical therapy, and diagnostic and therapeutic interventions.
Clinical presentation
Patients can present with the hemorrhagic manifestation of GI bleeding (table below) and/or other related symptoms that are suggestive of blood loss 1.
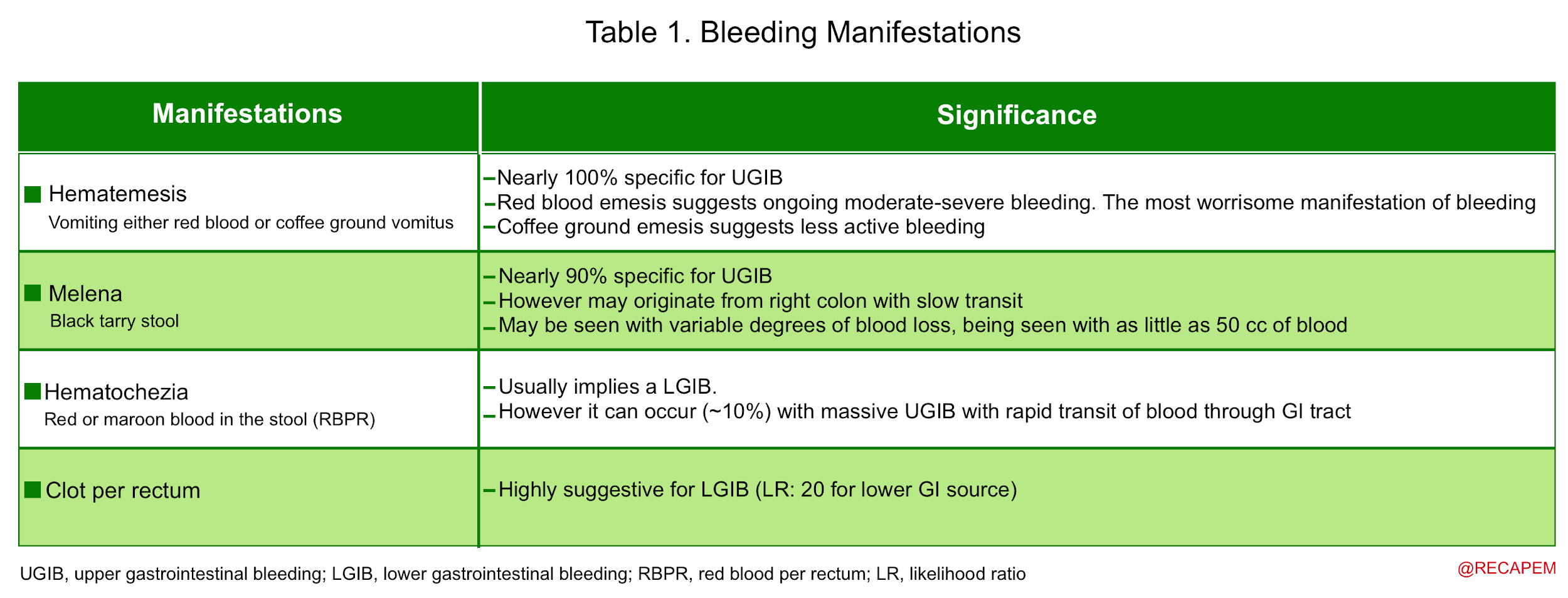
▪️Other related symptoms of GI bleeding
- Syncope or presyncope
- By definition, syncope is a transient complete loss of consciousness, associated with the inability to maintain postural tone, with rapid and spontaneous recovery.
- The presumed mechanism is cerebral hypoperfusion.
- There should not be clinical features of other non-syncope causes of loss of consciousness, such as seizure, antecedent head trauma, or apparent loss of consciousness (i.e. pseudosyncope). More on this here.
- Presyncope is the symptoms before syncope, and it warrants the same approach just like syncope 5.
- These symptoms could include extreme lightheadedness; visual sensations, such as “tunnel vision” or “graying out”; and variable degrees of altered consciousness without complete loss of consciousness. Presyncope could progress to syncope, or it could abort without syncope.
- By definition, syncope is a transient complete loss of consciousness, associated with the inability to maintain postural tone, with rapid and spontaneous recovery.
- Angina
- Severe hypovolemia and hypotension (or conditions other than a coronary arterial disease) can cause an imbalance between myocardial oxygen supply and/or demand, resulting in myocardial infarction (Type 2 Myocardial Infarction is secondary to ischemic imbalance) 6.
- Altered mental status
- Altered mental status is a continuum that begins with agitation, progresses to confusion or delirium, and ends in obtundation or coma.
- The altered sensorium in the setting of shock can be due to poor cerebral perfusion or metabolic encephalopathy *. However, altered mental status is neither sensitive nor specific for the diagnosis of shock.
- Altered mentation is not a specific symptom of cerebral hypoperfusion in a shock state *. ⭐️See here for different causes.
- For example, patients with advanced liver disease and hepatic encephalopathy are altered at their baseline.
- It is not a sensitive symptom of shock either. Normal mental status does not rule out a hypoperfusion state.
- For example, some patients in occult cardiogenic shock have normal mentation despite profoundly low cardiac output. In contrast, delirium is more commonly seen in patients with septic shock.
- Altered mentation is not a specific symptom of cerebral hypoperfusion in a shock state *. ⭐️See here for different causes.
- Abdominal pain
- The presence of abdominal pain is worrisome and in the presence of hemorrhagic manifestation of GI bleeding (table 1), it can suggest 1:
- Perforated peptic ulcer disease.
- Aortoenteric fistula.
- Mesenteric vein thrombosis.
- GI tumors, polyps.
- Colitis (Inflammatory, infectious, ischemic).
- The presence of abdominal pain is worrisome and in the presence of hemorrhagic manifestation of GI bleeding (table 1), it can suggest 1:
- Other bleeding symptoms
- Patients with coagulopathy may present with other bleeding symptoms such as hematuria, however, hematuria is rarely the cause of hemodynamic instability, and an astute clinician is prepared and will search for other possible sources of life-threatening bleeding which can explain the patient’s hemodynamic instability such as retroperitoneal hemorrhage, GI bleeding, etc.
- Unresponsiveness
- Severe exsanguinating hemorrhagic shock can present with pseudo-pulseless electrical activity (aka. PREM; pulseless with a rhythm and echocardiographic motion).
- This is a severe shock state and often is due to profound hypovolemia. If not recognized and promptly resuscitated progresses to asystole cardiac arrest.
- Severe exsanguinating hemorrhagic shock can present with pseudo-pulseless electrical activity (aka. PREM; pulseless with a rhythm and echocardiographic motion).
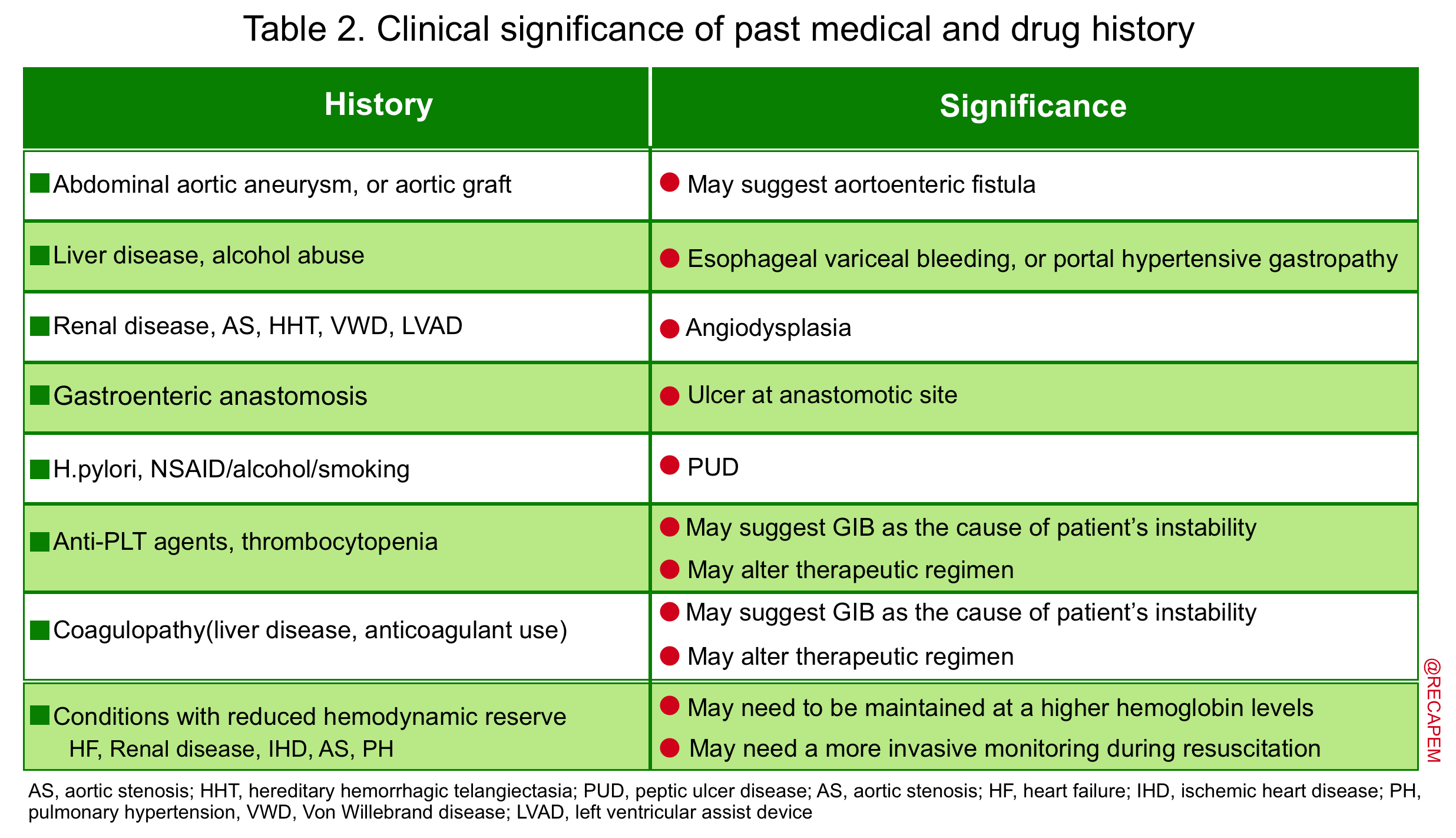
▪️Past medical and drug history
- Reviewing PMH and DH provides valuable diagnostic information that can potentially influence therapeutic decision-making (table 2).
- Studies have shown that up to 60% of patients with a prior history of GIB are bleeding from the same lesion.
- In patients with undifferentiated hypotension, wherein the cause of instability is less clinically apparent, this information is especially helpful and may alter the pretest probability of GIB.
Physical examination
- Hypotension
- Hypotension may be absolute (SBP <90 mmHg; MAP<65 mmHg), relative (eg, a drop in SBP >40 mmHg), or profound (eg, vasopressor-dependent).
- Patients with adequate hemodynamic reserve (e.g. young patients without comorbid conditions) may be initially hypertensive, or normotensive with mild-moderate volume loss. Conversely, not every patient with hypotension has shock. Patients with cirrhosis are moderately hypotensive at their baseline.
- Tachycardia (Sinus tachycardia or atrial fibrillation with rapid ventricular response in patients with pre-existing AF rhythm at their baseline).
- Tachycardia is not sensitive in patients who consume heart rate-lowering agents (e.g. beta blockers etc.). In patients at extremes (impending to crash), the paradoxical bradycardic response may supervene.
- Shock index (SI)
- The SI (HR/SBP) is a more helpful way to understand tachycardia within the context of blood pressure.
- A shock index > 1 suggests significant instability and possible shock.
- Pulse pressure (PP)
- Mild volume loss causes a high cardiac output state with ‘Wide PP’; whereas moderate to severe volume loss results in ‘Narrow PP’ (low cardiac output state).
- Tachypnea
- It is not a specific sign, but is highly sensitive for the presence of occult critical illness.
- Cold/clammy extremities
- Early signs of vasoconstriction with reduced cardiac output.
- Mottling
- This is a specific (but less sensitive) sign for hypoperfusion, implying endogenous vasoconstriction.
- Peripheral cyanosis and increased capillary refill time (in adults>4.5 sec): Imply poor peripheral perfusion.
- Low urine output
- Urine output below 0.5 cc/kg/hr is worrisome for renal malperfusion, however immediately following Foley catheter placement the urine output won’t be known. In this situation scanty and dark urine is worrisome.
- Patients with preexisting chronic kidney disease have low urine at their baseline.
- Urine output below 0.5 cc/kg/hr is worrisome for renal malperfusion, however immediately following Foley catheter placement the urine output won’t be known. In this situation scanty and dark urine is worrisome.
- Pulse oximetry
- Keep in mind that in patients with severe shock, pulse oximetry readings are not reliable.
- Orthostatic hypotension
- Patients with severe volume loss are recognizable! The expected diagnostic value of orthostatic measurements is whether it can recognize patients with moderate volume loss or not?!
- The answer is No.
- Roughly 50% of the healthy population have orthostatic changes in measurement at their baseline. In patients with moderate blood loss, PR change had 22% sensitivity, and BP change had 7-27% sensitivity 7. Orthostatic vital signs are unhelpful. (see more on this)
- The answer is No.
- Patients with severe volume loss are recognizable! The expected diagnostic value of orthostatic measurements is whether it can recognize patients with moderate volume loss or not?!
⚠️Caution: Hemorrhagic shock is notorious for rapid deterioration (in contrast to septic shock which has a slower pace)!
- Signs of peritonitis (acute abdomen)
- This should raise concern for surgical etiologies, therefore more evaluation may be required before endoscopy to exclude conditions like perforation.
- Ascites
- The presence of ascites and other stigmata of chronic liver disease (e.g. jaundice, palmar erythema, etc.) will raise the pretest probability of complications of portal hypertension (esophagogastric varices, portal hypertensive gastropathy).
- Keep in mind that patients with portal hypertension can have other etiologies for upper GI hemorrhage. In one study, approximately 40% of patients with cirrhosis and UGIB had a cause unrelated to portal hypertension 8.
- The presence of ascites and other stigmata of chronic liver disease (e.g. jaundice, palmar erythema, etc.) will raise the pretest probability of complications of portal hypertension (esophagogastric varices, portal hypertensive gastropathy).
- A pulsatile abdominal mass
- This may suggest an aortoenteric fistula as a cause for GIB (more on this below).
▪️Skin
- The presence of jaundice is suggestive of liver disease and possible variceal source for GIB.
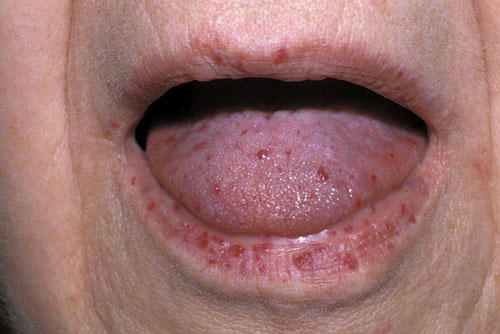
- Patients with hereditary hemorrhagic telangiectasia (HHT) may have skin lesions (telangiectasia shown below) and this will suggest possible GI vascular abnormalities as a cause of GIB.
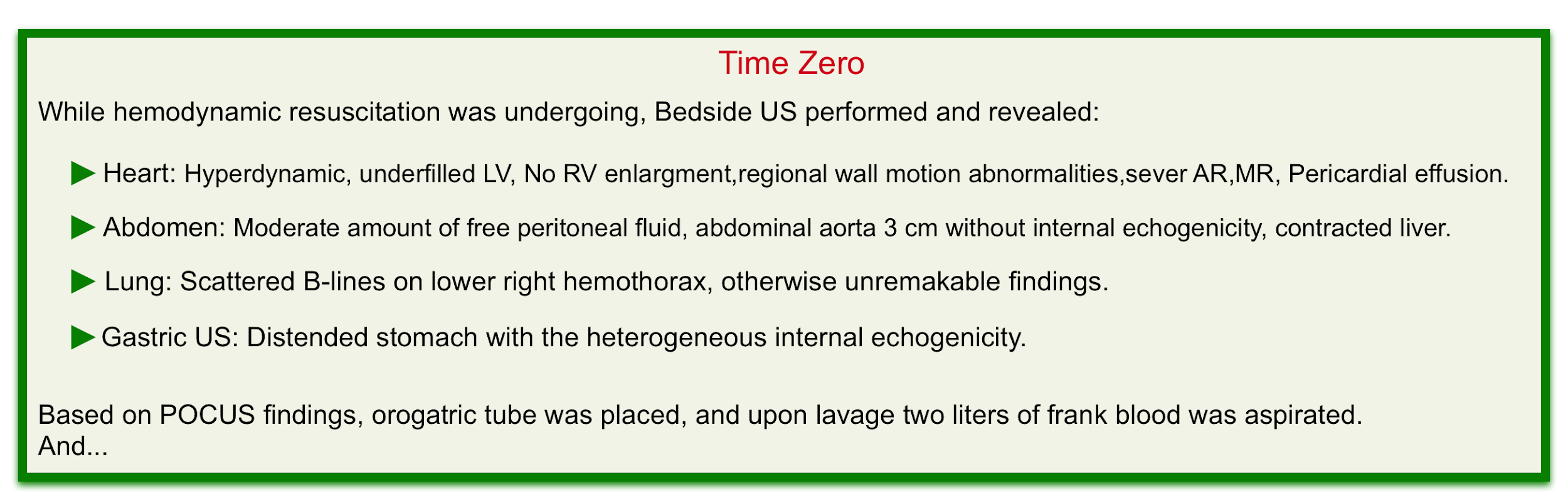
POCUS
Bedside ultrasound evaluation can help clinicians to rapidly diagnose life-threatening conditions and narrow the differential diagnosis (especially in undifferentiated hypotension where the state of shock is recognized but the cause is unknown, and in multifactorial shock, wherein a combination of more than one form of shock is present).
- Hemodynamic evaluation
- Hyperkinetic left ventricle plus virtual IVC can suggest absolute volume loss such as GIB or relative volume loss such as non-resuscitated septic shock.
- A normal or plumped IVC virtually rules out hemorrhagic shock as a cause of a patient’s instability unless there are coexisting conditions that increases the right atrial pressure such as pulmonary embolism, pulmonary hypertension, tricuspid regurgitation, or pericardial disease.
- Abdomen
- In patients with risk factors for abdominal aortic aneurysm presenting with GIB (especially UGIB) always consider aortoenteric fistula in your differential diagnosis.
- Examining the abdominal aorta for aneurysmal changes can shift the diagnostic paradigm in such patients.
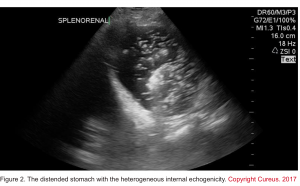
- Gastric ultrasonography
- This is a non-invasive widely available, and rapid method to evaluate both gastric size and content. (see scanning technique). Studies have demonstrated that various contents of the stomach (gas, fluid, and solid) can be differentiated based on their appearance on the ultrasound 9.
- An empty stomach is arguably against active bleeding in the esophagus or stomach (e.g. variceal hemorrhage). Alternatively, a distended stomach (especially if heterogeneous internal echogenicity is seen) may suggest upper GI hemorrhage if the patient hasn’t recently eaten (figure 2) 10.
- For patients undergoing intubation, gastric distension increases the risk of aspiration and NG lavage may be warranted here.
Nasogastric (NG) Lavage
NG tube insertion is one of the most unpleasant and inhumane procedures and should be avoided unless specifically indicated. Regarding the evaluation and treatment of GIB, indications for NG tubes differ widely among practitioners. Recent studies reveal that the true clinical value of an NG tube in patients with GI bleeding is probably less than what traditionally was believed {11, 12, 13}.
- Diagnostic indications
- Patients with very high clinical suspicion for UGIB (a significant amount of proven bloody vomitus) or those with presumptive upper source (e.g. melena, age <50 y/o; hematocrit <30) do not need NGT insertion for diagnosis. On the other hand, in the following conditions, NGT insertion may have diagnostic yields:
- Suspected history of bloody vomitus: a bloody aspirate may confirm UGIB.
- Hematochezia: Up to 10% of patients with hematochezia have an upper GI source of bleeding. In the presence of findings that are suggestive of upper GI bleeding (e.g. history of UGIB, cirrhosis, BUN/Cr >30, epigastric discomfort), insertion of NGT may be helpful diagnostically. In this situation, a bloody lavage signifies an upper source of bleeding and will determine the next appropriate diagnostic approach (upper endoscopy).
- Hypovolemic shock: In patients presenting with a hemodynamic profile of hypovolemic shock with minimal/no hematemesis, NG tubes can provide the earliest indication of ongoing high-volume esophageal or gastric bleeding and thus they may benefit from an early upper endoscopy.13
- Patients with very high clinical suspicion for UGIB (a significant amount of proven bloody vomitus) or those with presumptive upper source (e.g. melena, age <50 y/o; hematocrit <30) do not need NGT insertion for diagnosis. On the other hand, in the following conditions, NGT insertion may have diagnostic yields:
- Caveats
- An NG aspirate is diagnostically unhelpful unless frankly bloody fluid is obtained. Small bits of darkish material, bloody mucus, or positive guaiac tests probably represent sequelae of the procedure, whereas a clear appearance and negative tests still miss most bleeding distal to the stomach (sensitivity 50%). Patients with a bleeding pattern indicative of a Mallory-Weiss tear may need neither an NG tube nor an endoscopy.
- Therapeutic indications
- Reducing the risk of aspiration (when endotracheal intubation is anticipated): If intubation is indicated in patients with GI bleeding, NG lavage may help empty the gastric contents and reduce the risk of aspiration, especially if the stomach is full and vomiting is uncontrolled.
- Providing a clear endoscopic field (at the discerning of gastroenterologists)
- ⛔️Contraindication for NG tubes
- Midface injury, basilar skull fracture.
- Coagulopathy.
- In the presence of coagulopathy or midface injury; orogastric placement may be a better option.
- Notice: Esophageal varices are not a contraindication for NG tube insertion.
- History of gastric bypass.
- Esophageal stricture or alkali injury.
Laboratory evaluation
- CBC
- The initial Hb and HCT level in patients with acute GI bleeding is at the patient’s baseline because the patient is losing whole blood.
- ⚠️Worrisome findings
- A normal Hb in a patient with hemodynamic instability is a worrisome finding.
- A failure to respond appropriately to transfusion signifies ongoing bleeding and is assumed to be a worrisome finding.
- Remember that transfusion of a unit of packed cells should increase Hb by ~1 g/dL.
- ✅Reassuring finding
- A very low Hb (e.g. Hb < 6 g/dL) in an otherwise hemodynamically stable patient with minimal symptoms suggests chronic bleeding with a very low risk of rapid deterioration {a very low Hb in a stable patient should not invoke panic!}.
- These patients have chronic bleeding (e.g. for days, or weeks), meanwhile gradually retaining volume to compensate (isovolumic anemia).
- The only immediate threat to these patients is iatrogenic (i.e. giving blood products too rapidly will put them in a volume overload state).
- Ideal management may involve gradual replacement of blood often in combination with diuretics.
- A very low Hb (e.g. Hb < 6 g/dL) in an otherwise hemodynamically stable patient with minimal symptoms suggests chronic bleeding with a very low risk of rapid deterioration {a very low Hb in a stable patient should not invoke panic!}.
- BMP
- Bleeding from the upper GI tract may elevate BUN levels through the digestion and absorption of hemoglobin.
- A BUN/Cr ratio >30 may suggest UGIB.
- Bleeding from the upper GI tract may elevate BUN levels through the digestion and absorption of hemoglobin.
- LFTs
- Serum lactate
- The best-established cut-off for arterial lactate is 2.0 mmol/L *,*.
- Arterial lactate is preferable since venous lactate is generally higher than arterial lactate.
- Arterial lactate is preferable since venous lactate is generally higher than arterial lactate.
- An elevated serum lactate is an early indicator of shock * and is particularly useful in the identification of occult shock (those who are normotensive or even hypertensive). However, elevated lactate elevation has a broad differential diagnosis (more on this here).
- Normal lactate can be seen in a shock state!
- The best-established cut-off for arterial lactate is 2.0 mmol/L *,*.
- Coagulation studies {PT, PTT, and possibly Thromboelastography (TEG)}
- Type and Crossmatch
- Troponin and ECG
- Subsequent anemia from a GI bleed can reduce a patient’s oxygen-carrying capacity.
- Patients with GI bleeding who are more than 40 years old, patients with any symptoms of ischemia, and patients with known coronary artery disease should be screened for potential myocardial ischemia.
- Subsequent anemia from a GI bleed can reduce a patient’s oxygen-carrying capacity.
Differential diagnosis (DDx)
- DDx of hematemesis
- Posterior epistaxis
- Hemoptysis
- DDx of hematochezia
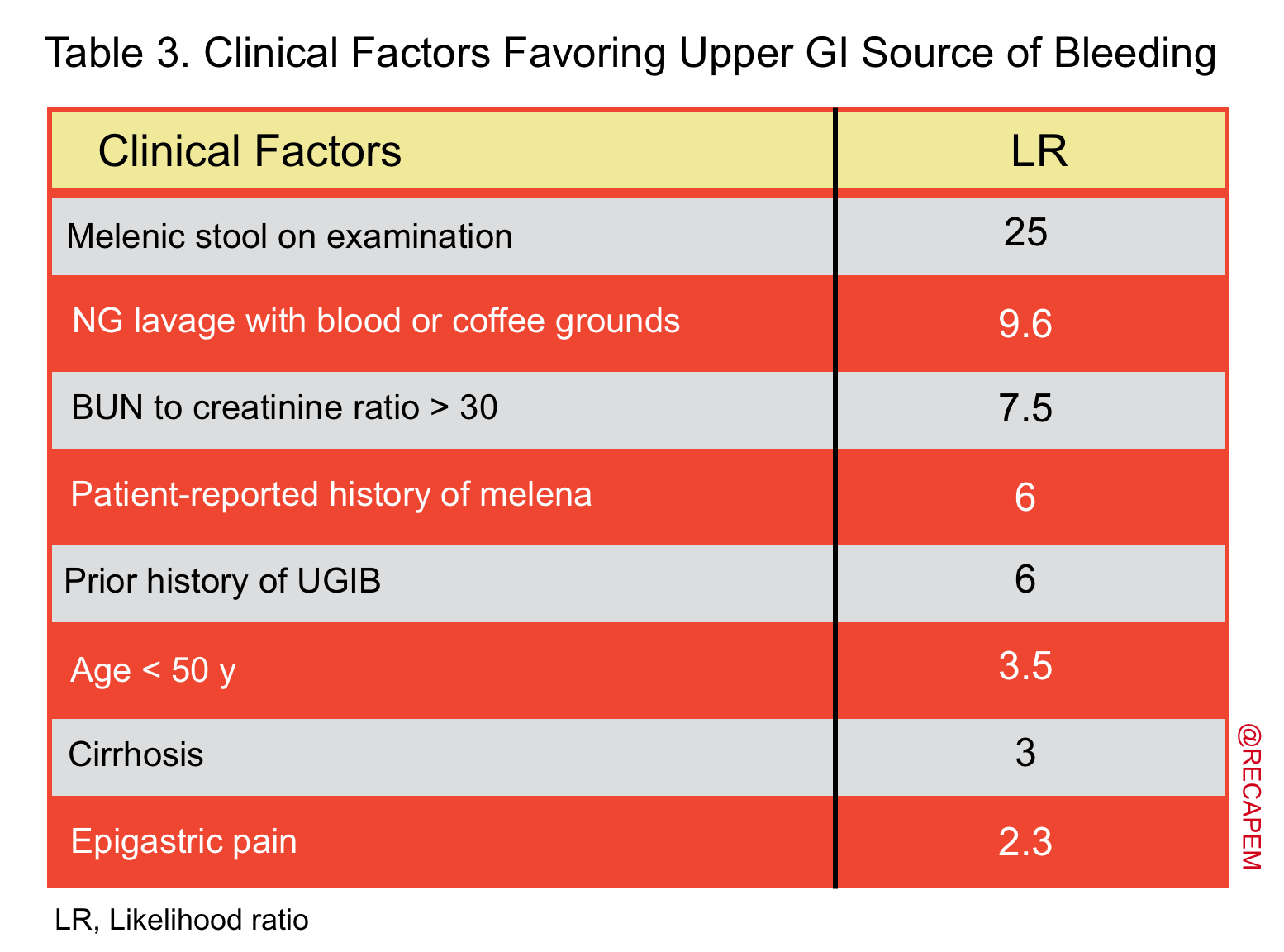
- UGIB: 10-15% of patients with severe hematochezia have an upper GI source 14.
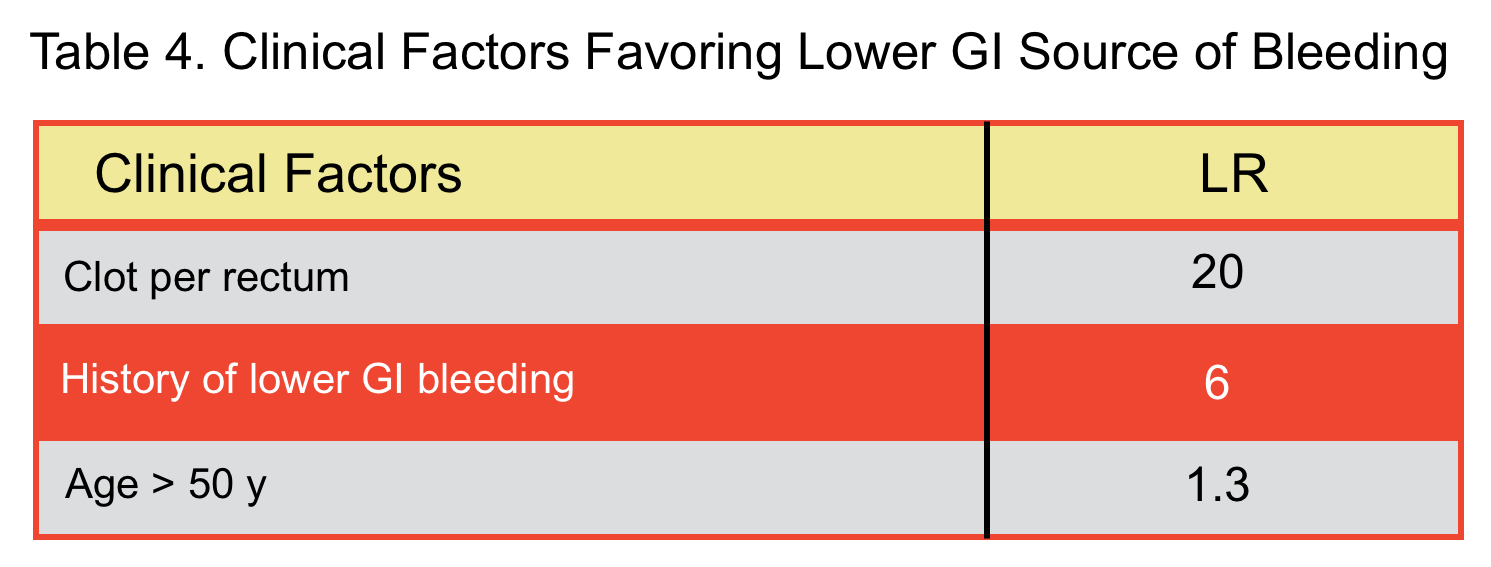
- In a meta-analysis, factors that were predictive of blood coming from upper GI source (table 3) and lower GI source (table 4) were identified 15.
- Bloody diarrhea: Technically it is GI bleeding, though bleeding is not the main problem.
- UGIB: 10-15% of patients with severe hematochezia have an upper GI source 14.
- The DDx of hypovolemic hemorrhagic shock (in non-traumatic patients) include:
- GI bleeding.
- Ruptured aortic aneurysm.
- Ruptured ectopic pregnancy.
- Uterine or vaginal hemorrhage.
- Postpartum hemorrhage.
- Spontaneous retroperitoneal or peritoneal hemorrhage due to bleeding diathesis.
- 💡Caution: The presence of free peritoneal fluid (on a physical or ultrasonographic exam) should also make you consider hemoperitoneum.
- Spontaneous rupture of the spleen has been reported in patients with severe portal hypertension 16.
- 💡Caution: The presence of free peritoneal fluid (on a physical or ultrasonographic exam) should also make you consider hemoperitoneum.
- Hemorrhagic pancreatitis.
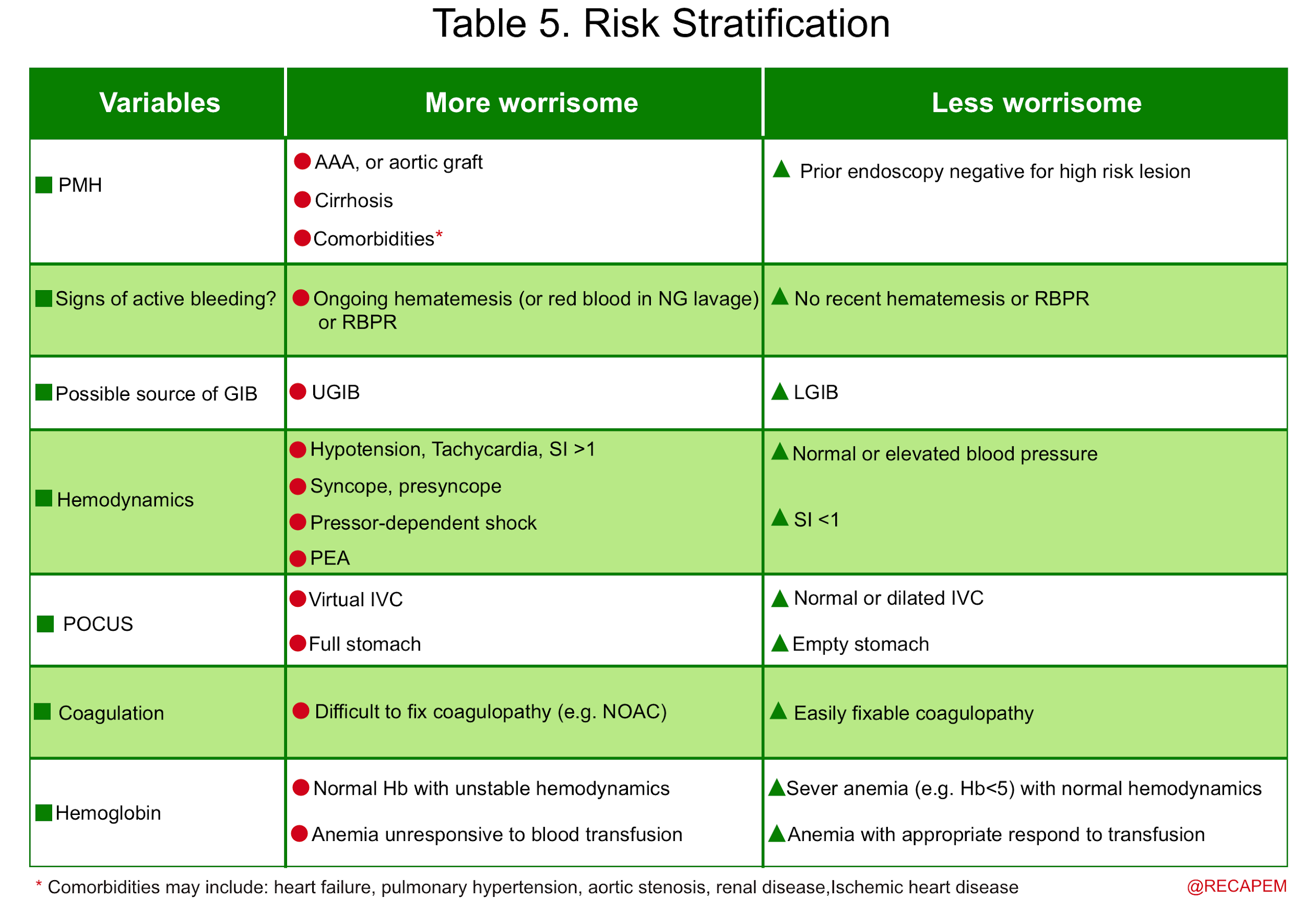
Risk stratification
Clinical, endoscopic, and laboratory features may be useful for risk stratification of patients who present with acute GI bleeding (table 5) {15 17 18 19 20 21 22}
Management
Resuscitation
The principles of management and basic resuscitation of patients with clinically significant GI bleeding and unstable hemodynamics are reviewed here. These patients require bedside cardiac monitoring, close monitoring of the airway, clinical status, urine output, and NGT output (if it has been placed).

- IV access
- Two large-bore peripheral IV lines (16 gauge or larger) and/or a central access.
- Patients predicted to need massive transfusion require central access via either a multi-lumen access catheter (MAC), or hemodialysis catheter, or the standard central line.
- Arterial line
- For patients undergoing massive transfusion, consider emergent placement of an arterial line (via femoral, or brachial artery).
- An arterial catheter is invaluable when running a massive transfusion to titrate vasopressors and avoid overshooting hypertension (which may encourage re-bleeding).
- The goal of hemodynamic resuscitation is to protect effective tissue perfusion. Keep in mind that this does not necessarily translate to (optimizing MAP).
- Currently, most experts target a MAP of 60 to 65 mmHg.
- ⭐️Studies have shown that higher blood pressure targets were not associated with lower mortality in critically ill hypotensive patients. In contrast, a higher MAP (e.g. >70 mmHg) may contribute to the worsening of GI bleeding by establishing a higher driving pressure behind the bleeding site.
- Anecdotally patient is given whatever he or she is losing, in this case, the blood. Filling the tank with fluid instead of blood can cause several undesired effects such as:
- Dilutional anemia
- The administration of excessive fluids may cause a relative, but not an absolute reduction in Hb concentration, RBC count, or Hct, which is termed ‘dilutional anemia’. This will result in a loss of RBC-filled capillaries, leading to a reduction in oxygen-carrying capacity and worsening the effective tissue perfusion. {23 24}
- Dilutional coagulopathy
- The adverse effect of liberal fluid administration on coagulation has been well-studied in trauma literature. The fluid causes dilutional coagulopathy and clot disruption from increased blood flow; resulting in worsening bleeding diathesis 25.
- Rebound portal hypertension
- Patients with cirrhosis are in a sodium-avid state (volume overload) at their baseline. Overzealous fluid administration will transiently increase (mean systemic filling pressure; i.e. the driving pressure within the venous system). A fraction of this increased venous pressure is translated to the portal vein and contributes to worsening portal hypertension 26.
- Dilutional anemia
- Patients with frankly severe ongoing blood loss should be transfused immediately with type O blood (women of childbearing age should be transfused with O-negative blood) while being typed and cross-matched for blood transfusion.
- If you choose crystalloid, just give a small amount!
- Extrapolated from trauma literature, volume replacement of 1.5 L or more in the emergency department has been shown to be an independent risk factor for mortality in trauma patients.27
- GI bleeding is a frequent indication for red cell transfusion because acute blood loss can decrease oxygen carrying capacity of the blood and therefore impairs tissue perfusion.
- Indications
- Unstable massive (exsanguinating) GI bleeding
- These patients require a blood transfusion from the very early of their presentation.
- Stable but active GI bleeding
- In patients with active bleeding who become hemodynamically stable, indications for blood transfusion is hemoglobin < 7 mg/dL, except for patients with active acute coronary syndrome, where the Hb threshold for transfusion is < 9 mg/dL.
- ⭐️This recommendation is based on a randomized trial that showed liberal transfusion (transfusion threshold with Hb <9mg/dL) is associated with increased mortality compared to a restrictive strategy (transfusion threshold with Hb < 7mg/dL).{28, 29}
- Unstable massive (exsanguinating) GI bleeding
- ⚠️GI bleeding can rapidly decompensate. If you’re worried that the patient will deteriorate, request 4 units of PRBC on hold in the blood bank.
- Does balanced transfusion (1:1:1 ratio of PRBC:FFP: PLT) improve the outcome in patients who require < 3 units of blood within an hour?
- No. In patients who by definition are not candidates for massive transfusion protocol (see below), administration of FFP, and PLT does not improve morbidity and mortality. Therefore if a patient requires two units of blood, administration of FFP, and PLT is not warranted.
- It is historically defined as the transfusion of > 10 units of packed red blood cells per 24 hours and was shown to be associated with multiple hemostatic and metabolic complications.
- This definition is retrospective and arbitrary. Alternative definitions accounting for rate and timing will provide a more accurate conceptual framework.
- The critical administration threshold (CAT) redefined MT as 3 units of red blood cells over one hour 30.
- This allows early identification of critical patients at greater risk of death from bleeding compared to traditional MT.
- The critical administration threshold (CAT) redefined MT as 3 units of red blood cells over one hour 30.
- Massive transfusion protocol is activated in situations such as:
- More than 3-4 units of blood are administered within an hour.
- Massive exsanguinating bleeding from the sites that is difficult to access immediately.
- Severe instability (vasopressor-dependent) from hemorrhagic shock.
- When MTP is activated, blood products will be provided with a 1:1:1 ratio of PRBC:FFP: PLT (balanced resuscitation) 31.
- Preparation and considerations for patients requiring massive transfusion include:
- Central access preferentially via multi-lumen access catheters or hemodialysis catheters.
- Arterial line to closely monitor blood pressure, since overly aggressive resuscitation can increase the risk of rebleeding.
- Cryoprecipitate, calcium (IV) may be considered.
Coagulopathy
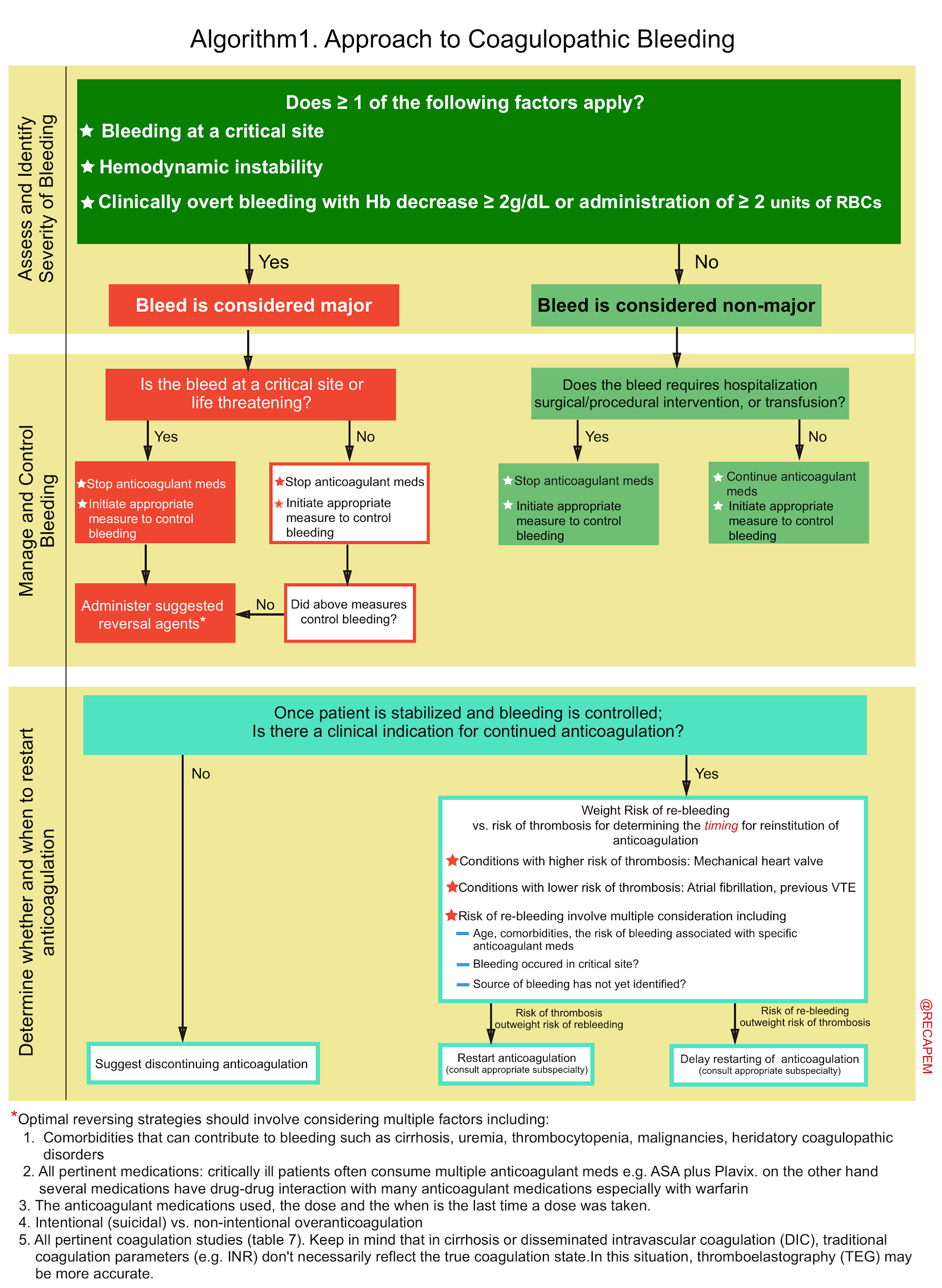
▪️Key considerations. There are several key points to consider in the approach to patients with coagulopathy and major bleeding (algorithm 1):
- 1# Assess severity of bleeding: By definition, if there were ≥ 1 of the following factors, the bleed is classified as ‘Major’ 32:
- Critical site bleeds
- These are bleeds that compromise the organ’s function.
- Intracranial hemorrhage and other central nervous system bleeds (e.g. intraocular, spinal) and thoracic, intra-abdominal, retroperitoneal, intra-articular, and intramuscular bleeds are considered critical as they may cause severe disability and require surgical procedures for hemostasis.
- Intraluminal gastrointestinal (GI) bleeding is not considered a critical site bleed; however, it can result in hemodynamic compromise.
- These are bleeds that compromise the organ’s function.
- Hemodynamic instability.
- Overt bleeding with hemoglobin drop ≥2 g/dL or administration of ≥2 units of packed RBCs.
- Critical site bleeds
- 2# Determine pertinent comorbidities and all medications that can contribute to bleeding: Critically ill patients often have multiple coagulopathies. Obtain full history, physical exam, and coagulation studies to get a global sense of how coagulopathic the patient is.
- Comorbidities that can contribute to bleeding may include uremia, thrombocytopenia, cirrhosis, malignancies, and hereditary coagulopathic disorder.
- The coagulopathy of cirrhosis is more complicated and is discussed separately (more on this below).
- Review all medications that the patient is taking which may affect coagulation.
- Determine the doses of medications and the last time a dose was taken.
- Comorbidities that can contribute to bleeding may include uremia, thrombocytopenia, cirrhosis, malignancies, and hereditary coagulopathic disorder.
- 3# Determine the etiology for which the patient was initially anticoagulated: As a principal, always consider the indication for which the patient is consuming anticoagulants (e.g. atrial fibrillation) and weight against the severity of the bleeding (major GIB, vs non-major GI bleeds) to determine how aggressively to reverse anticoagulation.
- Most patients are anticoagulated for atrial fibrillation or deep vein thrombosis. These are low-risk conditions wherein short-term interruption is not problematic.
- Some patients are anticoagulated for higher-risk conditions such as mechanical valve prosthesis (which has a higher risk for thrombosis). This may shift the risk/benefit ratio.
- 4# Assess for the requirement of reversing anticoagulation: Life-threatening bleeding requires aggressive normalization of coagulation parameters, but minor bleeding may respond to local measures.
▪️The algorithmic approach to coagulopathic bleeding
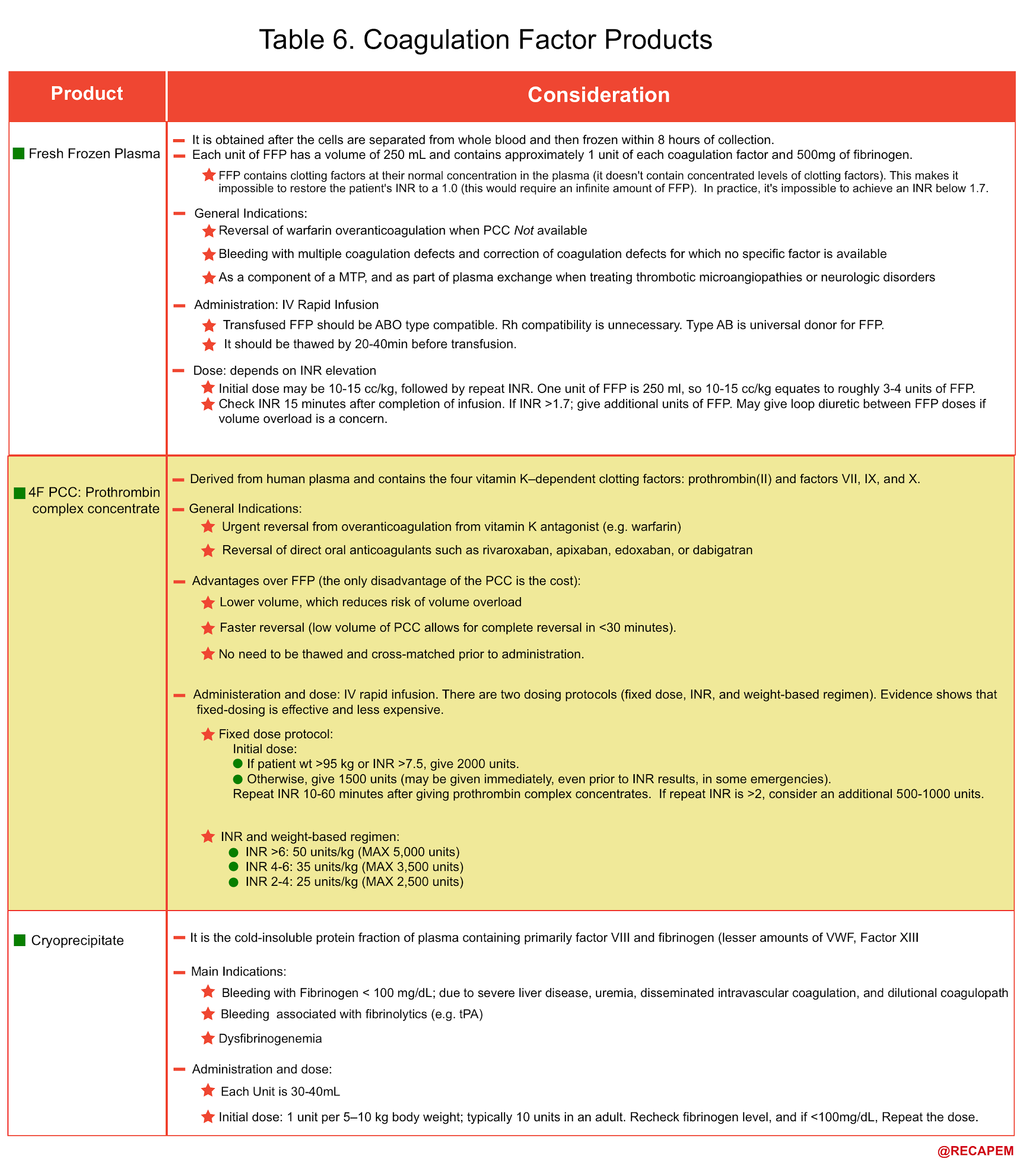
- The common coagulation factor products that may be used in coagulopathic patients with life-threatening bleeding are mentioned below (table 6).
- Non-cirrhotic coagulopathic patients with life-threatening bleeding and INR > 2 should generally be transfused with prothrombin complex concentrate (PCC) or FFP (Plus vitamin K in the case of warfarin over-anticoagulation) 33. See table 6,7.
- For patients with thrombocytopenia, uremia, and antiplatelet consumption: Consider DDAVP, and platelet transfusion (if PLT < 50.000u/L).
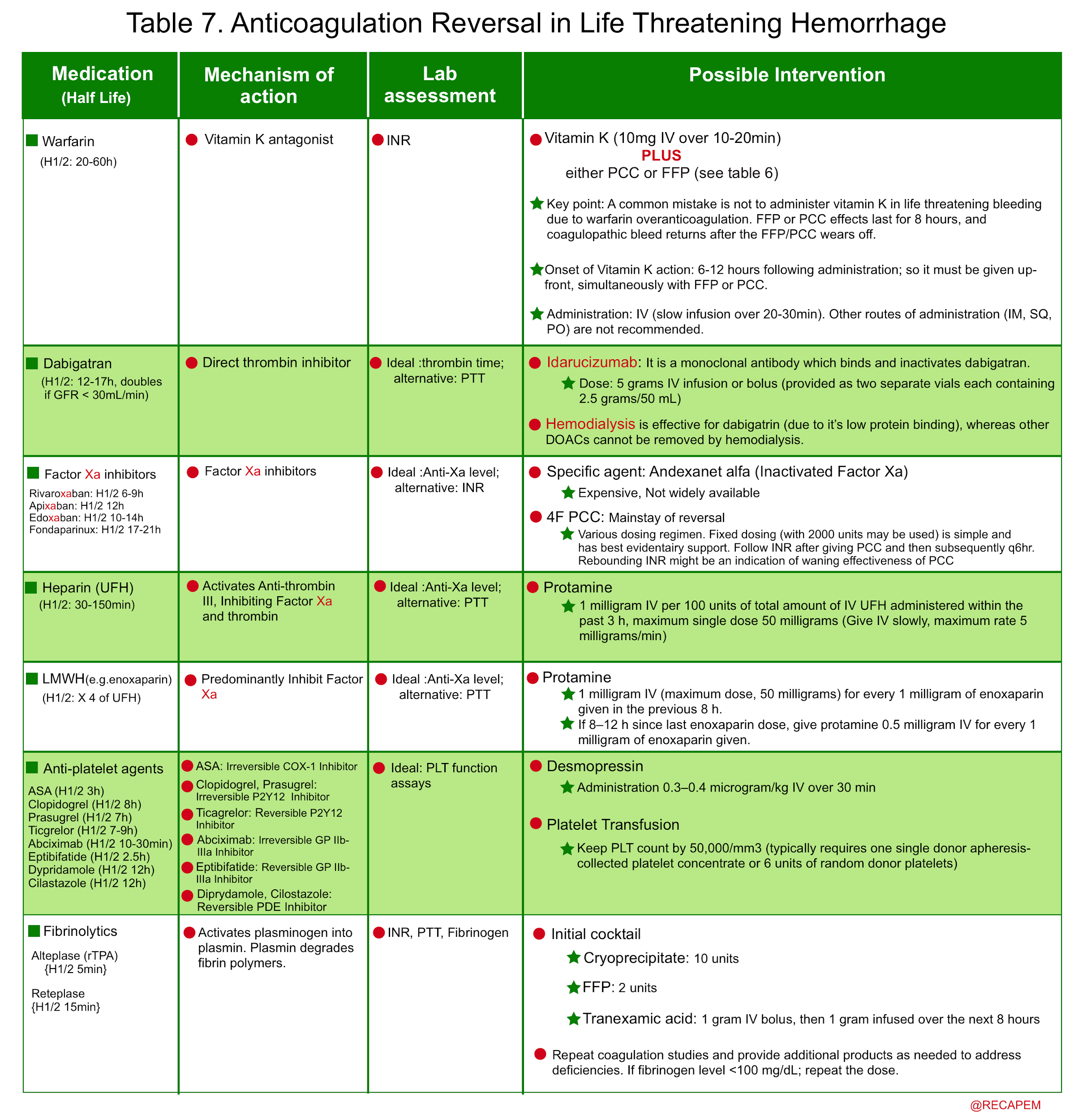
- The principles of anticoagulation reversal in life-threatening hemorrhage is summarized below.
Medications
- Proton Pump Inhibitors (PPI)
- Patients with possible acute upper GI bleeding are given PPI. However the benefit of PPI administration ‘before endoscopy’ is still unclear (Does patients with variceal bleeding benefit from PPI too?).
- At the current time, most experts agree on the administration of PPI for acute UGIB.
- Administration protocol: Evidence showed that intermittent IV bolus is comparable to continuous infusion 34. The recommended dose for patients with acute UGIB is to give a high dose IV bolus (e.g. 80 mg pantoprazole), then 40mg IV q12hr.
- Patients with possible acute upper GI bleeding are given PPI. However the benefit of PPI administration ‘before endoscopy’ is still unclear (Does patients with variceal bleeding benefit from PPI too?).
- Octreotide
- The benefit of octreotide administration in variceal GI bleeding is relatively clear. It decreases portal vein pressure and, therefore theoretically reduces variceal bleeding. In non-variceal upper GI hemorrhage, there is some weak evidence that octreotide administration was associated with a reduced risk of continued bleeding 35.
- In patients with possible variceal bleeding, octreotide is given as a 50 mcg bolus followed by a continuous infusion at a rate of 50 mcg/hr.
- Tranexamic acid
- There is no compelling evidence for its role in the treatment of GI bleeding. In a recent randomized trial (HALT-IT) use of tranexamic acid did not improve death due to bleeding within 5 days in patients with acute GI bleeding 36.
- Antibiotics
- Patients with cirrhosis and portal hypertension have a favorite milieu for gastrointestinal bacterial translocation and infection, especially during the decompensated stage 37. Several trials have shown that antibiotic administration is associated with reduced mortality in patients with variceal hemorrhage 38.
- The recommended agent is usually ceftriaxone 1 gram IV daily.
Intervention
Upper endoscopy is often the first-line option for both diagnostic and therapeutic purposes in patients with acute UGIB. The severity of bleeding may determine the optimal time for endoscopy. Patients with initial hemodynamic instability deserve more urgent intervention. (EM:RAP: timing of endoscopy for acute UGIB)
Risks of upper endoscopy
- It’s relatively a safe procedure. The complications include pulmonary aspiration, GI perforation, and increasing bleeding while attempting therapeutic intervention.
- Patients should be optimally resuscitated and hemodynamically stable before undergoing endoscopy (since they may crash during the procedure). Data suggests that most patients do not need to have normal hematocrit in order to safely undergo endoscopy 39. Anticoagulation reversal should not delay urgent endoscopy. Evidence shows that endoscopy is safe in patients who are mild to moderately anticoagulated (e.g. INR< 2.5) 40.
Endotracheal intubation
- Intubation may be indicated when there are high-risk features for aspiration such as for unconscious patients unable to protect their airway or patients with massive upper GI bleeding (possibly variceal hemorrhage). Studies have shown that endotracheal intubation is not a benign procedure in patients with GI bleeding and is associated with worse outcomes 41. Be prepared for hemodynamic decompensation and massive aspiration.
- First resuscitate: Optimize hemodynamics. Consider initiation of vasopressor before intubation, to avoid hemodynamic collapse.
- Reduce the risk of aspiration: If gastric ultrasound shows distention, the following measures may reduce the risk of aspiration:
- NG suction
- Prokinetic agents: may consider erythromycin or metoclopramide to promote gastric emptying before the procedure. Erythromycin can be given as a 3 mg/kg IV infusion over 5 minutes, 15 minutes before the procedure.42
Procedural sedation for upper endoscopy in non-intubated patients
- If you were asked to provide sedation for endoscopist, consider the following:
- Pretreatment with antiemetics such as ondansetron.
- Provide short-acting titratable drugs with both analgesic properties (fentanyl) and sedative properties (midazolam or propofol) 43
- Consider cardiostable agents such as ketamine, and etomidate in unstable patients.
- Patients with alcoholism may be ketamine resistant, which can be overcome by using higher doses.
Management of re-bleeding
- This will depend on the lesion seen initially. However, the usual sequence of events is as follows:
- 1st line: Repeat endoscopy.
- 2nd line: Interventional radiology.
- 3rd line: Surgery.
Bleeding in cirrhosis
Portal hypertension
▪️Portal hypertension develops when there is resistance to portal blood flow.
▪️The etiologies for portal hypertension (figure 3) 44 can be classified as:
- Prehepatic (e.g. portal vein thrombosis).
- Intrahepatic (e.g. cirrhosis).
- Post-hepatic (eg, Budd-Chiari syndrome).
▪️Portal hypertension is often asymptomatic until complications such as ascites and variceal bleeding develop 45.
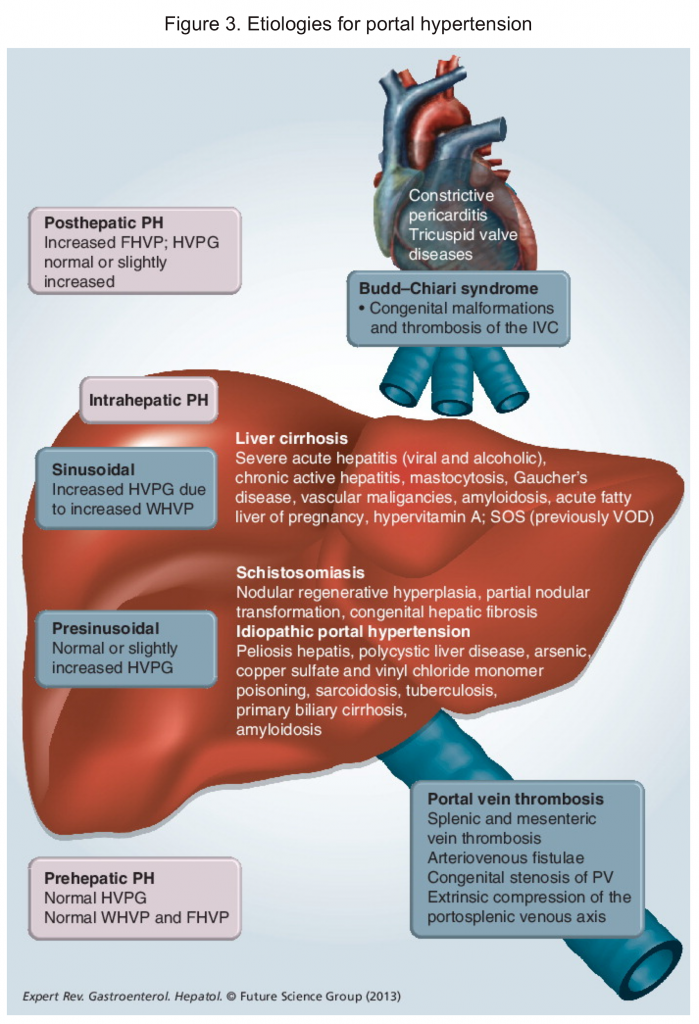
▪️Portal hypertension can cause upper GI bleeding via several distinct pathologic lesions including
- Esophageal varices
- Portal hypertensive gastropathy
- Gastric varices
- Ectopic varices.
▪️It should be kept in mind that patients with portal hypertension and cirrhosis can develop UGIB from sources unrelated to portal hypertension (eg, peptic ulcer disease). Since the most common cause of portal hypertension is cirrhosis, the focus of the following discussion is centered on cirrhotic patients.
Esophagogastric variceal hemorrhage
Patients with variceal hemorrhage present with hematemesis, melena, and hematochezia (brisk bleeders) similar to that seen in patients with GI bleeding from non-variceal lesions. Successful resuscitation of these patients requires understanding the basic mechanism and pathophysiology of variceal hemorrhage.
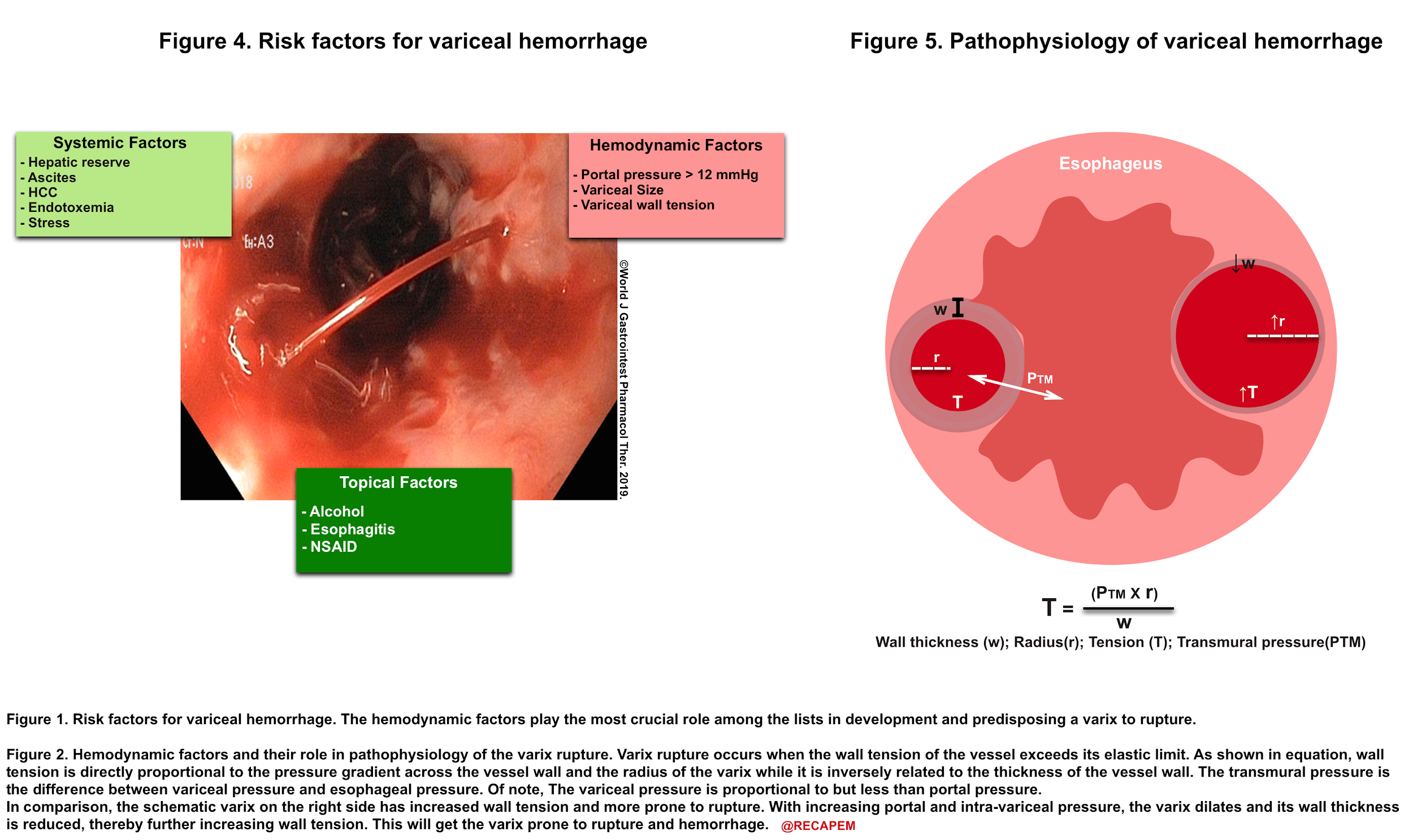
▪️Pathophysiologic principles of portal hypertension and varix formation and rupture in patients with cirrhosis
- In cirrhotic patients portal hypertension results from the combination of both abnormally high portal venous blood flow and increased resistance to flow through the cirrhotic liver. Over time the high-pressure system within the portal vein gives rise to the formation of varices by dilating portosystemic venous connections 46.
- Once varices are formed; the variceal hemorrhage can be caused by a wide range of factors including topical, portal hemodynamic, and systemic factors (figure 4). Portal pressure plays the most crucial role in the pathophysiology of variceal hemorrhage among the list (figure 5).
- Clinical implication:
- The increasing intra-variceal pressure (which is approximately equal to portal pressure) plays a pivotal role in variceal hemorrhage (explained in Figure 5). Unlike most other GI bleeders, the variceal one is from the venous side.
- Over-resuscitation of patients with variceal bleeding will increase the central venous pressure and this will directly increase portal venous pressure and risk of bleeding 47.
- Keeping in mind that cirrhotic patients usually have low MAP at their baseline (chronic vasodilation); so a borderline hypotension is preferable to higher MAPs (avoiding large-volume resuscitation) 48.
- Blackmore tube placement (media below)
- Patients with UGIB due to esophageal variceal hemorrhage often have advanced liver disease and can arrive at the ED with airway compromise, hemodynamic instability, thrombocytopenia, and coagulopathy. Managing these patients can be a formidable challenge in ED. Endoscopy by a gastroenterologist remains the “gold standard” for the diagnosis and treatment of acute variceal hemorrhage. However, sometimes endoscopy cannot be performed in unstable patients with exsanguinating bleeding or the consultant physicians are unavailable, and medical therapy has failed to stop the bleeding and stabilize the patient’s hemodynamics.
- Therefore, it is significantly important that clinicians have the required skills to temporarily stop the bleeding. Balloon tamponade of esophageal varices often can be done quickly in ED with high success rates in controlling the initial hemorrhage.
- Abdominal paracentesis
- It has been shown that total volume paracentesis in patients with tense ascites will improve portal hemodynamics and decrease variceal pressure, size, and wall tension 60.
Coagulopathy in cirrhosis
▪️Pathophysiologic principles of coagulopathy in cirrhotic patients
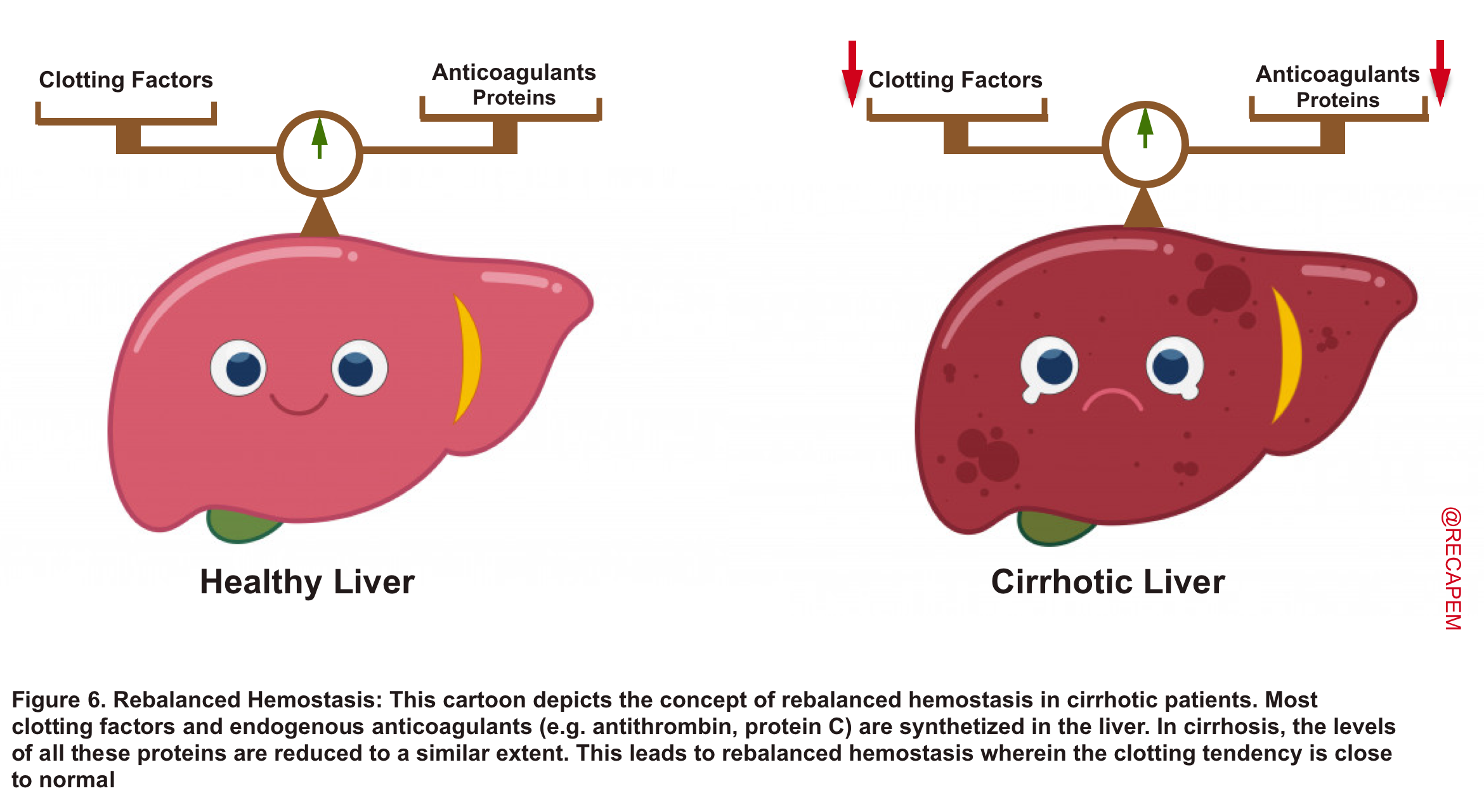
- The coagulopathies in cirrhosis are complex. Most procoagulants (clotting factors) and endogenous anticoagulants are synthesized in the liver. In advanced liver diseases, levels of all these proteins are reduced to a similar extent. This leads to ‘rebalanced hemostasis’ wherein the clotting tendency is close to normal (figure 6).
- The INR measures the level of several clotting factors (fibrinogen, factors II, V, VII, X; involved in the extrinsic pathway).
- Since most of these proteins are synthesized in the liver, it is obvious that INR will be elevated. However, the INR is only evaluating half of the picture!
- An increased INR in this setting gives the false impression of heightened bleeding risk (reduced clotting factors), while in fact anticoagulant protein synthesis is reduced as well, and a rebalanced hemostasis is reached.
- The INR measures the level of several clotting factors (fibrinogen, factors II, V, VII, X; involved in the extrinsic pathway).
- Hyperfibrinolysis in cirrhosis.
- The liver synthesizes fibrinogen, and also clears the blood from endogenous fibrinolytic (e.g. tissue plasminogen activator).
- In advanced liver disease, both fibrinogen level is reduced and clearance of endogenous fibrinolytics is elevated, resulting in hyperfibrinolysis 49.
- Clinical implication
- The concept of rebalanced hemostasis explains some otherwise puzzling findings in cirrhosis. This explains why the INR fails to predict bleeding following procedures and also how patients may experience portal vein thrombosis or deep vein thrombosis despite an elevated INR.
- In patients with cirrhosis, INR is not an accurate measure for hemostasis, and therefore INR elevation in cirrhosis is meaningless and doesn’t necessarily correlate with actual coagulation tendency. Thromboelastography (TEG) is more accurate here.
- In hemodynamically stable cirrhotic patients with inactive GI bleeding (e.g. melena): Perform ‘TEG’. Elevated INR is meaningless. The routine administration of PCC or FFP is not recommended (50 51).
- In unstable cirrhotic patients with active massive bleeding (e.g. massive hematemesis, hematochezia), regardless of INR; one may be more likely to administer FFP or PCC (especially when there is evidence of true hypocoagulopathy on TEG).
- No recommendations can be given regarding platelet transfusion in patients with variceal hemorrhage, however, some experts recommend keeping PLT count > 50.000 in patients with active bleeding 52.
- May consider cryoprecipitate transfusion for bleeding cirrhotic patients to increase the fibrinogen level over 150 mg/dL 53.
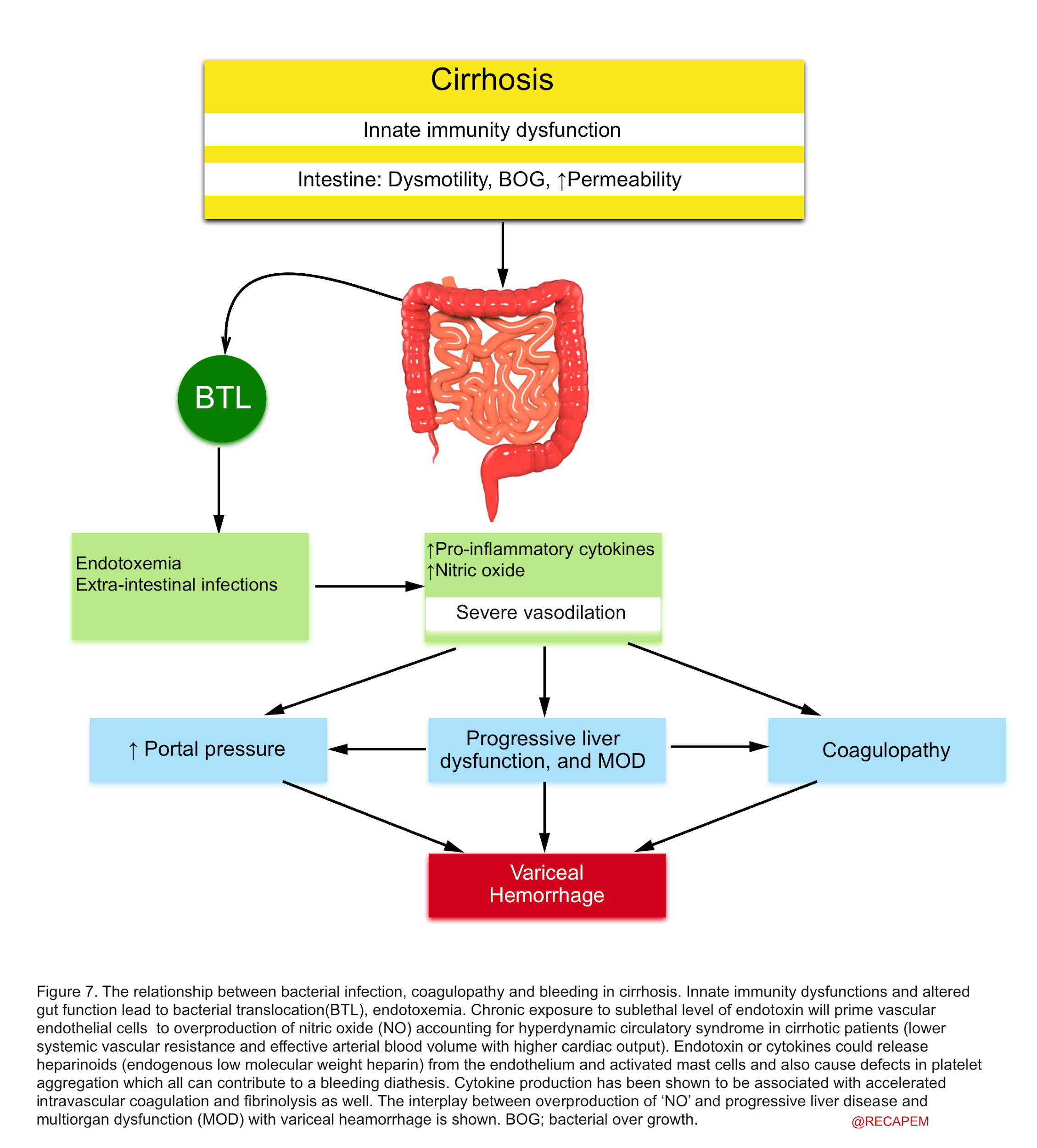
▪️Pathophysiologic principles of infection in cirrhotic patients and its relation with bleeding and coagulopathy
- Liver dysfunction will impose cirrhotic patients at increased risk of bacterial infection 54.
- Several studies have shown the importance of infection in pathophysiologic mechanisms in cirrhosis and its association with variceal hemorrhage {55,56}, abnormalities in coagulation57, vasodilatation of the systemic vasculature 58, and worsening liver function 59.
- Patients with cirrhosis and portal hypertension have a favorite milieu for gastrointestinal bacterial translocation and infection, especially during the decompensated stage 37. There’s a close relationship between bacterial infection, bleeding, and coagulopathy (figure 7).
- Clinical implication
- Trials have shown that antibiotic administration is associated with reduced mortality in patients with variceal hemorrhage 38.
- Patients require a thorough workup to determine possible etiologies for decompensated chronic liver disease (e.g. infection, mesenteric vein thrombosis, etc.).

Management of variceal bleeding in patients with cirrhosis
▪️In patients with exsanguinating variceal hemorrhage and unstable hemodynamics; aggressive mindful resuscitation is warranted to optimize tissue perfusion.
- Emergent endoscopy for unstable actively bleeding patients is recommended.
- Transjugular Intrahepatic Portosystemic Shunting (TIPS) is often the second step if endoscopy fails.
- Blackmore tube may be used as a last-ditch effort (e.g. to bridge an exsanguinating patient to TIPS) 61.
- Hematologic management
- Activate MTP for actively bleeding patients who require 3-4 unit PRBC per hour.
- In hemodynamically stable patients, the trigger of red cell transfusion is hemoglobin < 7mg/dL 62.
- ⚠️INR is misleading in cirrhotic patients and oftentimes trying to normalize the INR in hemodynamically stable patients with FFP, PCC is futile and a wrong move 63.
- Platelet transfusion may be considered in actively bleeding patients with a PLT count < 50.000.
- May consider cryoprecipitate transfusion for bleeding cirrhotic patients to increase the fibrinogen level over 150 mg/dL.
- Adjuvant measures that reduce portal vein pressure help stop bleeding in patients with active variceal hemorrhage:
- Octreotide: Initial IV bolus of 50 micrograms (can be repeated in the first hour if ongoing bleeding), then continuous IV infusion of 50 µg/hr for two to five days.
- Vasopressin: Continuous IV infusion: 0.2‐0.4 U/min; can be increased to 0.8 U/min for 24 hours 64.
- Abdominal paracentesis. More on this, here.
- Antibiotics: Ceftriaxone 1gr IV.
- Evaluate for infection
- Be prepared for hepatic encephalopathy as UGIB is the common precipitator.
- Have a low threshold to initiate lactulose +/- rifaximin for patients who develop delirium.
Approach to lower GI bleeding (LGIB)
- The hemorrhagic manifestations of LGIB are clot per rectum and hematochezia. As explained earlier, hematochezia is due to lower GI bleeding almost 85% of the time 65.
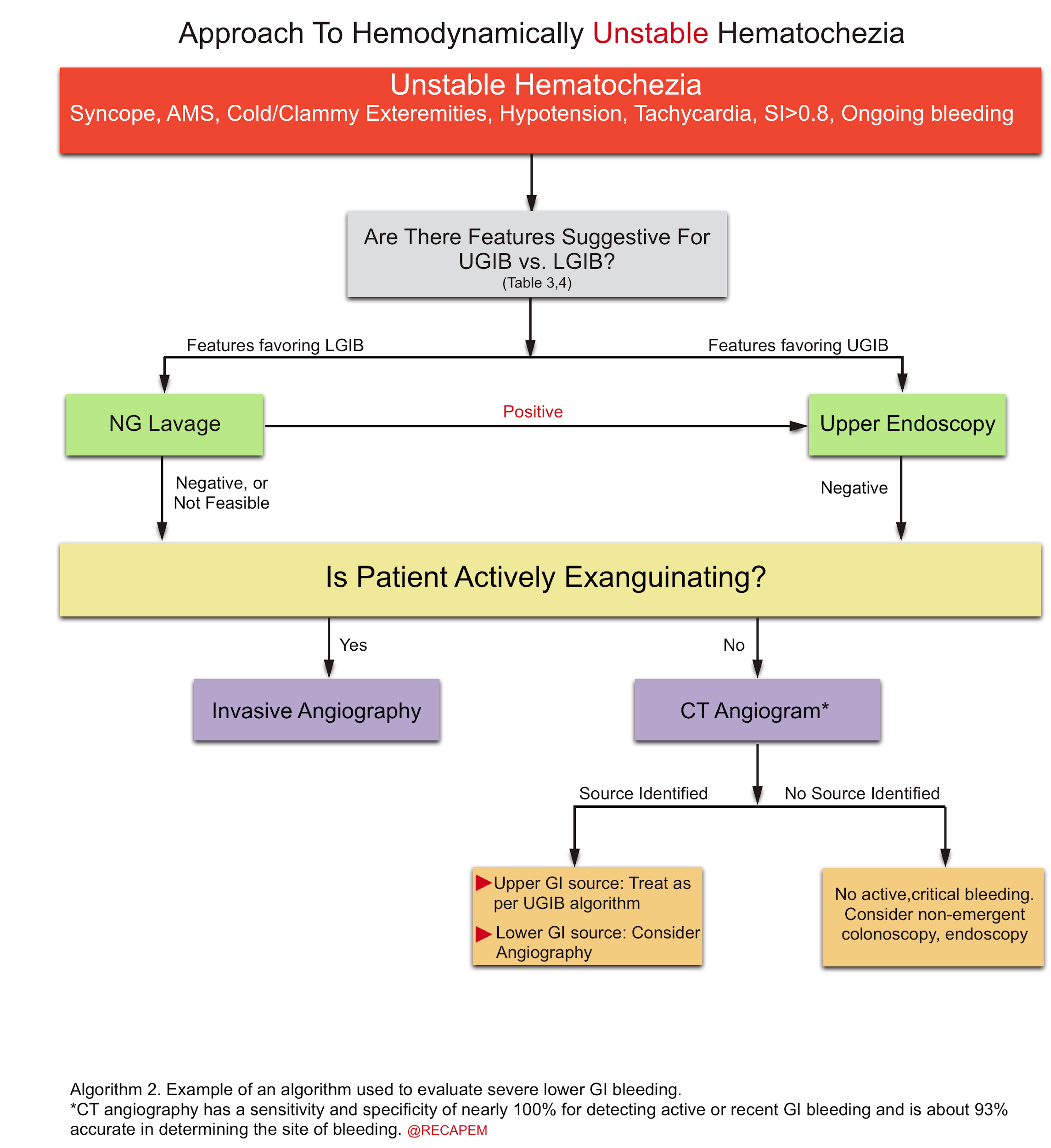
- Hematochezia originates from an upper GI source in 10-15% of patients, whereas it indicates brisk upper GI bleeding and may be associated with unstable hemodynamic and or hematemesis. The general diagnostic approach to LGIB is explained (algorithm 1) 66.
- Hematochezia in the presence of favoring features for upper GI source (table 3): NGT lavage, and upper endoscopy should be performed.
- Hematochezia in the presence of features favoring lower GI source (table 4):
- Exsanguinating bleeding (extremely severe bleeding): Invasive angiography should be performed (is both diagnostic and therapeutic).
- Less severe bleeding: Multidetector CT angiography has replaced the traditional technetium-labeled red cell scans. CT angiography has a sensitivity and specificity of nearly 100% for detecting active or recent GI bleeding and is about 93% accurate in determining the site of bleeding {67 68}
- Advantages of CT Angiography:
- Can be performed rapidly.
- Localizes bleeding lesions anywhere within the GI tract with high diagnostic accuracy for a wide range of pathologic lesions.
- Provides a map before treatment with conventional angiography especially in unstable patients.
- Advantages of CT Angiography:
▪️Diagnostic algorithm for lower GI bleeding
▪️Therapeutic intervention
- Interventional radiology: It plays a pivotal role in exsanguinating lower GI bleeding.
- Colonoscopy: is less useful in an active bleeding state, as active bleeding impairs the ability to clear the bowel, making colonoscopy difficult.
- Surgery: May be considered for refractory bleeding. The reported proportion of patients requiring surgery varies from 5% to 25% 69.
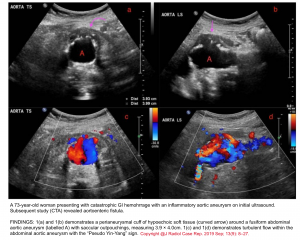
Aortoenteric fistula (AEF)
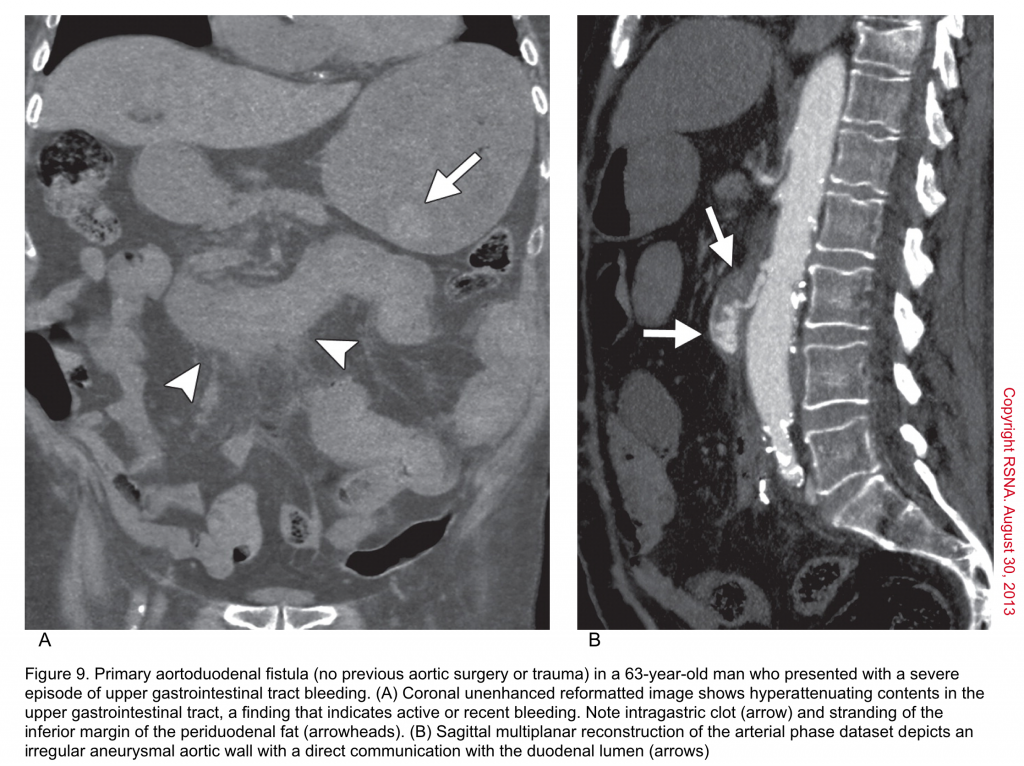
▪️Aortoenteric fistula (AEF) is a devastating and life-threatening condition, which is as difficult to diagnose as it is to treat. It is an abnormal communication between the aorta and an adjacent loop of the bowel.
▪️Types
- Two types are recognized: primary and secondary.
- Primary: fistulas occur de novo between the aorta and bowel, most commonly duodenum. However, it may involve other portions of the GI tract such as the stomach (aortogastric fistula), jejunum (aortojejunal fistula), ileum (aortoiliac fistula), or sigmoid colon (aortosigmoid fistula)
- Secondary: fistulas occur between an aortic graft and a segment of the bowel.
▪️Risk factor
- Abdominal aortic aneurysm (AAA) remains the most common risk factor of AEF.
- The AEF must be kept in mind as a possible etiology of gastrointestinal bleeding in any patient with known AAA or prior aortic intervention, no matter how long ago.
▪️Clinical manifestation
- GIB: is the most common initial manifestation, but may not always be present. It may be a minor episode of bleeding (herald bleed), or a life-threatening overt bleed. GIB may be accompanied by abdominal pain. The most common hemorrhagic presentations are hematemesis, and hematochezia 70.
- The classic triad of GIB, abdominal pain, and palpable mass occurs in only 6% to 12% of patients found to have AEF.
- Other presentations: Graft thrombosis with lower extremity ischemia, overt sepsis, malaise, weight loss.
▪️Diagnosis
- It requires a high index of suspicion in patients who present with either signs of GI bleeding (especially UGIB) or infection. Early diagnosis is essential for a successful outcome because of the lethal nature of AEF. The management approach to patients with high suspicion for AEF (known as AAA, prior aortic intervention) is as follows:
- Hemodynamically unstable patients with massive bleeding and known AAA should be taken directly to the operating room to make the diagnosis and perform the repair.
- For patients with acute GIB, who become stabilized, the first-line diagnostic study is CT angiography.
💡For those in whom a diagnosis of AAA is unknown, but suspected by risk factors, ultrasonography may identify the presence of the aneurysm but will not identify the AEF.
▪️Differential Diagnosis
- The differential diagnosis of AEF includes other pathologies causing GIB. Keep in mind the following points:
- Patients with a known history of AAA, or aortic intervention can develop GIB due to other common pathologies e.g. peptic ulcer disease, etc.
- If another etiology for GIB is found, this does not necessarily rule out the presence of AEF.
▪️Management: It is a uniformly fatal disease if not rapidly recognized and treated.
- Hemodynamic resuscitation
- Consultation: Immediately consult the appropriate surgical subspecialist if the patient has a history of known AAA or aortic intervention and there is concern for AEF.
- Aortic repair: via open surgical or endovascular options 71.
- Antibiotics: Consult with infectious disease service. A reasonable regimen includes vancomycin plus an agent with activity against gram-negative organisms (ceftriaxone or fluoroquinolone or piperacillin-tazobactam).
RECAP
- Orthostatic measurements are not sensitive for identifying patients with moderate volume loss.
- Treatment modalities that ‘Improve Mortality’ in UGIB are:
- Antibiotic prophylaxis in cirrhotic patients
- Restrictive transfusion strategy (transfuse if Hb < 7 g/dL)
- Treatments that ‘Do Not’ Improve Mortality in UGIB:
- NG lavage
- Somatostatin analogue: It does not reduce mortality in UGIB from esophageal varices, but may reduce blood transfusion requirements.
- PPIs: It does not improve mortality either in undifferentiated UGIB or in peptic ulcer bleeding. PPI treatment in peptic ulcer bleeding reduces rebleeding and surgical intervention rates.
- PPIs drip have not been proven to be more beneficial than bolus.
- Patients with cirrhosis and GIB are considered to have variceal bleeding until proven otherwise. When they develop hemorrhagic shock, their hemodynamic indices may not best fit the typical hemorrhagic shock profile. This is due to their chronic vasodilation and volume overload state at their baseline.
- In cirrhotic patients in shock state: Consider hemorrhagic shock and septic shock.
- Overesuscitation with fluid and blood products increases mortality and complications. In hemodynamically stable patients transfuse to a hemoglobin >7 g/dL.
- The coagulopathy in non-cirrhotic patients with life-threatening GI bleeding should be corrected emergently. FFP, PCC, cryoprecipitate, and platelets are considered as indicated. In patients with life-threatening GIB who use warfarin, administration of vitamin K is probably the key therapeutic measure, since the coagulation factor products wear off by 6-8 hours. Once bleeding has been controlled, risk assessment for thrombosis will be done and if required, anticoagulants can be restarted.
- Efforts to correct INR in cirrhotic patients with FFP or PCC are a wrong move.
- Tagged RBC scan is essentially replaced by CT angiography in the assessment of hematochezia.
- In patients with a history of aortic repair or known AAA, always consider aortoenteric fistula if they present with GI bleeding (especially UGIB) or look.
Media
Checklist
Going further
GIB review
- Upper GI Bleed(EM:RAP)
- C3 – Massive GI Bleed(EM:RAP)
- GI Bleed Emergencies Part 1(Emergency Medicine Cases)
- GI Bleed Emergencies Part 2(Emergency Medicine Cases)
- EMU 365: Niagara Falls of GI Bleeding(Emergency Medicine Cases)
- GI Bleeding(IBCC)
Intubation:
Coagulopathy:
Medications:
- Octreotide for non-variceal bleeding (RebelEM)
- The Good, The Bad, and The Ugly of Proton Pump Inhibitors in UGIB (RebelEM)
Gastric ultrasound:
CTA:
References
1. Kim BS, Li BT, Engel A, Samra JS, Clarke S, Norton ID, Li AE. Diagnosis of gastrointestinal bleeding: A practical guide for clinicians. World J Gastrointest Pathophysiol. 2014 Nov 15;5(4):467-78. doi: 10.4291/wjgp.v5.i4.467. PMID: 25400991; PMCID: PMC4231512.
2. Wuerth BA, Rockey DC. Changing Epidemiology of Upper Gastrointestinal Hemorrhage in the Last Decade: A Nationwide Analysis. Dig Dis Sci. 2018 May;63(5):1286-1293. doi: 10.1007/s10620-017-4882-6. Epub 2017 Dec 27. PMID: 29282637
3. Strate LL. Lower GI bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am. 2005 Dec;34(4):643-64. doi: 10.1016/j.gtc.2005.08.007. PMID: 16303575
4. Wuerth BA, Rockey DC. Changing Epidemiology of Upper Gastrointestinal Hemorrhage in the Last Decade: A Nationwide Analysis. Dig Dis Sci. 2018 May;63(5):1286-1293. doi: 10.1007/s10620-017-4882-6. Epub 2017 Dec 27. PMID: 29282637
5. Shen WK, Sheldon RS, Benditt DG, Cohen MI, Forman DE, Goldberger ZD, Grubb BP, Hamdan MH, Krahn AD, Link MS, Olshansky B, Raj SR, Sandhu RK, Sorajja D, Sun BC, Yancy CW. 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients With Syncope: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2017 Aug 1;136(5):e25-e59. doi: 10.1161/CIR.0000000000000498. Epub 2017 Mar 9. Erratum in: Circulation. 2017 Oct 17;136(16):e269-e270. PMID: 28280232.
6. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018 Oct 30;72(18):2231-2264. doi: 10.1016/j.jacc.2018.08.1038. Epub 2018 Aug 25. PMID: 30153967
7. McGee S, Abernethy WB 3rd, Simel DL. The rational clinical examination. Is this patient hypovolemic?. JAMA. 1999;281(11):1022-1029. doi:10.1001/jama.281.11.1022
8. Lyles T, Elliott A, Rockey DC. A risk scoring system to predict in-hospital mortality in patients with cirrhosis presenting with upper gastrointestinal bleeding. J Clin Gastroenterol. 2014;48(8):712-720. doi:10.1097/MCG.0000000000000014
9. Perlas A, Chan VW, Lupu CM, Mitsakakis N, Hanbidge A. Ultrasound assessment of gastric content and volume. Anesthesiology. 2009 Jul;111(1):82-9. doi: 10.1097/ALN.0b013e3181a97250. PMID: 19512861
10. Perlas A, Chan VW, Lupu CM, Mitsakakis N, Hanbidge A. Ultrasound assessment of gastric content and volume. Anesthesiology. 2009 Jul;111(1):82-9. doi: 10.1097/ALN.0b013e3181a97250. PMID: 19512861
11. Pallin DJ, Saltzman JR. Is nasogastric tube lavage in patients with acute upper GI bleeding indicated or antiquated? Gastrointest Endosc. 2011 Nov;74(5):981-4. doi: 10.1016/j.gie.2011.07.007. PMID: 22032314
12. Karakonstantis S, Tzagkarakis E, Kalamaki D, Lydakis C, Paspatis G. Nasogastric aspiration/lavage in patients with gastrointestinal bleeding: a review of the evidence. Expert Rev Gastroenterol Hepatol. 2018 Jan;12(1):63-72. doi: 10.1080/17474124.2018.1398646. Epub 2017 Nov 10. PMID: 29098897.
13. Huang ES, Karsan S, Kanwal F, Singh I, Makhani M, Spiegel BM. Impact of nasogastric lavage on outcomes in acute GI bleeding. Gastrointest Endosc. 2011 Nov;74(5):971-80. doi: 10.1016/j.gie.2011.04.045. Epub 2011 Jul 7. PMID: 21737077
14. Farrell JJ, Friedman LS. Review article: the management of lower gastrointestinal bleeding. Aliment Pharmacol Ther. 2005;21(11):1281-1298. doi:10.1111/j.1365-2036.2005.02485.x
15. Srygley FD, Gerardo CJ, Tran T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed?. JAMA. 2012;307(10):1072-1079. doi:10.1001/jama.2012.253
16. Thijs JC, Schneider AJ, van Kordelaar JM. Spontaneous rupture of the spleen complicating portal hypertension. Intensive Care Med. 1983;9(5):299-300. doi:10.1007/BF01691260
17. Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356(9238):1318-1321. doi:10.1016/S0140-6736(00)02816-6
18. Kollef MH, O’Brien JD, Zuckerman GR, Shannon W. BLEED: a classification tool to predict outcomes in patients with acute upper and lower gastrointestinal hemorrhage. Crit Care Med. 1997;25(7):1125-1132. doi:10.1097/00003246-199707000-00011
19. Aoki T, Nagata N, Shimbo T, et al. Development and Validation of a Risk Scoring System for Severe Acute Lower Gastrointestinal Bleeding. Clin Gastroenterol Hepatol. 2016;14(11):1562-1570.e2. doi:10.1016/j.cgh.2016.05.042
20. Das A, Ben-Menachem T, Cooper GS, et al. Prediction of outcome in acute lower-gastrointestinal haemorrhage based on an artificial neural network: internal and external validation of a predictive model. Lancet. 2003;362(9392):1261-1266. doi:10.1016/S0140-6736(03)14568-0
21. Strate LL, Orav EJ, Syngal S. Early predictors of severity in acute lower intestinal tract bleeding. Arch Intern Med. 2003;163(7):838-843. doi:10.1001/archinte.163.7.838
22. Savage SA, Sumislawski JJ, Zarzaur BL, Dutton WP, Croce MA, Fabian TC. The new metric to define large-volume hemorrhage: results of a prospective study of the critical administration threshold. J Trauma Acute Care Surg. 2015;78(2):224-230. doi:10.1097/TA.0000000000000502
23. Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care. 2015;19 Suppl 3(Suppl 3):S8. doi:10.1186/cc14726
24. Haupt MT, Gilbert EM, Carlson RW. Fluid loading increases oxygen consumption in septic patients with lactic acidosis. Am Rev Respir Dis. 1985 Jun;131(6):912-6. doi: 10.1164/arrd.1985.131.6.912. PMID: 4003944
25. Chatrath V, Khetarpal R, Ahuja J. Fluid management in patients with trauma: Restrictive versus liberal approach. J Anaesthesiol Clin Pharmacol. 2015;31(3):308-316. doi:10.4103/0970-9185.161664
26. Cerqueira RM, Andrade L, Correia MR, Fernandes CD, Manso MC. Risk factors for in-hospital mortality in cirrhotic patients with esophageal variceal bleeding. Eur J Gastroenterol Hepatol. 2012;24(5):551-557. doi:10.1097/MEG.0b013e3283510448
27. Ley EJ, Cloned MA, Srour MK, Barnajian M, Mirocha J, Margulies DR, Salim A. Emergency department crystalloid resuscitation of 1.5 L or more is associated with increased mortality in elderly and nonelderly trauma patients. J Trauma. 2011 Feb;70(2):398-400. doi: 10.1097/TA.0b013e318208f99b. PMID: 21307740.
28. Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez-Gea V, Aracil C, Graupera I, Poca M, Alvarez-Urturi C, Gordillo J, Guarner-Argente C, Santaló M, Muñiz E, Guarner C. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013 Jan 3;368(1):11-21. doi: 10.1056/NEJMoa1211801. Erratum in: N Engl J Med. 2013 Jun 13;368(24):2341. PMID: 23281973
29. Odutayo A, Desborough MJ, Trivella M, Stanley AJ, Dorée C, Collins GS, Hopewell S, Brunskill SJ, Kahan BC, Logan RF, Barkun AN, Murphy MF, Jairath V. Restrictive versus liberal blood transfusion for gastrointestinal bleeding: a systematic review and meta-analysis of randomised controlled trials. Lancet Gastroenterol Hepatol. 2017 May;2(5):354-360. doi: 10.1016/S2468-1253(17)30054-7. Epub 2017 Mar 23. PMID: 28397699
30. Lal DS, Shaz BH. Massive transfusion: blood component ratios. Curr Opin Hematol. 2013;20(6):521-525. doi:10.1097/MOH.0b013e3283653982
31. Sachar, Hamita & Vaidya, Keta & Laine, Loren. (2014). Intermittent vs Continuous Proton Pump Inhibitor Therapy for High-Risk Bleeding Ulcers. JAMA internal medicine. 174. 10.1001/jamainternmed.2014.4056
32. Tomaselli, G. F., Mahaffey, K. W., Cuker, A., Dobesh, P. P., Doherty, J. U., Eikelboom, J. W., Florido, R., Hucker, W., Mehran, R., Messé, S. R., Pollack, C. V., Rodriguez, F., Sarode, R., Siegal, D., & Wiggins, B. S. (2017). 2017 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. Journal of the American College of Cardiology, 70(24), 3042–3067. https://doi.org/10.1016/j.jacc.2017.09.1085
33. ASGE Standards of Practice Committee, Acosta RD, Abraham NS, Chandrasekhara V, Chathadi KV, Early DS, Eloubeidi MA, Evans JA, Faulx AL, Fisher DA, Fonkalsrud L, Hwang JH, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Shaukat A, Shergill AK, Wang A, Cash BD, DeWitt JM. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc. 2016 Jan;83(1):3-16. doi: 10.1016/j.gie.2015.09.035. Epub 2015 Nov 24. Erratum in: Gastrointest Endosc. 2016 Mar;83(3):678. PMID: 26621548
34. Imperiale TF, Birgisson S. Somatostatin or octreotide compared with H2 antagonists and placebo in the management of acute nonvariceal upper gastrointestinal hemorrhage: a meta-analysis [published correction appears in Ann Intern Med 1998
35. Thalheimer U, Triantos CK, Samonakis DN, Patch D, Burroughs AK. Infection, coagulation, and variceal bleeding in cirrhosis. Gut. 2005;54(4):556-563. doi:10.1136/gut.2004.048181
36. HALT-IT Trial Collaborators. Effects of a high-dose 24-h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT-IT): an international randomised, double-blind, placebo-controlled trial. Lancet. 2020;395(10241):1927-1936. doi:10.1016/S0140-6736(20)30848-5
37. Thalheimer U, Triantos CK, Samonakis DN, Patch D, Burroughs AK. Infection, coagulation, and variceal bleeding in cirrhosis. Gut. 2005 Apr;54(4):556-63. doi: 10.1136/gut.2004.048181. PMID: 15753544; PMCID: PMC1774431
38. Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila F, et al. Meta-analysis: antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding – an updated Cochrane review. Aliment Pharmacol Ther. 2011;34(5):509-518. doi:10.1111/j.1365-2036.2011.04746.x
39. Balderas V, Bhore R, Lara LF, Spesivtseva J, Rockey DC. The hematocrit level in upper gastrointestinal hemorrhage: safety of endoscopy and outcomes. Am J Med. 2011;124(10):970-976. doi:10.1016/j.amjmed.2011.04.032
40. Wolf AT, Wasan SK, Saltzman JR. Impact of anticoagulation on rebleeding following endoscopic therapy for nonvariceal upper gastrointestinal hemorrhage. Am J Gastroenterol. 2007;102(2):290-296. doi:10.1111/j.1572-0241.2006.00969.
41. Hayat U, Lee PJ, Ullah H, Sarvepalli S, Lopez R, Vargo JJ. Association of prophylactic endotracheal intubation in critically ill patients with upper GI bleeding and cardiopulmonary unplanned events. Gastrointest Endosc. 2017;86(3):500-509.e1. doi:10.1016/j.gie.2016.12.008
42. Czarnetzki C, Elia N, Frossard JL, et al. Erythromycin for Gastric Emptying in Patients Undergoing General Anesthesia for Emergency Surgery: A Randomized Clinical Trial. JAMA Surg. 2015;150(8):730-737. doi:10.1001/jamasurg.2015.0306
43. Amornyotin S. Sedation and monitoring for gastrointestinal endoscopy. World J Gastrointest Endosc. 2013;5(2):47-55. doi:10.4253/wjge.v5.i2.47
44. Berzigotti A, Seijo S, Reverter E, Bosch J. Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol. 2013 Feb;7(2):141-55. doi: 10.1586/egh.12.83. PMID: 23363263
45. Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017 Jan;65(1):310-335. doi: 10.1002/hep.28906. Epub 2016 Dec 1. Erratum in: Hepatology. 2017 Jul;66(1):304. PMID: 27786365
46. Herman J, Baram M. Blood and volume resuscitation for variceal hemorrhage. Ann Am Thorac Soc. 2015 Jul;12(7):1100-2. doi: 10.1513/AnnalsATS.201502-095CC. PMID: 26203612
47. Cerqueira RM, Andrade L, Correia MR, Fernandes CD, Manso MC. Risk factors for in-hospital mortality in cirrhotic patients with oesophageal variceal bleeding. Eur J Gastroenterol Hepatol. 2012 May;24(5):551-7. doi: 10.1097/MEG.0b013e3283510448. PMID: 22356784.
48. Boregowda U, Umapathy C, Halim N, Desai M, Nanjappa A, Arekapudi S, Theethira T, Wong H, Roytman M, Saligram S. Update on the management of gastrointestinal varices. World J Gastrointest Pharmacol Ther. 2019 Jan 21;10(1):1-21. doi: 10.4292/wjgpt.v10.i1.1. PMID: 30697445; PMCID: PMC6347650
49. Leebeek FW, Rijken DC. The Fibrinolytic Status in Liver Diseases. Semin Thromb Hemost. 2015 Jul;41(5):474-80. doi: 10.1055/s-0035-1550437. Epub 2015 Jun 6. PMID: 26049070
50. Bosch J, Thabut D, Bendtsen F, D’Amico G, Albillos A, González Abraldes J, Fabricius S, Erhardsen E, de Franchis R; European Study Group on rFVIIa in UGI Haemorrhage. Recombinant factor VIIa for upper gastrointestinal bleeding in patients with cirrhosis: a randomized, double-blind trial. Gastroenterology. 2004 Oct;127(4):1123-30. doi: 10.1053/j.gastro.2004.07.015. PMID: 15480990
51. Bosch J, Thabut D, Albillos A, Carbonell N, Spicak J, Massard J, D’Amico G, Lebrec D, de Franchis R, Fabricius S, Cai Y, Bendtsen F; International Study Group on rFVIIa in UGI Hemorrhage. Recombinant factor VIIa for variceal bleeding in patients with advanced cirrhosis: A randomized, controlled trial. Hepatology. 2008 May;47(5):1604-14. doi: 10.1002/hep.22216. PMID: 18393319
52. Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017 Jan;65(1):310-335. doi: 10.1002/hep.28906. Epub 2016 Dec 1. Erratum in: Hepatology. 2017 Jul;66(1):304. PMID: 27786365
53. Levy JH, Goodnough LT. How I use fibrinogen replacement therapy in acquired bleeding. Blood. 2015 Feb 26;125(9):1387-93. doi: 10.1182/blood-2014-08-552000. Epub 2014 Dec 17. PMID: 25519751
54. Borzio M, Salerno F, Piantoni L, et al. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33(1):41-48. doi:10.1016/s1590-8658(01)80134-1
55. North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319(15):983-989.
56. Goulis J, Armonis A, Patch D, Sabin C, Greenslade L, Burroughs AK. Bacterial infection is independently associated with failure to control bleeding in cirrhotic patients with gastrointestinal hemorrhage. Hepatology. 1998;27(5):1207-1212. doi:10.1002/hep.510270504
57. Montalto P, Vlachogiannis J, Cox DJ, Pastacaldi S, Patch D, Burroughs AK. Bacterial infection in cirrhosis impairs coagulation by a heparin effect: a prospective study. J Hepatol. 2002;37(4):463-470. doi:10.1016/s0168-8278(02)00208-8
58. Rasaratnam B, Kaye D, Jennings G, Dudley F, Chin-Dusting J. The effect of selective intestinal decontamination on the hyperdynamic circulatory state in cirrhosis. A randomized trial. Ann Intern Med. 2003;139(3):186-193.
59. Brandtzaeg P. Significance and pathogenesis of septic shock. Curr Top Microbiol Immunol. 1996;216:15-37. doi:10.1007/978-3-642-80186-0_2
60. Kravetz D, Romero G, Argonz J, et al. Total volume paracentesis decreases variceal pressure, size, and variceal wall tension in cirrhotic patients. Hepatology. 1997;25(1):59-62. doi:10.1053/jhep.1997.v25.pm0008985265
61. Panés J, Terés J, Bosch J, Rodés J. Efficacy of balloon tamponade in treatment of bleeding gastric and esophageal varices. Results in 151 consecutive episodes. Dig Dis Sci. 1988;33(4):454-459. doi:10.1007/BF01536031
62. Wang J, Bao YX, Bai M, Zhang YG, Xu WD, Qi XS. Restrictive vs liberal transfusion for upper gastrointestinal bleeding: a meta-analysis of randomized controlled trials. World J Gastroenterol. 2013;19(40):6919-6927. doi:10.3748/wjg.v19.i40.6919
63. Tripodi A, Chantarangkul V, Primignani M, et al. Thrombin generation in plasma from patients with cirrhosis supplemented with normal plasma: considerations on the efficacy of treatment with fresh-frozen plasma. Intern Emerg Med. 2012;7(2):139-144. doi:10.1007/s11739-011-0528-4
64. Wells M, Chande N, Adams P, et al. Meta-analysis: vasoactive medications for the management of acute variceal bleeds. Aliment Pharmacol Ther. 2012;35(11):1267-1278. doi:10.1111/j.1365-2036.2012.05088.x
65. Byers SE, Chudnofsky CR, Sorondo B, Dominici P, Parrillo SJ. Incidence of occult upper gastrointestinal bleeding in patients presenting to the ED with hematochezia. Am J Emerg Med. 2007 Mar;25(3):340-4. doi: 10.1016/j.ajem.2006.09.005. PMID: 17349911
66. Strate LL, Gralnek IM. ACG Clinical Guideline: Management of Patients With Acute Lower Gastrointestinal Bleeding. Am J Gastroenterol. 2016 Apr;111(4):459-74. doi: 10.1038/ajg.2016.41. Epub 2016 Mar 1. Erratum in: Am J Gastroenterol. 2016 May;111(5):755. PMID: 26925883; PMCID: PMC5099081
66. Yoon W, Jeong YY, Shin SS, Lim HS, Song SG, Jang NG, Kim JK, Kang HK. Acute massive gastrointestinal bleeding: detection and localization with arterial phase multi-detector row helical CT. Radiology. 2006 Apr;239(1):160-7. doi: 10.1148/radiol.2383050175. Epub 2006 Feb 16. PMID: 16484350
67. Martí M, Artigas JM, Garzón G, Alvarez-Sala R, Soto JA. Acute lower intestinal bleeding: feasibility and diagnostic performance of CT angiography. Radiology. 2012 Jan;262(1):109-16. doi: 10.1148/radiol.11110326. Epub 2011 Nov 14. PMID: 22084211
68. Strate LL. Lower GI bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am. 2005 Dec;34(4):643-64. doi: 10.1016/j.gtc.2005.08.007. PMID: 16303575
69. Lindsay TF. Ruptured aortoiliac aneurysms and their management. In: Sidawy AN, Perler BA, eds. Rutherford’s Vascular Surgery and Endovascular Therapy. 9th ed. Philadelphia, PA: Elsevier; 2019:944-960e4.
70. Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, Mastracci TM, Mell M, Murad MH, Nguyen LL, Oderich GS, Patel MS, Schermerhorn ML, Starnes BW. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018 Jan;67(1):2-77.e2. doi: 10.1016/j.jvs.2017.10.044. PMID: 29268916




Add comment