1 December 2022, by Shahriar Lahouti. Last Update 3 August 2025.
CONTENTS
- Preface
- General consideration
- Diagnosis
- Stages of shock
- Epidemiology and causes of shock
- Investigating the cause of shock
- Initial Approach to Diagnosis and Treatment
- Refractory shock
- Vasoactive agents
- Going further
- References
Preface
Shock is the clinical expression of circulatory failure. It is defined as a state of systemic hypoperfusion causing inadequate oxygen delivery to the tissues to meet cellular metabolic needs, resulting in cellular and tissue hypoxia. The effects of shock are initially reversible, but it rapidly becomes irreversible, resulting in multiorgan failure (MOF) and death. In this post, the classification, etiologies, and approach to diagnosis and initial stabilization of undifferentiated shock are reviewed.
General consideration
◾️Importance of early recognition of shock state
- The effects of shock are initially reversible, but can rapidly become irreversible, resulting in multiorgan failure (MOF) and death.
- Early detection and intervention are critical for improving a patient’s chance of survival.
◾️Pitfalls of diagnosis and management of shock
- Shock has no single definitive diagnostic marker.
- There is no standalone test that can definitively diagnose or exclude shock. For example,
- Normal vital signs don’t guarantee stability. A patient may have normal blood pressure or mental status and still be in shock.
- Lactate isn’t everything. A normal lactate level does not exclude the presence of shock.
- There is no standalone test that can definitively diagnose or exclude shock. For example,
- Heterogeneous presentation of shock
- The clinical presentation of shock is highly variable and influenced by severity, underlying etiology, and the patient’s physiologic reserve. Some key points:
- Shock is classically associated with hypotension and low cardiac output, but it can also occur with normal or elevated blood pressure, and even increased cardiac output (e.g., early sepsis).
- ⎮⚠️ Shock is not synonymous with hypotension.
- Multifactorial shock is common.
- For example, a patient with a pulmonary embolism (obstructive shock) who is also bleeding from the GI tract (hypovolemic shock).
- Pre-existing conditions may cloud acute presentation.
- Many patients have complex medical backgrounds (e.g., chronic heart failure, CKD, cognitive impairment), which can obscure the acute picture.
- For example, a patient with known severe systolic heart failure who now presents with fever and sepsis. Is the shock primarily cardiogenic or septic?
- ⎮💡Reviewing archival data (e.g., prior ECGs, echos) can help distinguish chronic vs. new pathology.
- Many patients have complex medical backgrounds (e.g., chronic heart failure, CKD, cognitive impairment), which can obscure the acute picture.
- Several shock phenotypes overlap in appearance, especially in late stages.
- For instance, late-phase septic shock may mimic advanced cardiogenic shock.
- Even with a full workup, shock may remain undifferentiated.
- ⎮💡When the cause of shock remains unclear, sepsis is the most likely culprit, until proven otherwise.
- The clinical presentation of shock is highly variable and influenced by severity, underlying etiology, and the patient’s physiologic reserve. Some key points:
Diagnosis
Recognition of shock requires clinical judgment based on a constellation of signs and symptoms augmented by bedside imaging and laboratory analysis.
The diagnosis of shock should be highly suspected in the presence of ≥ 2 of the following features:
🟥Hemodynamics
- Hypotension may be absolute (e.g., SBP <90 mm Hg; MAP<65 mm Hg), relative (e.g., a drop in SBP >40 mm Hg), or profound (e.g., vasopressor-dependent).
- Tachycardia. Tachycardia is an early compensatory mechanism in patients with shock.
- It can be isolated or occur in association with hypotension.
- Shock index (HR/SBP) is a more helpful way to understand tachycardia within the context of blood pressure (in certain types of shock).
- Shock index > ~0.8 suggests significant instability and possible shock *.
- ⎮💡However, the shock index is not applicable in primarily bradycardia shock.
- Bradycardia: Severe bradycardia (e.g., HR< 30 bpm) should always raise concern for shock (even in normotensive patients).
- Blood pressure can be maintained by compensatory systemic vasoconstriction, while cardiac output and perfusion may still be poor.
- Tachypnea. It is an early compensatory mechanism in patients with shock and metabolic acidosis, specifically.
- It is a useful tool to identify patients at risk of clinical deterioration, as evidenced by its incorporation into the qSOFA score.
🟥Oliguria: A urine output <0.5 mL/kg/h is worrisome for renal malperfusion *.
- Immediately following Foley catheter placement, the urine output won’t be known. In this situation, scanty and dark urine is worrisome.
- Urine output should be monitored by Foley catheter placement early in the ED course, as an accurate estimation requires at least 30 minutes of collection.
- During early resuscitation, here are considerations for urine production (urine output, “UOP):
- Normal if UOP >1 mL/kg/h.
- Mildly reduced if UOP 0.5-1 mL/kg/h.
- Severely reduced if UOP< 0.5 mL/kg/h.
- During early resuscitation, here are considerations for urine production (urine output, “UOP):
🟥Mental status changes.
- New onset of change in baseline sensorium can be a sign of shock state *.
- It is a continuum that begins with agitation, progresses to confusion or delirium, and ends in obtundation or coma.
- However, altered mental status is neither sensitive nor specific.
- Delirium is not a specific symptom of shock. The list of etiologies causing altered mentation is broad. In fact, most new-onset delirium isn’t due to shock.
- Delirium is not a sensitive symptom for shock. Normal mental status does not rule out a hypoperfusion state.
- Most patients with cardiogenic shock often have normal mentation despite poor perfusion of other organs.
- In contrast, patients with sepsis more often develop delirium (delirium tends to be a feature of septic shock rather than of cardiogenic shock).
🟥Skin perfusion status
- Cool, clammy skin is due to compensatory peripheral vasoconstriction that redirects blood centrally, to maintain vital organ perfusion (i.e., coronary, cerebral, and splanchnic flow) *. Caveats:
- A cool, clammy, or cyanotic skin may also be due to the underlying peripheral arterial vascular disease.
- A warm, hyperemic skin does not exclude the absence of shock because such an appearance may be present in patients with early distributive shock (before the onset of compensatory vasoconstriction) or terminal shock (due to failure of compensatory vasoconstriction).
- Mottling is less sensitive, but more specific for hypoperfusion and elevated mortality *. A cyanotic, mottled appearance is a late and worrisome feature of shock.
- Mottling suggests active endogenous vasoconstriction, implying that the patient would benefit from an increase in cardiac output (e.g., an inotrope), rather than additional exogenous vasoconstrictor.
- Urticaria, angioedema, and pruritus are suggestive of anaphylaxis; when in doubt, start empiric therapy for anaphylaxis immediately.
🟥Elevated Lactate
- Elevated lactate (arterial lactate > 2 mmol/L) is suggestive of shock *, although this has a broad differential diagnosis *.
- An elevated serum lactate is an early indicator of shock * and is particularly useful in identification of occult shock (those who are normotensive or even hypertensive).
- High lactate level is worrisome.
- This should be interpreted in an appropriate clinical context. It can represent shock or some other impending disaster until proven otherwise *.
- Normal lactate isn’t necessarily reassuring (can occur in shock).
- In practice, lactate usually doesn’t reflect oxygen deficiency, but rather endogenous epinephrine in response to physiologic stress. This explains why lactate can be normal in shocked patients who have inadequate sympathetic nervous function.
- 📚Read more on lactate👉 here
Stages of shock
- Shock is a physiologic continuum, from compensatory early phases to profound irreversible failure.
- Early detection and physiologically guided interventions are the keys to turning the tide.
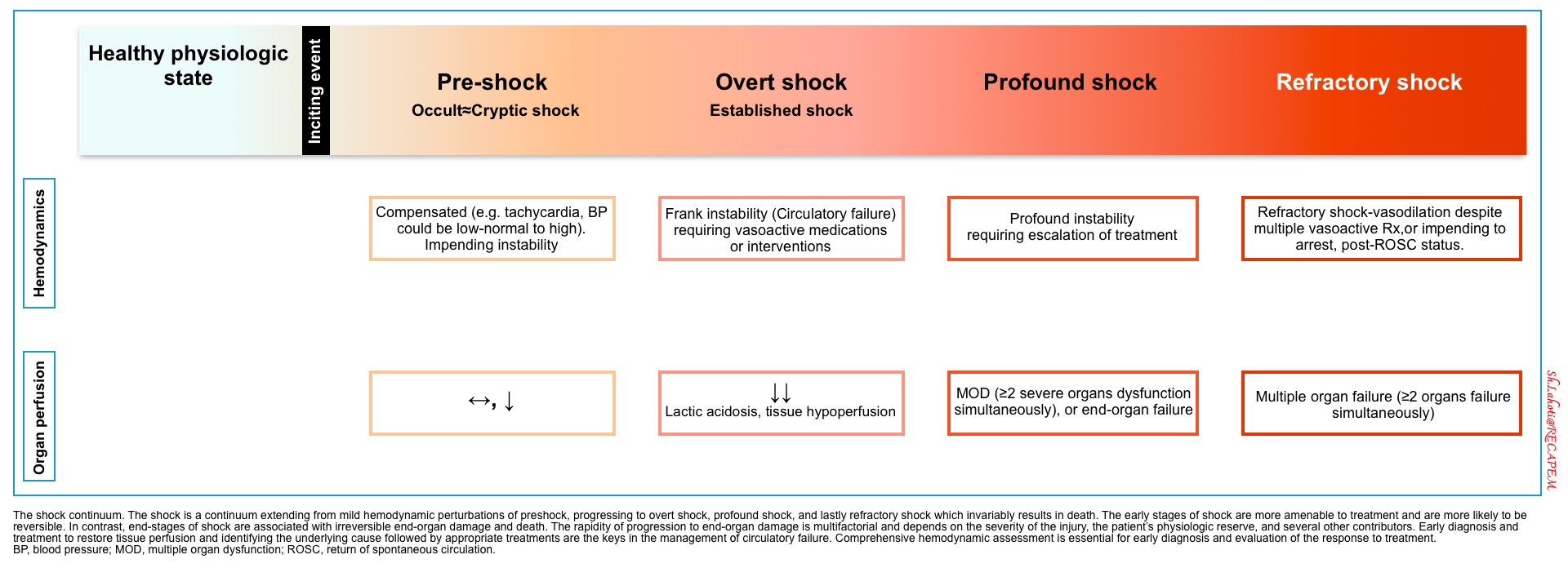
⬛️ Compensated shock (aka. occult shock, impending shock, cryptic shock)
- ⚙️ Compensatory response to circulatory derangement is working.
- Hemodynamics: Compensated (e.g., tachycardia); BP may be normal or elevated.
- Perfusion: Early reduction in perfusion; subtle clinical signs.
- ⎮⚠️ Impending instability — often under-recognized.
- ⎮💡It is potentially important to recognize this early stage, as early management can prevent further deterioration.
⬛️ Frank (overt) shock
- ⚙️ Compensatory mechanisms are overwhelmed, and overt manifestation of shock is evident.
- Hemodynamics: Frank circulatory failure (hypotensive).
- Requires vasoactive support or immediate intervention.
- Perfusion: Lactic acidosis, overt tissue hypoperfusion, e.g., altered mentation, oliguria.
⬛️ Profound Shock
- Hemodynamics: Severe instability despite standard interventions.
- Escalation of therapies is required.
- Perfusion: Multi-Organ Dysfunction (MOD ≥2 systems).
⬛️ Refractory Shock
- ⚙️Progressive shock leads to irreversible organ damage, Multiorgan Failure (MOF), and death.
- Hemodynamics: Persistent hypotension despite multiple vasopressors.
- Perfusion: Irreversible multi-organ failure.
- Prognosis: Often associated with imminent cardiac arrest or post-ROSC state.
- Typically, patients with impending cardiorespiratory arrest (from any cause) present with:
- Obtundation
- Agonal breathing, or RR < 10.
- Mottling, cyanosis, diaphoresis.
- MAP < 40 mm Hg.
- HR < 30 bpm.
- VT/VF.
- Typically, patients with impending cardiorespiratory arrest (from any cause) present with:
Severity of illness
◾️Several scoring systems help predict outcomes in critically ill patients. They use key clinical parameters to estimate the risk of mortality.
- Common examples include:
⎮Note: These scoring systems are designed to assess how sick a patient is. They help identify those at higher risk of death.
⎮💡 Pearl: While they aren’t intended to diagnose specific conditions like septic shock, scores such as qSOFA and SOFA are commonly used as proxies to help identify sepsis *.
⎮🔍 Recent evidence shows that the NEWS 2 score outperforms qSOFA when screening for sepsis with organ dysfunction *.
Epidemiology and causes of shock
◾️It is estimated that more than 1.2 million annual ED visits are for patients in shock *. Shock is common in the critically ill population, occurring in a third of patients admitted to the ICU.
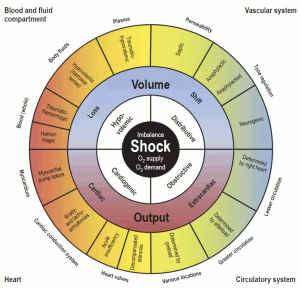
◾️Classically, four categories of shock have been described *:
- Distributive shock (vasodilatory shock): The most common class of shock.
- Septic shock (~62%)*
- Non-septic (~4%)
- Inflammatory shock (severe systemic inflammatory response syndrome)
- Pancreatitis, post-cardiac arrest, burns, trauma, postmyocardial infarction, post coronary bypass, post cardiac arrest, amniotic fluid embolism, fat embolism.
- Anaphylaxis
- Neurogenic shock (severe CNS/spinal trauma, spinal anesthesia)
- Others:
- Adrenal crisis.
- Thyroid storm.
- Liver failure
- Toxic shock syndrome.
- Transfusion reactions.
- Vasoplegia: Excess vasodilatory drug, cardiopulmonary bypass.
- Inflammatory shock (severe systemic inflammatory response syndrome)
- Hypovolemic (~16%)
- Hypovolemic (e.g., vomiting, diarrhea, over-diuresis, post-ATN or postobstructive polyuria, DKA).
- Hemorrhagic
- Gastrointestinal bleeding (e.g., varices, peptic ulcer)
- Retroperitoneal bleeding (e.g, ruptured aortic aneurysm)
- Aortoenteric fistula
- Ruptured ectopic pregnancy
- Spontaneous peritoneal hemorrhage from bleeding diathesis
- Postpartum bleeding
- Hemothorax
- Cardiogenic (~16%)
- Arrhythmogenic
- Tachyarrhythmia (usually >170 bpm)
- Bradyarrhythmia (usually < 30-40 bpm)
- LV failure (non-mechanical)
- Myocardial infarction (involving > 40% of the left ventricle or with extensive ischemia)
- Myocarditis, postpartum cardiomyopathy
- Drug-induced: Beta blockers and calcium channel blocker overdose.
- LV failure (mechanical)
- Severe valvular insufficiency, e.g., AR, MR.
- LVOTO
- RV failure
- Severe right ventricular infarction
- Massive pulmonary embolism
- Decompensated chronic pulmonary hypertension
- Arrhythmogenic
- Obstructive (~2%)
- Tension pneumothorax
- Tamponade
- Abdominal compartment syndrome
- AutoPEEP or high mean airway pressures.
👉Mortality rates for patients in shock vary drastically based on the etiology, but the common causes of shock, including sepsis, trauma, and cardiac failure, have mortality rates of 20-50%.
Investigating the cause of shock
⬛️ History
- Current medications & changes in medication list.
- Cardiac history (cardiovascular risk factors, any prior information about cardiac structure/function, such as ECG, and echo).
- Risk factors for venous thromboembolism.
- Adrenal disease (patients chronically on oral steroids may be assumed to be insufficient).
- Risk factors for systemic infections such as
- Immunosuppression, invasive devices (e.g., hemodialysis catheters), IV drug users, and splenectomized patients.
- Previous procedures or trauma, for example:
- The aortoenteric fistula (AEF) must be kept in mind as a possible etiology of GI bleeding in any patient with known AAA or prior aortic intervention, no matter how long ago.
- AEF may present with abdominal pain, hematemesis, and hematochezia.
⬛️ Specific signs and symptoms
- Several signs and symptoms can potentially suggest certain causes of shock (in an appropriate clinical context).
- Chest pain, dyspnea (AMI, acute aortic syndrome, PE)
- Abdominal/back pain (ruptured AAA)
- Leg size discrepancy (PE)
- Fever, shaking chills, productive cough, lower urinary tract symptoms, peritoneal signs, skin rashes (sepsis)
- Hematemesis, hematochezia, melena (GI bleeding)
⬛️ Labs
- Lactate
- CBC and differentiation
- Renal and liver function tests.
- Extended electrolytes
- Coagulation profile
- Troponin (in patients suspected of AMI)
- D-dimer
- A value lower than the cut-off (adjusted for age *) in patients with a low to intermediate pretest probability of having a pulmonary embolism can rule out PE.
- BNP
- A BNP level < 100 pg/ml or N-terminal-proBNP < 300 pg/ml indicates that acute HF is unlikely *.
- Sepsis evaluation
- Infectious workup if sepsis is suspected (e.g., blood cultures, urinalysis, and culture)
- Procalcitonin: If a decision has been made to treat empirically with antibiotics, then check a procalcitonin as well.
- Type & cross-match blood if hemorrhage suspected
- Endocrine evaluation
- Cortisol level, if adrenal insufficiency is a possibility.
- TSH if thyroid storm is suspected.
⬛️ ECG is used to identify arrhythmias, ischemia, or changes consistent with certain toxidromes.
⬛️ Imaging
- CXR (e.g., may reveal pneumonia or cardiogenic edema, implying LV failure)
- CT, depending on clinical scenario (e.g., CTA to evaluate for PE, CT abdomen/pelvis to exclude septic focus in abdomen, CT angio of abdomen to look for hemorrhage).
- POCUS: is important for identifying the etiology of hypoperfusion * and monitoring responses to therapy at any point during resuscitation.
- Multiple POCUS protocols (ACES, BLUE, RUSH, FALLS, etc.) have been proposed for the early and rapid evaluation of patients in shock.
- It has been shown that early use of POCUS in the evaluation of patients in shock is helpful to identify the etiology or at least the category of shock *.
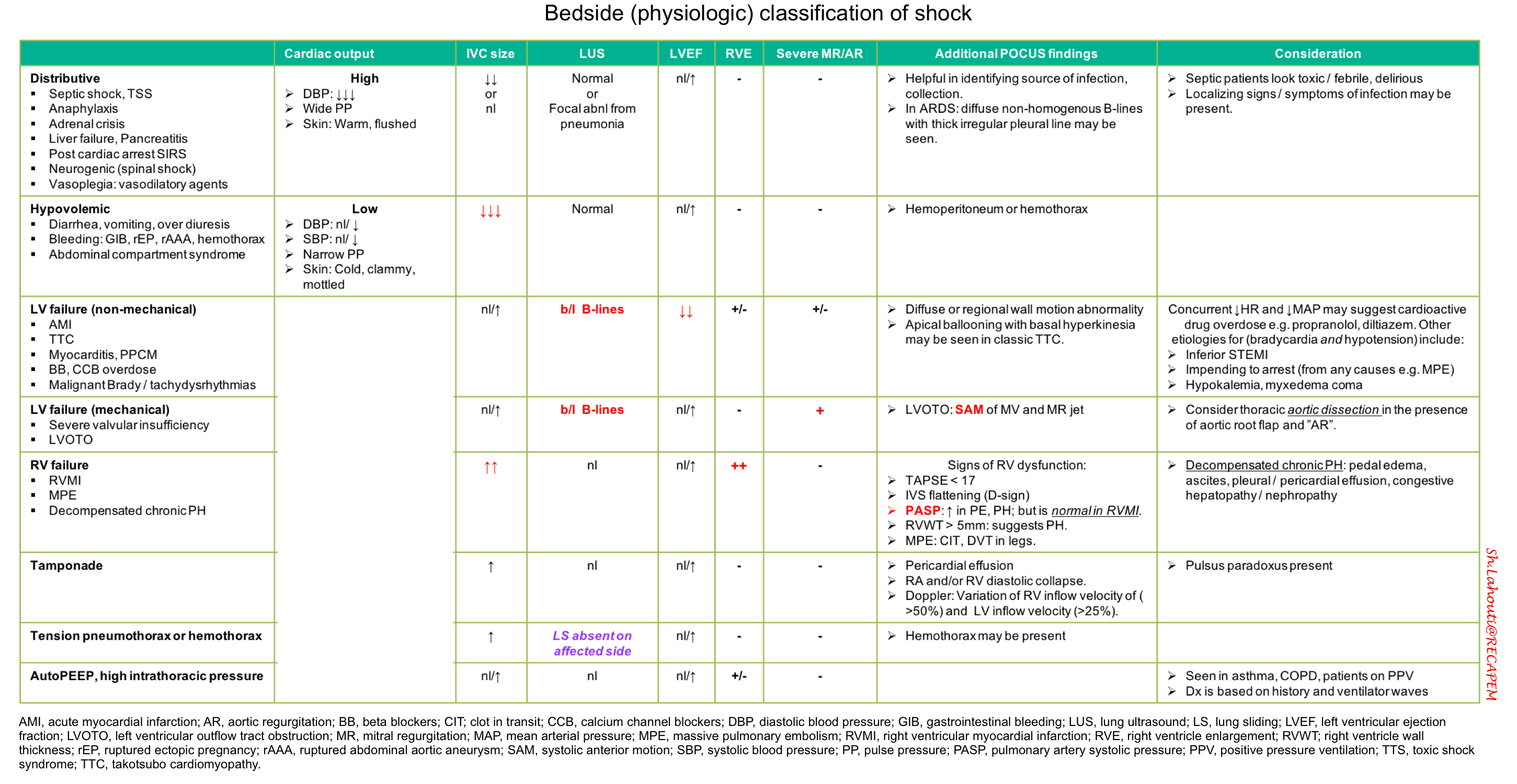
Bedside physiologic classification of shock
⬛️ Findings on ultrasonography and physical examination may be integrated for bedside physiologic classification of shock (table below).
- This works best in previously healthy patients with a single mechanism of shock.
-
- Patients with multiple chronic problems or multifactorial shock may defy classification.
- Septic shock is the most common type of shock in critically ill patients.
- If initial evaluation fails to reveal any obvious cause of shock, septic shock is likely, and it is imperative to treat empirically for sepsis.
Initial Approach to Diagnosis and Treatment
In unstable patients, diagnosis and stabilization should proceed simultaneously without further delay.
⬛️ Principle
- Determination of the category and etiology of shock can guide the appropriate therapy.
- In these critically ill patients, it is imperative to evaluate/think/act simultaneously.
- Act promptly—initiate resuscitation and formulate a differential diagnosis before completing the full history, exam, and investigations *.
- ⎮💡Time is Tissue: Promptly stabilize hemodynamics before identifying the cause of shock.
Resuscitation
Assess CABs
⬛️ Circulation (see below)
⬛️ Airway
- Correct hypotension with push-dose pressors before proceeding with intubation (if intubation is required).
- ⎮⚠️ Avoid intubation (and positive pressure ventilation) if possible in patients with suspected obstructive shock (pulmonary embolism, tension pneumothorax, cardiac tamponade).
⬛️ Breathing
-
Administer supplemental oxygen, targeting SpO2 of 90%-94%.
-
Consider non-invasive positive pressure ventilation (CPAP or BiPAP) for patients with cardiogenic pulmonary edema.
Circulation ⎯ Initial Stabilization
💡”Stabilization must start immediately, often before the cause of shock is known”.
- In the presence of multiple hemodynamic derangements, consider treatment strategies in the following sequence:
- First: Correct the “HR” first if dysrhythmia is suspected as the primary cause of hemodynamic instability.
- ⎮💡Dysrhythmia is likely the primary cause of hemodynamic instability if:
- HR < 30 bpm.
- AF with rapid ventricular response > 150 bpm.
- Non-sinus tachycardia > 170 bpm.
- ⎮💡Dysrhythmia is likely the primary cause of hemodynamic instability if:
- Second: Correct volume status (if appropriate) before correcting the resistance problem.
- Third: Correct resistance problems (e.g., by vasopressors) to rapidly improve tissue perfusion.
- Fourth: Consider inotropic support (if indicated).
- First: Correct the “HR” first if dysrhythmia is suspected as the primary cause of hemodynamic instability.
⬛️ Circulation ⎯ Resuscitative measures
The following are common interventions to consider.
- IV Access
- Establish ≥2 large-bore peripheral IVs, a central venous line, or intraosseous access to allow for high-volume fluid resuscitation and vasopressor administration.
- Fix the rate & rhythm if an abnormal rate or rhythm is driving the hemodynamic instability.
- Volume resuscitation
- Fluid is often provided in bolus fashion (e.g., 500 ml) with attention to patient response.
- Consider using a balanced crystalloid solution (e.g., Lactated Ringer’s solution) over normal saline.
- The total amount of fluid administered should generally be limited to <1-2 liters in the absence of a history suggesting substantial total-body volume depletion (e.g., severe diarrhea).
- Even patients with ESRD and severe CHF may need adequate volume resuscitation with frequent reassessments, especially if the shock etiology is hypovolemic or distributive.
- ⎮⚠️ Hold IV fluid if signs of pulmonary edema are present on exam or bedside ultrasound.
- Fluid administration can be diagnostic and therapeutic in confusing situations where hypovolemia is suspected:
- If fluid resuscitation alone resolves the shock, this supports a diagnosis of hypovolemia.
- If fluid resuscitation fails, this suggests an alternative diagnosis. This is especially true if fluid resuscitation results in adequate filling pressures (e.g., full IVC) without resolving the shock.
- Fluid is often provided in bolus fashion (e.g., 500 ml) with attention to patient response.
- Vasopressor
- Initiate vasopressor immediately if the blood pressure is inadequate (e.g., MAP< 65 mmHg) and in peri-cardiac (impending) arrest situation *.
- Pressors may be administered via a peripheral vein (except for vasopressin).
-
Norepinephrine
-
First-line vasopressor for undifferentiated shock with suspected septic, cardiogenic, and neurogenic shock.
-
Infusion (0.01-3 μg/kg/min)
-
-
Epinephrine
- It is an appropriate alternative to norepinephrine for undifferentiated shock, especially in bradycardic peri-arrest.
-
Infusion (0.01-0.1 μg/kg/min)
-
In cases of suspected anaphylaxis, epinephrine IM (1:1000) 0.1-0.5 mg (max 1 mg) followed by epinephrine infusion is the first-line treatment for ongoing shock.
- Initiate vasopressor immediately if the blood pressure is inadequate (e.g., MAP< 65 mmHg) and in peri-cardiac (impending) arrest situation *.
- Inotropic support
- Consider inotropic support if low cardiac output is suspected in patients with signs of ongoing hypoperfusion despite adequate intravascular volume and an appropriate dose of vasopressors.
- Dobutamine
- Infusion (2-20 μg/kg/min) (max 40 μg/kg/min)
- Dobutamine
- Consider inotropic support if low cardiac output is suspected in patients with signs of ongoing hypoperfusion despite adequate intravascular volume and an appropriate dose of vasopressors.
- Antibiotic
- ⎮💡Give an empirical dose of antibiotic for undifferentiated hypotension in the ED.
- If sepsis is possible, cultures should be performed, and empiric antibiotics should be started without delay.
- In patients with possible sepsis, you don’t necessarily need to go extremely broad with the antibiotics.
- A single broad-spectrum agent may be reasonable (e.g., piperacillin-tazobactam)..
- Steroid
- Indicated for patients whom you suspect have an adrenal crisis, for example:
- Patients with known adrenal insufficiency
- Patients taking chronic steroids who have recently missed doses.
- Indicated for patients whom you suspect have an adrenal crisis, for example:
Source control
No resuscitative efforts will improve patient outcomes if source control is not achieved in parallel. It is necessary to have excluded cardio-respiratory mechanical causes
of shock (following conditions) and other pathologies that have specific treatments.
- Acute myocardial infarction (AMI)
- Acute aortic syndrome
- Massive pulmonary embolism (MPE)
- Tamponade
- Tension pneumothorax
- Read more on this here.
- Ventricular free wall rupture, papillary muscle rupture.
- Abdominal compartment syndrome.
- Hemorrhage, for example
- Infection (septic shock)
- Acute valvular emergencies such as
Refractory Shock
Definition
- There is no single universally accepted definition, but across the literature, “refractory shock” generally refers to:
- Persistent hypotension and hypoperfusion despite adequate fluid resuscitation and escalating doses of vasopressors.
- Key features often include:
- MAP < 65 mmHg despite vasopressor support.
- High-dose vasopressor therapy, defined as ≥ 1 ug/kg/min norepinephrine-equivalent dose *, or the need for rescue vasopressor therapy.
- Evidence of end-organ hypoperfusion (e.g., oliguria, altered mental status, lactate > 2–4 mmol/L).
- Often associated with multiple organ dysfunction.
Diagnostic evaluation
◾️Patients in refractory shock often have a recognized cause, yet continued clinical re-evaluation may help refine management.
◾️Diagnostic evaluation includes
- #1. Earlier considerations
- Check for equipment malfunction.
- Placement of a central arterial line (femoral or axillary).
- Radial arterial lines may underestimate BP.
- If the patient is on a sedative, shift to a more hemodynamically neutral sedative.
- Trial of different pressor agents (e.g., epinephrine challenge, addition of vasopressin).
- Vasopressin has been demonstrated to be safe at doses of 0.06 U/min and could likely be titrated higher.
- High-dose norepinephrine (there is no maximal dose).
- For patients with right ventricular dysfunction and hypoxemia on mechanical ventilation, inhaled pulmonary vasodilators could be trialed (e.g., inhaled nitroglycerine).
- #2. Etiology and evaluation of refractory shock*
- Acidosis
- Test: Blood gas, basic metabolic panel
- Adrenal insufficiency
- Clue: Clinic, ↓Na, ↑K.
- Test: Cortisol level
- Hypocalcemia
- Dx: ↓Ionized Ca, ↑QTC
- Test: Ionized calcium level.
- Hypothyroid
- Clues: Clinic, TSH may be false negative or delayed.
- Occult bleeding
- Is there occult bleeding, e.g., retroperitoneal bleeding, ruptured EP, ruptured spleen, large vessel aneurysm?
- Test: POCUS, CTA of abdomen.
- Rx: Anticoagulation reversal, transfusion, Surgery/IR.
- Toxicologic
- Occult causes, e.g., beta blocker, calcium channel blocker, TCA.
- Rx: Hyperinsulin euglycemia therapy (HIT) for BB/CCB intox, Bicarbonate for TCA intox
- Pneumothorax
- Is there a pneumothorax after placement of central venous access?
- Test: POCUS
- Read more on the management of pneumothorax here.
- Anaphylaxis
- Patients with anaphylaxis may present with isolated hypotension.
- Rx: Epinephrine
- Second cause of shock:
- Shock often is multifactorial.
- Look for second causes such as pump failure or obstruction that is causing shock, such as:
- Cardiac tamponade in dialysis patients
- Pulmonary embolism
- Dynamic LVOTO
- Aortic dissection.
- Evaluation:
- Repeat POCUS evaluation to characterize hemodynamics.
- Formal echocardiography.
- Evaluate for unusual forms of shock, e.g., LVOTO.
- Review any available CT imaging of the heart.
- Acidosis
Approach to Refractory Shock: A Multimodal Strategy
Refractory shock requires layered, tailored interventions. Below is a streamlined framework of high-yield considerations:
- Review Medications
- Minimize hypotensive drugs:
- Prefer hemodynamically neutral sedatives (e.g., ketamine) over agents like propofol/dexmedetomidine.
- Minimize hypotensive drugs:
- Correct Metabolic Derangements
- Temperature control: Avoid extremes (fever causes ↑ metabolic demand and ↓SVR; hypothermia causes ↓cardiac function).
- Calcium replacement: If ionized Ca < 0.8–0.9 mmol/L.
- Acidosis correction:
- IV bicarbonate, ventilator adjustments, or dialysis if needed.
- ⎮💡A rapid, significant but transient bump in BP after bicarbonate administration proves acidosis as the cause of refractoriness of shock.
- IV Thiamine: Consider empirically for beriberi; may improve lactate clearance.
- Stress-dose steroids: Especially in septic or SIRS-related shock (may enhance vascular responsiveness to vasopressors), and adrenal insufficiency. More on this here.
- Suspected hypothyroidism, consider empiric levothyroxine (TSH level may be falsely negative or delayed).
- Define and Optimize MAP Targets
- Use central arterial lines (femoral or axillary) for more accurate BP measurement.
- ⎮Radial lines may underestimate MAP.
- Lower the MAP goal (e.g., ≥55–60 mmHg) if high-dose norepinephrine (>1 µg/kg/min) is required, to reduce vasopressor-related harm.
- Use central arterial lines (femoral or axillary) for more accurate BP measurement.
- Optimize Preload
- Reassess volume status using POCUS.
- Consider gentle fluid challenges only if still preload-responsive.
- Reduce PEEP or manage auto-PEEP to improve venous return in ventilated patients.
- Optimize Vasopressor Therapy
- Norepinephrine: No strict upper limit.
- Vasopressin: Add at 0.06 U/min; bolus may help in severe vasoplegia.
- Epinephrine: Especially in bradycardia or poor systolic function. Try a 4 mcg/min trial.
- Consider second-line agents in vasodilatory shock:
- Methylene blue
- Hydroxocobalamin
- Angiotensin II
- Heart Rate Management
- For bradycardia:
- Paced-patients
- Temporary transvenous pacemaker: Titrate heart rate to 100-120 bpm to determine if this improves perfusion.
- Permanent pacing: Consult electrophysiology to increase heart rate.
- For relative/absolute bradycardia, may consider
- Positive chronotropic agents (e.g., isoproterenol).
- Therapies for refractory bradycardia👉 here
- Paced-patients
- In atrial fibrillation, balance rate control with perfusion (individualized). Read more👉 here
- For bradycardia:
- Don’t Forget Source Control
- Ensure early and appropriate antibiotics in all cases of suspected sepsis.
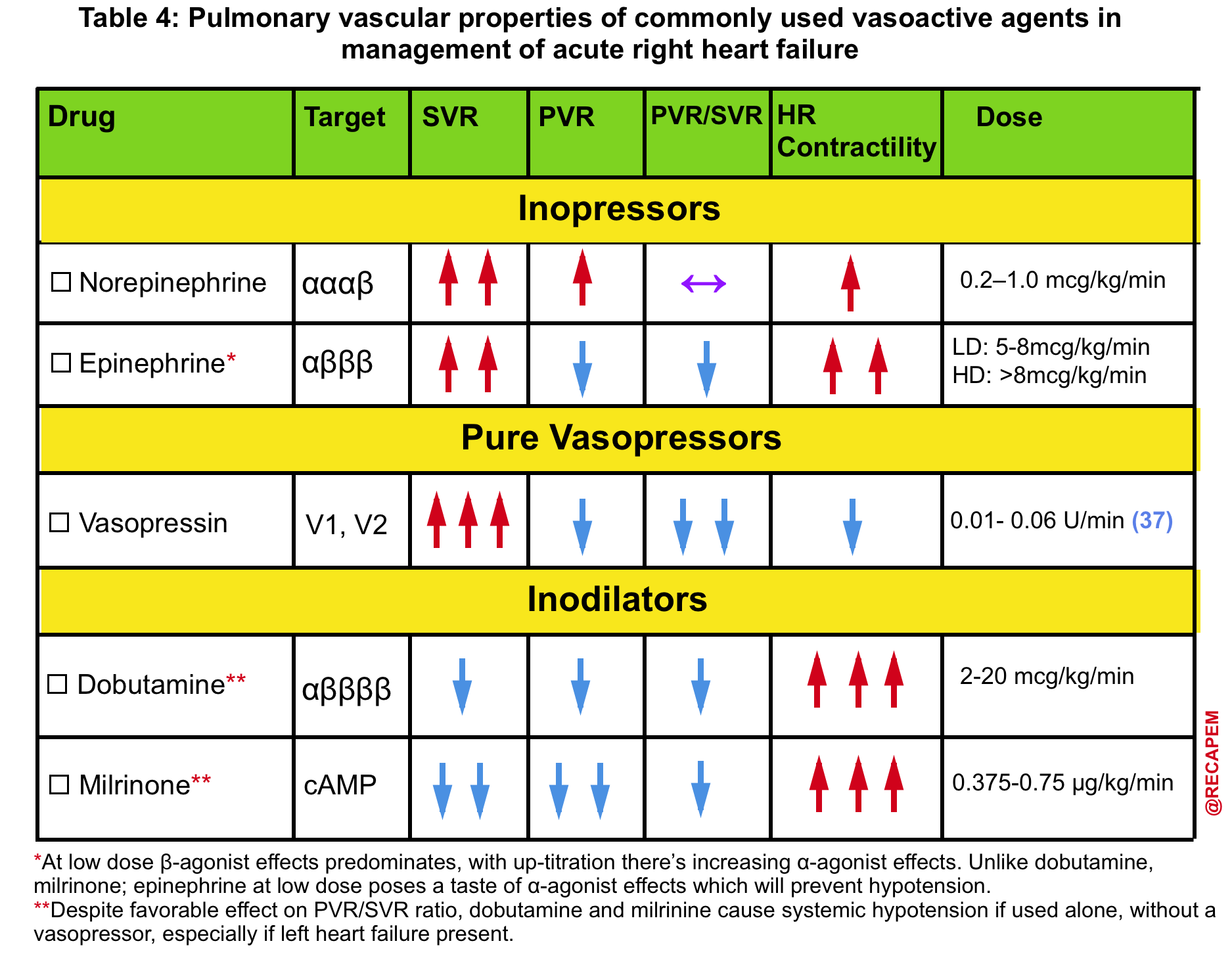
- Support the Right Ventricle
- In cases of RV dysfunction, consider inhaled pulmonary vasodilators (e.g., epoprostenol). More on RV failure management👉 here.
Vasoactive agents
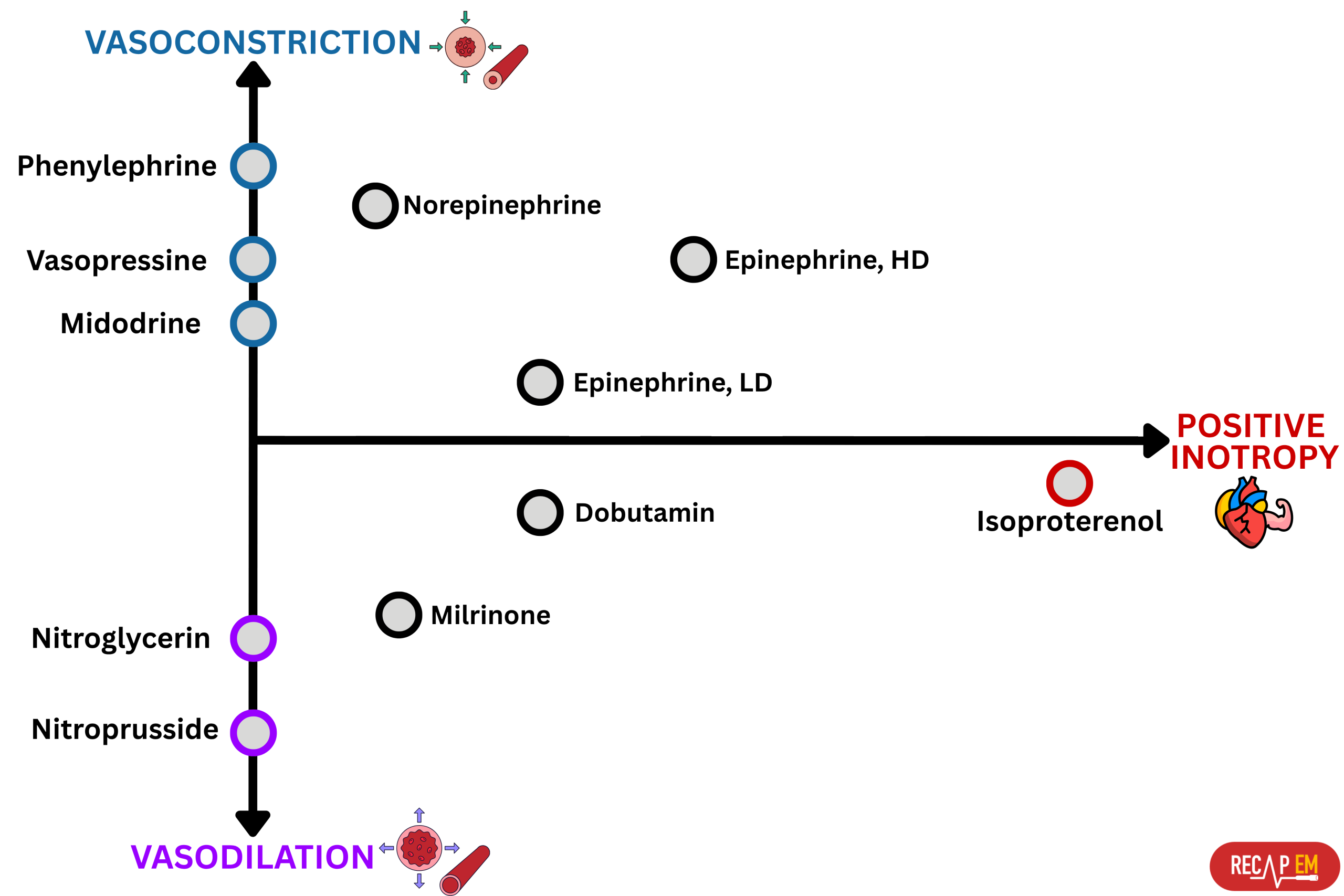
◾️Terminology
- Vasopressor: ↑SVR, e.g., phenylephrine.
- Inotropes: ↑ Myocardial contractility, e.g., dobutamine
- Inopressor: Combined effects of inotropic (↑ contractility) + vasopressor (↑ SVR); e.g., epinephrine
- Inodilator: Combined effects of inotropic (↑ cardiac contractility) and vasodilator (↓ SVR); e.g., dobutamine
- Chronotropic agents affect the heart rate:
- Positive chronotrope: Increases heart rate, e.g., atropine
- Negative chronotrope: Decreases heart rate, e.g., beta blockers
◾️Commonly used vasoactive drug class *
- Catecholamines
- Sympathetic amines are the most commonly administered class of vasopressor and inotropic medications in the critical care setting.
- These agents produce their physiologic effects through the stimulation of alpha-adrenergic (α1), beta-adrenergic (β1 and β2), and dopamine (D1) receptors.
- Phosphodiesterase Inhibitors
- Drug Class: Phosphodiesterase-3 (PDE3) inhibitor
- Primary Action: Inhibits PDE3, an enzyme responsible for breaking down cyclic adenosine monophosphate (cAMP)
- Effect in Cardiac Myocytes:
- ↑ cAMP levels
- ↑ Inotropy (improved contractility)
- Effect in Vascular Smooth Muscle:
- ↑ cAMP levels
- Vasodilation of systemic and pulmonary vessels
- Hemodynamic Benefits:
- ↑ Cardiac output
- ↓ Afterload
- ↓ Systemic vascular resistance
- ↓ Pulmonary vascular resistance
- Arginine Vasopressin Antagonists
- Drug Class: Endogenous nonapeptide hormone (Arginine Vasopressin)
- Receptor Targets:
- V1a Receptor (vascular smooth muscle)
- ↑ Cytosolic Ca²⁺
- → Vasoconstriction
- V2 Receptor (renal collecting tubules)
- → Water retention via distal convoluted tubule
- V1a Receptor (vascular smooth muscle)
- Primary Hemodynamic Effect:
- ↑ Systemic vascular resistance (SVR) (dose-dependent)
- Minimal Effects On:
- Cardiac output
- Pulmonary capillary wedge pressure (PCWP)
- Additional (Pleiotropic) Effects:
- ↓ Nitric oxide production → reverses vasoplegia in SIRS
- May reduce catecholamine resistance (by improving adrenergic receptor sensitivity)
- Pulmonary Circulation:
- May cause systemic vasoconstriction
- While producing pulmonary vasodilation → ↓ RV afterload
- Guanylate Cyclase and Nitric Oxide Synthase Inhibitors: Methylene Blue
- Drug Class: Guanylate cyclase inhibitor; repurposed agent (historically used for methemoglobinemia)
- Mechanism:
- Inhibits nitric oxide (NO)–mediated activation of guanylate cyclase
- ↓ cGMP production → increased vascular smooth muscle tone
- → Enhanced vasoconstriction
Vasopressors
🧠Factors that should be considered for choosing the appropriate vasopressor for patients
- IV access
- Only vasopressin can not be given through peripheral access *.
- Otherwise, in the presence of a proximal, well-functioning peripheral line, any catecholamine can be used (norepinephrine, epinephrine, or phenylephrine).
- Epinephrine or phenylephrine may offer the safest profile when peripheral IV lines are unreliable and extravasation is likely.
- Presence of hemodynamically significant RV failure (left below figure👇)
- ⚠️ Avoid phenylephrine for its potential pulmonary vasoconstriction and dopamine for its arrhythmogenic properties *.
- The agent of choice would be the one with optimal systemic vasopressor activity while having the least pulmonary vasoconstriction effect (↓PVR/SVR).
- ✅ Vasopressin may be a useful vasopressor (neutral effect on pulmonary vascular resistance).
- ✅ Epinephrine may be a useful inopressor (neutral effect on pulmonary vascular resistance; inotropic support for the right ventricle).
- Norepinephrine might not be ideal (it’s predominantly an alpha-agonist).
- Heart Rate (HR) and LVEF
- Inappropriately ⬇️HR and/or ⬇️ EF:
- Epinephrine.
- Norepinephrine.
- ⬆️HR, ↔️⬆️ EF, and pure vasodilatory shock:
- Vasopressin.
- Phenylephrine.
- ⎮💡Significantly elevated EF can be detrimental, as it may raise the risk of developing cardiomyopathy or LVOTO. Elevating afterload could help manage this.
- Inappropriately ⬇️HR and/or ⬇️ EF:
- Underlying structural/valvular heart disease (see right below table).
Vasopressors
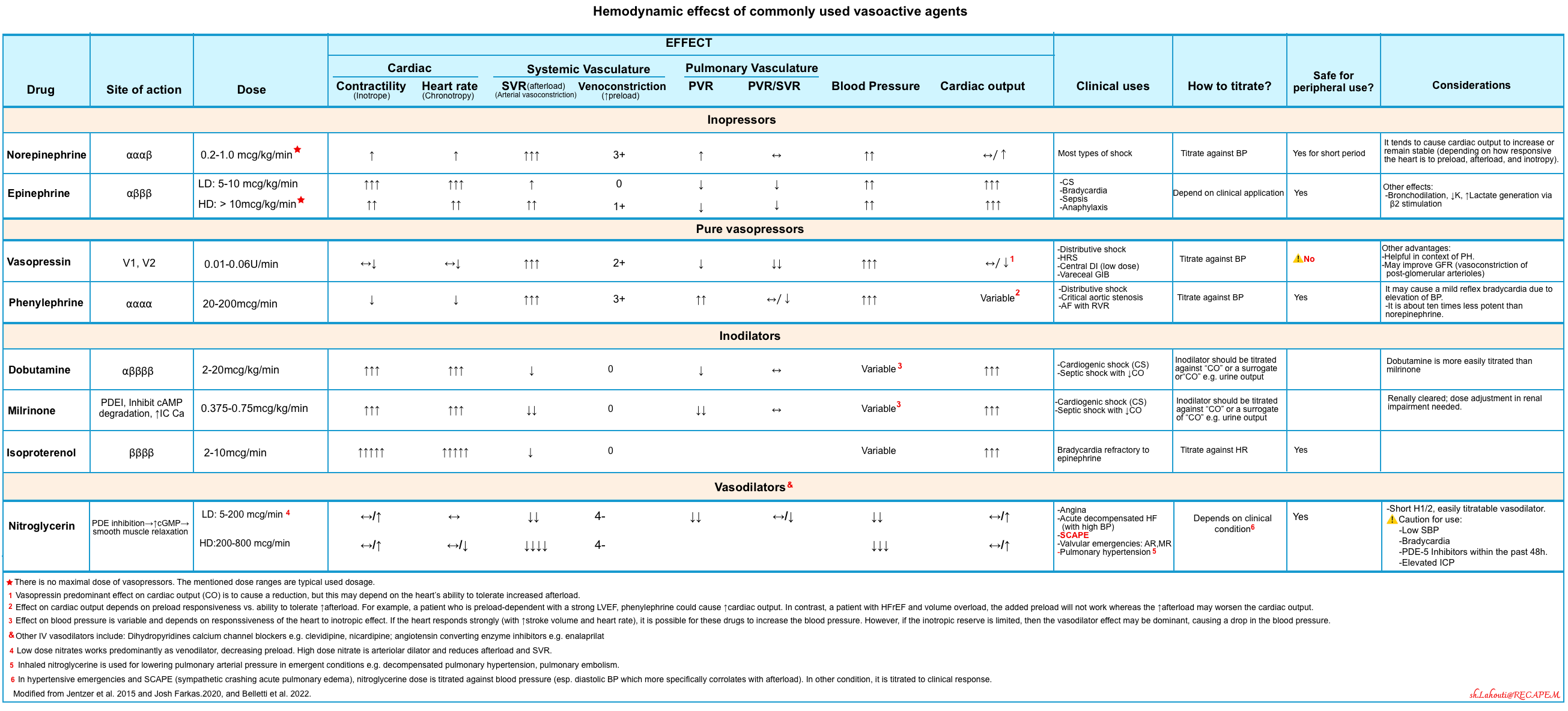
The following table provides a summary of commonly used vasopressors (scroll horizontally to view the full list).
| Category | Norepinephrine | Phenylephrine | Vasopressin | Epinephrine |
|---|---|---|---|---|
| Indications | Septic shock, vasodilatory shock, cardiogenic shock with hypotension | Vasodilatory shock with adequate cardiac output, atrial fibrillation with fast ventricular response | Septic shock, post-cardiac surgery vasoplegia, RV failure, HRS | Bradycardia, septic shock, anaphylaxis, asthma, cardiogenic shock |
| Contraindications | Sulfite sensitivity, vascular thrombosis (relative) | Bradycardia, pulmonary hypertension, profound hypotension | Systemic hypoperfusion, vascular disease, poor IV access | Tachycardia, arrhythmias, profound acidosis, LVOTO |
| Mechanism of Action | Alpha-1 agonist (↑SVR, preload), mild Beta-1 (inotropy/chronotropy) | Pure alpha-1 agonist (↑SVR, preload, reflex bradycardia) | V1: vasoconstriction; V2: water retention | Beta-1, Beta-2, Alpha-1 agonist (inotropy, vasoconstriction, bronchodilation) |
| Side Effects | Mesenteric ischemia, arrhythmia, hyperglycemia, extravasation | ↓ Cardiac output, reflex bradycardia, low potency | Digital ischemia, hyponatremia, skin necrosis, ↓ CO | Tachycardia, lactate elevation, hyperglycemia, stress response |
| Dosing | Up to 30 µg/min, titrate to MAP | ~40-180 µg/min, 10x less potent than NE | 0.03–0.06 U/min; avoid peripheral IV | <4–8 µg/min (low), higher doses for vasoconstriction |
| Half-life | ~2.5 minutes | ~5 minutes (short), terminal ~2–3 hrs | ~10–30 minutes | ~2–4 minutes |
◾️Vasopressin ⎯considerations
- A loading dose of vasopressin may be required in specific conditions. why?
- Vasopressin pharmacokinetics:
- Half-life: Approximately 15–20 minutes, which is longer than most other vasopressors.
- Clinical implication: Its longer half-life must be considered when starting or adjusting the dose.
- Delayed onset: Without a loading dose, the onset of action may be slow.
- Time to effect: Continuous infusion without a bolus may take up to ~30 minutes.
- Moreover, the vasopressin loading dose can determine the patient’s responsiveness.
- Vasopressin-responsive patients generally experienced an increase in MAP by >18-22 mm following a 1-unit loading dose.
- Patients who failed to respond to the 1-U loading dose were more likely to develop digital ischemia following vasopressin infusion.
- Vasopressin pharmacokinetics:
- Dosing *
- Septic shock: Infusion 0.03-0.04 U/min
- Refractory shock: Max infusion dose of 0.06 U/min
- Post-cardiotomy vasoplegic shock: Max infusion dose of 0.1 U/min
- Hemodynamic complications (share the same physiology as for phenylephrine)
- Reduced cardiac output in selected patients
- Bradycardia
◾️Phenylephrine⎯considerations
- Mechanism of action: Phenylephrine causes ↑afterload (↑SVR) and ↑Preload (venoconstriction).
- Hemodynamic complications
- Reduced cardiac output in selected patients.
- Reduced cardiac output may occur via two main mechanisms:
- Increased afterload without preload augmentation (in patients who are not preload-responsive), especially harmful in systolic heart failure (cause they can hardly tolerate excessive afterload).
- ⎮💡Patients who are preload-responsive with a stronger LVEF, phenylephrine could cause a net increase in cardiac output.
- Reflex bradycardia leads to a drop in heart rate, which is often the primary cause of reduced cardiac output.
- Increased afterload without preload augmentation (in patients who are not preload-responsive), especially harmful in systolic heart failure (cause they can hardly tolerate excessive afterload).
- Reduced cardiac output may occur via two main mechanisms:
- Bradycardia. Phenylephrine can cause mild reflex bradycardia due to an elevation in blood pressure.
- Heart rate trends can indicate phenylephrine’s impact on cardiac output.
- Reflex bradycardia often signals a decline in cardiac output.
- If heart rate remains unchanged, cardiac output may increase.
- A drop in heart rate suggests baroreceptor activation and suppression of the body’s natural sympathetic compensation for low blood pressure.
- Heart rate trends can indicate phenylephrine’s impact on cardiac output.
- Reduced cardiac output in selected patients.
◾️Norepinephrine ⎯considerations
- Norepinephrine is often used as a “broad-spectrum” vasoactive agent for undifferentiated hypotensive patients.
- Mechanism of action: NE causes ↑afterload (↑SVR) and ↑Preload (venoconstriction). Mild β-1 agonistic effect→ Mild ↑contractility & ↑HR
- Effects on cardiac output
- This is similar to those of phenylephrine, although some beta-adrenergic activity may allow norepinephrine to have a more favorable impact on cardiac output.
- Norepinephrine raises both venous return (preload) and systemic resistance (afterload).
- In preload-responsive hearts, this may preserve or enhance cardiac output.
- In non-responsive or failing hearts, the increased afterload may actually impair cardiac output.
- Mild beta-1 stimulation from norepinephrine can elevate heart rate, but this effect is often offset by reflex-mediated slowing of the heart (bradycardia) due to the rise in blood pressure.
◾️Epinephrine⎯considerations
◾️Dosing
- Low dose (LD) Epinephrine is inotropic dose (~ <4-8 ug/minute or <0.05-0.08 ug/kg/min).
- Inotropic effect dominates: Useful in low-output cardiogenic shock, especially at doses <4–8 µg/min.
- Minimal SVR impact: Beta effects lower SVR; mild alpha effects may balance it.
- The net result might be a pure inotrope that doesn’t cause systemic vasodilation.
- Alternative to NE + dobutamine: Can serve as monotherapy for hypotensive cardiogenic shock.
- Higher dose (HD) Epinephrine has a greater alpha-agonist effects.
◾️Specific side effect
- Epinephrine increases lactate production, which is usually benign and may be beneficial. However, it should be noted that, this effect has some implications:
- Limits lactate utility: Makes lactate unreliable for monitoring occult shock or tracking resuscitation.
- Use with caution in acidosis: May worsen severe lactic/metabolic acidosis (e.g., from metformin toxicity).
Inodilators
Selecting Dobutamine and/or Milrinone
◾️Clinical use of inodilators
- Inodilators should be adjusted based on cardiac output or indirect indicators (e.g., urine output).
- Avoid titrating them based on blood pressure, as their effect on blood pressure is variable and not reliable.
- Low-output cardiogenic shock.
- These are most useful for patients with reduced cardiac output and a high systemic vascular resistance (SVR).
- ⎮⚠️ Be aware for hypotension.
- These are most useful for patients with reduced cardiac output and a high systemic vascular resistance (SVR).
- Septic shock with inadequate cardiac output (as an add-on agent).
- ⎮💡Epinephrine is a better choice, if the BP is low.
- RV failure.
- Both agents improve RV systolic function.
- Milrinone additionally reduce the PVR (pulmonary vascular resistance).
◾️Mechanism of action
- Milrinone increases cardiac output primarily driven by reducing systemic vascular resistance (with less effect on inotropy).
- It works predominantly as a vasodilator (including venodilation, systemic arterial vasodilation, and pulmonary arterial vasodilation).
- More likely to cause hypotension
- Dobutamine
- Stimulates beta-1 receptors > beta-2 receptors, with a little stimulation of alpha-1 receptors.
- It primarily increases cardiac output by enhancing contractility, with a relatively modest increase in heart rate.
- Systemic vascular resistance is generally reduced due to beta-2 agonist stimulation and also reduced endogenous sympathetic tone in response to improved cardiac output.
| Category | Dobutamine | Milrinone |
|---|---|---|
| Best Use Cases | Low-output cardiogenic shock with high SVR; easier titration in renal dysfunction | RV failure, septic cardiomyopathy; patients on beta-blockers |
| Hemodynamic Target | ⚠️ Both should be titrated based on cardiac output (not blood pressure) | |
| Advantages |
|
|
| Limitations |
|
|
| Combination Therapy | Can be combined with milrinone (additive stroke volume & ↓ LVEDP) | Can be combined with dobutamine or epinephrine |
Inodilators Comparisson Table
| Category | Dobutamine | Milrinone | Isoproterenol |
|---|---|---|---|
| Contraindications | Hypotension, arrhythmias, LVOTO, sulfite sensitivity, congenital QT prolongation | Hypotension, severe aortic stenosis, arrhythmias, renal failure | Active myocardial ischemia, digoxin toxicity–induced heart block |
| Drug Interactions | MAO inhibitors, COMT inhibitors | Guanylate cyclase stimulators, cilostazol | COMT inhibitors |
| Side Effects | Hypotension or hypertension, arrhythmias, ischemia, hypersensitivity | Hypotension, arrhythmias, ischemia, thrombocytopenia | Tachycardia, arrhythmias, hypotension, hyperglycemia, hypokalemia, lactic acidosis |
| Indications | Inotropy in heart failure and shock states | Heart failure, RV failure (renal caution) | Bradycardia, torsades, RV failure, severe AV delay, asthma |
| Dosing | 2.5–10 µg/kg/min; higher doses may not help | 0.125–0.75 µg/kg/min; slow titration; renal adjustment | 1–10 µg/min (up to 20 µg/min); titrate to HR |
| Mechanism | Beta-1 > Beta-2 agonist; mild alpha-1 activity | PDE3 inhibitor → ↑cAMP → inotropy + vasodilation | Nonselective beta-1 and beta-2 agonist |
| Physiological Effects | ↑ CO via inotropy; modest HR ↑; mild vasodilation; BP unpredictable | ↓ SVR; ↑ CO via vasodilation; minimal inotropy; hypotension common | Potent chronotrope > inotrope; ↓ PVR; causes metabolic shifts |
| Pharmacology | IV; onset 1–2 min; peak ~10 min; half-life ~2 min; hepatic metabolism | IV; onset gradual; half-life ~2.5 hrs; renal elimination | IV; onset rapid; half-life 2.5–5 min; COMT metabolism |
◾️Isoproterenol⎯considerations
- Mechanism of action
- Isoproterenol is a pure, nonselective beta-agonist (it stumulates both beta-1 and beta-2 receptors).
- Isoproterenol is the most potent chronotrope (primarily increases heart rate).
- Also acts as a positive inotrope, but less impact on stroke volume than dobutamine.
- Dobutamine boosts stroke volume more, while isoproterenol mainly raises heart rate.
- Beta-2 agonist activity results in:
- Enhances chronotropy: Beta-2 stimulation amplifies isoproterenol’s heart rate–boosting effects.
- Decreases pulmonary vascular resistance: Helps reduce RV afterload.
- Triggers metabolic effects: May cause lactic acidosis, hyperglycemia, and other epinephrine-like.
- Primary Indications
- Bradycardia, even those refractory to epinephrine.
- Torsade de Pointes: It is often used to increase HR and thus avoid pause-dependent torsade in patients with torsade due to medication- or electrolyte-induced QT prolongation.
Methylene Blue (MB)
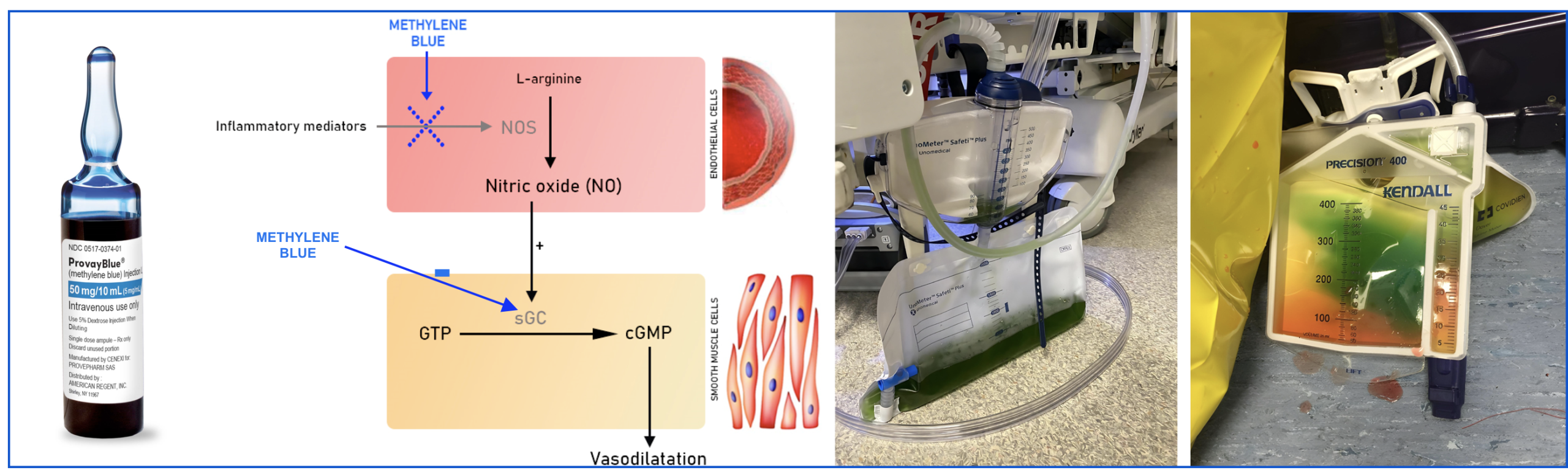
- Mechanism of action
- Inhibits nitric oxide synthase → causes vasoconstriction, but may impair microvascular perfusion.
- Blocks guanylate cyclase → reduces cGMP → decreases vasodilation.
- Mitochondrial effect: Acts as an electron carrier, potentially restoring mitochondrial function in dysfunction (e.g., metformin toxicity).
- ⛔️Contraindications
- If methylene blue is being use to treat shock
- Severe pulmonary hypertension (MB will increase pulmonary vascular resistance).
- Cardiogenic shock (↑afterload may promote pulmonary edema).
- Hypovolemic shock (↑afterload could reduce cardiac output).
- Risk of serotonin syndrome (e.g., recent use of selective serotonin reuptake inhibitors, high-dose fentanyl).
- MB inhibits monoamine oxidase A (MAO), increasing brain serotonin levels.
- G6PD deficiency (glucose-6-phosphate dehydrogenase deficiency)⎯personal or family history.
- MB works as an oxidizing agent at high doses (e.g., >7 mg/kg). This may cause methemoglobinemia.
- In patients with G6PD deficiency, this could also lead to hemolytic anemia.
- Poor IV access (relative contraindication):
- MB can cause tissue necrosis if it extravasates.
- Ideally, it should be infused via a central line.
- Dilution in larger volumes of carrier solution might also reduce this risk (e.g., 100 mg diluted in 500 ml saline).
- If methylene blue is being use to treat shock
- Side effects
- Serotonin syndrome.
- Hemolytic anemia.
- Methemoglobinemia.
- Blue discoloration of skin (may be confused with methemoglobinemia).
- Extravasation may cause skin necrosis.
- ⚠️Drug-Drug Interactions, see 👉 here
- Indications
- Refractory vasoplegic shock.
- Especially following cardiothoracic surgery.
- Possibly also: septic shock, anaphylaxis.
- Treatment of methemoglobinemia, with the following specific indications:
- Symptomatic methemoglobinemia.
- Methemoglobin level >30%.
- Possibly: Methemoglobin level >10-20% in patients who are less likely to tolerate reduced oxygen delivery.
- Metformin poisoning
- Refractory vasoplegic shock.
- Dosing
- 🦠 Septic Shock
- Typical dose: 1–2 mg/kg IV (e.g., 100 mg).
- Infusion time: 5 min to 6 hrs, depending on severity.
- Largest RCT: 100 mg in 500 mL saline over 6 hrs daily × 3 days *.
- Bolus effect may wane after 2–3 hrs.
- 🫀 Post-Cardiotomy Vasoplegic Shock
- Loading dose: 1–2 mg/kg IV over 15 min.
- Infusion: 0.5 mg/kg over 6 hrs.
- 🚨 Refractory Shock States
- 🩸 Methemoglobinemia
- Initial dose: 1–2 mg/kg IV over 5 min.
- Expect improvement within minutes.
- Cyanosis resolves within ~1 hr.
- Re-dosing:
- Repeat after 60 min if no response.
- Lack of response may suggest G6PD deficiency.
- Rebound methemoglobinemia: may need continuous infusion in severe cases.
- Initial dose: 1–2 mg/kg IV over 5 min.
- 🦠 Septic Shock
⎮⚠️ Important Warnings
- Rapid administration may cause false low pulse oximetry readings.
- Doses >7 mg/kg → risk of serious side effects:
- Nausea, vomiting, dyspnea, tremors, confusion, diaphoresis, hemolysis.
- Renal dysfunction: Consider dose adjustment.
Hydroxycobalamine
- Mechanism of action *
- Hydroxocobalamin is a synthetic B12 vitamin analog that is administered intravenously.
- Hydroxocobalamin inhibits nitric oxide directly and also inhibits nitric oxide synthase.
- It might also function by eliminating the endogenous vasodilator hydrogen sulfide (H2S).
- Indications
- Dosing
Angiotensin II
- Mechanism of action
- Angiotensin II binds to angiotensin II-receptor type-1 on the vascular smooth muscle cells → stimulates Ca/calmodulin-dependent phosphorylation of myosin → smooth muscle contraction and vasoconstriction.
- Angiotensin II stimulates the production of aldosterone.
- ⛔️Contraindications
- Non-vasodilatory shock states (e.g., cardiogenic shock).
- High risk for DVT/PE.
- Side effects
- Thrombotic events (both arterial and venous):
- DVT/PE.
- MI.
- Reduced cardiac output in selected patients: This share the same physiology as for phenylephrine and vasopressin.
- Bronchoconstriction may occur in susceptible patients.
- Peripheral edema
- Thrombotic events (both arterial and venous):
- Indication
- Refractory vasodilatory shock, especially:
- Post-cardiotomy vasoplegic syndrome (after the patient is on ≧15 mcg/min NE, ≧0.04 U/min vasopressin, methylene blue, and hydroxocobalamin).
- ACE-inhibitor overdose.
- The use of vasopressin appears to improve responsiveness to angiotensin II.
- In practice, angiotensin II should only be utilized among patients already on vasopressin.
- Refractory vasodilatory shock, especially:
- Dosing 💉
- Initial (Stabilization Phase):
- Start at 20 ng/kg/min.
- Titrate by 10–15 ng/kg/min every 5 min.
- Max initial dose: 80 ng/kg/min (up to 3 hours).
- Maintenance Phase:
- Continue only if responsive.
- Typical maintenance dose: ≤40 ng/kg/min.
- Can reduce to as low as 1.25 ng/kg/min.
- Initial (Stabilization Phase):
Top image: Synoptic view of the four types of shock (inner, white field) with the organ systems primarily associated with them (outer corners), sites and mechanisms of manifestation (outside the circle), and pathogenetic and pathophysiologic features (outer and middle sectors of the circle). To maintain clarity, mixed types of shock are not depicted. PMID: 30573009
Going further
- The Hypotensive ED Patient: A Sequential Systematic Approach (emDocs)
- Approach to shock (IBCC)
- An approach to undifferentiated hypotension (First 10 in EM)
- Algorithm for the Evaluation of Hypotension
- Undifferentiated shock (WikEM)
References
1.Tseng J, Nugent K. Utility of the shock index in patients with sepsis. Am J Med Sci. 2015 Jun;349(6):531-5. doi: 10.1097/MAJ.0000000000000444.
2. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: Lifeline. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 2017 Oct 17;136(16):e232-e268. doi: 10.1161/CIR.0000000000000525. Epub 2017 Sep 18.
3. Galbois A, Bigé N, Pichereau C, Boëlle PY, Baudel JL, Bourcier S, Maury E, Guidet B, Ait-Oufella H. Exploration of skin perfusion in cirrhotic patients with septic shock. J Hepatol. 2015 Mar;62(3):549-55. doi: 10.1016/j.jhep.2014.10.012. Epub 2014 Oct 19.
4. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017 Mar;45(3):486-552. doi: 10.1097/CCM.0000000000002255.
5. Kraut JA, Madias NE. Lactic acidosis. N Engl J Med. 2014 Dec 11;371(24):2309-19. doi: 10.1056/NEJMra1309483.
6. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016 Feb 23;315(8):801-10. doi: 10.1001/jama.2016.0287.
7. Mellhammar L, Linder A, Tverring J, Christensson B, Boyd JH, Sendi P, Åkesson P, Kahn F. NEWS2 is Superior to qSOFA in Detecting Sepsis with Organ Dysfunction in the Emergency Department. J Clin Med. 2019 Jul 29;8(8):1128. doi: 10.3390/jcm8081128.
8. Pitts SR, Niska RW, Xu J, Burt CW. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Natl Health Stat Report. 2008 Aug 6;(7):1-38.
9. Standl T, Annecke T, Cascorbi I, Heller AR, Sabashnikov A, Teske W. The Nomenclature, Definition and Distinction of Types of Shock. Dtsch Arztebl Int. 2018 Nov 9;115(45):757-768. doi: 10.3238/arztebl.2018.0757.
10. Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013 Oct 31;369(18):1726-34. doi: 10.1056/NEJMra1208943.
11. Righini M, Van Es J, Den Exter PL, Roy PM, Verschuren F, Ghuysen A, Rutschmann OT, Sanchez O, Jaffrelot M, Trinh-Duc A, Le Gall C, Moustafa F, Principe A, Van Houten AA, Ten Wolde M, Douma RA, Hazelaar G, Erkens PM, Van Kralingen KW, Grootenboers MJ, Durian MF, Cheung YW, Meyer G, Bounameaux H, Huisman MV, Kamphuisen PW, Le Gal G. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. 2014 Mar 19;311(11):1117-24. doi: 10.1001/jama.2014.2135. Erratum in: JAMA. 2014 Apr 23-30;311(16):1694.
12. Martindale JL, Wakai A, Collins SP, Levy PD, Diercks D, Hiestand BC, Fermann GJ, deSouza I, Sinert R. Diagnosing Acute Heart Failure in the Emergency Department: A Systematic Review and Meta-analysis. Acad Emerg Med. 2016 Mar;23(3):223-42. doi: 10.1111/acem.12878. Epub 2016 Feb 13.
13. Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: Rapid Ultrasound in SHock in the evaluation of the critically lll. Emerg Med Clin North Am. 2010 Feb;28(1):29-56, vii. doi: 10.1016/j.emc.2009.09.010
14. Atkinson PR, Milne J, Diegelmann L, Lamprecht H, Stander M, Lussier D, Pham C, Henneberry R, Fraser JM, Howlett MK, Mekwan J, Ramrattan B, Middleton J, van Hoving DJ, Peach M, Taylor L, Dahn T, Hurley S, MacSween K, Richardson LR, Stoica G, Hunter S, Olszynski PA, Lewis DA. Does Point-of-Care Ultrasonography Improve Clinical Outcomes in Emergency Department Patients With Undifferentiated Hypotension? An International Randomized Controlled Trial From the SHoC-ED Investigators. Ann Emerg Med. 2018 Oct;72(4):478-489. doi: 10.1016/j.annemergmed.2018.04.002. Epub 2018 Jun 2.
15. Gidwani H, Gómez H. The crashing patient: hemodynamic collapse. Curr Opin Crit Care. 2017 Dec;23(6):533-540. doi: 10.1097/MCC.0000000000000451.






Add comment