13 February 2024, by Shahriar Lahouti.
CONTENT
- Preface
- Definition
- Neuroanatomy of consciousness
- Pathophysiology
- Etiology
- Approach to diagnosis
- History
- Physical exam
- Classifying coma syndromes
- Bilateral hemispheric lesions
- Cerebral herniation syndromes
- Brainstem displacement from
- Intrinsic brainstem lesion
- Metabolic coma
- Coma mimics
- Evaluation
- Approach to management
- References
Preface
The term coma should be used to identify patients who cannot be aroused and cannot interact with the environment. Coma is a life-threatening emergency that requires immediate stabilization and a structured approach to diagnosis and management. The differential diagnosis for coma is long but is often divided into structural vs. diffuse
neuronal dysfunction. Correct diagnosis of the cause of coma has extensive implications for treatment and prognosis, as the definitive treatment of patients with coma is ultimately disease-specific.
Definition
Background
- Consciousness is the brain function that makes people who they are. It is composed of two inter-related domains *:
- Arousal
- Refers to wakefulness (e.g. eye opening) and responsiveness to the surrounding environment and stimuli.
- The level of consciousness refers to the degree of arousal *.
- Consciousness is mediated by the reticular activating system.
- Dysfunction of arousal is described by terms such as lethargy, drowsiness, and somnolence.
- Awareness (content of consciousness)
- The content of consciousness consists of cortex-level functions such as orientation, perception, executive functions, and memory.
- These processes are performed by widespread neuronal networks located in the cortical regions.
- Arousal
Definitions
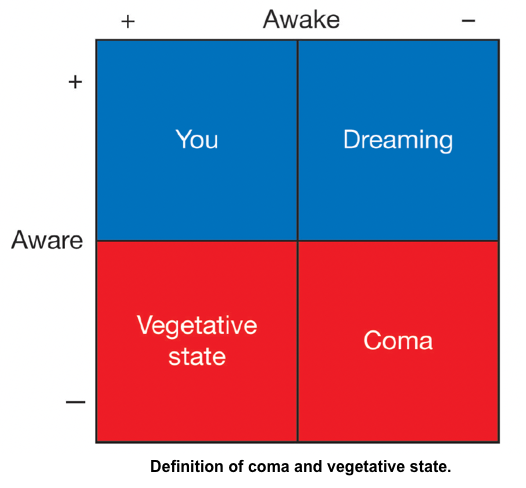
- Coma is a state of complete unresponsiveness to any stimuli. Patients are not awake, not aware, and not arousable *.
- Stupor/obtundation is a state of impaired arousal wherein patients have some responsiveness to vigorous and continuous painful stimuli.
- A vegetative state refers to a state of intact arousal (e.g. sleep/wake cycles) but without awareness*. A patient may open his or her eyes spontaneously but will not track or fixate. Reflexive movements and random movements of limbs, trunk, and even vocalizations with tears or smiles may occur, but no reproducible and purposeful responses will be seen *.
- Minimally conscious state (MCS): Variable arousal and inconsistent awareness, but one or more reproducible behaviors reveal some awareness (e.g. following simple commands, appropriate yes/no responses, or other purposeful behavior) *.
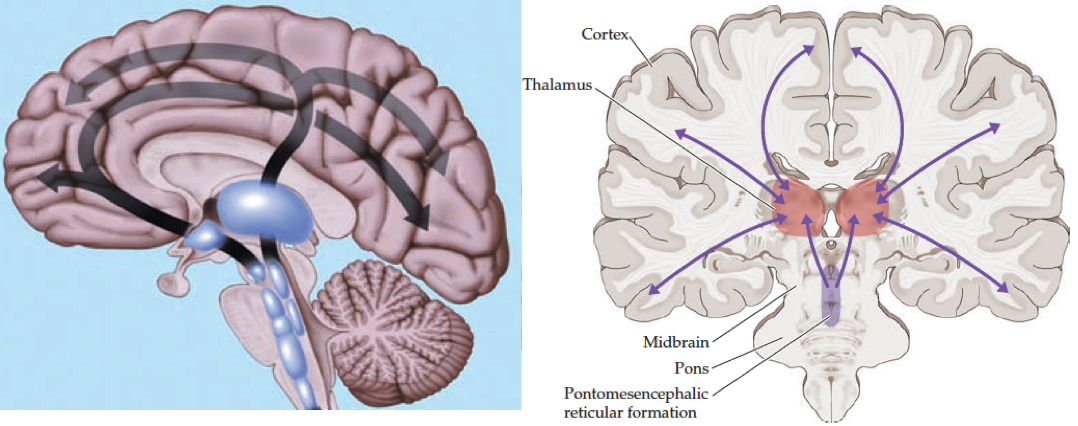
Neuroanatomy of consciousness
Key structures in maintaining an awake and awareness state involve the ascending reticular activating system (ARAS) which originates from the midbrain and upper pontine tegmentum rostrally and ascends to the thalamus and the anterior cingulate regions and subsequently diverges widely throughout the bilateral cortexes *. Coma results from the disruption of pathways from the ascending reticular activating system.
Pathophysiology of coma
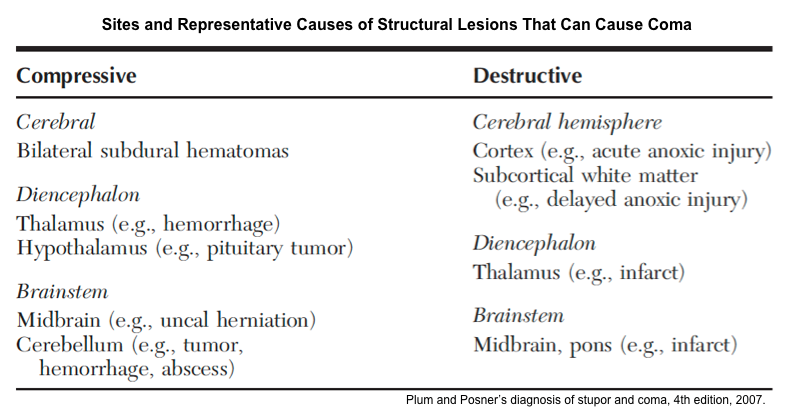
▪️Coma can be caused by structural lesions or global acute physiologic derangement of brain function.
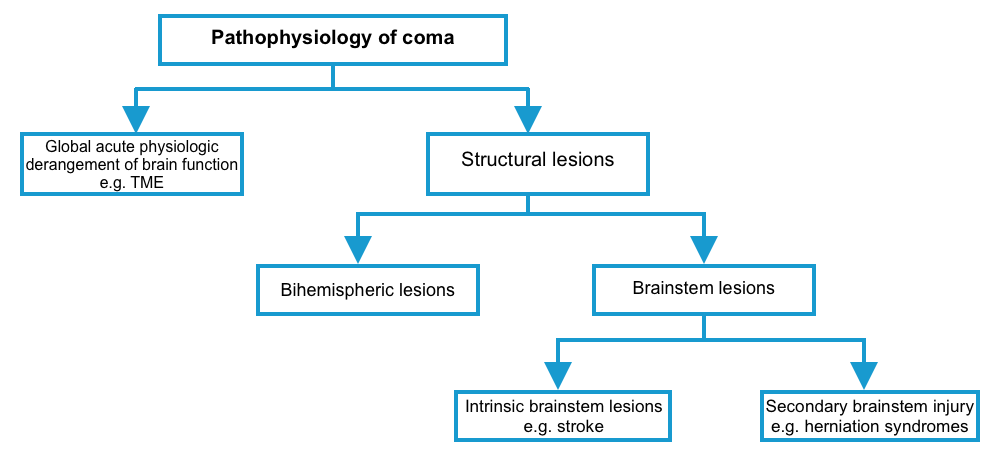
▪️Is the structural lesion bihemispheric, or brainstem lesion?
- If bihemispheric, it should cause diffuse bihemispheric damage or bilateral thalamic injury (e.g. vein of Galen thrombosis).
- ⭐️Coma cannot be attributed to a unilateral cortical lesion alone. For example, for a unilateral cortical lesion to cause coma it must cause a herniation syndrome that results in damage to the upper midbrain *.
- If brainstem lesion
- Primary brainstem lesion (e.g. brainstem infarction) vs. secondary brainstem injury (e.g. displacement)?
- If it is a primary brainstem lesion, it should be located at the dorsolateral upper-mid pontine or paramedian upper midbrain.
- Note that isolated sstructural lesions located more caudally such as in the lower pons or medulla typically do not affect consciousness.
- Similarly, consciousness is typically spared by lesions located at the ventral midbrain or pons that spare the reticular formation as in locked-in syndrome.
- If it is a primary brainstem lesion, it should be located at the dorsolateral upper-mid pontine or paramedian upper midbrain.
- If secondary brainstem injury e.g. displacement)
- Is it caused by central vs. lateral displacement? Note that both can be caused by a mass lesion in either hemisphere or cerebellum *.
- Primary brainstem lesion (e.g. brainstem infarction) vs. secondary brainstem injury (e.g. displacement)?
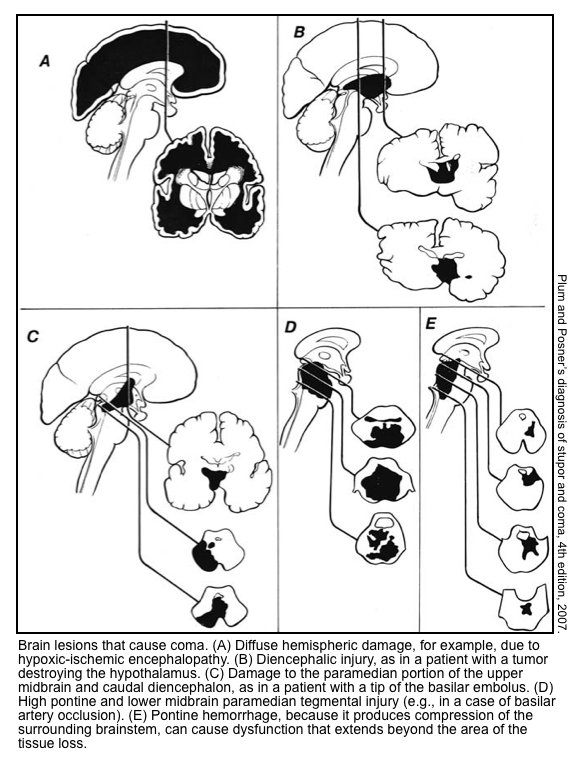
Etiology of coma
🟥Structural intracranial disease *
- Cerebrovascular disease
- Intracranial hemorrhage
- Large hemorrhage causing herniation.
- Intracerebral hemorrhage (typically pontine)
- Subarachnoid hemorrhage (coma often due to acute hydrocephalus; may improve with external ventricular drain placement).
- Ischemic stroke
- Most commonly, basilar artery stroke.
- Severe multifocal infarction (e.g. due to endocarditis).
- Artery of Percheron occlusion involving bilateral thalami (rare)
- Cerebral venous thrombosis
- Thrombosis of the straight sinus may cause bilateral thalamic dysfunction.
- Diffuse cerebral edema due to extensive thrombus burden.
- PRES (posterior reversible encephalopathy syndrome).
- Intracranial hemorrhage
- Trauma
- Hematoma (epidural, subdural, or parenchymal).
- Multifocal/bilateral contusions causing edema and elevated intracranial pressure.
- Diffuse axonal injury (DAI).
- Edema with intracranial hypertension
- Cerebral fat embolism
- Tumor
- Large supratentorial tumor with mass effect
- Cerebellar tumor with mass effect
- Brainstem tumor
- Infiltrative tumor (glioma, lymphoma)
- Pituitary apoplexy
- Acute hydrocephalus
- Infection/inflammatory
- Meningitis
- An abscess (epidural/subdural/cerebral) with mass effect
- Encephalitis (viral, autoimmune)
- Cerebral malaria
- ADEM (acute disseminated encephalomyelitis)
🟥Diffuse brain dysfunction
- Seizure
- Nonconvulsive status epilepticus (NCSE)
- Postictal state
- Shock (especially septic shock)
- Severe metabolic and endocrine disorder
- Dysthermia: Extremes of body temperature (temperature <28C or >40C) *.
- Dysglycemia (glucose <~40 mg/dL, or rapid rise >~900 mg/dL commonly seen in the hyperosmolar hyperglycemic state)*
- Hyponatremia/hypernatremia (e.g. sudden drop below ~110 mmol/L or sudden rise above ~160 mmol/L)
- Hypercalcemia (Total serum calcium > 14mg/dL, or ionized serum calcium > 3.5 mmol/L)
- Hypermagnesemia (serum magnesium >12 mg/dL, >5 mM, >10 mEq/L)*
- Hypercapnia (e.g. pCO2 > ~80-100 mm).
- Hypoxic/anoxic brain injury.
- Hepatic encephalopathy
- Myxedema coma
- Uremia
- Severe hyperammonemia (in the absence of liver failure)
- Addison crisis
- Thiamine deficiency
- Intoxications
- Carbon monoxide
- Cyanide
- Opiate
- Alcohol (ethanol, methanol, ethylene glycol).
- Anticholinergics, including tricyclics.
- Sedative hypnotics, including baclofen.
- Sympathomimetic intoxication.
- Serotonin syndrome.
- Malignant catatonia including neuroleptic malignant syndrome
- Salicylate poisoning.
- Lithium.
Approach to diagnosis
▪️Coma can be caused by structural brain injury or generalized brain dysfunction. Correct diagnosis of the cause of coma has huge therapeutic and prognostic implications.
- Despite that numerous etiologies can cause coma, the differential diagnoses can often narrowed down after initial evaluation, which includes history, physical examination, and basic investigations.
- Evaluation of coma should be methodical. It is useful to keep a mental checklist to avoid missing important elements of differential diagnosis, examination, and testing.
- When evaluating a comatose patient, it is imperative to concentrate first on identifying diagnoses amenable to specific emergency treatments.
▪️Most frequent causes of emergently treatable acute coma (surprising coma survivors) *:
- Hypoglycemia (Dextrose infusion)
- Severe hyponatremia (Gradual serum sodium correction)
- CO2 narcosis (Mechanical ventilation)
- CO intoxication (Hyperbaric oxygen)
- Opiate intoxication (Naloxone
- Intracranial hemorrhage (Surgical evacuation)
- Basilar artery occlusion (Reperfusion therapy (IV thrombolysis and/or mechanical thrombectomy)
- Acute hydrocephalus (Ventriculostomy)
- Cerebral venous thrombosis (Anticoagulation)
- Status epilepticus (Antiseizure drugs)
- Fulminant bacterial meningitis (Antibacterial drugs with CNS penetration and dexamethasone)
- Hypertensive encephalopathy (Gradual blood pressure reduction)
- Intoxications (Antidotes).
- Diabetic coma (Insulin)
- Myxoedema coma (Thyroid supplement).
- Uremic encephalopathy (Dialysis)
- Hyperammonemic encephalopathy (Lactulose, rifaximin)
- Sepsis (Antibiotics, hemodynamic resuscitation)
History
In the initial evaluation of the comatose patient with no apparent cause, the medical history becomes more important and often cues the physician towards a possible explanation *.
- The time course of the loss of consciousness?
- Abrupt (e.g. cerebrovascular causes, seizure).
- Gradual (eg, brain tumor, infection).
- Fluctuating (e.g. recurring seizures, subdural hematoma, metabolic encephalopathy).
- Preceding symptoms
- Did focal signs or symptoms precede the loss of consciousness? For example:
- Hemiparesis suggests a structural lesion, likely with mass effect.
- Transient visual symptoms (eg, diplopia or vertigo) may suggest ischemia in the posterior circulation.
- Headache and vomiting just before loss of consciousness could indicate an intracranial hemorrhage.
- Headache and fever may suggest intracranial infections.
- Abnormal movements/seizure?
- Did focal signs or symptoms precede the loss of consciousness? For example:
- Recent illness
- Altered behavior or function recently?
- Fever suggests an infection.
- An increasing headache suggests an expanding intracranial lesion, infection, or venous sinus thrombosis.
- Recent falls raise the possibility of a subdural hematoma.
- Recent confusion or delirium might indicate a metabolic or toxic cause.
- Medications? medical, neurologic, or psychiatric conditions? For example:
- Is the patient known to have atrial fibrillation or other cardiac disease and be using anticoagulation or has it recently been discontinued?
- Has the patient had prior episodes of diabetic ketoacidosis or severe hypoglycemia, and has there been a recent change in insulin medications?
- Has the patient had prior suicide attempts, a psychiatric consultation, and are there problems at work?
- History of alcohol or drug abuse?
- Has the patient used medications that may increase heat production, such as salicylate drugs with sympathomimetics such as cocaine, amphetamines, or ecstasy?
General exam
Vital signs
▪️Extreme hypertension. It may suggest *
- Reversible posterior leukoencephalopathy syndrome (PRES).
- Hypertensive intracerebral/cerebellar/brainstem hemorrhage.
- ↑ICP (Cushing syndrome: hypertension plus bradycardia)
▪️Hypotension
- May reflect circulatory failure from sepsis, hypovolemia, or cardiac failure, as well as certain drugs (e.g. beta blocker, calcium channel blocker) or Addison disease *.
▪️Tachycardia. In a comatose state, it may suggest *
- Intoxication
- Antidepressants
- Antipsychotic
- Ketamine intoxication
- Sympathomimetics
- Adrenergic hyperactivity from acute structural brain injury.
▪️Bradycardia
- Intoxications
- β blockers with membrane-stabilizing activity
- Clonidine
- Organophosphates
- Sedative-hypnotic agents
- γ-hydroxybutyrate
- Opioids
- ↑Intracerebral pressure (including hydrocephalus)
- Myxedema coma *
▪️Hyperthermia
- CNS disease: Hypothalamic injury (e.g. CVA or hemorrhage), massive pontine hemorrhage, subarachnoid hemorrhage.
- Heat stroke
- Intoxication: Neuroleptic malignant syndrome, serotonin syndrome, salicylates intoxication, anticholinergic intoxication, sympathomimetic overdose.
- Infection: Sepsis, focal infections (including CNS infections).
- Infection usually doesn’t cause profound temperature elevation, but this can occur especially in CNS infection (meningitis/encephalitis), or sepsis + other factors limiting heat loss (e.g. anticholinergics, phenothiazines).
- Endocrinopathy: Adrenal crisis, thyroid storm, pheochromocytoma.
▪️Hypothermia
- Accidental (cold exposure).
- Metabolic: Hypoglycemia, diabetic ketoacidosis, myxedema, adrenal crisis, thiamine deficiency, anorexia nervosa, pituitary apoplexy.
- Toxicologic: Ethanol, sedatives, opioids, phenothiazines, cyclic antidepressants, sympatholytics (beta-blockers, alpha-blockers), central alpha-agonists (e.g. clonidine), carbon monoxide poisoning, lithium toxicity.
- Neurologic: Thalamic injury (e.g. stroke), spinal cord injury, multiple sclerosis, Parkinson’s disease.
- Distributive shock: Sepsis, pancreatitis.
Neurologic exam
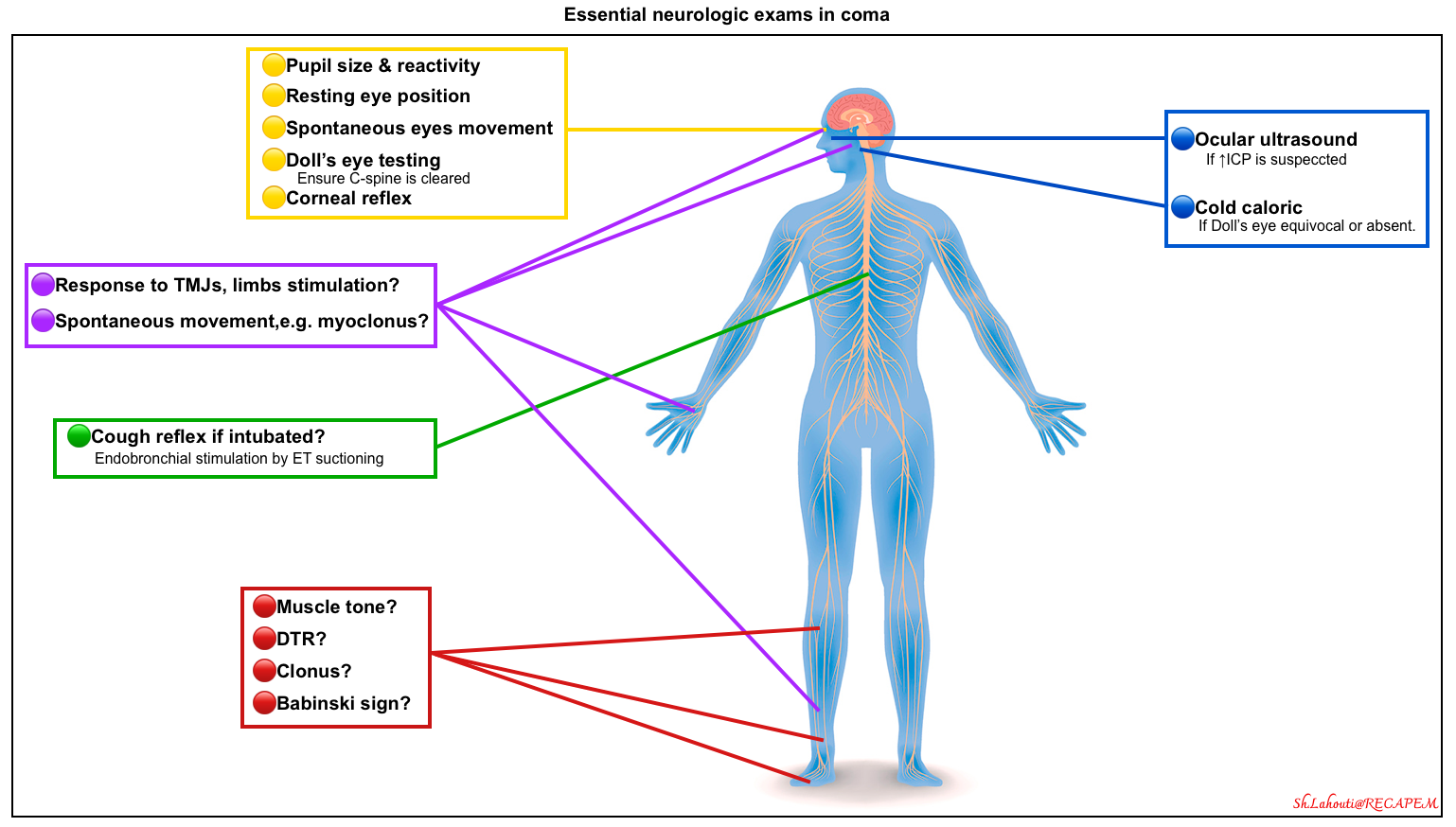
The main purpose of the neurologic examination is to recognize telltale signs that determine not only the location of the lesion but also the direction of further diagnostic laboratory evaluation *. The examination provides critical information regarding which patients should be risk-stratified for emergent or specialized neuroimaging (e.g. emergent CT angiography to evaluate for basilar artery occlusion).
Mental status
Background
- In stuporous/comatose patients assessment of the level of arousal is the most important component of the mental status examination.
- 🎯Goal: To determine the intensity of the stimulus necessary to arouse the patient as well as the quality of the arousal.
- A description of whether the patient’s eyes are open, whether commands are followed, and if verbal output is possible immediately alerts the clinician as to the presence of coma.
- Always begin by addressing the patient verbally.
- If the patient does not respond, more vigorous attempts at stimulation should be attempted including speaking louder or applying increasing degrees of painful stimuli.
- ⭐️Before proceeding with the application of painful stimuli, it is appropriate to open the patient’s eyes and assess whether commands to direct gaze can be followed. This brief assessment prevents one from proceeding to applying painful stimuli to an awake patient in a locked-in state, a rare condition, which can result from brainstem infarction, severe polyneuropathy, or neuromuscular blockade.
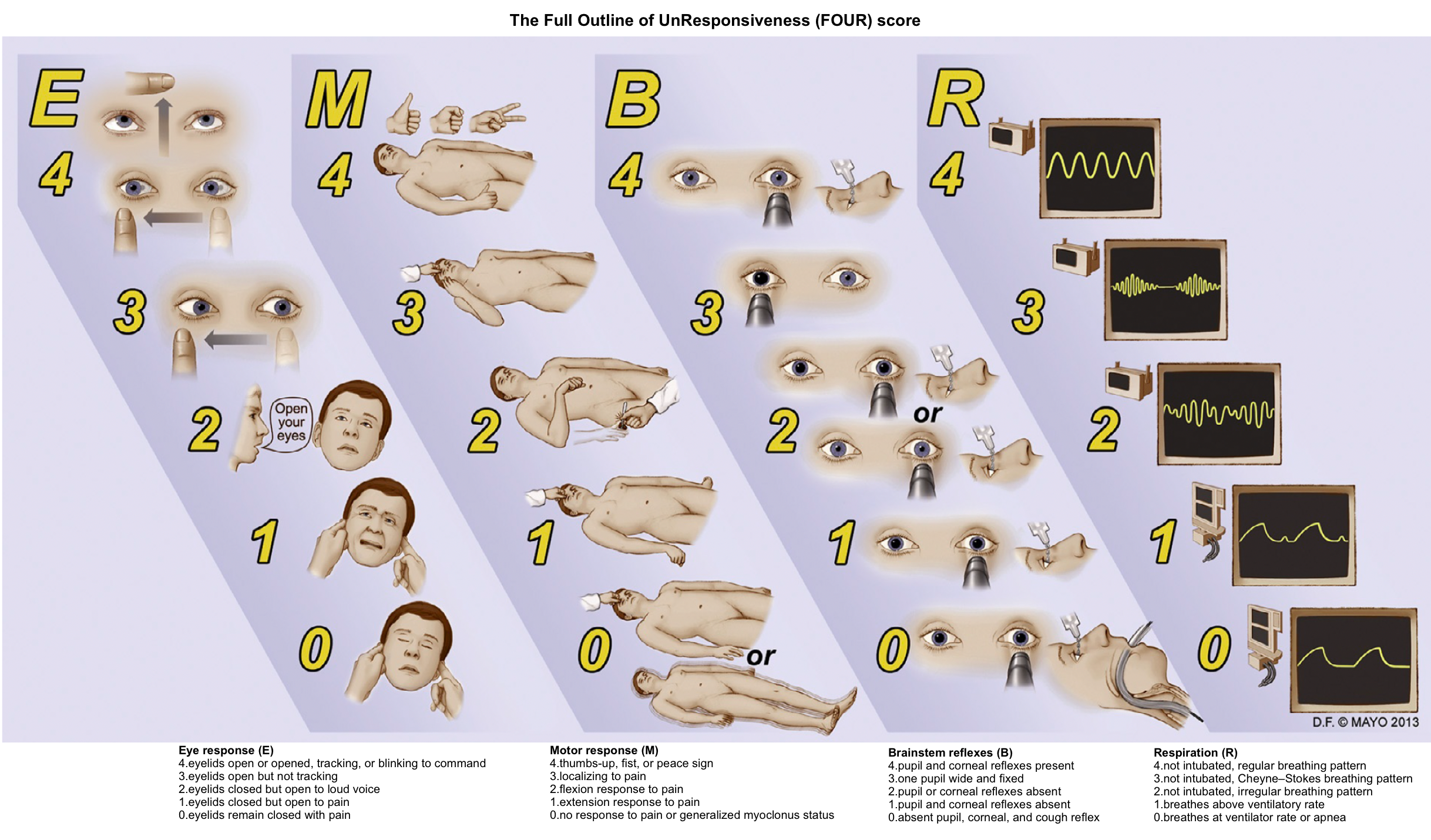
Tools: Coma scales
- The Glasgow Coma Scale is a widely used clinical scoring system for alterations in consciousness (🧮MDCalc).
- Advantages are the simplicity of the scoring system and the assessment of separate verbal, motor, and eye-opening functions.
- Disadvantages include a lack of acknowledgment of hemiparesis or other focal motor signs and a lack of testing of higher cognitive functions.
- The FOUR (Full Outline of Unresponsiveness) score has been used in intensive care units and has the advantage of assessing simple brainstem functions and respiratory patterns, as well as eye and motor responses (🧮MDCalc).
Motor system
▪️Motor system assessment in unresponsive patients involves:
-
- Motor response to painful stimuli.
- This can be tested by applying a painful stimulus to four extremities and a bilateral face (e.g. supraorbital ridge, temporomandibular joints).
- 🎯Goal: Localizing information can be gleaned from observing spontaneous motor responses or eliciting responses to stimuli.
- Spontaneous movements.
- Tremor, asterixis, and multifocal myoclonus strongly suggest metabolic coma, especially if these are symmetric *.
- Reflexes: Patellar reflex? ankle clonus? Babinski sign?
- Motor response to painful stimuli.
▪️Motor response to painful stimuli. Look for:
- Localizing response (withdrawal), in which the patient reaches toward the stimulus.
- Asymmetric response: The presence of any asymmetry (e.g. an asymmetric grimace or motor response) implies the presence of a focal lesion.
- Grimacing.
- Grimacing without withdrawal may suggest an intact sensory response, with motor paralysis.
- Extremity flexion without grimacing in the lower extremity may be seen in the presence of brain death, due to a spinal reflex known as triple flexion.
- Triple flexion is also suggested if the patient responds the same way, regardless of where their foot is stimulated (e.g., dorsum vs. sole).
- A reflexive response such as decorticate or decerebrate posturing.
- Decorticate (flexor posturing) and decerebrate (extensor posturing) have been loosely associated with damage at the level of the thalamus and midbrain in sequence. However, they can be seen with either focal or global conditions as well as some severe toxic/metabolic etiologies.
- It is also not uncommon for both responses to be present in the same patient at the same time *.
- Given this, the presence of decerebrate or decorticate posturing has relatively little utility in prognostication until the underlying etiology is found *.
- Absence of any response at all.
Cranial nerves
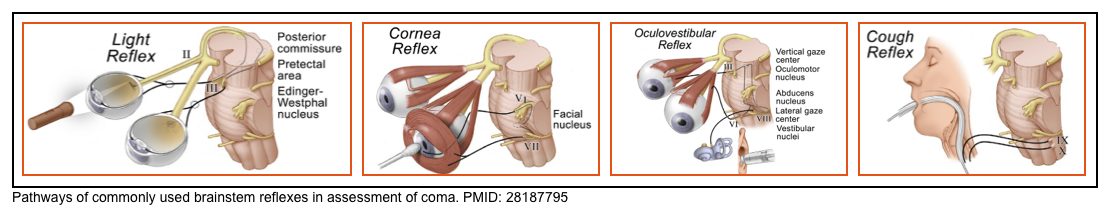
- In unresponsive patients, most cranial nerves can be examined easily. Asymmetry in responses could be concerning for pathology in the midbrain, pons, or medulla.
- Essential examinations involve (brainstem reflexes are shown in the figure above):
- Pupil size & reactivity 📖
- Eye movement
- Primary eye position (resting eye position).
- Horizontal deviation of the eyes may indicate an ipsilateral hemispheric lesion affecting the frontal eye fields or a contralateral pontine lesion.
- Horizontal deviation can also be seen with nonconvulsive status epilepticus (NCSE).
- Spontaneous eye movements 📖
- Roving eye movements (slow, conjugate, lateral, and to-and-fro excursions) can be caused by bihemispheric, metabolic, and various toxicities.
- Roving eye movement indicates that the brainstem is relatively intact.
- These movements do not support a particular localization or a specific disease process.
- Roving eye movements (slow, conjugate, lateral, and to-and-fro excursions) can be caused by bihemispheric, metabolic, and various toxicities.
- Reflex eye movements 📖
- Doll’s Eyes reflex (oculocephalic reflex)
- Cold caloric testing (vestibuloocular response)
- Primary eye position (resting eye position).
- Corneal reflex 📖
- Cough reflex (if intubated). 📖
- Pupil size & reactivity 📖
- Additional exams may include the fundoscopic examination or ocular ultrasound.
Classifying coma syndromes
Combinations of physical findings can be used to characterize herniation syndromes, toxic syndrome, and certain causes of coma. These are discussed here.
Bilateral hemispheric lesions
Clinical features *
- Spontaneous eye movements (roving, dipping, ping-pong, nystagmoid jerks).
- Upward or downward eye deviation.
- Intact oculovestibular reflexes.
- Intact pupillary and corneal reflexes.
- Variable motor responses.
- Adventitious limb movements (subtle manifestations of seizures, myoclonus, asterixis).
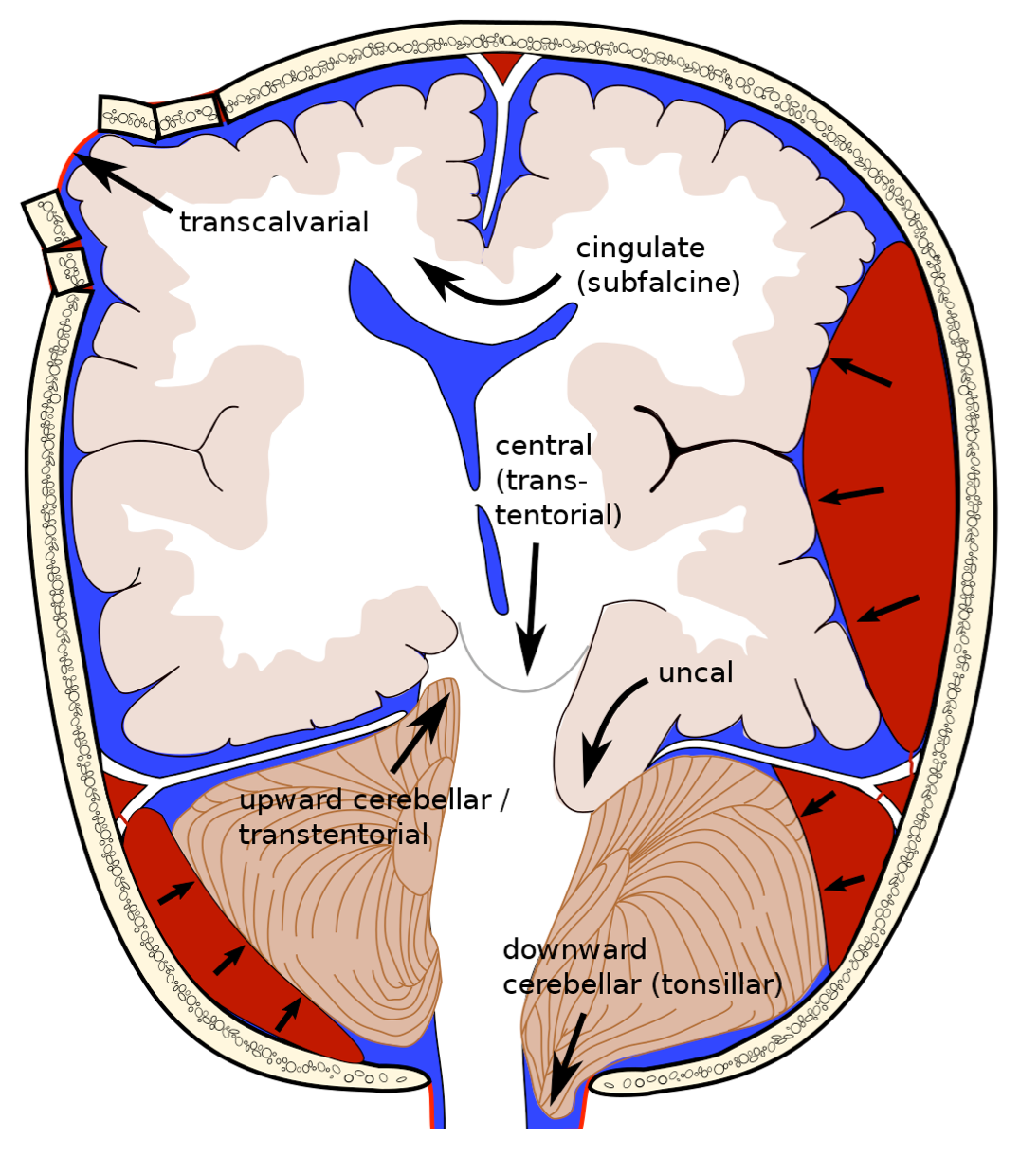
Cerebral Herniation Syndromes
▪️Background
- Cerebral herniation is defined as a shift of cerebral tissue from its normal location into an adjacent space, and it is a life-threatening condition that requires prompt diagnosis.
- Brain herniation may produce brain damage (brain pressure necrosis), and compress cranial nerves and vessels causing hemorrhage or ischemia, or it may obstruct the normal circulation of cerebrospinal fluid producing hydrocephalus.
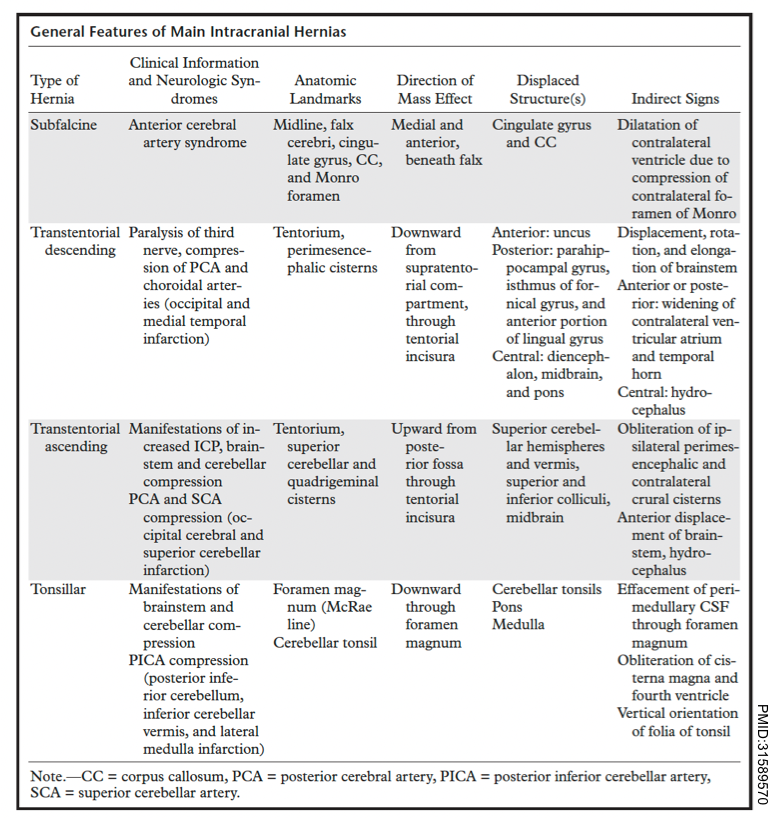
- Brain herniation can be classified into two broad categories: intracranial and extracranial. The intracranial hernias can be subdivided into three basic types:
- Subfalcine hernia
- Transtentorial hernia, which can be descending (lateral and central), or ascending.
- Tonsillar hernia.
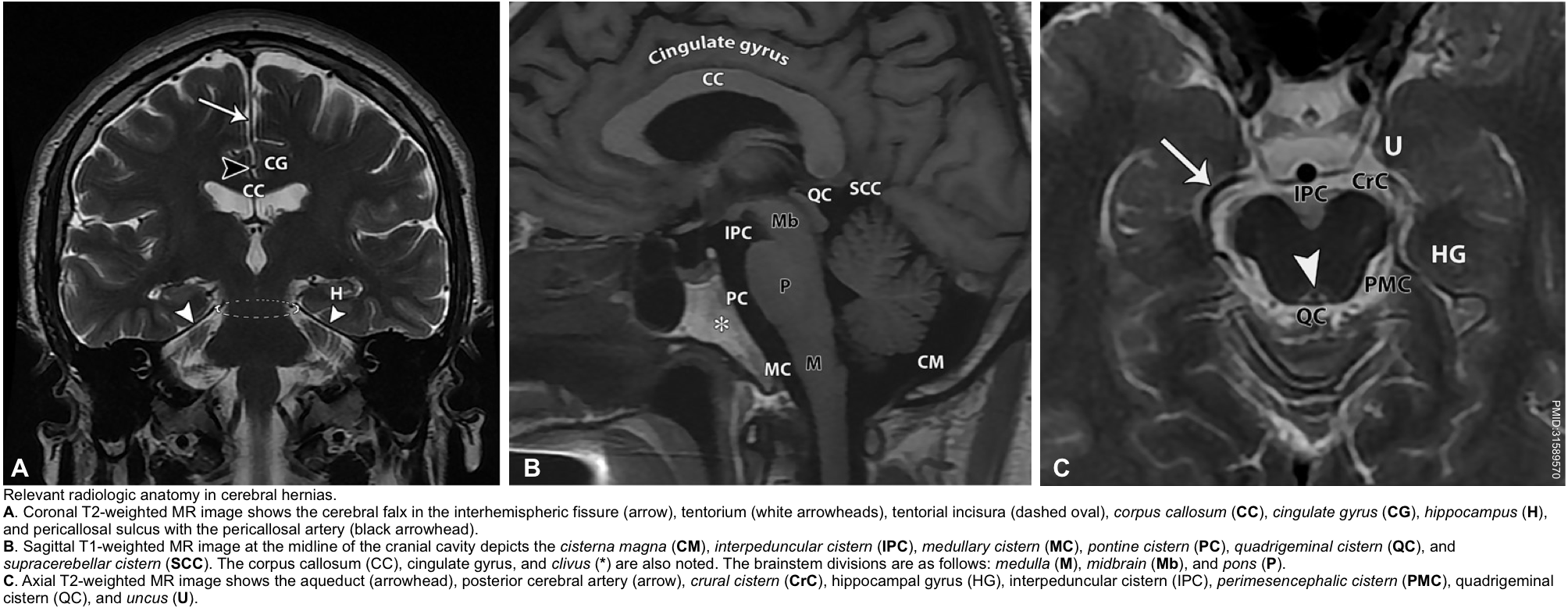
▪️The relevant radiologic neuroanatomy in cerebral hernias is shown below.
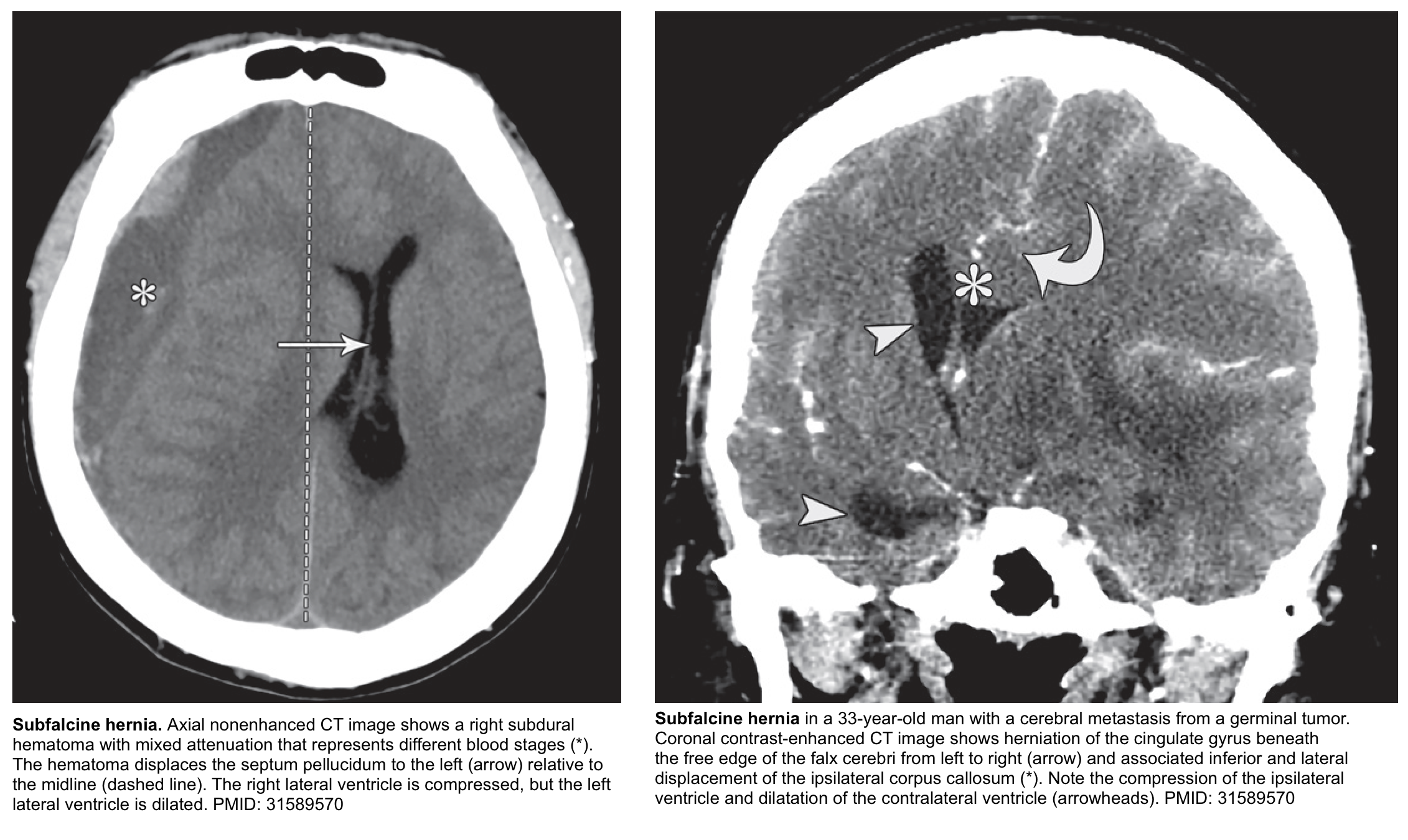
Subfalcine hernia
Subfalcine hernia (aka. midline shift or cingulate hernia) is the most common type of cerebral hernia *.
Clinical manifestation
- Contralateral leg weakness is a central finding, due to compression of the ipsilateral anterior cerebral artery. Posturing of the leg may also be seen.
- Mental status is relatively unaffected, as compared to other herniation syndromes. However, there may be apathy and indifference.
⭐️In subfalcine hernias, the degree of midline shift correlates with the prognosis; less than 5 mm deviation has a good prognosis, whereas a shift of >15 mm is related to a poor outcome.
Other features
- The cingulate gyrus herniates underneath the falx cerebri. This may eventually cause necrosis of the ipsilateral cingulate gyrus.
- ACA (anterior cerebral artery) infarction may occur (causing contralateral leg weakness).
- Falcine herniation may compromise both foramina of Monro, causing dilation of the contralateral ventricle (the ipsilateral ventricle tends to be directly compressed).
- Ultimately, this may progress to central downward herniation over time (due to increasing edema of herniated tissue).
Descending Transtentorial Hernia
▪️Descending transtentorial hernia (DTH) is the second most common type of cerebral hernia. It occurs when brain tissue is displaced downward through the tentorial notch. DTH may be divided into 2 types:
- Lateral hernias
- Lateral hernias involve the medial temporal lobe. Lateral hernias have 2 subtypes:
- Anterior: The uncus is herniated downward into the ipsilateral crural cistern. See Uncal herniation.
- Posterior: The parahippocampal gyrus is displaced downward into the posterolateral part of the tentorial incisura.
- Lateral hernias involve the medial temporal lobe. Lateral hernias have 2 subtypes:
- Central hernias
- There is a descent of the diencephalon, midbrain, and pons. More on this below.
💡Keep in mind that different types of cerebral hernias can be present at the same time.
- In DTH, if there is further descent of brain tissue, a tonsillar hernia might occur.
- Also, a subfalcine hernia may be present, depending on the location of the disease.
▪️Clinical features
- In this type of hernia, the pressure caused by the crowding of tissue within the incisura compromises the following structures *:
- Cranial nerve 3.
- Posterior cerebral artery.
- Midbrain.
- Hydrocephalus develops because of the compression of the cerebral aqueduct.
- Brainstem ischemia and hemorrhage
- Severe and abrupt downward displacement of the brainstem causes stretching and shearing of perforating branches of the basilar artery, resulting in ischemia and hemorrhage in the brainstem.
- However, this effect can be multiple or even extend into the cerebellar peduncles. This is called Duret hemorrhage; it is a late finding and portends a poor prognosis, usually death.
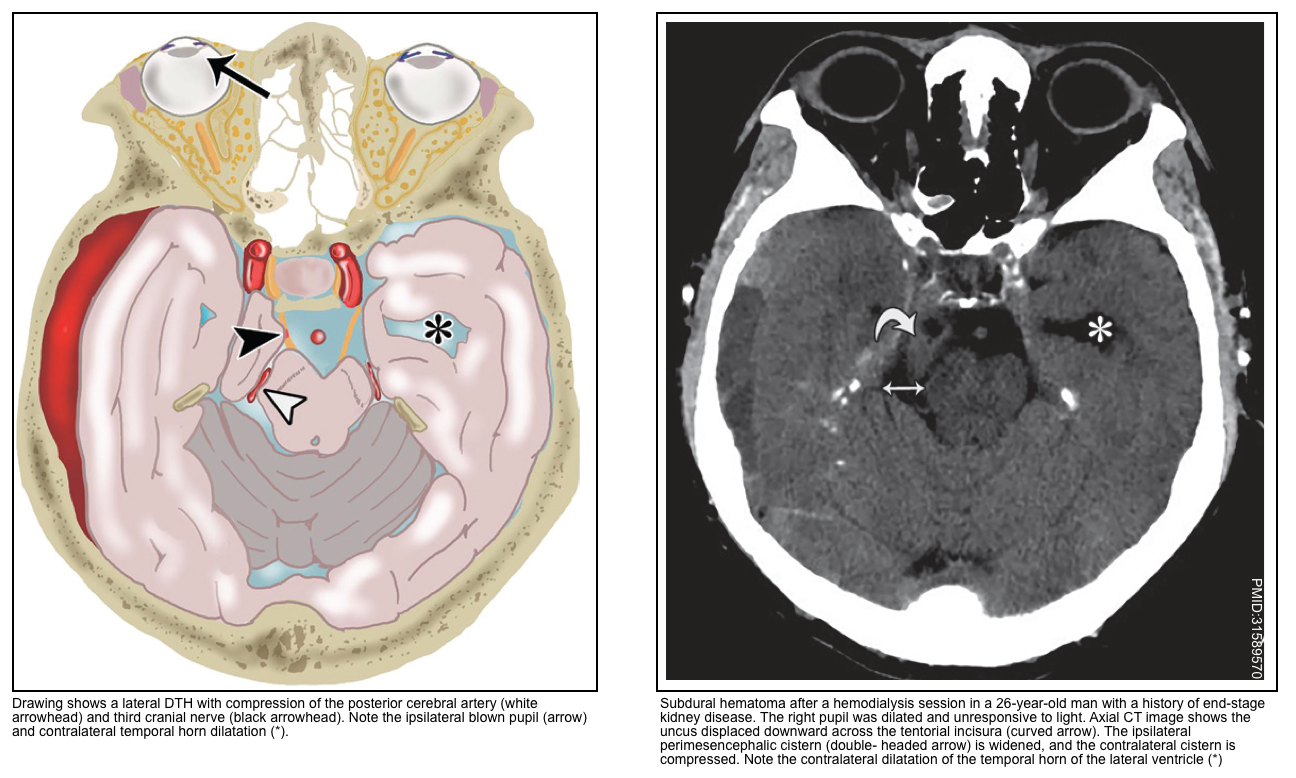
Uncal herniation syndrome
▪️Uncal herniation refers to downward herniation of the anterolateral portion of the temporal lobe.
▪️Clinical manifestations
- Ipsilateral blown pupil
- Initially, the pupil is dilated and sluggish.
- Parasympathetic fibers run outside CN3-oculomotor, so pupillary dilation can occur first *.
- With progression, the pupil becomes fixed and dilated. Impaired consciousness is almost always present by the time the pupil is fixed and dilated.
- Oculomotor paralysis occurs with the progression (causing the eye to be locked in a down-and-out orientation) *.
- Ipsilateral ptosis results from the denervation of the levator palpebrae superiors *.
- Initially, the pupil is dilated and sluggish.
- Contralateral hemiparesis due to compression of the cerebral pedicle.
- Hemiparesis may be accompanied by hyperreflexia and a positive Babinski’s sign.
- If untreated, this may eventually compress the midbrain (e.g. causing bilateral fixed & dilated pupils, with decorticate or decerebrate posturing).
▪️Kernohan’s notch phenomenon *
- Definition: Lateral displacement of the midbrain can compress the contralateral cerebral peduncle. This causes weakness ipsilateral to the herniation (a false localizing sign).
- The CN3-oculomotor palsy sometimes remains correctly localizing (ipsilateral to the herniation). Thus, Kernohan’s notch phenomenon could be suggested by ipsilateral pupillary dilation and weakness *.
▪️Neuroimaging findings
- The earliest finding may be the effacement of the suprasellar cistern.
- Widening of the ipsilateral perimesencephalic cistern (since the midbrain is pushed away from the hernia).
- Hydrocephalus may be seen (especially involving the contralateral lateral ventricle).
▪️Relevant anatomical features
- Uncal herniation is often the first event in downward transtentorial herniation, followed by herniation of more posteriorly located brain tissue.
- Compression of the PCA (posterior cerebral artery) may cause infarction of the medial temporal and occipital lobes *.
- Compression of the aqueduct of Sylvius may cause hydrocephalus.
- Compression of the reticular activating system (RAS) in the midbrain can cause unconsciousness.
Posterior lateral descending transtentorial herniation
- Refers to herniation of posterior portions of the temporal lobe (e.g. parahippocampal gyrus) downward into the posterolateral part of the tentorial incisura.
- In patients with occipital and posterior temporal disease, the herniation of the medial temporal lobe occurs more posteriorly.
- This may cause impingement on the lateral part of the quadrigeminal plate cistern and cause displacement, rotation, and compression of the brainstem (figure below) *.
- Clinical manifestations may include:
- Parinaud syndrome may occur due to the involvement of the tectum at the level of the superior colliculus.
- Cranial nerve 3 or PCA (posterior cerebral artery) can be involved, but less commonly compared to uncal herniation *.
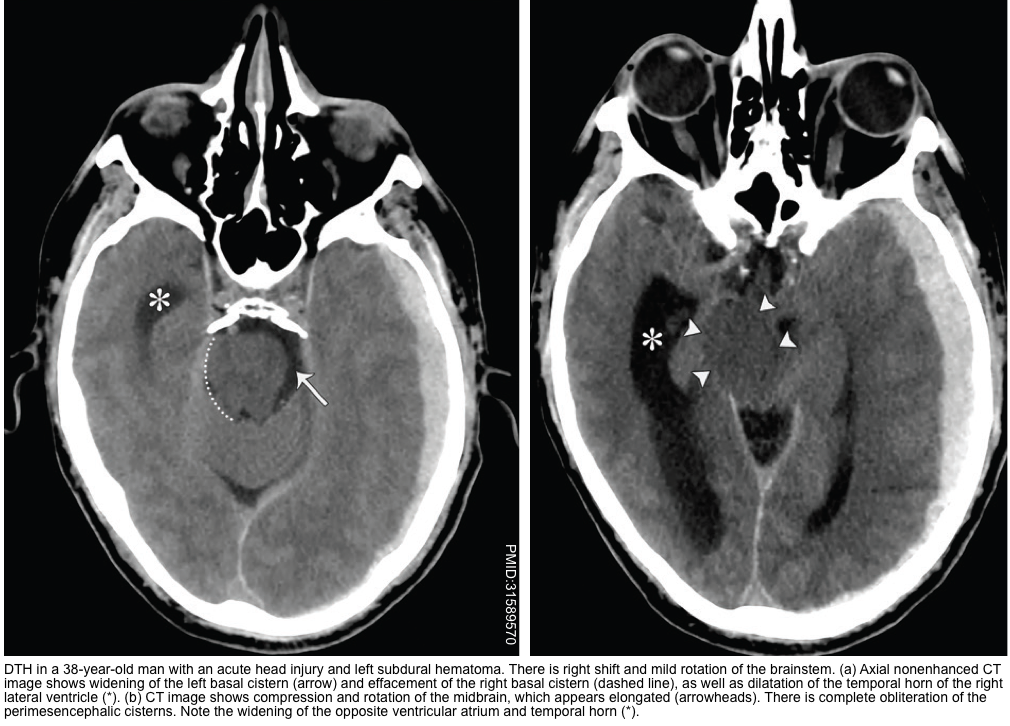
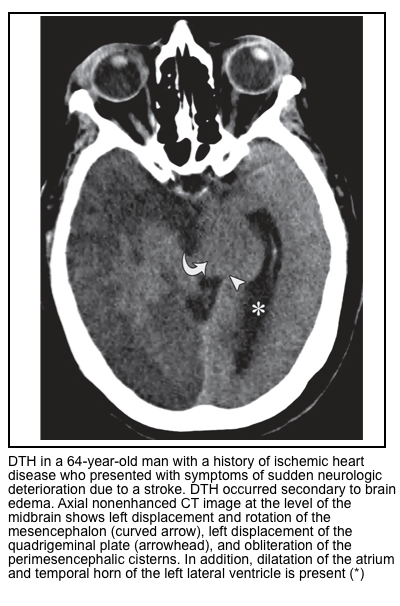
Central Descending Transtentorial Herniation
- In the central hernia, there is descent of the diencephalon, midbrain, and pons. It usually manifests along with other types of DTH (discussed above).
- Central DTH can be caused by bilateral supratentorial disease causing mass effect, midline masses, severe brain edema, or supratentorial hydrocephalus.
- The signs and symptoms are bilateral (in contrast to uncal herniation which often causes unilateral signs) and progress rostrally to caudally.
- The initial impairment of consciousness with supratentorial mass lesions usually relates to lateral rather than downward displacement.
- Horizontal shifts of midline structures, especially the pineal body of greater than 8 mm, are associated with some impairment of consciousness; patients with shifts of >11 mm are usually comatose *.
- Other signs of increased ICP, papilledema, and Cushing triad (hypertension, bradycardia, irregular respiration) may be observed in this setting.
▪️Clinical manifestations
- Diencephalic stage (localized to the thalamus)
- Drowsiness and confusion (due to disruption of the reticular activating system).
- Miotic (small) pupils are initially a prominent sign, often with restricted upgaze (Parinaud syndrome).
- Uncommonly, a unilateral Horner syndrome may occur early on (before transitioning to bilateral small pupils).
- ⚠️ Initially, this may resemble an intoxication causing small pupils (e.g. cholinergic agonists, opioids, clonidine, ACE-inhibitors). There may initially be conjugate roving eye movements, simulating a toxic/metabolic coma.
- Midbrain-upper pons stage (mesencephalic stage)
- Coma deepens.
- Midbrain dysfunction emerges: Pupils may become fixed & mid-position.
- Abnormal motor responses (e.g. posturing) may be an early clue to a structural etiology of the coma. Decorticate and later decerebrate posturing occur, with bilateral extensor Babinski reflexes.
- Pontine dysfunction emerges, with loss of vestibulo-ocular reflexes and corneal reflexes.
- Pathological breathing patterns may emerge (e.g. central neurogenic hyperventilation).
- Medullary stage
- Breathing may slow and become irregular (ataxic breathing).
- Cushing’s reflex may occur with hypertension and bradycardia. Pupils may temporarily dilate due to a surge of epinephrine release.
▪️Neuroimaging findings
- Effacement of the perimesencephalic cisterns is the most useful and consistent finding *.
- Hydrocephalus *.
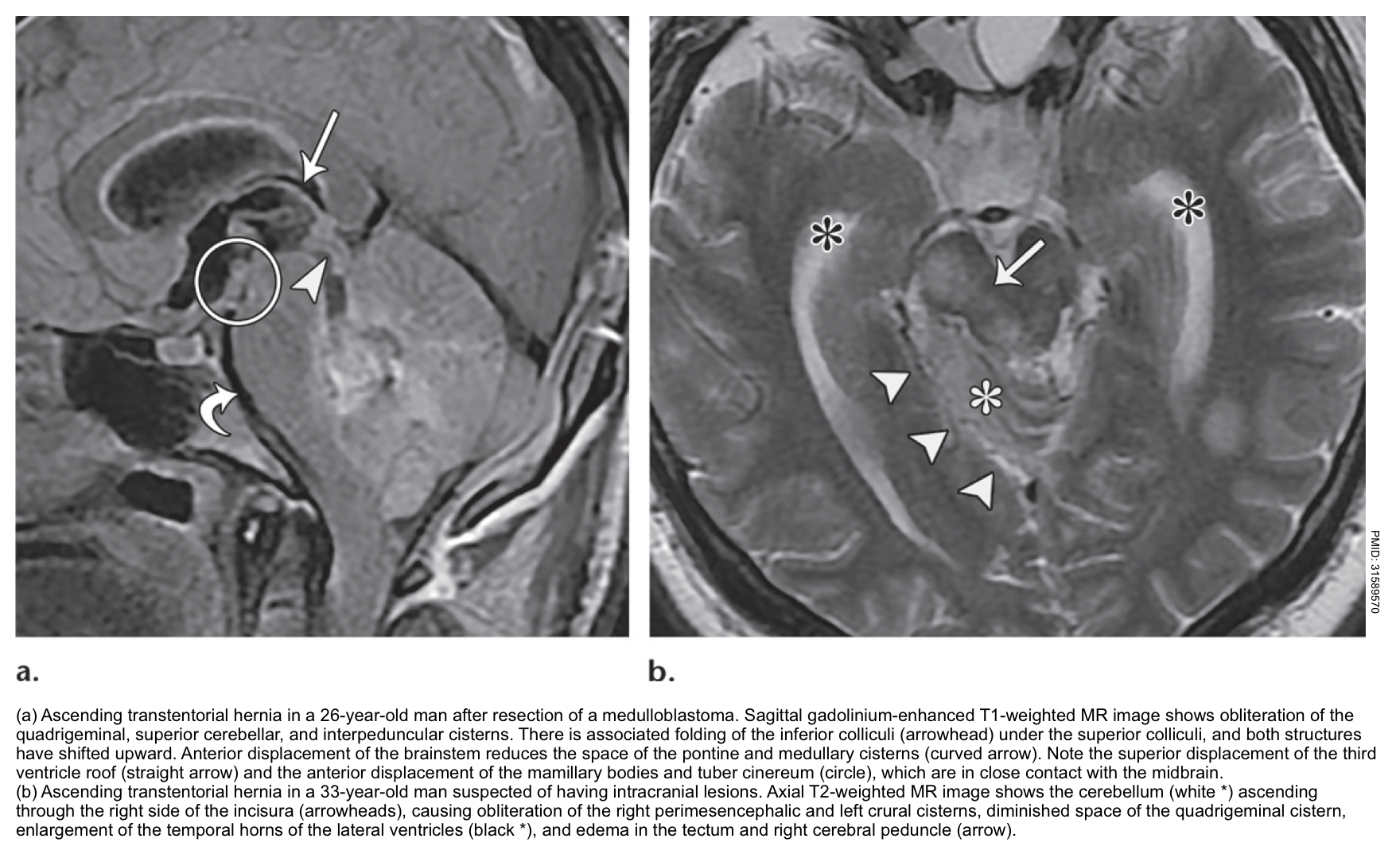
Ascending transtentorial herniation
- Mass effect from the posterior cranial fossa, especially a mass originating near the opening of the tentorium cerebelli (e.g. within the cerebellar vermis).
- Sudden relief of supratentorial intracranial hypertension (e.g. due to placement of an external ventricular drain) *.
▪️Clinical manifestations
- Vertical gaze palsy may be followed by stupor/coma *.
- Pupils may be fixed and either midposition or dilated.
- Cerebral aqueduct compression may result in acute hydrocephalus.
- Patients may also have impaired swallowing and aspiration, due to compression of the brainstem.
- Branches of the SCA (superior cerebellar arteries) and the PCA (posterior cerebral arteries) may be compressed, causing infarction of the superior portion of the cerebellar hemisphere and the occipital lobe.
▪️Neuroimaging findings
- The quadrigeminal cistern is distorted.
- Compression of the aqueduct of Sylvius may cause hydrocephalus.
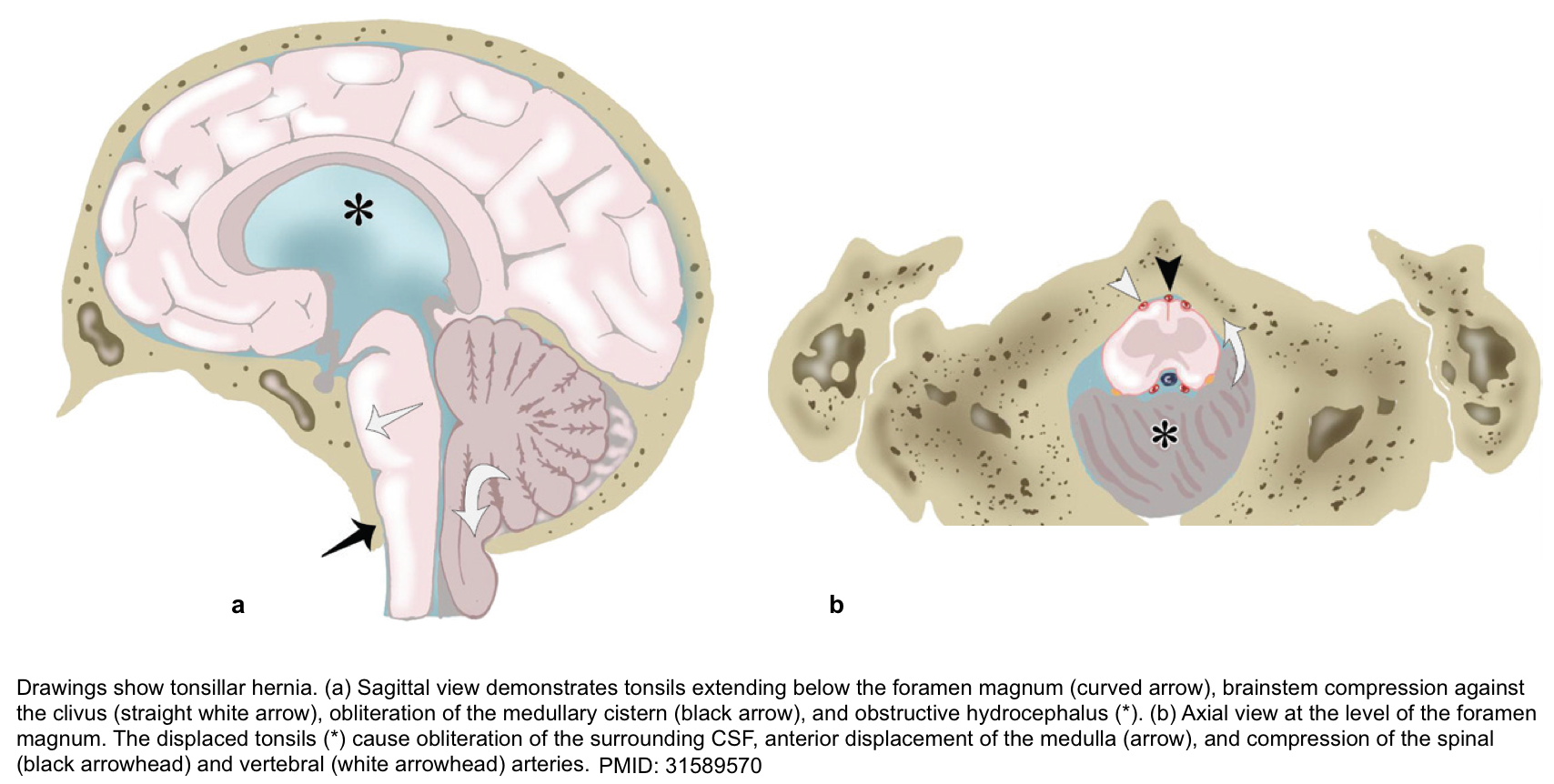
Tonsillar Herniation
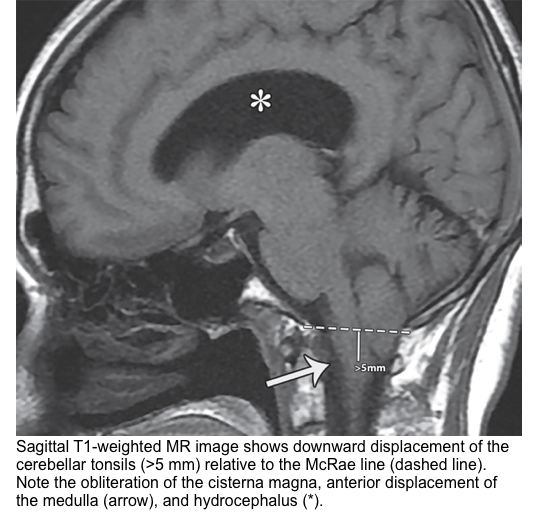
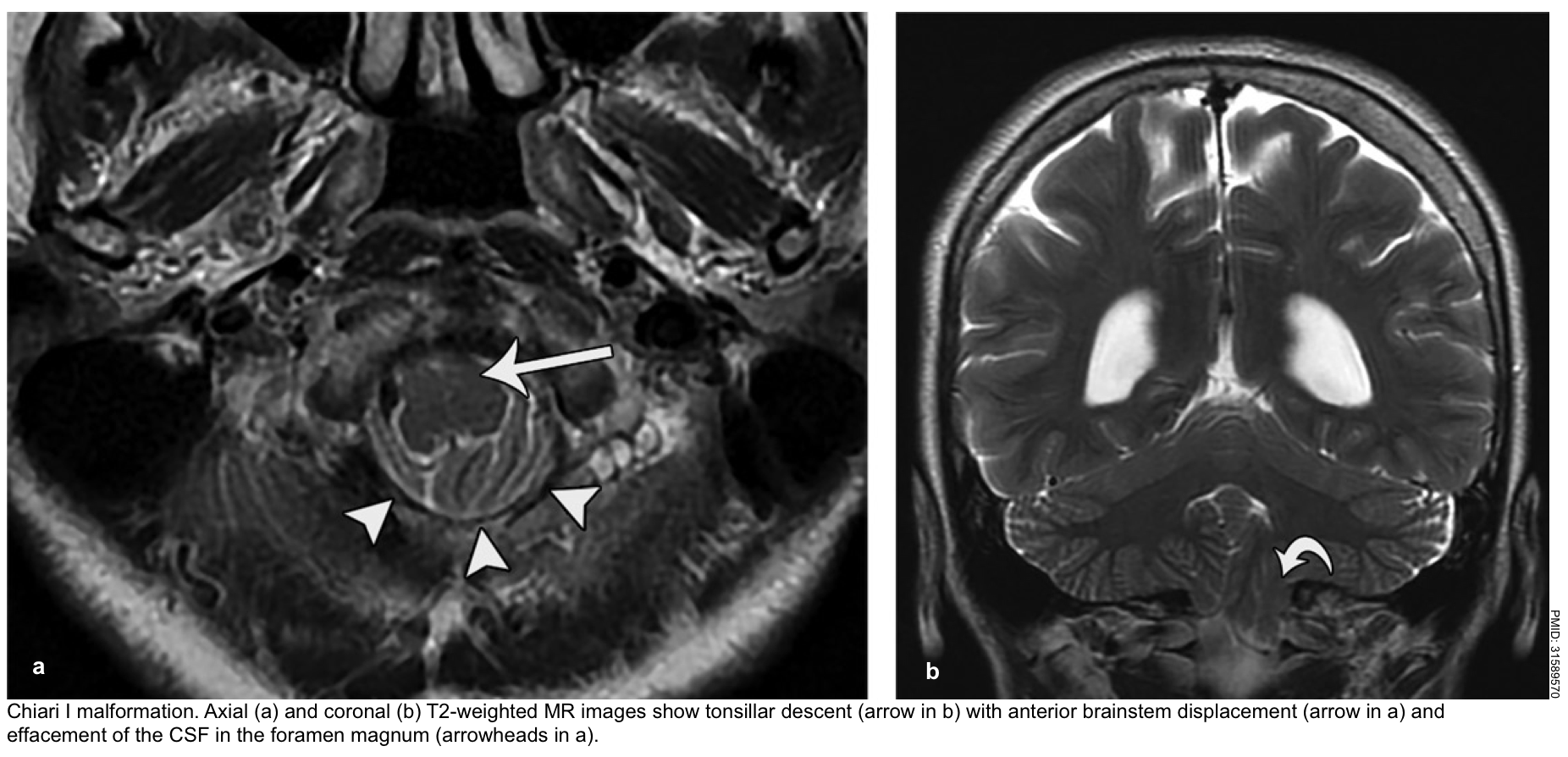
The tonsillar hernia is an inferior displacement of the cerebellar tonsils through the foramen magnum into the cervical spinal canal. It may be congenital (Chiari spectrum) or acquired.
Normal tonsillar position relative to the foramen magnum varies with age. The normal position of tonsils below the foramen magnum for different age groups.
- In the 1st decade of life, the presence of cerebellar tonsils > 6 mm below the foramen magnum is considered abnormal.
- In the next 2 decades, the reference value is 5 mm; for the 4th to 8th decades, the threshold is >4 mm; and at age 80 years or older, 3 mm is the limit *.
The McRae line is used as a reference for this measurement. It is obtained by drawing a line from the basion to the opisthion. The degree of tonsillar herniation is the perpendicular length from the McRae line to the tip of the displaced tonsil (figure below).
▪️Clinical manifestation *
- A stiff neck may be a very early sign.
- Cranial nerve palsies and stupor/coma result from brainstem compression.
- Decerebrate posturing may occur initially, but eventually, flaccid paralysis occurs.
- Cushing reflex may occur including hypertension, bradycardia, and slow respirations. Compression of the medulla may eventually lead to central hypoventilation with respiratory arrest.
▪️Other features
- Acute hydrocephalus may occur (due to compression of the fourth ventricle). This may accelerate the process of herniation and consciousness impairment.
- Tonsillar herniation may cause infarction of the PICA (posterior inferior cerebellar artery), causing infarction of the cerebellum and lateral medulla *.
Brainstem displacement from a hemispheric mass
Clinical features *
- Anisocoria or unilateral fixed and dilated pupil (predominant lateral displacement).
- Mid-position fixed pupils (predominant downward displacement).
- Extensor or flexor posturing.
- Central hyperventilation (diencephalic).
Brainstem displacement from a cerebellar mass
Clinical features *
- Direction-changing or vertical nystagmus from the cerebellar lesion.
- Ocular bobbing, described as a rapid downward deviation of the eyes followed by a slow upward movement, typically implies a pontine lesion.
- The skew deviation may occur (vertical misalignment of eyes).
- Internuclear ophthalmoplegia can happen.
- Cranial nerves:
- Pupillary reflexes are often intact (since these are located in the midbrain, which is above the level of compression).
- Absent corneal reflexes and abnormal vestibulo-ocular reflexes are often seen.
- Facial or abducens nerve palsy
- Extensor or flexor posturing can occur.
▪️Etiology *
- Coma from a primary brainstem process can occur in the following conditions
- Infarction or hemorrhage of the upper pons and/or midbrain.
- Osmotic demyelination syndrome (formerly called central pontine myelinolysis).
- Brainstem encephalitis.
⭐️It is critical to ensure that these patients are not a locked-in syndrome.
▪️General Features
- Bilateral long-tract involvement is usual and may manifest with flaccid quadriparesis or decerebrate posturing.
- Eye movements may be notably asymmetric or absent and pupils are classically small *.
▪️Clinical manifestations *
- Vertical nystagmus or bobbing.
- Miosis (with pontine lesions).
- Internuclear ophthalmoplegia.
- Variable pupillary and corneal reflexes (can both be absent).
- Absent oculocephalic and oculovestibular responses.
- Extensor or flexor posturing.
- Ataxic breathing (pontomedullary damage).
⚠️Patients with an intrinsic brainstem syndrome and a normal CT scan most likely have an embolus to the basilar artery; an abnormal CT scan will likely show a hemorrhage into the brainstem, traumatic brain injury to the brainstem, or a brainstem tumor or infectious mass.
Metabolic coma
▪️Helpful diagnostic clues *
- The neurologic deficit is almost always symmetrical.
- For example, in a toxic-metabolic coma, if the patient demonstrates either spontaneous movements or reflex posturing, the movements are symmetric without evidence of hemiparesis. Muscle stretch reflexes, if present, are symmetric.
- 💡Exceptions occur; in particular, hypo- and hyperglycemia are frequently associated with lateralized motor findings.
- Fluctuations in the examination are common.
- Tremor, asterixis, and multifocal myoclonus strongly suggest metabolic coma.
- Decreased muscle tone is usually present.
- Decerebrate posturing is less common in metabolic coma but may occur.
- The pupillary response is generally preserved.
- Pupils may appear abnormal but almost always are symmetric and constrict with light.
- Typically, the pupils are small but reactive.
- Extraocular eye movements: They are symmetric (if present) *.
- ⚠️If extraocular movements are absent, however, this sign is of no value in differentiating toxic-metabolic from the structural coma.
- ⚠️Suppression of VORs and corneal reflex occurs with profoundly deep metabolic coma.
💡A notable exception is severe sedative poisoning such as from barbiturates; the pupils may be large, extraocular movements absent, muscles flaccid, and the patient apneic, which simulates the appearance of brain death.
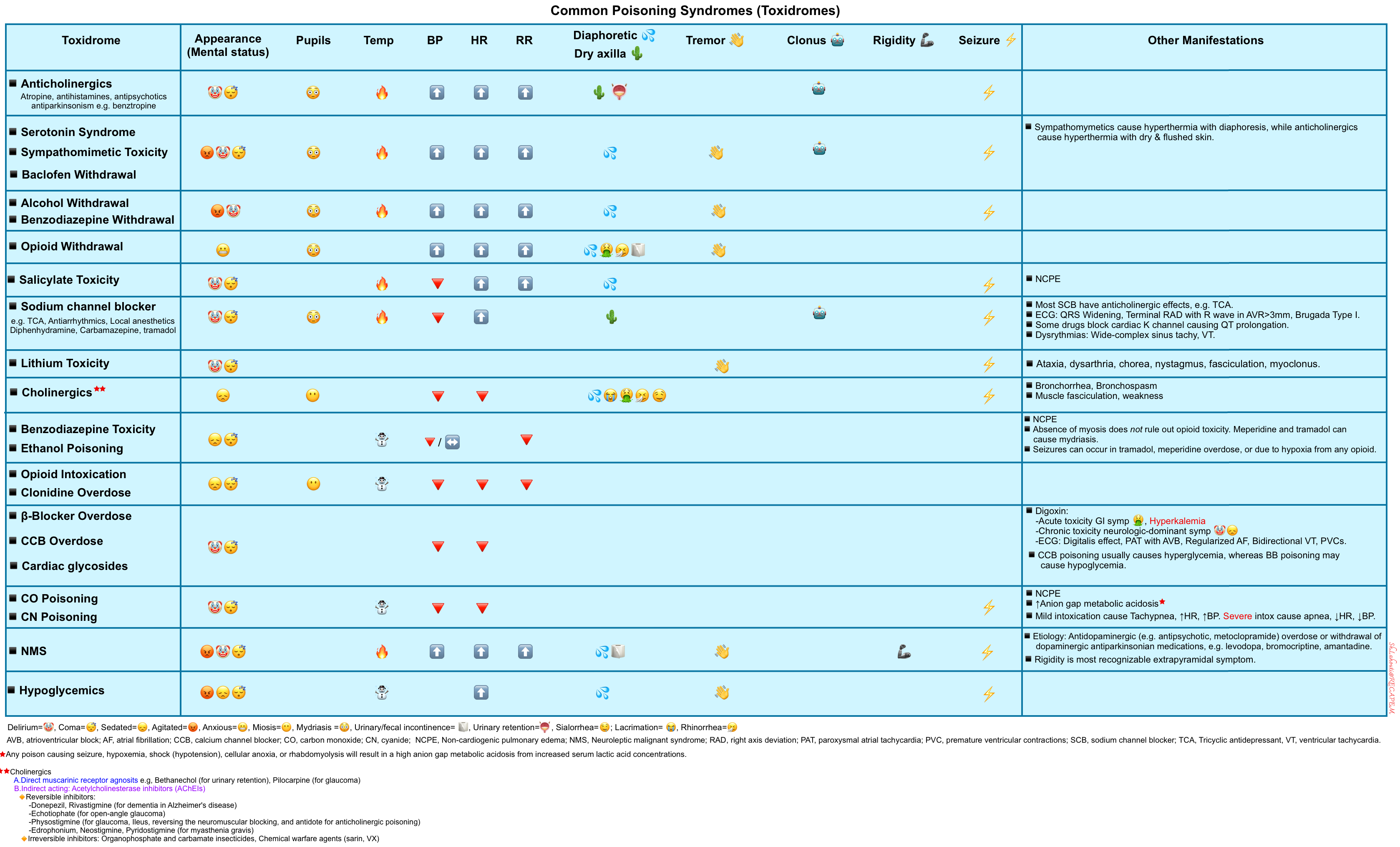
Toxic Syndrome
Drug overdoses or poisonings often appear similar to metabolic coma but may be associated with distinctive clinical features. Common poisoning syndromes (toxidromes) are outlined below.
Coma mimics
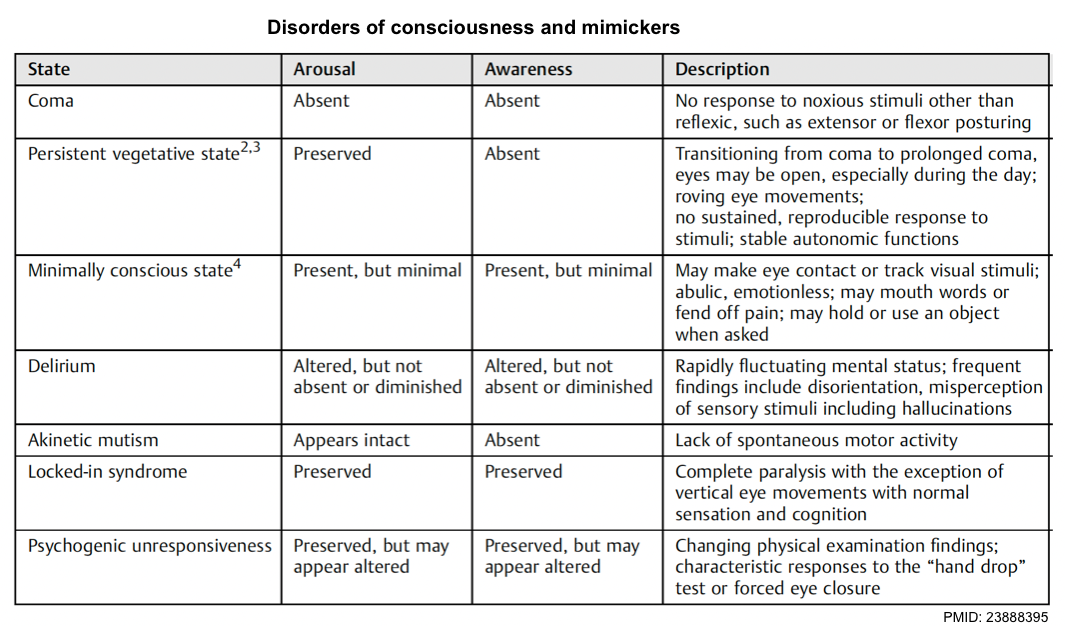
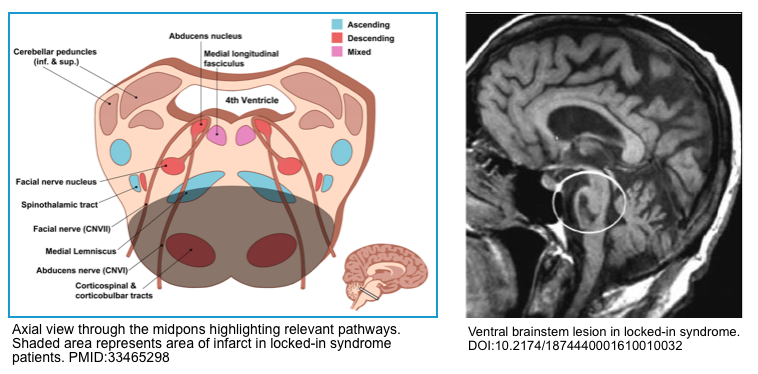
Locked-in syndrome
Locked-in syndrome is caused by an injury to bilateral anterior (ventral) pons which damages the bilateral corticobulbar and corticospinal tracts. Clinically patients are awake, cognitively intact with preserved sensation, but paralyzed (only with the ability to blink or move their eyes vertically) *.
- Damage to this area is responsible for complete motor tetraplegia and paralysis of the bilateral face.
- The fascicles for the abducens nerve and the paramedian pontine reticular formation are also injured resulting in impaired bilateral horizontal gaze.
The following neurologic functions are preserved:
- The sensation is usually preserved, because the medial lemniscus and the spinothalamic pathways (which sit laterally) are spared.
- Vertical eye movement and blinking are also spared as they are primarily controlled by cranial nerve III (oculomotor), cranial nerve IV (trochlear), the rostral midbrain reticular formation, and pretectal areas of the midbrain.
- Arousal and consciousness remain intact as they are modulated by the ascending reticular activating system, which is largely located in the midbrain and cerebral peduncle
Etiology
- An acute ischemic stroke involving the basilar artery territory.
- Pontine hemorrhage,
- Central pontine myelinolysis.
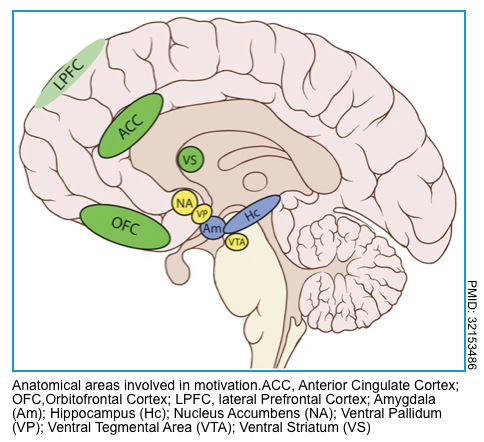
Abulia and akinetic mutism
Disorders of diminished motivation are characterized by impairments in goal-directed executive function (potentially including failure to speak, move, or eat), thought, and emotion and include akinetic mutism, abulia, and apathy *.
- Akinetic mutism is characterized by an inability to voluntarily initiate motor or verbal responses, in the presence of preserved arousal and sensorimotor functions.
- Abulia is a disorder of the will. Although individuals with abulia show less severe symptoms than persons with akinetic mutism, these symptoms are qualitatively identical: passivity reduced spontaneous behavior and speech, lack of initiative, and psycho-motor slowing, combined with reduced emotional responsiveness and spontaneity.
Clinical manifestation
- Profound failure of executive function (potentially including failure to speak, move, or eat).
- Patients may respond to questions, but only in a very delayed fashion.
- Visual tracking may occur, with conjugate eye movements.
- These persons still retain some degree of alertness.
Etiology
- Often reflects damage to the medial frontal lobe(s).
- May result from infarction of the ACA (anterior cerebral artery), or vasospasm involving the ACA territory
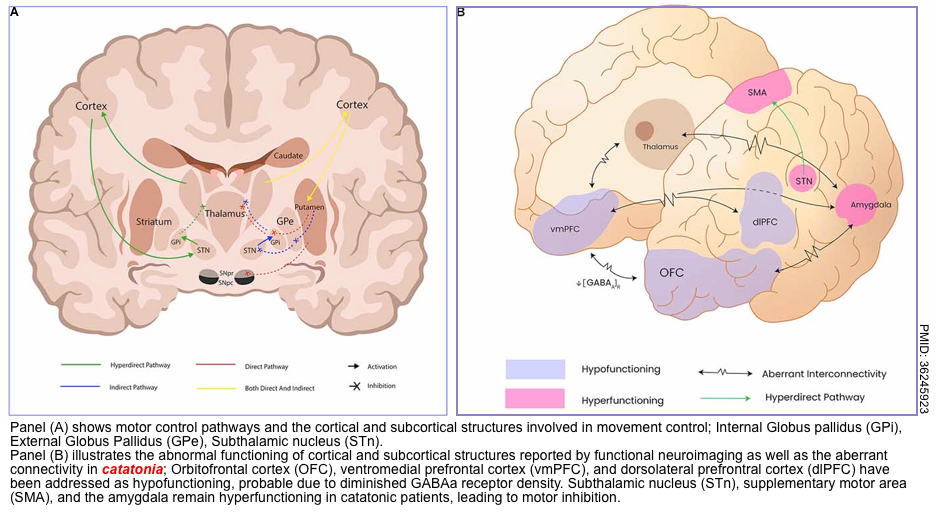
Hypokinetic catatonia
Background
- Catatonia is a psychomotor dysregulation syndrome, and its diverse symptomatology can include motor (difficulty initiating or terminating actions), mental, behavioral, and vegetative manifestations.
- Catatonia is a spectrum disorder that varies in severity (rather than a binary, dichotomous condition).
- Generally, catatonia seems to reflect an abnormality of neural circuitry that regulates movement (involving the basal ganglia, thalamus, and cortex; figure below). This shares some similarities with Parkinson’s disorder.
- Catatonia seems to involve several neurotransmitter imbalances (perhaps with different imbalances occurring in different patients)
- Inadequate dopamine activity at D2 receptors, inadequate GABA signaling, and excessive signaling via glutaminergic NMDA receptors.
- Catatonia variants: It has 3 types. Hypokinetic catatonia (most common type), hyperkinetic catatonia, and malignant catatonia *.
- Note that individual patients can transition back and forth between hypokinetic and hyperkinetic types.
- Either hypokinetic or hyperkinetic catatonia may lead to autonomic instability. Catatonia which causes autonomic instability is defined as malignant catatonia.
Hypokinetic catatonia *
- Generally appear obtunded, rather than completely comatose.
- The most common symptoms include immobility, mutism, withdrawal, refusal to eat, and staring (some patients may have closed eyes that are resistant to passive eye-opening).
- Motor symptoms (e.g. rigidity, posturing) may occur less commonly (~50%).
- Patients may fail to respond to painful stimuli.
- Patients may be incontinent of urine or stool or may retain urine and require catheterization.
Distinguishing features of catatonia
- Catatonia may be suggested by bizarre features that aren’t generally seen in the context of medical illness. The presence of these distinctive features of catatonia may be useful if observed (e.g., cataplexy, waxy flexibility).
Psychogenic coma
- It is a similar state to functional (non-epileptic) seizures and is commonly triggered by psychological stress.
- The diagnosis is generally based on 2 features:
- Exclusion of alternative medical/neurologic emergencies (e.g. hypoglycemia).
- Demonstration of positive features on neurological examination. The use of positive features is extremely important to secure a confident diagnosis.
- Examination features suggestive of psychogenic coma (positive signs) * :
- Open the eyelids and look for three signs of functional unresponsiveness:
- Tightly shut eyelids. Patients with functional unresponsiveness may resist eye-opening, whereas in an organic coma, the eyes can be easily opened.
- Eye movements. Patients with functional unresponsiveness may move their eyes in the usual ways (e.g. eyes roll upwards). In an organic coma, the eyes should often remain still or move in a stereotyped fashion (e.g. conjugate horizontal roving, ocular bobbing, or ocular dipping).
- Rapid eyelid closure may occur in functional unresponsiveness. Alternatively, in an organic coma, the eyes will close in a smooth, gradual movement that cannot be duplicated by an awake person simulating unconsciousness.
- Response to stimuli
- Eyelash tickling or gentle nasal swabbing may elicit eyelid opening or fluttering in a patient with functional unresponsiveness.
- ⚠️Patients with functional unresponsiveness may fail to respond to highly painful stimuli, so avoid the repeated use of highly aversive stimuli (e.g., sternal rub and nailbed pressure).
- ⚠️Dropping the patient’s hand over their face is a popular technique, but this carries a risk of trauma and may be ambiguous to interpret.
- Open the eyelids and look for three signs of functional unresponsiveness:
Evaluation
Labs
- Initial investigations *
- Fingerstick glucose
- Electrolytes including calcium, especially in older patients.
- CBC with differential.
- Coagulation studies (INR, PTT).
- Liver function tests & ammonia.
- TSH (thyroid stimulating hormone).
- Other labs to consider
- ABG/VBG, if hypercapnia is suspected.
- Creatinine kinase, if found down.
- Pregnancy test, if relevant.
- Infectious workup as appropriate
- Blood cultures (if sepsis or meningitis is suspected) *.
- Chest X-ray, urinalysis.
- HIV screen.
- Toxicology evaluation as appropriate
- Carboxyhemoglobin level.
- Serum ethanol level.
- Serum salicylate & acetaminophen levels.
Neuroimaging
▪️CT scan
- Non-contrast head CT: This may reveal traumatic lesions, hemorrhagic stroke, and other space-occupying lesions. Possible indications include:
- Elderly
- Evidence of trauma
- Focal neurological signs
- Unclear etiology.
- CT angiography (CTA) may be employed in cases of suspected stroke.
- CT venogram (CTV) should be considered if there is suspicion of venous sinus thrombosis (thrombosis of the straight sinus or Vein of Galen may cause thalamic dysfunction, leading to coma).
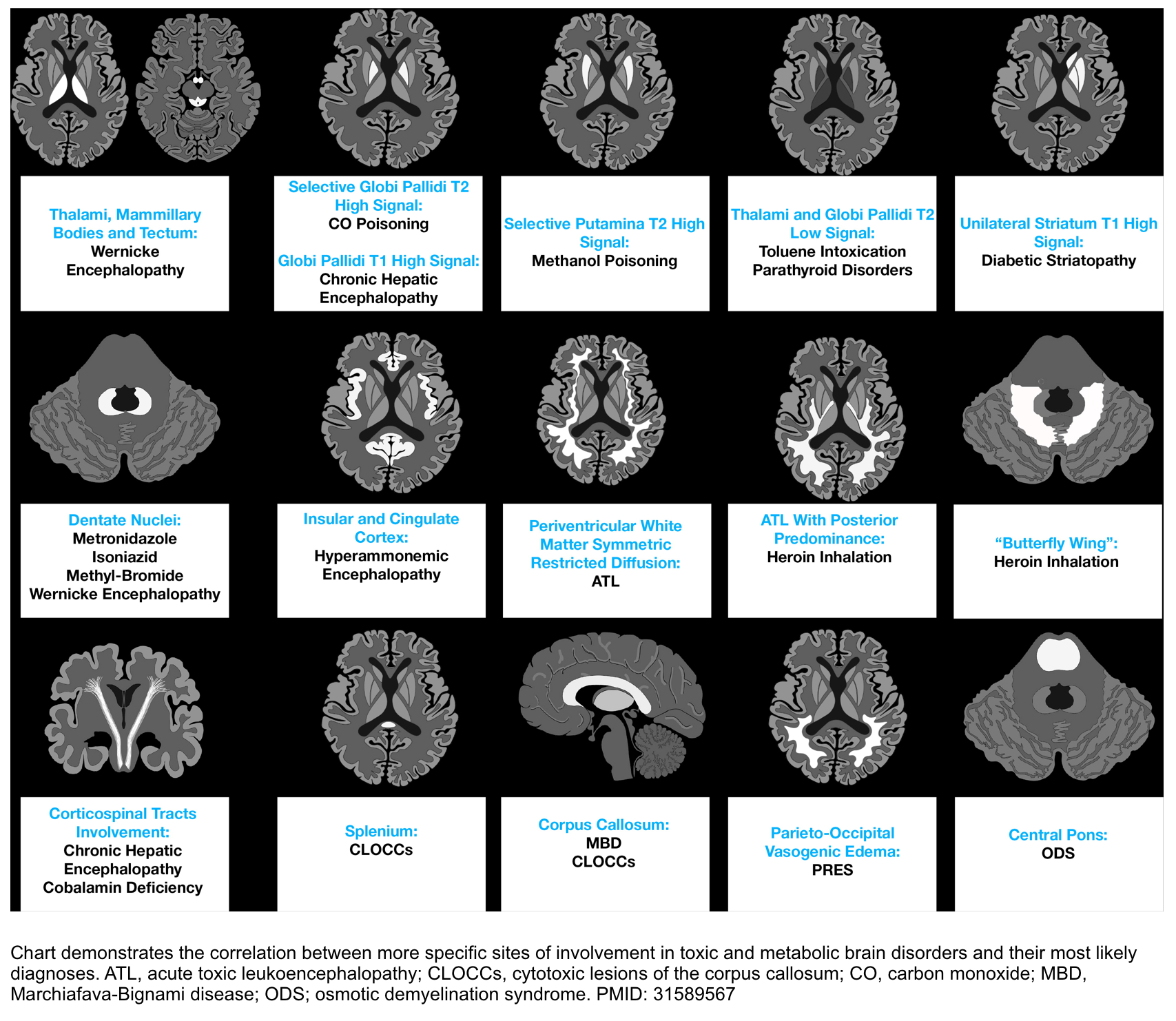
▪️MRI
- MRI is indicated if the above workup fails to reveal a diagnosis.
- MRI has been diagnostic in demonstrating PRES, fat embolization syndrome, infarcts in the brainstem and thalamus, or acute demyelination diseases such as acute disseminated encephalomyelitis (ADEM) or osmotic demyelination. The most common site of involvement in TME on MRI is shown below.
- MR angiography (MRA) and/or MR venography (MRV) may be considered, similarly to CT angiography and CT venography (e.g. based on the index of suspicion for cerebral venous sinus thrombosis).
- Indications
- Clinical suspicion of meningitis or encephalitis.
- Lack of any alternative explanations for the coma following a review of neurologic examination, labs, and neuroimaging.
- In situations where there is a low index of suspicion for meningitis or higher risk from lumbar puncture (e.g. coagulopathy), a contrast-enhanced MRI may be considered as an alternative evaluation for meningitis or encephalitis.
EEG
▪️Indications
- Seizure history (current or prior).
- Clinical indications of possible nonconvulsive status, for example:
- Focal twitching of the hands or face, nystagmus, eye blinking, or chewing movements.
- Recurrent pupillary hippus (pupils dilating/constricting spontaneously), pupillary dilation.
- Focal neurologic findings despite a normal CT scan (e.g. gaze deviation) *.
- Elevated lactate that rapidly normalizes.
Approach to management
- Immediate stabilization.
- The initial management of a comatose patient is to correct abnormal vital signs and laboratory abnormalities.
- The initial management of a comatose patient is to correct abnormal vital signs and laboratory abnormalities.
- Differential diagnosis and initial diagnoses based on history, exam, and ancillary sources of information.
- Differentiate coma from coma mimics.
- Is coma caused by structural lesions or is it due to global acute physiologic derangement of brain function?
- Definitive treatment is disease-specific.
| Stabilization of vital functions |
Airway and Breathing
▪️General indications for intubation in comatose patients *:
- The patient cannot protect the airway (i.e. increased work of breathing, pooling secretions, gurgling sounds).
- Gas exchange is poor, with low oxygen saturation or hypercapnia, and irregular ineffective respiratory drive.
- There is a significant risk of aspiration.
- Breathing pattern is inefficient (note: Cheyne-Stokes breathing and central neurogenic hyperventilation do not always require mechanical ventilation)
▪️However, not all patients with one or more above conditions require intubation *.
- Decisions regarding airway management should be driven by gestalt rather than algorithmic decision-making, and are based on several factors:
- The mechanism of coma is important.
- Although a GCS of <8 is an indication for intubation in a trauma patient, poisoned patients, or those patients with rapidly reversible causes of coma e.g. hypoglycemia can be managed without intubation and with low levels of complications.
- A postictal patient who does not show compromise of airway patency maintains a regular breathing pattern and does not have major oxygen desaturation may be carefully observed without harm.
- Expected clinical course (particularly in poisoned patients).
- The patient with an isolated alprazolam ingestion will likely do well without intubation. In contrast, a patient with carbamazepine ingestion is more likely to have a complicated course and require airway intervention.
- Monitoring concerns.
- Patients who require significant time out of the department for diagnostic imaging, as may occur during a CT scan, may require intubation; whereas patients who remain in an acute care area might be managed expectantly, even at the same level of consciousness
- The mechanism of coma is important.
▪️Note that noninvasive ventilation is not a safe option for comatose patients because of a heightened risk of aspiration. If mechanical ventilation is deemed necessary for a comatose patient, tracheal intubation is indicated.
Circulation
▪️IV Access
▪️Ensure adequate blood pressure and sufficient organ perfusion.
- Hypotension
- Obtain ECG.
- If malignant dysrhythmias seem to be the cause of hypotension, follow advanced cardiac life support guidelines to correct dysrhythmia.
- Vasopressor often should be promptly started rather than fluid loading. Immediately correct critical hypotension (since it is detrimental to the injured brain).
- Vasoactive medications: 📖
- Obtain ECG.
- Hypertension
- Some patients may have posterior reversible encephalopathy syndrome (PRES), in which hypertension is the cause of the coma. This is usually associated with MAP >140 mm. Blood pressure should be promptly but carefully controlled. 📖
- In other patients (e.g. with ischemic stroke), hypertension may be a physiologic compensatory mechanism that helps perfuse the injured brain. In these patients, aggressive reduction of the blood pressure can be detrimental. ICH 📖, SAH 📖 Acute ischemic stroke 📖.
- Ultimately, clinical judgment is required to determine optimal management for the undifferentiated patient. If the blood pressure is profoundly elevated (e.g. mean arterial pressure >140 mm), then a gentle reduction may be reasonable. Hypertensive emergency 📖
- ⭐️IV Antihypertensive medication: 📖
Temperature management
▪️Have a broad differential diagnosis for hyperthermia or hypothermia, If the temperature is significantly abnormal.
▪️Normothermia should generally be targeted *.
- Hyperthermia (>40°C)
- Cooling measures such as evaporative cooling are achieved by continuous cold water application to exposed patient skin with convection cooling by having a fan directly blowing over the patient, ice packs to the axilla, groin, neck, and torso, or bladder irrigation with cold saline through the foley catheter.
- Hypothermia (<35°C)
- Warming measures such as warm blankets, forced air convection (eg, Bair Hugger blanket), and warm intravenous fluids.
- More aggressive measures are appropriate in patients with cardiovascular instability who are severely hypothermic.
| Other therapeutic considerations |
Coma Cocktail
▪️The practice of empirically administering a battery of potential antidotes to patients presenting in a coma is no longer recommended *.
- The so-called coma cocktail, consisting of some combination of an opiate reversal agent such as naloxone, thiamine, and hypertonic dextrose, has more risks than benefits.
- Flumazenil, the reversal agent for midazolam, was also briefly included in some regimens. Flumazenil is not recommended in undifferentiated coma and may result in intractable seizures
🟢Dextrose
- IV Dextrose is given only if glucose is low, the result is pending, or a glucometer is not available. 📖
- Although the risks are largely theoretical, it is appropriate to administer an empiric dose of glucose if a glucometer is unavailable, however, if there is no clinical response, further boluses may be harmful.
🟢Naloxone
- Primary indication: reversal of life-threatening respiratory depression (eg, apnea, severe bradypnea, hypoxia, and/or severe hypercapnia) *.
- Naloxone administration is not recommended for altered mental status alone in patients with adequate respiration as it may precipitate acute opioid withdrawal or unmask another toxidrome, e.g. sympathomimetic.
- More on opioid intoxication and naloxone dosing, here.
🟢Thiamine
- A high dose of IV thiamine (500 mg IV q8h *) in patients with at least 2 of 3 features of Wernicke encephalopathy (ataxia, ocular palsy, confusion) should be given, as it has been proven efficacious and safe *.
- A lower dose of thiamine (eg, 100 mg intravenous) is appropriate for patients with undifferentiated altered mental status.
- A common teaching is that thiamine must be administered before or simultaneously with glucose in patients with potential thiamine deficiency (eg, those with malnutrition, chronic alcohol abuse, and signs of Wernicke’s encephalopathy) is debunked.
- A recent literature review examines the issue in detail and concludes that “the available evidence neither supports nor refutes the belief that glucose administration without prior thiamine precipitates Wernicke’s encephalopathy” *.
Empiric management for CNS infection
If there is a significant concern for CNS infection (meningitis, encephalitis), empiric therapy should be given following blood cultures (before CT or lumbar puncture).
- A reasonable regimen is often 10 mg dexamethasone, ceftriaxone 2 grams IV q12hr, and acyclovir 10 mg/kg IV q8hr *. The added benefit of vancomycin and ampicillin here is usually low because most of these patients don’t actually have meningitis. More on this, here.
Antiepileptic drug therapy
- Potential signs of nonconvulsive status epilepticus are discussed here 📖.
- If there is a high index of suspicion for ongoing seizures:
- For intubated patients: increase propofol to a relatively high dose (e.g. ~80 mcg/kg/min), even if this requires vasopressor support with norepinephrine.
- Consider a levetiracetam loading dose (e.g. 60 mg/kg up to a maximal dose of 4.5 grams).
Correct Lab abnormalities
- Severe hyponatremia: 3% Saline 100mL IV bolus over 10min (may repeat x2)
- Severe hypercalcemia: IV Fluid + parenteral bisphosphonate pamidronate.
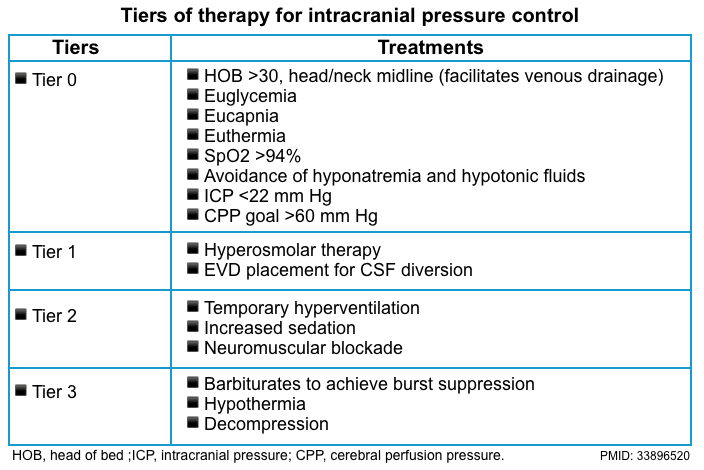
Osmotherapy for suspected ↑ICP
▪️Potential indications for semi-empiric therapy
- Neurologic examination is suggestive of a herniation syndrome.
- The optic disc nerve sheath diameter is unequivocally elevated.
- A history that suggests elevated ICP (eg, coma after head trauma, sudden severe headache typical of subarachnoid hemorrhage)
▪️Management (more on this, here 📖).
- Elevate the head of the bed to >30°
- Hypertonic saline vs. manitol
- With growing familiarity of use, hypertonic saline has increasingly been employed as a first-line agent, supplanting mannitol at numerous institutions.
- 3% saline may be given as a bolus of 250 ml. It is safe to administer 3% saline via a peripheral line *.
- Hypertonic bicarbonate ampules (typically 50 ml of 1 mEq/ml bicarbonate).
- This has the same tonicity as “6% saline”, making it roughly twice as potent as 3% saline.
- 2-3 ampules of hypertonic bicarbonate (100-150 ml) may be used as emergent therapy for elevated ICP.
- Hypertonic bicarbonate may be contraindicated in patients with preexisting metabolic alkalosis.
- Mannitol 0.5-1 g/kg intravenous
- With growing familiarity of use, hypertonic saline has increasingly been employed as a first-line agent, supplanting mannitol at numerous institutions.
⚠️Prolonged hypoventilation (PaCO2 OF ~25-30 mmHg) should be avoided.
- Although hyperventilation will decrease the ICP, it will also reduce cerebral perfusion by causing cerebral vasoconstriction which can lead to cerebral hypoperfusion, secondary ischemic injury, and subsequently worsening cerebral edema *.
- Hyperventilation should be reserved for use as a short-term bridge to immediate neurosurgical intervention.
An overview of general and specific treatment of intracranial hypertension is summarized below *.
| Disease-specific treatment |
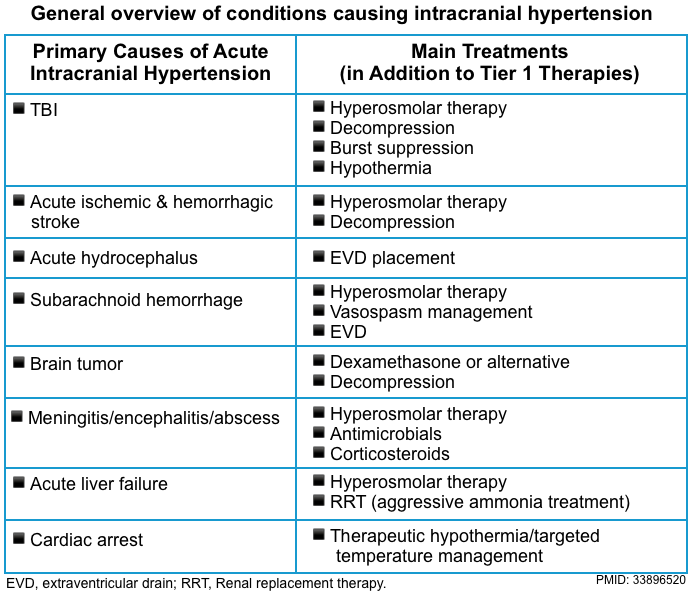
These should be guided by the results of the diagnostic work-up, which should always be geared toward identifying treatable neurologic and neurosurgical emergencies. Some critical diagnoses are mentioned below
▪️Brainstem stroke (basilar artery conclusion)
- Key initial diagnostic investigation: CTA/CT perfusion.
-
Treatment
-
Intravenous thrombolytics per stroke guideline.
-
Discuss mechanical thrombolysis with neuro-interventional consultants.
-
▪️CVT
- Key initial diagnostic investigation: CT venogram, MR venogram.
- Treatment: anticoagulation 📖
▪️Intracranial mass
- If any hydrocephalus, ventriculostomy to reduce raised intracranial pressure.
- Consider evacuation of any mass to relieve increased intracranial pressure.
- If a large mass cannot be removed decompressive craniectomy should be considered (the swollen brain will move out and decompress the mass effect on the deeper diencephalic structures).
References
1. PMID: 30743288. Smith AT, Han JH. Altered Mental Status in the Emergency Department. Semin Neurol. 2019 Feb;39(1):5-19. doi: 10.1055/s-0038-1677035. Epub 2019 Feb 11.
2. PMID: 33896518. Sakusic A, Rabinstein AA. Acute Coma. Neurol Clin. 2021 May;39(2):257-272. doi: 10.1016/j.ncl.2021.01.001.
3. PMID: 27741988. Traub SJ, Wijdicks EF. Initial Diagnosis and Management of Coma. Emerg Med Clin North Am. 2016 Nov;34(4):777-793. doi: 10.1016/j.emc.2016.06.017. Epub 2016 Sep 3.
4. PMID: 20130300. Wijdicks EF. The bare essentials: coma. Pract Neurol. 2010 Feb;10(1):51-60. doi: 10.1136/jnnp.2009.200097.
5. PMID: 10025420. Young GB, Pigott SE. Neurobiological basis of consciousness. Arch Neurol. 1999 Feb;56(2):153-7. doi: 10.1001/archneur.56.2.153.
6. Eelco F. M. Wijdicks. THE COMATOSE PATIENT, 2nd edition, 2014.
7. PMID: 30220246. Van Laecke S. Hypomagnesemia and hypermagnesemia. Acta Clin Belg. 2019 Feb;74(1):41-47. doi: 10.1080/17843286.2018.1516173. Epub 2018 Sep 17.
8. PMID: 31537635. Wijdicks EFM. Predicting the outcome of a comatose patient at the bedside. Pract Neurol. 2020 Feb;20(1):26-33. doi: 10.1136/practneurol-2019-002359. Epub 2019 Sep 19.
9. PMID: 28187795. Wijdicks EF. Management of the comatose patient. Handb Clin Neurol. 2017;140:117-129. doi: 10.1016/B978-0-444-63600-3.00008-8.
10. PMID: 33154707. Zakaria Z, Abdullah MM, Halim SA, Ghani ARI, Idris Z, Abdullah JM. The Neurological Exam of a Comatose Patient: An Essential Practical Guide. Malays J Med Sci. 2020 Oct;27(5):108-123. doi: 10.21315/mjms2020.27.5.11. Epub 2020 Oct 27.
11. PMID: 31589570. Riveros Gilardi B, Muñoz López JI, Hernández Villegas AC, Garay Mora JA, Rico Rodríguez OC, Chávez Appendini R, De la Mora Malváez M, Higuera Calleja JA. Types of Cerebral Herniation and Their Imaging Features. Radiographics. 2019 Oct;39(6):1598-1610. doi: 10.1148/rg.2019190018.
12. PMID: 26704760. Lin AL, Avila EK. Neurologic Emergencies in the Patients With Cancer. J Intensive Care Med. 2017 Feb;32(2):99-115. doi: 10.1177/0885066615619582. Epub 2016 Jul 9.
13. PMID: 24684337. Safavi-Abbasi S, Maurer AJ, Archer JB, Hanel RA, Sughrue ME, Theodore N, Preul MC. From the notch to a glioma grading system: the neurological contributions of James Watson Kernohan. Neurosurg Focus. 2014 Apr;36(4):E4. doi: 10.3171/2014.1.FOCUS13575.
14. PMID: 32938557. Carlstrom LP, Perry A, Puffer RC, Graffeo CS, Reuter PJ, Fogelson JL, Wijdicks EF. A Puzzling Exam: Kernohan’s Notch Reimaged. J Clin Neurosci. 2020 Sep 14:S0967-5868(20)31328-X. doi: 10.1016/j.jocn.2020.06.017. Epub ahead of print.
15. PMID: 7677003. Laine FJ, Shedden AI, Dunn MM, Ghatak NR. Acquired intracranial herniations: MR imaging findings. AJR Am J Roentgenol. 1995 Oct;165(4):967-73. doi: 10.2214/ajr.165.4.7677003.
16. PMID: 30516597. Koenig MA. Cerebral Edema and Elevated Intracranial Pressure. Continuum (Minneap Minn). 2018 Dec;24(6):1588-1602. doi: 10.1212/CON.0000000000000665.
17. PMID: 33465298. Farr E, Altonji K, Harvey RL. Locked-In Syndrome: Practical Rehabilitation Management. PM R. 2021 Dec;13(12):1418-1428. doi: 10.1002/pmrj.12555. Epub 2021 Feb 20.
18. PMID: 32153486. Palmisano S, Fasotti L, Bertens D. Neurobehavioral Initiation and Motivation Problems After Acquired Brain Injury. Front Neurol. 2020 Feb 21;11:23. doi: 10.3389/fneur.2020.00023.
19. PMID: 19884605. Fink M, Taylor MA. The catatonia syndrome: forgotten but not gone. Arch Gen Psychiatry. 2009 Nov;66(11):1173-7. doi: 10.1001/archgenpsychiatry.2009.141.
20. PMID: 27719852. Ludwig L, McWhirter L, Williams S, Derry C, Stone J. Functional coma. Handb Clin Neurol. 2016;139:313-327. doi: 10.1016/B978-0-12-801772-2.00028-X.
21. PMID: 26469689. Sivilotti ML. Flumazenil, naloxone and the ‘coma cocktail’. Br J Clin Pharmacol. 2016 Mar;81(3):428-36. doi: 10.1111/bcp.12731. Epub 2015 Sep 21.
22. PMID: 32551830. Kohnke S, Meek CL. Don’t seek, don’t find: The diagnostic challenge of Wernicke’s encephalopathy. Ann Clin Biochem. 2021 Jan;58(1):38-46. doi: 10.1177/0004563220939604. Epub 2020 Jul 13.
23. PMID: 28064230. Nishimoto A, Usery J, Winton JC, Twilla J. High-dose Parenteral Thiamine in Treatment of Wernicke’s Encephalopathy: Case Series and Review of the Literature. In Vivo. 2017 Jan 2;31(1):121-124. doi: 10.21873/invivo.11034.
24. PMID: 22104258. Schabelman E, Kuo D. Glucose before thiamine for Wernicke encephalopathy: a literature review. J Emerg Med. 2012 Apr;42(4):488-94. doi: 10.1016/j.jemermed.2011.05.076. Epub 2011 Nov 21.
25. PMID: 22784117. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012 Jul 12;367(2):146-55. doi: 10.1056/NEJMra1202561.
26. PMID: 31402154. Hoen B, Varon E, de Debroucker T, Fantin B, Grimprel E, Wolff M, Duval X; expert and reviewing group. Management of acute community-acquired bacterial meningitis (excluding newborns). Long version with arguments. Med Mal Infect. 2019 Sep;49(6):405-441. doi: 10.1016/j.medmal.2019.03.009. Epub 2019 Aug 8.
27. PMID: 12097540. Laffey JG, Kavanagh BP. Hypocapnia. N Engl J Med. 2002 Jul 4;347(1):43-53. doi: 10.1056/NEJMra012457.





Add comment